Abstract
Drugs of abuse, such as psychostimulants and opiates, are generally considered as exerting their locomotor and rewarding effects through an increased dopaminergic transmission in the nucleus accumbens. Noradrenergic transmission may also be implicated because most psychostimulants increase norepinephrine (NE) release, and numerous studies have indicated interactions between noradrenergic and dopaminergic neurons through α1-adrenergic receptors. However, analysis of the effects of psychostimulants after either destruction of noradrenergic neurons or pharmacological blockade of α1-adrenergic receptors led to conflicting results. Here we show that the locomotor hyperactivities induced by d-amphetamine (1–3 mg/kg), cocaine (5–20 mg/kg), or morphine (5–10 mg/kg) in mice lacking the α1b subtype of adrenergic receptors were dramatically decreased when compared with wild-type littermates. Moreover, behavioral sensitizations induced by d-amphetamine (1–2 mg/kg), cocaine (5–15 mg/kg), or morphine (7.5 mg/kg) were also decreased in knock-out mice when compared with wild-type. Ruling out a neurological deficit in knock-out mice, both strains reacted similarly to novelty, to intraperitoneal saline, or to the administration of scopolamine (1 mg/kg), an anti-muscarinic agent. Finally, rewarding properties could not be observed in knock-out mice in an oral preference test (cocaine and morphine) and conditioned place preference (morphine) paradigm.
Because catecholamine tissue levels, autoradiography of D1 and D2 dopaminergic receptors, and of dopamine reuptake sites and locomotor response to a D1 agonist showed that basal dopaminergic transmission was similar in knock-out and wild-type mice, our data indicate a critical role of α1b-adrenergic receptors and noradrenergic transmission in the vulnerability to addiction.
Keywords: α1b-adrenergic receptors, knock-out mice, locomotor activity, d-amphetamine, cocaine, morphine, behavioral sensitization, oral test, CPP
Psychostimulants, such asd-amphetamine and cocaine, and opiates, such as morphine and heroin, share the ability to cause addiction in humans and to increase release of dopamine (DA) in the nucleus accumbens (Di Chiara and Imperato, 1988; Wise and Rompre, 1989; Pontieri et al., 1995; Koob et al., 1998; Robbins and Everitt, 1999). The same drugs also induce locomotor hyperactivity in rodents and trigger behavioral sensitization after repeated injections (Kalivas and Duffy, 1987; Vezina, 1993;Robinson and Berridge, 2001).
Psychostimulants are thought to increase extracellular DA in the nucleus accumbens by blocking or reversing the DA transporter (DAT) located on dopaminergic nerve terminals. However, a number of studies in mice and rats revealed the existence of interactions between ascending noradrenergic and dopaminergic systems (Antelman and Caggiula, 1977; Kokkinidis and Anisman, 1978, 1979; Tassin et al., 1982, 1986; Ogren et al., 1983; Taghzouti et al., 1988; Lategan et al., 1990; Shi et al., 2000). These interactions may pass through the stimulation of α1-adrenergic receptors (α1-ARs) (Davis et al., 1985; Tessel and Barrett, 1986; Trovero et al., 1992a,b). Indeed, prazosin, an α1-adrenergic antagonist, injected either systemically or locally into the prefrontal cortex, hampers the locomotor hyperactivity induced by d-amphetamine (Snoddy and Tessel, 1985; Dickinson et al., 1988; Blanc et al., 1994; Darracq et al., 1998). Furthermore, microdialysis studies indicated that the stimulation of cortical α1-adrenergic receptors is required to obtain the release, by d-amphetamine, of the functional component of extracellular DA in the nucleus accumbens (Darracq et al., 1998).
At the opposite, earlier studies led to consider that noradrenergic neurons are not implicated in the behavioral effects of psychostimulants. For example, chemical depletion of ascending noradrenergic neurons only slightly affected acute locomotor response to d-amphetamine (Ogren et al., 1983; Mohammed et al., 1986), and Woolverton (1987) found that prazosin failed to systematically alter the reinforcing effects of cocaine in rhesus monkeys.
To further test the involvement of noradrenergic neurons in addictive processes and identify the α1-AR subtype eventually involved in this phenomenon, we used mice knock-out (KO) for the α1b-AR subtype (Cavalli et al., 1997). As previously reported, these KO mice have no apparent phenotype changes except a decreased phenylephrine-induced blood pressure response. Although neurodevelopmental modifications may occur in KO mice, such a model has the advantage to avoid the use of pharmacological compounds that may be unspecific or, as it is the case for prazosin, may not readily cross the blood–brain barrier (Hess, 1975; Trovero et al., 1992b; Stone et al., 2001).
After different biochemical and behavioral controls aimed at verifying that basal dopaminergic activity and motor behavior were similar in α1b-AR KO and wild-type (WT) littermates, locomotor responses and behavioral sensitizations induced by d-amphetamine and cocaine were analyzed in both strains. Because a coupling between noradrenergic and dopaminergic transmission may also affect responses to opiates, similar experiments were performed with morphine. Finally, rewarding properties of cocaine and morphine were estimated. Data indicate a critical role of α1b-ARs in the development of addictive processes to both psychostimulants and opiates.
MATERIALS AND METHODS
Animals
Animals were adult male mice bred at the Institut de Pharmacologie et Toxicologie (Lausanne, Switzerland), weighing 35–45 gm when experiments took place. As described by Cavalli et al. (1997), the genetic background of the mice was a 129/SvXC57BL/6J mixture for both the WT and α1b-AR KO mice. Two of seven chimerical mice, which were mated, gave rise to germ line transmission of the disrupted allele generating heterozygous mice. Heterozygous mice were mated to obtain the homozygous α1b-AR +/+ (WT) and −/− (KO) progeny. For each genotype, mice from different litters were randomly intercrossed to obtain the WT and KO progeny used in this study. Since 1997, this makes at least 40 intercrosses. The mice were never intercrossed with other strains or mated with those from the same litters. Animal experimentation was conducted in accordance with the guidelines for care and use of experimental animals of the European Economic Community (86/809; DL27.01.92, number 116).
Drugs
d-amphetamine sulfate, cocaine hydrochloride, scopolamine hydrobromide, and [±]-6-chloro-7,8-dihydroxy-3-allyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine (chloro-APB) hydrobromide were purchased from Sigma (L'isle d'Abeau, France) and morphine chlorhydrate from Francopia (Paris, France). Doses are expressed as salts.
Locomotor activity
Mice were introduced in a circular corridor (4.5 cm width, 17 cm external diameter) crossed by four infrared beams (1.5 cm above the base) placed at every 90° (Imetronic, Pessac, France). The locomotor activity was counted when animals interrupted two successive beams and, thus, had traveled a 1/4 of the circular corridor. In each session, the spontaneous activity was recorded for 90 min, before mice received saline or drugs, and their activity was recorded for an additional 60 or 120 min period. The first session was a session of habituation to the experimental procedure during which animals received saline (∼3 ml/kg, i.p.). The locomotor response to a drug administration was measured on the next day. Tests were performed between 12:00 and 6:00 P.M. in stable conditions of temperature and humidity.
Autoradiography
Brains were rapidly removed after animal death and frozen in isopentane (−30°C). Sections (20 μm) were cut with a cryostat, mounted onto gelatin-coated glass slides, and stored at −20°C until incubation. For D1 binding sites, sections were incubated with 3H-SCH23390 as previously described (Trovero et al., 1992a). For D2 binding sites, sections were incubated for 60 min at 20°C in Tris–HCl buffer (50 mm, pH 7.4) containing 0.2 nm125I-iodosulpride (NEN DuPont, Paris, France), washed five times in ice-cold Tris-HCl buffer (50 mm, pH 7.4), dried, and exposed to3H-hyperfilm for 10 d. For DAT binding sites, sections were incubated for 2 hr at 20°C in NaH2PO4 buffer (50 mm, pH 7.4) containing 7.5 nm3H-WIN35,428 (NEN DuPont), washed two times in ice-cold NaH2PO4buffer (50 mm, pH 7.4), dried, and exposed to3H-hyperfilm for 15 d. For VMAT binding sites, sections were incubated for 1 hr at 20°C in HEPES buffer (20 mm, pH 8.0) containing 0.3 m sucrose and 2 nm3H-dihydrotetrabenazine (Amersham, Orsay, France), washed two times in ice-cold Tris–HCl buffer (50 mm, pH 7.4), rinsed in distilled water, dried, and exposed to 3H-hyperfilm for 2 months. Autoradiograms were digitized and quantified with a video-imager (ImageQuest video software).
Monoamine tissue contents
Brains were rapidly extracted after animal death and split into two parts in a frontal plane. Anterior parts were frozen in dry ice. Tissue samples were punched out from frontal slices (300 μm obtained with a microtome refrigerated at −12°C) with cooled stainless tubes (an equilateral triangular shape of 3.7 mm side for both sides of prefrontal cortex, and circular shape of 0.9 mm diameter for nucleus accumbens and of 1.4 mm diameter for striatum). Samples were dissolved and sonicated in 150 μl of perchloric acid (0.1 N), sodium metabisulfite (0.05%). After centrifugation, supernatants were used to simultaneously estimate DOPAC, DA, and NE via a column of HPLC coupled to electrochemical detection previously described (Vezina et al., 1992). Protein quantities were determined from the pellets with the bicinchoninic acid-based method (Smith et al., 1985).
