Abstract
Sensory hair cells in the inner ears of nonmammalian vertebrates can regenerate after injury. In many species, replacement hair cells are produced by the proliferation of epithelial supporting cells. Thus, the ability of supporting cells to undergo renewed proliferation is a key determinant of regenerative ability. The present study used cultures of isolated inner ear sensory epithelia to identify cellular signals that regulate supporting cell proliferation. Small pieces of sensory epithelia from the chicken utricle were cultured in glass microwells. Under those conditions, cell proliferation was inversely related to local cell density. The signaling molecules N-cadherin, β-catenin, and focal adhesion kinase were immunolocalized in the cultured epithelial cells, and high levels of phosphotyrosine immunoreactivity were present at cell–cell junctions and focal contacts of proliferating cells. Binding of microbeads coated with a function-blocking antibody to N-cadherin inhibited ongoing proliferation. The growth of epithelial cells was also affected by the density of extracellular matrix molecules. The results suggest that cell density, cell–cell contact, and the composition of the extracellular matrix may be critical influences on the regulation of sensory regeneration in the inner ear.
Keywords: auditory, vestibular, regeneration, hair cell, adhesion molecules, cell culture
Sensory hair cells in the cochlea and vestibular organs of birds can regenerate after injury caused by acoustic trauma or treatment with aminoglycoside antibiotics (Cotanche, 1987; Cruz et al., 1987; Weisleder and Rubel, 1993; Cotanche 1999). Although a variety of cellular mechanisms are thought to contribute to repair in the vertebrate ear (Baird et al., 1996; Corwin and Oberholtzer, 1997; Forge et al., 1998; Stone et al., 1998), the principal regenerative mechanism in the avian ear involves the renewed proliferation of epithelial supporting cells (Corwin and Cotanche, 1988; Ryals and Rubel, 1988; Weisleder and Rubel, 1993). A more limited regenerative response occurs in the vestibular organs of mammals (Forge et al., 1993; Warchol et al., 1993; Kuntz and Oesterle, 1998), but spontaneous regeneration has not been demonstrated in normal mammalian cochlea (Roberson and Rubel, 1994). Because intrinsic limitations on cell proliferation appear to be a key determinant of the potential for hair cell regeneration, it is of great interest to identify factors that regulate proliferation in the postembryonic ear.
Regenerative proliferation is triggered by the death of hair cells and their removal from sensory epithelia (Balak et al., 1990; Stone and Cotanche, 1994; Warchol and Corwin, 1996), but the cellular signals that mediate this response are not known. Proliferation can be induced by activation of the cAMP signaling pathway (Navaratnam et al., 1996; Montcouquiol and Corwin, 2001). Also, many studies have examined the possible role of mitogenic growth factors in otic regeneration and have reported that the proliferation of vestibular supporting cells can be enhanced by treatment with fibroblast growth factor 2, glial growth factor-2, insulin-like growth factor 1, insulin, transforming growth factor α, and tumor necrosis factor α (Lambert, 1994; Yamashita and Oesterle, 1995; Gu et al., 1996; Oesterle et al., 1997; Zheng et al., 1997; Kuntz and Oesterle, 1998; Warchol, 1999). It is notable, however, that treatment with mitogens does not lead to large increases in supporting cell proliferation, and cultured avian supporting cells continue to proliferate at high levels even in the absence of added mitogens (Warchol and Corwin, 1993; Warchol, 1995). These results suggest that exogenous mitogens are not the sole regulators of proliferation and sensory regeneration in the ear.
Studies of other types of nontransformed cells have identified a number of intrinsic and environmental conditions that influence proliferation. For example, entry into the S-phase of the cell cycle is regulated by changes in cell spreading and cytoskeletal conformation (Folkman and Moscona, 1978; Ingber, 1997; Aplin et al., 1999). The proliferation of epithelial cells can also be influenced by cell–cell contact and by interactions between cell adhesion molecules (St. Croix et al., 1998;Levenberg et al., 1999). The composition of the extracellular matrix (ECM) is another critical influence on cell proliferation and differentiation (Ingber and Folkman, 1989). The present study examined the influence of these factors on the proliferation of supporting cells from the sensory epithelium of the avian utricle. The mature avian vestibular organs exhibit ongoing supporting cell proliferation (Jørgensen and Mathiesen, 1988; Roberson et al., 1992; Warchol and Corwin, 1993; Kil et al., 1997; Stone et al., 1999) and have a robust capacity for sensory regeneration (Weisleder and Rubel, 1993). Results presented here suggest that cell density, cadherin-mediated interactions, and the composition of the ECM may all interact to regulate ongoing and regenerative proliferation in the inner ear.
MATERIALS AND METHODS
Preparation of epithelial cultures. Chicks (White Leghorn strain, 7–21 d after hatching) were killed by CO2 asphyxiation and decapitated. After removal of the lower jaw and the skin, heads were immersed in 70% ethanol for 5–10 min. All further dissection was performed under aseptic conditions in a laminar flow tissue culture hood (Baker). The labyrinths were exposed laterally, and utricles were quickly removed and transferred to medium 199 with HBSS and HEPES (Invitrogen, Gaithersburg, MD). The otoconia were removed using fine forceps, and utricles were incubated for 60 min in 500 μg/ml thermolysin (Sigma, St. Louis, MO) (dissolved in medium 199 with Earle's salts, 2200 mg/l sodium bicarbonate, 25 mm HEPES, and 0.69 mml-glutamine) at 37°C in a 5% CO2environment (Germain et al., 1993; Corwin et al., 1995). Specimens were then transferred to medium 199 with HBSS, and iridectomy scissors were used to trim away the edges and peripheral regions of the utricles, leaving only the central sensory region (the utricular cotillus;Jørgensen, 1989). A 30-gauge needle was used to gently remove the sensory epithelium from the basement membrane and associated connective tissue. Isolated epithelia were then placed on a small spatula and transferred into fibronectin- or laminin-coated culture wells that contained 50 μl of medium 199 (with Earle's salts, 2200 mg/l sodium bicarbonate, 25 mm HEPES, and 0.69 mml-glutamine), supplemented with 10% fetal bovine serum (FBS; Invitrogen). A single utricular epithelium was placed in each well. Once in the wells, the epithelia were cut into 10–12 small (∼200 × 200 μm) pieces using iridectomy scissors. The cultures were then incubated at 37°C in 5% CO2and 95% air.
Preparation of culture substrates. The glass surfaces of culture wells (P35G-0-10-G; Mat Tek, Ashland, MA) were coated with either bovine fibronectin or murine laminin (Sigma) for 2 hr at room temperature. Most cultures wells were coated with 10 μg of fibronectin (dissolved in 100 μl of medium 199 with HBSS and 25 mm HEPES), quickly rinsed with fresh medium 199, and used immediately. Other culture wells were coated with 0.1–10.0 μg of fibronectin or laminin (in 100 μl of medium 199) and used in experiments that quantified the effects of attachment factor density on epithelial cell outgrowth (see Results).
Culture in defined media. All cultures were initially maintained for 3 d in medium 199 and 10% FBS to allow time for attachment to the substrate and for the initial outgrowth of epithelial cells. Most cultures were then rinsed three times with serum-free medium 199 and incubated for an additional 2–5 d in defined media. The precise formulation of the medium and the total time in culture depended on the particular experiment. Data on the relationship between cell density and proliferation were obtained from cultures that were maintained for 5 d in medium 199 with N2 supplement (Bottenstein and Sato, 1979; Invitrogen). Experiments on the effects of fibronectin and laminin on cell growth, as well as immunolocalization of phosphotyrosine, N-cadherin, β-catenin, and focal adhesion kinase were performed on cultures that were maintained for 2 d in medium 199 and N2. Experiments on the effects of retinoic acid were performed in cultures that were maintained for 2–5 d in medium 199 and N2 and all-trans-retinoic acid (Sigma). Retinoic acid was prepared as a 1 mg/ml stock solution (in DMSO) and stored at −20°C. Control cultures were maintained in medium 199 and N2 with comparable concentrations of DMSO.
Treatment with neutralizing antibody to N-cadherin. The role of N-cadherin interactions in regulating proliferation was tested by incubating cultures with microbeads that were coated with a function-blocking antibody to N-cadherin (NCD-2; Hatta and Takeichi, 1986). Latex microbeads (4.5 μm, 1 mg, precoated with anti-rat IgG; 110.07/08; Dynal, Lake Success, NY) were suspended in 100 μl of medium 199 (with 0.1% BSA) and incubated for 2 hr with 2 μg of NCD-2 (R & D Systems, Minneapolis, MN) at room temperature and with gentle agitation. The NCD-2-coated beads were then rinsed three times with fresh medium 199 and added to epithelial cultures. Control cultures received beads that had been coated with nonspecific rat IgG. Individual culture wells each received ∼4 × 106 beads in 50 μl of medium.
