Abstract
The monocular deprivation model of amblyopia is characterized by a reduction in cortical responses to stimulation of the deprived eye. Although the effects of monocular deprivation on the primary visual cortex have been well characterized physiologically and anatomically, the molecular mechanisms underlying ocular dominance plasticity remain unknown. Previous studies have indicated that the transcription factor adenosine cAMP/Ca2+ response element-binding protein (CREB) is activated during monocular deprivation. However, it remains unknown whether CREB function is required for the loss of cortical responses to the deprived eye. To address this issue, we used the herpes simplex virus (HSV) to express a dominant negative form of CREB (HSV-mCREB) containing a single point mutation that prevents its activation. Quantitative single-unit electrophysiology showed that cortical expression of this mutated form of CREB during monocular deprivation prevented the loss of responses to the deprived eye. This effect was specific and not related to viral toxicity, because overexpression of functional CREB or expression of β-galactosidase using HSV injections did not prevent the ocular dominance shift during monocular deprivation. Additional evidence for specificity was provided by the finding that blockade of ocular dominance plasticity was reversible; animals treated with HSV-mCREB recovered ocular dominance plasticity when mCREB expression declined. Moreover, this effect did not result from a suppression of sensory responses caused by the viral infection because neurons in infected cortex responded normally to visual stimulation. These findings demonstrate that CREB function is essential for ocular dominance plasticity.
Keywords: CREB, ocular dominance plasticity, primary visual cortex, ferret, herpes simplex virus, viral-mediated gene transfer, monocular deprivation
Degradation of the visual input to one eye during development leads to amblyopia, which is characterized by a decrease in visual acuity that cannot be improved with corrective lenses and constitutes the major cause of visual disability in children (National Eye Institute, 1984; Rutstein and Daum, 1998). The monocular deprivation model of amblyopia shows a reduction in cortical responsiveness to stimulation of the deprived eye and subsequent loss of connections relaying information from this eye to the primary visual cortex (Wiesel and Hubel, 1965; Hubel et al., 1977). Previous studies have indicated that the NMDA type of glutamate receptor plays a major role in ocular dominance plasticity during monocular deprivation (Bear et al., 1990; Rauschecker et al., 1990; Roberts et al., 1998). However, the specific contribution of the NMDA receptor to ocular dominance plasticity has not been characterized. One approach to defining this role is to examine what mechanisms are activated downstream from the NMDA receptor. Increased calcium influx through the NMDA receptor-associated channel and other membrane calcium channels probably contributes to long-term synaptic changes after monocular deprivation by activating protein kinases (Malinow et al., 1988;Malenka et al., 1989) that could, in turn, activate nuclear transcription factors. One of the best candidate targets of these events is the cAMP/Ca2+ response element-binding protein (CREB) (Deisseroth et al., 1996; Silva et al., 1998), which binds to a consensus sequence known as CRE (cAMP response element) (Montminy et al., 1990).
Gene activation by CREB has been shown to be important for several experimental models of learning and long-term memory (Silva et al., 1998). Consistent with a role for CREB in ocular dominance plasticity, monocular deprivation has been shown to induce CREB activation in mouse visual cortex (Pham et al., 1999). However, CREB activation does not necessarily imply a role in sensory plasticity. One example of this is seen in the mouse somatosensory cortex, in which CREB is also activated during whisker deprivation (Barth et al., 2000), yet mutations targeting the creb gene did not block barrel cortex plasticity of juvenile animals (Glazewski et al., 1999). Therefore, it remains unknown whether CREB function is required for the loss of cortical responses to the deprived eye during monocular deprivation. Alternative possibilities are that local protein synthesis at the synapse is sufficient for loss or strengthening of synapses (Stewart et al., 1996; Huber et al., 2000) or that other transcription factors are involved.
To elucidate the participation of CREB in ocular dominance plasticity, we used a herpes simplex virus (HSV) vector (Lim et al., 1996) to express a dominant negative mutant form of CREB (mCREB) in the primary visual cortex of ferrets during monocular deprivation. The dominant negative mutant contains a single point mutation (Ala for Ser at residue 133) that prevents phosphorylation and activation of CREB (Gonzalez and Montminy, 1989; Carlezon et al., 1998). Quantitativein vivo electrophysiology was used to measure alterations in the ocular dominance shift during monocular deprivation after injections of HSV containing cDNAs for mCREB and, as controls, CREB or β-galactosidase.
MATERIALS AND METHODS
Ferrets at postnatal day 50, the peak of ocular dominance plasticity (Issa et al., 1999), received two intracortical injections (left hemisphere) of HSV containing cDNA for mCREB, CREB, or thelacZ reporter gene (HSV-lacZ). In some injections, HSV vectors expressing mCREB or CREB tagged with green fluorescent protein (HSV-mCREB-GFP or HSV-CREB-GFP) were used (Olson et al., 1999). The HSV was used to express these transgenes because it is capable of carrying large transgenes and preferentially infects postmitotic cells, especially neurons (Neve and Lim, 1999). One or 7 d after injection (see below), the lid of the contralateral eye (right eye) was sutured closed to prevent patterned visual stimulation. After 3 d of monocular deprivation, the animals were anesthetized, and quantitative single-unit in vivo electrophysiology was performed to assess changes in cortical ocular dominance. Ferrets were used for this study because they have a highly developed visual cortex characterized by ocular dominance columns, orientation columns, and orientation-selective neurons (Chapman and Stryker, 1993; Weliky et al., 1996; Ruthazer et al., 1999; White et al., 1999). The ferret model is also advantageous over rodent models because the frontally placed eyes of the ferret allows for a large binocular representation in the primary visual cortex (Law et al., 1988). The Institutional Animal Care and Use committee at Virginia Commonwealth University approved all procedures described in this paper.
HSV-1 application. Animals were anesthetized using intraperitoneal injection of sodium pentobarbital (35 mg/kg) and acepromazine maleate (1.1 mg/kg) and fixed in a stereotaxic frame. Using a Dremel drill, a small craniotomy was performed to expose a small area of the primary visual cortex. Next, the tip of a 31 gauge Hamilton (Reno, NV) syringe containing the HSV (average titer of the purified virus stocks, 4.0 × 107infectious units/ml) was stereotaxically positioned at an angle of 15° from the midline and lowered ∼1 mm into the binocular region of the primary visual cortex. A volume of ∼2 μl was injected over 10 min. This volume was selected because it infects a restricted area of cortex (see Fig. 1), leaving a large uninfected region available for control recordings in the same hemisphere. Animals in the mCREB-, CREB-, and HSV-lacZ-treated groups were monocularly deprived ∼24 hr after injection for a period of 3 d. To examine reversibility of effects, one subgroup of HSV-mCREB-treated animals was monocularly deprived for 3 d starting 7 d after injection. This allows for dissipation of mCREB expression (Carlezon et al., 1998).
Fig. 1.
