Abstract
Cholinergic agents elicit prominent smooth muscle contractions via stimulation of muscarinic receptors that comprise five distinct subtypes (M1–M5). Although such contractions are important for autonomic organs, the role of each subtype has not been characterized precisely because of the poor selectivity of the currently available muscarinic ligands. Here, we generated a mutant mouse line (M2−/−M3−/− mice) lacking M2 and M3 receptors that are implicated in such cholinergic contractions. The relative contributions of M2 and M3 receptors in vitrowas ∼5 and 95% for the detrusor muscle contraction and ∼25 and 75% for the ileal longitudinal muscle contraction, respectively. Thus, M1, M4, or M5receptors do not seem to play a role in such contractions. Despite the complete lack of cholinergic contractions in vitro, M2−/−M3−/− mice were viable, fertile, and free of apparent intestinal complications. The urinary bladder was distended only in males, which excludes a major contribution by cholinergic mechanisms to the urination in females. Thus, cholinergic mechanisms are dispensable in gastrointestinal motility and female urination. After 10 Hz electrical field stimulation, noncholinergic inputs were found to be increased in the ileum of M2−/−M3−/− females, which may account for the lack of apparent functional deficits. Interestingly, the M2−/−M3−/− mice had smaller ocular pupils than M3-deficient mice. The results suggest a novel role of M2 in the pupillary dilation, contrary to the well known cholinergic constriction. These results collectively suggest that an additional mechanism operates in the control of pupillary constriction–dilatation.
Keywords: acetylcholine, muscarinic receptors, smooth muscle contraction, gene targeting, intestinal motility, ocular pupils
Acetylcholine (ACh) is the most common neurotransmitter at the parasympathetic nerve ending to induce smooth muscle contractions. In the gastrointestinal tract, ACh is released from the primary excitatory motor neurons and mediates an immediate smooth muscle contraction (Goyal and Hirano, 1996; Furness, 2000). Activities of the motor neurons in the gut wall are coordinated by the enteric nervous system to generate functional movement, such as peristalsis. Although the excitatory enteric neurons corelease other transmitters, such as tachykinins (Holzer and Holzer-Petsche, 1997), ACh is believed to be functionally predominant in inducing contractions (Goyal and Hirano, 1996; Furness, 2000).
The cholinergic signaling is mediated by the muscarinic ACh receptor expressed on the surface of the smooth muscle cells. To date, five subtypes (M1–M5) of muscarinic receptors have been identified, which are variable in both the tissue distribution and signal transduction mechanisms (Caulfield and Birdsall, 1998). Delineating the role of these receptors has been a matter of considerable interest, because they are promising therapeutic targets for various diseases (Eglen et al., 2001). However, their pharmacological characterization remains inconclusive because of the poor subtype selectivity of available ligands. In the intestine, for example, M3 is considered to be predominant in eliciting cholinergic contraction, whereas the role of the more abundant M2 remains unclear (Ehlert et al., 1999;Eglen, 2001).
To overcome such technical problems, mutant mice deficient in each individual subtype have been constructed (Hamilton et al., 1997; Gomeza et al., 1999a,b; Matsui et al., 2000; Yamada et al., 2001a,b). Notably, all five mutant mouse lines are viable, which suggests a functional redundancy among the subtypes. The mice deficient in either M2 or M3 receptors show decreased responses to cholinergic stimuli (Matsui et al., 2000;Stengel et al., 2000), but precise mechanisms of the residual contractions remain to be elucidated.
Here, we generated mutant mice lacking both M2and M3 receptors and studied the role of these subtypes in various smooth muscle organs. In addition, we used electrical field stimulation (EFS) and evaluated the in vivo significance of cholinergic smooth muscle contraction.
MATERIALS AND METHODS
Construction of a targeting vector for disruption of M2 receptor gene. A targeting vector, pChrm2-N1 (Fig. 1A), was constructed using the mouse M2 genomic fragments (Matsui et al., 1999). The BamHI–EcoRI (1.0 kb) and SmaI–BamHI (8.5 kb) fragments were placed upstream and downstream of the PGK-neo-bpA cassette (Soriano et al., 1991), respectively. The PGK-DTA cassette (Yagi et al., 1990) was inserted at the upstream end in reverse orientation.
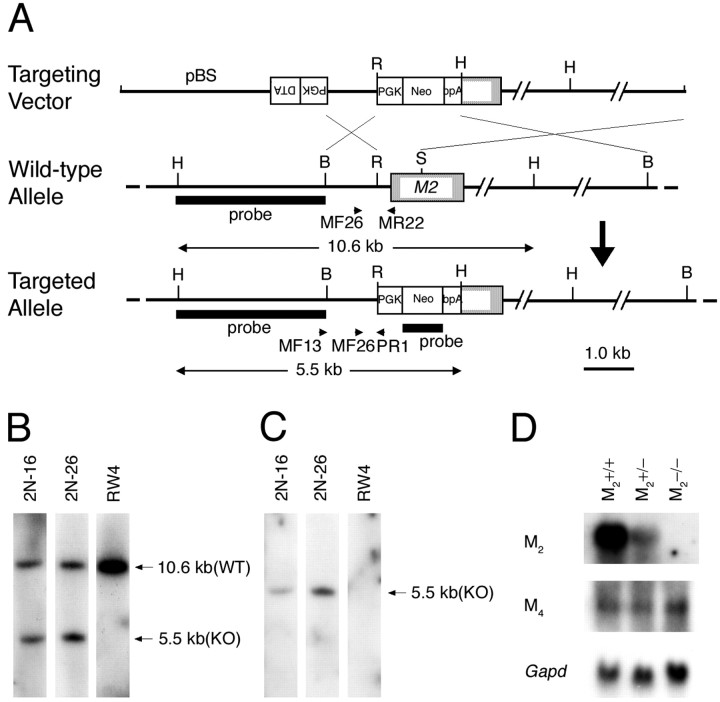
Fig. 1.
