Abstract
Individual axon arbors within developing neural circuits are remodeled during restricted sensitive periods, leading to the emergence of precise patterns of connectivity and specialized adaptive behaviors. In male zebra finches, the circuit connecting the medial dorsolateral nucleus of the thalamus (DLM) and its cortical target, the lateral magnocellular nucleus of the anterior neostriatum (lMAN), is crucial for the acquisition of a normal vocal pattern during the sensitive period for song learning. The shell subregion of lMAN as well as the entire terminal field of DLM axons within lMAN undergo a striking increase in overall volume during early stages of vocal learning followed by an equally substantial decrease by adulthood, by which time birds have acquired stable song patterns. Because the total number of DLM neurons remains stable throughout this period, the dramatic changes within the overall DLM→lMAN circuit are presumably attributable to dynamic rearrangements at the level of individual DLM axon arbors over the course of vocal learning. To study such rearrangements directly, we reconstructed individual DLM axon arbors in three dimensions at different stages during vocal learning. Unlike axon arbors in other model systems, in which the number of branches increases during development, DLM arbors are unusual in that they have the greatest number of branches at the onset of vocal learning and undergo large-scale retraction during the sensitive period for song learning. Decreases in the degree of overlap between DLM arbors apparently contribute to the increased overall volume of the DLM→lMAN circuit during vocal learning. These developmental changes in DLM axon arbors occur at the height of the sensitive period for vocal learning, and hence may represent either a morphological correlate of song learning or a necessary prerequisite for acquisition of song.
Keywords: axons, songbirds, vocal learning, topographic, remodeling, terminals
Highly ordered patterns of connectivity within different neural circuits and the specialized behaviors that they subserve emerge during restricted sensitive periods of development. The initial formation of connections between groups of presynaptic and postsynaptic neurons is characterized by considerable specificity; early patterns of axonal connectivity appear to be genetically predetermined and do not depend on sensory experience (Catalano et al., 1991; Crair et al., 1998; Crowley and Katz, 1999). Adult patterns of connectivity emerge at the culmination of sensitive periods during a later activity-dependent phase when sensory experience is thought to refine these neural circuits at the level of individual axon arbors and synapses (Goodman and Shatz, 1993;Katz and Shatz, 1996; Tessier-Lavigne and Goodman, 1996; Weliky and Katz, 1999).
The projection connecting the medial dorsolateral nucleus of the thalamus (DLM) and its cortical target, the lateral magnocellular nucleus of the anterior neostriatum (lMAN), underlies song learning in male zebra finches (Bottjer and Arnold, 1997; Nordeen and Nordeen, 1997; Bottjer, 2001). Juvenile birds learn their songs from a tutor (normally their father) during a specific sensitive period in development by memorizing their tutor's song (∼20–40d) (Immelman, 1969; Böhner, 1990). During an overlapping phase of auditory-motor integration (25–90d), these birds perfect their own vocalizations by matching them to the tutor's song. Lesions of lMAN disrupt song only during the initial period of song learning (20–55d) and have little or no effect on the stereotyped song patterns of adult birds (Bottjer et al., 1984; Scharff and Nottebohm, 1991). Recent studies have suggested that the DLM→lMAN circuit may function during this period by generating an error signal that is used to compare the vocal motor output of juvenile birds with a copy of their tutor's song (Williams and Mehta, 1999; Brainard and Doupe, 2000a,b; Troyer and Bottjer, 2001).
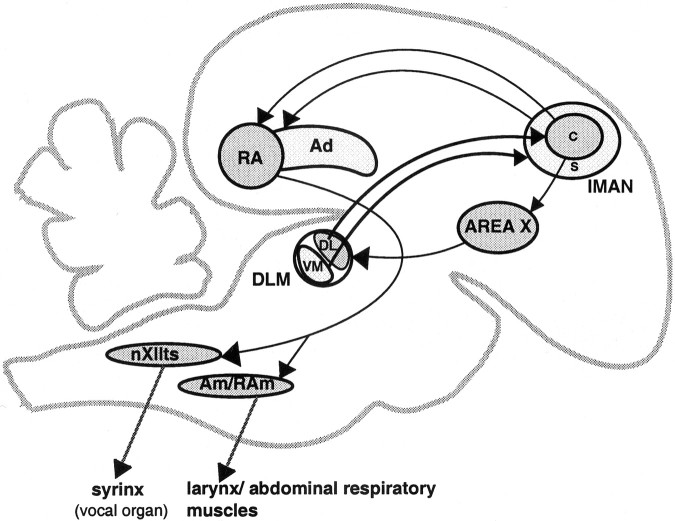
lMAN consists of a central core of predominantly magnocellular neurons surrounded by a shell of parvicellular neurons (Johnson and Bottjer, 1992) (see Fig. 1). Core and shell subregions of lMAN receive projections from distinct parts of DLM and project in turn to different postsynaptic targets: neurons in lMANcore project to the robust nucleus of the archistriatum (RA), and those in lMANshell project to the dorsal archistriatum (Ad), an area of motor cortex adjacent to RA. These pathways form distinct parallel projections within the song control system and, with the exception of the lMANcore→RA circuit, are topographically organized throughout development (Johnson et al., 1995;Iyengar et al., 1999). The shell subregion of lMAN and the DLM terminal field within this region show an almost threefold increase in overall volume between 20 and 35 d at the height of the sensitive period for vocal learning, followed by an equally dramatic decrease between 35 d and adulthood. However, because the total number of neurons within DLM remains constant throughout vocal learning, growth and regression of the overall volume of the DLM terminal field are unlikely to be attributable to changes in the number of individual axons. Rather, expansion and retraction of the DLM→lMAN projection presumably involve dynamic rearrangements in the structure of a fixed population of DLM axons (Johnson and Bottjer, 1992).
Fig. 1.
Sagittal schematic of the song control system of male zebra finches demonstrating parallel “core” and “shell” connections of lMAN. Area X (a nucleus of the avian basal ganglia) projects to the thalamic nucleus DLM. The dorsolateral (DL) subregion of DLM projects to a central core of magnocellular neurons within lMAN (lMANcore), whereas the ventromedial (VM) subdivision of DLM projects to a surrounding shell of parvicellular neurons within lMAN (lMANshell) (Johnson and Bottjer, 1992). The core region of lMAN projects to the motor cortical nucleus RA, whereas lMANshell projects specifically to Ad, an area adjacent to RA (Bottjer et al., 1989; Johnson et al., 1995; Vates and Nottebohm, 1995; Iyengar et al., 1999). Ad, Dorsal archistriatum;Am/RAm, nucleus ambiguus/retroambigualis;DLM, medial portion of the dorsolateral nucleus of the anterior thalamus; lMAN, lateral magnocellular nucleus of the anterior neostriatum (c, core; s, shell); LPO, lobus parolfactorius (medial portion of avian striatum); RA, robust nucleus of the archistriatum; X, area X of avian striatum;nXIIts, tracheosyringeal part of the hypoglossal nucleus.
To study these changes in DLM axon arbors, we made small iontophoretic injections of the anterograde tracer biocytin into DLM and reconstructed individual axons in three dimensions. We found large-scale pruning of the branches of these axons over the course of vocal learning, unlike axon arbors that have been described in other developing systems (Crepel et al., 1976, 1980; Lichtman, 1977; Smolen and Raisman, 1979; Mariani and Changeux, 1980, 1981; Johnson and Purves, 1981; Sretavan and Shatz, 1984, 1986; Rubin, 1985; Young and Rubel, 1986; Callaway and Katz, 1991; Agmon et al., 1993; Antonini and Stryker, 1993; Catalano et al., 1996; Gan and Lichtman, 1998). Additionally, the dramatic changes in overall volume of the DLM terminal field within lMAN could be explained by changes in spatial extent and degree of overlap between individual DLM axon arbors. These striking developmental differences in DLM axon arbors may be a prerequisite for vocal learning, or conversely may be an experience-dependent consequence of song acquisition.
MATERIALS AND METHODS
All birds used in this study were bred in group aviaries and had received normal exposure to song up until the time of the experiment. Surgical procedures were in accordance with National Institutes of Health guidelines and the Animal Care and Use Committee at the University of Southern California.
Biocytin injections
Adult (>90 d), 35d (33–37 d), and 20d (18–22 d) male zebra finches (n = 4 per group) (see Tables 1, 2) were anesthetized with the barbiturate anesthetic Equithesin (0.04 ml/10 gm) and placed in a stereotaxic apparatus. Micropipettes (25–30 μm outer diameter) filled with the anterograde tracer biocytin (5% in 0.05m Tris HCl, pH 8.0) were lowered into DLM. Iontophoretic injections of biocytin were made into different subregions of right and left DLM by pulsing positive current through a silver wire immersed in the biocytin solution (6 sec on/6 sec off, 4–8 μA) for 3–6 min. Twenty-four hours after surgery, birds were deeply anesthetized and perfused transcardially with 0.7% saline followed by a solution of 4% paraformaldehyde and 0.4% glutaraldehyde, pH 7.8. Brains were removed, post-fixed in 4% paraformaldehyde for 5–7 d, and then cryoprotected in 25% sucrose overnight. Brains were sectioned coronally at a thickness of 60 μm, and sections were stored in free-floating wells in a solution of 0.02 m PBS with 0.1% sodium azide at 4°C.
Table 1.
Number of injections used for analysis of individual DLM axon arbors within core and shell regions of lMAN
| Age of birds used | Number of birds | Number of injections | Position of injections in DLM | Number of arbors | |||
|---|---|---|---|---|---|---|---|
| DLMDL | DLMVM | DLMDL + DLMVM | DLMDL | DLMVM | |||
| 20 d | 4 | 5 | 1 | 0 | 4 | 9 | 7 |
| 35 d | 4 | 5 | 2 | 1 | 2 | 10 | 6 |
| Adult | 4 | 6 | 0 | 0 | 6 | 6 | 8 |
| Total | 12 | 16 | 3 | 1 | 12 | 25 | 21 |
Table 2.