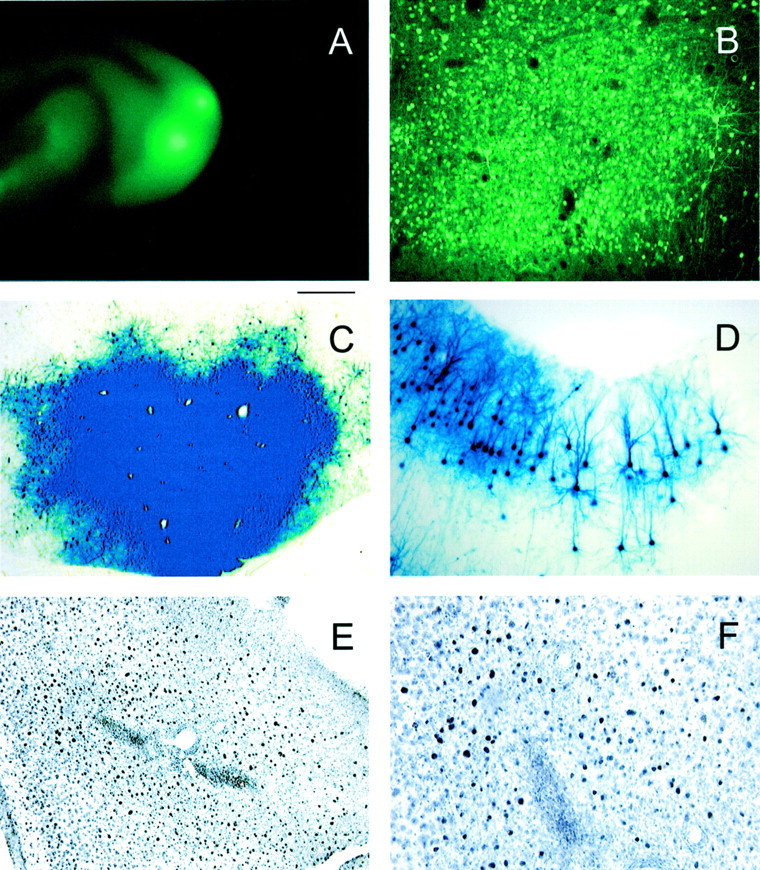
Histological examination shows successful expression of transgenes in the primary visual cortex.A, Epifluorescence shows the distribution of mCREB tagged with GFP resulting from 2 and 5 μl injections made 3 d earlier. B, The photomicrograph shows a large number of cells expressing mCREB-GFP near the site of injection.C, Staining for β-galactosidase conducted 3 d after injection of HSV-lacZ revealed many infected cells near the site of injection. D, The photomicrograph taken at high power shows the dendritic trees of labeled neurons.E, F, A large number of darkly labeled cells stained with CREB-specific antibodies surround an electrolytic lesion made during an electrode penetration near the injection site. The immunohistochemical procedures used for the CREB-specific antibodies were designed to minimize detection of endogenous CREB. For this reason, the number of immunopositive neurons overexpressing CREB is probably underrepresented. Scale bar (in A):A, 4 mm; B, D,E, 200 μm; C, 500 μm;F, 100 μm.
In vivo electrophysiology. Ferrets were anesthetized as above, and levels of anesthesia were ascertained by loss of withdrawal and corneal-blink reflexes. Eyelid sutures were then removed, a tracheotomy was performed, and the animal was placed in a stereotaxic frame. Expired CO2 was maintained at 4.0% and temperature at 38°C with a homeostatic blanket. The animal was then paralyzed using pancuronium bromide (1.5 mg/kg), and supplemental doses of sodium pentobarbital (10–30 mg/kg, i.p.) and acepromazine (0.05 mg/kg, s.c.) were given every hour to maintain levels of anesthesia or whenever heart rate or expired CO2 increased. The nictitating membranes were retracted using 2.5% phenylephrine hydrochloride, the pupils were dilated using 2% atropine sulfate, and contact lenses of appropriate refractive power were placed in the eyes to protect the cornea. After exposing the primary visual cortex, a tungsten in glass microelectrode was used to make single-unit recordings from both the treated and untreated portions of the hemisphere contralateral to the deprived eye. Recordings of treated cells were made in electrode penetrations near (up to 400 μm away) the injection site, whereas control recordings were made ∼2 mm away. Histological examination confirmed that the recordings of treated cells were made within the area of infection, whereas control recordings were outside of this region. Once a single unit was isolated, its receptive field was mapped, and the preferred orientation, direction, and velocity of the light bar stimulus was determined. A computer-controlled light bar stimulus was then presented to each eye 10 times, and the evoked responses were recorded using Spike2 software (Cambridge Electronics Design, Cambridge, UK). Spontaneous activity was recorded for 2 sec in the absence of stimulation and averaged over the course of 10 runs. At the end of each penetration, electrolytic lesions were made by passing 4 μA intensity current for 5 sec.
Quantification of ocular dominance. Using peristimulus histograms generated for each cell in Spike2, the number of spikes evoked for each eye was determined by measuring the total neuronal response during stimulus presentation. The spontaneous activity was then subtracted out of the total number of spikes. Ocular dominance was then quantified for each cell by calculating a binocularity index (BI = EE/(EE + DE), where EE stands for responses to the experienced eye, and DE stands for responses to the deprived eye. A binocularity index of 1.0 indicates that a cell is responsive only to the experienced eye (left eye), and a binocularity index of 0.0 indicates that a cell is responsive only to the deprived eye (right eye). A binocularity index of 0.5 indicates that a cell is equally responsive to stimulation of both eyes.
To analyze the results statistically, we obtained for each animal the median of the binocularity indices for recordings performed in treated cortex and the median of the binocularity indices for recordings performed in untreated control cortex in the same hemisphere. In this analysis, we only used data from animals that had at least one penetration in each control and treated region of the same hemisphere. A Wilcoxon Mann–Whitney rank sum test was then used to determine for each experimental group whether the medians of the binocularity indices obtained in the treated and untreated regions were significantly different from each other.
Immunohistochemistry. At the conclusion of the electrophysiology experiment, ferrets were killed with euthanol (125 mg/kg) and perfused transcardially with cold 0.9% saline, pH 7.2, followed by cold 4% paraformaldehyde in 0.4 mPBS, pH 7.2. The brains were then removed from the skull and post-fixed overnight at 4°C in the 4% paraformaldehyde solution. Serial vibratome sections of the caudal portion of the brain containing the primary visual cortex were collected at 50 μm intervals. Alternating sections were then used for cresyl violet staining and immunohistochemistry. The cresyl violet staining was used to reconstruct electrode recording tracts, and immunohistochemistry was used to confirm expression of transgenes in the primary visual cortex.
For immunohistochemistry, free-floating tissue sections were placed in 24-well cell culture plates (Corning, Corning, NY), and the tissue was washed (three times for 10 min each) in a stock solution of 0.3% Triton X-100 (Sigma, St. Louis, MO) in 0.1 m PBS to permeabilize the tissue. Next, endogenous peroxidases were quenched by incubating the sections in 1% H202 in stock solution for 20 min and then rinsed in the stock solution (three times for 10 min each). Sections were then incubated for 30 min in a 2% blocking solution of bovine serum albumin (Sigma) in stock solution and incubated overnight at 4°C in anti-CREB rabbit polyclonal IgG (1:1000 in blocking solution) (Upstate Biotechnology, Lake Placid, NY). On the following day, sections were once again rinsed in stock solution (three times for 10 min each), followed by incubation in biotinylated anti-rabbit IgG (1:200 in blocking solution) (Vector Laboratories, Burlingame CA). Next, sections were rinsed and incubated in ABC solution for 1 hr (Vectastain Elite ABC kit; Vector Laboratories), followed by DAB peroxidase substrate (Sigma Fast 3,3′-diaminobenzidine tetrahydrochloride with metal enhancer tablet set; Sigma) for visualization. lacZ staining was done using a β-galactosidase staining kit (Roche Diagnostics, Indianapolis, IN). All incubations were performed on a shaker table at the lowest speed setting. After immunohistochemistry, sections were mounted on chrom-alum-subbed slides and dehydrated with graded alcohol solutions (70, 95, and 100% ethanol) and citrus-based clearing solvent (Stephens Scientific, Riverdale, NJ). Slides were then coverslipped using Permount (Fisher Scientific, Fair Lawn, NJ). Sections from each animal were always processed and analyzed together.
