Abstract
In Drosophila melanogaster, ebony andtan, two cuticle melanizing mutants, regulate the conjugation (ebony) of β-alanine to dopamine or hydrolysis (tan) of the β-alanyl conjugate to liberate dopamine. β-alanine biosynthesis is regulated byblack. ebony and tan also exert unexplained reciprocal defects in the electroretinogram, at ON and OFF transients attributable to impaired transmission at photoreceptor synapses, which liberate histamine. Compatible with this impairment, we show that both mutants have reduced histamine contents in the head, as measured by HPLC, and have correspondingly reduced numbers of synaptic vesicles in their photoreceptor terminals. Thus, the histamine phenotype is associated with sites of synaptic transmission at photoreceptors. We demonstrate that when they receive microinjections into the head, wild-type Sarcophaga bullata (in whose larger head such injections are routinely possible) rapidly (<5 sec) convert exogenous [3H]histamine into its β-alanine conjugate, carcinine, a novel metabolite. Drosophila tan has an increased quantity of [3H]carcinine, the hydrolysis of which is blocked; ebony lacks [3H]carcinine, which it cannot synthesize. Confirming these actions, carcinine rescues the histamine phenotype ofebony, whereas β-alanine rescues the carcinine phenotype of black;tan double mutants. The equilibrium ratio between [3H]carcinine and [3H]histamine after microinjecting wild-typeSarcophaga favors carcinine hydrolysis, increasing to only 0.5 after 30 min. Our findings help resolve a longstanding conundrum of the involvement of tan andebony in photoreceptor function. We suggest that reversible synthesis of carcinine occurs in surrounding glia, serving to trap histamine after its release at photoreceptor synapses; subsequent hydrolysis liberates histamine for reuptake.
Keywords: HPLC; carcinine (β-alanyl histamine); histamine; neurotransmitter action, termination; Drosophila melanogaster; Sarcophaga bullata; mutant, black; transmitter precursor; β-alanyl conjugation
Neurotransmitter released from a presynaptic terminal is removed from the synaptic cleft by mechanisms that include metabolic breakdown, as by acetylcholinesterase at cholinergic synapses (Massoulié et al., 1993) with subsequent uptake of choline into the terminal (Blusztajn and Wurtman, 1983), or reuptake of the transmitter molecule itself, as for noradrenaline (Iversen, 1971) and many other amines and fast transmitters, by means of transporters (Amara and Arriza, 1993). These mechanisms assume particular importance at high-output synapses, such as at photoreceptors, where transmitter output is tonic (Juusola et al., 1996; Witkovsky et al., 2001), even in the dark (Dowling and Ripps, 1973; Uusitalo et al., 1995). Histamine is a transmitter at photoreceptor synapses of the compound eye (Hardie, 1987; Callaway and Stuart, 1989, 1999), synthesized from histidine (Morgan et al., 1999) under the control of histidine decarboxylase (Burg et al., 1993). The rate of synthesis is slow (Morgan et al., 1999), and the transmitter pool is maintained by histamine reuptake (Stuart et al., 1996; Melzig et al., 1998). Reuptake is presumed to occur and to be rapid, but there is no direct evidence that reuptake terminates histamine action as a transmitter, and little is known about how residual histamine is metabolized.
Two mutants of the fruit fly Drosophila melanogaster,ebony and tan, act reciprocally on the pathway for metabolism of dopamine by β-alanine conjugation (Wright, 1987), involved in sclerotization and melanization of the body cuticle (Wright, 1987). Closely related to microbial peptide synthetases (Hovemann et al., 1998), ebony is the structural gene for β-alanyl-dopamine synthase and controls dopamine removal by conjugation to β-alanine (Wright, 1987); β-alanine in turn is synthesized under the control of black (Hodgetts, 1972).tan is the structural gene for β-alanyl-dopamine-hydrolase, catalyzing the re-formation of dopamine. Not only do ebony and tan produce reciprocal defects in cuticle melanization, they also act reciprocally on transients of the electroretinogram (ERG) (Hotta and Benzer, 1969;Heisenberg, 1971) of the compound eye. The transients are associated with synaptic transmission in the first neuropile, the lamina, at the chief photoreceptor target neurons, the monopolar cells L1 and L2 (Coombe, 1986).
The defects in the lamina transients of ebony andtan imply that transmission to L1 and L2 is somehow abnormal. This is altogether paradoxical, however, because evidence for dopaminergic neurons in the lamina is entirely lacking (Nässel et al., 1988), although dopamine possibly exerts a direct effect on the development of the transients (Neckameyer et al., 2001). Hardie (1989)first pointed out this anomaly, suggesting that, in addition to effects on body dopamine, these mutants might also have abnormal histamine levels. Recently, immunoreactivity to Ebony protein has been localized to glia at two sites, the lamina and distal medulla (see Fig.1B), corresponding to sites of photoreceptor histamine release (Richardt et al., 2002) and consistent with the role of ebony in histamine metabolism by β-alanine conjugation. Yet the ERG defects of ebony and tan await explanation. In this study, we help resolve this paradox by demonstrating that these genes regulate the metabolism of not only dopamine but also histamine.
Fig. 1.
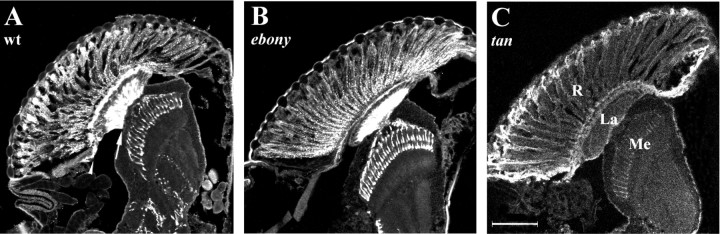
Distribution of histamine immunoreactivity in the lamina and distal medulla neuropiles of the optic lobe from wild-type (wt; A) and red-eye stocks ofebony1 (B) andtan1 (C)D. melanogaster. Confocal images of representative 10-μm-thick cryostat sections immunolabeled with antihistamine, revealing immunopositive labeling of photoreceptors, are shown. As shown in C, axons R1–R6 terminate in the lamina (La) or, for the long visual fibers, R7 and R8, in the distal medulla (Me), after crossing their positions in the external chiasma. Strong immunoreactivity is localized to the photoreceptor somata and their terminals (wt,ebony1), in neurons of the central brain (wt, ebony1), as well as in fenestration (arrowhead) and marginal (arrow) glia in the lamina (wt). Scale bar: C, 100 μm.
MATERIALS AND METHODS
Animals. D. melanogaster, Oregon R wild-type, tan1,ebony1,ebony11, andIn(3R)eAFA, an ebonychromosomal break leading to an inversion (Caizzi et al., 1987), were from stocks held at 24°C in a 12 hr light/dark cycle. Wild-type flesh flies, Sarcophaga bullata, also held at 24°C in a 12 hr light/dark cycle, were reared from larvae grown on commercial granulated laboratory rat food. Adult flies were fed with sugar and skimmed milk powder and had water ad libitum. All flies were sampled in the morning.
To create black;tan double mutants, virgin female X X/Y;black/CyO carrying two X chromosomes joined at the centromere (denoted X X) were crossed to maletan1/Y, and X X/Y;black/CyO virgins were then crossed to maletan1/Y;black/+. From the second cross,tan1/Y;black/black flies were collected, and the stock was maintained using X X/Y;black/black females. The X chromosome carryingtan was transmitted to all males in the stock through the centromere-linked X chromosomes of the females. Double-mutant males with thetan1/Y;black/blackgenotype were used.
Histamine determinations. The histamine content in theDrosophila head was measured by HPLC with electrochemical detection as reported previously (Borycz et al., 2000). Flies were quickly killed in the early day by freezing on dry ice, and samples of ∼50 heads that had been sifted from the bodies using a mesh size of 425 μm and stored at −80°C were prepared. For each determination, we ran 3-methylhistamine internal standards to check recovery, as well as a histamine standard to confirm the retention time. In this way, we could be certain to accurately identify the histamine peak.
