Abstract
Sympathetic neurons innervate the heart early in postnatal development, an event that is crucial for proper modulation of blood pressure and cardiac function. However, the axon guidance cues that direct sympathetic neurons to the heart, and the neuronal receptors that recognize those cues, are poorly understood. Here we present evidence that interactions between the α4β1 integrin on sympathetic neurons and vascular cell adhesion molecule-1 (VCAM-1) in the heart plays a role in cardiac innervation.
The α4 subunit was detected on postnatal rat superior cervical ganglion (SCG) neurons in culture and in cryosections of SCG and heart. VCAM-1 immunoreactivity was detected on cardiac myocytes that associate with invading sympathetic neurons. Purified recombinant soluble VCAM-1 (rsVCAM-1) stimulated SCG neurite outgrowth at levels comparable with laminin 2/4 and fibronectin (Fn), and outgrowth on rs-VCAM-1 and Fn was blocked by antibodies specific for the α4 and β1 integrin subunits. Intrathoracic injection of function-blocking antibodies to α4 and VCAM-1, as well as a small molecule inhibitor of α4 integrins, significantly reduced sympathetic innervation of the heart. These results indicate that the interaction between α4 integrin and VCAM-1 is important for sympathetic innervation of the heart.
Keywords: integrins, vascular cell adhesion molecule-1, sympathetic neurons, heart, neurite outgrowth, neural development
Sympathetic neurons with cell bodies in the superior, middle, and inferior cervical and thoracic sympathetic chain ganglia innervate the heart and, during stimulation from higher centers, release norepinephrine (NE) (Pappano, 1977; Ganguly and Sherwood, 1991), which increases the rate and strength of cardiac contractions. The path followed by developing sympathetic axons starts at the ganglia and follows the basolateral surface of the common carotid artery to the aorta, through the cardiac plexus down into the heart. Growth cones begin to emerge from the ganglia at approximately embryonic day 18 (E18) and innervate the myocardium and precapillary arterioles during the first three postnatal weeks [postnatal day 0 (P0) through P22] (Berthoud and Powley, 1996). Innervation of the heart starts with the right atrium (P2), followed by the right ventricle (P4), blood vessels (P8), and the left ventricle (P22) (Iversen et al., 1967; Lipp and Rudolph, 1972; Nyquist-Battie et al., 1994).
Generally, axons are thought to find their way to targets via a combination of attractive and repulsive soluble factors and extracellular matrix and cell surface substrates in the microenvironment of the growth cone (Tessier-Lavigne and Goodman, 1996). ECM molecules that induce sympathetic axon outgrowth in vitro include laminins (Lns), fibronectins (Fns), collagens, and thrombospondin 1 (Reichardt et al., 1990; Lein et al., 1991). Dendrite outgrowth apparently relies on different factors, such as osteogenic protein-1 (Lein et al., 1995). Cell–cell interactions involved in sympathetic neurite outgrowth are less well characterized, but several cell adhesion molecules have been identified on sympathetic neurons, including GP130/F11, N-cadherin, DM-Grasp, Nr-CAM, and Thy-1 (Lustig et al., 1999). However, the mechanisms responsible for axon outgrowth in vivo remain poorly understood.
α4 integrins (α4β1 and α4β7) play crucial roles in inflammation and hematopoeisis (Lobb and Hemler, 1994; Arroyo et al., 1996). The α4β1 integrin binds multiple ligands, including vascular cell adhesion molecule-1 (VCAM-1) (Osborn et al., 1989), connecting sequence-1 of fibronectin (Guan and Hynes, 1990), thrombospondin-1 (Yabkowitz et al., 1993), other α4 integrins (Altevogt et al., 1995), the propolypeptide of von Willebrand factor (Isobe et al., 1997), intercellular adhesion molecule-4 (Spring et al., 2001), transglutaminase C (Isobe et al., 1999), and osteopontin (Bayless et al., 1998). α4 integrins are also expressed by neural cells, including neural crest cells (Kil et al., 1998), retinal cells (Sheppard et al., 1994; Cann et al., 1996), and dorsal root ganglion neurons (Vogelezang et al., 2001).
VCAM-1, an IgG superfamily member, is best known as a cytokine-induced protein expressed on vascular endothelium in proximity to inflamed tissue in which it is recognized by lymphoid cells via integrin α4β1 (Osborn et al., 1989; Aplin et al., 1998). However, VCAM-1 is also expressed in early development in many regions, including heart tissue that is contacted by sympathetic neurons (Sheppard et al., 1994).
In this study, we show that recombinant soluble VCAM-1 (rsVCAM-1) promotes robust sympathetic neurite outgrowth that is dependent on α4β1integrins. We examine the expression pattern of these counter receptors and demonstrate that immunological blockage of both α4 integrin and VCAM-1 results in a decrease in sympathetic innervation of the heart.
MATERIALS AND METHODS
Immunohistochemistry of rat superior cervical ganglion and heart tissue. Superior cervical ganglion (SCG) and heart tissue were dissected from P1 Long–Evans rats (Charles River Laboratories, Wilmington, MA) and briefly fixed in 4% paraformaldehyde at 4°C (5 min for SCG and 30 min for heart tissue). After rising in PBS for 20 min and overnight cryoprotection in 20% sucrose and 0.1m sodium phosphate, pH 7.4, at 4°C, the tissue was embedded in 15% gelatin in phosphate buffer, frozen on dry ice, and 16 μm sections were cut using a Leica (Nussloch, Germany) cryostat. SCG and heart sections were first blocked with 3% bovine serum albumin (BSA), 1% goat serum, and 0.01% Triton X-100 in PBS and then stained with the mouse monoclonal anti-rat α4 antibody TA-2 (10 μg/ml; Chemicon, Temecula, CA) or with the mouse monoclonal anti-rat VCAM-1 antibody MR106 (10 μg/ml; PharMingen, San Diego, CA). A polyclonal rabbit antibody against tyrosine hydroxylase (TH) (1:100;Chemicon) was used to identify sympathetic neurons. Staining was visualized using the following secondary antibodies: goat anti-mouse Cy3 (1:800; Jackson ImmunoResearch, West Grove, PA) and goat anti-rabbit Alexa-488 (1:200; Molecular Probes, Eugene, OR). The secondary antibody alone was used as the negative control, and it produced no detectable signal. Micrographs were captured on a Zeiss(Oberkochen, Germany) microscope equipped for epifluorescence or on a Bio-Rad (Hercules, CA) 1024 laser scanning confocal microscope.
SCGs were dissected from P1 and P6 rats and prepared for culture as above. On the following day, the cells were fixed with 4% paraformaldehyde in PBS for 5 min at 4°C and then stained with one of the following antibodies: TA-2 and HMβ-1 (hamster anti-β1 integrin; PharMingen) or the secondary antibody alone (goat anti-mouse or hamster; Jackson ImmunoResearch). The cells were then mounted with Prolong (Molecular Probes) and viewed with an epifluorescence microscope.