Immunocytochemical identification of cells and signaling molecules. Epithelial cultures were fixed for 15 min in 4% paraformaldehyde. After thorough rinsing in PBS, nonspecific antibody binding was reduced by incubation for 60–120 min in 2% normal horse serum, 1% BSA, and 0.2% Triton X-100 (in PBS). Primary antibodies were then used to label the following molecules: calretinin (clone Ab-6C, 1:1000; a generous gift from J. H. Rogers, Cambridge University, Cambridge UK), N-cadherin (clone NCD-2, 10 μg/ml; R & D Systems), β-catenin (clone CAT-5H10, 5 μg/ml; Zymed, South San Francisco, CA), focal adhesion kinase (clone 2A7, 10 μg/ml; Upstate Biotechnology, Lake Placid, NY), and phosphotyrosine (clone PT-66, 1:200; Sigma). Primary antibodies were applied overnight at 4°C. The next day, cultures were rinsed five times with PBS and treated with secondary antibodies (anti-mouse, anti-rabbit, or anti-rat IgG, as appropriate). Peroxidase labeling was achieved using biotinylated secondary antibodies and avidin–biotinylated enzyme complex (ABC; Vector Laboratories, Burlingame, CA). Specimens that had been cultured in the NCD-2 (N-cadherin) antibody were processed using a rat-absorbed anti-mouse IgG (Vector). Epifluorescence labeling was performed using secondary antibodies that were conjugated to Cy3 (Amersham Biosciences, Arlington Heights, IL). Specimens were viewed and photographed on a Nikon Diaphot inverted microscope. Photographic images were processed for publication using Adobe Photoshop.
Quantification of cell proliferation. Cells in the S-phase of the cell cycle were labeled by the addition of bromodeoxyuridine (BrdU, 3 μg/ml) to the cultures for the final 4 hr in vitro. Processing for the immunocytochemical labeling of BrdU-labeled cells was performed by following a previously published protocol (Warchol, 1995; Warchol and Corwin, 1996). Most quantification of cell proliferation was performed in regions of known density (between 20 and 120 cells/10,000 μm2). Randomly selected regions of the cultures were viewed with differential interference contrast microscopy (Axiovert 135; Zeiss, Thornwood, NY) and displayed on a video monitor via a Cohu (San Diego, CA) CCD camera. The total number of cells and the number of BrdU-labeled cells in 10,000 μm2 (100 × 100 μm) region were counted, and a proliferation index (defined as the number of BrdU+ cells/total cells) was computed. Typically, three to six measurements of cell proliferation were obtained from each individual culture. All counts were performed “blind,” such that the identity and previous treatment of individual specimens were not revealed until all data had been obtained.
Statistical tests. Unless otherwise noted, all data are expressed as mean ± SEM. Statistical significance of results was determined by use of the two-tailed Student's t test, as implemented in Microsoft Excel (Office 98, Macintosh version).
RESULTS
Morphology of epithelial cultures
Cultures originated from individual pieces of mature sensory epithelium that were ∼200 × 200 μm and had cellular densities of ∼200 cells/10,000 μm2. After 3 d of growth on fibronectin substrates in medium 199 and 10% FBS, newly divided epithelial cells were visible, growing outward from the original explants. After an additional 5 d in defined medium (medium 199 and N2), individual cultures were approximately circular and had diameters of 1500–2000 μm. At this point, the central regions had densities of ∼100–200 cells/10,000 μm2 (Fig.1A). Cell density decreased with increasing distance from the center (Fig.1B), so that the outermost regions of the cultures (which comprised newly produced and proliferating cells) had densities of ∼10 cells/10,000 μm2 (Fig.1C). Cells in all regions expanded to completely fill available space, resulting in a confluent, epithelium-like morphology in which all cells (except those at the outermost edges) were completely contacted by neighboring cells.
Fig. 1.
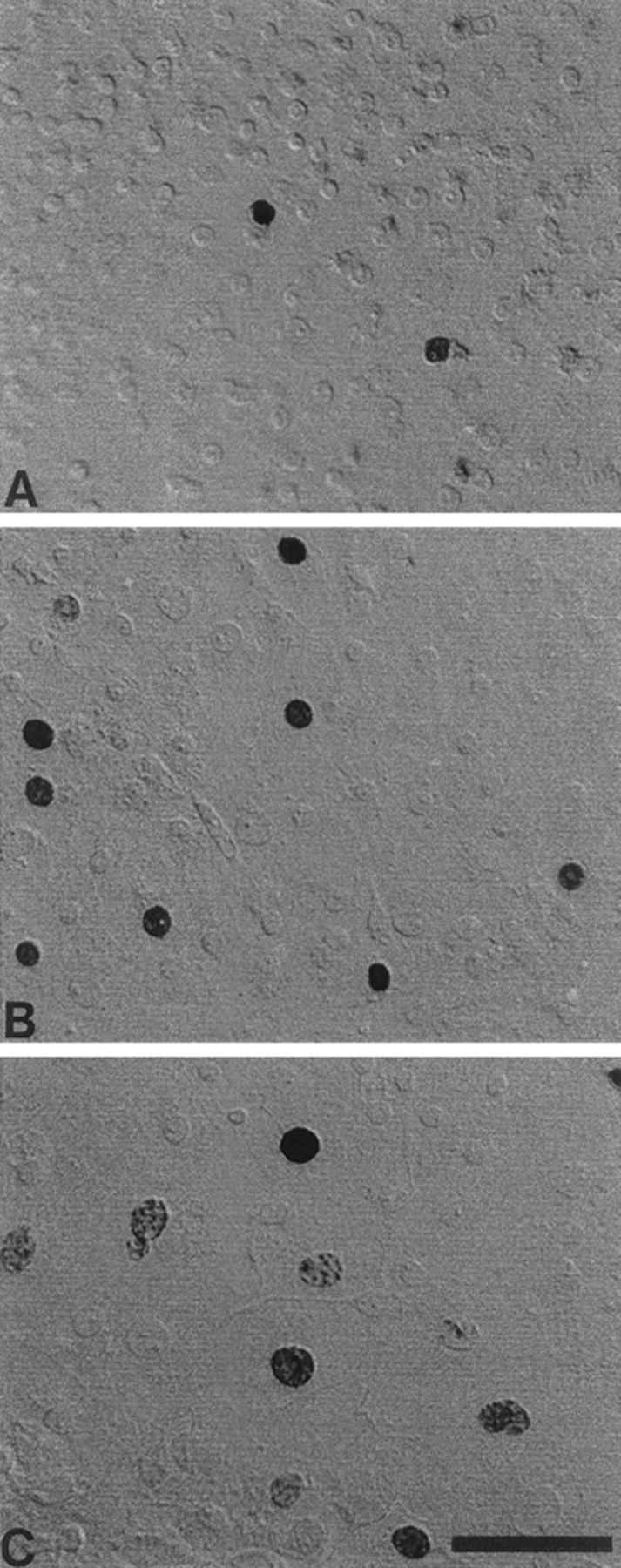
Morphology of supporting cells in selected regions of epithelial cultures. Cultured cells grew as confluent monolayers on fibronectin and laminin substrates. Photographs illustrate the high-density (A), moderate-density (B), and low-density (C) regions of the cultures. Darkly stained BrdU+ nuclei were present in all regions, but the relative numbers of BrdU+ cells were higher in regions of low cell density. Scale bar, 50 μm.
Identification of hair cells in the epithelial cultures
Previous studies have demonstrated that vestibular hair cells can be identified by immunoreactivity to calretinin (Rogers, 1989; Zheng and Gao, 1997). After culture for 8 d (3 d in medium 199 and 10% FBS, followed by 5 d in medium 199 and N2), a subpopulation of epithelial cells were immunoreactive for calretinin. Numerous calretinin-positive cells were observed in the central regions of the cultures. Those cells probably represent surviving hair cells from the original explants. Appreciable numbers of calretinin-labeled cells were also present in the peripheral regions, which were created by cell proliferation while in culture (Fig. 2). In low-density regions (20–40 cells/10,000 μm2), 10.7 ± 0.8% of all cells were calretinin-positive (n = 23 samples from eight specimens), whereas at moderate density (41–80 cells/10,000 μm2), the percentage of calretinin-labeled cells was 11.0 ± 0.7% (n = 39 samples from eight specimens).
Fig. 2.
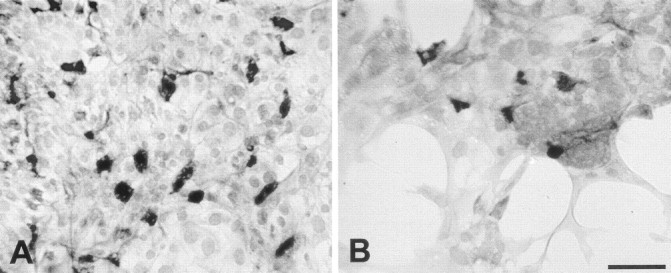
Immunoreactivity for calretinin in high-density (A) and low-density (B) regions of epithelial cultures. Calretinin-positive cells (presumptive hair cells) were present in all regions. Scale bar, 50 μm.