RESULTS
Intracortical injections of HSV result in successful expression of the transgenes
To examine whether CREB function is required in ocular dominance plasticity, ferrets received an injection of HSV containing cDNA for CREB, mCREB, or β-galactosidase. Figure1 shows that intracortical injections of HSV resulted in the successful expression of the transgenes. To visualize the distribution of infected cells in the brain, some animals were injected with HSV vectors expressing mCREB tagged with green fluorescent protein (HSV-mCREB-GFP). Figure 1A shows the distribution of label resulting from two separate injections (2 μl for medial injection and 5 μl for lateral injection) of HSV-mCREB-GFP. In both cases, the label spread from the injection site to include a portion of the primary visual cortex, which is located at the caudal pole of the hemisphere in ferrets. An injection size of ∼2 μl was considered ideal for our studies because the infected cells were distributed over a relatively small area of cortex, ensuring that sufficient uninfected cortex was available for control recordings in the same hemisphere of each animal. Figure 1B shows that a very large number of striate cortical cells expressed the fluorescent label, indicating feasibility of using viral-mediated gene transfer in studies of ocular dominance plasticity. Similar results were observed with β-galactosidase staining in the case of animals injected with HSV containing the corresponding cDNA (Fig.1C). Moreover, these cells were confirmed to be neurons by the characteristic dendritic morphology seen at the edge of the region of infection in which fewer neurons were infected (Fig.1D). Closer to the injection, a very large number of cells were infected; however, the resulting staining was so dense that it precluded the visualization of the dendritic morphology (Fig.1C). Antibody staining with a CREB-specific antibody (Fig.1E,F) in animals injected with HSV-CREB showed a large number of darkly labeled infected cells surrounding an electrolytic lesion made at the end of a recording penetration ∼400 μm from the point of injection. The number of infected cells is likely to be underrepresented by the use of CREB-specific antibodies, because the immunohistochemical procedures used were designed to minimize detection of endogenous CREB. The position of the electrolytic lesion demonstrates that extracellular recordings were made within the area of infection. Similar electrolytic lesions made at the end of penetrations ∼2 mm from the point of injection were outside of the region of viral infection (data not shown) and therefore rendered control penetrations. In the following studies, we only used animals that had one or more penetrations in each of the control and treated regions of the same hemisphere.
Having established that successful expression of transgenes occurs after intracortical injection of HSV, we examined the effects of expression of different transgenes on ocular dominance plasticity. Animals were monocularly deprived 1 d after injection of HSV containing the cDNA for CREB, mCREB, or β-galactosidase. An additional group of animals injected with HSV-mCREB was monocularly deprived 7 d after injection, when mCREB expression had declined. In every case, monocular deprivation lasted for 3 d, and electrophysiological recordings were conducted at the end of this period to measure alterations in the ocular dominance shift. Extracellular recordings were made from 673 cells with receptive fields located in the binocular region of the visual field representation in striate cortex.
Intracortical HSV-mCREB injection blocks ocular dominance plasticity
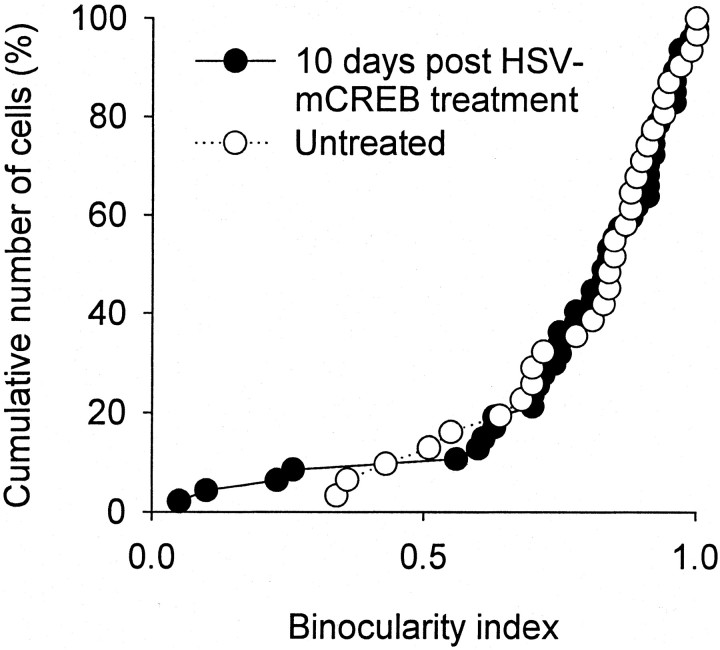
The histograms showing the distribution of neurons into five binocularity ranges are shown in Figure 2for animals that received an intracortical injection of HSV-mCREB and were monocularly deprived. Results show that the ocular dominance distribution is markedly shifted toward the nondeprived eye in control neurons (Fig. 2B). The results observed in control penetrations located medially and laterally from the injection site were similar and have been pooled together. In contrast, the expected ocular dominance shift was reduced markedly in cells recorded within the area of infection; the ocular dominance distribution of cells near the injection site has a flat profile (Fig. 2A). The results for each individual neuron are shown in Figure 2C, which plots the cumulative percentage of neurons as a function of the binocularity index. The distribution of indices for neurons recorded near the injection site is compared with the distribution for control neurons recorded away from the injection site. As expected, the majority (84%) of control cells had a binocularity index >0.5, indicating that they responded preferentially to the nondeprived eye. In contrast, the distribution of binocularity indices for neurons located near the injection site shows a similar number of cells dominated by the experienced and deprived eye. The binocularity indices in the group of neurons near the injection site differed significantly from those observed in the group of untreated neurons (p < 0.01; Wilcoxon Mann–Whitney test). These findings indicate that the loss of responses to the deprived eye was prevented by expression of mCREB.
Fig. 2.
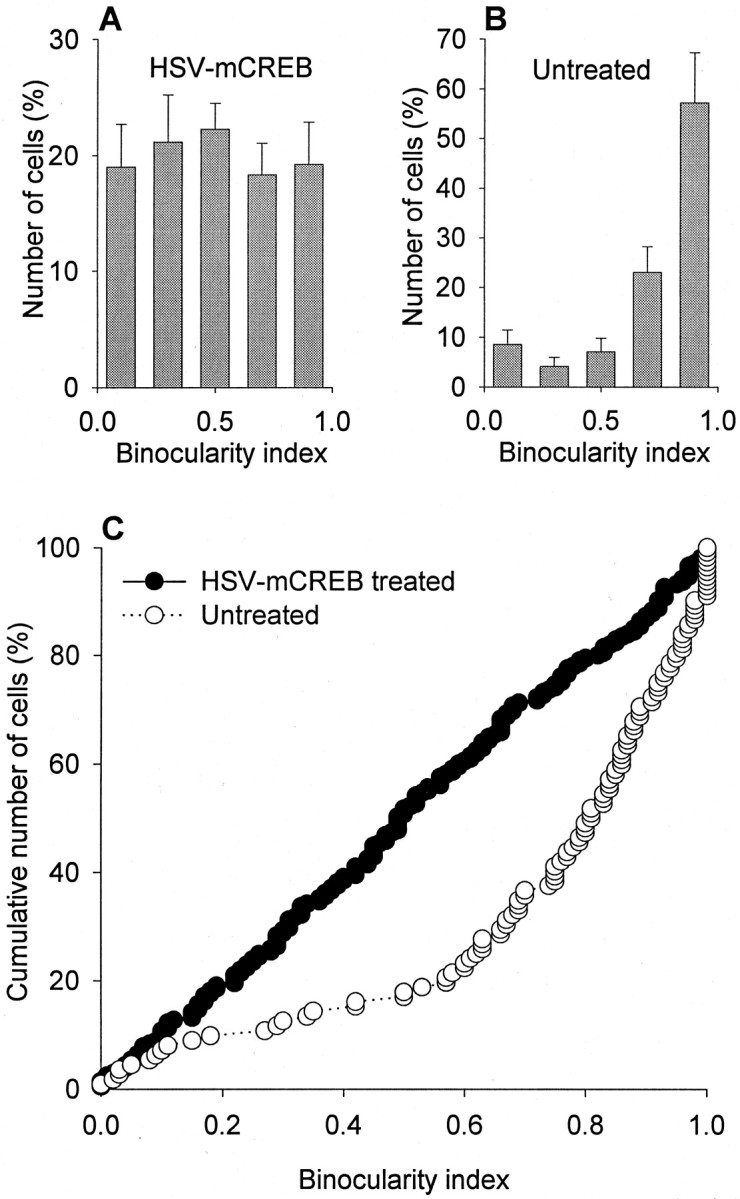
Intracortical injection of HSV-mCREB blocked the loss of neuronal response to the deprived eye during monocular deprivation. A and B (with error bars) show that neurons located in treated cortex near the injection site (A) had a nonshifted distribution of ocular dominance groups after 3 d monocular deprivation, whereas untreated cells (B) away from the injection site had a markedly shifted distribution of ocular dominance in the direction of the nondeprived eye. C, The cumulative plots show that most (84% of 112) untreated neurons had a binocularity index >0.5 after 3 d of monocular deprivation, indicating that they responded preferentially to the nondeprived eye. In contrast, recordings from 205 neurons located in treated cortex indicated that an equal number of cells responded preferentially to stimulation of the deprived (n = 103) and experienced (n = 102) eyes after 3 d of monocular deprivation. For each animal, treated and untreated neurons were located in the same hemisphere, contralateral to the deprived eye. The results obtained from animals injected with HSV-mCREB (n = 5 animals) and HSV-mCREB-GFP (n = 2 animals) were indistinguishable and were pooled together.
