Abstract
Dorsolateral prefrontal cortex has an essential role in the cognitive process of working memory, dysfunction of which is considered to be a core deficit in schizophrenia. Although this cortical region is densely innervated with 5-HT2A receptors to which atypical antipsychotic drugs bind with high affinity, little is known of the influence of this serotonin receptor subtype on prefrontal function. We addressed this issue by examining the effects of iontophoresis of selective receptor ligands on prefrontal neurons possessing spatially tuned delay activity, or “memory fields,” in monkeys performing a delayed-response task. Memory fields of putative pyramidal cells were attenuated by iontophoresis of 5-HT2A antagonists, which primarily produced a reduction in delay activity for preferred target locations. Conversely, 5-HT2A stimulation by α-methyl-5-HT or 5-HT itself, accentuated the spatial tuning of these neurons by producing a modest increase in activity for preferred target locations and/or a reduction in activity for nonpreferred locations. The agonist effects could be reversed by the selective antagonist MDL100,907, and were dose-dependent, such that high levels attenuated spatial tuning by profoundly reducing delay activity. A role for feedforward inhibitory circuitry in these effects was supported by the finding that 5-HT2A blockade also attenuated the memory fields of putative interneurons. We conclude that prefrontal 5-HT2A receptors have a hitherto unrecognized role in the cognitive function of working memory, which involves actions at both excitatory and inhibitory elements within local circuitry.
Keywords: prefrontal cortex, monkey, iontophoresis, 5-HT2A receptor, working memory, single unit, spatial tuning, fast-spiking, interneuron, pyramidal cell, schizophrenia
The prefrontal cortex is substantially innervated by serotonergic fibers from the dorsal raphe nucleus in both primates and rodents (Porrino and Goldman-Rakic, 1982;Morrison et al., 1982; Takeuchi and Sano, 1983; Smiley and Goldman-Rakic, 1996). Of all the 5-HT receptor subtypes found in cortex, the G-protein-coupled 5-HT2A receptor has received extensive attention in both physiological and pharmacological experiments. Immunocytochemistry has revealed abundant 5-HT2A receptors on the dendrites of prefrontal pyramidal cells as well as in large and medium-sized calbindin- and parvalbumin-positive interneurons (Jakab and Goldman-Rakic, 1998,2000). These receptors have been shown to have a facilitatory action on cortical pyramidal cells (Araneda and Andrade, 1991; Tanaka and North, 1993), which includes a presynaptic action on glutamate release (Aghajanian and Marek, 1997). In primate prefrontal cortex, serotonergic fibers terminate mainly in association with the smooth dendrites of putative interneurons (Smiley and Goldman-Rakic, 1996). Accordingly, 5-HT2A facilitation of this cell type (Gellman and Aghajanian, 1993, 1994) has been shown to produce considerable IPSCs in neighboring pyramidal cells (Zhou and Hablitz, 1999). Therefore, the 5-HT2A-mediated influence of serotonin on cortical function would be expected to involve an interaction of facilitatory and feedforward inhibitory components of intrinsic circuitry. Despite these insights into the physiology of cortical 5-HT2A receptors, the consequences of serotonergic stimulation or depletion for cognitive function remains unclear. Although the study of Luciana et al. (1998)suggests that supranormal levels of serotonin may be deleterious for spatial working memory, others have demonstrated that depletion of serotonin leads to deficits in the cognitive process of decision-making, similar to that seen after damage to orbitofrontal cortex in humans (Rogers et al., 1999). The 5-HT2A receptor has also been shown to be particularly involved in the action of hallucinogens such as lysergic acid diethylamide (LSD) in the cortex (Marek and Aghajanian, 1996) as well as in the therapeutic efficacy of antipsychotic medications (Meltzer, 1989, 1999).
The circuitry of the dorsolateral prefrontal cortex has an established role in the working memory processes essential to human cognition (Goldman-Rakic, 1987; McCarthy et al., 1994, 1996). Deficiency in these processes has been associated not only with the negative symptoms and cognitive deficits that are prominent in schizophrenia (Weinberger et al., 1986; Goldman-Rakic, 1991, 1994; Liddle, 1987; Liddle and Morris, 1991; Park and Holzman, 1992), but also with the positive symptoms of the disorder (Andreasen et al., 1997; Sabri et al., 1998; Lennox et al., 2000). Single cell recordings of dorsolateral prefrontal neurons in nonhuman primates have revealed profiles of neuronal activation correlated with sensory, mnemonic, and response processes in both manual (Fuster, 1973) and oculomotor delayed-response tasks (ODRs) (Funahashi et al., 1989). The delay activity observed in many prefrontal neurons has been shown to be spatially dependent, giving rise to “memory fields” with excitatory responses to targets in preferred directions and null or inhibitory responses to other targets in nonpreferred directions. A recent study of regular-spiking (RS) and fast-spiking (FS) neurons in prefrontal cortex has indicated that putative interneurons can also exhibit memory fields, which have similar tuning properties to those of neighboring pyramidal cells (Rao et al., 1999). This cellular basis of working memory in prefrontal cortex provides an ideal model for testing the influence of different neurotransmitters on cognitive function at the cortical level. A previous study, using iontophoresis of selective dopamine D1 receptor agonists and antagonists, revealed an important relationship between the level of D1 receptor occupancy and the strength of pyramidal cell memory fields (Williams and Goldman-Rakic, 1995). Here, we used a similar approach to investigate 5-HT2A receptor modulation of delay activity of both RS and FS units in the ODR task, to determine the contribution of this particular receptor subtype to the influence of prefrontal serotonergic input on cognitive function.
MATERIALS AND METHODS
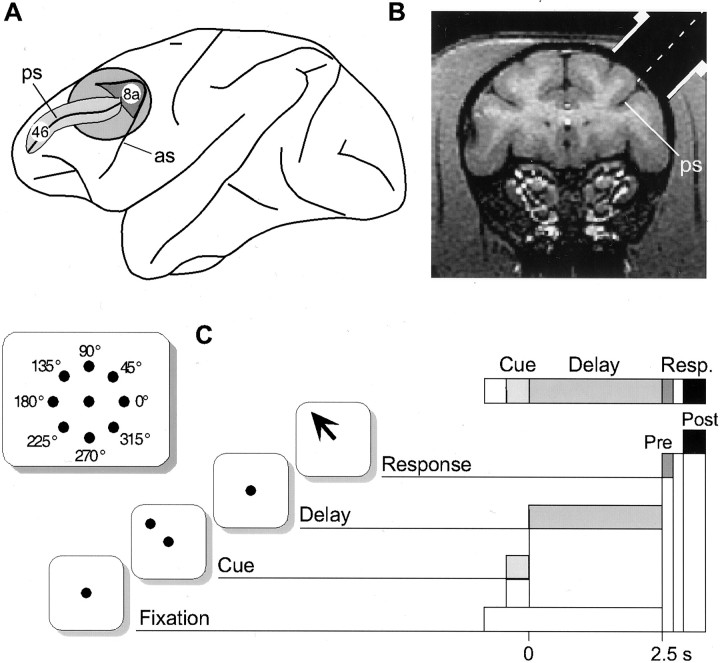
Use of animals. Studies were performed on two adult male rhesus monkeys (Macaca mulatta), which were cared for under the guidelines of the National Institutes of Health and Yale Animal Care Committee. They were prepared for chronic daily recording as previously described (Funahashi et al., 1989). The recording cylinders were centered over the caudal principal sulcus using x-ray imaging and stereotaxic coordinates (Fig.1A) (Rao et al., 1999). Magnetic resonance imaging in a number of recent monkeys has shown a high level of concordance in the coordinates used. Single-unit recordings at this site (Fig. 1B, central position) frequently reveal spatially tuned delay activity: the hallmark of response properties in caudal area 46 and rostral area 8a of Walker (1940).
Fig. 1.
Region of recording and experimental paradigm.A, Left hemisphere view of the macaque brain showing the calculated position of the recording chamber (gray circle) over caudal areas 46 and 8a (as, arcuate sulcus; ps, principal sulcus). B,Anatomical MRI at the same rostrocaudal level as the center of the circle shown in A (∼ 27 mm anterior to ear, bar zero). The estimated lateral position of the chamber is shown inwhite with the center marked by a dashed line. C, Schematic view of the ODR task.Top left panel shows the position of the central fixation point and the possible position of the eight peripheral cues. One trial is depicted below and to the right, where the target at 135° is displayed during the cue period and the correct response is portrayed by the arrow in the response (Resp.) epoch (Pre, presaccadic epoch;Post, postsaccadic epoch).
ODR task. The animals were trained in a spatial ODR task shown in Figure 1C (Funahashi et al., 1989). In this task the monkey commences each trial by fixating a central stimulus (small square on monitor, or a light-emitting diode) within 2° for 0.5 sec and must continue to fixate while one of 8 peripheral stimuli (45° separation in circumference, 13° eccentricity) is illuminated for 0.5 sec (cue period). There then follows a delay period of 2.5 or 3.0 sec during which the monkey must maintain fixation. At the end of this time the central stimulus is extinguished, and the monkey must make a saccade, within 0.5 sec (response period), to the position of the peripheral stimulus shown earlier (again within 2°) to be rewarded with fruit juice. The peripheral cues were presented in a semirandom order across trials such that, during the delay period, the monkey had to remember the cue location shown within the present trial to make the correct response. Note that the response period can be divided into a presaccadic epoch (lasting 250 msec from the end of the delay) because this incorporates the typical time required to initiate a saccade in this task (Funahashi et al., 1991), and a postsaccadic epoch (lasting 500 msec) starting 500 msec after the end of the delay and therefore, by definition, after the performance of a successful saccade.
