Abstract
Acute inescapable stress dramatically affects the inducibility of plasticity at glutamatergic synapses in the intact hippocampus. The present study examined the involvement of serotonergic mechanisms in mediating and modulating the block of long-term potentiation (LTP) in the CA1 area of anesthetized rats after exposure to an elevated platform stress. Fluoxetine and fenfluramine, agents that raise hippocampal extracellular 5-HT concentration, blocked the induction of LTP in nonstressed animals, thus mimicking the effect of stress. In contrast, (±)-tianeptine, a drug that decreases 5-HT levels, had no effect on LTP induction in nonstressed animals. Remarkably, (±) administration of tianeptine after the stress rapidly overcame the block of LTP induction without affecting baseline excitatory transmission. Consistent with a reduction of 5-HT levels being responsible for this effect of tianeptine, the (−) enantiomer, which is associated with the 5-HT uptake enhancing action of (±)-tianeptine, also caused a recovery of the induction of LTP in previously stressed animals, whereas the relatively inactive (+) enantiomer had no effect. Furthermore, fluoxetine prevented the effect of tianeptine in stressed animals. These findings show that antidepressants have rapid and powerful interactions with the mechanisms controlling the persistence of the block of LTP by inescapable stress.
Keywords: acute stress, synaptic plasticity, long-term potentiation, 5-hydroxytryptamine, fluoxetine, fenfluramine, tianeptine, in vivo, antidepressant
Stress has long been recognized to strongly influence learning and memory (Izquierdo and Medina, 1997;McGaugh, 2000). In the case of the performance of hippocampal-dependent learning tasks, stress has been reported to either facilitate or block the acquisition, consolidation, and/or recall of such tasks, depending on experimental conditions (Diamond et al., 1996; Healy and Drugan, 1996; de Quervain et al., 1998; Roozendaal, 2000; Kim et al., 2001).
Stress dramatically affects synaptic plasticity, a putative hippocampal memory mechanism (Kim and Yoon, 1998; McEwen, 1999; Martin et al., 2000; Garcia, 2001). Acute inescapable stress can produce a change in both the susceptibility to, and the direction of, plasticity at glutamatergic synapses in the CA1 area without affecting baseline transmission (Shors et al., 1997; Xu et al., 1997). Such stress blocks high-frequency stimulation-induced persistent increases in synaptic efficacy [long-term potentiation (LTP)] (Shors et al., 1989; Diamond et al., 1990; Xu et al., 1997), whereas low-frequency stimulation-induced long-term depression (LTD) can be facilitated (Kim et al., 1996; Xu et al., 1997). These changes in the inducibility of synaptic plasticity can be observed several hours after the stress episode in anesthetized animals, whereas they are rapidly reversed in awake animals that are allowed to behaviorally adapt to the aversive event (Xu et al., 1997).
A wide variety of neurotransmitter and neuroendocrine systems are activated by stress that can potentially affect synaptic plasticity. Evidence for NMDA and opioid receptor-dependent mechanisms was provided by the prevention of the stress block of LTP by pretreatment with the receptor antagonists CGP 39551 (Kim et al., 1996) and naloxone (Shors et al., 1990), respectively. Consistent with an involvement of corticosteroid-dependent mechanisms in stress modification of plasticity, an antagonist of glucocorticoid receptors (RU38486) and a protein synthesis inhibitor (emetine) prevented the block of LTP induction when given just before or soon after the inescapable stress (Xu et al., 1998). Significantly, apart from emetine, which was inactive, none of these agents was administered at the time of high-frequency conditioning stimulation to determine possible mechanisms maintaining, or ways of overcoming, the block.
Recently, the involvement of serotonergic mechanisms in mediating some of the persistent effects of inescapable stress has gained support (Edwards et al., 1993; Graeff et al., 1996; Chaouloff et al., 1999; de Kloet, 2000; Joëls, 2001). Many aversive stressors have been reported to increase 5-hydroxytryptamine (5-HT) release and levels in both the ventral and dorsal hippocampus (Joseph and Kennett, 1983;Vahabzadeh and Fillenz, 1994; Wilkinson et al., 1996; Matsuo et al., 1996; Ge et al., 1997; Kirby et al., 1997). In the case of inescapable stress, the increase has been found to be greater and more persistent (Amat et al., 1998). Furthermore, 5-HT can inhibit LTP in the CA1 area of the hippocampus (Corradetti et al., 1992; Passani et al., 1994;Stäubli and Otaky, 1994; Stäubli and Xu, 1995). The present experiments investigated the effects of agents that regulate endogenous 5-HT on the ability of high-frequency stimulation to induce LTP in the CA1 area in vivo. An agent that lowers endogenous 5-HT levels was found to reverse the block of LTP induction in anesthetized rats previously exposed to an inescapable raised platform stress (Xu et al., 1997). In contrast, in nonstressed animals the stress-evoked block of LTP was mimicked by compounds that increase extracellular 5-HT concentration. These results point to a possible key role of endogenous 5-HT in mediating and overcoming the effects of inescapable stress on plasticity at glutamatergic synapses.
MATERIALS AND METHODS
Animals and surgery. Adult (280–350 gm) male Wistar rats (inbred strain; Bio-Resources Unit, Trinity College, Dublin) were used in all experiments. Animals were group-housed, six or less to a cage, under a 12 hr light/dark cycle and allowed ad libitum access to food and water. During surgery, the rats were anesthetized with urethane (ethyl carbamate; 2.1 gm/kg, i.p.) and lignocaine (10 mg, 1% adrenaline) was injected subcutaneously over the area of the skull where the electrodes were to be implanted. The body temperature was maintained at 36.8–37.5°C for the duration of the experiments. At the end of each experiment the animal was killed with a lethal dose of sodium pentobarbione (800 mg/kg, i.p.).
Electrode implantation. Electrodes were made and implanted as described previously (Xu et al., 1998). Briefly, twisted wire bipolar electrodes were constructed from Teflon-coated tungsten wires (625 μm tungsten inner core diameter/750 μm external diameter). Recordings of field EPSPs were made from the stratum radiatum in the CA1 area of the right hippocampal hemisphere in response to stimulation of the ipsilateral Schaffer collateral–commissural pathway. The electrode implantation sites were identified using stereotaxic coordinates, with the recording site located 3.4 mm posterior to bregma and 2.5 mm lateral to the midline, and stimulating electrodes 4.2 mm posterior to bregma and 3.8 mm lateral to midline. Stainless steel screws mounted in the skull served as ground (7.0 mm posterior and 5 mm right of midline) and reference (8.0 mm anterior and 0.5 mm lateral of midline) electrodes. The final placement of electrodes the CA1 region was optimized using electrophysiological criteria (Leung, 1979).