HSV-mCREB infection preserves visual responses
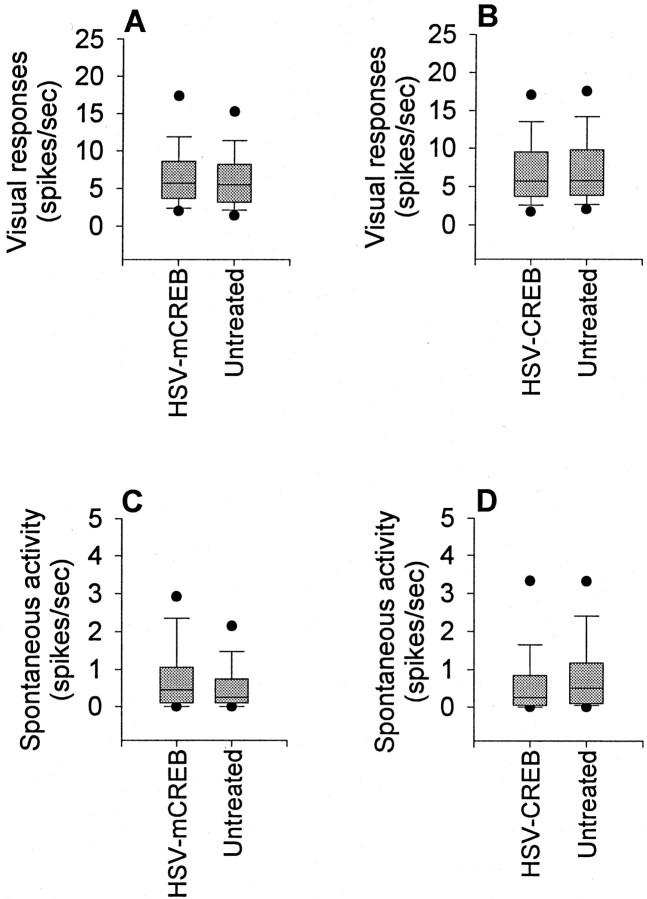
Finding that mCREB expression blocks ocular dominance plasticity raises the question of whether the effects result from a disruption of visual responses. Qualitative assessment revealed that most neurons studied near the injection site responded strongly to visual stimulation and were selective to stimulus orientation and direction of movement. Quantitative analysis also revealed that mCREB injection did not affect visual responsiveness of the neurons that were examined in the above studies. Figure 3Ashows that the maximum response (in spikes per second) to stimulation at the optimal orientation was not significantly different for the control and treated neurons (p > 0.05). An increased spontaneous activity could conceivably also disrupt activity-dependent mechanisms of ocular dominance plasticity by decreasing the signal-to-noise ratio. However, expression of mCREB was found not to increase spontaneous activity, as shown in Figure3C. These findings indicate that the effects of HSV-mCREB treatment on ocular dominance plasticity did not result from disruption of sensory responses or spontaneous activity.
Fig. 3.
HSV infection did not suppress visual cortical responses. A shows that injection of HSV-mCREB did not affect the visual responsiveness of neurons examined in these studies. The maximum response (in spikes per run) to stimulation at the optimal orientation was not significantly different for the control (n = 112 cells) and treated (n= 205) neurons (p > 0.05). Expression of mCREB also did not increase spontaneous activity, as shown inC. Similar results were seen in neurons near the injection of HSV-CREB (n = 102 cells) and control (n = 105) neurons away from the injection (B, D). The box plots show the median, 10th, 25th, 75th, and 90th percentiles as vertical boxeswith error bars. The fifth and 95th percentiles are shown asdots.
Effects of HSV-mCREB on ocular dominance plasticity are reversible
In view of the profound effect of HSV-mCREB treatment on ocular dominance plasticity, we asked whether the effect was correlated with the expression of mCREB. HSV-mediated expression of mCREB has been reported to be restricted to 1 week after injection (Carlezon et al., 1998). Similarly, we found that mCREB expression dissipates in primary visual cortex after the first week after injection, as illustrated in Figure 4. For this reason, some animals were monocularly deprived starting 1 week after injection of HSV-mCREB. Quantitative in vivo electrophysiology at the end of 3 d of monocular deprivation revealed a clear ocular dominance shift in these animals; Figure 5 shows that the nondeprived eye dominated most cells in the treated and untreated cortex. In conclusion, animals treated with HSV-mCREB recovered ocular dominance plasticity when mCREB expression had declined. These findings support our conclusion that the effects obtained with the HSV injections were specific and related to the expression of mCREB.
Fig. 4.
