Abstract
A variety of Ca2+ binding proteins are known to play an integral role in catecholamine release from synapses as well as secretory cells, such as chromaffin cells. TheDrosophila protein frequenin and its mammalian homolog neuronal Ca2+ sensor-1 (NCS-1) belong to a family of Ca2+ sensors with EF hands that bind Ca2+ and then interact with other proteins. Frequenin/NCS-1 has been shown to enhance exocytotic activity in addition to altering Ca2+ channel regulation. To better understand how NCS-1 regulates stimulus–secretion coupling, bovine chromaffin cells were infected with Semliki Forest virus (SFV) vectors containing the rat NCS-1 gene. Cells were studied in the perforated whole-cell patch-clamp configuration. Membrane capacitance was monitored as an indicator of exocytosis–endocytosis. Exocytosis elicited by membrane depolarization was not significantly different between cells infected with SFV expressing green fluorescent protein (GFP) or GFP plus NCS-1, except that the overexpression of NCS-1 resulted in a faster rundown in exocytosis. When cells were stimulated with histamine, NCS-1 overexpression led to higher exocytosis, as well as [Ca2+]i elevation. Immunocytochemistry showed a similar distribution of NCS-1 and phosphatidylinositol 4-kinase β (PI4Kβ). NCS-1 and PI4Kβ coimmunoprecipitate, opening up the possibility that the two proteins directly interact. These results suggest that NCS-1 may regulate cellular activity through the modulation of the phosphatidylinositol signaling pathway.
Keywords: neuronal calcium sensor, NCS-1, chromaffin, exocytosis, calcium, histamine, phosphatidylinositol, IP3, Semliki Forest virus vector
Ca2+ions regulate a variety of physiological processes, including muscle contraction, neurotransmitter release, and gene expression. These processes are regulated by proteins, which bind calcium and sense elevations in [Ca2+]i. Many Ca2+-binding molecules have been identified, reflecting the importance of [Ca2+]i and its regulatory function within the cell (Burgoyne and Morgan, 1995;Benfenati et al., 1999; Brunger, 2000). So far, >250 proteins with the EF hand, a helix–loop–helix Ca2+-binding motif, have been identified. They fall into two distinct families that can be categorized on either their Ca2+-buffering or Ca2+-sensing properties (Braunewell and Gundelfinger, 1999). In contrast to Ca2+buffers, Ca2+ sensors, such as calmodulin, change their conformation during binding Ca2+, thereby enabling their interaction with intracellular targets.
The neuronal calcium sensor (NCS) protein family includes a variety of intracellular Ca2+-binding proteins expressed primarily in neurons. These proteins are 185–205 amino acid residues long, contain four potential EF hand Ca2+-binding motifs, and share a consensus motif for N-terminal myristoylation. NCS family members are highly conserved in different species.
Frequenin, which was first cloned from Drosophila, has been shown to be able to enhance neuromuscular junction activity (Pongs et al., 1993). Recently, McFerran et al. (1998) using PCR methodology confirmed the presence of NCS-1, the mammalian homolog of frequenin, in bovine chromaffin cells; they also showed that overexpression of NCS-1 in chromaffin or pheochromocytoma (PC12) cells enhanced Ca2+-dependent exocytosis from large dense-core vesicles (LDCV). Overexpression of NCS-1 in NG108–15 cells resulted in enhanced synapse formation and neurotransmission (Chen et al., 2001). When a dominant negative mutant of NCS-1 (E120Q) was overexpressed in chromaffin cells, Ca2+current was increased, suggesting that NCS-1 may be involved in Ca2+ channel modulation (Weiss et al., 2000).
To identify possible physiological functions, NCS-1 was overexpressed in bovine chromaffin cells using Semliki Forest virus (SFV) vectors, shown previously to efficiently infect these cells (Ashery et al., 1999; Duncan et al., 1999; Knight, 1999). To identify SFV-infected cells, the SFV vector contained the green fluorescent protein (GFP) gene behind an internal ribosomal entry site (IRES). When compared with uninfected cells, both the Ca2+ current and exocytosis were smaller in SFV-mediated expression of either GFP alone or NCS-1-IRES-GFP based on membrane depolarization-elicited secretion. During the first train of depolarizations, there was no difference in peak exocytosis observed in the SFV-mediated expression of either GFP alone or NCS-1-IRES-GFP cells. Interestingly, the NCS-1 overexpressed cells showed a faster rundown in subsequent secretory responses compared with GFP-infected cells, although the Ca2+ influxes were similar throughout the experiment. In contrast, when histamine was used to stimulate cells, NCS-1 overexpressed cells exhibited a significant higher [Ca2+]i elevation and exocytotic response than did GFP cells. These responses were mediated by Ca2+ released from intracellular Ca2+ stores because pretreatment with thapsigargin (TG) blocked the exocytosis elicited by histamine. Immunostaining revealed that the distribution of NCS-1 and phosphatidylinositol-4-kinase β (PI4Kβ) were similar. In addition, NCS-1 and PI4Kβ coimmunoprecipitate with each other. These results suggest that NCS-1 may interact with the phosphatidylinositol (PtdIns) pathway, thereby regulating stimulus–secretion coupling.
MATERIALS AND METHODS
Cell culture. Bovine adrenal chromaffin cells were prepared from animals ∼18 weeks old. Adrenal glands, obtained from a local abattoir, were digested with collagenase and purified by density gradient centrifugation as described previously (Artalejo et al., 1992). Baby hamster kidney (BHK-21) cells, used for generation of recombinant SFV particles, were cultured in a 1:1 mixture of Dulbecco's modified F-12 medium (Invitrogen, San Diego, CA) and Iscove's modified DMEM (Invitrogen) supplemented with 4 mm glutamine and 10% fetal calf serum.
Solutions. The bath solution used for recordings contained (in mm): 130 NaCl, 20 glucose, 10 Na-HEPES, 1 MgCl2, 2 KCl, and 5 CaCl2, pH 7.3 with NaOH. The perforated patch pipette solution contained (in mm): 135 Cs-glutamate, 10 Na-HEPES, 9.5 NaCl, 0.5 TEACl, and 0.5 CaCl2, pH7.3 with CsOH; 0.5% amphotericin B (Calbiochem, La Jolla, CA) was added into the internal solution before the start of the experiment. The preparation of amphotericin B was done as reported previously (Pan and Fox, 2000). The initial pipette resistance was 1.5–2.5 MΩ. After perforation, the access resistance was 8–20 MΩ. Typically, ∼60% of the access resistance was compensated by the compensation circuitry of the patch clamp.
Histamine (10 μm) in bath solution was applied onto the cell with a fast perfusion system (DAD-12 Superfusion System; ALA Scientific Instruments, Westbury, NY). Cells were first perfused with a control solution containing no histamine and then rapidly switched to a solution with 10 μm histamine for 1.5 min. In TG treatment experiments, cells were incubated for >30 min in 2 μm TG before initiating whole-cell conditions.
For [Ca2+]imeasurements, an HBSS (Invitrogen) was used as the extracellular solution. For these experiments, fura-2 AM (Molecular Probes, Eugene, OR) was the intracellular indicator as described previously (Harkins et al., 2000). Ratios were converted to free [Ca2+]i by comparing ratiometric data from cells with in vitro fura-2 calibration curves made by adding fura-2 (50 μmfree acid) to solutions containing known concentrations of Ca2+ (0 nm to 2000 nm).
