Abstract
NMDA receptors, an ionotropic subtype of glutamate receptors (GluRs), play an important role in excitatory neurotransmission, synaptic plasticity, and brain development. They are composed of the GluRζ subunit (NR1) combined with any one of four GluRε subunits (GluRε1–GluRε4; NR2A–NR2D). Although the GluRζ subunit exists in the majority of the CNS throughout all stages of development, the GluRε subunits are expressed in distinct temporal and spatial patterns. In the present study, we investigated neuronal functions in mice lacking the embryonic GluRε4 subunit. GluRε4 mutant mice exhibited reductions of [3H]MK-801 [(+)-5-methyl-10,11-dihydro-5H-dibenzo [a,d] cyclohepten-5,10-imine maleate] binding and45Ca2+ uptake through the NMDA receptors. The expression of GluRζ subunit protein, but not GluRε1 and GluRε2 subunit proteins, was reduced in the frontal cortex and striatum of the mutant mice. A postmortem examination in GluRε4 mutant mice revealed that tissue contents of norepinephrine, dopamine, serotonin, and their metabolites were reduced in the hippocampus and that dopamine, as well as serotonin, metabolism was upregulated in the frontal cortex, striatum, hippocampus, and thalamus. To clarify the phenotypical influences of the alteration in neuronal functions, performances in various behavioral tests were examined. GluRε4 mutant mice showed reduced spontaneous locomotor activity in a novel environment and less sensitivity to stress induced by the elevated plus-maze, light–dark box, and forced swimming tests. These findings suggest that GluRε4 mutant mice have dysfunctional NMDA receptors and altered emotional behavior probably caused by changes in monoaminergic neuronal activities in adulthood.
Keywords: NMDA receptor, GluRε4 subunit, GluRζ subunit, monoaminergic neuronal systems, locomotor activity, emotional behavior
Glutamate receptors (GluRs) play an important role in excitatory neurotransmission, synaptic plasticity, neuronal development, and neurodegenaration of the CNS. They are divided in two general groups: ionotropic GluRs form ligand-gated cation channels and are subdivided into NMDA, AMPA, and kainate receptors, whereas metabotropic GluRs link G-protein that modulates the production of intracellular messengers (Hollmann and Heinemann, 1994;Nakanishi and Masu, 1994; Pin and Duvoisin, 1995).
NMDA receptors are thought to be a critical subtype mediating glutamate actions in the CNS. They are coupled with inherent high Ca2+-permeable channels, which are formed by assembly of the GluRζ subunit (NR1) and any one of four GluRε subunits (GluRε1–GluεR4; NR2A–NR2D). Although the GluRζ subunit exists in the brain at all developmental stages, the GluRε subunits are expressed in distinct temporal and spatial patterns (Mayer and Westbrook, 1987; Hollmann and Heinemann, 1994; Nakanishi and Masu, 1994). Prenatal NMDA receptors include the GluRε2 and/or GluRε4 subunit, whereas the GluRε1 and GluRε3 subunits appear only after birth, the former being expressed predominantly in the forebrain and the latter mainly in the cerebellum (Watanabe et al., 1992; Monyer et al., 1994). Thus, the GluRε subunits provide the molecular diversity in NMDA receptors during development and in various regions of the brain.
The physiological significance of NMDA receptor subunits has been demonstrated in knock-out mice. Although disruption of the GluRε2 subunit caused perinatally death (Kutsuwada et al., 1996), mice lacking the embryonic GluRε4 subunit are viable and exhibit reduced spontaneous locomotor activity (Ikeda et al., 1995). The impairment of spatial and contextual learning was demonstrated in GluRε1 mutant mice (Sakimura et al., 1995; Kiyama et al., 1998), but no deficits were observed in GluRε3 mutant mice (Ebralidze et al., 1996). GluRζ mutant mice showed a deficit of all NMDA receptors and perinatal death (Forrest et al., 1994; Li et al., 1994). These findings suggest that the GluRε subunits are major determinants of the functional properties of NMDA receptors, whereas the GluRζ subunit is an essential molecule in functional NMDA receptors and in brain development.
Accordingly, we investigated the alteration of neuronal functions in mice lacking NMDA receptor subunits. In GluRε1 mutant mice, NMDA receptor function was significantly reduced, but the dopaminergic neuronal activity was increased because of disinhibition of inhibitory GABAergic input. Furthermore, GluRε1 mutant mice showed an enhancement of locomotor activity in a novel environment, which is attributed to hyperfunction of dopaminergic neuronal system (Miyamoto et al., 2001). In the present study, we investigated the alteration of neuronal functions in adult mice lacking the embryonic GluRε4 subunit. [3H]MK-801 [(+)-5-methyl-10,11-dihydro-5H-dibenzo [a,d] cyclohepten-5,10-imine maleate] binding and45Ca2+ uptake through the NMDA receptors were measured to elucidate the role of the GluRε4 subunit in NMDA receptor function. Because the functional alteration of NMDA receptors in vivo has been reported to affect monoaminergic neuronal function and behavior (Hiramatsu et al., 1989; Imperato et al., 1990; Miller and Abercrombie, 1996), monoamine metabolism, locomotor activity, and emotional behavior in GluRε4 mutant mice were also assessed.
MATERIALS AND METHODS
Animals. Mutant mice lacking the GluRε4 subunit of NMDA receptors were provided by Prof. M. Mishina (Ikeda et al., 1995). The homozygous GluRε4 mutant (−/−; 3 months old) and the wild-type (+/+; 3 months old) mice used in this study were obtained by crossing F11 heterozygous GluRε4 mutant mice (+/−) having a 99.99% pure C57BL/6 genetic background. The genotypes of mice were determined by Southern blotting analyses of tail DNA as described by Ikeda et al. (1995). The animals were housed in plastic cages and were kept in a regulated environment (24 ± 1°C, 50 ± 5% humidity), with a 12 hr light/dark cycle (lights on at 9:00 A.M.). Food and tap water were available ad libitum. All experiments were performed in accordance with the Guidelines for Animal Experiments of the Nagoya University School of Medicine. The procedures involving animals and their care were conducted in conformity with the international guidelines Principles of Laboratory Animal Care (National Institutes of Health publication 85-23, revised 1985).
