Abstract
Ras proteins are small GTPases with well known functions in cell proliferation and differentiation. In these processes, they play key roles as molecular switches that can trigger distinct signal transduction pathways, such as the mitogen-activated protein kinase (MAPK) pathway, the phosphoinositide-3 kinase pathway, and the Ral–guanine nucleotide dissociation stimulator pathway. Several studies have implicated Ras proteins in the development and function of synapses, but the molecular mechanisms for this regulation are poorly understood. Here, we demonstrate that the Ras–MAPK pathway is involved in synaptic plasticity at the Drosophila larval neuromuscular junction. Both Ras1 and MAPK are expressed at the neuromuscular junction, and modification of their activity levels results in an altered number of synaptic boutons. Gain- or loss-of-function mutations in Ras1 and MAPK reveal that regulation of synapse structure by this signal transduction pathway is dependent on fasciclin II localization at synaptic boutons. These results provide evidence for a Ras-dependent signaling cascade that regulates fasciclin II-mediated cell adhesion at synaptic terminals during synapse growth.
Keywords: mitogen-activated protein kinase, Ras, neuromuscular junction, internalization, cell adhesion, synapse plasticity
Synapse formation and modification are highly complex processes that include the activation of gene expression, cytoskeletal reorganization, and signal transduction activation (Koh et al., 2000; Lee and Sheng, 2000). A pathway involved in these processes is the Ras–mitogen-activated protein kinase (MAPK) signal transduction cascade (Lowy and Willumsen, 1993; Mazzucchelli and Brambilla, 2000). Ras proteins are highly localized in developing and adult brains (Leon et al., 1987), and maintenance of long-term potentiation is critically dependent on MAPK activation (English and Sweatt, 1997). Mutations in genes encoding members of the MAPK pathway, such as MAPK kinase (MEK), Ras–guanine nucleotide-releasing factor, and H-Ras, cause defects in learning and long-term potentiation (Brambilla et al., 1997; Atkins et al., 1998; Manabe et al., 2000).
In Aplysia, ApMAPK, the homolog of P44/42 extracellular signal-regulated kinase (ERK), plays a major role in long-term facilitation (LTF) (Bailey et al., 1997). LTF elicits translocation of activated ApMAPK into the neuronal nucleus and the internalization of ApCAM, a homolog of neuronal cell-adhesion molecule in mice, and fasciclin II (FasII) in flies (Mayford et al., 1992). Mutations in MAPK or MAPK phosphorylation targets in ApCAM block internalization of ApCAM, preventing synaptic growth (Bailey et al., 1997; Martin et al., 1997).
The Drosophila neuromuscular junction (NMJ) is a powerful system to understand the mechanisms underlying synaptic plasticity. Larval NMJs continuously increase in size to compensate for muscle growth during development. This form of plasticity is controlled by electrical activity and FasII-mediated cell adhesion (Budnik et al., 1990; Schuster et al., 1996a,b; Koh et al., 1999). Discs-Large (DLG), a member of the postsynaptic density-95 protein family, associates with the synaptic cytoskeleton, where it clusters FasII (Thomas et al., 1997). With increased electrical activity, Ca2+/calmodulin protein kinase II (CaMKII) phosphorylates DLG, decreasing its FasII-clustering ability and promoting NMJ growth (Koh et al., 1999).
The studies in Aplysia raise the possibility that the MAPK pathway may also regulate the synaptic localization of FasII. Ras1, theDrosophila homolog of N-, K-, and H-Ras, is activated upon GTP binding (Gaul et al., 1992) and activates the phosphoinositide-3 kinase (PI3-K) (p110), Ral–guanine nucleotide dissociation stimulator (GDS), and MAPK pathways (Fig.1A) (Bergmann et al., 1998). As in mammals, in Drosophila Ras1 activity can be manipulated by mutations in ras1 and by ras1transgenic variants. Substitution of glycine-12 to valine (Ras1V12) renders a constitutively active form of Ras1, and all three pathways can be activated. Additional mutations in the effector loop of Ras1V12 can result in the activation of a single pathway, because the interaction between Ras1 and the other two effectors is blocked (Fig.1A) (White et al., 1995; Rodriguez-Viciana et al., 1997; Bergmann et al., 1998; Karim and Rubin, 1998; Halfar et al., 2001).
Fig. 1.
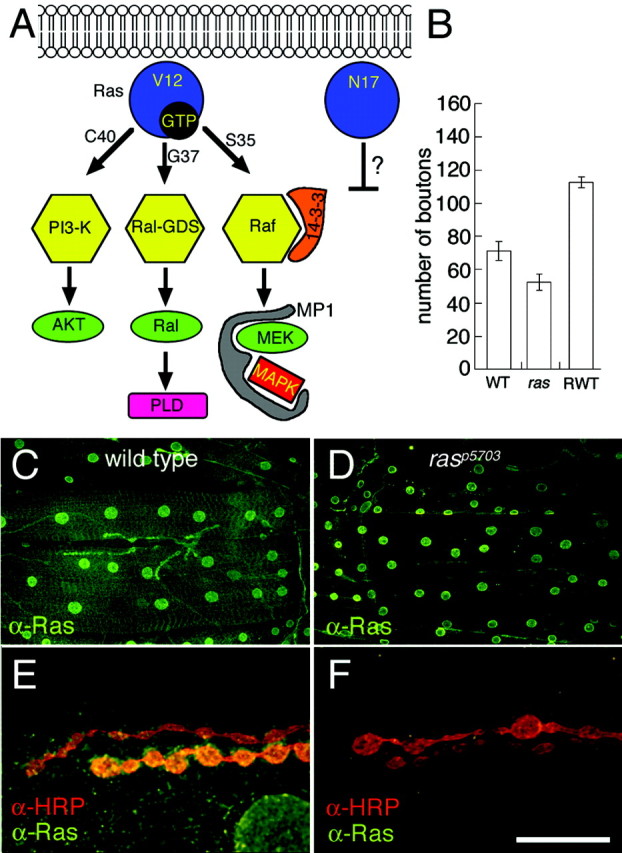
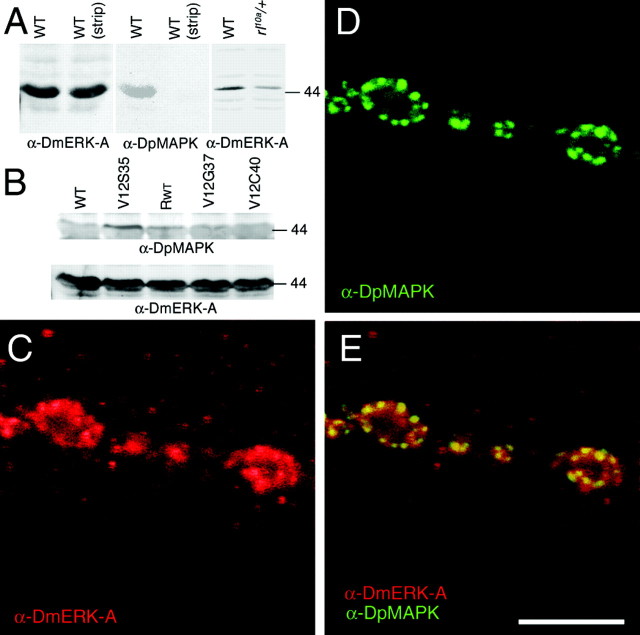
Ras signal transduction pathways and Ras1 expression at the Drosophila larval NMJ.A, Diagram of signal transduction pathways constitutively activated by Ras1 mutant constructs. Substitution of glycine-12 to valine (Ras1V12) renders Ras1 constitutively active. Additional substitution of threonine-35 to serine (Ras1V12S35), glutamic acid-37 to glycine (Ras1V12G37), or tyrosine-40 to cysteine (Ras1V12C40) results in the activation of a single pathway (Bergmann et al., 1998; Karim and Rubin, 1998). Substitution of serine-17 into asparagine (Ras1N17) has been reported in some studies to result in a dominant-negative form of Ras1, but not in others.AKT, Cell-derived AKT8 virus oncogene; PLD, phospholipase D. B, Number of synaptic boutons at muscles 6 and 7 in wild-type (WT),rasP5703 (ras), and wild-type overexpressing transgenic wild-type Ras1 (Ras1WT;RWT) in motor neurons and muscles. C, D, Anti-Ras1 immunoreactivity at the larval body wall muscles of wild type (C) andrasP5703 mutant (D). Note that in wild type, immunoreactivity is concentrated at synaptic boutons and muscle nuclei as well as at low levels throughout the muscle surface. In the ras mutant, immunoreactivity at NMJs and at the muscle surface is severely reduced, but the signal at the nuclei persists. E, F, High-magnification view of synaptic boutons double-stained with anti-HRP (red) and anti-Ras (green) in wild type (E) and rasP5703 mutant (F). Scale bar, 20 μm.