Virus preparation and infection. Recombinant SFV particles were generated as described previously (Lundstrom et al., 1994). Briefly, in vitro transcribed RNA from pSFV-GFP or pSFV-NCS-1-IRES-GFP were coelectroporated into BHK-21 cells with pSFV-Helper2 RNA. Virus stocks were harvested 24 hr later and activated with α-chymotrypsin before infection studies. Approximate titers were estimated by infection of known numbers of BHK-21 cells with serial dilutions of SFV stocks, and the GFP-positive cells were counted. Generally, titers in the range of 5 × 108 infectious particles/ml were obtained.
SFV particles (30 μl/dish) were added to 35 mm Petri dishes containing freshly prepared chromaffin cells (2 × 105 per dish) and incubated overnight. The infected cells were recorded in 48 hr after removal of virus by careful repeated washes. Infected cells were identified by their GFP fluorescence.
Capacitance recordings. Capacitance measurements were performed using an Axopatch-1C patch-clamp amplifier (Axon Instruments, Foster City, CA) and a computer-based phase tracking algorithm as described previously (Pan and Fox, 2000). Data acquisition was initiated when the uncompensated series resistance was <20 MΩ and stable. Unbalancing the slow capacitance compensation by 100 fF provided the calibration signal for capacitance trace.
Stimulation protocols. To elicit exocytosis, cells were stimulated with trains of 10 depolarizations to +20 mV from a holding potential of −80 mV. Each depolarization was 150 msec in duration, with an interpulse interval of 400 msec. There was a 5 min interval between each train of stimuli. In experiments that used small depolarizations, cells were depolarized 100 times to −10 mV from −80 mV; each depolarization was 150 msec in duration, with an interpulse interval of 1 sec.
Immunocytochemistry. Chromaffin cells were fixed with 3.7% formaldehyde in PBS buffer for 2 hr and permeabilized by incubation in PBT solution (0.5% BSA and 0.1% Triton X-100 in PBS buffer) for 30 min. After washing out the PBT solution with PBS buffer three times, cells were incubated with the PBS-diluted primary antibody (chicken anti-NCS-1 at 1:100 dilution) (Weisz et al., 2000), or rabbit anti-PI4Kβ (Upstate Biotechnology, Lake Placid, NY) or rabbit anti-secretogranin II (SgII) (Biogenesis, Poole, UK) for 1 hr. The primary antibody was washed out by three successive washes in PBT solution for 5 min each time. The cells were then incubated in PBS-diluted secondary antibody [rhodamine-conjugated donkey anti-chicken (Jackson ImmunoResearch, West Grove, PA) or FITC-conjugated goat anti-rabbit (Santa Cruz Biotechnology, Santa Cruz, CA)] for 1 hr. The secondary antibody was washed out by three successive washes in PBT solution for 5 min each time and then incubated in PBS buffer overnight before mounting onto glass. The staining was examined with an Olympus Optical (Tokyo, Japan) Scanning Laser Confocal Microscope.
Immunoprecipitation. To obtain protein extracts from bovine chromaffin cells, the adrenal medullary chromaffin cells were lysed with a lysis buffer [20 mm HEPES, pH 7.5, 0.1m NaCl, 2.5 mmMgCl2, 2 mm EDTA, 40 mm β-glycerophosphate, 1% Nonidet P-40, 0.5 mmNa3VO4, 1 mm dithiothreitol, 0.1 mm4-(2-aminoethyl)-benzenesulfonyl fluoride, 20 μg/ml aprotinin, and 20 μg/ml leupeptin]. After a brief sonication, the samples were centrifuged at 19,300 × g for 20 min, and the chromaffin cell protein extracts were collected in the supernatant. For immunoprecipitation experiments, 2 μg of NCS-1 antibody or 4 μg of PI4Kβ antibody was added to 200 μg of protein extracts and incubated overnight at 4°C. The immunoprecipitated proteins were then collected by protein G-Sepharose for 1 hr and washed six times with 1 ml of lysis buffer each. The immunoprecipitated proteins were then separated on 12% SDS-polyacrylamide gels and examined for the presence of NCS-1 and PI4Kβ by immunoblot analysis.
Data analysis. For each stimulation, Ca2+ entry was determined by integrating the Ca2+ currents using limits that excluded the early influx attributable to Na+ current. Before integration, currents were leak and capacitance subtracted. To determine the capacitance change, the difference before and after each stimulation was calculated using the 100 fF calibration signal. Single comparisons were made using Student's t test.
RESULTS
NCS-1-expressing cells showed faster rundown of exocytosis
Infected chromaffin cells were identified by GFP fluorescence. Typically, ∼80% of cells showed GFP fluorescence. For SFV-NCS-1-IRES-GFP-infected cells, the overexpression of NCS-1 was confirmed by immunostaining and then comparing these results with cells showing no GFP fluorescence (data not shown). Figure1, A and B, plots membrane capacitance elicited by three consecutive trains of depolarizations from two representative cells infected with either SFV-GFP alone or SFV-NCS-1-IRES-GFP. The currents recorded during the first depolarization of each train are plotted immediately below. Please note that the current traces include an early peak attributable to Na+-influx, followed by a slow maintained Ca2+ current. Whereas the GFP-expressing cells showed significant rundown by the third train of depolarizations, the rundown for the NCS-1-expressing cells was faster and more dramatic. Figure 1C, a plot of averaged capacitance data, makes the point that the rundown observed in NCS-1-infected cells was more rapid. Please note that the Ca2+influx was not significantly different in both groups of cells (Fig.1C). We showed previously that three successive trains of stimulations in non-infected cells did not cause any significant rundown in secretion with identical stimulation conditions (Pan and Fox, 2000). The results shown in Figure 1 suggest that viral infection alone can alter stimulus–secretion coupling in chromaffin cells. The more rapid rundown observed in NCS-1-expressing cells suggests that NCS-1 may play a role in the modulation of secretion.
Fig. 1.