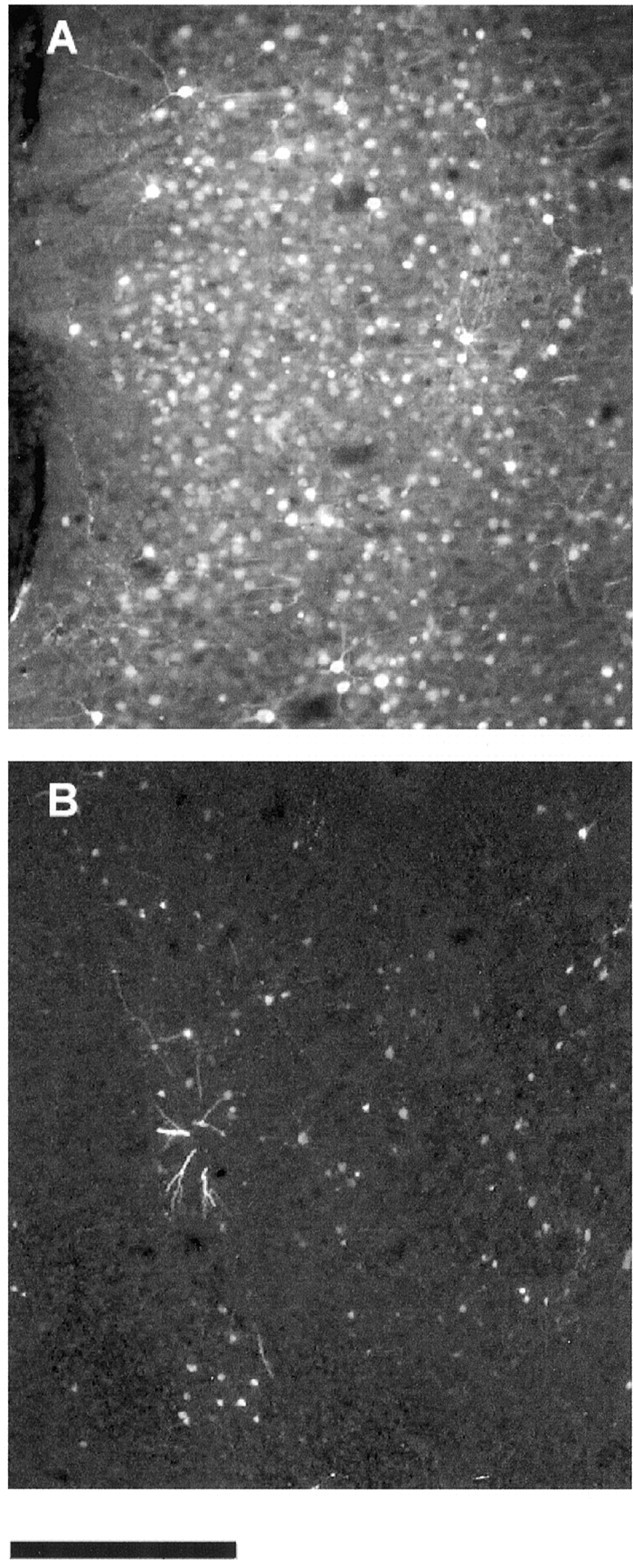
Expression of cortical mCREB decreased markedly after the first week after injection. The photomicrograph inA shows a large number of cells labeled with fluorescence in the primary visual cortex 3 d after injection of HSV-mCREB-GFP. In contrast, B shows relatively few cells labeled with mCREB tagged with GFP 10 d after injection. Both photomicrographs were obtained from cells located near the injection site (i.e., within 400 μm distance). Scale bar, 300 μm.
Fig. 5.
The effects of mCREB on ocular dominance plasticity were reversible. Animals in this study were subjected to 3 d of monocular deprivation of the contralateral eye starting 7 d after injection of HSV-mCREB in the left hemisphere. The cumulative plots for neurons studied in treated and untreated cortex were remarkably similar. Approximately 90% of 31 untreated neurons away from the injection site and 91% of 47 neurons (n = 2 animals) near the injection site were driven preferentially by the nondeprived eye (i.e., had a binocularity index >0.5).
Injections of HSV-CREB and HSV-lacZ do not block ocular dominance plasticity
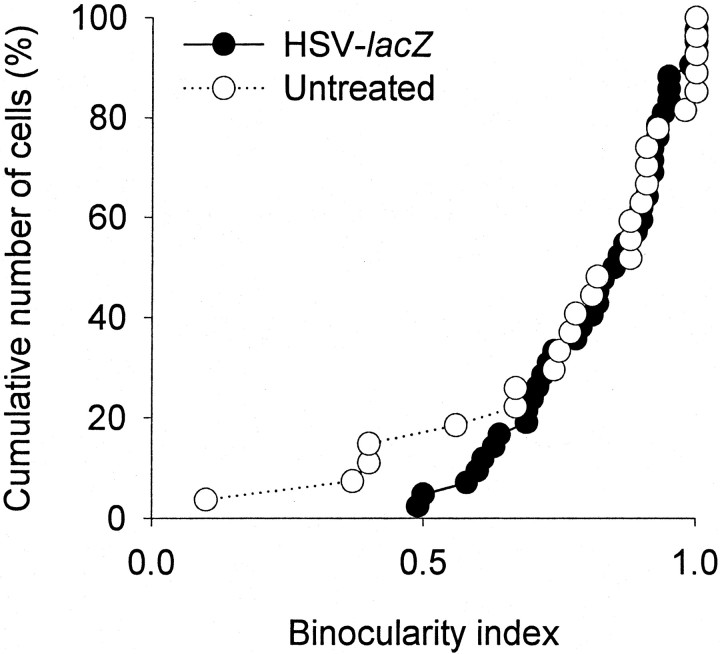
To examine further the specificity of the effects reported here, we studied cortical binocularity in animals treated with intracortical injection of HSV containing the cDNA for CREB or the lacZreporter gene. Figure 6 compares the cumulative plots observed for the HSV-CREB-treated and untreated cortex. Microelectrode recordings in the regions of treated and untreated primary visual cortex revealed that cortical neurons had a marked preference to stimulation of the experienced eye; the nondeprived eye dominated the majority (∼75%) of cells in HSV-CREB-treated and control recording sites. The binocularity indices observed in the two groups of neurons were not different (p > 0.05). Similar results were observed in animals treated with HSV-lacZ. As shown in Figure7, similar binocularity indices were observed for neurons in the treated and control recording sites. These results indicate the following: (1) viral infection by HSV alone does not cause loss of ocular dominance plasticity; (2) overexpression of the endogenous protein CREB does not lead to loss of ocular dominance plasticity; and (3) expression of the foreign protein β-galactosidase does not cause loss of ocular dominance plasticity.
Fig. 6.
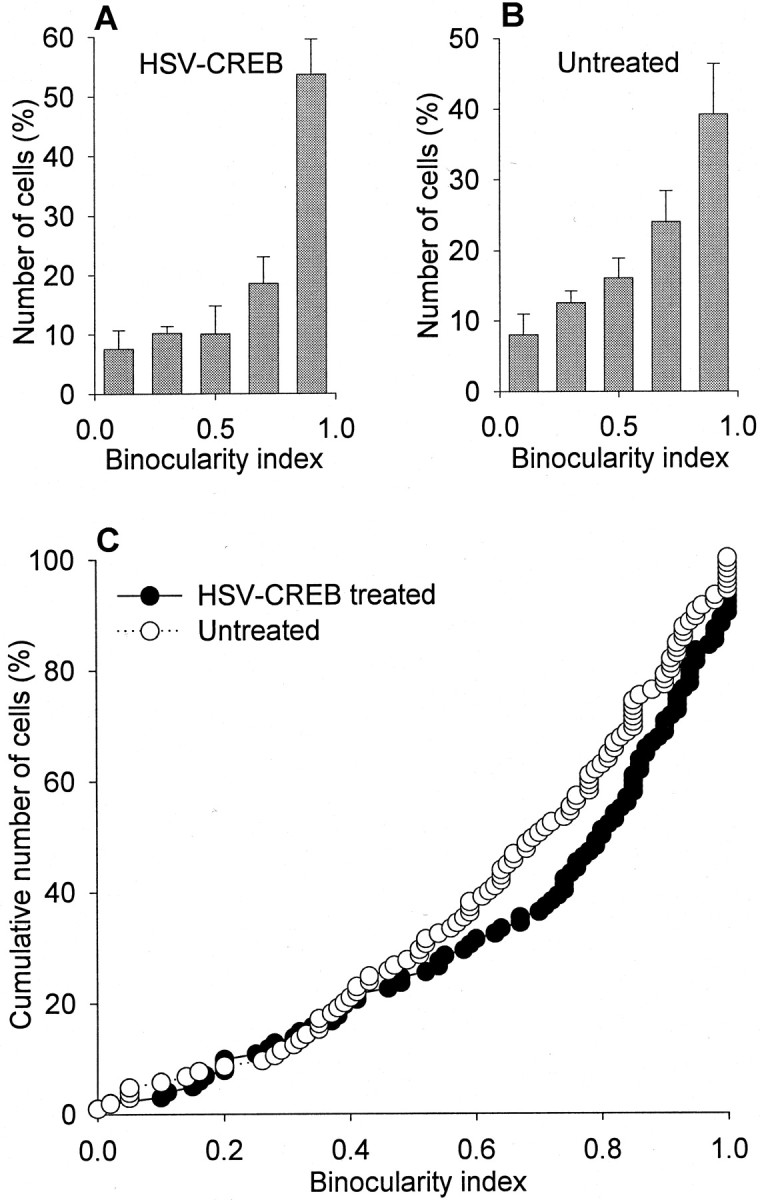
Intracortical injection of HSV-CREB did not block ocular dominance plasticity. Neurons recorded near (A) and away (B) from the injection site showed a markedly shifted distribution of ocular dominance in the direction of the nondeprived, ipsilateral eye. The cumulative plots (C) show that cells in both groups responded preferentially to the nondeprived eye after 3 d of monocular deprivation. Approximately 72% of 105 neurons in untreated cortex and 75% of 102 neurons in treated cortex had a binocularity index >0.5. The results obtained using HSV-CREB (n = 4 animals) and HSV-CREB-GFP (n = 1 animal) were indistinguishable and were pooled together. For each animal, the two groups of neurons were located in the same hemisphere (left hemisphere).
