Abstract
Studies in nonhuman primates documented that appropriate stimulation of dopamine (DA) D1 receptors in the dorsolateral prefrontal cortex (DLPFC) is critical for working memory processing. The defective ability of patients with schizophrenia at working memory tasks is a core feature of this illness. It has been postulated that this impairment relates to a deficiency in mesocortical DA function. In this study, D1 receptor availability was measured with positron emission tomography and the selective D1 receptor antagonist [11C]NNC 112 in 16 patients with schizophrenia (seven drug-naive and nine drug-free patients) and 16 matched healthy controls. [11C]NNC 112 binding potential (BP) was significantly elevated in the DLPFC of patients with schizophrenia (1.63 ± 0.39 ml/gm) compared with control subjects (1.27 ± 0.44 ml/gm; p = 0.02). In patients with schizophrenia, increased DLPFC [11C]NNC 112 BP was a strong predictor of poor performance at the n-back task, a test of working memory. These findings confirm that alteration of DLPFC D1 receptor transmission is involved in working memory deficits presented by patients with schizophrenia. Increased D1 receptor availability observed in patients with schizophrenia might represent a compensatory (but ineffective) upregulation secondary to sustained deficiency in mesocortical DA function.
Keywords: dopamine, D1 receptors, schizophrenia, prefrontal cortex, working memory, positron emission tomography
Impairment in higher cognitive functions is one of the most enduring symptoms of schizophrenia and a strong predictor of poor clinical outcome (Green, 1996). Among these deficits, defective performance at tasks involving working memory, i.e., the ability to retain and manipulate information over a brief period of time, has been reliably observed in these patients (Park and Holzman, 1992; Fleming et al., 1995; Morice and Delahunty, 1996; Gold et al., 1997; Keefe et al., 1997; Park, 1997; Conklin et al., 2000). Functional brain imaging studies documented the engagement of the dorsolateral prefrontal cortex (DLPFC) in the execution of working memory tasks in humans (Cohen et al., 1994; McCarthy et al., 1994;Courtney et al., 1996; Braver et al., 1997; Carlson et al., 1998;D'Esposito et al., 1998; Callicott et al., 1999; Jansma et al., 2000). Alterations in DLPFC activation during completion of working memory tasks has been reported in patients with schizophrenia by numerous investigators, suggesting that pathology of the DLPFC or its connectivity is implicated in working memory deficits in schizophrenia (Callicott et al., 1998, 2000; Stevens et al., 1998; Honey et al., 1999; Manoach et al., 1999, 2000; Barch et al., 2001; Perlstein et al., 2001).
The mesocortical DA system, ascending form the ventral tegmental area, provides a widespread innervation to the neocortical areas (Levitt et al., 1984; Lewis et al., 1987; Oades and Halliday, 1987). D1 receptors are the most expressed DA receptors in the neocortex (Lidow et al., 1991; Hall et al., 1994; Hurd et al., 2001). Studies in nonhuman primates have shown that working memory, studied during delayed response paradigms, is critically dependent on prefrontal DA function and appropriate stimulation of D1 receptors in the DLPFC (Brozoski et al., 1979;Sawaguchi and Goldman-Rakic, 1991, 1994; Arnsten et al., 1994; Arnsten and Goldman-Rakic, 1998). These observations led to the suggestion that altered DA transmission at D1 receptors in DLPFC might be involved in the pathophysiology of working memory in schizophrenia (Weinberger, 1987; Davis et al., 1991; Goldman-Rakic, 1994; Goldman-Rakic et al., 2000).
The aim of the study reported here was to measure DLPFC D1 receptor availability in untreated patients with schizophrenia and matched healthy controls and to assess the relationship between DLPFC D1 receptor availability and working memory performance. (+)-5-(7-Benzofuranyl)-8-chloro-7-hydroxy-3-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine (NNC 112) is a potent and selective D1 receptor antagonist (Andersen et al., 1992). [11C]NNC 112 was recently introduced as a new and superior radiotracer to image D1receptors (Halldin et al., 1998). Because of its high specific to nonspecific binding ratio, [11C]NNC 112 is well suited for quantification of D1 receptors in extrastriatal areas such as the neocortex (Halldin et al., 1998), where the density of these receptors is much lower than in the striatum (Hall et al., 1994). We recently demonstrated that [11C]NNC 112 provides a reproducible measurement of D1 receptor parameters in several regions of the PFC, including the DLPFC (Abi-Dargham et al., 2000). Working memory was assessed in both patients and controls, using a verbal n-back paradigm and three working memory load levels (1-back, 2-back, 3-back).
MATERIALS AND METHODS
Subjects
The protocol was approved by the Institutional Review Boards of the New York State Psychiatric Institute and Columbia Presbyterian Medical Center. Patients were recruited after voluntary admission to a research ward (Schizophrenia Research Unit, New York State Psychiatric Institute) or from the affiliated outpatient research clinic. Capacity to provide informed consent was evaluated by a psychiatrist not associated with the study. Subjects provided written informed consent after detailed explanation of the nature and risks of the study. According to the recommendations of the National Alliance for the Mentally Ill (Arlington, VA), assent from involved family members was also obtained when appropriate.
Inclusion criteria for patients were as follows: (1) diagnosis of schizophrenia or schizophreniform disorder (provisional) according to the Diagnostic and Statistical Manual (DSM-IV); (2) no other DSM-IV axis I diagnosis; (3) no lifetime history of alcohol or substance abuse or dependence; (4) absence of any psychotropic medication for at least 14 d before the study (with the exception of lorazepam, which was allowed at a maximal dose of 3 mg per day up to 24 hr before the study); (5) no concomitant or past severe medical conditions; (6) no pregnancy; (7) no current suicidal or homicidal ideation; and (8) capacity to provide informed consent. Subjects with schizophreniform disorder (provisional) were included in the study only if the diagnosis of schizophrenia was confirmed after 6 months.
Inclusion criteria for the control group were (1) absence of past or present neurological or psychiatric illnesses, including substance abuse; (2) no concomitant or past severe medical conditions; (3) no pregnancy; and (4) informed consent. Groups were matched for age, gender, race, parental socioeconomic level (Hollingshead, 1975), and nicotine smoking.
A total of 17 patients with schizophrenia and 20 controls were enrolled in the study. All sequentially enrolled subjects were included in the study sample, with the following exceptions. One patient with schizophrenia was excluded because of failure of arterial sampling during the scan. Three controls subjects were excluded for the following reasons: failure to obtain magnetic resonance image (MRI) (n = 1), discovery of a meningioma at the MRI (n = 1), and technical failure of the PET scanner during the experiment (n = 2). Thus, the final sample includes 16 patients with schizophrenia and 16 healthy controls. Patients and controls were studied over a 2 year period (June 1999 to June 2001).
Clinical assessment
Diagnosis was assessed with the Structured Clinical Interview for DSM-IV (SCID) (Spitzer et al., 1992), followed by consensus diagnosis conference. For controls, a trained rater administered the SCID nonpatient version (SCID-NP). Medical evaluation included a detailed medical and neurological history, complete physical and neurological examination, EKG, and routine laboratory tests. Severity of symptoms was assessed with the Positive and Negative Symptoms Scale (PANSS) (Kay et al., 1987), obtained on the day of the PET study, i.e., after at least 14 d without antipsychotic medications.
Working memory assessment
A large number of tasks involving working memory have been used in schizophrenia research (Goldman-Rakic, 1994). These tasks might vary in complexity, from pure maintenance tasks (allowing to vary the delay and the extent of information to be maintained) to tasks involving increasing levels of information manipulation (ranging from simple matching to more elaborate decision making processes, such as set shifting in the Wisconsin card sorting task). We selected the n-back paradigm because: (1) it is a reliable method to activate DLPFC, irrespective of the presentation modality or the nature of the information, (2) it allows to vary the working memory load of the task, and (3) DLPFC activation during n-back tasks has been demonstrated to be load-sensitive by several investigators (Cohen et al., 1994; Barch et al., 1997; Braver et al., 1997; Carlson et al., 1998; Carter et al., 1998; Callicott et al., 1999; Rama et al., 2001).
The n-back task used here required subjects to monitor a series of letters presented sequentially on a computer screen and to respond when a letter is identical to the one that immediately preceded it (1-back condition), the one presented two trials back (2-back), or three trials back (3-back) (Cohen et al., 1994). Sixty letters were presented in each condition. Each presentation lasted 500 msec, with 2500 msec intervals (blank screen). A total of 12, 10, and 10 targets were presented for the 1-, 2-, and 3-back conditions, respectively. The hit rate (HR) was calculated as the number of correct responses divided by the number of targets (maximum is 1, minimum is 0). The error rate (ER) was calculated as the number of errors divided by the number of nontargets (maximum is 1, minimum is 0). The adjusted HR (AHR) was calculated as HR − ER. AHR ranges from 1 (if the subject provides all the correct responses and no incorrect response) to −1 (if the subject provides no correct response and all the incorrect responses). Operating at chance level corresponds to an AHR of 0. d′ was calculated for 2 and 3 back as inv(HR) − inv(ER), where inv is the inverse of the standard normal cumulative distribution.
D1 receptor measurement
Radiochemistry. The desmethyl precursor (+)-5-(7-benzofuranyl)-8-chloro-7-hydroxy-3-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine was kindly provided by Christer Halldin (Karolinska Institute, Stockholm, Sweden). [11C]NNC 112 was prepared by N-methylation of the precursor using [11C]methyl triflate as previously described (Halldin et al., 1998). Specific radioactivity at the time of injection was 949 ± 533 Ci/mmol (mean ± SD;n = 32). Injected dose was 12.5 ± 4.8 mCi, and injected mass was 4.8 ± 1.3 μg (range from 1.8 to 6.5 μg).
PET protocol. PET imaging sessions were conducted as previously described (Abi-Dargham et al., 2000). An arterial catheter was inserted in the radial artery after completion of the Allen test and infiltration of the skin with 2% lidocaine. A venous catheter was inserted in a forearm vein on the opposite side. A polyurethane head immobilizer system (Soule Medical, Tampa, FL) was used to minimize head movement (Mawlawi et al., 1999). PET imaging was performed with the ECAT EXACT HR+ (Siemens, Knoxville, TN) [63 slices covering an axial field of view of 15.5 cm, axial sampling of 2.46 mm, in-plane and axial resolution of 4.4 and 4.1 mm full width half-maximum at the center of the field of view in the three-dimensional mode (3-D), respectively]. A 10 min transmission scan was obtained before radiotracer injection. [11C]NNC 112 was injected intravenously over a 45 sec period. Emission data were collected in the 3-D mode for 90 min as 18 successive frames of increasing duration (3 × 20 sec, 3 × 1 min, 3 × 2 min, 2 × 5 min, 7 × 10 min). Images were reconstructed with attenuation correction using the transmission data and a Sheppe 0.5 filter (cutoff 0.5 cycles per projection rays).