Western blot of P1 rat SCG tissue. SCGs from P1 rats were dissected and frozen on dry ice-chilled glass, and crude membranes were isolated (0.5 μg/ganglia) (Bono et al., 1983). After determining the protein concentration using the amido-schwarz assay (Schaffner and Weissmann, 1973), equal amounts of protein (2.8 μg) from each sample were separated by SDS-PAGE and transferred to nitrocellulose for Western blotting. The rat α4 integrin antibody TA-2 (1 μg/ml) was used to detect the presence of α4 integrins, and a goat-anti mouse-HRP secondary was used to visualize bands using the ECL unit from Amersham Biosciences (Piscataway, NJ).
Neurite outgrowth assay. Primary cultures of chicken lumbar sympathetic ganglia (LSG) (embryonic day 12) and mouse and rat SCG (P1) were isolated as before and plated on ECM-coated 96-well culture plates (Corning-Costar, Acton, MA) and incubated as described by Choi et al. (1994). Briefly, the cells were manually dissociated in media, trypsinized (0.05% trypsin), and triturated to eliminate clumped cells. The cells were cultured in serum-free media supplemented with insulin–transferin–selenium (Invitrogen, Carlsbad, CA), penicillin–streptomycin–fungizone (Invitrogen), and 100 ng/ml 7s-NGF (Sigma, St. Louis, MO). The wells were coated overnight with either merosin (Ln-2/4; Invitrogen) as a positive control, 1% BSA as a negative control, plasma Fn (Invitrogen), or rsVCAM-1 (a kind gift from Roy Lobb, Biogen, Cambridge, MA). The function blocking antibodies TA-2 (α4 integrin), 1 mm GRGDNP, 1 mm GRADSP (Biomol, Plymouth Meeting, PA) and HMβ-1 (β1 integrin) were added to the culture media before incubation. (The blocking activity of the RGD peptide was confirmed in assays of chick embryo fibroblast adhesion to vitronectin.) Cells were incubated overnight at 37°C in a humidified atmosphere containing 5% CO2. The neurons and neurites were visualized by staining with the vital dye calcein AM (fluorescein diacetate; Molecular Probes) and viewed with an epifluorescence microscope (Culley et al., 2001). Three images of the best neurite outgrowth from each condition were projected onto a magnetized bit pad for analysis. The percentage of cells with processes of any length and the neurite lengths were scored to quantify the response (Burns et al., 1991; Choi et al., 1994; Culley et al., 2001). A neurite is defined as a visible process emanating from the cell body. Statistical analysis was performed using a Student's two-tailedt test.
Intrathoracic injection of antibodies and α4 antagonist. At P1 and P3, function-blocking antibodies against α4 (TA-2, 20 μg), VCAM-1 (5F10, 20 μg), or IgG isotype controls were injected intrathoracically into Long–Evans rats. The isotype-matched IgG controls were injected into littermates of those animals injected with blocking antibody. The α4 antagonist [similar to the Genentech (San Francisco, CA) example compound shown by Jackson, 2002, his Table 12] was dissolved in 50% PEG400 (Sigma) and injected into the intrathoracic cavity of rats at a concentration of 40 mg/kg animal weight on postnatal days 2–4. This concentration was ∼10-fold greater than the amount required for a complete block of neurite outgrowth on rsVCAM-1 in vitro (data not shown). The antagonist has been shown to be specific for α4 integrins in binding assays using purified integrins and ligands and in cell adhesion experiments as described by Jackson (2002). The drug carrier (50% PEG400) was injected into littermates as a control. At P6, heart, SCG, and spleen tissues were harvested and frozen immediately in liquid nitrogen for biochemistry (TA-2, n = 23; animals, 5F10,n = 11; IgG1, n = 18; IgG2a, n = 3) or fixed for sectioning (TA-2, n = 11; animals, 5F10,n = 3; IgG1, n = 8; IgG2a, n = 3; G-016390,n = 6; PEG400, n = 7) as before. Sections were stained with a goat anti-mouse secondary antibody alone (to visualize the injected antibody) and with a primary goat anti-TH described above. The number of TH-positive fibers in the ventricles was quantified by directly counting in the microscope using a double-blind procedure. From each animal, three 16 μm transverse sections (246 μm apart) from the medial heart, at the depth of the atrioventricular valves, were stained with anti-TH antibodies. TH-positive fibers were counted two to three times for each microscopic field of the section (∼25 fields at 400×) by two to three observers. Fibers were defined as linear, fine-caliber TH immunoreactivity longer than ∼5 μm. Examples of what was and was not counted are shown in Figure 6. Fibers per section were recorded for each condition. In some experiments, digital images were captured, and pixel analysis was performed using the Scion (Frederick, MD) Image program. The average ± SEM value for the control animals was 791 ± 267 fibers per three sections. The amount of NE in the heart was quantified by a radioenzymatic assay (Coyle and Henry, 1973) and a radioimmunoassay (Analytics, Gaithersburg, MD). For the first assay, catecholamines were labeled using catechol-O-methyltransferase andS-adenosyl-l-[methyl-3H] methionine and were solvent extracted, and the counts were determined. For the second assay, NE was quantified using the RIA kit from Alpco Diagnostics (Windham, NH). A standard curve of NE allowed the calculation of nanograms of NE per gram of heart tissue. Hematocrit measurement was performed as described by Bozzini et al. (1989). Statistical analysis was performed using a Student's two-tailedt test.
Fig. 6.

Injection of anti-α4 reduces sympathetic fiber density in the heart. Cardiac cryosections from animals injected with anti-α4 (B, D, F,H) or mouse IgG1 control (A, C, E,G) were stained with anti-TH to reveal sympathetic fibers. Filled arrowheads indicate examples of TH immunoreactivity scored as a fiber; open arrowheadsindicate examples of faint immunofluorescence that was not scored as a fiber. Scale bar, 50 μm.
Terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling of SCG sections from TA-2- and α4 antagonist-treated animals. Terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL) of SCG sections was used to detect apoptotic cells within the SCG. The assay was performed according to Johnson et al. (1999). Briefly, 16 μm sections (160 μm apart) from the central portion of the SCGs were collected. Sections were incubated in PBS (1 hr) and 70% ethanol (30 min), permeabilized, and incubated with dUTP–biotin and terminal transferase. TUNEL-positive nuclei were visualized with avidin–Cy3 (1:250; Jackson ImmunoResearch), three sections from two to six animals at P6 (TA-2, n = 9 sections; 5F10, n = 6 sections; control IgG, n = 18 sections) were counted, and TUNEL-positive cells per square millimeter were quantified.
SCG neuron numbers were counted as described by Smolen et al. (1983), with modifications. Briefly, 16 μm serial cryosections were collected from the entire SCG. Every 16th section was stained with methylene blue and eosin (HEMA 3 stain; Biochemical Sciences, Swedesboro, NJ), and nuclei were counted in the entire section. Cell counts for the entire SCG were then determined using the algorithm described by Smolen et al. (1983).
RESULTS
α4 integrins are expressed by sympathetic neurons
To determine whether α4 integrins were expressed by SCG neurons, cryosections of P1 SCG were immunolabeled with the anti-α4 monoclonal antibody TA-2 (Fig. 1). Immunoreactivity was observed on both cell bodies and processes (inset) of SCG neurons, which were identified by staining for TH. Not all TH-positive cells stained for α4, however (Fig. 1C,green). In addition, non-neuronal TH-negative cells and processes within the ganglia also expressed α4. Judging from the morphology of these cells, they include both glial cells and ED1-positive macrophages (data not shown).