Adenylyl cyclase assay in vitro
Four microdisks (diameter, 0.9 mm) were punched bilaterally from the central striatum, blown into 200 μl of 1 mmTris-maleate, pH 7.2, containing 2 mm EGTA, pH 7.2, and 300 mm sucrose, and gently homogenized in a Potter Elvehjem apparatus (10 strokes). Adenylyl cyclase activity was assayed by measuring conversion of [α-32P]ATP into [α-32P]cAMP in the presence or absence of 10−4m DA. [α-32P]cAMP was purified according toSalomon et al. (1974).
Oral consumption
Fluid intake was measured daily by weighing bottles, mice being housed individually. Tested solutions were replaced twice weekly.
Cocaine and morphine. For 2 weeks, bottles were filled with cocaine or morphine solution of decreasing concentration (one dose per week; 0.3 mg/ml then 0.2 mg/ml for cocaine and 0.2 mg/ml and 0.15 mg/ml for morphine) instead of water, so that mice would become accustomed to the bitter taste. Replacing water with cocaine produced no significant alteration of mice fluid intake. Replacing water with morphine significantly increased WT fluid intake [97.5 ± 5.0 ml · kg−1 · d−1for water vs 117.2 ± 3.9 for morphine (0.15 mg/ml),p < 0.001; paired Student's t test), but not α1b-AR KO fluid intake. Then, to determine mean water consumption and eliminate basal side preference, two bottles of water were given for 3 d to each animal, and basal consumption was calculated as the mean consumption from one bottle on day 2 and the other on day 3. Preference for cocaine (0.2 mg/ml) or morphine (0.15 mg/ml) was then measured over two 12 d sessions, drug and water sides being exchanged between the two sessions. Means of drug and water consumptions were estimated for the last 5 d of both 12 d periods.
Sucrose and quinine. In this case, mice were first exposed for 3 d to two bottles of water to measure basal preference as described above. Then on one side, the bottle was filled with either quinine or sucrose solution, and bottles were exchanged on the next day, the mean consumptions of water and either sucrose or quinine being estimated on a 2 d period. Several concentrations of either quinine or sucrose were tested with the same mice in random order.
Morphine-induced conditioned place preference
Conditioned place preference was measured in a Y maze. Mice were habituated to the experimental apparatus (Imetronic, Pessac, France) for 3 d (1 hr of exploration of the maze with neutral cues, i.e., smooth gray walls and floor). On the next day, animals were allowed to freely explore the two compartments (with different visual and tactile cues) of the maze for 20 min corresponding to a preconditioning test. The total amount of time spent in each compartment was recorded and analyzed as previously described (Valverde et al., 1996). For the next 8 d corresponding to the conditioning session, mice alternatively received morphine (5 mg/kg, s.c.) in one compartment and saline in the other compartment the next day. According to the unbiased method, morphine was equally associated to both compartments and was given either first or second, mice being confined in one compartment for the 30 min after each injection. One day after the end of the conditioning session, mice were submitted to a postconditioning test identical to the test performed before conditioning, i.e., each mice being allowed to explore both compartments for 20 min. A 4 d conditioning session was added, and a second postconditioning test was performed similar to the first one. Locomotor activities were recorded with electronic cells in each conditioning session.
Data analysis
Data were analyzed with Student's t test or ANOVA. For behavioral sensitization experiments, correlation between the number of drug injections and the amplitude of the locomotor responses was analyzed with linear regression and the influence of genotype on this correlation with an analysis of covariance. Genotype and prazosin treatments were between-subjects factors. Time, sucrose, and quinine concentration, chloro-APB doses, and number of injections (for behavioral sensitization and morphine-induced CPP) were within-subjects factors. Differences were considered significant when p< 0.05.
RESULTS
Prazosin binding sites in the brain of WT and α1b-AR KO mice
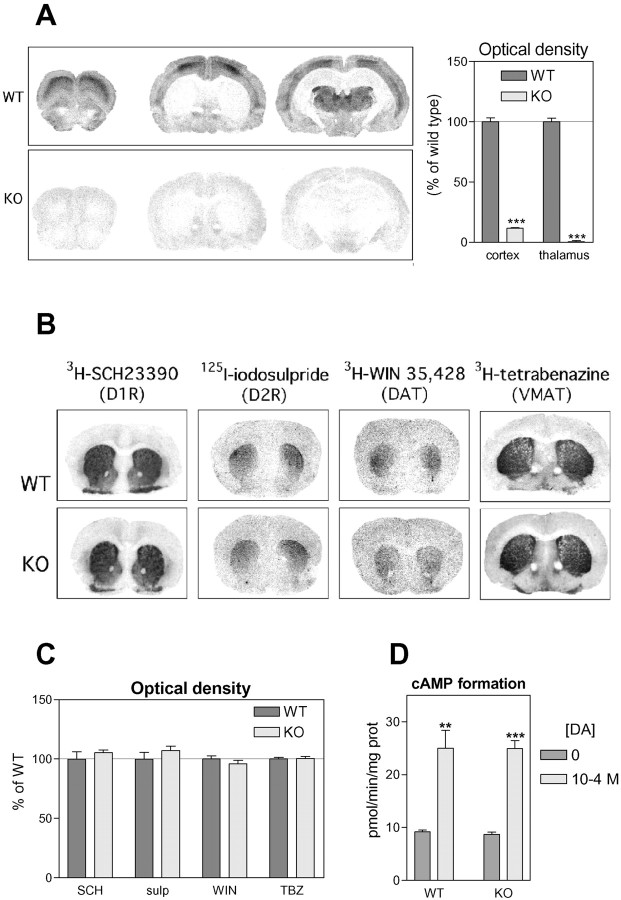
Distributions of 3H-prazosin binding sites on coronal brain sections were compared between WT and α1b-AR KO mice (Fig. 1A).3H-prazosin binding pattern in WT brains was similar to those previously described (Trovero et al., 1992b), with particularly high densities in the layer III of the cerebral cortex and in the thalamus. For α1b-AR KO mice, binding densities were dramatically decreased in these regions (−88% in cortical layer III and −97% in thalamus, compared with WT, p < 0.001, Student's t test), and the typical pattern of prazosin binding was lost.
Fig. 1.
Catecholamine transmission markers in WT and α1b-AR KO mice. A, Autoradiograms show the localization of α1-adrenergic receptors revealed by3H-prazosin (1 nm). Binding densities were quantified in the cortex (layer III) and the thalamus (N = 4 animals per group). B,Autoradiograms show the localization of D1 and D2 dopamine receptors (D1R and D2R), dopamine transporter (DAT), and vesicular monoamine transporter (VMAT) revealed, respectively, by3H-SCH23390, 125I-iodosulpride,3H-WIN35,428, and 3H-tetrabenazine.C, Binding densities were quantified in the striatum (N = 4 animals per group). D,Histograms show the formation of cAMP in striatal membranes under basal conditions or in response to DA (100 μm) (N = 4 animals per group).
Equivalent basal dopaminergic transmission in the brain of WT and α1b-AR KO mice
Striatal distributions (Fig. 1B) and densities (Fig. 1C) of D1 and D2 DA receptors, as well as DA and vesicular monoamine transporters measured by autoradiography with specific radioactive ligands revealed no significant difference between WT and α1b-AR KO animals. Furthermore, the sensitivity of striatal D1 DA receptor to DA measured by in vitro adenylyl cyclase assay was unaltered in α1b-AR KO mice (Fig. 1D). Finally, tissue contents of NE, DA, and DOPAC in the prefrontal cortex, nucleus accumbens, and striatum of WT and α1-AR KO mice were equivalent (Table 1). DOPAC–DA ratios were unmodified, suggesting that basal DA utilization was the same in WT and α1b-AR KO brains.
Table 1.
Tissue contents of DOPAC, DA, and norepinephrine in wild-type (WT) and α1b-AR knock-out (KO) mice
| DOPAC (μg/gm protein) | Dopamine (μg/gm protein) | DOPAC/dopamine | Norepinephrine (μg/gm protein) | ||
|---|---|---|---|---|---|
| Prefrontal cortex | WT | 0.585 ± 0.084 | 1.07 ± 0.23 | 0.571 ± 0.062 | 4.31 ± 0.33 |
| KO | 0.458 ± 0.116 | 1.03 ± 0.16 | 0.451 ± 0.061 | 3.70 ± 0.12 | |
| Nucleus accumbens | WT | 18.3 ± 2.2 | 113 ± 12 | 0.160 ± 0.009 | ND |
| KO | 15.4 ± 2.5 | 95 ± 14 | 0.159 ± 0.008 | ND | |
| Striatum | WT | 14.8 ± 1.5 | 172 ± 25 | 0.117 ± 0.015 | ND |
| KO | 13.4 ± 0.9 | 195 ± 25 | 0.090 ± 0.009 | ND |
N = 4 animals per group. ND, Not determined.