Input function measurement. After radiotracer injection, arterial samples were collected every 10 sec with an automated sampling system for the first 2 min, and manually thereafter at longer intervals. A total of 30 samples was obtained per experiment. After centrifugation (10 min at 1800 × g), a 200 μl aliquot of plasma was collected, and activity was measured in a gamma counter (1480 Wizard 3M automatic gamma counter; LKB-Wallac, Gaithersburg, MD). Gamma counter efficiency was calibrated at regular intervals with the PET camera using an18F solution. In addition, a long-lived source (22Na) was counted with each set of samples, to control for between run variance in counting efficiency. Six selected samples (collected at 2, 8, 16, 30, 50, and 70 min) were further processed by protein precipitation using acetonitrile followed by HPLC to measure the fraction of plasma activity representing unmetabolized parent compound, as previously described (Abi-Dargham et al., 2000).
A biexponential function was fitted to the six measured fractions parent and used to interpolate values between and after the measurements. The smallest exponential of the fraction parent curve, λpar, was constrained to the difference between λcer, the terminal rate of washout of cerebellar activity, and λtot, the smallest elimination rate constant of the total plasma (Abi-Dargham et al., 1999). The input function was calculated by the product of total counts and interpolated fraction parent at each time. The measured input function values (Ca(t), μCi/ml) were fitted to a sum of three exponentials, and the fitted values were used as input to the kinetic analysis of the regional brain uptake. The clearance of the parent compound (CL, l/hr) was calculated as the ratio of the injected dose to the area under the curve of the input function (Abi-Dargham et al., 1994).
For the determination of the plasma-free fraction (f1), triplicate 200 μl aliquots of plasma collected before injection were mixed with radiotracer, pipetted into ultrafiltration units (Centrifree; Amicon, Danvers, MA), and centrifuged at room temperature (20 min at 4000 rpm). At end of centrifugation, plasma and ultrafiltrate activities were counted, and f1 was calculated as the ratio of ultrafiltrate to total activity concentrations (Gandelman et al., 1994).
MRI acquisition and segmentation procedures. MRIs were acquired on a GE 1.5 T Signa Advantage system. After a sagittal scout (1 min), performed to identify the anterior commissure (AC)–posterior commissure (PC) plane, a transaxial T1-weighted sequence with 1.5 mm slice thickness was acquired in a coronal plane orthogonal to the AC–PC plane over the whole brain with the following parameters: three-dimensional spoiled gradient recalled acquisition in the steady state; repetition time, 34 msec; echo time, 5 msec; flip angle of 45°; slice thickness 1.5 mm and zero gap; 124 slices; field of view, 22 × 16 cm; with 256 × 192 matrix, reformatted to 256 × 256, yielding a voxel size of 1.5 × 0.9 × 0.9 mm; and time of acquisition, 11 min. MRI segmentation was performed within MEDx (Sensor Systems, Inc., Sterling, VA), with original subroutines implemented in MATLAB (Math Works, Natick, MA). Steps for MRI segmentation included correction for field inhomogeneities, fitting of the voxel distribution to a combination of three Gaussian functions, voxel classification, and post filtering (Abi-Dargham et al., 2000).
Image analysis. Image analysis was performed blind to the subject diagnosis with MEDx (Sensor Systems, Inc., Sterling, VA). To correct for head movement during the acquisition, all frames were coregistered to a frame of reference, using a least-square algorithm for within modalities coregistration [automated image registration (AIR)] (Woods et al., 1992). After frame-to-frame registration, the 18 frames were summed, and the summed PET image was coregistered and resampled to the MRI, using AIR (Woods et al., 1993). The summed PET image was used for the coregistration because it contains counts from the initial, flow-dependent, activity distribution, which enhances detection of boundaries of regions with low receptor density, such as the cerebellum. The parameters of the spatial transformation matrix of the summed PET data set were then applied to each individual frame. Thus, each PET frame was resampled in the coronal plane to a voxel volume of 1.5 × 0.9 × 0.9 mm3.
The boundaries for 14 regions of interest (ROIs) and one region of reference were drawn on the MRI according to criteria based on brain atlases (Talairach and Tournoux, 1988; Duvernoy, 1991) and on published reports (Pani et al., 1990; Kates et al., 1997; Killiany et al., 1997). Cortical regions (n = 8) included DLPFC, medial prefrontal cortex (MPFC), orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), parietal cortex (PC), temporal cortex (TC), parahippocampal gyrus (PHG), and occipital cortex (OC).
The prefrontal cortex was sampled from the most rostral plane to the plane corresponding to the rostral boundary of the genu of the corpus callosum. Prefrontal area ventral to the AC–PC plane was labeled OFC and included the inferior rostral gyrus, the gyrus rectus, the orbital gyrus, and the inferior frontal gyrus (orbital part). Prefrontal area dorsal to the AC–PC plane was divided into a lateral and medial section (medial section defined as cortex adjacent to the interhemispheric fissure). The lateral section (dorsal part of superior frontal gyrus, middle frontal gyrus and inferior frontal gyrus, triangular part) was labeled DLPFC. This region includes Brodmann areas 9 and 46 (Rajkowska and Goldman-Rakic, 1995). The medial section excluded the anterior cingulate and corresponded to the MPFC (areas 8 and 9, medial part of the superior frontal gyrus).
Subcortical regions (n = 6) included dorsal caudate (DCA), dorsal putamen (DPU), ventral striatum (VST), thalamus (THA), amygdala (AMY), and hippocampus (HIP). Criteria used to delineate striatal subregions (DCA, DPU, and VST) are found in Mawlawi et al. (2001), and criteria used for other regions are available on request. Right and left regions for bilateral ROIs were averaged. The cerebellum (CER), a region with negligible density of D1receptors, was used as region of reference to define the distribution volume of the nonspecific compartment.
Two methods were used for final ROI definition. A segmentation-based method was used for cortical regions, and a direct identification method was used for subcortical regions. For cortical regions, “large” regions were first drawn to delineate the boundaries of the ROIs. Within these regions, only the voxels classified as gray matter voxels by the MRI segmentation procedure were sampled to measure activity distribution (see details in Abi-Dargham et al., 2000). The steps involved in this method are illustrated in Figure1. Because of the mixture of gray and white matter in central gray structures (especially thalamus), the segmentation-based approach was not used to define subcortical ROIs, and the boundaries of these regions were identified by anatomical criteria. The CER region included the whole structure (both gray and white matter).
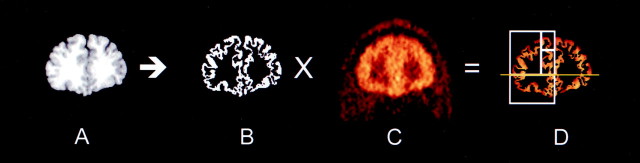
Fig. 1.
Illustration of the steps involved in the sampling of activity from cortical regions. A coronal section 0.5 mm anterior to the rostral part of the corpus callosum is displayed. The MRI (A) is segmented into white matter, gray matter, and CSF voxels. Gray matter voxels are assigned a value of 1, and all other pixels are assigned a value of 0 to form a gray inverted mask image (B). The coregistered PET image (C) is multiplied by the mask (B) to form a gray matter PET image (D). Regional activities are sampled onD in nonzero voxels. Region boundaries (white lines) are illustrated on the right. Theyellow line corresponds to the AC–PC plane. The region ventral to this plane is the OFC. Regions dorsal to the AC–PC plane are divided into a lateral region (DLPFC) and medial regions, which, at this level, include the MPFC and ACC, dorsal and ventral to the cingulate gyrus, respectively.
Derivation of regional total distribution volumes.Derivation of [11C]NNC 112 regional distribution volumes was performed using kinetic analysis and a three compartment model in the ROIs and a two compartment model in the cerebellum (Mintun et al., 1984; Abi-Dargham et al., 2000). The three compartment configuration included the arterial plasma compartment (Ca), the intracerebral free and nonspecifically bound compartment (nondisplaceable compartment,C2), and the specifically bound compartment (C3). Brain activity was corrected for the contribution of plasma activity assuming a 5% blood volume (Mintun et al., 1984).
The total regional distribution volume (VT, milliliters of plasma per gram of tissue) was defined as the ratio of the tracer concentration in this region (CT) to the metabolite-corrected plasma concentration at equilibrium:
| Equation 1 |
VT is equal to the sum of the distribution volumes for the second (nondisplaceable,V2) and third (specific,V3) compartments.VT was derived from the kinetic rate constants as:
| Equation 2 |
where K1 (in milliliters per gram per minute) and k2(min−1) are the unidirectional fractional rate constants for the transfer betweenCa andC2, andk3(min−1) andk4(min−1) are the unidirectional fractional rate constants for the transfer betweenC2 andC3. The termsK1 andk2 include the regional blood flow:K1 = F(1 − expPS/F) where F is regional blood flow, and PS is the permeability surface area product of the tracer; k2 =K1/V2f1. Thus, distribution volumes (V2,V3, andVT) are flow independent (the flow is present in both numerator and denominator and cancels out).
Kinetic parameters were derived by nonlinear regression using a Levenberg–Marquardt least-squares minimization procedure (Levenberg, 1944) implemented in MATLAB (Math Works) as previously described (Laruelle et al., 1994b). Given the unequal sampling over time (increasing frame acquisition time from beginning to end of the study), the least squares minimization procedure was weighed by the square root of the frame acquisition time.
Derivation of binding potential. The binding potential (BP, milliliters per gram) was derived as the difference between total (VT) and nonspecific (V2) distribution volumes, with cerebellum VT used as a measure ofV2:
| Equation 3 |
Under these conditions, BP is equal to (Laruelle et al., 1994a):
| Equation 4 |
where Bmax is the regional concentration of D1 receptors,KD is the affinity of [11C]NNC 112 for D1 receptors, andf1 is the free fraction of [11C]NNC 112 in the plasma. Althoughf1 was measured in this study, the derivation of BP was not corrected forf1, given the low free fraction of [11C]NNC 112 in the plasma (<1%) and the lack of reliability of f1measurement when f1 is <10% (Abi-Dargham et al., 2000).
A second outcome measure of interest was the BP normalized to cerebellum distribution volume, termedV3".V3" is equal to the ratio of BP toV2 (Laruelle et al., 1994a):
| Equation 5 |
where f2 is the free fraction in the nondisplaceable compartment (f2 =f1/V2). Comparing Equations 4 and 5 informs on the nature of BP andV3", respectively. Both outcome measures are related to receptor parametersBmax andKD plus a term unrelated to receptor parameters, f1 andf2, respectively. Thus, BP corrects for between-subject differences in nonspecific binding in the brain, but is affected by between-subject differences in nonspecific binding in the plasma. V3" corrects for between-subject differences in nonspecific binding in the plasma, but is affected by between-subject differences in nonspecific binding in the brain. [11C]NNC 112 test–retest studies demonstrated that BP and V3" are affected by similar test–retest variability, but that the intraclass correlation coefficient of BP was superior toV3" (Abi-Dargham et al., 2000). Thus, BP as defined by Equation 4 was a priori selected as the primary outcome measure for this study.
Statistical analysis
Between-group comparisons were performed using two-tailed unpaired t tests. Relationships between continuous variables were analyzed with the Pearson product moment correlation coefficient. A probability value of 0.05 was selected as significance level.