Fig. 1.
Immunolocalization of α4 integrins on SCG neurons. Cryostat sections from P1 rat SCG were double immunolabeled with the anti-rat α4 antibody TA-2 (red,A) and a polyclonal anti-TH antibody (green, B). A composite image ofA and B is shown in C.Insets show a magnified neuronal cell and process from a different micrograph. In cultured P1 neurons, integrin α4 (red, D) and β1 (green, E) immunoreactivity was observed on neuronal cell bodies and neurites (arrowhead), as well as on non-neuronal cells (curved arrow), and punctate α4 staining was also observed on isolated growth cones (F, open arrows). G, Immunoblot of P1 SCG membranes probed with the anti-α4 antibody TA-2. Scale bars:A–E, 50 μm; insets inA–C, 25 μm; F, 10 μm.
In cultured P1 SCG neurons, α4 immunoreactivity was observed on cell bodies, neurites, and growth cones (Fig.1D,F). Flat non-neuronal cells also expressed α4 integrins (Fig. 1D,curved arrow). The integrin β1 subunit, which we believe pairs with α4, was also observed on cell bodies and processes on both neurons and glia (Fig. 1E). Immunoblots of SCG membranes probed with the same α4 antibody detected faint but reproducible bands at 140 and 80 kDa, which is consistent with known α4 banding patterns under nonreducing conditions (Vogelezang et al., 2001). These data show that integrins are distributed over the entire surface of SCG neurons, including growth cones, in which they could play a role in axon extension.
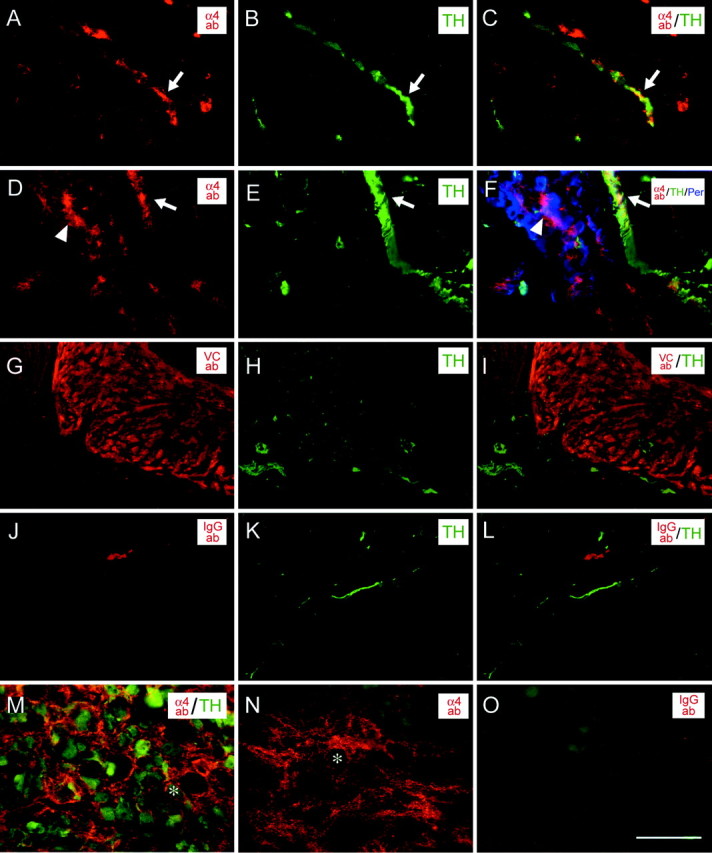
α4 integrins were also detected on SCG fibers in sections of rat heart (Fig. 2A), which were identified by labeling for TH (Fig. 2B). In the first postnatal week, most of these fibers are restricted to the atrial regions. In addition, local interneurons, with cell bodies in the intracardiac ganglia, also displayed α4 immunoreactivity (Fig.2D). These neurons, which relay electrical impulses to control contractions, are TH negative (Fig. 2E) but do express choline acetyl transferase (ChAT) and peripherin (Per) (Fig. 2G–I) (Horackova et al., 1999). Some α4 immunoreactivity was also detected on myocardial cells, as has been reported for E13 mouse heart tissue (Sheppard et al., 1994). These experiments show that α4 integrins are found in growing sympathetic axons, as well as other cell types.
Fig. 2.
Immunolocalization of α4 integrins and VCAM-1 in heart tissue. P1 (A–C, G–L) and P6 (D–F) heart cryosections were stained with TA-2 (red, A,D) and anti-TH (green,B, E, I). InA–C, a portion of the medial wall of the right atrium is shown. C is a composite image of A andB. TH-positive fibers express α4 (arrows). In D–F, a section through an intracardiac ganglia is shown (F is a composite of D andE). Cell bodies of TH-negative neurons express α4 (open arrowheads, D). These local interneurons also express ChAT (red, G) and peripherin (blue, H).J–L show a section of the right atrium stained with the anti-VCAM-1 antibody MR106 (red,J) and anti-TH (green,K), with a composite of J andK shown in L. VCAM-1 immunoreactivity was observed in close proximity to TH-positive sympathetic fibers (filled arrowheads), which sometimes follow VCAM-1-positive blood vessels (curved arrow).Insets show a magnified view of the blood vessel indicated by the curved arrow. Scale bars:A–L, 50 μm; insets, 25 μm.Ch, ChAT; VC, VCAM-1.
VCAM-1 is expressed in heart
To determine whether the α4 ligand–counter receptor VCAM-1 is expressed in regions of the heart contacted by sympathetic axons, heart sections were double immunolabeled with anti-VCAM-1 (MR106) and anti-TH (Fig. 2J–L). VCAM-1 immunoreactivity was observed throughout the myocardium but was especially evident in regions surrounding blood vessels (Fig. 2J, curved arrow). This is consistent with previous work that found VCAM-1 to be expressed on the basolateral surface of the smooth muscle lining around precapillary arterioles (Duplaa et al., 1997). Both the precapillary arterioles and myocardium are innervated by sympathetic neurons, and VCAM-1 immunoreactivity could be detected in the vicinity of TH-positive fibers within the myocardium (Fig.2J–L, filled arrowheads). Thus, VCAM-1 is expressed at the right time and place to play a role in sympathetic innervation.
rsVCAM-1 induces neurite outgrowth by sympathetic neurons
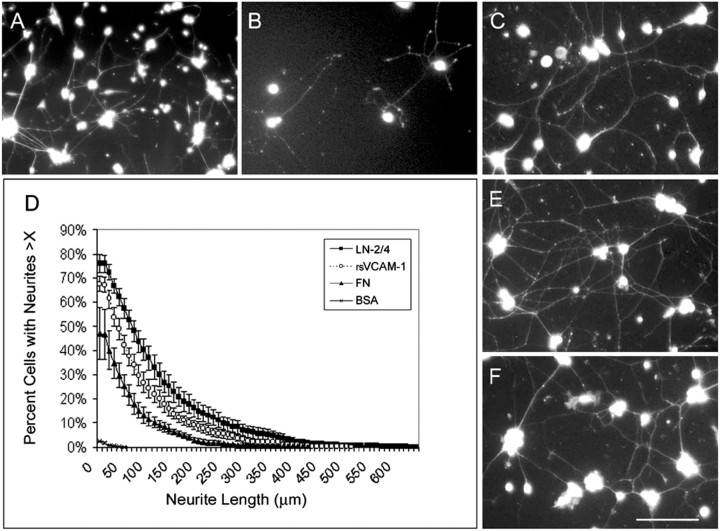
To see whether α4 integrins expressed by SCG neurons were active, we cultured sympathetic neurons from rat, mouse, and chick on a carpet of rsVCAM-1 and stained them with calcein AM (Fig.3). Neurons from all three species mounted a vigorous response to rsVCAM-1, intermediate between the response elicited by Ln-2/4 and Fn. Seventy percent of rat SCG neurons extended neurites on rsVCAM-1 compared with 80% on Ln-2/4 and ∼50% on Fn.