Equivalent locomotor responses to novelty, saline injection, scopolamine, and chloro-APB in WT and α1b-AR KO mice
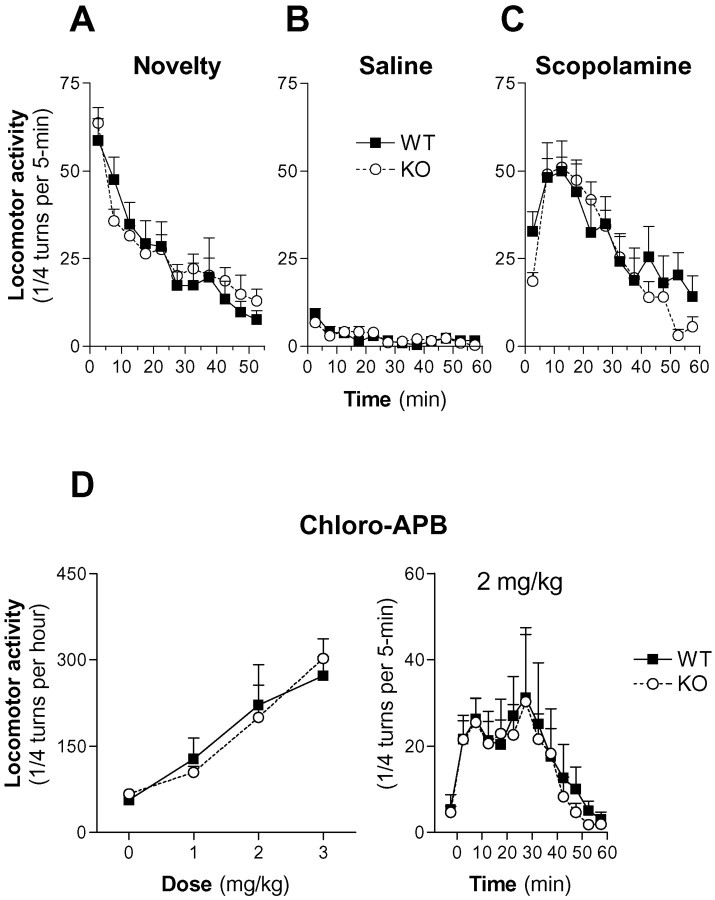
No significant difference was observed between WT and α1b-AR KO mice when their locomotor activity was recorded immediately after their first introduction in the experimental apparatus [time × genotype:F(10,220) = 0.92,p = 0.52; genotype:F(1,220) = 0.03, p = 0.86; two-way repeated measures (RM) ANOVA] (Fig.2A).
Fig. 2.
Locomotor responses to novelty, saline, scopolamine, and chloro-APB in WT and α1b-AR KO mice.A, Locomotor response to novelty was measured every 5 min during the first 50 min mice spent in the experimental apparatus.B, C, Animals were placed in the experimental apparatus for 90 min, received a saline injection, and were replaced in the apparatus for 60 min. On the next day, animals were placed in the experimental apparatus for 90 min, they received an intraperitoneal injection of either saline or scopolamine (1 mg/kg), and their locomotor response was measured every 5 min for 60 min.D, Animals were placed in the experimental apparatus for 90 min, received a saline injection, and were replaced in the apparatus for 60 min. On the following days, animals were placed in the experimental apparatus for 90 min, they received an intraperitoneal injection of chloro-APB, and their locomotor response was measured every 5 min for 60 min. Several doses of chloro-APB were tested on consecutive days in a random order. Groups of 8–14 animals were used in all of these experiments (A–D).
Furthermore, basal locomotor responses of WT and α1b-AR KO mice to an intraperitoneal saline injection were similar (Fig.2B) (time × genotype:F(11,242) = 1.21, p = 0.283; genotype: F(1,242) = 0.01,p = 0.92; two-way RM ANOVA).
The stimulatory effect of scopolamine (1 mg/kg, i.p.), a centrally acting muscarinic antagonist known to act independently from catecholaminergic transmission (Joyce and Koob, 1981; Blanc et al., 1994) (Fig. 2C) was equivalent in WT and α1b-AR KO mice (time × genotype: F(11,209) = 1.63, p = 0.092; genotype:F(1,209) = 0.32, p = 0.58; two-way RM ANOVA).
Finally, chloro-APB, a D1 receptor agonist, dose-dependently increased locomotor activity of WT mice (F(3,21) = 9.08; p < 0.001; one-way RM ANOVA). Similar effects were observed in α1b-AR KO mice (F(3,21) = 18.7;p < 0.0001; one-way RM ANOVA). No significant differences were observed in the amplitudes of locomotor responses to chloro-APB between WT and α1b-AR KO mice (dose × genotype:F(3,28) = 0.66, p = 0.58; genotype: F(1,28) = 0.0,p = 0.95; two-way RM ANOVA) (Fig.2D).
Altogether, these data suggested that α1b-AR KO mice were devoid of gross neurological deficits and were therefore suitable to analyze the role of α1b-ARs in the responses to psychostimulants and opiates.
Reduced locomotor response of α1b-AR KO mice tod-amphetamine, cocaine, and morphine
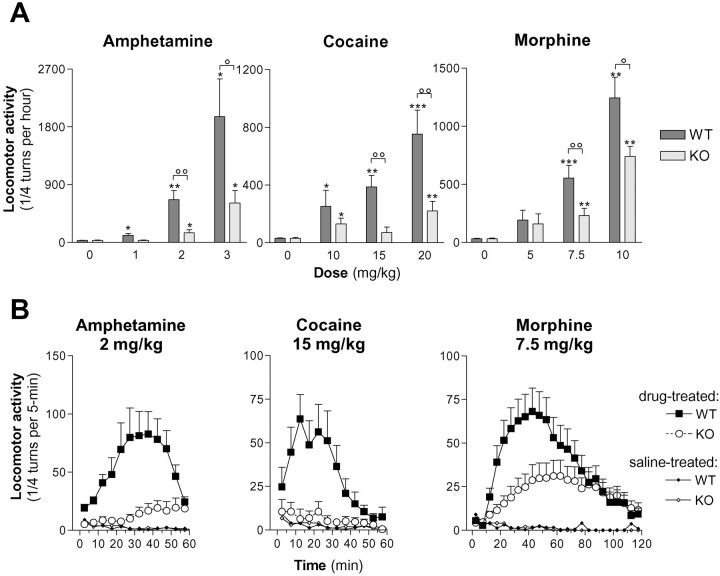
d-amphetamine, cocaine, and morphine induced a dose-dependent stimulation of locomotor activity in WT mice (d-amphetamine: F(3,30) = 14.9, p < 0.0001; cocaine:F(3,33) = 9.3, p = 0.0001; morphine: F(3,30) = 19.0,p < 0.0001; one-way ANOVA) (Fig.3A). In α1b-AR KO mice, these drugs also increased locomotor activity (d-amphetamine:F(3,32) = 9.19, p < 0.001; cocaine: F(3,28) = 3.65,p < 0.05; morphine:F(3,32) = 14.0, p < 0.0001; one-way ANOVA). However, amplitudes of locomotor responses were significantly lower in α1b-AR KO mice compared with WT animals Ford-amphetamine, amplitude of locomotor response was significantly altered by genotype, depending on the dose ofd-amphetamine (genotype × dose:F(2,47) = 5.63, p < 0.05; genotype: F(1,47) = 17.26,p < 0.0001; two-way ANOVA), and locomotor activity of α1b-AR KO were significantly lower in response to 2 and 3 mg/kgd-amphetamine (p < 0.01 and p < 0.05, respectively; Student's ttest).
Fig. 3.
Locomotor responses tod-amphetamine, cocaine, and morphine in WT and α1b-AR KO mice. Locomotor responses to different doses ofd-amphetamine, cocaine, and morphine were measured every 5 min under conditions similar to Figure 2Bexcept that locomotor response to morphine was measured for 120 min. Independent groups of animals were used for each treatment to avoid eventual behavioral sensitization (N = 6–16 per group). A, Total locomotor responses measured during the first hour after the drug administration are presented in function of the dose. *p < 0.05 and **p < 0.01 when WT and α1b-AR KO mice locomotor responses were significantly different from basal locomotor responses. °p < 0.05 and °°p < 0.01 when WT and α1b-AR KO mice locomotor responses were significantly different (Student's t test).B, Time courses of locomotor responses measured every 5 min are illustrated for d-amphetamine (2 mg/kg), cocaine (15 mg/kg), and morphine (7.5 mg/kg).
For cocaine, amplitude of locomotor response was significantly altered by the genotype, and the effect was independent of the dose of cocaine (genotype × dose: F(2,46) = 2.16p > 0.05; genotype:F(1,46) = 14.3, p < 0.001; two-way ANOVA).
For morphine, amplitude of locomotor response was significantly altered by the genotype, and the effect was independent of the dose of morphine (genotype × dose: F(2,40) = 1.22p < 0.05; genotype:F(1,40) = 6.9, p < 0.05; two-way ANOVA). In the course of these experiments we observed that, in KO mice, morphine induced a stereotyped walking behavior identical to that described after local perfusion of opiates in the nucleus accumbens. These responses, considered to be independent of the increased local release of DA (Castellano et al., 1976; Pert and Sivit, 1977; Kalivas et al., 1983), suggest the existence of at least two components in morphine-induced locomotor hyperactivity.
