Abstract
The α5 subunit of the GABAA receptor is localized mainly to the hippocampus of the mammalian brain. The significance of this rather distinct localization and the function of α5-containing GABAA receptors has been explored by targeted disruption of the α5 gene in mice. The α5 −/− mice showed a significantly improved performance in a water maze model of spatial learning, whereas the performance in non-hippocampal-dependent learning and in anxiety tasks were unaltered in comparison with wild-type controls. In the CA1 region of hippocampal brain slices from α5 −/− mice, the amplitude of the IPSCs was decreased, and paired-pulse facilitation of field EPSP (fEPSP) amplitudes was enhanced. These data suggest that α5-containing GABAA receptors play a key role in cognitive processes by controlling a component of synaptic transmission in the CA1 region of the hippocampus.
Keywords: GABAA receptor, mouse, hippocampus, learning and memory, water maze, elevated plus maze, active avoidance, synaptic transmission, inhibitory postsynaptic current, paired pulse facilitation, long-term potentiation, benzodiazepine
GABAAreceptors are ligand-gated ion channels that are the major modulators of the inhibitory tone throughout the CNS. They are the site of action of a number of clinically important drugs, including benzodiazepines (BZs), barbiturates, and anesthetics (Whiting et al., 2001). GABAA receptors exist as a number of subtypes formed by the coassembly of gene family subunit polypeptides, the majority of which contain α, β, and γ subunits (McKernan and Whiting, 1996; Barnard et al., 1998). Each receptor subtype has a distinct pattern of expression within the mammalian brain, suggesting a defined physiological role (Wisden et al., 1992; Fritschy and Möhler, 1995).
Gene-targeting approaches in mice proved to be useful to gain clues about the function of individual GABAA receptor subunits. For example, it has been demonstrated that GABAA receptors containing an α1 subunit mediate sedative–muscle relaxant effects of benzodiazepines, whereas those containing an α2 or α3 subunit mediate the anxiolytic–anticonvulsant effects (Rudolph et al., 1999; Löw et al., 2000; McKernan et al., 2000). These results were obtained using knock-in mice in which individual GABAA receptor subunits were rendered diazepam-insensitive, but left normal otherwise, by introducing a His101 to Arg101 codon change into the murine α subunits genes.
Gene knock-outs of entire GABAA receptor subunits also contributed to our knowledge of their physiological role. Homozygous mice lacking the γ2 subunit of the GABAA receptor, which is the major γ subunit and is widely distributed throughout the brain, die shortly after birth. However, heterozygotes have a normal life expectancy and demonstrate neophobia when being exposed to a novel environment (Crestani et al., 1999). β3 −/− mice have cleft palate, epilepsy, and behavioral characteristics resembling Angelman syndrome, providing evidence that the lack of β3-containing GABAAreceptor subtypes contributes strongly to the overall severity of that human disease (Culiat et al., 1995; Homanics et al., 1997; DeLorey et al., 1998). Considering their great abundance in the brain, it was surprising to find that α1, β2, and α6 −/− mice, respectively, do not demonstrate major phenotypic abnormalities, which could be attributable to compensatory adaptations in the mutant animals (Jones et al., 1997; Sur et al., 2000; Brickley et al., 2001). Mice lacking the δ subunit are viable but show epileptic seizures and an attenuated sensitivity to neuroactive steroids (Mihalek et al., 1999).
The role of GABAA receptors containing an α5 subunit have remained primarily undefined. α5-containing receptors have a particularly restricted distribution, being primarily expressed in the dendritic fields of the hippocampus where they account for ∼20% of all GABAA receptors (Sur et al., 1998,1999). The localization to this region of the brain suggests that this GABAA receptor subtype may be involved in the physiological processes underlying learning and memory. In this study we have generated a mouse line with a disrupted α5 gene (α5 −/− mice) and investigated the performance of this mouse in models of spatial learning, as well as characterized the changes in hippocampal neurophysiology that result from the loss of α5-containing GABAA receptors.
MATERIALS AND METHODS
Generation of α5 −/− mice. A bacterial artificial chromosome (BAC) library (Research GeneticsInc.) containing genomic DNA sequences of 129SvEv line was screened using a DNA fragment as a probe, which was amplified by PCR using the following oligonucleotides: 5′-GAGCGAATCACGCAGGTGCGAACAGAC-3′ and 5′-GGTGCTGATGTTCTCAGTGCCTACTGT-3′. PCR conditions were described earlier (Soriano et al., 1991; Rosahl et al., 1993). Six positively hybridizing BAC clones were identified, and a 7.8 kbXbaI, a 6 kb BamHI/KpnI, and a 7.6 kbKpnI DNA fragment were subcloned into pBluescriptII. A multiple utility targeting vector was generated by cloning the 1.65-kb-long NotI/EcoRI cut pPGKneo cassette blunt-ended into the EcoRV site of the loxP site containing plasmid pBS246 (Sauer, 1993) and the 2-kb-long XbaI cut thymidine kinase (TK) cassette blunt-ended into the ScaI site of pBS246. A novel polylinker containing a BstXI,NheI, and HpaI recognition site was introduced into the EcoRI site of the multiple utility vector pBS246-neo-tk-1 (T. W. Rosahl and R. Cothliff, unpublished observations). The 7 kb long arm and 1.5 kb short arm were introduced via the ClaI and NheI cloning sites of pBS246-neo-tk-1.
The targeting vector was linearized with HpaI and introduced into AB2.1 embryonic stem (ES) cells as described (Rosahl et al., 1993). Homologous recombinants were identified by using the following PCR primers: P1: 5′-CCCTACTAGCTATGGGGGCTCCATTCT-3′ and P2: 5′-GGATGCGGTGGGCTCTATGGCTTCTGA-3′. Correctly targeted ES clones were injected into blastocysts derived from C57/BL6 mice. Two chimeric males derived from one of the three injected ES clones gave rise to germline transmission of the targeted mutation. After further breeding, a colony of wild-type (WT) and homozygous α5 −/− mice were generated in a mixed 50% C57BL6 and 50% 129SvEv genetic background. Animals of the F3 generation only were used for this study.
Radioligand binding and Western blot analysis. For [3H]L-655,708 autoradiography, mice were killed by decapitation, and brains were removed and frozen in cold isopentane. Coronal sections (10–14 μm) were cut using a cryostat, air-dried, thaw-mounted, and stored at −80°C. Slide-mounted sections were washed 30 min in ice-cold Tris–EDTA (10:1 mm), pH 7.4, buffer and incubated at 4°C for 2 hr in buffer containing 4 nm[3H]L-655,708 (Quirk et al., 1996; Sur et al., 1998, 1999) plus 10 μm zolpidem. Labeled sections were washed two times for 1 min each in Tris–EDTA buffer, dipped in distilled water, dried in cold air, and then exposed for 8 weeks to [3H]Hyperfilm (Amersham Biosciences, Arlington Heights, IL). Image analyses were performed with MCID device (Imaging Research Inc., St. Catharines, Ontario, Canada).