Electrode design and construction. Electrodes were constructed either from 33 μm carbon fiber (AVCO, Lowell MA) inside quad-barreled glass (Clark Electromedical Instruments, Reading, UK) or, more commonly, from 20 μm pitch carbon fiber (ELSI, San Diego, CA) inserted into the center of seven barrel nonfilamented capillary glass (Friedrich and Dimmock, Millville, NJ). The assembly was pulled through a heating coil element (nichrome wire; Narashige, Tokyo, Japan) using a proprietary, computer-controlled electrode puller. The latter allowed for the precise control of the heating-coil temperature, the time and velocity required to pull the shaft of the electrode (60–62 mm in length), and the timing of the solenoid used to pull the tip of the electrode. The tip was further fashioned by a combination of spark etching and beveling on diamond (Stoelting, Wood Dale, IL) and stone (polishing) wheels. This helped to produce sharp tips, 20–40 μm, in length with impedances ranging from 250 kΩ to 1.2 MΩ (at 1 kHz) and a noise level of <15 μV peak to peak. Extracellular voltage was recorded with a custom (SKYLAB) low-noise preamplifier and was bandpass filtered between 180 Hz and 8 kHz (four pole Butterworth; Krohn-Hite, Avon, MA). At the beginning of each track, the dura was punctured with a 25 gauge hypodermic stainless steel guide tube (projecting from a 21 gauge guide tube), within which the electrode was lowered slowly into the brain using a MD-2 motorized hydraulic drive mounted on an MO-95 micromanipulator (Narishige).
Pharmacological agents and iontophoresis. In these and previous studies, we have focused on the effects of ligands that act as antagonists at particular receptors rather than agonist drugs. This is because the physiological activation by neurotransmitters at their designated receptors at particular locations on different neurons, and with a particular time course, can be best studied by examining the consequences of removing that action. Iontophoresis of an agonist may level the normal spatial and temporal profile of receptor action and, consequently, could obscure its functional significance. The drugs used in this study have well documented affinities at 5-HT2A receptors. MDL 100,907 (Aventis Pharmaceuticals, Bridgewater, NJ) has high affinity (<1 nm) at 5-HT2A receptors (Johnson et al., 1996), whereas ritanserin (Janssen Pharmaceutical, Titusville, NJ) and LY53857 (Sigma/RBI, Natick, MA) have an order of magnitude higher affinity at 5-HT2A receptors (<10 nm) than at 5-HT2Creceptors (Schreiber et al., 1995; Mazzola-Pomietto et al., 1996). The partial agonist α-methyl-5-HT (Sigma/RBI), which has high affinity at 5-HT2A receptors and moderate affinity at 5-HT2C receptors (Garnovskaya et al., 1995), was used to compare with, or reverse, the action of the antagonists. Serotonin (HCl salt; Sigma/RBI) was also used in some experiments to compare the actions of endogenous and exogenous agonists. The drugs were dissolved in 1 ml of triple distilled water (adjusted with HCl to pH 3.5–4.0) at a concentration of ∼ 0.01m and stored in aliquots of 50 μl at −70°C. Immediately before use, the drugs were sonicated briefly and drawn up into fine, fused-silica glass pipette fillers (WPI, Sarasota, FL), each instilled into one barrel of quad electrodes or two adjacent barrels of seven barrel electrodes, and forced to the tip by compressed air. Thus, three drugs could be tested with one electrode, typically one agonist and two antagonists. Teflon-coated platinum–iridium wires (Medwire, Mt. Vernon, NY) were then fitted inside each drug barrel and connected to a Neurophore BH2 iontophoretic system (Medical Systems Corp., Greenvale, NY) such that one channel (IP-2) of the device controlled the delivery of one drug. The results presented here are taken from findings with ejection currents ranging from 5 to 100 nA. Retaining currents of −3 to −5 nA were used in a cycled manner (1 sec on, 1 sec off) when not applying drugs, and current balancing was not required because of the low impedance of the electrode. Drug ejection did not create noise in the recording, and there was no systematic change in either spike amplitude or time course at any ejection current. Iontophoresis was started after a sufficient number of trials (≥8) had been collected for each target position in the task under the control condition.
Data acquisition and analysis. Eye movements were monitored with a magnetic search coil system (CNC Engineering, Seattle, WA) or by infrared pupil tracking (ISCAN, Burlington, MA). These data were incorporated into task control, performed by a PDP-11 running MONK software or by a personal computer running TEMPO (Reflective Computing, St. Louis, MO). Spike waveform-sorting and data acquisition was run on a micro1401 using Spike2 software (Cambridge Electronic Design, Cambridge, UK). Waveform sorting (template matching algorithm) made it possible to isolate up more than one unit at the same recording site. The waveform templates constructed in the sorting were of sufficient range in amplitude that they could incorporate any moment by moment change in the magnitude of the spikes or slow drift in spike amplitude over time. The data collected from each unit was time-stamped (and the spike waveform stored) to precisely determine when each spike occurred relative to task events, and output via a text file for subsequent analysis. Unit activity was measured in spikes per second during each epoch of the trial (Fig. 1C). Data were first collected from the cell under a control condition, followed by a drug condition in which one of the 5-HT2A ligands was applied, and then typically a recovery condition after drug application had been terminated. Because the synthetic, high-affinity drugs used in these experiments take many seconds to act, they can also take a long time to wear off. Thus, although the general activity level of the cell may fully return to normal by the end of the recovery condition, the value obtained for spatial tuning over the entire condition can only approach that in control (because multiple trials for each target direction are required in the analysis). However, in some recordings we used a post-drug condition immediately after drug application to ensure an optimal recovery condition afterwards. Occasionally dose-dependent effects of the drug were tested in two or more consecutive conditions, or an agonist was applied in the condition immediately after application of an antagonist (and vice versa) to detect reversal or opposing effects. Data were obtained from at least five trials (typically 10 or more) at each cue location for each condition. The first 30–90 sec of data (or originally data from the first trial at each cue location) from noncontrol conditions was omitted from the text file to allow for the time taken for drug action. The text file data were processed by a proprietary C++ program for statistical analysis using the Student's two-tailed t test with unequal variance and an α of 5%. In this way, data with a statistical probability level of < 0.05 were obtained for neuronal activity within each epoch of the task in comparison to baseline activity, and the effects of drug application on unit activity within each period of the task in comparison with the previous control condition. Population analysis was performed on normalized data, derived by first aligning the preferred directions of the population of tuned cells to 0°. The mean firing rate for each target location for each unit was then taken as a ratio to the mean activity over all target locations and plotted relative to the preferred direction. A similar analysis was performed for the percentage change in activity between drug and control conditions for each target location. One-way and two-way ANOVAs were performed on this normalized data, and the results were analyzed with post hoc Scheffe tests.
Identification of single units. As previously reported (Mountcastle et al., 1969; Wilson et al., 1994; Rao et al., 1999,2000), it was possible to identify two distinct putative cell typesin vivo by measuring the time course of their spike waveforms. Fast-spiking (FS) neurons had relatively low-amplitude spikes (typically <50 μV), biphasic action potentials, relatively high firing rates, and short spike durations of < 0.9 msec. Regular-spiking (RS) neurons typically had more complex triphasic waveforms with a larger initial negative deflection, relatively low basal firing rates, and long durations of typically >1 msec. FS units could only be tracked for typically <20 μm, whereas RS units could often be tracked for >100 μm, a distinction that probably arises from the larger dendritic field of pyramidal cells. Using a cutoff point of 0.9 msec spike-based width (the extracellular impulse being a close corollary to the first order differential of the action potential recorded intracellularly) (McCormick et al., 1985; Kawaguchi, 1993,1995), the two cell types could be readily segregated, in accordance with their other spike properties. A recent report by Gur et al. (1999), recording from macaque V1 neurons, provides support for the assumption that units with different spike properties are likely to originate from cells of different types, which also show different physiological properties, as recognized in many previous in vivo rodent studies (Simons, 1978; Swadlow et al., 1998; Shimegi et al., 1999; Dantzker and Callaway, 2000; Morris and Henderson, 2000;Baeg et al., 2001; Timofeev et al., 2001). For further details on the unit isolation and spike segregation used, see Rao et al. (1999).
Assessment of spatial tuning. Spatial signal strength and direction in neuronal response was analyzed by a vector algorithm (Rao et al., 1999). Briefly, vectors were computed for loops constructed from firing rates for each target direction in order of occurrence (for five or more trials), and their dot-products were determined, relative to the resultant vector. A statistical comparison (Wilcoxon signed-rank test, p < 0.05; Conover, 1971) of these scalar values was then made with arbitrary thresholds to yield an integer tuning factor (TF) ranging from 0 (untuned) to 10. The effect of a drug on this tuning was assessed by a statistical comparison (Wilcoxon sum-rank test, p < 0.05) of the final scalar values between the drug and control conditions. The angle of tuning, θ, varying continuously between 0° and 360°,was determined by taking the median angle of the individual loop vectors (Fisher, 1993).