Epithelial cell proliferation is regulated by local cell density
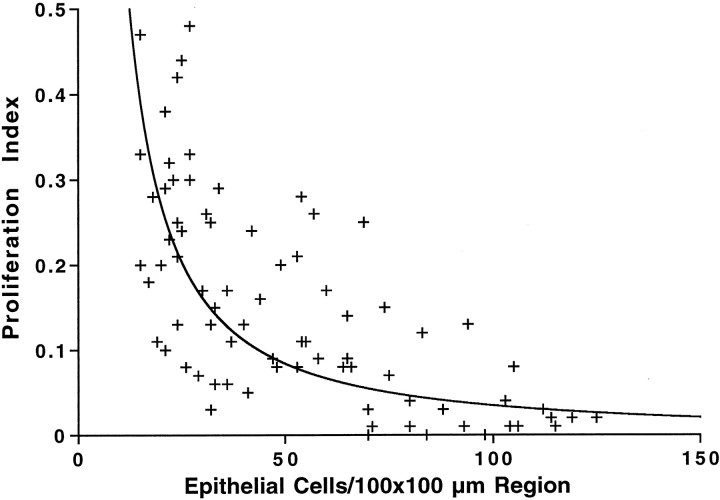
Decreased epithelial cell density was accompanied by an increase in the percentage of cells that were mitotically active. A series of experiments examined the quantitative relationship between local cell density and cell proliferation. Epithelial cultures were maintained for 3 d in medium 199 and 10% FBS, followed by 5 d in medium 199 and N2. The mitotic tracer BrdU was added for the final 4 hr in vitro. After immunohistochemical processing, the total numbers of cells in randomly selected 10,000 μm2(100 × 100 μm) regions of the cultures and the numbers of BrdU-labeled cells in these same regions were quantified (n = 76 sampled regions from 12 cultures). For each region, a proliferation index (BrdU-labeled cells/total cells) was calculated and plotted as a function of cell density (Fig.3). The mean proliferation index at high densities (81–120 cells/10,000 μm2) was 0.03 ± 0.01 (mean ± SEM; n = 15 sampled regions), whereas at moderate densities (41–80 cells/10,000 μm2), the index was 0.12 ± 0.02 (n = 27). At low cell densities (20–40 cells/10,000 μm2), the mean proliferation index was 0.21 ± 0.02 (n = 34). The proliferation index in each of these three regions was significantly different from those of the other two regions (p < 0.005).
Fig. 3.
Plot of proliferation index (BrdU+ cells/total cells in a 100 × 100 μm region) as a function of local cell density. Cultures were maintained in medium 199 and 10% FBS for 3 d, followed by medium 199 and N2 for 5 d, and received BrdU for the final 4 hr in vitro. Although all cells in the sampled regions were maintained in the same environment and were completely contacted by adjoining cells, the relative numbers of proliferating cells decreased with increased cell density.
Phosphotyrosine activity varies with cell density
Phosphorylation of tyrosine residues in certain regulatory molecules is an early signaling event during entry into the cell cycle (Bray, 1998). Immunocytochemical techniques were used to visualize the patterns of phosphotyrosine (pTyr) activity in both rapidly proliferating and more quiescent regions of cultured utricular epithelia. In low-density (high-proliferation) regions, intense pTyr immunoreactivity was observed at most cell–cell junctions (Fig.4A). Rapidly dividing cells at the growing edges of the epithelial explants also showed intense pTyr labeling at points where the cells appeared to contact the fibronectin substrates (Fig. 4B). In contrast, the high-density (low-proliferation) regions of the cultures contained mainly diffuse cytoplasmic pTyr immunoreactivity, with only rare labeling present at cell–cell junctions (Fig. 4C).
Fig. 4.
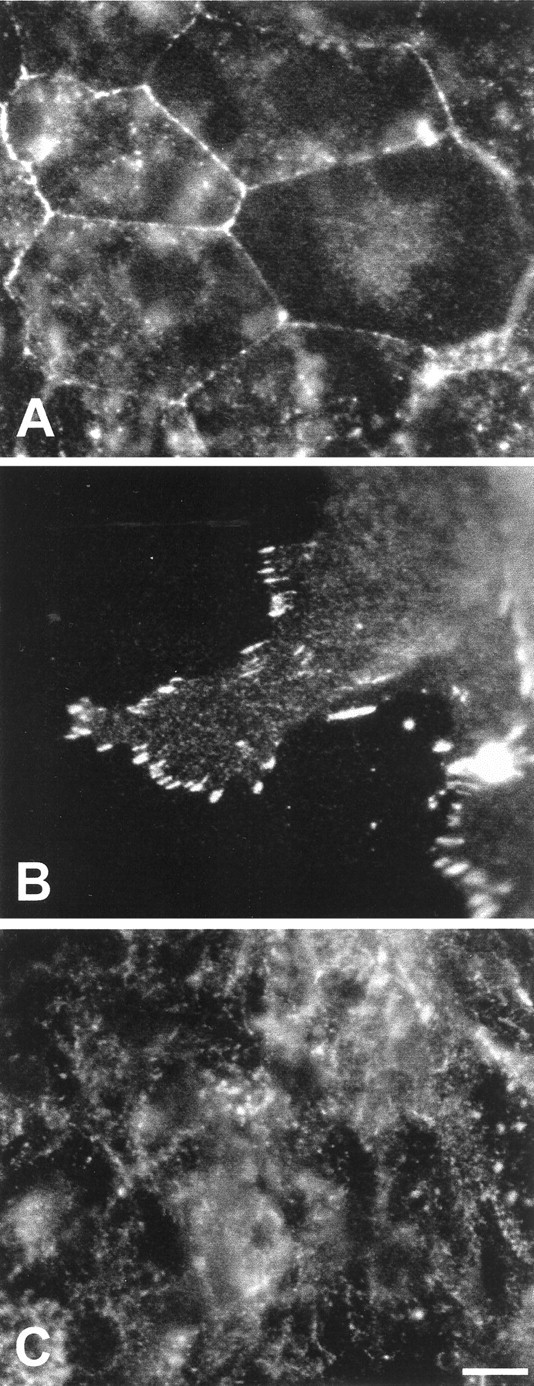
Patterns of pTyr immunoreactivity in the epithelial cultures. The location and intensity of pTyr labeling varied with cell density. In low-density regions of the cultures (where proliferation levels were high), intense pTyr activity was observed at cell–cell junctions (A) and focal contacts between the cells and the substrate (B). In contrast, pTyr labeling in the more quiescent high-density regions of the cultures was mainly diffuse and cytoplasmic (C). Scale bar, 10 μm.
N-cadherin, β-catenin, and focal adhesion kinase are present in cultured epithelia
The previous results indicate the presence of signaling events at cell–cell junctions and focal contacts of proliferating cells and suggest that molecules located at those sites may be involved in the regulation of proliferation. Epithelial cells are linked by molecules of the cadherin family, and N-cadherin mediates cell–cell junctions in the avian cochlea (Raphael et al., 1988). Cadherins are joined to the cytoskeleton via a complex that includes β-catenin (Vleminckx and Kemler, 1999). Signaling at focal contacts is likely to be mediated (at least in part) by focal adhesion kinase (pp125FAK or FAK; Burridge et al., 1992;Kornberg et al., 1992). Monoclonal antibodies were used to localize N-cadherin, β-catenin, and FAK in epithelial explants. Epithelia were cultured on fibronectin substrates for 5 d (3 d in medium 199 and 10% FBS, followed by 2 d in medium 199 and N2). Labeling for N-cadherin and β-catenin was observed at cell–cell junctions throughout the cultures (Fig.5A,B). Immunoreactivity for β-catenin was also present in epithelial cell nuclei, and diffuse labeling was occasionally present in epithelial cell cytoplasm (Fig.5B). Labeling for FAK was confined to points where the cultured epithelial cells appeared to contact the fibronectin substrates and was most apparent at the growing edges of the cultures (Fig. 5C).
Fig. 5.
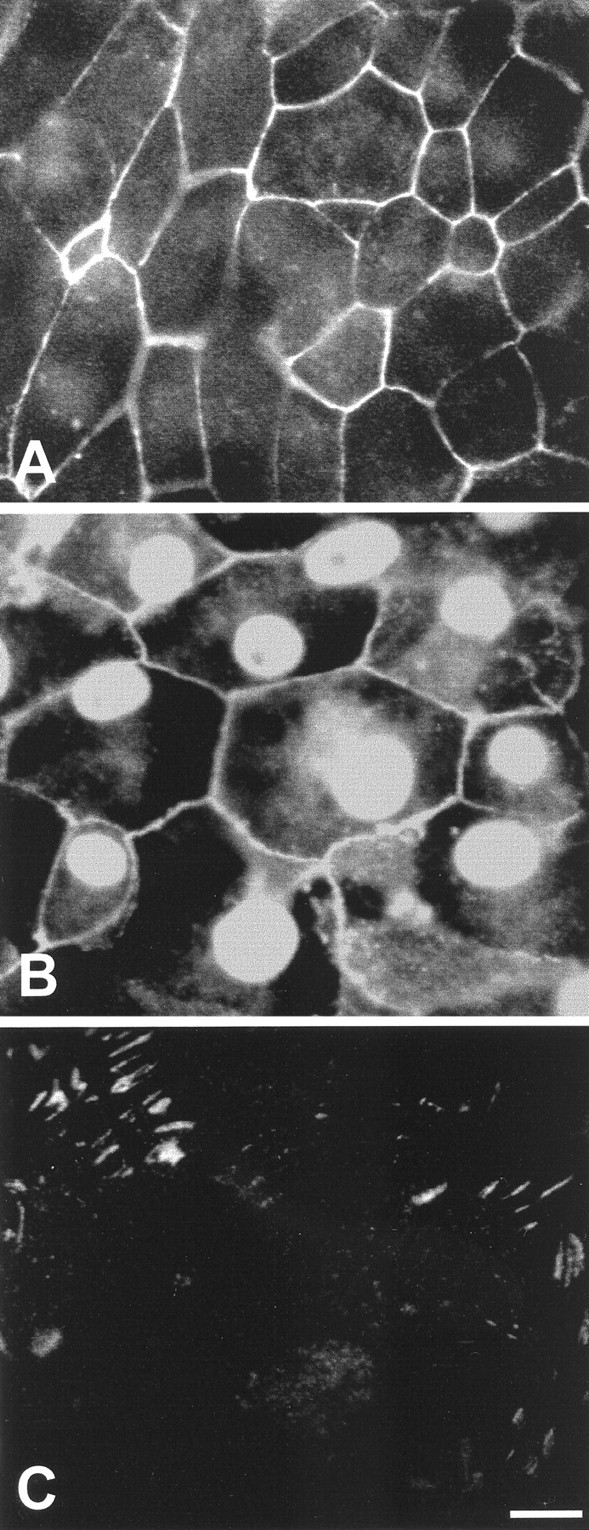
Immunoreactivity for selected signaling molecules in the epithelial cultures. A, N-cadherin immunoreactivity was present at cell–cell junctions. B, β-Catenin immunoreactivity was observed at the cell–cell junction and in cell nuclei. Some cells also contained diffuse cytoplasmic β-catenin immunoreactivity. C, FAK immunoreactivity was observed at points of contact between cells and the fibronectin substrate. Scale bar, 10 μm.