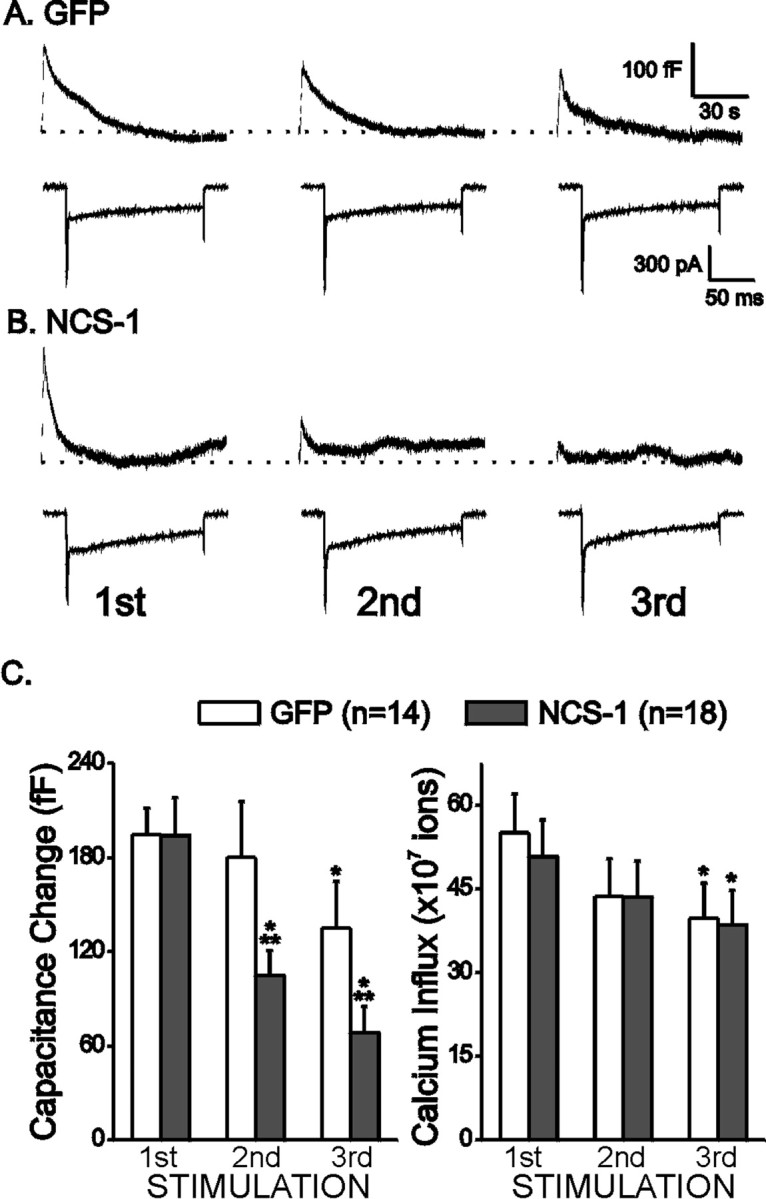
SFV-infected cells exhibit rundown of depolarization-evoked exocytosis. Exocytosis was evoked with a train of 10 depolarizations to +20 mV from a −80 mV holding potential; each lasts 150 msec with an interval of 400 msec. Stimulations were separated by 5 min intervals. Representative capacitance traces (top) and the first inward current elicited by the train of depolarizations (bottom) are plotted.A plots data from a cell expressing GFP alone (GFP), and B plots data from a cell expressing both NCS-1 and GFP (NCS-1). The dotted line indicates the baseline capacitance level before stimulation. C plots the averaged peak capacitance changes observed, as well as Ca2+ influx for all 10 depolarizations for SFV-GFP cells (GFP; white bars) and SFV-NCS-1-IRES-GFP cells (NCS-1;gray bars). Data are means ± SEM. *p < 0.05 when compared with the first stimulations with paired Student's t test; **p < 0.05 when compared with the GFP cells at the same stimulations with independent Student's ttest.
The rundown in exocytosis was not attributable to alterations in the release machinery
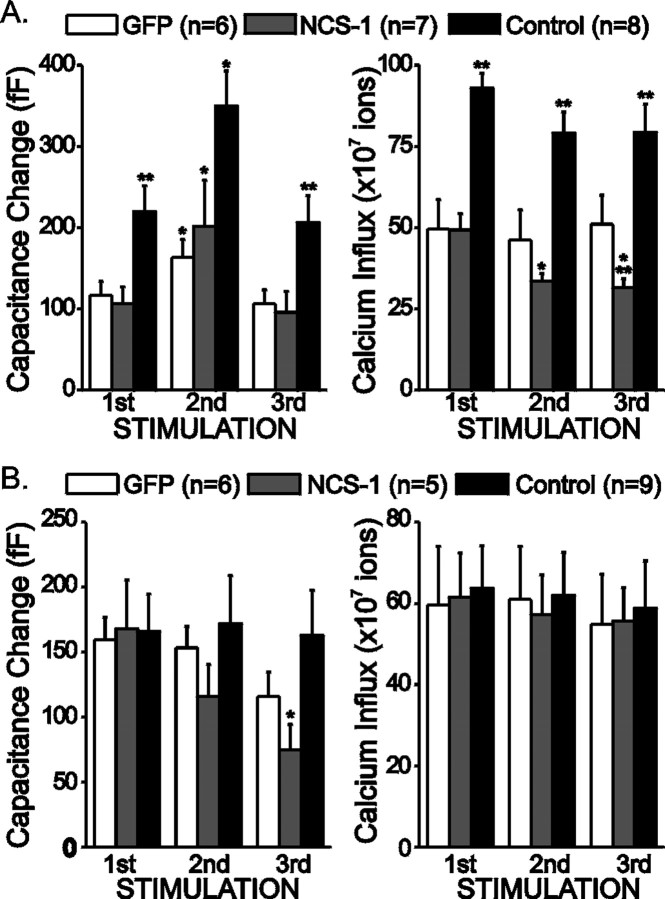
It is possible that the rundown in exocytosis observed was attributable to an impairment of the secretory machinery. To test this possibility, a series of 100 depolarizations to −10 mV from a holding potential of −80 mV was applied between the first and the second stimulation trains. These depolarizations were large enough to allow some Ca2+ influx, but they were not sufficient to trigger exocytosis. This strategy elevates [Ca2+]i and helps mobilize vesicles to the release sites (Pan and Fox, 2000). In Figure2A, averaged results are plotted from cells stimulated with trains of 100 small depolarizations between the first and the second stimulation trains. Control cells exhibited a larger Ca2+influx and larger exocytosis than SFV-infected cells. This suggests that viral infection by itself decreased both Ca2+ current and exocytosis. Application of the small depolarizations transiently enhanced exocytosis in all three groups. These results suggest that the secretory apparatus was relatively unchanged in the infected cells. However, in these experiments, we observed a significant rundown in Ca2+ influx in the NCS-1-expressing cells.
Fig. 2.
Vesicle replenishment is still functional in SFV-infected cells. A, A series of 100 depolarizations from −80 to −10 mV for 150 msec each with an interval of 1 sec was applied between the first and second stimulations. Plotted are averaged peak capacitance changes and Ca2+ influx for SFV-GFP cells (GFP; white bars), SFV-NCS-1-IRES-GFP cells (NCS-1; gray bars), or without SFV (Control; black bars). B, The extracellular Ca2+ concentration for control cells was lowered to 2 mm (from 5 mm) to produce currents comparable in size with SFV-infected cells. Plotted are averaged peak capacitance changes and Ca2+ influx. Data are means ± SEM. *p < 0.05 when compared with the first stimulations with paired Student's t test; **p < 0.05 when compared with the GFP cells at the same stimulations with independent Student's ttest.
Because uninfected cells have a larger Ca2+ influx than SFV-infected cells, it is possible that the rundown in exocytosis is attributable to an inability to support vesicle mobilization, which is required to maintain exocytosis. To test this possibility, the extracellular Ca2+ concentration in uninfected cells was lowered to 2 mm to generate similar levels of Ca2+ influx in both infected and uninfected cells. In these experiments, three trains of stimulations were applied as before. Averaged results are plotted in Figure2B. Although Ca2+ influx was similar in all three groups of cells during these three trains, control cells were able to maintain the same level of secretion with no significant rundown, whereas the NCS-1-expressing cells showed a significant rundown. These results suggest that the rundown in exocytosis after NCS-1 expression is not simply attributable to decreased Ca2+ influx.