Generation of M2−/− mice.A, Targeting strategy. Arrows MF13 andPR1 indicate the PCR primers used for homologous recombinant screening, and arrows MF26,MR22, and PR1 indicate PCR primers used for genotyping. BamHI (B),HindIII (H),EcoRI (R), and SmaI (S) sites relevant to the identification of homologous recombinant clones are shown together with the expected sizes hybridizable to the M2 and the neoprobes. B, Hybridization with the M2 probe showing a 5.5 kb band specific to the targeted allele (KO) and a 10.6 kb band derived from the wild-type allele (WT). C, Hybridization with the neo probe showing a 5.5 kb band specific to the targeted allele. D, Northern analysis showing mRNA levels of M2 and M4 in the brains of wild-type, M2+/−, and M2−/− mice. Note that the M2 mRNA in the M2+/− brain decreased to approximately half of the wild-type brain and was absent in the M2−/− brain. The mRNA levels of M4 are not different among the three genotypes. Bottom, Signals hybridized with a Gapd probe used as an internal control.
Gene targeting in embryonic stem cells. The gene targeting in embryonic stem cells (RW4; Genome Systems, St. Louis, MO) was performed as described previously (Matsui et al., 2000). G418-resistant cells were screened by PCR using primers MF13 (5′-CCC TGC TTC AAA TAC TTG TCC-3′) and PR1 (5′-CAG ACT GCC TTG GGA AAA GC-3′) to amplify a 1.2 kb fragment. Two independent clones (2N-16 and 2N-26) were verified for the homologous recombination (Fig.1B,C). The 2N-26 clone contributed to chimeric males that transmitted the targeted allele to the germ line. A mutant mouse line was established in a mixed background between 129/SvJ and C57BL/6.
Genotyping. The genotyping protocol for the M3 allele was described previously (Matsui et al., 2000). The procedure for the M2 allele was similar, except that the primers used were MF26 (5′-GGT TGG GTG CAT TGG TTA GT-3′), PR1 (5′-CAG ACT GCC TTG GGA AAA GC-3′), and MR22 (5′-GTG TTC AGT AGT CAA GTG GC-3′).
Northern analysis. An M2 probe was prepared from a 510 bp genomic DNA fragment (SmaI–SmaI) corresponding to the mouse M2-encoding region (129/SvJ origin). For M4 and the glyceraldehyde-3-phosphate dehydrogenase gene (Gapd) probes, DNA fragments were amplified by PCR from cloned genomic DNA fragments of a 129/SvJ mouse and from a genomic DNA of a C57BL/6 mouse, respectively. The primers used were as follows: for M4, M4F (5′-AGC CGC AGC CGT GTT CAC AA-3′) and M4R (5′-TGG GTT GAG GGT TCG TGG CT-3′); and forGapd, GapdF (5′-GCG TCC TGC ACC AAC TG-3′) and GapdR (5′-ATG GTC CTT TAC TCG AAG TG-3′).
Generation of M2−/−M3−/− mice. The mouse colony was maintained in a specific pathogen-free area, and the lights in the animal room were turned on between 7:00 A.M. and 7:00 P.M. The M2−/− mice (N3 generation) and M3−/− mice (N2 generation) were crossed to obtain the M2+/−M3+/− mice. Intercross between these mice yielded pups of various genotypes for M2 and M3 alleles, including M2−/−M3−/− mutants. The descendants of these mutant mice were used for phenotypic analysis in this study. To improve the growth of the pups lacking M3, hydrated paste food was fed as described previously (Matsui et al., 2000).
Blood chemistry. Blood samples were taken intracardially from anesthetized animals and analyzed with a Fuji Dri-Chem system (model 5500) at Fujimoto Biomedical Laboratories (Osaka, Japan).
Histological analyses. All organs were fixed in 10% Formalin, embedded in paraffin, and sectioned at 4 μm. Sections were stained with hematoxylin and eosin and examined under a light microscope.
In vitro responsiveness of ileal and urinary bladder smooth muscles. The urinary bladder was prepared free of serosal connective tissue and cut into four longitudinal unfolded sections. The ileum was removed, and longitudinal muscles were isolated from the segments of the ileum (5 mm long). The preparations were placed in 5 ml organ baths containing modified Krebs–Henseleit solution maintained at 32°C, aerated continuously with 95% O2 and 5% CO2, and connected to isometric transducers with sutures.
Mechanical responses were recorded isometrically by a multichannel polygraph. The tissue was equilibrated for at least 60 min with initial tension of 0.5 gm, and then contractions were induced by adding 50 mm KCl twice. The second contractile response to KCl was taken as a reference. The concentration–contraction curves for carbachol were obtained by cumulative addition of carbachol to the organ bath.
Trains of EFS (pulses at 1 or 10 Hz) were given for 10 sec at 5 min intervals. Each pulse was 45 V, 0.5 msec (bladder) or 40 V, 1 msec (ileum). After the muscle strips were contracted by 50 mmKCl for a reference, EFS was applied to cause basal contraction. To obtain noncholinergic contraction of wild-type muscle, atropine (10 μm) was applied subsequently.
RESULTS
Generation of M2−/−M3−/− mice
To establish a mouse line lacking both M2and M3 receptors, we first constructed a mutant mouse line deficient in M2(M2−/− mice) (Fig. 1). The M2−/− mice seemed healthy, consistent with another report on a similar mutant line lacking M2 (Gomeza et al., 1999a). We crossed our M2−/− mice with the M3−/− mice (Matsui et al., 2000) to produce M2+/−M3+/− mutants and further intercrossed these M2+/−M3+/− mice to obtain M2−/−M3−/− mutants. Among the pups, the M2−/−M3−/− mutants were found at the Mendelian ratio (data not shown), excluding lethalityin utero.