N-cadherin interactions influence the proliferation of epithelial cells
Cadherin-mediated interactions can influence the proliferation of other types of epithelial cells (St. Croix et al., 1998; Levenberg et al., 1999). To examine the role of N-cadherin in regulation of proliferation in sensory epithelia, cultures were treated with a blocking antibody to chicken N-cadherin. Latex beads (4.5 μm diameter) were coated with the NCD-2 antibody, a rat IgG that binds to the extracellular region of N-cadherin and prevents homophilic cadherin binding (Hatta and Takeichi, 1986). Epithelial cultures (n = 10) were incubated with antibody-coated beads for 48 hr. During this time, numerous beads bound to epithelial cells at the growing edges of the epithelial cultures (Fig.6A). Control cultures (n = 10) received equal numbers of microbeads that were coated with nonspecific rat IgG, but those beads did not appear to interact with epithelial cells (Fig. 6B). Proliferating cells were labeled by the addition of BrdU for the final 4 hr in vitro, and the total number of BrdU-labeled cells in each well was counted. Cultures treated with NCD-2-coated beads contained 548 ± 81 BrdU+ cells per well (n = 10) compared with 1226 ± 268 BrdU+ cells per well in control cultures (n = 10; Fig. 6C). Thus, treatment with anti-N-cadherin-coated beads reduced total cell proliferation to ∼45% of control levels (p < 0.05).
Fig. 6.
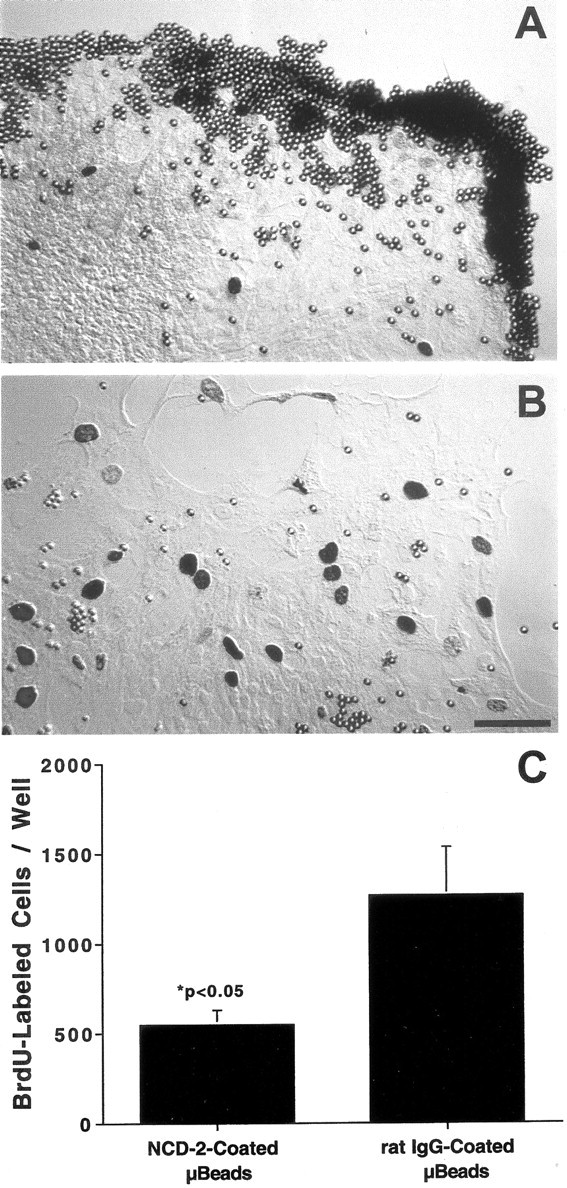
Interfering with the function of N-cadherin inhibited the proliferation of cultured supporting cells. Cultures were incubated with latex microbeads that were coated with anti-N-cadherin (A) or nonspecific rat IgG (B). Beads coated with anti-N-cadherin bound to the growing edges of the epithelial explants, resulting in reduced numbers of proliferating cells (C). Scale bar, 20 μm.
Substrate-bound fibronectin and laminin enhance the growth of epithelial cells
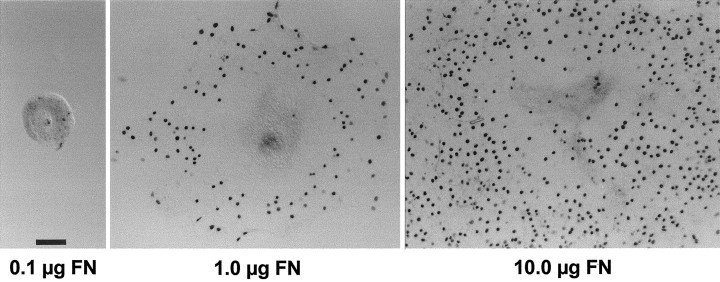
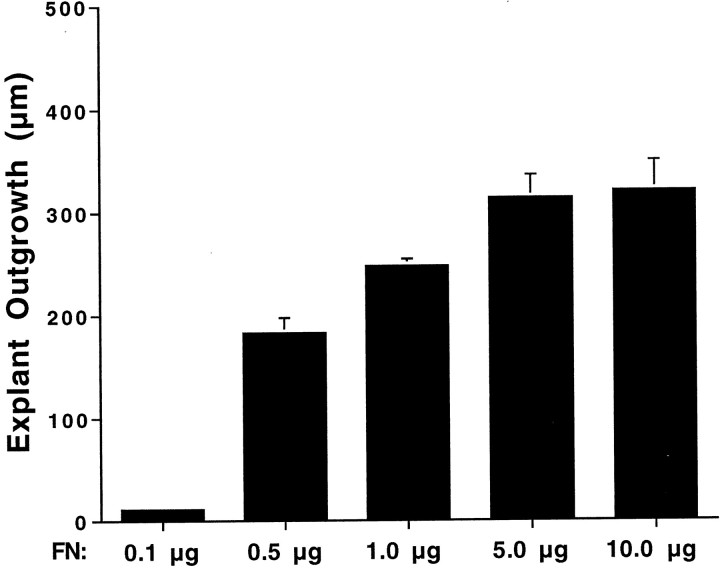
The presence of phosphotyrosine and focal adhesion kinase at points of contact between epithelial cells and the ECM suggests that signaling between cells and the ECM might also influence proliferation. To examine the effects of ECM composition on the growth of epithelial cells, epithelia were cultured on substrates that were prepared with various concentrations of either fibronectin (0.1, 0.2, 0.5, 1.0, 2.5, 5.0, or 10 μg) or laminin (0.1, 1.0, or 10 μg). Cultures were maintained for 3 d in medium 199 with 10% FBS, followed by 2 d in medium 199 and N2. After fixation, cell outgrowth was quantified by measuring the radial extent of the sensory epithelium from four orthogonal directions on each individual explant (Fig.7). Mean outgrowth from epithelia that were cultured on 10 μg of fibronectin was 321 ± 29 μm (n = 24 cultures). Reducing the coating density of fibronectin to 1 μg reduced outgrowth to 77 ± 14% of control values (p < 0.001). Further reduction in the amount of fibronectin (from 1.0 to 0.1 μg) resulted in a nearly complete inhibition of epithelial cell outgrowth and proliferation (Fig. 8). Very similar results were obtained using laminin substrates. Mean epithelial outgrowth on wells coated with 10 μg of laminin was 329 ± 22 μm (n = 12 specimens), and reduction of the coating concentration to 1.0 and 0.1 μg reduced outgrowth to 161 ± 15 and 131 ± 15 μm, respectively (n = 12 and 10 specimens; p < 0.001). The overall morphology of epithelia that were cultured on laminin appeared indistinguishable from that of epithelia that were cultured on fibronectin.
Fig. 7.
Effects of varying the coating density of fibronectin (FN) on the outgrowth of cultured supporting cells. Small pieces of sensory epithelium were plated onto substrates that had been coated with various concentrations of fibronectin. Supporting cell proliferation and outgrowth were strongly influenced by the density of the fibronectin substrate. Darkly stained cell nuclei are BrdU+. Scale bar, 100 μm.
Fig. 8.