The primary hypothesis of this study related to DLPFC D1 receptor, and no correction for multiple testing was applied to this region. Other regions were analyzed and compared between groups, to test the regional specificity of the finding in DLPFC. To correct for multiple testing and explore the covariance structure among regions, principal component analysis with varimax transformation was performed on the complete sample. Individual regional BP values were transformed into z scores and reduced to an appropriate number of factors using the factor weights derived by the principal component analysis. Repeated measure ANOVA was performed with factors as repeated measure and diagnosis as grouping variable to test the existence of a factor by diagnosis interaction, followed by unpaired t test on each factor.
RESULTS
Sample composition
Table 1 lists the demographic and clinical variables of the samples. Groups were matched for age, gender, race, socioeconomic status of the family of origin, and nicotine smoking. The socioeconomic status of patients was lower than controls. In the patient group, seven subjects were experiencing a first episode of illness and had never been treated with antipsychotic medications at the time of the scan. Nine subjects were chronic patients who had been previously treated with antipsychotic drugs. At the time of the scan, these patients were off antipsychotics for 164 ± 173 d (range from 15 to 360 d, with the latter value used for patients off antipsychotic drugs for >1 year). Twelve patients were studied as inpatients, and four patients were studied as outpatients. Duration of illness was 6.8 ± 7.1 years (range from 4 weeks to 26 years). Patients displayed a moderate level of symptom severity. PANSS-positive symptoms subscale (seven positive symptoms rated from 1 to 7) score was 18.6 ± 7.5 (range from 7 to 33). PANSS-negative symptoms subscale (seven negative symptoms rated from 1 to 7) was 18.4 ± 5.7 (range from 11 to 30). PANSS general psychopathology subscale (16 symptoms rated from 1 to 7) was 33.6 ± 7.6 (18–48).
Table 1.
Demographic and clinical variables of the samples
| Variables | Controls | Patients | p |
|---|---|---|---|
| n | 16 | 16 | |
| Age | 34 ± 10 | 33 ± 12 | 0.85 |
| Gender (F/M)1-a | 5/11 | 3/13 | 0.29 |
| Race (C/AA/H/AS)1-b | 6/4/5/1 | 6/4/4/2 | 0.95 |
| Subject SES1-c | 35 ± 16 | 18 ± 3 | <0.01 |
| Family SES | 42 ± 13 | 43 ± 17 | 0.87 |
| Nicotine smoking (N/Y/E)1-d | 9/4/3 | 12/1/3 | 0.42 |
| DN/DF1-e | 7/9 | ||
| Drug-free interval (DF patients) | 164 ± 173 | ||
| PANSS Positive symptoms subscale | 19 ± 7 | ||
| PANSS Negative symptoms subscale | 18 ± 6 | ||
| PANSS General psychopathology subscale | 34 ± 7 |
F, Female; M, male.
C, Caucasian; AA, African-American; H, Hispanic; AS, Asian.
SES, Socioeconomic status, measured with Hollingshead scale (Hollingshead, 1975).
N, Nonsmoker; Y, currently smoking; E, ex-smoker.
DN, Drug (antipsychotic) naive patient, experiencing a first episode of illness; DF, drug (antipsychotic)-free patients, i.e. patients previously treated with antipsychotic drugs but not currently taking antipsychotic medications for at least 14 d.
Working memory assessment
The n-back task was administered to 14 patients and 15 controls (three subjects were not available for testing). Twelve patients completed the n-back during the drug-free interval preceding the PET study, and two completed it after the PET study, while on antipsychotic medications (for scheduling reasons, not for clinical reasons).
Figure 2 presents the AHR for the 1-, 2-, and 3-back conditions for controls and patients with schizophrenia. Controls performed almost perfectly at the 1- back with an AHR of 0.99 ± 0.03. The AHR in controls decreased to 0.86 ± 0.10 at 2-back and 0.72 ± 0.18 at 3-back. The AHR in patients with schizophrenia was 0.88 ± 0.16 at 1-back, 0.61 ± 0.30 at 2-back, and 0.50 ± 0.28 at 3-back. Each of these distributions had a mean significantly different from zero (one sample ttest; p < 0.001), indicating that patients performed significantly above chance levels.
Fig. 2.
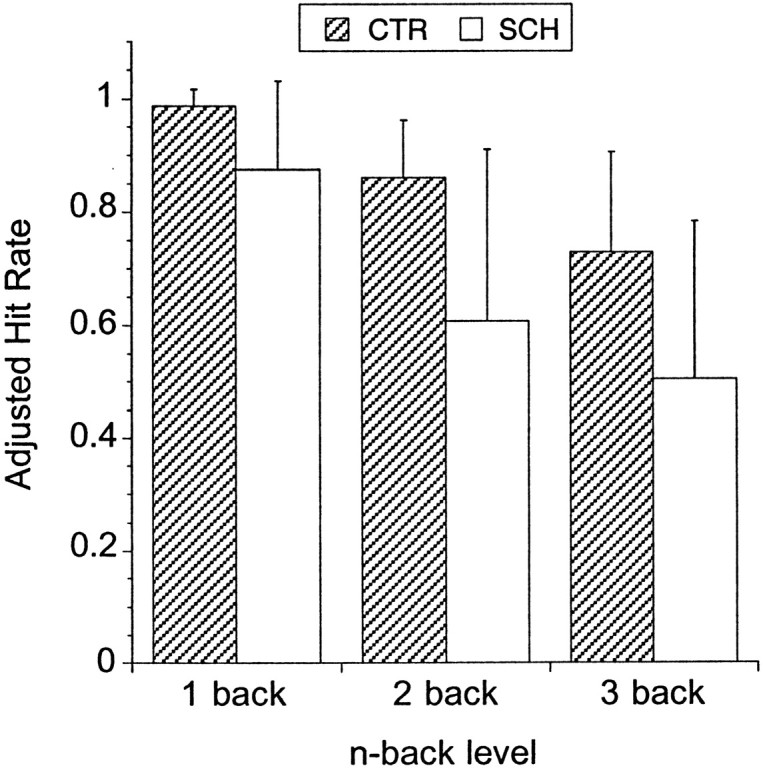
Adjusted hit rate (mean ± SD) for the 1-, 2-, and 3-back conditions in controls (CTR;n = 15) and patients with schizophrenia (SCH;n = 14). The adjusted hit rate is the hit rate (number of correct responses divided by number of targets) corrected for the error rate (number of incorrect responses divided by number of nontargets) and ranges from +1 to −1. Patients performed significantly worse than controls at each level of the task, but above chance level (score of zero). Increasing task difficulty results in similar relative decrements in performance in patients and controls (repeated measures ANOVA, task level, p < 0.0001; diagnosis factor, p = 0.0017; diagnosis by task level interaction, p = 0.27).
The effect of working memory load (1- vs 2- vs 3-back) and diagnosis on the AHR was evaluated with repeated measure ANOVA, with load level as repeated factor. This test indicated a significant effect of task level (p < 0.0001), diagnosis (p = 0.001), but no task level by diagnosis interaction (p = 0.23). Patients performed worse than controls at each level of the task (1-back, p = 0.011; 2-back, p = 0.005; 3-back, p = 0.015). AHR variance was larger in patients compared with controls at the 1-back (p < 0.0001), the 2-back (p = 0.003), but not the 3-back (p = 0.10). d′ was calculated for 2-back and 3-back. For both conditions, d′ in the patient group (2-back d′, 2.07 ± 1.04; 3-back d′, 1.75 ± 1.01) was significantly lower than d′ in the control group (2-back d′, 3.10 ± 0.60,p = 0.005; 3-back d′, 2.57 ± 0.75,p = 0.003). AHR at 1-, 2-, and 3-back conditions were not associated with age (r2 < 0.01 for all three correlations).
Among patients with schizophrenia, no differences were noted in AHR at any level of the test between first episode patients never previously exposed to antipsychotics (n = 7; AHR at 1-, 2-, and 3-back of 0.90 ± 0.10, 0.64 ± 0.28, and 0.49 ± 0.31, respectively) and chronic patients previously treated with antipsychotics (n = 7; AHR at 1-, 2-, and 3-back of 0.85 ± 0.20, 0.57 ± 0.33, and 0.52 ± 0.26, respectively). Severity of positive, negative, or general symptoms measured with the PANSS subscales were not predictive of performance at 1-back, 2-back, or 3-back conditions (r2 < 0.15, p> 0.05 for all correlations).
D1 receptor measurements
[11C]NNC 112 injection parameters
No significant between-group differences were observed in the [11C]NNC 112-injected dose (controls: 13.0 ± 4.4 mCi; patients with schizophrenia: 11.9 ± 5.2 mCi; p = 0.53), specific activity at time of injection (controls: 1022 ± 545 Ci/mmol; patients with schizophrenia: 876 ± 528 Ci/mmol; p = 0.44), or injected mass (controls: 4.7 ± 1.3 μg; patients with schizophrenia: 5.0 ± 1.2 μg; p = 0.47).
[11C]NNC 112 input function
No significant between-group differences were observed in the clearance rate of [11C]NNC 112 from the plasma compartment (controls: 83 ± 21 l/hr; patients with schizophrenia: 85 ± 32 l/hr; p = 0.81). The plasma-free fraction (f1) was similar in control subjects (0.81 ± 0.34%) and patients with schizophrenia (0.83 ± 0.38%; p = 0.88), supporting the use of BP as outcome measure (Eq. 4) for between-group comparisons.
[11C]NNC 112 cerebellum distribution volume
The cerebellum distribution volume (V2) was derived using a two compartment model and was not significantly different between control subjects (1.92 ± 0.43 ml/gm) and patients with schizophrenia (1.81 ± 0.46 ml/gm; n = 0.51). The free fraction of the nonspecific distribution volume (f2) was not different between groups (controls, 0.43 ± 0.18%; patients, 0.47 ± 0.21%;p = 0.55).
ROI volumes
Table 2 lists the ROI volumes in healthy controls and patients with schizophrenia. No significant between-group differences were found in DLPFC volumes, nor in volumes of the other regions. A trend was observed for the hippocampus (p = 0.05) and parahippocampal gyrus (p = 0.07) volumes to be smaller in patients with schizophrenia compared with controls, by 13 and 9%, respectively.
Table 2.
ROI volumes in controls and patients with schizophrenia (cm3)
| Region | Subregion | Controls | Patients |
|---|---|---|---|
| Neocortex | DLPFC | 33.2 ± 6.3 | 35.2 ± 5.8 |
| MPFC | 9.1 ± 2.4 | 10.8 ± 4.8 | |
| OFC | 11.3 ± 4.8 | 11.8 ± 4.1 | |
| TC | 50.8 ± 9.3 | 54.9 ± 12.1 | |
| PC | 61.2 ± 13.1 | 69.0 ± 13.9 | |
| OC | 43.3 ± 7.8 | 40.0 ± 7.6 | |
| Limbic/paralimbic | ACC | 6.7 ± 1.6 | 6.8 ± 1.1 |
| AMY | 3.6 ± 0.4 | 3.4 ± 0.8 | |
| HIP | 7.0 ± 1.2 | 6.1 ± 1.3 | |
| PHG | 11.9 ± 1.5 | 10.8 ± 1.7 | |
| Striatum | DCA | 5.2 ± 0.7 | 5.1 ± 0.7 |
| DPU | 7.8 ± 0.9 | 8.3 ± 1.1 | |
| VST | 2.1 ± 0.9 | 2.2 ± 0.6 | |
| Thalamus | 8.3 ± 0.9 | 7.7 ± 1.3 |
Values are mean ± SD; n = 16 per group. DLPFC, Dorsolateral prefrontal cortex; MPFC, medial prefrontal cortex; OFC, orbitofrontal cortex; TC, temporal cortex; PC, parietal cortex; OC, occipital cortex; ACC, anterior cingulate cortex; AMY, amygdala; HIP, hippocampus; PHG, parahippocampal gyrus; DCA, dorsal caudate; DPU, dorsal putamen; VST, ventral striatum. No significant between group differences were observed. HIP and PHG were smaller in patients with schizophrenia at trend level (p = 0.055 and 0.068, respectively).