Western blot analysis was performed using membranes prepared from hippocampi from WT and α5 −/− mice. Membrane preparations (Sur et al., 1998) (30 μg of protein) were electrophoresed on 4–12% gradient acrylamide gel (NuPage Bis-Tris gel, Novex), transferred to nitrocellulose membrane (Hybond; Amersham Pharmacia Biotech) and incubated with a specific polyclonal α5 subunit antibody (Sur et al., 1998) (5 μg/ml) overnight at 4°C. Antibody binding was visualized with a goat anti-rabbit antibody coupled to peroxidase and ECL chemiluminescence (Amersham Biosciences).
Radioligand binding to membranes prepared from hippocampi from WT and α5 −/− mice using [3H]Ro15–1788 (NEN, Boston, MA) and [3H]L-655,708 was performed as described previously (Quirk et al., 1996; Sur et al., 1998).
Water maze tests. The water maze used is a 1-m-diameter circular pool filled with an opaque mixture of water and white dye (E308, Morton) maintained at 26–28°C. The three-dimensional spatial extra maze cues were fixed to the walls and hung with string from the structural framework. Animal movements were captured by closed-circuit video camera mounted directly above the center of the pool and analyzed using a Hampton Video Systems Image software system (Hampton Video Systems, Buckingham, UK). In the “matching-to-place” version of the water maze test, the position of the platform was altered daily. Animals were given four trials daily for 10 d, and the position of the hidden platform in order of days was as follows: northeast (NE), southwest (SW), Center, east (E), northwest (NW), northeast (NE), west (W), SW, Center, NE. For the first two trials on each day, the animals were placed in the pool at the most distal points from the platform position. The maximum trial length was 60 sec. If by that time the mouse had not climbed on to the platform, the trial ended automatically, the experimenter placed the mouse on the platform, and an escape latency of 60 sec was recorded. The mouse remained on the platform for 30 sec and was then removed to a high-sided opaque plastic container for a further 30 sec [intertrial interval (ITI)]. At the end of the ITI the mouse was placed into the pool again, but at a different location and after release the next trial began. This procedure was repeated until four trials had been completed. The improvement in the animals' memory was quantified by subtracting the trial 2 latency from the trial 1 latency to give the savings score. Statistically significant differences were determined using two-way ANOVA.
Elevated plus-maze. The apparatus consisted of a square partitioned off area measuring 160 × 160 cm with the walls painted white. The elevated plus-maze consisted of four arms (5 × 27.5 cm) joined by a central area (5 × 5 cm). Two opposite arms were enclosed by 30-cm-high walls, and the other two arms were open. The maze was elevated to a height of 50 cm. The floor of the maze was covered with a single piece of white rubber sheeting. The maze was placed in the center of the room, and fluorescent strip lights covered with polarizing film illuminated the apparatus. A camera fitted with a wide angle lens and covered with a polarizing lens cap was mounted above the maze. This relayed images to a VP200 advanced tracker (Hampton Video Systems Image), which in turn relayed digitized data to a personal computer running Hampton Video Systems Image plus-maze software.
Mice were placed on the central area of the maze facing an open arm and were allowed to explore the maze for 5 min. After the 5 min trial, the mouse was returned to its home cage. The maze was wiped clean using water and a paper towel. Statistically significant differences were determined using two-way ANOVA.
Two-way active avoidance. Each training session consisted of placing individual animals in a two-compartment shuttle box with a grid floor that operates on a tilt mechanism to detect the location of the animal. Each compartment had its own light source, and both halves were connected by an open doorway. At the beginning of each trial, the animal is exposed to light [conditional stimulus (CS)], which signals an unconditional stimulus (US) (footshock: 0.4 mA for 10 sec) delivered 10 sec later. Animals can actively avoid receiving footshock by crossing into the opposite (dark) compartment during the conditional stimulus (light). Alternatively, crossing compartments after initiation of footshock terminates shock delivery and is recorded as an escape. If an animal did not move during the 10 sec CS or the following 10 sec US, an unmoved response was recorded. Each session consisted of 15 light–shock trials with an ITI of 25–40 sec. Training was conducted in this manner for 12 d. Statistically significant differences were determined using two-way ANOVA.
Electrophysiology. Brain slices were prepared from WT and α5 −/− mice (5- to 6-month-old for long-term potentiation (LTP) studies, postnatal 14- to 32-d-old for kinetics studies), in accordance with the UK Animals (Scientific Procedures) Act (1986), as previously described (Seabrook et al., 1999). To eliminate bias, the experimenters remained blind to the genotype of the animal during recording and all analysis.
For the LTP studies, field EPSPs were recorded using extracellular recording as previously described (Seabrook et al., 1999). Briefly, test stimuli were applied every 30 sec, slopes were calculated on-line and allowed to stabilize so that baseline values varied by not >5% for a minimum of 30 min. LTP was induced by a theta-burst protocol (four pulses at 100 Hz repeated 10 times at an interval of 200 msec).
For the kinetics studies, evoked and spontaneous IPSCs were recorded using whole-cell patch clamp recording (Vh = −60 mV at 22°C) as previously described (Jarolimek and Misgeld, 1997). A patch pipette filled with (in mm): 165 NaCl, 2 KCl, 1.67 CaCl2, 1 MgCl2, 17d-glucose, and 10 HEPES, pH 7.3, was used to electrically stimulate inhibitory axons close to the pyramidal cell body layer (5–30 V for 0.02–0.1 msec). To minimize current spread to distal dendrites, the stimulation intensity was set just above the value that evoked ∼50% failures. Recording electrodes were filled with (in mm): 150 KCl, 2 MgCl2, 0.1 CaCl2, 11 EGTA, 10 HEPES, and 2 MgATP, 5 lidocaine N-ethyl bromide (QX-314), pH adjusted to 7.3 with KOH. Peak amplitudes, rise and decay time constants of IPSCs were measured from individual and 6–10 averaged traces using pClamp 8.0 software (Clampfit; Axon Instruments, Foster City, CA) as previously described (Jarolimek and Misgeld, 1997). Briefly, the decay of IPSCs was fitted with a double-exponential function using the Levenberg–Marquardt nonlinear least square algorithm. A decay was considered monoexponential when τ1 was similar to τ2(± 10%), the weighting factor of τ1 was 10× larger than the weighting factor of τ2 or τ1 was >500 msec. Data are presented as the mean and SEM.
