Abstract
Second messenger systems mediate neuronal responses to extracellular factors that elicit axon branching, turning, and guidance. We found that mutations in Caenorhabditis elegans that affect components of second messenger systems, a G-protein subunit, phospholipase Cβ, diacylglycerol (DAG) kinase, and calcium/calmodulin-dependent protein kinase (CaMKII), have no obvious effect on axon responses to UNC-6 except in animals in which the N-terminal fragment, UNC-6ΔC, is expressed. In these animals, the mutations enhance or suppress ectopic branching of certain axons. Netrin UNC-6 is an extracellular protein that guides circumferential migrations, and UNC-6ΔC has UNC-6 guidance activity. We propose that the guidance response elicited by the UNC-6 N-terminal domains involves mechanisms that can induce branching that is sensitive to CaMKII- and DAG-dependent signaling, and that the UNC-6 C domain is required incis to the N-terminal domains to silence the branching and to maintain proper axon morphology.
Keywords: netrin, UNC-6, Caenorhabditis elegans, guidance, axon branching, genetics, G-protein subunit, Gqα, phospholipase Cβ, PLCβ, diacylglycerol kinase, DAK, calcium/calmodulin-dependent protein kinase, CaMKII, neuropeptide Y receptor
The regulation of axon guidance and branching is critical for the proper development of the nervous system. Recent studies suggest that guidance and branching share common mechanisms (Brose et al., 1999; Lim et al., 1999; Kalil et al., 2000). For example, fragments of proteins known to mediate axon guidance can promote axon branching. In Caenorhabditis elegans, netrin UNC-6 guides circumferential migrations, and the expression of an N-terminal fragment has been shown to cause additional axon branches from ventral cord motor neurons (Lim et al., 1999). In vertebrates, Slit2 has been implicated in axon guidance and the N-terminal fragment has been shown to promote axon elongation and branching in an in vitro collagen assay system (Wang et al., 1999). Aside from such protein fragments, there are a number of other secreted factors, such as neurotrophins, that have been shown to influence axon guidance and promote branching (for review, see Acebes and Ferrus, 2000; Kalil et al., 2000). Although such factors have been identified, little is known about the underlying mechanisms by which such molecules dictate axon morphology.
The netrins are a family of extracellular guidance proteins that can function in vivo to attract and repel axons from sources that secrete the molecule (Hedgecock et al., 1990; Ishii et al., 1992;Serafini et al., 1994; Colamarino and Tessier-Lavigne, 1995). InC. elegans, netrin UNC-6 guides circumferential cell and axon migrations (Hedgecock et al., 1990; Ishii et al., 1992; Wadsworth et al., 1996). Axons that express the UNC-5 and UNC-40 netrin receptors migrate away from UNC-6 sources; axons that express UNC-40 migrate toward UNC-6 sources. UNC-6 is expressed in changing patterns by 12 types of cells and is predicted to create a stable global cue peaking near the ventral midline and to create local cues on cell surfaces (Wadsworth and Hedgecock, 1996; Wadsworth et al., 1996).
UNC-6 was the first characterized member of the netrin family, and residues 1–437 were designated domains VI, V-1, V-2, and V-3 based on the similarity of the domains to the N-terminal domains of laminin subunits (Ishii et al., 1992). Residues 438–591 were designated domain C, and it was observed that the same motif is found in C3, C4, and C5 complement proteins but not in a paralogous protein, α2macroglobulin (Ishii et al., 1992). Recently, a modified domain C (C′) was found in a functionally divergent form of vertebrate netrin designated netrin-G1 (Nakashiba et al., 2000). These observations indicate that UNC-6 C is a conserved structural module; they suggest that UNC-6 C has a biological function.
We have shown previously that in unc-6ΔC transgenic animals, which express UNC-6 without UNC-6 C, the ventral nerve cord motor neurons extend additional processes circumferentially (see Fig.3B) (Lim et al., 1999). This activity requires the netrin receptors UNC-5 and UNC-40 (Lim et al., 1999). The expression of UNC-6ΔC provides a means to explore the mechanisms by which axons respond to secreted factors. We have examined the morphology of neurons in unc-6ΔC animals and have uncovered mutations that modulate these morphologies. From these results, we are able to make predictions regarding the mechanisms by which morphological changes to axons might be controlled.
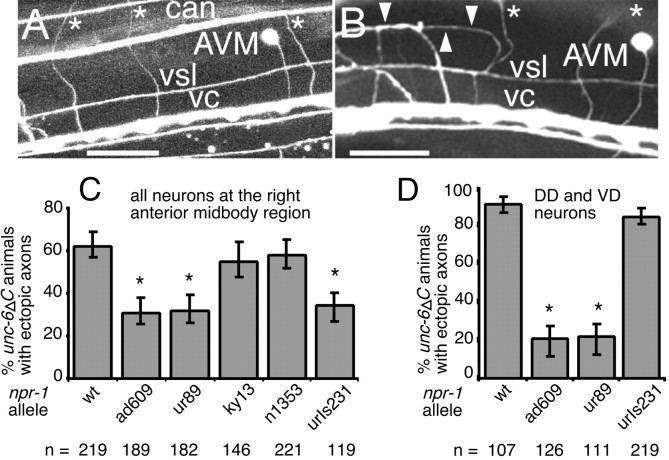
Fig. 3.
Alleles of npr-1 suppress the UNC-6ΔC-elicited outgrowth of ventral nerve cord motor neuron processes. A–D, The percentage of animals that had any additional processes induced by unc-6ΔC expression was measured by scoring for displaced axons at the anterior midbody right ventral sublateral–lateral boundary using pan-neural expression of GFP (Lim et al., 1999) (A–C) or for all DD and VD axons using anti-GABA antibodies (D).A, In the wild type, ventral nerve cord motor neuron processes migrate longitudinally in the ventral nerve cord (vc) and circumferentially (asterisks) past the ventral sublateral nerve (vsl), the lateral canal-associated nerve (can), and toward the dorsal midline. The AVM axon migrates to the vc. B, Expression of unc-6ΔC induces the outgrowth of additional processes that migrate from the ventral nerve cord motor neurons, past the vsl, and to the boundary (arrowheads) between the ventral sublateral and lateral epidermal cells (Lim et al., 1999). No axons are present in wild-type animals at this position. As in wild-type animals, the AVM axon migrates ventrally and circumferential motor neuron axons (asterisks) migrate to the dorsal midline (out of the plane of view). Scale bar: A, B, 25 μm.C, npr-1 alleles were examined for the suppression of all additional outgrowth induced byunc-6ΔC expression. npr-1(ky13)introduces a stop codon after the first of the seven transmembrane domains of the neuropeptide Y receptor homolog and is most likely a loss-of-function allele (de Bono and Bargmann, 1998).urIs231 is an integrated transgene that expresses thenpr-1(ur89) sequence under a promoter that drives expression in the DA and DB neurons. These animals were scored in thenpr-1(ky13) background. D, Expression ofnpr-1(ad609) and npr-1(ur89) was tested for the ability to suppress the UNC-6ΔC-elicited outgrowth of DD and VD motor neuron processes. Asterisks indicate values that differ from control unc-6ΔC animals atp < 0.001; error bars indicate the SE of proportion.
MATERIALS AND METHODS
Strains. The following mutations and strains were used for mapping and double-mutant constructions: N2; RW7000; IM145urIs77[IM#183 IM#175 pRF4] II; IM222npr-1(ur89); CX4056 npr-1(ad609)lon-2(e678) X; CX3048 npr-1(ky13) X; DA658npr-1(n1353)lon-2(e678) X; and MT1434egl-30(n686) I. The following strains were generated in this study: IM342 lon-2(e678)unc-6(ev400) X; IM290npr-1(ur89)lon-2(e678) X; IM337npr-1(ky13)lon-2(e678) X; IM234lon-2(e678);unc-6ΔC(urIs77); IM289npr-1(ur89)lon-2(e678);unc-6ΔC(urIs77); IM288npr-1(ad609)lon-2(e678);unc-6ΔC(urIs77); IM341npr-1(ky13)lon-2(e678);unc-6ΔC(urIs77); IM235npr-1(n1353)lon-2(e678);unc-6ΔC(urIs77); IM338egl-30 (n686);npr-1(ur89)lon-2(e678); IM339egl-30(n686);npr-1(ad609)lon-2(e678); IM340egl-30(n686);unc-6ΔC(urIs77); IM357 urIs193 [npr-1p:: npr-1:: GFP]; IM403unc-6ΔC(urIs77);npr-1(ky13);urIs231 [unc-129p:: npr-1(ur89):: gfp]; IM405unc-129(ev554);npr-1(ur89)lon-2(e678); IM406unc-129(ev554);npr-1(ad609)lon-2(e678); and IM409unc-129(ev554);npr-1(ky13).