Electrophysiology. Test field EPSPs were evoked at a frequency of 0.033 Hz and intensity evoking a response that was 50–60% of maximum amplitude. High-frequency stimulation (HFS) consisted of square pulses (0.2 msec duration) of 10 trains of 20 stimuli with an interstimulus interval of 5 msec (200 Hz) and an intertrain interval of 2 sec.
Stress protocol. Animals were placed on a platform (30 × 27 cm) that was 130 cm above ground level. This protocol was chosen because it has been found to raise serum corticosterone levels and to reliably block the induction of LTP in our laboratory (Xu et al., 1997; see Results). All stressed rats were left on the platform for 30 min followed immediately by anesthesia. Control, nonstressed rats were anesthetized immediately after transfer from the animal house.
Corticosterone assay. Plasma corticosterone levels were assessed using radioimmunoassay (IDS Ltd., Boldon, UK). Plasma samples (∼1 ml) were taken in series by cardiac puncture in separate groups of rats undergoing similar surgical procedures to those used for the electrophysiology experiments. Samples were taken at the time of anesthesia and at the time of application of HFS protocol. All samples were heparinized and centrifuged at room temperature for 5 min. The plasma was then frozen until the day of the assay.
Compounds. All drugs were dissolved in distilled water. (±)-Tianeptine, (−)-tianeptine (S-16190–1) and (+)-tianeptine (S-16191–1) were provided by Servier. (±)-Fluoxetine HCl was purchased from Sigma (St. Louis, MO).
Data analysis. Field EPSP amplitude was measured as the potential difference between the baseline immediately before stimulation and the peak negative response. All data points are expressed as the percentage of the mean response over a 30 min baseline period and presented as the mean ± SEM for 10 min epochs at the times indicated. Statistical comparisons were carried out using repeated measures ANOVA or two-tailed paired and unpaired ttests where appropriate. The probability level interpreted as significant was p < 0.05.
RESULTS
LTP induction in nonstressed animals
First, the ability of agents that raise endogenous 5-HT levels to modulate the induction of LTP in the intact hippocampus of nonstressed rats was investigated (Fig. 1). Both fenfluramine and fluoxetine increase hippocampal extracellular 5-HT concentration by blocking its reuptake but fenfluramine also acts by promoting the release of 5-HT, the levels rising ∼2–3 fold for several hours at the doses tested (Sabol et al., 1992; Hervas and Artigas, 1998; Rocher and Gardier, 2001). Neither drug affected baseline glutamatergic transmission at the doses used to examine their effects on LTP.
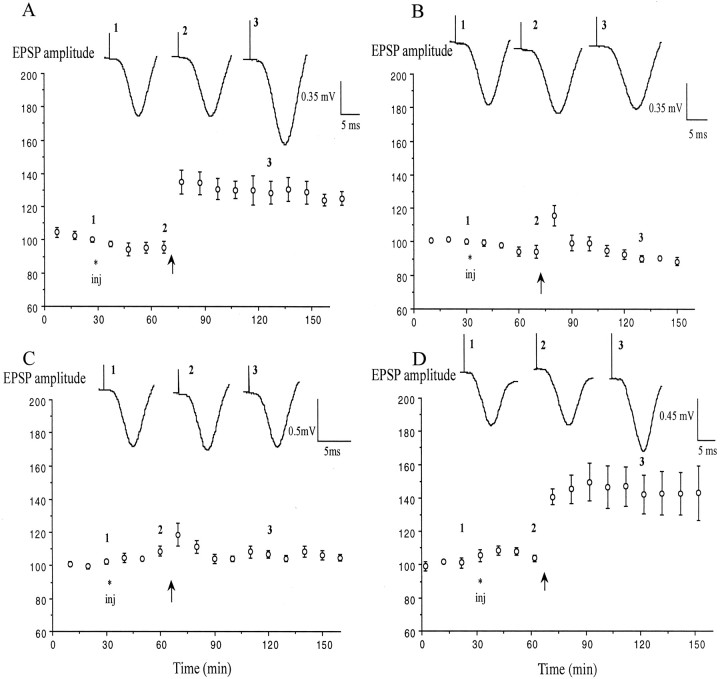
Fig. 1.
Block of LTP induction in the CA1 area of nonstressed anesthetized rats by agents that raise hippocampal 5-HT levels. A, Application of high-frequency stimulation (arrow) after the intraperitoneal injection (inj) of water vehicle (n = 5) induced stable LTP of the field EPSP. B,C, The same tetanus after injection of either fenfluramine (5 mg/kg; n = 5; B) or fluoxetine (10 mg/kg; n = 5; C) failed to induce LTP. D, Pretreatment with the 5-HT uptake enhancer tianeptine (1 mg/kg; n = 5) did not affect the induction of LTP. Values are the mean ± SEM percentage of baseline EPSP amplitude. Insets show typical traces of EPSPs at the times indicated.
Both drugs mimicked the effects of stress on LTP induction. Injection of fenfluramine (5 mg/kg, i.p.) 30 min before the conditioning stimulation prevented the induction of LTP, leaving a residual, nonsignificant short-term potentiation (STP). Thus, the EPSP amplitude did not significantly increase above baseline after the tetanus (118.6 ± 6.8, 107 ± 2.1, and 105.5 ± 3.5% at 10, 60, and 120 min after HFS; p > 0.05 compared with baseline; p < 0.05 compared with water-injected controls, 135 ± 7.3, 130.6 ± 6.3 and 126.1 ± 6.3%, respectively; n = 5 per group) (Fig.1A,B).
Similarly, fluoxetine (10 mg/kg, i.p.) blocked the induction of both STP and LTP when injected 40 min before the HFS. There was no significant potentiation of the synaptic responses at 10, 60, or 90 min after HFS (115.6 ± 6.2, 90.5 ± 1.3, and 89.5 ± 4%, respectively; n = 5) (Fig. 1C) (p > 0.05 compared with baseline,p < 0.05 compared with water injected controls).