RESULTS
Generation and validation of α5 −/− mice
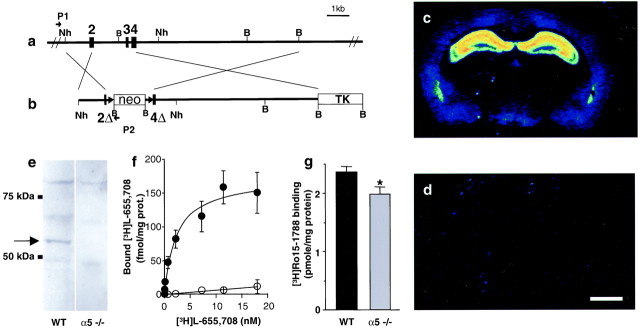
Mice deficient for the α5 subunit of the GABAA receptor were generated by gene targeting (Fig. 1a,b). Quantitative autoradiographic analyses with [3H]L-655,708, an α5-selective BZ site ligand (Quirk et al., 1996; Sur et al., 1998, 1999), demonstrated the absence of α5 BZ binding site containing GABAAreceptors in the brains of α5 −/− mice (Fig. 1c,d). Furthermore, Western blot analysis demonstrated the absence of an α5-specific band at 55 kDa in membranes from hippocampi of α5 −/− mice (Fig. 1e). Finally, saturation experiments confirmed the lack of high-affinity [3H]L-655,708 binding sites (WT, Kd, 3.25 ± 1.26 nm, mean ± SEM, n = 5) in gene-targeted animals (Fig. 1f). Radioligand binding experiments with [3H]Ro15–1788, which binds with high affinity to the BZ site of α1-, α2-, α3-, and α5-containing receptors, revealed a significant reduction (−16%; Student's t test, p < 0.034) in the total number of BZ sites in α5 −/− hippocampus (WT,Bmax, 2.36 ± 0.09 pmol/mg protein; α5 −/−, Bmax, 1.98 ± 0.12 pmol/mg protein; mean ± SEM, n = 6). This reduction in total [3H]Ro15–1788 binding sites is consistent with the proportion of α5 receptors present in rat hippocampus (Sur et al., 1998) and suggests that the lack of α5 subunit in α5 −/− mice is not compensated by an upregulation of α1, α2, or α3 subunits (Fig. 1g). The pharmacology of hippocampal BZ sites remaining in α5 −/− mice was unchanged, with similar affinity for Ro15–1788 (WT,Kd, 1.93 ± 0.35 nm; α5 −/−,Kd, 1.98 ± 0.14 nm; mean ± SEM, n = 6) and flunitrazepam, another BZ site ligand (WT,Ki, 4.9 ± 0.85 nm; α5 −/−,Ki, 4.6 ± 0.82 nm; mean ± SEM, n = 3).
Fig. 1.
Generation and validation of α5 −/− mice.a, b, Schematic representation of the WT α5 allele of the GABAA receptor and the targeting vector, respectively.2, 3,4, Exons 2, 3, and 4; 2Δ,4Δ, partial deletion of exons 2 and 4; neo, neomycin resistance gene;TK, thymidine kinase gene; Nh,NheI restriction site; B,BamHI restriction site; P1,P2, PCR primers. c–g, Pharmacological and biochemical characterization of α5-deficient mice. c, d, Color-coded autoradiograms for [3H]L-655,708 binding to mouse brain sections reveals binding to the hippocampus in the WT (c) and absence of signal in the α5 −/− mice (d). Scale bar, 1 mm. e, Western blot shows the absence of a specific α5 subunit band at 55 kDa in membranes from α5 −/− hippocampus. f, Saturation experiment demonstrating the absence of high-affinity [3H]L-655,708 binding sites in α5 −/− mice (open circles) compared with WT mice (closed circles). g, Total number of [3H] BZ sites labeled by [3H]Ro15–1788 is reduced (−16%) in α5 −/− mice compared with WT controls.
Enhanced performance of α5 −/− mice in the water maze
Homozygous α5 −/− mice display no overt phenotypic abnormalities, have a normal life span, breed normally, and do not exhibit spontaneous seizures (data not shown). In tests evaluating motor performance and coordination (by testing their ability to balance on beams of various sizes and their ability to stay on a rotating rod) α5 −/− mice were indistinguishable from WT mice (data not shown). To investigate the role of α5-containing GABAAreceptors in learning and memory, we determined the performance of the α5 −/− mice in the water maze, a hippocampal-dependent test of cognition (Morris 1984).
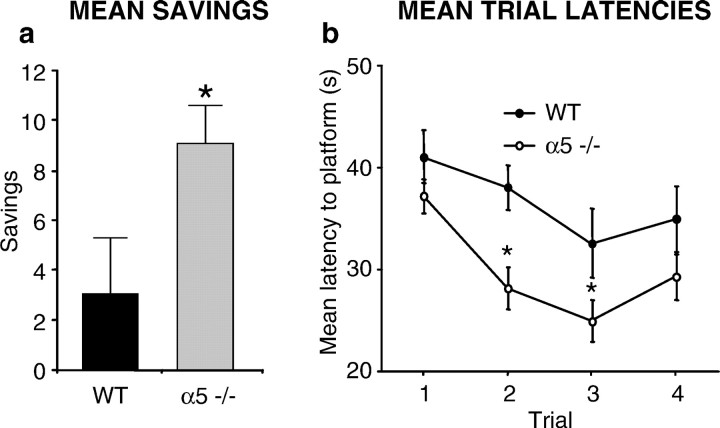
The “matching-to-place” version of the water maze was used, in which the position of the submerged platform in the pool varies each day, but remains in the same location for the duration of that day (Steele and Morris, 1999). The mouse is required to find the platform four times each day. On the first trial of each day the mouse must search the 1-m-diameter pool for the hidden platform. If it locates the hidden platform more rapidly on trials 2, 3, and 4, this indicates that the mouse has remembered the platform position on trial 1. The improvement in memory can be quantified by calculating the difference between the time taken to find the platform on trial 1 compared with subsequent trials. Figure 2ashows a significant improvement in performance between trial 1 and trial 2 for the α5 −/− compared with WT mice (p < 0.05). Data from a 10 d period of testing shows a significant difference in performance in trial 2 (p < 0.005) and trial 3 (p < 0.05) between α5 −/− and WT mice (Fig.2b). There was no significant effect of swim speed between α5 −/− and WT mice (F(1.36) = 0.54; p = 0.465), and no significant differences in the performances between males and females of both genotype groups were observed.
Fig. 2.
Enhanced performance of α5 −/− mice in the matching-to-place version of the water maze test. Eighteen WT and 20 α5 −/− mice were used in this test. a, The difference in time taken between trial 1 and trial 2 (savings) to find the hidden platform over the 10 d testing period is shown. α5 −/− mice made significantly higher savings compared with WT mice.b, α5 −/− mice were significantly quicker (*) at finding the hidden platform for both trial 2 and trial 3.