[3H]MK-801 binding. The GluRε4 mutant mice and the wild-type mice were killed by decapitation, and brains were quickly removed and placed on an ice-cold glass plate. The forebrain (whole brain minus the cerebellum and brainstem) was rapidly dissected out, frozen, and stored in a deep freezer at −80°C until assayed. [3H]MK-801 binding was measured as described previously (Yoneda and Ogita, 1989, 1991), with a minor modification (Miyamoto et al., 2001). Briefly, frozen samples were thawed at room temperature and homogenized in 40 vol of 50 mm Tris-acetate buffer, pH 7.4, containing 1 mm EDTA using a Physcotron homogenizer. All additional procedures were performed at 4°C. The homogenates were centrifuged at 40,000 × g for 30 min, and resultant pellets were washed three times with the same volume of 50 mm Tris-acetate buffer. The final pellets were suspended in 30 vol of 0.32 m sucrose, and the suspensions were frozen at −80°C for no longer than 1 week until use. On the day of the experiments, the frozen suspensions were thawed at room temperature and treated with 0.08% Triton X-100 at 4°C (an approximate protein concentration of 0.32 mg/ml) for 10 min with gentle stirring. The treatment was terminated by centrifugation at 40,000 × g for 30 min, and pellets were washed five times with 40 vol of 50 mm Tris-acetate buffer, followed by centrifugation at 40,000 × g for 30 min. For determination of [3H]MK-801 binding, an aliquot (0.3 mg of protein) of the membrane preparations was incubated, in the presence or absence of glutamate (10 μm), glycine (10 μm), and spermidine (1 mm), with 5 nm (+)[3-3H]MK-801 (22.5 Ci/mmol; NEN Life Science Products, Boston, MA) in a total volume of 0.5 ml of 50 mm Tris-acetate buffer at 30°C for 16 hr. The incubation was terminated by the addition of 3 ml of ice-cold 50 mm Tris-acetate buffer and subsequent filtration through a Whatman GF/B glass filter under a constant vacuum. The filter was rinsed with the same volume of ice-cold 50 mm Tris-acetate buffer three times within 10 sec. The radioactivity retained on the filter was measured by liquid scintillation spectrophotometry, at a counting efficiency of 57–59%. Nonspecific binding was determined with 0.1 mmcold (+)MK-801 (Sigma, St. Louis, MO), and the specific binding accounted for >60% of the total binding found in the absence of cold (+)MK-801.
45Ca2+uptake.45Ca2+ uptake through the NMDA receptors was measured as described by Miyamoto et al. (2001). The GluRε4 mutant mice and the wild-type mice were killed by decapitation, the brains were quickly removed, and the forebrain (whole brain minus the cerebellum and brainstem) was dissected out on an ice-cold glass plate. The forebrains were homogenized in 20 vol of ice-cold 0.32 m sucrose at 4°C in a Teflon glass homogenizer. All additional procedures were performed at 4°C. The homogenates were centrifuged at 1000 × g for 10 min. The supernatants were collected and then diluted 1:1 with basal buffer of the following composition (in mm): 135 NaCl, 5 KCl, 1 CaCl2, and 10 HEPES, pH adjusted to 7.4 with Tris base, and centrifuged at 10,000 × gfor 15 min. The pellets were resuspended in basal buffer and used for the 45Ca2+uptake assay. The synaptosome suspension (0.5 mg of protein) was preincubated in a total volume of 450 μl of basal buffer, in the presence or absence of (+)MK-801 (100 μm), at 37°C for 10 min. The45Ca2+ uptake assay was initiated by adding 50 μl of prewarmed basal buffer containing 1 μCi/ml45CaCl2 (18.1 mCi/mg; NEN Life Science Products), in the presence or absence of NMDA (100 μm), glycine (10 μm), and spermidine (1 mm) or high K+ (45 mm; isomolar replacement of NaCl with KCl). The reaction was terminated after 5 min by adding 3 ml of ice-cold basal buffer. The mixture was rapidly filtered under vacuum over Whatman GF/B glass filters, and the filters were rinsed twice with 3 ml of basal buffer. The radioactivity was determined by liquid scintillation spectrophotometry at a counting efficiency of 90%. Ca2+ uptake was defined by subtracting the uptake at 4°C.
Western blot analysis. The GluRε4 mutant mice and the wild-type mice were killed by decapitation, the brains were quickly removed, and the frontal cortex, striatum, hippocampus, and thalamus were dissected out on an ice-cold glass plate according to the method of Glowinski and Iversen (1966). Each section was homogenized with an ultrasonic processor in 10 vol of buffer (10 mmTris-Cl, pH 7.2, 5 mm EDTA, 0.32m sucrose, 1 mmphenylmethylsulfonyl fluoride, and 10 mg/l leupeptin) within 3 min of decapitation. The homogenates were centrifuged at 1000 ×g for 10 min at 4°C to obtain a postnuclear fraction. Protein determinations were made by the method of Lowry et al. (1951). The supernatants of 50 μg of protein were electrophoresed in a 7.5% SDS-polyacrylamide gel, transferred to polyvinylidene difluoride membrane, and incubated with a 1:1000 dilution of GluRζ, GluRε1, or GluRε2 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Antibody binding was detected by peroxidase-labeled secondary antibodies and the ECL detection kit (Amersham Biosciences, Arlington Heights, IL). The films were quantitated with a densitometer.
Contents of monoamines and their metabolites. The GluRε4 mutant mice and the wild-type mice were killed by focused microwave irradiation for 1.5 sec at 5 kW, the brains were quickly removed, and the frontal cortex, striatum, hippocampus, and thalamus were dissected out on an ice-cold glass plate according to the method of Glowinski and Iversen (1966). Each section was rapidly frozen and stored in a deep freezer at −80°C until assayed. The contents of monoamines and their metabolites were determined using an HPLC system with an electrochemical detector (Eicom, Kyoto, Japan), as described by Noda et al. (1998), with a minor modification (Miyamoto et al., 2001). Briefly, each frozen brain sample was weighed and homogenized with an ultrasonic processor in 350 μl of 0.2 m perchloric acid containing isoproterenol as an internal standard. The homogenates were placed on ice for 30 min and centrifuged at 20,000 × gfor 15 min at 4°C. The supernatants were mixed with 1m sodium acetate to adjust the pH to 3.0 and injected into an HPLC system equipped with a reversed-phase ODS-column (Eicompak MA-5 ODS; 4.6 × 150 mm; Eicom) and an electrochemical detector. The column temperature was maintained at 25°C, and the detector potential was set at +750 mV. The mobile phase was 0.1m citric acid and 0.1 msodium acetate, pH 3.6, containing 14% methanol, 180 mg/l sodium-l-octanesulfonate, and 5 mg/l EDTA, and the flow rate was set at 1 ml/min. The turnover of monoamines was calculated from the concentrations of each monoamine and its metabolite.
Behavioral analyses. To measure locomotor activity in a novel environment, a mouse was placed in a transparent acrylic cage with a black frosting Plexiglas floor (45 × 26 × 40 cm), and locomotion and rearing were measured every 5 min for 30 min using digital counters with infrared sensors (Scanet SV-10; Toyo Sangyo Co. Ltd., Toyama, Japan).