Histamine elicited more exocytosis in NCS-1 overexpressed cells
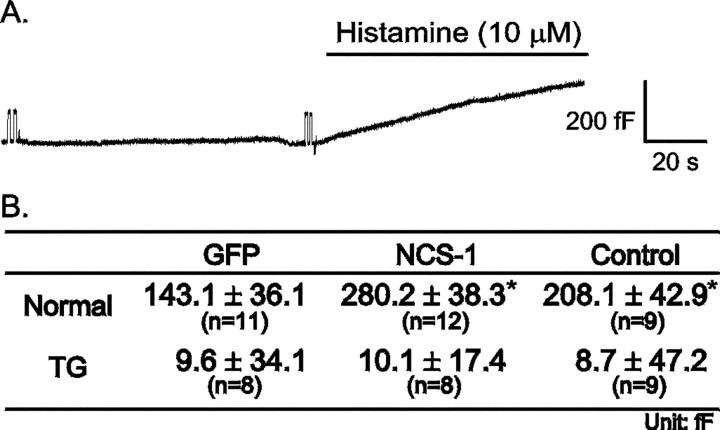
A previous study reported that NCS-1 upregulates PI4K (Hendricks et al., 1999), which produces an increase in the concentration of PtdIns 4,5-bisphosphate (PIP2). In bovine chromaffin cells, histamine activates phospholipase C, which catalyzes the production of IP3 from PIP2. Elevation of IP3levels results in the release of Ca2+ from intracellular stores (Brini and Carafoli, 2000). It is well documented that histamine elevates [Ca2+]i and induces exocytosis in bovine chromaffin cell (Finnegan et al., 1996). To determine whether NCS-1 plays a role in the IP3 pathway, histamine (10 μm) was perfused onto chromaffin cells to elicit exocytosis. Figure3A shows a representative capacitance trace from a control cell. Histamine was perfused onto the cells as indicated. Before switching to the histamine-containing solution, the membrane capacitance trace was stable; after histamine was perfused into the bath, membrane capacitance increased gradually. Figure 3B is the averaged capacitance increase observed 1.5 min after histamine application. Although histamine elicited significantly more exocytosis from NCS-1-expressing cells than from GFP-expressing cells, the overall levels were quite similar to those observed for control cells.
Fig. 3.
NCS-1 enhances histamine-evoked exocytosis. For these experiments, cells were was perfused with a solution without histamine and then switched to a solution containing histamine (10 μm) for 1.5 min. A, Plotted is a representative capacitance recording from a control cell that was not infected with SFV. Histamine application is indicated. Bshows the averaged capacitance change 1.5 min after histamine was applied. *p < 0.05 when compared with the GFP cells with independent Student's t test.
To verify the role of the intracellular Ca2+ store in the histamine response, TG was used to deplete the store (Pan and Fox, 2000). Figure 3Bshows that, after TG treatment, histamine no longer elicited any capacitance change. These results suggest that histamine elicits more exocytosis from NCS-1-expressing cells than from GFP-expressing cells and that Ca2+ release from the intracellular Ca2+ stores plays an important role in this response.
Histamine produces a larger elevation of [Ca2+]i peak in NCS-1-expressing cells
To monitor [Ca2+]i changes during histamine perfusion, [Ca2+]i was measured by the fura-2 fluorescence ratio method. Figure4A plots representative [Ca2+]imeasurements from different cells. After histamine perfusion, [Ca2+]i increased sharply in all cases. Some cells exhibited oscillations in [Ca2+]i, followed by a decay to a plateau. After histamine was washed out of the bath, the [Ca2+]idecreased slowly to baseline. The averaged results are shown in Figure4B, which shows that NCS-1-expressing and control cells have similar peak values (571.1 ± 39.4 and 550.8 ± 29.4 nm, respectively), and both are significantly higher than that of GFP-expressing cells (485.9 ± 22.8 nm). In the plateau phase, [Ca2+]i in control cells is higher than that of SFV-infected cells. These results suggest that cells expressing NCS-1 reach higher [Ca2+]i than cells expressing GFP do when stimulated with histamine.
Fig. 4.
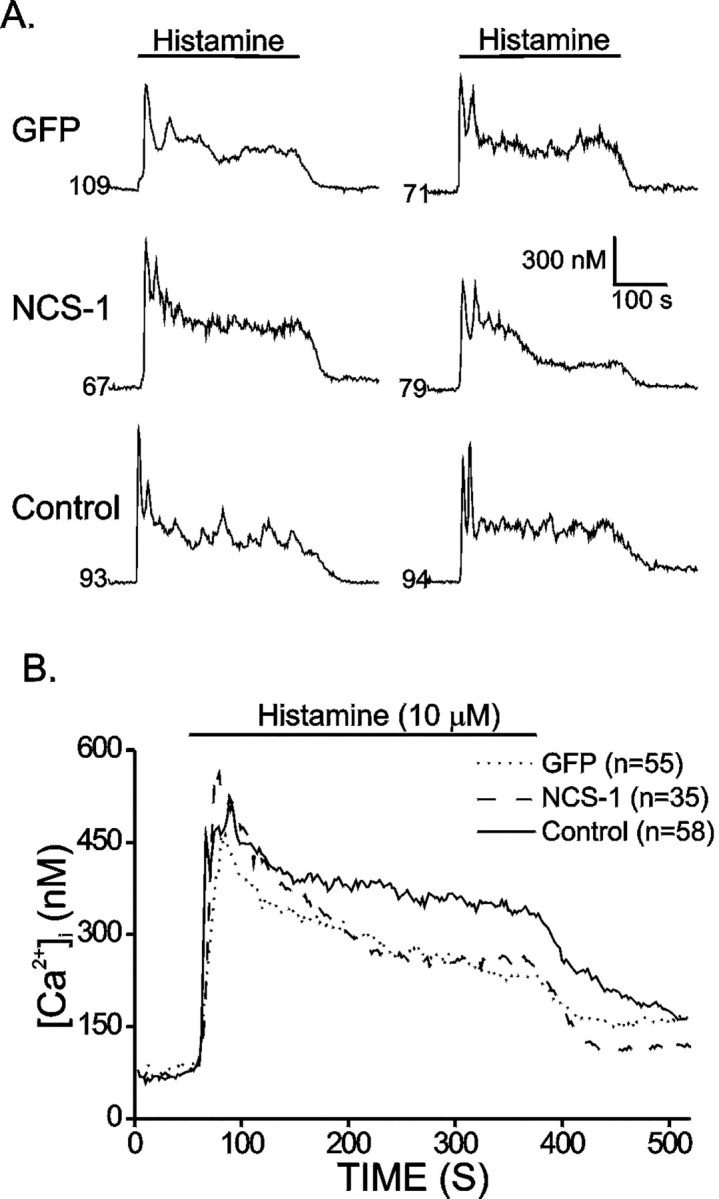
NCS-1 overexpression enhances the [Ca2+]i peak response induced by histamine. Cells was loaded with fura-2 to monitor [Ca2+]i. Fluorescence ratios were converted to free [Ca2+]i by comparing data with fura-2 calibration curves made in vitro by adding fura-2 (50 μm free acid) to solutions that contained known concentrations of calcium (0 to 2000 nm).A, Plotted are representative [Ca2+]i recordings from SFV-GFP (GFP), SFV-NCS-1-IRES-GFP (NCS-1), and control (Control) cells. Cells were perfused with histamine (10 μm) for 5 min as indicated. Thenumbers indicate resting [Ca2+]i before histamine application.B, Plotted are averaged [Ca2+]i recordings from SFV-GFP (GFP; dotted line), SFV-NCS-1-IRES-GFP (NCS-1; dashed line), and control (Control; solid line) cells stimulated by histamine. The number of cells in each group is indicated in the figure.