Histamine metabolism via carcinine biosynthesis. Our histamine HPLC procedure did not clearly separate carcinine from other histamine metabolites (Borycz et al., 2000), which were studied instead using exogenous tritiated histamine ([3H]histamine, 1 mCi/ml and 23.2 Ci/mmol; NEN, Boston, MA).
Drosophila flies were dehydrated for 3 hr, after which they were given a droplet of 25% [3H]histamine (1 mCi/ml and 23.2 Ci/mmol) in 4% aqueous glucose. After 40 min, flies were frozen, and their heads were collected and prepared for HPLC separation as above. After samples were run through the HPLC system, fractions of the mobile phase were collected at 1 min intervals; 0.9 ml of mobile phase was mixed with 5 ml of scintillation cocktail (Ready Safe; Beckman Coulter) and counted for 5 min in a scintillation counter (Beckman Coulter LS 6500). The retention time for [3H]histamine and [3H]carcinine in these fractions was confirmed exactly from the retention time for the histamine and carcinine peaks seen by electrochemical detection. The quantity of3H in histamine was measured by summing the two adjacent 3H fractions, whereas the amount of 3H in carcinine was measured from the larger of the two 3H fractions.
Flies were also pressure microinjected (Nanoject; Drummond Scientific) with [3H]histamine from glass micropipettes broken to an approximate tip diameter of 3 μm, using a Leitz (Wetzlar, Germany) joystick micromanipulator, as follows.S. bullata were injected with 10 μl of [3H]histamine (as above), one part in four in 0.9% NaCl, and frozen in liquid nitrogen either immediately (<5 sec) or at 5, 10, 15, 30, 45, or 60 min intervals after the injection. Drosophila were injected with 70 nl of 17% [3H]histamine (as above) in 0.9% NaCl, and their heads were frozen 20 min after the injection. Samples were prepared and run as for flies, which drank [3H]histamine.
Histamine metabolism via alternative pathways. Sarcophaga were injected as described above with the monoamine oxidase (MAO) inhibitors pargyline, deprenyl, or clorgyline and with the semicarbazide-sensitive amine oxidase (SSAO) inhibitors semicarbazide or hydroxylamine. Each of these inhibitors was dissolved together with [3H]histamine, and the flies were frozen 30 min after injection; controls received an injection of [3H]histamine only. The [3H]histamine solution for injections was one part isotope (1 mCi/ml and 23.2 Ci/mmol) in four parts 0.9% NaCl.
Mutant rescue of the ebony and tanpathway. Double-mutant flies (black;tan) were fed a 5% solution of β-alanine dissolved in 4% glucose for 24 hr. Next, they were left for 5 hr to dehydrate, after which they received a droplet of [3H]histamine dissolved in 4% glucose (as above), which they drank for 40 min. Mutantebony flies drank a 0.5% aqueous solution of carcinine in 4% aqueous glucose for 24 hr.
Histamine immunolabeling. The probosces were removed from flies, the flies were decapitated, and their heads were fixed in 4% 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide in 0.1m phosphate buffer, pH 7.4. Cryostat sections of fixed heads, 10 μm thick, were immunolabeled using reported methods (Pollack and Hofbauer, 1991) with a rabbit polyclonal antibody (PAN19C; Immunostar, Stillwater, MN) at 1:500 and a Cy3-conjugated goat anti-rabbit secondary antibody (The Jackson Laboratory, Bar Harbor, ME) at 1:400. To confirm that immunolabeling was absent in the null allele JK910 of the gene for histidine decarboxylase, which regulates histamine synthesis (Burg et al., 1993), flies were taken off medium for 2 d and fed only a 4% sucrose solution. This eliminated bacterial sources for the synthesis of histamine in, and the fly's uptake of histamine from (Melzig et al., 1998), fly medium. Images of immunolabeled sections were collected by confocal microscopy (LSM410; Zeiss, Oberkochen, Germany) using fixed parameters for all image settings to ensure comparability of final image intensities.
Counts of synaptic vesicles. The numbers of synaptic vesicle profiles were sampled using single-section quantitative EM methods (Meinertzhagen, 1996). In each condition, either 10 cartridges (see Fig. 1A) in each of three flies were sampled or 20 cartridges in each of six flies were sampled.
Statistical analysis. Determinations from HPLC samples were tabulated as mean ± SEM (n = 10) for each mutant. Statistical significance between differences in the histamine contents of Drosophila mutants was assessed using the ttest.
RESULTS
Histamine content is reduced in ebonyand tan
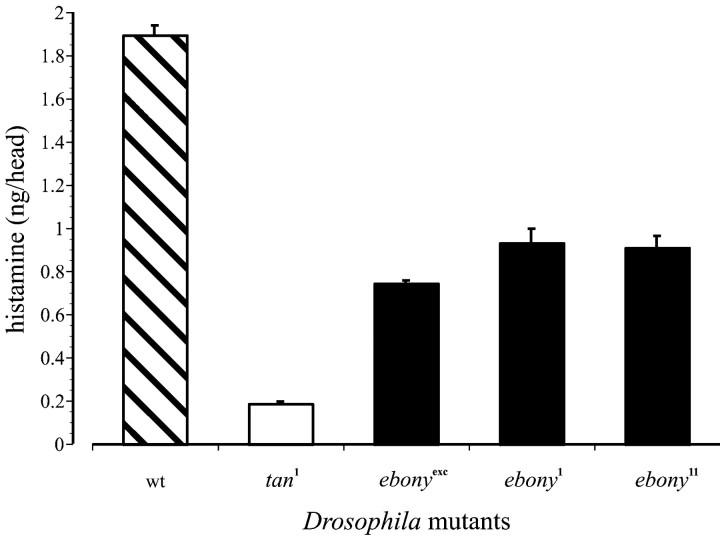
The total head content of histamine, ∼2 ng in wild-typeDrosophila (Borycz et al., 2000), was reduced in bothtan1 and two ebonyalleles, ebony1 andebony11, as well as in anIn(3R)eAFA (Caizzi et al., 1987), which also served as an ebony null. Compared with Oregon R wild type, the extents of the reductions were considerable, to 47–49% in the twoebony mutants, to 39% inIn(3R)eAFA, and to 9.8% intan (Fig. 2). Head histamine contents are reduced by ∼70% in the eyeless mutant sine oculis (Borycz et al., 2000), compatible with the reductions intan and ebony mutants having an origin in the compound eye but not restricted thereto. The differences between wild type and ebony were significant (p < 0.01; t test), as was the difference betweenIn(3R)eAFA andtan1 (p < 0.01; t test).
Fig. 2.
Histamine contents for theDrosophila head. Total head histamine fortan and three alleles of ebony, compared with the Oregon R wild-type contents of ∼2 ng.tan1 has ∼0.2 ng;ebonyexc, the ebonyexcision allele In(3R)eAFA has ∼0.7 ng; and ebony1 has ∼0.9 ng. Values are mean ± SEM for 10 samples per value. wt, Wild type.