Genetic screen. A second filial generation (F2) screen was performed using ethylmethanesulfonate as a mutagen. The starting strain, IM145, carries a transgene, urIs77, which encodes hemagglutinin (HA) epitope-tagged UNC-6 that does not include domain C (Lim et al., 1999). The urIs77 transgene was made by removing the sequence that encodes for domain C from anunc-6:: HA clone that had been shown to rescue all mutant phenotypes in the unc-6 null genetic background (Wadsworth et al., 1996). In unc-6 null mutants, expression of urIs77 exhibits rescue of the unc-6(−)uncoordinated behavior. In unc-6(+) animals, expression causes a slightly uncoordinated phenotype. The transgene also expresses green fluorescent protein (GFP) throughout the nervous system and confers a rolling phenotype to the animals (Lim et al., 1999). F2 animals were screened for wild-type movement; the selected mutants were examined for wild-type axon branching by epifluorescence microscopy. The isolated mutant strains were outcrossed against wild-type N2 six times to remove other possible mutations. Expression of the transgene was confirmed by the rolling phenotype as well as by GFP expression; the presence of the UNC-6 protein was further confirmed by Western blot analysis using monoclonal antibody 12CA5 (Boehringer Mannheim, Mannheim, Germany) to detect the HA epitope.
npr-1 analysis. ur89 was mapped to thenpr-1 region. From the cross ur89 ×lon-2(e678)unc-6(ev400) X, 1/50 Lon non-Unc recombinants were clumping. Noncomplementation between ur89 and othernpr-1 alleles was tested by scoring for clumping oftrans heterozygotes (non-Lon progeny) from a cross such asur89 × n1353 lon-2. To characterizenpr-1(ur89) animals, the allele was linked withlon-2(e678) on linkage group X by crossingur89 × lon-2(e678)unc-6(ev400) X, 1/50 Lon non-unc recombinants segregated clumping of npr-1(ur89) lon-2(e678). Transgenic npr-1 strains were generated by standard methods (Mello and Fire, 1995).npr-1(ur89) animals were injected with pM4, a plasmid that contains an insert of npr-1 genomic DNA derived from the solitary strain N2 (de Bono and Bargmann, 1998). The npr-1insert contains a 7.4 kb promoter region and 2.3 kb of the coding region. pM4 was coinjected with the markers pRF4, which expressesrol-6(su1006) and causes a dominant rolling phenotype, and pPD118.33, which expresses GFP under the myo-2 promoter in pharyngeal muscles (Mello and Fire, 1995). Four independently derived strains were found to rescue the ur89 phenotype in the clumping assay. To test whether the results of the assay were affected by the rolling phenotype conferred by pRF4, the assay was repeated using animals that also carried the dpy-11(e224) allele, which suppresses the rol-6(su1006) rolling phenotype.npr-1(ur89);dpy-11(e224) males were crossed withnpr-1(ur89);urEx162[pM4,pRF4,pPD118.33] hermaphrodites;ur89/ur89;dpy-11/+ progeny that carried theurEx162 transgene were chosen. Dpy non-Rol F2 progeny with pharyngeal-muscle GFP expression were selected and the assay was repeated. There was no significant difference in the assay results. To identify the molecular lesion in npr-1(ur89), PCR fragments including the entire coding sequence and intron region were amplified from the genomic DNA of npr-1(ur89) animals. PCR fragments were cloned into a pBluescript SK+ vector (Stratagene, La Jolla, CA), and both PCR fragments and the subclones were sequenced by an automated sequencer. The mutation was confirmed by sequencing two independent PCR fragments.
The npr-1 lon-2;unc-6ΔC(urIs77) animals were constructed by crossing npr-1 lon-2 males withunc-6ΔC(urIs77) hermaphrodites and selecting Lon Rol F2 progeny. The urIs77 transgene maps to LGII and is followed by the Rol phenotype and by the pan-neural expression ofunc-119:: GFP. For the branching assay, theunc-6ΔC(urIs77) transgene was crossed intourEx162 animals by standard methods for unlinked genes.
Transgenic animals expressing an NPR-1:: GFP reporter were obtained by microinjection of pIM#200, a plasmid constructed by inserting 7 kb of upstream regulatory sequence and 1 kb ofnpr-1 coding sequence from pM4 in frame with the GFP coding sequence of vector pPD95.79 (supplied by A. Fire, Carnegie Institution of Washington, Baltimore, MD). Transgenes were integrated by γ-ray irradiation and four independent lines were established. To express npr-1(ur89) ectopically in DD and VD motor neurons, the unc-129 motor neuron-specific promoter (Colavita et al., 1998) was placed upstream of thenpr-1 coding sequence, the npr-1 sequence was altered to encode the C178Y change, and the GFP coding sequence was inserted in frame immediately before the stop codon sequence. The pIM#201 plasmid was used to create integrated transgenic strains. Expression of the transgene was monitored by the motor neuron expression of GFP.
Axon outgrowth and aldicarb sensitivity assays. For the outgrowth assay, animals were scored by epifluorescence microscopy. Living animals were mounted on a slide in a small drop of M9 buffer on a 5% agar pad. L4 larvae or young adults were randomly picked and scored for the presence of ectopic processes at the ventral sublateral–lateral boundary on the right side of the body wall between the vulva and the retrovesicular ganglion (Lim et al., 1999). Theunc-6ΔC(urIs77) animals were picked at random from plates in this study, whereas in the study by Lim et al. (1999), animals with the strongest roller phenotype were selected. Animals with a strong roller phenotype have a slightly lower percentage of ectopic axons.
For scoring the DD and VD motor neurons, immunofluorescence histochemistry was used to stain animals for GABA as described byMcIntire et al. (1992) using rabbit anti-GABA antiserum (Sigma, St. Louis, MO) and Cytm3-conjugated AffiniPure goat anti-rabbit antiserum (Jackson ImmunoResearch, West Grove, PA).
Acute sensitivity to aldicarb (Chem Services Inc., West Chester, PA) was determined as described previously (Lackner et al., 1999). Briefly, in each experiment, 20 L4 worms were placed on 1 mm Aldicarb plates and prodded every 10 min over a 2 hr period to determine whether they retained the ability to move; worms that failed to respond to this harsh touch were classified as paralyzed. Each experiment was repeated a minimum of three times.
RESULTS
Netrin UNC-6 and UNC-6ΔC affect axon guidance and branching of the DD and VD motor neurons
Netrin UNC-6 is required to guide circumferential cell and axon migrations (Hedgecock et al., 1990; Ishii et al., 1992). To investigate the relationship between axon guidance and axon branching that is influenced by UNC-6, we examined the DD and VD motor neurons (Fig.1). The guidance of the circumferential axons of these particular neurons was shown by McIntire et al. (1992)to require UNC-6. We reasoned that the branching of these axons might be especially susceptible to the effects of UNC-6, because each neuron extends circumferentially an UNC-6 responsive axon from an UNC-6 nonresponsive axon that runs longitudinally in the ventral nerve cord. The DD axons develop in the embryo and the VD axons develop in the larva. Together these neurons circumferentially extend 17 axons along the right body wall and 2 axons along the left body wall in wild-type animals (White et al., 1986). The axons are GABAergic and can be specifically visualized using anti-GABA antibodies (McIntire et al., 1992).
Fig. 1.