Normal performance of α5 −/− mice in the two-way active avoidance and elevated plus maze tasks
Elevated plus-maze
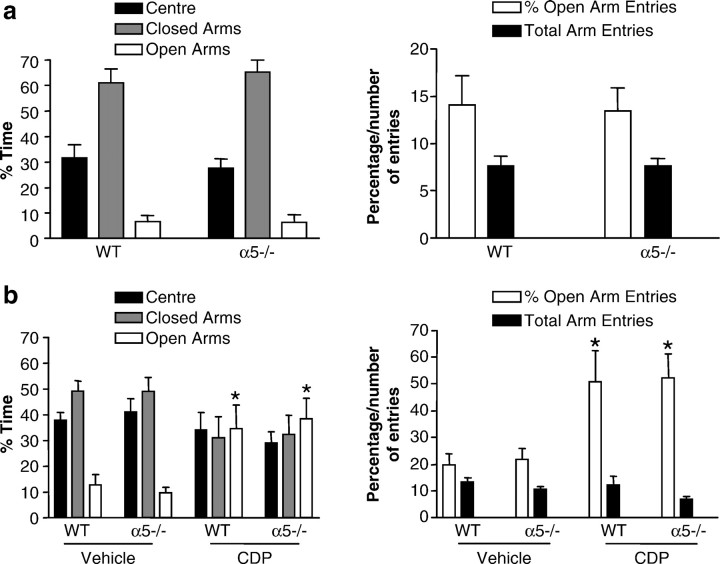
Differences in anxiety levels could potentially serve as confounding factors during studies of cognition and memory. To determine whether α5 −/− animals have a higher predisposition to elevated anxiety levels, the mice were evaluated using the elevated plus-maze, an unconditioned model of anxiety (Lister, 1987). This apparatus consists of two open arms and two enclosed arms in a “+” shape with an open roof and elevated at a height of 50 cm. Mice are allowed to explore the apparatus for 5 min. Usually, mice prefer the closed arms of the maze to the open arms, and they spend a significantly greater amount of time in the closed arms, and enter them more frequently than the open arms. Clinically effective anxiolytics such as chlordiazepoxide (CDP) increase the time spent in an the entries made to the open arms (Pellow et al. 1985).
The α5 −/− and WT mice were tested on the elevated plus-maze (Fig.3a). The results demonstrated that α5 −/− mice do not appear to have elevated background anxiety levels on the plus-maze, spending comparable times in the open and closed arms to the WT mice. A second experiment was designed to determine whether α5 −/− mice showed altered sensitivity to the anxiolytic-like effects of the BZ CDP (which does not have selectivity for any of the major GABAA receptor subtypes). CDP lead to anxiolytic-like effects in both α5 −/− and WT mice on the plus-maze (Fig. 3b). There was no significant effect of sex in any of the plus-maze studies, and thus this grouping factor was collapsed.
Fig. 3.
Normal performance of the α5 −/− mice in the elevated plus maze. a, Data shown are mean (±SEM) values for percentage time spent in (left panel) and number of entries made to (right panel) different areas of the elevated plus-maze by WT and α5 −/− mice (n = 20–23). There were no significant differences between WT and α5 −/− mice on any of the measures. b, Data shown are mean (± SEM) values for percentage time spent in (left panel) and number of entries made to (right panel) different areas of the elevated plus-maze by 10.0 mg/kg CDP or vehicle-treated WT and α5 −/− mice (n = 13–20). CDP had a significant anxiolytic-like effect of the same magnitude in both WT and α5 −/− mice (*). This is indicated by the increase in the percentage of time spent in (p < 0.01) and percentage of entries made to (p < 0.01) the open arms of the plus-maze in comparison with vehicle-treated WT and α5 −/− mice.
Two-way active avoidance
In two-way active avoidance studies, dangerous and safe areas are constantly interchanged, most commonly using a shuttle box. In a typical experiment the animal is on one side of the two-compartment apparatus when a warning signal (light or tone) is presented. At some time after the onset of this stimulus a shock is delivered, unless the animal has by then moved to the other side of the apparatus. If it is still on the original side, it can escape shock by running to the other side. The warning signal is itself normally terminated by the animal crossing to the other side, whether by successful avoidance or on escape trials. The avoidance response then, consists of crossing to the other side of the box, taking the animal back to the side from which it ran away on the previous trial. The successful performance of animals with hippocampal lesions in two-way active avoidance (Gray, 1982) suggests that this task is not hippocampal-dependent. α5 −/− and WT mice were tested in a two-way active avoidance task over a period of 12 d (Fig. 4). The performance of α5 −/− mice was not different than that of WT mice. However, there was a significant effect of sex with female mice performing at a level inferior to that of the male mice (p < 0.05). Subsequent studies revealed that this could be attributable to an increase in sensitivity to footshock in and/or a decrease in spontaneous locomotor activity in the female mice (data not shown).
Fig. 4.
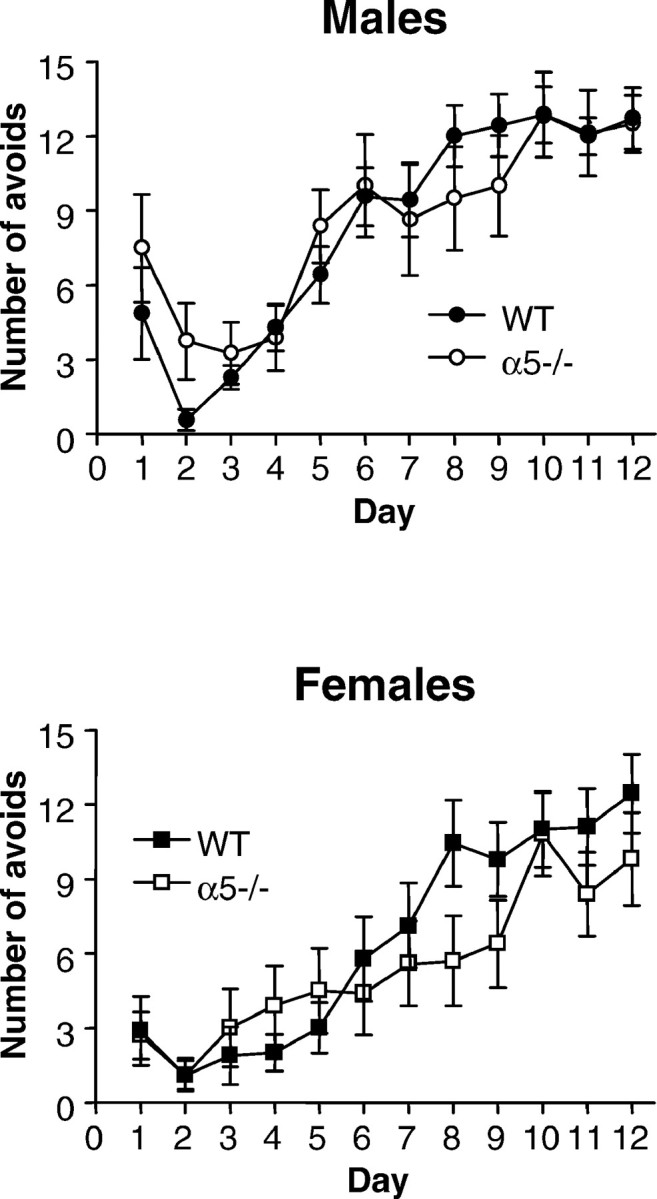
Normal performance of the α5 −/− mice in the non-hippocampal-dependent two-way active avoidance. Data shown are mean (± SEM) avoid responses recorded on each of 12 d by untreated male (top) and female (bottom) wild-type (WT) and GABAA receptor α5 knock-out (α5 −/−) mice (n = 7–10). There was no significant difference between WT and α5 −/− mice but a significant effect of sex (p < 0.05).