Although cholinergic signals are involved in the control of vital organs, such as gastrointestinal (Goyal and Hirano, 1996; Furness, 2000) and urinary (Andersson, 1998) tracts, the M2−/−M3−/− pups survived to adulthood after a transient postweaning growth retardation (data not shown). The growth of M2−/−M3−/− pups was improved by feeding paste food, reflecting their impaired salivation as a result of the loss of M3-mediated signals (Matsui et al., 2000). The M3−/− mice of either sex were fertile (Matsui et al., 2000), despite the proposed roles of the muscarinic receptor in genital organs (Lepor and Kuhar, 1984; Eglen et al., 1989). Because M2 and M3 receptors are coexpressed in the uterus (Choppin et al., 1999) and prostate (Pontari et al., 1998), it was conceivable that M2 receptors compensated for the lost function of M3. However, the M2−/−M3−/− mice of either sex were still fertile. Together, the M2−/−M3−/− mice were unexpectedly healthy, despite the proposed importance of these receptors in the functions of various autonomic organs.
Sex difference in urinary retention phenotype
In the urinary bladder, muscarinic contraction of the detrusor muscle is considered to be the main source of voiding power (Andersson, 1998), and muscarinic antagonists have been applied for the treatment of bladder hyperactivity in humans (de Groat and Yoshimura, 2001). In our previous report (Matsui et al., 2000), the cholinergic contractility of the M3−/− detrusor muscle corresponded to only 5% of that of the wild-type muscle. Although there was no sex difference in the degree of such in vitrodeficits, urinary disturbance was severe in males but only mild in females, suggesting that the contribution of M2is significantly larger in females. However, the urinary bladder of the M2−/− mice seemed normal in both sexes. Furthermore, the bladder diameter of the female M2−/−M3−/− mice (5.9 ± 0.7 mm; n = 4) was not significantly different (p = 0.81) from that of the female M3−/− mice (5.7 ± 0.5 mm;n = 7) reported by Matsui et al. (2000). Consistent with a minor urinary retention phenotype, the kidney histology was normal (data not shown). In males, the bladder distension in the M2−/−M3−/− mice (14.5 ± 1.4 mm; n = 4) was not significantly different (p = 0.29) from that in the M3−/− mice (12.3 ± 1.3 mm;n = 6) reported by Matsui et al. (2000). Urinalysis and blood chemistry data for renal functions were normal in both sexes (data not shown). Thus, M3, but not M2, is essential for voiding in males, but the contribution by M2 or M3 in female urination is rather small. Although the reason for the sex difference is unclear, it is conceivable that the roles of the central muscarinic receptors in urination (Ishiura et al., 2001) are different between the sexes. An alternative and more plausible explanation is that there is an additional, nonmuscarinic postjunctional mechanism in females that mediates urination in the M2−/−M3−/− mice (see Discussion).
Normal appearance of digestive tracts
In the digestive tract, both M2 and M3 mediate the intestinal smooth muscle contraction (Ehlert et al., 1999; Eglen, 2001), which is considered the basis of functional gut movements, including peristalsis (Goyal and Hirano, 1996; Furness, 2000). Loss of peristalsis should be fatal, as exemplified by a clinical condition called acute colonic pseudo-obstruction, in which cholinergic dysfunction is implicated (Ponec et al., 1999). Therefore, we anticipated severe malfunctions in the gut of the M2−/−M3−/− mice. Unexpectedly, they showed no signs of abnormal abdominal distension or constipation. At necropsy, the whole digestive tract of the M2−/−M3−/− mice seemed normal (data not shown). Histological examinations revealed no abnormalities in the gastric, jejunal, or ileal sections (Fig.2). Other visceral organs, such as the heart, lung, and spleen, appeared normal at gross and histological examinations (data not shown). Muscarinic receptors are implicated in gall bladder contraction (Parkman et al., 1999a) and in pancreatic endocrine (Boschero et al., 1995) and exocrine (Schmid et al., 1998) secretions. However, blood biochemistry data were normal, including those representing the hepatic and pancreatic functions (data not shown).
Fig. 2.
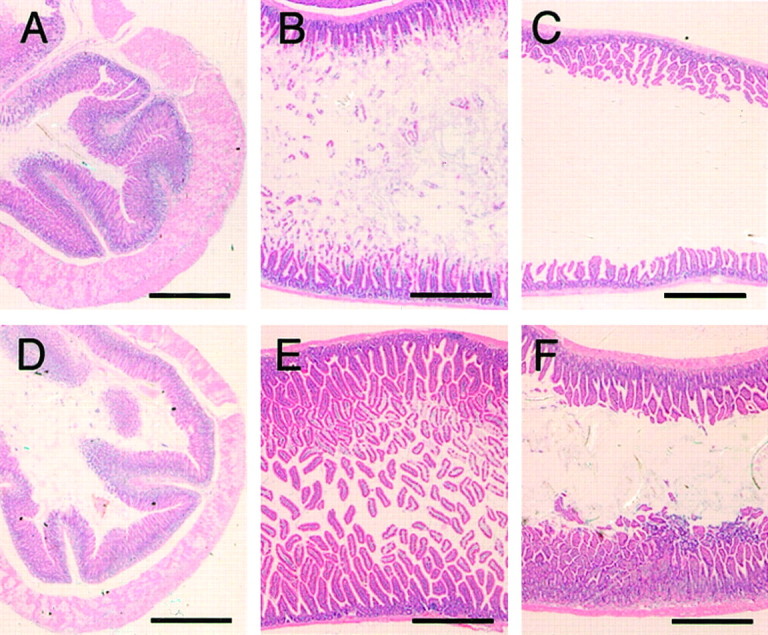
Normal histology of the gastrointestinal tract of M2−/−M3−/− mice. Sections of the stomach (A, D), jejunum (B, E), and ileum (C,F), stained with hematoxylin and eosin. Note that the diameter of the lumen and morphology of the muscular or mucosal layers are indistinguishable between the wild-type (A–C) and the M2−/−M3−/− (D–F) mice. Scale bars, 0.5 mm.
