Abstract
Nicotine addiction is a complex behavioral phenomenon comprising effects on several neural systems. Recent studies have expanded initial observations that the actions of nicotine on dopaminergic systems increase dopaminergic activity and release, leading to nicotine-induced reinforcement. Indeed, the actions of nicotine on many systems, including brainstem cholinergic, GABAergic, noradrenergic, and serotonergic nuclei, may help to mediate nicotine effects related to addiction. Furthermore, studies of mice lacking nicotinic acetylcholine receptor subunits or expressing supersensitive forms of these subunits have begun to tie together the molecular, neurochemical, and behavioral effects of nicotine. The use of multiple techniques by many laboratories provides optimism that the field is advancing toward elucidating the basic mechanisms of nicotine dependence.
The mesolimbic dopamine (DA) projection from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) is a central element in drug-reinforced behaviors and associated conditioned phenomena (Robinson and Berridge, 1993). Nicotine is not an exception (Di Chiara, 2000); however, recent evidence suggests that several other neurochemical systems also mediate the addiction-related behaviors of nicotine (Watkins et al., 2000). In parallel, contemporary molecular genetics, specifically the availability of lines of knock-out (KO) mice lacking specific subunits of the nicotinic acetylcholine receptors (nAChRs) and knock-in (KI) mice with mutations in these subunits, has allowed investigation of the physiological and behavioral actions of nicotine and determination of the nAChR subtypes responsible for its various effects. These domains, which inform our understanding of nicotine addiction, are reviewed here.
Novel anatomical targets and circuits
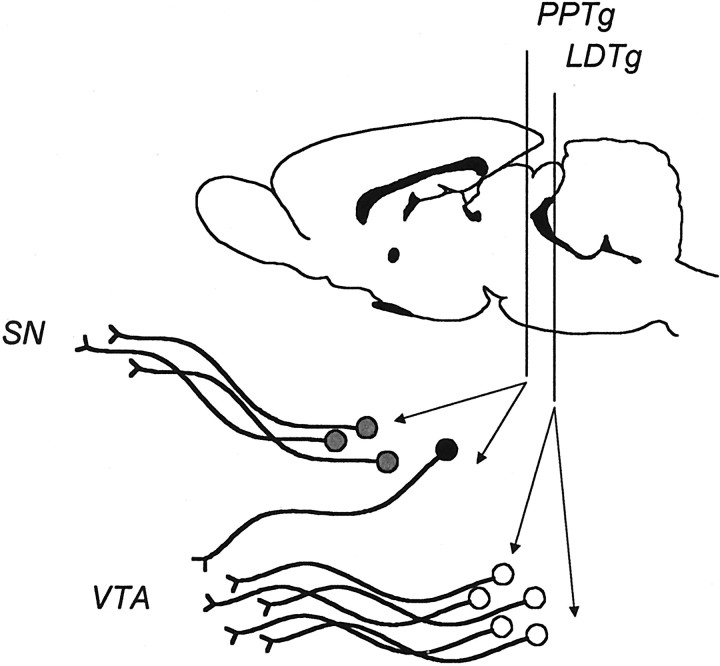
One brain region receiving recent attention is the brainstem pedunculopontine tegmental nucleus (PPTg), which has been implicated in the acquisition of drug-taking, conditioned behaviors, and brain stimulation reward (Olmstead et al., 1998; Yeomans et al., 2000). However, interest in PPTg with respect to nicotine self-administration derives primarily from the observation that this behavior is reduced by microinfusion of the high-affinity nAChR antagonist dihydro-β−erythroidine (DHβE) into the VTA (Corrigall et al., 1994). Consistent with dialysis studies, this observation suggests that the VTA is a “unified” target for the behavioral and neurochemical effects of nicotine on midbrain DA. Logically, these observations question the role of the cholinergic input to VTA nAChRs, which arises from the PPTg and the adjacent laterodorsal tegmental nucleus (LDTg) (Fig. 1) (Oakman et al., 1995).
Fig. 1.
This schematic represents the rat brain in sagittal section, showing the anterior–posterior locations for the pedunculopontine tegmental nucleus (PPTg) and laterodorsal tegmental nucleus (LDTg). The cholinergic input to substantia nigra arises from neurons in the rostral PPTg (gray circles), whereas the input to the VTA comes from the LDTg (open circles), with a component from the caudal PPTg (filled circles). GABA- and glutamate-containing neurons also found in PPTg and LDTg may synapse locally as well as project to other brain regions.
