Abstract
Chondroitin sulfate proteoglycan (CS-PG) expression is increased in response to CNS injury and limits the capacity for axonal regeneration. Previously we have shown that neurocan is one of the CS-PGs that is upregulated (Asher et al., 2000). Here we show that another member of the aggrecan family, versican, is also upregulated in response to CNS injury. Labeling of frozen sections 7 d after a unilateral knife lesion to the cerebral cortex revealed a clear increase in versican immunoreactivity around the lesion. Western blot analysis of extracts prepared from injured and uninjured tissue also revealed considerably more versican in the injured tissue extract. In vitrostudies revealed versican to be a product of oligodendrocyte lineage cells (OLCs). Labeling was seen between the late A2B5-positive stage and the O1-positive pre-oligodendrocyte stage. Neither immature, bipolar A2B5-positive cells, nor differentiated, myelin-forming oligodendrocytes were labeled. The amount of versican in conditioned medium increased as these cells differentiated. Versican and tenascin-R colocalized in OLCs, and coimmunoprecipitation indicated that the two exist as a complex in oligodendrocyte-conditioned medium. Treatment of pre-oligodendrocytes with hyaluronidase led to the release of versican, indicating that its retention at the cell surface is dependent on hyaluronate (HA). In rat brain, approximately half of the versican is bound to hyaluronate. We also provide evidence of a role for CS-PGs in the axon growth-inhibitory properties of oligodendrocytes. Because large numbers of OLCs are recruited to CNS lesions, these results suggest that OLC-derived versican contributes to the inhospitable environment of the injured CNS.
Keywords: chondroitin sulfate, extracellular matrix, glial scar, hyaluronate, proteoglycan, regeneration, tenascin-R
Chondroitin sulfate proteoglycans (CS-PGs) inhibit axon growth in vitro (Snow et al., 1990;Dou and Levine, 1994; Friedlander et al., 1994; Milev et al., 1994;Yamada et al., 1997; Schmalfeldt et al., 2000) and are thought to create exclusion zones for growing axons in the embryo (Snow et al., 1990; Oakley and Tosney, 1991; Brittis et al., 1992; Emerling and Lander, 1996). Their increased expression in CNS injuries may therefore limit the capacity for axon regeneration (McKeon et al., 1991; Pindzola et al., 1993; Lips et al., 1995; Gates et al., 1996; Davies et al., 1997, 1999; Fitch and Silver, 1997). Various CS-PGs have been shown to be present at sites of CNS injury, including NG2 (Levine, 1994), phosphacan (Laywell and Steindler, 1991; McKeon et al., 1995; Barker et al., 1996; Deller et al., 1997; McKeon et al., 1999), neurocan (Haas et al., 1999; McKeon et al., 1999; Asher et al., 2000), and brevican (Jaworski et al., 1999; Thon et al., 2000). Definitive evidence of an inhibitory role for these molecules in axon regeneration has been lacking. However, recent work has shown that infusion of the enzyme chondroitinase ABC, which denudes the core protein of chondroitin sulfate, enables severed axons of the nigrostriatal tract to regrow, and in some cases to reach their original target, the ipsilateral striatum (Moon et al., 2001). Chondroitinase also promoted regeneration of sensory axons in the dorsal columns of the spinal cord (Bradbury et al., 2000).
Versican belongs to the aggrecan family of hyaluronate (HA)-binding CS-PGs. It was first identified in the adult CNS as the glial hyaluronate-binding protein (GHAP) (Perides et al., 1989), which was later shown to constitute the N-terminal (hyaluronate-binding) domain of versican (Zimmermann and Ruoslahti, 1989). GHAP is a naturally occurring fragment that is thought to be generated by the action of a matrix metalloproteinase(s) on versican (Perides et al., 1995). Versican is highly expressed in white matter tracts, and its expression is closely linked to myelination in the rat (Bignami et al., 1993). Four mRNA splice variants have been identified, the three largest of which, V0, V1, and V2, carry chondroitin sulfate (Dours-Zimmermann and Zimmermann, 1994). V0, V1, and V2 have been identified in the human (Dours-Zimmermann and Zimmermann, 1994) and bovine brain (Schmalfeldt et al., 1998). In the adult bovine brain, the V2 isoform is by far the most abundant (Schmalfeldt et al., 1998).
Versican interacts primarily with other components of the extracellular matrix (ECM). Versican binds to hyaluronate via its N-terminal globular domain (LeBaron et al., 1992). The C-terminal globular domain mediates binding to tenascin-R (Aspberg et al., 1995), fibulin-1 (Aspberg et al., 1999), and certain sulfated glycolipids (Miura et al., 1999).
Recently, bovine spinal cord-derived versican has been shown to inhibit axon growth in vitro (Niederöst et al., 1999;Schmalfeldt et al., 2000). Oligodendrocytes in vitrolabeled for versican and treatment with proteoglycan synthesis inhibitors led to the loss of cell surface versican and abolished the growth cone collapsing activity of these cells (Niederöst et al., 1999). Clearly, these findings point to an inhibitory role for versican in axon growth. In this study, we set out to establish (1) whether versican is upregulated in the damaged CNS, (2) which cells might be responsible for its presence, (3) what factors affect its expression, (4) with what does it associate in the ECM, and (5) whether CS-PGs contribute to the axon growth-inhibitory properties of oligodendrocytes.
Some of these data have been published previously in abstract form (Asher et al., 1999).
MATERIALS AND METHODS
Surgical procedures
Adult female Sprague Dawley rats (Charles River, Margate, Kent, UK; approximate body weight 200 gm) were anesthetized under Fluorothane, and the head was held in a stereotaxic apparatus with the incisor bar 2.5 mm below the interaural line. A fine scalpel blade was inserted stereotaxically and vertically into the brain, through a parasagittal dorsal craniotomy, to a depth of 3 mm below the dura and 2–3 mm lateral to the midline. The knife was then moved in the horizontal plane to produce a lesion 4–5 mm in length, midway between lambda and bregma. The operation was performed unilaterally (on the right-hand side) with the other hemisphere serving as a control. After 7–28 d, the animals were terminally anesthetized with an intraperitoneal injection of 0.7 ml of sodium pentabarbitone and decapitated. The brain was rapidly removed and immediately frozen on dry ice or prepared for frozen sectioning.
Immunolabeling of frozen sections
Frozen, coronal sections (10 μm) were cut from unfixed tissue 7 and 14 d post-lesion (dpl). Labeling was performed without fixation. Nonspecific binding was blocked with PBS containing 3% BSA and 20 mml-lysine (PBS/BSA/Lys). The sections were incubated with the anti-versican monoclonal antibody (mAb) 12C5 (undiluted hybridoma-conditioned medium) (Asher et al., 1991) or a mouse myeloma-derived IgG1 (Sigma, Poole, Dorset, UK; 11 μg/ml in PBS/BSA/Lys) for 1 hr. Bound antibodies were visualized with biotinylated anti-mouse immunoglobulins (1:100; Amersham, Little Chalfont, Bucks, UK) and Cy3-streptavidin (1.0 μg/ml; Amersham) in the manner described previously (Asher et al., 1995).
Sample preparation for SDS-PAGE
Brain tissue. Dissection was performed as rapidly as possible while the brain was still semifrozen. A piece of tissue ∼2 × 4 × 6 mm containing the lesion was excised. A similar sized piece was dissected from the same location on the uninjured side. The tissue was immediately placed in 1.0 ml of ice-cold extraction buffer [0.05 m Tris-HCl, pH 7.0, 0.15 M NaCl, 1% SDS and Complete protease inhibitor mixture (Roche, Lewes, East Sussex, UK)], and homogenized in a Teflon-glass Dounce homogenizer. The homogenates were centrifuged at 13,000 ×g for 10 min at 4°C. Protein measurements were made with the BCA Protein Assay Kit (Pierce, Chester, Cheshire, UK).
Cultured cells. Serum-free conditioned medium was removed from the cells, and Complete protease inhibitors were added immediately. The conditioned medium was then centrifuged (1000 ×g for 5 min) and concentrated in a Centricon 100 (Millipore, Watford, Herts, UK) to approximately one-tenth of its initial volume. The protein content was determined using the Coomassie Plus Protein Assay Reagent (Pierce). Chondroitinase ABC (protease-free; Roche) digestion was performed for 3 hr at 37°C using 0.01 U of enzyme per milliliter of conditioned medium.
For the preparation of detergent lysates, cells were washed twice with PBS and then solubilized in a small volume (∼0.25 ml/25 cm2 flask) of 0.05 m Tris-HCl, pH 7.0, 0.15 m NaCl containing 1% NP-40 (Calbiochem, Nottingham, Notts, UK), and Complete protease inhibitors. The cells were removed with a cell scraper and placed on a gyro-rocker at 4°C for 1 hr. The lysate was then centrifuged at 13,000 ×g for 10 min at 4°C. The supernatant is henceforth referred to as the detergent lysate. The protein content was determined with the BCA Protein Assay Kit (Pierce).
Electrophoresis and Western blotting
SDS-PAGE was performed in the manner described previously (Asher et al., 2000). Proteins were transferred to nitrocellulose (Hybond-C pure, Amersham) or polyvinyldifluoride (Hybond-P, Amersham) at 4°C for 15–18 hr, at a constant current of 150 mA.
All subsequent procedures were performed at room temperature. The blots were rinsed twice in Tris-buffered saline (TBS; 0.9% NaCl, 10 mm Tris-HCl, pH 7.5) containing 0.05% Tween 20 (TBS/Tween) and incubated for a further 40 min in TBS/Tween. All washes and antibody incubations were performed in TBS/Tween. The blots were then incubated with the anti-versican mAb 12C5 (hybridoma-conditioned medium diluted 1:60), the anti-neurocan mAb 1G2 (hybridoma-conditioned medium diluted 1:60) (Oohira et al., 1994), an anti-brevican mAb (250 ng/ml; Transduction Labs, San Diego, CA), an anti-tenascin-R mAb (1.5 μg/ml) (Pesheva et al., 1989), mouse IgG1 (1.1 μg/ml), or rabbit anti-NG2 proteoglycan (1.0 μg/ml) (gift of J. Levine, State University of New York, Stony Brook, NY) for 2 hr. Reactive species were visualized with peroxidase-conjugated anti-mouse or anti-rabbit IgG (100 ng/ml; Vector, Peterborough, Cambs, UK) and a chemiluminescent substrate (ECL, Amersham).