Number of DLMDL and DLMVM arbors reconstructed from birds at different ages
| Age | Birds | Number of DLMDL arbors reconstructed | Birds | Number of DLMVM arbors reconstructed |
|---|---|---|---|---|
| 20 d | Lg522 | 4 | Lg522 | 4 |
| W295 | 4 | W518 | 3 | |
| Y360 | 1 | |||
| 35 d | R676 | 5 | Y251 | 1 |
| Y251 | 2 | Y328 | 1 | |
| Y328 | 3 | R683 | 1 | |
| R676 | 3 | |||
| Adult | Dg915 | 4 | Dg915 | 4 |
| Dg949 | 1 | Dg969 | 1 | |
| Dg969 | 1 | Lg995 | 3 |
The immunohistochemical reaction used to visualize biocytin has been published previously (Foster et al., 1997; Foster and Bottjer, 1998;Bottjer et al., 2000) (cf. Bernard et al., 1993). Briefly, sections were rinsed in 0.02 m PBS and transferred into a 1% solution of hydrogen peroxide (H2O2) for 30–40 min to quench endogenous peroxidase activity. Sections were immersed in normal rabbit serum (5% in 0.3% Triton-X, blocking step) for 1 hr and then in goat anti-biotin antibody (1:20,000 in 0.3% Triton-X; Vector Labs, Burlingame, CA) overnight at room temperature. The next day they were incubated in anti-goat IgG (1:200 in 0.3% Triton-X solution; Vector Labs) and then transferred into avidin–biotin conjugate (1:100; Vector Elite Kits); each step lasted 1 hr. After a preincubation step in chromagen 3, 3′-diaminobenzidine (DAB) (0.05% solution in 0.02m PBS) for 15 min, sections were transferred into 0.05% DAB-PBS containing 0.003% H2O2 for 5 min, and then into a 0.015% H2O2/0.05% DAB solution until reaction product of the desired intensity was achieved. After further rinses in 0.02 m PBS, sections were mounted onto gelatin-coated slides, air dried overnight, and cleared in xylene and coverslipped using Permount.
Arbor reconstructions
A total of 25 DLMDL axons within lMANcore and 21 DLMVM axons within lMANshell were selected for serial reconstruction in three dimensions on the basis of careful visual inspection (Fig. 1, Tables1, 2). Major branches as well as fine terminal arborizations of these axons were completely labeled with biocytin, with no gaps or fading of the reaction product (Fig. 2). The cut ends of all axonal branches could also be reliably identified and matched in serial sections of lMAN. Most of the axon arbors reconstructed in this study were located in areas of lMAN with few other arbors such that they could be visualized and traced easily. In cases in which the density of anterograde label over lMAN was relatively high, only those arbors that could be unambiguously discerned in their entirety were reconstructed to avoid errors in tracing (average ± SD = 3 ± 2 axon arbors; range, 1–7 arbors for each injection site).
Fig. 2.
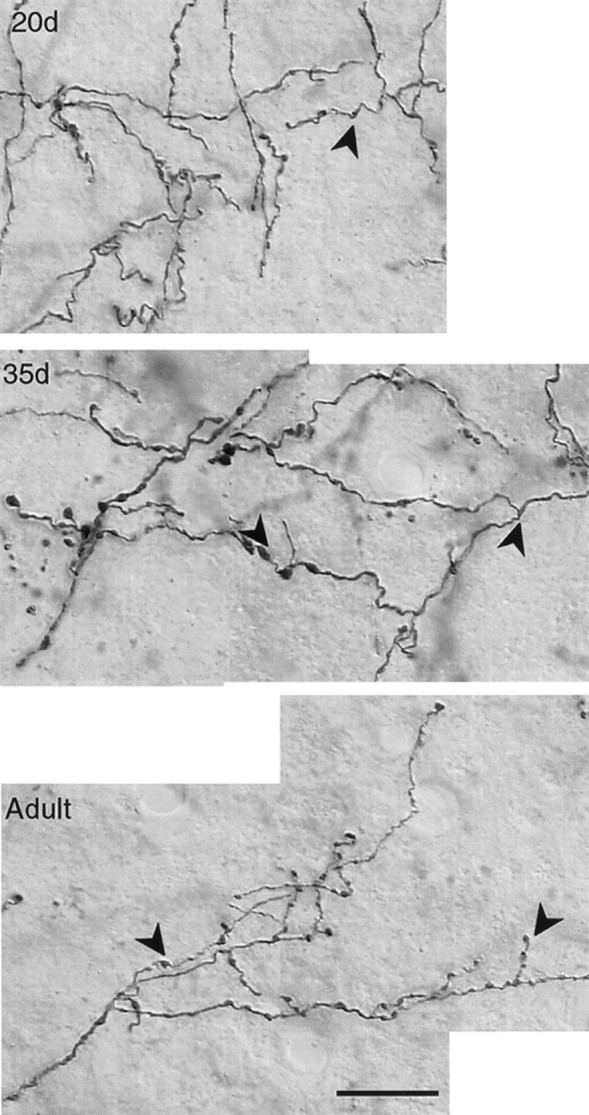
Photomicrographs demonstrating the presence of varicosities (examples marked by arrowheads) that may represent sites of synaptic contact along the length of biocytin-labeled DLMVM axon arbors within lMAN in20d, 35d, and Adult birds. There was no difference in the pattern of varicosities along DLMVM and DLMDL arbors at any age. Scale bar, 10 μm.
All DLM axon arbors were reconstructed in three dimensions using an image analysis system with a depth encoder interfaced with a microscope at a final magnification of 1000× (Neurotrace, InterAction, Boston, MA) (cf. Passera et al., 1988; Antonini and Stryker, 1993, 1996;Antonini et al., 1998). After traversing area X and the surrounding basal ganglia, the main axon of each DLM arbor entered the ventral part of lMAN and divided into branches. To have a consistent starting point for all reconstructions, each arbor was traced starting at the first branch point of the axon within lMAN. Although the caudoventral portions of the main axons were not included in our reconstructions, they were followed as far back as possible to ensure that individual axons did not branch as they traversed area X and the surrounding basal ganglia en route to lMAN. None of the axons that we examined ever extended side branches before entering lMAN.
The actual thickness of each section was measured using a depth encoder attached to the fine focus of the microscope and by carefully focusing at the top and bottom of each section. Although brains had been sectioned at a thickness of 60 μm, individual sections underwent significant shrinkage in thickness during subsequent processing. A scaling factor was calculated to correct for shrinkage in thez-axis by dividing 60 μm by the actual thickness of that section after processing. The average scaling factor for different sections in which each DLM axon was present was used for the entire arbor to correct for shrinkage in the z-axis (average ± SD = 6.0 ± 0.8 over all arbors). Shrinkage in the medial-lateral and dorsal-ventral (x-y) axes was minor and was not corrected (cf. Antonini and Stryker, 1993). After all arbors were traced, individual arbor reconstructions were flattened along the anterior-posterior axis (that is, the depth of these reconstructions was collapsed) and viewed as two-dimensional drawings that were used for obtaining measures of the medial-lateral and dorsal-ventral extent of each arbor (see below).
Although the outer borders of DLM and lMAN were visible in the immunostained sections, the distinction between lMANcore and lMANshell was not obvious in the absence of Nissl staining. To confirm the location of injection sites in DLM and the resulting anterograde label as being within the core or shell of lMAN, the outer borders of DLM and lMAN in the immunostained sections were traced initially using a camera lucida. Tracings at the level of lMAN also included landmarks such as outlines of blood vessels and two major fiber tracts, the hyperstriatal lamina and dorsomedial lamina, which are located dorsal and ventral to lMAN, respectively, as reference points. Slides were then immersed in xylene overnight to remove coverslips, Nissl stained with thionin, and coverslipped again. The exact location of each DLM arbor within lMAN core or shell was then confirmed in each section by aligning the outlines of blood vessels and fiber tracts with the camera lucida tracings that had been drawn before Nissl staining. In addition, the biocytin label was still visible in the Nissl-stained sections, thereby allowing direct observation of axon arbors as being within core or shell. The boundaries of lMAN core and shell were defined on the basis of the somal size of neurons; that is, lMANcorecontains a high density of magnocellular neurons whereas lMANshell contains a high density of parvicellular neurons (Johnson and Bottjer, 1992; Johnson et al., 1995).
Analysis
Measures of complexity of arbors. DLM axon arbors varied in complexity, but all axons divided into a large number of branches arranged in several orders of branching beyond the first branch point. The Neurotrace program was used to obtain measurements of the number of branches, orders of branching, average branch length, and total length of each arbor, which were used as measures of complexity to compare across different ages. The number of branches is the sum of all branches for each axon, whereas order of branching measures how many tiers of branches each axon has. Average branch length was obtained directly from the Neurotrace program and is equivalent to the total length of single axons divided by the number of branches. The total length of each arbor is the sum of the length of all branches of each arbor and also provides a measure of the net growth or retraction of axon arbors during development (cf. Antonini and Stryker, 1993,1996).
Measures of spatial extent Of arbors. One measure of spatial extent was assessed by determining the maximal extent of each arbor along each axis. The maximum anterior-posterior extent of each reconstructed DLM arbor was obtained directly from the Neurotrace program and was multiplied by the scaling factor for that arbor to get the corrected depth. Software from Media Cybernetics (Image Pro Plus) was used to determine the maximum medial-lateral and dorsal-ventral extent of each arbor from two-dimensional drawings of these arbors flattened along their anterior-posterior extent.