Next, the ability of an agent that lowers the endogenous extracellular concentration of 5-HT to modulate the induction of LTP by HFS in nonstressed animals was examined (Fig. 1D). The 5-HT reuptake enhancer tianeptine (Dresse and Scuvee-Moreau, 1988;Fattaccini et al., 1990; De Simoni et al., 1992; Labrid et al., 1992;Wilde and Penfield, 1995) (see also Pineyro et al., 1995; Malagie et al., 2000) was injected at a dose of 1 mg/kg, intraperitoneally, because this did not affect baseline excitatory transmission, whereas a dose of 5 mg/kg increased it (data not shown, see Spedding et al., 1998). In contrast with the agents that raise 5-HT levels, tianeptine, injected 40 min before the HFS, did not significantly affect the magnitude of LTP (n = 5; 140.6 ± 4.5, 142.9 ± 12.8, and 141.1 ± 13.1% at 10, 60, and 120 min post-HFS) (Fig. 1D) (values similar to those observed in vehicle-injected animals; p > 0.05).
LTP induction in stressed animals
The inescapable stress procedure (Xu et al., 1997) entailed placing the rat on the elevated platform for a period of 30 min, after which they were immediately anesthetized with urethane. During the period on the elevated platform the rats showed signs of stress including “behavioral freezing”, piloerection, defecation, and urination. The plasma corticosterone levels were elevated in the stressed animals (176.3 ± 30.3 ng/ml at time of anesthesia,n = 4, p < 0.05, compared with116.7 ± 5.8 ng/ml in nonstressed animals, n = 8).
To investigate the role of elevated endogenous 5-HT in the block of LTP by stress, the effect of the 5-HT reuptake enhancer tianeptine (1 mg/kg, i.p.) was examined in stressed animals (Fig.2). The effectiveness of the stress protocol to block LTP induction was established in each animal first by applying conditioning stimulation in the absence of drug. Thus, tetanic stimulation (HFS1) failed to induce a persistent change in synaptic strength (105.5 ± 4.8% at 1 hr; n = 6;p > 0.05 compared with baseline) in these previously stressed rats. However, the application of a second HFS (HFS2) 40 min after the administration of tianeptine now induced stable LTP (155.9 ± 13.6 and 159.5 ± 17.2% at 60 and 120 min after HFS2; p < 0.05 compared with pre-HFS2 baseline) (Fig.2B). The recovery of the ability to induce LTP with HFS after tianeptine treatment was not caused by a time-dependent recovery from the stress because a second HFS failed to elicit LTP in stressed, water-injected controls (102.1 ± 3 and 98.7 ± 2.1%, respectively; n = 5; p < 0.05 compared with tianeptine) (Fig. 2A). Furthermore, the ability of tianeptine to reverse the effect of stress was not caused by a reduction in plasma corticosterone because this was not affected (249.3 ± 37.7 and 254.3 ± 54.8 ng/ml at the time of HFS2 in water, n = 6, and tianeptine-treated animals,n = 7, p > 0.05).
Fig. 2.
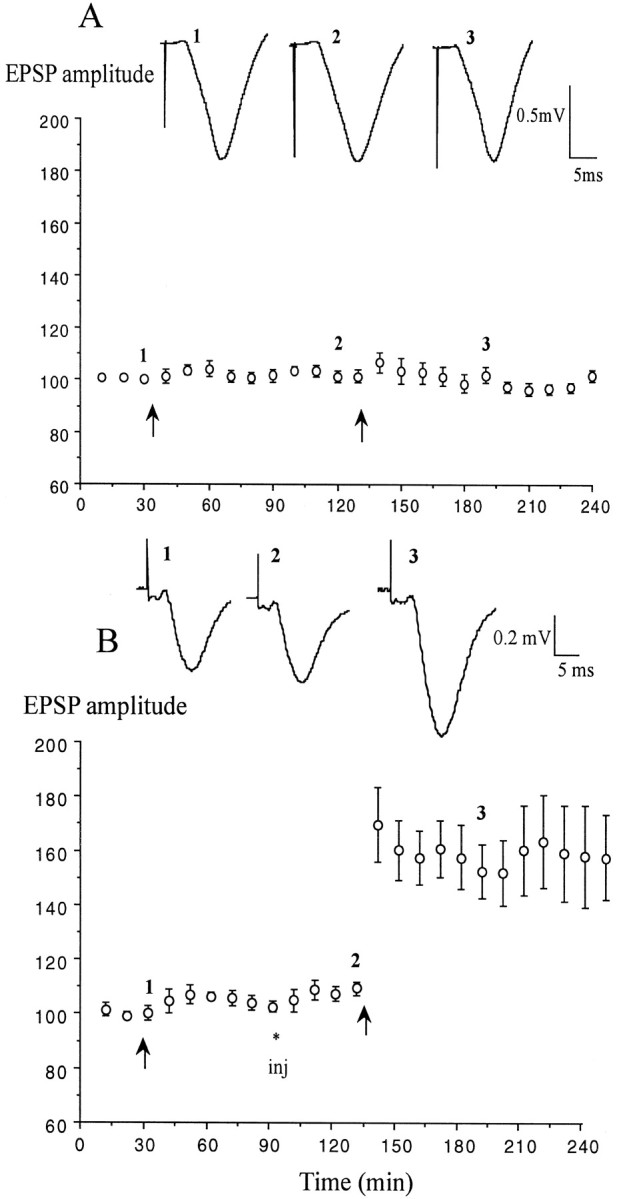
The 5-HT uptake enhancer tianeptine overcomes the stress-induced block of LTP. A, In animals that had been stressed by placing them on an elevated platform for 30 min before anesthesia, high-frequency stimulation (arrows) failed to induce LTP either before or after intraperitoneal injection (inj) of water vehicle (n = 5).B, Injection of (±)-tianeptine (1 mg/kg;n = 6) before the second tetanus enabled the induction of LTP in stressed animals. Values are the mean ± SEM percentage of baseline EPSP amplitude. Insets show typical traces of EPSPs at the times indicated.
