Abstract
Hermissenda CSP24 (cytoskeletal-related protein 24) is a 24 kDa β-thymosin-like protein that is associated with intermediate memory. We showed previously that one-trial conditioning resulted in a significant increase in the phosphorylation of CSP24 detected in lysates of the pathway supporting the conditioned stimulus (CS). Here we report the association of the protein with the actin cytoskeleton and the distribution of CSP24-immunoreactive neurons in two sensory structures and the circumesophageal nervous system. Identified photoreceptors, hair cells, and neurons in the cerebropleural and pedal ganglia were immunoreactive for CSP24. Immunoprecipitation experiments with32PO4-labeled lysates of the circumesophageal nervous system identified a 44 kDa protein band (consistent with actin) that coprecipitates with CSP24. An analysis of immunoprecipitates on Western blots probed with anti-actin antibody also showed that actin coprecipitates with CSP24. Laser confocal microscopy of photoreceptors costained with fluorescently labeled anti-actin antibody and anti-CSP24 antibody, or fluorescent phalloidin and anti-CSP24 antibody showed that CSP24 is localized with actin in the cytosol of photoreceptor cell bodies and colocalized with presumed G-actin, but not F-actin, in regions adjacent to the plasma membrane. Although CSP24 is widely distributed in the Hermissenda nervous system, its regulation by one-trial conditioning was observed only in the CS pathway. Our findings suggest that CSP24 may interact with components of the actin cytoskeleton that contribute to structural changes underlying the formation and maintenance of enduring forms of memory.
Keywords: Hermissenda, pavlovian conditioning, cytoskeleton, actin, phosphorylation, β-thymosin repeat protein
One-trial conditioning ofHermissenda produces suppression of normal light-elicited locomotion (Crow and Forrester, 1986). One-trial conditioning and its analog (in vitro conditioning) also result in distinct stages of memory for enhanced excitability that are detected in identified sensory neurons of the conditioned stimulus pathway (CSP) (Crow and Forrester, 1990, 1993; Crow et al., 1991, 1997, 1999; Crow and Siddiqi, 1997). In addition to excitability changes, one-trialin vitro conditioning results in an increase in phosphorylation of several proteins in lysates of the CSP 1–2 hr after conditioning (Crow et al., 1996). One of the proteins designated as CSP24 (cytoskeletal-related protein 24), is a 24 kDa apparent molecular weight phosphoprotein that is associated with intermediate memory, distinct from short-term memory, produced by one-trial conditioning (Crow and Xue-Bian, 2000). Amino acid sequences of peptides derived from CSP24 exhibited varying degrees of sequence identity to the β-thymosin family of actin-binding proteins (Nachmias, 1993;Carpintero et al., 1995; Safer and Chowrashi, 1997; Stoeva et al., 1997) and of actin-binding proteins consisting of multiple β-thymosin repeats (Hertzog et al., 2002). Here we show the distribution of CSP24 immunoreactivity in sections of the circumesophageal nervous system using anti-CSP24 antibody. CSP24 was detected in neuronal cell bodies of identified photoreceptors, statocyst hair cells, and the cell bodies of neurons in both the cerebropleural (CP) and pedal (P) ganglia and in lysates of both the CP and P ganglia. In addition, immunoreactivity was detected in the neuropil of both ganglia, including the area proximal to photoreceptor somas in which the optic nerve forms before entering into the CP ganglion. The association of CSP24 with actin was indicated by results showing the coimmunoprecipitation of CSP24 and actin from lysates of the CSP and circumesophageal nervous system. In addition, double-fluorescent labeling of CSP24 and actin or CSP24 and phalloidin in conjunction with confocal microscopy revealed that CSP24 is localized with actin in the cytoplasm of photoreceptor cell bodies and colocalized with presumed G-actin, but not F-actin, in regions of the cytoplasm near the plasma membrane. A statistically significant increase in phosphorylation of CSP24 immunoprecipitates relative to unpaired controls was observed after in vitro conditioning only in the CSP compared with immunoprecipitates from either the P or CP ganglia.
MATERIALS AND METHODS
One-trial in vitro conditioning. AdultHermissenda crassicornis (Sea Life Supply, Sand City, CA) were maintained in artificial sea water (ASW) aquaria at 14 ± 1°C on a 12 hr light/dark cycle. The one-trial in vitroconditioning procedure has been described in detail previously (Crow et al., 1991, 1996, 1997) and will be discussed only briefly in this report. The conditioning trial consisted of a 5 min presentation of light, the CS (10−4W/cm2) paired with the application of serotonin (5-HT) to the isolated circumesophageal nervous system. The final concentration of 5-HT in the ASW was 10−4m. Unpaired control groups received the CS and 5-HT (10−4m) separated by 5 min. For the unpaired control group, the 5-HT was applied in the dark (infrared illumination) and washed out after the 5 min exposure. Fifteen minutes after the conditioning trial, the circumesophageal nervous system was isolated into three areas consisting of components of the CSP (eye and proximal optic nerve), the CP ganglia, the P ganglia, and was prepared for32PO4 labeling of proteins. In vitro conditioning involved five independent replications for the conditioned and unpaired procedures with three nervous systems in each sample.
