Abstract
The ovarian hormone estradiol (E2) and insulin-like growth factor-I (IGF-I) interact in the CNS to regulate neuroendocrine function and synaptic remodeling. Previously, our laboratory showed that 2 d E2 treatment induces α1B-adrenoceptor expression and promotes IGF-I enhancement of α1-adrenoceptor potentiation of cAMP accumulation in the preoptic area (POA) and hypothalamus (HYP). This study examined the hypothesis that E2-dependent aspects of female reproductive function, including α1B-adrenoceptor expression and function in the POA and HYP, are mediated by brain IGF-I receptors (IGF-IRs) in female rats. Ovariohysterectomized rats were implanted with a guide cannula aimed at the third ventricle and treatedin vivo with vehicle or E2 daily for 2 d before experimentation. Intracerebroventricular infusions of JB-1, a selective IGF-IR antagonist, were administered every 12 hr beginning 1 hr before the first E2 injection. Administration of JB-1 during E2 priming completely blocks hormone-induced luteinizing hormone release and partially inhibits hormone-dependent reproductive behavior. Reproductive behavior is restored by intracerebroventricular infusion of 8-bromo-cGMP, the second messenger implicated in α1-adrenergic facilitation of lordosis. In addition, blockade of IGF-IRs during E2priming prevents E2-induced increases in α1B-adenoceptor binding density and abolishes acute IGF-I enhancement of NE-stimulated cAMP accumulation in HYP and POA slices. These data document the existence of a novel mechanism by which IGF-I participates in the remodeling of noradrenergic receptor signaling in the HYP and POA after E2 treatment. These events may help coordinate the timing of ovulation with the expression of sexual receptivity.
Keywords: estradiol, IGF-I, adrenoceptor, hypothalamus, preoptic area, reproduction
Estradiol (E2), the main estrogenic hormone produced and secreted by the ovaries, acts on the hypothalamus (HYP) and preoptic area (POA), brain regions responsible for female reproductive function (Barfield and Chen, 1977; Chappel, 1985). E2actions in these brain regions enhance reproductive success by ensuring that female sexual receptivity (lordosis) coincides with the release of pituitary luteinizing hormone (LH), which triggers ovulation (Pfaff, 1980; Etgen et al., 1992; Freeman, 1994).
E2 action in the HYP and POA modifies several cellular and molecular components of the noradrenergic system. Norepinephrine (NE) has been implicated as a major neurochemical mediator of both sexual receptivity and the preovulatory LH surge (Kalra and Kalra, 1983; Crowley, 1986; Etgen et al., 1992; Freeman, 1994; Herbison, 1997). NE actions in the HYP–POA can either facilitate or inhibit lordosis behavior and LH release, depending on the hormonal status of the animal (Etgen et al., 1992). NE activation of α1-adrenoceptors facilitates lordosis behavior and LH release only if animals have been exposed to E2 (Crowley, 1986; Kow et al., 1992; Weesner et al., 1993; Herbison, 1997). E2 increases α1B-adrenoceptor binding and mRNA levels in both HYP and POA (Petitti et al., 1992; Karkanias et al., 1996). These NE receptors are believed to mediate NE facilitation of sexual receptivity and preovulatory LH release (Kow et al., 1992; Hosny and Jennes, 1998). Despite evidence that E2 remodels the biochemical responses of the HYP and POA to NE, the mechanism or mechanisms by which E2 modulates the noradrenergic system are still unclear.
Cross-talk or interactions between E2 and insulin-like growth factor-I (IGF-I) have been demonstrated in the CNS, in various reproductive tissues and in cell cultures. For example, E2 can regulate the expression of IGF-I, IGF-I binding proteins, and IGF-I receptors (IGF-IRs) (Dickson et al., 1986; Pons and Torres-Aleman, 1993; Wimalasena et al., 1993; Sahlin et al., 1994). Likewise, IGF-I can regulate the expression and function of E2 receptors (Aronica and Katzenellenbogen, 1993;Stoica et al., 2000). In addition, IGF-I has been implicated in certain effects of E2 on synaptic structure and on neuroprotection (Duenas et al., 1996; Garcia-Segura et al., 1996;Patrone et al., 1996; Azcoitia et al., 1999; Fernandez-Galaz et al., 1999). Recently, we showed that IGF-I enhances α1-adrenoceptor function in the HYP and POA, but only in E2-primed rats (Quesada and Etgen, 2001). Because these observations suggest that E2effects on NE receptor function may involve IGF-I, we hypothesized that E2-dependent sexual receptivity, LH release and remodeling of the biochemical responses of HYP and POA slices to NE may be mediated via IGF-IR activity. Present data show that in vivo blockade of brain IGF-IRs during E2priming prevents increased expression of α1B-adrenoceptor in the HYP–POA and completely blocks E2-dependent LH release. In vivo blockade of IGF-IRs during E2 priming also blocks IGF-I enhancement of α1-adrenoceptor signaling in POA and HYP slices and partially inhibits E2-dependent sexual receptivity. These novel findings demonstrate that brain IGF-IRs are involved in the cellular and molecular actions underlying E2 regulation of female reproductive function.
MATERIALS AND METHODS
Animal treatments. Female Sprague Dawley rats (Taconic Farms, Germantown, NY) weighing 175–200 gm were anesthetized with ketamine (80 mg/kg body weight) and xylazine (4 mg/kg body weight), placed into a stereotaxic apparatus, and secured with ear bars and a nosepiece set at +5.0 mm. A 26 gauge guide cannula (Plastics One, Roanoke, VA) was implanted into the third ventricle using coordinates from the atlas of Pellegrino et al. (1979). Animals were bilaterally ovariohysterectomized (OVX) to remove the primary source of estrogen and progesterone immediately after stereotaxic surgery. Four to seven days after stereotaxic surgery and OVX, all rats were injected subcutaneously with either peanut oil (control) or with 2 μg of E2 benzoate (EB) (Steraloids Inc., Wilton, NH) in peanut oil 24 and 48 hr before killing. In most cases, multiple infusions of 1 μl of saline or 4 μg of JB-1 (Bachem, San Carlos, CA), a selective antagonist for IGF-IR, in 1 μl of saline were given intracerebroventricularly 1 hr before and 12 hr after both EB injections. Experimental use of animals was in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. The Institutional Animal Care and Use Committee of the Albert Einstein College of Medicine approved all animal protocols.
NE-stimulated cAMP accumulation. The animals were rapidly killed, and the brains were placed in artificial CSF (aCSF) (Yamamoto, 1972). In all experiments, brain slices were prepared between 1000 and 1100 hr to eliminate potential diurnal variation in cAMP (Kant et al., 1981) and adrenoceptor (Krauchi et al., 1984;Jhanwar-Uniyal et al., 1986) content. HYP and POA were dissected, and 350 μm slices were made on a McIlwain tissue chopper beginning ∼2 mm anterior to the optic chiasm and ending 1 mm anterior to the mammillary bodies. The first four slices containing the medial and lateral POA, suprachiasmatic nucleus, and supraoptic nucleus were taken and designated POA. The next slice was discarded, and the following four slices containing the anterior, lateral, ventromedial, paraventricular, arcuate, and dorsomedial nuclei of the HYP were kept and designated HYP. Individual slices were incubated at 35°C in 300 μl of oxygenated aCSF, which included a phosphodiesterase (PDE) inhibitor, 1 mm 3-isobutyl-1-methylxanthine (IBMX). IBMX was dissolved in absolute ethanol and added to slices such that the final concentration of ethanol was 1%. After a 75 min equilibration period slices were exposed for 15 min to 10 nm IGF-I (National Institutes of Health, National Hormone and Pituitary Program) dissolved in distilled water. Slices were then stimulated with either vehicle or 100 μm NE (Research Biochemicals, Natick, MA) dissolved in 0.01 N HCl. Each experiment included POA and HYP slices from EB-treated animals infused with either saline or JB-1, and each experiment was repeated four times. cAMP determination was done as previously described (Quesada and Etgen, 2001) using a modified Gilman cAMP assay (Brostrom and Kon, 1974).