In vitro contractility of smooth muscles to carbachol
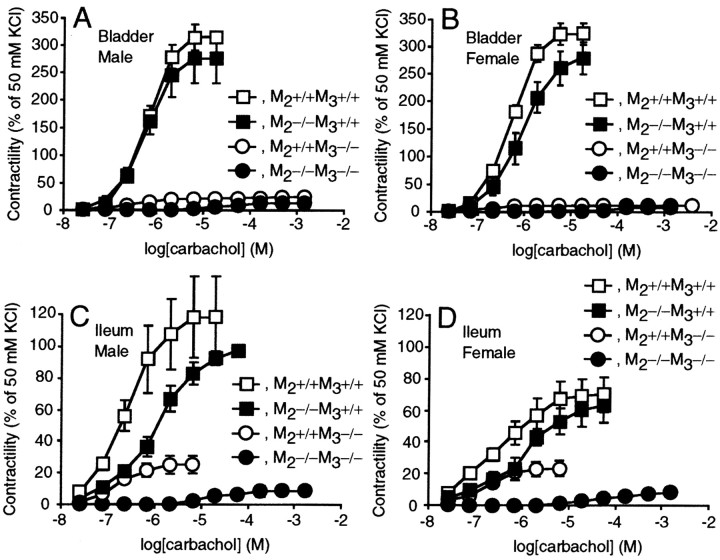
Although M2 and M3receptors have been implicated in cholinergic contraction (Sawyer and Ehlert, 1998; Matsui et al., 2000; Stengel et al., 2000; Giglio et al., 2001), the mice lacking both receptors showed no apparent abnormalities in the autonomic organs, except for the urinary retention in males. Because pharmacological analyses in these reports were inconclusive, it was conceivable that cholinergic signaling remained intact through other muscarinic receptor subtypes. To address this issue, we examinedin vitro contractility of the detrusor muscle (Fig.3A,B) and of the ileal longitudinal smooth muscle (Fig.3C,D). Their contractility in response to 50 mm KCl was comparable among wild-type, M2−/−, M3−/−, and M2−/−M3−/− tissues. Strikingly, however, the M2−/−M3−/− muscles did not respond to carbachol, even at concentrations (e.g., 10−5m) that evoked maximal contractions in the wild-type muscles. Although application of higher dosages induced small contractions, these responses were blocked by hexamethonium, which indicated a slight contribution by nicotinic receptors (data not shown). Regarding the role of each subtype in mediating contractions, the proportions reduced by the M3 loss (95% in the bladder, 72–77% in the ileum) reported by Matsui et al. (2000) were essentially the same as those that remained in the M2−/− tissues (Fig.3) (Stengel et al., 2000). Thus, we conclude that M2 and M3 receptors are entirely responsible for the cholinergic contraction of smooth muscles in an additive manner.
Fig. 3.
Abolished bladder (A,B) and ileal (C, D) smooth muscle contractions in response to carbachol in the M2−/−M3−/− mice. Responses to carbachol are shown (mean ± SEM) as percentages of those to 50 mmKCl in the male (A, C) wild-type, M2−/−, M3−/−, and M2−/−M3−/− mice and the female (B, D) wild-type, M2−/−, M3−/−, and M2−/−M3−/− mice. Each symbol represents the data of four experiments. The data of the M3−/− mice have been published previously (Matsui et al., 2000).
In vitro contractility of smooth muscles to EFS
When stimulated in an electrical field, intramural neurons of the smooth muscle tissue release multiple endogenous neurotransmitters that elicit muscle contractions. To estimate the possible difference in the noncholinergic component of the EFS-induced contraction, we compared the responses between the mutant tissues with those of the wild type treated with atropine.
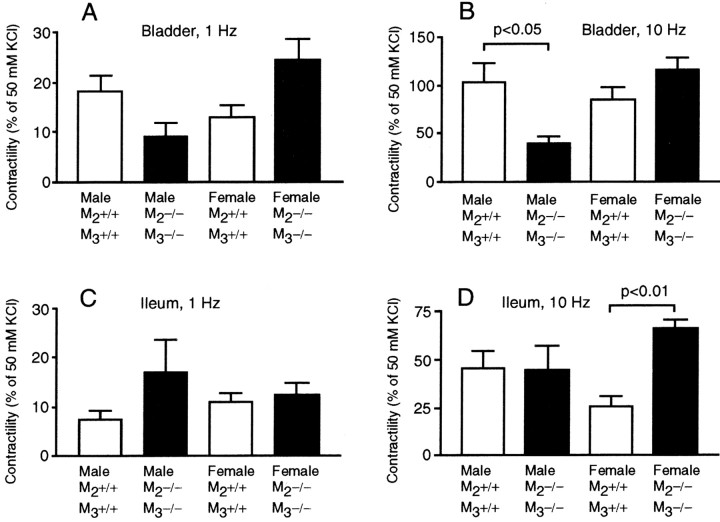
When the male bladder strips were stimulated in an electrical field at 10 Hz frequency, the noncholinergic (i.e., atropine-resistant) contraction was significantly weaker in the mutants than in the wild type (40.3 vs 102.3%; p < 0.05) (Fig.4B). A similar tendency was detected in the stimulation at 1 Hz frequency (8.9 vs 18.2%;p = 0.065) (Fig. 4A). This reduction may reflect a functional damage to the intramural noncholinergic neurons, which may be caused by the severe extension of the bladder wall. In the female mutant tissue, in contrast, noncholinergic components were substantially larger (24.3 vs 12.9% at 1 Hz and 115.6 vs 84.3% at 10 Hz; statistically not significant) (Fig.4A,B). This may reflect a weak compensatory upregulation of the noncholinergic contraction mechanism.
Fig. 4.
Noncholinergic contractile responses to EFS of the bladder (A, B) and ileal (C, D) smooth muscles. Responses to EFS (1 Hz, A, C; 10 Hz, B,D) are shown (mean with SEM; each bar represents the data from four mice) as percentages of those to 50 mmKCl.