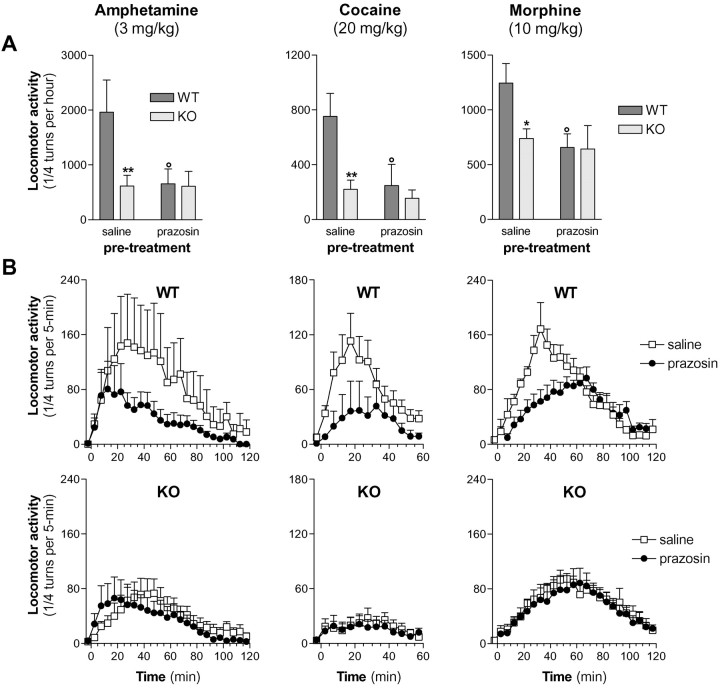
Effects of prazosin on the locomotor responses of WT and α1b-AR KO mice to d-amphetamine, cocaine, and morphine
Because highest doses tested of d-amphetamine (3 mg/kg), cocaine (20 mg/kg), and morphine (10 mg/kg) significantly increased locomotor activity in KO mice, effects of prazosin (1 mg/kg) were measured in these conditions in WT and KO mice (Fig.4). Prazosin significantly reduced the locomotor responses of WT mice to d-amphetamine (p < 0.01, Student's t test), cocaine (p < 0.01, Student's ttest) and morphine (p < 0.05, Student'st test) but failed to modify the locomotor responses observed in α1b-AR KO mice. Moreover, prazosin pretreatment abolished the locomotor differences between WT and α1b-AR KO mice (Fig. 4). This suggests that the inhibitory influence of prazosin observed in WT mice is solely attributable to the blockade of α1b-ARs and that locomotor differences between WT and KO mice are directly caused by the absence of α1b-AR in KO mice rather than to secondary neurodevelopmental deficits. Interestingly, after morphine administration, the stereotyped walking behavior previously described in KO mice was also observed in WT pretreated with prazosin.
Fig. 4.
Prazosin effect on the locomotor responses of WT and α1b-AR KO mice to d-amphetamine, cocaine, and morphine. Animals were placed in the experimental apparatus for 150 min, and they received two saline injections after 60 and 90 min spent in the corridor. On the next day, animals were placed in the experimental apparatus for 60 min, they received an intraperitoneal injection of either saline or prazosin (1 mg/kg), and they were replaced in the corridor for 30 min. Then, they received an intraperitoneal injection of d-amphetamine, cocaine, or morphine, and their locomotor response was measured every 5 min for 60 or 120 min. Independent groups of animals were used for each treatment to avoid eventual behavioral sensitization (N = 6–12 per group). Locomotor responses measured during the first hour after the injection are presented in histograms in A,and time courses are illustrated in B. *p < 0.05 and **p < 0.01 when locomotor responses after prazosin pretreatment were significantly different from locomotor responses after saline pretreatment (Student's t test). °p < 0.05 significantly different from WT mice locomotor responses (Student'st test).
Locomotor sensitization induced by repeated administration of d-amphetamine, cocaine, or morphine in WT and α1b-AR KO mice
For saline, locomotor responses decreased significantly with repeated injections in both WT (−5.3 ± 2.1,F(1,46) = 6.4, p = 0.015) and α1b-AR KO mice (−5.9 ± 2.2,F(1,45) = 7.4, p= 0.009) . The rates of decrease were similar in both strains (F(1,91) = 0.039 ,p = 0.84).
Repeated treatments with d-amphetamine (1–2 mg/kg), cocaine (5–15 mg/kg), or morphine (7.5 mg/kg) led to a progressive increase in the locomotor responses of WT animals that was correlated with the number of drug administrations. The rate of sensitization was evaluated by determining the slope of the response per number of injections curve (Fig. 5).
Fig. 5.

Induction of locomotor sensitizations by repeated administration of d-amphetamine, cocaine, and morphine in WT and α1b-AR KO mice. Animals spent 90 min in the experimental apparatus, received a saline injection, and were replaced in the apparatus for 60 min. On the next day, they spent 90 min in the experimental apparatus, they received an intraperitoneal injection of saline, morphine, cocaine, or d-amphetamine, and their locomotor response was measured for 60 or 120 min. Four similar sessions took place every other day. The sixth session took place after a 10 d withdrawal. Locomotor responses measured during the first hour after each injection are presented in function of the number of injections, and slope values are given in Results.N = 6–15 animals per group.
For morphine (7.5 mg/kg), locomotor response increased significantly with repeated injections in WT mice (146.7 ± 51.4,F(1,63) = 8.11, p = 0.006), but not in α1b-AR KO mice (38.17 ± 19.48,F(1,81) = 3.840, p = 0.0535).
For cocaine (5 mg/kg), locomotor response increased significantly with repeated injections in WT mice (slope: 101.0 ± 47.4,F(1,27) = 4.52, p = 0.042), but not in α1b-AR KO mice (−0.155 ± 20.96,F(1,40) <0.0001, p = 0.99). For cocaine (15 mg/kg), locomotor response increased significantly with repeated injections in both WT (198.2 ± 48.19, F(1,38) = 16.92,p = 0.0002) and α1b-AR KO mice (102.8 ± 19.37,F(1,47) = 28.14, p < 0.0001), and the rates of sensitization differed significantly between strains (F(1,85) = 3.96,p = 0.049).
For d-amphetamine (1 mg/kg), locomotor response increased significantly with repeated injections in WT mice (179.8 ± 69.1,F(1,33) = 6.8, p = 0.014) but not in α1b-AR KO mice (33.4 ± 17.7,F(1,34) =3.5, p = 0.07). For d-amphetamine (2 mg/kg), locomotor response increased significantly with repeated injections in both WT (265 ± 71, F(1,46) =13.69,p = 0.0006) and α1b-AR KO mice (104.4 ± 31,F(1,46) = 10.98, p = 0.0018). However, the rate of sensitization was lower in α1b-AR KO than in WT mice (F(1,92) = 4.23,p = 0.042).
For both WT and α1b-AR KO mice, we also compared the locomotor responses of naive and drug-treated mice (Fig.6). In WT mice, locomotor responses of animals pretreated with drugs were higher than locomotor responses of naïve animals, for all drugs tested. In KO mice, there was no significant difference in the locomotor responses of naive and drug-treated mice for cocaine (5 mg/kg). For other doses and other drugs tested, locomotor responses of drug-treated mice were higher than locomotor responses of naive animals. However, locomotor responses of α1-AR KO drug-treated mice were significantly lower than those of WT drug-treated mice.
Fig. 6.
Expression of locomotor sensitizations induced by repeated administration of d-amphetamine, cocaine, and morphine in WT and α1b-AR KO mice. Locomotor responses tod-amphetamine, cocaine, and morphine were measured in naive mice and in mice having previously received five drug injections, as described in Figure 5. N = 6–12 animals per group.A, Total locomotor responses measured during the first hour after the drug injection. *p < 0.05, **p < 0.01, and ***p < 0.001 significantly different between WT and α1b-AR KO mice. °p < 0.05, °°p < 0.01, and °°°p < 0.001 significantly different from respective naive mice. B, Time course of the locomotor responses measured every 5 min.
Rewarding properties of cocaine and morphine in WT and α1b-AR KO mice
Rewarding properties of cocaine and morphine were assessed in a two-bottle choice paradigm adapted from the method described by Ferraro et al. (2000) for cocaine and Borg and Taylor (1994) for morphine. In this test, WT and α1b-AR KO mice consumptions of cocaine and morphine were different. WT mice exhibited a preference for cocaine (p < 0.05) but not for morphine, whereas KO mice displayed an aversion for both drugs (p < 0.01 and p < 0.05, respectively for cocaine and morphine) (Fig. 7, top).
Fig. 7.
Oral consumption of cocaine, morphine, sucrose, and quinine in a two-bottle choice paradigm in WT and α1b-AR KO mice. Consumption of water, cocaine (0.2 mg/ml), morphine (0.15 mg/ml), and different concentrations of sucrose and quinine were measured in a two-bottle choice paradigm, as described in Materials and Methods, and were expressed in percentage of total fluid intake.N = 7–9 animals per group. *p< 0.05, **p < 0.01 when cocaine or morphine consumption significantly differed from water consumption (paired Student's t test). °p < 0.05, °°°p < 0.001 when consumption of α1b-AR KO mice significantly differed from consumption of WT mice (unpaired Student's t test).
No significant difference could be found between the two groups of animals for either sucrose preference (genotype:F(1,64) = 1.04, p= 0.31; concentration × genotype:F(2,64) = 0.11, p = 0.89; two-way RM ANOVA), or quinine aversion (genotype:F(1,64) = 0.4, p = 0.53; concentration × genotype:F(2,64) = 0.28, p = 0.75; two-way RM ANOVA) (Fig. 7, bottom), indicating that differences observed between genotypes were not related to differences in taste perception.