Enhanced paired pulse facilitation, but normal long-term potentiation, in α5 −/− mice
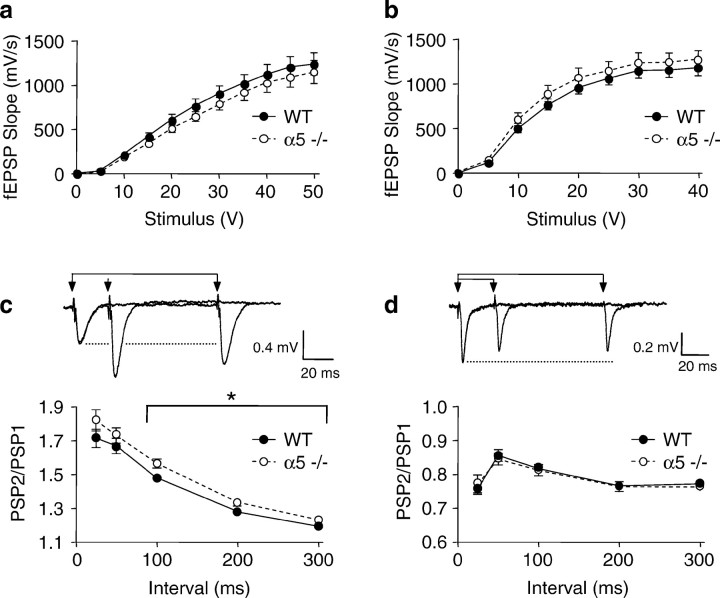
To determine whether α5 −/− mice have alterations in synaptic function within the hippocampus we examined the effect on low-frequency synaptic transmission, on paired-pulse facilitation (PPF) and depression (PPD) in the CA1 region and dentate gyrus, respectively, and the ability of high-frequency stimuli to induce LTP. No difference was found in the ability of low-frequency stimuli to activate fEPSPs in either the CA1 or dentate gyrus. The maximal fEPSP strength in WT and α5 −/− mice was 1241 ± 127 and 1155 ± 132 mV/sec, respectively, in the CA1 region (Fig.5a), and 1188 ± 93 and 1270 ± 100 mV/sec, respectively, in the dentate gyrus (Fig.5b). However, the ability of paired-pulse stimuli to facilitate the amplitude of synaptic potentials was significantly enhanced in α5 −/− mice (ANOVA with repeated measures for intervals 100–300 msec: p = 0.02,F(1.92) = 5.58, n = 46 and 48 slices from WT and α5 −/− mice, respectively) (Fig.5c). This effect was specific to the amplitude of the postsynaptic potential, a parameter that is regulated by postsynaptic GABAA receptors (Pananceau et al., 1997), because the enhancement of fEPSP slopes over the same paired-pulse interval range of field EPSP slopes was unaffected (ratio of PSP2/PSP1; PPF at 100 msec = 1.52 ± 0.03 in WT and 1.52 ± 0.03 in α5 −/− mice). This alteration was specific to the CA1 region, because deletion of the α5 subunit had no effect on the ability of paired-pulse stimuli to induce synaptic depression in the dentate gyrus (Fig. 5d), when either the amplitude or slope of fEPSPs were measured (PPD at 100 msec = 0.82 ± 0.01 in WT and 0.81 ± 0.02 in α5 −/− mice for amplitude, and for slopes the ratios were 0.83 ± 0.01 in WT and 0.83 ± 0.01 in α5 −/− mice;n = 41 and 42 slices, respectively). This may reflect the regional localization of α5-containing receptors, which are considerably more abundant in the CA1–CA3 regions than in the dentate gyrus where other GABAA receptor subtypes predominate (Sperk et al., 1997). In contrast with the enhancement of PPF in the CA1 region of α5 −/− mice, there was no significant enhancement of LTP induced by either theta burst stimulation alone (LTP at 60 min after stimulus = 128 ± 6% of control in WT and 141 ± 7% of control in α5 −/− mice; n = 32 and 38 slices, respectively) (Fig. 6) or by a brief tetanus followed by a theta burst (LTP at 60 min after stimulus = 235 ± 20% of control in WT and 195 ± 11% of control in α5 −/− mice; n = 10 and 9 slices, respectively).
Fig. 5.
Synaptic strength in hippocampal brain slices of α5 −/− mice compared with its effect on paired-pulse facilitation in CA1 region and paired-pulse depression in the dentate gyrus. In all figures the filled circles represent data from age-matched WT mice, and open circles are from α5 −/− mice. No significant difference was found in the maximal fEPSP amplitude in the CA1 region. a, Forty-six slices from 16 WT controls and 48 slices from 16 α5 −/− mice or dentate gyrus.b, Forty-one slices from 16 WT controls and 42 slices from 16 α5 −/− mice. In the CA1 region the amplitude of fEPSPs was significantly enhanced during paired-pulse stimulus intervals of 100–300 msec. c, Filled circles, 46 slices from 16 WT controls; open circles, 48 slices from 16 α5 −/− mice. In comparison, paired-pulse depression of fEPSPs in the dentate gyrus remained unaffected. d, Filled circles, 41 slices from 16 WT controls; open circles, 42 slices from 16 α5 −/− mice.
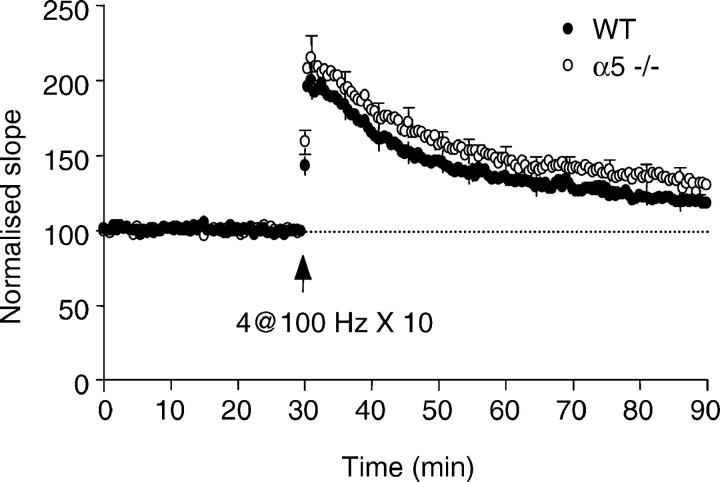
Fig. 6.
Long-term potentiation in CA1 region of hippocampal brain slices from α5 −/− mice. LTP was induced by theta burst (4 stimuli at 100 Hz repeated 10 times every 200 msec). The amount of LTP was not different between WT and α5 −/− mice (filled circles, 32 slices from 8 WT mice;open circles, 38 slices from α5 −/− mice) at 1 hour after the theta burst (p = 0.18;F(1,68) = 1.82).