There are fewer synaptic vesicles in ebonyand tan
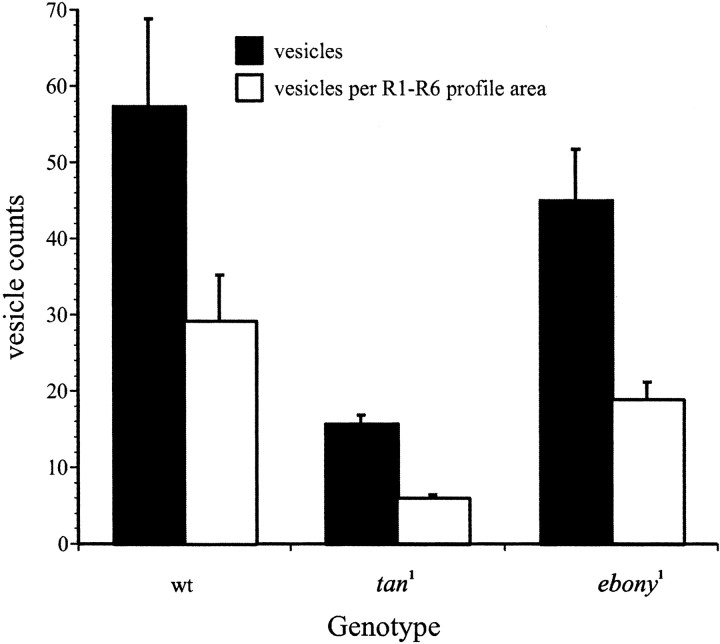
To validate the reduction in head histamine in tan andebony and localize its likely origin in the photoreceptors, we counted the number of synaptic vesicle profiles, presumed sites of histamine storage, in electron micrographs of photoreceptor terminal cross sections. Here, too, there were reductions, compared with wild type, to 78% in ebony1 and to 28% in tan1 (Fig.3). The difference between wild-type andtan1 terminals was significant (p < 0.001; t test). The terminals of ebony had fewer profiles as well, reduced to 69% of the value for wild-type terminals, but this difference was not significant. To ascertain that these differences did not result from a simple reduction in the diameter of the terminals, we also measured the cross-sectional area to calculate the packing density of vesicle profiles. These, too, paralleled the changes in head histamine contents, because the terminals were reduced very little in size. The density differences between ebony1and wild type and between tan1 and wild type were then both significant (p < 0.01;t test).
Fig. 3.
Synaptic vesicle counts per R1–R6 photoreceptor terminal profile in Oregon R wild-type (wt),tan1, andebony1Drosophila. Counts (n) and their densities (N/μm2) in terminal cross sections are shown.
Regional differences in histamine immunolabeling
Given the difference in head histamine in both mutantebony and tan from wild type, we subsequently examined the distribution of histamine immunoreactivity within the photoreceptors, as well as in other histamine-immunoreactive neurons. Relative to the wild type (Fig. 1A), as reported previously (Pollack and Hofbauer, 1991), ebony showed a rather similar pattern of labeling, except that labeling in the central brain was less pronounced, and a prominent band of label beneath the basement membrane, as well as a less prominent band proximal to the lamina neuropile, were both absent (Fig. 1B). These bands have the same locations as the fenestration (and possibly pseudocartridge) glia and marginal glia, respectively (Saint Marie and Carlson, 1983). In contrast, tan showed altogether weaker labeling, commensurate with its reduced head histamine, with only moderate immunoreactivity in the photoreceptor cell bodies, very little in their terminals, and none in the neurons of the central brain (Fig.1C).
[3H]histamine is converted to [3H]carcinine in flies
The β-alanyl conjugate of histamine, carcinine, is an identified metabolite of the crab's heart (Arnould, 1985), and a peak with the same retention time as carcinine appears in HPLC chromatograms of the fly's head (Borycz et al., 2000). Neither N-acetyl histamine nor imidazol-4-acetic acid, both identified previously as histamine metabolites in insect brains (Elias and Evans, 1983), were detected with our HPLC methods (Borycz et al., 2000), so we are unable to exclude these two metabolites from the carcinine peak. The peak does overlap another, however, making determination of the content of carcinine in the fly's head incomplete. To confirm the relationship between histamine and carcinine, we therefore gaveDrosophila water to drink that was laced with [3H]histamine and measured3H with a scintillation counter. The duration of the flies' drinking bout, which lasted 40 min, and the clearance of [3H]histamine from the gut into the hemolymph and from the hemolymph into the brain, all filter the dynamics of the transfer between histamine and carcinine.
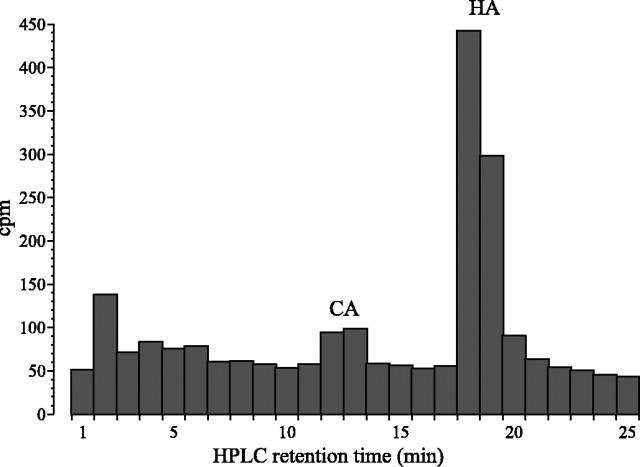
A histogram of successive 1 min fractions separated by HPLC reveals not only a peak of 3H at the same retention time as histamine but also at the retention time corresponding to that for carcinine. This peak was unmistakable fortan1 (Fig.4), consistent with the lack of hydrolase activity required to break down carcinine and liberate histamine. The [3H]histamine peak itself declined with time after [3H]histamine intake, whereas the 3H peak corresponding to carcinine persisted for ≥48 hr after tan1flies drank [3H]histamine for 40 min and even after the [3H]histamine peak itself had largely disappeared. A 3H peak corresponding to carcinine was, in contrast, lacking inebony1 (Fig. 4), consistent with the inability of this mutant to synthesize carcinine. That interpretation is ambiguous, however, because a clear 3H peak corresponding to carcinine was also lacking in wild-type flies (Fig. 4).
Fig. 4.
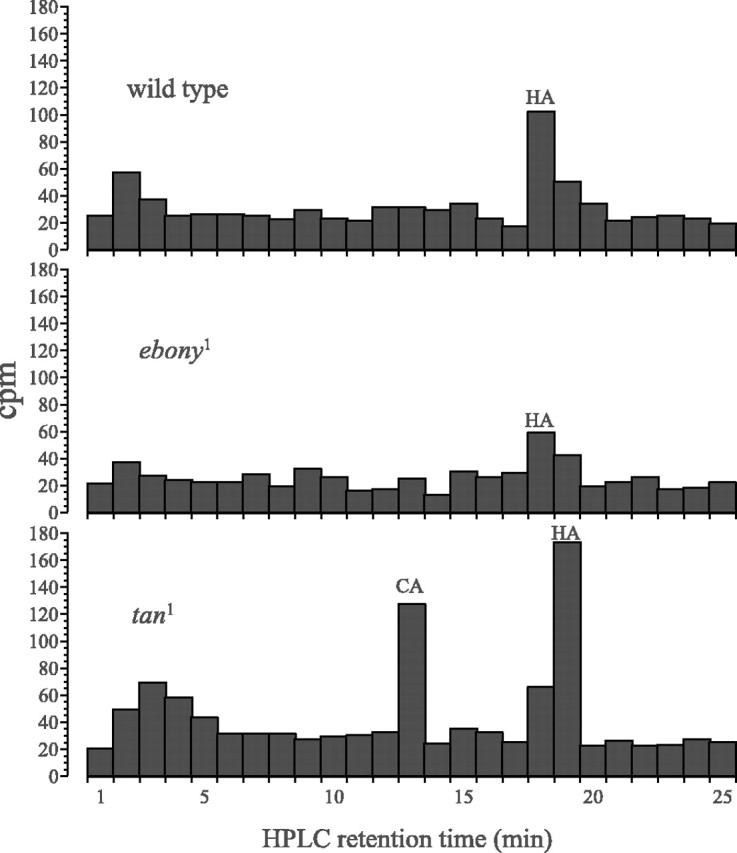
Distribution of 3H cpm in chromatographs obtained after the separation by HPLC of head extracts from tan1,ebony1, and wild-type Oregon R flies, after they had been permitted to drink [3H]histamine for 40 min. A clear 3H peak with the same retention time as carcinine (CA) appears in tan but not in the other two. Note the smaller overall 3H (HA) head content inebony1.
