Abstract
Lesions of the amygdala impair acquisition of a food conditioned place preference (CPP) task. In contrast, lesions of the fornix facilitate acquisition on this task, showing that an intact hippocampal system can interfere with learning an amygdala-dependent task. Our recent findings indicate that acetylcholine (ACh) release in the hippocampus increases while rats perform a hippocampus-dependent spontaneous alternation task. To the extent that ACh output in the hippocampus reflects activation of that brain area in learning and memory, the results obtained with fornix lesions suggest that ACh release in the hippocampus might be negatively correlated with learning on a CPP task. Using in vivo microdialysis, release of ACh was measured in the hippocampus while rats learned and were tested on an amygdala-dependent CPP task and a hippocampus-dependent spontaneous alternation task. Release of ACh in the hippocampus increased when rats were tested on either task. The magnitude of the increase in release of hippocampal ACh was negatively correlated with good performance on the amygdala-dependent CPP task. These findings suggest that ACh release may reflect activation and participation of the hippocampus in learning and memory, but in a manner that can be detrimental to performance on a task dependent on another brain area.
Keywords: competition, memory systems, amygdala, hippocampus, microdialysis, conditioned place preference, acetylcholine
Experimental evidence suggests that memory processing is mediated by parallel, and somewhat independent, neural systems. Much of the evidence for multiple systems of memory stems from studies of the neuropsychology of human memory (Cohen and Squire, 1980; Warrington and Wieskrantz, 1982; Willingham, 1997), showing that damage to different brain regions impairs different kinds of cognitive functions with sometimes extraordinary specificity. Similar region-specific cognitive effects have been found in rats (Hirsh, 1974; O'Keefe and Nadel; 1978) (cf. Kesner, 1998;White and McDonald, 2002).
Because different memory systems appear to acquire different classes of information, processing of different attributes of memory may at times come into conflict with one another (Squire et al., 1993; Matthews and Best, 1995; Matthews et al., 1999; Gold et al., 2001; Packard, 2001;White and McDonald, 2001). For example, whereas lesions of the amygdala impair performance on a conditioned place preference (CPP) task in which rats receive a large food reward in one place versus no food in another location, lesions of the fornix facilitate acquisition of the same task. Such findings suggest that an intact hippocampal system, or information acquired by the hippocampal system, may interfere with performance on the amygdala-dependent task (McDonald and White, 1995). One hypothesis is that acquisition of a knowledge base involving the hippocampal system may promote the use of that information during acquisition of the CPP task, even if the information does not provide an effective means of acquiring the CPP task (McDonald and White, 1995). According to this view, with impaired hippocampal function, a parallel system including the amygdala is free to control what is learned, and acquisition of the CPP task proceeds more efficiently.
When the brain is intact, learning most likely involves an interaction of these parallel systems. One way to examine involvement of a neural system in a particular learned behavior without lesioning that system is to measure release of neurotransmitters during training and testing. For example, release of acetylcholine (ACh) in the hippocampus is positively correlated with training on a working memory task (Fadda et al., 1996) and with good performance on a hippocampus-dependent, spontaneous alternation task (Ragozzino and Gold, 1995). These results are compatible with the extensive literature demonstrating involvement of cholinergic contributions, particularly the septohippocampal cholinergic system, to learning and memory (Chrobak et al., 1989; Brioni et al., 1990; Mizumori et al., 1990; Olton et al., 1991;Givens and Olton, 1995; Markowska et al., 1995; Dougherty et al., 1998).
In the present study, release of ACh in the hippocampus was measured while rats were trained and tested on the amygdala-dependent CPP task, as well as during testing on a hippocampus-dependent spontaneous alternation task. Given the evidence that hippocampal involvement may be detrimental to acquisition of such amygdala-dependent learning, but is necessary for good spontaneous alternation performance, the experiments described here tested the hypothesis that release of ACh in the hippocampus would be negatively correlated with good performance on the CPP task and positively correlated with performance on the hippocampus-dependent spontaneous alternation task.
MATERIALS AND METHODS
Subjects. Twenty-eight adult male Sprague Dawley rats (Hilltop Breeders), weighing between 275 and 300 gm, were housed individually. All rats were maintained on a 12 hr light/dark cycle (on at 7:00 A.M.) and allowed to adjust to their new environment withad libitum access to food and water for 1 week after arrival.
Surgery. All rats undergoing surgery received atropine sulfate (0.4 mg/kg, i.p.) before being anesthetized with sodium pentobarbital (50 mg/kg, i.p.). After anesthesia, using standard stereotaxic procedures, a plastic guide cannula (CMA/12 type; Carnegie Medicine, Stockholm, Sweden) was lowered into the hippocampal formation. The stereotaxic coordinates were 3.8 mm posterior to bregma, 5.0 mm lateral, and 3.8 mm ventral from dura. The nose bar was set at 5.0 mm above the interaural line according to the atlas of Pellegrino et al. (1979).
Microdialysis procedure. A 3 mm dialysis probe (CMA/12; Carnegie Medicine, Chelmsford, MA) was inserted through the guide cannula. The dialysis probe was connected to plastic tubing and driven by a microinfusion system (CMA/100; Carnegie Medicine). The dialysis probe was perfused continuously at a rate of 2.1 μl/min with artificial CSF (in mm: 128 NaCl, 2.5 KCl, 1.3 CaCl2, 2.1 MgCl2, 21 NaH2PO4, 1.3 Na2HPO4, and 3.3 glucose, brought to pH 7.0 by NaOH), which contained the acetylcholinesterase inhibitor neostigmine (1 μm).