Protein phosphorylation and immunoprecipitation. Protein phosphorylation after one-trial in vitro conditioning was examined in immunoprecipitates of the CSP, the CP and P ganglia. The preparations were incubated for 2 hr in 200 μl of oxygenated ASW containing 11 mm glucose and 0.125 mCi of32PO4 (carrier-free; NEN, Boston, MA). After the 2 hr incubation, the samples were rinsed with PBS and lysed in ice-cold lysis buffer [radioiummunoprecipitation assay (RIPA) buffer–PBS, 1% NP-40, 0.5% deoxycholate, 0.1% SDS, 0.1 mg/ml 4-(2-aminoethyl)-benzenesulfonylfluoride (Calbiochem, La Jolla, CA), 0.6 U/ml aprotinin, and 1 mm sodium orthovanadate]. All steps were conducted at 4°C. Lysates were centrifuged for 20 min, and supernatants were incubated with rabbit polyclonal anti-CSP24 for 1 hr. Protein A/G-agarose (Santa Cruz Biotechnology, Santa Cruz, CA) was then added for overnight incubation with rotation. Immunoprecipitates were collected by centrifugation for 10 min in a microfuge, and the agarose pellets were carefully resuspended and washed in the RIPA buffer four times. The washed pellets were then rinsed two additional times with the sample buffer described above. After the final wash, 40 μl of SDS sample buffer (0.5 M Tris, 23% SDS, 10% glycerol, and 5% β mercaptoethanol) was added to the agarose pellet and boiled for 3 min. After boiling and centrifugation, samples were loaded for one-dimensional (1-D) PAGE. Gels containing32PO4-labeled proteins were exposed to storage phosphor screens for a period of 24 hr. Phosphor screens were computer scanned and analyzed using ImageQuant software (Molecular Dynamics, Sunnyvale, CA) for quantitative analysis. Densitometric analysis of Coomassie blue or SYPRO Ruby-stained CSP24 from immunoprecipitates and 44 kDa bands from lysates provided for normalization of32PO4 levels of CSP24 for conditioned groups and unpaired controls. Therefore, differences in 32PO4incorporation were not attributable to between-group differences in protein loading or amount of precipitated protein.
Identification of actin on Western blots involved lysates of the circumesophageal nervous system or CSP resolved in SDS gels and transferred to polyvinylidene difluoride (PVDF) membranes that were probed with mouse anti-actin monoclonal antibody (Santa Cruz Biotechnology). Coimmunoprecipitation experiments involved immunoprecipitation of lysates with anti-CSP24 antibody. In some experiments,32PO4-labeled immunoprecipitates from preparations exposed to 10−4m 5-HT (15 min) were resolved with 1-D PAGE, and storage phosphor screens were scanned for coprecipitated phosphoprotein bands. The immunoprecipitates were resolved as described or, for some experiments in SDS nonreducing gels (without β-mercaptoethanol), transferred to PVDF membranes and probed with anti-actin antibody. Use of the nonreducing gels resulted in a shift of the heavy and light chains of the antibody to a higher molecular weight that did not interfere with the detection of the 44 kDa protein band. The immunocomplexes were detected with enhanced chemiluminescence reagent (Amersham Biosciences, Piscataway, NJ) following the procedures of the manufacturer.
Immunohistochemistry. Circumesophageal nervous systems were removed and fixed in 4% paraformaldehyde in 0.1 m PBS with 30% sucrose overnight at 4°C. Nervous systems were rinsed in 30% sucrose in 0.1 m PBS, and frozen preparations were sectioned on a cryostat at a nominal thickness of 16 μm. Slides were air dried for 1–2 hr at room temperature, followed by incubation with 0.3% Triton X-100 in 0.1 m PBS for 10 min. Sections were incubated at 4°C overnight in the primary antibody (anti-CSP24 at 1:500) in the blocking solution (3% normal goat serum and 0.3% Triton X-100 in 0.1 m PBS). Slides were incubated in the secondary antibody consisting of biotinylated anti-rabbit IgG for anti-CSP24 for 1 hr at room temperature, followed by incubation in ABC-HRP or ABC-alkaline phosphatase (Vector Laboratories, Burlingame, CA) and exposure to 0.05% DAB or Vector Red. Slides were rinsed in PBS, dehydrated in increasing concentrations of ethanol, cleared in xylene, and mounted. Control sections were processed and photographed under identical conditions, with the exception of exposure to the primary antibody. For double labeling of CSP24 and actin, slides were incubated in the primary antibody (anti-CSP24 or anti-actin), followed by incubation in the secondary antibody consisting of biotinylated anti-rabbit IgG for anti-CSP24 or anti-mouse IgG for anti-actin, washed in PBS, followed by exposure to streptavidin Alexa 488 or 594. For detection of F-actin, slides were incubated with phalloidin Alexa Fluor 488 conjugate for F-actin and streptavidin Alexa Fluor 594 conjugate for anti-CSP24 (Molecular Probes, Eugene, OR). Double-labeled sections were viewed at 60 or 100× magnification on anOlympus Optical (Tokyo, Japan) BX-50 upright microscope with attached epifluorescence. A serial stack (z-plane) of images with 0.25μm steps between images were collected using a three-line, laser scanning confocal microscope (Radiance 2000 system; Bio-Rad, Hercules, CA). The sample fluorophores were stimulated at 488 and 568 nm using a mixed-gas krypton–argon laser. At the completion of image acquisition, the image stack was converted into a three-dimensional projection using Bio-Rad LaserSharp 2000 software.