In the ileum of females, the noncholinergic response elicited by EFS at 10 Hz frequency was stronger in the mutants than in the wild type (65.6 vs 25.2%; p < 0.01) (Fig. 4D). A similar tendency was detected in males as well when stimulated at 1 Hz (16.7 vs 7.3%; p = 0.23) (Fig. 4C). These findings may suggest that the noncholinergic excitatory stimulations were upregulated in a compensatory manner.
Finally, we analyzed the sex difference in the proportion of cholinergic contraction to the total contraction in the wild-type tissues. Interestingly, cholinergic components in the urinary bladder contraction were larger in males than in females. In males, the contributions of cholinergic contraction were 28% (1 Hz stimulation) to 45% (10 Hz stimulation), whereas they were only 18% (1 Hz stimulation) to 27% (10 Hz stimulation) in females. This is consistent with our interpretation of the sex difference in the urinary retention phenotype that the cholinergic mechanism in vivo is more important in males than in females.
Pupillary constriction by M2 loss
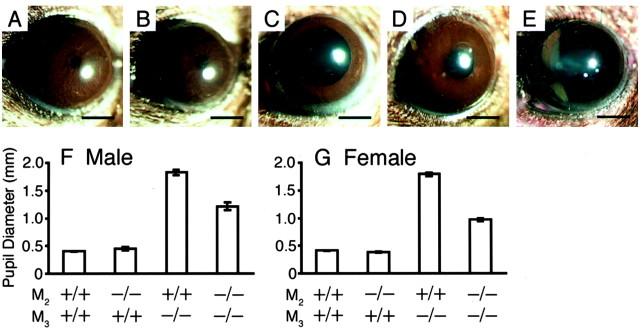
Muscarinic stimulation is known to cause strong constriction of the pupil (Gil et al., 2001). As reported previously (Matsui et al., 2000), the M3−/− mice showed partially dilated pupils (Fig. 5C). In contrast, the pupil size of the M2−/− mice looked normal (Fig. 5B), suggesting that M2 is dispensable for normal pupillary function. Strikingly, however, the M2−/−M3−/− mice had smaller pupils (Fig. 5D) than the M3−/− mice (Fig. 5C). These results suggest a role of M2 in dilation, rather than constriction, of pupils. An instillation of 1% atropine solution resulted in additional mydriasis (Fig. 5E), suggesting that M1, M4, and/or M5 receptors might play some additional roles in constricting the pupils in the M2−/−M3−/− mouse (see Discussion).
Fig. 5.
Appearance of pupils of the wild-type (A), M2−/− (B), M3−/− (C), and M2−/−M3−/− (D) mice. The pupils of the M2−/−M3−/− mice (D) are smaller than those of the M3−/− mice (C). Full mydriasis was achieved in the M2−/−M3−/− mouse after instillation of atropine solution (E). The pupils of the M2−/− mice (B) are indistinguishable from those of the wild-type mice (A). Scale bars, 1 mm. F,G, Pupil diameter (mean ± SEM;n = 4–30) of the mutant mice, measured in a bright room (∼1000 lux). The M2−/−M3−/− mice have smaller pupils than the M3−/− mice in both sexes.
DISCUSSION
Role of muscarinic receptor subtypes in cholinergic contractions
Whereas M3 is known to play a dominant role in eliciting smooth muscle contractions, the significance of other colocalizing subtypes has been understood poorly. Thus far, the following indirect roles of M2 have been suggested. (1) Relaxation of smooth muscles induced by isoproterenol could be reversed by the stimulation of M2through the inhibition of adenylate cyclase (Ehlert et al., 1999). (2) Stimulation of M2 may have a modulatory role on the intracellular signaling of M3 (Ehlert et al., 1999). Here, we demonstrated that M2 has considerable potency to contract the muscle “directly,” i.e., independent of the coexisting M3 receptors or accompanying stimuli to increase cAMP levels. From the response curves shown in Figure 3, we estimated the proportion of contribution by each subtype. Contributions by M1, M2, M3, M4, and M5 receptors were ∼0, 5, 95, 0, and 0%, respectively, in detrusor muscle contraction and ∼0, 25, 75, 0, and 0%, respectively, in ileal longitudinal muscle contraction. The mechanism of the direct contraction through M2 remains unclear, but it may involve the opening of nonselective cation channels (Kotlikoff et al., 1999).
In vivo significance of cholinergic contractions in visceral organs
In humans, systemic administration of atropine delays gastric emptying (Parkman et al., 1999b) and reduces colonic motility (O'Brien et al., 1997). Therefore, muscarinic antagonists, such as atropine, are used widely as a premedication to reduce gastrointestinal movements. However, an elaborate study (Waxman et al., 1991) showed the clinical efficacy of such anticholinergic pretreatment in colonoscopy to be limited, raising a question about the role of cholinergic signaling in smooth muscle contraction in vivo.
Loss of key molecules in gastrointestinal motility can lead to the apparent bowel obstruction in the relevant mutant mice, as exemplified by enlarged stomachs of the mice deficient in neuronal nitric oxide synthase (Huang et al., 1993). However, we found no such obstruction phenotype in the gastrointestinal or female urinary tracts, despite the complete loss of cholinergic contractions in these tissues. Thus, cholinergic contractions cannot be regarded as a prerequisite for the basal function of these organs. It is worth noting that the phenotypes are essentially identical between our gene-targeted mice and thein vivo effects caused by an acute pharmacological block of the muscarinic receptors (Hardman et al., 2001). Low doses of atropine cause dryness of the mouth, and moderate doses result in mydriasis. We reported a similar phenotype in the M3−/− mice (Matsui et al., 2000). The urinary disturbance and inhibition of peristalsis become evident at relatively high dosages. Consistent with the intact gut appearance of M2−/−M3−/− mutant mice, it was reported that acute administration of a high dose of atropine did not block gut motility completely (Ruwart et al., 1979; Calignano et al., 1992).