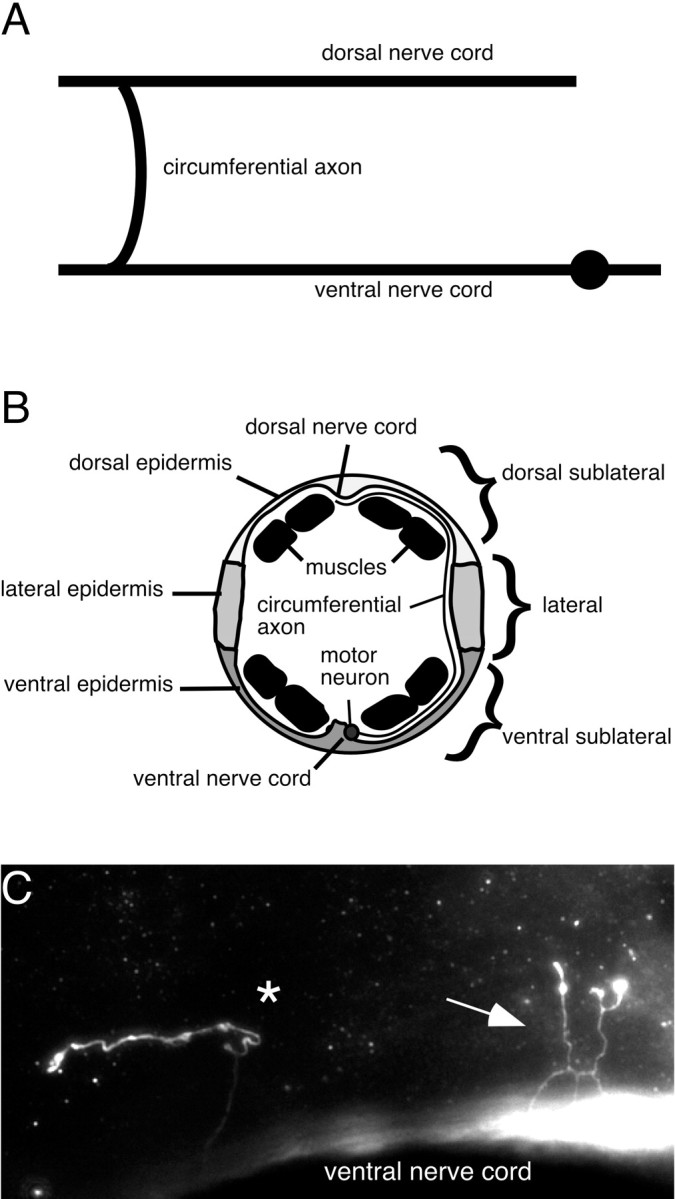
The morphology of DD and VD motor neurons is shown. A, The morphology of the DD and VD neurons. The circumferential axons extend from the distal end of the longitudinal axon in the ventral nerve cord and migrate dorsally to the dorsal nerve cord. B, Schematic transverse section of the adult hermaphrodite body wall. The DD and VD circumferential axons migrate between the basal surface of the epidermis and the basement membranes. Axon morphology was scored at the different dorsoventral positions indicated. C, The DD and VD neurons were visualized using anti-GABA immunocytochemistry. In unc-6 null mutants, the circumferential axons wander laterally. Some axons terminate without branching (asterisk), whereas others produce ectopic branches before terminating (arrow).
We scored the total number of DD and VD motor neuron axons leaving from the right side of ventral nerve cord in unc-6 null mutants. We found that an average of 16.3 ± 0.7 (n = 23) axons leave in wild-type animals, whereas an average of 8.0 ± 1.8 (n = 33) axons leave in unc-6 null mutants (Fig. 2B). This raises the possibility that DD/VD neurons may fail to extend circumferential axons in the unc-6 null mutant. It is also possible that these branches form but simply stay within the ventral nerve cord; however, analyses of the ventral nerve cord ofunc-6 mutants by electron microscopy show that the average number of axons in the cord is equal to that in the wild-type animals (Hedgecock et al., 1990; McIntire et al., 1992). Axons that do dorsally extend often wander laterally and may branch and terminate at lateral positions (Fig. 1C). We analyzed the morphology of these circumferential DD/VD axons by scoring the position at which they terminate and the number of branch points (Fig.2B,C). In unc-6 null mutants, most axons wander and terminate in the ventral sublateral region after leaving the dorsal nerve cord (Fig. 2B); 67% (n= 166) have ectopic lateral branches (Fig. 2A). Likewise, in unc-5 and unc-40 mutants the frequency of ectopic branching is similar, although inunc-40 mutants the majority of the circumferential axons are guided to the dorsal midline. These results indicate that UNC-6 plays a role in the dorsal extension of the circumferential axons from the ventral nerve cord and in preventing the ectopic branching of the axons once they leave the cord.
Fig. 2.
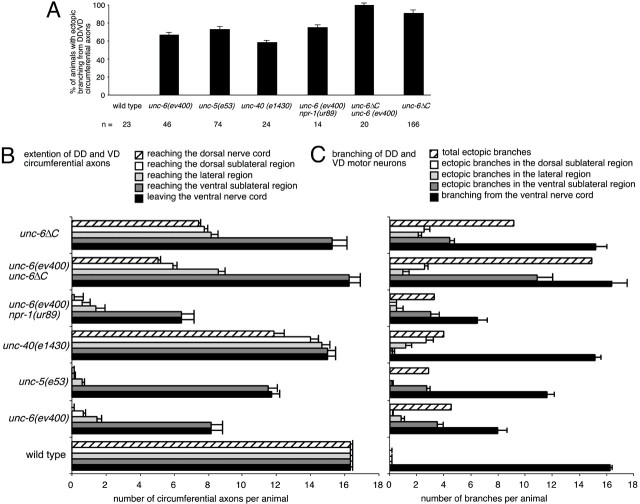
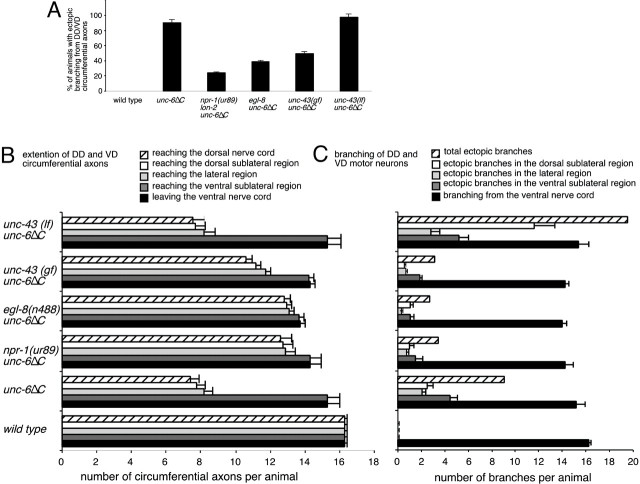
Netrin UNC-6 and UNC-6ΔC affect the morphology of the DD and VD motor neurons. A, For each strain, the percentage of the population that had ectopic axon branching of the circumferential axons was measured by scoring for ectopic branches anywhere along the entire length of an animal. The ectopic branching occurs in unc-6, unc-5, andunc-40 loss-of-function mutants as well as in animals expressing unc-6ΔC. B, The number of circumferential axons extending from the ventral nerve cord and reaching each of the different dorsoventral positions was measured. For each axon, the trajectory that projected farthest dorsally was scored; the dorsal extension of ectopic branches was not included. A significant fraction of the axons reaches the dorsal nerve cord inunc-40, unc-6(−);unc-6ΔC, andunc-6(+);unc-6ΔC animals. C, The number of axon branch points occurring at each dorsoventral position was measured. For each axon, only the branch points that occurred along the trajectory that projected farthest dorsally were scored. The ectopic branching is enhanced in unc-6(+);unc-6ΔC animals. The means ± SEM are reported.