Fig. 7.
Expression of β-galactosidase did not block ocular dominance plasticity. The cumulative plots are similar for neurons recorded near and away from the injection site; ∼85% of 27 untreated neurons and 98% of 42 neurons near the injection of HSV-lacZ (n = 2 animals) had a binocularity index >0.5, indicating that both groups of cells responded preferentially to the deprived, contralateral eye.
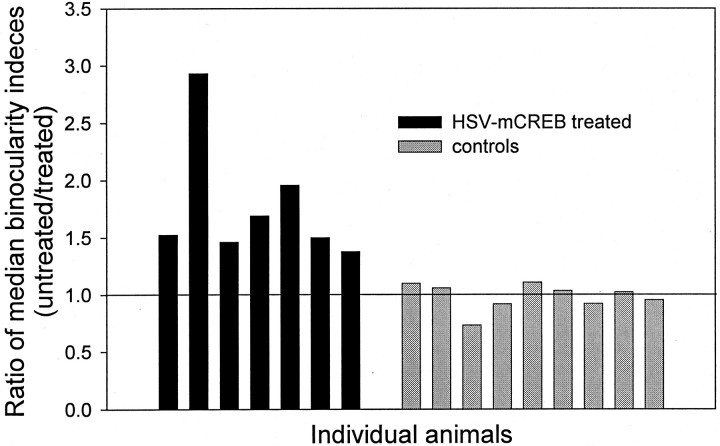
To summarize the findings of this study, the median binocularity indices were computed for the penetrations located in the treated and untreated region for each animal. Then a ratio of the binocularity indices (untreated/treated) was calculated for every animal, and the results are shown in Figure 8. As expected, all control animals had a ratio of approximately one, indicating that the median binocularity indices in the treated and untreated areas were similar. In contrast, all animals treated with HSV-mCREB had a ratio above one, indicating that the median binocularity index in the untreated region was greater than the median binocularity index in the treated region in every case. These findings show that all animals treated with HSV-mCREB had reduced ocular dominance plasticity, whereas control treatments did not reduce ocular dominance plasticity.
Fig. 8.
The ratios of median binocularity indices (untreated/treated) are shown for each animal. Every animal treated with HSV-mCREB had reduced ocular dominance plasticity, as revealed by ratios above one. In contrast, every control animal had a ratio of approximately one, indicating that the control treatments did not disrupt ocular dominance plasticity.
DISCUSSION
Requirement for CREB in ocular dominance plasticity
The present report shows that viral-mediated expression of a dominant negative form of CREB in the primary visual cortex prevents the loss of responses to the deprived eye during monocular deprivation. This effect was specific and did not result from viral toxicity, because HSV-CREB and HSV-lacZ injections failed to block ocular dominance plasticity. Moreover, the blockade of ocular dominance plasticity was temporally correlated with the expression of mCREB in cortical neurons, allowing the effects of mCREB infection to be reversed when expression had declined. This reversibility further supports the specificity of effects. Finally, the effects did not result from a suppression of sensory responses, because most neurons studied in infected cortex responded strongly to visual stimulation and had stimulus specificities (i.e., orientation and direction selectivity) characteristic of the primary visual cortex in ferrets (Chapman and Stryker, 1993). In conclusion, the present findings demonstrate that CREB-mediated transcription is required for ocular dominance plasticity during monocular deprivation.
The proposal that CREB may be involved in neural plasticity is not new. Gene activation by CREB has been shown to be important for several experimental models of learning and long-term memory (Silva et al., 1998), as well as for long-term synaptic plasticity in the hippocampus (Deisseroth et al., 1996). However, the role of CREB in visual cortical plasticity during development has remained unclear. CRE-mediated transcription in the visual cortex is increased during monocular deprivation, as revealed using transgenic mice carrying a CRE-lacZ reporter (Pham et al., 1999). However, this study did not test whether CREB function is required for the changes in binocularity that occur during monocular deprivation. CREB function could be involved with cellular regulatory mechanisms not directly related to the loss of response to the deprived eye. Alternative possibilities are that local protein synthesis at the synapse is sufficient for loss of synapses (Stewart et al., 1996; Huber et al., 2000) or that other transcription factors are involved. Moreover, an unexpected finding was the presence of lacZ immunoreactivity in the monocular zone of the visual cortex (Pham et al., 1999), in which binocular competition does not occur. This problem may, however, be related to the fact that mice have reduced binocular vision and are not the best model available to study ocular dominance plasticity.
We have taken a more direct approach to studying the role of CREB function in loss of binocularity during monocular deprivation. Use of viral-mediated gene transfer has allowed us to directly suppress CREB function in an animal model with visual cortical properties closely related to those found in higher mammals. The animal model that we chose, the ferret, is advantageous over rodent models because their frontally placed eyes allow for a large binocular representation in the primary visual cortex (Law et al., 1988). In addition, their visual cortex is characterized by the presence of ocular dominance columns and orientation columns (Weliky et al., 1996; Ruthazer et al., 1999; White et al., 1999) that are not present in mice. For all of the above reasons, our study provides unambiguous evidence for an essential role of CREB in ocular dominance plasticity.
Activation of CREB
Finding that CREB has a critical function in ocular dominance plasticity raises the question of how it may be regulated during monocular deprivation. CREB has been shown to be a target of a diverse number of signaling pathways whose kinases activate CREB by phosphorylation of Ser-133 (Gonzalez and Montminy, 1989). Some of these kinases include Ca2+/calmodulin-dependent kinases (CaMKs) (Sheng et al., 1991; Enslen et al., 1994; Matthews et al., 1994), cAMP-dependent protein kinase A (Montminy et al., 1990;Dash et al., 1991), and Ras/mitogen-activated protein kinase (MAPK) (Xing et al., 1996). CREB can also be activated indirectly through neurotrophin-mediated mechanisms (Ginty et al., 1994; Finkbeiner et al., 1997; Riccio et al., 1997; Xing et al., 1998; Pizzorusso et al., 2000).
In the visual system, studies involving transgenic mice lacking an isoform of protein kinase A show a robust ocular dominance shift after monocular deprivation (Hensch et al., 1998), suggesting a noncrucial role for this activation system in visual plasticity (but see Beaver et al., 2001). The role of CaMKs and MAPK-dependent activation of CREB in the visual cortex is still unknown. However, strong evidence for a role of NMDA receptors (Bear et al., 1990; Roberts et al., 1998; Ramoa et al., 2001) in visual cortical development and plasticity implicates Ca2+-dependent second-messenger systems. Activation of the NMDA receptor may lead to activation of CREB, via phosphorylation by CaMKs, when intracellular calcium exceeds a threshold level (Bito et al., 1996; Deisseroth et al., 1996). Additional studies are required to elucidate what pathways lead to activation of CREB after degradation of visual input through one eye.