Because the 5-HT uptake enhancing action of (±)tianeptine is believed to reside predominantly in one enantiomer, (−)-tianeptine (S 16190) (Oluyomi et al., 1997), a blind study of the effects of the two enantiomers on LTP induction in stressed animals was then undertaken. The dose chosen (0.5 mg/kg) was half that used in the study of the racemic mixture. Consistent with a role for a reduction in extracellular 5-HT concentration mediating the action of tianeptine, the (−)-enantiomer mimicked the ability of the racemate to reverse the block of LTP induction by stress, whereas (+)-tianeptine was inactive. Although the first HFS failed to elicit LTP in previously stressed animals, the injection of (−)-tianeptine enabled the induction of LTP by a second HFS (140.5 ± 9.7 and 127.9 ± 8.9% at 60 and 120 min; n = 5) (Fig.3A) (p< 0.05 compared with water-injected controls). In contrast, application of HFS in stressed animals receiving an injection of the same dose of (+)-tianeptine failed to induce LTP (102.2 ± 7.3 and 101.2 ± 7.4%; n = 5) (Fig. 3B) (p > 0.05 compared with water-injected controls).
Fig. 3.
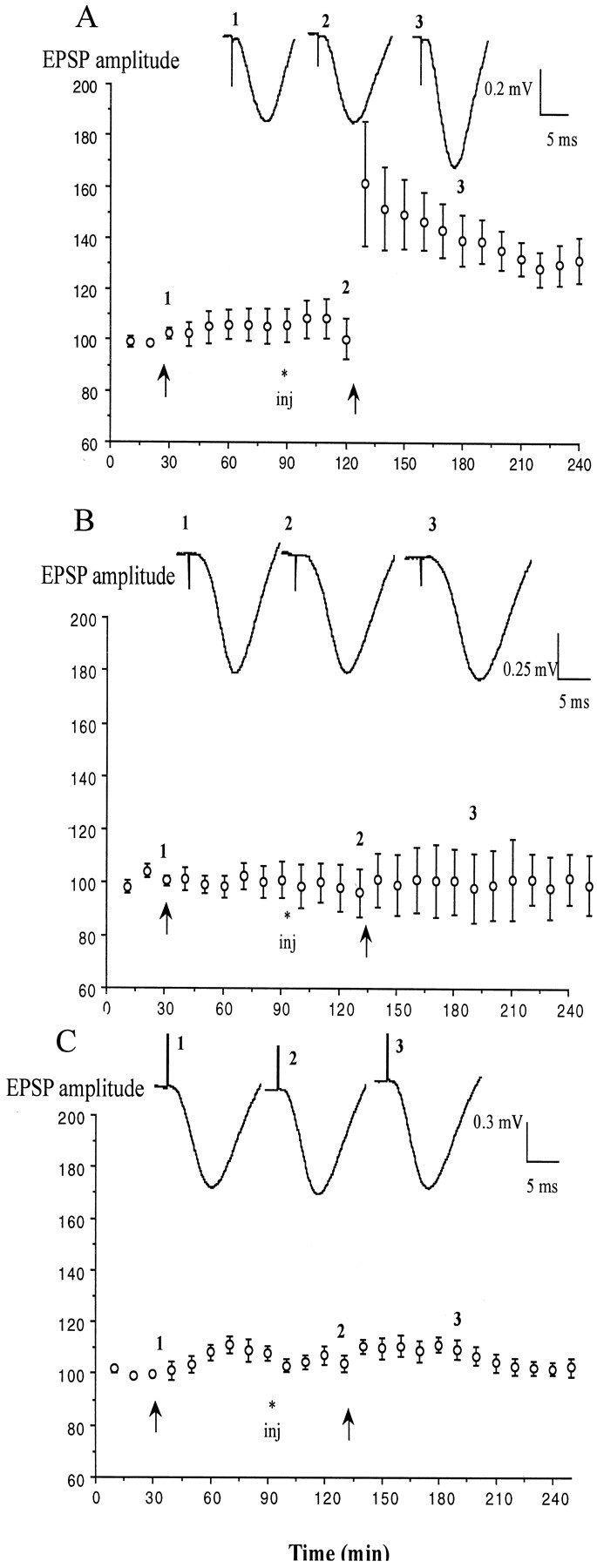
Enantiomer selectivity and fluoxetine sensitivity of the effect of tianeptine. A,B, Pretreatment with the uptake-enhancing (−)-enantiomer (A, open circles) but not the (+)-enantiomer (B, closed squares) of tianeptine (0.5 mg/kg; n = 5 per group) enabled the recovery of the ability to induce LTP in stressed rats. C, Dual injection of both fluoxetine (10 mg/kg) and (±)-tianeptine (1 mg/kg;n = 5) failed to overcome the block of LTP by stress. Values are the mean ± SEM percentage of baseline EPSP amplitude. Insets show typical traces of EPSPs at the times indicated.
There was no evidence to support the possibility that tianeptine facilitated LTP induction in stressed animals by altering the synaptic response during the HFS protocol. The difference between the response to HFS1 (before injection) and HFS2 (after injection) was assessed by examining the integrated amplitude of the negative wave of the field potential evoked by the first burst of each HFS train. There was no effect of tianeptine on the magnitude of this difference [3.4 ± 1.1 for (±)-tianeptine, −2.0 ± 2.2 for (−)-tianeptine, and −0.3 ± 0.9 for (+)-tianeptine; p > 0.05 compared with −0.2 ± 1.4 in water-treated animals;n = 5 per group).
Because fluoxetine exerts an opposite action on 5-HT uptake, it was reasoned that it should oppose the effect of tianeptine in stressed animals (Nowakowska et al., 2000). Consistent with this proposal, fluoxetine prevented the reversal of the effect of stress by tianeptine (Fig. 3C). Thus, when previously stressed animals were injected with both tianeptine and fluoxetine at doses that individually reversed the effects of stress, the HFS failed to induce LTP (104.5 ± 3.5 and 99 ± 2.5% compared with pre-HFS2 baseline; n = 5).
DISCUSSION
Evidence that endogenous 5-HT has a major inhibitory effect on LTP induction in the CA1 area in the intact hippocampus was provided byStäubli and Xu (1995). They reported that in freely behaving rats LTP was facilitated by blockade of 5-HT receptors using the selective 5-HT3 receptor antagonist ondansetron. Previousin vitro studies have shown that bath application of 5-HT can block the induction of LTP in the CA1 area of hippocampal slices (Corradetti et al., 1992; Passani et al., 1994; Stäubli and Otaky, 1994). Because there are many different 5-HT receptor subtypes with different sensitivities to 5-HT (Barnes and Sharp, 2000), raising endogenous levels of 5-HT would lead to activation of a large range of receptors. Many of these receptors have actions on cellular processes that regulate the induction of LTP. The block of LTP induction in vitro has been proposed to be a result of either a 5-HT3 receptor-mediated activation of inhibitory interneurons or a more direct 5-HT1Areceptor-mediated inhibition of pyramidal neuron excitability (Corradetti et al., 1992; Passani et al., 1994; Stäubli and Otaky, 1994). The present data strongly support the idea that endogenous 5-HT has a powerful suppressant action. In particular, the new data strongly implicate 5-HT mechanisms in the mediation and modulation of the block of LTP induction by acute inescapable stress.