RESULTS
Effects of 5-HT2A receptor blockade on the memory fields of RS units
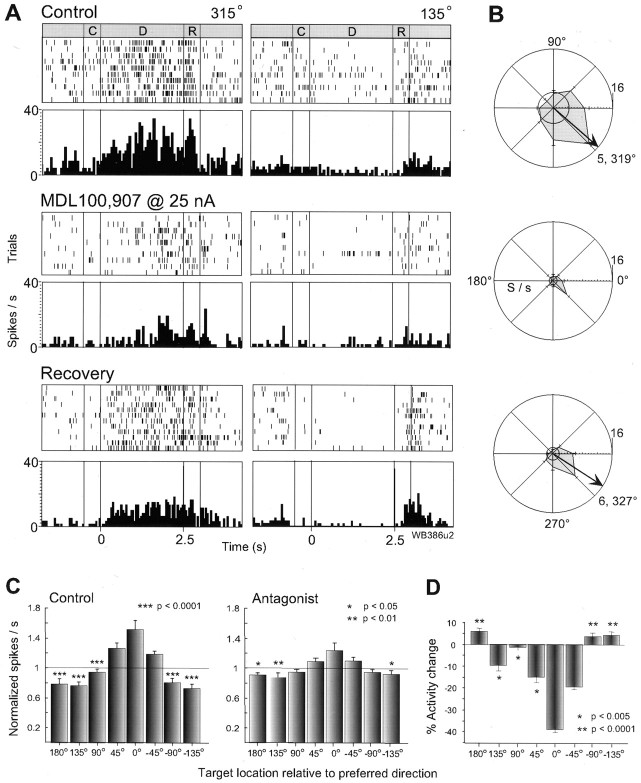
Of 75 RS units tested with 5-HT2Aantagonists, 31 (41%) displayed spatially tuned delay activity, firing maximally for one or two preferred directions and minimally for nonpreferred targets in the opposite region of space. When examined for their effects on the spatial-tuning of delay activity in RS units, iontophoresis of 5-HT2A antagonists, ritanserin, LY53857, and MDL100,907, at 15–50 nA ejection currents, attenuated tuning in nearly all (28 of 31; 90%) cells that were tuned under the control condition (Table 1). Attenuation of tuning between the drug and control conditions was detected by a significant reduction (p < 0.05, Wilcoxon rank-sum Test) in the vectors derived from the delay activity of the cell over all eight target directions (see Materials and Methods). Data are presented for one cell in Figure2A as rastergrams (top) and sum-histograms (below) for the preferred (315°) and nonpreferred (135°) target locations. In the control condition, it can be seen that the cell fired consistently for trials at 315° throughout the delay and the presaccadic epochs and showed a concomitant reduction in activity for trials at 135°. Iontophoresis of MDL100,907 at 25 nA produced a steady decline in the response of the cell for its preferred direction, which returned slowly during the recovery period after drug application. Note that the antagonist also reduced presaccadic activity (early response period) for the preferred direction and postsaccadic activity (late response period) for the nonpreferred direction of the cell (Fig. 2A, right panel). The polar plots of mean and SE of delay activity for all target locations in Figure 2B shows the full extent of the memory field (TF = 5; θ = 319°; see arrow) in the control condition, its destruction by the 5-HT2A antagonist, and its partial re-emergence (TF = 6) in recovery at a similar angle of tuning (θ = 327°).
Table 1.
Effects of iontophoresis of 5-HT2A agonists and antagonists on the spatial tuning of delay activity in RS neurons
| TUNED | UNTUNED | ||||
|---|---|---|---|---|---|
| Drug Effect | Enhanced | Unchanged | Diminished | Created | Unchanged |
| Antagonists | |||||
| MDL100907 | 0 | 1 | 20 | 5 | 25 |
| Ritanserin | 0 | 2 | 3 | 1 | 10 |
| LY53857 | 0 | 0 | 5 | 0 | 3 |
| % Effect | 0 | 10 | 90 | 14 | 86 |
| Agonists | |||||
| α-methyl-5-HT | 6 | 0 | 0 | 6 | 9 |
| 5-HT | 1 | 3 | 0 | 4 | 1 |
| % Effect | 70 | 30 | 0 | 50 | 50 |
The cells were categorized according to whether they were tuned or untuned in the control condition, and the results are given separately for each drug tested as well as for the combined effects of the agonists and antagonists. All three antagonists showed a high propensity (90%) to attenuate the memory fields of the 31 tuned RS neurons and, only in rare cases, created tuning in the 44 RS cells that were untuned in the control condition. Conversely, the agonists showed a high proclivity both to enhance existing tuning and to create tuning in RS neurons.
Fig. 2.
Effects of 5-HT2A blockade on RS neurons. A, Rastergrams and average histograms of the activity of one RS neuron (C, cue; D, delay; R, response period; bin = 50 msec). Thetop panel shows activity during the control condition in which an elevation of activity can be seen during the delay and early response period for the preferred direction at 315° (left), and only a postsaccadic response for the nonpreferred location at 135° (right). Iontophoresis of MDL100,907 (middle panel) attenuated the delay and presaccadic activity at 315° and the postsaccadic activity for the opposite target location. After drug application was ceased (bottom panel), the delay activity partially returns toward that in control. B, Mean and SE polar plots of the firing rate of the same cell during the delay period for each target location (arrow: vector angle of tuning;inner circle indicates background activity). A memory field can be seen in the control condition (top) that is diminished by the 5-HT2A antagonist (middle). In recovery (bottom), the memory field returns in a similar shape, although smaller in size.C, Population analysis of the delay activity of 28 tuned RS cells tested with 5-HT2A antagonists in the control (left) and drug (right) conditions. As described in Materials and Methods, the cells are first normalized for their preferred target location which is set to 0° and then the activity for each target location, relative to the preferred location is taken as a ratio to the mean for the delay activity of the cell across all target directions (shown byline). The histogram therefore depicts the dispersion of delay activity between preferred and nonpreferred target locations. A clear spatial profile can be seen in the control condition, which is highly diminished under 5-HT2A blockade (asterisks denote significant differences from the preferred direction). D, Histogram showing the percentage change in delay activity produced by 5-HT2Ablockade (for the same neuronal population), relative to the activity in the control condition for each target location (preferred direction again normalized to 0°). A reduction in activity can be seen for the preferred direction, greater than that for the two adjacentlocations, and a small increase in activity is evident for opposite locations in space.
The attenuation of spatial tuning in delay activity could also be seen at the population level by normalizing both the preferred direction of each cell to 0° and the activity for each target location relative to the mean activity over all target locations. The results are illustrated for 28 RS neurons in Figure 2C, which show that, in the control condition, there is a distinct elevation of activity for the preferred target location above the mean (scaled to unity), less elevation for the adjacent directions (±45°), and a clear depression below the mean for targets separated by 135–180° from the preferred location. ANOVA revealed a highly significant effect of location (F = 21.87; p < 0.0001) with significant differences between activity for the preferred direction and all locations separated by ≥90° (p < 0.0001; Scheffe post hoc test). A highly significant drug condition by direction interaction (F = 4.17;p = 0.0002) was found between the control and drug conditions. The effect of direction still remained (p < 0.0001), but its magnitude (F = 4.63) and the post hoc differences between preferred and nonpreferred directions were much reduced in the presence of 5-HT2A blockade (p < 0.05 at −135 and 180°;p < 0.01 at +135°). In rare instances (6 of 44 U), 5-HT2A antagonists induced tuning in RS cells that were not previously tuned in the control condition. However, these drugs never improved tuning in those neurons that were already tuned in the control condition.
To analyze further how delay activity was changed by 5-HT2A blockade, we examined the percentage change in activity from control for each target direction for the same population of tuned RS units tested with 5-HT2Aantagonists. From the results presented in Figure 2Dit can be seen that the deleterious effect of 5-HT2A blockade was produced by an overall selective reduction in activity within the memory field of the cell with a greater attenuation for the preferred target location (again normalized for each cell to 0°) than the two adjacent targets (+45°, p < 0.0001; −45°, p < 0.0001). This effect would be expected to produce a profound attenuation of spatial tuning in RS cells.