[11C]NNC 112 delivery to the brain
No significant between-group differences were observed in the regional rates of tracer delivery to the brain, as measured by the parameter K1. For example, DLPFCK1 was 0.14 ± 0.03 ml · gm−1 · min−1in control subjects and 0.15 ± 0.04 ml ·gm−1 · min−1in patients with schizophrenia (p = 0.46). Assuming that patients and controls have similar permeability–surface area product for [11C]NNC 112, this result indicates no significant between-group difference in regional blood flow.
[11C]NNC 112 regional BP
The regional uptake of [11C]NNC 112 was consistent with the known distribution of D1receptors in the human brain. Representative images and regional time–activity curves are presented in Figures 3 and 4, respectively.
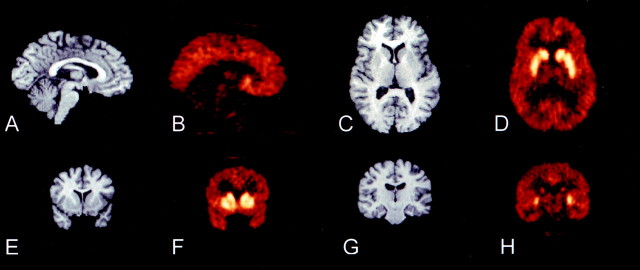
Fig. 3.
MRI and coregistered [11C]NNC 112 PET images. The PET image represents the activity recorded from 30 to 60 min after injection of 13.3 mCi in a 37-year-old healthy female volunteer. A, B, Sagittal view, illustrating the contrast between cortical and cerebellar activities.C, D, Transaxial view, at the level of the head of caudate, putamen, and thalamus. E, F,Coronal view, at the level of the anterior striatum, illustrating the lower level of activity in the ventral striatum compared with the caudate and putamen. G, H, Coronal view at the level of the hippocampus, illustrating low levels of activity in thalamus, hippocampus, and parahippocampal gyrus. Putamen and caudate activities are still visualized.
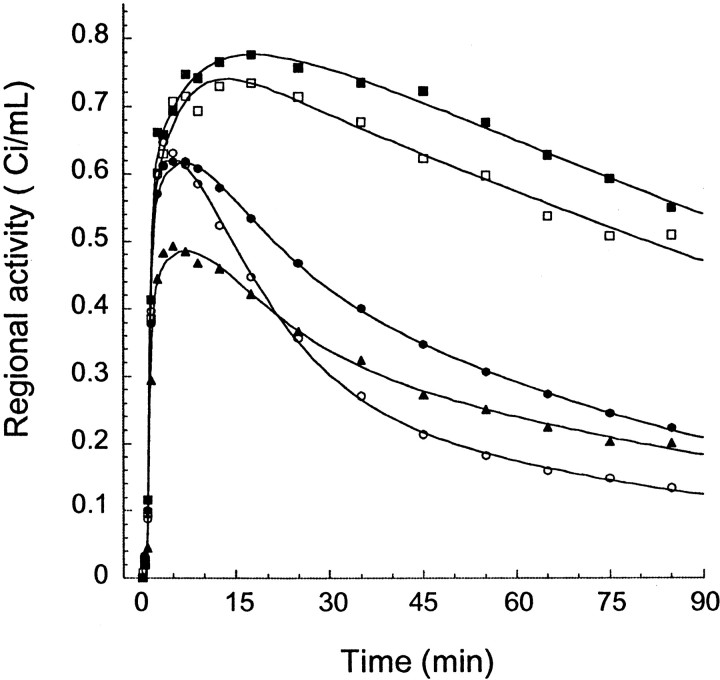
Fig. 4.
Regional time-activity curves after injection of 16.6 mCi [11C]NNC 112 in a 42-year-old male healthy volunteer. Only a subset of regions are represented: dorsal caudate (closed squares), ventral striatum (open squares), dorsolateral prefrontal cortex (closed circles), hippocampus (closed triangles), and cerebellum (open circles). Points are measured values for each frame, and lines are values fitted to a three compartment model.
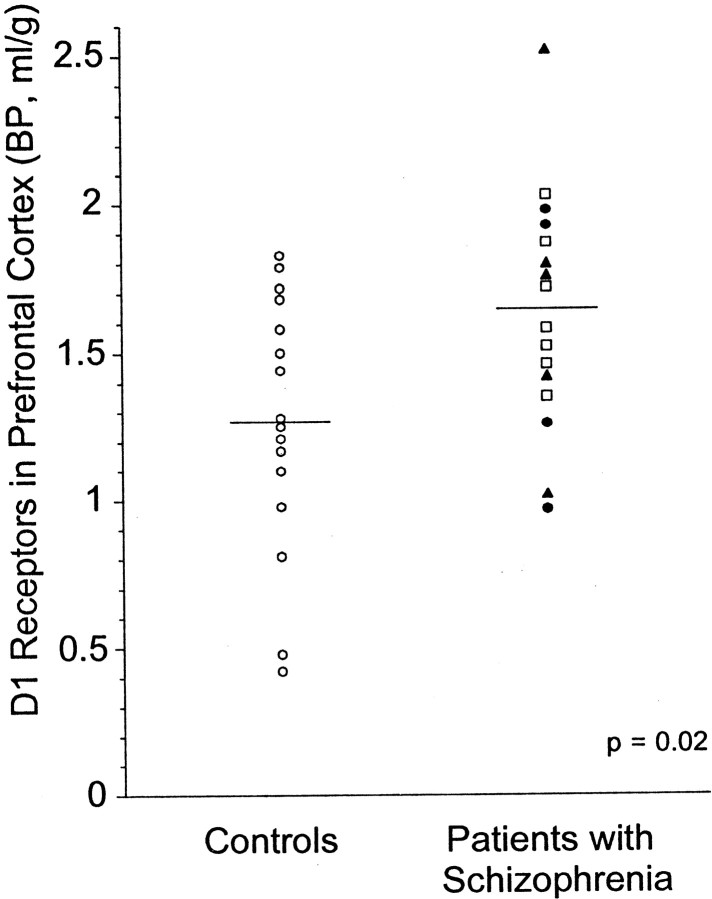
DLPFC [11C]NNC 112 BP was significantly higher in patients with schizophrenia compared with control subjects (p = 0.02) (Table3). The distribution of DLPFC [11C]NNC 112 BP values in each group is presented in Figure 5. The variance was not different between groups (F test; p = 0.70). One value in the patients group was an outlier, withz score of 2.26. No rationale was identified to exclude this observation a posteriori. Nonetheless, the analysis was repeated without this value, and provided similar results (p = 0.038). The absence of significant between-group differences in DLPFC volume indicates that the between-group difference in DLPFC [11C]NNC 112 BP was not attributable to partial voluming effects.
Table 3.
[11C]NNC 112 binding potential in controls and patients with schizophrenia (ml/gm)
| Region | Subregion | Controls | Patients |
|---|---|---|---|
| Neocortex | DLPFC | 1.27 ± 0.44 | 1.63 ± 0.393-150 |
| MPFC | 1.57 ± 0.33 | 1.82 ± 0.44 | |
| OFC | 1.41 ± 0.44 | 1.69 ± 0.72 | |
| TC | 1.55 ± 0.34 | 1.82 ± 0.49 | |
| PC | 1.46 ± 0.41 | 1.63 ± 0.44 | |
| OC | 1.51 ± 0.42 | 1.70 ± 0.38 | |
| Limbic/paralimbic | ACC | 1.74 ± 0.50 | 1.93 ± 0.37 |
| AMY | 1.42 ± 0.39 | 1.51 ± 0.48 | |
| HIP | 1.31 ± 0.86 | 1.18 ± 0.39 | |
| PHG | 1.14 ± 0.40 | 1.31 ± 0.43 | |
| Striatum | DCA | 5.93 ± 2.68 | 6.36 ± 1.35 |
| DPU | 6.72 ± 2.44 | 6.90 ± 1.49 | |
| VST | 5.71 ± 2.73 | 5.48 ± 1.57 | |
| Thalamus | 0.98 ± 0.43 | 0.91 ± 0.28 |
Values are mean ± SD; n = 16 per group. DLPFC, Dorsolateral prefrontal cortex; MPFC, medial prefrontal cortex; OFC, orbitofrontal cortex; TC, temporal cortex; PC, parietal cortex; OC, occipital cortex; ACC, anterior cingulate cortex; AMY, amygdala; HIP, hippocampus; PHG, parahippocampal gyrus; DCA, dorsal caudate; DPU, dorsal putamen; VST, ventral striatum.
F3-150: p<0.05.
Fig. 5.
Distribution of [11C]NNC 112 BP in DLPFC of healthy controls (n = 16; open circles) and patients with schizophrenia (n = 16; antipsychotic-naive patients, open squares; patients antipsychotic-free for >1 year, closed circles;patients with 2–3 weeks of antipsychotic-free interval, closed triangles). Patients with schizophrenia displayed increased D1 receptor availability compared with controls (p = 0.02).
Table 3 also lists [11C]NNC 112 BP values in other regions. A trend was observed for higher [11C]NNC 112 BP in the MPFC (p = 0.08) and temporal cortex (p = 0.08; values not corrected for multiple testing). Other regions did not show significant between-group differences in [11C]NNC 112 BP. Principal component analysis was applied to [11C]NNC 112 BP values from the 14 regions included in Table 3. This analysis returned three significant factors (eigenvalues higher than 1) that accounted for 87% of the variance. The regional weights associated with each factor are presented in Table 4. Factor 1 accounted for 54% of the variance, showed high loads for anterior neocortical regions, and was termed frontocortical factor. Factor 2 accounted for 19% of the variance and was essentially contributed to by the striatal regions. A third factor was extracted, accounting for 10% of the variance and showing high loads in hippocampus and thalamus only. Individual values on each factor were computed by multiplying thez score matrix of regional [11C]NNC 112 BP value by the factor weight matrix. Repeated measure ANOVA with factors as repeated measure and diagnosis as factor showed no diagnosis effect (p = 0.62), but a significant factor by diagnosis interaction (p = 0.036). Post hoc analysis revealed that patients with schizophrenia had significantly higher values on factor 1 compared with controls (p = 0.016). No significant differences were observed in factors 2 (p = 0.38) and 3 (p = 0.21). Together, these data indicated a relatively diffuse and significant increase in neocortical D1 receptor availability in patients with schizophrenia, most pronounced in DLPFC.