Immunoprecipitation
Formalin-fixed Staphylococcus aureus cells (Pansorbin, Calbiochem) were preloaded with either the 12C5 anti-versican mAb or an anti-tenascin-R mAb via rabbit anti-mouse IgGFC (Jackson, West Grove, PA). Oligodendrocyte-conditioned medium was concentrated and precleared with fixed S. aureus cells preloaded with rabbit anti-mouse IgGFC. The preloaded S. aureus cells were then resuspended in the precleared conditioned medium and shaken on ice for 1 hr. The cells were washed six times in 50 mm Tris buffer, pH 7.5, containing 0.5m sodium chloride and 0.1% Tween 20, and once in water. Immunoprecipitated proteins were eluted by boiling for 5 min in sample buffer and subjected to Western blot analysis.
Glial cell culture
All cell culture reagents were purchased from Invitrogen (Paisley, UK), unless stated otherwise. Primary glial cell cultures were prepared from the brains of newborn rats <3 d old, as described previously (Asher et al., 2000).
Cultures enriched for oligodendrocyte lineage cells (OLCs) were derived from the cells dislodged by shaking the primary culture. The resultant cell suspension was filtered through 35 and 15 μm nylon mesh and then preplated on non-tissue culture plastic to deplete it of macrophages/microglia. The nonattached cells were washed once in Ca2+- and Mg2+-free HBSS and dispensed into either 24-well plates containing poly-d-lysine-coated glass coverslips (for immunocytochemistry) or poly-d-lysine-coated 6-well plates or 25 cm2 flasks (for biochemistry) in DMEM containing 10% FCS. The cells were allowed to adhere for 1–2 hr, and the medium was changed to either DMEM containing 0.01% crystalline BSA, 5 μg/ml insulin, 50 μg/ml transferrin, 30 nmsodium selenite, 10 nmd-biotin (Sigma), 10 nm hydrocortisone (Collaborative Biomedical Products, Bedford, MA), and 3,3′5-triiodo-l-thyronine (Sigma) (=oligodendrocyte differentiation medium) (Gard et al., 1995), or DMEM containing BSA, insulin, transferrin, and sodium selenite (as above), and 10 ng/ml platelet-derived growth factor (PDGF-AB; R&D, Abingdon, Oxon, UK) and 10 ng/ml fibroblast growth factor 2 (FGF2; Roche) (=division medium).
Astrocyte and meningeal cell cultures were prepared in the manner described previously (Asher et al., 2000). For the preparation of meningeal cell-conditioned medium, the cells were grown for 3 d in DMEM containing BSA, insulin, transferrin, sodium selenite, and 1% FCS.
Functional assay
Oligodendrocyte progenitor cells were grown on poly-d-lysine-coated 13 mm glass coverslips in differentiation medium for 33 hr. Chondroitinase ABC (0.05 U/ml; Roche) or vehicle (HBSS) was then added to the medium, and the plates were returned to the incubator for 3 hr. The cells were washed in PBS and fixed in 4% paraformaldehyde for 20 min at 4°C. Sterile conditions were maintained throughout the digestion, fixation, and subsequent procedures. The cells were washed in PBS, and quenched with 50 mml-lysine in PBS. The coverslips were then used as a substrate for the culture of dissociated neonatal (postnatal day 0–1) rat dorsal root ganglion (DRG) neurons (Skaper et al., 1990). These cells were grown in serum-free DMEM containing BSA, insulin, transferrin, sodium selenite, and NGF (10 ng/ml) for 40 hr. The cells were fixed in cold methanol and labeled with the anti-neurofilament-associated protein mAb 3A10 (hybridoma-conditioned medium diluted 1:4; Developmental Studies Hybridoma Bank, Iowa City, IA) (Serafini et al., 1996), biotinylated anti-mouse Ig, and Cy3-streptavidin in the manner described below.
Quantification of axon growth
An estimate of the extent of axon growth on chondroitinase-treated and untreated oligodendrocytes was obtained as follows. The number of 3A10-positive (neuronal) processes intersecting two parallel lines 2 mm from the edge of the coverslip was counted. An assay consisted of 16 coverslips, 8 of which were treated with chondroitinase. Hence, n = 8 for each condition. To determine whether chondroitinase affected neuronal adhesion to the substrate, the number of 3A10-positive neurons was counted in a 1 mm band 2 mm from the edge of each coverslip (i.e., in the same region as the axon counts). Provided that the number of neurons is constant, this assay is a reliable guide to any difference in axon growth.
Immunocytochemistry
Oligodendrocyte lineage cells were grown on poly-d-lysine-coated glass coverslips. Versican labeling was performed at room temperature on living cells in Liebovitz's L-15 medium (Invitrogen) containing 2% FCS (L-15/FCS). The cells were washed once in L-15/FCS and incubated with the 12C5 anti-versican mAb (hybridoma-conditioned medium diluted 1:1) for 20 min. The cells were washed three times with L-15/FCS and then incubated with biotinylated anti-mouse IgG, Fcγ fragment specific (12 μg/ml; Jackson) for 20 min. The cells were washed as before and incubated with Cy3-streptavidin (1 μg/ml) for 20 min. The cells were washed as before and incubated with either the A2B5 (hybridoma-conditioned medium diluted 1:1; American Type Culture Collection, Manassas, VA) or O1 (hybridoma-conditioned medium diluted 1:1; European Collection of Animal Cell Cultures, Salisbury, Wilts, UK) mAb for 20 min. These antigens were visualized with FITC anti-mouse IgM (μ chain specific, 15 μg/ml; Jackson). Finally, the cells were washed three times in L-15/FCS, once in PBS, and fixed in cold (−20°C) methanol for 2 min. Nuclei were labeled with Hoechst 33342 (10 μg/ml; Sigma) for 30 min.
Double labeling for versican and hyaluronate and O1 and HA was performed on living cells as follows. The cells were washed once in L-15/FCS and incubated with 10 μg/ml biotinylated hyaluronate-binding protein (Seikagaku, Falmouth, MA) for 20 min. The cells were washed three times and incubated with Cy3-streptavidin (1.0 μg/ml) for 20 min. The cells were washed as before, labeled with 12C5 and FITC anti-mouse IgG (10 μg/ml; Dako, High Wycombe, Bucks, UK), or O1 and FITC anti-mouse IgM (μ chain specific), and fixed in the manner described above.
Labeling for tenascin-R was performed on living cells with either monoclonal or polyclonal antibodies (Pesheva et al., 1989). Double labeling for tenascin-R and A2B5 or O1 was performed in the manner described above for versican/A2B5 using an anti-tenascin-R mAb. Versican/tenascin-R double labeling was performed on living cells using rabbit antibodies against tenascin-R (20 μg/ml) and FITC anti-rabbit IgG (15 μg/ml; Jackson), and the 12C5 anti-versican mAb, biotinylated anti-mouse Ig, and Cy3-streptavidin. Doing the versican labeling before the tenascin-R labeling made no difference to the outcome. Glial fibrillary acidic protein (GFAP) labeling was performed on methanol-fixed cells with rabbit anti-GFAP (20 μg/ml; Dako) and FITC anti-rabbit IgG.
Sterile testicular hyaluronidase (1.0 mg/ml stock in DMEM; type IV; Sigma) or Streptomyces hyaluronidase [100 turbidity reducing units (TRU)/ml stock in DMEM; Seikagaku] was added directly to cell cultures to yield a final concentration of 20, 50, or 100 μg/ml and 20 TRU/ml, respectively, and incubation continued for 3 hr at 37°C in a CO2-containing atmosphere.
Hyaluronidase release of rat brain versican
Adult rat brains (Harlan Sera-Lab, Loughborough, Leics, UK) were finely chopped while still frozen and then homogenized, using a Teflon-glass Dounce homogenizer, in 0.1 m phosphate buffer, pH 5.3, containing 0.15 m NaCl, Complete protease inhibitors, and 2 μg/ml pepstatin A (Calbiochem), at a ratio of ∼1 gm of (wet) tissue per 7.0 ml of buffer. The homogenate was kept on ice for 10 min and then centrifuged at 3000 × g for 10 min at 4°C. The resultant supernatant constitutes the first saline extract. The pellet was rehomogenized four times in the manner described above, giving rise to supernatants 2, 3, 4, and 5. The final homogenate was incubated for 10 min at 37°C rather than 4°C. The homogenate was then divided into two equal parts, to only one of which was added testicular hyaluronidase (type IV; Sigma) to a final concentration of 50 μg/ml. Both homogenates were incubated at 37°C for 2 hr. An aliquot was removed from each after 30 min. The homogenates were then centrifuged for 10 min at 3000 ×g, and the pellets were washed twice. Testicular hyaluronidase was added only to the homogenate not previously exposed to the enzyme, and both homogenates were incubated for a further 2 hr at 37°C. All of the supernatants (i.e., the five saline extracts and those with and without hyaluronidase) were clarified by centrifugation at 13,000 × g for 10 min and frozen on dry ice. Chondroitinase digestion was performed by adding one-tenth volume 0.4m Tris-acetate, pH 8.0, and 0.025 U chondroitinase ABC (Roche) per milliliter of supernatant and incubating for 3 hr at 37°C.
RESULTS
Versican is upregulated in CNS injury
We investigated versican expression in the injured CNS by immunohistochemistry and Western blotting. The distribution of versican was examined in unfixed frozen sections cut 7 d after a unilateral knife cut lesion to the cerebral cortex. An increase in versican labeling was seen around the lesion, in comparison with the uninjured side (Fig. 1). A similar increase in versican labeling was seen at 14 dpl. This increase was restricted to a region within ∼100 μm of the knife cut. The appearance of the labeling was consistent with an extracellular matrix localization and could not be localized to a particular cell type. As reported previously, versican expression was low in the cerebral cortex but much higher in the subcortical white matter (Bignami et al., 1993). Versican labeling was also seen in the glia limitans and around some of the larger blood vessels.