RESULTS
CSP24 immunoreactivity in the circumesophageal nervous system
We examined sections of the circumesophageal nervous system ofHermissenda to determine the distribution of CSP24. Immunoreactive neurons were detected in the CP and P ganglia. Consistent with the identification of CSP24 in Western blots of lysates of components of the CSP (Crow and Xue-Bian, 2000), immunoreactivity was observed in identified photoreceptors of the eye (Fig.1A, double arrows). As shown in Figure 1, immunoreactivity was observed in cell bodies of statocyst hair cells, (single arrow) and in both the cytoplasm of neuronal cell bodies and neuropil of the CP and P ganglia. The immunoreactivity in the neuropil most likely represents labeling along the numerous neuronal processes. Figure 1 shows sections of immunoreactive cell bodies in the pedal (top arrow) and pleural ganglia (bottom arrow). Adjacent control sections that were exposed to the secondary, but not primary, antibody did not exhibit immunoreactive labeling (Fig.1B). Higher magnification of the eye revealed immunoreactivity in the cytoplasm of a lateral type B photoreceptor (Fig. 1C1, right arrow) and medial type B photoreceptor (Fig. 1C1, left arrow). In addition, immunoreactivity was observed in the base region of the eye, which may indicate labeling of photoreceptor axons converging to form the proximal segment of the optic nerve before entry into the cerebropleural ganglion (Fig. 1C1, arrow). As shown in Figure 1C2, an adjacent section of the eye exposed to the secondary, but not primary, antibody did not exhibit labeling. The results of the immunohistochemistry revealed that CSP24 is widely distributed throughout the Hermissenda nervous system and in the primary sensory neurons of the two central sensory organs (eyes and statocysts).
Fig. 1.
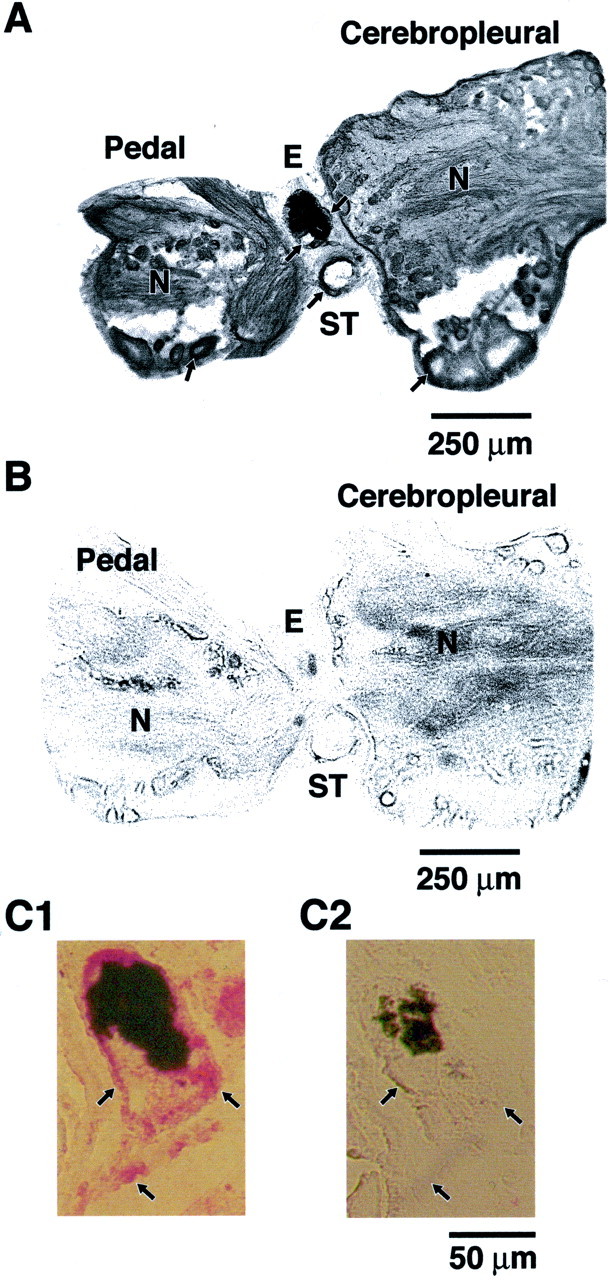
CSP24 is widely distributed in sensory structures and circumesophageal nervous system. CSP24 immunoreactivity in sections of the eye, statocyst, and circumesophageal nervous system.A, Immunoreactivity is present in neuronal cell bodies in the P and CP ganglion (arrows) and in the neuropil (N) of both ganglia. Immunoreactivity is also present in the perimeter region of the statocyst (ST;arrow). Photoreceptors in the eye (E) exhibit immunoreactivity as indicated by thetwo arrows on each side of the eye, indicating the location of identified type B photoreceptors. B, Control section adjacent to the section shown in A was exposed to secondary, but not primary, antibody. C1, Higher magnification of an eye section showing CSP24 immunoreactivity (red label) in the area of two type B photoreceptors (arrows) and the region of the optic nerve proximal to the base of the eye (arrow).C2, Control section adjacent to sections shown inC1 that was exposed to the secondary, but not primary, antibody does not show labeling. Arrows indicate location of B photoreceptors and proximal optic nerve. The black area in each section of the eye is screening pigment.