Altered sIPSCs kinetics in α5-deficient mice
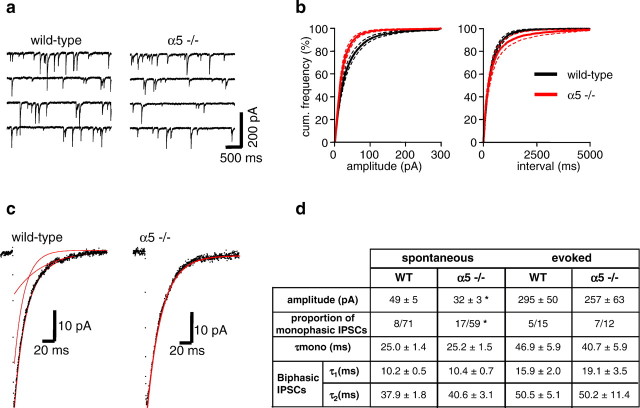
Spontaneous IPSCs (sIPSCs) were recorded from CA1 pyramidal neurons in brain slices in the presence of glutamate and GABAB receptor blockers (see Materials and Methods) (Fig. 7a). The amplitude of sIPSCs varied over a large range (8 to >200 pA), suggesting that both proximal and distal release sites were activated (Kapur et al., 1997). In WT mice, sIPSCs occurred at a mean frequency of 3.0 ± 0.3 Hz, a mean rise time of 1.4 ± 0.1 msec, a mean amplitude of 48.6 ± 5.4 pA, and had a reversal potential of ∼0 mV consistent with GABA-mediated chloride currents (n = 15 hippocampal slices). The characteristics of sIPSCs recorded in WT mice were entirely consistent with previous studies conducted in the rat hippocampus (Ropert et al., 1990). Deletion of the α5 subunit in α5 −/− mice had no effect on the frequency (3.2 ± 0.7 Hz) or rise time (1.5 ± 0.1 msec) of sIPSCs (n = 13 hippocampal slices). In contrast, the mean peak amplitude of sIPSCs were significantly smaller (67% of WT; p < 0.01 using Student's t test) in α5 −/− mice (32.4 ± 3.1 pA;n = 13 slices) (Fig. 7b,d). Similarly, local electrical stimulation within the pyramidal cell layer evoked IPSCs with a smaller mean peak amplitude (87% of WT) in α5 −/− mice (257 ± 63 pA in α5 −/− mice and 295 ± 50 pA in WT mice; n = 12 and 15 slices, respectively) (Fig.7d). This reduction in amplitude is consistent with the loss of α5-containing GABAA receptors in the hippocampus of these mice as determined by radioligand binding (discussed above).
Fig. 7.
Characteristics of IPSCs recorded from CA1 pyramidal neurons in hippocampal slices in vitroprepared from WT and α5 −/− mice. a, Examples of recordings of membrane current from CA1 neurons in WT and α5 −/− mice at a holding potential of −60 mV. The regular downward deflections are sIPSCs. b, Cumulative frequency distributions of the sIPSC amplitude and interevent interval in WT (black;n = 15 slices) and α5 −/− mice (red;n = 13 slices), showing a clear reduction in IPSC amplitude but no change in IPSC frequency in the α5 −/− mice. c, Representative sIPSCs recorded from CA1 pyramidal neurons in hippocampal brain slices from WT and α5 −/− mice. In the examples shown, the decay of the sIPSC recorded from the WT mouse was best fitted to a double-exponential function, whereas the decay of the sIPSC recorded from the α5 −/− mouse was best fitted to a monoexponential function (decay fits superimposed in red).d, Summary of the mean data of spontaneous and evoked IPSC mean peak amplitude and decay kinetics from WT and α5 −/− mice.
The rate of sIPSC decay has previously been shown to be highly sensitive to modulators of the GABAAreceptor (Segal and Barker, 1984; Strecker et al., 1999). To determine whether the rate of IPSC decay was affected by deletion of the α5 subunit, IPSCs were averaged and the decay fitted with a mono- or double-exponential function (Fig. 7c). Consistent with previous studies, the time course of the decay of IPSCs was usually best fit by a sum of two exponentials (Pearce, 1993). There were no significant differences between the time constants of mono-exponential decays and fast and slow double-exponential decays of sIPSCs or evoked IPSCs between WT and α5 −/− mice (Fig. 7d). However, in α5 −/− mice, 29% of sIPSCs were best fitted to a monoexponential function (17 of 59 averaged sIPSCs from 13 slices), whereas in the WT animals significantly fewer sIPSCs were monophasic (8 of 71 averaged sIPSCs from 15 slices; p < 0.02 using Fisher's exact test). Similarly, in α5 −/− mice 29% of evoked IPSCs were best fitted to a monoexponential function (7 of 12), whereas in the WT animals fewer sIPSCs were monophasic (5 of 15). Thus, fewer cells in α5 −/− mice exhibited a fast inactivating component to the IPSC compared with WT (Fig. 7d).
DISCUSSION
In this study we have used a gene disruption approach to investigate the function of α5-containing GABAAreceptors. The restricted localization of α5, primarily to the dendritic regions of the CA1–CA3 fields of the hippocampus, suggests the possibility that this subtype may influence or contribute to the molecular processes underlying learning and memory. Indeed, disruption of the α5 gene lead to an improved performance in the water maze model of spatial learning, suggesting that this is the case. In contrast, and in agreement with the mainly hippocampal localization of α5-containing GABAA receptors, the performance in a non-hippocampal-dependent learning task was unaltered in α5-deficient mice, as determined in the passive avoidance assay. Moreover, the shorter latency times to find the hidden platform in the Morris water maze were not caused by an increased anxiety level of the α5 −/− mice, as shown by the data on the elevated plus maze. Therefore, deletion of the α5 gene seems to affect specifically hippocampal-dependent learning and memory. However, it cannot be ruled out entirely, that potential abnormalities may exist in other relevant brain subregions such as the amygdala and neocortex, where the α5 subunit of the GABAA receptor is also expressed, but at a very low level compared with other GABAAreceptor α subunits.
What could be the underlying mechanism of altered synaptic transmission resulting in the enhanced performance of the α5−/− mice in the water maze model of spatial learning? The α5-containing GABAA receptors would be predicted to contribute to GABA-mediated inhibitory synaptic currents in the hippocampus, and so one possible effect of disruption of the α5 gene would be a reduction in the amplitude of IPSCs. Indeed, the amplitude of sIPSCs was significantly smaller in α5 −/− mice compared with WT, with a 33% reduction in the mean peak amplitude (from 48.6 to 32.4 pA). This value is in accord with the reduction of GABAAreceptors in the hippocampus of α5 −/− mice, as determined by a radioligand binding assay. An additional characteristic of inhibitory synaptic currents that may be altered after the disruption of a GABAA receptor subunit gene is the current inactivation time constant. The decay of spontaneous IPSCs in CA1 pyramidal cells can be fitted with mono- or double-exponential algorithm (Roepsdorff and Lambert, 1994; Pearce et al., 1995). The two time constants may reflect populations of GABAAreceptors that have different channel inactivation time constants (Roepsdorff and Lambert, 1994). Because the lack of α5-containing receptors does not change the average decay time constants for either mono- or bi-exponential sIPSCs, it can be concluded that the overall decay of hippocampal sIPSCs is not dominated by α5-containing GABAA receptors, in line with their relative abundance. Interestingly, however, the relative proportion of cells with inhibitory synaptic currents that had a fast component to the IPSC inactivation was significantly less in α5−/− mice (71%) compared with WT mice (89%). This, coupled with a significant reduction in the peak amplitude of sIPSCs, is consistent with the enhanced ability of paired pulse stimuli to facilitate the amplitude rather than the initial slope of synaptic potentials in the α5 −/− mice.