NCS-1 localization and interaction
To identify the subcellular localization of NCS-1 in chromaffin cell, antibodies against NCS-1, PI4Kβ, and SgII were used to stain uninfected bovine chromaffin cells. As shown in Figure5, A and D, antibodies against NCS-1 stains the plasma membrane and the cytosol. SgII has been used as a marker for LDCV. As shown in Figure5B, the staining is mainly cytosolic, producing some large spots. PI4Kβ has been shown to be associated with synaptic-like microvesicles; its staining pattern as shown in Figure 5E is on the plasma membrane, in the cytosol, and in the nucleus. Superimposing the results showed that NCS-1 has a similar distribution to that of PI4Kβ, with the exception of nuclear localization (Fig.5F). In contrast, the NCS-1 immunofluorescence appears to overlap poorly with SgII (Fig. 5C).
Fig. 5.
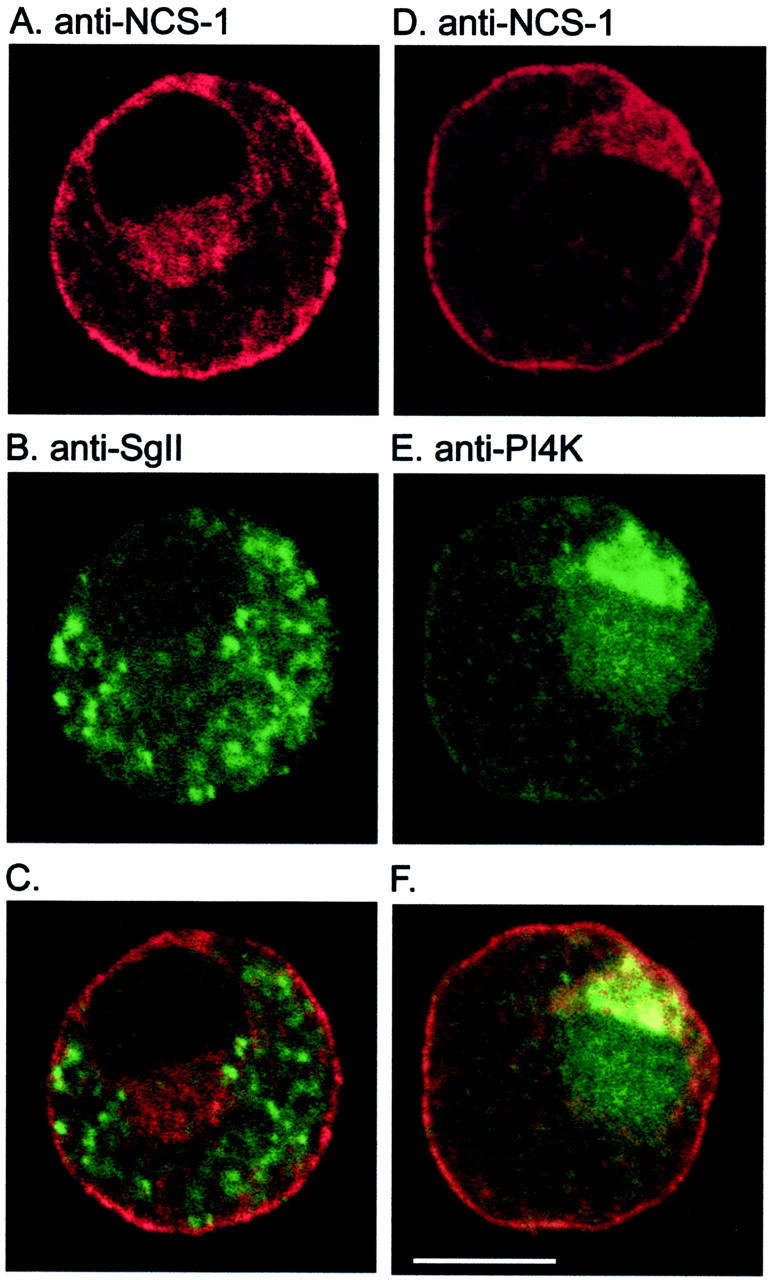
NCS-1 is colocalized with PI4K but not SgII. Images of endogenous NCS-1 in chromaffin cells were obtained by confocal microscopy after incubation with chicken (A,D) polyclonal antibody against NCS-1 and after double labeling with rabbit polyclonal antiserum against SgII (B) or rabbit monoclonal antibody against PI4Kβ (E). C and F, respectively, represent the combined images of NCS-1 with SgII and NCS-1 with PI4Kβ. Areas of colocalization appearyellow. Scale bar, 5 μm.
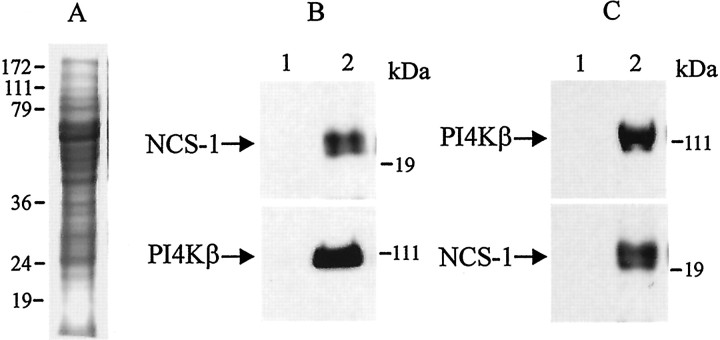
In agreement with the colocalization data, Figure6 demonstrates a direct interaction between NCS-1 and PI4Kβ. For this experiment, protein extracts from chromaffin cells were first immunoprecipitated with anti-NCS-1 antibody, separated on a 12% SDS gel, and then were immunoblotted with anti-NCS-1 antibody (Fig. 6B, top,NCS-1) and PI4Kβ antibody (Fig. 6B,bottom, PI4Kβ). Similar results were obtained when these extracts were first immunoprecipitated with anti-PI4Kβ antibody and immunoblotted with anti-PI4Kβ antibody (top) and NCS-1 antibody (bottom). Preimmune IgG was used as control antibody (lane 1). Additional evidence for interaction between NCS-1 and PI4Kβ can be found elsewhere (Zhao et al., 2001).
Fig. 6.