CSP24 is associated with the actin cytoskeleton
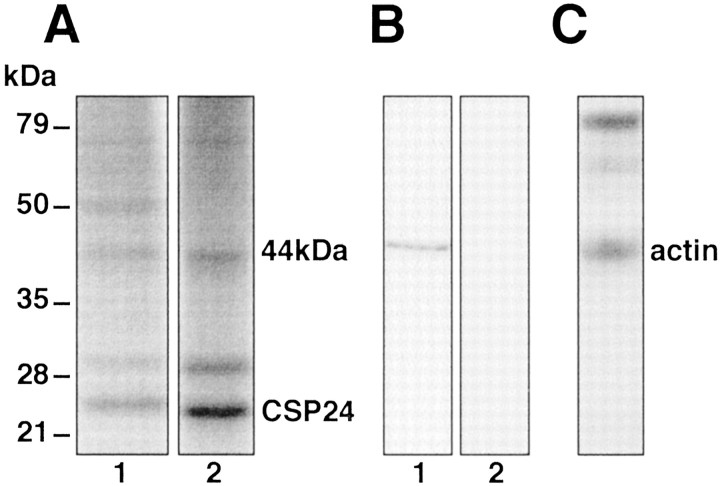
We examined the relationship between CSP24 and actin using coimmunoprecipitation procedures and colabeling of CSP24 and actin. The 1-D gels in Figure 2Ashow 32PO4incorporation into proteins in lysates from preparations exposed to 10−4m 5-HT (15 min) (lane 1). The32PO4-labeled immunoprecipitates revealed a 44 kDa protein band (consistent with actin), CSP24, and its 29 kDa splice variant (lane 2). To verify that the 44 kDa protein band contained actin, lysates of the CSP were resolved in SDS-PAGE, transferred to PVDF membranes, and exposed to an anti-actin antibody. As shown in Figure 2B, the anti-actin antibody recognized a single 44 kDa protein band on Western blots. The detection of the 44 kDa protein band was blocked by preabsorption with the peptide antigen (blocking peptide) (Fig.2B, lane 2). Because a 44 kDa phosphoprotein band coimmunoprecipitates with CSP24, we next determined whether immunoprecipitation of CSP24 with anti-CSP24 antibody would result in the coprecipitation of actin. A Western blot from a nonreducing gel of anti-CSP24 immunoprecipitates probed with an anti-actin antibody shows that actin coimmunoprecipitates with CSP24 (Fig. 2C).
Fig. 2.
Association of actin and CSP24 in components of the CSP. A, 32PO4 incorporation into proteins in a sample of lysates from preparations exposed to 10−4m 5-HT (lane 1).32PO4 incorporation into anti-CSP24 immunoprecipitates revealed a 44 kDa protein band that coprecipitated with CSP24 and the 29 kDa splice variant of CSP24 (lane 2). B, Anti-actin antibody recognized a single 44 kDa protein band on Western blots (lane 1). The detection of the 44 kDa protein was blocked by preabsorption exposure with the peptide antigen (blocking peptide) on the Western blot (lane 2). C, Coimmunoprecipitation of actin and CSP24 shown by the blot generated from a nonreducing gel of anti-CSP24 immunoprecipitates probed with anti-actin antibody. Western blot probed with anti-actin antibody detected a 44 kDa protein band (actin) from anti-CSP24 immunoprecipitates without exposure to a reducing agent (β-mercaptoethanol). The dark bands at higher molecular weight represent the antibody used for immunoprecipitation.