Table 4.
[11C]NNC 112 binding potential: principal component analysis
| Region | Subregion | Factor 1 (frontocortical) | Factor 2 (striatal) | Factor 3 (thalamolimbic) |
|---|---|---|---|---|
| Neocortex | DLPFC | 0.83 | 0.00 | −0.40 |
| MPFC | 0.73 | 0.07 | −0.09 | |
| OFC | 0.67 | −0.05 | −0.22 | |
| TC | 0.65 | 0.07 | 0.11 | |
| PC | 0.59 | 0.00 | 0.21 | |
| OC | 0.49 | 0.04 | 0.35 | |
| Limbic/paralimbic | ACC | 0.43 | 0.06 | 0.32 |
| AMY | 0.31 | 0.04 | 0.42 | |
| HIP | −0.19 | −0.13 | 0.82 | |
| PHG | 0.44 | −0.11 | 0.40 | |
| Striatum | DCA | 0.07 | 0.81 | −0.18 |
| DPU | 0.00 | 0.81 | 0.13 | |
| VST | −0.19 | 0.89 | −0.04 | |
| Thalamus | −0.16 | 0.01 | 0.89 |
Values are regional weights for the three factors derived from principal component analysis of the entire sample (n = 32). DLPFC, Dorsolateral prefrontal cortex; MPFC, medial prefrontal cortex; OFC, orbitofrontal cortex; TC, temporal cortex; PC, parietal cortex; OC, occipital cortex; ACC, anterior cingulate cortex; AMY, amygdala; HIP, hippocampus; PHG, parahippocampal gyrus; DCA, dorsal caudate; DPU, dorsal putamen; VST, ventral striatum.
[11C]NNC 112 regional V3"
Results obtained with V3" (=BP/V2) were essentially similar to results obtained with BP, which was expected from the absence of between-group differences in V2. Thus, DLPFC was the only region showing a significant difference in [11C]NNC 112 V3" (higher in patients compared with controls; p = 0.03).
DLPFC D1 receptors BP and clinical variables
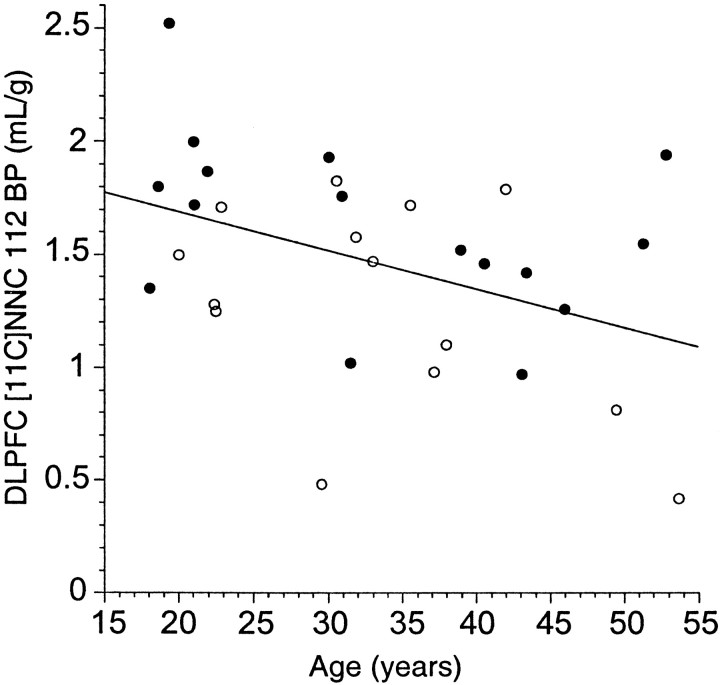
A significant age-related decline in DLPFC [11C]NNC 112 BP was observed in the entire sample (r2 = 0.13;p = 0.02) (Fig. 6). This relationship was present in both groups, but failed to reach significance when groups were analyzed separately (control subjects: r2 = 0.11,p = 0.08; patients with schizophrenia,r2 = 0.17, p = 0.11). No age by diagnosis interaction was observed for DLPFC [11C]NNC 112 BP (p = 0.62), suggesting that age affected both groups similarly.
Fig. 6.
Effect of aging on [11C]NNC 112 BP in DLPFC of healthy controls (open circles) and patients with schizophrenia (closed circles). A significant age-related decline in DLPFC [11C]NNC 112 BP was observed in the entire sample (r2 = 0.13;p = 0.02). No age by diagnosis interaction was observed for DLPFC [11C]NNC 112 BP (p = 0.62), suggesting that age affected both groups similarly.
In the patient group, DLPFC [11C]NNC 112 BP was not significantly different between first episode–antipsychotic drug-naive patients (n = 7; age = 30 ± 13 years; DLPFC [11C]NNC 112 BP = 1.64 ± 0.23 ml/gm) and chronic patients previously exposed to antipsychotic medications (n = 9; age = 35 ± 12 years; DLPFC [11C]NNC 112 BP = 1.62 ± 0.50 ml/gm; p = 0.94). Among the previously treated patients, five underwent inpatient washout and had a relatively brief drug-free interval before the PET scan (19 ± 3 d), and four were not taking antipsychotic drugs for at least 1 year before the PET scan. DLPFC [11C]NNC 112 BP was not different between previously treated patients with short (1.70 ± 0.55 ml/gm) or long drug-free interval (1.52 ± 0.40 ml/gm; p = 0.62). In previously treated patients, no relationship was observed between duration of the drug-free interval and DLPFC [11C]NNC 112 BP (r2 = 0.03; p = 0.66). Together, these data indicate that the upregulation of D1 receptors in the DLPFC in patients with schizophrenia was not related to previous exposure to antipsychotic medications.
In the patient group, DLPFC [11C]NNC 112 BP was not significantly associated with severity of positive symptoms as assessed by the PANSS-positive subscale (r2 = 0.02; p = 0.65), nor with severity of negative symptoms (PANSS-negative subscale;r2 = 0.04; p = 0.21), nor with general psychopathology (PANSS general psychopathology scale; r2 = 0.04;p = 0.46). DLPFC [11C]NNC 112 BP was not associated with duration of illness (r2 = 0.03;p = 0.49).
DLPFC D1 receptors BP and working memory performance
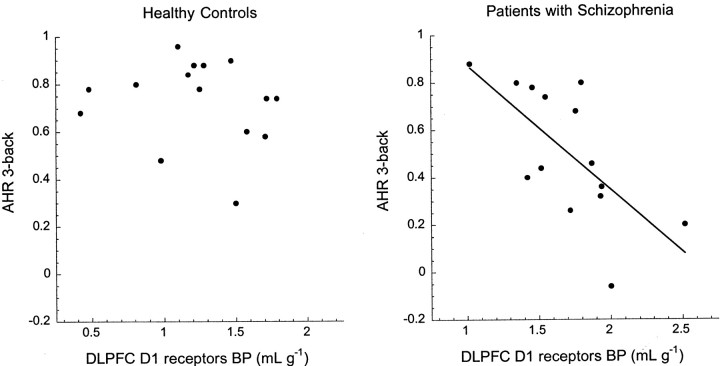
The hypothesis of an association between D1receptor availability in DLPFC and n-back performance was tested in the entire sample and in each group separately (Table5). When analyzing both groups together, an association was observed between low working memory performance at 1-, 2-, and 3-back and high DLPFC [11C]NNC 112 BP. This effect was accounted for by the patients with schizophrenia. Within the control group, there was no relationship between performance on the n-back and D1 receptor availability. In patients with schizophrenia, high DLPFC D1 receptor availability was associated with low AHR at 2-back (r2 = 0.31; p = 0.04) and at 3-back (r2 = 0.45;p = 0.008). Similar results were observed with d′ (2-back, r2 = 0.29,p = 0.04; 3-back,r2 = 0.43, p = 0.01). The relationship between DLPFC [11C]NNC 112 BP and AHR at the 3-back condition is presented in Figure 7.
Table 5.
Association between DLPFC [11C]NNC 112 BP and performance at n-back (Adjusted Hit Rate)
| Task level | All subjects (n = 28) | Control subjects | Patients with schizophrenia | Interaction | |||
|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | ||
| p | |||||||
| 1-back | −0.44 | 0.02 | 0.04 | 0.88 | −0.43 | 0.12 | 0.044 |
| 2-back | −0.42 | 0.02 | 0.23 | 0.07 | −0.55 | 0.04 | 0.008 |
| 3-back | −0.62 | 0.01 | −0.05 | 0.41 | −0.67 | 0.008 | 0.094 |
Fig. 7.
Relationship between [11C]NNC 112 BP in DLPFC (x-axis) and performance (AHR) at the 3-back test (y-axis) in healthy controls (left) and in patients with schizophrenia (right). The AHR ranges from 1 (best performance) to −1 (worse performance), with a score of 0 corresponding to performance at chance level. In controls, DLPFC D1 receptor availability was not associated with performance at the task. In patients with schizophrenia, increased DLPFC D1 receptor availability was associated with low performance at the task (r2 = 0.45; p = 0.008). Note the difference inx-axis scales between controls and patients with schizophrenia. Similar findings were observed with the 2-back (Table5).
DISCUSSION
The main findings of this study are that DLPFC D1 receptor availability, measured by thein vivo binding of [11C]NNC 112, is increased in never-treated and currently untreated patients with schizophrenia, and that this increase is a strong predictor of decreased performance at the n-back task.
Because of the evidence implicating DLPFC DA transmission in working memory processes, evaluation of [11C]NNC 112 BP in the DLPFC was the primary focus of this study. Other regions were also investigated to assess the regional specificity of the results in the DLPFC. The DLPFC was the only region examined in which a significant difference in [11C]NNC 112 BP was found between patients with schizophrenia and controls. However, principal component analysis indicated that the alteration of DA transmission associated with increased [11C]NNC 112 BP might not be restricted to the DLPFC, because other cortical regions such as MPFC showed high covariance with DLPFC. Analysis of a larger sample is required to further explore this issue. In contrast, there was no indication for alterations in [11C]NNC 112 BP in striatal, limbic, and thalamic regions.
The absence of change in striatal [11C]NNC 112 BP observed in this study is consistent with postmortem studies that reported unaltered striatal binding of [3H]SCH 23390 in schizophrenia (Pimoule et al., 1985; Seeman et al., 1987; Joyce et al., 1988; Reynolds and Czudek, 1988), although one study reported decreased striatal binding of [3H]SCH 23390 in schizophrenia (Hess et al., 1987).
The significant increase in DLPFC [11C]NNC 112 BP observed in patients with schizophrenia contrasts with results of three previous postmortem studies. Two studies evaluated the binding of [3H]SCH 23390 in the PFC in schizophrenia. One homogenate binding study reported no change (Laruelle et al., 1990), and one autoradiography study reported a nonsignificant increase (Knable et al., 1996). PFC D1 receptor mRNA levels were also reported unchanged (Meador-Woodruff et al., 1997). These postmortem results might be affected by antemortem medications, because administration of antipsychotic drugs downregulate PFC D1 receptor mRNA and proteins (Lidow and Goldman-Rakic, 1994; Lidow et al., 1997).