The expression of UNC-6ΔC was shown to have partial UNC-6 guidance activity and to cause axon branching of ventral nerve cord motor neurons (Lim et al., 1999). Here the morphology of the DD and VD neurons was specifically examined. We find that the number of DD and VD axons that extend from the ventral nerve cord in animals that expressunc-6ΔC in the unc-6 wild-type andunc-6(−) background is nearly normal (Fig.2B). Furthermore, the percentage of circumferential axons that reach the dorsal midline is greater in these animals than inunc-6 null animals (48% in unc-6(+);unc-6ΔC, 42% in unc-6(−);unc-6ΔC, and 1% in unc-6(−)animals) (Fig. 2B). However, despite the improved guidance of the axons, ectopic branching is not reduced in theunc-6ΔC animals (Fig. 2C). These results, together with the branching observed in unc-40 mutants, indicate that the ectopic branching is not simply the result of the failure of dorsal guidance; they raise the possibility that UNC-6, but not UNC-6ΔC, mediates another response that prevents inappropriate branching of the circumferential axons.
Alleles of npr-1 suppress the branching of the circumferential DD and VD axons in unc-6ΔC animals
The above model predicts separate UNC-6 activities that modulate the axon guidance and branching responses. Therefore, we reasoned that it might be possible to isolate mutations that affect the ectopic branching but not the guidance response to UNC-6ΔC. A genetic screen was performed to isolate mutations that suppress the branching of additional processes from ventral nerve cord motor neurons inunc-6ΔC animals (Fig.3A,B). This screen took advantage of the observation that the additional motor neuron branches caused by the expression of an unc-6ΔC transgene,urIs77, in an otherwise wild-type animal result in an uncoordinated phenotype (Lim et al., 1999). We reasoned that mutations that suppress the additional branching might restore wild-type movement. Because in general axon guidance mutants have an uncoordinated phenotype, selecting mutagenized animals with wild-type movement should isolate mutations that affect the ability to induce the extra motor neuron branches but not axon guidance. Thus, in principal, this screen isolates new mutations only if the axon branching response is separable from the axon guidance response. From a screen of 40,000 haploid genomes, we isolated six mutations that partly suppress the urIs77-induced motor neuron processes.
We have identified one suppressor, ur89, as an allele ofnpr-1. Without unc-6ΔC expression, theur89 allele causes a social behavior phenotype; that is, the mutants aggregate together on food to form clumps. The same phenotype was described for npr-1, a gene that encodes a predicted seven-transmembrane receptor of the neuropeptide Y receptor family (de Bono and Bargmann, 1998). We examined previously identified alleles ofnpr-1 and found that npr-1(ad609) also suppresses all additional motor neuron processes in a fraction ofunc-6ΔC animals; however, npr-1(ky13) andnpr-1(n1353) do not (Fig. 3C). The expression ofunc-6ΔC partially suppresses the clumping phenotype conferred by any of the npr-1 mutations (data not shown). Genetically, the ur89 allele fails to complementnpr-1(n1353) or npr-1(ky13), and the expression of an npr-1(+) transgene rescues the clumping phenotype ofur89 animals and the ur89 suppression of extra motor neuron processes in unc-6ΔC;ur89 animals. By DNA sequence analysis, we determined that the ur89 mutation changes residue 178 from a cysteine to a tyrosine. Cysteine 178 is conserved among neuropeptide Y receptors and is predicted to occur in the second extracellular loop between transmembrane domains 4 and 5 (de Bono and Bargmann, 1998). In comparison, npr-1(ky13)introduces a stop codon after the first of seven transmembrane domains, whereas substitutions occur in ad609 at transmembrane domains 2 and 4 and in n1353 at transmembrane domain 3 (de Bono and Bargmann, 1998).
npr-1 is expressed in ventral nerve cord motor neurons that produce additional branches in response to UNC-6ΔC
The DA, DB, DD, and VD motor neurons have been observed to have additional ventral nerve cord motor neuron branches inunc-6ΔC animals (Lim et al., 1999). To determine whether the npr-1 suppressors might act within any of these ventral nerve cord motor neurons, we made a reporter construct by fusing the sequence coding for GFP after the npr-1 sequence that encodes to the 10th amino acid of the fourth predicted transmembrane domain. We observed strong expression in DD and VD motor neurons (Fig.4). In unc-6ΔC animals, ectopic DD and VD branches are induced; these are completely suppressed in a large fraction of the npr-1(ad609);unc-6ΔC andnpr-1(ur89);unc-6ΔC animals (Fig. 3D). Expression in DA and DB neurons was weak or absent. Note that rather than scoring the number of processes directly, which is difficult because unambiguously identifying a branch can be difficult (Fig. 3B), we measured the proportion of animals that have ectopic processes. This was done by scoring for the presence of any processes at the sublateral–lateral boundary in a region of the animal in which normally no processes are observed (see Materials and Methods) (Lim et al., 1999) (Fig. 3A,B).
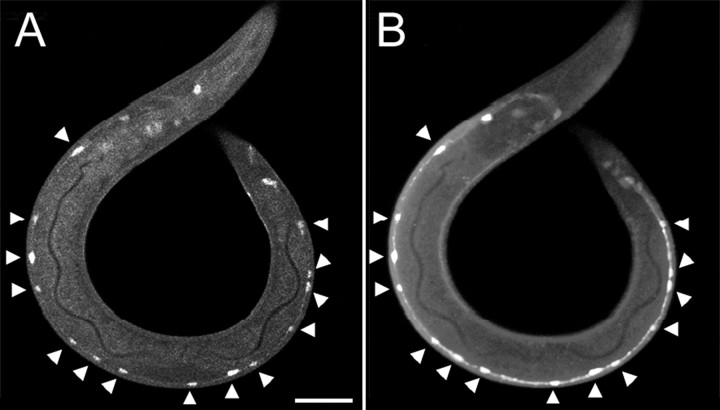
Fig. 4.
npr-1 is expressed in DD and VD ventral nerve cord motor neurons. A, B, Immunofluorescence micrographs of a larva expressing anpr-1:: gfp reporter. The larva was stained with anti-GFP antibodies (A) and anti-GABA antibodies (B). NPR-1:: GFP is coincident with the GABAergic motor neurons DD and VD of the ventral nerve cord (arrowheads). Scale bar, 25 μm.
The analysis of npr-1 provides evidence that signaling pathways within the DD and VD motor neurons mediate the development of the additional processes. For each strain, transcription of theunc-6ΔC transgene was confirmed by the expression of the GFP comarker; the presence of the UNC-6ΔC protein, which contains an HA epitope tag, was confirmed by Western blot analysis (data not shown). Our results indicate that different cells express NPR-1 and UNC-6ΔC. To further examine the cell-autonomous suppression of branching by npr-1(ur89), we ectopically expressed the mutant receptor NPR-1(C178Y) in the cholinergic DA and DB ventral nerve cord motor neurons, which are the other subset of motor neurons affected by unc-6ΔC expression. We observed that when the receptor was expressed in unc-6ΔC animals in thenpr-1 null background the combined ectopic branching of DD, VD, DA, and DB neurons was reduced, whereas the ectopic branching of DD and VD neurons alone was unchanged (Fig. 3C,D). By inference, we conclude that the mutant receptor can function cell autonomously even in the DA and DB motor neurons to suppress ectopic branching.
The npr-1(ur89) and npr-1(ad609)mutations may specifically affect the activity of components involved in regulation of ectopic branching in unc-6ΔC animals. Aside from suppressing the branching response and causing thenpr-1 phenotype of mutant social behavior, theur89 and ad609 alleles do not have other obvious phenotypes. Interestingly, other alleles of npr-1, including the predicted loss-of-function (lf) alleles, affect the social behavior but do not affect the number of motor neuron branches. Theur89 mutation changes one of the two cysteine residues that are thought to form a disulfide bond that governs the topology of the extracellular loops of G-protein-coupled receptors. This topology is predicted to be critical for receptor activation (Perlman et al., 1995; Le Gouill et al., 1997; Zhang et al., 1999). We speculate that the ur89 and ad609 alleles produce an altered NPR-1 protein that inactivates downstream effectors required to mediate the ectopic branching, perhaps by sequestering a factor.