Quantification of supporting cell outgrowth from explants after culture for 5 d on various densities of fibronectin. Near-maximal growth was observed on substrates that were coated with 5 μg of bovine fibronectin. A similar relationship between substrate density and supporting cell outgrowth was observed in epithelia cultured on laminin.
Retinoic acid inhibits epithelial cell proliferation but does not promote hair cell differentiation
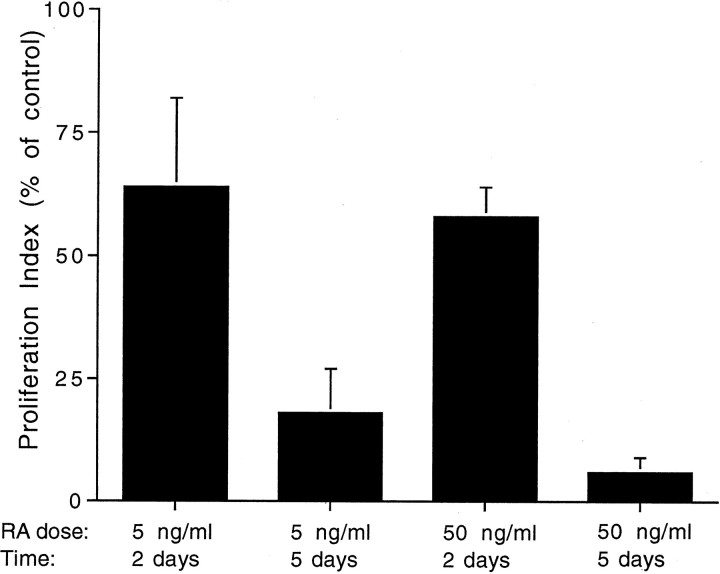
Retinoic acid has been shown to influence the level of cell proliferation in the developing ear (Represa et al., 1990). To determine the effects of retinoic acid on proliferation in mature sensory epithelia, cultures were maintained in defined medium (medium 199 and N2) that contained retinoic acid (5 or 50 ng/ml) for either 2 or 5 d. Proliferating cells were labeled by the addition of BrdU to the culture medium for the final 4 hr in vitro. In all cases, retinoic acid treatment inhibited proliferation (Fig.9). After 5 d of exposure to 5 or 50 ng/ml retinoic acid, cell proliferation was reduced to 18 ± 9 and 6 ± 3% of control levels, respectively (p< 0.001). Additional experiments examined whether retinoic acid promoted hair cell differentiation in the epithelial cultures. Cultures were treated for 5 d with 100 ng/ml retinoic acid or 0.1% DMSO (in medium 199 and N2) and processed for calretinin immunohistochemistry. Calretinin-labeled cells (presumptive hair cells) were counted in randomly selected 100,000 μm2 regions in each culture. Retinoic acid-treated cultures contained 79.7 ± 6.2 calretinin-positive cells/100,000 μm2 (n = 11 samples from seven cultures) versus 76.4 ± 5.6 calretinin-positive cells/100,000 μm2 in control cultures (n = 13 samples from eight cultures).
Fig. 9.
Effects of RA on the proliferation of epithelial supporting cells. Explants were cultured for 2 or 5 d in medium 199 and N2 and all-trans-retinoic acid (5 or 50 ng/ml). Inhibition of proliferation was observed in all RA-treated cultures. Treatment with RA did not, however, lead to increased numbers of calretinin+ hair cells (see Results).
DISCUSSION
Phenotypic identity of proliferating cells
The present data demonstrate that cells in the sensory epithelia of the avian inner ear can sustain considerable levels of proliferation when maintained in culture in defined medium and on identified substrates. The observed proliferation index in the low-density regions of the epithelial cultures (20–40 cells/10,000 μm2) was 0.21 ± 0.02, indicating that ∼20% of the cells in that region were in the S-phase of the cell cycle during the 4 hr BrdU pulse. As in previous studies of cultured inner ear epithelia (Stone et al., 1996; Zheng et al., 1997), the proliferating cells were assumed to be supporting cells. The present study did not address the broader issue of whether all epithelial supporting cells were capable of proliferating. Epithelia in other somatic tissues often contain a specialized class of precursor cells (Slack, 2000). Previous studies have shown morphological evidence for subclasses of supporting cells in the avian cochlea (Oesterle et al., 1992; Fekete et al., 1998), but there is no direct evidence that these subclasses of supporting cells play unique roles in hair cell regeneration. Instead, injury to the avian cochlea results in the expression of early cell cycle proteins in nearly all epithelial supporting cells (Bhave et al., 1995), whereas an undefined fraction of those cells progresses to S-phase (Roberson et al., 1996). Large numbers of proliferating cells are observed within 48 hr of hair cell lesions in the avian utricle (Matsui et al., 2000), and ongoing proliferation in that organ does not appear to be restricted to a subset of epithelial supporting cells (Wilkins et al., 1999). All proliferating cells in the present study appeared to have similar morphologies, and all cells displayed identical immunoreactivity for N-cadherin. It is not known, however, whether all epithelial cells (other than hair cells) form a single phenotypic class and are capable of proliferation.
Regulatory influence of cell density
The observed level of proliferation within the epithelial cultures was inversely related to local cell density. The specific signaling pathways that regulate proliferation are not known, but they might be of two general classes: (1) local mechanical signals and (2) diffusible chemical signals. Studies of other types of cells have demonstrated that mechanical factors, such as changes in cytoskeletal tension or configuration, can activate signal transduction molecules and pathways involved in the early phases of proliferation (Cheng et al., 1996;Chicurel et al., 1998; Huang and Ingber, 1999). Also, the ability of a cell to extend its cytoskeleton appears to be necessary for entry into S-phase (Folkman and Moscona, 1978; Ingber et al., 1995; Iwig et al., 1995; Chen et al., 1997). In the avian cochlea, expansion and spreading of the apical surfaces of supporting cells occur shortly after the loss of hair cells but before the onset of regenerative proliferation (Cotanche and Dopyera, 1990; Raphael, 1993). It is possible that the resulting change in cytoskeletal conformation may activate intracellular signaling molecules that trigger renewed proliferation.
Previous studies have also suggested that regenerative proliferation in the avian ear can be regulated by endogenously produced diffusible factors. Supporting cells in cultures of the avian vestibular organs continue to proliferate in the absence of added mitogens, indicating that those tissues produce whatever mitogens are necessary for proliferation (Warchol and Corwin, 1993; Warchol, 1995). In addition, regenerative proliferation in the avian cochlea is limited to regions within 200 μm of hair cell lesions, which is consistent with the release of a diffusible mitogen from the lesion site (Warchol and Corwin, 1996). It is possible that the relatively low levels of proliferation that were observed in the high-density regions of epithelial cultures (Fig. 3) resulted from competition between cells for a locally released mitogen. However, the observed relationship between cell density and proliferation might also indicate that each epithelial cell releases a diffusible inhibitor of proliferation. In that case, high concentrations of the inhibitor would accumulate in high-density regions, resulting in local inhibition of proliferation. A previous study has reported evidence for the release of an inhibitory substance from the avian utricle (Tsue et al., 1994). Also, retinoic acid has been shown to be present in the vestibular maculae of birds (Kelley et al., 1993), and the present results show that retinoic acid inhibits proliferation in the epithelial cultures. Future experiments that use primary cultures of epithelial supporting cells should be able to distinguish between these possibilities.
Phosphotyrosine signaling in cultured epithelial cells
Immunoreactivity for pTyr activity was present at cell–cell junctions in the lower-density (high-proliferation) regions of the epithelial cultures. Because those regions display high levels of proliferation (Fig. 3), junctional pTyr activity appears to be correlated with local cell proliferation. Very similar results have been reported from studies of cultured endothelial cells (Lampugnani et al., 1997). Phosphotyrosine activity was also observed at points of contact between epithelial cells and the fibronectin substrate, and additional antibody labeling revealed the presence of pp125FAK at focal adhesions. The involvement of FAK in regulating cell proliferation is well established (Zhao et al., 1998), and mechanically induced cell spreading stimulates phosphorylation of FAK (Hamasaki et al., 1995; Tang et al., 1999) and other tyrosine kinases (Sadoshima et al., 1996). Transcripts for FAK are also present in the mammalian utricle (Corwin et al., 2000). The observation that both fibronectin and laminin stimulate the growth of epithelial cells is consistent with the suggestion that signaling at the focal adhesion complex can regulate supporting cell proliferation.
Role of N-cadherin and β-catenin in the regulation of proliferation
Additional results support the hypothesis that signaling at cell–cell junctions can influence proliferation in epithelial cultures. The presence of N-cadherin was detected at cell–cell junctions in the epithelial cultures, and the binding of microbeads coated with anti-N-cadherin inhibited epithelial cell proliferation. Those findings suggest that some form of contact inhibition (Dulbecco and Stoker, 1970; Gradl et al., 1995) may play a role in regulating the proliferation of epithelial cells. Neutralization of cadherins has been shown to mimic contact inhibition and to block the proliferation of other types of epithelial cells (St. Croix et al., 1998; Levenberg et al., 1999). The precise mechanism by which N-cadherin regulates cell proliferation in the ear is not known, but the loss of hair cells will break N-cadherin-mediated bonds at adherins junctions, and this may be an early signal of epithelial injury (Corwin and Warchol, 1991). The observation that β-catenin is localized at cell–cell junctions and in the cytoplasm of epithelial cells may also indicate a role for that molecule in the regulation of proliferation. Previous data have shown that β-catenin (armadillo) is a key messenger in the wnt signaling pathway (Peifer and Polakis, 2000). Breakage of adherins junctions will release β-catenin into the cytoplasm. Under such conditions, β-catenin can enter cell nuclei and associate with the lymphocyte enhancer factor-1 transcription factor, leading to the expression of cyclin D1 (Shtutman et al., 1999).