Because WT mice did not exhibit a clear preference for morphine in the oral consumption test, rewarding properties of morphine (5 mg/kg, s.c.) were also tested in the conditioned place preference (CPP) paradigm. A significant CPP was induced by morphine in WT (p < 0.05) but not in KO mice (Fig.8, top).
Fig. 8.
Conditioned place preference induced by morphine in WT and α1b-AR KO mice. WT and α1b-AR KO mice were conditioned to receive morphine (5 mg/kg, s.c.) in a compartment and a saline injection in the other compartment. The saline group received saline injections in both compartments. N = 6–11 animals per group. Top, Left graph shows the time spent in the morphine-associated compartment before conditioning (pre-test), after four morphine injections and four saline injections (post-test 1), and after two supplementary morphine and two supplementary saline injections (post-test 2). *p < 0.05 and **p < 0.01 when time spent in the drug compartment was different between pre-test and post-test 1 or 2 (paired Student'st test). On the right graph, scores correspond to the time spent in the morphine compartment during the post-test (1 and 2) minus time spent in the morphine compartment during the pre-test. °p< 0.05 when scores were higher for morphine-treated mice than for saline-treated mice (unpaired Student's t test).Bottom, Graphs show the locomotor activity measured during the 30 min of each of the 12 conditioning sessions corresponding either to morphine or to saline injection. ***p < 0.001 locomotor activity measured during the morphine session was higher than during the corresponding saline session (paired Student'st test).
Locomotor activity was recorded during the conditioning sessions after either saline or morphine injection. In WT animals, differences between locomotor responses to saline and morphine were significantly influenced by the number of injections (treatment × number of injections: F(5,50) = 7.316,p < 0.001; two-way RM ANOVA). In KO mice, no significant difference between locomotor responses to saline and morphine was observed (treatment:F(1,60) = 0.0155, p = 0.903; treatment × number of injections:F(5,60) = 1.581, p = 0.179). This indicated that, in these conditions, repeated morphine injections induced a behavioral sensitization in WT but not in KO mice (Fig. 8, bottom).
DISCUSSION
Our data show that α1b-ARs control acute and sensitized locomotor effects, as well as rewarding properties, of compounds belonging to the two main classes of drugs of abuse, psychostimulants and opiates, and considered as exerting their addictive properties through an increased release of DA in the nucleus accumbens. These conclusions can be drawn from differences obtained in the locomotor hyperactivity, behavioral sensitization, and rewarding processes induced by d-amphetamine, cocaine, and morphine in WT and α1b-AR KO mice.
Locomotor responses and behavioral sensitization tod-amphetamine, cocaine, and morphine
Locomotor effects of acute d-amphetamine, cocaine, and morphine were dramatically reduced in α1b-AR KO mice. This decrease directly resulted from the absence of α1b-AR in KO mice because pretreatment with prazosin abolished differences observed between WT and α1b-AR KO mice. Locomotor activities measured in basal conditions or in response to novelty exposure, scopolamine, or a D1 agonist were identical in both strains. This suggests that α1b-AR KO mice do not suffer from a nonspecific locomotor deficit. Nevertheless, the possibility that these KO mice have developed compensatory mechanisms cannot be completely excluded.
d-amphetamine, cocaine and morphine, however, still induced locomotor responses in α1b-AR KO mice. This indicates that α1b-adrenergic transmission greatly enhances but is not absolutely required to obtain locomotor responses to psychostimulants and opiates.
Locomotor responses to psychostimulants observed in KO mice could be attributed to a direct action of the drugs in the nucleus accumbens onto the dopaminergic nerve terminals (Delfs et al., 1990; Vezina et al., 1991; Darracq et al., 1998). In the case of morphine, experiments performed in WT mice in presence of prazosin suggest that a specific locomotor hyperactivity independent of the increased release of catecholamines (Pert and Sivit, 1977; Kalivas et al., 1983) is induced by local stimulation of opioid receptors in the nucleus accumbens (Tempel and Zukin, 1987; Dilts and Kalivas, 1989). After that line, only the morphine-induced locomotor hyperactivity related to an increased release of DA would be dependent on the stimulation of α1b-ARs.
Our results also show that, during the repeated administration of either psychostimulants or morphine, locomotor responses of α1b-AR KO mice became less sensitized than those of WT animals, suggesting that α1b-adrenergic transmission is also involved in the induction and/or in the expression of the sensitized locomotor response. These data are in agreement with previous experiments performed in rats showing that prazosin blocks not only the expression but also the induction of behavioral sensitization to low doses of d-amphetamine (0.75 mg/kg) and cocaine (5 mg/kg) (Drouin et al., 2002) and reduces the expression of the behavioral sensitization induced by morphine (Drouin et al., 2001).
Altogether, these data indicate that expression of behavioral sensitization to psychostimulants and opiates is affected in α1b-AR KO mice but that, at least in these KO mice, other pathways than those involving α1b-ARs allow a partial development of this process.
Rewarding effects of cocaine and morphine in α1b-AR KO mice
Wild-type and KO mice were submitted to an oral preference test, a procedure independent of drug-induced locomotor hyperactivity. KO mice exhibited an aversion for cocaine and morphine, and WT mice exhibited a preference for cocaine and no choice for morphine. This suggests that cocaine and morphine exert a combination of rewarding and aversive effects and that rewarding effects of both drugs are suppressed in KO mice. Indeed, subcutaneous administration of morphine induced a conditioned place preference in WT mice, but not in KO mice, indicating that α1b-AR KO mice may be less sensitive to the rewarding effects of morphine.
How may α1b-ARs interact with dopaminergic neurons?
Electrophysiological observations showed that systemic prazosin hampers bursting activities of ventral tegmental area (VTA) dopaminergic neurons (Grenhoff and Svensson, 1993). More recently, it was shown that systemic reboxetine, a specific inhibitor of the NE transporter (NET), increases the burst firing of VTA–DA cells (Linner et al., 2001). Excitatory input may be provided by glutamatergic afferents, regulated by α1b-ARs, and originating from several areas, including the prefrontal cortex (Gariano and Groves, 1988; Sesack and Pickel, 1992; Chergui et al., 1993; Darracq et al., 1998). This effect, however, is likely to be indirect (Carr and Sesack, 2000) and could pass through the nucleus accumbens (Darracq et al., 2001).d-amphetamine and cocaine increase NE release in the prefrontal cortex (Florin et al., 1994) and may facilitate indirectly dopaminergic transmission in the nucleus accumbens by increasing the stimulation of prefronto-cortical α1b-ARs.
It has also been shown that α1b-ARs are highly expressed in medial and dorsal raphe nuclei (Pieribone et al., 1994; Day et al., 1997), where cell bodies of serotonergic neurons are located. Therefore, it cannot be excluded that the serotonin system serves as an intermediate between psychostimulant-induced NE release and observed behavioral effects.
Behavioral sensitization to psychostimulants can be partly attributable to stimulation of α1b-ARs located either in the VTA (Vezina, 1993;Miner et al., 2001) or in the prefrontal cortex, the latter interacting with cortical D1 receptors (Gioanni et al., 1998) present on the same efferent glutamatergic neurons than those that innervate VTA (Trovero et al., 1994; Lu et al., 1997). Indeed, Li et al. (1999) recently indicated that the cocaine-induced sensitization is under the control of cortical glutamatergic efferents.
Morphine does not increase NE release but, at the opposite, partly inhibits the electrical activity of locus coeruleus noradrenergic neurons (Korf et al., 1974) and stimulates VTA–DA neurons via an inhibition of the GABAergic interneurons in contact with dopaminergic cells in the VTA (Johnson and North, 1992). Our data therefore indicate that, even when VTA–DA neurons are disinhibited by morphine, the functional effect of DA is still controlled by α1b-ARs. This was recently confirmed in experiments showing that local injection of prazosin into the rat prefrontal cortex inhibits morphine-induced locomotor hyperactivity (Drouin et al., 2001).
Previous microdialysis experiments performed in rats in presence of prazosin (Darracq et al., 1998) suggest that behavioral deficits observed in mice lacking α1b-ARs in response tod-amphetamine, cocaine, and morphine are caused by an absence of increased release of functional DA in the nucleus accumbens (Darracq et al., 2001). Indeed, data obtained in α1b-AR KO mice do not result from an alteration in basal dopaminergic transmission mice because no significant differences were detected between both strains in the distribution and striatal densities of D1 and D2 receptors, DAT and VMAT, in the DA and DOPAC tissue contents in the prefrontal cortex, the nucleus accumbens and the striatum, in the DA-induced adenylyl cyclase stimulation in the striatum, or in the locomotor response to a D1 agonist.
Interestingly, Carboni et al. (2001) have recently shown in microdialysis experiments that reboxetine increases extracellular DA levels in the nucleus accumbens of mice lacking DAT. Although these authors relate this effect to the blockade of NET located on noradrenergic fibers innervating the nucleus accumbens, it cannot be excluded that part of this increase in extracellular DA levels is caused by an increased stimulation of cortical α1b-ARs by NE. Such a hypothesis may also explain why mice lacking DAT still self-administer cocaine, a potent inhibitor of NET (Giros et al., 1996; Rocha et al., 1998). Similarly, the hyper-responsiveness to psychostimulants observed in mice lacking NET (Xu et al., 2000) may be linked to the coupling between noradrenergic and dopaminergic systems that we propose.