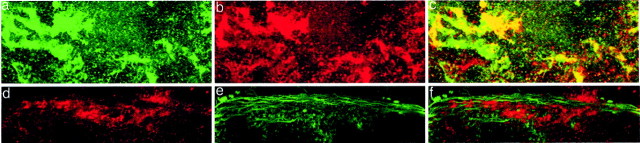
We next examined the colocalization of actin and CSP24 in confocal images generated from sections of the eye using double labeling with streptavidin Alexa 488 conjugate for anti-CSP24 and streptavidin Alexa 594 for anti-actin, or streptavidin Alexa 594 conjugate for anti-CSP24 and phalloidin Alexa 488 conjugate for F-actin. Figure3 shows confocal images of immunofluorescent labeling of CSP24 (green) and actin (red) and fluorescent labeling of CSP24 (red) and F-actin (green) in identified photoreceptors within the eye. Labeling of both CSP24 and actin was detected in the cytoplasm of photoreceptor cell bodies. The merged images shown in Figure3c show regions of colocalization of CSP24 and presumed G-actin denoted by yellow–orange. In contrast, colocalization of CSP24 and F-actin was not detected in the merged image of fluorescently labeled phalloidin and CSP24 (Fig.3f).
Fig. 3.
CSP24 and actin are colocalized in type B photoreceptors. Confocal images of CSP24 and actin in sections of a B photoreceptor. a, Immunofluorescent-labeled anti-CSP24; streptavidin, Alexa Fluor 488 conjugate (green).b, Immunofluorescent-labeled anti-actin; streptavidin, Alexa Fluor 594 conjugate (red). c, Merged images show CSP24 and presumed G-actin localized in the photoreceptor cytoplasm and in regions of colocalization adjacent to the plasma membrane. d, Immunofluorescent-labeled anti-CSP24; streptavidin, Alexa Fluor 594 conjugate (red). e, F-Actin labeling; phalloidin Alexa Fluor 488 conjugate (green).f, Merged image shows that CSP24 and F-actin do not colocalize. All images are magnified at 100×.
One-trial conditioning regulates phosphorylation of CSP24 in components of the CS pathway
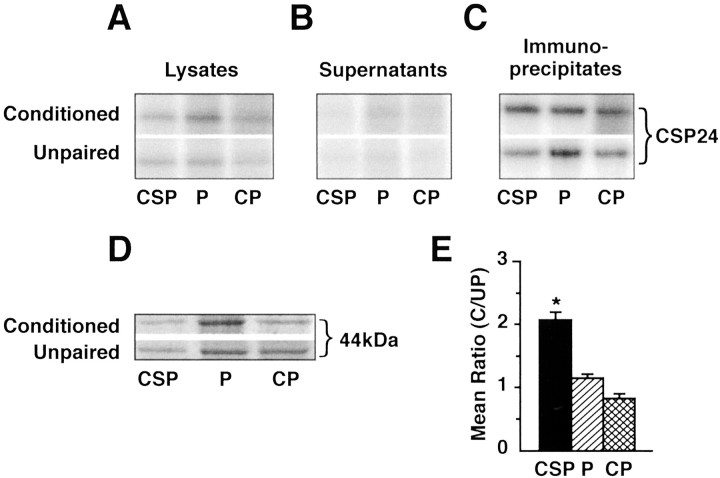
Because CSP24 is widely distributed throughout the circumesophageal nervous system, its regulation by one-trial conditioning may occur in regions other than the CS pathway. We examined this issue by determining whether one-trial in vitro conditioning produced increased phosphorylation of CSP24 in immunoprecipitates of the P or CP ganglia. The prints in Figure4 from a storage phosphor screen shows32PO4-labeled CSP24 from lysates (A), supernatants (B), and immunoprecipitates (C) of the CSP, P, and CP ganglia of conditioned animals and unpaired controls. The analysis of immunoprecipitates shown in Figure 4C revealed more32PO4 labeling of CSP24 from the CSP in the conditioned group compared with the unpaired controls. The examination of the supernatants revealed that immunoprecipitation with anti-CSP24 antibody dramatically reduced labeling of CSP24 from the CSP, P, and CP ganglia of both groups (Fig.4B). Figure 4D shows an example of Coomassie blue-stained 44 kDa bands from CSP, P, and CP lysates of conditioned and unpaired controls, indicating similar loading of protein for conditioned (C) and unpaired controls (UP). The group data depicting the mean ratio (C/UP) of densitometric measurements for CSP24 is shown in Figure 4E. The one-way ANOVA revealed an overall difference between the groups (F(2,12) = 31.4; p<0.001). Multiple comparisons using Tukey's tests showed that the CSP samples were significantly different from the CP samples (q = 10.7; p < 0.05) and the P samples (q = 8.2; p < 0.05), whereas the CP and P samples were not different from each other. An analysis of the ratios showed that the difference between conditioned and unpaired controls in 32PO4incorporation in the immunoprecipitates of the CSP was statistically significant (t(4) = 6.2; p<0.003). In contrast, the C/UP ratios of32PO4 incorporation in the immunoprecipitates from the CP ganglia and P ganglia were not significantly different. These results show that the increased phosphorylation of CSP24 by one-trial in vitro conditioning is specific to components of the CS pathway.