Apparatus. An eight-arm radial maze, made of wood and painted gray, was used for training. The maze was elevated 63 cm from the floor. The center platform was 40 cm in diameter. Each arm was 9 cm wide and 60 cm long. The room contained various extramaze cues (bookcase, another maze covered with a black sheet, shelves containing laboratory supplies, and a counter with a sink).
Behavioral procedures. Unoperated control rats were food deprived and handled daily (5 min/d) for 7 d. Rats receiving surgery were given 5 d to recover before being handled and food deprived. After 1 week of food restriction, each rat was approximately at a target weight of 85% of its free-feeding body weight.
Conditioned place preference. The procedures were similar to those described by White and McDonald (1993). Two arms of an eight-arm radial maze were made available, and the other six arms were removed from the center platform (Fig.1A). The arms were not adjacent or directly across from one another, and the position of the arms was constant relative to the room over days of training and testing. On a habituation trial, 1 d before training, rats were allowed to explore both arms freely for 10 min. Time spent in each of the arms was recorded to determine original arm preference.
Fig. 1.
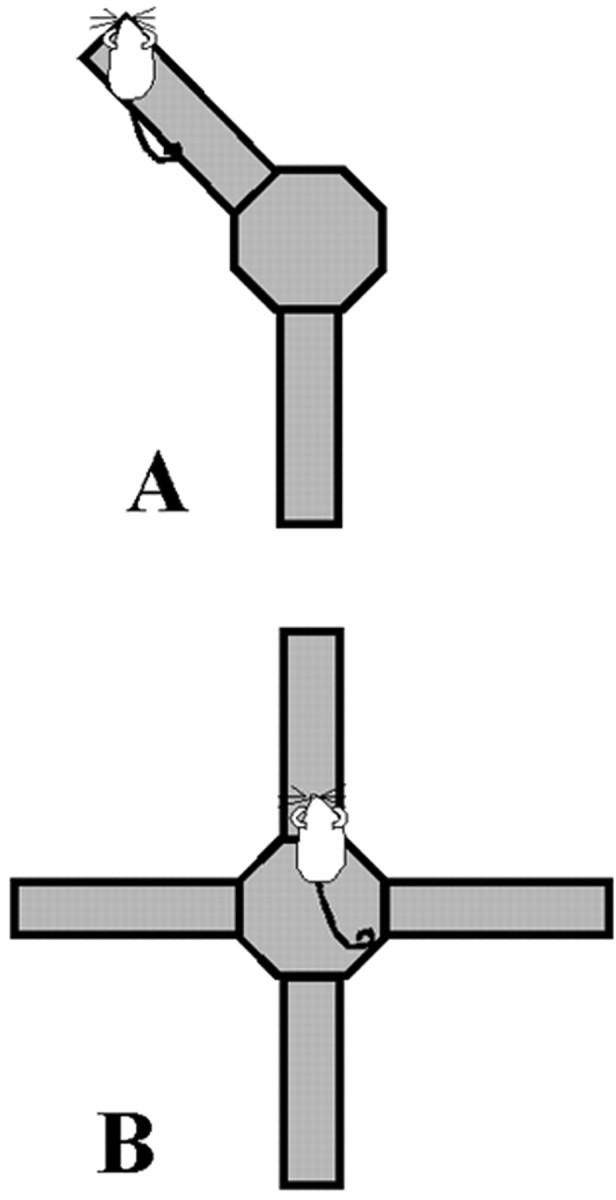
A, CPP configuration of an eight-arm radial maze. Two arms are accessible, during habituation and training. The two arms are never adjacent or directly across from one another. During the training trials, one arm is consistently paired with food and the other is never baited. On day 1 of a trial, only one arm is accessible and rats are blocked in for 30 min. On day 2 of the same trial, only the other arm is accessible and rats are again blocked in for 30 min. B, Spontaneous alternation configuration of an eight-arm radial maze. Four arms in the configuration of a cross are accessible for training and testing. No food is on the maze.
Training consisted of four trials; each trial occurred across 2 consecutive days. On one day of a trial, rats were only allowed to enter an arm that was “paired” with 14 gm of wheat puffs and were blocked in the arm for 30 min. On the other day of the same trial, rats were blocked in the “unpaired” arm. Assignment of paired versus unpaired arms was determined for each rat based on original preference. The paired arm for an individual rat during training was the arm not preferred on the habituation trial by that rat. Paired and unpaired arms were consistent for each rat across all four training trials. The order of presentation of paired and unpaired arms varied between rats but remained the same for a given rat. A retention test was given 24 d after the last training trial. On the test trial, both arms were open and no food was on the maze. Rats were free to move between the two arms for 20 min. Percentage of time spent in each arm was recorded.
According to White and McDonald (1993) and as seen previously in our laboratory (McIntyre et al., 1998), rats first show a significant preference for the paired arm after receiving four training trials. Microdialysis samples were collected from operated rats during training on both days of trial 4 and during the test day. Twenty-four hours before the first day of training, a dialysis probe was inserted into the guide cannula and removed. The dialysis probe was inserted again 105 min before training on trial 4. Perfusate collected for the first 45 min was not analyzed, allowing time for equilibration between the sample tissue and perfusion solution. Subsequently, four baseline samples were collected at 15 min intervals while rats remained in their home cages. After collection of the last baseline sample, rats were placed on the maze and two perfusate samples were collected during the 30 min of training. After the training session, rats were returned to their home cages in which two more dialysis samples were collected. Microdialysis procedures were repeated on day 2 of trial 4. On the test day, dialysis probes were again inserted 105 min before behavioral testing. Four baseline samples were taken during the last 60 min of this time. After the last baseline sample, rats were placed on the maze and tested behaviorally for 20 min. One dialysis sample was collected during the first 15 min of testing. After the test session, rats were returned to their home cages in which two more dialysis samples were collected.