Lesions that reduce the number of PPTg cholinergic neurons and intra-PPTg microinfusion of DHβE both reduce nicotine self-administration (Lança et al., 2000a). Fos-immunoreactive neuronal nuclei are readily observed in PPTg and LDTg after experimenter-administered nicotine (Lança et al., 2000b) and are located not in the cholinergic neurons but almost exclusively in identified GABA and glutamate neurons (W. A. Corrigall, A. J. Lança, and D. J. Martens, unpublished observations). Intracranial self-stimulation also induces Fos expression in GABAergic neurons of the mesopontine tegmentum (Nakahara et al., 2001). GABA mechanisms are implicated in nicotine self-administration as well. Of several agents microinfused into the PPTg, only GABA agonists reduce nicotine self-administration selectively, compared with cocaine (Corrigall et al., 2001, 2002). Although glutamate mechanisms remain to be tested, these observations suggest that GABA systems in PPTg may be a key element in nicotine-rewarded behavior.
Nicotine action in the PPTg may not regulate VTA DA, however. PPTg and LDTg cholinergic projections to midbrain DA are topographic; VTA input arises mainly from the LDTg and caudal PPTg (Oakman et al., 1995). Function appears to follow anatomy. VTA DA neurons and DA release in the NAc are influenced by LDTg cholinergic cells (Forster and Blaha, 2000). Presumed nAChR-bearing neurons in PPTg, the GABA and glutamate cells, might project independently. Establishing DA dependence or independence of PPTg mechanisms will be an important next step in elucidating its role in nicotine addiction.
Other observations suggest additional targets for nicotine dependence, but in general have received less attention. For example, although they have not been a major focus in drug abuse, norepinephrine (NE) mechanisms may be relevant because they are able to modulate midbrain DA function (Linnér et al., 2001). Moreover, nicotine releases NE in various CNS regions (Summers and Giacobini, 1995; Fu et al., 1997) by action at distinct sites and through several nAChR subtypes (Fu et al., 1998; Léna et al., 1999). Recent evidence on two fronts implicates NE more directly in nicotine reinforcement. First, the NE reuptake inhibitor reboxetine attenuates nicotine self-administration (Bardo et al., 2001), an observation with added interest given that the smoking cessation treatment bupropion has an NE component to its action. Second, NE secretion in the hypothalamic paraventricular nucleus (PVN), measured with microdialysis, has been shown to increase during continuous nicotine self-administration (Fu et al., 2001); nAChRs in the nucleus tractus solitarius may be the locus for this effect (Fu et al., 1997). These observations suggest an important NE target for investigation. Perhaps the links among hypothalamic function, stress responses, and nicotine self-administration may be profitably explored in the future.
Serotonin (5HT) too might be expected to influence nicotine reward: nicotine alters 5HT release and neuronal activity (Li et al., 1998), reward-related nicotine effects are modified by 5HT manipulations [e.g., behavioral sensitization (Olausson et al., 2001)], and DA neurons are influenced by 5HT processes (Kalivas, 1993). Yet there is no direct evidence for distinct 5HT circuitry in nicotine reinforcement. It may be that 5HT processes underlie the co-morbidity of nicotine dependence and psychiatric disorders (Balfour and Ridley, 2000). For instance, the overlap of 5HT mechanisms in the anxiolytic/anxiogenic effects of nicotine (Cheeta et al., 2001) and the presence of such effects during nicotine self-administration (Irvine et al., 2001) is an avenue for future exploration.
In overview we can say that PPTg mechanisms of yet-to-be-determined nature, as well as NE mechanisms, appear to be directly involved in nicotine reinforcement. Systems such as 5HT and others not discussed here could also be implicated. In consequence, the most compelling commentary about these studies is that they implicate previously unexplored CNS processes in nicotine addiction. As these processes are plumbed in the future, they will no doubt expand our knowledge of how drugs gain control of behavior.
Studies of nicotine-modulated systems in KO mice
Recent developments in molecular genetics have also contributed greatly to the understanding of systems underlying nicotine reinforcement and related behaviors. The nAChR subtypes responsible for nicotine-mediated stimulation of the DA system have been identified, in part, in studies using KO mice. Nicotine increases the firing rate of DA neurons (Grenhoff et al., 1986; Pidoplichko et al., 1997) and increases DA release from synaptic terminals (Rowell et al., 1987). Mice lacking the β2 subunit lack all high-affinity binding sites for nicotine in the VTA and SN (Picciotto et al., 1998), whereas most of these sites are absent in α4 subunit KO mice (Marubio et al., 1999).