In contrast with the finding of Stäubli and Xu (1995) that the 5-HT3 receptor antagonist ondansetron increased the amplitude and duration of theta burst-induced LTP in freely moving animals, tianeptine, an agent that lowers 5-HT levels, had no effect on LTP induced by HFS in nonstressed anesthetized animals. This may be attributable to a greater ability of 5-HT to inhibit the response to short burst conditioning stimulation (Stäubli and Otaky, 1994). Consistent with this, Corradetti et al. (1992) found that 5-HT blocked primed burst-induced LTP but did not affect the induction of LTP by long (100 pulses) trains of HFS. The present study used an intermediate burst duration (20 pulses). The lack of effect in nonstressed animals in the present experiments may also be attributable to the use of anesthesia because background output of 5-HT increases with increased motor activity and arousal (Jacobs and Fornal, 1999; Park et al., 1999).
The discovery that fluoxetine and fenfluramine, agents that increase hippocampal extracellular 5-HT levels, blocked LTP induction in the intact hippocampal CA1 area of nonstressed animals indicates that endogenously released 5-HT can have a strong inhibitory action on synaptic plasticity without affecting basal synaptic transmission. Thus, raising endogenous 5-HT appears to mimic the effect of acute inescapable stress on LTP. Consistent with a key role of raised endogenous 5-HT levels in mediating the effects of stress, tianeptine, an agent that reduces 5-HT levels, was able to overcome the block of LTP induction by inescapable stress at a dose level that did not affect LTP in nonstressed animals. Furthermore, the reversal of the effect of stress by tianeptine was enantiomer-selective, the uptake enhancing (−)-enantiomer (Oluyomi et al., 1997) accounting for the activity of the racemic mixture in our model. That tianeptine was effective in enabling LTP induction several hours after the stress is consistent with evidence that inescapable stress triggers a persistent increase in 5-HT tone (Amat et al., 1998). The latter study, performed in the ventral hippocampus, found an approximately twofold increase that lasted for several hours after exposure to inescapable tailshock. This appears to be caused by a persistent restricted activation of certain serotonergic neurons, in particular, those in the middle and caudal parts of the dorsal raphe nucleus (Grahn et al., 1999). Different groups of these neurons supply the ventral and dorsal hippocampus and the medial septum, which innervates the hippocampus extensively (Azmitia, 1981; Köhler and Steinbusch, 1982; Imai et al., 1986;Vertes, 1991; Acsady et al., 1996).
Clearly, in vivo, the involvement of extrahippocampal actions of 5-HT might also indirectly contribute to the regulation of hippocampal LTP induction by stress or systemically administered agents. For example, tianeptine opposes stress-induced reductions in 5-HT uptake not only in the hippocampus but also in the cortex and hypothalamus (Mennini et al., 1993), and under some conditions can block stress-evoked elevations in corticosterone (Broqua et al., 1992;Labrid et al., 1992; Delbende et al., 1994). Given the intricate inter-relationship between 5-HT and the limbic–hypothalamic–pituitary–adrenal axis (Chaouloff et al., 1999;Vollmayr et al., 2000), it was important to determine if tianeptine reduced corticosterone levels and thereby lead to a recovery of LTP induction in stressed animals. The lack of an effect in the present study, in which the drug was administered under anesthesia after the stress, shows that this is not the case.
Although the present findings strongly support an inhibitory role of endogenous 5-HT on LTP induction in the CA1 area, they do not exclude the likelihood of opposing actions of 5-HT via different receptor subtypes in this or other hippocampal subregions. Thus, 5-HT4 receptors mediate excitation of pyramidal neurons and are positively coupled to adenylyl cyclase, and activation of these receptors has been shown to promote LTP induction in the CA1 area in vivo (Matsumoto et al., 2001). Furthermore, unlike the CA1 area, in the dentate gyrus endogenous 5-HT may have a predominantly facilitatory role in the full elaboration of LTPin vivo (Bliss et al., 1983). Intriguingly, fluoxetine has been reported to increase basal excitatory synaptic transmission and thereby occlude LTP at perforant path to granule cell synapses after repeated treatment (Stewart and Reid, 2000). These authors also reported that acquisition of a hippocampal-dependent spatial memory task (Morris water maze) was not affected by repeated fluoxetine treatment. It would be interesting to determine if the acute block of LTP in the CA1 area seen in the present study is sustained with more prolonged exposure. Recently, chronic treatment with the nonselective 5-HT reuptake inhibitor imipramine was reported to partly reverse the block of LTP by social stress (Von Frijtag et al., 2001)
The findings reported here have potentially important implications for how drugs may affect aspects of affective disorders that are linked to hippocampal dysfunction, particularly cognitive impairment (Duman et al., 2000; Levkovitz et al., 2001; McEwen and Magarinos, 2001; Reid and Stewart, 2001; Sapolsky, 2001). Intriguingly, repeated tianeptine treatment has been reported to prevent chronic stress-induced reduction in hippocampal volume (Czéh et al., 2001) and dendritic atrophy in the CA3 area, a major source of input to the CA1 region (Magarinos et al., 1999). The latter effect was associated with a recovery of stress-impaired learning in a hippocampal-dependent task (Conrad et al., 1996).
An important aspect of the present model is the ability to study the persistent effects of stress on hippocampal function and ways of overcoming it independent of behavior. The finding that tianeptine was able to reverse the effect of stress when administered several hours after the stress and anesthesia onset is remarkable. This is consistent with the report that tianeptine can reverse stress-suppressed exploration of a novel environment when injected after the stress (Whitton et al., 1991). Thus, tianeptine has the capacity to reverse the neurophysiological effects of stress in a behaviorally independent manner and thereby may boost neural coping–adaptive mechanisms that may be deficient in affective disorders.
Footnotes
This research was supported by the Wellcome Trust, Enterprise Ireland, and the Health Research Board of Ireland. We thank Professor Michael Spedding for providing tianeptine and for his advice and encouragement.