A Drosophila MAPK gene, rolled(rl), with homology to P42/44-ERKs has also been identified (Biggs et al., 1994; Oellers and Hafen, 1996). The functions of MAPK in Drosophila have been investigated in eye and wing development (Bergmann et al., 1998; Karim and Rubin, 1998; Treisman and Heberlein, 1998), but its synaptic function is unknown.
Here, we assessed the role of the Ras–MAPK signal transduction pathway in synaptic plasticity at the larval NMJ. We find that both Ras1 and MAPK are expressed at presynaptic terminals, where they regulate synaptic bouton number. Mutational and transgenic analyses indicate that this regulation is likely to be mediated by local changes in FasII at the presynaptic terminal.
MATERIALS AND METHODS
Flies. Flies were reared in standard conditions between 22 and 25°C. We used the following strains: a hypomorphic mutation in ras1, ras15703(Schnorr and Berg, 1996), upstream activation sequence (UAS)-RafF179, UAS-RalA72L, and UAS-Ras85DV12, obtained from the Bloomington Stock Center (Bloomington, IN); UAS-Ras1WT/CyO, UAS-Ras185DV12S35/CyO, UAS-Ras185DV12G37, UAS-Ras185DV12C40, UAS-Ras185DN17, andrl10a/CyO, obtained fromBergmann et al. (1998); andrlSEM/+ (Oellers and Hafen, 1996), obtained from Dr. L. Zipursky (University of California, Los Angeles, CA). We also used the severe hypomorphsfasIIe76, reported to contain ∼10% FasII levels (Schuster et al., 1996a), anddlgXI-2, which expresses DLG at very low levels and contains a deletion of the guanylate kinase domain (Woods et al., 1996), and the Gal4 driver strains C380 and Sca-Gal4 (presynaptic) and BG487 (postsynaptic) (Koh et al., 1999). The wild-type strain Canton-S (CS) was used as a control.
Immunocytochemistry. The immunocytochemical procedure for body wall muscle preparations was described by Thomas et al. (1997). The following antibodies were used for this study: rabbit and rat anti-DLGPDZ (1:40,000 and 1:500, respectively) (Thomas et al., 1997), rabbit anti-Ras (1:200; Calbiochem, San Diego, CA), rabbit anti-Drosophila melanogaster (Dm)ERK-A (Biggs and Zipursky, 1992; Gabay et al., 1997b), mouse anti-diphospho-MAPK monoclonal (DpMAPK) (1:20; Sigma, St. Louis, MO) (Gabay et al., 1997a), mouse anti-FasII 1D4 monoclonal (1:2 dilution; gift from Dr. C. Goodman, University of California at Berkeley, Berkeley, CA), rabbit anti-FasII (1:3000; Thomas et al., 1997), and anti-HRP-FITC (1:400 dilution; Sigma). As secondary antibodies, we used FITC- or Texas Red-conjugated donkey anti-rabbit IgG, donkey anti-rat IgG, or donkey anti-mouse IgG (1:200 dilution;Jackson ImmunoResearch, West Grove, PA).
The number of type I boutons at muscles 6 and 7 (abdominal segment 2) was counted under an epifluorescence microscope at 63× magnification in preparations stained with FITC- or Texas Red-conjugated anti-HRP to stain the presynaptic terminal. The intensity of FasII and DpMAPK immunoreactivity at synapses of CS,rl10a/+, andrlSEM/+ was determined as inThomas et al. (1997) using the NIH Image program (version 1.57). Larvae used for intensity analysis (Table 1) were processed simultaneously for immunocytochemistry, and confocal images were acquired under identical conditions. Briefly, a line beginning from the center of a synaptic bouton and extending through the outer limit occupied by DLG immunoreactivity was traced. Next, the maximum intensity along the line (on a linear relative scale of 0–255) was determined using the plot profile function of NIH Image. Four measurements at right angles were taken for each bouton, averaged, and expressed as a percentage of maximum relative intensity.
Table 1.
FasII and DpMAPK immunoreactivity levels at larval NMJs with altered MAPKinase activity
| Anti-dpMAPK (n) | Anti-FasII (n) | |
|---|---|---|
| CS | 143 ± 5 (65) | 106 ± 3 (80) |
| rl10a/+ | 102 ± 4 (66) | 135 ± 3 (68) |
| rlSEM/+ | 194 ± 6 (59) | 80 ± 2 (84) |
Numbers in parentheses represent the number of scored boutons out of ∼20 preparations for each genotype and antibody.
Western blots and immunoprecipitations.Dissected body wall muscles (without CNS) were homogenized inDrosophila buffer 2% buffer (50 mm Tris-HCl, pH 6.8, 25 mmKCl, 2 mm EDTA, 0.3 msucrose, 2% SDS) in a 75°C bath. The homogenate was prepared for SDS-PAGE and separated in an 8% gel. Blots were probed with anti-DmERK-A (1:10,000) or anti-DpMAPK monoclonal (1:500) peroxidase-conjugated secondary antibodies and enhanced with chemiluminescent reagents (Amersham Biosciences, Piscataway, NJ). Coimmunoprecipitations were performed essentially as described byThomas et al. (1997). Briefly, 10 dissected body wall muscle preparations (consisting of body wall muscles and CNS) were homogenized in 100 μl of radioimmunoprecipitation buffer containing protease inhibitors at 4°C. After centrifugation at 3000 ×g for 5 min, the supernatant was precleared with preimmune serum and protein A+G beads for 1 hr. The cleared homogenate was then incubated with rat anti-DLGPDZ (5 μl of crude serum) at 4°C for 1 hr. Immunoprecipitates were collected with protein A+G-Sepharose, separated in a 7.5% SDS-PAGE gel, and immunoblotted sequentially with anti-FasII and anti-DLGPDZ (1:2000 dilution). Bands were visualized with peroxidase-conjugated secondary antibodies and enhanced with chemiluminescent reagents (Amersham). Quantification of band intensities was performed by scanning the radiographic film on a linear response scanner (UMAX-Powerlook III; UMAX, Dallas, TX). The intensity of the bands was measured by using the NIH Image 1.54 software for densitometric analysis of one-dimensional gels.
Statistical analysis. The Mintab program (Minitab Inc., State College, PA; www.minitab.com) was used for statistical analysis. A two-sample Student's t test was used to determine differences between samples. Numbersrepresent mean ± SEM throughout.
RESULTS
Ras1 is expressed at presynaptic terminals and is involved in the regulation of bouton number
To determine the presence of Ras1 at larval NMJs, we used an anti-peptide antibody generated using a conserved Ras1 sequence (Sawada et al., 1989). We found that Ras immunoreactivity was concentrated at type I synaptic boutons and in the nuclei of muscle cells (Fig.1C,E). Low levels of immunoreactivity were also observed throughout the surface of the muscle membrane. The immunoreactivity at synaptic boutons and at the muscle surface was specific, because in the severe hypomorph rasP5703, Ras immunoreactivity was dramatically reduced (Fig.1D,F). In contrast, immunoreactivity at the muscle nuclei was similar to wild type, suggesting that the nuclear staining might be cross-reactivity or that the mutant protein is still able to localize at this site.
To investigate the role of Ras1 at the NMJ, we manipulated Ras1 activity at presynaptic and postsynaptic terminals by using therasP5703 allele and by expressing transgenic wild-type and mutant Ras1 variants using the UAS/Gal4 system (Brand and Perrimon, 1993). For the transgenic experiments, we expressed Ras proteins at both presynaptic and postsynaptic sites by using the Gal4 drivers C380 and Sca-Gal4 (presynaptic) and BG487 (postsynaptic) (Koh et al., 1999). Synaptic morphology was examined by labeling presynaptic arbors using anti-HRP, an insect neuronal marker. We found that decreased levels of Ras1 in the hypomorphrasP5703 mutant resulted in a significant reduction in the number of type I synaptic boutons compared with wild type (Fig. 1B). In contrast, overexpressing transgenic wild-type Ras1 (Ras1WT) at both presynaptic and postsynaptic cells or at presynaptic cells alone caused an increase in the number of boutons (Fig. 1B). An even greater increase was seen by expressing a constitutively active Ras1, Ras1V12 (Fig.2A). These results suggest a Ras-dependent signal transduction pathway in the regulation of synaptic bouton number. The effect in Ras1WT flies also implies that the endogenous pathway, which presumably is activated by Ras during synapse growth, is overstimulated by increasing levels of wild-type Ras. This hypothesis was confirmed by examining the levels of MAPK activation (see below).