An elegant study combining RT-PCR and patch-clamp physiology in wild-type (WT) mice and mice lacking the α4, β2, or α7 subunits showed that most DA neurons in the midbrain express two nAChR subtypes (Klink et al., 2001). Both types are sensitive to DHβE, and the second subtype is also sensitive to low concentrations of α-conotoxin MII and methyllycaconitine (MLA), although neither subtype contains the α7 subunit. These two classes of nAChRs are thought to be made up of the α4/α5/β2 and the α4/α5/α6/β2 subunits, respectively. An α7 subunit-containing nAChR is also expressed in somewhat less than half of the DA neurons. The continued presence of a slow, MLA-sensitive current in DA neurons in α7 subunit KO mice, and its absence in β2 subunit KO mice (Klink et al., 2001), suggests that MLA-sensitive nAChRs on VTA neurons that are stimulated by low concentrations of nicotine (Pidoplichko et al., 1997) may not contain the α7 subunit. In slices through the SN and VTA, Ca2+ influx could be evoked with either nicotine or choline, suggesting that both β2 and α7 subunit-containing nAChRs contribute to this effect (Tsuneki et al., 2000).
The α6 and β3 subunits have been shown to combine with the β2 subunit in vitro, with or without the α4 subunit, to form functional nAChRs (Kuryatov et al., 2000), suggesting that these subunits may contribute to the observed nicotine-sensitive currents in DA neurons (Klink et al., 2001). The β2 subunit is critical not only for currents in DA cell bodies but also for nicotine-induced DA release from synaptosomes or as measured by microdialysis, both of which are abolished in β2 subunit KO mice (Picciotto et al., 1998; Grady et al., 2001). Cyclic voltammetric studies also confirm that nicotine- and ACh-evoked DA release are abolished in β2 subunit KO mice (Zhou et al., 2001).
Importantly, nicotine can enhance synaptic plasticity in glutamatergic inputs to VTA neurons via an MLA-sensitive nAChR (Mansvelder and McGehee, 2000). This effect could underlie plastic changes in the DA system that lead to the development of addiction. The ability of nicotine to affect synaptic strength in both excitatory and inhibitory inputs to the DA cell bodies is one of the critical areas for future research on the physiological basis of nicotine addiction.
Behavioral phenotypes in mice with mutations in nAChR subunits
Mutations in nAChR subunits that contribute to nicotine-induced DA release, including the α4, α6, α7, β2, and β3 subunits, might be expected to affect nicotine self-administration. WT mice trained to self-administer cocaine and then switched to nicotine continue to self-administer nicotine, whereas β2 subunit KO mice do not self-administer nicotine and extinguish their response as if they had been given saline (Picciotto et al., 1998), suggesting that this subunit may be involved in nicotine reinforcement. The β2 subunit of the nAChR also modulates the reinforcing effects of cocaine in the conditioned place preference (CPP) paradigm. KO mice lacking the β2 subunit show reduced CPP to a threshold dose of cocaine, do not show the alterations in DA turnover in the striatum seen in WT mice after administration of cocaine, and show attenuated induction of the chronic fos-related antigens after cocaine treatment (Zachariou et al., 2001). These results suggest that nicotine can modulate cocaine reinforcement by increasing DA tone and support the idea that endogenous ACh, acting through nAChRs containing the β2 subunit, modulates DA neurotransmission.
Drug-induced locomotor activation also may contribute to psychostimulant-induced reinforcement. Nicotine increases locomotion, via activation of DA pathways (Clarke et al., 1988), in a familiar environment. β2 subunit KO mice show reduced locomotion in a familiar environment (Picciotto et al., 1998), whereas KI mice expressing a low level of a super-sensitive α4 subunit show increased locomotion in a novel environment (Labarca et al., 2001). This suggests that α4/β2-containing nAChRs are important for spontaneous locomotor activity. One line of α4 subunit KO mice shows reduced habituation to a novel environment (Ross et al., 2000), whereas a second line of α4 subunit KO mice showed no differences in locomotor activity in a novel environment (Marubio et al., 1999), suggesting that different genetic backgrounds or different methods of measuring locomotor activity may contribute to phenotypic heterogeneity. Infusion of α6 antisense oligonucleotides could also attenuate the locomotor-activating effects of nicotine in rats (Le Novère et al., 1999). Taken together, these results suggest that the α4, α6, and β2 subunits are involved in the ability of nicotine to stimulate both the DA system and locomotor activity.