Regulation of the ocular dominance shift by CREB
The strong evidence for a role of neurotrophic factors in ocular dominance plasticity in the developing cortex (Riddle et al., 1995;Gillespie et al., 2000; Lodovichi et al., 2000) and in activation of CREB (see above) raises the possibility that neurotrophic factors released by the postsynaptic cells contribute to the regulation of CREB activity during monocular deprivation. During monocular deprivation, activity-dependent competition for scarce neurotrophic factors such as BDNF released by the target neuron could participate in strengthening–weakening of synapses through mechanisms that require transcription of other genes by CREB. Therefore, blocking CREB function using HSV-mCREB may indirectly block synaptic changes mediated by neurotrophic factors.
What genes activated by CREB may have a role in ocular dominance plasticity? CREB has been shown to be a mediator of neuronal neurotrophin responses (Finkbeiner et al., 1997) and may also enhance the transcription of other genes, such as nitric oxide synthase (Sasaki et al., 2000), somatostatin (Gonzalez and Montminy, 1989), and immediate early genes (Sgambato et al., 1998) that could be involved in regulating ocular dominance plasticity. Additional information about the pool of genes regulated by CREB in visual cortex will be required before we can fully understand how CREB contributes to the ocular dominance shift during monocular deprivation.
Use of viral-mediated gene transfer in studies of visual cortical plasticity
This is the first time that viral-mediated gene transfer has been used to study mechanisms of visual cortical plasticity. HSV-1 was used in these studies because it is currently one of the best vectors available for genetic interventions in the CNS. HSV is capable of carrying large transgenes and infecting nondividing cells, especially neurons, with high efficiency (Neve and Lim, 1999). Moreover, it is episomal and thereby does not cause integration effects, and HSV-1 particles can be concentrated to relatively high titers. The high infectivity rate obtained in this study allowed us to observe an almost complete blockade of the ocular dominance shift. An alternative approach would be to use transgenic mice null of thecreb gene altogether, but these mice have been found to die perinatally (Rudolph et al., 1998). Another alternative would be to use homozygous mutant mice whose mutations target the creb gene (Hummler et al., 1994). However, homozygous mutant mice still retain active isoforms of CREB, which have been found to be strikingly upregulated in these animals (Blendy et al., 1996). Moreover, the animals typically exhibited increased levels of CREM, a transcriptional repressor that is another transcription factor belonging to the same family as CREB (Hummler et al., 1994). Together, these findings indicate that diverse feedback mechanisms compensate for the long-term absence of CREB in transgenic mice, and these compensatory mechanisms may lead to a lack of effects on developmental cortical plasticity despite targeted mutation of the creb gene (Glazewski et al., 1999).
Conclusions
Because viral-mediated gene transfer allows temporally and spatially restricted expression of selected genes, it currently provides the best method for investigating the role of different proteins in cortical ocular dominance plasticity of higher mammals. In the present study, the endogenous CREB could not induce an ocular dominance shift because of competition with the dominant negative mutant for the CRE binding site. More specifically, this manipulation provides a mechanism by which the activity of the three main CREB isoforms can be suppressed. Importantly, whereas other cortical manipulations used in the study of visual plasticity may result in disruption of sensory responses (Bear et al., 1990; Rauschecker et al., 1990), the use of HSV did not. These findings are of particular importance, because depression of sensory responses and disruption of functional properties may block ocular dominance plasticity in a nonspecific manner (for discussion, see Roberts et al., 1998). Our finding that CREB function is required for ocular dominance plasticity, together with the advantages of viral-mediated gene transfer, provides the foundation needed to elucidate the molecular mechanisms leading to amblyopia in higher mammals.
Footnotes
This work was supported by National Institutes of Health Grants EY-11508 and AA-13023 to A.S.R.
Correspondence should be addressed to Dr. Ary S. Ramoa, Department of Anatomy, Virginia Commonwealth University School of Medicine, 1101 E. Marshall Street, Sanger Hall, Room 12-042, Richmond, VA 23298-0709. E-mail: aramoa@hsc.vcu.edu.
REFERENCES
- 1.Barth AL, McKenna M, Glazewski S, Hill P, Impey S, Storm D, Fox K. Upregulation of cAMP response element-mediated gene expression during experience-dependent plasticity in adult neocortex. J Neurosci. 2000;20:4206–4216. doi: 10.1523/JNEUROSCI.20-11-04206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bear MF, Kleinschmidt A, Gu Q, Singer W. Disruption of experience-dependent synaptic modifications in striate cortex by infusion of an NMDA receptor antagonist. J Neurosci. 1990;10:909–925. doi: 10.1523/JNEUROSCI.10-03-00909.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beaver CJ, Ji Q, Fischer QS, Daw NW. Cyclic AMP-dependent protein kinase mediates ocular dominance shifts in cat visual cortex. Nat Neurosci. 2001;4:159–163. doi: 10.1038/83985. [DOI] [PubMed] [Google Scholar]
- 4.Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca++- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- 5.Blendy JA, Kaestner KH, Schmid W, Gass P, Schutz G. Targeting of the CREB gene leads to up-regulation of a novel CREB mRNA isoform. EMBO J. 1996;15:1098–1106. [PMC free article] [PubMed] [Google Scholar]
- 6.Carlezon IW, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- 7.Chapman B, Stryker MP. Development of orientation selectivity in ferret visual cortex and effects of deprivation. J Neurosci. 1993;13:5251–5262. doi: 10.1523/JNEUROSCI.13-12-05251.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dash PK, Karl KA, Colicos MA, Prywes R, Kandel ER. cAMP response element-binding protein is activated by Ca2+/calmodulin- as well as cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1991;88:5061–5065. doi: 10.1073/pnas.88.11.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deisseroth K, Bito H, Tsien RW. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron. 1996;16:89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- 10.Enslen H, Sun P, Brickey D, Soderling SH, Klamo E, Soderling TR. Characterization of Ca++/calmodulin-dependent protein kinase IV. Role in transcriptional regulation. J Biol Chem. 1994;269:15520–15527. [PubMed] [Google Scholar]