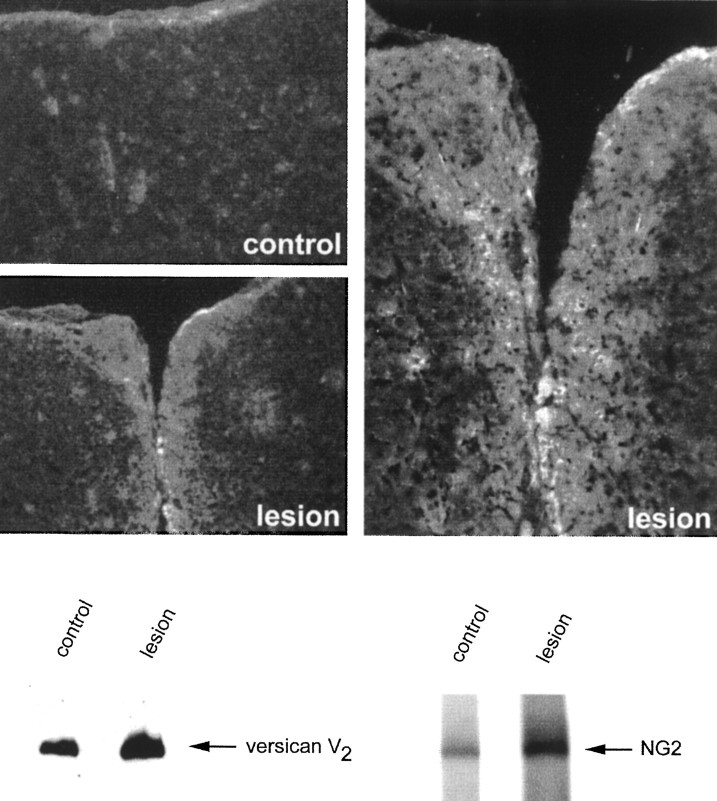
Fig. 1.
Versican is upregulated in the injured CNS.Top, Immunolocalization of versican in the injured CNS. Coronal frozen sections were labeled with the anti-versican mAb 12C5 7 d after a knife cut lesion to the cerebral cortex. The dorsal surface of the brain is topmost. Labeling is apparent around the injury (lesion), which is clearly lacking on the uninjured side (control).Bottom, Western blot analysis of versican in the injured brain. SDS extracts were prepared from injured and uninjured cerebral cortex. The extracts were equalized for total protein (162 μg), run in a 4% gel under nonreducing conditions, and transferred to polyvinylidene difluoride. The blot was labeled with the anti-versican mAb 12C5 and then with rabbit antibodies against the NG2 proteoglycan. An upregulation of both CS-PGs was clearly evident in the injured brain extracts.
For biochemical analysis, a piece of tissue ∼2 × 4 × 6 mm was dissected from around the lesion and from the equivalent region on the uninjured (contralateral) side. Care was taken to avoid the underlying white matter, in which versican is highly expressed. SDS extracts were prepared from the two pieces of tissue, and equivalent amounts of protein were run under nonreducing conditions in a 4% gel. Western blot analysis with the anti-versican mAb 12C5 revealed a single high Mr species of ∼400 kDa in injured and uninjured cortex (Fig. 1). This species comigrated with the versican isoform (V2) found in normal rat CNS tissue and is the smallest of the three high Mr species recognized by 12C5, all of which bear chondroitin sulfate (see Fig.3). Quantification with NIH Image software revealed there to be two to three times more versican in the injured tissue extract at 7 dpl (Fig. 1). A similar increase was evident at 14 dpl but not at 28 dpl. This is likely to underestimate the extent of the upregulation, because the piece of tissue dissected from around the lesion was considerably larger than the region in which versican was seen to be upregulated in frozen sections.
Fig. 3.
Oligodendrocytes produce the same versican isoform (V2) as that found in the rat CNS. The versican in adult rat brain (the 0.5 m NaCl fraction from DEAE-bound material; see Fig. 5) was compared with that in the conditioned medium of OLCs, astrocytes, meningeal cells, and the OPC-like cell line CG4. A testicular hyaluronidase (test. hyal.) extract of rat brain was overloaded to show that low levels of V0 and V1 are present in adult rat brain. OLCs make only the V2 isoform, which is unable to enter the gel without chondroitinase treatment. Astrocytes do not produce versican. Meningeal cells make only V0 and V1, both of which require chondroitinase digestion to enter the gel. As expected, CG4 cells make mostly V2, although small amounts of V0 and V1 were also detected.
To further characterize the GAG content of CNS versican, DEAE cellulose, an anion exchange resin, was used to partially purify CS-PGs from adult rat brain. The bound proteins were eluted with increasing concentrations of sodium chloride. These fractions were then assayed for versican by Western blotting. Most of the versican (V2) was found in the 0.5 and 0.75 mNaCl fractions (see Fig. 5, bottom). For a given protein, the length and number of GAG chains will have a major influence on how well it binds to the anion exchange resin. An increase in the overall amount of GAG will lead to stronger binding and consequently require a higher salt concentration to remove it. Hence, the shift brought about by chondroitinase is more pronounced in the higher salt fractions, because they contain versican core proteins with a higher relative GAG content (see Fig. 5, bottom). Compared with other CNS CS-PGs, such as neurocan (Asher et al., 2000), versican carries relatively little chondroitin sulfate.
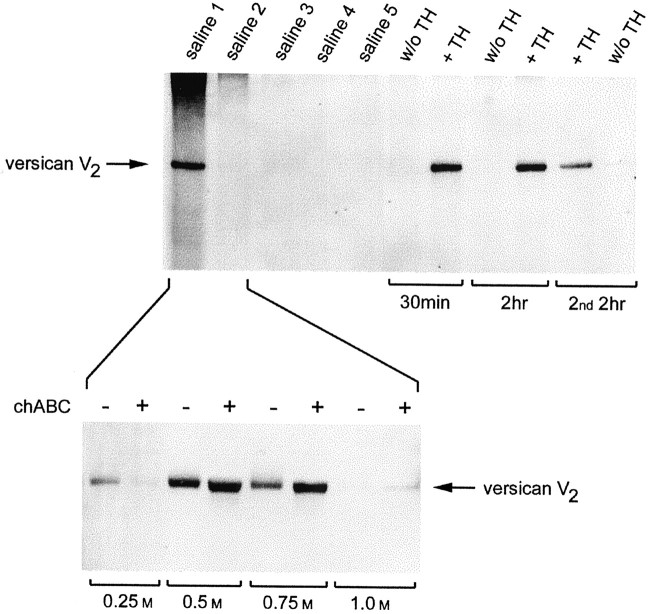
Fig. 5.
Versican can be released from adult rat brain with hyaluronidase. Top, Serial extracts were prepared from adult rat brain with PBS, pH 5.3 (saline 1–5). After the fifth PBS extract, the homogenate was divided into two equal parts, to only one of which was added testicular hyaluronidase (TH). The homogenates were incubated at 37°C for 2 hr. An aliquot was removed from each after 30 min. A second 2 hr incubation was then set up, in which hyaluronidase was added to the homogenate not previously exposed to the enzyme. All extracts were treated with chondroitinase ABC. The volume of each extract was adjusted according to the amount of tissue from which it derived, run in a 4% gel under nonreducing conditions, and transferred to nitrocellulose. The blot was labeled with the anti-versican mAb 12C5. Versican was present in the first saline extract, but the release of additional amounts required hyaluronidase. Bottom, The first and second saline extracts were pooled and DEAE cellulose was added. The bound proteins were eluted with increasing concentrations of NaCl. Versican (V2) bound to the anion exchange resin and was eluted between 0.5 and 0.75 msalt. Chondroitinase brought about a small but discrete shift, indicating that versican V2 carries little chondroitin sulfate.
Versican is a product of oligodendrocyte lineage cellsin vitro
A recent report has shown that in vitro, differentiated oligodendrocytes label with antibodies against versican (Niederöst et al., 1999). However, such cells could not account for the upregulation of versican in the gray matter of the injured cerebral cortex. We therefore examined versican expression in cells of the oligodendrocyte lineage at different stages of differentiation, and in astrocytes and meningeal cells, using immunocytochemistry and biochemical techniques.
Immunocytochemistry
Oligodendrocyte lineage cells
Oligodendrocyte lineage cells were removed from mixed glial cultures by shaking, and either plated directly in medium supportive of oligodendrocyte differentiation (Gard et al., 1995) or grown first for 2 d in medium containing PDGF and FGF2, which drives their division, before being switched to differentiation medium. The cells were double labeled with the anti-versican mAb 12C5, and with either the A2B5 or O1 mAb, after 1–5 d in culture. Such cultures contained cells at various stages of differentiation. For comprehensibility, we describe the labeling patterns by stage of differentiation.
Oligodendrocyte progenitor cells (A2B5+, O4−, O1−). Bipolar, A2B5-positive oligodendrocyte progenitor cells (OPCs) were invariably versican negative (Fig.2a–c).
Fig. 2.

Pro-oligodendroblasts and pre-oligodendrocytes, but not bipolar oligodendrocyte precursor cells or myelin-forming oligodendrocytes, label for versican. OPCs were grown for 1 d (a–c), 2 d (d–f), or 5 d (j–l) in differentiation medium, or in division medium for 2 d and then in differentiation medium for 2 d (g–i). The cells were double labeled for A2B5 (a) or O1 (d, g, j) and versican (b, e, h, k). The two are shown together in theright-hand column (c, f, i, l). The labeling was performed on living cells, and the cells were post-fixed in cold methanol. In OPCs plated directly in differentiation medium, versican is first seen to be associated with weakly A2B5-positive, multipolar cells (a–c,arrowheads) and subsequently with O1-positive cells (d–f). In OPCs grown initially in medium containing PDGF and FGF2 and then in differentiation medium, versican appears as a ring on the dorsal (top) cell surface (g–i). Neither bipolar, A2B5-positive OPCs (a–c, arrows) nor myelin-forming oligodendrocytes (j–l) label for versican. Scale bars, 20 μm.