Immunoblotting for phosphorylated extracellular receptor-activated kinase 1/2. HYP slices from E2-primed rats that were also infused with JB-1 or saline were incubated at 35°C in 300 μl of oxygenated aCSF for 75 min, then exposed for 15 min to vehicle or 10 nm IGF-I dissolved in distilled water. After vehicle or IGF-I treatments, two slices were pooled and prepared by Teflon homogenization in ice-cold 1% Triton X-100, 1 mm sodium vanadate, 1 mmphenylmethylsulfonyl fluoride, and 10 mmTris-HCl, pH 7.4. After homogenization, samples were spun for 15 min at 14,000 × g to remove insoluble material. Protein concentrations were determined by a modified Lowry assay. Fifty micrograms of protein were applied to 12.5% SDS-polyacrylamide minigels and resolved at 150 V for 1.5 hr. Proteins were transferred electrophoretically onto polyvinylidenediflouride membranes (Renaissance; New England Nuclear, Boston, MA) at 150 amps for 1 hr. Membranes were then blocked for 1 hr in 5% bovine albumin serum (BSA) and 0.1% Tween 20 in Tris-buffered saline (TBS) at 37°C. Membranes were incubated for 1 hr at 37°C with an antibody for phosphorylated extracellular receptor-activated kinase 1/2 (ERK1/2) (Tyr 204; 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA). Blots were stripped by incubating the membranes for 30 min at 50°C in stripping solution (0.0625 m Tris-HCl, pH 6.8; 0.2% SDS, 0.1m β-mercaptoethanol), washed three or four times in TBS for 10 min, and reprobed with rabbit anti-ERK2 (1:1000; Santa Cruz Biotechnology) for total ERK2 protein assessment. Binding of the primary antibody was detected by anti-rabbit secondary antibodies conjugated to horseradish peroxidase (1:10,000) in 5% BSA. Peroxidase activity was visualized by means of chemiluminescence. Blots were exposed to FUJI medical x-ray film (Fisher Scientific, Pittsburgh, PA). Band intensity was obtained by scanning autoradiograms on a DC-120 digital camera (Eastman Kodak, Rochester, NY) with a +3 diopter lens and analyzing the image using the Kodak 1D gel analysis program. Immunoblots were quantitatively analyzed by taking the ratio of the optical density (OD) of the phospho-ERK2 band to the OD of the total ERK2 band. That ratio was used to calculate the percentage of change in ERK2 activation of IGF-I versus vehicle-treated HYP slices run on the same gel.
Radioligand binding assays. To provide sufficient material for Scatchard analysis, tissue from two rats given identical hormone injections and drug treatments was combined. POA and HYP samples were homogenized separately in 5 ml of ice-cold Tris-MgCl2 buffer (50 mmTris HCl and 10 mm MgCl2, pH 7.4) using a Polytron at speed 5–6 for 20 sec. The homogenates were centrifuged for 10 min at 20,000 × g, the supernatant was discarded, and the pellet containing the membrane fraction was frozen at −70°C until assay. Freezing of the crude membrane fraction does not result in a measurable loss of any NE receptor subtype (Etgen and Karkanias, 1990).
The radioligand 3H-prazosin (87 Ci/mmol; New England Nuclear) was used to measure total α1-adrenoceptor binding in brain membranes. To distinguish α1A and α1B-adrenoceptor subtypes, chlorethylclonidine (CEC; Research Biochemicals), a selective, irreversible inactivator of the α1B-adrenoceptor, was used. Frozen membranes were resuspended in 6 ml of Na-HEPES buffer and split into two equal fractions. Each fraction was preincubated for 10 min at 37°C with vehicle or with 10 μm CEC. Reactions were stopped by addition of 6 ml of ice-cold Na-HEPES buffer and centrifugation for 10 min at 20,000 × g. The supernatant was discarded, and the pellet was resuspended in 6 ml of Tris-MgCl2 buffer. For Scatchard analysis, duplicate 200 μl aliquots were incubated for 20 min at 37°C with 0.05–5 nm3H-prazosin with and without a 2000-fold excess of phentolamine to assess nonspecific binding. Specific3H-prazosin binding after CEC inactivation reflects the α1A-adrenergic receptor population. The α1B-adrenoceptor population was determined by subtracting the binding of3H-prazosin after CEC inactivation from total specific 3H-prazosin binding. Bound and free 3H-prazosin were separated by rapid filtration through glass fiber filters (FPB-148 Whatman GF/B) on a Brandel (Gaithersburg, MD) cell harvester as described previously (Petitti et al., 1992). Ligand affinities (Kd), apparent receptor numbers (Bmax), and Hill coefficients were calculated using the EBDA ligand program (Elsevier-Biosoft, Cambridge, UK). Experiments used tissue from OVX control and EB-primed female rats infused chronically with either saline or JB-1 and were repeated four times.
LH radioimmunoassay. Concentration of LH in serum was determined using primary antibody for rat LH (1:30,000; National Hormone and Pituitary Program) and 125I-LH (3000–5000 cpm/100 μl; Covance Laboratories, Inc., Vienna, VA). Secondary antibody, goat anti-rabbit IgG, was obtained from Sigma (St. Louis, MO). Concentration of rat LH was expressed as ng RP-3 per ml, provided by the National Hormone and Pituitary Program. Animals were maintained on a reverse 14:10 light/dark cycle with the lights off at 11:00 A.M. Trunk blood samples were collected when the animals were decapitated at 8:00 P.M., allowed to clot overnight at 4°C, then centrifuged at 2000 × g for 30 min. Serum was decanted into polypropylene tubes and frozen at −20°C until analysis. Concentrations of LH in 100 μl aliquots of serum were determined in triplicate. Incubation periods between additions of primary antibody, radioiodinated hormone, and second antibody were 24 hr at room temperature. The sensitivity of the assay was 0.05 ng/tube, and samples were run in two separate assays. The intra-assay and inter-assay variances were 6 and 18%, respectively.
Reproductive behavior testing. Animals were maintained on a reverse 14:10 light/dark cycle with the lights off at 11:00 A.M. OVX female rats were primed with EB for 48 hr as described previously followed by 500 μg of progesterone 4 hr before behavior testing. Animals were tested once a week for 3 weeks after receiving one of three treatments in random order: (1) multiple infusions of JB-1 (4 μg/1 μl) dissolved in saline, (2) multiple infusions of 1 μl of saline, or (3) multiple infusions of JB-1 (4 μg/1 μl) followed by a single infusion of 8-bromo-cGMP (1 μg/2 μl) (Calbiochem, La Jolla, CA) administered 4 hr before behavior testing. Separate groups of rats received a single acute infusion of JB-1 (4 μg/1 μl) given either at 12 or 4 hr before testing (12 hr, n = 6; 4 hr,n = 4). Experienced stimulus male rats were placed in 20 gallon glass arenas and allowed to adapt for 10 min. Females were then placed in the arenas with a male until they received 10 mounts with pelvic thrusting. A lordosis quotient (LQ = number of lordosis responses/number of mounts × 100) was used as a measure of behavioral receptivity. The quality of lordosis was also scored on a scale of 0–3 (0, no lordosis; 1, shallow lordosis; 2, definitive dorsiflexion of the spine; 3, exaggerated lordosis). Anatomical verification of the cannula placement was made according to the atlas of Pellegrino et al. (1979).
Statistics. cAMP levels were analyzed with one-way ANOVA using the Statview statistical program with drug treatment as the only factor. Significant differences between means were determined for main effects by Fisher's PLSD. Radioligand binding and LH data were analyzed using two-way ANOVA with drug treatment and hormone as the two factors. Significant differences between means were determined for main effects by Fisher's PLSD. Behavioral data were analyzed using repeated measures one-way ANOVA with drug as the repeated factor, followed by Fisher's PLSD. Differences were considered significant ifp < 0.05.
RESULTS
E2 induction of α1B-adrenoceptor in the POA and HYP requires IGF-IR activity
Receptor tyrosine kinases such as IGF-I and insulin can increase α1D-adrenoceptor gene expression and function in vascular smooth muscle cells (Hu et al., 1996), and general tyrosine kinase inhibitors can block NE upregulation of α1B-adrenoceptors in clonal H cells (Bird et al., 1997). We examined the possibility that the E2-dependent increase in α1B-adrenoceptor density in the POA and HYP in female rats may require IGF-I activity. E2-treated female rats received multiple intracerebroventricular infusions of JB-1 or saline, and receptor density for total α1-adrenoceptor, α1A- and α1B-adrenoceptor was examined in HYP and POA membranes. JB-1 is a peptide analog of IGF-I based on the amino acid sequence of the C-terminal, D domain of IGF-I, which is involved in binding to the IGF-IR. It is a potent, highly selective, competitive antagonist of IGF-I-dependent receptor autophosphorylation and cellular proliferation (Pietrzkowski et al., 1992).