Spontaneous alternation. Two days after CPP testing, rats were tested for spontaneous alternation performance on the same maze and in the same room. On the spontaneous alternation task, four arms in the configuration of a cross were accessible, and the other four arms were removed from the center platform. Microdialysis procedures were the same as on the CPP test. Rats were placed on the center platform and allowed to navigate the maze for 15 min. The sequence and number of arm entries was recorded. Entry into four different arms of five consecutive arm choices (overlapping sets of five) was considered an alternation. Therefore, a set of arm choices such as B,A,D,B,C was recorded as an alternation, but a set such as A,B,A,C,B was not. With this procedure, possible alternation sequences are equal to the number of total arm entries minus 4. The percentage of alternation score is equal to the ratio of (actual alternations/possible alternations) × 100. Chance performance on this task is 44%. After the testing session, rats were returned to their home cages in which two more dialysis samples were collected.
Acetylcholine assay. Perfusate samples (20 μl) were assayed for ACh by using HPLC with electrochemical detection. ACh was separated from choline by a reverse-phase analytical column (Chromspher 5 C18, 100 × 3 mm; Chrompack, Middleburg, The Netherlands). An enzymatic postcolumn reactor containing acetylcholinesterase (EC3.1.1.7; type VI-S; Sigma, St. Louis, MO) and choline oxidase (EC 1.1.3.17; Sigma) converted the ACh to choline and acetate and the choline to betaine. The final conversion to hydrogen peroxide was electrochemically detected by a platinum electrode held at a potential of +525 mV. Mobile phase containing 0.2 mmdibasic potassium phosphate, 1.0 mmtetramethylammonium hydroxide, 0.3 mm EDTA, and 0.005% Kathon CG (to prevent bacterial growth) was delivered at a rate of 0.6 ml/min by a solvent delivery system (PM-80; Bioanalytical Systems, West Lafayette, IN). The detection limit was 50 fmol. ACh peaks were quantified by comparison with peak heights of ACh standard solutions and corrected for in vitro recovery of the probe.
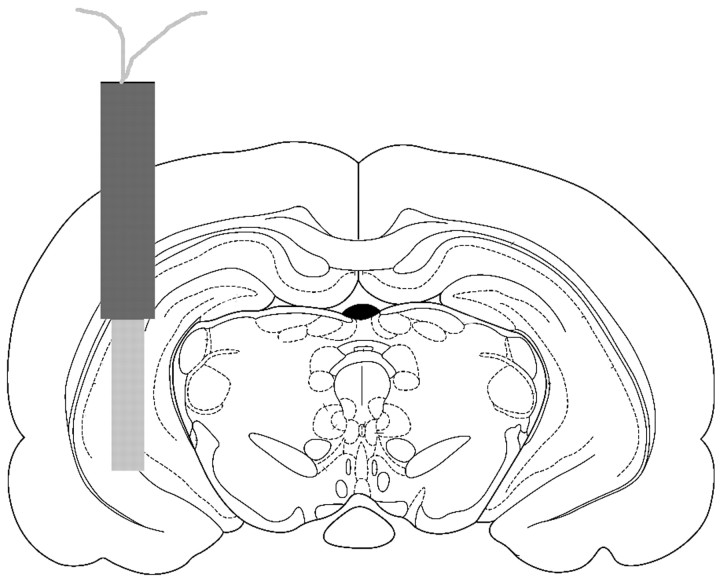
Histology. After the completion of both behavioral tasks, rats were given a lethal dose of sodium pentobarbital, followed by intracardial perfusion with 0.9% saline and a 10% formalin solution. Brains were removed and placed in a 30% sucrose–formalin solution for storage. In preparation for sectioning, brains were frozen at −20°C and mounted on a Reichert Jung cryostat. Forty micrometer sections were taken beginning at the anterior hippocampus, mounted onto slides, and stained with cresyl violet. Figure 2illustrates an example of a placement that would be considered acceptable. Rats with probe placements outside of this area or with extensive visible signs of damage were not included in data analysis.
Fig. 2.
An example of an acceptable hippocampal probe placement. Rat brain section was taken from the stereotaxic atlas ofPaxinos and Watson (1986).
Hippocampal microdialysis probes were inserted into each rat four times. Because repeated probing could create more tissue damage than a single microdialysis session, baseline values were compared across days. One rat was excluded from analysis based on a decrease in ACh output during baseline measurements on the spontaneous alternation test. Using these measures, we found that repeated probing provides better baseline stability than does maintaining a probe in the brain for 6 d (our unpublished observation). Others have reported that repeated probing causes less damage than leaving a probe in the brain for an extended period of time (Bruno, 1999).
Statistical analysis. Two-tailed Student's ttests were used to compare the percentage of the total time on the maze spent in the paired arm for operated and unoperated rats performing the CPP task. Baseline values of ACh, in picomoles, were compared across days using a single-factor ANOVA. Microdialysis data were converted to percentages from each rat's baseline output. Baseline scores were derived from the mean of the first four samples (taken while rats were in the home cage).