Conclusions
In spite of, or perhaps because of, the relative newness of these studies, research into the neuronal systems underlying nicotine dependence has in many ways capitalized on emerging knowledge from different domains. Perhaps most impressive has been the degree to which researchers interested in nAChRs have tested the consequences of their manipulations in behaving animals, using models that have been developed by behavioral neuroscientists to understand drug-taking behavior itself and other related phenomena. For its part, behavioral neuroscience research in the nicotine field has begun to elucidate novel circuitry in drug reinforcement, an advance that permits analysis at the molecular level. Even from the still early data described in this review, we can surmise that nicotine addiction is based on the action of the drug at several systems. One can extrapolate with hope to a near future in which the profitability of these efforts is fully evident in discovering the basic mechanisms of nicotine dependence.
Footnotes
This work has been supported by National Institute on Drug Abuse (NIDA) Grants DA00436, DA10455, and DA84733 (M.R.P.), and NIDA Grant DA09577 (W.A.C.).
Correspondence should be addressed to Marina Picciotto, Department of Psychiatry, Yale University School of Medicine, 34 Park Street, Third Floor Research, New Haven, CT 06508. E-mail:marina.picciotto@yale.edu.
REFERENCES
- 1.Balfour DJ, Ridley DL. The effects of nicotine on neural pathways implicated in depression: a factor in nicotine addiction? Pharmacol Biochem Behav. 2000;66:79–85. doi: 10.1016/s0091-3057(00)00205-7. [DOI] [PubMed] [Google Scholar]
- 2.Bardo MT, Rauhut AS, Mullins SN, Dwoskin LP. Effect of reboxetine on nicotine self-administration and reinstatement: preclinical evidence for a novel smoking cessation pharmacotherapy. Soc Neurosci Abstr. 2001;31:344. [Google Scholar]
- 3.Cheeta S, Irvine EE, Kenny PJ, File SE. The dorsal raphe nucleus is a crucial structure mediating nicotine's anxiolytic effects and the development of tolerance and withdrawal responses. Psychopharmacology. 2001;155:78–85. doi: 10.1007/s002130100681. [DOI] [PubMed] [Google Scholar]
- 4.Clarke PB, Fu DS, Jakubovic A, Fibiger HC. Evidence that mesolimbic dopaminergic activation underlies the locomotor stimulant action of nicotine in rats. J Pharmacol Exp Ther. 1988;246:701–708. [PubMed] [Google Scholar]
- 5.Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- 6.Corrigall WA, Coen KM, Zhang J, Adamson KL. GABA mechanisms in the pedunculopontine tegmental nucleus influence particular aspects of nicotine self-administration selectively in the rat. Psychopharmacology. 2001;158:190–197. doi: 10.1007/s002130100869. [DOI] [PubMed] [Google Scholar]
- 7.Corrigall WA, Coen KM, Zhang J, Adamson KL. Pharmacological manipulations of the pedunculopontine tegmental nucleus in the rat reduce self-administration of both nicotine and cocaine. Psychopharmacology. 2002;160:198–205. doi: 10.1007/s00213-001-0965-2. [DOI] [PubMed] [Google Scholar]
- 8.Di Chiara G. Behavioural pharmacology and neurobiology of nicotine reward and dependence. In: Clementi F, Fornasari D, Gotti C, editors. Handbook of experimental pharmacology. Springer; New York: 2000. pp. 603–750. [Google Scholar]
- 9.Forster GL, Blaha CD. Laterodorsal tegmental stimulation elicits dopamine efflux in the rat nucleus accumbens by activation of acetylcholine and glutamate receptors in the ventral tegmental area. Eur J Neurosci. 2000;12:3596–3604. doi: 10.1046/j.1460-9568.2000.00250.x. [DOI] [PubMed] [Google Scholar]
- 10.Fu Y, Matta SG, Valentine JD, Sharp BM. Adrenocorticotropin response and nicotine-induced norepinephrine secretion in the rat paraventricular nucleus are mediated through brainstem receptors. Endocrinology. 1997;138:1935–1943. doi: 10.1210/endo.138.5.5122. [DOI] [PubMed] [Google Scholar]