As reported previously, E2 modestly increases the density of 3H-prazosin binding sites (total α1-adrenoceptor) in POA and HYP membranes from saline-infused animals (p < 0.05). Multiple intracerebroventricular infusions of JB-1 block E2-induced increases in total α1-adrenoceptor binding in POA and HYP (Fig.1A). When the α1A- and α1B-adrenoceptor subtypes were pharmacologically distinguished, POA and HYP membranes from saline-infused, E2-treated females demonstrate a fourfold to fivefold fold increase in α1B-adrenoceptor density when compared with OVX control animals (p < 0.05). The density of α1A-adrenoceptors is not affected by E2 treatment (Fig. 1B). Multiple intracerebroventricular infusions of JB-1 prevent E2 induction of α1B-adrenoceptor expression in POA and HYP (Fig. 1C). Neither E2 treatment nor infusions of JB-1 affect the binding affinity of3H-prazosin in POA and HYP membranes (Table 1).
Fig. 1.
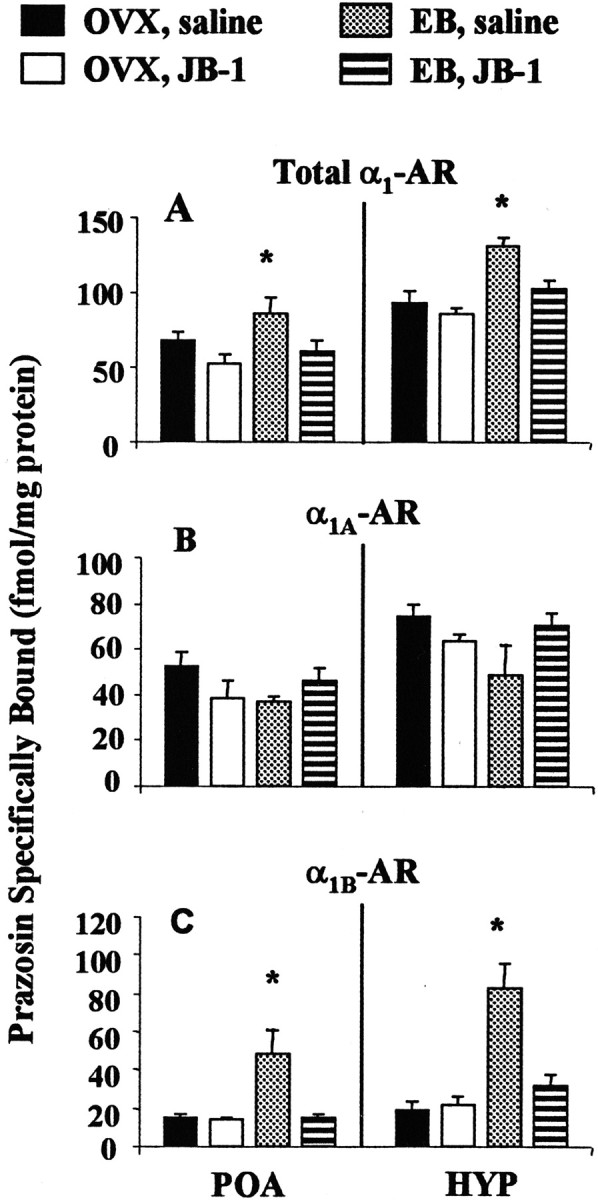
Effect of JB-1 infused into the third ventricle on E2-induced α1B-adrenoceptor (AR) density in the POA and HYP. Membranes from POA and HYP were prepared from OVX control and E2-treated (EB) female rats infused with saline or JB-1 as described in Materials and Methods. A, Total3H-prazosin binding corrected for nonspecific binding reflects both α1-AR subtypes. B, The α1A-AR population is measured after CEC inactivation.C, The α1B-AR population is determined by subtracting the binding of 3H-prazosin after CEC inactivation from total 3H-prazosin binding.Bmax values were obtained by Scatchard analysis. The data presented are the means ± SEM from four independent replications. *p < 0.05.
Table 1.
Effects of JB-1 on 3H-prazosin binding affinity
| KD (nm ± SEM) | ||||
|---|---|---|---|---|
| OVX | EB | |||
| Saline | JB-1 | Saline | JB-1 | |
| POA | 0.45 ± 0.08 | 0.70 ± 0.11 | 0.66 ± 0.11 | 0.82 ± 0.17 |
| HYP | 0.48 ± 0.10 | 0.54 ± 0.10 | 0.78 ± 0.15 | 0.64 ± 0.10 |
OVX female rats were primed with vehicle (OVX), or with EB (2 μg, 24 and 48 hr before killing). Multiple infusions of saline or JB-1 were given 1 hr before the first EB or vehicle injection and every 12 hr thereafter. KD values were obtained from Scatchard analysis of 3H-prazosin binding. Each value represents the mean of four independent replications.Bmax values are shown in Figure1A.
E2-induced LH surge is dependent on IGF-IR activity
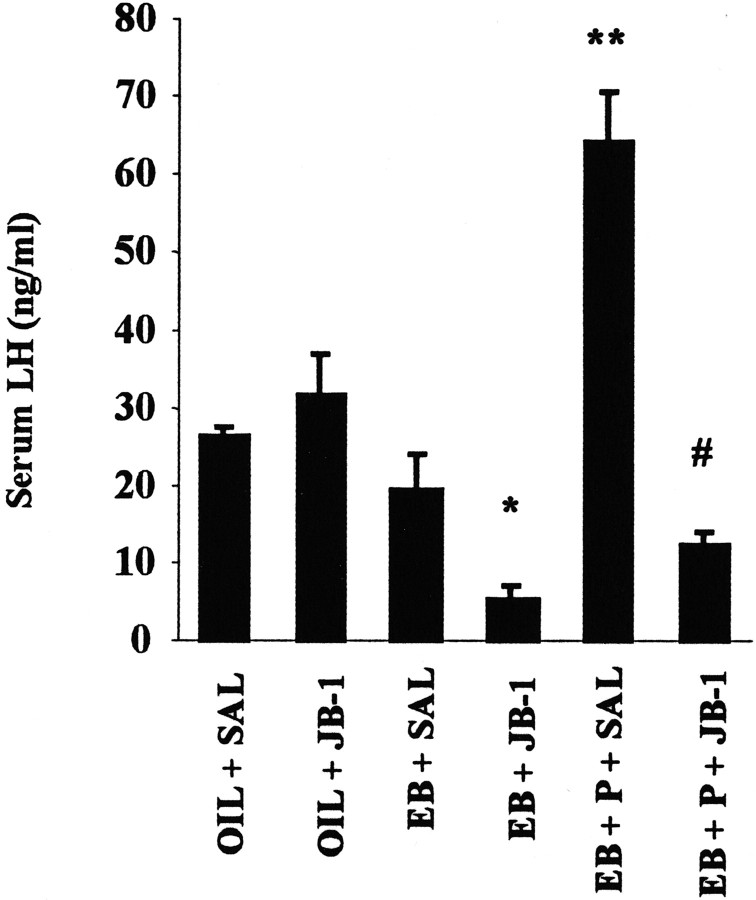
Administration of E2 to OVX female rats causes a daily LH surge (Caligaris et al., 1971). IGF-I can also affect LH release by altering gonadotropin-releasing hormone (GnRH) release from the HYP (Hiney et al., 1991; Soldani et al., 1994; Wilson, 1995;Hiney et al., 1996). We tested the hypothesis that steroid-induced LH release requires IGF-IR activation. OVX rats were given one of three hormone treatments (Oil, EB, or EB + progesterone; N = 4–8) and were infused every 12 hr with either JB-1 or saline. The estrogen priming consisted of two injections of EB (2 μg) 24 and 48 hr before killing. Progesterone (500 μg) was injected 44 hr after the first EB injection and 4 hr before killing. LH levels in OVX rats are chronically elevated because of the loss of steroid negative feedback (Ortmann et al., 1988). Multiple intracerebroventricular infusions of JB-1 have no effect on these elevated LH levels in OVX rats given no hormone replacement (Fig. 2). Treatment of OVX, saline-infused rats with E2 alone tends to lower LH levels, but this effect is not statistically significant. However, LH levels in E2-treated rats given multiple intracerebroventricular infusions of JB-1 are significantly lower than in OVX controls (p < 0.05). Because this result does not conclusively indicate if blockade of IGF-IR activity enhances E2 negative feedback or inhibits E2 positive feedback on LH secretion, we treated OVX rats with both E2 and progesterone. LH release is further enhanced when E2-treated, OVX female rats are subsequently given progesterone (Caligaris et al., 1971). The administration of E2 plus progesterone significantly increases LH levels when compared with OVX controls (p < 0.05). Intracerebroventricular infusions of JB-1 during estrogen priming abolish the LH surge produced by administration of EB plus progesterone to OVX female rats (p < 0.05) (Fig. 2).