Effects of 5-HT2A receptor stimulation on the memory fields of RS units
If the effect observed on the spatially tuned delay activity of RS cells was, indeed, a direct effect of 5-HT2Ablockade, then we would expect that the agonist would produce an enhancement of the memory fields of these neurons. However, it should be recognized that iontophoretic application of the agonist onto a neuron under its natural conditions with an operational level of endogenous serotonin will not always produce a functionally significant influence (as discussed in Materials and Methods). In contrast to the effect of the 5-HT2A antagonists, we saw no effect on most of the 21 RS cells tested with application of α-methyl-5-HT at 20–50 nA (Table 1). Nevertheless, 12 RS cells (57% of those tested) showed an elevated spatial tuning in the delay period with iontophoresis of the drug. Attenuation of the memory field was never found in RS units that previously showed any tuning in the control condition (n = 6; 29%). The enhancement of spatial tuning in delay activity by the agonist is illustrated for one RS cell in Figure 3A. Here it can be seen that there is a modest differentiation of delay period activity between the preferred target location at 135° and the nonpreferred location at 315°. The relationship of this activity to the memory field of the cell is apparent from the adjacent polar plot in Figure 3B. This neuron had a TF = 2 and a θ = 124° (see arrow) in the control condition. Iontophoresis of α-methyl-5-HT at 40 nA increased delay activity in trials with the target at 135° but actually reduced it for those at 315°, increasing TF to 3, with θ remaining at 127°. When MDL100,907 was coapplied at 25 nA subsequently, the previous agonist-induced enhancement was reversed and the TF was reduced to 1, with θ at 130° (data not shown). Finally, when agonist application was terminated, continued iontophoresis of the antagonist destroyed the memory field completely, without any overall decrease in activity of the cell (bottom panel). Reversal of the deleterious effect of MDL100,907 on the memory field by the agonist was also observed in three additional RS cells. Population analysis of the agonist effect on 11 RS neurons that were tuned in the control condition, or became tuned in the drug condition, revealed a highly significant effect of the drug on the differentiation between activity for the preferred and nonpreferred target locations (Fig. 3C). The preferred target location (again normalized to 0°) shows a small elevation of activity above mean, whereas the activity of nonpreferred target locations is moderately submerged. Even so, ANOVA revealed a distinct effect of direction (F = 7.09; p < 0.0001) with significant post hoc differences at −90°, −135°, and 180°. Application of the agonist dramatically increased the effect of direction (F = 29.32; p< 0.0001) with a significant drug by direction interaction (F = 4.72; p < 0.0001). This effect was particularly prominent for the preferred target location of the cell, which created large post hoc differences not only with target locations 135° and 180° distant but also with the adjacent target locations (±45°). In this way, the spatial tuning of this population was considerably sharpened. Note that the distribution of activity about the mean for the agonist condition (Fig. 3C) is even more polarized between preferred and nonpreferred directions than the population of tuned cells in the control condition that were tested with the antagonist (Fig. 2C). Thus, not only is it possible for a population of cells with moderate or no tuning to become considerably tuned because of increased 5-HT2Astimulation (above the endogenous level), but these cells appear to be more tuned than a separate sample of neurons recorded in the control condition. To investigate further the mechanism by which the agonist may exert its beneficial effects, we analyzed the percentage change in activity from the control condition at each target location for the same population of RS units tested with the agonist. As shown in Figure3D, the agonist produced an overall decrease in delay activity which was significant for nonpreferred target locations (p < 0.001; two-tailed t test) and significantly less for the preferred target location (0°) than the adjacent (±45°) locations (p = 0.003,p < 0.001; two-tailed paired t test). Thus, it appears that increasing 5-HT2A receptor stimulation can enhance tuning in RS cells, primarily by producing a net reduction in their activity, both for the opponent, nonpreferred target locations and the two locations adjacent to the preferred target.
Fig. 3.
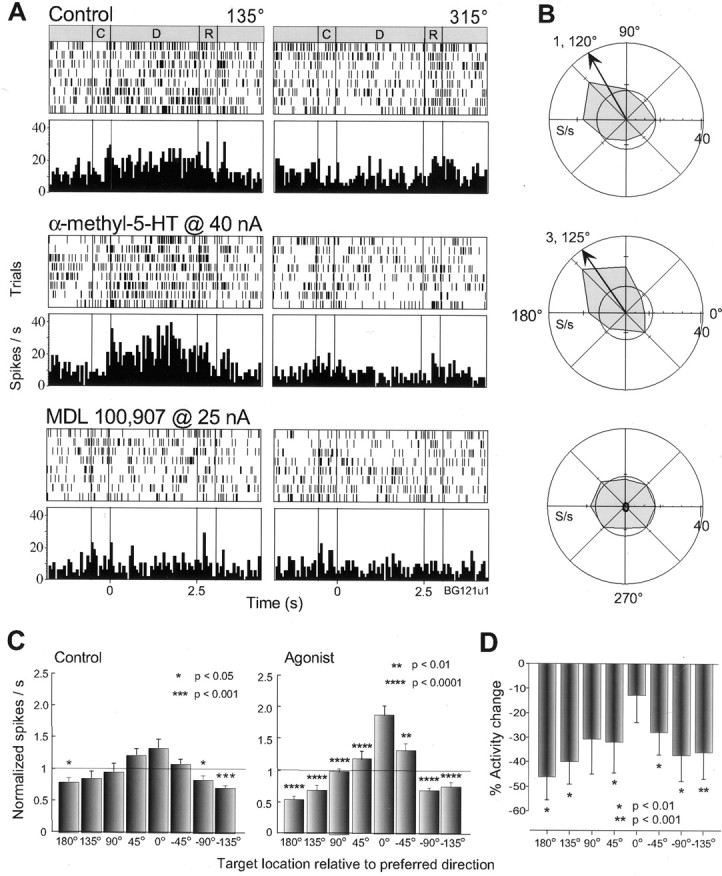
Effects of 5-HT2A stimulation on RS neurons. A, Neuronal activity of an RS neuron showing a small response during the delay period for targets at 135° but not at 315° in the control condition. Iontophoresis of α-methyl-5-HT boosts the delay activity for the preferred direction while, at the same time, it depresses activity for the opposite location. The same cell tested with MDL100,907 (bottom panel) shows a complete abolition of its previous response. B, The memory field of this cell exhibits modest tuning during the control condition (top), which is sharpened by application of the agonist (middle), but delay activity loses spatial specificity altogether after application of MDL100,907.C, Population analysis for 11 RS cells tested with the agonist reveals signs of a spatial profile in response in control (left) that is dramatically augmented by the agonist (right). Note that one cell was excluded from this analysis because it showed changes in activity for opposite directions in space between the first and second half of the delay period.D, Overall, the agonist produces a larger reduction in the delay activity for nonpreferred target locations than that for the preferred location in this population of cells. Conventions as in previous figure.
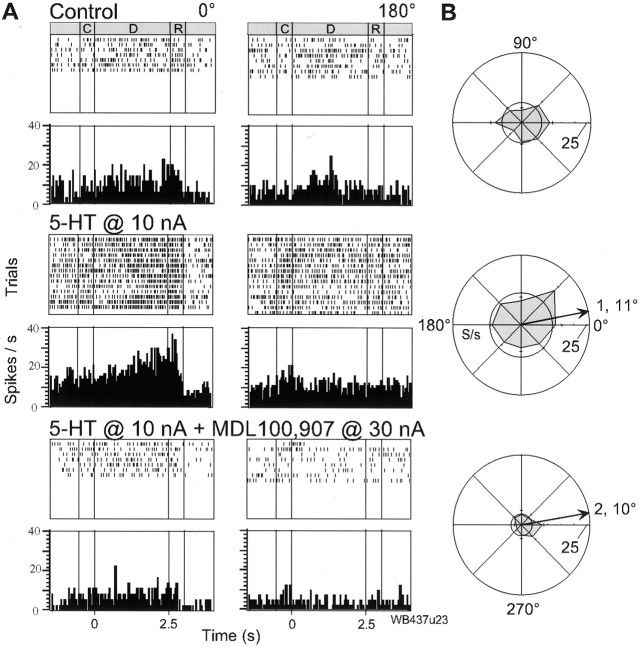
Given the above results for the effect of an agonist with some selectivity for the 5-HT2A receptor, we were interested to see whether elevation of serotonergic stimulation, by iontophoresis of 5-HT itself, could also have advantageous effects on the memory fields of putative pyramidal cells. Serotonin at 4–10 nA improved tuning in five of nine (56%) RS cells, in three cases by significantly increasing the delay activity of the cell (Table 1). As illustrated in Figure 4, 5-HT, at just 10 nA, produced a dramatic, spatially dependent increase in the delay activity of an RS cell exhibiting no apparent tuning in control. However, the modest increase in activity for the nonpreferred target locations partially offset this effect, such that the cell becomes only weakly tuned (TF = 1; θ = 11°). Although coapplication of MDL100,907 clearly reverses this increase in delay activity, its combined effect is distributed over all target locations such as to improve the signal to noise in the spatially-dependent firing of the cell and significantly enhance tuning (TF = 2; θ = 14°). Thus, 5-HT is capable of facilitating the spatially tuned excitatory input to the cell but, in the absence of producing any substantial inhibition, may not be so effective as α-methyl-5-HT in enhancing memory fields of RS neurons. In keeping with this supposition, it was observed that iontophoresis of α-methyl-5-HT at levels of >50 nA could produce a profound depression in the delay activity of RS neurons, as can be seen in the dose-dependent response of one RS neuron in Figure 5. Thus, “excessive” stimulation of 5-HT2A receptors by an exogenous agonist can result in attenuation of the memory fields of putative pyramidal cells.
Fig. 4.
Effect of serotonin on delay activity in an RS cell. A, In the control condition the cell shows barely any distinction in its response for the 0° and 180° targets. However, on application of 5-HT at just 10 nA there is a marked enhancement of the firing of the cell that particularly accentuates the delay activity on 0° trials. Subsequent coapplication of MDL100,907 (bottom panel) dramatically reduces the firing rate and attenuates the previous selective response in the delay period. B, The delay activity of this RS cell does not show any spatial specificity in control but it develops into a significant memory field (TF = 1) when 5-HT is applied (middle panel). Coapplication of MDL100,907 produces a substantial reduction in the delay activity but sharply limits firing to a small region of space and, as a consequence, improves spatial tuning (TF = 2). Conventions as in previous figures.
Fig. 5.