The elevated plus-maze consisted of two open (25 × 8 × 0.5 cm) and two closed (25 × 8 × 20 cm) arms emanating from a common central platform (8 × 8 cm) to form a plus shape (Yamada et al., 2000). The entire apparatus was elevated to a height of 50 cm above floor level. The test was started by placing a mouse on the central platform of the maze facing an open arm, and a 5 min test duration was used. Conventional parameters consisted of the frequency of entry to open and closed arms. These data were used to calculate the percentage of open arms entries [i.e., (open arms entries/open and closed arms entries) × 100].
The light–dark box consisted of two compartments: a transparent Plexiglas box with a white frosting Plexiglas floor and a black Plexiglas box with a black frosting Plexiglas floor (both 15 × 15 × 15 cm). Each box could be divided by a sliding door (10 × 5 cm high). The test was started by placing a mouse in the black Plexiglas box. The amounts of time spent in the transparent and black Plexiglas boxes was measured for 10 min using digital counters with infrared sensors (Scanet SV-10 LD; Toyo Sangyo Co. Ltd.). These data were used to calculate the percentage of time spent in the light box [i.e., (time spent in transparent Plexiglas box/time spent in transparent and black Plexiglas boxes) × 100].
In the forced swimming test, a mouse was placed in a transparent glass cylinder (8 cm in diameter × 20 cm high), which contained water at 25°C to a depth of 8 cm, and was forced to swim for 10 min. The duration of immobility was measured every 1 min using digital counters with infrared sensors (Scanet MV-10 AQ; Toyo Sangyo Co. Ltd.), as described previously (Noda et al., 1995).
Statistical analysis. All data were expressed as the mean ± SEM. Statistical differences between the GluRε4 mutant mice and the wild-type mice were determined with Student'st comparison test. In the analysis of locomotion and rearing curves, statistical differences between the GluRε4 mutant mice and the wild-type mice were determined by an ANOVA with repeated measures.
RESULTS
Function of NMDA receptors in adult GluRε4 mutant mice
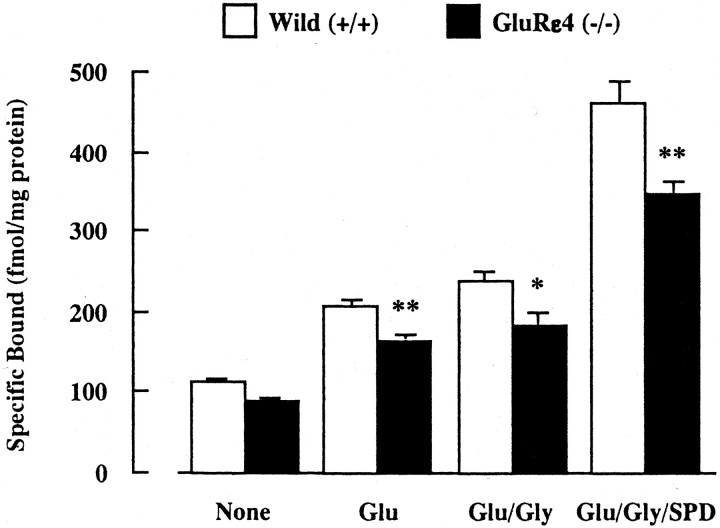
To demonstrate the functional changes in NMDA receptors of adult GluRε4 mutant mice, we first measured the binding activity of [3H]MK-801, which is known as a noncompetitive antagonist for NMDA receptors, in synaptic membranes of the forebrain (whole brain minus the cerebellum and brainstem) treated with Triton X-100 to deplete endogenous amino acids (Fig.1). The specific binding of [3H]MK-801 in both wild-type and mutant mice was increased when the assay was performed in the presence of activators for NMDA receptors such as glutamate (10 μm), glycine (10 μm), and spermidine (1 mm). In the presence of glutamate, glutamate plus glycine, or glutamate plus glycine plus spermidine, the specific binding of [3H]MK-801 was significantly lower in GluRε4 mutant mice than wild-type mice. Treatment with glycine or spermidine alone did not affect the basal-specific binding of [3H]MK-801 in either of the mice (data not shown).
Fig. 1.
[3H]MK-801 binding in forebrain synaptic membranes of adult GluRε4 mutant mice. Triton X-100-treated forebrain synaptic membranes were incubated with 5 nm [3H]MK-801 at 30°C for 16 hr, in the presence or absence of 10 μm glutamate (Glu), Glu plus 10 μm glycine (Gly), or Glu plus Gly plus 1 mm spermidine (SPD). Each column represents the mean ± SEM (n = 6). *p < 0.05 and **p < 0.01 versus wild-type (+/+).
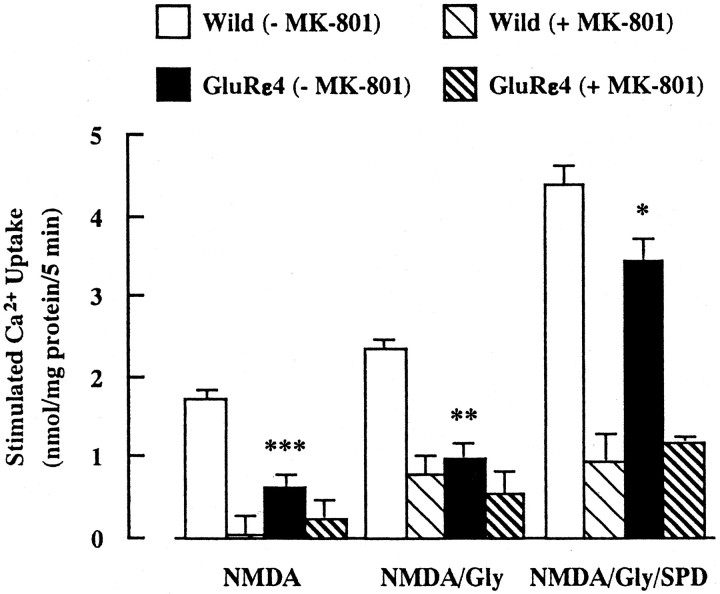
We next measured NMDA-stimulated45Ca2+ uptake into synaptosomes of the forebrain (whole brain minus the cerebellum and brainstem) (Fig. 2). There was no difference in the basal level of45Ca2+ uptake between wild-type (12.7 ± 0.1 nmol/mg protein per 5 min) and GluRε4 mutant (12.8 ± 0.2 nmol/mg protein per 5 min) mice. When the assay was performed in the presence of NMDA (100 μm), NMDA plus glycine (10 μm), or NMDA plus glycine plus spermidine (1 mm),45Ca2+ uptake was increased in both groups. However, there was significantly less45Ca2+ uptake in GluRε4 mutant mice than in wild-type mice under these stimulated conditions. The NMDA-, glycine-, and/or spermidine-stimulated45Ca2+ uptake in both groups was antagonized by the pretreatment with MK-801 (100 μm). On the other hand, there was no difference in high K+ (45 mm)-stimulated45Ca2+ uptake between wild-type (6.5 ± 0.2 nmol/mg protein per 5 min) and GluRε4 mutant (6.8 ± 0.4 nmol/mg protein per 5 min) mice. The results on [3H]MK-801 binding and NMDA-stimulated45Ca2+ uptake suggest that the function of NMDA receptors is reduced in adult GluRε4 mutant mice.