Correspondence should be addressed to Dr. Michael J. Rowan, Department of Pharmacology and Therapeutics, Zoology Building, Trinity College, Dublin 2, Ireland. E-mail: mrowan@tcd.ie.
REFERENCES
- 1.Acsady L, Arabadzisz D, Katona I, Freund TF. Topographical distribution of dorsal and median raphe neurons with hippocampal, septal and dual projection. Acta Biol Hung. 1996;47:9–19. [PubMed] [Google Scholar]
- 2.Amat J, Matus-Amat P, Watkins L, Maier S. Escapable and inescapable stress differentially and selectively alter extracellular levels of 5-HT in the ventral hippocampus and dorsal periaqueductal gray of the rat. Brain Res. 1998;797:12–22. doi: 10.1016/s0006-8993(98)00368-0. [DOI] [PubMed] [Google Scholar]
- 3.Azmitia EC. Bilateral serotonergic projections to the dorsal hippocampus of the rat: simultaneous localization of 3H–5HT and HRP after retrograde transport. J Comp Neurol. 1981;203:737–743. doi: 10.1002/cne.902030410. [DOI] [PubMed] [Google Scholar]
- 4.Barnes N, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 2000;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 5.Bliss T, Goddard G, Riives M. Reduction of long-term potentiation in the dentate gyrus of the rat following selective depletion of monoamines. J Physiol (Lond) 1983;334:475–491. doi: 10.1113/jphysiol.1983.sp014507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broqua P, Baudrie V, Laude D, Chaouloff F. Influence of the novel antidepressant tianeptine on neurochemical, neuroendocrinological, and behavioral effects of stress in rats. Biol Psychiatry. 1992;31:391–400. doi: 10.1016/0006-3223(92)90232-o. [DOI] [PubMed] [Google Scholar]
- 7.Chaouloff F, Berton O, Mormede P. Serotonin and stress. Neuropsychopharmacology. 1999;21:28S–32S. doi: 10.1016/S0893-133X(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 8.Conrad C, Galea L, Kuroda Y, McEwen B. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- 9.Corradetti R, Ballerini L, Pugliese A, Pepeu G. Serotonin blocks the long-term potentiation induced by primed burst stimulation in the CA1 region of rat hippocampal slices. Neuroscience. 1992;46:511–518. doi: 10.1016/0306-4522(92)90140-w. [DOI] [PubMed] [Google Scholar]
- 10.Czéh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci USA. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Kloet ER. Stress in the brain. Eur J Pharmacol. 2000;405:187–198. doi: 10.1016/s0014-2999(00)00552-5. [DOI] [PubMed] [Google Scholar]
- 12.de Quervain D-F, Roozendaal B, McGaugh J. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- 13.De Simoni M, De Luigi A, Clavenna A, Manfridi A. In vivo studies on the enhancement of serotonin uptake by tianeptine. Brain Res. 1992;574:93–97. doi: 10.1016/0006-8993(92)90804-i. [DOI] [PubMed] [Google Scholar]
- 14.Delbende C, Tranchand Bunel D, Tarozzo G, Grino M, Oliver C, Mocaer E, Vaudry H. Effect of chronic treatment with the antidepressant tianeptine on the hypothalamic-pituitary-adrenal axis. Eur J Pharmacol. 1994;251:245–251. doi: 10.1016/0014-2999(94)90406-5. [DOI] [PubMed] [Google Scholar]
- 15.Diamond DM, Bennett MC, Stevens KE, Wilson RL, Rose GM. Exposure to a novel environment interferes with the induction of hippocampal primed burst potentiation in the behaving rat. Psychobiology. 1990;18:273–281. [Google Scholar]
- 16.Diamond DM, Fleshner M, Ingersoll N, Rose RM. Psychological stress impairs spatial working memory: Relevance to electrophysiological studies of hippocampal function. Behav Neurosci. 1996;110:661–672. doi: 10.1037//0735-7044.110.4.661. [DOI] [PubMed] [Google Scholar]
- 17.Dresse A, Scuvee-Moreau J. Electrophysiological effects of tianeptine on rat locus coeruleus, raphe dorsalis and hippocampus activity. Clin Neuropharmacol [Suppl 2] 1988;11:S55–S58. [PubMed] [Google Scholar]
- 18.Duman R, Malberg J, Nakagawa S, D'Sa C. Neuronal plasticity and survival in mood disorders. Biol Psychiat. 2000;48:732–739. doi: 10.1016/s0006-3223(00)00935-5. [DOI] [PubMed] [Google Scholar]
- 19.Edwards E, Kornrich W, Houtten P, Henn F. Presynaptic serotonin mechanisms in rats subjected to inescapable shock. Neuropharmacology. 1993;31:323–330. doi: 10.1016/0028-3908(92)90063-u. [DOI] [PubMed] [Google Scholar]
- 20.Fattaccini C, Bolaños-Jimenez F, Gozlan H, Hamon M. Tianeptine stimulates uptake of 5-hydroxytryptamine in vivo in the rat brain. Neuropharmacology. 1990;29:1–8. doi: 10.1016/0028-3908(90)90076-4. [DOI] [PubMed] [Google Scholar]
- 21.Garcia R. Stress, hippocampal plasticity, and spatial learning. Synapse. 2001;40:180–183. doi: 10.1002/syn.1040. [DOI] [PubMed] [Google Scholar]
- 22.Ge J, Barnes NM, Costall B, Naylor RJ. Effect of aversive stimulation on 5-hydroxytryptamine and dopamine metabolism in the rat brain. Pharmacol Biochem Behav. 1997;58:775–783. doi: 10.1016/s0091-3057(97)00024-5. [DOI] [PubMed] [Google Scholar]
- 23.Graeff F, Guimaraes F, DeAndrade T, Deakin J. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- 24.Grahn R, Will M, Hammack S, Maswood S, McQueen M, Watkins L, Maier S. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- 25.Healy D, Drugan R. Escapable stress modulates retention of spatial learning in rats: preliminary evidence for involvement of neurosteroids. Psychobiology. 1996;24:110–117. [Google Scholar]
- 26.Hervas I, Artigas F. Effect of fluoxetine on extracellular 5-hydroxytryptamine in rat brain. Role of 5-HT autoreceptors. Eur J Pharmacol. 1998;358:9–18. doi: 10.1016/s0014-2999(98)00579-2. [DOI] [PubMed] [Google Scholar]