Role of noradrenergic neurons in the development of addiction
A role of noradrenergic neurons in addiction to opiates has already been suggested, because clonidine, an α2-adrenergic receptor agonist, alleviates opiate-withdrawal symptoms in humans and experimental animals (Delfs et al., 2000). The stimulation of α2-adrenergic somatodendritic autoreceptors would inhibit the electrical activity of noradrenergic neurons. However, this indicates an involvement of noradrenergic neurons in the consequences of opiate abuse but does not implicate these neurons, nor α1-ARs, in the establishment of addictive processes. Moreover, some studies have led to the conclusion that noradrenergic neurons do not play a primary role in the reinforcing properties of psychostimulants. One of the evidence concerns the preserved locomotor response to d-amphetamine in NE-depleted animals (Ogren et al., 1983; Mohammed et al., 1986). However, because it was suggested that DA could stimulate α1-ARs (U'Prichard and Snyder, 1977; Ruffolo et al., 1984; Paladini et al., 2001) and cortical NE and DA depletion abolishedd-amphetamine-induced locomotor responses (A. S. Villégier, G. Blanc, C. Drouin, and J. P. Tassin, unpublished observation), it can be proposed that the DA released byd-amphetamine from the remaining cortical dopaminergic nerve terminals can compensate for NE depletion. Similarly, equivocal results have been obtained when prazosin was used to block behavioral effects of d-amphetamine or cocaine. Indeed, prazosin did not block cocaine discriminative stimulus or rate altering in some studies (Howell and Byrd, 1991; Berthold et al., 1992; Kleven and Koek, 1998; Kleven et al., 1999), whereas prazosin did block the behavioral effects of cocaine in other studies (Poncelet et al., 1983; Tessel and Barrett, 1986; van Haaren, 1992; Sasaki et al., 1995; Spealman, 1995). These varying effects can be related either to the peripheral antihypertensive properties of prazosin or to its difficulty entering brain (Hess, 1975; Trovero et al., 1992b; Stone et al., 2001). The use of mice lacking α1b-ARs allowed to unravel these observations.
Actually, our findings may provide new insights to the problem of the great variability in the individual sensitivity to drugs of abuse observed on humans and animals (O'Brien et al., 1986; Piazza et al., 1989; Hooks et al., 1991; Shiffman, 1991). α1b-ARs are physiologically stimulated by NE and locus coeruleus noradrenergic neurons are extremely sensitive to environmental stimuli (Aston-Jones and Bloom, 1981). Genetic or epigenetic variations in the reactivity of noradrenergic neurons to environmental cues may affect the activation of VTA dopaminergic neurons and, more generally, the sensitivity to drugs of abuse. Similarly, because numerous studies indicate a strong link between glucocorticoids, corticotropin-releasing factor, and NE release (Valentino et al., 1993; Pavcovich and Valentino, 1997; Page and Abercrombie, 1999), it cannot be excluded that the effects of glucocorticoids on VTA dopaminergic cells and drug abuse behavior (Piazza et al., 1989, 1996; Marinelli et al., 1998; Sillaber et al., 1998) are mediated in part through locus coeruleus neurons.
Footnotes
This work was supported by Institut National de la Santé et de la Recherche Médicale, Ministère de la Recherche et de la Technologie, and Fonds National Suisse de la Recherche Scientifique (Grant 31-51043.97). We thank Jean-Antoine Girault for his advice and Denis Hervé and Jean-Christophe Corvol for their help in biochemical experiments.
Correspondence should be addressed to Jean-Pol Tassin, Institut National de la Santé et de la Recherche Médicale U.114, Collège de France, 11 place Marcelin Berthelot, 75231 Paris Cedex 05, France. E-mail:jean-pol.tassin@college-de-france.fr.
REFERENCES
- 1.Antelman SM, Caggiula AR. Norepinephrine-dopamine interactions and behavior. Science. 1977;195:646–653. doi: 10.1126/science.841304. [DOI] [PubMed] [Google Scholar]
- 2.Aston-Jones G, Bloom FE. Nonrepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci. 1981;1:887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berthold CW, Gonzales RA, 3rd, Moerschbaecher JM. Prazosin attenuates the effects of cocaine on motor activity but not on schedule-controlled behavior in the rat. Pharmacol Biochem Behav. 1992;43:111–115. doi: 10.1016/0091-3057(92)90646-w. [DOI] [PubMed] [Google Scholar]
- 4.Blanc G, Trovero F, Vezina P, Herve D, Godeheu AM, Glowinski J, Tassin JP. Blockade of prefronto-cortical alpha 1-adrenergic receptors prevents locomotor hyperactivity induced by subcortical d-amphetamine injection. Eur J Neurosci. 1994;6:293–298. doi: 10.1111/j.1460-9568.1994.tb00272.x. [DOI] [PubMed] [Google Scholar]
- 5.Borg PJ, Taylor DA. Voluntary oral morphine self-administration in rats: effect of haloperidol or ondansetron. Pharmacol Biochem Behav. 1994;47:633–646. doi: 10.1016/0091-3057(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 6. Carboni E, Spielewoy C, Vacca C, Nosten-Bertrand M, Giros B, Di Chiara G. Cocaine and amphetamine increase extracellular dopamine in the nucleus accumbens of mice lacking the dopamine transporter gene. J Neurosci 21 2001. RC141:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castellano C, Filibeck L, Oliverio A. Effects of heroin, alone or in combination with other drugs, on the locomotor activity in two inbred strains of mice. Psychopharmacology (Berl) 1976;49:29–31. doi: 10.1007/BF00427467. [DOI] [PubMed] [Google Scholar]
- 9.Cavalli A, Lattion AL, Hummler E, Nenniger M, Pedrazzini T, Aubert JF, Michel MC, Yang M, Lembo G, Vecchione C, Mostardini M, Schmidt A, Beermann F, Cotecchia S. Decreased blood pressure response in mice deficient of the alpha1b-adrenergic receptor. Proc Natl Acad Sci USA. 1997;94:11589–11594. doi: 10.1073/pnas.94.21.11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chergui K, Charlety PJ, Akaoka H, Saunier CF, Brunet JL, Buda M, Svensson TH, Chouvet G. Tonic activation of NMDA receptors causes spontaneous burst discharge of rat midbrain dopamine neurons in vivo. Eur J Neurosci. 1993;5:137–144. doi: 10.1111/j.1460-9568.1993.tb00479.x. [DOI] [PubMed] [Google Scholar]
- 11.Darracq L, Blanc G, Glowinski J, Tassin JP. Importance of the noradrenaline-dopamine coupling in the locomotor activating effects of d-amphetamine. J Neurosci. 1998;18:2729–2739. doi: 10.1523/JNEUROSCI.18-07-02729.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darracq L, Drouin C, Blanc G, Glowinski J, Tassin JP. Stimulation of metabotropic but not ionotropic glutamatergic receptors in the nucleus accumbens is required for the d-amphetamine-induced release of functional dopamine. Neuroscience. 2001;103:395–403. doi: 10.1016/s0306-4522(00)00578-9. [DOI] [PubMed] [Google Scholar]
- 13.Davis M, Kehne JH, Commissaris RL. Antagonism of apomorphine-enhanced startle by alpha 1-adrenergic antagonists. Eur J Pharmacol. 1985;108:233–241. doi: 10.1016/0014-2999(85)90445-5. [DOI] [PubMed] [Google Scholar]
- 14.Day HE, Campeau S, Watson SJ, Jr, Akil H. Distribution of alpha 1a-, alpha 1b- and alpha 1d-adrenergic receptor mRNA in the rat brain and spinal cord. J Chem Neuroanat. 1997;13:115–139. doi: 10.1016/s0891-0618(97)00042-2. [DOI] [PubMed] [Google Scholar]
- 15.Delfs JM, Schreiber L, Kelley AE. Microinjection of cocaine into the nucleus accumbens elicits locomotor activation in the rat. J Neurosci. 1990;10:303–310. doi: 10.1523/JNEUROSCI.10-01-00303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- 17.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickinson SL, Gadie B, Tulloch IF. Alpha 1- and alpha 2-adrenoreceptor antagonists differentially influence locomotor and stereotyped behaviour induced by d-amphetamine and apomorphine in the rat. Psychopharmacology (Berl) 1988;96:521–527. doi: 10.1007/BF02180034. [DOI] [PubMed] [Google Scholar]
- 19.Dilts RP, Kalivas PW. Autoradiographic localization of mu-opioid and neurotensin receptors within the mesolimbic dopamine system. Brain Res. 1989;488:311–327. doi: 10.1016/0006-8993(89)90723-3. [DOI] [PubMed] [Google Scholar]
- 20.Drouin C, Blanc G, Trovero F, Glowinski J, Tassin JP. Cortical α1-adrenergic regulation of acute and sensitized morphine locomotor effects. NeuroReport. 2001;12:3483–3486. doi: 10.1097/00001756-200111160-00022. [DOI] [PubMed] [Google Scholar]