Only 10 rats were used in the statistical analysis for the spontaneous alternation test. Baseline levels of ACh were markedly reduced in one rat on the test day, two rats failed to make the minimum number (eight) of arm entries, and a fourth rat lost its guide cannula in the time between CPP and spontaneous alternation testing.
Two-tailed Student's t tests were used to compare the percentage of change between baseline extracellular levels of ACh and mean percentage of ACh output during behavior samples. Correlation and regression analyses were used to analyze the relationship between percentage of increase in ACh during behavior and performance for each group.
RESULTS
CPP
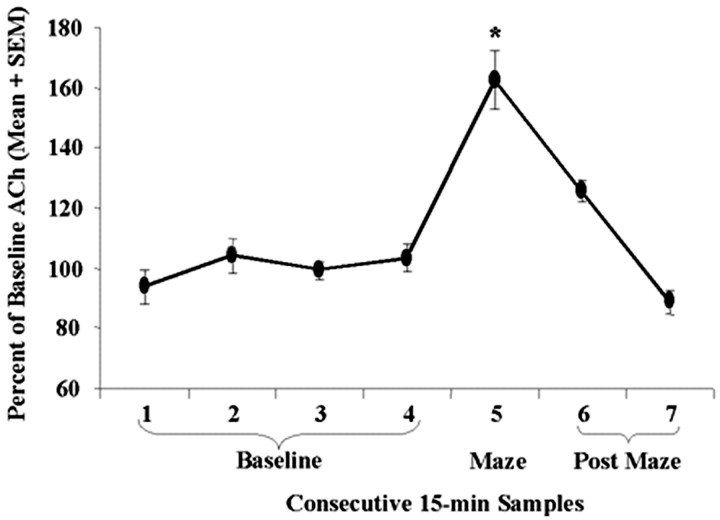
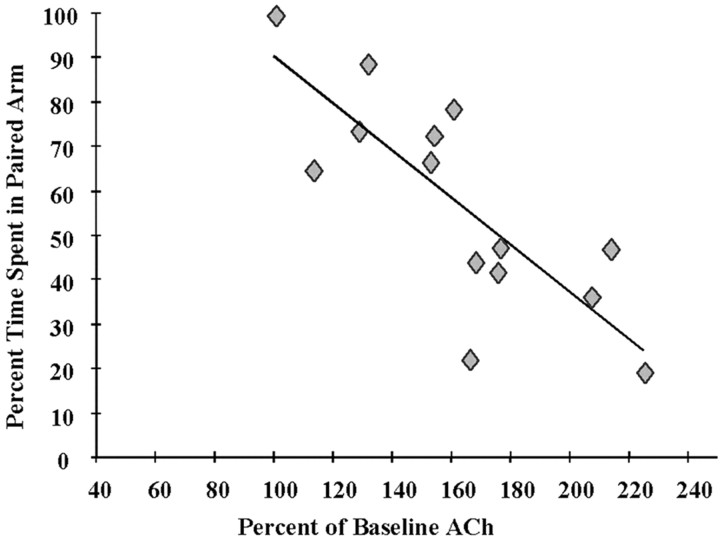
Analysis of ACh output levels during the test day revealed significant increases during the sample taken while rats were being tested on the maze (p < 0.0001), as seen in Figure 3. Importantly, the magnitude of increase of ACh within the hippocampus during testing was inversely related to test performance (r = −0.80;p < 0.0007). This negative correlation is illustrated in Figure 4. Of the total time spent in both of the arms, rats in the CPP groups spent a mean of 57.29 ± 6.57% in the paired arm. Unoperated control rats spent 67.57 ± 4.42% of their time in the paired arm. This difference in percentage of time spent in the paired arm was not significant (p = 0.206).
Fig. 3.
Mean percentage of baseline ACh levels in dialysate samples during baseline and CPP testing. ACh release increased significantly above baseline while rats were tested (*p < 0.0001 vs baseline).
Fig. 4.
Negative correlation between mean percentage of increase in ACh during CPP testing and mean percentage of time spent in the paired arm (r = −0.80; p = 0.0007).
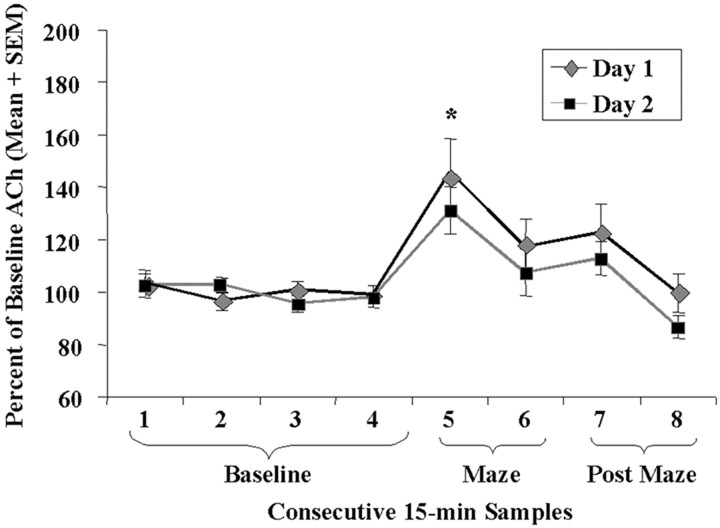
Figure 5 shows the biochemical results of CPP training for all cannulated rats on day 1 and day 2 of training trial 4. Extracellular levels of ACh in the hippocampus increased from baseline in all groups when rats were placed on the maze for CPP training (p = 0.003, day 1; p = 0.015, day 2). The magnitude of increase in release of ACh in the hippocampus during training and later performance on the test day were not significantly correlated (r = 0.19,p = 0.521, Day 1; r = 0.36,p = 0.22; Day 2). A repeated-measures ANOVA revealed no significant differences in baseline values of ACh across days (p = 0.49). Absolute baseline values of ACh in the hippocampus across days were estimated to be a mean of 4.1 ± 0.20 pmol.