Fig. 2.
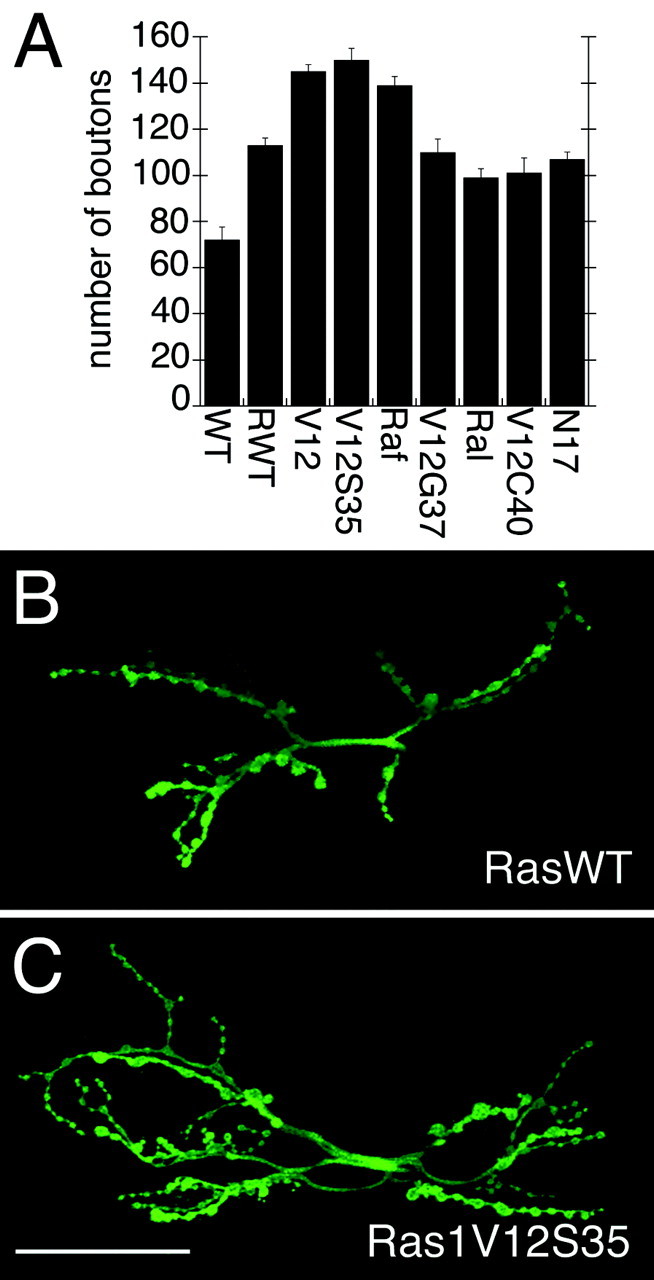
Morphology of NMJs in larvae expressing Ras1 variants. A, Number of type I synaptic boutons in wild-type larvae (WT) and in larvae overexpressing wild-type Ras1 (RWT), constitutively active Ras (RasV12; V12), Ras1V12S35 (V12S35), constitutively active Raf (RafF179; Raf), Ras1V12G37 (V12G37), constitutively active Ral (RalA72L; Ral), Ras1V12C40 (V12C40), and Ras1N17 (N17) using the C380 Gal4 driver. B, C, Anti-HRP immunoreactivity in larvae expressing transgenic wild-type Ras1 (RasWT) (B) or the Ras1V12S35 variant (C), which constitutively activates the MAPK pathway. Note that MAPK pathway activation results in a significantly increased number of synaptic boutons. Scale bar, 50 μm.
To determine which of the signal transduction pathways known to be activated by Ras1 is involved in the regulation of bouton number, we expressed different constitutively active Ras1V12 variants that selectively activate one of the following pathways: Ral-GDS, PI3-K, or MAPK at the NMJ (Fig. 1A). We found that expression of Ras1V12S35, which activates the MAPK pathway only, induced a striking increase in the number of type I boutons at muscles 6 and 7 compared with controls expressing Ras1WT and compared with wild-type larvae (Fig. 2). This increase was indistinguishable from the increase in bouton number observed in Ras1V12. In contrast, expression of Ras1V12G37 (which activates the Ral pathway), Ras1V12C40 (which activates the PI3-K pathway), and Ras1N17 [which has been demonstrated to block Ras1 activation in certain cases (Feig and Cooper, 1988) but not in others (Malumbres and Pellicer, 1998; Marais et al., 1998)] did not alter NMJ morphology compared with RasWT (Fig.2A). However, expression of these transgenes did result in a significant increase in bouton number compared with wild-type controls, suggesting that these pathways may also influence NMJ growth or that the activation of the MAPK pathway is not completely eliminated in these Ras variants. Thus, activation of the MAPK pathway appears to be most effective in increasing bouton number, and activating this pathway alone (Ras1V12S35) mimics the effects of constitutive Ras activation (Ras1V12).
We also studied the effect of constitutively active forms of proteins downstream of Ras: RalA72L, a constitutively active RalA protein (Lee et al., 1996), and RafF179, which activates the MAPK pathway (Brand et al., 1994). We found that in RafF179 there was a dramatic increase in bouton number that was similar to Ras1V12 and Ras1V12S35 (Fig.2A), consistent with the model that the Ras–MAPK pathway is involved in increasing bouton number. However, constitutively active RalA also enhanced bouton number compared with wild-type controls, although to a lesser extent than Ras1V12, Ras1V12S35, and RafF179, in agreement with the idea that activation of other Ras-dependent pathways may also influence synapse growth. Because activation of the Ras–MAPK pathway was the most robust in enhancing bouton number, we centered on the analysis of this pathway.
Similar results were obtained by using either of the presynaptic Gal4 drivers C380 (Fig. 2A) or ScaGal4 alone, or C380 in conjunction with a postsynaptic driver, BG487. In addition, no changes in bouton number were observed when the transgenes were expressed using BG487 alone or in Gal4/+ heterozygotes (data not shown). These results imply that the increase in bouton number is the result of manipulating Ras1 activity at the presynaptic terminals. The increased number of synaptic boutons observed by expressing Ras1V12S35 was not attributable to different levels of expression of the Ras transgenes, as determined by comparing levels of Ras immunoreactivity in the CNS and NMJ in the different Ras transgenic flies (data not shown).
Activated double phosphorylated MAPK is expressed at NMJs, where it regulates the levels of FasII
The above observations suggested that Ras1 is expressed at NMJs, where it can regulate synaptic bouton number through activation of the MAPK pathway. A prediction of this hypothesis is that MAPK also should be expressed at the NMJ. This was tested by using a polyclonal antibody, anti-DmERK-A, against the Drosophila MAPK, Rolled (Biggs and Zipursky, 1992; Gabay et al., 1997b). In addition, we used an anti-MAPK monoclonal antibody, DpMAPK, which recognizes the active, double-phosphorylated form (Gabay et al., 1997a). Western blot analysis of body wall muscle extracts using DmERK-A and DpMAPK antibodies confirmed the presence of a band at ∼44 kDa, as expected inDrosophila. The intensity of the DmERK-A band was decreased to 56 ± 13% in the heterozygous hypomorphrl10a/+ with regard to wild-type controls, demonstrating the specificity of the DmERK-A antibody (Fig. 3A). Notably, activated MAPK was slightly enhanced in flies overexpressing presynaptic Ras1WT. An additional increase was observed in Ras1V12S35 but not in Ras1V12G37 or Ras1V12C40, corroborating the specificity of RasV12S35 in MAPK pathway activation. Levels of total MAPK protein were similar in all genotypes (Fig. 3B).
Fig. 3.