Fig. 2.
NMDA-stimulated45Ca2+ uptake into forebrain synaptosomes of adult GluRε4 mutant mice. The forebrain synaptosomes were preincubated at 37°C for 10 min, in the presence or absence of 100 μm MK-801. The assay was initiated by adding prewarmed buffer containing 1 μCi/ml 45CaCl2for 5 min, in the presence of 100 μm NMDA, NMDA plus 10 μm glycine (Gly), or NMDA plus Gly plus 1 mm spermidine (SPD). Eachcolumn represents the mean ± SEM (n = 8 for −MK-801 group; n = 6 for +MK-801 group). *p < 0.05, **p < 0.01, and ***p < 0.001 versus wild-type (−MK-801).
Expression of NMDA receptor subunit in adult GluRε4 mutant mice
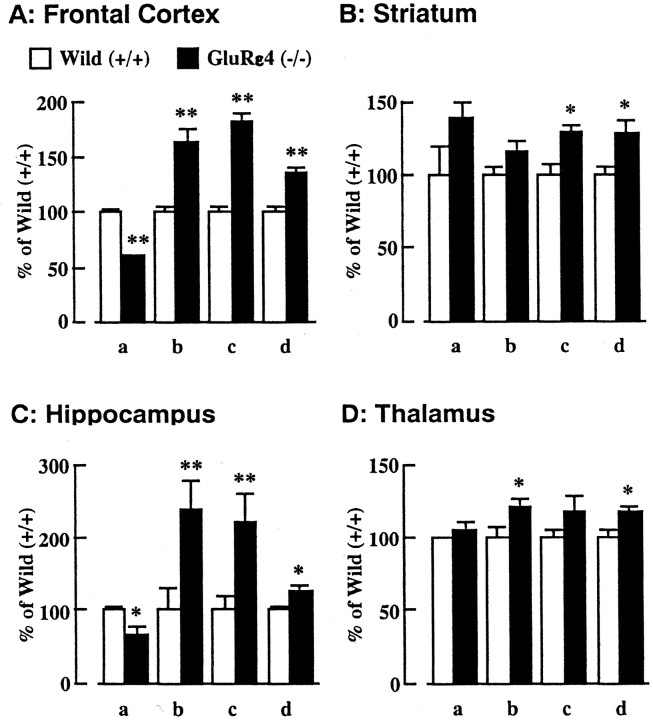
As demonstrated above, a defect in the embryonic GluRε4 subunit results in an impairment of NMDA receptor function in adulthood (3 months old). Because the GluRε4 subunit is expressed predominantly at the prenatal stage, but not at the postnatal stage, these findings suggest that disruption of the GluRε4 subunit affects the expression of other GluR subunits composing NMDA receptors in adult animals. To test this possibility, the expression of other subunit proteins forming NMDA receptors was analyzed by Western blotting in adult GluRε4 mutant mice (Fig. 3). The amount of GluRζ subunit protein, an essential molecule for functional NMDA receptors in vivo, was selectively reduced in the frontal cortex and striatum, but not in the hippocampus and thalamus, of GluRε4 mutant mice (Fig. 3A). There was no significant difference in GluRε1 and GluRε2 subunit protein levels in any region between wild-type and GluRε4 mutant mice (Fig.3B,C). These findings indicate that NMDA receptor subunit composition or the amount of functional NMDA receptors may be altered in adult GluRε4 mutant mice, which results in the malfunction of NMDA receptors.
Fig. 3.
Expression of NMDA receptor subunit proteins in various regions of the adult GluRε4 mutant mouse brain. The amount of each NMDA receptor subunit was determined by Western blot analysis using antibodies against GluRζ, GluRε1, or GluRε2. The synaptosomal protein samples were prepared from various regions of the adult GluRε4 mutant mouse brain. FC, Frontal cortex;HIP, hippocampus; STR, striatum;THA, thalamus. Each column represents the mean ± SEM (n = 4). *p < 0.05 versus wild-type (+/+).
Monoaminergic neuronal function in adult GluRε4 mutant mice
To investigate whether targeted disruption of the embryonic GluRε4 subunit gene would affect the function of monoaminergic neuronal systems, the tissue contents of monoamines and their metabolites in various regions of the adult GluRε4 mutant mouse brain were measured (Table 1). A marked reduction of norepinephrine (NE), dopamine (DA), and serotonin (5-hydroxytryptamine; 5-HT) contents, being 53, 22, and 50% of that in wild-type mice, respectively, was evident in the hippocampus of the mutant mice. Metabolite levels were also reduced in this region. In addition, DA in the frontal cortex and NE in the striatum decreased significantly, whereas 5-HT in the striatum increased in the mutant mice compared with the wild-type mice. To address the functional alterations of monoaminergic neuronal systems, monoamine metabolism was evaluated by calculating the ratio of the tissue content of the monoamines to their metabolites (Fig. 4). The ratio of 3,4-dihydroxyphenylacetic acid (DOPAC) to DA and/or of homovanillic acid (HVA) to DA in the frontal cortex, striatum, hippocampus, and thalamus was significantly increased in GluRε4 mutant mice compared with wild-type mice (Fig.4A–D). In the same regions, the ratio of 5-hydroxyindoleacetic acid (5-HIAA) to 5-HT was also significantly increased in the mutant mice (Fig. 4A–D). In contrast, the ratio of 3-methoxy-4-hydroxyphenylglycol (MHPG) to NE decreased in the frontal cortex and hippocampus (Fig.4A,C), but not in the striatum and thalamus (Fig. 4B,D), of GluRε4 mutant mice. These findings suggest that the activities of monoaminergic neuronal systems are altered in adult GluRε4 mutant mice as a result of the disruption of the GluRε4 subunit gene.
Table 1.