Fig. 5.
Mean percentage of baseline ACh levels in dialysate samples during baseline and CPP training on trial 4. ACh release increased significantly above baseline when rats were being trained on the maze (*p = 0.003, day 1 vs baseline; p = 0.015, day 2 vs baseline).
Spontaneous alternation
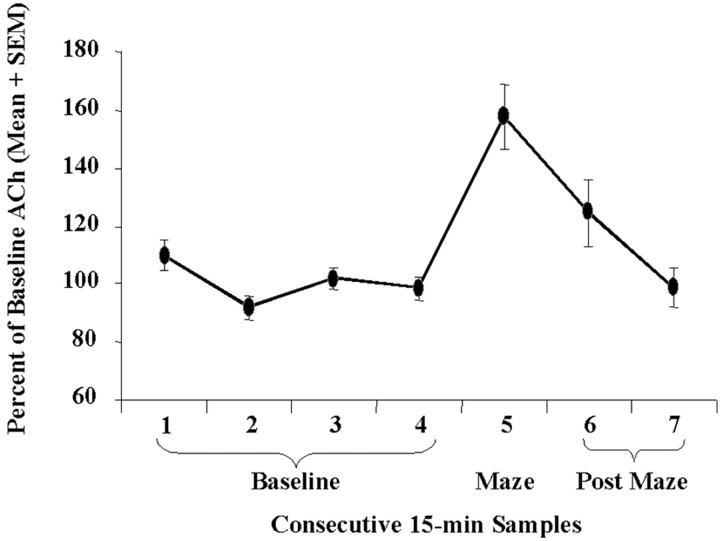
Unoperated and operated rats alternated at mean rates of 64.9 ± 5.52 and 67 ± 3.81%, respectively. Hippocampal ACh output during the spontaneous alternation task is shown in Figure6. ACh output increased significantly when the rats were on the maze (p < 0.005); the increases above baseline were not correlated significantly with alternation scores (r = 0.11, p = 0.77).
Fig. 6.
Mean percentage of baseline ACh levels in dialysate samples during baseline and spontaneous alternation behavior. ACh release increased significantly during spontaneous alternation (p = 0.005).
DISCUSSION
Extracellular levels of ACh increased in the hippocampus when rats were performing either the amygdala-dependent CPP task or the hippocampus-dependent spontaneous alternation task. A striking inverse correlation was observed between the magnitude of the increase in ACh release in the hippocampus and test performance on the CPP task. These results suggest that hippocampal activation, as marked by ACh release, was detrimental for performance of learned behavior dependent on the amygdala. The magnitude of increase in ACh release in the hippocampus was not significantly correlated with alternation scores in individual rats, although, consistent with previous reports, hippocampal ACh release increased significantly above baseline during spontaneous alternation testing (Ragozzino et al., 1996; Stefani and Gold, 2001).
The different relationships observed between ACh release and performance on the two tasks could be based on the order of behavioral test procedures rather than task demands, because all rats were trained on the CPP task first. This order was based on previous reports that habituation to the room and maze affects the rate of acquisition of CPP (McDonald and White, 1995). Results of spontaneous alternation performance and ACh release were no different from those seen in studies in which rats were exposed to the apparatus and room for the first time (Stefani and Gold, 2001), suggesting that previous CPP training did not affect ACh release or behavioral measures on the spontaneous alternation test.
On the CPP test day, ACh release in the hippocampus may mark activation related to use of hippocampus-dependent information remaining from the habituation trial. McDonald and White (1995) reported that rats trained on the CPP task without a habituation trial, or habituated in another room, learned at a rate comparable with that of rats with fornix lesions, i.e., a rate significantly faster than habituated controls. These findings suggest that information acquired during the habituation phase of training is a key element in the suppressed acquisition of CPP demonstrated by control animals. The number of training trials required for an individual rat to acquire the CPP task may depend on the degree to which hippocampus-dependent information is used by that individual rat. This view resembles the well established latent inhibition theory (Lubow, 1972), in which non-reinforced preexposure to the conditioned stimulus interferes with the later conditioned association between that stimulus and reinforcement. Such interference has been reported to be hippocampus dependent when the nature of the conditioned stimulus is contextual (Lubow, 1972; Honey and Good, 1993). Although the data are not shown here, previous findings indicate that ACh levels increase in the hippocampus during the habituation period, but this increase does not correlate with performance on the test trial (our unpublished observation).
The current findings complement those reported by McDonald and White (1995) in supporting the view that activation of the hippocampus, via the fimbria fornix, has an adverse affect on amygdala-dependent processing during the retention test. Recent findings indicate that lesions of the ventral, but not dorsal, hippocampus result in facilitation of the CPP task (White and Wallet, 2000; Ferbinteanu and McDonald, 2001). It may be noteworthy that the major connections between the hippocampus and amygdala involve the ventral hippocampus (cf. Pitkänen et al., 2000), perhaps contributing to the interactions between these brain areas in processing learning and memory for the CPP task. In the present experiment, the extracellular sampling domain of the 3 mm microdialysis probes most likely included ACh release in both dorsal and ventral portions of the hippocampus.