Mutations that affect second messenger systems enhance or suppress ectopic branching in unc-6ΔC animals
The evidence that signaling pathways within the motor neurons influence the branching of additional motor neuron processes led us to examine whether known neuromodulatory pathway mutations also have an effect. We first tested whether altering G-protein signaling could affect the induction of ectopic axon branches, because neuropeptide Y receptors are G-protein-coupled and because it was reported that adenosine A2b receptor, also a G-protein-coupled receptor, is a netrin-1 receptor (Corset et al., 2000). We found thategl-30(n686), which affects one of the G-protein α-subunits (Gqα) (Brundage et al., 1996), suppresses all induced motor neuron processes in a significant fraction ofegl-30(n686);unc-6ΔC animals (Fig.5A). Whereas EGL-30 is required for viability, egl-30(n686) is a splice acceptor site mutation that causes reduced copies of full-length EGL-30 and is not lethal (Brundage et al., 1996).
Fig. 5.
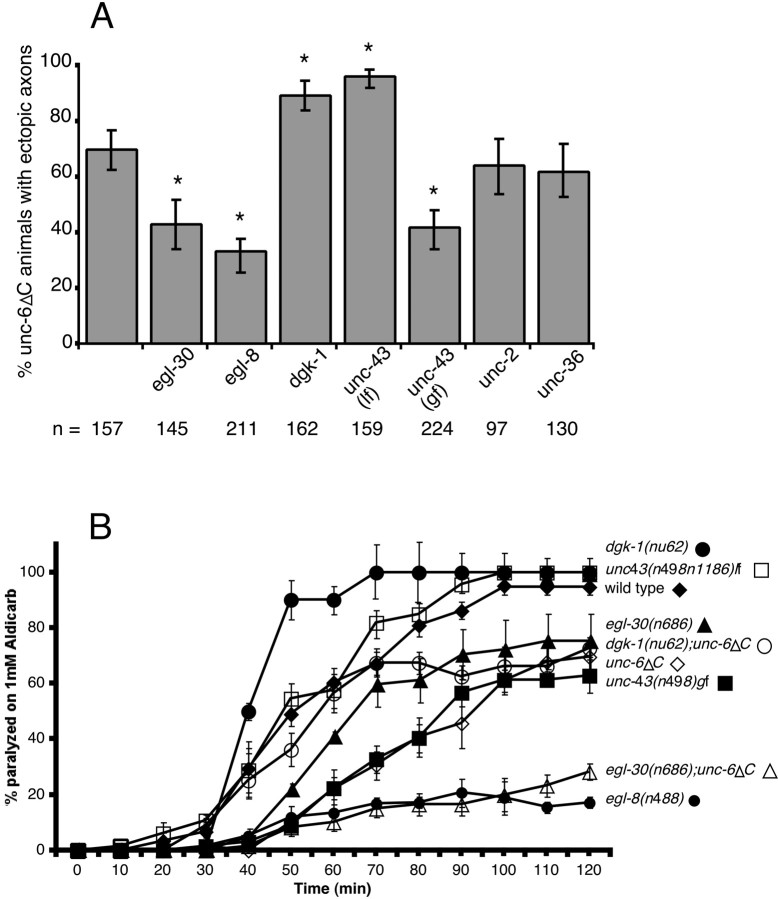
Mutations that affect neuromodulatory pathways enhance or suppress the UNC-6ΔC-elicited outgrowth of ventral nerve cord motor neuron processes. A, Seven different mutations affecting Gqα, (egl-30), phospholipase Cβ (elg-8), DAK (dgk-1), CaMKII (unc-43 lf and gf), the α1subunit of voltage-dependent calcium channels (unc-2), and the α2 subunit of voltage-dependent calcium channels (unc-36) were examined for the ability to suppress or enhance the UNC-6ΔC-elicited outgrowth of ventral nerve cord motor neuron processes.Asterisks indicate values that differ from controlunc-6ΔC animals at p < 0.001; error bars indicate the SE of proportion. B, The synaptic release of acetylcholine was assayed by the degree of paralysis induced by the cholinesterase inhibitor aldicarb. Expression of unc-6ΔC, egl-8(sa47),dgk-1(nu62);unc-6ΔC,egl-8(sa47);unc-6ΔC,egl-30(n686);unc-6ΔC, and unc-43(n498) gf decreases the release of acetylcholine, making animals more resistant to aldicarb. Expression ofdgk-1(nu62) enhances the release of acetylcholine and makes the animals less resistant. Data points are the mean ± SEM of at least three trials.
EGL-30 is a component of a signaling network that is present in most, if not all, neurons in C. elegans. In the ventral nerve cord motor neurons, activation of the Gqα pathway stimulates neurotransmitter release (Lackner et al., 1999; Miller et al., 1999). EGL-8, a homolog of phospholipase Cβ (PLCβ), is predicted to be a downstream effector of Gqα EGL-30 (Lackner et al., 1999; Miller et al., 1999); we found that egl-8(n488), a loss-of-function allele, also suppresses all motor neuron processes induced byunc-6ΔC in a significant fraction ofegl-8(n488);unc-6ΔC animals (Fig. 5A). Activated PLCβ cleaves phosphatidylinositol 4,5-biphosphate to produce inositol 1,4,5-triphosphate and diacylglycerol (DAG). In turn, these two second messengers may modulate intracellular events through their respective regulation of intracellular free Ca2+ and protein kinase C isozymes (Singer et al., 1997). In addition, DAG binds the presynaptic protein UNC-13 and recruits it to release sites (Nurrish et al., 1999). DGK-1, a diacylglycerol kinase (DAK), acts antagonistically to EGL-30 and EGL-8, presumably by converting DAG to phosphatidic acid and thereby reducing DAG levels (Miller et al., 1999). Consistent with this antagonistic role, dgk-1(nu62), a loss-of-function allele (Nurrish et al., 1999), increases the proportion ofunc-6ΔC animals with extra ventral nerve cord motor neuron branches (Fig. 5A).
Calcium/calmodulin-dependent protein kinase (CaMKII) is an enzyme that is thought to be critical for regulating synaptic strength and other neural functions. In C. elegans, there is one CaMKII gene, unc-43, and mutations affect neuronal gene expression and the density of ventral nerve cord synapses (Reiner et al., 1999; Rongo and Kaplan, 1999; Troemel et al., 1999). CaMKII activity is reduced by the loss-of-function mutationunc-43(n498n1186), whereas constitutive calcium-independent CaMKII activity is caused by the gain-of-function (gf) mutationunc-43(n498). Compared with unc-6ΔCanimals, a greater proportion of animals with additional ventral nerve cord motor neuron branches is observed in theunc-43(lf);unc-6ΔC strain and all of the additional motor neuron branches are suppressed in a significant fraction ofunc-43(gf);unc-6ΔC animals (Fig. 5A). These results suggest that the level of UNC-43 CaMKII activity is important for regulating the branching response to UNC-6ΔC.
Calcium influx through voltage-gated calcium channels appears to be one means by which UNC-43 CaMKII is activated (Troemel et al., 1995; Rongo and Kaplan, 1999). Moreover, cytoplasmic Ca2+ levels affect growth cone extensions and can regulate the turning response of cultured Xenopusaxons to netrin-1 (Gomez and Spitzer, 1999; Hong et al., 2000; Zheng, 2000). Therefore, we examined whether unc-2 andunc-36, genes that encode α1- and α2-subunits of voltage-dependent calcium channels, respectively (Schafer and Kenyon, 1995; Lee et al., 1997), influence the branching. We found that the loss-of-function allelesunc-2(e55) and unc-36(e251) do not affect the number of ventral nerve cord motor neuron branches (Fig.5A), suggesting that the branching response to UNC-6ΔC is independent of calcium influx through these channels.