Fig. 3.
rsVCAM-1 induces neurite outgrowth by sympathetic neurons. E10 Chicken lumbar sympathetic ganglion neurons (A) and P1 mouse (B) and rat (C) SCG neurons were visualized by calcein AM staining after overnight culture on rsVCAM-1. Rat SCG neurite morphology on rsVCAM-1 was similar to that on Ln-2/4 (E) and Fn (F). Neurite lengths on rsVCAM-1, Ln-2/4, Fn, and BSA are quantified inD. Scale bar, 50 μm.
The outgrowth on rsVCAM-1 was blocked by antibodies to the integrin α4 and β1 subunits (Fig. 4). The anti-α4 antibody TA-2 inhibited neurite outgrowth on both rsVCAM-1 (Fig. 4D) and Fn (Fig. 4F) but had no effect on outgrowth on Ln-2/4 (Fig. 4E). MRα4–1 (a nonfunction-blocking α4 integrin antibody) did not inhibit neurite outgrowth on any of the substrates (data not shown). Outgrowth on Fn was not inhibited by 1 mm RGD peptide (Fig.4J). We confirmed that the RGD peptide was active in assays of chick embryo fibroblast adhesion to vitronectin. The β1 function-blocking antibody (HMβ-1) inhibited SCG outgrowth on rsVCAM-1 as well (Fig. 4G,H). Because antibodies to either α4 or β1 reduced the outgrowth to the minimal level observed on BSA (<10%), we interpret this as a complete block.
Fig. 4.

Neurite outgrowth on rsVCAM-1 is mediated by α4β1 integrins. P1 rat SCG neurons were cultured on rsVCAM-1 (A, D, G,H), Ln-2/4 (B, E), Fn (C, F), and BSA (I). Neurites were visualized by staining with calcein AM. Cultures were treated with the anti-α4 antibody TA-2 at 50 μg/ml (D–F), anti-β1 antibody at 5 μg/ml (G) or 10 μg/ml (H), or 1 mm RGD or RAD peptide (J). Scale bar, 50 μm. Percentage of cells with neurites for each condition is indicated inJ. The level of outgrowth on BSA was <10% (data not shown). * indicates 95% confidence level; ** indicates 99% confidence level; Student's two-tailed t test. No AB, No antibody.
Although the α4 subunit is capable of pairing with β7 as well as β1, we were unable to detect any β7 immunoreactivity in sections of mouse SCG tissue (data not shown). Although we cannot rule out the presence of small amounts of α4β7, the blocking data indicate that the integrin heterodimer α4β1 is responsible for outgrowth on rsVCAM-1 by sympathetic neurons.
α4 integrin and VCAM-1 immunological blockadein vivo
To test whether α4 integrins and VCAM-1 play a role in sympathetic innervation of the heart in vivo, we injected purified, function-blocking anti-α4 and anti-VCAM-1 antibodies into the thoracic cavity of neonatal rats. As a control, we injected equivalent amounts of isotype-matched non-immune IgG. After two rounds of injections at P1 and P3, hearts, SCG, and spleen tissues were processed for either histology or NE quantification. NE has proved to be a reliable biochemical marker for sympathetic neurons (Clegg et al., 1989).
To determine the distribution of injected antibodies, sections of tissue were incubated with a goat anti-mouse secondary antibody (Fig.5). The anti-α4 antibody bound to TH-positive fibers in the heart (Fig. 5A–C). We also saw a significant accumulation of the antibody on peripherin-positive intracardiac ganglion cells (Fig. 5D–F). Injected α4 antibody was able to penetrate into the SCG as well (Fig.5M,N). The injected anti-VCAM-1 antibody could be detected in the atria, but we could not detect antibody in all regions that contained TH-positive fibers (Fig.5G–I). We do not know whether this is attributable to poor penetration or retention or whether it is attributable to inefficient detection of the antibody. This anti-VCAM-1 antibody does not work well for immunochemistry (data not shown).
Fig. 5.
Penetration of injected antibodies. Heart (A–L) and SCG (M–O) of animals that received intrathoracic injections were stained with goat anti-mouse secondary antibody to detect injected antibodies. Injected anti-α4 antibody (red, A,D) bound to fibers in the atrium and ventricles of the heart that were positive for TH (green,B, E) and peripherin (blue, F) immunoreactivity. The VCAM-1 antibody localized to the atria in heart tissue, but not all areas with the TH-positive fibers were stained (G–I). Injected mouse IgG1 or IgG2a control antibodies did not bind to TH-positive fibers (J–L). Injected anti-α4 antibody diffused to the SCG, in which it bound to TH-positive cell bodies (M) and fibers (N). Injected mouse IgG control antibody was not detected in the SCG (red, O). (The backgroundgreen fluorescence in O is attributable to background staining of the goat anti-rabbit IgG secondary that was included in the experiment). Scale bar, 50 μm. ab, Antibody; VC, VCAM-1.
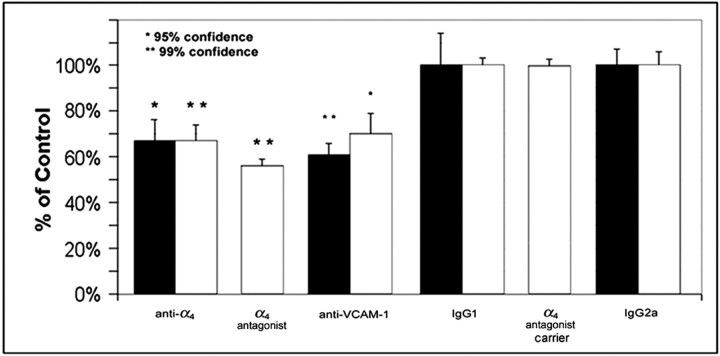
We assayed sympathetic innervation of the hearts of injected animals by both immunohistochemical and biochemical assays. First, we quantified the number of TH-positive fibers in heart ventricles. Using a double-blind protocol, the numbers of fibers were counted directly in the microscope for animals injected with anti-α4, anti-VCAM-1, and control antibodies. Typical sections for anti-α4-injected animals and controls are shown in Figure 6. α4 blockade reduced ventricular TH-positive fibers by 33%, and VCAM-1 blockade resulted in a 39% reduction (Fig.7). The IgG control antibody-injected animals were used to establish a baseline of P6 ventricular innervation. The same results were obtained when digital images were captured and subjected to pixel analysis using the Scion Image software (see Materials and Methods).
Fig. 7.