Consistent with recent findings (Ragozzino et al., 1996; Stefani and Gold, 2001), ACh release increased above baseline in the hippocampus during performance of the hippocampus-dependent, spontaneous alternation task. The extent of the increase in individual rats was not correlated with performance on the spontaneous alternation task. Others have reported positive correlations between hippocampal ACh and performance on alternation and other spatial memory tasks (Ragozzino et al., 1994; Fadda et al., 2000), although these correlations generally included rats across treatment conditions. In the present experiment, the relatively high alternation rate, and narrow range of scores, may have interfered with reasonable ability to see correlations of individual differences in behavior with differences in release of ACh.
The present results match well findings obtained with previous examinations of ACh release in the hippocampus during learning and memory (cf. Gold et al., 2001). The present findings also fit well with a large literature showing that, when administered near the time of training or testing, pharmacological agents that augment cholinergic functions in the hippocampus enhance learning and memory and agents that interfere with cholinergic functions in the hippocampus impair learning and memory (cf. Carli et al., 1997; Felix and Levin, 1997;Ohno et al., 1997; Mishima et al., 2000). Thus, the results obtained with measurements and manipulations of an intact septohippocampal cholinergic system quite consistently support a role for hippocampal cholinergic mechanisms in learning and memory.
Because lesions of ACh projections to the hippocampus generally do not impair learning and memory (Baxter et al., 1996; Perry et al., 2001) (but see Wrenn and Wiley, 1998), the role of ACh in memory formation is likely to be one of modulating memory formation rather than contributing to memory substrate formation in a direct and essential manner.
The results obtained here suggest that ACh might act to modulate memory formation by biasing the relative contributions of different neural systems to learning and memory. The present findings might equally well be described in other terms, for example, that release of ACh provides a measure of selective attention, arousal, or modulation of memory across hippocampus and amygdala processing of information. Other findings point to involvement of forebrain cholinergic projections to neocortex in particular, but also to hippocampus, in arousal and attentional mechanisms, as well as in learning and memory mechanisms (Hasselmo, 1995; Acquas et al., 1996; Sarter et al., 1996; Dalley et al., 2001).
With the information gained from the use of in vivomicrodialysis during training and testing, it is possible to visualize the interactions that occur between neural systems in a relatively intact brain as memories are consolidated and retrieved. Furthermore, findings from the current study are consistent with the general hypothesis that neurochemical modulators of learning and memory may mediate competition between systems to determine which attributes of information control learned performance (Gold, 1995; Gold et al., 2001;Packard, 2001). For example, the ratio of ACh release in hippocampus and striatum is associated with preferential learning of a t-maze using place or response strategies, respectively (C. K. McIntyre, L. K. Marriott, and P. E. Gold, unpublished observations).
In summary, we found that ACh release in the hippocampus is associated with performance on an amygdala-dependent learning and memory task. These results support the hypothesis that (1) acetylcholine and perhaps other modulators of memory formation are good candidates for coordinating the relative contributions and participation of discrete memory systems during learning and (2) activation of the septohippocampal system, marked here by increased release of acetylcholine, adversely affects expression of a type of memory supported by the amygdala. These findings suggest further that a balance of activity within separate memory systems, at times revealed by ACh release, may contribute to individual differences in learning.
Footnotes
This work was supported by National Institute on Aging Grant AG 07648, National Institute of Neurological Disorders and Stroke Grant NS 32914, United States Department of Aging Grant 00-35200-9839, and the Alzheimer's Association.
Correspondence should be addressed to Dr. Paul E. Gold, Department of Psychology, University of Illinois at Urbana-Champaign, 603 East Daniel Street, Champaign, IL 61820. E-mail: pgold@uiuc.edu.
REFERENCES
- 1.Acquas E, Wilson C, Fibiger HC. Conditioned and unconditioned stimuli increase frontal cortical and hippocampal acetylcholine release: effects of novelty, habituation and fear. J Neurosci. 1996;16:3089–3096. doi: 10.1523/JNEUROSCI.16-09-03089.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baxter MG, Bucci DJ, Sobel TJ, Williams MJ, Gorman LK, Gallagher M. Intact spatial learning following lesions of basal forebrain cholinergic neurons. NeuroReport. 1996;7:1417–1420. doi: 10.1097/00001756-199605310-00019. [DOI] [PubMed] [Google Scholar]
- 3.Brioni JD, Decker MW, Gamboa LP, Izquierdo I, McGaugh JL. Muscimol injections in the medial septum impair spatial learning. Brain Res. 1990;522:227–234. doi: 10.1016/0006-8993(90)91465-s. [DOI] [PubMed] [Google Scholar]
- 4.Bruno JP (1999) Afferent regulation of basal forebrain cholinergic neurons: the use of multiple microdialysis probes and repeated perfusions in the same animal. Paper presented at the 8th International Conference on In Vivo Methods, Stonybrook, NY, June.