Expression of MAPK at the NMJ. A, Western blot of body wall muscle extracts stained with anti-DmERK-A (left) and reprobed with anti-DpMAPK (middle) after stripping. To show that the blot was completely stripped after staining with DmERK-A, the second lane of the middle panel was incubated with secondary antibody and chemiluminescent reagent before exposure to film. Each lane in the right andmiddle panels was loaded with an equal amount of protein from body wall muscle extracts (∼5 body wall muscle preparations perlane). In the Western blot shown in the left panel, equal amounts of protein (∼2.5 body wall muscle preparations per lane) from extracts of wild-type andrl10A/+ heterozygotes were loaded, and the membrane was probed with anti-DmERK-A.B, Western blots of body wall muscle extracts from wild type (WT) and from larvae overexpressing Ras1V12S35 (V12S35), wild-type Ras1 (Rwt), Ras1V12G37 (V12G37), and Ras1V12C40 (V12C40) using C380, probed sequentially with anti-DpMAPK and anti-DmERK-A. Equal amounts of protein were loaded in each lane (3.5 body wall muscle preparations perlane). The molecular masses given at theright of the blots are in kilodaltons. C, Anti-DmERK-A immunoreactivity at type I synaptic boutons.D, Antibodies against activated MAPK (DpMAPK) result in highly immunoreactive hot spots at synaptic boutons. E, DpMAPK hot spots colocalize with DmERK-A patches at the boutons, but DpMAPK staining is more restricted than DmERK-A, as shown by mergingA and B. Scale bar, 10 μm.
Using the DmERK-A antibody, we found that MAPK was expressed at synaptic boutons of the NMJ both diffusely and in immunoreactive hot spots (Fig. 3C). Because the DmERK-A antibody recognizes both inactive and active MAPK forms, we subsequently used the DpMAPK antibody to determine whether it was similarly localized at synaptic boutons. Remarkably, the active, double-phosphorylated MAPK had a more restricted localization than DmERK-A at synaptic boutons, being localized at well defined hot spots (Fig. 3D) that generally colocalized with areas of high DmERK-A immunoreactivity (Fig.3E). The wider localization of DmERK-A with regard to DpMAPK suggests that active MAPK is selectively recruited to restricted domains at synaptic boutons or that MAPK activation is spatially restricted at the boutons.
In Aplysia, LTF of the gill withdrawal reflex results in an increase in the number of sensory synaptic boutons, an observation that can be replicated in dissociated neuronal cultures by application of serotonin (Mayford et al., 1992). These studies suggest that the decrease of cell adhesion induced by downregulation of ApCAM allows the expansion of sensory processes, resulting in the formation of new synaptic sites. Similarly, at the Drosophila NMJ, manipulations that lead to a decrease in FasII, the homolog of ApCAM, lead to an increase in synaptic bouton number (Budnik et al., 1990;Schuster et al., 1996a; Koh et al., 1999). Notably, inDrosophila, FasII is also required for the maintenance of synaptic boutons. Therefore, increases in bouton number are observed only when FasII is within permissive levels, which are required to maintain the NMJ (Schuster et al., 1996a).
The studies in Aplysia suggest that activation of MAPK during LTF is crucial for the internalization of ApCAM from the surface of sensory neurons and therefore for structural synaptic plasticity during LTF (Martin et al., 1997). In flies, neuronal activity can regulate the localization of FasII by influencing its clustering at the NMJ by DLG (Koh et al., 1999).
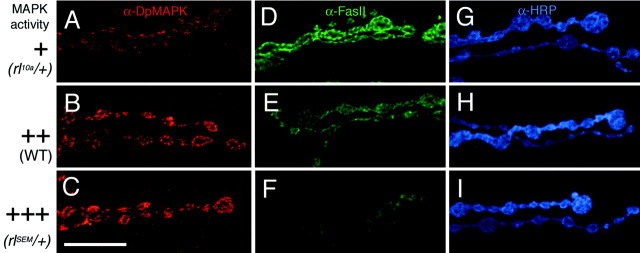
We hypothesized that as in Aplysia, FasII levels at theDrosophila NMJ could be additionally regulated by MAPK activity. This was tested by examining the expression of FasII immunoreactivity in the partial-loss-of-function MAPK mutant (rl10a/+) and in a gain-of-function mutant (rlSEM/+), in which a single amino acid substitution in the kinase domain causes a twofold to threefold increase in levels of MAPK activity (Brunner et al., 1994;Oellers and Hafen, 1996). Consistent with our hypothesis, the intensity of FasII immunoreactivity at the NMJ of the MAPK loss-of-function mutant was 135 ± 3, an ∼30% increase compared with wild-type controls (106 ± 3; Table 1; Fig.4). In contrast, in the gain-of-function mutant, there was an ∼30% reduction in the levels of FasII at the NMJ (81 ± 2; Table 1; Fig. 4). Alteration in MAPK activity levels, however, did not seem to affect the distribution of active MAPK (Fig. 4A–C) or the general morphology of the boutons (Fig. 4G–I), although it did alter bouton number (see below).
Fig. 4.
FasII levels at the NMJ are inversely correlated to MAPK activity. A–C, NMJs immunolabeled with anti-DpMAPK antibody in a hypomorphic MAPK mutant (rl10a/+) (A), wild type (B), and a gain-of-function MAPK mutant (rlSEM/+) (C). D–I, Equivalent views of type I boutons as A–C, but showing anti-FasII labeling (D–F) and anti-HRP labeling (G–I). Note that increased MAPK activity results in decreased levels of FasII and decreased MAPK activity results in increased levels of FasII. Bouton morphology is not affected, as shown by anti-HRP staining. Scale bar, 20 μm.
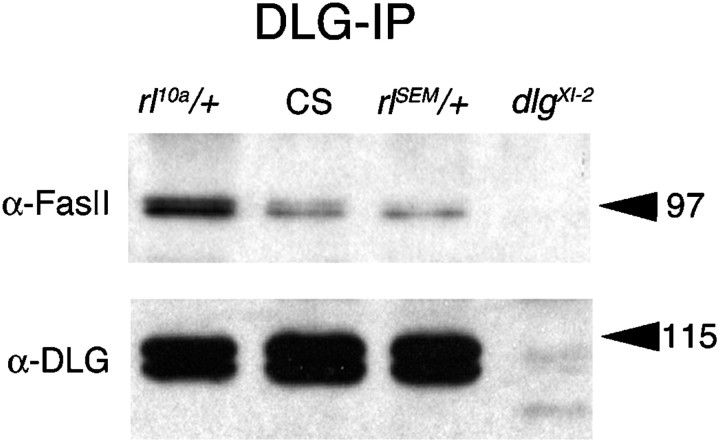
Additional evidence that alterations in MAPK activity result in changes in FasII localization at synapses was obtained by performing immunoprecipitation of body wall muscle extracts using anti-DLG antibodies (Fig. 5). FasII is expressed at all neuromuscular junctions, but it interacts with DLG only at type I boutons (Thomas et al., 1997). Therefore, we expected that immunoprecipitation with anti-DLG would result in coimmunoprecipitation of the transmembrane FasII isoform that is expressed at type I boutons. As expected, the amount of FasII at type I synapses was dependent on MAPK activity. Increased MAPK activity in the gain-of-function MAPK mutant resulted in a 27% reduction in the amount of FasII that was coimmunoprecipitated by DLG compared with wild-type controls (Fig. 5). Conversely, reduced MAPK activity in the loss-of-function MAPK allele resulted in a 170% increase in the amount of FasII coimmunoprecipitated by anti-DLG.
Fig. 5.
Synaptic FasII levels are altered in a MAPK activity-dependent manner. Body wall muscle extracts fromrl10a/+, wild-type (CS), rlSEM/+, anddlgXI-2 were immunoprecipitated with anti-DLG antibody and the Western blots were probed sequentially with anti-FasII and anti-DLG. Note that wild-type,rlSEM/+, andrl10a/+ have similar DLG levels, but DLG-associated FasII is decreased inrlSEM/+ and increased in rl10a/+. The molecular masses given at the right of the blots are in kilodaltons.
We subsequently examined the distribution of active MAPK in relation to FasII to determine whether any change in FasII could be observed at the sites of the synaptic boutons at which active MAPK was concentrated (Fig. 6). As documented by Sone et al. (2000), we found that FasII was not homogeneously distributed at type I synaptic boutons but rather formed an irregular network on their surface, interrupted by nonstaining areas (Fig. 6B). When viewed in rotating Z-series, these nonimmunoreactive areas appeared as little windows through which the opposite side of the bouton was visible. Interestingly, these windows of low FasII immunoreactivity coincided with the highly immunoreactive active MAPK hot spots (Fig. 6A,C). Thus, the decrease in FasII distribution at synaptic boutons corresponds to sites of active MAPK localization. However, whether there is a causal relationship between active MAPK and the regions of low FasII remains to be established.
Fig. 6.