Pro-oligodendroblast (A2B5+, O4+, O1−). Versican immunoreactivity first appeared on multipolar, weakly A2B5-positive cells. Two different labeling patterns were distinguishable, one of which was substrate associated and the other cell associated. The former took the form of slightly fuzzy, stellate profiles, the topography of which did not always correspond to the location of the processes of the accompanying cell (Fig. 2a–c). This material must be extracellular, because the labeling was performed on living cells. To determine whether the 12C5-reactive material is present on the cells or the substrate, the cells were removed with 1% NP-40 in water. The same 12C5-positive stellate profiles were seen in the absence of the cells. Much of this material is therefore associated with the substrate, rather than the cells themselves. The substrate-associated versican was also unaffected by treatment with 1 mm EDTA for 1 hr.
The other, cell-associated labeling pattern took the form of a ring, partly or wholly encircling the cell body. Again, this labeling is extracellular, because it was performed without any previous fixation. The ring-type labeling was seen in OPCs plated directly in differentiation medium, but at a much lower frequency than the substrate-associated labeling. The two types of labeling were occasionally seen in the same cell. The ring-type labeling was the predominant pattern, however, in cells expanded in PDGF and FGF2, and subsequently switched to differentiation medium (see below).
Pre-oligodendrocytes (A2B5−, O4+, O1+). Both types of versican labeling described above were also seen in multipolar, O1-positive, A2B5-negative cells. The substrate-associated labeling took the form of an irregular, circular patch, at the periphery of which fuzzy processes were discernible (Fig.2d–f). In growth factor-exposed cells, the ring-type labeling was the predominant pattern, in terms of both intensity and the number of cells involved (Fig.2g–i). The processes of such cells were generally not labeled for versican. Growth factor-expanded, chondroitinase ABC-treated pre-oligodendrocytes were labeled with the 2B6 and 3B3 mAbs, which react with the “stubs” remaining after chondroitinase digestion of chondroitin-4-sulfate and chondroitin-6-sulfate, respectively. The 2B6 mAb, but not the 3B3 mAb, labeled O1-positive pre-oligodendrocytes in a manner identical to that seen with the anti-versican mAb, and it may therefore be concluded that the pre-oligodendrocyte-associated versican carries chondroitin-4-sulfate. The CS-56 mAb, which reacts with an epitope peculiar to certain chondroitin sulfate chains, did not react with these cells.
Myelin-forming oligodendrocytes (A2B5−, O4−, O1+). Myelin-forming oligodendrocytes (i.e., O1-positive cells with large membranous sheets) were not labeled for versican (Fig.2j–l). Without previous fixation, no labeling of the membranous sheets was seen, although vestiges of the ring-type labeling were occasionally seen on the cell body. A similar picture emerged in cells permeabilized by fixation in cold methanol before labeling (data not shown).
Astrocytes
Oligodendrocyte cultures inevitably contain astrocytes, some of which derive from OPCs (so-called type 2 astrocytes) and some of which result from contamination with monolayer-derived cells (type 1 astrocytes). No labeling for versican was ever seen on, or in the environs of, type 1 astrocytes (data not shown). Some versican labeling was seen, however, in a small proportion of what by morphological criteria were type 2 astrocytes. This labeling colocalized with the GFAP-positive processes of these cells.
Meningeal cells
The 12C5 mAb labeled fibronectin-positive cells derived from newborn rat brain meninges (data not shown). This labeling took the form of a fine reticular meshwork on the dorsal (upper) cell surface.
Biochemistry
The conditioned medium of purified cultures of oligodendrocyte lineage cells, astrocytes, meningeal cells, and microglia was assayed for the presence of versican by Western blotting with the 12C5 mAb.
Oligodendrocyte lineage cells
Western blot analysis of chondroitinase-treated OLC-conditioned medium with the 12C5 mAb revealed a single, very large (∼400 kDa) species (Fig. 3). In contrast to the versican found in CNS tissues (Fig. 3), the OLC-derived species was unable to enter a 4% gel without previous chondroitinase ABC treatment (Fig. 3). It may therefore be concluded that the OLC-derived versican bears more chondroitin sulfate than rat brain versican. The OLC-derived versican core protein (i.e., after chondroitinase treatment) comigrated with the versican isoform found in the adult rat CNS, i.e., V2 (Fig. 3). The amount of versican present in the conditioned medium of cells grown in differentiation medium was greater than that in cells in PDGF- and FGF2-containing medium (Fig.4), supporting the conclusion that versican expression increases when these cells exit the division cycle or commence differentiation or both.
Fig. 4.

Versican binding to pre-oligodendrocytes is hyaluronate-dependent. Pre-oligodendrocytes were double labeled for O1 and versican (a, b), hyaluronate and versican (c, d), and O1 and versican (e, f). The cells shown in e and f were digested withStreptomyces hyaluronidase before labeling. Versican labeling takes the form of a ring, wholly or partly encircling the cell body of O1-positive pre-oligodendrocytes (b, d). The distribution of hyaluronate is identical to that of versican (c, d). Strepto-myces hyaluronidase abolished versican labeling (f, shows Hoechst-labeled nuclei). Scale bar, 20 μm. g, Two 25 cm2 flasks of OPCs were grown for 2 d in medium containing PDGF and FGF2 (div). This medium was then changed to one supportive of oligodendrocyte differentiation (diff), and the cells were grown for a further 24 hr. This medium was then removed and replaced with the same medium, either with (#1) or without (#2) testicular hyaluronidase (50 μg/ml) for 1 hr. This medium was removed and replaced with the same medium, except that the enzyme was added to the flask not previously treated with hyaluronidase (#2), but not to flask #1. The conditioned media were concentrated and treated with chondroitinase ABC. An equal volume of each was run under nonreducing conditions in a 4% gel (forversican and neurocan) or under reducing conditions in a 7% gel (brevican) and transferred to nitrocellulose. The blots were labeled with the anti-versican mAb 12C5, the anti-neurocan mAb 1G2, or an anti-brevican mAb. Versican, but not neurocan or brevican, was released intact from pre-oligodendrocytes by hyaluronidase. The amount of versican detected in the conditioned medium of differentiating OLCs was greater than that in dividing cells. This was not the case for either neurocan or brevican.
The OPC-like cell line CG4 was also tested for versican expression and found to be capable of producing all three large versican isoforms (Fig. 3). The V2 isoform was by far the most abundant, indicating that the cells have remained primarily true to type as far as versican expression is concerned. Our ability to detect low levels of the V0 and V1isoforms may be related to the high overall level of versican expression in these cells.
Meningeal cells
The 12C5 mAb recognized two species of Mrhigher than 400 kDa in the conditioned medium of cultured meningeal cells, both of which failed to enter a 4% gel without previous chondroitinase treatment (Fig. 3). These species correspond to the V0 and V1 isoforms.
Astrocytes and microglia
No form of versican was detected in the conditioned medium of type 1 astrocytes (Fig. 3) (Asher et al., 2000). Neither was versican detectable in the conditioned medium of newborn rat brain-derived microglia (data not shown).
The 12C5 mAb therefore recognizes three large, chondroitin sulfate-bearing species. On the basis of their Mr, the two larger species produced by meningeal cells and CG4 cells correspond to the V0 and V1 isoforms, and the smaller (∼400 kDa) form, produced by cells of the oligodendrocyte lineage and CG4 cells, and the predominant form in the adult rat CNS, corresponds to the V2 isoform.
Versican is bound to hyaluronate in vitro andin vivo
Versican binds hyaluronate in vitro (LeBaron et al., 1992), and there is evidence that it is associated with HAin vivo (Asher et al., 1991). We therefore investigated whether the binding of versican to oligodendrocyte lineage cells is mediated by HA. We also examined in greater detail the association of versican with HA in vivo.
Oligodendrocyte lineage cells in vitro
The biotinylated hyaluronate-binding domain of cartilage aggrecan was used to localize HA in oligodendrocyte lineage cells. In OLCs expanded in PDGF- and FGF2-containing medium and then allowed to differentiate, labeling for HA gave rise to a cell body-associated ring very similar to that seen with the anti-versican mAb. Double labeling for HA and versican revealed the two to be almost entirely coincident (Fig. 4c,d). In OLCs plated directly in oligodendrocyte differentiation medium, in which the predominant form of versican labeling is substrate associated, HA labeling gave rise only to the cell-associated, ring-like pattern (in a small proportion of the cells) and not to the substrate-associated type.
To determine whether the attachment of versican to the surface of these cells is mediated by HA, we pretreated the cells with hyaluronidase before versican labeling. The ring-type versican labeling in pre-oligodendrocytes was found to be sensitive to both testicular andStreptomyces hyaluronidases (Fig.4e,f). HA labeling was also completely abolished by pretreatment of the cells with Streptomyceshyaluronidase. This enzyme is HA specific (Ohya and Kaneko, 1970), unlike testicular hyaluronidase, which also degrades chondroitin sulfate. Versican immunoreactivity was unaffected by chondroitinase ABC. These findings imply that the binding of versican to the surface of these cells is mediated by HA. The substrate-associated labeling, however, was unaffected by hyaluronidase, implying that HA is not involved in the attachment of versican to the substrate.