Physiological state of the neurons potentiate branching inunc-6ΔC but not unc-6 wild-type animals
Our genetic analyses indicate that the branching of the ventral nerve cord motor neuron elicited by UNC-6ΔC can be enhanced or suppressed by certain neuromodulatory mutations. However, by themselves these mutations do not induce additional motor neuron branches (0%,n = 100 for each). Although altering Ca2+ and cAMP levels in vitroaffects axon responses to netrin (Ming et al., 1997; Song and Poo, 1999; Hong et al., 2000), axon guidance defects are not observed in the mutants we tested. However, in some cases there are defects in the positioning of neuronal cell bodies (Tam et al., 2000) (our unpublished observations). To verify that the physiological state of the neurons is altered by the mutations and to establish the relationship between the branching response and second messenger signaling activity, we determined the sensitivity of animals to the acetylcholinesterase inhibitor aldicarb. In this assay, sensitivity is a measure of acetylcholine release; reduced acetylcholine release confers resistance to aldicarb, whereas increased acetylcholine release causes hypersensitivity (Lackner et al., 1999; Miller et al., 1999). As noted previously, acetylcholine release can be altered by dgk-1,egl-8, and egl-30 mutations (Lackner et al., 1999; Miller et al., 1999), and we now show that alleles ofunc-43 and unc-6ΔC also affect release (Fig.5B). However, ectopic circumferential axon branches are observed only in unc-6ΔC animals, indicating that an altered physiological state induced by the mutations is not sufficient to modulate branching in unc-6 wild-type animals. However, the physiological changes caused by these mutations can affect branching in unc-6ΔC animals. Theegl-30(n686);unc-6ΔC animals have less release than unc-6ΔC animals and fewer of these mutants have additional branches of the ventral nerve cord motor neuron. In contrast, dgk-1(nu62);unc-6ΔC animals have more release than unc-6ΔC animals and more of these mutants have additional motor neuron branches. We conclude that the second messenger signaling networks affected by these mutations are altering the physiological state of the neurons, and that these changes can potentiate branching in unc-6ΔC animals but not in wild-type animals.
It is interesting that unc-6ΔC expression and the mutations that affect the patterning of axon branching inunc-6ΔC animals affect acetylcholine release. This establishes a connection between acetylcholine release, axon branching, and the responses to an extracellular protein that guides migrations.
Guidance mediated by UNC-6ΔC is affected by ectopic branching
Several of the mutations inhibit the ectopic branching of the DD and VD circumferential axons. We investigated whether this inhibition affects the dorsal guidance of the axons (Fig.6). In unc-6ΔC animals, which also express endogenous UNC-6, ectopic axon branches are observed across all lateral regions, with nearly one-half of the axons reaching the dorsal midline. In comparison, unc-6ΔC animals with the npr-1(ur89), egl-8(n488), orunc-43(gf) mutation have fewer ectopic axon branches across all lateral regions and the axons extend further dorsally, with a majority reaching the dorsal midline (Fig. 6B,C). Thus, inhibiting ectopic branching improves the directed axon extension. However, stimulation of ectopic branching by theunc-43(lf) mutation does not alter the ability of the axons to be dorsally guided relative to the expression ofunc-6ΔC alone (Fig. 6B,C). Taken together, these results suggest that the ability of UNC-6ΔC to mediate dorsal guidance is affected by the mutations, but dorsal guidance mediated by the endogenous UNC-6 is not. This further supports the idea that the ectopic branching is a direct consequence of UNC-6ΔC rather than a consequence of disrupting endogenous UNC-6 functions.
Fig. 6.
Mutations that affect second messenger systems affect axon morphology in unc-6ΔC animals.A, For each strain, the percentage of the population that had ectopic axon branching of the circumferential axons was measured by scoring for ectopic branches anywhere along the entire length of an animal. The percentage of unc-6ΔC animals with ectopic branches is reduced by the presence of thenpr-1, egl-8, orunc-43(gf) mutations. B, The number of circumferential axons extending from the ventral nerve cord and reaching each of the different dorsoventral positions was measured. For each axon, the trajectory that projected farthest dorsally was scored; the dorsal extension of ectopic branches was not included. Inunc-6ΔC animals, the presence of thenpr-1, egl-8, orunc-43(gf) mutations improves dorsal guidance, whereasunc-43(lf) does not. C, The number of axon branch points occurring at each dorsoventral position was measured. For each axon, only the branch points that occurred along the trajectory that projected farthest dorsally were scored. Inunc-6ΔC animals, the presence of thenpr-1, egl-8, orunc-43(gf) mutations suppressed ectopic branching, whereas unc-43(lf) enhanced branching. The means ± SEM are shown.
DISCUSSION
Both UNC-6 and UNC-6ΔC can guide circumferential DD and VD axons. Compared with unc-6(−) animals,unc-6(−);unc-6ΔC or unc-6(+);unc-6ΔC animals have better extension of circumferential axons from the nerve cord and better dorsal guidance of the circumferential axons; however, the penetrance of the ectopic branching phenotype is not reduced. In comparison with unc-6(−);unc-6ΔC animals,unc-6(+);unc-6ΔC animals show slightly improved dorsal guidance and less ectopic branching. These results suggest that UNC-6ΔC competes with the endogenous UNC-6, which can suppress the ectopic branching. This was also suggested from the results of the expression of different unc-6ΔC transgenes inunc-6(−) and unc-6(+) backgrounds (Lim et al., 1999). We also show that mutations that affect CaMKII- and DAG-dependent signaling modulate the ectopic branching phenotype inunc-6ΔC animals but do not affect axon morphology in UNC-6 wild-type animals. Our interpretation of these results is that UNC-6 C is responsible for inhibiting the effects of CaMKII- and DAG-dependent signaling, which, if not silenced, can modulate axon morphology.
The molecular mechanism by which UNC-6ΔC triggers ectopic branching is unknown. Netrin is thought to induce receptor complexes that can trigger different types of axon responses depending on the components they contain (Hong et al., 1999; Stein and Tessier-Lavigne, 2001). UNC-6ΔC may allow the formation of UNC-6 receptor complexes that can promote directed extension of growth cones but cannot inhibit responses that lead to branching. This inhibition requires the UNC-6 C domain working in cis to the N-terminal domains within the receptor complex. It is interesting that the UNC-6 C module has been found in a number of proteins, including the complement C345 protein family, frizzled related proteins, type I C-proteinase enhancer proteins (PCOLCEs), and tissue inhibitors of metalloproteinases (TIMPs) (Ishii et al., 1992; Leyns et al., 1997; Banyai and Patthy, 1999). In PCOLCE and TIMP proteins, the UNC-6 C module is involved in the regulation of metalloproteinase activity (Murphy et al., 1991; Hulmes et al., 1997; Langton et al., 1998). This raises the possibility that without UNC-6 C the UNC-6ΔC-containing complexes are more susceptible to regulation by proteases. It has been found that chemical inhibitors of metalloproteinases potentiate netrin-mediated axon outgrowthin vitro and that the netrin receptor homolog of UNC-40, deleted in colorectal cancer (DCC), is a substrate for metalloproteinase-dependent ectodomain shedding (Galko and Tessier-Lavigne, 2000).
Models of UNC-6/netrin guidance predict axon responses to gradients of the molecule. Our results indicate that the ectopic branching inunc-6ΔC animals is caused by a separate branching mechanism that is sensitive to UNC-6 C function, rather than by guidance errors caused by a novel distribution of UNC-6ΔC. First, expression of UNC-6ΔC causes the ectopic branching in only a subset of UNC-6 responsive neurons that extends along the entire body wall (Lim et al., 1999). A novel distribution of UNC-6ΔC would be expected to affect all of the UNC-6-responsive axons that are present along the body wall. Second, when UNC-6 is ectopically expressed, the branching phenotype is not observed, although the guidance of axons is severely disrupted in such animals (Ren et al., 1999). This indicates that a novel distribution of UNC-6 is not sufficient to cause the ectopic branching phenotype. Third, circumferential axon migrations are partially rescued when unc-6ΔC is expressed inunc-6 null animals, indicating that the proposed gradient and guidance information of UNC-6ΔC is not significantly different from that of UNC-6 in wild-type animals (Lim et al., 1999). Finally, we have uncovered mutations in genes that enhance or suppress the ectopic branching by acting within the branching neurons themselves. It is more likely that the mutations affect the cellular machinery that mediates an axon branching response than the extracellular distribution of UNC-6ΔC.