As a second measure of spatial extent, we calculated the volume of each arbor. The two-dimensional area encompassed by each arbor was determined by tracing around the flattened outlines of the branches of each DLM axon. Larger gaps between major branches were excluded from this measure by tracing around these branches, whereas very small gaps (<300 μm2) between fine branches of dense terminal arborizations were included in this measure (cf. Cline and Constantine-Paton, 1990; Hata et al., 1999). This two-dimensional area was multiplied by the corrected depth of each DLM arbor to estimate the volume of lMAN encompassed by that arbor. We also divided the volume of each DLMDL and DLMVM arbor for 20d, 35d, and adult birds by the average volumes of lMANcore and lMANshell at these ages, respectively (Johnson et al., 1995, their Fig. 14), to estimate the proportion of lMAN occupied by single arbors. Assuming that the number of DLM axons does not change over the course of song learning (because the total number of DLM neurons does not change over song learning) (Johnson and Bottjer, 1992), this measure estimates the degree of overlap between arbors within subregions of lMAN at different ages.
All quantitative measures were evaluated by parametric analyses of variance unless the data failed tests for either normality or homogeneity of variance, in which case a nonparametric ANOVA (Kruskal–Wallis) was used where indicated.
RESULTS
Analysis of individual DLM axon arbors within lMAN core and shell
Labeled DLM axons traveled rostrally in the lateral forebrain bundle and then continued laterally and rostrally within area X and surrounding basal ganglia before entering lMAN (cf. Bottjer et al., 1989; Johnson and Bottjer, 1992). Most of the axons that arborized in medial core or shell regions of lMAN traversed the medial part of area X or lobus parolfactorius, whereas axons that arborized within lateral lMAN core or shell ascended within the lateral part of area X. However, some axons ascended within the intermediate part of area X and then turned medially or laterally before forming a terminal arborization. In addition, a few axons entered medial lMAN and then turned laterally to arborize within intermediate or lateral lMAN, and vice versa. Thus, axons of DLM neurons occasionally traversed different subregions of area X and lMAN before arborizing within a topographically appropriate location in lMAN core or shell (cf. Simon and O'Leary, 1992).
Individual DLM axon arbors within both lMANcoreand lMANshell were highly variable in shape at each age (Figs. 3,4). Careful observation revealed that axons that arborized within lMANcorenever extended branches into lMANshell at any age and vice versa. This segregation at the level of single arbors confirms earlier results indicating that the DLMDL→lMANcore and DLMVM→lMANshell circuits remain as separate, parallel pathways throughout the course of song learning (cf. Johnson et al., 1995; Iyengar et al., 1999). A large number of varicosities were distributed along branches and at branch tips of all DLM axons (Fig. 2, arrowheads). These varicosities may represent boutons en passant and terminal boutons [i.e., sites of synaptic contact between DLM and lMAN; but see LeVay and Stryker (1979), Antonini and Stryker (1993), Seki and Arai (1999), and Jacoby and Marshak (2000)].
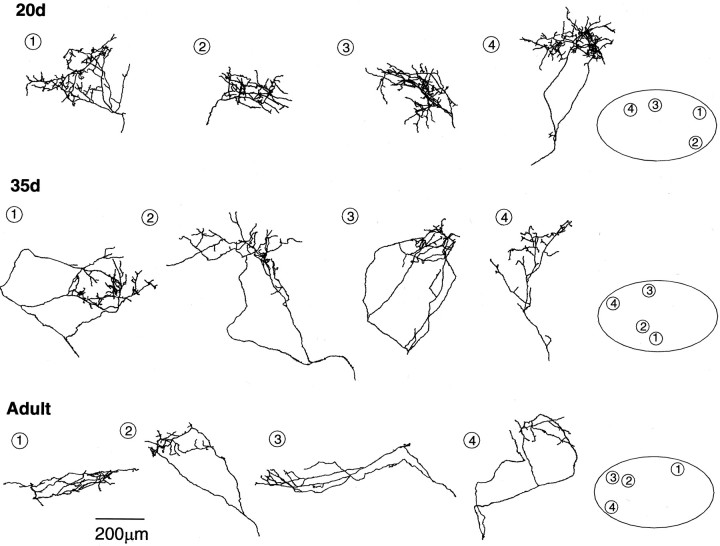
Fig. 3.
Reconstructions of biocytin-labeled DLMDL axon arbors within different subregions of lMANcore in 20d, 35d, andAdult zebra finches. The relative positions of each arbor within different subregions of lMANcore (depicted byoval outlines on the right; medial =left, dorsal = up) are represented bynumbers. The density of branching of DLMDLarbors is highest at 20 d and lowest in adults, indicating that these arbors undergo retraction between 20 d and adulthood. Scale bar, 200 μm.
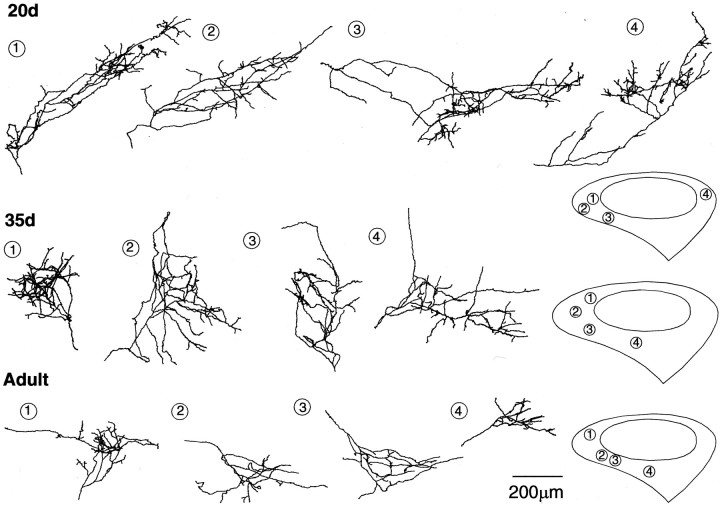
Fig. 4.
Reconstructions of biocytin-labeled DLMVM axon arbors within different subregions of lMANshell in 20d, 35d, andAdult zebra finches. Schematics on the rightdepict the outlines of lMANshell and lMANcore(medial = left, dorsal = up), andnumbers represent the positions of DLMVMarbors within lMANshell. DLMVM arbors are similar to DLMDL arbors in that the density of branching of DLMVM axon arbors is highest at 20 d and lowest at adulthood, demonstrating that these arbors undergo substantial retraction over the course of song learning. The spatial extent of DLMVM axon arbors also decreases significantly over song learning. Scale bar, 200 μm.
Analysis of DLMDL axon arbors in lMANcore
Changes in complexity of DLMDL axon arbors during song learning
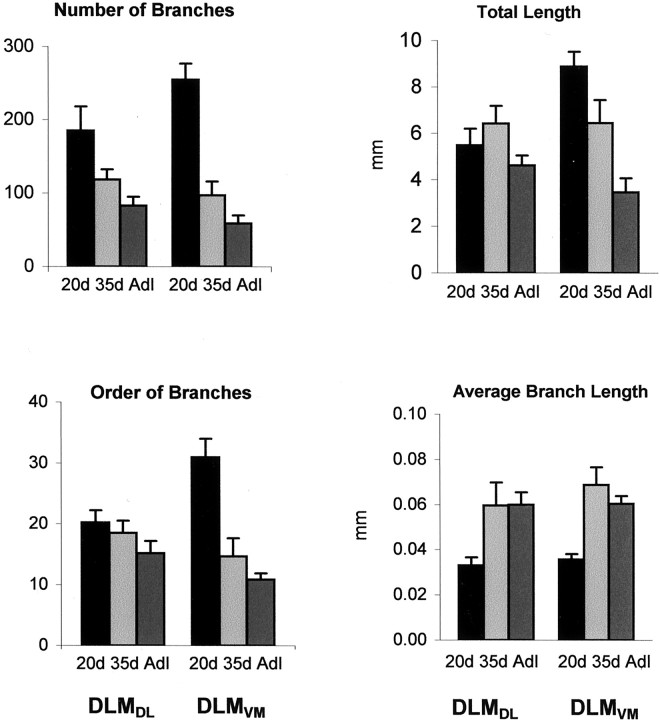
DLMDL axons elaborated a large number of branches arranged in several tiers in 20d birds, giving rise to dense terminal arborizations within different regions of lMANcore (Fig. 3). Branch number decreased throughout the course of vocal learning (F(2,22) = 5.09; p = 0.02) (Figure 5, Table3), such that DLMDLaxons had sparse terminal arborizations with relatively few branches in adult birds compared with the more complex arbors in juvenile birds. Planned comparisons revealed that DLMDL axons had significantly more branches at 20 d compared with 35 d (p = 0.03). Branch number decreased further between 35 d and adulthood, although this difference was not significant (p = 0.29).
Fig. 5.
Quantitative analysis demonstrating the decrease in complexity of DLM axon arbors within lMANcore(left side of each graph) and lMANshell(right side of each graph) during vocal learning.Top left, The number of DLMDL branches in lMANcore as well as of DLMVM branches in lMANshell decreased significantly between 20 and 35 d.Bottom left, DLMVM arbors had substantially fewer tiers of branches in 35d birds compared with20d and adult (Adl) birds. The decrease in this measure in DLMDL arbors over the course of song learning was not significant. Top right, The total length of DLMVM arbors decreased significantly over the course of vocal learning. Changes in total length of DLMDL arbors were not significant. Bottom right, The average length of branches of DLMDL and DLMVM arbors increased between 20 and 35 d and did not change substantially thereafter, showing that branches remaining after retraction tended to be longer in both sets of arbors. Average length of branches and total length of DLM arbors are measured in millimeters, and error bars represent SEMs.