Intrathoracic injection of anti-α4 and anti-VCAM-1 antibodies reduces sympathetic innervation of the heart. Cardiac norepinephrine levels (black bars) from animals injected with anti-α4 and anti-VCAM-1 antibodies showed a 33 ± 9% (n = 23 animals) and 39 ± 5% (n = 9 animals) reduction compared with controls. Quantification of the TH-positive fibers within the ventricles (average number of fibers per section of the experimental animal divided by the average number of fibers per section of the control animals;white bars) revealed a 33 ± 7% (n = 11 animals) and 30 ± 9% (n = 3 animals) reduction, respectively. Treatment with the α4 antagonist (n = 6 animals) yielded a 43 ± 5% reduction of fiber density compared with control animals injected with carrier (n = 7 animals). Error bars represent the SEM. * indicates 95% confidence level; ** indicates 99% confidence level; Student's two-tailed ttest.
A small molecule inhibitor of α4 integrins was also injected into neonatal rats. This treatment reduced ventricular fibers to a similar extent (43 ± 5%).
As a biochemical measure of sympathetic innervation, we assayed NE levels in injected hearts using two different NE assay protocols. Blocking α4 integrins reduced cardiac NE by 33%, and blockade of VCAM-1 resulted in a 39% reduction (Fig. 7). These data agree with the fiber quantification above. Immunological blockade significantly reduced the density of sympathetic fibers and NE levels in the heart (95–99% confidence) (Fig. 7).
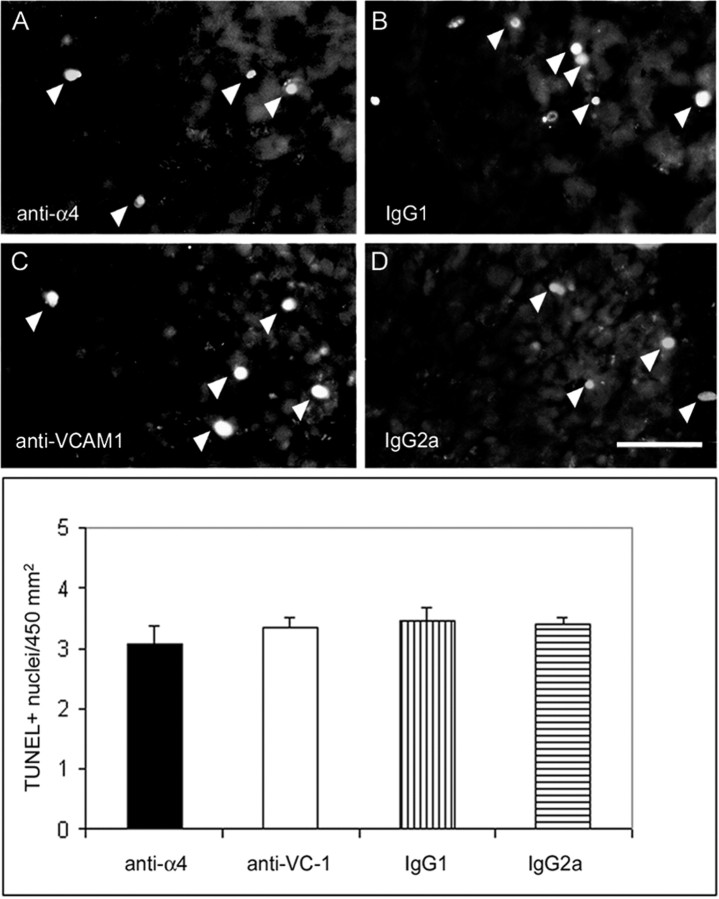
To determine whether the blockade of α4 and VCAM-1 caused an increase in apoptosis in the SCG, we assayed tissue from antibody-injected animals for the presence of TUNEL-positive nuclei. An increase in SCG cell death could account for the decrease in sympathetic tissue in the heart. However, there was no difference in the number of TUNEL-positive cells in the SCG tissue from animals injected with the function-blocking antibodies compared with the non-immune antibody controls (Fig. 8). Because apoptotic cells might be too rapidly cleared to be detected by TUNEL, we also counted total SCG neuron numbers in animals treated with the α4 small molecule inhibitor. The treated animals had an average of 24,264 ± 2824 neurons per ganglia, and the control animals had 22,670 ± 5215 neurons per ganglia. Thus, we could not detect any cell death induced by perturbation of α4–VCAM-1 interactions.
Fig. 8.
Injection of anti-α4 and anti-VCAM-1 does not alter apoptosis in the SCG. No difference was detected in the number of TUNEL-positive nuclei (arrowhead) within SCG tissue of animals injected with anti-α4, anti-VCAM-1, or control antibodies. Scale bar, 50 μm. Results were not significantly different, as analyzed using the Student's two-tailed ttest.
As would be predicted from previous work (Arroyo et al., 1996), blockade of the α4 integrin would be expected to cause improper development of reticulocytes in the bone marrow and lead to anemia. The TA-2-injected animals had a hematocrit of 16% compared with the control and anti-VCAM-1-injected animals, which had hematocrits of 35%. Thus, the reduction in sympathetic innervation brought about by blockade of α4 could be a secondary consequence of anemia. As a control, another experiment was conducted to determine whether this reduction of red blood cells contributed to the decrease in sympathetic fibers in the heart. Animals were injected with phenyl hydrazine (25 mg/kg) to induce anemia at P1. This treatment resulted in a hematocrit of 16% at P6, similar to the α4-injected animals (Chen et al., 1986). Hearts were sectioned and stained for the presence of TH-positive fibers that were quantified as before. There was no apparent reduction in the TH-positive fibers within the hearts of the anemic animals (data not shown). We do not think that the anemia induced by the blockade of α4 integrins contributes to the reduction of sympathetic tissue in the heart (see Discussion).
Consistent with this interpretation, treatment with the small molecule inhibitor only marginally decreased hematocrit (from 35 to 29%) yet decreased sympathetic fibers as much as the antibody treatments.
DISCUSSION
We demonstrated that α4 integrins on sympathetic neurons mediate neurite outgrowth on VCAM-1 in vitro and that this interaction is important in vivo during innervation of the heart. Although many previous studies have implicated integrins in axon outgrowth (Clegg et al., 2000), the involvement of α4 in sympathetic outgrowth was unexpected. The α4β1 heterodimer is well known for its role in inflammation, but experiments presented here, along with several other recent studies, suggest a significant role in neural development and regeneration.
Previous studies showed that α4 integrins are abundantly expressed on neural crest cells (Sheppard et al., 1994; Stepp et al., 1994; Kil et al., 1998). This expression is maintained on developing sensory neurons into adulthood, in which it can mediate interactions with Fn fragments that are upregulated during injury (Vogelezang et al., 2001). As shown here, expression is also maintained on neonatal sympathetic neurons. α4 integrins were detected all over the SCG neuronal surface, including on growth cones, in which they are positioned to play a role in axon outgrowth. We could detect α4 immunoreactivity on sympathetic fibers in the heart at P1 and P6, during a time of active sympathetic axon extension. Furthermore, we show that the α4 integrins are active in that they mediate outgrowth on Fn and rsVCAM-1. Our data suggest that neuronal α4 is pairing with the β1 subunit on neurons, because β7 expression has not been detected on neurons (Brezinschek et al., 1996; Wagner et al., 1996) and because anti-β1 antibody completely blocks α4-mediated neurite outgrowth (Fig. 4). Thus, α4β1 is present at the right place and time to mediate sympathetic axon extension.