Influence of extracellular matrix molecules
The demonstration that the cultured epithelia can attach and grow on fibronectin and laminin substrates indicates that vestibular epithelial cells can express the correct integrin receptors for those ECM ligands. In addition, increasing the coating density of fibronectin and laminin strongly increased the growth of the cultured cells. The extent of growth enhancement in response to increased ECM density exceeds previously reported growth increases after application of mitogenic growth factors (Lambert, 1994; Yamashita and Oesterle, 1995;Gu et al., 1996; Oesterle et al., 1997; Zheng et al., 1997; Warchol, 1999). These results suggest that the composition of the ECM may be an important regulator of regeneration in the ear. Numerous studies of other cell types have suggested that the composition of the ECM can regulate cell proliferation, differentiation, and survival (Bitterman et al., 1983; Mooney et al., 1992; Meredith et al., 1993;Lukashev and Werb, 1998). Changes in ECM composition are also an early feature of epidermal wound healing (Gailit and Clark, 1994; Martin, 1997). Although injury-evoked changes in the ECM of the avian inner ear have not yet been extensively studied (Cotanche et al., 1996), developmental changes in ECM composition have been documented (Hemond and Morest, 1991; Woolf et al., 1992; Cosgrove and Rodgers, 1997). In particular, fibronectin is present below the sensory epithelium of the avian and mammalian cochlea (Richardson et al., 1987; Santi et al., 1989). Injury-evoked changes in the ECM of inner ear sensory organs might occur if either epithelial cells or cells below the sensory epithelium were to secrete particular ECM components in response to the loss of hair cells. The ECM may also serve as a reservoir for mitogens, which could be released after hair cell injury. Finally, modification of the ECM might be performed by macrophages. Recent studies have shown that macrophages are recruited to sites of hair cell lesions in the avian cochlea and vestibular organs, but their contribution to the process of regeneration is not clear (Warchol, 1997; Bhave et al., 1998, 1999). Injury to other epithelial structures can result in the local secretion of fibronectin by activated macrophages (Brown et al., 1993) as well as proteases that modify the structure of the ECM (Shapiro, 1998).
Regulatory role of retinoic acid
The present data show that retinoic acid (RA) can inhibit the proliferation of vestibular supporting cells. Although RA and its binding proteins serve diverse roles during embryonic development, RA generally acts to inhibit cell proliferation and to promote differentiation (Maden, 2001). Notably, application of exogenous RA stimulates the production of supernumerary hair cells in the developing mammalian cochlea (Kelley et al., 1993). Retinoic acid and cellular retinol-binding protein I (CRBP I) are present in the mature vestibular organs of birds and mammals but not in the mature mammalian cochlea (Kelley et al., 1993; Ylikoski et al., 1994). Thus, the presence of RA and CRBP I correlates with the ability to regenerate hair cells. Supporting cells in the undamaged utricle proliferate at a moderate rate (Jørgensen and Mathiesen, 1988; Roberson et al., 1992; Kil et al., 1997), and the present data raise the possibility that endogenous RA may act as a negative regulator of supporting cell proliferation. In addition, the observation that application of RA did not increase the numbers of calretinin-positive cells in the epithelial cultures suggests that RA alone is not sufficient to induce mature supporting cells to differentiate as hair cells.
Summary
The results presented here indicate that a key determinant of supporting cell proliferation is local cell density. Low- and moderate-density regions of epithelial cultures had differing mean levels of proliferation, although those regions contained approximately equal numbers of surviving hair cells (∼10%). These data are consistent with the notion that cell shape and cytoskeletal conformation influence cell proliferation (Huang and Ingber, 1999) or that epithelial cells secrete a diffusible regulator of proliferation. Contact-mediated cell–cell interactions via the adhesion molecule N-cadherin appear to regulate epithelial cell proliferation. Breakage and reformation of those junctions after hair cell injury may be an early trigger for regenerative proliferation. The data also suggest that cell–matrix interactions and the composition of the ECM are important influences on the proliferation of supporting cells, but it is unclear how molecules at focal adhesions interact with molecules at adherins junctions. Future studies should determine how signaling events at cell–cell and cell–matrix junctions regulate sensory regeneration in the inner ears of birds and mammals.
Footnotes
This work was supported by Grants DC00291 and DC03576 from the National Institute on Deafness and Other Communicative Disorders, National Institutes of Health. I thank J. H. Rogers for providing the calretinin antibody, Jaci Lett for assistance with preparation of the figures, and J. Matsui and two reviewers for valuable comments on this manuscript.
Correspondence should be addressed to Mark E. Warchol, Central Institute for the Deaf, 4560 Clayton Road, St. Louis, MO 63110-1549. E-mail: mwarchol@cid.wustl.edu.
REFERENCES
- 1.Aplin AE, Howe AK, Juliano RL. Cell adhesion molecules, signal transduction, and cell growth. Curr Opin Cell Biol. 1999;11:737–744. doi: 10.1016/s0955-0674(99)00045-9. [DOI] [PubMed] [Google Scholar]
- 2.Baird RA, Steyger PS, Shuff NR. Mitotic and nonmitotic mechanisms of hair cell regeneration in the bullfrog. Ann NY Acad Sci. 1996;781:59–70. doi: 10.1111/j.1749-6632.1996.tb15693.x. [DOI] [PubMed] [Google Scholar]
- 3.Balak KJ, Corwin JT, Jones JE. Regenerated hair cells can originate from supporting cell progeny: evidence from phototoxicity and laser ablation experiments in the lateral line system. J Neurosci. 1990;10:2502–2512. doi: 10.1523/JNEUROSCI.10-08-02502.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhave SA, Stone JS, Rubel EW, Coltrera MD. Cell cycle progression in gentamicin-damaged avian cochleas. J Neurosci. 1995;15:4618–4628. doi: 10.1523/JNEUROSCI.15-06-04618.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhave SA, Oesterle EC, Coltrera MD. Macrophage and microglia-like cells in the avian inner ear. J Comp Neurol. 1998;398:241–256. doi: 10.1002/(sici)1096-9861(19980824)398:2<241::aid-cne6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Bitterman PB, Rennard SI, Adelberg S, Crystal RG. Role of fibronectin as a growth factor for fibroblasts. J Cell Biol. 1983;97:1925–1932. doi: 10.1083/jcb.97.6.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bottenstein JE, Sato GH. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci USA. 1979;76:514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bray D. Signaling complexes: biophysical constraints on intracellular communication. Annu Rev Biophys Biomol Struct. 1998;27:59–75. doi: 10.1146/annurev.biophys.27.1.59. [DOI] [PubMed] [Google Scholar]
- 9.Brown LF, Dubi D, Lavigne L, Logan B, Dvorak HF, Van De Water L. Macrophages and fibroblasts express embryonic fibronectins during wound healing. Am J Pathol. 1993;142:793–801. [PMC free article] [PubMed] [Google Scholar]
- 10.Burridge K, Turner CE, Romer LH. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to the extracellular matrix. J Cell Biol. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 12.Cheng GC, Libby P, Grodzinsky AJ, Lee RT. Induction of DNA synthesis by a single transient mechanical stimulus of human vascular muscle cells. Circulation. 1996;93:99–105. doi: 10.1161/01.cir.93.1.99. [DOI] [PubMed] [Google Scholar]
- 13.Chicurel ME, Chen CS, Ingber DE. Cellular control lies in the balance of forces. Curr Opin Cell Biol. 1998;10:232–239. doi: 10.1016/s0955-0674(98)80145-2. [DOI] [PubMed] [Google Scholar]
- 14.Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1772–1774. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- 15.Corwin JT, Oberholtzer JC. Fish n' chicks: model recipes for hair cell regeneration? Neuron. 1997;19:951–954. doi: 10.1016/s0896-6273(00)80386-4. [DOI] [PubMed] [Google Scholar]
- 16.Corwin JT, Warchol ME. Auditory hair cells: structure, function, development, and regeneration. Annu Rev Neurosci. 1991;14:301–333. doi: 10.1146/annurev.ne.14.030191.001505. [DOI] [PubMed] [Google Scholar]
- 17.Corwin JT, Finley JE, Safer L, Gu R, Cunningham LR, Xia B, Warchol ME. Isolation of pure living hair cell epithelia by use of thermolysin. Assoc Res Otolaryngol Abstr. 1995;18:345. [Google Scholar]