Table 3.
Quantitative analysis of DLMDL arbors in lMANcore
| Age | Number of branches | Order of branches | Total length (mm) | Average branch length (mm) | Medial-lateral extent (mm) | Dorsal-ventral extent (mm) | Anterior-posterior extent (mm) | Arbor volume (mm3) | Volume of lMANcore(mm3)3-a | % of lMANcore occupied |
|---|---|---|---|---|---|---|---|---|---|---|
| 20 d | 185 ± 92 | 20 ± 7 | 5.48 ± 2.15 | 0.033 ± 0.011 | 0.40 ± 0.12 | 0.31 ± 0.14 | 0.13 ± 0.05 | 0.0033 ± 0.002 | 0.22 ± 0.05 | 0.015 ± 0.009 |
| 35 d | 118 ± 45 | 19 ± 5 | 6.41 ± 2.42 | 0.060 ± 0.032 | 0.64 ± 0.30 | 0.45 ± 0.11 | 0.26 ± 0.19 | 0.0134 ± 0.018 | 0.21 ± 0.04 | 0.064 ± 0.085 |
| Adult | 83 ± 30 | 15 ± 4 | 4.62 ± 1.04 | 0.060 ± 0.014 | 0.60 ± 0.30 | 0.31 ± 0.17 | 0.21 ± 0.11 | 0.0050 ± 0.005 | 0.12 ± 0.01 | 0.042 ± 0.042 |
All measures are expressed as average ± SD; see Results for details.
Nissl-defined volumes of lMANcore after Johnson and Bottjer (1992).
Two other measures of complexity of DLMDL arbors, orders of branching (F(2,22) = 1.33;p = 0.29), and total length of DLMDL arbors (F(2,22) = 1.44; p = 0.26) did not change significantly during song learning (Fig. 5, Table3). The total length of DLMDL arbors was slightly greater at 35 d than at 20 d, although individual arbors had fewer branches at 35 d. This pattern was caused by an increase in the average length of axonal branches at 35 d compared with 20 d (F(2,22) = 4.14;p = 0.03), indicating that although a large number of branches were retracted between 20 and 35 d, those that remained were longer. Taken together, the overall pattern of results suggests that DLMDL axon arbors are most complex with elaborate terminal arborizations at the onset of song learning and then undergo net retraction during the sensitive period for vocal learning.
Changes in spatial extent of DLMDL axon arbors during song learning
DLMDL arbors were compact at 20 d and then expanded somewhat by 35 d of age (Fig. 3). In adult birds, the spatial extent of these arbors was slightly smaller than those at 35 d. DLMDL axons tended to divide into several tiers of smaller branches immediately after entering lMANcore in 20d birds, whereas initial branches of DLMDL axons were apt to traverse a larger spatial extent within lMANcore before forming dense terminal arbors in 35d and adult birds. When analyzed quantitatively, there was no significant difference in the tangential extent of DLMDL arbors along any axis during song learning (medial-lateral: F(2,22) = 2.38, p = 0.12; dorsal-ventral:F(2,22) = 2.88, p = 0.08; anterior-posterior: F(2,22) = 2.10, p = 0.15). However, all three measures tended to increase between 20 and 35 d and then decrease between 35 d and adulthood (Fig. 6, Table 3).
Fig. 6.
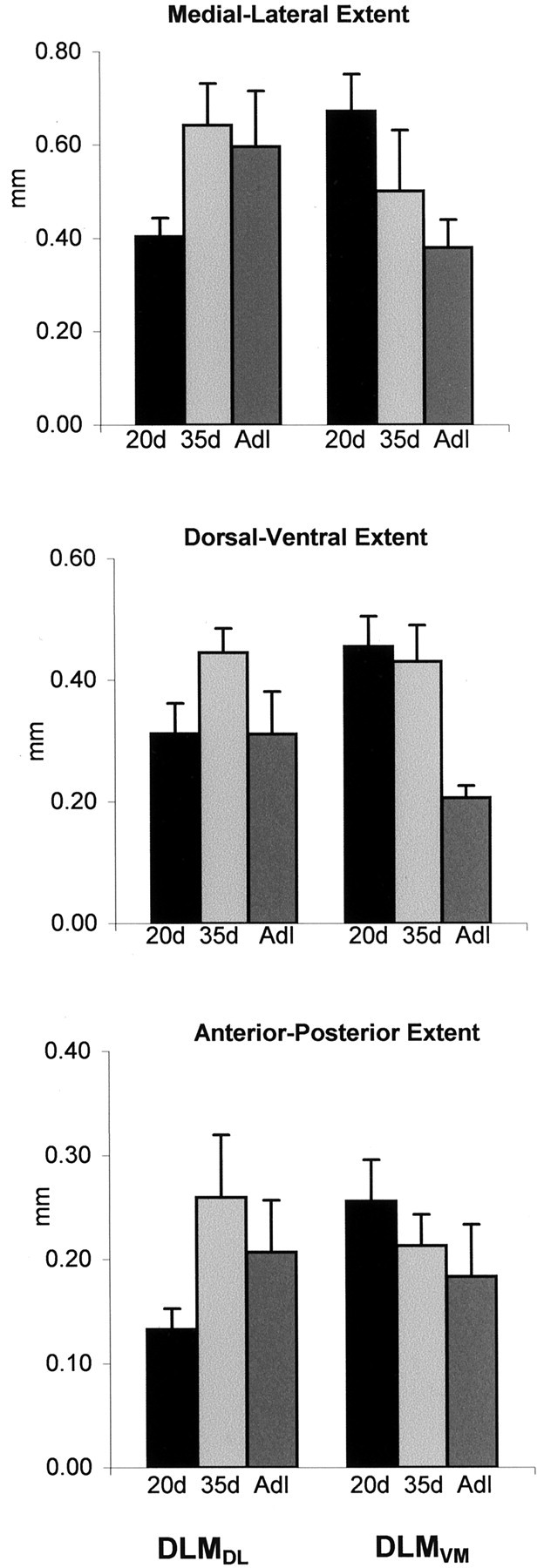
Changes in spatial extent along different axes of DLM axon arbors within lMANcore and lMANshell over the course of vocal learning measured in millimeters; error bars represent SEMs. The maximal extent of medial-lateral, dorsal-ventral, and anterior-posterior DLMDL axons increased between 20 and 35 d and then decreased between 35 d and adulthood, although these differences were not significant. All three measures of spatial extent decreased over the course of song learning in DLMVM arbors. This decrease was significant in the medial-lateral extent of DLMVM arbors between 20 d and adulthood and in the dorsal-ventral extent of these arbors between 35 d and adulthood.
The increase in spatial extent along all axes of DLMDL arbors between 20 and 35 d contributed to an increase in the volume encompassed by individual arbors, although the volume of lMANcore does not change during this period of song learning (Johnson and Bottjer, 1992) (Fig.7, Table 3). The increase in volume encompassed by DLMDL arbors and corresponding lack of change in the size of their postsynaptic target led to an increase in the proportion of lMANcore occupied by individual arbors in 35d birds. The volume encompassed by DLMDL arbors then decreased between 35 d and adulthood. The volume of lMANcore also decreased during this interval, although less than that of individual arbors, thereby producing a modest decrease in the proportion of lMANcore occupied by DLMDLarbors (Fig. 7, Table 3). The changes in volume encompassed by individual arbors during song learning were not significant as judged by a Kruskal–Wallis ANOVA (H(2) = 3.70; p = 0.16), whereas changes in the proportion of lMANcore occupied by individual arbors were marginally significant (H(2) = 5.93;p = 0.05). This latter difference was entirely attributable to the increase in proportion of lMANcore occupied by individual arbors between 20 and 35 d (p = 0.04; 35d vs adult:p = 0.96). These measures of spatial extent suggest a trend toward increased overlap between DLMDL axon arbors within lMANcore between 20 and 35 d, followed by a decrease in overlap between 35 d and adulthood. These results also suggest that DLMDL axons contact lMANcore neurons over a greater spatial extent at 35 d than at 20 d or adulthood.
Fig. 7.