Evidence for direct interaction between NCS-1 and PI4Kβ. A, Protein extracts (25 μg) from bovine chromaffin cells were separated on a 12% SDS gel and were visualized with Coomassie blue staining. B, The same protein extracts were immunoprecipitated with anti-NCS-1 antibody, separated on a 12% SDS gel, and immunoblotted with anti-NCS-1 antibody (top; NCS-1) and PI4Kβ antibody (bottom; PI4Kβ). C, The same extracts were immunoprecipitated with anti-PI4Kβ antibody and immunoblotted with anti-PI4Kβ antibody (top) and NCS-1 antibody (bottom). Preimmune IgG was used as control antibody (lane 1).
DISCUSSION
We examined the function of NCS-1, a neuronal Ca2+ sensor protein, in stimulus–secretion coupling in bovine chromaffin cells. Secretion was elicited by the direct activation of voltage-dependent Ca2+ channels or alternatively by activation of the PI pathway. Our data suggest that NCS-1 may be involved in PtdIns-related exocytosis. Depolarization-evoked exocytosis does not show much difference in GFP and NCS-1 cells at the beginning, but an accelerated rundown was observed in NCS-1 cells during each experiment. This rundown in exocytosis was not attributable to decreased Ca2+ influx because comparable Ca2+ influx levels in control cells showed no rundown, nor is the difference between SFV-infected cells and control cells likely to be attributable to alterations in vesicle replenishment because small depolarizations that elevate [Ca2+]i without triggering exocytosis can transiently augment exocytosis observed during a subsequent stimulation. These results are in agreement with those reported by Ashery et al. (1999), which showed that the size of the readily releasable pool of vesicles and refilling kinetics were not significantly altered in SFV-infected cells. When histamine was used to elicit exocytosis, NCS-1 cells exhibited increased levels of exocytosis compared with GFP cells. [Ca2+]imeasurement also showed higher peak values in NCS-1 cells. Immunocytochemical staining suggested that NCS-1 colocalized with PI4Kβ. Finally, NCS-1 and PI4Kβ coimmunoprecipitate with each other.
Virus infection
SFV is a positive stranded RNA virus and has been modified as a carrier to express recombinant proteins in mammalian cells (Liljestrom and Garoff, 1991). It has been reported that SFV can infect bovine chromaffin cells with a high efficiency and can be accessed a few hours after infection (Knight, 1999). Although it has been reported that this infection at an early stage does not significantly alter the physiological condition of the cell, this was not true in our studies. Chromaffin cells infected with SFV always exhibited a diminished Ca2+ influx and reduced exocytosis when compared with uninfected cells under the same conditions. In experiments that measured intracellular Ca2+, application of histamine induced [Ca2+]i peak and plateau phases that were lower than those observed in infected cells. Although it is not clear how viral infection changes cellular properties, basic behaviors, such as vesicle replenishment mechanisms, histamine-induced [Ca2+]ioscillation and exocytosis were similar in infected and control cells. These suggest that the infection may subtly alter cellular responses but that basic physiological mechanisms still remain intact. We believe our study sounds an important cautionary note for the continued use of SFV, including the second generation of SFV vectors (Lundstrom et al., 2001).
Depolarization-evoked exocytosis
Our previous data showed that there was no rundown in exocytosis during multiple stimulations in uninfected cells (Pan and Fox, 2000), results that were confirmed in the current study. In contrast, infected cells showed significant rundown of exocytosis, which was even more dramatic in SFV-NCS-1-IRES-GFP cells. Because Ca2+ influx remained unchanged during the course of each experiment and it was similar between SFV-GFP and SFV-NCS-1-IRES-GFP cells, one possible explanation for the observed rundown may come from the availability of release-ready vesicles. Compromises in this system would result in decreased secretion with time. As shown in Figure 2, the replenishment mechanism is still functional because the rundown was rescued by multiple small depolarizations. Another possible explanation for the rundown of secretion is that the diminished Ca2+influx observed in SFV-infected cells was insufficient for vesicle replenishment. Control cells in which Ca2+influx was reduced to comparable levels showed no such rundown, suggesting that a diminished Ca2+ influx did not account for the rundown in secretion observed. Another possible explanation is that overexpressed NCS-1 may function as a Ca2+ buffer to limit the elevation in [Ca2+]i, which may inhibit vesicle movement to the release-ready pool. If so, we would expect a reduced [Ca2+]i elevation in SFV-NCS-1-IRES-GFP cells when compared with SFV-GFP cells. Contrary to expectations, SFV-NCS-1-IRES-GFP showed higher [Ca2+]i peak elevation and a similar plateau level when compared with SFV-GFP cells after stimulation with histamine. Yet another possibility, which we have not yet addressed, is that NCS-1 alters the Ca2+ dependence of vesicle mobilization.
It has been suggested that an elevated PIP2concentration can increase the adhesion energy between plasma membrane and cytoskeleton, which may control the cell shape (Raucher et al., 2000). SFV-NCS-1-IRES-GFP cells may have an elevated plasma membrane PIP2 concentration, which would increase the interaction between plasma membrane and cytoskeleton, thereby limiting the fusion of vesicles with the membrane. This hypothesis might explain the faster rundown of exocytosis. On the other hand, when cells were stimulated with histamine, more IP3 and DAG should have been released, and the subsequent elevation in [Ca2+]i should work together with PKC, activated by DAG, to induce more exocytosis.
Histamine-evoked exocytosis
It has been reported that the NCS-1 homolog in Saccharomyces cerevisiae can bind PI4K and stimulates its activity in vitro (Hendricks et al., 1999). PI4K phosphorylates PtdIns to produce PtdIns 4-P, which then serves as a substrate for PI5K, resulting in the production of PIP2. In cells, activation of phospholipase C results in the generation of two second messengers, diacylglycerol and IP3 (Zhang and Majerus, 1998; Toker, 1998). It has been shown that a granule-associated PI4K activity is required for the priming of secretory vesicles in bovine chromaffin cells (Wiedemann et al., 1996). In chromaffin cells, histamine treatment can activate PLC and release DAG and IP3. The generation of IP3 results in the elevation of [Ca2+]i, which augments exocytosis (Finnegan et al., 1996; Zerbes et al., 1998). DAG activates protein kinase C, which increases secretion in chromaffin cells by increasing the size of the release-ready vesicle pool (Vitale et al., 1995; Warashina, 1997). Histamine exposure induces an immediate [Ca2+]i peak elevation attributable to the release of Ca2+ from IP3-sensitive Ca2+stores, followed by a sustained [Ca2+]i elevation produced by Ca2+ influx from the extracellular space (Cheek et al., 1993). Our results show that histamine-evoked exocytosis from SFV-NCS-1-IRES-GFP cells is approximately the same as that from uninfected cells and significantly higher than that from SFV-GFP cells. [Ca2+]imeasurement shows that NCS-1 overexpressed cells have higher averaged peak than SFV-GFP cells, which may explain why exocytosis is higher than SFV-GFP cells. Histamine-induced exocytosis requires release from an intracellular Ca2+ store because TG-pretreated cells showed no exocytosis when stimulated with histamine.