- 5.Carli M, Luschi R, Samanin R. Dose-related impairment of spatial learning by intrahippocampal scopolamine: antagonism by ondansetron, a 5-HT3 receptor antagonist. Behav Brain Res. 1997;82:185–194. doi: 10.1016/s0166-4328(97)80988-6. [DOI] [PubMed] [Google Scholar]
- 6.Chrobak JJ, Stackman RW, Walsh TJ. Intraseptal administration of muscimol produces dose-dependent memory impairments in the rat. Behav Neural Biol. 1989;52:357–369. doi: 10.1016/s0163-1047(89)90472-x. [DOI] [PubMed] [Google Scholar]
- 7.Cohen NJ, Squire LR. Preserved learning and retention of pattern-analyzing skill in amnesia: dissociation of knowing how and knowing that. Science. 1980;210:207–210. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- 8.Dalley JW, McGaughy J, O'Connell MT, Cardinal RN, Levita L, Robbins TW. Distinct changes in cortical acetylcholine and noradrenaline efflux during contingent and noncontingent performance of a visual attention task. J Neurosci. 2001;21:4908–4914. doi: 10.1523/JNEUROSCI.21-13-04908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dougherty KD, Turchin PI, Walsh TJ. Septocingulate and septohippocampal cholinergic pathways: involvement in working/episodic memory. Brain Res. 1998;810:59–71. doi: 10.1016/s0006-8993(98)00870-1. [DOI] [PubMed] [Google Scholar]
- 10.Fadda F, Mellis F, Stancampiano R. Increased hippocampal acetylcholine release during a working memory task. Eur J Pharmacol. 1996;307:R1–R2. doi: 10.1016/0014-2999(96)00289-0. [DOI] [PubMed] [Google Scholar]
- 11.Fadda F, Cocco S, Stancampiano R. Hippocampal acetylcholine release correlates with spatial learning performance in freely moving rats. NeuroReport. 2000;11:2265–2269. doi: 10.1097/00001756-200007140-00040. [DOI] [PubMed] [Google Scholar]
- 12.Felix R, Levin ED. Nicotinic antagonist administration into the ventral hippocampus and spatial working memory in rats. Neuroscience. 1997;81:1009–1017. doi: 10.1016/s0306-4522(97)00224-8. [DOI] [PubMed] [Google Scholar]
- 13.Ferbinteanu J, McDonald RJ. Dorsal/ventral hippocampus, fornix, conditioned place preference. Hippocampus. 2001;11:187–200. doi: 10.1002/hipo.1036. [DOI] [PubMed] [Google Scholar]
- 14.Givens B, Olton DS. Bidirectional modulation of scopolamine induced working memory impairments by muscarinic activation of the medial septal area. Neurobiol Learn Mem. 1995;63:269–276. doi: 10.1006/nlme.1995.1031. [DOI] [PubMed] [Google Scholar]
- 15.Gold PE. Modulation of emotional and non-emotional memories: same pharmacological system, different neuroanatomical systems. In: McGaugh JL, Weinberger NM, Lynch GS, editors. Brain and memory: modulation and mediation of neuroplasticity. Oxford UP; New York: 1995. pp. 41–74. [Google Scholar]
- 16.Gold PE, McIntyre C, McNay E, Stefani MR, Korol DL. Neurochemical referees of dueling memory systems. In: Gold PE, Greenough WT, editors. Memory consolidation: essays in honor of James L. McGaugh: a time to remember. Am Psychological Association; Washington, DC: 2001. pp. 219–248. [Google Scholar]
- 17.Hasselmo ME. Neuromodulation and cortical function: modeling the physiological basis of behavior. Behav Brain Res. 1995;67:1–27. doi: 10.1016/0166-4328(94)00113-t. [DOI] [PubMed] [Google Scholar]
- 18.Hirsh R. The hippocampus and contextual retrieval of information from memory: a theory. Behav Biol. 1974;12:421–444. doi: 10.1016/s0091-6773(74)92231-7. [DOI] [PubMed] [Google Scholar]
- 19.Honey RC, Good M. Selective hippocampal lesions abolish the contextual specificity of latent inhibition and conditioning. Behav Neurosci. 1993;107:23–33. doi: 10.1037//0735-7044.107.1.23. [DOI] [PubMed] [Google Scholar]
- 20.Kesner RP. Neurobiological views of memory. In: Martinez JS, Kesner RP, editors. Neurobiology of learning and memory, Ed 3. Academic; San Diego: 1998. pp. 361–416. [Google Scholar]
- 21.Lubow RE. Latent inhibition. Psychol Bull. 1972;79:398–407. doi: 10.1037/h0034425. [DOI] [PubMed] [Google Scholar]
- 22.Markowska AL, Olton DS, Givens B. Cholinergic manipulations in the medial septal area: age-related effects on working memory and hippocampal electrophysiology. J Neurosci. 1995;15:2063–2073. doi: 10.1523/JNEUROSCI.15-03-02063.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matthews DB, Best PJ. Fimbria/fornix lesions facilitate the learning of a nonspatial response task. Psychon Bull Rev. 1995;2:113–116. doi: 10.3758/BF03214415. [DOI] [PubMed] [Google Scholar]
- 24.Matthews DB, Ilgen M, White AM, Best PJ. Acute ethanol administration impairs spatial performance while facilitating nonspatial performance in rats. Neurobiol Learn Mem. 1999;72:167–179. doi: 10.1006/nlme.1998.3900. [DOI] [PubMed] [Google Scholar]
- 25.McDonald RJ, White NM. Information acquired by the hippocampus interferes with acquisition of the amygdala-based conditioned cue preference (CCP) in the rat. Hippocampus. 1995;5:189–197. doi: 10.1002/hipo.450050305. [DOI] [PubMed] [Google Scholar]