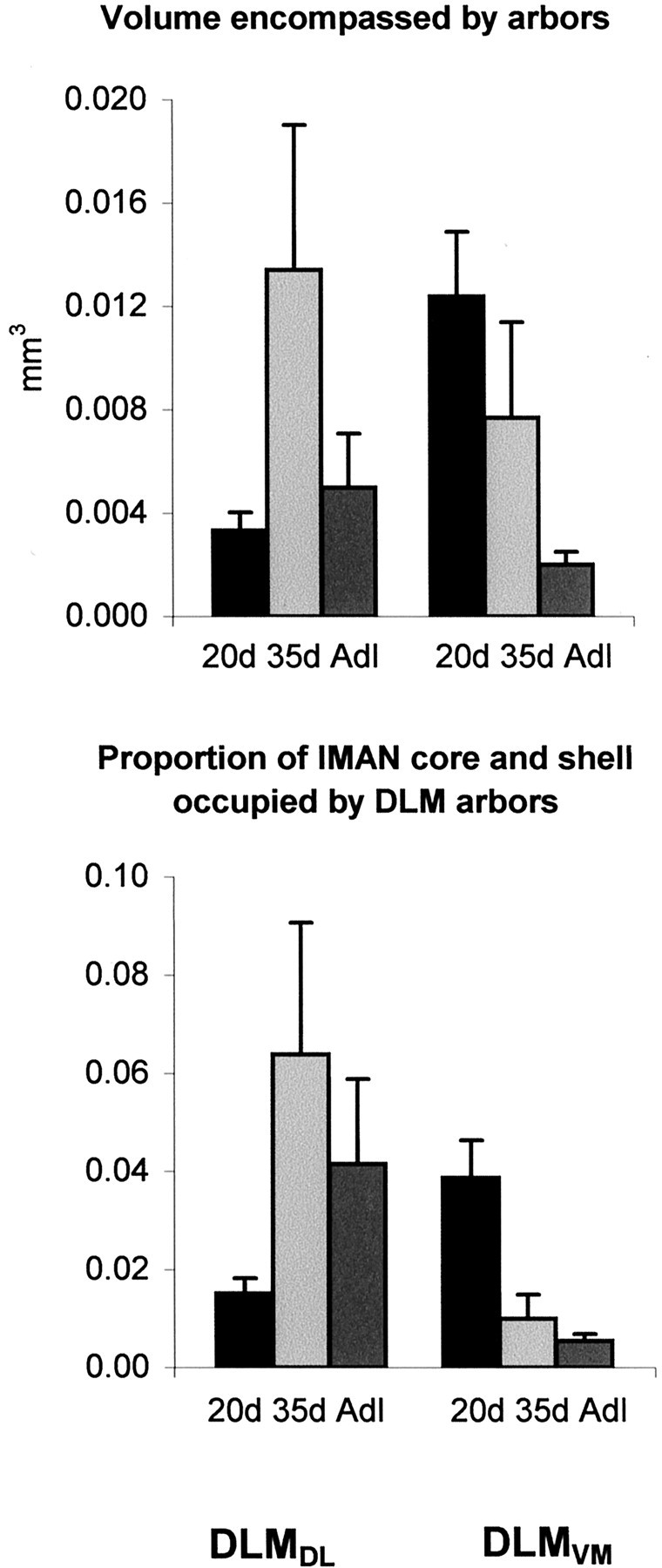
The volume encompassed by single arbors (inmm3; top panel) and the proportion of lMAN occupied by DLM arbors (bottom panel); means ± SEM. The volume encompassed by DLMDL arbors increased between 20 and 35 d and then decreased between 35 d and adulthood, although these changes were not significant. However, the proportion of lMANcoreoccupied by individual arbors increased between 20 and 35 d followed by a slight decrease between 35 d and adulthood. The volume encompassed by DLMVM arbors decreased significantly between 20 d and adulthood. The decrease in this measure, accompanied by the dramatic increase in overall volume of lMANshell between 20 and 35 d, produced a significant decrease in the proportion of lMANshell occupied by single arbors, suggesting that the degree of overlap between DLMVMarbors decreases substantially during the sensitive period for song learning. There was no significant change in the proportion of lMANshell occupied by DLMVM arbors between 35 d and adulthood.
Analysis of DLMVM axon arbors within lMANshell
Changes in complexity of DLMVM axon arbors during song learning
The terminal arborizations of DLMVM axons within lMANshell were similar to those of DLMDL axons in lMANcore in that they had the largest number of branches arranged in several tiers at 20 d (Fig. 4). Branch number of DLMVMarbors decreased over the course of song learning (F(2, 18) = 38.60; p< 0.0001), such that axons of adult birds had relatively sparse terminal arbors within lMANshell (Fig. 5, Table4). Planned comparisons showed that DLMVM arbors had substantially more branches at 20 d than at 35 d (p < 0.0001). Branch number decreased further between 35 d and adulthood, although this difference was not significant (p= 0.13). Orders of branching of DLMVM arbors also decreased significantly over the course of song learning (F(2, 18) = 23.80; p< 0.0001). Branch order decreased between 20 and 35 d (p < 0.01), but not between 35 d and adulthood (p = 0.228). The total length of DLMVM arbors also decreased significantly during song learning (F(2, 18) = 14.88;p = 0.0002). Arbors of DLMVMneurons were longer at 20 d than at 35 d (p = 0.04) and were also longer in 35d birds than in adults (p = 0.01). Because the number of branches decreased sharply between 20 and 35 d, whereas the total length of arbors showed a more modest decrease, the average branch length increased during this interval (F(2, 18) = 13.01; p = 0.0003). Overall, these results reveal that DLMVM arbors undergo large-scale retraction during vocal learning, particularly between 20 and 35 d, and that regression of DLMVMarbors is greater than that of DLMDL axons between 20 and 35 d.
Table 4.
Quantitative analysis of DLMVM arbors in lMANshell
| Age | Number of branches | Order of branches | Total length (mm) | Average branch length (mm) | Medial-lateral extent (mm) | Dorsal-ventral extent (mm) | Anterior-posterior extent (mm) | Arbor volume (mm3) | Volume of lMANshell (mm3)4-a | % of lMANshell occupied |
|---|---|---|---|---|---|---|---|---|---|---|
| 20 d | 255 ± 59 | 31 ± 7 | 8.88 ± 1.70 | 0.036 ± 0.006 | 0.67 ± 0.22 | 0.46 ± 0.13 | 0.26 ± 0.11 | 0.0124 ± 0.006 | 0.32 ± 0.09 | 0.039 ± 0.020 |
| 35 d | 97 ± 41 | 15 ± 7 | 6.45 ± 2.42 | 0.069 ± 0.019 | 0.50 ± 0.31 | 0.43 ± 0.15 | 0.21 ± 0.08 | 0.0077 ± 0.009 | 0.77 ± 0.15 | 0.010 ± 0.011 |
| Adult | 59 ± 31 | 11 ± 4 | 3.47 ± 1.69 | 0.060 ± 0.01 | 0.38 ± 0.16 | 0.21 ± 0.06 | 0.18 ± 0.15 | 0.0020 ± 0.002 | 0.37 ± 0.15 | 0.005 ± 0.004 |
All measures are expressed as average ± SD; see Results for details.
Nissl-defined volumes of lMANshell after Johnson and Bottjer (1992).
Changes in spatial extent of DLMVM axon arbors during song learning
Axon arbors of DLMVM neurons decreased in spatial extent over the course of vocal learning such that they were more compact in adult birds compared with those in juvenile birds (Fig.6, Table 4). Unlike initial branches of DLMDLaxons at 35 d and adulthood, which typically extended for some distance within lMANcore before dividing into finer terminals, the majority of DLMVM axons tended to branch immediately after entering lMANshell at all three ages studied (compare Figs. 3 and 4). Quantitative analysis revealed that the dorsal-ventral extent of DLMVM arbors decreased significantly during song learning (F(2, 18) = 10.5;p < 0.001). Although there was no difference in the dorsal-ventral extent of DLMVM arbors between 20 and 35 d (p > 0.50), this measure decreased significantly between 35 d and adulthood (p = 0.002). There was no difference in anterior-posterior extent of DLMVM arbors across ages (F < 1), whereas the medial-lateral extent of these arbors tended to decrease over the course of song learning (F(2, 18) = 3.08; p > 0.07). Planned comparisons showed that this latter difference was entirely attributable to the overall decrease seen between 20 d and adulthood (p = 0.02).
The decrease in spatial extent of DLMVM axon arbors was reflected in a significant decrease in the volume encompassed by these arbors over the course of song learning (Kruskal–Wallis: H(2) = 9.39;p = 0.01) (Fig. 7, Table 4). Planned comparisons showed that the decrease in volume encompassed by individual arbors was not significant between 20 and 35 d (p = 0.14) or between 35 d and adulthood (p = 0.11). However, the sixfold decrease in volume encompassed by DLMVM arbors within lMANshell between 20 d and adulthood was highly significant (p = 0.002). The proportion of lMANshell occupied by individual DLMVM axon arbors also decreased significantly over the course of song learning (H(2)= 11.00; p = 0.004). The volume of lMANshell is small at 20 d relative to 35 d (Johnson and Bottjer, 1992), whereas the volume encompassed by DLMVM arbors is maximal at 20 d. Therefore, the proportion of lMANshell occupied by individual arbors is greatest at 20 d. The large decrease in the volume encompassed by individual DLMVMarbors, accompanied by the almost threefold increase in the overall volume of lMANshell between 20 and 35 d, led to a significant decrease in the proportion of lMANshell occupied by DLMVMarbors during this period (p = 0.01). The proportion of lMANshell occupied by DLMVM axon arbors did not change between 35 d and adulthood (p = 0.57), because the decrease in volume encompassed by DLMVM arbors is offset by a comparable decrease in the overall volume of lMANshell during this period. Our results suggest that the increase in volume of lMANshellcoincides with the decrease in volume encompassed by DLMVM axon arbors between 20 and 35 d, such that the degree of overlap between these arbors decreases greatly between 20 and 35 d and does not change appreciably thereafter.
DISCUSSION
The present results demonstrate that individual thalamocortical axons in the song-control pathway from DLM to lMAN are highly complex and have the greatest number of branches at 20 d and then undergo large-scale pruning during the sensitive period for song learning. Interestingly, the regression of individual arbors was particularly pronounced in the shell region of lMAN during early stages of song learning, when lMANshell is growing dramatically (including a substantial increase in neuron number) (Johnson and Bottjer, 1992; S. W. Bottjer, unpublished observations). Thus, smaller arbors with a reduced spatial extent come to encompass a greatly expanded postsynaptic target space in this pathway during the sensitive period for vocal learning. This pattern results in a substantial decrease in the proportion of lMANshell occupied by single DLMVM arbors in 35d birds, suggesting a concomitant decrease in overlap between these arbors and a greater level of specificity within the topographic organization of this pathway at the height of the sensitive period for vocal learning. Thereafter the overall volume of lMANshellregresses between 35 d and adulthood, by which time vocal learning is complete. Thalamic arbors within the shell region also continue to regress, such that the proportion of lMANshelloccupied by single arbors is not substantially different in 35d and adult birds. Thus, specificity of axonal connections may not change appreciably after 35 d in this pathway, although active arbor remodeling must be occurring continuously as the overall size of lMANshell decreases (presumably because of cell death).