The results presented here contrast with the results of a previous PET study that reported lower [11C]SCH 23390k3/k4 ratio (V3" in our notation) in the PFC in patients with schizophrenia (Okubo et al., 1997). In addition to potential clinical differences in clinical populations and symptoms severity, several critical technical factors limit the comparability of these studies: (1) the PFC–cerebellum distribution volume ratio of [11C]SCH 23390 is in the 1.2–1.4 range, versus 1.7–2 for [11C]NNC 112, indicating that [11C]NNC 112 is a superior ligand for the measurement of D1receptors in the PFC; (2) the PET camera used by Okubo et al. (1997)was a seven slices device, with limited field of view and limited resolution compared with the camera used in this study; (3) [11C]SCH 23390 displays a relatively low selectivity against 5HT2A/2C receptors (Laruelle et al., 1991), whereas [11C]NNC 112in vivo binding in the PFC is not affected by pretreatment with selective 5HT2A/2C antagonists (Halldin et al., 1998); (4) several lines of evidence suggest that the cellular localization of D1 receptors differentially influence the in vivo binding of [11C]SCH 23390 and [11C]NNC 112 (for review, see Laruelle, 2000). Acute DA depletion has no effect on the in vivobinding of [11C]NNC 112, but decreases the in vivo binding of [3H]SCH 23390 (Guo et al., 2000), an effect that might be attributable to receptor translocation from the endosomial compartment to the cell surface and lower in vivoaffinity of SCH 23390 for externalized compared with internalized receptors (Dumartin et al., 2000; Laruelle, 2000). In addition, sustained DA depletion induced by chronic reserpine treatment (21 d) is associated with increased in vivo[11C]NNC 112 binding in the rat PFC (presumably reflecting increase in D1 receptor expression) (Guo et al., 2001). However, the same treatment failed to produce detectable changes in the in vivo binding of [3H]SCH 23390 in the PFC (Guo et al., 2001), maybe because the opposite effects of receptor upregulation and externalization on [3H]SCH 23390in vivo binding cancel each other. Further research is warranted to elucidate the role of these factors in the discrepant findings between these two studies.
Patients enrolled in this study performed significantly worse than controls at each working memory load, but, even at the challenging 3-back load, performed significantly above chance level. This observation might reflect a selection bias toward patients with moderate pathology, because of the rigor of the capacity to consent evaluation. In these patients, increased DLPFC D1receptor availability was a strong predictor of poor performance at the 2-back and 3-back conditions (approximately one-third of the variance in 2- and 3-back AHR was accounted for by the variance in DLPFC [11C]NNC 112 BP). This relationship supports the involvement of altered DLPFC D1receptor transmission in the working memory deficits presented by these patients. Although it has been argued that working memory deficit might be the fundamental cognitive impairment in schizophrenia (Goldman-Rakic, 1994), patients with schizophrenia are impaired on multiple cognitive dimensions such as attention, learning and memory, executive function, and general intelligence (Braff et al., 1991;Saykin et al., 1994). Studies in a larger sample of subjects characterized with a comprehensive neuropsychological battery are warranted to test the specificity of the association between increased D1 receptor availability and working memory deficits.
The in vivo binding of [11C]NNC 112 is not affected by acute changes in endogenous DA (Abi-Dargham et al., 1999; Chou et al., 1999;Guo et al., 2000). It is therefore reasonable to assume that the increased [11C]NNC 112 binding observed in this study reflects increased concentration of D1 receptors in DLPFC of patients with schizophrenia. This increased concentration might represent a primary phenomenon or a compensatory upregulation secondary to chronic deficiency in D1 receptor stimulation by DA. At this point, both possibilities must be entertained, but the second hypothesis is favored by several lines of evidence.
The first interpretation (increase in DLPFC D1receptor is a primary phenomenon) might suggest that the alteration in working memory performance seen in these patients results from increased postsynaptic sensitivity to DA released in the DLPFC during performance of the task (Watanabe et al., 1997). This view is consistent with the evidence that excessive stimulation of D1 receptors, either because of excessive DA release or to high doses of DA agonists (Arnsten et al., 1994; Murphy et al., 1996a,b; Cai and Arnsten, 1997; Zahrt et al., 1997), is associated with a deterioration of working memory function in primates. This interpretation would predict that administration of D1 antagonists should improve working memory function in patients with schizophrenia. While we are not aware of studies that specifically evaluated the effect of D1 receptor antagonists on working memory function in schizophrenia, limited therapeutic trials with selective D1 receptor antagonists in schizophrenia showed a lack of efficacy or even worsening of clinical conditions (de Beaurepaire et al., 1995; Den Boer et al., 1995; Karle et al., 1995;Karlsson et al., 1995).
The second interpretation (increase in DLPFC D1receptors is a compensatory response to deficit in presynaptic DA function) is consistent with several indirect lines of evidence suggesting that schizophrenia might be associated with a deficit in prefrontal DA function. This hypothesis was proposed based on the relationship between low CSF homovanillic acid and poor performance at tasks involving the DLPFC in schizophrenia (Weinberger et al., 1988; Kahn et al., 1994), and on the beneficial effect of DA agonists on the pattern of DLPFC activation measured with PET during these tasks (Daniel et al., 1989, 1991; Dolan et al., 1995). More direct evidence for such a deficit was recently provided by one postmortem study suggesting a decrease in DA innervation in the DLPFC (Akil et al., 1999). This interpretation is also consistent with the performance deficits at delayed-response tasks observed in nonhuman primate models of prefrontal DA deficiency (selective 6-OHDA-induced DA depletion in the PFC, aged monkeys, and monkeys chronically treated with haloperidol). These deficits are reversed by indirect DA agonists and D1 agonists (Brozoski et al., 1979; Arnsten et al., 1994; Cai and Arnsten, 1997; Castner et al., 2000). This view is also supported by the observation that chronic phencyclidine exposure, which induces in humans symptoms reminiscent of schizophrenia (for review, see Javitt and Zukin, 1991), is associated with both impaired working memory performance and decreased DA turnover in the PFC in rodents and primates (for review, see Jentsch and Roth, 1999).
The observation that chronic DA depletion is associated with increasedin vivo binding of [11C]NNC 112 in the PFC supports the plausibility of this interpretation of the PET findings (Guo et al., 2001). If this hypothesis is correct, both the increase in DLPFC [11C]NNC 112 BP and the decrease in n-back performance would be related to a common cause, i.e., a deficit in mesocortical presynaptic DA function. The similarity in DLPFC [11C]NNC 112 BP between first episode and chronic patients and the absence of association between this parameter and duration of illness are consistent with the hypothesis that this mesocortical DA function deficiency might be of neurodevelopmental origin (Weinberger, 1987). This interpretation suggests that working memory function in patients with schizophrenia might be improved by DA agonists.
A third possible interpretation of the data that combines elements of the first and second interpretations should also be discussed. A persistent decrease in prefrontal DA activity might induce upregulation of D1 receptors. This upregulation, which could be associated with increased sensitivity to agonists, might create conditions in which the increase in DA associated with stress or cognitive demands would result in an overstimulation of these upregulated D1 receptors. This model would predict that acute administration of a D1receptor agonist might be detrimental, although repeated administration of a D1 agonist might lead to desensitization of the receptors and thus have long term therapeutic effects. The development of an effective D1 receptor agonist suitable for human administration is critical to test these predictions.
Conclusions
In this study, D1 receptor availability was measured in vivo with PET and [11C]NNC 112 in untreated patients with schizophrenia and controls. [11C]NNC 112 BP was significantly elevated in the DLPFC, as well as, to a lower extent, in other anterior cortical regions. This increase was not caused by previous antipsychotic medications and was not associated with the severity of clinical symptomatology. However, excessive expression of D1 receptor in the DLPFC was strongly associated with impaired performance at the n-back task, a test of working memory function, confirming in humans the critical role of prefrontal D1 receptor transmission in delayed-response tasks observed in animal studies. We propose that both D1 receptor upregulation and impaired working memory performance might be caused by a chronic deficit in presynaptic DA function in the DLPFC of patients with schizophrenia. Additional imaging studies are warranted to confirm these data in an extended sample, to study the specificity of the relationship between cortical D1 receptor expression and working memory relative to other cognitive impairments, and to evaluate the relationship between prefrontal presynaptic DA function and D1 receptor availability in patients with schizophrenia.
Footnotes
This work was supported by United States Public Health Service Grant RO1 MH59144-01 from the National Institute of Mental Health, a Charles A. Dana Foundation grant, and the Lieber Center for Schizophrenia Research. We thank the subjects who participated in the study, Christer Halldin (Karolinska Institute, Stockholm, Sweden), who provided the desmethyl precursor of [11C]NNC 112, Drs. Janine Rodenhiser-Hill, Mark Slifstein, and Eric Zarahn, and the expert technical assistance of Marcella Bonjovi, Nicole Eftychiou, Ingrid Gelbard, David Amstel, Heather Lawson, Jennifer Bae, Mohamed Ali, Julie Montoya, Kim Ngo, Norman Simpson, and Kris Wolff as well as the staff of the Schizophrenia Research Center at the New York State Psychiatric Institute.
Correspondence should be addressed to Dr. Anissa Abi-Dargham, New York State Psychiatric Institute, 1051 Riverside Drive, Box 31, New York, NY 10032. E-mail: aa324@columbia.edu.