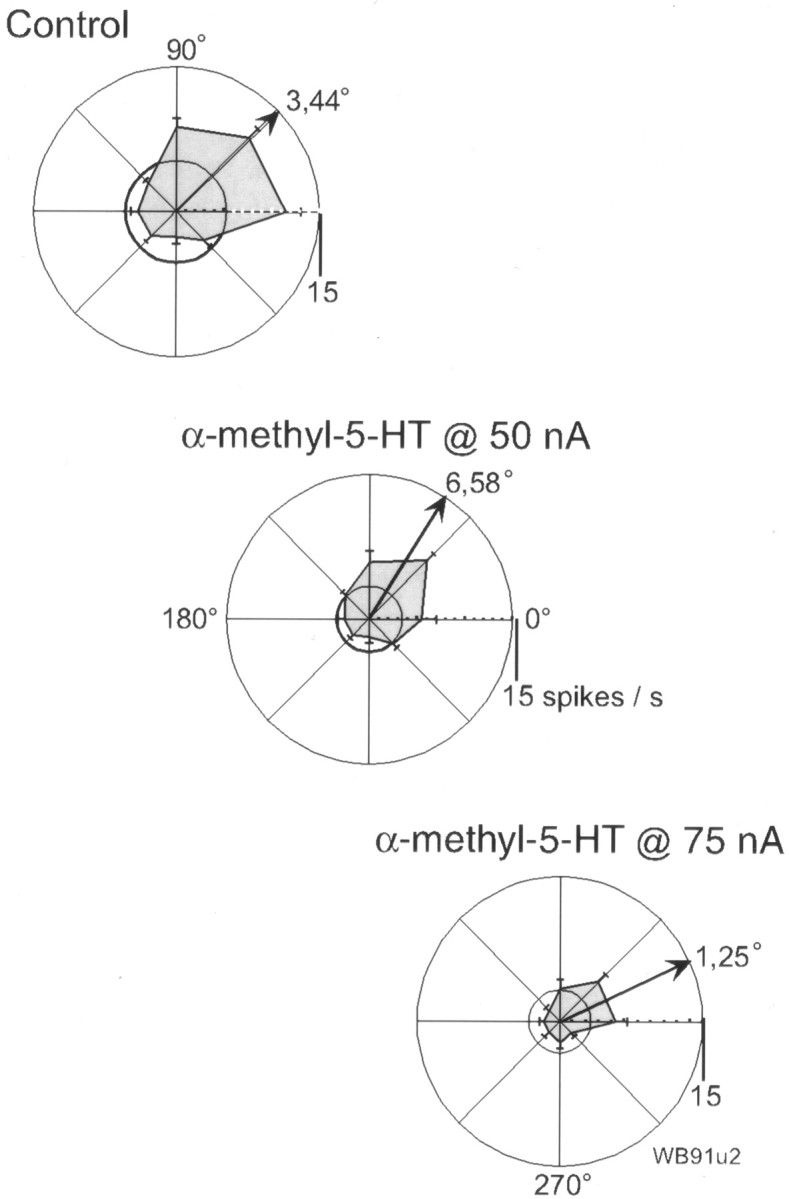
Dose-dependent effects of α-methyl-5-HT. This RS cell possessed a rather broad memory field in the control condition (top), which is clearly sharpened by iontophoresis of α-methyl-5-HT at 50 nA, despite some reduction in the overall level of delay activity. When agonist application was increase to 75 nA (bottom), the delay was further reduced to the extent that the memory field was dramatically attenuated.
Effects of 5-HT2A receptor blockade on the memory fields of FS units
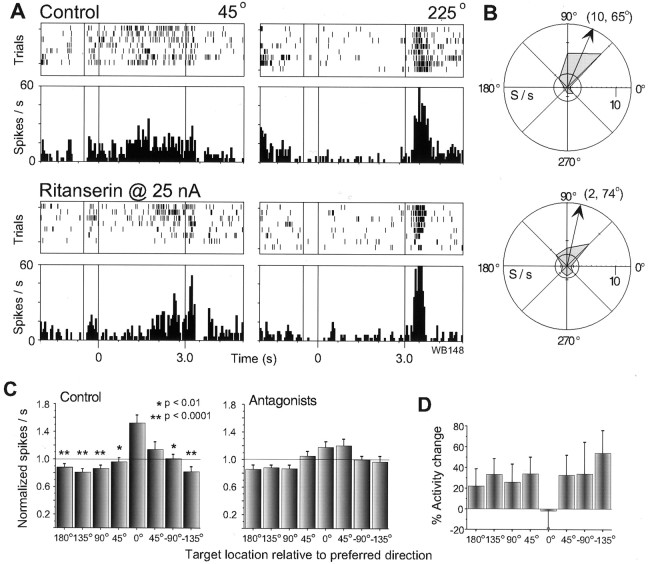
The above findings raised the possibility that α-methyl-5-HT raised the level of feedforward inhibition onto the RS cell being recorded from by activating surrounding inhibitory interneurons that also posses 5-HT2A receptors. To more directly examine whether feedforward inhibition could be recruited by serotonergic transmission in the regulation of spatial tuning in pyramidal cells, we next investigated 5-HT2Areceptor modulation of mnemonic responses in FS neurons. Like RS units, most FS cells that were tuned in the control condition showed an attenuation of their memory fields (13 of 16; 81%) during application of a 5-HT2A antagonist at 15–50 nA. As illustrated for one FS cell in Figure 6, a clear directional specificity can be seen in the control condition with a maximal response for the 45° target location (TF = 10; θ = 65°), but this is lost after 5-HT2Ablockade (ritanserin, 25 nA) because of reduction in delay activity for the preferred direction of the cell. Note that the postsaccadic response for the nonpreferred target (225°) withstood 5-HT2A blockade much more robustly. Population analysis of delay tuning in 13 of these cells (Fig. 6C) showed a similar flattening of spatial profile (two-way ANOVA; drug–target location: p = 0.0308) such that there were no significant post hoc differences between target locations in the drug condition. The percentage change in activity produced by 5-HT2A blockade in this population of FS neurons did show an important difference to that found for RS neurons, such that, overall, activity increased for all target locations other than the preferred target direction (Fig. 6D). The possibility that endogenous 5-HT2A stimulation might help to sculpt tuning in FS neurons by exerting a “surrounding” inhibition on their activity, was supported by two further observations. First, α-methyl-5-HT (25–50 nA) produced only attenuation in tuning of FS units (6 of 13), reducing their response for preferred target locations. Second, delay tuning could be induced by 5-HT2A blockade in a number of previously untuned neurons in this spike class (7 of 31) by the “unmasking” of responses for preferred target locations. Thus, the effect of blocking 5-HT2A receptor stimulation by the endogenous ligand on spatial tuning appears to be very similar between both major classes of cortical neurons that are positive for this particular serotonin receptor subtype and that already posses spatial tuning, but the effect on their actual firing rates was quite different.
Fig. 6.
Effects of 5-HT2A blockade on FS neurons. A, An FS neuron which, in the control condition (top panel), fired during the delay period on trials in which the target was at 45°, and in the postsaccadic epoch for the opponent target location (225°). Application of ritanserin gradually abolished the delay activity for the preferred target location but cue and presaccadic activity for this location, as well as the postsaccadic activity for the opposite target location, persisted longer during drug application. B, The memory field of this FS cell included responses to two adjacent targets at 45° and 90° that were both diminished after the application of ritanserin.C, A population of 13 FS cells that were tuned in the control condition showed a clear spatial profile in their delay activity that was practically abolished by 5-HT2A blockade.D, Rather than generally reducing the activity of this cell type, the 5-HT2A antagonists produced an overall increase in delay activity for all targets other than the preferred location in this cell population. Conventions as in previous figures.
DISCUSSION
Neuromodulation of the mnemonic process
Previous studies in vitro on the actions of 5-HT2A receptors have sought to determine their excitatory and inhibitory actions on different cell types in systems in which many of the normal functional inputs to these cells are absent. In the present study, we used iontophoresis to examine the function of the 5-HT2A receptor on-line during behavior and, therefore, during the actual cognitive process in which these neurons operate. The major finding of this work is the demonstration that 5-HT2A receptor stimulation leads to an augmentation of spatial tuning in putative prefrontal pyramidal cells while blockade of this receptor consistently attenuates existent tuning of this cell type. Consequently, it can be concluded that 5-HT2A receptor stimulation is facilitatory for the mnemonic process occurring in prefrontal pyramidal cells participating in spatial working memory.
The 5-HT2A receptor has been shown in numerous studies to have direct facilitatory actions on both pyramidal cells and interneurons. Sheldon and Aghajanian (1991) and Marek and Aghajanian (1996) have shown that these receptors can powerfully activate interneurons in the piriform cortex, which in turn produce a profound inhibition of neighboring pyramidal cells. Likewise, Araneda and Andrade (1991) as well as Tanaka and North (1993) have shown that pyramidal cells and interneurons in the neocortex are facilitated in their excitatory responses by 5-HT2A receptors. The significance of 5-HT2A recruitment of cortical inhibitory interneurons has been demonstrated by Zhou and Hablitz, (1999), who observed a dramatic induction of IPSCs in cortical pyramidal cells by α-methyl-5-HT which was reversible by coapplication of the 5-HT2A antagonist ketanserin. Finally, Aghajanian and Marek (1997) have recently shown that 5-HT2A receptors directly facilitate pyramidal neurons in prefrontal cortex by a powerful effect at the “trigger zone” on their primary apical dendrites, which has now been shown to contain intense immunoreactivity for 5-HT2A receptors (Jakab and Goldman-Rakic, 1998,2000). These findings, from work in vitro, point to direct facilitation and indirect feedforward inhibition of pyramidal cells by 5-HT2A receptor activation. Our present work demonstrates that these mechanisms of facilitation and feedforward inhibition are operative in vivo, where they are integral to the construction of spatially tuned memory fields in putative pyramidal cells.