- 11.Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME. CREB: a major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–1047. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- 12.Gillespie DC, Crair MC, Stryker MP. Neurotrophin-4/5 alters responses, blocks the effect of monocular deprivation in cat visual cortex during the critical period. J Neurosci. 2000;20:9174–9186. doi: 10.1523/JNEUROSCI.20-24-09174.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ginty DD, Bonni A, Greenberg ME. Nerve growth factor activates a ras-dependent protein kinase that stimulates c-fos transcription via phosphorylation of CREB. Cell. 1994;77:713–725. doi: 10.1016/0092-8674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 14.Glazewski S, Barth AL, Wallace H, McKenna M, Silva M, Fox K. Impaired experience-dependent plasticity in barrel cortex of mice lacking the alpha and delta isoforms of CREB. Cereb Cortex. 1999;9:249–256. doi: 10.1093/cercor/9.3.249. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 16.Hensch TK, Gordon JA, Brandon EP, McKnight GS, Idzerda RL, Stryker MP. Comparison of plasticity in vivo and in vitro in the developing visual cortex of normal and protein kinase A RIβ-deficient mice. J Neurosci. 1998;18:2108–2117. doi: 10.1523/JNEUROSCI.18-06-02108.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977;278:377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- 18.Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGLUR-dependent long-term depression. Science. 2000;288:1254–1256. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- 19.Hummler E, Cole TJ, Blendy JA, Ganss R, Aguzzi A, Schmid W, Beerman F, Schutz G. Targeted mutation of the CREB gene: compensation within the CREB/ATF family of transcription factors. Proc Natl Aca Sci USA. 1994;91:5647–5651. doi: 10.1073/pnas.91.12.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Issa NP, Trachtenberg JT, Chapman B, Zahs KR, Stryker MP. The critical period for ocular dominance plasticity in the ferret's visual cortex. J Neurosci. 1999;19:6965–6978. doi: 10.1523/JNEUROSCI.19-16-06965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Law MI, Zahs KR, Stryker MP. Organization of primary visual cortex (Area 17) in the ferret. J Comp Neurol. 1988;278:157–180. doi: 10.1002/cne.902780202. [DOI] [PubMed] [Google Scholar]
- 22.Lim F, Hartley D, Starr P, Lang P, Song S, Yu L, Wang Y, Geller AI. Generation of high-titer defective HSV-1 vectors using an IE 2 deletion mutant and quantitative study of expression in cultured cortical cells. Biotechniques. 1996;20:460–469. doi: 10.2144/19962003460. [DOI] [PubMed] [Google Scholar]
- 23.Lodovichi C, Berardi N, Pizzorusso T, Maffei L. Effects of neurotrophins on cortical plasticity: same or different? J Neurosci. 2000;20:2155–2165. doi: 10.1523/JNEUROSCI.20-06-02155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malenka RC, Kauer JA, Perkel DJ, Nicoll RA. The impact of postsynaptic calcium on synaptic transmission—its role in long-term potentiation. Trends Neurosci. 1989;12:444–450. doi: 10.1016/0166-2236(89)90094-5. [DOI] [PubMed] [Google Scholar]
- 25.Malinow R, Madison DV, Tsien RW. Persistent protein kinase activity underlying long-term potentiation. Nature. 1988;335:820–824. doi: 10.1038/335820a0. [DOI] [PubMed] [Google Scholar]
- 26.Matthews RP, Guthrie CR, Wailes LM, Zhao X, Means AR, McKnight GS. Calcium/calmodulin-dependent protein kinase types II and IV differentially regulate CREB-dependent gene expression. Mol Cell Biol. 1994;14:6107–6616. doi: 10.1128/mcb.14.9.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montminy MR, Gonzalez GA, Yamamoto KK. Regulation of cAMP-inducible genes by CREB. Trends Neurosci. 1990;13:184–188. doi: 10.1016/0166-2236(90)90045-c. [DOI] [PubMed] [Google Scholar]
- 28.National Eye Institute. Visual acuity impairment survey pilot. National Eye Institute; Bethesda, MD: 1984. [Google Scholar]
- 29.Neve RL, Lim F. Overview of gene delivery into cells using HSV-1 based vectors. In: Crawley JN, Gerfen CR, McKay R, Rogawski MA, Sibley DR, Skolnick P, editors. Current protocols in neuroscience: gene cloning, expression, and mutagenesis. Wiley; New York: 1999. pp. 4.12.1–4.12.6. [Google Scholar]
- 30.Olson VG, Hughes T, Wolf DH, Russell DS, Neve RL, Carlezon WA, Nestler EJ. Regulation of cocaine reward by CREB in the ventral tegmental area. Soc Neurosci Abstr. 1999;25:812. [Google Scholar]
- 31.Pham TA, Impey S, Storm DR, Stryker MP. CRE-mediated gene transcription in neocortical neuronal plasticity during the developmental critical period. Neuron. 1999;22:63–72. doi: 10.1016/s0896-6273(00)80679-0. [DOI] [PubMed] [Google Scholar]
- 32.Pizzorusso T, Ratto GM, Putignano E, Maffei L. Brain-derived neurotrophic factor causes cAMP response element-binding protein phosphorylation in absence of calcium increases in slices and cultured neurons from rat visual cortex. J Neurosci. 2000;20:2809–2816. doi: 10.1523/JNEUROSCI.20-08-02809.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramoa AS, Mower AF, Liao D, Jafri SIA. Suppression of cortical NMDA receptor function prevents development of orientation selectivity in the primary visual cortex. J Neurosci. 2001;21:4299–4309. doi: 10.1523/JNEUROSCI.21-12-04299.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rauschecker JP, Egert V, Kossel A. Effects of NMDA antagonists on developmental plasticity in kitten visual cortex. Int J Dev Neurosci. 1990;8:425–435. doi: 10.1016/0736-5748(90)90075-d. [DOI] [PubMed] [Google Scholar]
- 35.Riccio A, Pierchala BA, Ciarallo CL, Ginty DD. An NGF-TrkA-mediated retrograde signal to transcription factor CREB in sympathetic neurons. Science. 1997;277:1097–1100. doi: 10.1126/science.277.5329.1097. [DOI] [PubMed] [Google Scholar]
- 36.Riddle DR, Lo DC, Katz LC. NT-4-mediated rescue of lateral geniculate neurons from effects of monocular deprivation. Nature. 1995;378:189–191. doi: 10.1038/378189a0. [DOI] [PubMed] [Google Scholar]
- 37.Roberts EB, Meredith AM, Ramoa AS. Suppression of NMDA receptor function using antisense DNA blocks ocular dominance plasticity while preserving visual responses. J Neurophysiol. 1998;80:1021–1032. doi: 10.1152/jn.1998.80.3.1021. [DOI] [PubMed] [Google Scholar]
- 38.Rudolph D, Tafuri A, Gass P, Hammerling GJ, Arnold B, Schutz G. Impaired fetal T cell development and perinatal lethality in mice lacking the cAMP response element binding protein. Proc Natl Acad Sci USA. 1998;95:4481–4486. doi: 10.1073/pnas.95.8.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruthazer ES, Baker GE, Stryker MP. Development and organization of ocular dominance bands in primary visual cortex of the sable ferret. J Comp Neurol. 1999;407:151–165. [PMC free article] [PubMed] [Google Scholar]
- 40.Rutstein RP, Daum KM. Anomalies of binocular vision: diagnosis and management, p 8. Mosby; St. Louis: 1998. [Google Scholar]
- 41.Sasaki M, Gonzalez-Zulueta M, Huang H, Herring WJ, Ahn S, Ginty DD, Dawson VL, Dawson TM. Dynamic regulation of neuronal NO synthase transcription by calcium influx through a CREB family transcription factor-dependent mechanism. Proc Natl Acad Sci USA. 2000;97:8617–8622. doi: 10.1073/pnas.97.15.8617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sgambato V, Pages C, Rogard M, Besson MJ, Caboche J. Extracellular signal-regulated kinase (ERK) controls immediate early gene induction on corticostriatal stimulation. J Neurosci. 1998;18:8814–8825. doi: 10.1523/JNEUROSCI.18-21-08814.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheng M, Thompson MA, Greenberg ME. CREB, a Ca++-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science. 1991;274:1672–1677. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- 44.Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- 45.Stewart O, Falk OM, Torre ER. Ultrastructural basis for gene expression at the synapse: synapse-associated polyribosome complexes. J Neurocytol. 1996;25:717–734. doi: 10.1007/BF02284837. [DOI] [PubMed] [Google Scholar]
- 46.Weliky M, Bosking WH, Fitzpatrick D. A systematic map of direction preference in primary visual cortex. Nature. 1996;379:725–728. doi: 10.1038/379725a0. [DOI] [PubMed] [Google Scholar]
- 47.White LE, Bosking WH, Williams SM, Fitzpatrick D. Maps of central visual space in ferret V1 and V2 lack matching inputs from the two eyes. J Neurosci. 1999;19:7089–7099. doi: 10.1523/JNEUROSCI.19-16-07089.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiesel TN, Hubel DH. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965;28:1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- 49.Xing J, Ginty DD, Greenberg ME. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science. 1996;273:959–963. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- 50.Xing J, Kornhauser JM, Xia Z, Thiele EA, Greenberg ME. Nerve growth factor activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways to stimulate CREB serine 133 phosphorylation. Mol Cell Biol. 1998;18:1946–1955. doi: 10.1128/mcb.18.4.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]