Monoamines and their metabolites contents in various regions of the adult GluRε4 (−/−) mutant mouse brain
| NE | MHPG | DA | DOPAC | HVA | 5-HT | 5-HIAA | |
|---|---|---|---|---|---|---|---|
| FC | |||||||
| Wild (+/+) | 683.9 ± 28.8 | 0.63 ± 0.03 | 21.8 ± 1.09 | 27.4 ± 1.23 | 74.3 ± 3.19 | 189.7 ± 8.35 | 195.0 ± 10.3 |
| GluRε4 (−/−) | 740.1 ± 27.4 | 0.47 ± 0.021-160 | 15.7 ± 0.551-160 | 30.2 ± 1.36 | 96.6 ± 2.641-160 | 210.0 ± 8.28 | 282.1 ± 11.01-160 |
| STR | |||||||
| Wild (+/+) | 293.4 ± 24.2 | 11.3 ± 0.79 | 12335.5 ± 113.2 | 2034.3 ± 96.2 | 1494.6 ± 62.4 | 370.3 ± 17.3 | 1168.7 ± 63.3 |
| GluRε4 (−/−) | 195.9 ± 17.61-160 | 11.4 ± 0.64 | 12371.8 ± 100.8 | 2407.5 ± 87.7* | 1840.0 ± 43.51-160 | 438.0 ± 19.8* | 1747.9 ± 67.81-160 |
| HIP | |||||||
| Wild (+/+) | 732.1 ± 22.4 | 4.01 ± 0.26 | 2.70 ± 0.43 | 10.2 ± 2.28 | 38.4 ± 3.24 | 581.8 ± 23.0 | 1269.0 ± 50.2 |
| GluRε4 (−/−) | 390.1 ± 27.21-160 | 1.28 ± 0.181-160 | 0.59 ± 0.021-160 | 3.60 ± 0.84* | 23.9 ± 2.031-160 | 290.5 ± 24.41-160 | 806.9 ± 24.21-160 |
| THA | |||||||
| Wild (+/+) | 1403.6 ± 39.6 | 5.15 ± 0.17 | 216.5 ± 21.3 | 223.8 ± 14.7 | 329.5 ± 21.5 | 887.0 ± 16.6 | 1530.3 ± 76.1 |
| GluRε4 (−/−) | 1356.5 ± 69.8 | 5.55 ± 0.22 | 192.7 ± 12.9 | 264.4 ± 22.0 | 390.2 ± 21.6 | 831.7 ± 22.0 | 1756.7 ± 78.5 |
Values are expressed as nanograms per gram of wet tissues (n = 8). FC, Frontal cortex; STR, striatum; HIP, hippocampus; THA, thalamus.
p < 0.05 and
F1-160: p < 0.01 versus wild-type (+/+).
Fig. 4.
Monoamine metabolism in various regions of the adult GluRε4 mutant mouse brain. The tissue contents of monoamines and their metabolites in various regions were measured by HPLC with an electrochemical detector. a, MHPG/NE; b, DOPAC/DA; c, HVA/DA; d, 5-HIAA/5-HT. Each column represents the mean ± SEM (n = 8). *p < 0.05 and **p < 0.01 versus wild-type (+/+).
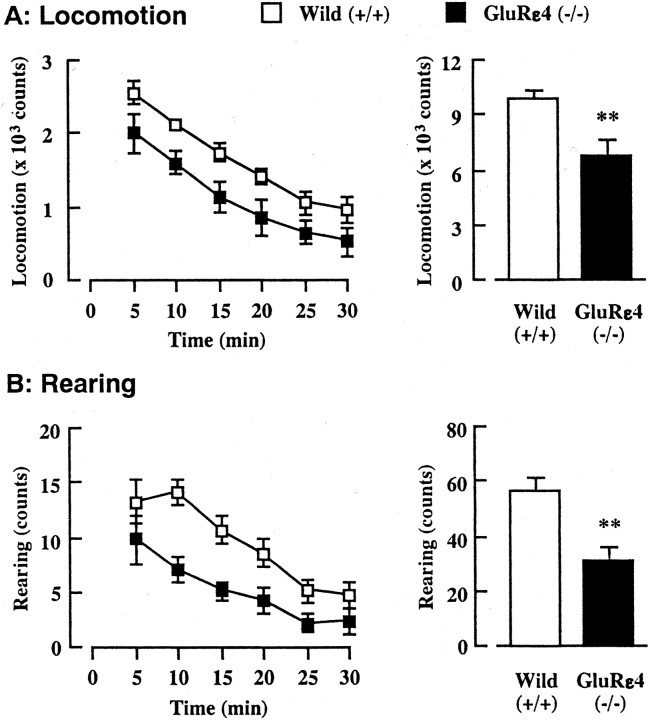
Locomotor activity in adult GluRε4 mutant mice
Monoaminergic neuronal systems, particularly dopaminergic and serotonergic neuronal activities, are thought to regulate locomotor activity in animals (Geyer, 1996; Giros et al., 1996; Lucki, 1998;Gainetdinov et al., 1999). To clarify the behavioral influences of altered monoaminergic neuronal function in adult GluRε4 mutant mice, the locomotor activity in a novel environment was measured for 30 min using digital counters with infrared sensors to record horizontal (locomotion) (Fig. 5A) and vertical (rearing) (Fig. 5B) activity. Although a time course analysis of locomotor activity revealed no significant difference between wild-type and GluRε4 mutant mice [F(1,19) = 0.192, p = 0.9651 (locomotion); F(1,19) = 1.180;p = 0.3253 (rearing)], total counts for both locomotion and rearing during a 30 min observation period were significantly lower in the mutant mice. These results suggest that GluRε4 mutant mice show reduced locomotor activity in a novel environment.
Fig. 5.
Locomotor activity in a novel environment in adult GluRε4 mutant mice. Locomotor activity and the number of rearing events in a novel environment were measured every 5 min for 30 min. Each column represents the mean ± SEM (n = 10–11). An ANOVA with repeated measures revealed no difference in the time course of locomotion (F(1,19) = 0.192; p= 0.9651) and rearing (F(1,19) = 1.180;p = 0.3253). **p < 0.01 versus wild-type (+/+).
Emotional behavior of adult GluRε4 mutant mice
We examined the performance of adult GluRε4 mutant mice in three different paradigms, the elevated plus-maze, light–dark box, and forced swimming tests (Fig. 6), because monoaminergic neuronal systems are also implicated in the regulation of emotional behavior (Schildkraut, 1965; Kim et al., 1997). In the elevated plus-maze test, GluRε4 mutant mice spent significantly more time exploring the open arms (Fig. 6A) and had more entries into the open arms (GluRε4 mutant mice, 7.0 ± 0.5 entries per 5 min; wild-type mice, 9.3 ± 0.6 entries per 5 min) than did wild-type mice. In the light–dark box test, GluRε4 mutant mice spent significantly more time in the light box than wild-type mice (Fig. 6B). The results in the elevated plus-maze and light–dark box tests suggest a reduced psychological anxiety in GluRε4 mutant mice. The immobility time in the forced swimming test decreased in GluRε4 mutant mice compared with wild-type mice (Fig.6C). Because immobility in this test is considered to reflect behavioral despair to escape from water (Porsolt et al., 1977a,b, 1978), GluRε4 mutant mice may have an increased motivation or a reduced susceptibility to stress. It is unlikely that the reduced immobility is attributable to a hyperactivity of the mutant mice because their locomotor activity is rather reduced in a novel environment. Thus, our findings on emotional behavior indicate that GluRε4 mutant mice may have a reduced susceptibility to stress and/or reduced psychological anxiety.