Mechanisms of drug action
Three distinctly different antagonists were used to demonstrate that 5-HT2A blockade attenuated memory fields in prefrontal cortex. However, despite their differences in chemical structure and receptor affinity, all three compounds produced the same overall effect on putative pyramidal neurons with spatially tuned delay activity, as evidenced by the data from both RS and FS population analysis. Not only did the agonist produce the opposite effects on the spatial tuning of delay activity to the antagonists at the population level, but it was possible to see the competitive action of both drug classes on the memory fields of individual cells. As expected, the antagonist effects, when looked at in terms of actual changes in activity for preferred and nonpreferred target locations, are primarily consistent with an attenuation of response of the neuron to its spatially tuned excitatory inputs as well as some minor reduction in inhibition for nonpreferred target locations. The effects of the agonist are not so directly interpretable, as the primary effect appears to be an overall reduction in activity of RS neurons, primarily for their nonpreferred target locations. This reduction in activity is reasonably explained by the findings of Zhou and Hablitz (1999) that α-methyl-5-HT drives substantial feedforward inhibition in neocortex, just as 5-HT2A stimulation does in piriform cortex (Gellman and Aghajanian, 1994). Thus, the agonist may facilitate excitatory spatially tuned inputs to pyramidal cells while at the same time activating inhibitory mechanisms that preserve the spatial resolution of their mnemonic response. Moreover, we now show that the memory fields of these putative parvalbumin-containing interneurons are also 5-HT2A-dependent. We therefore postulate that α-methyl-5-HT diffuses a sufficient distance to facilitate multiple surrounding interneurons with similar spatial tuning, which feedforward onto the pyramidal cells from which we recorded. This hypothesis is supported by the finding that iontophoresis of 5-HT itself at very low ejection currents boosted the delay activity of the cells without producing pronounced enhancement of spatial tuning. In this case, we would not expect the endogenous ligand to diffuse a significant distance from the recording site (because of selective processes of metabolism and reuptake), so low-level application of serotonin should not evoke considerable feedforward inhibition. Therefore, it can be postulated that the synergistic action of the 5-HT2A receptor on both pyramidal cells, and the interneurons which innervate them, may be important for the expression of significant spatially tuned delay activity in prefrontal cortex. The outcome of this interaction would obviously depend on the level of serotonin release, reuptake and the sensitivity of the 5-HT2A receptor on the two cell types. Despite this complexity, it is clear that serotonin recruits inhibitory networks that are integral components of the local circuits involved in modulating the construction of spatial tuning by excitatory afferents in prefrontal pyramidal cells.
Comparison of 5-HT2A and D1receptor effects
In a previous study, we have shown that D1receptor blockade can dramatically enhance the tuning of prefrontal pyramidal cells during the delay period by directly boosting the strength of their memory fields, and in some cases, reducing activity even further in the opponent memory field (Williams and Goldman-Rakic, 1995). This was suggested to be a direct action at the level of the spines on the distal dendrites of pyramidal cells where the majority of D1 receptors are located. Thus, there appears to be a critical concentration range of cortical dopamine required for cellular function in working memory (Arnsten et al., 1994; Murphy et al., 1996; Zahrt et al., 1997; Lidow et al., 1998; Castner et al., 2000). In contrast, the 5-HT2A receptor appears to operate in a more linear range in the enhancement of prefrontal memory fields than the D1 receptor. Stimulation of this receptor would be expected to increase the ability of EPSPs arriving at the proximal dendrites to reach sufficient magnitude for action potential generation. As such, it could preferentially increase the response of the cell to weaker excitatory inputs and in theory reduce the spatial tuning of its activity. Why this does not happen in pyramidal cells is most likely attributable to the strength of their excitatory input related to the preferred target direction (Funahashi et al., 1989) and the counteractive effect of increased inhibitory input to the cell for nonpreferred directions. Therefore, serotonin acting at 5-HT2A receptors might provide a tonic facilitation of cortical pyramidal cells and interneurons that sets their level of responsiveness to their direct excitatory inputs as well as the degree to which they are held under the influence of inhibitory local circuits. Preliminary data indicate that this tonic facilitation appears to be consistent for neuronal responses in all epochs of the task, in contrast to the apparently selective suppression of mnemonic activity ascribed to D1 receptor stimulation in our previous report.
Functional and clinical relevance
From the evidence above it would be expected that increased serotonin release might unilaterally benefit working memory performance. However there is little or no data to support this case (Jakala et al., 1993; Curran and Travill, 1997; Ruotsalainen et al., 1997), and the physiological findings from the present study might appear to be inconsistent with those from behavioral studies. One possible explanation for this is that under most normal conditions, the effects of 5-HT2A receptor activation interact strongly with the effects of dopamine receptor activation, as suggested by a number of clinical and experimental studies (Kuroki et al., 1999;Ichikawa et al., 2001). We propose an alternative hypothesis that may provide a better insight into the functions of serotonin in prefrontal cortex. In our experiments, only the stimuli relevant to the spatial working memory task are present, and the animal is highly motivated to engage in this task rather than any other behavior. When 5-HT2A receptor activation results in facilitation of the inputs to the prefrontal cortex, only the relevant inputs are boosted, and therefore the signal-to-noise ratio in the system can only improve. However, in the presence of real world environmental stimuli, when there is motivation to engage in multiple behaviors, 5-HT2A receptor activation of prefrontal neurons may cause the contents of working memory to become submerged in “noise” related to many alternative interoceptive and exteroceptive stimuli. Accordingly, a recent fMRI study has shown that increasing task load on human cognition leads to increasing activation in dorsolateral prefrontal cortex as more and more information is required to be held on-line (Manoach et al., 1997). Secondly, hallucinogens have high affinities at the 5-HT2Areceptor (Aghajanian and Marek, 1999) and, although they may have their major actions in sensory systems, they may also have similar actions on cognitive systems. In a recent positron emission tomography (PET) study the 5-HT2/5-HT1 agonist psilocybin was found to produce marked increases in cerebral metabolism in frontomedial and frontolateral cortex (Vollenweider et al., 1997), which correlated positively with psychotic symptom formation. These effects could be reversed by the 5-HT2Aantagonist ketanserin, suggesting that sufficient activation of this hallucinoceptor can disrupt prefrontal function.
In clinical studies, there is accumulating evidence that 5-HT2A receptor blockade may help to ameliorate both the positive and negative symptoms, and to some extent, the cognitive deficits in schizophrenia (Meltzer, 1999; Meltzer and McGurk, 1999). Clozapine and other atypical neuroleptics have been shown to occupy 5-HT2A receptors considerably more than D2 receptors in PET studies of patients with schizophrenia (Farde et al., 1994, 1995; Lundberg et al., 1996). Although emphasis has been placed on the ability of 5-HT2A antagonists to enhance dopamine release in prefrontal cortex as a possible antipsychotic mechanism (Iyer and Bradberry, 1996), there is obviously a case for the direct involvement of these receptors in the manifestation of cognitive disorder in schizophrenia (Aghajanian and Marek, 2000). Prefrontal dysfunction is also implicated in depression, in which there is evidence that stimulation of 5-HT receptors may be so low as to result in reduced cerebral blood flow in prefrontal cortex (Bremner et al., 1997; Smith et al., 1997). Treatment of this insufficiency by serotonin reuptake blockers can reinstate normal blood flow in the frontal lobes, indicating the requirement for serotonergic facilitation of neuronal activity for proper function of this brain region. Therefore, our results support the proposal that 5-HT2Asignaling may also play an important role in the amelioration of cognitive function in this mental disorder (Degl'Innocenti et al., 1999; Hindmarch et al., 2000; Rajkowska, 2000)
The present findings point to a beneficial role for 5-HT2A receptors in the working memory process in primates performing a well learned task, although it remains to be seen whether increased activation of this serotonin receptor subtype could actually lead to disruption of mnemonic processing when task demands increase. Hence, our results support the assertion that alterations in 5-HT2A receptor signaling may be a contributing factor to the development of cognitive dysfunction in mental disorders such as schizophrenia and depression, and thus, may provide an important target for drug therapy.
Footnotes
This work was supported by National Institute of Mental Health Grants P50 MH44866 and R37 MH38546 (P.S.G.-R.). Further support was provided by the Medical Scientist Training Program of the National Institute of Health (S.G.R.). We thank Chris Muly for helpful discussion, Susheel Vijayraghavan and Peter Vosler for assistance in data analysis, and Gary Leydon for his help in developing Spike2 scripts.
Correspondence should be addressed to Dr. Graham V. Williams, Section of Neurobiology, Yale University Medical School, 333 Cedar Street, New Haven, CT 06510. E-mail: graham.williams@yale.edu.