Enhancement of learning and memory coincident with increased LTP has been described for transgenic mice overexpressing the NR2B subunit of the NMDA-type glutamate receptor (Tang et al., 1999) and also in mice lacking the nociceptin receptor (Manabe et al., 1998). However, two different versions to induce theta burst long-term potentiation failed to reveal significant abnormalities in the α5 −/− mice, although there was a tendency toward an enhancement of LTP (Fig. 6). One interpretation is that the increase in PPF and/or decrease of the peak amplitude of sIPSCs alone is sufficient to support some forms of enhanced learning and memory. However, other forms of synaptic plasticity within the hippocampus may also contribute to the enhanced cognitive performance observed in this study. There is considerable contention regarding the relationship between LTP and learning and memory, particularly in the context of transgenic models, and this study adds to that controversy. Although some studies show coordinate deficits in cognitive tasks and LTP (Grant et al., 1992; Abeliovich et al., 1993; Tsien et al., 1996), others fail to show such a relationship (Silva et al., 1996; Meiri et al., 1998; Migaud et al., 1998; Zamanillo et al., 1999). Similarly, deficits in PPF in α calcium calmodulin kinase II knock-out mice have been reported to be associated with impairment in spatial learning (Silva et al., 1996), whereas others have described enhanced PPF in synapsin I knock-out mice (Rosahl et al., 1993) in the absence of any concomitant change in cognitive performance (Silva et al., 1996).
Therefore, we suggest here that the decreased GABA-mediated synaptic inhibition in the hippocampus, as shown by the reduced peak amplitudes and slower decay times of sIPSCs, is the most likely underlying mechanism for the enhanced performance of α5-deficient mice in the learning and memory task. Our behavioral and electrophysiological evidence suggest a central role of the α5 subtype of the GABAA receptor in cognitive processes. The discrete localization of the α5 subtype mainly in the hippocampus and the excellent sensitivity of the inhibitory GABAergic system to pharmacological intervention make the α5-containing GABAA receptor an attractive novel target for treatment of disorders associated with cognitive defects.
N.C., F.M.K., W.J., K.A.M., and R.C. contributed equally to different aspects of this work.
Correspondence should be addressed to Dr. Thomas W. Rosahl, Merck Sharp and Dohme, Neuroscience Research Center, Terlings Park, Eastwick Road, Harlow, Essex, CM20 2QR, UK. E-mail: thomas_rosahl@merck.com.
C. Sur's present address: Merck & Co., Department of Neuroscience, West Point WP26A-3000, Sumneytown Pike, P.O. Box 4, West Point, PA 19486.
REFERENCES
- 1.Abeliovich A, Paylor R, Chen C, Kim JJ, Wehner JM, Tonegawa S. PKC gamma mutant mice exhibit mild deficits in spatial and contextual learning. Cell. 1993;75:1263–1271. doi: 10.1016/0092-8674(93)90614-v. [DOI] [PubMed] [Google Scholar]
- 2.Barnard EA, Skolnick P, Olson RW, Möhler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- 3.Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent K+ conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- 4.Crestani F, Lorez M, Baer K, Essrich C, Benke D, Laurent JP, Belzung C, Fritschy J-M, Luscher B, Möhler H. Decreased GABAA receptor clustering results in enhanced anxiety and a bias for threat cues. Nat Neurosci. 1999;2:833–839. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- 5.Culiat CT, Stubbs L, Woychik RP, Russell LB, Johnson DK, Rinchik EM. Deficiency of the β3 subunit of the type A γ-aminobutyric acid receptor causes cleft palate in mice. Nat Genet. 1995;11:344–346. doi: 10.1038/ng1195-344. [DOI] [PubMed] [Google Scholar]
- 6.DeLorey TM, Handforth A, Anagnostaras SG, Homanics GE, Minassian BA, Asatourian A, Fanselow MS, Delgado-Escueta A, Ellison GD, Olson RW. Mice lacking the β3 subunit of the GABAA receptor have the epilepsy phenotype and many of the behavioral characteristics of Angelman syndrome. J Neurosci. 1998;18:8505–8514. doi: 10.1523/JNEUROSCI.18-20-08505.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fritschy JM, Möhler H. GABAA receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 8.Grant SG, O'Dell TJ, Karl KA, Stein PL, Soriano P, Kandel ER. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992;258:1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- 9.Gray JA. The behavioural effects of septal and hippocampal lesions. In: Broadbent DE, McGaugh JL, Mackintosh NJ, Posner MI, Tulving E, Weiskrantz L, editors. The neuropsychology of anxiety: an inquiry into the functions of the septo-hippocampal system. Oxford UP; Oxford: 1982. pp. 115–183. [Google Scholar]
- 10.Homanics GE, DeLorey TM, Firestone LL, Quinlan JJ, Handforth A, Harrison NL, Krasowski MD, Rick CEM, Korpi ER, Makela R, Brilliant MH, Hagiwara N, Ferguson C, Snyder K, Olsen RW. Mice devoid of γ-aminobutyrate type A receptor β3 subunit have epilepsy, cleft palate, and hypersensitive behavior. Proc Natl Acad Sci USA. 1997;94:4143–4148. doi: 10.1073/pnas.94.8.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarolimek W, Misgeld U. GABAB receptor-mediated inhibition of tetrodotoxin-resistant GABA release in rodent hippocampal CA1 pyramidal cells. J Neurosci. 1997;17:1025–1032. doi: 10.1523/JNEUROSCI.17-03-01025.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones A, Korpi ER, McKernan RM, Pelz R, Nusser Z, Makela R, Mellor JR, Pollard S, Bahn S, Stephenson FA, Randall AD, Sieghart W, Somogyi P, Smith AJH, Wisden W. Ligand-gated ion channel subunit partnerships: GABAA receptor α6 subunit gene inactivation inhibits δ subunit expression. J Neurosci. 1997;17:1350–1362. doi: 10.1523/JNEUROSCI.17-04-01350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapur A, Pearce RA, Lytton WW, Haberly LB. GABAA-mediated IPSCs in piriform cortex have fast and slow components with different properties and locations on pyramidal cells. J Neurophysiol. 1997;78:2531–2545. doi: 10.1152/jn.1997.78.5.2531. [DOI] [PubMed] [Google Scholar]
- 14.Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology. 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 15.Löw K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy J-M, Rulicke T, Bluethmann H, Möhler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- 16.Manabe T, Noda Y, Mamiya T, Katagiri H, Houtani T, Nishi M, Noda T, Takahashi T, Sugimoto T, Nabeshima T, Takeshima H. Facilitation of long-term potentiation and memory in mice lacking nociceptin receptors. Nature. 1998;394:577–581. doi: 10.1038/29073. [DOI] [PubMed] [Google Scholar]