- 11.Fu Y, Matta SG, James TJ, Sharp BM. Nicotine-induced norepinephrine release in the rat amygdala and hippocampus is mediated through brainstem nicotinic cholinergic receptors. J Pharmacol Exp Ther. 1998;284:1188–1196. [PubMed] [Google Scholar]
- 12.Fu Y, Matta SG, Brower VG, Sharp BM. Norepinephrine secretion in the hypothalamic paraventricular nucleus of rats during unlimited access to self-administered nicotine: an in vivo microdialysis study. J Neurosci. 2001;21:8979–8989. doi: 10.1523/JNEUROSCI.21-22-08979.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grady SR, Meinerz NM, Cao J, Reynolds A, Picciotto MR, Changeux J-P, McIntosh MJ, Marks MJ, Collins AC. Nicotinic agonists stimulate acetylcholine release from mouse interpeduncular nucleus: a function mediated by a different nAChR than dopamine release from striatum. J Neurochem. 2001;76:258–268. doi: 10.1046/j.1471-4159.2001.00019.x. [DOI] [PubMed] [Google Scholar]
- 14.Grenhoff J, Aston-Jones G, Svensson TH. Nicotinic effects on the firing pattern of midbrain dopamine neurons. Acta Physiol Scand. 1986;128:351–358. doi: 10.1111/j.1748-1716.1986.tb07988.x. [DOI] [PubMed] [Google Scholar]
- 15.Irvine EE, Bagnalasta M, Marcon C, Motta C, Tessari M, File SE, Chiamulera C. Nicotine self-administration and withdrawal: modulation of anxiety in the social interaction test in rats. Psychopharmacology. 2001;153:315–320. doi: 10.1007/s002130000586. [DOI] [PubMed] [Google Scholar]
- 16.Kalivas PW. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res Rev. 1993;18:75–113. doi: 10.1016/0165-0173(93)90008-n. [DOI] [PubMed] [Google Scholar]
- 17.Klink R, de Kerchove d'Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuryatov A, Olale F, Cooper J, Choi C, Lindstrom J. Human alpha 6 AChR subtypes: subunit composition, assembly, and pharmacological responses. Neuropharmacology. 2000;39:2570–2590. doi: 10.1016/s0028-3908(00)00144-1. [DOI] [PubMed] [Google Scholar]
- 19.Labarca C, Schwarz J, Deshpande P, Schwarz S, Nowak MW, Fonck C, Nashmi R, Kofuji P, Dang H, Shi W, Fidan M, Khakh BS, Chen Z, Bowers BJ, Boulter J, Wehner JM, Lester HA. Point mutant mice with hypersensitive alpha 4 nicotinic receptors show dopaminergic deficits and increased anxiety. Proc Natl Acad Sci USA. 2001;98:2786–2791. doi: 10.1073/pnas.041582598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lança AJ, Adamson KL, Coen KM, Chow BLC, Corrigall WA. The pedunculopontine tegmental nucleus and the role of cholinergic neurons in nicotine self-administration in the rat: a correlative neuroanatomical and behavioral study. Neuroscience. 2000a;96:735–742. doi: 10.1016/s0306-4522(99)00607-7. [DOI] [PubMed] [Google Scholar]
- 21.Lança JA, Sanelli TR, Corrigall WA. Nicotine-induced fos expression in the pedunculopontine mesencephalic tegmentum in the rat. Neuropharmacology. 2000b;39:2808–2817. doi: 10.1016/s0028-3908(00)00129-5. [DOI] [PubMed] [Google Scholar]
- 22.Léna C, de Kerchove D'Exaerde A, Cordero-Erausquin M, Le Novère N, del Mar Arroyo-Jimenez M, Changeux JP. Diversity and distribution of nicotinic acetylcholine receptors in the locus ceruleus neurons. Proc Natl Acad Sci USA. 1999;96:12126–12131. doi: 10.1073/pnas.96.21.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Novère N, Zoli M, Léna C, Ferrari R, Picciotto MR, Changeux J-P. Involvement of alpha 6 nicotinic receptor subunit in nicotine-elicited locomotion, demonstrated by in vivo antisense oligonucleotide infusion. NeuroReport. 1999;10:2497–2501. doi: 10.1097/00001756-199908200-00012. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Rainnie DG, McCarley RW, Greene RW. Presynaptic nicotinic receptors facilitate monoaminergic transmission. J Neurosci. 1998;18:1904–1912. doi: 10.1523/JNEUROSCI.18-05-01904.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linnér L, Endersz H, Ohman D, Bengtsson F, Schalling M, Svensson TH. Reboxetine modulates the firing pattern of dopamine cells in the ventral tegmental area and selectively increases dopamine availability in the prefrontal cortex. J Pharmacol Exp Ther. 2001;297:540–546. [PubMed] [Google Scholar]