- 27.Imai H, Steindler DA, Kitai ST. The organization of divergent axonal projections from the midbrain raphe nuclei in the rat. J Comp Neurol. 1986;243:363–380. doi: 10.1002/cne.902430307. [DOI] [PubMed] [Google Scholar]
- 28.Izquierdo I, Medina JH. The biochemistry of memory formation and its regulation by hormones and neuromodulators. Psychobiology. 1997;25:1–9. [Google Scholar]
- 29.Jacobs BL, Fornal CA. Activity of serotonergic neurons in behaving animals. Neuropsychopharmacology. 1999;21:9S–15S. doi: 10.1016/S0893-133X(99)00012-3. [DOI] [PubMed] [Google Scholar]
- 30.Joëls M. Corticosteroid actions in the hippocampus. J Neuroendocrinol. 2001;13:657–669. doi: 10.1046/j.1365-2826.2001.00688.x. [DOI] [PubMed] [Google Scholar]
- 31.Joseph MH, Kennett GA. Stress-induced release of 5-HT in the hippocampus and its dependence on increased tryptophan availability: an in vivo electrochemical study. Brain Res. 1983;270:251–257. doi: 10.1016/0006-8993(83)90598-x. [DOI] [PubMed] [Google Scholar]
- 32.Kim J, Yoon K. Stress: metaplastic effects in the hippocampus. Trends Neurosci. 1998;21:505–509. doi: 10.1016/s0166-2236(98)01322-8. [DOI] [PubMed] [Google Scholar]
- 33.Kim J, Lee H, Han J, Packard M. Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation and learning. J Neurosci. 2001;21:5222–5228. doi: 10.1523/JNEUROSCI.21-14-05222.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JJ, Foy MR, Thompson RF. Behavioral stress modifies hippocampal plasticity through N-methyl-d-aspartate receptor activation. Proc Natl Acad Sci USA. 1996;93:4750–4753. doi: 10.1073/pnas.93.10.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirby LG, Chou-Green JM, Davis K, Lucki I. The effects of different stressors on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res. 1997;760:218–230. doi: 10.1016/s0006-8993(97)00287-4. [DOI] [PubMed] [Google Scholar]
- 36.Köhler C, Steinbusch H. Identification of serotonin and non-serotonin-containing neurons of the mid-brain raphe projecting to the entorhinal area and the hippocampal formation. A combined immunohistochemical and fluorescent retrograde tracing study in the rat brain. Neuroscience. 1982;7:951–975. doi: 10.1016/0306-4522(82)90054-9. [DOI] [PubMed] [Google Scholar]
- 37.Labrid C, Mocaër E, Kamoun A. Neurochemical and pharmacological properties of tianeptine, a novel antidepressant. Br J Psychiat [Suppl 15] 1992;160:56–60. [PubMed] [Google Scholar]
- 38.Leung LS. Orthodromic activation of hippocampal CA1 region of the rat. Brain Res. 1979;176:49–63. doi: 10.1016/0006-8993(79)90869-2. [DOI] [PubMed] [Google Scholar]
- 39.Levkovitz Y, Grisaru N, Segal M. Transcranial magnetic stimulation and antidepressive drugs share similar cellular effects in rat hippocampus. Neuropsychopharmacology. 2001;24:608–616. doi: 10.1016/S0893-133X(00)00244-X. [DOI] [PubMed] [Google Scholar]
- 40.Magarinos A, Deslandes A, BS M. Effects of antidepressants and benzodiazepines on the dendritic structure of CA3 pyramidal neurons after chronic stress. Eur J Pharmacol. 1999;371:113–122. doi: 10.1016/s0014-2999(99)00163-6. [DOI] [PubMed] [Google Scholar]
- 41.Malagie I, Deslandes A, Gardier A. Effects of acute and chronic tianeptine administration on serotonin outflow in rats: comparison with paroxetine by using in vivo microdialysis. Eur J Pharmacol. 2000;403:55–65. doi: 10.1016/s0014-2999(00)00486-6. [DOI] [PubMed] [Google Scholar]
- 42.Martin S, Grimwood P, Morris R. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 43.Matsumoto M, Togashi H, Mori K, Ueno K, Ohashi S, Kojima T, Yoshioka M. Evidence for involvement of central 5-HT(4) receptors in cholinergic function associated with cognitive processes: behavioral, electrophysiological, and neurochemical studies. J Pharmacol Exp Ther. 2001;296:676–682. [PubMed] [Google Scholar]
- 44.McEwen B. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 45.McEwen B, Magarinos A. Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum Psychopharmacol Clin Exp. 2001;16:S7–S19. doi: 10.1002/hup.266. [DOI] [PubMed] [Google Scholar]
- 46.McGaugh J. Memory: a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 47.Matsuo M, Kataoka Y, Mataki S, Kato Y, Oi K. Conflict situation increases serotonin release in rat dorsal hippocampus: in vivo study with microdialysis and Vogel test. Neurosci Lett. 1996;215:197–200. doi: 10.1016/0304-3940(96)12982-7. [DOI] [PubMed] [Google Scholar]
- 48.Mennini T, Taddei C, Codegoni A, Gobbi M, Garattini S. Acute noise stress reduces [3H]5-hydroxytryptamine uptake in rat brain synaptosomes: protective effects of buspirone and tianeptine. Eur J Pharmacol. 1993;14:255–260. doi: 10.1016/0014-2999(93)90211-y. [DOI] [PubMed] [Google Scholar]
- 49.Nowakowska E, Kus K, Chodra A, Rybakowski J. Behavioural effects of fluoxetine and tianeptine, two antidepressants with opposite action mechanisms, in rats. Arzneim Forsch. 2000;50:5–10. doi: 10.1055/s-0031-1300156. [DOI] [PubMed] [Google Scholar]
- 50.Oluyomi A, Datla K, Curzon G. Effects of (+) and (-) enantiomers of the antidepressant drug tianeptine on 5-HT-induced behaviour. Neuropharmacology. 1997;36:383–387. doi: 10.1016/s0028-3908(97)00016-6. [DOI] [PubMed] [Google Scholar]
- 51.Park SP, Lopez-Rodriguez F, Wilson CL, Maidment N, Matsumoto Y, Engel J. In vivo microdialysis measures of extracellular serotonin in the rat hippocampus during sleep-wakefulness. Brain Res. 1999;833:291–296. doi: 10.1016/s0006-8993(99)01511-5. [DOI] [PubMed] [Google Scholar]