Fig. 6.
Emotional behavior of adult GluRε4 mutant mice.A, Elevated plus-maze test; the frequencies of entries to open and closed arms were recorded for 5 min. B, Light–dark box test; the amounts of time spent in the light and dark boxes were measured for 10 min. C, Forced swimming test; the duration of immobility was measured for 10 min. The data represent the mean ± SEM (n = 8–10). **p < 0.01 and ***p < 0.001 versus corresponding wild-type (+/+).
DISCUSSION
NMDA receptors are heteromeric assemblies of the GluRζ and GluRε subunits, which exhibit characteristic differences in activities and properties depending on the GluRε subunits involved, such as affinity for agonists, channel gating kinetics, unitary conductance, and sensitivity to Mg2+, Zn2+, and antagonist block (Kutsuwada et al., 1992; Monyer et al., 1992; Ishii et al., 1993). Among GluRε/ζ heteromeric NMDA receptors, the GluRε4/ζ1 assembly has the highest apparent affinities for glutamate and glycine (Ikeda et al., 1992), has a very slow channel gating (Monyer et al., 1994), and is less sensitive to Mg2+ block than the GluRε1/ζ1 and GluRε2/ζ1 assemblies (Mishina et al., 1993). These findings suggest that the GluRε4 subunit plays an important role in synaptic transmission in the early stages of brain development, because this subunit is abundant in the embryonic diencephalon and abruptly disappears in the postnatal forebrain (Watanabe et al., 1992; Monyer et al., 1994). To demonstrate the physiological significance of the GluRε4 subunit in vivo, Ikeda et al. (1995) generated mutant mice without this subunit by a gene-targeting recombination technique. In the present study, we investigated the altered neuronal functions of the mutant mice in adulthood.
In [3H]MK-801 binding and45Ca2+ uptake through the NMDA receptors under various conditions, an impairment of NMDA receptor function was demonstrated in the forebrain (whole brain minus the cerebellum and brainstem) of adult GluRε4 mutant mice. Furthermore, a reduction in GluRζ subunit protein, but not GluRε1 and GluRε2 subunit proteins, in the frontal cortex and striatum of the mutant mice was also evident by Western blot analysis. The expression of the GluRζ subunit did not change, however, in the hippocampus and thalamus. A similar modification of the GluR subunit expression has been demonstrated in mice lacking the GluRζ subunit (Forrest et al., 1994) or overexpressing the GluRε4 subunit (Okabe et al., 1998). Therefore, it is suggested that the impairment of NMDA receptor function in adult GluRε4 mutant mice is associated with an alteration of NMDA receptor subunit composition, that is, a reduction of GluRζ subunit expression in the frontal cortex and striatum attributable to the disruption of the embryonic GluRε4 subunit. Alternatively, because the GluRζ subunit is an essential molecule for functional NMDA receptors in vivo, which are composed by assembly of four or five GluR subunits, including at least two of the GluRζ subunits (Laube et al., 1998; Hawkins et al., 1999), the reduction of GluRζ subunit protein might result in fewer functional NMDA receptors per se, without alterations of subunit composition in the receptors. In addition, our findings suggested that the expression of NMDA receptor subunits is regulated interdependently during the development of the brain in a region-specific manner. Thus, the embryonic GluRε4 subunit may play a role in the regulation of NMDA receptor function in adulthood. To ascertain precisely where in the forebrain the NMDA receptor function is altered, an autoradiographic analysis of [3H]MK-801 binding is necessary. Moreover, it remains to be determined, however, why the expression of the GluRζ subunit in GluRε4 mutant mice was downregulated in the frontal cortex and striatum but not other regions of the brain.
In the postmortem brain analysis, GluRε4 mutant mice showed reduced monoamines and their metabolite contents in the hippocampus. They also showed an increase in both DA and 5-HT metabolism in the frontal cortex, striatum, hippocampus, and thalamus. The alteration to monoaminergic neuronal systems was greatest in the hippocampus among the regions examined, including the frontal cortex, striatum, and thalamus. Thus, in monoaminergic neuronal systems of adult GluRε4 mutant mice, it is likely that the hippocampus is most severely influenced by a lack of the embryonic GluRε4 subunit.
The mechanisms responsible for the functional alteration of monoaminergic neuronal systems in adult GluRε4 mutant mice remain to be determined. It is of interest that rats with neonatal excitotoxic lesions in the ventral hippocampus exhibit hyperfunction of dopaminergic and hypofunction of glutamatergic neuronal systems in adulthood (Schroeder et al., 1999), which resemble the increase of DA metabolism and malfunction of NMDA receptors in adult GluRε4 mutant mice. It has been proposed that hyperfunction of dopaminergic neuronal system in the hippocampus-lesioned rats is attributable to an impairment of neuronal development induced by the loss of synaptic connections between the medial prefrontal cortex and ventral hippocampus as a result of excitotoxic lesions of the hippocampus during the neonatal period (Lipska et al., 1993; Schroeder et al., 1999). In GluRε4 mutant mice, there are no alteration of NMDA receptor subunit levels in the hippocampus (present study) and no obvious histological abnormalities in any region, including the hippocampus on Nissl staining (Ikeda et al., 1995). Therefore, we consider that there may be an impairment of synapse formation connecting the hippocampus and other brain regions in GluRε4 mutant mice, because previous studies have suggested a role of NMDA receptors in neuronal development (Scheetz and Constantine-Paton, 1994;Contestabile, 2000). Additional study is required to address this possibility.
Alternatively, it may be attributable to the results of NMDA receptor malfunction in adult GluRε4 mutant mice, because the pharmacological blockade of NMDA receptors in vivo either directly or indirectly results in an altered neuronal activity in monoaminergic neuronal systems, particularly dopaminergic and serotonergic neuronal systems (Hiramatsu et al., 1989; Imperato et al., 1990; Miller and Abercrombie, 1996). Moreover, we provided recently genetic evidence that GluRε1 mutant mice with reduced NMDA receptor function exhibit an increase in dopaminergic and serotonergic neuronal activities in the frontal cortex and striatum (Miyamoto et al., 2001). In fact, an impairment of NMDA receptor function in the forebrain was evident as a significant decrease of [3H]MK-801 binding and45Ca2+ uptake through the NMDA receptors in adult GluRε4 mutant mice.