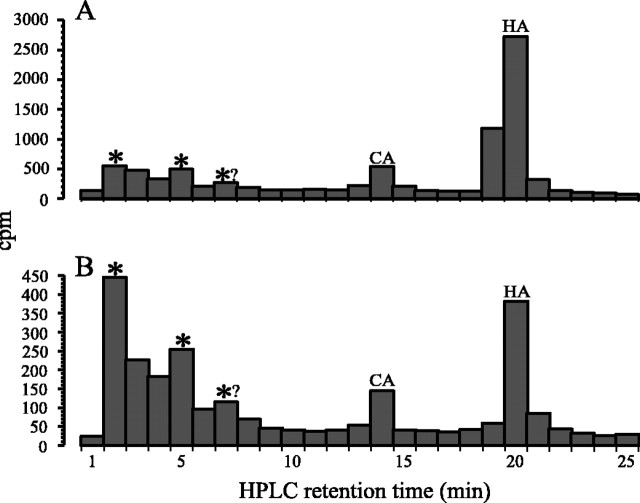
Given the failure to see a clear wild-type [3H]carcinine peak after drinking [3H]histamine, we decided to seek a clearer peak in flies that were injected with [3H]histamine directly into the head. This required making individual injections into flies, which was technically challenging because 50 were required to survive for us to be able to make HPLC determinations. A small but clear3H peak corresponding to carcinine was seen, however, after making such injections (Fig.5). To obtain a larger peak, we would have had to select the correct injection dose and recovery time, and this would have required us to undertake the experiment repeatedly, which was not possible. We therefore adopted a different strategy and injected [3H]histamine into the head of another fly species, S. bullata. In this large fly, such injections are technically possible, and samples can be obtained from fewer animals. A 3H peak with the same retention time as carcinine was clearly visible even when flies were killed immediately after their injection by freezing on dry ice (i.e., <5 sec after injection). The peak became larger 5 min after the injection and was clearest between 10 and 30 min (Fig.6). The presence of this peak strongly suggested that carcinine was a metabolite of histamine inSarcophaga, supporting the presence of carcinine in the wild type of smaller Drosophila.
Fig. 5.
Distribution of 3H (cpm) in chromatographs obtained after the separation by HPLC of head extracts from wild-type Oregon R Drosophila, 20 min after microinjecting [3H]histamine individually into the head. Note the retention times for histamine (HA) and carcinine (CA).
Fig. 6.
Distribution of 3H (cpm) in chromatographs obtained after the separation by HPLC of head extracts from wild-type Sarcophaga microinjected into the head with [3H]histamine. A, At 5 min after injecting [3H]histamine (HA), 1 min fractions reveal a small peak at the same retention time as carcinine (CA), which is larger. B, After 30 min, two additional peaks (*) and a possible third (*?) with shorter retention times also appear.
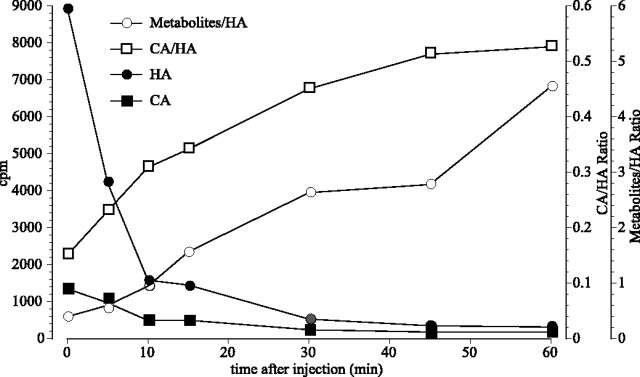
Similar injections repeated for different recovery intervals showed a gradually changing ratio between the heights of the [3H]histamine and the [3H]carcinine peaks (Fig.7). The carcinine/histamine ratio increased from ∼0.15 immediately after the injection to a value of ∼0.55 after 30 min. The trace of carcinine seen even immediately after the injection (t = 0) indicates its rapid formation from [3H]histamine.
Fig. 7.
The ratio between the peaks for histamine (HA) and carcinine (CA) at different times after injections into Sarcophaga. Each point is the mean, or the ratio between the indicated mean, of the corresponding fractions from at least two individually injected flies, from a batch carefully selected for similar age and weight. Values for [3H]carcinine and [3H]histamine from the two flies differed by <20%. Total [3H]histamine declines, presumably first by diluting into the hemolymph of the body, and then by excretion. The [3H]carcinine/[3H]histamine ratio is initially low but increases; [3H]carcinine does not accumulate, however, indicating that it is lost in parallel to [3H]histamine, presumably by back conversion, thereby establishing a dynamic equilibrium between histamine and its metabolite. The ratio between the major peak of the earlier3H retention peaks and the peak for [3H]histamine changes in a similar manner to, but is much larger than, the [3H]carcinine/[3H]histamine ratio (note different ordinate scales).
Carcinine rescues the histamine phenotype ofebony mutants
To confirm that ebony controls the β-alanyl conjugation pathway of histamine, we examined whether the reduced content of histamine in the head of ebony flies could be rescued by the administration of carcinine to mutant flies. The rationale was that because ebony flies, we propose, are unable to synthesize carcinine, they therefore lack this metabolite. We found that after drinking a 0.5% aqueous solution of carcinine for 24 hr, such flies had a histamine content of 41.4 ± 1.82 ng/head. This was fully 20 times the normal wild-type content. In comparison, wild-type flies fed with the same solution of carcinine increased their head histamine content only to 25.4 ± 1.58 ng, whereas the histamine level remained unchanged when tan flies were treated in the same way. The latter observation provides additional evidence that tan affects the hydrolysis not only of β-alanyl-dopamine but also of carcinine. The difference between the increase in head histamine seen in wild-type and ebony flies when both were fed with carcinine may indicate that the pathway for carcinine hydrolysis is upregulated in ebony, when the pathway for carcinine biosynthesis is lacking.
β-alanine rescues the carcinine phenotype ofblack;tan double mutants
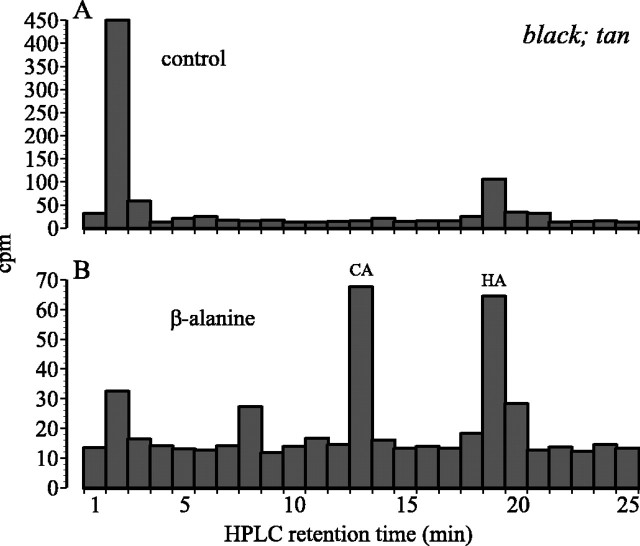
Flies mutant for black, a glutamate or aspartate decarboxylase, are defective in the biosynthesis of β-alanine (Hodgetts, 1972), the substrate for β-alanyl conjugation of dopamine. As a result, they have increased body cuticle melanization (Hodgetts and Choi, 1974). The mutants also have a reduced content of histamine, 1.32 ± 0.06 ng/head, significantly less than wild type (p < 0.05). Like Ebony, the product ofblack localizes to the epithelial glial cells of the lamina (Phillips et al., 1993) (A. M. Phillips, personal communication), where it is well placed to synthesize the β-alanine required for conjugation to histamine released from photoreceptor terminals. To confirm that ebony and tan control the β-alanyl conjugation for histamine, we therefore sought to examine whetherblack;tan double mutants were able to synthesize carcinine from β-alanine and histamine but unable to hydrolyze it, like the single mutant tan. After drinking [3H]histamine, control double-mutantblack;tan flies lacked a [3H]carcinine peak (Fig.8A), as predicted from their failure to synthesize β-alanine. However, they had a large peak at a shorter HPLC retention time, possibly reflecting an alternative metabolite. The head chromatographs of flies given medium containing 5% β-alanine before drinking [3H]histamine showed, in contrast, a clear carcinine peak (Fig. 8B) like that seen intan. The head content of histamine reflected this uptake of3H. Control black;tan double mutants had a significantly reduced histamine content of 1.34 ± 0.14 ng/head, like the single mutant black, with 1.32 ± 0.06 ng (as above), whereas double mutants fed with 5% β-alanine had 0.26 ± 0.03 ng, significantly decreased from control flies not fed with β-alanine (t test; p < 0.01). The very small difference between total head histamine content for the double black;tan mutant rescued with β-alanine (0.26 ng) and that for the single mutant tan (0.18 ± 0.02 ng) was nevertheless significant (p < 0.01; t test). Thus, β-alanine rescues the tanphenotype almost completely in black;tan double mutants. When black single-mutant flies were fed with 5% β-alanine, they had 1.87 ± 0.07 ng of histamine per head, which did not differ statistically from wild type. In contrast, when similarly fed β-alanine, neither tan nor ebonyincreased their head content of histamine.