- 18.Corwin JT, Montcouquil M, Shi Q, Karaoli T, Karimi K, Whitley M, Byrne Molecular changes associated with the loss of proliferation in mammalian hair cell epithelia. Assoc Res Otolaryngol Abstr. 2000;23:272. [Google Scholar]
- 19.Cosgrove D, Rodgers KD. Expression of the major basement membrane-associated proteins during postnatal development in the murine cochlea. Hear Res. 1997;105:159–170. doi: 10.1016/s0378-5955(96)00203-1. [DOI] [PubMed] [Google Scholar]
- 20.Cotanche DA. Regeneration of hair cell stereociliary bundles in the chick cochlea following severe acoustic trauma. Hear Res. 1987;30:181–196. doi: 10.1016/0378-5955(87)90135-3. [DOI] [PubMed] [Google Scholar]
- 21.Cotanche DA. Structural recovery from sound and aminoglycoside damage in the avian cochlea. Audiol Neurootol. 1999;4:271–285. doi: 10.1159/000013852. [DOI] [PubMed] [Google Scholar]
- 22.Cotanche DA, Dopyera CEJ. Hair cell and supporting cell response to acoustic trauma in the chick cochlea. Hear Res. 1990;46:29–40. doi: 10.1016/0378-5955(90)90137-e. [DOI] [PubMed] [Google Scholar]
- 23.Cotanche DA, Messana EP, Riedl A. Hyaline cell migration into the basilar papilla involves the secretion of a new layer of extracellular matrix. Assoc Res Otolaryngol Abstr. 1996;19:796. [Google Scholar]
- 24.Cruz RM, Lambert PR, Rubel EW. Light microscopic evidence of hair cell regeneration after gentamicin toxicity in chick cochlea. Arch Otolaryngol Head Neck Surg. 1987;113:1058–1062. doi: 10.1001/archotol.1987.01860100036017. [DOI] [PubMed] [Google Scholar]
- 25.Dulbecco R, Stoker MGP. Conditions determining initiation of DNA synthesis in 3T3 cells. Proc Natl Acad Sci USA. 1970;66:204–210. doi: 10.1073/pnas.66.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fekete DM, Muthukumar S, Karagogeos D. Hair cells and supporting cells share a common progenitor in the avian inner ear. J Neurosci. 1998;18:7811–7821. doi: 10.1523/JNEUROSCI.18-19-07811.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;273:345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- 28.Forge A, Li L, Corwin JT, Nevill G. Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science. 1993;259:1616–1619. doi: 10.1126/science.8456284. [DOI] [PubMed] [Google Scholar]
- 29.Forge A, Kin L, Nevill G. Hair cell recovery in the vestibular sensory epithelia of mature guinea pigs. J Comp Neurol. 1998;397:69–88. [PubMed] [Google Scholar]
- 30.Gailit J, Clark RAF. Wound repair in the context of extracellular matrix. Curr Opin Cell Biol. 1994;6:717–725. doi: 10.1016/0955-0674(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 31.Germain L, Rouabhia M, Guignard R, Carrier L, Bouvard V, Auger FA. Improvement of human keratinocyte isolation and culture using thermolysin. Burns. 1993;19:99–104. doi: 10.1016/0305-4179(93)90028-7. [DOI] [PubMed] [Google Scholar]
- 32.Gradl G, Faust D, Oesch F, Wieser RJ. Density-dependent regulation of cell growth by contactinhibin and the contactinhibin receptor. Curr Biol. 1995;5:526–535. doi: 10.1016/s0960-9822(95)00105-9. [DOI] [PubMed] [Google Scholar]
- 33.Gu R, Marchionni M, Corwin JT. Glial growth factor enhances supporting cell proliferation in rodent vestibular epithelia cultured in isolation. Soc Neurosci Abstr. 1996;22:520. [Google Scholar]
- 34.Hamasaki K, Mimura T, Furuya H, Morino N, Yamazaki T, Komuro I, Yazaki Y, Nojima Y. Stretching mesangial cells stimulates tyrosine phosphorylation of focal adhesion kinase pp125FAK. Biochem Biophys Res Commun. 1995;212:544–549. doi: 10.1006/bbrc.1995.2004. [DOI] [PubMed] [Google Scholar]
- 35.Hatta K, Takeichi M. Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature. 1986;320:447–449. doi: 10.1038/320447a0. [DOI] [PubMed] [Google Scholar]
- 36.Huang S, Ingber DE. The structural and mechanical complexity of cell-growth control. Nat Cell Biol. 1999;1:E131–E138. doi: 10.1038/13043. [DOI] [PubMed] [Google Scholar]
- 37.Hemond SG, Morest DK. Formation of the cochlea in the chicken embryo: sequence of innervation and localization of basal lamina-associated molecules. Dev Brain Res. 1991;61:87–96. doi: 10.1016/0165-3806(91)90117-2. [DOI] [PubMed] [Google Scholar]
- 38.Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- 39.Ingber DE, Folkman J. Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: role of extracellular matrix. J Cell Biol. 1989;109:317–330. doi: 10.1083/jcb.109.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ingber DE, Prusty D, Sun Z, Betensky H, Wang H. Cell shape, cytoskeletal mechanics, and cell cycle control in angiogenesis. J Biomech. 1995;28:1471–1484. doi: 10.1016/0021-9290(95)00095-x. [DOI] [PubMed] [Google Scholar]
- 41.Iwig M, Czeslick E, Muller A, Gruner M, Spindler M, Glaesser D. Growth regulation by cell shape alteration and organization of the cytoskeleton. Eur J Cell Biol. 1995;67:145–157. [PubMed] [Google Scholar]
- 42.Jørgensen JM. Number and distribution of hair cells in the utricular macula of some birds. J Morphol. 1989;201:187–204. doi: 10.1002/jmor.1052010208. [DOI] [PubMed] [Google Scholar]
- 43.Jørgensen JM, Mathiesen C. The avian inner ear: continuous production of hair cells in the vestibular sensory organs but not in the auditory papilla. Naturwissenschaften. 1988;75:319–320. doi: 10.1007/BF00367330. [DOI] [PubMed] [Google Scholar]
- 44.Kelley MW, Xu X-M, Wagner MA, Warchol ME, Corwin JT. The developing organ of Corti contains retinoic acid and forms supernumerary hair cells in response to exogenous retinoic acid in culture. Development. 1993;119:1041–1053. doi: 10.1242/dev.119.4.1041. [DOI] [PubMed] [Google Scholar]
- 45.Kil J, Warchol ME, Corwin JT. Cell death, cell proliferation, and estimates of hair cell life spans in the vestibular organs of chicks. Hear Res. 1997;114:117–126. doi: 10.1016/s0378-5955(97)00166-4. [DOI] [PubMed] [Google Scholar]
- 46.Kornberg L, Earp HS, Parsons JT, Schaller M, Juliano RL. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem. 1992;267:23439–23442. [PubMed] [Google Scholar]
- 47.Kuntz AL, Oesterle EC. Transforming growth factor-α with insulin stimulates cell proliferation in vivo in adult rat vestibular sensory epithelium. J Comp Neurol. 1998;399:413–423. [PubMed] [Google Scholar]
- 48.Lambert PR. Inner ear regeneration in a mammal: identification of a triggering factor. Laryngoscope. 1994;104:701–718. doi: 10.1288/00005537-199406000-00010. [DOI] [PubMed] [Google Scholar]
- 49.Lampugnani MG, Corada M, Andriopoulou P, Esser S, Risau W, Dejana E. Cell confluence regulates tyrosine phosphorylation of adherins junction components in endothelial cells. J Cell Sci. 1997;110:2065–2077. doi: 10.1242/jcs.110.17.2065. [DOI] [PubMed] [Google Scholar]
- 50.Levenberg S, Yarden A, Kam Z, Geiger B. p27 is involved in N-cadherin-mediated contact inhibition of cell growth and S-phase entry. Oncogene. 1999;18:869–876. doi: 10.1038/sj.onc.1202396. [DOI] [PubMed] [Google Scholar]
- 51.Lukashev ME, Werb Z. ECM signaling: orchestrating cell behavior and misbehavior. Trends Cell Biol. 1998;8:437–441. doi: 10.1016/s0962-8924(98)01362-2. [DOI] [PubMed] [Google Scholar]
- 52.Maden M. Role and distribution of retinoic acid during CNS development. Int Rev Cytol. 2001;209:1–77. doi: 10.1016/s0074-7696(01)09010-6. [DOI] [PubMed] [Google Scholar]
- 53.Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 54.Matsui JI, Oesterle EC, Stone JS, Rubel EW. Characterization of damage and regeneration in cultured avian utricles. J Assoc Res Otolaryngol. 2000;1:46–63. doi: 10.1007/s101620010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meredith JE, Fazeli B, Schwartz MA. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993;4:953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montcouquiol M, Corwin JT. Brief treatments with forskolin enhance S-phase entry in balance epithelia from the ears of rats. J Neurosci. 2001;21:974–982. doi: 10.1523/JNEUROSCI.21-03-00974.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mooney D, Hansen L, Vacanti J, Langer R, Farmer S, Ingber D. Switching from differentiation to growth in hepatocytes: control by extracellular matrix. J Cell Physiol. 1992;151:497–505. doi: 10.1002/jcp.1041510308. [DOI] [PubMed] [Google Scholar]
- 58.Navaratnam DS, Su HS, Scott S-P, Oberholtzer JC. Proliferation in the auditory receptor epithelium mediated by cyclic AMP-dependent signaling pathway. Nat Med. 1996;2:1136–1139. doi: 10.1038/nm1096-1136. [DOI] [PubMed] [Google Scholar]
- 59.Oesterle EC, Cunningham DE, Rubel EW. Ultrastructure of hyaline, border, and vacuole cells in chick inner ear. J Comp Neurol. 1992;318:64–82. doi: 10.1002/cne.903180105. [DOI] [PubMed] [Google Scholar]