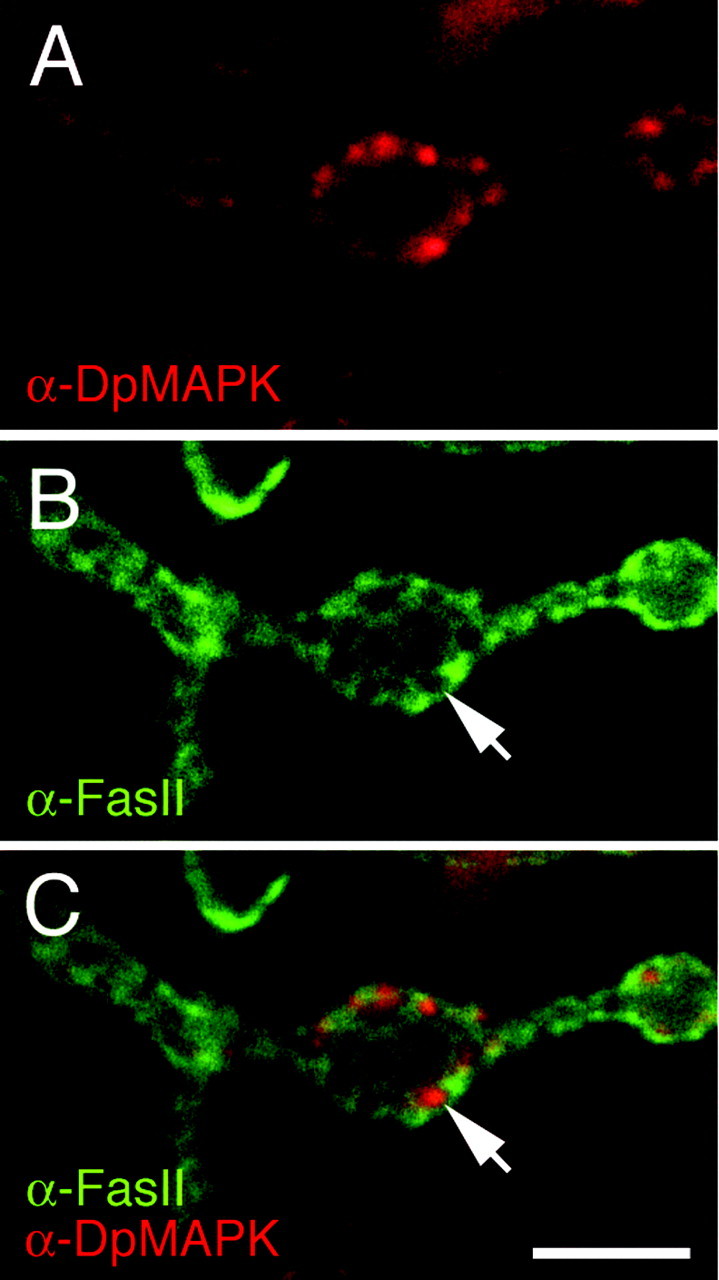
Active MAPK expression coincides with synaptic areas with reduced FasII immunoreactivity. A, Anti-DpMAPK staining at type I synaptic boutons in wild-type.B, The same preparation has been double-labeled with anti-FasII antibodies. C, Images in A andB have been merged to show that areas containing active MAPK have decreased FasII (arrows). Scale bar, 5 μm.
MAPK activity regulates the number of boutons through FasII-mediated adhesion
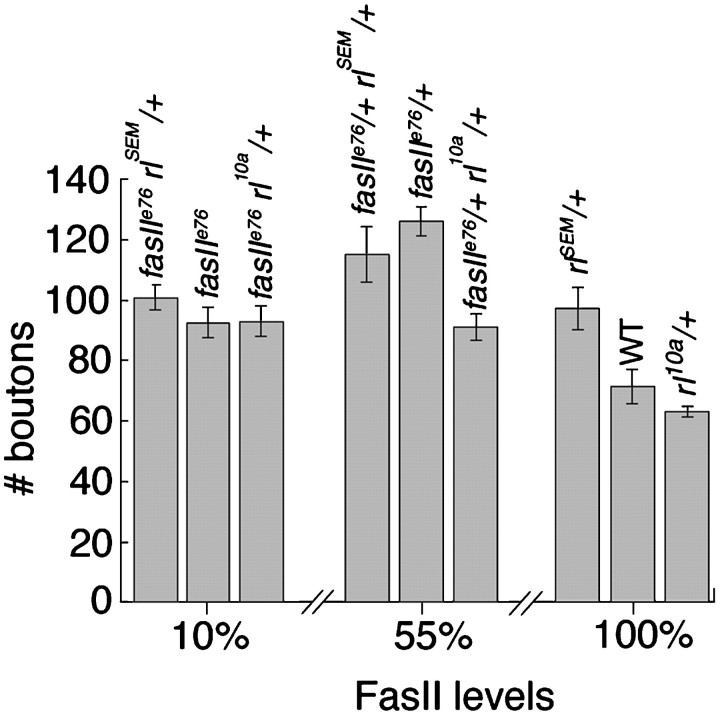
In Drosophila, as in Aplysia, a decrease in levels of FasII results in an increase in the number of synaptic boutons (Schuster et al., 1996a,b). To demonstrate that activated MAPK can affect FasII levels, leading to an increase in the number of boutons, in this report we used the mutant strains Ras1V12S35 and RafF179, which selectively activate the MAPK pathway (Fig. 1), and quantified the number of boutons in the MAPK mutants. Figure 7 shows the number of synaptic boutons in relation to approximate FasII levels at the NMJ. In a wild-type background (100% FasII levels), enhanced MAPK activity in the gain-of-function allele (rlSEM/+) resulted in a decrease in FasII levels (Fig. 4; Table 1) that was accompanied by a significant (p < 0.01) increase in the number of boutons (Fig. 7). However, reduction in MAPK activity in the loss-of-function mutant (rl10a/+) did not significantly affect the bouton number (Fig. 7), although FasII levels were enhanced (Fig. 4; Table 1). Thus, an increase in MAPK activity causes an increase in the number of synaptic boutons, which was accompanied by a decrease in synaptic FasII.
Fig. 7.
Number of synaptic boutons in mutants with alterations in both FasII and MAPK levels. Background levels of FasII in wild-type (100%), fasIIe76homozygotes (10%), andfasIIe76/+heterozygotes (55%) are indicated on the x-axis. Otherbars are positioned on the basis of the influence of the rolled alleles on FasII expression as seen in Figure 4.
To determine whether the increase in synaptic bouton number observed inrlSEM/+ was likely to be mediated by a decrease in FasII, we examined genetic interactions between fasII and rl by generating fasII rl double mutants. Previous studies have shown that the homophilic cell-adhesion molecule FasII is localized both presynaptically and postsynaptically, where it serves two related roles: it is required for synapse maintenance, and it regulates synaptic growth (Schuster et al., 1996a). The first role was demonstrated by the observation that infasII-null mutants, synaptogenesis is normal, but motor endings subsequently retract. The second role was established by usingfasII mutant alleles with different levels of FasII, in which the motor endings showed an increase in bouton number depending on FasII levels. A decrease in FasII levels to ∼50% of normal (e.g., in the heterozygote fasIIe76/+) results in a striking increase in bouton number, presumably because the strong cell adhesion that stabilizes synaptic endings in the wild type is partially lifted, allowing for sprouting. However, decreases in FasII levels much below 50% (e.g., infasIIe76 homozygotes, which are reported to have on the order of 10% wild-type FasII levels) do not result in an additional increase in bouton number, because FasII-mediated cell adhesion becomes compromised, and this interferes with the maintenance of a large arbor (Schuster et al., 1996a).
To ascertain whether MAPK and FasII function in the same pathway during the regulation of bouton number, we generated the double-mutant combinations shown in Figure 7. The relative positions of the bars in the histograms correspond to the approximate change in FasII levels that MAPK mutants are expected to have (Fig. 4; Table 1) in the different fasII mutant backgrounds. If rl andfasII interact genetically, then the effects that each one has on NMJ growth should be nonadditive. We initially generated the mutant combination fasIIe76/+; rl10a/+. We expected that if MAPK and FasII function in the same pathway, then a decrease in MAPK activity should suppress the increased number of boutons resulting from a decreased cell adhesion in thefasIIe76/+ heterozygote. Indeed, we found that the increase in bouton number observed infasIIe76/+ heterozygotes was partially suppressed by rl10a/+(Fig. 7). We also generated the combinationfasIIe76/+; rlSEM/+. We expected that if the increase in bouton number observed inrlSEM/+ was attributable to a decrease in FasII at the NMJ, then we should see an enhancement of the increase in bouton number phenotype infasIIe76/+, provided that FasII levels do not drop below the levels required to maintain a large arbor. However, we found that infasIIe76/+; rlSEM/+ the number of boutons was not significantly different from that in thefasIIe76/+ heterozygote alone (Fig. 7). The observation that the effects were nonadditive in these double mutants indicates that Rl and FasII interact genetically and act in the same pathway. In addition, we found that the moderate increase in bouton number observed in fasIIe76 was neither enhanced nor suppressed by changes in MAPK activity. However, the effects of rl and fasII were nonadditive in every double mutant combination (Fig. 7). The possible interpretation of these results is presented in the Discussion.