Fig. 2.
Effect of JB-1 infused into the third ventricle on hormone-induced LH secretion. Serum for LH radioimmunoassay was prepared as described in Materials and Methods. OVX rats were given one of the following treatments: OIL, EB, or EB + progesterone (P). Multiple intracerebroventricular infusions of vehicle saline (SAL) or JB-1 were given 1 hr before the first EB injection and every 12 hr thereafter. The data presented are the means ± SEM from four to eight independent replications. *OIL + SAL, OIL + JB-1, EB + SAL, and EB + P + SAL (p < 0.05); **all other groups (p < 0.05), #OIL + SAL, OIL + JB-1, and EB + P + SAL (p < 0.05).
E2-dependent sexual behavior is partially dependent on IGF-IR activity
To examine the possibility that E2-dependent sexual behavior requires IGF-IR activity, E2 plus progesterone-treated, OVX female rats received multiple intracerebroventricular infusions of JB-1 during estrogen priming. The E2 priming dosages used in our experimental paradigm are subthreshold for lordosis behavior; administration of progesterone is required to facilitate sexual receptivity in these animals (Pfaff et al., 1994). JB-1 modestly but significantly (p < 0.05) decreases lordosis behavior (Fig.3A,B). Recently, acute intracerebroventricular infusion of IGF-I was shown to facilitate female sexual behavior independent of E2 priming (Apostolakis et al., 2000). Therefore, we assessed whether acute JB-1 infusion to block IGF-IRs near the time of lordosis testing would inhibit the behavior. Acute intracerebroventricular infusion of JB-1 at either 12 hr (Fig. 3C) or 4 hr (LQ: saline = 70 ± 4.0; JB-1 = 75 ± 2.9) before testing has no effect on lordosis behavior.
Fig. 3.

Effect of JB-1 infused into the third ventricle on lordosis behavior of E2- and progesterone-primed female rats. A, Lordosis quotient (LQ) after chronic infusions of saline (SAL) or JB-1 alone or JB-1 followed by intracerebroventricular infusion of 1 μg of 8-bromo-cGMP 4 hr before behavior testing. B, Quality of lordosis (QL) in same animals shown in A. Values presented are the means ± SEM (n = 9). *p < 0.05. C, LQ after acute infusion of 4 μg of JB-1 or SAL 12 hr before behavior testing. Values presented are the means ± SEM (n = 6).
A likely mechanism by which α1-adrenoceptors facilitate female sexual behavior is by stimulating cGMP synthesis, which in turn activates protein kinase G (Chu and Etgen, 1999). For example, intracerebroventricular infusion of 8-bromo-cGMP 4 hr before lordosis behavior testing reversed α1-adrenoceptor antagonist inhibition of lordosis behavior (Chu and Etgen, 1999). Thus, we examined whether the inhibitory effects of multiple intracerebroventricular infusions of JB-1 on sexual behavior can be rescued by intracerebroventricular infusion of 8-bromo-cGMP. During one of their three weekly tests, E2-primed animals receiving multiple intracerebroventricular infusions of JB-1 were infused with 1 μg of 8-bromo-cGMP at the time of progesterone injection. Intracerebroventricular infusion of 8-bromo-cGMP reverses the inhibitory effects of multiple intracerebroventricular infusions of JB-1 on sexual behavior (p < 0.05) (Fig.3A,B).
Blockade of IGF-IR during E2 priming prevents E2-dependent, IGF-I enhancement of NE-stimulated cAMP accumulation
We previously demonstrated that acute application of IGF-I enhanced NE-stimulated cAMP accumulation in POA and HYP slices, via modulation of α1-adrenoceptor function, only if the animal is E2-primed (Quesada and Etgen, 2001). The present study showed that blockade of IGF-IR during estrogen priming prevents E2-dependent increases in α1B-adrenoceptor binding in the HYP and POA. We therefore examined the hypothesis that E2-dependent, IGF-I modulation of NE signaling in the POA and HYP of female rats requires IGF-IR activation during E2 priming.
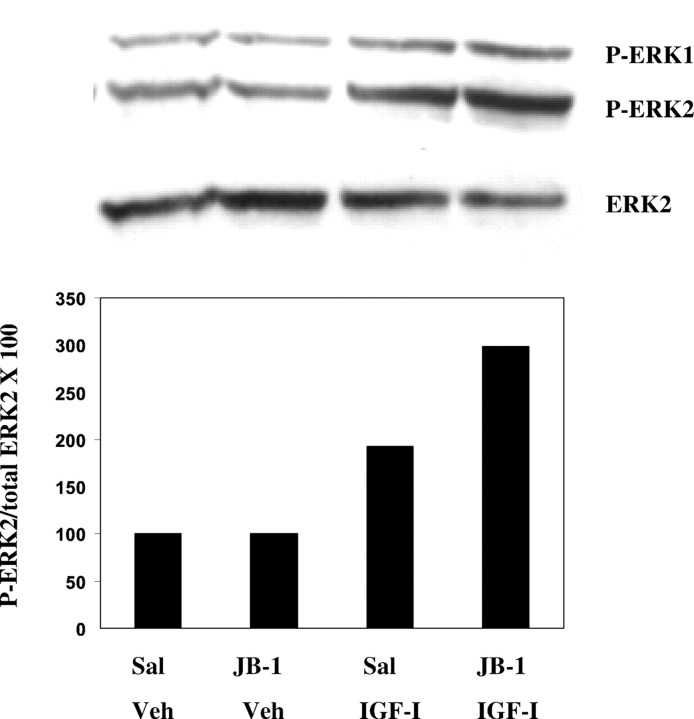
E2-treated, OVX female rats received multiple intracerebroventricular infusions of JB-1 or saline every 12 hr during the 2 d E2 priming period. IGF-I enhances NE-stimulated cAMP accumulation in POA and HYP slices of E2-treated female rats infused with saline. Multiple intracerebroventricular infusions of JB-1 abolish IGF-I enhancement of NE-stimulated cAMP accumulation in POA and HYP slices of E2-primed animals (Fig.4). It is possible that residual JB-1 from the multiple intracerebroventricular infusions was present in the slices and blocked IGF-I potentiation of NE-stimulated cAMP accumulation. To determine if IGF-IRs could be activated by application of IGF-I onto brain slices, phosphorylation of ERK1/2 in response to acute application of IGF-I onto slices from JB-1 infused rats was monitored. The mitogen-activated protein kinase cascade, including ERK1 and ERK2, are known downstream substrates of IGF-IR activation (Zumkeller and Schwab, 1999). Figure 5shows that multiple infusions of JB-1 do not interfere with the ability of acute IGF-I application to activate IGF-IRs, as measured by IGF-I-induced phosphorylation of ERK1/2 in HYP slices from E2-treated rats.
Fig. 4.