- 52.Passani M, Pugliese A, Azzurrini M, Corradetti R. Effects of DAU 6215, a novel 5-hydroxytryptamine3 (5-HT3) antagonist on electrophysiological properties of the rat hippocampus. Br J Pharmacol. 1994;112:695–703. doi: 10.1111/j.1476-5381.1994.tb13132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pineyro G, Deveault L, Blier P, Dennis T, de Montigny C. Effect of acute and prolonged tianeptine administration on the 5-HT transporter: electrophysiological, biochemical and radioligand binding studies in the rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1995;351:111–118. doi: 10.1007/BF00169324. [DOI] [PubMed] [Google Scholar]
- 54.Reid I, Stewart C. How antidepressants work - New perspectives on the pathology of depressive disorder. Br J Psychiat. 2001;178:299–303. doi: 10.1192/bjp.178.4.299. [DOI] [PubMed] [Google Scholar]
- 55.Rocher C, Gardier AM. Effects of repeated systemic administration of d-fenfluramine on serotonin and glutamate release in rat ventral hippocampus: comparison with methamphetamine using in vivo microdialysis. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:422–428. doi: 10.1007/s002100000381. [DOI] [PubMed] [Google Scholar]
- 56.Roozendaal B. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology. 2000;25:213–238. doi: 10.1016/s0306-4530(99)00058-x. [DOI] [PubMed] [Google Scholar]
- 57.Sabol K, Richards J, LS S. Fenfluramine-induced increases in extracellular hippocampal serotonin are progressively attenuated in vivo during a four-day fenfluramine regimen in rats. Brain Res. 1992;571:64–72. doi: 10.1016/0006-8993(92)90509-8. [DOI] [PubMed] [Google Scholar]
- 58.Sapolsky RM. Depression, antidepressants, and the shrinking hippocampus. Proc Natl Acad Sci USA. 2001;98:12320–12232. doi: 10.1073/pnas.231475998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shors TJ, Seib T, Levine S, Thompson R. Inescapable versus escapable shock modulates long-term potentiation in the rat hippocampus. Science. 1989;244:224–226. doi: 10.1126/science.2704997. [DOI] [PubMed] [Google Scholar]
- 60.Shors TJ, Levine S, Thompson RF. Opioid antagonist eliminates the stress-induced impairment of long-term potentiation (LTP). Brain Res. 1990;506:316–318. doi: 10.1016/0006-8993(90)91270-q. [DOI] [PubMed] [Google Scholar]
- 61.Shors T, Gallegos R, Breindl A. Transient and persistent consequences of acute stress on long-term potentiation (LTP), synaptic efficacy, theta rhythms and bursts in area CA1 of the hippocampus. Synapse. 1997;26:209–217. doi: 10.1002/(SICI)1098-2396(199707)26:3<209::AID-SYN2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 62.Spedding M, Deslandes A, Netzer R, Roeper J, Szabo G, Egyed A. Potentiation of CA1 hippocampal population spike potential by the atypical antidepressant tianeptine. Soc Neurosci Abstr. 1998;24:685. [Google Scholar]
- 63.Stäubli U, Otaky N. Serotonin controls the magnitude of LTP induced by theta bursts via an action on NMDA-receptor-mediated responses. Brain Res. 1994;643:10–16. doi: 10.1016/0006-8993(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 64.Stäubli U, Xu F. Effects of 5-HT3 receptor antagonism on hippocampal theta rhythm, memory, and LTP induction in the freely moving rat. J Neurosci. 1995;15:2445–2452. doi: 10.1523/JNEUROSCI.15-03-02445.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stewart C, Reid I. Repeated ECS and fluoxetine administration have equivalent effects on hippocampal synaptic plasticity. Psychopharmacology. 2000;148:217–223. doi: 10.1007/s002130050045. [DOI] [PubMed] [Google Scholar]
- 66.Vahabzadeh A, Fillenz M. Comparison of stress-induced changes in noradrenergic and serotonergic neurons in the rat hippocampus using microdialysis. Eur J Neurosci. 1994;6:1205–1212. doi: 10.1111/j.1460-9568.1994.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 67.Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- 68.Vollmayr B, Keck S, Henn F, Schloss P. Acute stress decreases serotonin transporter mRNA in the raphe pontis but not in other raphe nuclei of the rat. Neurosci Lett. 2000;25:109–112. doi: 10.1016/s0304-3940(00)01346-x. [DOI] [PubMed] [Google Scholar]
- 69.Von Frijtag JC, Kamal A, Reijmers LGJE, Schrama LH, van den Bos R, Spruijt BM. Chronic imipramine treatment partially reverses the long-term changes of hippocampal synaptic plasticity in socially stressed rats. Neurosci Lett. 2001;309:153–156. doi: 10.1016/s0304-3940(01)02062-6. [DOI] [PubMed] [Google Scholar]
- 70.Whitton P, Sarna G, Datla K, Curzon G. Effects of tianeptine on stress-induced behavioural deficits and 5-HT dependent behaviour. Psychopharmacology (Berl) 1991;104:81–85. doi: 10.1007/BF02244558. [DOI] [PubMed] [Google Scholar]
- 71.Wilde M, Penfield P. Tianeptine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in depression and coexisting anxiety and depression. Drugs. 1995;49:411–439. doi: 10.2165/00003495-199549030-00007. [DOI] [PubMed] [Google Scholar]
- 72.Wilkinson LS, Humby T, Killcross S, Robbins TW, Everitt BJ. Dissociations in hippocampal 5-hydroxytryptamine release in the rat following Pavlovian aversive conditioning to discrete and contextual stimuli. Eur J Neurosci. 1996;8:1479–1487. doi: 10.1111/j.1460-9568.1996.tb01610.x. [DOI] [PubMed] [Google Scholar]
- 73.Xu L, Anwyl R, Rowan MJ. Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature. 1997;387:497–500. doi: 10.1038/387497a0. [DOI] [PubMed] [Google Scholar]
- 74.Xu L, Holscher C, Anwyl R, Rowan MJ. Glucocorticoid receptor and protein/RNA synthesis-dependent mechanisms underlie the control of synaptic plasticity by stress. Proc Natl Acad Sci USA. 1998;95:3204–3208. doi: 10.1073/pnas.95.6.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]