In contrast, although the branch number of thalamic arbors within lMANcore also decreases during early stages of vocal learning, overall arbor regression is much less pronounced (the volume encompassed by individual arbors tends to increase between 20 and 35 d), and the overall volume (and neuron number) of lMANcore does not change during this period. The proportion of lMANcore that is occupied by individual DLMDL arbors actually increases between 20 and 35 d, suggesting that the specificity of axonal connectivity might decrease somewhat over this interval. Of course, we cannot rule out the possibility that the increased proportion of postsynaptic space occupied by arbors in 35d birds could represent axonal remodeling that simply contributes to a different or enhanced unit of functional connectivity within lMANcore. Interestingly, spine frequencies on dendrites of lMANcore neurons increase until 35 d, after which spine density and the radial extent of distal dendrites both decrease as the overall volume of lMANcoreundergoes a small but significant regression (Johnson et al., 1995;Nixdorf-Bergweiler et al., 1995). Thus, the spatial expansion of presynaptic DLMDL arbors that we observed between 20 and 35 d, followed by regression, correlates well with the postsynaptic pattern. Overall, these results suggest that the degree of overlap between DLMDL arbors and hence precision of matching between subgroups of presynaptic and postsynaptic neurons within DLMDL and lMANcoremay decrease somewhat between 20 and 35 d and then improve between 35 d and adulthood.
Functional implications of changes in DLM axon arbors during the sensitive period for vocal learning
What do changes within individual DLM axon arbors during the sensitive period for song learning represent? Lesions of lMAN in juvenile birds have demonstrated that this nucleus is critical for the acquisition of a normal song during early stages of vocal learning, from ∼20 to 60 d of age (Bottjer et al., 1984; Bottjer and Arnold, 1986; Scharff and Nottebohm, 1991). During this time, neurons within lMAN respond to auditory playback of tutor song as well as to other zebra finch songs but gradually become selectively tuned to their own self-produced songs by ∼60 d (Solis and Doupe, 1997, 1999) (cf.Doupe and Solis, 1997). Thus, an internal representation of the bird's song may develop within lMAN as a result of learning and be used to generate an error signal required to correct vocal output during song learning (cf. Williams and Mehta, 1999; Brainard and Doupe, 2000a,b). Once lMAN neurons become tuned to the bird's own song, the error signal may decrease to the point at which lMAN is no longer playing an active role in normal song development. The changes in complexity and spatial extent of DLM axon arbors described in this study indicate synaptic remodeling within the DLM→lMAN circuit, which may represent structural correlates of vocal learning. For example, arbor regression may represent a morphological correlate of the auditory tuning of lMAN neurons to the bird's own song and the decreased involvement of lMAN neurons in vocal learning. Future studies will be needed to determine whether remodeling of individual DLM arbors is guided by song-related experience.
It is interesting that changes in individual DLM arbors were particularly profound in the DLMVM→lMANshell pathway. Previous work (and this study) has indicated that axonal connections of core and shell pathways are separate and parallel as they traverse the forebrain. However, it seems likely that there is cross-talk between these two pathways. Possible sources of integration between core and shell pathways are in feedback loops and convergent and reciprocal projections made between core and shell circuits (Johnson et al., 1995;Vates and Nottebohm, 1995; Bottjer and Johnson, 1997). For example, lMANshell projects directly onto Ad (an area within motor cortex immediately adjacent to RA), and Ad projects onto a dorsal thalamic zone, which potentially completes feedback loops to core and shell regions of lMAN and to medial magnocellular nucleus of anterior neostriatum (Foster et al., 1997; Iyengar et al., 1999;Bottjer et al., 2000). Cross-talk between core and shell pathways provides a potential mechanism whereby the DLMVM→ lMANshell→Ad circuit could trigger changes within the DLMDL→lMANcore→RA circuit during the sensitive period for vocal learning. For example, perhaps feedback signals (such as auditory feedback and efference copy) are mapped within topographic connections of the shell pathway and serve as an instructive signal for the refinement of connectional specificity in the core pathway.
Axon arbor regression: pruning as an atypical feature of remodeling?
One of the most unusual features of the developmental changes within individual DLM axon arbors is the net retraction that they undergo, such that the number of branches decreases substantially during song learning. It had traditionally been thought that the initial specificity of axonal connections in developing systems was imprecise, at least in part because of exuberant growth of axon arbors, and that specificity of brain wiring was achieved by pruning of such neural overgrowth. However, subsequent studies have demonstrated for the most part that early development of axon arbors involves a substantial net addition of branches or collaterals, and a resultant increase in total length, with no net regression (Katz and Shatz, 1996). For example, adult patterns of connectivity within developing sensory systems emerge as a result of extensive elaboration of axonal branches within restricted regions of postsynaptic targets. This increase in the number of axonal branches is accompanied by the elimination of a relatively small number of branches within “inappropriate” areas (Sretavan and Shatz, 1984, 1986; Young and Rubel, 1986; Callaway and Katz, 1991; Agmon et al., 1993; Antonini and Stryker, 1993; Catalano et al., 1996; Snider et al., 1999). Thus, nascent axon arbors tend to be simple in shape and restricted in extent, and initial axonal connections can be extremely specific (Crowley and Katz, 1999). As development proceeds, adult patterns of connectivity emerge as a result of a large increase in number of branches and total length of arbors. In contrast, the net decrease in number of branches of DLM axons over the course of vocal learning may represent the diminishing role of lMAN during this period, as juvenile birds acquire and consolidate a stereotyped song pattern (Bottjer et al., 1984; Scharff and Nottebohm, 1991). To our knowledge, large-scale retraction of single arbors has not been reported previously [but see Sur et al. (1984) and Florence and Casagrande (1990)]. However, it is possible that net retraction of arbors might occur at later ages in other systems and thus yield results comparable to those observed here.
How might this net retraction relate to specificity of brain wiring? The overall volume of both the DLMVM terminal field and that of its postsynaptic target, lMANshell, undergoes an almost threefold increase between 20 and 35 d that corresponds to the height of the sensitive period for learning syllables from a tutor (cf. Immelman, 1969; Böhner, 1990; Zann, 1990; Slater et al., 1993). The present study shows that individual DLMVM arbors retract substantially during this period, and it is highly unlikely that the increase in overall size of the DLMVM terminal field can be attributed to the addition of new DLMVM axon arbors within lMANshell because the total number of DLM neurons remains constant throughout song learning (Johnson and Bottjer, 1992). Thus smaller arbors are spread across an expanded target space in 35d birds, thereby contributing to the increase in overall volume of the DLMVM terminal field and presumably to a refinement in the grain of the topographic map caused by the consequent decrease in overlap between single arbors.
This pattern of results is a bit surprising in light of our previous report that the pattern of retrograde label in DLMVM after small tracer injections into lMANshell does not change during vocal development (Iyengar et al., 1999). Considering the extent of changes within individual arbors, the stability in the pattern of retrograde label is remarkable. That is, the greater spatial extent of DLMVM arbors at 20 d seems to predict that retrogradely labeled somata should be spread over a greater extent of DLMVM after tracer injections into lMANshell, although we observed no such tendency. This apparent discrepancy may be explained by the fact that the size of retrograde tracer injections in our previous study was much larger than those used in the present study. Thus, perhaps smaller injections of retrograde tracers would reflect structural changes within DLMVM axon arbors during song learning (cf. Agmon et al., 1995). In addition, retrograde tracers may be transported even by single branches of arbors that extend through the injection site, even if such processes do not make functional connections or the vast majority of the arbor is localized elsewhere. Therefore, retrograde tracers will tend to label a greater number of neurons than those that specifically project to the injection site (Trachtenberg and Stryker, 2001), and this tendency might contribute preferentially to the pattern seen in older birds. Future studies should examine patterns of anterograde label in the DLMVM→lMANshell pathway as a more sensitive assay of overall patterns of axonal connectivity.
Possible mechanisms underlying the formation of specific connections between DLM axon arbors and lMAN neurons during the sensitive period for song learning
Structural changes within developing neural circuits produced by sensory experience during the sensitive period are mediated by activity-dependent mechanisms. One mechanism that bridges both development and learning is alterations of synaptic strength thought to depend on detection of coincident patterns of synaptic input by NMDA receptors (NMDARs) (Cline and Constantine-Paton, 1990; Feldman et al., 1996, 1998; Bear and Rittenhouse, 1999). NMDARs decrease in density within lMAN during song learning, and the duration of NMDAR-mediated synaptic currents in lMAN neurons becomes significantly shorter (Aamodt et al., 1995; Livingston and Mooney, 1997) (cf. Basham et al., 1996, 1999; Singh et al., 2000). In addition, lMAN contains a population of neurons with “silent” (pure NMDAR) synapses in juvenile but not adult birds (Grammer and Bottjer, 2001). These changes indicate that NMDARs carry a greater proportion of the synaptic current in juveniles than adults, and maturation of NMDAR-mediated currents correlates with functional changes such as the loss in effectiveness of lMAN lesions and the increase in auditory tuning of lMAN neurons. Relative to normal age-matched controls, juvenile birds that are raised in acoustic isolation or deprived of normal auditory input are delayed in achieving faster NMDAR-mediated currents at DLM→lMAN synapses, decreased numbers of dendritic spines on lMANcoreneurons, and normal topographic patterning within the lMANcore→RA circuit (Wallhäusser-Franke et al., 1995; Livingston et al., 2000; Iyengar and Bottjer, 2002). This pattern suggests that NMDARs may serve as correlation detectors for convergent patterns of auditory and/or motor feedback that match the tutor template and lead to corresponding strengthening or weakening of synapses, thereby leading to refinement of individual DLM axon arbors within lMAN. In general, specific patterns of synaptic activity may mediate the refinement of individual DLM axon arbors and their synaptic connections within lMAN, thereby contributing to the engram for specific vocal patterns (Bottjer, 2001). Boettiger and Doupe (2001)have recently described forms of activity-dependent potentiation and depression within lMAN that are unique to juvenile birds and could contribute to synaptic pruning of this connection.