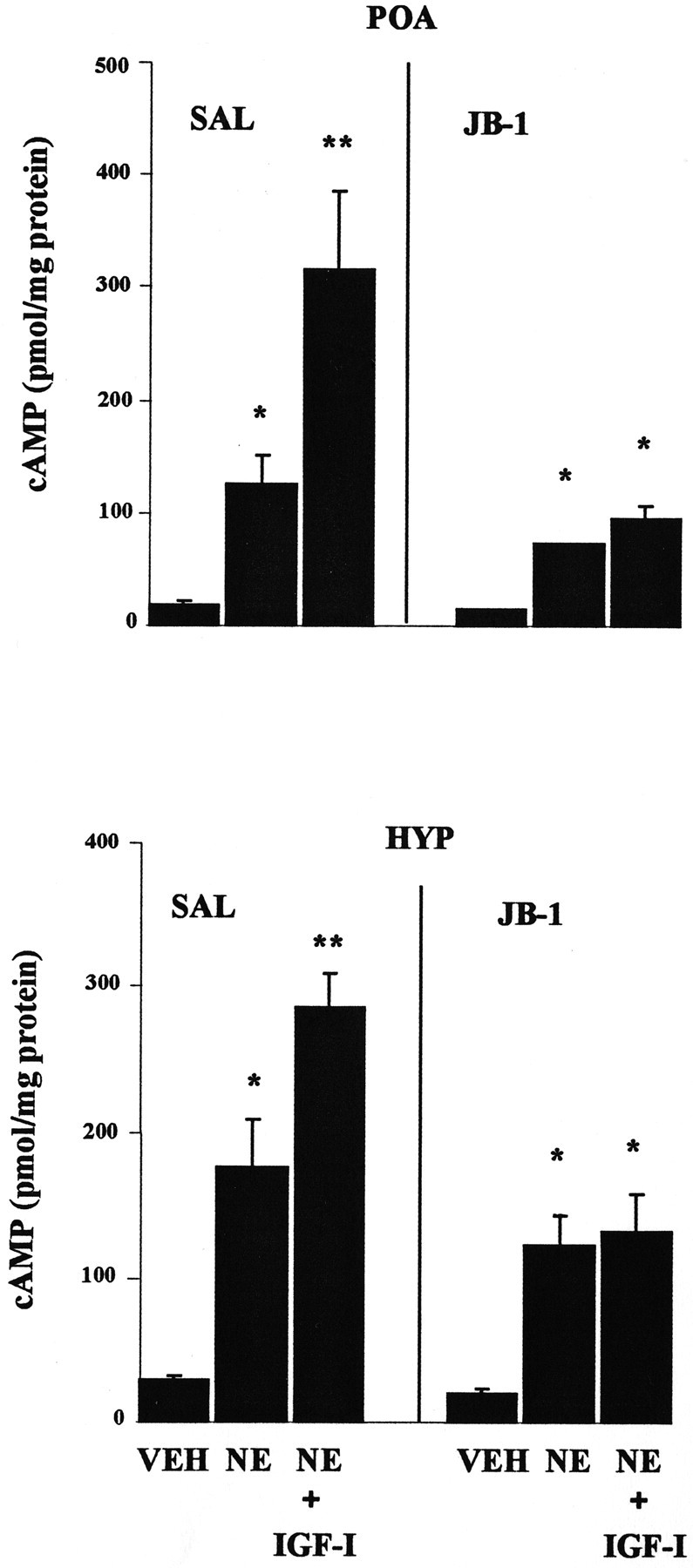
Effect of JB-1 infused into the third ventricle on E2-dependent, IGF-I enhancement of NE-stimulated cAMP. POA and HYP slices from E2-treated female rats were prepared from animals infused chronically with JB-1 or saline (SAL) as described in Materials and Methods. Slices were incubated for 15 min with 10 nm IGF-I followed by a 20 min incubation with 0.01 N HCl vehicle (VEH) or 100 μm NE. The PDE inhibitor 1 mm IBMX was included. The data presented are the means ± SEM from four independent replications. *VEH; **all other groups (p < 0.05).
Fig. 5.
Effect of JB-1 infused into the third ventricle on acute IGF-I activation of extracellular receptor activated kinase 2 (ERK2). HYP slices from E2-treated female rats were prepared from animals infused chronically with JB-1 or saline (Sal) as described in Materials and Methods and incubated for 15 min with 10 nm IGF-I or vehicle (Veh). Immunoblots were done using a monoclonal antibody for phosphorylated ERK1/2 (P-ERK1/2) and for total ERK2. Phosphotyrosine immunoblots were quantitatively analyzed by taking the ratio of the OD of the P-ERK2 band to the OD of the total ERK2 band. The immunoblot shown is representative of two independent experiments.
DISCUSSION
The data presented herein demonstrate that the positive feedback effects of E2 on LH release require IGF-IR activity in the brain and that E2 priming of sexual receptivity also involves IGF-I action. Present results also show that E2-induced increases in α1-adrenoceptor binding and function in the HYP and POA require IGF-IR activity. Thus, our findings provide the first evidence that IGF-IRs in the brain are physiological mediators of multiple aspects of estrogen action on the hypothalamic–pituitary–gonadal axis and sexual behavior.
E2 and IGF-I regulation of gene transcription
E2 actions in the POA and HYP facilitate reproductive success by ensuring that the period of female sexual receptivity coincides with the release of LH, which triggers ovulation (Pfaff, 1980; Etgen et al., 1992; Freeman, 1994). These effects of E2 require gene transcription and protein synthesis in target HYP-POA neurons that control reproductive function (Pfaff et al., 1994). We have shown that E2increases α1B-adrenoceptor mRNA levels and binding density in the HYP-POA (Petitti et al., 1992; Karkanias et al., 1996). We now demonstrate that chronic blockade of brain IGF-IRs with the competitive antagonist JB-1 prevents the E2-induced increase in the density of α1B-adrenoceptor binding sites in the HYP and POA. JB-1 treatment does not have nonspecific effects on brain NE receptors, because neither basal α1B-adrenoceptor nor α1A-adrenoceptor densities are affected by the drug. It is also unlikely that the actions of JB-1 are attributable to interference with other growth factor signaling pathways (e.g., epidermal growth factor, platelet-derived growth factor), because this peptide analog of IGF-1 does not block the actions of other growth factors (Pietrzkowski et al., 1992).
The mechanism or mechanisms by which IGF-IR and E2 interact to regulate α1B-adrenoceptor expression in the HYP-POA remain unknown, although there are several possibilities. Downstream mitogenic signals activated by IGF-IR may increase the expression or transcription activating function of E2 receptors (Aronica and Katzenellenbogen, 1993; Stoica et al., 2000). Therefore, blockade of IGF-IRs could result in downregulation of either the expression and/or transcription activation function of E2 receptors. This possibility is consistent with the observation that IGF-IRs are colocalized with both E2 receptor-α and -β in neurons and glia in various brain regions, including HYP and POA (Cardona-Gomez et al., 2000). Alternatively, IGF-I may promote secretion of other trophic factors or signaling molecules from HYP-POA glial cells in an E2-dependent manner, resulting in increased expression of α1B-adrenoceptor in the HYP-POA.
The increased expression of α1B-adrenoceptor in the HYP-POA, observed in response to E2treatment, is believed to mediate the facilitatory component of NE action on sexual receptivity and LH secretion (Kow et al., 1992; Hosny and Jennes, 1998). Both α1B-adrenoceptor mRNA and protein are expressed in the POA and HYP (Blendy et al., 1990;Pieribone et al., 1994; Acosta-Martinez et al., 1999). In the POA, GnRH cell bodies show the highest density of α1B-adrenoceptor-immunostaining, whereas GnRH nerve terminals in the median eminence show moderate immunostaining (Hosny and Jennes, 1998). Many cells and fibers in the arcuate nucleus–median eminence, the site of GnRH release, demonstrate robust α1B-adrenoceptor immunostaining (Acosta-Martinez et al., 1999). This is also the primary neural site at which IGF-I and E2 interact to regulate synaptic plasticity (Fernandez-Galaz et al., 1999). The ventromedial HYP, the major site of E2 facilitation of sexual receptivity, also contains both mRNA and protein for α1B-adrenoceptor (Pieribone et al., 1994;Acosta-Martinez et al., 1999). Thus, E2regulation of the hypothalamic-pituitary-gonadal axis and sexual behavior may involve IGF-IR-dependent increases in the expression of α1B-adrenoceptors in target regions of the HYP-POA.