DISCUSSION
Previous work in Drosophila demonstrates that synapse stability and synapse expansion during muscle growth are regulated by changes in FasII expression at presynaptic and postsynaptic membranes and that FasII expression is in part controlled by electrical activity (Schuster et al., 1996a). One mechanism through which electrical activity alters FasII levels is by regulating its synaptic clustering via CaMKII-dependent phosphorylation of DLG (Thomas et al., 1997; Koh et al., 1999). In this report, we provide an additional mechanism by which the levels of FasII at the presynaptic terminal are modified: the activation of the Ras–MAPK pathway. This redundant mechanism may serve the differential regulation of FasII localization at the presynaptic and postsynaptic site or may represent FasII regulation in response to different signals. Whereas activation of CaMKII is elicited by an increase in electrical activity, activation of the MAPK pathway may be triggered by activity or by an as yet unknown but different signaling mechanism.
Studies in Aplysia (Bailey et al., 1992, 1997; Martin et al., 1997) indicate that activity-dependent endocytosis of ApCAM results in an increase in the number of synaptic contacts during long-term facilitation (Bailey et al., 1992). Studies by Martin et al. (1997) suggest that ApMAPK is likely to induce ApCAM internalization in a process that depends on ApMAPK activity in dissociated neurons. However, its involvement in the intact organism has not been tested.
In this study, we used Drosophila larval neuromuscular synapses to determine the involvement of the Ras–MAPK pathway in the regulation of synaptic FasII levels and in morphological synaptic plasticity. We demonstrate that both Ras and MAPK are expressed at the NMJ, where they regulate presynaptic expansion. We also showed that this regulation is accomplished by altering FasII levels at synaptic boutons.
Ras proteins are conserved from yeast to humans (Cohen, 1997). InDrosophila, Ras1 has been involved in several processes, including the mechanisms of cell proliferation, eye development, and apoptosis (Grether et al., 1995; Bergmann et al., 1998; Kurada and White, 1998; Malumbres and Pellicer, 1998; Prober and Edgar, 2000). In this study, we used a ras hypomorph mutant and anti-Ras antibodies to determine that Ras1 is specifically expressed at the larval NMJ. Although Ras1 immunoreactivity at synapses and muscles was severely reduced in ras1 hypomorphic mutants, nuclear staining persisted.
Drosophila MAPK has also been studied intensively for its role in cell proliferation and apoptosis. In particular, antibodies directed against the activated form of MAPK (DpMAPK) have shown it to be present in proliferating tissues, such as embryos and imaginal disks (Gabay et al., 1997b). In this study, we used two antibodies to demonstrate the synaptic localization of MAPK at the NMJ, an antibody that recognizes all forms of the MAPK Rolled (DmERK-A) and an antibody that exclusively labels active, double-phosphorylated MAPK (DpMAPK). Interestingly, although both antibodies labeled synaptic boutons, their distribution was not identical. In particular, the antibody against active MAPK-labeled hot spots was more restricted in its localization than general MAPK staining. This suggests that active MAPK is recruited to specific domains within the synaptic bouton or that MAPK activation occurs at discrete regions within the boutons. Interestingly, the same domain that is occupied by active MAPK has lower levels of FasII, consistent with the idea that MAPK activation might be involved in the downregulation of FasII. A recent report (Sone et al., 2000) suggested that the regions of low FasII concentration correspond to the active zone, suggesting that active MAPK is localized to the active zone. The localization pattern of Ras1 and MAPK at synapses is also consistent with the localization protein 14-3-3, another protein that has been involved in the Ras1–Drosophila Raf–MAPK signal transduction pathway (Chang and Rubin, 1997; Zou and Cline, 1999).
Ras1–MAPK signal transduction pathway regulates the number of synaptic boutons
In this study, we found that expression of constitutively active Ras (Ras1V12) drastically increased the number of synaptic boutons. This change was indistinguishable from the increase in boutons observed in the Ras1V12S35 variant and the constitutively activated RafF179, suggesting that these changes were induced by activation of the MAPK pathway. Consistent with these results was the observation that a hypomorphic mutation inras1, ras15703, had the opposite phenotype, a decrease in bouton number, and that a gain-of-function mutation in rl led to an increase in bouton number. Our finding that Ras1V12 and Ras1V12S35 elicited identical phenotypes at the NMJ is consistent with findings in other tissues, such as in the retina, in which the epidermal growth factor receptor–Ras1 pathway is involved in photoreceptor survival (Bergmann et al., 1998), or in the wing disks, where the Ras pathway is involved in hyperplastic growth (Karim and Rubin, 1998).
Notably, expression of Ras variants that activate the PI3-K and Ral signal transduction pathways and a constitutively active RalA also induced an increase in bouton number that was similar in extent to RasWT and considerably lower than Ras1V12. These results raise the possibility that Ras1V12G37 and Ras1V12C40 may still retain some degree of affinity for Raf or, alternatively, that other Ras-mediated pathways might also influence NMJ development. All known ras genes encode a protein region, the effector loop, that is highly conserved in all species. Mutations in this loop interfere with the ability of Ras to bind to specific effectors without altering its catalytic activity. A series of mutations in the effector loop that allow almost exclusive activation of a single effector have been isolated in mammals. The specificity of these mutants has been tested by in vitrobinding assays as well as by genetic and biochemical approaches in cell culture (White et al., 1995; Khosravi-Far et al., 1996;Rodriguez-Viciana et al., 1997). In Drosophila, a genetic approach has been used to demonstrate specificity. These studies suggest that Ras1V12 and RasV12S35 phenotypes are emulated by a hyperactivated form of Raf and suppressed by Raf, MEK, and MAPK mutants (Karim et al., 1996; Karim and Rubin, 1998; Halfar et al., 2001).
Studies in vertebrate cells and in Drosophila suggest that although Ras activation by receptor tyrosine kinases is blocked by the putative dominant-negative RasN17 (Feig and Cooper, 1988; Bergmann et al., 1998; Prober and Edgar, 2000), Ras activation by PKC and the Ras1V12C40/PI3-K effect on cytoskeletal reorganization in fibroblasts are not (Malumbres and Pellicer, 1998; Marais et al., 1998). We found that at the NMJ, Ras1N17 did not behave as a dominant negative. Thus, taken together, our analysis of NMJ structure in the different Ras strains suggests that Ras1 regulates the number of type I glutamatergic synapses in Drosophila and that this regulation depends to a considerable extent on the activation of the MAPK pathway. Although activation of PI3-K and Ral-GDS–Ral by presumably PKC activation also points to a role for these pathways, their effect on NMJ growth was less prominent than the MAPK pathway.
MAPK regulates FasII levels at synaptic boutons
Immunocytochemical studies of FasII immunoreactivity at synaptic terminals of MAPK gain- and loss-of-function mutants suggest that MAPK regulates levels of synaptic FasII, a cell-adhesion molecule that plays a key role in the maintenance and expansion of NMJs inDrosophila (Schuster et al., 1996a,b). This model was supported by experiments in which only type I synaptic FasII was immunoprecipitated. This was accomplished by using anti-DLG antibodies, because DLG binds directly to FasII at type I boutons but not at other bouton types (Thomas et al., 1997). The immunoprecipitation experiments demonstrated that enhancing the levels of MAPK activity at synaptic terminals resulted in a reduction of type I synaptic FasII. Conversely, decreasing levels of MAPK activity resulted in an increase in type I synaptic FasII levels. These results are in agreement with the studies in Aplysia dissociated neurons, which show that ApMAPK is involved in the internalization of ApCAM (Bailey et al., 1997; Martin et al., 1997).
Additional support for the idea that the changes in bouton number elicited by alterations in Ras1 and MAPK activity are mediated by alterations in FasII levels was demonstrated by examining the overall expression of FasII in MAPK gain- or loss-of-function alleles, examining the distribution of FasII within single synaptic boutons in relation to active MAPK, and using hypomorphic fasIImutants. The studies with rl mutants demonstrated that there was an inverse relationship between levels of synaptic FasII and MAPK activity. Furthermore, active MAPK localization coincided with regions of the bouton that have no or low FasII levels.