Fig. 8.
Distribution of 3H (cpm) in chromatographs obtained after the separation by HPLC of head extracts from black;tan double-mutant Drosophila, after they had been permitted to drink [3H]histamine (HA) for 40 min.A, Control double-mutant flies. B, Double mutants previously fed 5% β-alanine. CA, Carcinine.
Other metabolites
In addition to the 3H peak corresponding to carcinine, there were also peaks at earlier retention times (Figs. 4-7), one of which corresponded to the peak seen inblack;tan double-mutant flies (Fig. 8A). Unlike the wild-type peaks for both [3H]histamine and [3H]carcinine, which disappeared with time, these peaks increased with time after the injection and persisted, attaining a plateau ratio approximately five times higher than [3H]histamine. We cannot address the possibility that they contained either N-acetyl histamine or imidazol-4-acetic acid, two common histamine metabolites of histamine (Elias and Evans, 1983), which we are unable to detect with our HPLC method. On a more positive note, the first of the peaks does clearly coincide with the retention time for γ-glutamyl histamine, which in our chromatographs has a retention time of ∼3 min, making this a possible alternative metabolite (see Discussion).
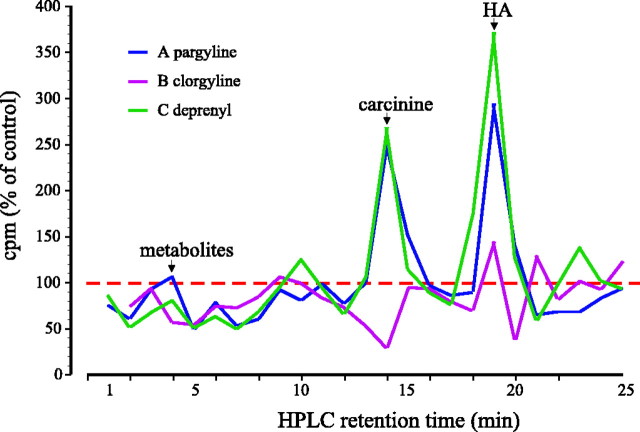
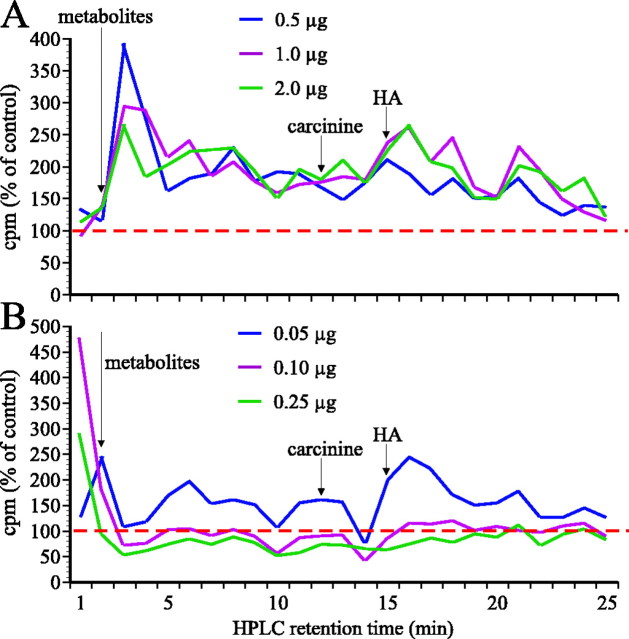
Additional evidence clearly suggests that these early HPLC retention peaks do in fact reflect alternative metabolic pathways. The peaks had different temporal characteristics than that for carcinine, progressively increasing in size, suggesting that the corresponding metabolites accumulated and were not converted back to histamine or were so converted only slowly. The ratio between the3H peaks for histamine and the major peak of these earlier retention peaks changed with time after injecting [3H]histamine into Sarcophagain a manner similar to the histamine/carcinine ratio, but it already exceeded 1 after 10 min (Fig. 7). The metabolites that eluted at these earlier retention times were sensitive to MAO inhibitors. Deprenyl and pargyline potently slowed the clearance of both [3H]histamine and [3H]carcinine from the fly. Pargyline and deprenyl (MAO-B inhibitors) both decreased the early HPLC peaks and increased the carcinine peak (Fig. 9). In parallel, the histamine peak increased. Clorgyline (a MAO-A inhibitor) had a similar action on the metabolites with earlier retention times but increased neither the histamine nor the carcinine peaks. This pattern of differential sensitivity could also be characteristic of SSAOs (Jalkanen and Salmi, 2001). Sarcophaga injected with semicarbazide exhibited increased peaks at retention times corresponding to histamine and carcinine and at early HPLC retention times. The effect was already seen at a dose of 0.5 μg (Fig.10A). Flies injected with another SSAO blocker, hydroxylamine, showed an increased histamine peak at 0.05 μg but decreases for higher doses (Fig.10B). In parallel, carcinine peaks were also decreased, and the early peaks were increased. Given that the relationship between histamine and its metabolites was unchanged after SSAO blockers, we can apparently exclude the involvement of this class of enzyme in histamine metabolism in Sarcophaga. Another possible enzyme we can also apparently eliminate is diamine oxidase, because aminoguanidine (10–50 μg) did not alter the early HPLC peaks and thus did not block the synthesis of these metabolites (data not shown). The identity of these early peaks is under further investigation.
Fig. 9.
Percentage changes relative to controls of3H (cpm) in chromatographs obtained after the separation by HPLC of head extracts from wild-type Sarcophaga, 30 min after they were injected with [3H]histamine (HA). Plotted are the ratios between control flies and flies that were pretreated with MAO inhibitors: pargyline at 10 μg, deprenyl at 0.5 μg, and clorgyline at 5 μg. The horizontal dashed line indicates control values.
Fig. 10.
Percentage changes relative to controls of3H (cpm) in Sarcophaga, 30 min after injecting [3H]histamine (HA).A, Flies pretreated with semicarbazide at concentrations between 0.5 and 2 μg/10 μl, relative to controls. B, Flies pretreated with hydroxylamine at concentrations between 0.05 and 0.25 μg/10 μl, relative to controls. The horizontal dashed lines indicate control values.