If hyaluronidase indirectly liberates versican from the cell surface by degrading HA, then it ought to be possible to detect intact versican in the medium of hyaluronidase-treated cells. OLCs were expanded in PDGF- and FGF2-containing medium and then allowed to differentiate to the pre-oligodendrocyte stage. The culture medium was removed and replaced with either medium containing testicular hyaluronidase (flask 1) or medium alone (flask 2) for 1 hr. This medium was then removed, and a second 1 hr incubation was initiated in which hyaluronidase was added to the flask (flask 2) that had not previously received it. The various media were then assayed for the presence of versican by Western blotting with the 12C5 mAb. The addition of testicular hyaluronidase brought about the release of intact versican into the medium of pre-oligodendrocytes (Fig. 4g). No versican was released in the absence of the enzyme. That the hyaluronidase-released versican comigrated with the versican normally present in the conditioned medium is important, because it indicates that the release did not occur as a result of proteolysis of the versican core protein (Fig.4g). Versican was not detected in detergent lysates prepared after exposure to hyaluronidase, indicating that all of the cell surface versican is removed by the enzyme. Two other CS-PGs with HA-binding domains, neurocan and brevican, were both detectable in the conditioned medium of these cells, yet neither was released by hyaluronidase (Fig. 4g). This phenomenon is not connected with link protein, which stabilizes the binding of aggrecan to HA in cartilage, because no link protein was detectable in these cells (data not shown).
Adult rat brain
We then asked whether versican is bound to HA in the adult rat brain. Serial extracts were prepared from whole rat brains in PBS, pH 5.3, in the manner described in Materials and Methods. The pellet was resuspended in the same buffer and divided into two equal parts, to only one of which was added testicular hyaluronidase (50 μg/ml). Both homogenates, one with and the other without the enzyme, were incubated for 2 hr at 37°C. An aliquot was removed from each after 30 min. The homogenates were then washed twice and incubated for a further 2 hr, this time with hyaluronidase added to the homogenate not previously exposed to the enzyme. All the resultant supernatants were then tested for the presence of versican by Western blotting with the 12C5 mAb.
Substantial amounts of versican (V2) were detected in the first PBS extract (Fig.5, top). The subsequent four PBS extracts were essentially devoid of versican. The release of (additional) versican from the PBS-insoluble material required hyaluronidase. The release of versican was essentially complete after 30 min at 37°C, because the amount released after 2 hr was not greater. Importantly, the addition of hyaluronidase to the homogenate initially incubated without the enzyme (and from which little versican had been released) led to the release of versican, indicating that versican release was not simply a consequence of prolonged incubation at 37°C. Because the amount of versican released by the enzyme was approximately equal to that detected in the first PBS extract, we may conclude that approximately half of the versican in the adult rat brain is bound to HA. As was the case with the cultured cells, the hyaluronidase-released versican comigrated with the versican present in the first PBS extract, implying that the release is mediated by HA degradation and not by proteolysis of the versican core protein.
Versican colocalizes with and is physically associated with tenascin-R in oligodendrocytes
Immunocytochemistry
At all stages of oligodendrocyte differentiation, labeling for tenascin-R appeared very similar to that for versican. Labeling for tenascin-R gave rise to both the ring-type and the substrate-associated stellate profiles. Double labeling of OPCs maintained for 1 d in differentiation medium (i.e., pro-oligodendroblasts) with the 12C5 anti-versican mAb and rabbit anti-tenascin-R revealed an almost identical distribution (Fig.6a,b). Doing the double labeling in the opposite direction (i.e., the rabbit anti-tenascin-R first) made no difference to the outcome (i.e., the tenascin-R antibodies did not inhibit the binding of the versican antibody). As was the case for versican, myelin-forming cells generally labeled poorly, if at all, for tenascin-R, and there was no labeling of the membranous sheets.
Fig. 6.
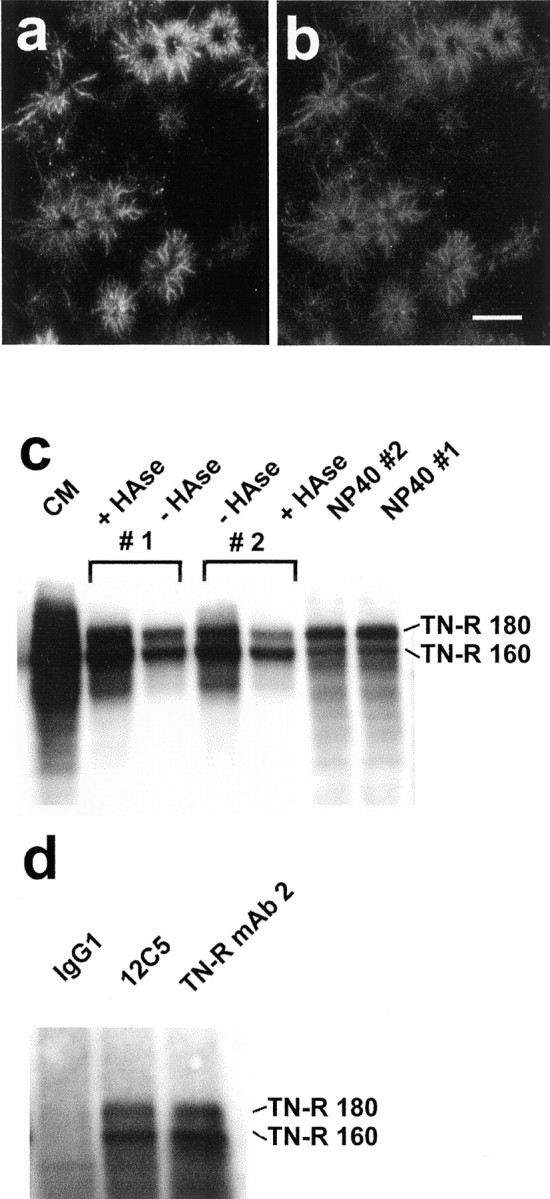
Versican colocalizes with tenascin-R, and the two exist as a complex in oligodendrocyte-conditioned medium. OPCs were grown for 1 d in differentiation medium and then double labeled with the 12C5 anti-versican mAb (a) and a polyclonal (rabbit) anti-tenascin-R (b). The distributions of the two appear identical. Scale bar, 25 μm.c, Two flasks of OPCs were grown for 2 d in differentiation medium. This was then removed and replaced with the same medium, either with (#1) or without (#2) testicular hyaluronidase (50 μg/ml) for 1 hr. This medium was removed and replaced with the same medium, except that the enzyme was added to the flask not previously treated with the enzyme (#2), but not to flask #1. The conditioned media were concentrated and equalized according to the protein content of the (NP-40) cell lysate. The samples were run under reducing conditions in a 7% gel, transferred to nitrocellulose, and labeled with an anti-tenascin-R mAb. Large amounts of tenascin-R were detected in the conditioned medium of oligodendrocytes (CM). Although tenascin-R was present in the hyaluronidase-treated cells (#1 +HAse), the amount was no greater than that present in the conditioned medium of the untreated cells (#2 −HAse). The failure of hyaluronidase to release tenascin-R is also evidenced by the presence of tenascin-R in the cell lysates (NP40). Note that the smaller tenascin-R 160 predominates in the conditioned media, whereas the 180 kDa form predominates in the cell lysates. d, Immunoprecipitation was performed on oligodendrocyte-conditioned medium with an isotype-matched control antibody (IgG1), the anti-versican mAb 12C5, or an anti-tenascin-R mAb. The products were run in a 7% gel under reducing conditions, transferred to nitrocellulose, and labeled with the same anti-tenascin-R mAb. The anti-versican mAb immunoprecipitated tenascin-R, indicating that the two are physically associated in oligodendrocyte-conditioned medium.
The tenascin-R labeling of growth factor-exposed pre-oligodendrocytes, although identical to that for versican, was essentially unaltered by testicular hyaluronidase, although there was some loss of intensity. This was confirmed by Western blot analysis (Fig. 6c). Although tenascin-R was detected in the medium of hyaluronidase-treated oligodendrocytes (presumably because of continued secretion), the amount was no greater than that found in medium lacking hyaluronidase (Fig. 6c). Furthermore, substantial amounts of tenascin-R were detectable in cell lysates made after hyaluronidase treatment (Fig. 6c). Hence, although the distribution of tenascin-R is identical to that of versican in these cells, its retention at the cell surface does not depend on hyaluronate.
Coimmunoprecipitation
Their overlapping distributions in OLCs in vitro and the known ability of (the C-type lectin domain of) versican to bind tenascin-R (Aspberg et al., 1995) led us to ask whether the two are physically associated in oligodendrocytes. The anti-versican mAb 12C5 was found to immunoprecipitate tenascin-R from oligodendrocyte-conditioned medium and from the medium of hyaluronidase-treated oligodendrocytes (Fig. 6d), indicating that the two do exist as a complex. The existence of such a complex in the medium of hyaluronidase-treated oligodendrocytes presumably means that some tenascin-R was released from the cells by hyaluronidase (accounting for the loss of intensity), but that the majority remained cell associated. Antibodies against tenascin-R were also found to immunoprecipitate versican (data not shown).
Control of versican expression in oligodendrocyte lineage cells
Our studies on versican expression in cultured CNS glia pointed to oligodendrocyte lineage cells as the likely source of versican in the injured CNS. The increased expression must be brought about, directly or indirectly, by cytokines and growth factors released after injury. Cytokines and growth factors known to be upregulated in response to CNS injury were therefore screened for their effect on versican expression in OLCs. The amount of versican in OLC-conditioned medium was assessed by Western blotting with the 12C5 mAb. We examined versican expression in both dividing (i.e., in the presence of PDGF and FGF2) and differentiating OLCs. The samples were equalized according to the protein content of the (NP-40) cell lysate from which the conditioned media were collected.
Dividing OLCs
OLCs were grown for 24 hr in medium containing PDGF and FGF2. The medium was then changed, and the cells were cultured for a further 2 d in the presence of PDGF and FGF2 and the cytokine or growth factor under investigation. In the presence of PDGF and FGF2, TGFβ clearly led to an increase in the amount of versican present (Fig.7a). The effects of TGFβ and EGF were investigated in more detail, and those of TGFβ were clearly reproduced (Fig. 7b). Versican expression was unaffected by EGF, however.
Fig. 7.