The studies by Schuster et al. (1996a,b) demonstrate two main functions of FasII in the regulation of synapse number. First, FasII is critically required for synapse maintenance: below threshold FasII levels, synaptic boutons are not maintained. Second, FasII operates by constraining synaptic growth, similar to the Aplysia system (Abel et al., 1998). Therefore, a decrease in FasII to a level still sufficient for maintenance results in an increase in synaptic arbor size (Schuster et al., 1996a). On the basis of this model, we propose the following interpretation of our results. The dramatic decrease in FasII levels in the homozygous fasII mutant did not allow any influence of MAPK activity changes on NMJ structure. Similarly, when FasII levels were decreased to approximately one-half the wild-type levels (fasIIe76/+), an increase in MAPK activity did not induce an additional increase in bouton number, probably because an additional decrease in FasII compromises synaptic maintenance, thus preventing NMJ growth. However, the increase in FasII levels induced by a reduction of MAPK activity (rl10a/+) in afasIIe76/+ background suppressed the increase in boutons observed infasIIe76/+ alone. This result suggested that MAPK regulates FasII levels and exists upstream of FasII at signal transduction pathways that regulate the number of type I synaptic boutons.
Notably, the hypomorph rl10a/+had no significant decrease in bouton number, although these mutants had a striking increase in FasII levels compared with wild-type controls. An explanation for this result is that FasII is a homophilic cell-adhesion molecule that is required both in the presynaptic and in the postsynaptic cell for function (Schuster et al., 1996a; Thomas et al., 1997). If the Ras–MAPK pathway functions to regulate FasII at the presynaptic cell, as suggested by our studies with cell-specific Gal4 drivers, then an asymmetric increase in FasII levels in the presynaptic cell alone may not have much of an effect. Previous studies also show that although the NMJ is very sensitive to a decrease in FasII levels, an increase in FasII over wild-type levels does not have much of an effect (Schuster et al., 1996a).
Although our results are consistent with a regulation of FasII-mediated synapse growth by the Ras–MAPK pathway, it is important to note that several other molecules in addition to FasII are involved in the regulation of synapse growth (Torroja et al., 1999; Sone et al., 2000;Parnas et al., 2001). Moreover, several studies suggest that many changes at the fly NMJ are compensated by yet unknown homeostatic mechanisms (Davis and Goodman, 1998). Therefore, further understanding of these regulatory and compensatory signals will be necessary to fully explain our observations.
In conclusion, we have identified a signaling pathway intimately involved in the regulation of synaptic growth at the NMJ. Identification of the mechanisms involved in the activation of this pathway may provide valuable clues toward understanding the plasticity of this synapse.
Footnotes
This work was supported by National Institutes of Health Grants RO1NS37061 and RO1NS30072 and by a Human Frontier Science Program grant. We thank the imaging facilities at the Department of Biology, University of Massachusetts and Smith College. We also thank Dr. John Roche for helpful comments on this manuscript and the Budnik laboratory members for insightful discussions. We thank Drs. C. Goodman and L. Zipursky for their generous gift of anti-FasII monoclonal and anti-DmERK-A antibodies, respectively.
Correspondence should be addressed to Dr. Vivian Budnik, Department of Biology, Morrill Science Center, University of Massachusetts, Amherst, MA 01003. E-mail: vbudnik@bio.umass.edu.
REFERENCES
- 1.Abel T, Martin KC, Bartsch D, Kandel ER. Memory suppressor genes: inhibitory constraints on the storage of long-term memory. Science. 1998;279:338–341. doi: 10.1126/science.279.5349.338. [DOI] [PubMed] [Google Scholar]
- 2.Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- 3.Bailey CH, Chen M, Keller F, Kandel ER. Serotonin-mediated endocytosis of apCAM: an early step of learning-related synaptic growth in Aplysia. Science. 1992;256:645–649. doi: 10.1126/science.1585177. [DOI] [PubMed] [Google Scholar]
- 4.Bailey CH, Kaang BK, Chen M, Martin KC, Lim CS, Casadio A, Kandel ER. Mutation in the phosphorylation sites of MAP kinase blocks learning-related internalization of apCAM in Aplysia sensory neurons. Neuron. 1997;18:913–924. doi: 10.1016/s0896-6273(00)80331-1. [DOI] [PubMed] [Google Scholar]
- 5.Bergmann A, Agapite J, McCall K, Steller H. The Drosophila gene hid is a direct molecular target of Ras-dependent survival signaling. Cell. 1998;95:331–341. doi: 10.1016/s0092-8674(00)81765-1. [DOI] [PubMed] [Google Scholar]
- 6.Biggs WH, III, Zipursky SL. Primary structure, expression, and signal-dependent tyrosine phosphorylation of a Drosophila homolog of extracellular signal-regulated kinase. Proc Natl Acad Sci USA. 1992;89:6295–6299. doi: 10.1073/pnas.89.14.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biggs WH, III, Zavitz KH, Dickson B, van der Straten A, Brunner D, Hafen E, Zipursky SL. The Drosophila rolled locus encodes a MAP kinase required in the sevenless signal transduction pathway. EMBO J. 1994;13:1628–1635. doi: 10.1002/j.1460-2075.1994.tb06426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brambilla R, Gnesutta N, Minichiello L, White G, Roylance AJ, Herron CE, Ramsey M, Wolfer DP, Cestari V, Rossi-Arnaud C, Grant SG, Chapman PF, Lipp HP, Sturani E, Klein R. A role for the Ras signalling pathway in synaptic transmission and long-term memory. Nature. 1997;390:281–286. doi: 10.1038/36849. [DOI] [PubMed] [Google Scholar]
- 9.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 10.Brand AH, Manoukian AS, Perrimon N. Ectopic expression in Drosophila. Methods Cell Biol. 1994;44:635–654. doi: 10.1016/s0091-679x(08)60936-x. [DOI] [PubMed] [Google Scholar]
- 11.Brunner D, Oellers N, Szabad J, Biggs WH, III, Zipursky SL, Hafen E. A gain-of-function mutation in Drosophila MAP kinase activates multiple receptor tyrosine kinase signaling pathways. Cell. 1994;76:875–888. doi: 10.1016/0092-8674(94)90362-x. [DOI] [PubMed] [Google Scholar]
- 12.Budnik V, Zhong Y, Wu CF. Morphological plasticity of motor axons in Drosophila mutants with altered excitability. J Neurosci. 1990;10:3754–3768. doi: 10.1523/JNEUROSCI.10-11-03754.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang HC, Rubin GM. 14-3-3 epsilon positively regulates Ras-mediated signaling in Drosophila. Genes Dev. 1997;11:1132–1139. doi: 10.1101/gad.11.9.1132. [DOI] [PubMed] [Google Scholar]
- 14.Cohen DM. Mitogen-activated protein kinase cascades and the signaling of hyperosmotic stress to immediate early genes. Comp Biochem Physiol A Physiol. 1997;117:291–299. doi: 10.1016/s0300-9629(96)00266-6. [DOI] [PubMed] [Google Scholar]
- 15.Davis GW, Goodman CS. Genetic analysis of synaptic development and plasticity: homeostatic regulation of synaptic efficacy. Curr Opin Neurobiol. 1998;8:149–156. doi: 10.1016/s0959-4388(98)80018-4. [DOI] [PubMed] [Google Scholar]
- 16.English JD, Sweatt JD. A requirement for the mitogen-activated protein kinase cascade in hippocampal long term potentiation. J Biol Chem. 1997;272:19103–19106. doi: 10.1074/jbc.272.31.19103. [DOI] [PubMed] [Google Scholar]
- 17.Feig LA, Cooper GM. Inhibition of NIH 3T3 cell proliferation by a mutant ras protein with preferential affinity for GDP. Mol Cell Biol. 1988;8:3235–3243. doi: 10.1128/mcb.8.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabay L, Seger R, Shilo BZ. In situ activation pattern of Drosophila EGF receptor pathway during development. Science. 1997a;277:1103–1106. doi: 10.1126/science.277.5329.1103. [DOI] [PubMed] [Google Scholar]
- 19.Gabay L, Seger R, Shilo BZ. MAP kinase in situ activation atlas during Drosophila embryogenesis. Development. 1997b;124:3535–3541. doi: 10.1242/dev.124.18.3535. [DOI] [PubMed] [Google Scholar]