It has been demonstrated that GluRε4 mutant mice show a reduction of spontaneous locomotor activity (Ikeda et al., 1995). Consistent with this finding, reduced locomotor activity in the mutant mice was observed in the present study. Moreover, we found a change in the emotional behavior of GluRε4 mutant mice. The mutant mice exhibited less susceptibility to psychological and physiological stress induced in the elevated plus-maze, light–dark box, and forced swimming tests. It is considered that locomotor activity and emotional behavior are regulated by the homeostatic balance of activity among monoaminergic neuronal systems (Schildkraut, 1965; Geyer, 1996; Giros et al., 1996;Kim et al., 1997; Lucki, 1998; Gainetdinov et al., 1999). In GluRε4 mutant mice, a significant increase in DA and 5-HT metabolism in the frontal cortex, striatum, hippocampus, and thalamus was evident. Thus, the reduction in locomotor activity and susceptibility to stress may be attributed to the altered monoaminergic, especially dopaminergic and serotonergic, neuronal functions in the mutant mice. However, alteration of neuronal functions in GABAergic neuronal system must be considered, because agonistic modulators of GABAAreceptors are effective in reducing emotional behaviors in the elevated plus-maze and light–dark box tests (Lister, 1987; Dalvi and Rodgers, 1996; Chaouloff et al., 1997).
Interestingly, a previous study demonstrated that the performance of GluRε4 mutant mice was not different from that of heterozygous mice as control group in the elevated plus-maze and light–dark box tests (Ikeda et al., 1995). It is possible that significant changes in emotional behavior could not be detected because homozygous GluRε4 mutant mice were compared with heterozygous littermates. Alternatively, the inconsistency between the two studies is attributable to the difference in the age of the mutant mice used. We used GluRε4 mutant mice at 3 months of age in this study, whereas the previous study examined mutant mice at postnatal day 26 (P26) or P28. The GluRε4 subunit mRNA is mainly expressed from embryonic day 13 through P14 in mouse brain (Watanabe et al., 1992). Considering that disruption of the GluRε4 subunit may affect the maturation of brain, the function of the CNS in mutant mice is more stable at 3 months than at 4 weeks, which is just after the weaning period. Because different behavioral effects of the GluRε4 subunit gene disruption in juvenile and adult mice are of special interest, additional studies are required to examine whether there is a critical age when GluRε4 mutant mice exhibit less susceptibility to psychological and physiological stress.
In summary, adult mice lacking the embryonic GluRε4 subunit had dysfunctional NMDA receptors, possibly attributable to changes in the receptor subunit composition or reduction of functional NMDA receptors without alterations of subunit composition. The mutant mice showed altered emotional behavior, which is probably attributable to a change in monoaminergic neuronal activities.
Footnotes
This work was supported in part by Grant-in-Aid for Scientific Research 10044260 from the Ministry of Education, Science, Sports, and Culture of Japan and by Special Coordination Funds for Promoting Science and Technology, Target-Oriented Brain Science Research Program from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Correspondence should be addressed to Dr. Toshitaka Nabeshima, Department of Neuropsychopharmacology and Hospital Pharmacy, Nagoya University Graduate School of Medicine, 65 Tsuruma-cho, Showa-ku, Nagoya 466-8560, Japan. E-mail: tnabeshi@med.nagoya-u.ac.jp.
Y. Miyamoto's present address: Department of Molecular Genetics, National Institute for Longevity Sciences, Gengo 36-3, Morioka-cho, Oobu, Aichi 474-8522, Japan.
REFERENCES
- 1.Chaouloff F, Durand M, Mormede P. Anxiety- and activity-related effects of diazepam and chlordiazepoxide in the rat light/dark and dark/light tests. Behav Brain Res. 1997;85:27–35. doi: 10.1016/s0166-4328(96)00160-x. [DOI] [PubMed] [Google Scholar]
- 2.Contestabile A. Roles of NMDA receptor activity and nitric oxide production in brain development. Brain Res Rev. 2000;32:476–509. doi: 10.1016/s0165-0173(00)00018-7. [DOI] [PubMed] [Google Scholar]
- 3.Dalvi A, Rodgers RJ. GABAergic influences on plus-maze behaviour in mice. Psychopharmacology. 1996;128:380–397. doi: 10.1007/s002130050148. [DOI] [PubMed] [Google Scholar]
- 4.Ebralidze AK, Rossi DJ, Tonegawa S, Slater NT. Modification of NMDA receptor channels and synaptic transmission by targeted disruption of the NR2C gene. J Neurosci. 1996;16:5014–5025. doi: 10.1523/JNEUROSCI.16-16-05014.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forrest D, Yuzaki M, Soares HD, Hg L, Luk DC, Sheng M, Stewart CL, Morgan JI, Connor JA, Curran T. Targeted disruption of NMDA receptor 1 gene abolishes NMDA response and results in neonatal death. Neuron. 1994;13:325–338. doi: 10.1016/0896-6273(94)90350-6. [DOI] [PubMed] [Google Scholar]
- 6.Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- 7.Geyer MA. Serotonergic functions in arousal and motor activity. Behav Brain Res. 1996;73:31–35. doi: 10.1016/0166-4328(96)00065-4. [DOI] [PubMed] [Google Scholar]
- 8.Giros B, Jaber M, Jones SR, Wightmann RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 9.Glowinski J, Iversen LL. Regional studies of catecholamines in the rat brain. I. The disposition of [3H] norepinephrine, [3H] dopamine and [3H]dopa in various regions of the brain. J Neurochem. 1966;13:655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- 10.Hawkins LM, Chazot PL, Stephenson FA. Biochemical evidence for the co-association of three N-methyl-d-aspartate (NMDA) R2 subunits in recombinant NMDA receptors. J Biol Chem. 1999;274:27211–27218. doi: 10.1074/jbc.274.38.27211. [DOI] [PubMed] [Google Scholar]
- 11.Hiramatsu M, Cho AK, Nabeshima T. Comparison of the behavioral and biochemical effects of the NMDA receptor antagonists, MK-801 and phencyclidine. Eur J Pharmacol. 1989;166:359–366. doi: 10.1016/0014-2999(89)90346-4. [DOI] [PubMed] [Google Scholar]
- 12.Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda K, Nagasawa M, Mori H, Araki K, Sakimura K, Watanabe M, Inoue Y, Mishina M. Cloning and expression of the ε4 subunit of the NMDA receptor channel. FEBS Lett. 1992;313:34–38. doi: 10.1016/0014-5793(92)81178-o. [DOI] [PubMed] [Google Scholar]
- 14.Ikeda K, Araki K, Takayama C, Inoue Y, Yagi T, Aizawa S, Mishina M. Reduced spontaneous activity of mice defective in the ε4 subunit of the NMDA receptor channel. Mol Brain Res. 1995;33:61–71. doi: 10.1016/0169-328x(95)00107-4. [DOI] [PubMed] [Google Scholar]
- 15.Imperato A, Scrocco MG, Bacchi S, Angelucci L. NMDA receptors and in vivo dopamine release in the nucleus accumbens and caudatus. Eur J Pharmacol. 1990;187:555–556. doi: 10.1016/0014-2999(90)90387-l. [DOI] [PubMed] [Google Scholar]