Footnotes
This research was supported by National Institutes of Health Grant NS37547. We thank Linh Ho for excellent technical assistance.
Correspondence should be addressed to Sarah W. Bottjer, Department of Biology, HNB 218, University of Southern California, Los Angeles, CA 90089-2520. E-mail: bottjer@usc.edu.
S. Iyengar's present address: Department of Psychology, Vanderbilt University, Nashville, TN 37203.
REFERENCES
- 1.Aamodt SM, Nordeen EJ, Nordeen KW. Early isolation from conspecific song does not affect the normal developmental decline of N-methyl-d-aspartate receptor binding in an avian song nucleus. J Neurobiol. 1995;27:76–84. doi: 10.1002/neu.480270108. [DOI] [PubMed] [Google Scholar]
- 2.Agmon A, Yang LT, O'Dowd DK, Jones EG. Organized growth of thalamocortical axons from the deep tier of terminations into layer IV of developing mouse barrel cortex. J Neurosci. 1993;13:5365–5382. doi: 10.1523/JNEUROSCI.13-12-05365.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agmon A, Yang LT, Jones EG, O'Dowd DK. Topological precision in the thalamic projection to neonatal mouse barrel cortex. J Neurosci. 1995;15:549–561. doi: 10.1523/JNEUROSCI.15-01-00549.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonini A, Stryker MP. Development of individual geniculocortical arbors in cat striate cortex and effects of binocular impulse blockade. J Neurosci. 1993;13:3549–3573. doi: 10.1523/JNEUROSCI.13-08-03549.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonini A, Stryker MP. Plasticity of geniculocortical afferents following brief or prolonged monocular occlusion in the cat. J Comp Neurol. 1996;369:64–82. doi: 10.1002/(SICI)1096-9861(19960520)369:1<64::AID-CNE5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 6.Antonini A, Gillespie DC, Crair MC, Stryker MP. Morphology of single geniculocortical afferents and functional recovery of the visual cortex after reverse monocular deprivation in the kitten. J Neurosci. 1998;18:9896–9909. doi: 10.1523/JNEUROSCI.18-23-09896.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basham ME, Nordeen EJ, Nordeen KW. Blockade of NMDA receptors in the anterior forebrain impairs sensory acquisition in the zebra finch (Poephila guttata). Neurobiol Learn Mem. 1996;66:295–304. doi: 10.1006/nlme.1996.0071. [DOI] [PubMed] [Google Scholar]
- 8.Basham ME, Sohrabji F, Singh TD, Nordeen EJ, Nordeen KW. Developmental regulation of NMDA receptor 2B subunit mRNA and ifenprodil binding in the zebra finch anterior forebrain. J Neurobiol. 1999;39:155–167. [PubMed] [Google Scholar]
- 9.Bear MF, Rittenhouse CD. Molecular basis for induction of ocular dominance plasticity. J Neurobiol. 1999;41:83–91. doi: 10.1002/(sici)1097-4695(199910)41:1<83::aid-neu11>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 10.Bernard JF, Alden M, Besson JM. The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: a Phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. J Comp Neurol. 1993;329:201–229. doi: 10.1002/cne.903290205. [DOI] [PubMed] [Google Scholar]
- 11.Boettiger CA, Doupe AJ. Developmentally restricted synaptic plasticity in a songbird nucleus required for song learning. Neuron. 2001;31:809–818. doi: 10.1016/s0896-6273(01)00403-2. [DOI] [PubMed] [Google Scholar]
- 12.Böhner J. Early acquisition of song in the zebra finch. Anim Behav. 1990;39:369–374. [Google Scholar]
- 13.Bottjer SW. Neural signatures of vocal learning in songbirds: brain-behavior relationships during a sensitive period. In: McClelland JL, editor. International encyclopedia of the social and behavioral sciences, Vol 2. Elsevier; Oxford: 2001. pp. 1245–1252. [Google Scholar]
- 14.Bottjer SW, Arnold AP. The ontogeny of bird song: neural mechanisms of vocal learning. In: Blass EM, editor. Handbook of behavioral neurobiology: developmental processes in psychobiology and neurobiology. Plenum; New York: 1986. pp. 129–161. [Google Scholar]
- 15.Bottjer SW, Arnold AP. Developmental plasticity in neural circuits for a learned behavior. Annu Rev Neurosci. 1997;20:459–481. doi: 10.1146/annurev.neuro.20.1.459. [DOI] [PubMed] [Google Scholar]
- 16.Bottjer SW, Johnson F. Circuits, hormones, and learning: vocal behavior in songbirds. J Neurobiol. 1997;33:602–618. doi: 10.1002/(sici)1097-4695(19971105)33:5<602::aid-neu8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224:901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- 18.Bottjer SW, Halsema KA, Brown SA, Miesner EA. Axonal connections of a forebrain nucleus involved with vocal learning in zebra finches. J Comp Neurol. 1989;279:312–326. doi: 10.1002/cne.902790211. [DOI] [PubMed] [Google Scholar]
- 19.Bottjer SW, Brady JD, Cribbs B. Connections of a motor cortical region in zebra finches: relation to pathways for vocal learning. J Comp Neurol. 2000;420:244–260. [PubMed] [Google Scholar]
- 20.Brainard MS, Doupe AJ. Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature. 2000a;404:762–766. doi: 10.1038/35008083. [DOI] [PubMed] [Google Scholar]
- 21.Brainard MS, Doupe AJ. Auditory feedback in learning and maintenance of vocal behaviour. Nat Rev Neurosci. 2000b;1:31–40. doi: 10.1038/35036205. [DOI] [PubMed] [Google Scholar]
- 22.Callaway EM, Katz LC. Effects of binocular deprivation on the development of clustered horizontal connections in cat striate cortex. Proc Natl Acad Sci USA. 1991;88:745–749. doi: 10.1073/pnas.88.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catalano SM, Robertson RT, Killackey HP. Early ingrowth of thalamocortical afferents to the neocortex of the prenatal rat. Proc Natl Acad Sci USA. 1991;88:2999–3003. doi: 10.1073/pnas.88.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Catalano SM, Robertson RT, Killackey HP. Individual axon morphology and thalamocortical topography in developing rat somatosensory cortex. J Comp Neurol. 1996;367:36–53. doi: 10.1002/(SICI)1096-9861(19960325)367:1<36::AID-CNE4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 25.Cline HT, Constantine-Paton M. NMDA receptor agonist and antagonists alter retinal ganglion cell arbor structure in the developing frog retinotectal projection. J Neurosci. 1990;10:1197–1216. doi: 10.1523/JNEUROSCI.10-04-01197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cline HT, Constantine-Paton M. LTP and activity-dependent synaptogenesis: the more alike they are, the more different they become. Curr Opin Neurobiol. 1998;8:139–148. doi: 10.1016/s0959-4388(98)80017-2. [DOI] [PubMed] [Google Scholar]
- 27.Crair MC, Gillespie DC, Stryker MP. The role of visual experience in the development of columns in cat visual cortex. Science. 1998;279:566–570. doi: 10.1126/science.279.5350.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crepel F, Mariani J, Delhaye-Bouchaud N. Evidence for a multiple innervation of Purkinje cells by climbing fibers in the immature rat cerebellum. J Neurobiol. 1976;7:576–578. doi: 10.1002/neu.480070609. [DOI] [PubMed] [Google Scholar]
- 29.Crepel F, Delhaye-Bouchaud N, Guastiavino JM, Sampaio I. Multiple innervation of cerebellar Purkinje cells by climbing fibers in staggerer mutant mouse. Nature. 1980;283:483–484. doi: 10.1038/283483a0. [DOI] [PubMed] [Google Scholar]
- 30.Crowley JC, Katz LC. Development of ocular dominance columns in the absence of retinal input. Nat Neurosci. 1999;2:1125–1130. doi: 10.1038/16051. [DOI] [PubMed] [Google Scholar]
- 31.Doupe AJ, Solis MM. Song- and order-selective neurons develop in the songbird anterior forebrain during vocal learning. J Neurobiol. 1997;33:694–709. [PubMed] [Google Scholar]
- 32.Feldman DE, Brainard MS, Knudsen EI. Newly learned auditory responses mediated by NMDA receptors in the owl inferior colliculus. Science. 1996;271:525–528. doi: 10.1126/science.271.5248.525. [DOI] [PubMed] [Google Scholar]
- 33.Florence SL, Casagrande VA. Development of geniculocortical axon arbors in a primate. Vis Neurosci. 1990;5:291–309. doi: 10.1017/s0952523800000365. [DOI] [PubMed] [Google Scholar]
- 34.Foster EF, Bottjer SW. Axonal connections of the high vocal center and surrounding cortical regions in juvenile and adult male zebra finches. J Comp Neurol. 1998;397:118–138. [PubMed] [Google Scholar]
- 35.Foster EF, Mehta RP, Bottjer SW. Axonal connections of the medial magnocellular nucleus of the anterior neostriatum in zebra finches. J Comp Neurol. 1997;382:364–381. doi: 10.1002/cne.903820305. [DOI] [PubMed] [Google Scholar]
- 36.Gan WB, Lichtman JW. Synaptic segregation at the developing neuromuscular junction. Science. 1998;282:1508–1511. doi: 10.1126/science.282.5393.1508. [DOI] [PubMed] [Google Scholar]
- 37.Grammer MK, Bottjer SW. Silent synapses at neural substrates for song learning in zebra finches. Soc Neurosci Abstr. 2001;27:1424. [Google Scholar]