In the intestines and female bladder, neurotransmitters other than ACh should contribute significantly to the smooth muscle contractionsin vivo and compensate for the loss of cholinergic signaling sufficiently. In fact, neurotransmission in the enteric nervous system is characterized by functional redundancy through multiple parallel pathways (Goyal et al., 1996; Furness, 2000). In our EFS experiment, a substantial increase in noncholinergic (i.e., atropine-resistant) contraction was detected in the female M2−/−M3−/− tissues. These results suggest that excitatory inputs other than the cholinergic stimuli were upregulated in the mutant tissue and contributed to the functional compensation in vivo under certain conditions. In the intestines, such inputs may be mediated by tachykinins (Holzer and Holzer-Petsche, 1997) or novel neurotransmitters yet to be identified. In female urination, other parasympathetic neurotransmitters, such as purine (Burnstock et al., 1972), should be of primary importance, because neurotransmission blockade at the parasympathetic ganglion results in severe urinary retention (Xu et al., 1999a,b).
M2 as a pupil dilator
We found that the pupils of the M2−/−M3−/− mice are unexpectedly smaller than those of the M3−/− mice. It is worth noting that muscarinic autoreceptors inhibiting ACh release were found in the parasympathetic nerve terminals in the iris, and the subtype was presumed to be M2 (Bognar et al., 1990). Lack of such autoreceptors would result in ACh over-release. Because topical applications of atropine caused full mydriasis in the M3−/− (Matsui et al., 2000) and M2−/−M3−/− (Fig.5E) pupils, it is conceivable that the over-released ACh stimulated the residual subtypes (M1, M4, and/or M5) and caused the relative mydriasis. However, precise mechanisms of the phenomenon remain inconclusive, because atropine interferes with other receptors, such as histamine receptor H1 at high concentrations in the range of 1–10 μm(Arunlakshana and Schild, 1959). Because the actual concentration at the smooth muscle of the iris cannot be determined, it is uncertain whether the effects of atropine are selective only to the muscarinic receptors (1% solution is ∼0.03 m).
It should be noted that small amounts of M2 are expressed in other tissues of the iris, such as the pupillary sphincter muscle (Ishizaka et al., 1998) and the sympathetic nerve terminals at the dilator muscle (Jumblatt and Hackmiller, 1994). However, our results are unlikely to be caused by the effects in such tissues. It is also possible that loss of M2 altered cholinergic neurotransmission in the CNS and influenced the reflex control of the pupil. In fact, a tonic muscarinic inhibitory input to the Edinger-Westphal nucleus was found in the dog (Sharpe and Pickworth, 1981).
Finally, it may be speculated that other subtypes, particularly M1 and M5, were upregulated as a result of M2 loss and contributed to the relative miotic phenotype of the M2−/−M3−/− animals. However, this seems rather unlikely, because no compensatory changes in other subtypes have been found in any previous reports on muscarinic receptor-deficient mice (Hamilton et al., 1997; Gomeza et al., 1999a,b;Matsui et al., 2000; Yamada et al., 2001a,b).
Conclusion
We discovered that cholinergic contractions are not necessarily required for gastrointestinal or female urinary functions. These pieces of information should facilitate additional studies on other mediators and help establish a valid rationale for developing better drugs for diseases with autonomic dysregulation. Such diseases include urinary incontinence, irritable bowel syndrome, and posttraumatic stress disorder. Thus far, M2 receptors are considered to play only minor roles in smooth muscles, despite its abundant expression. Our gene-targeted mice should be useful to further unravel the functions of this subtype. In fact, we demonstrated an unexpected role of M2 in pupillary dilation that may help develop a novel class of muscarinic drugs for ocular diseases.
Footnotes
This research was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture (M.M., M.M.T.), by Industrial Technology Research Grant Programs in 2000 and 2002 from the New Energy and Industrial Technology Development Organization of Japan (M.M.), by a grant from the Organization for Pharmaceutical Safety and Research, Japan (M.M.T.), and by Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Science, Sports, Culture, and Technology of Japan (T.M.). We thank T. Tamai, T. Ishikawa, and K. Takaku for technical advice; A. M. Watabe and H. Morikawa for critical reading of this manuscript; I. Ishii, A. Matsunaga, and A. Yokoi for blastocyst injections; Y. Araki, S. Kobayashi, N. Matsubara, and H. Karasawa for technical assistance; and S. Ishikawa and his staff for animal care.
Correspondence should be addressed to Dr. Minoru Matsui, Division of Neuronal Network, Department of Basic Medical Sciences, The Institute of Medical Science, The University of Tokyo, 4-6-1 Shirokanedai, Minato-ku, Tokyo 108-8639, Japan. E-mail: mmatsui@dd.iij4u.or.jp.