The α4β1 integrin is a multifunctional receptor capable of binding a number of ECM and cell surface ligands. In considering growth cones that enter heart tissue, a number of these ligands might be potential binding partners. Fibronectin and transglutaminase C are both expressed in heart tissue during early development (Sheppard et al., 1994;Rongish et al., 1996; Lee et al., 2000). Fibronectin is localized primarily in the atria in the adult rat (Mamuya and Brecher, 1992). The expression pattern of transglutaminase C in postnatal heart has not been examined. Osteopontin has been detected around macrophages and fibroblasts but only during wound healing (Murry et al., 1994). Here we show that VCAM-1 is expressed in heart tissue, especially on blood vessels that are followed by sympathetic growth cones. Although apical expression of VCAM-1 by endothelial cells in vessels would be expected from previous studies, it has also been shown to be on the basolateral side of smooth muscle (Duplaa et al., 1997). The staining we observed was on the outside of the vessels, and it coincided with staining for smooth muscle actin (data not shown). This VCAM-1 would be accessible to sympathetic growth cones.
We showed that a genetically engineered form of the seven IgG domain VCAM-1 (lacking the transmembrane and cytoplasmic domains) possesses a neurite outgrowth-promoting activity for sympathetic neurons in vitro. To our knowledge, this is the first demonstration of such an activity for VCAM-1. Alternatively, spliced mRNAs encode transmembrane forms of VCAM-1 with six, seven, and eight extracellular IgG domains, and a three-domain glycosylphosphatidylinositol-linked form is also known (Osborn et al., 1989; Hession et al., 1991; Moy et al., 1993). The seven-domain form is the most abundantly expressed (Hession et al., 1991). This form of VCAM-1 was able to induce outgrowth from 70% of P1 SCG neurons, a level intermediate between Ln-2/4 and Fn. The morphology of processes and growth cones on these substrates was indistinguishable. VCAM-1 is expressed in other locations during early development and may support outgrowth by other neurons (Sheppard et al., 1994). We found that retinal neurons and DRG neurons can also respond to rsVCAM-1 in vitro (data not shown).
α4 integrins also mediate neurite outgrowth on Fn (Fig. 3). The almost complete block of neurite outgrowth by anti-α4 antibodies was surprising, because other integrins, such as α5β1, which bind the RGD site in Fn, might be expected to play a role. However, RGD peptides did not inhibit the outgrowth of sympathetic neurons on Fn. The full range of integrins expressed by SCG neurons is not yet clear.
Because mice lacking α4 or VCAM-1 die at early embryonic times (Gurtner et al., 1995; Kwee et al., 1995; Yang et al., 1995), it has been difficult to study their functions in later development, and, as with other inquiries, genetic strategies and other perturbations have given conflicting results. For example, peptide and antibody perturbation experiments suggest a role in neural crest cell migration (Kil et al., 1998). However, α4 null crest cells appear to migrate normally (Haack and Hynes, 2001). Crest-derived glial cells lacking α4 do show increased apoptosis, however, indicating a role in glial cell survival. We used function-blocking antibodies to investigate functions of α4 and VCAM-1 in sympathetic innervation. Antibodies to both proteins inhibited the formation of fibers and the resulting accumulation of NE within the heart. Although only a 30–40% reduction was observed, these results are significant, and even a bit remarkable, given the wide number of adhesive interactions that might contribute to axon extension. Sympathetic neurons express a number of integrins, including the α1 and α3subunits, and many integrin ligands and cell adhesion molecules are present in pathways followed by sympathetic axons (Reichardt et al., 1990; DeFreitas et al., 1995; Murase and Hayashi, 1998).
The mechanism of inhibition in these experiments is still under investigation. One possible explanation was that the neurons were simply not surviving, because α4 and other integrins have been implicated in preventing apoptosis (Haack and Hynes, 2001). However, we could not detect any increase in TUNEL-positive neurons in the SCGs of treated animals, nor were any differences in neuronal numbers or pyknotic nuclei observed (data not shown). Another explanation was that the anemia caused by anti-α4 antibodies decreased sympathetic innervation by some secondary means. However, induction of anemia using phenyl hydrazine did not decrease sympathetic innervation. Furthermore, the anti-VCAM-1 antibody decreased sympathetic density but did not cause anemia. Because α4 integrins are expressed on cardiac myocytes, the blocking antibody could affect these cells in some way that would lead indirectly to an alteration in innervation. It is possible that the axons are not growing at all, or are rerouted to a different location, or are not maintained once reaching the target. Experiments are underway to further investigate this question.
Our data support a role for α4β1–VCAM-1 interactions in sympathetic innervation of the heart. By studying the molecules involved in the proper development of the sympathetic nervous system, it may be possible to elucidate the origin of pathologies involving overdevelopment and underdevelopment of these neurons (Chen et al., 2001).
Footnotes
This work was supported by California Tobacco Related Disease Research Program Grant 9RT-0212 and National Institutes of Health/National Eye Institute Grant EY06916. We thank Roy Lobb for the generous gift of rsVCAM-1.
Correspondence should be addressed to Dennis O. Clegg, Neuroscience Research Institute, University of California, Santa Barbara, Santa Barbara, CA 93106. E-mail: clegg@lifesci.ucsb.edu.