REFERENCES
- 1.Abi-Dargham A, Laruelle M, Seibyl J, Rattner Z, Baldwin RM, Zoghbi SS, Zea-Ponce Y, Bremner JD, Hyde TM, Charney DS, Hoffer PB, Innis RB. SPECT measurement of benzodiazepine receptors in human brain with [123-I]iomazenil: kinetic and equilibrium paradigms. J Nucl Med. 1994;35:228–238. [PubMed] [Google Scholar]
- 2.Abi-Dargham A, Simpson N, Kegeles L, Parsey R, Hwang DR, Anjilvel S, Zea-Ponce Y, Lombardo I, Van Heertum R, Mann JJ, Foged C, Halldin C, Laruelle M. PET studies of binding competition between endogenous dopamine and the D1 radiotracer [11C]NNC 756. Synapse. 1999;32:93–109. doi: 10.1002/(SICI)1098-2396(199905)32:2<93::AID-SYN3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 3.Abi-Dargham A, Martinez D, Mawlawi O, Simpson N, Hwang DR, Slifstein M, Anjilvel S, Pidcock J, Guo NN, Lombardo I, Mann JJ, Van Heertum R, Foged C, Halldin C, Laruelle M. Measurement of striatal and extrastriatal dopamine D1 receptor binding potential with [11C]NNC 112 in humans: validation and reproducibility. J Cereb Blood Flow Metab. 2000;20:225–243. doi: 10.1097/00004647-200002000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, Lewis DA. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry. 1999;156:1580–1589. doi: 10.1176/ajp.156.10.1580. [DOI] [PubMed] [Google Scholar]
- 5.Andersen PH, Gronvald FC, Hohlweg R, Hansen LB, Guddal E, Braestrup C, Nielsen EB. NNC-112, NNC-687 and NNC-756, new selective and highly potent dopamine D1 receptor antagonists. Eur J Pharmacol. 1992;219:45–52. doi: 10.1016/0014-2999(92)90578-r. [DOI] [PubMed] [Google Scholar]
- 6.Arnsten AF, Goldman-Rakic PS. Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch Gen Psychiatry. 1998;55:362–368. doi: 10.1001/archpsyc.55.4.362. [DOI] [PubMed] [Google Scholar]
- 7.Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology. 1994;116:143–151. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- 8.Barch DM, Braver TS, Nystrom LE, Forman SD, Noll DC, Cohen JD. Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia. 1997;35:1373–1380. doi: 10.1016/s0028-3932(97)00072-9. [DOI] [PubMed] [Google Scholar]
- 9.Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, III, Noll DC, Cohen JD. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- 10.Braff DL, Heaton R, Kuck J, Cullum M, Moranville J, Grant I, Zisook S. The generalized pattern of neuropsychological deficits in outpatients with chronic schizophrenia with heterogeneous Wisconsin Card Sorting Test results. Arch Gen Psychiatry. 1991;48:891–898. doi: 10.1001/archpsyc.1991.01810340023003. [DOI] [PubMed] [Google Scholar]
- 11.Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. NeuroImage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- 12.Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- 13.Cai JX, Arnsten AFT. Dose-dependent effects of the dopamine D1 receptor agonists A77636 or SKF81297 on spatial working memory in aged monkeys. J Pharmacol Exp Ther. 1997;283:183–189. [PubMed] [Google Scholar]
- 14.Callicott JH, Ramsey NF, Tallent K, Bertolino A, Knable MB, Coppola R, Goldberg T, vanGelderen P, Mattay VS, Frank JA, Moonen CTW, Weinberger DR. Functional magnetic resonance imaging brain mapping in psychiatry: methodological issues illustrated in a study of working memory in schizophrenia. Neuropsychopharmacology. 1998;18:186–196. doi: 10.1016/S0893-133X(97)00096-1. [DOI] [PubMed] [Google Scholar]
- 15.Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, Goldberg TE, Weinberger DR. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9:20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- 16.Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, Weinberger DR. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- 17.Carlson S, Martinkauppi S, Rama P, Salli E, Korvenoja A, Aronen HJ. Distribution of cortical activation during visuospatial n-back tasks as revealed by functional magnetic resonance imaging. Cereb Cortex. 1998;8:743–752. doi: 10.1093/cercor/8.8.743. [DOI] [PubMed] [Google Scholar]
- 18.Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD. Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry. 1998;155:1285–1287. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- 19.Castner SA, Williams GV, Goldman-Rakic PS. Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science. 2000;287:2020–2022. doi: 10.1126/science.287.5460.2020. [DOI] [PubMed] [Google Scholar]
- 20.Chou YH, Karlsson P, Halldin C, Olsson H, Farde L. A PET study of D1-like dopamine receptor ligand binding during altered endogenous dopamine levels in the primate brain. Psychopharmacology. 1999;146:220–227. doi: 10.1007/s002130051110. [DOI] [PubMed] [Google Scholar]
- 21.Cohen JD, Forman SD, Braver TS, Casey BJ, Servan-Schreiber D, Noll DC. Activation of the prefrontal cortex in a nonspatial working memory task with functional MRI. Human Brain Mapp. 1994;1:293–304. doi: 10.1002/hbm.460010407. [DOI] [PubMed] [Google Scholar]
- 22.Conklin HM, Curtis CE, Katsanis J, Iacono WG. Verbal working memory impairment in schizophrenia patients and their first-degree relatives: evidence from the digit span task. Am J Psychiatry. 2000;157:275–277. doi: 10.1176/appi.ajp.157.2.275. [DOI] [PubMed] [Google Scholar]
- 23.Courtney SM, Ungerleider LG, Keil K, Haxby JV. Object and spatial visual working memory activate separate neural systems in human cortex. Cereb Cortex. 1996;6:39–49. doi: 10.1093/cercor/6.1.39. [DOI] [PubMed] [Google Scholar]
- 24.D'Esposito M, Ballard D, Aguirre GK, Zarahn E. Human prefrontal cortex is not specific for working memory: a functional MRI study. NeuroImage. 1998;8:274–282. doi: 10.1006/nimg.1998.0364. [DOI] [PubMed] [Google Scholar]
- 25.Daniel DG, Berman KF, Weinberger DR. The effect of apomorphine on regional cerebral blood flow in schizophrenia. J Neuropsychiatry Clin Neurosci. 1989;1:377–384. doi: 10.1176/jnp.1.4.377. [DOI] [PubMed] [Google Scholar]
- 26.Daniel DG, Weinberger DR, Jones DW, Zigun JR, Coppola R, Handel S, Bigelow LB, Goldberg TE, Berman KF, Kleinman JE. The effect of amphetamine on regional cerebral blood flow during cognitive activation in schizophrenia. J Neurosci. 1991;11:1907–1917. doi: 10.1523/JNEUROSCI.11-07-01907.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- 28.de Beaurepaire R, Labelle A, Naber D, Jones BD, Barnes TR. An open trial of the D1 antagonist SCH 39166 in six cases of acute psychotic states. Psychopharmacology (Berl) 1995;121:323–327. doi: 10.1007/BF02246070. [DOI] [PubMed] [Google Scholar]
- 29.Den Boer JA, van Megen HJ, Fleischhacker WW, Louwerens JW, Slaap BR, Westenberg HG, Burrows GD, Srivastava ON. Differential effects of the D1-DA receptor antagonist SCH39166 on positive and negative symptoms of schizophrenia. Psychopharmacology (Berl) 1995;121:317–322. doi: 10.1007/BF02246069. [DOI] [PubMed] [Google Scholar]
- 30.Dolan RJ, Fletcher P, Frith CD, Friston KJ, Frackowiak RS, Grasby PM. Dopaminergic modulation of impaired cognitive activation in the anterior cingulate cortex in schizophrenia. Nature. 1995;378:180–182. doi: 10.1038/378180a0. [DOI] [PubMed] [Google Scholar]
- 31.Dumartin B, Jaber M, Gonon F, Caron MG, Giros B, Bloch B. Dopamine tone regulates D1 receptor trafficking and delivery in striatal neurons in dopamine transporter-deficient mice. Proc Natl Acad Sci USA. 2000;97:1879–1884. doi: 10.1073/pnas.97.4.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duvernoy H. The human brain. Surface, three-dimensional sectional anatomy and MRI. Springer-Verlag Wien; New York: 1991. [Google Scholar]
- 33.Fleming K, Goldberg TE, Gold JM, Weinberger DR. Verbal working memory dysfunction in schizophrenia: use of a Brown-Peterson paradigm. Psychiatry Res. 1995;56:155–161. doi: 10.1016/0165-1781(95)02589-3. [DOI] [PubMed] [Google Scholar]
- 34.Gandelman MS, Baldwin RM, Zoghbi SS, Zea-Ponce Y, Innis RB. Evaluation of ultrafiltration for the free fraction determination of single photon emission computerized tomography (SPECT) radiotracers: β-CIT, IBF and iomazenil. J Pharm Sci. 1994;83:1014–1019. doi: 10.1002/jps.2600830718. [DOI] [PubMed] [Google Scholar]
- 35.Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry. 1997;54:159–165. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- 36.Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- 37.Goldman-Rakic PS, Muly EC, III, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- 38.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 39.Guo N, Waterhouse RN, Hwang DR, Zea-Ponce Y, Huang Y, Simpson N, Castillon J, Abi-Dargham A, Laruelle M. Effect of acute endogenous dopamine depletion on in vivo binding of dopamine D1 and D2 radiotracers. NeuroImage. 2000;11:S4. [Google Scholar]
- 40.Guo N, Hwang D, Abdellhadi S, Abi-Dargham A, Zarahn E, Laruelle M. The effect of chronic DA depletion on D1 ligand binding in rodent brain. Soc Neurosci Abstr. 2001;27:238.10. [Google Scholar]
- 41.Hall H, Sedvall G, Magnusson O, Kopp J, Halldin C, Farde L. Distribution of D1- and D2-dopamine receptors, and dopamine and its metabolites in the human brain. Neuropsychopharmacology. 1994;11:245–256. doi: 10.1038/sj.npp.1380111. [DOI] [PubMed] [Google Scholar]
- 42.Halldin C, Foged C, Chou YH, Karlsson P, Swahn CG, Sandell J, Sedvall G, Farde L. Carbon-11-NNC 112: a radioligand for PET examination of striatal and neocortical D1-dopamine receptors. J Nucl Med. 1998;39:2061–2068. [PubMed] [Google Scholar]
- 43.Hess EJ, Bracha HS, Kleinman JE, Creese I. Dopamine receptor subtype imbalance in schizophrenia. Life Sci. 1987;40:1487–1497. doi: 10.1016/0024-3205(87)90381-x. [DOI] [PubMed] [Google Scholar]
- 44.Hollingshead AB (1975) Four factor index of social status. New Haven, CT: Working paper published by the author.