- 21.Drouin C, Blanc G, Villegier A-S, Glowinski J, Tassin JP. Critical role of alpha1-adrenergic receptors in acute and sensitized locomotor effects of d-amphetamine, cocaine and GBR 12783: Influence of pre-exposure conditions and pharmacological characteristics. Synapse. 2002;43:51–61. doi: 10.1002/syn.10023. [DOI] [PubMed] [Google Scholar]
- 22.Ferraro TN, Golden GT, Berrettini WH, Gottheil E, Yang CH, Cuppels GR, Vogel WH. Cocaine intake by rats correlates with cocaine-induced dopamine changes in the nucleus accumbens shell. Pharmacol Biochem Behav. 2000;66:397–401. doi: 10.1016/s0091-3057(00)00187-8. [DOI] [PubMed] [Google Scholar]
- 23.Florin SM, Kuczenski R, Segal DS. Regional extracellular norepinephrine responses to amphetamine and cocaine and effects of clonidine pretreatment. Brain Res. 1994;654:53–62. doi: 10.1016/0006-8993(94)91570-9. [DOI] [PubMed] [Google Scholar]
- 24.Gariano RF, Groves PM. Burst firing induced in midbrain dopamine neurons by stimulation of the medial prefrontal and anterior cingulate cortices. Brain Res. 1988;462:194–198. doi: 10.1016/0006-8993(88)90606-3. [DOI] [PubMed] [Google Scholar]
- 25.Gioanni Y, Thierry AM, Glowinski J, Tassin JP. Alpha1-adrenergic, D1, and D2 receptors interactions in the prefrontal cortex: implications for the modality of action of different types of neuroleptics. Synapse. 1998;30:362–370. doi: 10.1002/(SICI)1098-2396(199812)30:4<362::AID-SYN3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 26.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 27.Grenhoff J, Svensson TH. Prazosin modulates the firing pattern of dopamine neurons in rat ventral tegmental area. Eur J Pharmacol. 1993;233:79–84. doi: 10.1016/0014-2999(93)90351-h. [DOI] [PubMed] [Google Scholar]
- 28.Hess HJ (1975) Prazosin: biochemistry and structure-activity studies. Postgrad Med Spec: 9–17. [PubMed]
- 29.Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr Individual differences in locomotor activity and sensitization. Pharmacol Biochem Behav. 1991;38:467–470. doi: 10.1016/0091-3057(91)90308-o. [DOI] [PubMed] [Google Scholar]
- 30.Howell LL, Byrd LD. Characterization of the effects of cocaine and GBR 12909, a dopamine uptake inhibitor, on behavior in the squirrel monkey. J Pharmacol Exp Ther. 1991;258:178–185. [PubMed] [Google Scholar]
- 31.Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joyce EM, Koob GF. Amphetamine-, scopolamine- and caffeine-induced locomotor activity following 6-hydroxydopamine lesions of the mesolimbic dopamine system. Psychopharmacology (Berl) 1981;73:311–313. doi: 10.1007/BF00426456. [DOI] [PubMed] [Google Scholar]
- 33.Kalivas PW, Duffy P. Sensitization to repeated morphine injection in the rat: possible involvement of A10 dopamine neurons. J Pharmacol Exp Ther. 1987;241:204–212. [PubMed] [Google Scholar]
- 34.Kalivas PW, Widerlov E, Stanley D, Breese G, Prange AJ., Jr Enkephalin action on the mesolimbic system: a dopamine-dependent and a dopamine-independent increase in locomotor activity. J Pharmacol Exp Ther. 1983;227:229–237. [PubMed] [Google Scholar]
- 35.Kleven MS, Koek W. Discriminative stimulus properties of cocaine: enhancement by monoamine reuptake blockers. J Pharmacol Exp Ther. 1998;284:1015–1025. [PubMed] [Google Scholar]
- 36.Kleven MS, Kamenka JM, Vignon J, Koek W. Pharmacological characterization of the discriminative stimulus properties of the phencyclidine analog, N-[1-(2-benzo(b)thiophenyl)-cyclohexyl]piperidine. Psychopharmacology (Berl) 1999;145:370–377. doi: 10.1007/s002130051070. [DOI] [PubMed] [Google Scholar]
- 37.Kokkinidis L, Anisman H. Involvement of norepinephrine in startle arousal after acute and chronic d-amphetamine administration. Psychopharmacology (Berl) 1978;59:285–292. doi: 10.1007/BF00426636. [DOI] [PubMed] [Google Scholar]
- 38.Kokkinidis L, Anisman H. Circling behavior following systemic d-amphetamine administration: potential noradrenergic and dopaminergic involvement. Psychopharmacology (Berl) 1979;64:45–54. doi: 10.1007/BF00427344. [DOI] [PubMed] [Google Scholar]
- 39.Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- 40.Korf J, Bunney BS, Aghajanian GK. Noradrenergic neurons: morphine inhibition of spontaneous activity. Eur J Pharmacol. 1974;25:165–169. doi: 10.1016/0014-2999(74)90045-4. [DOI] [PubMed] [Google Scholar]
- 41.Lategan AJ, Marien MR, Colpaert FC. Effects of locus coeruleus lesions on the release of endogenous dopamine in the rat nucleus accumbens and caudate nucleus as determined by intracerebral microdialysis. Brain Res. 1990;523:134–138. doi: 10.1016/0006-8993(90)91646-x. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Hu XT, Berney TG, Vartanian AJ, Stine CD, Wolf ME, White FJ. Both glutamate receptor antagonists and prefrontal cortex lesions prevent induction of cocaine sensitization and associated neuroadaptations. Synapse. 1999;34:169–180. doi: 10.1002/(SICI)1098-2396(19991201)34:3<169::AID-SYN1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 43.Linner L, Endersz H, Ohman D, Bengtsson F, Schalling M, Svensson TH. Reboxetine modulates the firing pattern of dopamine cells in the ventral tegmental area and selectively increases dopamine availability in the prefrontal cortex. J Pharmacol Exp Ther. 2001;297:540–546. [PubMed] [Google Scholar]
- 44.Lu XY, Churchill L, Kalivas PW. Expression of D1 receptor mRNA in projections from the forebrain to the ventral tegmental area. Synapse. 1997;25:205–214. doi: 10.1002/(SICI)1098-2396(199702)25:2<205::AID-SYN11>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 45.Marinelli M, Aouizerate B, Barrot M, Le Moal M, Piazza PV. Dopamine-dependent responses to morphine depend on glucocorticoid receptors. Proc Natl Acad Sci USA. 1998;95:7742–7747. doi: 10.1073/pnas.95.13.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miner LA, Liprando LA, Blakely RD, Sesack SR (2001) Ultrastructural interactions between terminals expressing the norepinephrine transporter and dopamine neurons in the rat ventral tegmental area. Soc Neurosci Abstr 27;373.2. [DOI] [PubMed]
- 47.Mohammed AK, Danysz W, Ogren SO, Archer T. Central noradrenaline depletion attenuates amphetamine-induced locomotor behavior. Neurosci Lett. 1986;64:139–144. doi: 10.1016/0304-3940(86)90089-3. [DOI] [PubMed] [Google Scholar]
- 48.O'Brien C, Ehrman R, Terns J. Classical conditionning in human. In: Goldberg S, Stlerman I, editors. Behavioral analysis of drug dependence. Academic; London: 1986. pp. 329–338. [Google Scholar]
- 49.Ogren SO, Archer T, Johansson C. Evidence for a selective brain noradrenergic involvement in the locomotor stimulant effects of amphetamine in the rat. Neurosci Lett. 1983;43:327–331. doi: 10.1016/0304-3940(83)90209-4. [DOI] [PubMed] [Google Scholar]
- 50.Page ME, Abercrombie ED. Discrete local application of corticotropin-releasing factor increases locus coeruleus discharge and extracellular norepinephrine in rat hippocampus. Synapse. 1999;33:304–313. doi: 10.1002/(SICI)1098-2396(19990915)33:4<304::AID-SYN7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 51.Paladini CA, Firillo CD, Morikawa H, Williams JT. Amphetamine selecticvely blocks inhibitory glutamate transmission in dopamine neurons. Nat Neurosci. 2001;4:275–281. doi: 10.1038/85124. [DOI] [PubMed] [Google Scholar]
- 52.Pavcovich LA, Valentino RJ. Regulation of a putative neurotransmitter effect of corticotropin-releasing factor: effects of adrenalectomy. J Neurosci. 1997;17:401–408. doi: 10.1523/JNEUROSCI.17-01-00401.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pert A, Sivit C. Neuroanatomical focus for morphine and enkephalin-induced hypermotility. Nature. 1977;265:645–647. doi: 10.1038/265645a0. [DOI] [PubMed] [Google Scholar]
- 54.Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- 55.Piazza PV, Marinelli M, Rouge-Pont F, Deroche V, Maccari S, Simon H, Le Moal M. Stress, glucocorticoids, and mesencephalic dopaminergic neurons: a pathophysiological chain determining vulnerability to psychostimulant abuse. NIDA Res Monogr. 1996;163:277–299. [PubMed] [Google Scholar]