DISCUSSION
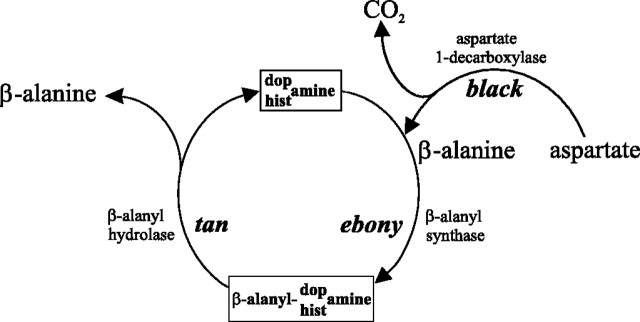
Our findings indicate that a novel histamine phenotype underlies the reciprocal actions of ebony and tan. Unlike other aspects of their phenotypes, however, the mutants do not act reciprocally; both reduce the histamine contents of the head. Associated with reduced histamine in both mutants are parallel decreases in the number and packing of synaptic vesicles in the photoreceptor terminals, implying that the histamine phenotype is at least partly of photoreceptor origin. Our HPLC data are consistent with the conversion into [3H]carcinine of exogenous [3H]histamine, either taken up by or injected into the fly's head. Such uptake has been demonstrated previously in mutant hdc flies (Melzig et al., 1998), which are unable to synthesize histamine (Hotta and Benzer, 1969; Burg et al., 1993) and lack vision (Melzig et al., 1996). The amount of [3H]carcinine converted inebony and tan is consistent with the action ofebony in regulating β-alanyl conjugation of histamine and of tan in regulating the hydrolysis of carcinine back to histamine (Fig. 11). The equilibrium between the actions of both enzymes evidently favors the hydrolysis of carcinine to liberate histamine, so that the wild-type carcinine content is normally low. Confirming this pathway, ebonyflies fed carcinine have increased head histamine, as does the wild type. In addition, feeding β-alanine to double-mutantblack;tan flies rescues their ability to synthesize carcinine by providing β-alanine as a substrate (Fig. 11). Finally, we provide evidence for the existence of alternative metabolic pathways, with metabolites that convert back to histamine only slowly, if at all.
Fig. 11.
Tan and Ebony regulate the β-alanine conjugation and hydrolysis of dopamine to β-alanyl dopamine. In the lamina and possibly in the distal medulla, they also regulate the comparable conjugation of histamine to β-alanyl histamine (ebony) and its subsequent hydrolysis (tan) to yield free histamine. In both cases, conjugation requires β-alanine, which is also liberated during hydrolysis, along with the corresponding amine. Synthesis of β-alanine from aspartate or uracil by decarboxylation is under the control of the gene black.
Carcinine as a metabolite of histamine
The evidence for in vivo carcinine biosynthesis is novel for the visual system but has previously been reported biochemically for CNS extracts from the crab Carcinus maenas(Arnould, 1987a), which accumulates carcinine in the heart. Not allCarcinus tissues are able to metabolize carcinine (Arnould, 1987b), however, suggesting that the hydrolysis pathway represented bytan in Drosophila is either lacking or of low activity. The universal histamine immunoreactivity of (Nässel, 1999), and likely prevalence of histaminergic transmission at, arthropod photoreceptors suggests that a carcinine biosynthesis pathway could be widely used at this site. Carcinine was not sought in a previous study of insect histamine metabolites (Elias and Evans, 1983) but is certainly not restricted to arthropods. It is also a minor metabolite in mammals (Flancbaum et al., 1990), in which it exerts a positive inotropic action at the heart (Brotman et al., 1990).
The simultaneous action of both Ebony and Tan proteins in wild-type flies indicates that carcinine forms rapidly. Within 5 sec of injectingSarcophaga, [3H]histamine gains access to Ebony and is already converted to carcinine. The independent regulation of synthase and hydrolase activity means that the rates for carcinine biosynthesis and hydrolysis can be independently regulated by differential transcription under different physiological conditions. For example, ebony transcription exhibits a circadian modulation (Claridge-Chang et al., 2001). The significance of carcinine as a metabolite may in fact lie not as much in the identity of the metabolite itself as in the rates of its biosynthesis and reversible hydrolysis, which our [3H]histamine evidence indicates are normally adjusted to a 2:1 equilibrium ratio in favor of hydrolysis. Although it is clear that Ebony acts rapidly, our methods do not allow us to say whether it contributes to the termination of histamine action at the cleft. Resolution of this question is crucial to understand how insects, especially fast-flying diurnal flies (Laughlin and Weckström, 1993), are able to use the high-temporal resolution of their photoreceptors. The latter depends on the rapid clearance of released histamine at photoreceptor terminals.
Exact sites of histamine metabolism in the lamina and distal medulla are still not clear. The photoreceptor terminals R1–R6 that surround the axons of L1 and L2 within a cartridge are wrapped in turn by three epithelial glial cells (Saint Marie and Carlson, 1983; Meinertzhagen and O'Neil, 1991; Eule et al., 1995). These are well placed to metabolize histamine from the synaptic cleft and thereby regulate its postsynaptic action at sites on L1 and L2. Moreover, the epithelial glia do indeed express Ebony strongly (Richardt et al., 2002). Carcinine biosynthesis by Ebony in the epithelial glia would remove histamine released into the lamina, presumably from the synaptic cleft, but would store histamine in a form that can then rapidly liberate it by hydrolysis. The site of that hydrolysis is unknown in detail, but mosaic studies indicate that tan acts in or close to the eye (Hotta and Benzer, 1970), compatible with its action in the photoreceptors.
The histamine phenotypes of ebonyand tan
The accumulation of [3H]carcinine in tan, but its lack in ebony, can be explained by the reciprocal regulation of β-alanyl conjugation of histamine in the two mutants. However, this still fails to explain how a reduction in head histamine results from the reciprocal action of the two genes. We propose that histamine content is reduced in tan because of the failure to liberate histamine from accumulated carcinine, a function that is autonomous to the mutant eye (Hotta and Benzer, 1970), but is reduced in ebony because carcinine fails to trap histamine after it is released, leaving the histamine free to diffuse away from the compound eye. The fate of histamine after diffusion is unclear but could finally be loss, to the thorax, thence by excretion. We propose that this loss is the primary reason for the reduced head content of histamine in ebony. In the absence of functional Ebony protein, mutant flies also fail to trap exogenous [3H]histamine, much of which is likewise lost by excretion. As a result, not only is total head histamine reduced but also the amount of 3H incorporation. In tan, a reciprocal effect occurs, with [3H]histamine incorporation increasing with respect to wild type. We believe that this may signify increased efficiency in the histamine uptake mechanisms in response to the greater reduction in the head histamine of tan. InDrosophila gynandromorphs with a single mutantebony eye, the defect in the ERG transients is nonautonomous (R. Hodgetts, personal communication). One interpretation of this difference from tan is that a mutant ebony lamina is unable to convert released histamine to carcinine, so that the histamine remains extracellular and may be free to diffuse to other sites, including the other eye, where it is converted to carcinine by functional Ebony. The fact that such sites can rescue the ERG defect in the mutant eye suggests that the lack of transients when both eyes are mutant for ebony could reflect the presence in the synaptic cleft of residual histamine, even that released in the dark (Uusitalo et al., 1995). We propose that carcinine that accumulates by the action of functional Ebony in a mutant tan eye is localized initially to the epithelial glia and is not free to diffuse. Therefore, it sequesters much of the histamine pool. In that case, the ERG defect in the mutant tan eye may be attributed to insufficient release of histamine. Our findings thus help shed light on the involvement in lamina function of tan and ebonyand offer a possible explanation for why both, albeit for different reasons, result in the loss of the ON transients of the ERG. An alternative interpretation, offered without reference to histamine metabolism and possibly an independent effect, is that the loss of the lamina transients of ERG in tan could result from the decreased availability of dopamine during larval development (Neckameyer et al., 2001), with ebony showing reciprocal defects to those shown by tan. Still left to be resolved is whether the carcinine pathway operates at other sites. These include (1) terminals of head mechanoreceptors, which also contain histamine (Pollack and Hofbauer, 1991) and in which function is both lost (Melzig et al., 1996) and rescued by exogenous histamine (Melzig et al., 1998) in flies mutant for hdc; (2) wide-field histamine-like immunoreactive neurons in the central brain (Pollack and Hofbauer, 1991); and (3) dopaminergic neurons in the brain (Nässel et al., 1988).