- 26.McIntyre CK, Ragozzino ME, Gold PE. Intra-amygdala infusions of scopolamine impair performance on a conditioned place preference task but not a spatial radial maze task. Behav Brain Res. 1998;2:219–226. doi: 10.1016/s0166-4328(97)00161-7. [DOI] [PubMed] [Google Scholar]
- 27.Mishima K, Iwasaki K, Tsukikawa H, Matsumoto Y, Egashira N, Abe K, Egawa T, Fujiwara M. The scopolamine-induced impairment of spatial cognition parallels the acetylcholine release in the ventral hippocampus in rats. Jpn J Pharmacol. 2000;84:163–173. doi: 10.1254/jjp.84.163. [DOI] [PubMed] [Google Scholar]
- 28.Mizumori SJY, Perez GM, Alvarado MC, Barnes CA, McNaughton BL. Reversible inactivation of the medial septum differentially affects two forms of learning in rats. Brain Res. 1990;528:12–20. doi: 10.1016/0006-8993(90)90188-h. [DOI] [PubMed] [Google Scholar]
- 29.Ohno M, Yoshimatsu A, Kobayashi M, Watanabe S. Noradrenergic DSP-4 lesions aggravate impairment of working memory produced by hippocampal muscarinic blockade in rats. Pharmacol Biochem Behav. 1997;57:257–261. doi: 10.1016/s0091-3057(96)00353-x. [DOI] [PubMed] [Google Scholar]
- 30.O'Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford UP; Oxford: 1978. [Google Scholar]
- 31.Olton DS, Wenk GL, Markowska AM. Basal forebrain, memory and attention. In: Richardson RT, editor. Activation to acquisition: functional aspects of the basal forebrain cholinergic system. Birkhaüser; Boston: 1991. pp. 247–262. [Google Scholar]
- 32.Packard MG (2001) On the neurobiology of multiple memory systems: Tolman versus Hull, system interactions, and the emotion-memory link. J Cog Proc, in press.
- 33.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; San Diego: 1986. [DOI] [PubMed] [Google Scholar]
- 34.Pellegrino LJ, Pellegrino AS, Cushman AJ. A stereotaxic atlas of the rat brain. Plenum; New York: 1979. [Google Scholar]
- 35.Perry T, Hodges H, Gray JA. Behavioural, histological, immunocytochemical consequences following 192 IgG-saporin immunolesions of the basal forebrain cholinergic system. Brain Res Bull. 2001;54:29–48. doi: 10.1016/s0361-9230(00)00413-5. [DOI] [PubMed] [Google Scholar]
- 36.Pitkänen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann NY Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- 37.Ragozzino ME, Gold PE. Glucose injections into the medial septum reverse the effects of intraseptal morphine infusions on hippocampal acetylcholine output and memory. Neuroscience. 1995;68:981–988. doi: 10.1016/0306-4522(95)00204-v. [DOI] [PubMed] [Google Scholar]
- 38.Ragozzino ME, Wenk GL, Gold PE. Glucose attenuates morphine-induced decrease in hippocampal acetylcholine output: an in vivo microdialysis study in rats. Brain Res. 1994;655:77–82. doi: 10.1016/0006-8993(94)91599-7. [DOI] [PubMed] [Google Scholar]
- 39.Ragozzino ME, Unick KE, Gold PE. Hippocampal acetylcholine release during memory testing in rats: augmentation by glucose. Proc Natl Acad Sci USA. 1996;93:4693–4698. doi: 10.1073/pnas.93.10.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarter M, Bruno JP, Givens B, Moore H, McGaughy J, McMahon K. Neuronal mechanisms mediating drug-induced cognition enhancement: cognitive ability as a necessary intervening variable. Brain Res Cogn Brain Res. 1996;3:329–343. doi: 10.1016/0926-6410(96)00018-3. [DOI] [PubMed] [Google Scholar]
- 41.Squire LR, Knowlton B, Musen G. The structure and organization of memory. Annu Rev Psychol. 1993;44:453–495. doi: 10.1146/annurev.ps.44.020193.002321. [DOI] [PubMed] [Google Scholar]
- 42.Stefani MR, Gold PE. Intra-hippocampal infusions of K-ATP channel modulators influence spontaneous alternation performance: relationships to acetylcholine release in the hippocampus. J Neurosci. 2001;21:609–614. doi: 10.1523/JNEUROSCI.21-02-00609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warrington EK, Weiskrantz L. Amnesia: a disconnection syndrome? Neuropsychologia. 1982;20:233–248. doi: 10.1016/0028-3932(82)90099-9. [DOI] [PubMed] [Google Scholar]
- 44.White NM, McDonald RJ. Acquisition of a spatial conditioned place preference is impaired by amygdala lesions and improved by fornix lesions. Behav Brain Res. 1993;55:269–281. doi: 10.1016/0166-4328(93)90122-7. [DOI] [PubMed] [Google Scholar]
- 45.White NM, McDonald RJ (2002) Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem, in press. [DOI] [PubMed]
- 46.White NM, Wallet PA. Dorsal hippocampal function in unreinforced spatial learning. Hippocampus. 2000;10:226–235. doi: 10.1002/1098-1063(2000)10:3<226::AID-HIPO3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 47.Willingham D. Systems of memory in the human brain. Neuron. 1997;18:5–8. doi: 10.1016/s0896-6273(01)80040-4. [DOI] [PubMed] [Google Scholar]
- 48.Wrenn CC, Wiley RG. The behavioral functions of the cholinergic basal forebrain: lessons form 192 IgG-saporin. Int J Devel Neurosci. 1998;16:595–602. doi: 10.1016/s0736-5748(98)00071-9. [DOI] [PubMed] [Google Scholar]