Fig. 4.
One-trial conditioning regulates CSP24 phosphorylation in immunoprecipitates of the CSP (eyes and proximal optic nerve). A, 32PO4incorporation into CSP24 in lysates from the in vitroconditioned group and unpaired controls. B,32PO4 incorporation into CSP24 of the supernatants from the same groups as shown in A. Immunoprecipitation with anti-CSP24 reduced labeling of CSP24 in the supernatants of all groups compared with the lysates. C,32PO4 incorporation into anti-CSP24 immunoprecipitates from the conditioned group and unpaired controls.D, Examples of Coomassie blue-stained 44 kDa protein bands from the CSP, P, and CP lysates of conditioned and unpaired controls. The examples show approximately equal loading of protein for the experimental and control groups. E, Group data showing mean ± SE. C/UP ratios of densitometric measurements for CSP24 from the immunoprecipitates. One-trial conditioning resulted in a significant increase in 32PO4 incorporation in the immunoprecipitates from the CSP compared with the unpaired control group CSP; *p < 0.003. However, the P and CP immunoprecipitates were not significantly different from their respective unpaired controls or each other.
DISCUSSION
We showed that CSP24, a protein identified previously with intermediate memory after one-trial conditioning, is distributed throughout the circumesophageal nervous system, including identified type B photoreceptors within the eyes. In addition, CSP24 immunoreactivity was observed in the cytoplasm of P and CP neuronal cell bodies and in the neuropil, suggesting some labeling of neuronal processes. Immunoprecipitation experiments revealed that one-trialin vitro conditioning resulted in a statistically significant increase in32PO4 labeling of immunoprecipitated CSP24 from the CSP compared with immunoprecipitates from unpaired control samples. However, conditioning did not significantly increase the phosphorylation of CSP24 in immunoprecipitates of CP or P ganglia relative to unpaired controls. Thus, whereas CSP24 is widely distributed in the circumesophageal nervous system, its significantly increased phosphorylation by one-trial conditioning is found only in the CSP.
We were initially drawn to study the relationship between CSP24 and the actin cytoskeleton because CSP24 exhibits a sequence homology to the β-thymosin family of actin-binding proteins and actin-binding proteins with β-thymosin repeats (Crow and Xue-Bian, 2000). Our immunoprecipitation experiments showed that actin coimmunoprecipitates with CSP24. In addition, we showed, using confocal microscopy of images of fluorescently labeled CSP24 and actin from sections of the eye, that the CSP24 is colocalized with presumed monomeric actin in the cytoplasm of type B photoreceptor cell bodies. In contrast, the analysis of sections costained for CSP24 and phalloidin-labeled F-actin indicated that CSP24 did not colocalize with F-actin. Studies of regenerating retinal ganglion cells have shown that growth cones and varicosities that are strongly β-thymosin positive exhibit weak F-actin labeling (Roth et al., 1999).
The assembly and disassembly of actin filaments regulated by extracellular signals may play a role in cellular and synaptic plasticity (for review, see Fifkova and Morales, 1992; Halpain, 2000). Studies of changes in the morphology of dendritic spines has been implicated in examples of learning and synaptic plasticity. Actin-dependent shape changes of dendritic spines can be inhibited by activation of glutamate receptors (Fischer et al., 2000), and morphological changes in Aplysia mechanoreceptors associated with 5-HT-induced long-term facilitation can be blocked by cytochalasin D (Hatada et al., 2000). A potential role for actin filament in hippocampal long-term potentiation (LTP) has been proposed (Kim and Lisman, 1999), and trafficking of AMPA receptors associated with synaptic plasticity involves the actin cytoskeleton (Zhou et al., 2000). In addition, blocking the expression of a cytoskeletal associated protein (Arc) in the hippocampus impairs the maintenance of LTP and memory consolidation for a spatial learning task (Guzowski et al., 2000).