E2 and IGF-I regulation of LH release
The ability of chronic JB-1 treatment to reduce E2 and progesterone-dependent increases in plasma LH suggests that IGF-IR blockade suppresses estrogen positive feedback regulation of LH release. JB-1 treatment did not interfere with LH synthesis or release, because JB-1 infusion into OVX rats given no hormone replacement does not reduce the elevated plasma LH levels observed in OVX rats. If JB-1 acts on pituitary gonadotropes to block LH synthesis or secretion, LH levels in the OVX control rats infused with JB-1 should have been reduced. This was clearly not the case. Nonetheless, it is possible that treatment with the IGF-IR antagonist influenced pituitary sensitivity to GnRH. Future experiments could evaluate this issue by measuring the LH response to exogenously administered GnRH in JB-1-infused animals.
Although we cannot rule out the possibility that the IGF-IR antagonist interferes with progesterone action as well, it is more likely that JB-1 acted primarily to interfere with estrogen-positive feedback. First, progesterone facilitation of LH release requires E2 priming; in the absence of previous exposure to estrogen, progesterone inhibits LH secretion (Freeman, 1994). Second, at the time blood samples were taken from E2-treated rats infused with saline, plasma LH levels were not significantly lower than in OVX control animals (Fig.2). Blood was collected 48 hr after the first estrogen injection, ∼9 hr into the dark phase of the reverse light/dark cycle. Thus, it is likely that the LH values observed in E2-treated, saline-infused rats reflect positive feedback caused by estrogen alone. Plasma LH levels in E2-treated rats were significantly reduced by JB-1, suggesting that antagonism of IGF-IR activity influences estrogen-positive feedback.
E2-induced synaptic remodeling in the arcuate nucleus, which is also dependent on IGF-IR activity (Fernandez-Galaz et al., 1999), is believed to be critical for the preovulatory release of GnRH (Perez et al., 1993; Garcia-Segura et al., 1994). Thus, JB-1 treatment in our experimental paradigm may also inhibit E2-induced synaptic remodeling in the arcuate nucleus, resulting in inhibition of E2-induced positive feedback on GnRH release, and ultimately inhibition of LH release. Our results add to an increasing body of evidence suggesting that IGF-I and E2 work together to modulate LH secretion. Furthermore, they implicate the α1B-adrenoceptor as a mediator of E2/IGF-I enhancement of gonadotropin release.
Interaction between IGF-I and E2 may also be involved in changes in estrogen-negative feedback believed to be essential for the initiation of female puberty. Peripheral administration of IGF-I to OVX, adolescent female monkeys attenuates E2 negative feedback on LH release (Wilson, 1995). Likewise, intracerebroventricular administration of IGF-I enhances LH release in both juvenile and peripubertal female rats, accelerating the initiation of puberty (Hiney et al., 1996).
E2 and IGF-I regulation of reproductive behavior
IGF-IR blockade during E2 priming partially attenuates E2-dependent sexual behavior. The effects of JB-1 on lordosis are caused by blockade of IGF-IRs during E2 priming rather than by residual JB-1 remaining in the brain after multiple infusions. This conclusion is supported by the observation that acute intracerebroventricular infusion of JB-1 between 4 and 12 hr before behavior testing has no effect on lordosis. We also showed that infusion of 8-bromo-cGMP, a cell-permeable analog of cGMP, reverses the partial inhibition of sexual behavior induced by multiple intracerebroventricular infusions of JB-1. This observation is in accordance with previous results from our laboratory demonstrating that inhibition of sexual behavior by systemic administration of an α1-adrenoceptor antagonist is reversed by intracerebroventricular administration of 8-bromo-cGMP (Chu and Etgen, 1999). Moreover, administration of progesterone to E2-primed rat switches α1-adrenoceptor signaling from phospholipase C activation and potentiation of adenylyl cyclase activity to stimulation of nitric oxide-dependent activation of cGMP synthesis (Chu and Etgen, 1999; Chu et al., 1999). Hence, the ability of 8-bromo-cGMP to rescue the partial inhibition of E2-dependent sexual behavior by JB-1 indirectly suggests that interference with α1-adrenoceptor signaling pathways may be responsible for the observed lordosis inhibition.
There are several reasons why JB-1 may produce only partial attenuation of sexual behavior. First, It is possible that multiple intracerebroventricular infusions of JB-1 did not completely block brain IGF-IRs. Therefore, future experiments using higher doses or constant intracerebroventricular delivery of JB-1 might produce a more complete inhibition of lordosis. Second, JB-1 treatment blocks the E2-dependent increase of α1B-adrenoceptor binding without affecting basal α1B-adrenoceptor in the HYP-POA. Hence, the remaining α1B-adrenoceptor present might be sufficient to support partial expression of sexual behavior. Third, E2 is thought to produce maximal sexual receptivity in part by induction of progesterone receptor expression in the HYP (Mac-Lusky and McEwen, 1978; Blaustein, 1982). Thus, JB-1 administration may have incompletely blocked E2induction of progesterone receptors in the HYP. Fourth, because the survival of species is dependent on successful reproduction, there are likely to be redundant neural elements (neurotransmitters, neurohormones and neuropeptides) on which E2 and progesterone act to coordinate reproductive physiology. Therefore, IGF-IRs may mediate the actions of E2 on only a subset of these neural targets of hormone action.
E2 and IGF-I modulation of α1-adrenoceptor signaling
Previously, we demonstrated that acute application of IGF-I onto HYP and POA slices in vitro enhances NE-stimulated cAMP accumulation, via α1-adrenoceptor potentiation of adenylyl cyclase activation, only in E2-primed female rats (Quesada and Etgen, 2001). We now show that blockade of IGF-IR activation during E2 priming prevents both the increased expression of α1B-adrenoceptors and IGF-I enhancement of NE-stimulated cAMP accumulation in the HYP and POA. Thus, the E2-dependent effect of acute application of IGF-I on α1-adrenoceptor signaling, like E2 induction of α1B-adrenoceptors and LH release, relies on brain IGF-IR activity during E2 priming. The E2 dependence of IGF-I potentiation of NE-stimulated cAMP synthesis might be attributable to the induction of α1B-adrenoceptor expression in HYP and POA cells that also express IGF-IRs. Because in vitroapplication of IGF-I onto HYP slices from JB-1-infused rats induces ERK1/2 phosphorylation, the effects of JB-1 on NE signaling must be caused by blockade of IGF-IR during E2 priming rather than residual JB-1 remaining in the brain after the multiple intracerebroventricular infusions. In addition, E2 can increase125I-IGF-I binding density (Quesada and Etgen, 2001) and IGF-IR content in the HYP (Michels et al., 1993; Pons and Torres-Aleman, 1993; Wimalasena et al., 1993). Therefore, E2 dependence of the interaction between IGF-I and NE may involve upregulation of IGF-IR, the α1B-adrenoceptor or both.
In summary, these data indicate that brain IGF-IR activity is necessary for long-term effects of E2 on α1B-adrenoceptor expression and function in the HYP and POA as well as for hormone-dependent sexual receptivity and positive feedback regulation of LH release. These results demonstrate a novel mechanism by which changes in noradrenergic signal transduction resulting from E2 and IGF-I action in the brain control GnRH release and the expression of reproductive behavior.
Footnotes
This work was supported by National Institutes of Health Grants R37 MH 41414, RO1 HD 29856, and T32 DK 07513 and by the Department of Neuroscience, Albert Einstein College of Medicine. We thank Chioma Oyeamalu for technical assistance and Dr. Jorge Larocca for critical review of this manuscript. The human IGF-I and antibody for rat LH were obtained through National Hormone and Pituitary Program, National Institute of Diabetes and Digestive and Kidney Diseases, and Dr. A. F. Parlow (Harbor-UCLA Research and Education Institute).
Correspondence should be addressed to Anne M. Etgen, Department of Neuroscience, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Bronx, NY 10461. E-mail: Etgen@aecom.yu.edu.