Versican is upregulated by TGFβ in dividing OPCs and by IL-1β and CNTF in differentiating oligodendrocytes. Dividing cells were maintained for 2 d in medium containing PDGF and FGF2 and the cytokine/growth factor (10 ng/ml) being tested (a, b). Differentiating cells were grown for 2 d in medium containing PDGF and FGF2, and then for a further 2 d in differentiation medium containing the cytokine/growth factor (10 ng/ml) under investigation (c). The conditioned media were collected, concentrated, and treated with chondroitinase ABC. The samples were equalized according to the protein content of the cell lysates, run under nonreducing conditions in a 4% gel, and transferred to nitrocellulose. The blots were labeled with the anti-versican mAb 12C5. TGFβ led to an increase in the amount of versican present in the conditioned media of dividing OPCs (a, b). In differentiating cells, IL-1β and CNTF, but not TGFβ, brought about an increase in the amount of versican in the conditioned medium (c).
Differentiating OLCs
OLCs were grown for 2 d in medium containing PDGF and FGF2. The medium was then changed to differentiation medium containing the cytokine/growth factor to be tested. Only IL-1β and CNTF consistently brought about an increase in versican expression under these conditions (Fig. 7c).
Chondroitin sulfate proteoglycans contribute to the axon growth-inhibitory properties of oligodendrocytes
Recently, it has been suggested that chondroitin sulfate proteoglycans are responsible for the growth cone-collapsing activity of oligodendrocytes (Niederöst et al., 1999). Because versican is one of the major CS-PGs expressed by these cells, the implication is that versican is at least partly responsible for this activity. We have investigated whether the axon growth-inhibitory effects of oligodendrocytes, grown under conditions shown to promote maximal versican expression, can be alleviated by the removal of chondroitin sulfate.
The enzyme chondroitinase ABC was used to remove chondroitin sulfate from the environs of differentiating oligodendrocytes. Oligodendrocyte progenitor cells were grown for 36 hr in differentiation medium, resulting in the deposition of versican on the substrate. It should be noted that these cells do not form a confluent monolayer and that versican is deposited on the substrate in the spaces between the cells. The cells were treated with chondroitinase ABC for the final 3 hr and then fixed in 4% paraformaldehyde. The cells were fixed to prevent further differentiation of the OPCs and concomitant loss of versican immunoreactivity during the course of the axon growth assay (2 d). The effectiveness of the chondroitinase digestion was assessed with the 2B6 mAb, which recognizes the stubs remaining on the core protein after chondroitinase digestion of chondroitin sulfate GAG chains. The digested and fixed cells were used as a substrate for the growth of dissociated DRG neurons. Removal of the chondroitin sulfate, much of which will be carried by versican, led to a significant increase in neurite outgrowth (Fig. 8). Neuronal adhesion to the substrate was not affected by chondroitinase treatment (Fig. 8). This assay was performed on three separate occasions, with the same outcome. Hence, the removal of chondroitin sulfate alleviates the axon growth-inhibitory properties of immature oligodendrocytes.
Fig. 8.

Chondroitin sulfate proteoglycans contribute to the axon growth-inhibitory properties of oligodendrocytes. Dorsal root ganglion neurons growing on untreated (a, c) and chondroitinase ABC-treated (b, d) paraformaldehyde-fixed pro-oligodendroblasts were labeled with 3A10 (a, b) and Hoechst 33342 to visualize nuclei (c, d). Scale bar, 100 μm. e, Quantification of neurite outgrowth on pro-oligodendroblasts. Chondroitinase treatment led to a significant increase in DRG neurite outgrowth (**p < 0.001; Student's t test) without affecting neuronal adhesion to the substrate. These findings suggest that axon growth in an oligodendroglial environment is impaired by chondroitin sulfate.
DISCUSSION
Versican is upregulated in the injured CNS
In the adult rat brain, immunohistochemistry has shown there to be high levels of versican in white matter tracts (Bignami et al., 1993). In gray matter, versican expression is more variable. Although it is readily detectable in spinal cord gray matter, very little versican is detectable in the cerebral cortex (Bignami et al., 1993). Here, we demonstrate a substantial increase in versican immunoreactivity around a knife lesion to the cerebral cortex, and Western blot analysis of extracts prepared from injured and uninjured tissue revealed there to be two to three times more versican in the injured tissue. The injury-induced versican comigrated (at 400 kDa) with that in the normal, uninjured rat brain and is therefore the V2 isoform. Rat CNS versican has been shown to carry little chondroitin sulfate (Asher et al., 1995). The injury-induced versican migrated as a single, discrete band without chondroitinase treatment and comigrated with normal rat brain versican, indicating that it too carries little chondroitin sulfate. The axon growth-inhibitory properties of brain-derived versican have been shown to reside in both the core protein and the chondroitin sulfate GAG chains (Schmalfeldt et al., 2000). Despite its relatively low level of glycanation, therefore, the injury-induced versican would be expected to exert an inhibitory effect on regenerating axons in the injured CNS.
Versican is a product of oligodendrocyte lineage cells
The distribution of versican in the normal (Bignami et al., 1993) and injured CNS is indicative of a glial origin. We have screened various glial cell types for versican expression. Versican was present in the conditioned media of OLCs and meningeal cells, but not in that of astrocytes or microglia. However, although meningeal cells produced the V0 and V1 isoforms of versican, OLCs were only capable of producing the smaller V2 isoform. OLCs, then, are uniquely capable of producing the isoform that predominates in the normal and injured CNS, and this suggests that they are the major source of CNS versican. Although cultured OLCs make the same isoform of versican as that found in the CNS, OLC-derived versican was unable to enter a 4% gel without previous chondroitinase treatment. OLC-derived versican therefore carries large amounts of chondroitin sulfate. The pattern of versican expression in the normal and injured CNS is entirely consistent with it being produced by OLCs, which are recruited to lesions in large numbers (Levine et al., 2001).
Versican expression increases in differentiating OLCs
Western blot analysis of conditioned medium revealed there to be higher levels of versican in differentiating oligodendrocytes than in dividing progenitor cells. Furthermore, no versican labeling was seen in dividing, bipolar OPCs. The labeling of large numbers of cells occurred only after the removal of the growth factors and was seen only on or around multipolar cells. These observations suggest that versican expression in OLCs requires some differentiation or their exit from the division cycle or both. Versican labeling was first seen at the late A2B5-positive stage, suggesting that versican expression begins at this early stage. Previously, it has been reported that oligodendrocytes label for versican V2(Niederöst et al., 1999). Our data are in agreement with this conclusion and show, furthermore, that cells of the oligodendrocyte lineage acquire the ability to produce versican at an early stage in their differentiation.
Versican is bound to hyaluronate in vitro andin vivo
Any attempt to remove versican from the injured CNS will require an understanding of how, and with what, it interacts in the ECM. In common with the other members of the aggrecan family, versican possesses a hyaluronate-binding domain at its N-terminal (Zimmermann and Ruoslahti, 1989) and has been shown to bind to hyaluronate in vitro (LeBaron et al., 1992).
Two types of labeling were seen in OLCs in vitro: substrate-associated, stellate profiles and ring-like labeling of the cell body. As noted above, both first appeared at the same stage (i.e., late A2B5 positive). The substrate-associated labeling was the predominant pattern in OLCs plated directly in differentiation medium (i.e., not exposed to growth factors), whereas the ring-type labeling was the predominant pattern in differentiating cells that had been exposed to growth factors. The question arises then as to why the ring-type labeling becomes more prevalent in growth factor-treated cells. The labeling of the cells is indicative not only of the ability of the cell to make versican, but also its ability to retain it at the cell surface. The ability of OLCs to retain versican is dependent on HA, because (1) labeling for HA gave rise to a pattern identical to that for versican, (2) the ring-type versican labeling was sensitive to hyaluronidase, and (3) hyaluronidase brought about the release of intact versican into the medium. Hyaluronate synthesis increases in dividing cells (Laurent and Fraser, 1992), and this explains why growth factor-driven OLCs retain versican at their surface. Although versican binding to these growth factor-driven cells is entirely HA dependent, versican binding to the substrate is not.
It was possible to solubilize approximately half of the versican in the adult rat brain with PBS. Hyaluronidase brought about the release of additional versican, after which no further versican could be extracted with SDS. The amount released by the enzyme was roughly equal to that solubilized by PBS. Hence, approximately half of the versican in the adult rat brain exists in association with HA. Preliminary data suggest that HA itself is upregulated in the injured CNS (R. A. Asher, D. A. Morgenstern, and J. W. Fawcett, unpublished observations) and could therefore play a pivotal role in anchoring versican and other HA-binding CS-PGs (e.g., neurocan) in the glial scar.
A versican/tenascin-R complex exists in oligodendrocytes
Versican also has the ability to interact with tenascin-R, via its C-type lectin domain (Aspberg et al., 1995). Here we have shown that tenascin-R colocalizes with versican in OLCs and that antibodies against one can immunoprecipitate the other. Hence, some of the versican and tenascin-R produced by these cells exists as a complex. Such a complex may well be present in the lesion environment, because transection of the postcommissural fornix led to an increase in tenascin-R expression (Probstmeier et al., 2000). The properties of the ECM as a whole may not be entirely explicable in terms of its constituent parts. For this reason, an understanding of how, and with what, each component interacts in the glial scar will be important.
Regulation of versican expression in OLCs
TGFβ brought about an increase in the amount of versican detected in the conditioned medium of dividing OPCs, and IL-1β and CNTF brought about an increase in differentiating cells. Such studies are complicated by the fact that versican expression increases as these cells differentiate. Because TGFβ is known to promote differentiation in these cells (McKinnon et al., 1993), it is possible that the increase in versican expression is an indirect consequence of TGFβ-induced differentiation, rather than a direct effect on versican expression. Nevertheless, the ability of TGFβ to bring about an increase in versican expression suggests that it may also play some part in the upregulation of versican in the injured CNS.