Other metabolites of histamine
The production of carcinine is not the sole metabolic pathway for photoreceptor histamine. In the horseshoe crab Limulus, histamine is also a putative photoreceptor transmitter (Battelle et al., 1991), and an additional or alternative metabolic pathway involves γ-glutamyl histamine (Battelle and Hart, 2002), a means of histamine inactivation reported previously in the opisthobranchAplysia (Stein and Weinreich, 1983). It is not clear what additional metabolites might also exist in Drosophila, but the presence of 3H peaks with HPLC retention times shorter than that for carcinine allows a number of candidates, possibly up to three. Our method is not able to detect acetyl-histamine or imidazol-4-acetic acid (Borycz et al., 2000), but a3H peak with the same retention time as γ-glutamyl histamine exists, so this metabolite could be present. Other metabolites probably exist as well (Borycz and Meinertzhagen, 2001). For example, the separate actions of pargyline and deprenyl and of clorgyline could indicate a role for monoamine oxidases. Therefore, it is surprising that the monoamine oxidase gene appears to have been lost from the Drosophila genome (Roelofs and Van Haastert, 2001), making the identity of these metabolites a topic for future clarification as well. Moreover, insensitivity to semicarbazide and hydroxylamine could indicate the lack of SSAO action.
In addition to their activities at one time and in one genetic background, the relative activities of the metabolic pathway for carcinine (regulated by ebony and tan) and for alternative metabolites indicate that each pathway can be differentially regulated. Regulation is seen in black;tandouble mutants, which have a large early retention peak suggesting that, in the congenital absence of a capacity to synthesize carcinine, histamine metabolism switches into another pathway, possibly for γ-glutamyl histamine. That pathway is not increased in the single mutant tan, possibly because tan is able to store released histamine as carcinine. Such shifts can also apparently occur in the short term, as for example in Sarcophaga injected with pargyline and deprenyl. Under the influence of these drugs, the early HPLC retention peaks are diminished, and the histamine peak is larger, suggesting that histamine metabolism via carcinine is upregulated.
Footnotes
This work was supported by a North Atlantic Treaty Organization Postdoctoral fellowship (J.B.), National Institutes of Health Grant EY-03592 and Medical Research Council Grant MOP 36453, and the Killam Trust of Dalhousie University (I.A.M.). We thank Dr. Bernd Hovemann for bringing the lamina expression pattern ofebony to our attention and Dr. Ross Hodgetts for discussing ERG phenotypes in ebony mosaics. We also thank Dr. Rima Porfir'evna Evstigneeva (Lomonsov Moscow State Academy of Fine Chemical Technology, Moscow, Russia) for synthesizing carcinine.
Correspondence should be addressed to I. A. Meinertzhagen, Life Sciences Centre, 1355 Oxford Street, Dalhousie University, Halifax, Nova Scotia, Canada B3H 4J1. E-mail: iam@is.dal.ca.
REFERENCES
- 1.Amara SG, Arriza JL. Neurotransmitter transporters: three distinct gene families. Curr Opin Neurobiol. 1993;3:337–344. doi: 10.1016/0959-4388(93)90126-j. [DOI] [PubMed] [Google Scholar]
- 2.Arnould J-M. Biosynthesis and metabolism of histamine in the central nervous system of Carcinus maenas. Arch Int Physiol Biochim. 1985;95:43–55. doi: 10.3109/13813458709075024. [DOI] [PubMed] [Google Scholar]
- 3.Arnould J-M. Demonstration of carcinine synthetase, a new enzyme catalysing the metabolism of histamine in the central nervous system of Carcinus maenas. J Neurochem. 1987a;48:1316–1324. doi: 10.1111/j.1471-4159.1987.tb05663.x. [DOI] [PubMed] [Google Scholar]
- 4.Arnould J-M. Beta-alanylation, a means for neutralization of histamine in the central nervous system of Carcinus maenas. Can J Physiol Pharmacol. 1987b;65:1898–1902. [PubMed] [Google Scholar]
- 5.Battelle B-A, Hart MK. Histamine metabolism in the visual system of the horseshoe crab Limulus polyphemus. Comp Biochem Physiol. 2002;133:135–142. doi: 10.1016/s1095-6433(02)00133-2. [DOI] [PubMed] [Google Scholar]
- 6.Battelle B-A, Calman BG, Andrews AW, Grieco FD, Mleziva MB, Callaway JC, Stuart AE. Histamine: a putative afferent neurotransmitter in Limulus eyes. J Comp Neurol. 1991;305:527–542. doi: 10.1002/cne.903050402. [DOI] [PubMed] [Google Scholar]
- 7.Blusztajn JK, Wurtman RJ. Choline and cholinergic neurons. Science. 1983;221:614–620. doi: 10.1126/science.6867732. [DOI] [PubMed] [Google Scholar]
- 8.Borycz J, Meinertzhagen IA. Histamine metabolism in the fly's visual system. Soc Neurosci Abstr. 2001;27:1902. [Google Scholar]
- 9.Borycz J, Vohra M, Tokarczyk G, Meinertzhagen IA. The determination of histamine in the Drosophila head. J Neurosci Methods. 2000;101:141–148. doi: 10.1016/s0165-0270(00)00259-4. [DOI] [PubMed] [Google Scholar]
- 10.Brotman DN, Flancbaum L, Kang Y-H, Merrill GF, Fisher H. Positive inotropic effect of carcinine in the isolated perfused guinea pig heart. Crit Care Med. 1990;18:317–321. doi: 10.1097/00003246-199003000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Burg MG, Sarthy PV, Koliantz G, Pak WL. Genetic and molecular identification of a Drosophila histidine decarboxylase gene required in photoreceptor transmitter synthesis. EMBO J. 1993;12:911–919. doi: 10.1002/j.1460-2075.1993.tb05732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caizzi R, Ritossa F, Ryseck R-P, Richter S, Hovemann B. Characterization of the ebony locus in Drosophila melanogaster. Mol Gen Genet. 1987;206:66–70. [Google Scholar]
- 13.Callaway JC, Stuart AE. Biochemical and physiological evidence that histamine is the transmitter of barnacle photoreceptors. Vis Neurosci. 1989;3:311–325. doi: 10.1017/s0952523800005502. [DOI] [PubMed] [Google Scholar]
- 14.Claridge-Chang A, Wijnen H, Naef F, Boothroyd C, Rajewsky N, Young MW. Circadian regulation of gene expression systems in the Drosophila head. Neuron. 2001;32:657–671. doi: 10.1016/s0896-6273(01)00515-3. [DOI] [PubMed] [Google Scholar]
- 15.Coombe PE. The large monopolar cells L1 and L2 are responsible for ERG transients in Drosophila. J Comp Physiol [A] 1986;159:655–665. [Google Scholar]
- 16.Dowling JE, Ripps H. Effect of magnesium on horizontal cell activity in the skate retina. Nature. 1973;242:101–103. doi: 10.1038/242101a0. [DOI] [PubMed] [Google Scholar]
- 17.Elias MS, Evans PD. Histamine in the insect nervous system: distribution, synthesis and metabolism. J Neurochem. 1983;41:562–568. doi: 10.1111/j.1471-4159.1983.tb04776.x. [DOI] [PubMed] [Google Scholar]
- 18.Eule E, Tix S, Fischbach K-F (1995) Glial cells in the optic lobe of Drosophila melanogaster Flybrain poster (http://www.flybrain.org/ Flybrain/html/poster/). Accession no. PP00004.