REFERENCES
- 1.Acosta-Martinez M, Fiber JM, Brown RD, Etgen AM. Localization of α1B-adrenergic receptor in female rat brain regions involved in stress and neuroendocrine function. Neurochem Int. 1999;35:383–391. doi: 10.1016/s0197-0186(99)00077-7. [DOI] [PubMed] [Google Scholar]
- 2.Apostolakis EM, Garai J, Lohmann JE, Clark JH, O'Malley BW. Epidermal growth factor activates reproductive behavior independent of ovarian steroids in female rodents. Mol Endocrinol. 2000;14:1086–1098. doi: 10.1210/mend.14.7.0490. [DOI] [PubMed] [Google Scholar]
- 3.Aronica S, Katzenellenbogen B. Stimulation of estrogen receptor-mediated transcription and alteration in the phosphorylation state of the rat uterine estrogen receptor by estrogen, cyclic adenosine monophosphate, and insulin-like growth factor-I. Mol Endocrinol. 1993;7:743–752. doi: 10.1210/mend.7.6.7689695. [DOI] [PubMed] [Google Scholar]
- 4.Azcoitia I, Sierra A, Garcia-Segura LM. Neuroprotective effects of estradiol in the adult rat hippocampus: interaction with insulin-like growth factor-I signalling. J Neurosci Res. 1999;58:815–822. doi: 10.1002/(sici)1097-4547(19991215)58:6<815::aid-jnr8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 5.Barfield RJ, Chen JJ. Activation of estrous behavior in ovariectomized rats by intracerebral implants of estradiol benzoate. Endocrinology. 1977;101:1716–1725. doi: 10.1210/endo-101-6-1716. [DOI] [PubMed] [Google Scholar]
- 6.Bird KS, Anderson JL, Toews ML. Modulation of α1B-adrenoceptor expression by agonist and protein kinase inhibitors. Eur J Pharmacol. 1997;340:267–275. doi: 10.1016/s0014-2999(97)01418-0. [DOI] [PubMed] [Google Scholar]
- 7.Blaustein JD. Alteration of sensitivity to progesterone facilitation of lordosis in guinea pigs by modulation of hypothalamic progestin receptors. Brain Res. 1982;243:287–300. doi: 10.1016/0006-8993(82)90252-9. [DOI] [PubMed] [Google Scholar]
- 8.Blendy JA, Grimm LJ, Perry DC, West-Johnsrud L, Kellar KJ. Electroconvulsive shock differentially increases binding to α1-adrenergic receptor subtypes in discrete regions of rat brain. J Neurosci. 1990;10:2580–2586. doi: 10.1523/JNEUROSCI.10-08-02580.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brostrom CO, Kon C. An improved protein binding assay for cyclic AMP. Anal Biochem. 1974;58:459–468. doi: 10.1016/0003-2697(74)90214-0. [DOI] [PubMed] [Google Scholar]
- 10.Caligaris L, Astrada JJ, Taleisnik S. Biphasic effect of progesterone on the release of gonadotropin in rats. Endocrinology. 1971;89:331–337. doi: 10.1210/endo-89-2-331. [DOI] [PubMed] [Google Scholar]
- 11.Cardona-Gomez GP, DonCarlos L, Garcia-Segura LM. Insulin-like growth factor I receptors and estrogen receptors colocalize in female rat brain. Neuroscience. 2000;99:751–760. doi: 10.1016/s0306-4522(00)00228-1. [DOI] [PubMed] [Google Scholar]
- 12.Chappel SC. Neuroendocrine regulation of luteinizing hormone and follicle stimulating hormone: a review. Life Sci. 1985;36:97–103. doi: 10.1016/0024-3205(85)90087-6. [DOI] [PubMed] [Google Scholar]
- 13.Chu HP, Etgen AM. Ovarian hormone dependence of α1-adrenoceptor activation of the nitric oxide-cGMP pathway: relevance for hormonal facilitation of lordosis behavior. J Neurosci. 1999;19:7191–7197. doi: 10.1523/JNEUROSCI.19-16-07191.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu HP, Morales JC, Etgen AM. Cyclic GMP may potentiate lordosis behaviour by progesterone receptor activation. J Neuroendocrinol. 1999;11:107–113. doi: 10.1046/j.1365-2826.1999.00298.x. [DOI] [PubMed] [Google Scholar]
- 15.Crowley WR. Reproductive neuroendocrine regulation in the female rat by central catecholamine-neuropeptide interactions: a local control hypothesis. Ann NY Acad Sci. 1986;474:423–436. doi: 10.1111/j.1749-6632.1986.tb28032.x. [DOI] [PubMed] [Google Scholar]
- 16.Dickson RB, McManaway ME, Lippman ME. Estrogen-induced factors of breast cancer cells partially replace estrogen to promote tumor growth. Science. 1986;232:1540–1543. doi: 10.1126/science.3715461. [DOI] [PubMed] [Google Scholar]
- 17.Duenas M, Torres-Aleman I, Naftolin F, Garcia-Segura LM. Interaction of insulin-like growth factor-I and estradiol signaling pathways on hypothalamic neuronal differentiation. Neuroscience. 1996;74:531–539. doi: 10.1016/0306-4522(96)00142-x. [DOI] [PubMed] [Google Scholar]
- 18.Etgen AM, Karkanias GB. Estradiol regulates the number of α1- but not β- or α2- noradrenergic receptors in hypothalamus of females rats. Neurochem Int. 1990;16:1–9. doi: 10.1016/0197-0186(90)90117-c. [DOI] [PubMed] [Google Scholar]
- 19.Etgen AM, Ungar S, Petitti N. Estradiol and progesterone modulation of norepinephrine neurotransmission: implications for the regulation of female reproductive behavior. J Neuroendocrinol. 1992;31:799–807. doi: 10.1111/j.1365-2826.1992.tb00167.x. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Galaz MC, Naftolin F, Garcia-Segura LM. Phasic synaptic remodeling of the rat arcuate nucleus during the estrous cycle depends on insulin-like growth factor-I receptor activation. J Neurosci Res. 1999;55:286–292. doi: 10.1002/(SICI)1097-4547(19990201)55:3<286::AID-JNR3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Freeman ME. The neuroendocrine control of the ovarian cycle in the rat. In: Knobil E, Neill JD, editors. The Physiology of reproduction, Vol 2. Raven; New York: 1994. pp. 613–658. [Google Scholar]
- 22.Garcia-Segura LM, Chowen JA, Parducz A, Naftolin F. Gonadal hormones as promoters of structural synaptic plasticity: cellular mechanisms. Prog Neurobiol. 1994;44:279–307. doi: 10.1016/0301-0082(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Segura LM, Duenas M, Fernandez-Galaz MC, Chowen JA, Argente J, Naftolin F, Torres-Aleman I. Interaction of the signalling pathways of insulin-like growth factor-I and sex steroids in the neuroendocrine hypothalamus. Horm Res. 1996;46:160–164. doi: 10.1159/000185016. [DOI] [PubMed] [Google Scholar]
- 24.Herbison AE. Noradrenergic regulation of cyclic GnRH secretion. Rev Reprod. 1997;2:1–6. doi: 10.1530/ror.0.0020001. [DOI] [PubMed] [Google Scholar]
- 25.Hiney JK, Ojeda SR, Dees WL. Insulin-like growth factor I: a possible metabolic signal involved in the regulation of female puberty. Neuroendocrinology. 1991;54:420–423. doi: 10.1159/000125924. [DOI] [PubMed] [Google Scholar]
- 26.Hiney JK, Srivastava V, Nyberg CL, Ojeda SR, Dees WL. Insulin-like growth factor I of peripheral origin acts centrally to accelerate the initiation of female puberty. Endocrinology. 1996;137:3717–3728. doi: 10.1210/endo.137.9.8756538. [DOI] [PubMed] [Google Scholar]
- 27.Hosny S, Jennes L. Identification of α1B-adrenergic receptor protein in gonadotropin releasing hormone neurones of the female rat. J Neuroendocrinol. 1998;10:687–692. doi: 10.1046/j.1365-2826.1998.00256.x. [DOI] [PubMed] [Google Scholar]