- 16.Ishii T, Moriyoshi K, Sugihara H, Sakurada K, Kadotani H, Yokoi M, Akazawa C, Shigemoto R, Mizuno N, Masu M, Nakanishi S. Molecular characterization of the family of the N-methyl-d-aspartate receptor subunits. J Biol Chem. 1993;268:2836–2843. [PubMed] [Google Scholar]
- 17.Kim JJ, Shih JC, Chen K, Chen L, Bao S, Maren S, Anagnostaras SG, Fanselow MS, De Maeyer E, Seif I, Thompson RF. Selective enhancement of emotional, but not motor, learning in monoamine oxidase A-deficient mice. Proc Natl Acad Sci USA. 1997;94:5929–5933. doi: 10.1073/pnas.94.11.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiyama Y, Manabe T, Sakimura K, Kawakami F, Mori H, Mishina M. Increased thresholds for long-term potentiation and contextual learning in mice lacking the NMDA-type glutamate receptor ε1 subunit. J Neurosci. 1998;18:6704–6712. doi: 10.1523/JNEUROSCI.18-17-06704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kutsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Meguro H, Masaki H, Kumanishi T, Arakawa M, Mishina M. Molecular diversity of the NMDA receptor channel. Nature. 1992;358:36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- 20.Kutsuwada T, Sakimura K, Manabe T, Takayama C, Katakura N, Kushiya E, Natsume R, Watanabe M, Inoue Y, Yagi T, Aizawa S, Arakawa M, Takahashi T, Nakamura Y, Mori H, Mishina M. Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor ε2 subunit mutant mice. Neuron. 1996;16:333–344. doi: 10.1016/s0896-6273(00)80051-3. [DOI] [PubMed] [Google Scholar]
- 21.Laube B, Kuhse J, Betz H. Evidence for a tetrameric structure of recombinant NMDA receptors. J Neurosci. 1998;18:2954–2961. doi: 10.1523/JNEUROSCI.18-08-02954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Erzurumlu RS, Chen C, Jhaveri S, Tonegawa S. Whisker-related neuronal patterns fail to develop in the trigeminal brainstem nuclei of NMDAR1 knock-out mice. Cell. 1994;76:427–437. doi: 10.1016/0092-8674(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 23.Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- 24.Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology. 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 25.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol regent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 26.Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 27.Mayer ML, Westbrook GL. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28:197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- 28.Miller DW, Abercrombie ED. Effects of MK-801 on spontaneous and amphetamine-stimulated dopamine release in striatum measured with in vivo microdialysis in awake rats. Brain Res Bull. 1996;40:57–62. doi: 10.1016/0361-9230(95)02144-2. [DOI] [PubMed] [Google Scholar]
- 29.Mishina M, Mori H, Araki K, Kushiya E, Meguro H, Kutsuwada T, Kashiwabuchi N, Ikeda K, Nagasawa M, Yamazaki M, Masaki H, Yamakura T, Morita T, Sakimura K. Molecular and functional diversity of the NMDA receptor channel. Ann NY Acad Sci. 1993;707:136–152. doi: 10.1111/j.1749-6632.1993.tb38049.x. [DOI] [PubMed] [Google Scholar]
- 30.Miyamoto Y, Yamada K, Noda Y, Mori H, Mishina M, Nabeshima T. Hyperfunction of dopaminergic and serotonergic neuronal systems in mice lacking the NMDA receptor ε1 subunit. J Neurosci. 2001;21:750–757. doi: 10.1523/JNEUROSCI.21-02-00750.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- 32.Monyer H, Burnashev N, Laurie D, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 33.Nakanishi S, Masu M. Molecular diversity and functions of glutamate receptors. Annu Rev Biophys Biomol Struct. 1994;23:319–348. doi: 10.1146/annurev.bb.23.060194.001535. [DOI] [PubMed] [Google Scholar]
- 34.Noda Y, Yamada K, Furukawa H, Nabeshima T. Enhancement of immobility in a forced swimming test by subacute or repeated treatment with phencyclidine: a new model of schizophrenia. Br J Pharmacol. 1995;116:2531–2537. doi: 10.1111/j.1476-5381.1995.tb15106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noda Y, Miyamoto Y, Mamiya T, Kamei H, Furukawa H, Nabeshima T. Involvement of dopaminergic system in phencyclidine-induced place preference in mice pretreated with phencyclidine repeatedly. J Pharmacol Exp Ther. 1998;286:44–51. [PubMed] [Google Scholar]
- 36.Okabe S, Collin C, Auerbach JM, Meiri N, Bengzon J, Kennedy MB, Segal M, McKay RDG. Hippocampal synaptic plasticity in mice overexpressing an embryonic subunit of the NMDA receptor. J Neurosci. 1998;18:4177–4188. doi: 10.1523/JNEUROSCI.18-11-04177.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- 38.Porsolt RD, Bertin A, Jalfre M. Behavioural despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977a;229:327–336. [PubMed] [Google Scholar]
- 39.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977b;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 40.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 41.Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H, Mishina M. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor ε 1 subunit. Nature. 1995;373:151–155. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- 42.Scheetz AJ, Constantine-Paton M. Modulation of NMDA receptor function: implications for vertebrate neural development. FASEB J. 1994;8:745–752. doi: 10.1096/fasebj.8.10.8050674. [DOI] [PubMed] [Google Scholar]
- 43.Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;122:509–522. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- 44.Schroeder H, Grecksch G, Becker A, Bogerts B, Hoellt V. Alterations of the dopaminergic and glutamatergic neurotransmission in adult rats with postnatal ibotenic acid hippocampal lesion. Psychopharmacology. 1999;145:61–66. doi: 10.1007/s002130051032. [DOI] [PubMed] [Google Scholar]
- 45.Watanabe M, Inoue Y, Sakimura K, Mishina M. Developmental changes in distribution of NMDA receptor channel subunit mRNAs. NeuroReport. 1992;3:1138–1140. doi: 10.1097/00001756-199212000-00027. [DOI] [PubMed] [Google Scholar]
- 46.Yamada K, Iida R, Miyamoto Y, Saito K, Sekikawa K, Seishima M, Nabeshima T. Neurobehavioral alterations in mice with a targeted deletion of the tumor necrosis factor-alpha gene: implications for emotional behavior. J Neuroimmunol. 2000;111:131–138. doi: 10.1016/s0165-5728(00)00375-1. [DOI] [PubMed] [Google Scholar]
- 47.Yoneda Y, Ogita K. Labeling of NMDA receptor channels by [3H]MK-801 in brain synaptic membranes treated with Triton X-100. Brain Res. 1989;499:305–314. doi: 10.1016/0006-8993(89)90779-8. [DOI] [PubMed] [Google Scholar]
- 48.Yoneda Y, Ogita K. Heterogeneity of the N-methyl-d-aspartate receptor ionophore complex in rat brain, as revealed by ligand binding techniques. J Pharmacol Exp Ther. 1991;259:86–96. [PubMed] [Google Scholar]