REFERENCES
- 1.Andersson KE. The importance of the cholinergic system in neurourology. Eur Urol. 1998;34:6–9. doi: 10.1159/000052266. [DOI] [PubMed] [Google Scholar]
- 2.Arunlakshana O, Schild HO. Some quantitative uses of drug antagonists. Br J Pharmacol. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bognar IT, Wesner MT, Fuder H. Muscarine receptor types mediating autoinhibition of acetylcholine release and sphincter contraction in the guinea-pig iris. Naunyn Schmiedebergs Arch Pharmacol. 1990;341:22–29. doi: 10.1007/BF00195053. [DOI] [PubMed] [Google Scholar]
- 4.Boschero AC, Szpak-Glasman M, Carneiro EM, Bordin S, Paul I, Rojas E, Atwater I. Oxotremorine-m potentiation of glucose-induced insulin release from rat islets involves M3 muscarinic receptors. Am J Physiol. 1995;268:E336–E342. doi: 10.1152/ajpendo.1995.268.2.E336. [DOI] [PubMed] [Google Scholar]
- 5.Burnstock G, Dumsday B, Smythe A. Atropine-resistant excitation of the urinary bladder: possibility of transmission via nerves releasing a purine nucleotide. Br J Pharmacol. 1972;44:451–461. doi: 10.1111/j.1476-5381.1972.tb07283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calignano A, Capasso A, Persico P, Mancuso F, Sorrentino L. Dexamethasone modifies morphine-, atropine-, verapamil-induced constipation in mice. Gen Pharmacol. 1992;23:753–756. doi: 10.1016/0306-3623(92)90161-c. [DOI] [PubMed] [Google Scholar]
- 7.Caulfield MP, Birdsall NJM. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- 8.Choppin A, Stepan GJ, Loury DN, Watson N, Eglen RM. Characterization of the muscarinic receptor in isolated uterus of sham operated and ovariectomized rats. Br J Pharmacol. 1999;127:1551–1558. doi: 10.1038/sj.bjp.0702696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Groat WC, Yoshimura N. Pharmacology of the lower urinary tract. Annu Rev Pharmacol Toxicol. 2001;41:691–721. doi: 10.1146/annurev.pharmtox.41.1.691. [DOI] [PubMed] [Google Scholar]
- 10.Eglen RM. Muscarinic receptors and gastrointestinal tract smooth muscle function. Life Sci. 2001;68:2573–2578. doi: 10.1016/s0024-3205(01)01054-2. [DOI] [PubMed] [Google Scholar]
- 11.Eglen RM, Michel AD, Whiting RL. Characterization of the muscarinic receptor subtype mediating contractions of the guinea-pig uterus. Br J Pharmacol. 1989;96:497–499. doi: 10.1111/j.1476-5381.1989.tb11843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eglen RM, Choppin A, Watson N. Therapeutic opportunities from muscarinic receptor research. Trends Pharmacol Sci. 2001;22:409–414. doi: 10.1016/s0165-6147(00)01737-5. [DOI] [PubMed] [Google Scholar]
- 13.Ehlert FJ, Sawyer GW, Esqueda EE. Contractile role of M2 and M3 muscarinic receptors in gastrointestinal smooth muscle. Life Sci. 1999;64:387–394. doi: 10.1016/s0024-3205(98)00584-0. [DOI] [PubMed] [Google Scholar]
- 14.Furness JB. Types of neurons in the enteric nervous system. J Auton Nerv Syst. 2000;81:87–96. doi: 10.1016/s0165-1838(00)00127-2. [DOI] [PubMed] [Google Scholar]
- 15.Giglio D, Delbro DS, Tobin G. On the functional role of muscarinic M(2) receptors in cholinergic and purinergic responses in the rat urinary bladder. Eur J Pharmacol. 2001;428:357–364. doi: 10.1016/s0014-2999(01)01286-9. [DOI] [PubMed] [Google Scholar]
- 16.Gil D, Spalding T, Kharlamb A, Skjaerbaek N, Uldam A, Trotter C, Li D, WoldeMussie E, Wheeler L, Brann M. Exploring the potential for subtype-selective muscarinic agonists in glaucoma. Life Sci. 2001;68:2601–2604. doi: 10.1016/s0024-3205(01)01058-x. [DOI] [PubMed] [Google Scholar]
- 17.Gomeza J, Shannon H, Kostenis E, Felder C, Zhang L, Brodkin J, Grinberg A, Sheng H, Wess J. Pronounced pharmacologic deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA. 1999a;96:1692–1697. doi: 10.1073/pnas.96.4.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomeza J, Zhang L, Kostenis E, Felder C, Bymaster F, Brodkin J, Shannon H, Xia B, Deng C, Wess J. Enhancement of D1 dopamine receptor-mediated locomotor stimulation in M4 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA. 1999b;96:10483–10488. doi: 10.1073/pnas.96.18.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goyal RK, Hirano I. The enteric nervous system. N Engl J Med. 1996;334:1106–1115. doi: 10.1056/NEJM199604253341707. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton SE, Loose MD, Qi M, Levey AI, Hille B, McKnight GS, Idzerda RL, Nathanson NM. Disruption of the m1 receptor gene ablates muscarinic receptor-dependent M current regulation and seizure activity in mice. Proc Natl Acad Sci USA. 1997;94:13311–13316. doi: 10.1073/pnas.94.24.13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardman JG, Limbird LE, Gilman AG. Goodman and Gilman's the pharmacological basis of therapeutics, Ed 10. McGraw-Hill; New York: 2001. [Google Scholar]
- 22.Holzer P, Holzer-Petsche U. Tachykinins in the gut. I. Expression, release and motor function. Pharmacol Ther. 1997;73:173–217. doi: 10.1016/s0163-7258(96)00195-7. [DOI] [PubMed] [Google Scholar]
- 23.Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- 24.Ishiura Y, Yoshiyama M, Yokoyama O, Namiki M, de Groat WC. Central muscarinic mechanisms regulating voiding in rats. J Pharmacol Exp Ther. 2001;297:933–939. [PubMed] [Google Scholar]
- 25.Ishizaka N, Noda M, Yokoyama S, Kawasaki K, Yamamoto M, Higashida H. Muscarinic acetylcholine receptor subtypes in the human iris. Brain Res. 1998;787:344–347. doi: 10.1016/s0006-8993(97)01554-0. [DOI] [PubMed] [Google Scholar]