REFERENCES
- 1.Aghajanian GK, Marek GJ. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997;36:589–599. doi: 10.1016/s0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- 2.Aghajanian GK, Marek GJ. Serotonin and hallucinogens. Neuropsychopharmacology. 1999;21:16.S–23S. doi: 10.1016/S0893-133X(98)00135-3. [DOI] [PubMed] [Google Scholar]
- 3.Aghajanian GK, Marek GJ. Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res Brain Res Rev. 2000;31:302–312. doi: 10.1016/s0165-0173(99)00046-6. [DOI] [PubMed] [Google Scholar]
- 4.Andreasen NC, O'Leary DS, Flaum M, Nopoulos P, Watkins GL, Boles Ponto LL, Hichwa RD. Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naive patients. Lancet. 1997;349:1730–1734. doi: 10.1016/s0140-6736(96)08258-x. [DOI] [PubMed] [Google Scholar]
- 5.Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology. 1994;116:143–151. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- 6.Araneda R, Andrade R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine 1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- 7.Baeg EH, Kim YB, Jang J, Kim HT, Mook-Jung I, Jung MW. Fast spiking and regular spiking neural correlates of fear conditioning in the medial prefrontal cortex of the rat. Cereb Cortex. 2001;11:441–451. doi: 10.1093/cercor/11.5.441. [DOI] [PubMed] [Google Scholar]
- 8.Bremner JD, Innis RB, Salomon RM, Staib LH, Ng CK, Miller HL, Bronen RA, Krystal JH, Duncan J, Rich D, Price LH, Malison R, Dey H, Soufer R, Charney DS. Positron emission tomography measurement of cerebral metabolic correlates of tryptophan depletion-induced depressive relapse. Arch Gen Psychiatry. 1997;54:364–374. doi: 10.1001/archpsyc.1997.01830160092012. [DOI] [PubMed] [Google Scholar]
- 9.Castner SA, Williams GV, Goldman-Rakic PS. Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science. 2000;287:2020–2022. doi: 10.1126/science.287.5460.2020. [DOI] [PubMed] [Google Scholar]
- 10.Conover WJ. Practical nonparametric statistics. Wiley; New York: 1971. [Google Scholar]
- 11.Curran HV, Travill RA. Mood and cognitive effects of +/−3,4-methylene-dioxymethamphetamine (MDMA, ecstasy): weekend high followed by mid-week low. Addiction. 1997;92:821–831. [PubMed] [Google Scholar]
- 12.Dantzker JL, Callaway EM. Laminar sources of synaptic input to cortical inhibitory interneurons and pyramidal neurons. Nat Neurosci. 2000;3:701–707. doi: 10.1038/76656. [DOI] [PubMed] [Google Scholar]
- 13.Degl'Innocenti A, Agren H, Zachrisson O, Backman L. The influence of prolactin response to d-fenfluramine on executive functioning in major depression. Biol Psychiatry. 1999;46:512–517. doi: 10.1016/s0006-3223(98)00325-4. [DOI] [PubMed] [Google Scholar]
- 14.Farde L, Nordstrom AL, Nyberg S, Halldin C, Sedvall G. D1-, D2-, and 5-HT2-receptor occupancy in clozapine-treated patients. J Clin Psychiatry. 1994;55:67–69. [PubMed] [Google Scholar]
- 15.Farde L, Nyberg S, Oxenstierna G, Nakashima Y, Halldin C, Ericsson B. Positron emission tomography studies on D2 and 5-HT2 receptor binding in risperidone-treated schizophrenic patients. J Clin Psychopharmacol. 1995;15:19S–23S. doi: 10.1097/00004714-199502001-00004. [DOI] [PubMed] [Google Scholar]
- 16.Fisher NI. Statistical analysis of circular data. Cambridge UP; Cambridge: 1993. [Google Scholar]
- 17.Fuster JM. Unit activity in prefrontal cortex during delayed-response performance: neuronal correlates of transient memory. J Neurophysiol. 1973;36:61–78. doi: 10.1152/jn.1973.36.1.61. [DOI] [PubMed] [Google Scholar]
- 18.Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- 19.Funahashi S, Bruce CJ, Goldman-Rakic PS. Neuronal activity related to saccadic eye movements in the monkey's dorsolateral prefrontal cortex. J Neurophysiol. 1991;65:1464–1483. doi: 10.1152/jn.1991.65.6.1464. [DOI] [PubMed] [Google Scholar]
- 20.Garnovskaya MN, Nebigil CG, Arthur JM, Spurney RF, Raymond JR. 5-Hydroxytryptamine2A receptors expressed in rat renal mesangial cells inhibit cyclic AMP accumulation. Mol Pharmacol. 1995;48:30–37. [PubMed] [Google Scholar]
- 21.Gellman RL, Aghajanian GK. Pyramidal cells in piriform cortex receive a convergence of inputs from monoamine activated GABAergic interneurons. Brain Res. 1993;600:63–73. doi: 10.1016/0006-8993(93)90402-9. [DOI] [PubMed] [Google Scholar]
- 22.Gellman RL, Aghajanian GK. Serotonin2 receptor-mediated excitation of interneurons in piriform cortex: antagonism by atypical antipsychotic drugs. Neuroscience. 1994;58:515–525. doi: 10.1016/0306-4522(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 23.Goldman-Rakic PS. Circuitry of the prefrontal cortex and the regulation of behavior by representational knowledge. In: Plum F, Mountcastle V, editors. Handbook of physiology, Vol 5. American Physiological Society; Bethesda, MD: 1987. p. 373. [Google Scholar]
- 24.Goldman-Rakic PS. Prefrontal cortical dysfunction in schizophrenia: the relevance of working memory. In: Carroll BJ, Barrett JE, editors. Psychopathology and the brain. Raven; New York: 1991. pp. 1–23. [Google Scholar]
- 25.Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- 26.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 27.Gur M, Beylin A, Snodderly DM. Physiological properties of macaque V1 neurons are correlated with extracellular spike amplitude, duration, and polarity. J Neurophysiol. 1999;82:1451–1464. doi: 10.1152/jn.1999.82.3.1451. [DOI] [PubMed] [Google Scholar]
- 28.Hindmarch I, Kimber S, Cockle SM. Abrupt and brief discontinuation of antidepressant treatment: effects on cognitive function and psychomotor performance. Int Clin Psychopharmacol. 2000;15:305–318. doi: 10.1097/00004850-200015060-00001. [DOI] [PubMed] [Google Scholar]
- 29.Ichikawa J, Ishii H, Bonaccorso S, Fowler WL, O'Laughlin IA, Meltzer HY. 5-HT2A and D-2 receptor blockade increases cortical DA release via 5-HT1A receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. J Neurochem. 2001;76:1521–1531. doi: 10.1046/j.1471-4159.2001.00154.x. [DOI] [PubMed] [Google Scholar]
- 30.Iyer RN, Bradberry CW. Serotonin-mediated increase in prefrontal cortex dopamine release: pharmacological characterization. J Pharmacol Exp Ther. 1996;277:40–47. [PubMed] [Google Scholar]
- 31.Jakab RL, Goldman-Rakic PS. 5-hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: Possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci USA. 1998;95:735–740. doi: 10.1073/pnas.95.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jakab RL, Goldman-Rakic PS. Segregation of serotonin 5-HT2A and 5-HT3 receptors in inhibitory circuits of the primate cerebral cortex. J Comp Neurol. 2000;417:337–348. doi: 10.1002/(sici)1096-9861(20000214)417:3<337::aid-cne7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 33.Jakala P, Sirvio J, Riekkinen P, Jr, Riekkinen Sr PJ. Effects of p-chlorophenylalanine and methysergide on the performance of a working memory task. Pharmacol Biochem Behav. 1993;44:411–418. doi: 10.1016/0091-3057(93)90483-a. [DOI] [PubMed] [Google Scholar]
- 34.Johnson MP, Siegel BW, Carr AA. [3H]MDL 100,907: a novel selective 5-HT2A receptor ligand. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:205–209. doi: 10.1007/BF00178722. [DOI] [PubMed] [Google Scholar]
- 35.Kawaguchi Y. Groupings of nonpyramidal and pyramidal cells with specific physiological and morphological characteristics in rat frontal cortex. J Neurophysiol. 1993;69:416–431. doi: 10.1152/jn.1993.69.2.416. [DOI] [PubMed] [Google Scholar]
- 36.Kawaguchi Y. Physiological subgroups of nonpyramidal cells with specific morphological characteristics in layer II/III of rat frontal cortex. J Neurosci. 1995;15:2638–2655. doi: 10.1523/JNEUROSCI.15-04-02638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuroki T, Meltzer HY, Ichikawa J. Effects of antipsychotic drugs on extracellular dopamine levels in rat medial prefrontal cortex and nucleus accumbens. J Pharmacol Exp Ther. 1999;288:774–781. [PubMed] [Google Scholar]
- 38.Lennox BR, Park SBG, Medley I, Morris PG, Jones PB. The functional anatomy of auditory hallucinations in schizophrenia. Psychiatry Res: Neuroimaging. 2000;100:13–20. doi: 10.1016/s0925-4927(00)00068-8. [DOI] [PubMed] [Google Scholar]
- 39.Liddle PF. Schizophrenic syndromes, cognitive performance and neurological dysfunction. Psychol Med. 1987;17:49–57. doi: 10.1017/s0033291700012976. [DOI] [PubMed] [Google Scholar]
- 40.Liddle PF, Morris DL. Schizophrenic syndromes and frontal lobe performance. Br J Psychiatry. 1991;158:340–345. doi: 10.1192/bjp.158.3.340. [DOI] [PubMed] [Google Scholar]
- 41.Lidow MS, Williams GV, Goldman-Rakic PS. The cerebral cortex: a case for a common site of action of antipsychotics. Trends Pharmacol Sci. 1998;19:136–140. doi: 10.1016/s0165-6147(98)01186-9. [DOI] [PubMed] [Google Scholar]
- 42.Luciana M, Collins PF, Depue RA. Opposing roles for dopamine and serotonin in the modulation of human spatial working memory functions. Cereb Cortex. 1998;8:218–226. doi: 10.1093/cercor/8.3.218. [DOI] [PubMed] [Google Scholar]