- 28.Hu ZW, Shi XY, Hoffman BB. Insulin and insulin-like growth factor I differentially induce α1-adrenergic receptor subtype expression in rat vascular smooth muscle cells. J Clin Invest. 1996;98:1826–1834. doi: 10.1172/JCI118983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jhanwar-Uniyal M, Roland CR, Leibowitz SF. Diurnal rhythm of α2-noradrenergic receptors in the paraventricular nucleus and other brain areas: relation to circulating corticosterone and feeding behavior. Life Sci. 1986;38:473–482. doi: 10.1016/0024-3205(86)90073-1. [DOI] [PubMed] [Google Scholar]
- 30.Kalra SP, Kalra PS. Neural regulation of luteinizing hormone secretion in the rat. Endocr Rev. 1983;4:311–351. doi: 10.1210/edrv-4-4-311. [DOI] [PubMed] [Google Scholar]
- 31.Kant GJ, Sessions GR, Lenox RH, Meyerhoff JL. The effects of hormonal and circadian cycles, stress, and activity on levels of cyclic AMP and cyclic GMP in pituitary, hypothalamus, pineal and cerebellum of female rats. Life Sci. 1981;29:2491–2499. doi: 10.1016/0024-3205(81)90704-9. [DOI] [PubMed] [Google Scholar]
- 32.Karkanias GB, Ansonoff MA, Etgen AM. Estradiol regulation of α1b-adrenoceptor mRNA in female rat hypothalamus-preoptic area. J Neuroendocrinol. 1996;8:449–455. doi: 10.1046/j.1365-2826.1996.04716.x. [DOI] [PubMed] [Google Scholar]
- 33.Kow LM, Weesner GD, Pfaff DW. α1-adrenergic agonists act on the ventromedial hypothalamus to cause neuronal excitation and lordosis facilitation: electrophysiological and behavioral evidence. Brain Res. 1992;588:237–245. doi: 10.1016/0006-8993(92)91581-x. [DOI] [PubMed] [Google Scholar]
- 34.Krauchi K, Wirz-Justice A, Morimasa T, Willener R, Feer H. Hypothalamic α2- and β-adrenoceptor rhythms are correlated with circadian feeding: evidence from chronic methamphetamine treatment and withdrawal. Brain Res. 1984;321:83–90. doi: 10.1016/0006-8993(84)90683-8. [DOI] [PubMed] [Google Scholar]
- 35.MacLusky NJ, McEwen BS. Oestrogen modulates progestin receptor concentrations in some rat brain regions but not in others. Nature. 1978;274:276–278. doi: 10.1038/274276a0. [DOI] [PubMed] [Google Scholar]
- 36.Michels KM, Lee WH, Seltzer A, Saavedra JM, Bondy CA. Up-regulation of pituitary [125]I insulin-like growth factor-I (IGF-I) binding and IGF binding protein-2 and IGF-I gene expression by estrogen. Endocrinology. 1993;132:23–29. doi: 10.1210/endo.132.1.7678216. [DOI] [PubMed] [Google Scholar]
- 37.Ortmann O, Emons G, Knuppen R, Catt KJ. Inhibitory actions of keoxifene on luteinizing hormone secretion in pituitary gonadotrophs. Endocrinology. 1988;123:962–968. doi: 10.1210/endo-123-2-962. [DOI] [PubMed] [Google Scholar]
- 38.Patrone C, Ma Z, Pollio G, Agrati P, Parker M, Maggi A. Cross-coupling between insulin and estrogen receptor in human neuroblastoma cells. Mol Endocrinol. 1996;10:499–507. doi: 10.1210/mend.10.5.8732681. [DOI] [PubMed] [Google Scholar]
- 39.Pellegrino L, Pellegrino A, Cushman A. A stereotaxic atlas of the rat brain. Plenum; New York: 1979. [Google Scholar]
- 40.Perez J, Luquin S, Naftolin F, Garcia-Segura LM. The role of estradiol and progesterone in phased synaptic remodelling of the rat arcuate nucleus. Brain Res. 1993;608:38–44. doi: 10.1016/0006-8993(93)90771-e. [DOI] [PubMed] [Google Scholar]
- 41.Petitti N, Karkanias GB, Etgen AM. Estradiol selectively regulates α1B-noradrenergic receptors in the hypothalamus and preoptic area. J Neurosci. 1992;12:3869–3876. doi: 10.1523/JNEUROSCI.12-10-03869.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfaff DW. Estrogens and Brain Function: Neural analysis of a hormone-controlled mammalian reproductive behavior. Springer; New York: 1980. [Google Scholar]
- 43.Pfaff DW, Schwartz-Giblin S, McCarthy MM, Kow LM. Cellular and molecular mechanisms of female reproductive behaviors. In: Knobil E, Neill JD, editors. The physiology of reproduction, Vol 2. Raven; New York: 1994. pp. 107–220. [Google Scholar]
- 44.Pieribone VA, Nicholas AP, Dagerlind A, Hokfelt T. Distribution of α1-adrenoceptors in rat brain revealed by in situ hybridization experiments utilizing subtype-specific probes. J Neurosci. 1994;14:4252–4268. doi: 10.1523/JNEUROSCI.14-07-04252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pietrzkowski Z, Wernicke D, Porcu P, Jameson BA, Baserga R. Inhibition of cellular proliferation by peptide analogues of insulin-like growth factor 1. Cancer Res. 1992;52:6447–6451. [PubMed] [Google Scholar]
- 46.Pons S, Torres-Aleman I. Estradiol modulates insulin-like growth factor I receptors and binding proteins in neurons from the hypothalamus. J Neuroendocrinol. 1993;5:267–271. doi: 10.1111/j.1365-2826.1993.tb00482.x. [DOI] [PubMed] [Google Scholar]
- 47.Quesada A, Etgen AM. IGF-1 regulation of α1-adrenergic receptor signaling is estradiol-dependent in the preoptic area and hypothalamus of female rats. Endocrinology. 2001;142:599–607. doi: 10.1210/endo.142.2.7946. [DOI] [PubMed] [Google Scholar]
- 48.Sahlin L, Norstedt G, Eriksson H. Estrogen regulation of the estrogen receptor and insulinlike growth factor-I in the rat uterus: a potential coupling between effects of estrogen and IGF-I. Steroids. 1994;59:421–430. doi: 10.1016/0039-128x(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 49.Soldani R, Cagnacci A, Yen SS. Insulin, insulin-like growth factor I (IGF-I) and IGF-II enhance basal and gonadotrophin-releasing hormone-stimulated luteinizing hormone release from rat anterior pituitary cells in vitro. Eur J Endocrinol. 1994;131:641–645. doi: 10.1530/eje.0.1310641. [DOI] [PubMed] [Google Scholar]
- 50.Stoica A, Saceda M, Fakhro A, Joyner M, Martin MB. Role of insulin-like growth factor-I in regulating estrogen receptor-α gene expression. J Cell Biochem. 2000;76:605–614. doi: 10.1002/(sici)1097-4644(20000315)76:4<605::aid-jcb9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 51.Weesner GD, Krey LC, Pfaff DW. α1-adrenergic regulation of estrogen-induced increases in luteinizing hormone-releasing hormone mRNA levels and release. Brain Res Mol Brain Res. 1993;17:77–82. doi: 10.1016/0169-328x(93)90075-z. [DOI] [PubMed] [Google Scholar]
- 52.Wilson ME. IGF-I administration advances the decrease in hypersensitivity to oestradiol negative feedback inhibition of serum LH in adolescent female rhesus monkeys. J Endocrinol. 1995;145:121–130. doi: 10.1677/joe.0.1450121. [DOI] [PubMed] [Google Scholar]
- 53.Wimalasena J, Meehan D, Dostal R, Foster JS, Cameron M, Smith M. Growth factors interact with estradiol and gonadotropins in the regulation of ovarian cancer cell growth and growth factor receptors. Oncol Res. 1993;5:325–337. [PubMed] [Google Scholar]
- 54.Yamamoto C. Activation of hippocampal neurons by mossy fiber stimulation in thin brain sections in vitro. Exp Brain Res. 1972;14:423–435. doi: 10.1007/BF00235037. [DOI] [PubMed] [Google Scholar]
- 55.Zumkeller W, Schwab M. Insulin-like growth factor system in neuroblastoma tumorigenesis and apoptosis: potential diagnostic and therapeutic perspectives. Horm Metab Res. 1999;31:138–141. doi: 10.1055/s-2007-978711. [DOI] [PubMed] [Google Scholar]