Second messenger signaling pathways, particularly cyclic nucleotides and Ca2+, are thought to play an important role in the regulation of axon responses to extracellular guidance molecules (for review, see Song and Poo, 1999; Gomez et al., 2001). For example, in vitro culture assays using Xenopusspinal neurons have shown that intracellular Ca2+ and cAMP levels are involved in dictating growth cone behavior in response to netrin-1 (Ming et al., 1997; Hong et al., 2000). Moreover, CaMKII, acting in a Ca2+-dependent manner, can mediate growth cone turning in response to acetylcholine (Zheng et al., 1994), and antagonist blocking of the acetylcholine receptor can inhibit the attractive response to netrin-1 (K. Hong, personal communication). This is interesting because the Gqα EGL-30–PLCβ EGL-8 pathway produces DAG in response to acetylcholine in C. elegans (Brundage et al., 1996; Lackner et al., 1999). Thus, observations in culture and in C. elegans are consistent with the notion that the CaMKII- and DAG-dependent signaling cascades are linked in the control of UNC-6/netrin responses.
CaMKII- and DAG-dependent signaling, which can modulate DD and VD axon morphology and cause ectopic branching, must be silenced during the dorsally directed migrations. In the unc-6 wild-type background, CaMKII- and DAG-dependent signaling are blocked by the activity mediated by UNC-6 C. In unc-6ΔC animals, the ability of CaMKII- and DAG-dependent signaling to alter axon morphology is not inhibited, and mutations such as dgk-1(lf) andunc-43(lf), which stimulate the signaling activity (as judged by their ability to elevate acetylcholine release), increase branching activity. Conversely, other mutations that inhibit signaling activity (by decreasing acetylcholine release), such asegl-8, egl-30, and unc-43(gf), diminish branching activity. The silencing effect of UNC-6 C is physiologically significant, because the suppression is required to prevent inappropriate responses that would cause erroneous morphological changes. Extracellular guidance molecules may have evolved strategies to counteract some process of the guidance machinery that tends to introduce branching. Although our results do not directly address what causes axons to branch at their normal stereotyped positions, they suggest that any mechanism that releases the inhibition mediated by UNC-6 C could trigger branching or turning responses.
Footnotes
This work was supported by National Institutes of Health Grant NS33156. We thank K. Hong for comments on this manuscript; Monica Driscoll, Diane Levitan, Claudio Pikielny, Ruth Steward, and Renping Zhou for helpful discussions; Zeynep Altun-Gultekin, Chen-Chen Huang, Gautam Kao, Yoo-Shick Lim, Xing-Cong Ren, and other members of the Wadsworth Laboratory for helpful discussions and technical assistance; Mario de Bono and Cori Bargmann for npr-1 strains,npr-1 DNA, and npr-1 sequencing primers, the Caenorhabditis Genetics Center for strains; and Andrew Fire for plasmid DNA.
Correspondence should be addressed to William G. Wadsworth, Department of Pathology, Robert Wood Johnson Medical School, 675 Hoes Lane, Piscataway, NJ 08854-5635. E-mail: william.wadsworth@umdnj.edu.
REFERENCES
- 1.Acebes A, Ferrus A. Cellular and molecular features of axon collaterals and dendrites. Trends Neurosci. 2000;23:557–565. doi: 10.1016/s0166-2236(00)01646-5. [DOI] [PubMed] [Google Scholar]
- 2.Banyai L, Patthy L. The NTR module: domains of netrins, secreted frizzled related proteins, and type I procollagen C-proteinase enhancer protein are homologous with tissue inhibitors of metalloproteases. Protein Sci. 1999;8:1636–1642. doi: 10.1110/ps.8.8.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96:795–806. doi: 10.1016/s0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- 4.Brundage L, Avery L, Katz A, Kim UJ, Mendel JE, Sternberg PW, Simon MI. Mutations in a C. elegans Gqα gene disrupt movement, egg laying, and viability. Neuron. 1996;16:999–1009. doi: 10.1016/s0896-6273(00)80123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colamarino SA, Tessier-Lavigne M. The axonal chemoattractant Netrin-1 is also a chemorepellent for trochlear motor axons. Cell. 1995;81:621–629. doi: 10.1016/0092-8674(95)90083-7. [DOI] [PubMed] [Google Scholar]
- 6.Colavita A, Krishna S, Zheng H, Padgett RW, Culotti JG. Pioneer axon guidance by UNC-129, a C. elegans TGF-β. Science. 1998;281:706–709. doi: 10.1126/science.281.5377.706. [DOI] [PubMed] [Google Scholar]
- 7.Corset V, Nguyen-Ba-Charvet KT, Forcet C, Moyse E, Chedotal A, Mehlen P. Netrin-1-mediated axon outgrowth and cAMP production requires interaction with adenosine A2b receptor. Nature. 2000;407:747–750. doi: 10.1038/35037600. [DOI] [PubMed] [Google Scholar]
- 8.de Bono M, Bargmann CI. Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell. 1998;94:679–689. doi: 10.1016/s0092-8674(00)81609-8. [DOI] [PubMed] [Google Scholar]
- 9.Galko MJ, Tessier-Lavigne M. Function of an axonal chemoattractant modulated by metalloprotease activity. Science. 2000;289:1365–1367. doi: 10.1126/science.289.5483.1365. [DOI] [PubMed] [Google Scholar]
- 10.Gomez TM, Spitzer NC. In vivo regulation of axon extension and pathfinding by growth-cone calcium transients. Nature. 1999;397:350–355. doi: 10.1038/16927. [DOI] [PubMed] [Google Scholar]
- 11.Gomez TM, Robles E, Poo M, Spitzer NC. Filopodial calcium transients promote substrate-dependent growth cone turning. Science. 2001;291:1983–1987. doi: 10.1126/science.1056490. [DOI] [PubMed] [Google Scholar]
- 12.Hedgecock EM, Culotti JG, Hall DH. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron. 1990;4:61–85. doi: 10.1016/0896-6273(90)90444-k. [DOI] [PubMed] [Google Scholar]
- 13.Hong K, Hinck L, Nishiyama M, Poo M, Tessier-Lavigne M, Stein E. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell. 1999;97:927–941. doi: 10.1016/s0092-8674(00)80804-1. [DOI] [PubMed] [Google Scholar]
- 14.Hong K, Nishiyama M, Henley J, Tessier-Lavigne M, Poo M. Calcium signalling in the guidance of nerve growth by netrin-1. Nature. 2000;403:93–98. doi: 10.1038/47507. [DOI] [PubMed] [Google Scholar]
- 15.Hulmes DJ, Mould AP, Kessler E. The CUB domains of procollagen C-proteinase enhancer control collagen assembly solely by their effect on procollagen C-proteinase/bone morphogenetic protein-1. Matrix Biol. 1997;16:41–45. doi: 10.1016/s0945-053x(97)90115-3. [DOI] [PubMed] [Google Scholar]
- 16.Ishii N, Wadsworth WG, Stern BD, Culotti JG, Hedgecock EM. UNC-6, a laminin-related protein, guides cell and pioneer axon migrations in C. elegans. Neuron. 1992;9:873–881. doi: 10.1016/0896-6273(92)90240-e. [DOI] [PubMed] [Google Scholar]
- 17.Kalil K, Szebenyi G, Dent EW. Common mechanisms underlying growth cone guidance and axon branching. J Neurobiol. 2000;44:145–158. [PubMed] [Google Scholar]
- 18.Lackner MR, Nurrish SJ, Kaplan JM. Facilitation of synaptic transmission by EGL-30 Gqα and EGL-8 PLCβ: DAG binding to UNC-13 is required to stimulate acetylcholine release. Neuron. 1999;24:335–346. doi: 10.1016/s0896-6273(00)80848-x. [DOI] [PubMed] [Google Scholar]