- 43.Lundberg T, Lindstrom L, Hartvig P, Reibring L, Agren H, Lundqvist H, Fasth KJ, Antoni G, Langstrom B. Serotonin-2 and dopamine-1 binding components of clozapine in frontal cortex and striatum in the human brain visualized by positron emission tomography. Psychiatry Res. 1996;67:1–10. doi: 10.1016/0925-4927(96)02653-4. [DOI] [PubMed] [Google Scholar]
- 44.Manoach DS, Schlaug G, Siewert B, Darby DG, Bly BM, Benfield A, Edelman RR, Warach S. Prefrontal cortex fMRI signal changes are correlated with working memory load. NeuroReport. 1997;8:545–549. doi: 10.1097/00001756-199701200-00033. [DOI] [PubMed] [Google Scholar]
- 45.Marek GJ, Aghajanian GK. LSD and the phenethylamine hallucinogen DOI are potent partial agonists at 5-HT2A receptors on interneurons in rat piriform cortex. J Pharmacol Exp Ther. 1996;278:1373–1382. [PubMed] [Google Scholar]
- 46.Mazzola-Pomietto P, Aulakh CS, Wozniak KM, Murphy DL. Evidence that m-chlorophenylpiperazine-induced hyperthermia in rats is mediated by stimulation of 5-HT2C receptors. Psychopharmacology (Berl ) 1996;123:333–339. doi: 10.1007/BF02246643. [DOI] [PubMed] [Google Scholar]
- 47.McCarthy G, Blamire AM, Puce A, Nobre AC, Bloch G, Hyder F, Goldman-Rakic PS, Shulman RG. Functional magnetic resonance imaging of human prefrontal cortex activation during a spatial working memory task. Proc Natl Acad Sci USA. 1994;91:8690–8694. doi: 10.1073/pnas.91.18.8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCarthy G, Puce A, Constable RT, Krystal JH, Gore JC, Goldman-Rakic P. Activation of human prefrontal cortex during spatial and nonspatial working memory tasks measured by functional MRI. Cereb Cortex. 1996;6:600–611. doi: 10.1093/cercor/6.4.600. [DOI] [PubMed] [Google Scholar]
- 49.McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985;54:782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- 50.Meltzer HY. Clinical studies on the mechanism of action of clozapine: the dopamine-serotonin hypothesis of schizophrenia. Psychopharmacology [Suppl] 1989;99:S18–S27. doi: 10.1007/BF00442554. [DOI] [PubMed] [Google Scholar]
- 51.Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21:106.S–115S. doi: 10.1016/S0893-133X(99)00046-9. [DOI] [PubMed] [Google Scholar]
- 52.Meltzer HY, McGurk SR. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull. 1999;25:233–255. doi: 10.1093/oxfordjournals.schbul.a033376. [DOI] [PubMed] [Google Scholar]
- 53.Morris NP, Henderson Z. Perineuronal nets ensheath fast spiking, parvalbumin-immunoreactive neurons in the medial septum/diagonal band complex. Eur J Neurosci. 2000;12:828–838. doi: 10.1046/j.1460-9568.2000.00970.x. [DOI] [PubMed] [Google Scholar]
- 54.Morrison JH, Foote SL, Molliver ME, Bloom FE, Lidow GW. Noradrenergic and serotonergic fibers innervate complementary layers in monkey visual cortex: an immunohistochemical study. Proc Natl Acad Sci USA. 1982;79:2401–2405. doi: 10.1073/pnas.79.7.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mountcastle VB, Talbot WH, Sakata H, Hyvarinen J. Cortical neuronal mechanisms in flutter-vibration studied in unanesthetized monkeys. Neuronal periodicity and frequency discrimination. J Neurophysiol. 1969;32:452–484. doi: 10.1152/jn.1969.32.3.452. [DOI] [PubMed] [Google Scholar]
- 56.Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Natl Acad Sci USA. 1996;93:1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Porrino L, Goldman-Rakic PS. Brainstem innervation of prefrontal and anterior cingulate cortex in the rhesus monkey revealed by retrograde transport of HRP. J Comp Neurol. 1982;205:63–76. doi: 10.1002/cne.902050107. [DOI] [PubMed] [Google Scholar]
- 58.Park S, Holzman PS. Schizophrenics show working memory deficits. Arch Gen Psychiat. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- 59.Rajkowska G. Histopathology of the prefrontal cortex in major depression: what does it tell us about dysfunctional monoaminergic circuits? Prog Brain Res. 2000;126:397–412. doi: 10.1016/S0079-6123(00)26026-3. [DOI] [PubMed] [Google Scholar]
- 60.Rao SG, Williams GV, Goldman-Rakic PS. Isodirectional tuning of adjacent interneurons and pyramidal cells during working memory: evidence for microcolumnar organization in PFC. J Neurophysiol. 1999;81:1903–1916. doi: 10.1152/jn.1999.81.4.1903. [DOI] [PubMed] [Google Scholar]
- 61.Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. J Neurosci. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JF, Sahakian BJ, Robbins TW. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- 63.Ruotsalainen S, Sirvio J, Jakala P, Puumala T, Macdonald E, Riekkinen P. Differential effects of three 5-HT receptor antagonists on the performance of rats in attentional and working memory tasks. Eur Neuropsychopharmacol. 1997;7:99–108. doi: 10.1016/s0924-977x(96)00389-6. [DOI] [PubMed] [Google Scholar]
- 64.Sabri O, Hellwig D, Schreckenberger M, Cremerius U, Schneider R, Kaiser HJ, Doherty C, Mull M, Ringelstein EB, Buell U. Correlation of neuropsychological, morphological and functional (regional cerebral blood flow and glucose utilization) findings in cerebral microangiopathy. J Nucl Med. 1998;39:147–154. [PubMed] [Google Scholar]
- 65.Schreiber R, Brocco M, Audinot V, Gobert A, Veiga S, Millan MJ. (1-(2,5-dimethoxy-4 iodophenyl)-2-aminopropane)-induced head-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT) 2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J Pharmacol Exp Ther. 1995;273:101–112. [PubMed] [Google Scholar]
- 66.Sheldon PW, Aghajanian GK. Excitatory responses to serotonin (5-HT) in neurons of the rat piriform cortex: evidence for mediation by 5-HT1C receptors in pyramidal cells and 5-HT2 receptors in interneurons. Synapse. 1991;9:208–218. doi: 10.1002/syn.890090307. [DOI] [PubMed] [Google Scholar]
- 67.Shimegi S, Ichikawa T, Akasaki T, Sato H. Temporal characteristics of response integration evoked by multiple whisker stimulations in the barrel cortex of rats. J Neurosci. 1999;19:10164–10175. doi: 10.1523/JNEUROSCI.19-22-10164.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simons DJ. Response properties of vibrissa units in rat SI somatosensory neocortex. J Neurophysiol. 1978;41:798–820. doi: 10.1152/jn.1978.41.3.798. [DOI] [PubMed] [Google Scholar]
- 69.Smiley JF, Goldman-Rakic PS. Serotonergic axons in monkey prefrontal cerebral cortex synapse predominantly on interneurons as demonstrated by serial section electron microscopy. J Comp Neurol. 1996;367:431–443. doi: 10.1002/(SICI)1096-9861(19960408)367:3<431::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 70.Smith KA, Fairburn CG, Cowen PJ. Relapse of depression after rapid depletion of tryptophan. Lancet. 1997;349:915–919. doi: 10.1016/s0140-6736(96)07044-4. [DOI] [PubMed] [Google Scholar]
- 71.Swadlow HA, Beloozerova IN, Sirota MG. Sharp, local synchrony among putative feed-forward inhibitory interneurons of rabbit somatosensory cortex. J Neurophysiol. 1998;79:567–582. doi: 10.1152/jn.1998.79.2.567. [DOI] [PubMed] [Google Scholar]
- 72.Takeuchi Y, Sano Y. Immunohistochemical demonstration of serotonin nerve fibers in the neocortex of the monkey (Macaca fuscata). Anat Embryol. 1983;166:155–168. doi: 10.1007/BF00305080. [DOI] [PubMed] [Google Scholar]
- 73.Tanaka E, North RA. Actions of 5-hydroxytryptamine on neurons of the rat cingulate cortex. J Neurophysiol. 1993;69:1749–1757. doi: 10.1152/jn.1993.69.5.1749. [DOI] [PubMed] [Google Scholar]
- 74.Timofeev I, Grenier F, Steriade M. Disfacilitation and active inhibition in the neocortex during the natural sleep-wake cycle: an intracellular study. Proc Natl Acad Sci USA. 2001;98:1924–1929. doi: 10.1073/pnas.041430398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vollenweider FX, Leenders KL, Scharfetter C, Maguire P, Stadelmann O, Angst J. Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology. 1997;16:357–372. doi: 10.1016/S0893-133X(96)00246-1. [DOI] [PubMed] [Google Scholar]
- 76.Walker AE. A cytoarchitectural study of the prefrontal area of the macaque monkey. J Comp Neurol. 1940;73:59–86. [Google Scholar]
- 77.Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I: Regional cerebral blood flow (rCBF) evidence. Arch Gen Psychiatry. 1986;43:114–125. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- 78.Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 79.Wilson FA, O' Scalaidhe SP, Goldman-Rakic PS. Functional synergism between putative gamma-aminobutyrate-containing neurons and pyramidal neurons in prefrontal cortex. Proc Natl Acad Sci USA. 1994;91:4009–4013. doi: 10.1073/pnas.91.9.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zahrt J, Taylor JR, Mathew RG, Arnsten AFT. Supranormal stimulation of D-1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou FM, Hablitz JJ. Activation of serotonin receptors modulates synaptic transmission in rat cerebral cortex. J Neurophysiol. 1999;82:2989–2999. doi: 10.1152/jn.1999.82.6.2989. [DOI] [PubMed] [Google Scholar]