- 38.Goodman C, Shatz CJ. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell. 1993;10[Suppl]:77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- 39.Hata Y, Tsumoto T, Stryker MP. Selective pruning of more active afferents when cat visual cortex is pharmacologically inhibited. Neuron. 1999;22:375–381. doi: 10.1016/s0896-6273(00)81097-1. [DOI] [PubMed] [Google Scholar]
- 40.Immelman K. Song development in the zebra finch and other estrildid finches. In: Hinde RA, editor. Bird vocalizations. Cambridge UP; Cambridge: 1969. pp. 61–77. [Google Scholar]
- 41.Iyengar S, Bottjer SW (2002) The role of auditory experience in the formation of neural circuits underlying vocal learning in zebra finches. J Neurosci, in press. [DOI] [PMC free article] [PubMed]
- 42.Iyengar S, Viswanathan SS, Bottjer SW. Development of topography within song control circuitry of zebra finches during the sensitive period for song learning. J Neurosci. 1999;19:6037–6057. doi: 10.1523/JNEUROSCI.19-14-06037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacoby RA, Marshak DW. Synaptic connections of DB3 diffuse bipolar cell axons in macaque retina. J Comp Neurol. 2000;416:19–29. doi: 10.1002/(sici)1096-9861(20000103)416:1<19::aid-cne3>3.0.co;2-h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson DA, Purves D. Postnatal reduction of neural unit size in the rabbit ciliary ganglion. J Physiol (Lond) 1981;318:143–159. doi: 10.1113/jphysiol.1981.sp013855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson F, Bottjer SW. Growth and regression of thalamic efferents in the song-control system of male zebra finches. J Comp Neurol. 1992;326:442–450. doi: 10.1002/cne.903260309. [DOI] [PubMed] [Google Scholar]
- 46.Johnson F, Sablan MM, Bottjer SW. Topographic organization of a forebrain pathway involved with vocal learning in zebra finches. J Comp Neurol. 1995;358:260–278. doi: 10.1002/cne.903580208. [DOI] [PubMed] [Google Scholar]
- 47.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 48.LeVay S, Stryker MP. The development of ocular dominance columns in the cat. In: Ferrebdelli JA, editor. Society for neuroscience symposia: aspects of developmental neurobiology. Society for Neuroscience; Bethesda, MD: 1979. pp. 83–98. [Google Scholar]
- 49.Lichtman JW. The reorganization of synaptic connections in the rat submandibular ganglion during post-natal development. J Physiol (Lond) 1977;302:121–130. doi: 10.1113/jphysiol.1977.sp012087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Livingston F, White SA, Mooney R. Slow NMDA-EPSCs at synapses critical for song development are not required for song learning in zebra finches. Nat Neurosci. 2000;3:482–488. doi: 10.1038/74857. [DOI] [PubMed] [Google Scholar]
- 51.Livingston FS, Mooney R. Development of intrinsic and synaptic properties in a forebrain nucleus essential to avian song learning. J Neurosci. 1997;17:8997–9009. doi: 10.1523/JNEUROSCI.17-23-08997.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mariani J, Changeux J-P. Mutiple innervation of cerebellar Purkinje cells by climbing fibers in the cerebellum of the adult staggerer mutant mouse. J Neurobiol. 1980;11:41–50. doi: 10.1002/neu.480110106. [DOI] [PubMed] [Google Scholar]
- 53.Mariani J, Changeux J-P. Ontogenesis of olivocerebellar relationships. I. Studies by intracellular recordings of the multiple innervation of Purkinje cells by climbing fibers in the developing rat cerebellum. J Neurosci. 1981;1:696–702. doi: 10.1523/JNEUROSCI.01-07-00696.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nixdorf-Bergweiler BE, Lips MB, Heinemann U. Electrophysiological and morphological evidence for a new projection of LMAN-neurones towards area X. NeuroReport. 1995;6:1729–1732. doi: 10.1097/00001756-199509000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Nordeen KW, Nordeen EJ. Anatomical and synaptic substrates for avian song learning. J Neurobiol. 1997;33:532–548. doi: 10.1002/(sici)1097-4695(19971105)33:5<532::aid-neu4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 56.Passera A, Fulks S, Schneider GE, Ayres S, Jhaveri S, Erzurumlu RS. The M.I.T. “Neurotrace” system for microcomputer-aided microscopy. Soc Neurosci Abstr. 1988;14:550. [Google Scholar]
- 57.Rubin E. Development of the rat superior cervical ganglion: initial stages of synapse formation. J Neurosci. 1985;5:697–704. doi: 10.1523/JNEUROSCI.05-03-00697.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seki T, Arai Y. Temporal and spatial relationships between PSA-NCAM-expressing, newly generated granule cells, and radial glia-like cells in the adult dentate gyrus. J Comp Neurol. 1999;410:503–513. doi: 10.1002/(sici)1096-9861(19990802)410:3<503::aid-cne11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 60.Simon DK, O'Leary DD. Development of topographic order in the mammalian retinocollicular projection. J Neurosci. 1992;12:1212–1232. doi: 10.1523/JNEUROSCI.12-04-01212.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh TD, Basham ME, Nordeen EJ, Nordeen KW. Early sensory and hormonal experience modulate age-related changes in NR2B mRNA within a forebrain region controlling avian vocal learning. J Neurobiol. 2000;44:82–94. doi: 10.1002/1097-4695(200007)44:1<82::aid-neu8>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 62.Slater PJB, Jones AE, ten Cate CJ. Can lack of experience delay the end of the sensitive phase for song learning? Netherlands J Zool. 1993;40:80–90. [Google Scholar]
- 63.Smolen AJ, Raisman G. Synapse formation in the superior cervical sympathetic ganglion of the rat during normal development and after neonatal deafferentation. Anat Rec. 1979;193:688. doi: 10.1016/0006-8993(80)90615-0. [DOI] [PubMed] [Google Scholar]
- 64.Snider CJ, Dehay C, Berland M, Kennedy H, Chalupa LM. Prenatal development of retinogeniculate axons in the macaque monkey during segregation of binocular inputs. J Neurosci. 1999;19:220–228. doi: 10.1523/JNEUROSCI.19-01-00220.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Solis MM, Doupe AJ. Anterior forebrain neurons develop selectivity by an intermediate stage of birdsong learning. J Neurosci. 1997;17:6447–6462. doi: 10.1523/JNEUROSCI.17-16-06447.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Solis MM, Doupe AJ. Contributions of tutor and bird's own song experience to neural selectivity in the songbird anterior forebrain. J Neurosci. 1999;19:4559–4584. doi: 10.1523/JNEUROSCI.19-11-04559.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sretavan DW, Shatz CJ. Prenatal development of individual retinogeniculate axons during the period of segregation. Nature. 1984;308:845–848. doi: 10.1038/308845a0. [DOI] [PubMed] [Google Scholar]
- 68.Sretavan DW, Shatz CJ. Prenatal development of retinal ganglion cell axons: segregation into eye-specific layers within the cat's lateral geniculate nucleus. J Neurosci. 1986;6:234–251. doi: 10.1523/JNEUROSCI.06-01-00234.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sur M, Weller RE, Sherman SM. Development of X- and Y-cell retinogeniculate terminations in kittens. Nature. 1984;310:246–249. doi: 10.1038/310246a0. [DOI] [PubMed] [Google Scholar]
- 70.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 71.Trachtenberg JT, Stryker MP. Rapid anatomical plasticity of horizontal connections in the developing visual cortex. J Neurosci. 2001;21:3476–3482. doi: 10.1523/JNEUROSCI.21-10-03476.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Troyer TW, Bottjer SW. Birdsong models and mechanisms. Curr Opin Neurobiol. 2001;11:721–726. doi: 10.1016/s0959-4388(01)00275-6. [DOI] [PubMed] [Google Scholar]
- 73.Vates GE, Nottebohm F. Feedback circuitry within a song-learning pathway. Proc Natl Acad Sci USA. 1995;92:5139–5143. doi: 10.1073/pnas.92.11.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wallhaüsser-Franke E, Nixdorf-Bergweiler BE, DeVoogd TJ. Song isolation is associated with maintaining high spine frequencies on zebra finch lMAN neurons. Neurobiol Learn Mem. 1995;64:25–35. doi: 10.1006/nlme.1995.1041. [DOI] [PubMed] [Google Scholar]
- 75.Weliky M, Katz LC. Correlational structure of spontaneous neuronal activity in the developing lateral geniculate nucleus in vivo. Science. 1999;285:599–604. doi: 10.1126/science.285.5427.599. [DOI] [PubMed] [Google Scholar]
- 76.Williams H, Mehta N. Changes in adult zebra finch song require a forebrain nucleus that is not necessary for song production. J Neurobiol. 1999;39:14–28. [PubMed] [Google Scholar]
- 77.Young SR, Rubel EW. Embryogenesis of arborization pattern and topography of individual axons in N. laminaris of the chicken brain stem. J Comp Neurol. 1986;254:425–459. doi: 10.1002/cne.902540402. [DOI] [PubMed] [Google Scholar]
- 78.Zann R. Song and call learning in wild zebra finches in south-east Australia. Anim Behav. 1990;40:811–828. [Google Scholar]