N. L. Goodman's present address: University of California, San Diego, La Jolla, CA 92093.
S. J. Rohan's present address: Medical College of Wisconsin, Milwaukee, WI 53226.
REFERENCES
- 1.Altevogt P, Hubbe M, Ruppert M, Lohr J, von Hoegen P, Sammar M, Andrew DP, McEvoy L, Humphries MJ, Butcher EC. The alpha 4 integrin chain is a ligand for alpha 4 beta 7 and alpha 4 beta 1. J Exp Med. 1995;182:345–355. doi: 10.1084/jem.182.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aplin AE, Howe A, Alahari SK, Juliano RL. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol Rev. 1998;50:197–263. [PubMed] [Google Scholar]
- 3.Arroyo AG, Yang JT, Rayburn H, Hynes RO. Differential requirements for alpha4 integrins during fetal and adult hematopoiesis. Cell. 1996;85:997–1008. doi: 10.1016/s0092-8674(00)81301-x. [DOI] [PubMed] [Google Scholar]
- 4.Bayless KJ, Meininger GA, Scholtz JM, Davis GE. Osteopontin is a ligand for the alpha4beta1 integrin. J Cell Sci. 1998;111:1165–1174. doi: 10.1242/jcs.111.9.1165. [DOI] [PubMed] [Google Scholar]
- 5.Berthoud HR, Powley TL. Interaction between parasympathetic and sympathetic nerves in prevertebral ganglia: morphological evidence for vagal efferent innervation of ganglion cells in the rat. Microsc Res Tech. 1996;35:80–86. doi: 10.1002/(SICI)1097-0029(19960901)35:1<80::AID-JEMT7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 6.Bono A, Cantoro G, Martorana A, Palermo R, Pandolfo L. Solubilization, gel filtration and sedimentation behaviour of prolactin receptors from human ovarian tissue. Biochim Biophys Acta. 1983;758:158–167. doi: 10.1016/0304-4165(83)90297-0. [DOI] [PubMed] [Google Scholar]
- 7.Bozzini CE, Alippi RM, Barcelo AC. Enhanced effect of increased erythrocyte production rate on plasma erythropoietin levels of mice during subsequent exposure to hypobaria. Adv Exp Med Biol. 1989;271:23–27. doi: 10.1007/978-1-4613-0623-8_4. [DOI] [PubMed] [Google Scholar]
- 8.Brezinschek RI, Brezinschek HP, Lazarovits AI, Lipsky PE, Oppenheimer-Marks N. Expression of the beta 7 integrin by human endothelial cells. Am J Pathol. 1996;149:1651–1660. [PMC free article] [PubMed] [Google Scholar]
- 9.Burns FR, von Kannen S, Guy L, Raper JA, Kamholz J, Chang S. DM-GRASP, a novel immunoglobulin superfamily axonal surface protein that supports neurite extension. Neuron. 1991;7:209–220. doi: 10.1016/0896-6273(91)90259-3. [DOI] [PubMed] [Google Scholar]
- 10.Cann GM, Bradshaw AD, Gervin DB, Hunter AW, Clegg DO. Widespread expression of β1 integrins in the developing chick retina: evidence for a role in migration of retinal ganglion cells. Dev Biol. 1996;180:82–96. doi: 10.1006/dbio.1996.0286. [DOI] [PubMed] [Google Scholar]
- 11.Chen LT, Chen MF, Porter VL. Increased bone marrow blood flow in rabbits with acute hemolytic anemia. Am J Hematol. 1986;22:35–41. doi: 10.1002/ajh.2830220106. [DOI] [PubMed] [Google Scholar]
- 12.Chen PS, Chen LS, Cao JM, Sharifi B, Karagueuzian HS, Fishbein MC. Sympathetic nerve sprouting, electrical remodeling and the mechanisms of sudden cardiac death. Cardiovasc Res. 2001;50:409–416. doi: 10.1016/s0008-6363(00)00308-4. [DOI] [PubMed] [Google Scholar]
- 13.Choi ES, Rettig WJ, Wayner EA, Srour ML, Clegg DO. Functional identification of integrin laminin receptors that mediate process outgrowth by human SY5Y neuroblastoma cells. J Neurosci Res. 1994;37:475–488. doi: 10.1002/jnr.490370407. [DOI] [PubMed] [Google Scholar]
- 14.Clegg DO, Large TH, Bodary SC, Reichardt LF. Regulation of nerve growth factor mRNA levels in developing rat heart ventricle is not altered by sympathectomy. Dev Biol. 1989;134:30–37. doi: 10.1016/0012-1606(89)90075-4. [DOI] [PubMed] [Google Scholar]
- 15.Clegg DO, Mullick LH, Wingerd KL, Lin H, Atienza JW, Bradshaw AD, Gervin DB, Cann GM. Adhesive events in retinal development and function: the role of integrin receptors. Results Probl Cell Differ. 2000;31:141–156. doi: 10.1007/978-3-540-46826-4_8. [DOI] [PubMed] [Google Scholar]
- 16.Coyle JT, Henry D. Catecholamines in fetal and newborn rat brain. J Neurochem. 1973;21:61–67. doi: 10.1111/j.1471-4159.1973.tb04225.x. [DOI] [PubMed] [Google Scholar]
- 17.Culley B, Murphy J, Babaie J, Nguyen D, Pagel A, Rousselle P, Clegg DO. Laminin-5 promotes neurite outgrowth from central and peripheral chick embryonic neurons. Neurosci Lett. 2001;301:83–86. doi: 10.1016/s0304-3940(01)01615-9. [DOI] [PubMed] [Google Scholar]
- 18.DeFreitas MF, Yoshida CK, Frazier WA, Mendrick DL, Kypta RM, Reichardt LF. Identification of integrin alpha 3 beta 1 as a neuronal thrombospondin receptor mediating neurite outgrowth. Neuron. 1995;15:333–343. doi: 10.1016/0896-6273(95)90038-1. [DOI] [PubMed] [Google Scholar]
- 19.Duplaa C, Couffinhal T, Dufourcq P, Llanas B, Moreau C, Bonnet J. The integrin very late antigen-4 is expressed in human smooth muscle cell. Involvement of alpha 4 and vascular cell adhesion molecule-1 during smooth muscle cell differentiation. Circ Res. 1997;80:159–169. doi: 10.1161/01.res.80.2.159. [DOI] [PubMed] [Google Scholar]
- 20.Ganguly PK, Sherwood GR. Noradrenaline turnover and metabolism in myocardium following aortic constriction in rats. Cardiovasc Res. 1991;25:579–585. doi: 10.1093/cvr/25.7.579. [DOI] [PubMed] [Google Scholar]
- 21.Guan JL, Hynes RO. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor alpha 4 beta 1. Cell. 1990;60:53–61. doi: 10.1016/0092-8674(90)90715-q. [DOI] [PubMed] [Google Scholar]
- 22.Gurtner GC, Davis V, Li H, McCoy MJ, Sharpe A, Cybulsky MI. Targeted disruption of the murine VCAM1 gene: essential role of VCAM-1 in chorioallantoic fusion and placentation. Genes Dev. 1995;9:1–14. doi: 10.1101/gad.9.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Haack H, Hynes RO. Integrin receptors are required for cell survival and proliferation during development of the peripheral glial lineage. Dev Biol. 2001;233:38–55. doi: 10.1006/dbio.2001.0213. [DOI] [PubMed] [Google Scholar]
- 24.Hession C, Tizard R, Vassallo C, Schiffer SB, Goff D, Moy P, Chi-Rosso G, Luhowskyj S, Lobb R, Osborn L. Cloning of an alternate form of vascular cell adhesion molecule-1 (VCAM1). J Biol Chem. 1991;266:6682–6685. [PubMed] [Google Scholar]
- 25.Horackova M, Armour JA, Byczko Z. Distribution of intrinsic cardiac neurons in whole-mount guinea pig atria identified by multiple neurochemical coding. A confocal microscope study. Cell Tissue Res. 1999;297:409–421. doi: 10.1007/s004410051368. [DOI] [PubMed] [Google Scholar]
- 26.Isobe T, Hisaoka T, Shimizu A, Okuno M, Aimoto S, Takada Y, Saito Y, Takagi J. Propolypeptide of von Willebrand factor is a novel ligand for very late antigen-4 integrin. J Biol Chem. 1997;272:8447–8453. doi: 10.1074/jbc.272.13.8447. [DOI] [PubMed] [Google Scholar]