Oligodendrocyte-derived chondroitin sulfate (proteoglycans) inhibit axon growth
Recently, it has been shown that the growth cone-collapsing activity of oligodendrocytes is related to the presence of CS-PGs (Niederöst et al., 1999). Growth of these cells in the presence of β-d-xyloside, an inhibitor of GAG synthesis, led to the disappearance of versican at the cell surface and to the loss of contact-mediated growth cone-collapse (Niederöst et al., 1999). Our data also point to an inhibitory role for CS-PG GAG chains in axon growth in an oligodendroglial environment, because the removal of chondroitin sulfate led to an increase in axon growth on oligodendrocytes grown under conditions shown to favor versican expression and glycanation. It has been reported that immature oligodendrocytes do not induce growth cone collapse (Schwab and Caroni, 1988). The cells used in our bioassay were mostly A2B5 negative and expressed versican at much higher levels than the (A2B5-positive) cells reported previously not to induce growth cone collapse. Regardless of their precise stage, our data suggest something rather different, namely that immature oligodendrocytes create an environment (i.e., ECM) that is unsupportive of axon growth, without the need to induce growth cone collapse.
Previously, we have shown that DRG neurons growing on a monolayer of living Neu7 cells do not avoid patches of versican (Fidler et al., 1999). However, these patches also contained laminin, and this may have been sufficient to override the inhibitory effects of versican.
Conclusions
Here we show that versican, an axon growth-inhibitory CS-PG, is upregulated in the injured CNS and is therefore present in the environment in which axon regeneration fails. We have identified oligodendrocyte lineage cells as the likely source of this versican and now believe these cells to be a major source of axon growth-inhibitory CS-PGs in the damaged CNS. Oligodendrocyte lineage cells are present throughout the normal adult CNS and are recruited to injuries in large numbers (Levine et al., 2001). These cells express the inhibitory CS-PG NG2 at their surface (Levine, 1994) and release proteolytically shed forms of it into the lesion environment (Morgenstern, 2000). These cells are also active in the synthesis of neurocan (Asher et al., 2000), phosphacan (Canoll et al., 1996), brevican (Seidenbecher et al., 1998; present work), and tenascin-R (Pesheva et al., 1997), all of which exert inhibitory effects on neurite outgrowth in vitro(Pesheva et al., 1993; Friedlander et al., 1994; Yamada et al., 1997;Garwood et al., 1999). A possible strategy for encouraging axon regeneration, therefore, would be to prevent the recruitment of OLCs to CNS injuries. Complement killing with gal-C antibodies, which would be expected to target versican-producing cells, has in fact been shown to promote axon regeneration in the rat spinal cord (Dyer et al., 1998). Also, the infusion of an anti-mitotic into the lesioned nigrostriatal tract prevented OLC recruitment and promoted axon regeneration (Rhodes et al., 2000).
Footnotes
This work was supported by the Medical Research Council, The Wellcome Trust, and the International Spinal Research Trust. We thank Dr. J. H. Rogers for helpful discussions, Dr. A. Oohira for the neurocan antibody, and Dr. J. M. Levine for the NG2 antibody.
Correspondence should be addressed to Dr. Richard A. Asher, Cambridge Centre for Brain Repair, Forvie Site, Robinson Way, Cambridge, CB2 2PY, UK. E-mail: raa24@cam.ac.uk.
REFERENCES
- 1.Asher R, Perides G, Vanderhaeghen J-J, Bignami A. Extracellular matrix of central nervous system white matter: demonstration of a hyaluronate-protein complex. J Neurosci Res. 1991;28:410–421. doi: 10.1002/jnr.490280314. [DOI] [PubMed] [Google Scholar]
- 2.Asher RA, Scheibe RJ, Keiser HD, Bignami A. On the existence of a cartilage-like proteoglycan and link proteins in the central nervous system. Glia. 1995;13:294–308. doi: 10.1002/glia.440130406. [DOI] [PubMed] [Google Scholar]
- 3.Asher RA, Morgenstern DA, Adcock KH, Rogers JH, Fawcett JW. Versican is up-regulated in CNS injury and is a product of O-2A lineage cells. Soc Neurosci Abstr. 1999;25:750. [Google Scholar]
- 4.Asher RA, Morgenstern DA, Fidler PS, Adcock KH, Oohira A, Braistead JE, Levine JM, Margolis RU, Rogers JH, Fawcett JW. Neurocan is upregulated in injured brain and in cytokine-treated astrocytes. J Neurosci. 2000;20:2427–2438. doi: 10.1523/JNEUROSCI.20-07-02427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aspberg A, Binkert C, Ruoslahti E. The versican C-type lectin domain recognizes the adhesion protein tenascin-R. Proc Natl Acad Sci USA. 1995;92:10590–10594. doi: 10.1073/pnas.92.23.10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aspberg A, Adam S, Kostka G, Timpl R, Heinegård D. Fibulin-1 is a ligand for the C-type lectin domains of aggrecan and versican. J Biol Chem. 1999;274:20444–20449. doi: 10.1074/jbc.274.29.20444. [DOI] [PubMed] [Google Scholar]
- 7.Barker RA, Dunnett SB, Faissner A, Fawcett JW. The time course of loss of dopaminergic neurons and the gliotic reaction surrounding grafts of embryonic mesencephalon to the striatum. Exp Neurol. 1996;141:79–93. doi: 10.1006/exnr.1996.0141. [DOI] [PubMed] [Google Scholar]
- 8.Bignami A, Perides G, Rahemtulla F. Versican, a hyaluronate-binding proteoglycan of embryonal precartilagenous mesenchyma, is mainly expressed postnatally in rat brain. J Neurosci Res. 1993;34:97–106. doi: 10.1002/jnr.490340110. [DOI] [PubMed] [Google Scholar]
- 9.Bradbury EJ, Bennett GS, Moon LDF, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC delivered to the site of a spinal cord injury upregulates GAP-43 expression in dorsal root ganglion neurons. Soc Neurosci Abstr. 2000;26:860. [Google Scholar]
- 10.Brittis PA, Canning DR, Silver J. Chondroitin sulfate as a regulator of neuronal patterning in the retina. Science. 1992;255:733–736. doi: 10.1126/science.1738848. [DOI] [PubMed] [Google Scholar]
- 11.Canoll PD, Petanceska S, Schlessinger J, Musacchio JM. Three forms of RPTP-β are differentially expressed during gliogenesis in the developing rat brain and during glial cell differentiation in culture. J Neurosci Res. 1996;44:199–215. doi: 10.1002/(SICI)1097-4547(19960501)44:3<199::AID-JNR1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 12.Davies SJA, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390:680–683. doi: 10.1038/37776. [DOI] [PubMed] [Google Scholar]
- 13.Davies SJA, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci. 1999;19:5810–5822. doi: 10.1523/JNEUROSCI.19-14-05810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deller T, Haas CA, Naumann T, Joester A, Faissner A, Frotscher M. Up-regulation of astrocyte-derived tenascin-C correlates with neurite outgrowth in the rat dentate gyrus after unilateral entorhinal cortex lesion. Neuroscience. 1997;81:829–846. doi: 10.1016/s0306-4522(97)00194-2. [DOI] [PubMed] [Google Scholar]
- 15.Dou C-L, Levine JM. Inhibition of neurite growth by the NG2 chondroitin sulfate proteoglycan. J Neurosci. 1994;14:7616–7628. doi: 10.1523/JNEUROSCI.14-12-07616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dours-Zimmermann MT, Zimmermann DR. A novel glycosaminoglycan attachment domain identified in two alternative splice variants of human versican. J Biol Chem. 1994;269:32992–32998. [PubMed] [Google Scholar]
- 17.Dyer JK, Bourque JA, Steeves JD. Regeneration of brain stem-spinal axons after lesion and immunological disruption of myelin in adult rat. Exp Neurol. 1998;154:12–22. doi: 10.1006/exnr.1998.6905. [DOI] [PubMed] [Google Scholar]
- 18.Emerling DE, Lander AD. Inhibitors and promoters of thalamic neuron adhesion and outgrowth in embryonic neocortex: functional association with chondroitin sulfate. Neuron. 1996;17:1089–1100. doi: 10.1016/s0896-6273(00)80242-1. [DOI] [PubMed] [Google Scholar]
- 19.Fidler PS, Schuette K, Asher RA, Dobbertin A, Thornton SR, Calle-Patino Y, Muir B, Levine JM, Geller HM, Rogers JH, Faissner A, Fawcett JW. Comparing astrocytic cell lines that are inhibitory or permissive for axon growth: the major axon-inhibitory proteoglycan is NG2. J Neurosci. 1999;19:8778–8788. doi: 10.1523/JNEUROSCI.19-20-08778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitch MT, Silver J. Activated macrophages and the blood-brain barrier: inflammation after CNS injury leads to increases in putative inhibitory molecules. Exp Neurol. 1997;148:587–603. doi: 10.1006/exnr.1997.6701. [DOI] [PubMed] [Google Scholar]
- 21.Friedlander DR, Milev P, Karthikeyan L, Margolis RK, Margolis RU, Grumet M. The neuronal chondroitin sulfate proteoglycan neurocan binds to the neural cell adhesion molecules Ng-CAM/L1/NILE and N-CAM, and inhibits neuronal adhesion and neurite outgrowth. J Cell Biol. 1994;125:669–680. doi: 10.1083/jcb.125.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gard AL, Williams WC, Burrell MR. Oligodendroblasts distinguished from O-2A glial progenitors by surface phenotype (O4+GalC-) and response to cytokines using signal transducer LIFRβ. Dev Biol. 1995;167:596–608. doi: 10.1006/dbio.1995.1051. [DOI] [PubMed] [Google Scholar]