Localization of NCS-1
NCS-1 has been shown to be colocalized with adaptin, which is localized in the trans-Golgi network and the late endosomes and with PI4Kβ in COS cells (Bourne et al., 2001). In PC12 cells, NCS-1 staining overlapped with the synaptic-like microvesicle marker synaptophysin but not with LDCV marker SgII (McFerran et al., 1998). SgII is one of the chromagranins stored mainly in LDCV in chromaffin cells (Winkler, 1993). As shown in Figure 5B, NCS-1 localization is primarily cytosolic, with some spots showing elevated levels. PI4K activity has been shown to be present on chromaffin granule (Wiedemann et al., 1996), in neurons, and in small synaptic vesicles (Wiedemann et al., 1998). The staining pattern of PI4Kβ in chromaffin cell shows that it appears in the plasma membrane, cytosol, and nucleus. PI4Kβ appears to be concentrated near the nucleus, and, unlike SgII, there are no large spots. Although it has been suggested that NCS-1 can bind to PI4Kβ to regulate its activity, colocalization has not yet been well documented. The colocalization results suggest that NCS-1 has the same distribution as PI4Kβ but does not appear in the nucleus. Overexpression of NCS-1 produced a similar staining pattern (everywhere but the nucleus; data not shown). Coimmunoprecipitation of NCS-1 and PI4Kβ from chromaffin cell extracts suggests direct interactions between the two proteins. A recent study by Zhao et al. (2001) reported that NCS-1 was able to interact with PI4Kβ. These results reinforce the suggestion that PI4Kβ may be one of the targets of NCS-1.
It has been reported that overexpression of frequenin homolog enhances synaptic efficacy in Drosophila motor neuron (Pongs et al., 1993; Rivosecchi et al., 1994), Xenopus embryonic spinal neuron (Olafsson et al., 1995), and ATP-induced LDCV exocytosis from PC12 cells (McFerran et al., 1998). Our results show that exocytosis was not enhanced in SFV-NCS-1-IRES-GFP cells when Ca2+ channel activation was used to elicit secretion. Activation of histamine receptors, in contrast, which results in alterations of PtdIns metabolism, enhanced exocytosis in SFV-NCS-1-IRES-GFP cells. The physiological activator of chromaffin cells, acetylcholine, activates both nicotinic and muscarinic ACh receptors. Activation of muscarinic receptors, and subsequent alterations in PtdIns metabolism, may result in an exocytotic response that is amenable to modulation by NCS-1. Our results suggest a possible role for NCS-1 in PI-mediated secretion in other systems, including secretory cells and neurons (Tse et al., 1997; Lysakowsi et al., 1999).
Footnotes
This research is supported by the Medical Research Council of Canada (A.J. and J.R.). We thank Dr. Kevin Currie for help in preparing bovine chromaffin cells.
Correspondence should be addressed to Chien-Yuan Pan, The University of Chicago, Department of Neurobiology, Pharmacology, and Physiology, 947 East 58th Street, Chicago, IL 60637. E-mail:cypan@drugs.bsd.uchicago.edu.
REFERENCES
- 1.Artalejo CR, Perlman RL, Fox AP. Omega-conotoxin GVIA blocks a Ca2+ current in bovine chromaffin cells that is not of the “classic” N type. Neuron. 1992;8:85–95. doi: 10.1016/0896-6273(92)90110-y. [DOI] [PubMed] [Google Scholar]
- 2.Ashery U, Betz A, Xu T, Brose N, Rettig J. An efficient method for infection of adrenal chromaffin cells using the Semliki Forest virus gene expression system. Eur J Cell Biol. 1999;78:525–532. doi: 10.1016/s0171-9335(99)80017-x. [DOI] [PubMed] [Google Scholar]
- 3.Benfenati F, Onofri F, Giovedi S. Protein-protein interactions and protein modules in the control of neurotransmitter release. Philos Trans R Soc Lond B Biol Sci. 1999;354:243–257. doi: 10.1098/rstb.1999.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourne Y, Dannenberg J, Pollmann V, Marchot P, Pongs O. Immunocytochemical localization and crystal structure of human frequenin (neuronal calcium sensor 1). J Biol Chem. 2001;276:11949–11955. doi: 10.1074/jbc.M009373200. [DOI] [PubMed] [Google Scholar]
- 5.Braunewell KH, Gundelfinger ED. Intracellular neuronal calcium sensor proteins: a family of EF-hand calcium-binding proteins in search of a function. Cell Tissue Res. 1999;295:1–12. doi: 10.1007/s004410051207. [DOI] [PubMed] [Google Scholar]
- 6.Brini M, Carafoli E. Calcium signalling: a historical account, recent developments and future perspectives. Cell Mol Life Sci. 2000;57:354–370. doi: 10.1007/PL00000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunger AT. Structural insights into the molecular mechanism of Ca2+-dependent exocytosis. Curr Opin Neurobiol. 2000;10:293–302. doi: 10.1016/s0959-4388(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 8.Burgoyne RD, Morgan A. Ca2+ and secretory-vesicle dynamics. Trends Neurosci. 1995;18:191–196. doi: 10.1016/0166-2236(95)93900-i. [DOI] [PubMed] [Google Scholar]
- 9.Cheek TR, Morgan A, O'Sullivan AJ, Moreton RB, Berridge MJ, Burgoyne RD. Spatial localization of agonist-induced Ca2+ entry in bovine adrenal chromaffin cells. Different patterns induced by histamine and angiotensin II, and relationship to catecholamine release. J Cell Sci. 1993;105:913–921. doi: 10.1242/jcs.105.4.913. [DOI] [PubMed] [Google Scholar]
- 10.Chen XL, Zhong ZG, Yokoyama S, Bark C, Meister B, Berggren PO, Roder J, Higashida H, Jeromin A. Overexpression of rat neuronal calcium sensor-1 in rodent NG108–15 cells enhances synapse formation and transmission. J Physiol (Lond) 2001;532:649–659. doi: 10.1111/j.1469-7793.2001.0649e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duncan RR, Don-Wauchope AC, Tapechum S, Shipston MJ, Chow RH, Estibeiro P. High-efficiency Semliki Forest virus-mediated transduction in bovine adrenal chromaffin cells. Biochem J. 1999;342:497–501. [PMC free article] [PubMed] [Google Scholar]
- 12.Finnegan JM, Borges R, Wightman RM. Comparison of cytosolic Ca2+ and exocytosis responses from single rat and bovine chromaffin cells. Neuroscience. 1996;71:833–843. doi: 10.1016/0306-4522(95)00459-9. [DOI] [PubMed] [Google Scholar]
- 13.Harkins AB, Dlouhy S, Ghetti B, Cahill AL, Won L, Heller B, Heller A, Fox AP. Evidence of elevated intracellular calcium levels in weaver homozygote mice. J Physiol (Lond) 2000;524:447–455. doi: 10.1111/j.1469-7793.2000.t01-2-00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hendricks KB, Wang BQ, Schnieders EA, Thorner J. Yeast homologue of neuronal frequenin is a regulator of phosphatidylinositol-4-OH kinase. Nat Cell Biol. 1999;1:234–241. doi: 10.1038/12058. [DOI] [PubMed] [Google Scholar]