- 56.Pieribone VA, Nicholas AP, Dagerlind A, Hökfelt T. Distribution of alpha 1 adrenoreceptors in rat brain revealed by in situ hybridization experiments utilizing subtype-specific probes. J Neurosci. 1994;14:4252–4268. doi: 10.1523/JNEUROSCI.14-07-04252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poncelet M, Chermat R, Soubrie P, Simon P. The progressive ratio schedule as a model for studying the psychomotor stimulant activity of drugs in the rat. Psychopharmacology. 1983;80:184–189. doi: 10.1007/BF00427967. [DOI] [PubMed] [Google Scholar]
- 58.Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci USA. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robbins TW, Everitt BJ. Drug addiction: bad habits add up. Nature. 1999;398:567–570. doi: 10.1038/19208. [DOI] [PubMed] [Google Scholar]
- 60.Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- 61. Rocha BA, Fumagalli F, Gainetdinov RR, Jones SR, Ator R, Giros B, Miller GW, Caron MG. Cocaine self-administration in dopamine-transporter knockout mice. Nat Neurosci 1 1998. 132 137 [Erratum (1998) 1:330] [DOI] [PubMed] [Google Scholar]
- 62.Ruffolo RR, Jr, Goldberg MR, Morgan EL. Interactions of epinephrine, noradrenaline, dopamine and their corresponding alpha-methyl-substituted derivatives with alpha and beta adrenoceptors in the pithed rat. J Pharmacol Exp Ther. 1984;230:595–600. [PubMed] [Google Scholar]
- 63.Salomon Y, Londos C, Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974;58:541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- 64.Sasaki JE, Tatham TA, Barrett JE. The discriminativ(1974) e stimulus effects of methamphetamine in pigeons. Psychopharmacology (Berl) 1995;120:303–310. doi: 10.1007/BF02311178. [DOI] [PubMed] [Google Scholar]
- 65.Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;320:145–160. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- 66.Shi WX, Pun CL, Zhang XX, Jones MD, Bunney BS. Dual effects of d-amphetamine on dopamine neurons mediated by dopamine and nondopamine receptors. J Neurosci. 2000;20:3504–3511. doi: 10.1523/JNEUROSCI.20-09-03504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shiffman S. Refining models of dependence: variations across persons and situations. Br J Addict. 1991;86:611–615. doi: 10.1111/j.1360-0443.1991.tb01817.x. [DOI] [PubMed] [Google Scholar]
- 68.Sillaber I, Montkowski A, Landgraf R, Barden N, Holsboer F, Spanagel R. Enhanced morphine-induced behavioural effects and dopamine release in the nucleus accumbens in a transgenic mouse model of impaired glucocorticoid (type II) receptor function: influence of long-term treatment with the antidepressant moclobemide. Neuroscience. 1998;85:415–425. doi: 10.1016/s0306-4522(97)00607-6. [DOI] [PubMed] [Google Scholar]
- 69.Smith P, Krohn R, Hermanson G, Mallia A, Gartner F, Provenzano M, Fujimoto E, Goeke N, Olson B, Klenk D (1985) Measurement of protein using bicinchoninic acid. Anal Biochem: 15076–15085. [DOI] [PubMed]
- 70.Snoddy AM, Tessel RE. Prazosin: effect on psychomotor-stimulant cues and locomotor activity in mice. Eur J Pharmacol. 1985;116:221–228. doi: 10.1016/0014-2999(85)90156-6. [DOI] [PubMed] [Google Scholar]
- 71.Spealman RD. Noradrenergic involvement in the discriminative stimulus effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 1995;275:53–62. [PubMed] [Google Scholar]
- 72.Stone EA, Rosengarten H, Lin Y, Quartermain D. Pharmacological blockade of brain alpha(1)-adrenoceptors as measured by ex vivo. Eur J Pharmacol. 2001;420:97–102. doi: 10.1016/s0014-2999(01)01003-2. [DOI] [PubMed] [Google Scholar]
- 73.Taghzouti K, Simon H, Herve D, Blanc G, Studler JM, Glowinski J, LeMoal M, Tassin JP. Behavioural deficits induced by an electrolytic lesion of the rat ventral mesencephalic tegmentum are corrected by a superimposed lesion of the dorsal noradrenergic system. Brain Res. 1988;440:172–176. doi: 10.1016/0006-8993(88)91172-9. [DOI] [PubMed] [Google Scholar]
- 74.Tassin JP, Simon H, Hervé D, Blanc G, Le Moal M, Glowinski J, Bockaërt J. Non-dopaminergic fibres may regulate dopamine-sensitive adenylate cyclase in the prefrontal cortex and nucleus accumbens. Nature. 1982;295:696–698. doi: 10.1038/295696a0. [DOI] [PubMed] [Google Scholar]
- 75.Tassin JP, Studler JM, Herve D, Blanc G, Glowinski J. Contribution of noradrenergic neurons to the regulation of dopaminergic (D1) receptor denervation supersensitivity in rat prefrontal cortex. J Neurochem. 1986;46:243–248. doi: 10.1111/j.1471-4159.1986.tb12953.x. [DOI] [PubMed] [Google Scholar]
- 76.Tempel A, Zukin RS. Neuroanatomical patterns of the mu, delta, and kappa opioid receptors of rat brain as determined by quantitative in vitro autoradiography. Proc Natl Acad Sci USA. 1987;84:4308–4312. doi: 10.1073/pnas.84.12.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tessel RE, Barrett JE. Antagonism of the behavioral effects of cocaine and d-amphetamine by prazosin. Psychopharmacology. 1986;90:436–440. doi: 10.1007/BF00174057. [DOI] [PubMed] [Google Scholar]
- 78.Trovero F, Herve D, Blanc G, Glowinski J, Tassin JP. In vivo partial inactivation of dopamine D1 receptors induces hypersensitivity of cortical dopamine-sensitive adenylate cyclase: permissive role of alpha 1-adrenergic receptors. J Neurochem. 1992a;59:331–337. doi: 10.1111/j.1471-4159.1992.tb08908.x. [DOI] [PubMed] [Google Scholar]
- 79.Trovero F, Blanc G, Herve D, Vezina P, Glowinski J, Tassin JP. Contribution of an alpha 1-adrenergic receptor subtype to the expression of the “ventral tegmental area syndrome.”. Neuroscience. 1992b;47:69–76. doi: 10.1016/0306-4522(92)90121-h. [DOI] [PubMed] [Google Scholar]
- 80.Trovero F, Marin P, Tassin JP, Premont J, Glowinski J. Accelerated resensitization of the D1 dopamine receptor-mediated response in cultured cortical and striatal neurons from the rat: respective role of alpha 1-adrenergic and N-methyl-d-aspartate receptors. J Neurosci. 1994;14:6280–6288. doi: 10.1523/JNEUROSCI.14-10-06280.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.U'Prichard DC, Snyder SH. Binding of 3H-catecholamines to alpha-noradrenergic receptor sites in calf brain. J Biol Chem. 1977;252:6450–6463. [PubMed] [Google Scholar]
- 82.Valentino RJ, Foote SL, Page ME. The locus coeruleus as a site for integrating corticotropin-releasing factor and noradrenergic mediation of stress responses. Ann NY Acad Sci. 1993;697:173–188. doi: 10.1111/j.1749-6632.1993.tb49931.x. [DOI] [PubMed] [Google Scholar]
- 83.Valverde O, Fournie-Zaluski MC, Roques BP, Maldonado R. The CCKB antagonist PD-134,308 facilitates rewarding effects of endogenous enkephalins but does not induce place preference in rats. Psychopharmacology (Berl) 1996;123:119–126. doi: 10.1007/BF02246168. [DOI] [PubMed] [Google Scholar]
- 84.van Haaren F. Effects of cocaine alone and in combination with prazosin or ondansetron on multiple fixed-interval fixed-ratio performance in pigeons. Pharmacol Biochem Behav. 1992;42:849–853. doi: 10.1016/0091-3057(92)90039-i. [DOI] [PubMed] [Google Scholar]
- 85.Vezina P. Amphetamine injected into the ventral tegmental area sensitizes the nucleus accumbens dopaminergic response to systemic amphetamine: an in vivo microdialysis study in the rat. Brain Res. 1993;605:332–337. doi: 10.1016/0006-8993(93)91761-g. [DOI] [PubMed] [Google Scholar]
- 86.Vezina P, Blanc G, Glowinski J, Tassin J. Opposed behavioral outputs of increased dopamine transmission in prefrontocortical and subcortical areas: a role for the cortical D-1 dopamine receptor. Eur J Neurosci. 1991;3:1001–1007. doi: 10.1111/j.1460-9568.1991.tb00036.x. [DOI] [PubMed] [Google Scholar]
- 87.Vezina P, Blanc G, Glowinski J, Tassin JP. Nicotine and morphine differentially activate brain dopamine in prefrontocortical and subcortical terminal fields: effects of acute and repeated injections. J Pharmacol Exp Ther. 1992;261:484–490. [PubMed] [Google Scholar]
- 88.Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 89.Woolverton WL. Evaluation of the role of norepinephrine in the reinforcing effects of psychomotor stimulants in rhesus monkeys. Pharmacol Biochem Behav. 1987;26:835–839. doi: 10.1016/0091-3057(87)90618-6. [DOI] [PubMed] [Google Scholar]
- 90.Xu F, Gainetdinov RR, Wetsel WC, Jones SR, Bohn LM, Miller GW, Wang YM, Caron MG. Mice lacking the norepinephrine transporter are supersensitive to psychostimulants. Nat Neurosci. 2000;3:465–471. doi: 10.1038/74839. [DOI] [PubMed] [Google Scholar]