- 19.Langton KP, Barker MD, McKie N. Localization of the functional domains of human tissue inhibitor of metalloproteinases-3 and the effects of a Sorsby's fundus dystrophy mutation. J Biol Chem. 1998;273:16778–16781. doi: 10.1074/jbc.273.27.16778. [DOI] [PubMed] [Google Scholar]
- 20.Lee RY, Lobel L, Hengartner M, Horvitz HR, Avery L. Mutations in the α1-subunit of an l-type voltage-activated Ca2+ channel cause myotonia in Caenorhabditis elegans. EMBO J. 1997;16:6066–6076. doi: 10.1093/emboj/16.20.6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Gouill C, Parent JL, Rola-Pleszczynski M, Stankova J. Role of the Cys90, Cys95 and Cys173 residues in the structure and function of the human platelet-activating factor receptor. FEBS Lett. 1997;402:203–208. doi: 10.1016/s0014-5793(96)01531-1. [DOI] [PubMed] [Google Scholar]
- 22.Leyns L, Bouwmeester T, Kim SH, Piccolo S, De Robertis EM. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell. 1997;88:747–756. doi: 10.1016/s0092-8674(00)81921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim YS, Mallapur S, Kao G, Ren XC, Wadsworth WG. Netrin UNC-6 and the regulation of branching and extension of motoneuron axons from the ventral nerve cord of Caenorhabditis elegans. J Neurosci. 1999;19:7048–7056. doi: 10.1523/JNEUROSCI.19-16-07048.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McIntire SL, Garriga G, White J, Jacobson D, Horvitz HR. Genes necessary for directed axonal elongation or fasciculation in C. elegans. Neuron. 1992;8:307–322. doi: 10.1016/0896-6273(92)90297-q. [DOI] [PubMed] [Google Scholar]
- 25.Mello C, Fire A. DNA transformation. In: Epstein HF, Shakes DC, editors. Caenorhabditis elegans: modern biological analysis of an organism. Academic; San Diego: 1995. pp. 451–482. [Google Scholar]
- 26.Miller KG, Emerson MD, Rand JB. Goα and diacylglycerol kinase negatively regulate the Gqα pathway in C. elegans. Neuron. 1999;24:323–333. doi: 10.1016/s0896-6273(00)80847-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ming GL, Song HJ, Berninger B, Holt CE, Tessier-Lavigne M, Poo MM. cAMP-dependent growth cone guidance by netrin-1. Neuron. 1997;19:1225–1235. doi: 10.1016/s0896-6273(00)80414-6. [DOI] [PubMed] [Google Scholar]
- 28. Murphy G, Houbrechts A, Cockett MI, Williamson RA, O'Shea M, Docherty AJ. The N-terminal domain of tissue inhibitor of metalloproteinases retains metalloproteinase inhibitory activity. Biochemistry 30 1991. 8097 8102[Erratum (1991) 30:10362]. [DOI] [PubMed] [Google Scholar]
- 29.Nakashiba T, Ikeda T, Nishimura S, Tashiro K, Honjo T, Culotti JG, Itohara S. Netrin-G1: a novel glycosyl phosphatidylinositol-linked mammalian netrin that is functionally divergent from classical netrins. J Neurosci. 2000;20:6540–6550. doi: 10.1523/JNEUROSCI.20-17-06540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nurrish S, Segalat L, Kaplan JM. Serotonin inhibition of synaptic transmission: Gα(0) decreases the abundance of UNC-13 at release sites. Neuron. 1999;24:231–242. doi: 10.1016/s0896-6273(00)80835-1. [DOI] [PubMed] [Google Scholar]
- 31.Perlman JH, Wang W, Nussenzveig DR, Gershengorn MC. A disulfide bond between conserved extracellular cysteines in the thyrotropin-releasing hormone receptor is critical for binding. J Biol Chem. 1995;270:24682–24685. doi: 10.1074/jbc.270.42.24682. [DOI] [PubMed] [Google Scholar]
- 32.Reiner DJ, Newton EM, Tian H, Thomas JH. Diverse behavioural defects caused by mutations in Caenorhabditis elegans unc-43 CaM kinase II. Nature. 1999;402:199–203. doi: 10.1038/46072. [DOI] [PubMed] [Google Scholar]
- 33.Ren XC, Kim S, Fox E, Hedgecock EM, Wadsworth WG. Role of netrin UNC-6 in patterning the longitudinal nerves of Caenorhabditis elegans. J Neurobiol. 1999;39:107–118. [PubMed] [Google Scholar]
- 34.Rongo C, Kaplan JM. CaMKII regulates the density of central glutamatergic synapses in vivo. Nature. 1999;402:195–199. doi: 10.1038/46065. [DOI] [PubMed] [Google Scholar]
- 35.Schafer WR, Kenyon CJ. A calcium-channel homologue required for adaptation to dopamine and serotonin in Caenorhabditis elegans. Nature. 1995;375:73–78. doi: 10.1038/375073a0. [DOI] [PubMed] [Google Scholar]
- 36.Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 37.Singer WD, Brown HA, Sternweis PC. Regulation of eukaryotic phosphatidylinositol-specific phospholipase C and phospholipase D. Annu Rev Biochem. 1997;66:475–509. doi: 10.1146/annurev.biochem.66.1.475. [DOI] [PubMed] [Google Scholar]
- 38.Song HJ, Poo MM. Signal transduction underlying growth cone guidance by diffusible factors. Curr Opin Neurobiol. 1999;9:355–363. doi: 10.1016/s0959-4388(99)80052-x. [DOI] [PubMed] [Google Scholar]
- 39.Stein E, Tessier-Lavigne M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science. 2001;291:1928–1938. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- 40.Tam T, Mathews E, Snutch TP, Schafer WR. Voltage-gated calcium channels direct neuronal migration in Caenorhabditis elegans. Dev Biol. 2000;226:104–117. doi: 10.1006/dbio.2000.9854. [DOI] [PubMed] [Google Scholar]
- 41.Troemel ER, Chou JH, Dwyer ND, Colbert HA, Bargmann CI. Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell. 1995;83:207–218. doi: 10.1016/0092-8674(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 42.Troemel ER, Sagasti A, Bargmann CI. Lateral signaling mediated by axon contact and calcium entry regulates asymmetric odorant receptor expression in C. elegans. Cell. 1999;99:387–398. doi: 10.1016/s0092-8674(00)81525-1. [DOI] [PubMed] [Google Scholar]
- 43.Wadsworth WG, Hedgecock EM. Hierarchical guidance cues in the developing nervous system of C. elegans. BioEssays. 1996;18:355–362. doi: 10.1002/bies.950180505. [DOI] [PubMed] [Google Scholar]
- 44.Wadsworth WG, Bhatt H, Hedgecock EM. Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron. 1996;16:35–46. doi: 10.1016/s0896-6273(00)80021-5. [DOI] [PubMed] [Google Scholar]
- 45.Wang KH, Brose K, Arnott D, Kidd T, Goodman CS, Henzel W, Tessier-Lavigne M. Biochemical purification of a mammalian slit protein as a positive regulator of sensory axon elongation and branching. Cell. 1999;96:771–184. doi: 10.1016/s0092-8674(00)80588-7. [DOI] [PubMed] [Google Scholar]
- 46.White J, Southgate E, Thompson J, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 47.Zhang P, Johnson PS, Zollner C, Wang W, Wang Z, Montes AE, Seidleck BK, Blaschak CJ, Surratt CK. Mutation of human mu opioid receptor extracellular “disulfide cysteine” residues alters ligand binding but does not prevent receptor targeting to the cell plasma membrane. Brain Res Mol Brain Res. 1999;72:195–204. doi: 10.1016/s0169-328x(99)00241-7. [DOI] [PubMed] [Google Scholar]
- 48.Zheng JQ. Turning of nerve growth cones induced by localized increases in intracellular calcium ions. Nature. 2000;403:89–93. doi: 10.1038/47501. [DOI] [PubMed] [Google Scholar]
- 49.Zheng JQ, Felder M, Connor JA, Poo MM. Turning of nerve growth cones induced by neurotransmitters. Nature. 1994;368:140–144. doi: 10.1038/368140a0. [DOI] [PubMed] [Google Scholar]