- 23.Garwood J, Schnädelbach O, Clement A, Schütte K, Bach A, Faissner A. DSD-1-proteoglycan is the mouse homolog of phosphacan and displays opposing effects on neurite outgrowth dependent on neuronal lineage. J Neurosci. 1999;19:3888–3899. doi: 10.1523/JNEUROSCI.19-10-03888.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gates MA, Fillmore H, Steindler DA. Chondroitin sulfate proteoglycan and tenascin in the wounded adult mouse neostriatum in vitro: dopamine neuron attachment and process outgrowth. J Neurosci. 1996;16:8005–8018. doi: 10.1523/JNEUROSCI.16-24-08005.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haas CA, Rauch U, Thon N, Merten T, Deller T. Entorhinal cortex lesion in adult rats induces the expression of the neuronal chondroitin sulfate proteoglycan neurocan in reactive astrocytes. J Neurosci. 1999;19:9953–9963. doi: 10.1523/JNEUROSCI.19-22-09953.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaworski DM, Kelly GM, Hockfield S. Intracranial injury acutely induces the expression of the secreted isoform of the CNS-specific hyaluronan-binding protein BEHAB/brevican. Exp Neurol. 1999;157:327–337. doi: 10.1006/exnr.1999.7062. [DOI] [PubMed] [Google Scholar]
- 27.Laurent TC, Fraser JRF. Hyaluronan. FASEB J. 1992;6:2397–2404. [PubMed] [Google Scholar]
- 28.Laywell ED, Steindler DA. Boundaries and wounds, glia and glycoconjugates. Cellular and molecular analyses of developmental partitions and adult brain lesions. Ann NY Acad Sci. 1991;633:122–141. doi: 10.1111/j.1749-6632.1991.tb15603.x. [DOI] [PubMed] [Google Scholar]
- 29.LeBaron RG, Zimmermann DR, Ruoslahti E. Hyaluronate binding properties of versican. J Biol Chem. 1992;267:10003–10010. [PubMed] [Google Scholar]
- 30.Levine JM. Increased expression of the NG2 chondroitin-sulfate proteoglycan after brain injury. J Neurosci. 1994;14:4716–4730. doi: 10.1523/JNEUROSCI.14-08-04716.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- 32.Lips K, Stichel CC, Müller HW. Restricted appearance of tenascin and chondroitin sulphate proteoglycans after transection and sprouting of adult rat postcommissural fornix. J Neurocytol. 1995;24:449–464. doi: 10.1007/BF01181606. [DOI] [PubMed] [Google Scholar]
- 33.McKeon RJ, Schreibe RC, Rudge JS, Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci. 1991;11:3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKeon RJ, Höke A, Silver J. Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp Neurol. 1995;136:32–43. doi: 10.1006/exnr.1995.1081. [DOI] [PubMed] [Google Scholar]
- 35.McKeon RJ, Jurynec MJ, Buck CR. The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. J Neurosci. 1999;19:10778–10788. doi: 10.1523/JNEUROSCI.19-24-10778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKinnon RD, Piras G, Ida JA, Dubois-Dalcq M. A role for TGF-β in oligodendrocyte differentiation. J Cell Biol. 1993;121:1397–1407. doi: 10.1083/jcb.121.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milev P, Friedlander DR, Sakurai T, Karthikeyan L, Flad M, Margolis RK, Grumet M, Margolis RU. Interactions of the chondroitin sulfate proteoglycan phosphacan, the extracellular domain of a receptor-type protein tyrosine phosphatase, with neurons, glia, and neural cell adhesion molecules. J Cell Biol. 1994;127:1703–1715. doi: 10.1083/jcb.127.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miura R, Aspberg A, Ethell IM, Hagihara K, Schnaar RL, Ruoslahti E, Yamaguchi Y. The proteoglycan lectin domain binds sulfated cell surface glycolipids and promotes cell adhesion. J Biol Chem. 1999;274:11431–11438. doi: 10.1074/jbc.274.16.11431. [DOI] [PubMed] [Google Scholar]
- 39.Moon LDF, Asher RA, Rhodes KE, Fawcett JW. Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nat Neurosci. 2001;4:465–466. doi: 10.1038/87415. [DOI] [PubMed] [Google Scholar]
- 40.Morgenstern DA. PhD thesis. University of Cambridge; 2000. Chondroitin sulphate proteoglycans in the peripheral and central nervous systems. [Google Scholar]
- 41.Niederöst BP, Zimmermann DR, Schwab ME, Bandtlow CE. Bovine CNS myelin contains neurite growth-inhibitory activity associated with chondroitin sulfate proteoglycans. J Neurosci. 1999;19:8979–8989. doi: 10.1523/JNEUROSCI.19-20-08979.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oakley RA, Tosney KW. Peanut agglutinin and chondroitin-6-sulfate are molecular markers for tissues that act as barriers to axon advance in the avian embryo. Dev Biol. 1991;147:187–206. doi: 10.1016/s0012-1606(05)80017-x. [DOI] [PubMed] [Google Scholar]
- 43.Ohya T, Kaneko Y. Novel hyaluronidase from Streptomyces. Biochim Biophys Acta. 1970;198:607–609. doi: 10.1016/0005-2744(70)90139-7. [DOI] [PubMed] [Google Scholar]
- 44.Oohira A, Matsui F, Watanabe E, Kushima Y, Maeda N. Developmentally regulated expression of a brain specific species of chondroitin sulfate proteoglycan, neurocan, identified with a monoclonal antibody 1G2 in the rat cerebrum. Neuroscience. 1994;60:145–157. doi: 10.1016/0306-4522(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 45.Perides G, Lane WS, Andrews D, Dahl D, Bignami A. Isolation and partial characterization of a glial hyaluronate-binding protein. J Biol Chem. 1989;264:5981–5987. [PubMed] [Google Scholar]
- 46.Perides G, Asher RA, Lark MW, Lane WS, Robinson RA, Bignami A. Glial hyaluronate-binding protein: a product of metalloproteinase digestion of versican? Biochem J. 1995;312:377–384. doi: 10.1042/bj3120377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pesheva P, Spiess E, Schachner M. J1–160 and J1–180 are oligodendrocyte-secreted nonpermissive substrates for cell adhesion. J Cell Biol. 1989;109:1765–1778. doi: 10.1083/jcb.109.4.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pesheva P, Gennarini G, Goridis C, Schachner M. The F3/11 cell adhesion molecule mediates the repulsion of neurons by the extracellular matrix glycoprotein J1–160/180. Neuron. 1993;10:69–82. doi: 10.1016/0896-6273(93)90243-k. [DOI] [PubMed] [Google Scholar]
- 49.Pesheva P, Gloor S, Schachner M, Probstmeier R. Tenascin-R is an intrinsic autocrine factor for oligodendrocyte differentiation and promotes cell adhesion by a sulfatide-mediated mechanism. J Neurosci. 1997;17:4642–4651. doi: 10.1523/JNEUROSCI.17-12-04642.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pindzola RR, Doller C, Silver J. Putative inhibitory extracellular matrix molecules at the dorsal root entry zone of the spinal cord during development and after root and sciatic nerve lesions. Dev Biol. 1993;156:34–48. doi: 10.1006/dbio.1993.1057. [DOI] [PubMed] [Google Scholar]
- 51.Probstmeier R, Stichel CC, Müller HW, Asou H, Pesheva P. Chondroitin sulfates expressed on oligodendrocyte-derived tenascin-R are involved in neural cell recognition. Functional implications during CNS development and regeneration. J Neurosci Res. 2000;60:21–36. doi: 10.1002/(SICI)1097-4547(20000401)60:1<21::AID-JNR3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 52.Rhodes KE, Moon LDF, Fawcett JW. Inhibiting proliferation in glial scar formation: effects on axon regeneration in the CNS. Soc Neurosci Abstr. 2000;26:856. doi: 10.1016/s0306-4522(03)00285-9. [DOI] [PubMed] [Google Scholar]
- 53.Schmalfeldt M, Dours-Zimmermann MT, Winterhalter KH, Zimmermann DR. Versican V2 is a major extracellular matrix component of the mature bovine brain. J Biol Chem. 1998;273:15758–15764. doi: 10.1074/jbc.273.25.15758. [DOI] [PubMed] [Google Scholar]
- 54.Schmalfeldt M, Bandtlow CE, Dours-Zimmermann MT, Winterhalter KH, Zimmermann DR. Brain derived versican V2 is a potent inhibitor of axonal growth. J Cell Sci. 2000;113:807–816. doi: 10.1242/jcs.113.5.807. [DOI] [PubMed] [Google Scholar]
- 55.Schwab ME, Caroni P. Oligodendrocytes and CNS myelin are nonpermissive substrates for neurite growth and fibroblast spreading in vitro. J Neurosci. 1988;8:2381–2393. doi: 10.1523/JNEUROSCI.08-07-02381.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seidenbecher CI, Gundelfinger ED, Böckers TM, Trotter J, Kreutz MR. Transcripts for secreted and GPI-anchored brevican are differentially distributed in rat brain. Eur J Neurosci. 1998;10:1621–1630. doi: 10.1046/j.1460-9568.1998.00166.x. [DOI] [PubMed] [Google Scholar]
- 57.Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 58.Skaper SD, Facci L, Milani D, Leon A, Toffano G. Culture and use of primary and clonal neural cells. In: Conn PM, editor. Methods in neurosciences, Vol 2. Academic; San Diego: 1990. pp. 17–33. [Google Scholar]
- 59.Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp Neurol. 1990;109:111–130. doi: 10.1016/s0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- 60.Thon N, Haas CA, Rauch U, Merten T, Fässler R, Frotscher M, Deller T. The chondroitin sulphate proteoglycan brevican is upregulated by astrocytes after entorhinal cortex lesions in adult rats. Eur J Neurosci. 2000;12:2547–2558. doi: 10.1046/j.1460-9568.2000.00109.x. [DOI] [PubMed] [Google Scholar]
- 61.Yamada H, Fredette B, Shitara K, Hagihara K, Miura R, Ranscht B, Stallcup WB, Yamaguchi Y. The brain chondroitin sulfate proteoglycan brevican associates with astrocytes ensheathing cerebellar glomeruli and inhibits neurite outgrowth from granule neurons. J Neurosci. 1997;17:7784–7795. doi: 10.1523/JNEUROSCI.17-20-07784.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zimmermann DR, Ruoslahti E. Multiple domains of the large fibroblast proteoglycan, versican. EMBO J. 1989;8:2975–2981. doi: 10.1002/j.1460-2075.1989.tb08447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]