- 20.Gaul U, Mardon G, Rubin GM. A putative Ras GTPase activating protein acts as a negative regulator of signaling by the Sevenless receptor tyrosine kinase. Cell. 1992;68:1007–1019. doi: 10.1016/0092-8674(92)90073-l. [DOI] [PubMed] [Google Scholar]
- 21.Grether ME, Abrams JM, Agapite J, White K, Steller H. The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes Dev. 1995;9:1694–1708. doi: 10.1101/gad.9.14.1694. [DOI] [PubMed] [Google Scholar]
- 22.Halfar K, Rommel C, Stocker H, Hafen E. Ras controls growth, survival and differentiation in the Drosophila eye by different thresholds of MAP kinase activity. Development. 2001;128:1687–1696. doi: 10.1242/dev.128.9.1687. [DOI] [PubMed] [Google Scholar]
- 23.Karim FD, Rubin GM. Ectopic expression of activated Ras1 induces hyperplastic growth and increased cell death in Drosophila imaginal tissues. Development. 1998;125:1–9. doi: 10.1242/dev.125.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Karim FD, Chang HC, Therrien M, Wassarman DA, Laverty T, Rubin GM. A screen for genes that function downstream of Ras1 during Drosophila eye development. Genetics. 1996;143:315–329. doi: 10.1093/genetics/143.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khosravi-Far R, White MA, Westwick JK, Solski PA, Chrzanowska-Wodnicka M, Van Aelst L, Wigler MH, Der CJ. Oncogenic Ras activation of Raf/mitogen-activated protein kinase-independent pathways is sufficient to cause tumorigenic transformation. Mol Cell Biol. 1996;16:3923–3933. doi: 10.1128/mcb.16.7.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koh YH, Popova E, Thomas U, Griffith LC, Budnik V. Regulation of DLG localization at synapses by CaMKII-dependent phosphorylation. Cell. 1999;98:353–363. doi: 10.1016/s0092-8674(00)81964-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koh YH, Gramates LS, Budnik V. Drosophila larval neuromuscular junction: molecular components and mechanisms underlying synaptic plasticity. Microsc Res Tech. 2000;49:14–25. doi: 10.1002/(SICI)1097-0029(20000401)49:1<14::AID-JEMT3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 28.Kurada P, White K. Ras promotes cell survival in Drosophila by downregulating hid expression. Cell. 1998;95:319–329. doi: 10.1016/s0092-8674(00)81764-x. [DOI] [PubMed] [Google Scholar]
- 29.Lee SH, Sheng M. Development of neuron-neuron synapses. Curr Opin Neurobiol. 2000;10:125–131. doi: 10.1016/s0959-4388(99)00046-x. [DOI] [PubMed] [Google Scholar]
- 30.Lee T, Feig L, Montell DJ. Two distinct roles for Ras in a developmentally regulated cell migration. Development. 1996;122:409–418. doi: 10.1242/dev.122.2.409. [DOI] [PubMed] [Google Scholar]
- 31.Leon J, Guerrero I, Pellicer A. Differential expression of the ras gene family in mice. Mol Cell Biol. 1987;7:1535–1540. doi: 10.1128/mcb.7.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowy DR, Willumsen BM. Function and regulation of ras. Annu Rev Biochem. 1993;62:851–891. doi: 10.1146/annurev.bi.62.070193.004223. [DOI] [PubMed] [Google Scholar]
- 33.Malumbres M, Pellicer A. RAS pathways to cell cycle control and cell transformation. Front Biosci. 1998;3:D887–D912. doi: 10.2741/a331. [DOI] [PubMed] [Google Scholar]
- 34.Manabe T, Aiba A, Yamada A, Ichise T, Sakagami H, Kondo H, Katsuki M. Regulation of long-term potentiation by H-Ras through NMDA receptor phosphorylation. J Neurosci. 2000;20:2504–2511. doi: 10.1523/JNEUROSCI.20-07-02504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marais R, Light Y, Mason C, Paterson H, Olson MF, Marshall CJ. Requirement of Ras-GTP-Raf complexes for activation of Raf-1 by protein kinase C. Science. 1998;280:109–112. doi: 10.1126/science.280.5360.109. [DOI] [PubMed] [Google Scholar]
- 36.Martin KC, Michael D, Rose JC, Barad M, Casadio A, Zhu H, Kandel ER. MAP kinase translocates into the nucleus of the presynaptic cell and is required for long-term facilitation in Aplysia. Neuron. 1997;18:899–912. doi: 10.1016/s0896-6273(00)80330-x. [DOI] [PubMed] [Google Scholar]
- 37.Mayford M, Barzilai A, Keller F, Schacher S, Kandel ER. Modulation of an NCAM-related adhesion molecule with long-term synaptic plasticity in Aplysia. Science. 1992;256:638–644. doi: 10.1126/science.1585176. [DOI] [PubMed] [Google Scholar]
- 38.Mazzucchelli C, Brambilla R. Ras-related and MAPK signalling in neuronal plasticity and memory formation. Cell Mol Life Sci. 2000;57:604–611. doi: 10.1007/PL00000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oellers N, Hafen E. Biochemical characterization of rolledSem, an activated form of Drosophila mitogen-activated protein kinase. J Biol Chem. 1996;271:24939–24944. doi: 10.1074/jbc.271.40.24939. [DOI] [PubMed] [Google Scholar]
- 40.Parnas D, Haghighi AP, Fetter RD, Kim SW, Goodman CS. Regulation of postsynaptic structure and protein localization by the Rho-type guanine nucleotide exchange factor dPix. Neuron. 2001;32:415–424. doi: 10.1016/s0896-6273(01)00485-8. [DOI] [PubMed] [Google Scholar]
- 41.Prober DA, Edgar BA. Ras1 promotes cellular growth in the Drosophila wing. Cell. 2000;100:435–446. doi: 10.1016/s0092-8674(00)80679-0. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez-Viciana P, Warne PH, Khwaja A, Marte BM, Pappin D, Das P, Waterfield MD, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 43.Sawada M, Shimizu S, Arai T, Konda S, Enomoto N, Date T. Point mutation in codon 61 of N-RAS genes in human myeloma cell lines. Nucleic Acids Res. 1989;17:8867. doi: 10.1093/nar/17.21.8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schnorr JD, Berg CA. Differential activity of Ras1 during patterning of the Drosophila dorsoventral axis. Genetics. 1996;144:1545–1557. doi: 10.1093/genetics/144.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuster CM, Davis GW, Fetter RD, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron. 1996a;17:641–654. doi: 10.1016/s0896-6273(00)80197-x. [DOI] [PubMed] [Google Scholar]
- 46.Schuster CM, Davis GW, Fetter RD, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. II. Fasciclin II controls presynaptic structural plasticity. Neuron. 1996b;17:655–667. doi: 10.1016/s0896-6273(00)80198-1. [DOI] [PubMed] [Google Scholar]
- 47.Sone M, Suzuki E, Hoshino M, Hou D, Kuromi H, Fukata M, Kuroda S, Kaibuchi K, Nabeshima Y, Hama C. Synaptic development is controlled in the periactive zones of Drosophila synapses. Development. 2000;127:4157–4168. doi: 10.1242/dev.127.19.4157. [DOI] [PubMed] [Google Scholar]
- 48.Thomas U, Kim E, Kuhlendahl S, Koh YH, Gundelfinger ED, Sheng M, Garner CC, Budnik V. Synaptic clustering of the cell adhesion molecule fasciclin II by discs-large and its role in the regulation of presynaptic structure. Neuron. 1997;19:787–799. doi: 10.1016/s0896-6273(00)80961-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torroja L, Packard M, Gorczyca M, White K, Budnik V. The Drosophila β-amyloid precursor protein homolog promotes synapse differentiation at the neuromuscular junction. J Neurosci. 1999;19:7793–7803. doi: 10.1523/JNEUROSCI.19-18-07793.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Treisman JE, Heberlein U. Eye development in Drosophila: formation of the eye field and control of differentiation. Curr Top Dev Biol. 1998;39:119–158. doi: 10.1016/s0070-2153(08)60454-8. [DOI] [PubMed] [Google Scholar]
- 51.White MA, Nicolette C, Minden A, Polverino A, Van Aelst L, Karin M, Wigler MH. Multiple Ras functions can contribute to mammalian cell transformation. Cell. 1995;80:533–541. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- 52.Woods DF, Hough C, Peel D, Callaini G, Bryant PJ. Dlg protein is required for junction structure, cell polarity, and proliferation control in Drosophila epithelia. J Cell Biol. 1996;134:1469–1482. doi: 10.1083/jcb.134.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zou DJ, Cline HT. Postsynaptic calcium/calmodulin-dependent protein kinase II is required to limit elaboration of presynaptic and postsynaptic neuronal arbors. J Neurosci. 1999;19:8909–8918. doi: 10.1523/JNEUROSCI.19-20-08909.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]