- 26.Jumblatt JE, Hackmiller RC. M2-type muscarinic receptors mediate prejunctional inhibition of norepinephrine release in the human iris-ciliary body. Exp Eye Res. 1994;58:175–180. doi: 10.1006/exer.1994.1005. [DOI] [PubMed] [Google Scholar]
- 27.Kotlikoff MI, Dhulipala P, Wang YX. M2 signaling in smooth muscle cells. Life Sci. 1999;64:437–442. doi: 10.1016/s0024-3205(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 28.Lepor H, Kuhar MJ. Characterization of muscarinic cholinergic receptor binding in the vas deferens, bladder, prostate and penis of the rabbit. J Urol. 1984;132:392–396. doi: 10.1016/s0022-5347(17)49635-2. [DOI] [PubMed] [Google Scholar]
- 29.Matsui M, Araki Y, Karasawa H, Matsubara N, Taketo MM, Seldin MF. Mapping of five subtype genes for muscarinic acetylcholine receptor to mouse chromosomes. Genes Genet Syst. 1999;74:15–21. doi: 10.1266/ggs.74.15. [DOI] [PubMed] [Google Scholar]
- 30.Matsui M, Motomura D, Karasawa H, Fujikawa T, Jiang J, Komiya Y, Takahashi S, Taketo MM. Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc Natl Acad Sci USA. 2000;97:9579–9584. doi: 10.1073/pnas.97.17.9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Brien MD, Camilleri M, Thomforde GM, Wiste JA, Hanson RB, Zinsmeister AR. Effect of cholecystokinin octapeptide and atropine on human colonic motility, tone, and transit. Dig Dis Sci. 1997;42:26–33. doi: 10.1023/a:1018868601475. [DOI] [PubMed] [Google Scholar]
- 32.Parkman HP, Pagano AP, Ryan JP. Subtypes of muscarinic receptors regulating gallbladder cholinergic contractions. Am J Physiol. 1999a;276:G1243–G1250. doi: 10.1152/ajpgi.1999.276.5.G1243. [DOI] [PubMed] [Google Scholar]
- 33.Parkman HP, Trate DM, Knight LC, Brown KL, Maurer AH, Fisher RS. Cholinergic effects on human gastric motility. Gut. 1999b;45:346–354. doi: 10.1136/gut.45.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ponec RJ, Saunders MD, Kimmey MB. Neostigmine for the treatment of acute colonic pseudo-obstruction. N Engl J Med. 1999;341:137–141. doi: 10.1056/NEJM199907153410301. [DOI] [PubMed] [Google Scholar]
- 35.Pontari MA, Luthin GR, Braverman AS, Ruggieri MR. Characterization of muscarinic cholinergic receptor subtypes in rat prostate. J Recept Signal Transduct Res. 1998;18:151–166. doi: 10.3109/10799899809047742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruwart MJ, Klepper MS, Rush BD. Evidence for noncholinergic mediation of small intestinal transit in the rat. J Pharmacol Exp Ther. 1979;209:462–465. [PubMed] [Google Scholar]
- 37.Sawyer GW, Ehlert FJ. Contractile roles of the M2 and M3 muscarinic receptors in the guinea pig colon. J Pharmacol Exp Ther. 1998;284:269–277. [PubMed] [Google Scholar]
- 38.Schmid SW, Modlin IM, Tang LH, Stoch A, Rhee S, Nathanson MH, Scheele GA, Gorelick FS. Telenzepine-sensitive muscarinic receptors on rat pancreatic acinar cells. Am J Physiol. 1998;274:G734–G741. doi: 10.1152/ajpgi.1998.274.4.G734. [DOI] [PubMed] [Google Scholar]
- 39.Sharpe LG, Pickworth WB. Pharmacologic evidence for a tonic muscarinic inhibitory input to the Edinger-Westphal nucleus in the dog. Exp Neurol. 1981;71:176–190. doi: 10.1016/0014-4886(81)90080-7. [DOI] [PubMed] [Google Scholar]
- 40.Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- 41.Stengel PW, Gomeza J, Wess J, Cohen ML. M2 and M4 receptor knockout mice: muscarinic receptor function in cardiac and smooth muscle in vitro. J Pharmacol Exp Ther. 2000;292:877–885. [PubMed] [Google Scholar]
- 42.Waxman I, Mathews J, Gallagher J, Kidwell J, Collen MJ, Lewis JH, Cattau EL, Jr, al-Kawas FH, Fleischer DE, Benjamin SB. Limited benefit of atropine as premedication for colonoscopy. Gastrointest Endosc. 1991;37:329–331. doi: 10.1016/s0016-5107(91)70725-6. [DOI] [PubMed] [Google Scholar]
- 43.Xu W, Gelber S, Orr-Urtreger A, Armstrong D, Lewis RA, Ou CN, Patrick J, Role L, De Biasi M, Beaudet AL. Megacystis, mydriasis, and ion channel defect in mice lacking the α3 neuronal nicotinic acetylcholine receptor. Proc Natl Acad Sci USA. 1999a;96:5746–5751. doi: 10.1073/pnas.96.10.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu W, Orr-Urtreger A, Nigro F, Gelber S, Sutcliffe CB, Armstrong D, Patrick JW, Role LW, Beaudet AL, De Biasi M. Multiorgan autonomic dysfunction in mice lacking the β2 and the β4 subunits of neuronal nicotinic acetylcholine receptors. J Neurosci. 1999b;19:9298–9305. doi: 10.1523/JNEUROSCI.19-21-09298.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yagi T, Ikawa Y, Yoshida K, Shigetani Y, Takeda N, Mabuchi I, Yamamoto T, Aizawa S. Homologous recombination at c-fyn locus of mouse embryonic stem cells with use of diphtheria toxin A-fragment gene in negative selection. Proc Natl Acad Sci USA. 1990;87:9918–9922. doi: 10.1073/pnas.87.24.9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamada M, Lamping KG, Duttaroy A, Zhang W, Cui Y, Bymaster FP, McKinzie DL, Felder CC, Deng CX, Faraci FM, Wess J. Cholinergic dilation of cerebral blood vessels is abolished in M5 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA. 2001a;98:14096–14101. doi: 10.1073/pnas.251542998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamada M, Miyakawa T, Duttaroy A, Yamanaka A, Moriguchi T, Makita R, Ogawa M, Chou CJ, Xia B, Crawley JN, Felder CC, Deng CX, Wess J. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature. 2001b;410:207–212. doi: 10.1038/35065604. [DOI] [PubMed] [Google Scholar]