- 17.McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- 18.McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor α1 subtype. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- 19.Meiri N, Sun MK, Segal Z, Alkon DL. Memory and long-term potentiation (LTP) dissociated: normal spatial memory despite CA1 LTP elimination with Kv1.4 antisense. Proc Natl Acad Sci USA. 1998;95:15037–15042. doi: 10.1073/pnas.95.25.15037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RG, Morrison JH, O'Dell TJ, Grant SG. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 1998;396:433–439. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- 21.Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi Z-P, Lagenaur C, Tretter V, Sieghart W, Anagnostaras G, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor delta subunit knock-out mice. Proc Natl Acad Sci USA. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris RGM. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 23.Pananceau M, Chen HX, Gustafsson B. Long-term potentiation induced by single volley activation: a mechanism for bicuculline-induced enhancement of synaptic field potentials in the CA1 hippocampal region. Neuroscience. 1997;79:95–101. doi: 10.1016/s0306-4522(96)00672-0. [DOI] [PubMed] [Google Scholar]
- 24.Pearce RA. Physiological evidence for two distinct GABAA responses in rat hippocampus. Neuron. 1993;10:189–200. doi: 10.1016/0896-6273(93)90310-n. [DOI] [PubMed] [Google Scholar]
- 25.Pearce RA, Grunder SD, Faucher LD. Different mechanisms for use-dependent depression of two GABAA-mediated IPSCs in rat hippocampus. J Physiol (Lond) 1995;484:425–435. doi: 10.1113/jphysiol.1995.sp020675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 27.Quirk K, Blurton P, Fletcher S, Leeson P, Tang F, Mellilo D, Ragan CI, McKernan RM. [3H]L-655,708, a novel ligand selective for the benzodiazepine site of GABAA receptors which contain the α5-subunit. Neuropharmacology. 1996;35:1331–1335. doi: 10.1016/s0028-3908(96)00061-5. [DOI] [PubMed] [Google Scholar]
- 28.Roepsdorff A, Lambert JDC. Factors contributing to the decay of the stimulus-evoked IPSC in rat hippocampal CA1 neurons. J Neurophysiol. 1994;72:2911–2926. doi: 10.1152/jn.1994.72.6.2911. [DOI] [PubMed] [Google Scholar]
- 29.Ropert N, Miles R, Korn H. Characteristics of miniature inhibitory postsynaptic currents in CA1 pyramidal neurones of rat hippocampus. J Physiol (Lond) 1990;428:707–722. doi: 10.1113/jphysiol.1990.sp018236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosahl TW, Geppert M, Spillane D, Herz J, Hammer RE, Malenka RC, Südhof TC. Short term synaptic plasticity is altered in mice lacking synapsin I. Cell. 1993;75:661–670. doi: 10.1016/0092-8674(93)90487-b. [DOI] [PubMed] [Google Scholar]
- 31.Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy J-M, Martin JR, Bluethmann H, Möhler H. Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 32.Sauer B. Manipulation of transgenes by site-specific recombination: use of Cre recombinase. Methods Enzymol. 1993;225:890–900. doi: 10.1016/0076-6879(93)25056-8. [DOI] [PubMed] [Google Scholar]
- 33.Seabrook GR, Smith DW, Bowery BJ, Easter A, Reynolds T, Fitzjohn SM, Morton RA, Zheng H, Dawson GR, Sirinathsinghji DJS, Davies CH, Collingridge GL, Hill RG. Mechanisms contributing to the deficits in hippocampal synaptic plasticity in mice lacking amyloid precursor protein. Neuropharmacology. 1999;38:340–359. doi: 10.1016/s0028-3908(98)00204-4. [DOI] [PubMed] [Google Scholar]
- 34.Segal M, Barker JL. Rat hippocampal neurons in culture: voltage-clamp analysis of inhibitory synaptic connections. J Neurophysiol. 1984;52:469–487. doi: 10.1152/jn.1984.52.3.469. [DOI] [PubMed] [Google Scholar]
- 35.Silva AJ, Rosahl TW, Chapman PF, Marowitz Z, Friedman E, Frankland PW, Cestari V, Cioffi D, Südhof TC, Bourtchuladze R. Impaired learning in mice with abnormal short-lived plasticity. Curr Biol. 1996;6:1509–1518. doi: 10.1016/s0960-9822(96)00756-7. [DOI] [PubMed] [Google Scholar]
- 36.Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- 37.Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. GABAA receptor subunits in the rat hippocampus: immunocytochemical distribution of 13 subunits. Neuroscience. 1997;80:987–1000. doi: 10.1016/s0306-4522(97)00146-2. [DOI] [PubMed] [Google Scholar]
- 38.Steele RJ, Morris RGM. Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist d-AP-5. Hippocampus. 1999;9:118–136. doi: 10.1002/(SICI)1098-1063(1999)9:2<118::AID-HIPO4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 39.Strecker GJ, Park WK, Dudek FE. Zinc and flunitrazepam modulation of GABA-mediated currents in rat suprachiasmatic neurons. J Neurophysiol. 1999;81:184–191. doi: 10.1152/jn.1999.81.1.184. [DOI] [PubMed] [Google Scholar]
- 40.Sur C, Quirk K, Dewar D, Atack J, McKernan RM. Rat and human hippocampal α5 subunit containing γ-aminobutyric acid-A receptors have α5β3γ2 pharmacological characteristics. Mol Pharmacol. 1998;54:928–933. doi: 10.1124/mol.54.5.928. [DOI] [PubMed] [Google Scholar]
- 41.Sur C, Fresu L, Howell O, McKernan RM, Atack JR. Autoradiographic localization of α5 subunit-containing GABAA receptors in rat brain. Brain Res. 1999;822:265–270. doi: 10.1016/s0006-8993(99)01152-x. [DOI] [PubMed] [Google Scholar]
- 42.Sur C, Wafford KA, Reynolds DS, Hadingham KL, Bromidge F, Macaulay A, Collinson N, O'Meara G, Howell O, Newman R, Myers J, Atack JR, Dawson GR, McKernan RM, Whiting PJ, Rosahl TW. Loss of the major GABAA receptor subtype in the brain is not lethal in mice. J Neurosci. 2000;21:3409–3418. doi: 10.1523/JNEUROSCI.21-10-03409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- 44.Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- 45.Whiting PJ, Wafford KA, McKernan RM. Pharmacological subtypes of GABAA receptors based upon subunit composition. In: Martin DL, Olsen RW, editors. GABA in the nervous system. Lippincott, Williams & Wilkins; New York: 2001. [Google Scholar]
- 46.Wisden W, Laurie DJ, Monyer HM, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain I Telencephalon, diencephalon and mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, Kaiser KMM, Koster HJ, Borchardt T, Worley P, Lubke J, Frotscher M, Kelly PH, Sommer B, Andersen P, Seeburg PH, Sakmann B. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284:1805–1811. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]