Many of the examples of synaptic and cellular plasticity underlying learning involve specific temporal components that can be dissociated based on the role of signal transduction pathways, protein synthesis, and gene induction (for review, see DeZazzo and Tully, 1995). The actin cytoskeleton could be a key contributor to the maintenance of cellular and synaptic plasticity underlying specific stages in the consolidation of memory. To perform functions supporting cellular plasticity and memory, the organization of the actin cytoskeleton requires both temporal and spatial regulation by proteins. The activity of these proteins is modulated by intracellular signals that recruit actin nucleation and polymerization to specific cellular sites (Schmidt and Hall, 1998). In examples of cellular motility, actin filament assembly and turnover is regulated by a diverse array of proteins (Higgs and Pollard, 2001). However, all known vertebrate and invertebrate β-thymosins bind actin monomers and promote disassembly of actin filaments (Nachmias, 1993; Safer and Chowrashi, 1997), although recent evidence suggests that β-thymosins are not just simple actin-buffering proteins (Sun et al., 1996). Homologs of the family of actin-binding proteins that consist of β-thymosin repeats are functionally similar to profilin because their complex with G-actin participates in filament barbed end growth (Hertzog et al., 2002). A number of actin-regulating proteins are directly controlled by second messengers. Profilin is phosphorylated by protein kinase C (Hansson et al., 1988), gelsolin is regulated by Ca2+and membrane polyphosphoinositides (Matsudaira and Janmey, 1988), and cofilin is regulated by Rac and LIM (Lin-11, Isl-1, and Mec-3) kinase 1 (Arber et al., 1998). Regulation of actin-binding proteins in the CSP by one-trial conditioning may amplify the effect of filament uncapping by creating a reservoir of G-actin that can be desequestered to supply actin to filament ends and facilitate assembly contributing to structural remodeling.
Footnotes
This research was supported by National Institutes of Health Grant MH40860 (T.C.). We thank R. Grill for assistance with the confocal microscopy, A. Bean and N. Waxham for helpful discussions, and D. Parker for typing this manuscript.
Correspondence should be addressed to T. Crow, Department of Neurobiology and Anatomy, University of Texas Medical School, Houston, TX 77225. E-mail: terry.crow@uth.tmc.edu.
REFERENCES
- 1.Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- 2.Carpintero P, Anadón R, Franco del Amo F, Gómez-Márquez J. The thymosin β4 gene is strongly activated in neural tissues during early postimplantation mouse development. Neurosci Lett. 1995;184:63–66. doi: 10.1016/0304-3940(94)11169-j. [DOI] [PubMed] [Google Scholar]
- 3.Crow T, Forrester J. Light paired with serotonin mimics the effects of conditioning on phototactic behavior in Hermissenda. Proc Natl Acad Sci USA. 1986;83:7975–7978. doi: 10.1073/pnas.83.20.7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crow T, Forrester J. Inhibition of protein synthesis blocks long-term enhancement of generator potentials produced by one-trial in vivo conditioning in Hermissenda. Proc Natl Acad Sci USA. 1990;87:4490–4494. doi: 10.1073/pnas.87.12.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crow T, Forrester J. Down-regulation of protein kinase C and kinase inhibitors dissociate short- and long-term enhancement produced by one-trial conditioning of Hermissenda. J Neurophysiol. 1993;69:636–641. doi: 10.1152/jn.1993.69.2.636. [DOI] [PubMed] [Google Scholar]
- 6.Crow T, Siddiqi V. Time-dependent changes in excitability after one-trial conditioning of Hermissenda. J Neurophysiol. 1997;78:3460–3464. doi: 10.1152/jn.1997.78.6.3460. [DOI] [PubMed] [Google Scholar]
- 7. Crow T, Xue-Bian JJ. Identification of a 24 kDa phosphoprotein associated with an intermediate stage of memory in Hermissenda. J Neurosci 20 2000. RC74(1–5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crow T, Forrester J, Williams M, Waxham MN, Neary JT. Down-regulation of protein kinase C blocks 5-HT-induced enhancement in Hermissenda B photoreceptors. Neurosci Lett. 1991;12:107–110. doi: 10.1016/0304-3940(91)90660-l. [DOI] [PubMed] [Google Scholar]