- 26.Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- 27.Marubio L, del Mar Arroyo-Jimenez M, Cordero-Erausquin M, Léna C, Le Novère N, de Kerchove d'Exaerde A, Huchet M, Damaj MI, Changeux J-P. Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature. 1999;398:805–810. doi: 10.1038/19756. [DOI] [PubMed] [Google Scholar]
- 28.Nakahara D, Ishida Y, Nakamura M, Furuno N, Nishimori T. Intracranial self-stimulation induces Fos expression in GABAergic neurons in the rat mesopontine tegmentum. Neuroscience. 2001;106:633–641. doi: 10.1016/s0306-4522(01)00298-6. [DOI] [PubMed] [Google Scholar]
- 29.Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK. Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. J Neurosci. 1995;15:5859–5869. doi: 10.1523/JNEUROSCI.15-09-05859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olausson P, Akesson P, Petersson A, Engel JA, Soderpalm B. Behavioral and neurochemical consequences of repeated nicotine treatment in the serotonin-depleted rat. Psychopharmacology. 2001;155:348–361. doi: 10.1007/s002130100710. [DOI] [PubMed] [Google Scholar]
- 31.Olmstead MC, Munn EM, Franklin KB, Wise RA. Effects of pedunculopontine tegmental nucleus lesions on responding for intravenous heroin under different schedules of reinforcement. J Neurosci. 1998;18:5035–5044. doi: 10.1523/JNEUROSCI.18-13-05035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Merlo Pich E, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta-2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- 33.Pidoplichko VI, Debiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- 34.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 35.Ross SA, Wong JYF, Clifford JJ, Kinsella A, Massalas JS, Horne MK, Scheffer IE, Kola I, Waddington JL, Berkovic SF, Drago J. Phenotypic characterization of an α4 neuronal nicotinic acetylcholine receptor subunit knock-out mouse. J Neurosci. 2000;20:6431–6441. doi: 10.1523/JNEUROSCI.20-17-06431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rowell PP, Carr LA, Garner AC. Stimulation of tritiated dopamine release by nicotine in rat nucleus accumbens. J Neurochem. 1987;49:1449–1454. doi: 10.1111/j.1471-4159.1987.tb01013.x. [DOI] [PubMed] [Google Scholar]
- 37.Summers KL, Giacobini E. Effects of local and repeated systemic administration of (−)nicotine on extracellular levels of acetylcholine, norepinephrine, dopamine, and serotonin in rat cortex. Neurochem Res. 1995;20:753–759. doi: 10.1007/BF01705545. [DOI] [PubMed] [Google Scholar]
- 38.Tsuneki H, Klink R, Léna C, Korn H, Changeux JP. Calcium mobilization elicited by two types of nicotinic acetylcholine receptors in mouse substantia nigra pars compacta. Eur J Neurosci. 2000;12:2475–2485. doi: 10.1046/j.1460-9568.2000.00138.x. [DOI] [PubMed] [Google Scholar]
- 39.Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob Res. 2000;2:19–37. doi: 10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]
- 40.Yeomans JS, Takeuchi J, Baptista M, Flynn DD, Lepik K, Nobrega J, Fulton J, Ralph MR. Brain-stimulation reward thresholds raised by an antisense oligonucleotide for the M5 muscarinic receptor infused near dopamine cells. J Neurosci. 2000;20:8861–8867. doi: 10.1523/JNEUROSCI.20-23-08861.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zachariou V, Caldarone BJ, Weathers-Lowin A, George TP, Elsworth JD, Roth RH, Changeux J-P, Picciotto MR. Nicotine receptor inactivation decreases sensitivity to cocaine. Neuropsychopharmacology. 2001;24:576–589. doi: 10.1016/S0893-133X(00)00224-4. [DOI] [PubMed] [Google Scholar]
- 42.Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci. 2001;4:1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]