- 45.Honey GD, Bullmore ET, Soni W, Varatheesan M, Williams SC, Sharma T. Differences in frontal cortical activation by a working memory task after substitution of risperidone for typical antipsychotic drugs in patients with schizophrenia. Proc Natl Acad Sci USA. 1999;96:13432–13437. doi: 10.1073/pnas.96.23.13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hurd YL, Suzuki M, Sedvall GC. D1 and D2 dopamine receptor mRNA expression in whole hemisphere sections of the human brain. J Chem Neuroanat. 2001;22:127–137. doi: 10.1016/s0891-0618(01)00122-3. [DOI] [PubMed] [Google Scholar]
- 47.Jansma JM, Ramsey NF, Coppola R, Kahn RS. Specific versus nonspecific brain activity in a parametric N-back task. NeuroImage. 2000;12:688–697. doi: 10.1006/nimg.2000.0645. [DOI] [PubMed] [Google Scholar]
- 48.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 49.Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- 50.Joyce JN, Lexow N, Bird E, Winokur A. Organization of dopamine D1 and D2 receptors in human striatum: receptor autoradiographic studies in Huntington's disease and schizophrenia. Synapse. 1988;2:546–557. doi: 10.1002/syn.890020511. [DOI] [PubMed] [Google Scholar]
- 51.Kahn RS, Harvey PD, Davidson M, Keefe RS, Apter S, Neale JM, Mohs RC, Davis KL. Neuropsychological correlates of central monoamine function in chronic schizophrenia: relationship between CSF metabolites and cognitive function. Schizophr Res. 1994;11:217–224. doi: 10.1016/0920-9964(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 52.Karle J, Clemmesen L, Hansen L, Andersen M, Andersen J, Fensbo C, Sloth-Nielsen M, Skrumsager BK, Lublin H, Gerlach J. NNC 01–0687, a selective dopamine D1 receptor antagonist, in the treatment of schizophrenia. Psychopharmacology (Berl) 1995;121:328–329. doi: 10.1007/BF02246071. [DOI] [PubMed] [Google Scholar]
- 53.Karlsson P, Smith L, Farde L, Harnryd C, Sedvall G, Wiesel FA. Lack of apparent antipsychotic effect of the D1-dopamine receptor antagonist SCH39166 in acutely ill schizophrenic patients. Psychopharmacology (Berl) 1995;121:309–316. doi: 10.1007/BF02246068. [DOI] [PubMed] [Google Scholar]
- 54.Kates WR, Abrams MT, Kaufmann WE, Breiter SN, Reiss AL. Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiatry Res. 1997;75:31–48. doi: 10.1016/s0925-4927(97)00019-x. [DOI] [PubMed] [Google Scholar]
- 55.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome scale (PANSS) for schizophrenia. Schizophrenia Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 56.Keefe RS, Lees-Roitman SE, Dupre RL. Performance of patients with schizophrenia on a pen and paper visuospatial working memory task with short delay. Schizophr Res. 1997;26:9–14. doi: 10.1016/S0920-9964(97)00037-6. [DOI] [PubMed] [Google Scholar]
- 57. Killiany RJ, Moss MB, Nicholson T, Jolez F, Sandor T. An interactive procedure for extracting features of the brain from magnetic resonance images: the lobes. Human Brain Mapp 5 1997. 355:363. [DOI] [PubMed] [Google Scholar]
- 58.Knable MB, Hyde TM, Murray AM, Herman MM, Kleinman JE. A postmortem study of frontal cortical dopamine D1 receptors in schizophrenics, psychiatric controls, and normal controls. Biol Psychiatry. 1996;40:1191–1199. doi: 10.1016/S0006-3223(96)00116-3. [DOI] [PubMed] [Google Scholar]
- 59.Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20:423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 60.Laruelle M, Casanova M, Weinberger D, Kleinman J. Postmortem study of the dopaminergic D1 receptors in the dorsolateral prefrontal cortex of schizophrenics and controls. Schizophr Res. 1990;3:30–31. [Google Scholar]
- 61.Laruelle M, Sidhu A, Casanova MF, Weinberger DR, Kleinman JE. Characterization of [125I]SCH23982 binding in human brain: comparison with [3H]SCH23390. Neurosci Lett. 1991;31:273–276. doi: 10.1016/0304-3940(91)90631-3. [DOI] [PubMed] [Google Scholar]
- 62.Laruelle M, van Dyck C, Abi-Dargham A, Zea-Ponce Y, Zoghbi SS, Charney DS, Baldwin RM, Hoffer PB, Kung HF, Innis RB. Compartmental modeling of iodine-123-iodobenzofuran binding to dopamine D2 receptors in healthy subjects. J Nucl Med. 1994a;35:743–754. [PubMed] [Google Scholar]
- 63.Laruelle M, Baldwin RM, Rattner Z, Al-Tikriti MS, Zea-Ponce Y, Zoghbi SS, Charney DS, Price JC, Frost JJ, Hoffer PB, Innis RB. SPECT quantification of [123I]iomazenil binding to benzodiazepine receptors in nonhuman primates. I. Kinetic modeling of single bolus experiments. J Cereb Blood Flow Metab. 1994b;14:439–452. doi: 10.1038/jcbfm.1994.55. [DOI] [PubMed] [Google Scholar]
- 64.Levenberg K. A method for the solution of certain problems in least squares. Quart Appl Math. 1944;2:164–168. [Google Scholar]
- 65.Levitt P, Rakic P, Goldman-Rakic P. Region-specific distribution of catecholamine afferents in primate cerebral cortex: a fluorescence histochemical analysis. J Comp Neurol. 1984;227:23–36. doi: 10.1002/cne.902270105. [DOI] [PubMed] [Google Scholar]
- 66.Lewis DA, Campbell MJ, Foote SL, Goldstein M, Morrison JH. The distribution of tyrosine hydroxylase-immunoreactive fibers in primate neocortex is widespread but regionally specific. J Neurosci. 1987;7:279–290. doi: 10.1523/JNEUROSCI.07-01-00279.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lidow MS, Goldman-Rakic PS. A common action of clozapine, haloperidol, and remoxipride on D1- and D2-dopaminergic receptors in the primate cerebral cortex. Proc Natl Acad Sci USA. 1994;91:4353–4356. doi: 10.1073/pnas.91.10.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lidow MS, Goldman-Rakic PS, Gallager DW, Rakic P. Distribution of dopaminergic receptors in the primate cerebral cortex: quantitative autoradiographic analysis using [3H]raclopride, [3H]spiperone and [3H]SCH23390. Neuroscience. 1991;40:657–671. doi: 10.1016/0306-4522(91)90003-7. [DOI] [PubMed] [Google Scholar]
- 69.Lidow MS, Elsworth JD, Goldman-Rakic PS. Down-regulation of the D1 and D5 dopamine receptors in the primate prefrontal cortex by chronic treatment with antipsychotic drugs. J Pharmacol Exp Ther. 1997;281:597–603. [PubMed] [Google Scholar]
- 70.Manoach DS, Press DZ, Thangaraj V, Searl MM, Goff DC, Halpern E, Saper CB, Warach S. Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biol Psychiatry. 1999;45:1128–1137. doi: 10.1016/s0006-3223(98)00318-7. [DOI] [PubMed] [Google Scholar]
- 71.Manoach DS, Gollub RL, Benson ES, Searl MM, Goff DC, Halpern E, Saper CB, Rauch SL. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry. 2000;48:99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- 72.Mawlawi OM, Weiss R, Shinn A, Pidcock J, Slifstien M, Laruelle M. Performance characteristics of a head immobilization device for PET imaging. J Nucl Med. 1999;40:281.P. [Google Scholar]
- 73.Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, Huang Y, Simpson N, Ngo K, Van Heertum R, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D2 receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 74.McCarthy G, Blamire AM, Puce A, Nobre AC, Bloch G, Hyder F, Goldman-Rakic P, Shulman RG. Functional magnetic resonance imaging of human prefrontal cortex activation during a spatial working memory task. Proc Natl Acad Sci USA. 1994;91:8690–8694. doi: 10.1073/pnas.91.18.8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meador-Woodruff JH, Haroutunian V, Powchik P, Davidson M, Davis KL, Watson SJ. Dopamine receptor transcript expression in striatum and prefrontal and occipital cortex. Focal abnormalities in orbitofrontal cortex in schizophrenia. Arch Gen Psychiatry. 1997;54:1089–1095. doi: 10.1001/archpsyc.1997.01830240045007. [DOI] [PubMed] [Google Scholar]
- 76.Mintun MA, Raichle ME, Kilbourn MR, Wooten GF, Welch MJ. A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann Neurol. 1984;15:217–227. doi: 10.1002/ana.410150302. [DOI] [PubMed] [Google Scholar]
- 77.Morice R, Delahunty A. Frontal/executive impairments in schizophrenia. Schizophr Bull. 1996;22:125–137. doi: 10.1093/schbul/22.1.125. [DOI] [PubMed] [Google Scholar]
- 78.Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Natl Acad Sci USA. 1996a;93:1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murphy BL, Arnsten AF, Jentsch JD, Roth RH. Dopamine and spatial working memory in rats and monkeys: pharmacological reversal of stress-induced impairment. J Neurosci. 1996b;16:7768–7775. doi: 10.1523/JNEUROSCI.16-23-07768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- 81.Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, Someya Y, Sassa T, Sudo Y, Matsushima E, Iyo M, Tateno Y, Toru M. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385:634–636. doi: 10.1038/385634a0. [DOI] [PubMed] [Google Scholar]
- 82.Pani L, Gessa GL, Carboni S, Portas CM, Rossetti ZL. Brain dialysis and dopamine: does the extracellular concentration of dopamine reflect synaptic release? Eur J Pharmacol. 1990;180:85–90. doi: 10.1016/0014-2999(90)90595-w. [DOI] [PubMed] [Google Scholar]
- 83.Park S. Association of an oculomotor delayed response task and the Wisconsin Card Sort Test in schizophrenic patients. Int J Psychophysiol. 1997;27:147–151. doi: 10.1016/s0167-8760(97)00045-7. [DOI] [PubMed] [Google Scholar]
- 84.Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- 85.Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158:1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- 86.Pimoule C, Schoemaker H, Reynolds GP, Langer SZ. [3H]SCH 23390 labeled D1 dopamine receptors are unchanged in schizophrenia and Parkinson's disease. Eur J Pharmacol. 1985;114:235–237. doi: 10.1016/0014-2999(85)90634-x. [DOI] [PubMed] [Google Scholar]
- 87.Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach coordinate system. Cereb Cortex. 1995;5:323–337. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
- 88.Rama P, Martinkauppi S, Linnankoski I, Koivisto J, Aronen HJ, Carlson S. Working memory of identification of emotional vocal expressions: an fMRI study. NeuroImage. 2001;13:1090–1101. doi: 10.1006/nimg.2001.0777. [DOI] [PubMed] [Google Scholar]
- 89.Reynolds GP, Czudek C. Status of the dopaminergic system in post-mortem brain in schizophrenia. Psychopharmacol Bull. 1988;24:345–347. [PubMed] [Google Scholar]
- 90.Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- 91.Sawaguchi T, Goldman-Rakic PS. The role of D1-dopamine receptor in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. J Neurophysiol. 1994;71:515–528. doi: 10.1152/jn.1994.71.2.515. [DOI] [PubMed] [Google Scholar]
- 92.Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC. Neuropsychological deficits in neuroleptic naive patients with first- episode schizophrenia. Arch Gen Psychiatry. 1994;51:124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- 93.Seeman P, Bzowej NH, Guan HC, Bergeron C, Reynolds GP, Bird ED, Riederer P, Jellinger K, Tourtelotte WW. Human brain D1 and D2 dopamine receptors in schizophrenia, Alzheimer's, Parkinson's, and Huntington's diseases. Neuropsychopharmacology. 1987;1:5–15. doi: 10.1016/0893-133x(87)90004-2. [DOI] [PubMed] [Google Scholar]
- 94.Spitzer R, Williams J, Gibbon M, First M. The Structured Clinical interview for DSM-III-R: 1. History, rationale and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 95.Stevens AA, Goldman-Rakic PS, Gore JC, Fulbright RK, Wexler BE. Cortical dysfunction in schizophrenia during auditory word and tone working memory demonstrated by functional magnetic resonance imaging. Arch Gen Psychiatry. 1998;55:1097–1103. doi: 10.1001/archpsyc.55.12.1097. [DOI] [PubMed] [Google Scholar]
- 96.Talairach J, Tournoux P. Co-planar stereotactic atlas of the human brain. Three-dimensional proportional system: an approach of cerebral imaging. Theime; New York: 1988. [Google Scholar]
- 97.Watanabe M, Kodama T, Hikosaka K. Increase of extracellular dopamine in primate prefrontal cortex during a working memory task. J Neurophysiol. 1997;78:2795–2798. doi: 10.1152/jn.1997.78.5.2795. [DOI] [PubMed] [Google Scholar]
- 98.Weinberger DR. Implications of the normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 99.Weinberger DR, Berman KF, Illowsky BP. Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia. III. A new cohort and evidence for a monoaminergic mechanism. Arch Gen Psychiatry. 1988;45:609–615. doi: 10.1001/archpsyc.1988.01800310013001. [DOI] [PubMed] [Google Scholar]
- 100.Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. J Comp Assist Tomogr. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- 101.Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J Comput Assist Tomogr. 1993;17:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 102.Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]