- 9.Crow T, Siddiqi V, Zhu Q, Neary JT. Time-dependent increases in protein phosphorylation following one-trial enhancement in Hermissenda. J Neurochem. 1996;66:1736–1741. doi: 10.1046/j.1471-4159.1996.66041736.x. [DOI] [PubMed] [Google Scholar]
- 10.Crow T, Siddiqi V, Dash PK. Long-term enhancement but not short-term in Hermissenda is dependent upon mRNA synthesis. Neurobiol Learn Mem. 1997;68:340–347. doi: 10.1006/nlme.1997.3779. [DOI] [PubMed] [Google Scholar]
- 11.Crow T, Xue-Bian JJ, Siddiqi V. Protein synthesis-dependent and mRNA synthesis-independent intermediate phase of memory in Hermissenda. J Neurophysiol. 1999;82:495–500. doi: 10.1152/jn.1999.82.1.495. [DOI] [PubMed] [Google Scholar]
- 12.DeZazzo J, Tully T. Dissection of memory formation from behavioral pharmacology to molecular genetics. Trends Neurosci. 1995;18:212–218. doi: 10.1016/0166-2236(95)93905-d. [DOI] [PubMed] [Google Scholar]
- 13.Fifkova E, Morales M. Actin matrix of dendritic spines, synaptic plasticity, and long-term potentiation. Int Rev Cytol. 1992;139:267–307. doi: 10.1016/s0074-7696(08)61414-x. [DOI] [PubMed] [Google Scholar]
- 14.Fischer M, Kaech S, Wagner V, Brinkhaus H, Matus A. Glutamate receptors regulate actin-based plasticity in dendritic spines. Nat Neurosci. 2000;3:887–894. doi: 10.1038/78791. [DOI] [PubMed] [Google Scholar]
- 15.Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halpain S. Actin and the agile spine: how and why do dendritic spines dance? Trends Neurosci. 2000;23:141–146. doi: 10.1016/s0166-2236(00)01576-9. [DOI] [PubMed] [Google Scholar]
- 17.Hansson A, Skoglund G, Lassing I, Linberg U, Ingelman-Sundberg M. Protein kinase C-dependent phosphorylation of profilin is specifically stimulated by phosphatidylinositol bisphosphate (PIP2). Biochem Biophys Res Commun. 1988;150:526–531. doi: 10.1016/0006-291x(88)90425-1. [DOI] [PubMed] [Google Scholar]
- 18.Hatada Y, Wu F, Sun Z-Y, Schacher S, Goldberg DJ. Presynaptic morphological changes associated with long-term synaptic facilitation are triggered by actin polymerization at preexisting varicosities. J Neurosci. 2000;20:1–5. doi: 10.1523/JNEUROSCI.20-13-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hertzog M, Yarmola EG, Didry D, Bubb MR, Carlier M-F. Control of actin dynamics by proteins made of β-thymosin repeats: the actobindin family. J Biol Chem. 2002;277:14786–14792. doi: 10.1074/jbc.M112064200. [DOI] [PubMed] [Google Scholar]
- 20.Higgs HN, Pollard TD. Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins. Annu Rev Biochem. 2001;70:649–676. doi: 10.1146/annurev.biochem.70.1.649. [DOI] [PubMed] [Google Scholar]
- 21.Kim C-H, Lisman J. A role of actin filament in synaptic transmission and long-term potentiation. J Neurosci. 1999;19:4314–4324. doi: 10.1523/JNEUROSCI.19-11-04314.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsudaira P, Janmey P. Pieces in the actin-severing protein puzzle. Cell. 1988;54:139–140. doi: 10.1016/0092-8674(88)90542-9. [DOI] [PubMed] [Google Scholar]
- 23.Nachmias VT. Small actin-binding proteins: the β-thymosin family. Curr Opin Cell Biol. 1993;5:56–62. doi: 10.1016/s0955-0674(05)80008-0. [DOI] [PubMed] [Google Scholar]
- 24.Roth LWA, Bormann P, Bonnet A, Reinhard E. β-thymosin is required for axonal tract formation in developing zebrafish brain. Development. 1999;126:1365–1374. doi: 10.1242/dev.126.7.1365. [DOI] [PubMed] [Google Scholar]
- 25.Safer D, Chowrashi PK. β-thymosins from marine invertebrates: primary structure and interaction with actin. Cell Motil Cytoskel. 1997;38:163–171. doi: 10.1002/(SICI)1097-0169(1997)38:2<163::AID-CM5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt A, Hall MN. Signaling to the actin cytoskeleton. Annu Rev Cell Dev Biol. 1998;14:305–338. doi: 10.1146/annurev.cellbio.14.1.305. [DOI] [PubMed] [Google Scholar]
- 27.Stoeva S, Hörger S, Voelter W. A novel β-thymosin from the Sea Urchin: extending the phylogenetic distribution of β-thymosins from mammals to echinoderms. J Pept Sci. 1997;3:282–290. doi: 10.1002/(SICI)1099-1387(199707)3:4%3C282::AID-PSC119%3E3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 28.Sun H-Q, Kwiatkowska K, Yin HL. β-thymosins are not simple actin monomer buffering proteins. J Biol Chem. 1996;271:9223–9230. [PubMed] [Google Scholar]
- 29.Zhou Q, Xiao M-Y, Nicoll R. Contribution of cytoskeleton to the internalization of AMPA receptors. Proc Natl Acad Sci USA. 2000;98:1261–1266. doi: 10.1073/pnas.031573798. [DOI] [PMC free article] [PubMed] [Google Scholar]