- 15.Knight DE. Secretion from bovine chromaffin cells acutely expressing exogenous proteins using a recombinant Semliki Forest virus containing an EGFP reporter. Mol Cell Neurosci. 1999;14:486–505. doi: 10.1006/mcne.1999.0793. [DOI] [PubMed] [Google Scholar]
- 16.Liljestrom P, Garoff H. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology (NY) 1991;9:1356–1361. doi: 10.1038/nbt1291-1356. [DOI] [PubMed] [Google Scholar]
- 17.Lundstrom K, Mills A, Buell G, Allet E, Adami N, Liljestrom P. High-level expression of the human neurokinin-1 receptor in mammalian cell lines using the Semliki Forest virus expression system. Eur J Biochem. 1994;224:917–921. doi: 10.1111/j.1432-1033.1994.00917.x. [DOI] [PubMed] [Google Scholar]
- 18.Lundstrom K, Rotmann D, Hermann D, Schneider EM, Ehrengruber MU. Novel mutant Semliki Forest virus vectors: gene expression and localization studies in neuronal cells. Histochem Cell Biol. 2001;115:83–91. doi: 10.1007/s004180000223. [DOI] [PubMed] [Google Scholar]
- 19.Lysakowsi A, Figuera H, Price SD, Peng YY. Dense-core vesicles, smooth endoplasmic reticulum, and mitochondria are closely associated with non-specialized parts of plasma membrane of nerve terminals: implications for exocytosis and calcium buffering by intraterminal organelles. J Comp Neurol. 1999;403:378–390. doi: 10.1002/(sici)1096-9861(19990118)403:3<378::aid-cne7>3.0.co;2-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McFerran BW, Graham ME, Burgoyne RD. Neuronal Ca2+ sensor 1, the mammalian homologue of frequenin, is expressed in chromaffin and PC12 cells and regulates neurosecretion from dense-core granules. J Biol Chem. 1998;273:22768–22772. doi: 10.1074/jbc.273.35.22768. [DOI] [PubMed] [Google Scholar]
- 21.Olafsson P, Wang T, Lu B. Molecular cloning and functional characterization of the Xenopus Ca2+-binding protein frequenin. Proc Natl Acad Sci USA. 1995;92:8001–8005. doi: 10.1073/pnas.92.17.8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan CY, Fox AP. Rundown of secretion after depletion of intracellular calcium stores in bovine adrenal chromaffin cells. J Neurochem. 2000;75:1132–1139. doi: 10.1046/j.1471-4159.2000.0751132.x. [DOI] [PubMed] [Google Scholar]
- 23.Pongs O, Lindemeier J, Zhu XR, Theil T, Engelkamp D, Krah-Jentgens I, Lambrecht HG, Koch KW, Schwemer J, Rivosecchi R, Mallart A, Galceran J, Canal I, Barbas JA, Ferrus A. Frequenin-a novel calcium-binding protein that modulates synaptic efficacy in the Drosophila nervous system. Neuron. 1993;11:15–28. doi: 10.1016/0896-6273(93)90267-u. [DOI] [PubMed] [Google Scholar]
- 24.Raucher D, Stauffer T, Chen W, Shen K, Guo S, York JD, Sheetz MP, Meyer T. Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton-plasma membrane adhesion. Cell. 2000;100:221–228. doi: 10.1016/s0092-8674(00)81560-3. [DOI] [PubMed] [Google Scholar]
- 25.Rivosecchi R, Pongs O, Theil T, Mallart A. Implication of frequenin in the facilitation of transmitter release in Drosophila. J Physiol (Lond) 1994;474:223–232. doi: 10.1113/jphysiol.1994.sp020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toker A. The synthesis and cellular roles of phosphatidylinositol 4,5-bisphosphate. Curr Opin Cell Biol. 1998;10:254–261. doi: 10.1016/s0955-0674(98)80148-8. [DOI] [PubMed] [Google Scholar]
- 27.Tse FW, Tse A, Hille B, Horstmann H, Almers W. Local Ca2+ release from internal stores controls exocytosis in pituitary gonadotrophs. Neuron. 1997;18:121–132. doi: 10.1016/s0896-6273(01)80051-9. [DOI] [PubMed] [Google Scholar]
- 28.Vitale ML, Seward EP, Trifaro JM. Chromaffin cell cortical actin network dynamics control the size of the release-ready vesicle pool and the initial rate of exocytosis. Neuron. 1995;14:353–363. doi: 10.1016/0896-6273(95)90291-0. [DOI] [PubMed] [Google Scholar]
- 29.Warashina A. Involvement of protein kinase C in homologous desensitization of histamine-evoked secretory responses in rat chromaffin cells. Brain Res. 1997;762:40–46. doi: 10.1016/s0006-8993(97)00346-6. [DOI] [PubMed] [Google Scholar]
- 30.Weiss JL, Archer DA, Burgoyne RD. Neuronal Ca2+ sensor-1/frequenin functions in an autocrine pathway regulating Ca2+ channels in bovine adrenal chromaffin cells. J Biol Chem. 2000;275:40082–40087. doi: 10.1074/jbc.M008603200. [DOI] [PubMed] [Google Scholar]
- 31.Weisz OA, Gibson GA, Leung SM, Roder J, Jeromin A. Overexpression of frequenin, a modulator of phosphatidylinositol 4-kinase, inhibits biosynthetic delivery of an apical protein in polarized madin-darby canine kidney cells. J Biol Chem. 2000;275:24341–24347. doi: 10.1074/jbc.M000671200. [DOI] [PubMed] [Google Scholar]
- 32.Wiedemann C, Schafer T, Burger MM. Chromaffin granule-associated phosphatidylinositol 4-kinase activity is required for stimulated secretion. EMBO J. 1996;15:2094–2101. [PMC free article] [PubMed] [Google Scholar]
- 33.Wiedemann C, Schafer T, Burger MM, Sihra TS. An essential role for a small synaptic vesicle-associated phosphatidylinositol 4-kinase in neurotransmitter release. J Neurosci. 1998;18:5594–5602. doi: 10.1523/JNEUROSCI.18-15-05594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winkler H. The adrenal chromaffin granule: a model for large dense core vesicles of endocrine and nervous tissue. J Anat. 1993;183:237–252. [PMC free article] [PubMed] [Google Scholar]
- 35.Zerbes M, Bunn ST, Powis DA. Histamine causes Ca2+ entry via both a store-operated and a store-independent pathway in bovine adrenal chromaffin cells. Cell Calcium. 1998;23:379–386. doi: 10.1016/s0143-4160(98)90094-x. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X, Majerus PW. Phosphatidylinositol signalling reactions. Semin Cell Dev Biol. 1998;9:153–160. doi: 10.1006/scdb.1997.0220. [DOI] [PubMed] [Google Scholar]
- 37.Zhao X, Varnai P, Tuymetova G, Balla A, Toth ZE, Oker-Blom C, Roder J, Jeromin A, Balla T. Interaction of neuronal calcium sensor-1 (NCS-1) with phosphatidylinositol 4-kinase beta stimulates lipid kinase activity and affects membrane trafficking in COS-7 cells. J Biol Chem. 2001;276:40183–40189. doi: 10.1074/jbc.M104048200. [DOI] [PubMed] [Google Scholar]