- 19.Flancbaum L, Brotman DN, Fitzpatrick JC, Van Es T, Kasziba E, Fisher H. Existence of carcinine, a histamine-related compound, in mammalian tissues. Life Sci. 1990;47:1587–1593. doi: 10.1016/0024-3205(90)90188-w. [DOI] [PubMed] [Google Scholar]
- 20.Hardie RC. Is histamine a neurotransmitter in insect photoreceptors? J Comp Physiol [A] 1987;161:201–213. doi: 10.1007/BF00615241. [DOI] [PubMed] [Google Scholar]
- 21.Hardie RC. Neurotransmitters in compound eyes. In: Stavenga DG, Hardie RC, editors. Facets of vision. Springer; Berlin: 1989. pp. 235–256. [Google Scholar]
- 22.Heisenberg M. Separation of receptor and lamina potentials in the electroretinogram of normal and mutant Drosophila. J Exp Biol. 1971;55:85–100. doi: 10.1242/jeb.55.1.85. [DOI] [PubMed] [Google Scholar]
- 23.Hodgetts RB. Biochemical characterization of mutants affecting the metabolism of β-alanine in Drosophila. J Insect Physiol. 1972;18:937–947. doi: 10.1016/0022-1910(72)90031-5. [DOI] [PubMed] [Google Scholar]
- 24.Hodgetts R, Choi A. β alanine and cuticle maturation in Drosophila. Nature. 1974;252:710–711. doi: 10.1038/252710a0. [DOI] [PubMed] [Google Scholar]
- 25.Hotta Y, Benzer S. Abnormal electroretinograms in visual mutants of Drosophila. Nature. 1969;222:354–356. doi: 10.1038/222354a0. [DOI] [PubMed] [Google Scholar]
- 26.Hotta Y, Benzer S. Genetic dissection of the Drosophila nervous system by means of mosaics. Proc Natl Acad Sci USA. 1970;67:1156–1163. doi: 10.1073/pnas.67.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hovemann BT, Ryseck R-P, Walldorf U, Störtkuhl KF, Dietzel ID, Dessen E. The Drosophila ebony gene is closely related to microbial peptide synthetases and shows specific cuticle and nervous system expression. Gene. 1998;221:1–9. doi: 10.1016/s0378-1119(98)00440-5. [DOI] [PubMed] [Google Scholar]
- 28.Iversen LL. Role of transmitter uptake mechanisms in synaptic neutotransmission. Br J Pharmacol. 1971;41:571–591. doi: 10.1111/j.1476-5381.1971.tb07066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jalkanen S, Salmi M. Cell surface monoamine oxidases: enzymes in search of a function. EMBO J. 2001;20:3893–3901. doi: 10.1093/emboj/20.15.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juusola M, French AS, Uusitalo RO, Weckström M. Information processing by graded-potential transmission through tonically active synapses. Trends Neurosci. 1996;19:292–297. doi: 10.1016/S0166-2236(96)10028-X. [DOI] [PubMed] [Google Scholar]
- 31.Laughlin SB, Weckström M. Fast and slow photoreceptors. A comparative study of the functional diversity of coding and conductances in the Diptera. J Comp Physiol [A] 1993;172:593–609. [Google Scholar]
- 32.Massoulié J, Pezzementi L, Bon S, Krejci E, Vallette FM. Molecular and cellular biology of cholinesterases. Prog Neurobiol. 1993;41:31–91. doi: 10.1016/0301-0082(93)90040-y. [DOI] [PubMed] [Google Scholar]
- 33.Meinertzhagen IA. Ultrastructure and quantification of synapses in the insect nervous system. J Neurosci Methods. 1996;69:59–73. doi: 10.1016/S0165-0270(96)00021-0. [DOI] [PubMed] [Google Scholar]
- 34.Meinertzhagen IA, O'Neil SD. Synaptic organization of columnar elements in the lamina of the wild type in Drosophila melanogaster. J Comp Neurol. 1991;305:232–263. doi: 10.1002/cne.903050206. [DOI] [PubMed] [Google Scholar]
- 35.Melzig J, Buchner S, Wiebel F, Wolf R, Burg M, Pak WL, Buchner E. Genetic depletion of histamine from the nervous system of Drosophila eliminates specific visual and mechanosensory behavior. J Comp Physiol [A] 1996;179:763–773. doi: 10.1007/BF00207355. [DOI] [PubMed] [Google Scholar]
- 36.Melzig J, Burg M, Gruhn M, Pak WL, Buchner E. Selective histamine uptake rescues photo- and mechanoreceptor function of histidine decarboxylase-deficient Drosophila mutant. J Neurosci. 1998;18:7160–7166. doi: 10.1523/JNEUROSCI.18-18-07160.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgan JR, Gebhardt KA, Stuart AE. Uptake of precursor and synthesis of transmitter in a histaminergic photoreceptor. J Neurosci. 1999;19:1217–1225. doi: 10.1523/JNEUROSCI.19-04-01217.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nässel DR. Histamine in the brain of insects: a review. Microsc Res Tech. 1999;44:121–136. doi: 10.1002/(SICI)1097-0029(19990115/01)44:2/3<121::AID-JEMT6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 39.Nässel DR, Elekes K, Johansson KUI. Dopamine-immunoreactive neurons in the blowfly visual system: light and electron microscopic immunocytochemistry. J Chem Neuroanat. 1988;1:311–325. [PubMed] [Google Scholar]
- 40.Neckameyer W, O'Donnell J, Huang Z, Stark W. Dopamine and sensory tissue development in Drosophila melanogaster. J Neurobiol. 2001;47:280–294. doi: 10.1002/neu.1035. [DOI] [PubMed] [Google Scholar]
- 41.Phillips AM, Salkoff LB, Kelly LE. A neural gene from Drosophila melanogaster with homology to vertebrate and invertebrate glutamate decarboxylases. J Neurochem. 1993;61:1291–1301. doi: 10.1111/j.1471-4159.1993.tb13621.x. [DOI] [PubMed] [Google Scholar]
- 42.Pollack I, Hofbauer A. Histamine-like immunoreactivity in the visual system and brain of Drosophila melanogaster. Cell Tissue Res. 1991;266:391–398. doi: 10.1007/BF00318195. [DOI] [PubMed] [Google Scholar]
- 43.Richardt A, Rybak J, Störtkuhl KF, Meinertzhagen IA, Hovemann BT. Ebony protein in the Drosophila nervous system: optic neuropile expression in glial cells. J Comp Neurol. 2002;452:93–102. doi: 10.1002/cne.10360. [DOI] [PubMed] [Google Scholar]
- 44.Roelofs J, Van Haastert PJM. Genes lost during evolution. Nature. 2001;411:1013–1014. doi: 10.1038/35082627. [DOI] [PubMed] [Google Scholar]
- 45.Saint Marie RL, Carlson SD. The fine structure of neuroglia in the lamina ganglionaris of the housefly, Musca domestica. J Neurocytol. 1983;12:213–241. doi: 10.1007/BF01148463. [DOI] [PubMed] [Google Scholar]
- 46.Stein C, Weinreich D. Metabolism of histamine in the CNS of Aplysia californica: cellular distribution of γ-glutamylhistamine synthetase. Comp Biochem Physiol. 1983;74C:79–83. doi: 10.1016/0742-8413(83)90153-6. [DOI] [PubMed] [Google Scholar]
- 47.Stuart AE. From fruit flies to barnacles, histamine is the neurotransmitter of arthropod photoreceptors. Neuron. 1999;22:431–433. doi: 10.1016/s0896-6273(00)80699-6. [DOI] [PubMed] [Google Scholar]
- 48.Stuart AE, Morgan JR, Mekeel HE, Kempter E, Callaway JC. Selective, activity-dependent uptake of histamine into an arthropod photoreceptor. J Neurosci. 1996;16:3178–3188. doi: 10.1523/JNEUROSCI.16-10-03178.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uusitalo RO, Juusola M, Kouvalainen E, Weckström M. Tonic transmitter release in a graded potential synapse. J Neurophysiol. 1995;74:470–473. doi: 10.1152/jn.1995.74.1.470. [DOI] [PubMed] [Google Scholar]
- 50.Witkovsky P, Thoreson W, Tranchina D. Concepts and challenges in retinal biology: a tribute to John E. Dowling. Prog Brain Res. 2001;131:145–159. [Google Scholar]
- 51.Wright TRF. The genetics of biogenic amine metabolism, sclerotization, and melanization in Drosophila melanogaster. Molecular genetics of development. Adv Genet. 1987;24:127–222. [PubMed] [Google Scholar]