- 27.Isobe T, Takahashi H, Ueki S, Takagi J, Saito Y. Activity-independent cell adhesion to tissue-type transglutaminase is mediated by alpha4beta1 integrin. Eur J Cell Biol. 1999;78:876–883. doi: 10.1016/s0171-9335(99)80089-2. [DOI] [PubMed] [Google Scholar]
- 28.Iversen LL, De Champlain J, Glowinski J, Axelrod J. Uptake, storage and metabolism of norepinephrine in tissues of the developing rat. J Pharmacol Exp Ther. 1967;157:509–516. [PubMed] [Google Scholar]
- 29.Jackson DY. Alpha 4 integrin antagonists. Curr Pharm Des. 2002;8:1229–1253. doi: 10.2174/1381612023394737. [DOI] [PubMed] [Google Scholar]
- 30.Johnson PT, Williams RR, Cusato K, Reese BE. Rods and cones project to the inner plexiform layer during development. J Comp Neurol. 1999;414:1–12. [PubMed] [Google Scholar]
- 31.Kil SH, Krull CE, Cann G, Clegg D, Bronner-Fraser M. The alpha4 subunit of integrin is important for neural crest cell migration. Dev Biol. 1998;202:29–42. doi: 10.1006/dbio.1998.8985. [DOI] [PubMed] [Google Scholar]
- 32.Kwee L, Baldwin HS, Shen HM, Stewart CL, Buck C, Buck CA, Labow MA. Defective development of the embryonic and extraembryonic circulatory systems in vascular cell adhesion molecule (VCAM-1) deficient mice. Development. 1995;121:489–503. doi: 10.1242/dev.121.2.489. [DOI] [PubMed] [Google Scholar]
- 33.Lee SK, Chi JG, Park SC, Chung SI. Transient expression of transglutaminase C during prenatal development of human muscles. J Histochem Cytochem. 2000;48:1565–1574. doi: 10.1177/002215540004801113. [DOI] [PubMed] [Google Scholar]
- 34.Lein P, Johnson M, Guo X, Rueger D, Higgins D. Osteogenic protein-1 induces dendritic growth in rat sympathetic neurons. Neuron. 1995;15:597–605. doi: 10.1016/0896-6273(95)90148-5. [DOI] [PubMed] [Google Scholar]
- 35.Lein PJ, Higgins D, Turner DC, Flier LA, Terranova VP. The NC1 domain of type IV collagen promotes axonal growth in sympathetic neurons through interaction with the alpha 1 beta 1 integrin. J Cell Biol. 1991;113:417–428. doi: 10.1083/jcb.113.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipp JA, Rudolph AM. Sympathetic nerve development in the rat and guinea-pig heart. Biol Neonate. 1972;21:76–82. doi: 10.1159/000240497. [DOI] [PubMed] [Google Scholar]
- 37.Lobb RR, Hemler ME. The pathophysiologic role of alpha 4 integrins in vivo. J Clin Invest. 1994;94:1722–1728. doi: 10.1172/JCI117519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lustig M, Sakurai T, Grumet M. Nr-CAM promotes neurite outgrowth from peripheral ganglia by a mechanism involving axonin-1 as a neuronal receptor. Dev Biol. 1999;209:340–351. doi: 10.1006/dbio.1999.9250. [DOI] [PubMed] [Google Scholar]
- 39.Mamuya WS, Brecher P. Fibronectin expression in the normal and hypertrophic rat heart. J Clin Invest. 1992;89:392–401. doi: 10.1172/JCI115598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moy P, Lobb R, Tizard R, Olson D, Hession C. Cloning of an inflammation-specific phosphatidyl inositol-linked form of murine vascular cell adhesion molecule-1. J Biol Chem. 1993;268:8835–8841. [PubMed] [Google Scholar]
- 41.Murase S, Hayashi Y. Integrin alpha1 localization in murine central and peripheral nervous system. J Comp Neurol. 1998;395:161–176. doi: 10.1002/(sici)1096-9861(19980601)395:2<161::aid-cne2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 42.Murry CE, Giachelli CM, Schwartz SM, Vracko R. Macrophages express osteopontin during repair of myocardial necrosis. Am J Pathol. 1994;145:1450–1462. [PMC free article] [PubMed] [Google Scholar]
- 43.Nyquist-Battie C, Cochran PK, Sands SA, Chronwall BM. Development of neuropeptide Y and tyrosine hydroxylase immunoreactive innervation in postnatal rat heart. Peptides. 1994;15:1461–1469. doi: 10.1016/0196-9781(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 44.Osborn L, Hession C, Tizard R, Vassallo C, Luhowskyj S, Chi-Rosso G, Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989;59:1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- 45.Pappano AJ. Ontogenetic development of autonomic neuroeffector transmission and transmitter reactivity in embryonic and fetal hearts. Pharmacol Rev. 1977;29:3–33. [PubMed] [Google Scholar]
- 46.Reichardt LF, Bossy B, Carbonetto S, de Curtis I, Emmett C, Hall DE, Ignatius MJ, Lefcort F, Napolitano E, Large T, Neugebauer KM, Tomasilli KJ. Neuronal receptors that regulate axon growth. Cold Spring Harb Symp Quant Biol. 1990;55:341–350. doi: 10.1101/sqb.1990.055.01.035. [DOI] [PubMed] [Google Scholar]
- 47.Rongish BJ, Hinchman G, Doty MK, Baldwin HS, Tomanek RJ. Relationship of the extracellular matrix to coronary neovascularization during development. J Mol Cell Cardiol. 1996;28:2203–2215. doi: 10.1006/jmcc.1996.0212. [DOI] [PubMed] [Google Scholar]
- 48.Schaffner W, Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973;56:502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- 49.Sheppard AM, Onken MD, Rosen GD, Noakes PG, Dean DC. Expanding roles for alpha 4 integrin and its ligands in development. Cell Adhes Commun. 1994;2:27–43. doi: 10.3109/15419069409014200. [DOI] [PubMed] [Google Scholar]
- 50.Smolen AJ, Wright LL, Cunningham TJ. neuron numbers in the superior cervical sympathetic ganglion of the rat: a critical comparison of methods for cell counting. J Neurocytol. 1983;12:739–750. doi: 10.1007/BF01258148. [DOI] [PubMed] [Google Scholar]
- 51.Spring FA, Parsons SF, Ortlepp S, Olsson ML, Sessions R, Brady RL, Anstee DJ. Intercellular adhesion molecule-4 binds alpha(4)beta(1) and alpha(V)-family integrins through novel integrin-binding mechanisms. Blood. 2001;98:458–466. doi: 10.1182/blood.v98.2.458. [DOI] [PubMed] [Google Scholar]
- 52.Stepp MA, Urry LA, Hynes RO. Expression of alpha 4 integrin mRNA and protein and fibronectin in the early chicken embryo. Cell Adhes Commun. 1994;2:359–375. doi: 10.3109/15419069409014210. [DOI] [PubMed] [Google Scholar]
- 53.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 54.Vogelezang MG, Liu Z, Relvas JB, Raivich G, Scherer SS, ffrench-Constant C. Alpha4 integrin is expressed during peripheral nerve regeneration and enhances neurite outgrowth. J Neurosci. 2001;21:6732–6744. doi: 10.1523/JNEUROSCI.21-17-06732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wagner N, Lohler J, Kunkel EJ, Ley K, Leung E, Krissansen G, Rajewsky K, Muller W. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature. 1996;382:366–370. doi: 10.1038/382366a0. [DOI] [PubMed] [Google Scholar]
- 56.Yabkowitz R, Dixit VM, Guo N, Roberts DD, Shimizu Y. Activated T-cell adhesion to thrombospondin is mediated by the alpha 4 beta 1 (VLA-4) and alpha 5 beta 1 (VLA-5) integrins. J Immunol. 1993;151:149–158. [PubMed] [Google Scholar]
- 57.Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]