- 60.Oesterle EC, Tsue TT, Rubel EW. Induction of cell proliferation in avian inner ear sensory epithelia by insulin-like growth factor 1 and insulin. J Comp Neurol. 1997;380:262–274. doi: 10.1002/(sici)1096-9861(19970407)380:2<262::aid-cne8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 61.Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 62.Raphael Y. Reorganization of the chick basilar papilla after acoustic trauma. J Comp Neurol. 1993;330:521–532. doi: 10.1002/cne.903300408. [DOI] [PubMed] [Google Scholar]
- 63.Raphael Y, Volk T, Crossin KL, Edelman GM, Geiger B. The modulation of cell adhesion molecule expression and intercellular junction formation in the developing avian inner ear. Dev Biol. 1988;128:222–235. doi: 10.1016/0012-1606(88)90284-9. [DOI] [PubMed] [Google Scholar]
- 64.Represa J, Sanchez A, Miner C, Lewis J, Giraldez F. Retinoic acid modulation of the early development of the inner ear is associated with the control of c-fos activation. Development. 1990;110:1081–1090. doi: 10.1242/dev.110.4.1081. [DOI] [PubMed] [Google Scholar]
- 65.Richardson GP, Crossin KL, Chuong C-M, Edelman GM. Expression of cell adhesion molecules during embryonic induction. III. Development of the otic placode. Dev Biol. 1987;119:217–230. doi: 10.1016/0012-1606(87)90223-5. [DOI] [PubMed] [Google Scholar]
- 66.Roberson DW, Rubel EW. Cell division in the gerbil cochlea after acoustic trauma. Am J Otolaryngol. 1994;15:28–34. [PubMed] [Google Scholar]
- 67.Roberson DW, Weisleder P, Bohrer P, Rubel EW. Ongoing production of sensory cells in the vestibular epithelium. Hear Res. 1992;57:166–174. doi: 10.1016/0378-5955(92)90149-h. [DOI] [PubMed] [Google Scholar]
- 68.Roberson DW, Kreig CS, Rubel EW. Light microscopic evidence that direct transdifferentiation gives rise to new hair cells in regenerating avian auditory epithelium. Aud Neurosci. 1996;2:196–205. doi: 10.1002/jnr.20271. [DOI] [PubMed] [Google Scholar]
- 69.Rogers JH. Two calcium-binding proteins mark many chick sensory neurons. Neuroscience. 1989;31:697–709. doi: 10.1016/0306-4522(89)90434-x. [DOI] [PubMed] [Google Scholar]
- 70.Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240:1774–1776. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- 71.Sadoshima J, Qiu Z, Morgan JP, Izumo S. Tyrosine kinase activation is an immediate and essential step in hypotonic cell swelling-induced ERK activation and c-fos gene expression in cardiac myocytes. EMBO J. 1996;15:5535–5546. [PMC free article] [PubMed] [Google Scholar]
- 72.Santi PA, Larson JT, Furcht LT, Economou TS. Immunological localization of fibronectin in the chinchilla cochlea. Hear Res. 1989;39:91–102. doi: 10.1016/0378-5955(89)90084-1. [DOI] [PubMed] [Google Scholar]
- 73.Shapiro SD. Matrix metalloproteinase degradation of extracellular matrix: biological consequences. Curr Opin Cell Biol. 1998;10:602–608. doi: 10.1016/s0955-0674(98)80035-5. [DOI] [PubMed] [Google Scholar]
- 74.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the β-catenin/LEF-1 pathway. Proc Natl Acad Sci USA. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Slack JMW. Stem cells in epithelial tissues. Science. 2000;287:1431–1433. doi: 10.1126/science.287.5457.1431. [DOI] [PubMed] [Google Scholar]
- 76.St. Croix B, Sheehan C, Rak JW, Flørenes VA, Slingerland JM, Kerbel RS. E-cadherin-dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p27KIP1. J Cell Biol. 1998;142:557–571. doi: 10.1083/jcb.142.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stone JS, Cotanche DA. Identification of the timing of S phase and the patterns of cell proliferation during hair cell regeneration in the chick cochlea. J Comp Neurol. 1994;341:50–67. doi: 10.1002/cne.903410106. [DOI] [PubMed] [Google Scholar]
- 78.Stone JS, Leano SG, Baker LP, Rubel EW. Hair cell differentiation in chick cochlear epithelium after aminoglycoside toxicity: in vivo and in vitro observations. J Neurosci. 1996;16:6157–6174. doi: 10.1523/JNEUROSCI.16-19-06157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stone JS, Oesterle EC, Rubel EW. Recent insights into regeneration of auditory and vestibular hair cells. Curr Opin Neurol. 1998;11:17–24. doi: 10.1097/00019052-199802000-00004. [DOI] [PubMed] [Google Scholar]
- 80.Stone JS, Choi Y-S, Woolley SMN, Yamashita H, Rubel EW. Progenitor cell cycling during hair cell regeneration in the vestibular and auditory epithelia of the chick. J Neurocytol. 1999;28:863–876. doi: 10.1023/a:1007022205821. [DOI] [PubMed] [Google Scholar]
- 81.Tang D, Mehta D, Gunst SJ. Mechanosensitive tyrosine phosphorylation of paxillin and focal adhesion kinase in tracheal smooth muscle. Am J Physiol. 1999;276:C250–C258. doi: 10.1152/ajpcell.1999.276.1.C250. [DOI] [PubMed] [Google Scholar]
- 82.Tsue TT, Oesterle EC, Rubel EW. Diffusible factors regulate hair cell regeneration in the avian inner ear. Proc Natl Acad Sci USA. 1994;91:1584–1588. doi: 10.1073/pnas.91.4.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vleminckx K, Kemler R. Cadherins and tissue formation: integrating adhesion and signaling. BioEssays. 1999;21:211–220. doi: 10.1002/(SICI)1521-1878(199903)21:3<211::AID-BIES5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 84.Warchol ME. Supporting cells in isolated sensory epithelia of avian utricles proliferate in serum-free culture. NeuroReport. 1995;6:981–984. doi: 10.1097/00001756-199505090-00008. [DOI] [PubMed] [Google Scholar]
- 85.Warchol ME. Macrophage activity in organ cultures of the avian cochlea: demonstration of a resident population and recruitment to sites of hair cell lesions. J Neurobiol. 1997;33:724–734. [PubMed] [Google Scholar]
- 86.Warchol ME. Immune cytokines and dexamethasone influence sensory regeneration in the avian vestibular periphery. J Neurocytol. 1999;28:889–900. doi: 10.1023/a:1007026306730. [DOI] [PubMed] [Google Scholar]
- 87.Warchol ME, Corwin JT. Supporting cells in avian vestibular organs proliferate in serum-free culture. Hear Res. 1993;71:28–36. doi: 10.1016/0378-5955(93)90018-v. [DOI] [PubMed] [Google Scholar]
- 88.Warchol ME, Corwin JT. Regenerative proliferation in organ cultures of the avian cochlea: identification of the initial progenitors and determination of the latency of the proliferative response. J Neurosci. 1996;16:5466–5477. doi: 10.1523/JNEUROSCI.16-17-05466.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Warchol ME, Lambert PR, Goldstein BJ, Forge A, Corwin JT. Regenerative proliferation in inner ear sensory epithelia of adult guinea pigs and humans. Science. 1993;259:1619–1622. doi: 10.1126/science.8456285. [DOI] [PubMed] [Google Scholar]
- 90.Weisleder P, Rubel EW. Hair cell regeneration after streptomycin toxicity in the avian vestibular epithelium. J Comp Neurol. 1993;331:97–110. doi: 10.1002/cne.903310106. [DOI] [PubMed] [Google Scholar]
- 91.Wilkins HR, Presson JC, Popper AN. Proliferation of vertebrate inner ear supporting cells. J Neurobiol. 1999;39:527–535. doi: 10.1002/(sici)1097-4695(19990615)39:4<527::aid-neu6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 92.Woolf NK, Koehrn FJ, Ryan AF. Immunohistochemical localization of fibronectin-like protein in the inner ear of the developing gerbil and rat. Dev Brain Res. 1992;65:21–33. doi: 10.1016/0165-3806(92)90004-g. [DOI] [PubMed] [Google Scholar]
- 93.Yamashita H, Oesterle EC. Induction of cell proliferation in mammalian inner-ear sensory epithelia by transforming growth factor-alpha and epidermal growth factor. Proc Natl Acad Sci USA. 1995;92:3152–3155. doi: 10.1073/pnas.92.8.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ylikoski J, Pirvola U, Eriksson U. Cellular retinol-binding protein type I is prominently and differentially expressed in the sensory epithelium of the rat cochlea and vestibular organs. J Comp Neurol. 1994;349:596–602. doi: 10.1002/cne.903490407. [DOI] [PubMed] [Google Scholar]
- 95.Zhao JH, Reiske H, Guan JL. Regulation of the cell cycle by focal adhesion kinase. J Cell Biol. 1998;143:1997–2008. doi: 10.1083/jcb.143.7.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zheng JL, Gao W-Q. Analysis of rat vestibular hair cell development and regeneration using calretinin as an early marker. J Neurosci. 1997;17:8270–8282. doi: 10.1523/JNEUROSCI.17-21-08270.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zheng JL, Helbig C, Gao W-Q. Induction of cell proliferation by fibroblast and insulin-like growth factors in pure rat inner ear epithelium cell cultures. J Neurosci. 1997;17:216–226. doi: 10.1523/JNEUROSCI.17-01-00216.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]