Abstract
The central complex is a topographically ordered neuropil structure in the center of the insect brain. It consists of three major subdivisions, the upper and lower divisions of the central body and the protocerebral bridge. To further characterize the role of this brain structure, we have recorded the responses of identified neurons of the central complex of the desert locust Schistocerca gregaria to visual stimuli. We report that particular types of central complex interneurons are sensitive to polarized light. Neurons showed tonic responses to linearly polarized light with spike discharge frequencies depending on e-vector orientation. For all neurons tested, e-vector response curves showed polarization opponency. Receptive fields of the recorded neurons were in the dorsal field of view with some neurons receiving input from both compound eyes and others, only from the ipsilateral eye. In addition to responses to polarized light, certain neurons showed tonic spike discharges to unpolarized light. Most polarization-sensitive neurons were associated with the lower division of the central body, but one type of neuron with arborizations in the upper division of the central body was also polarization-sensitive. Visual pathways signaling polarized light information to the central complex include projections via the anterior optic tubercle. Considering the receptive fields of the neurons and the biological significance of polarized light in insects, the central complex might serve a function in sky compass-mediated spatial navigation of the animals.
Keywords: polarized light, polarization vision, central complex, compass navigation, head direction, insect brain, locust, Schistocerca gregaria
Insects can detect the polarization pattern of the blue sky and use it as a sensory cue for spatial navigation (for review, see Wehner 1992, 1994). The biological significance of polarization vision for compass orientation has been most thoroughly studied in desert ants (Wehner, 1994) and honeybees (Rossel and Wehner, 1986; Rossel, 1993), but polarized light-dependent orientation has also been demonstrated in several other insect species (for review, see Stockhammer, 1959; Waterman, 1981). Photoreceptors in a small dorsal rim area in the compound eye of many insects are particularly adapted for polarized light detection and show high polarization sensitivity (for review, see Labhart and Meyer, 1999).
Polarization-sensitive interneurons in the insect brain were first described in the optic lobe of the cricket, Gryllus campestris (Labhart, 1988; Labhart and Petzold, 1993; Labhart et al., 2001; Petzold, 2001). These neurons, like the photoreceptors of the dorsal rim area, are most sensitive to blue light. The neurons show polarization opponency, i.e., e-vector orientations causing maximal excitation (Φmax) are oriented perpendicularly to e-vectors causing maximal inhibition (Φmin). This feature indicates that the neurons receive antagonistic inputs from perpendicularly orientede-vector analyzers. Recently, polarization-opponent interneurons were also found in the optic lobe of the desert locust (Homberg and Würden, 1997), the Madeira cockroach (Loesel and Homberg, 2001), and the desert ant (Labhart, 2000).
In search for higher brain areas involved in sky compass orientation, we report here that certain interneurons in the locust central complex are polarization-sensitive. The central complex is a group of interconnected neuropils in the center of the insect brain and includes the protocerebral bridge, the upper and lower divisions of the central body, and the paired noduli (Homberg, 1987) (see Fig.1A). Its most striking feature is a highly stratified internal organization consisting of well defined layers in the central body and, perpendicularly, an arrangement into sets of sixteen columns. Columnar neurons provide precise interhemispheric connections and are the main output pathway from the central complex to the adjacent lateral accessory lobes. While the anatomical organization of the central complex has been unraveled in some detail (Hanesch et al., 1989; Homberg, 1985, 1987, 1991; Wendt and Homberg, 1992; Vitzthum et al., 1996; Müller et al., 1997; Vitzthum and Homberg, 1998), its functional role is little understood. In moths, descending neurons from the lateral accessory lobes are involved in motor control such as steering maneuvers during walking and flight (Kanzaki et al., 1991,1994), and behavioral analysis of Drosophila melanogastermutants with structural defects in the central complex also support a role of the central complex in motor control (Strauss and Heisenberg, 1993; Ilius et al., 1994).
Fig. 1.
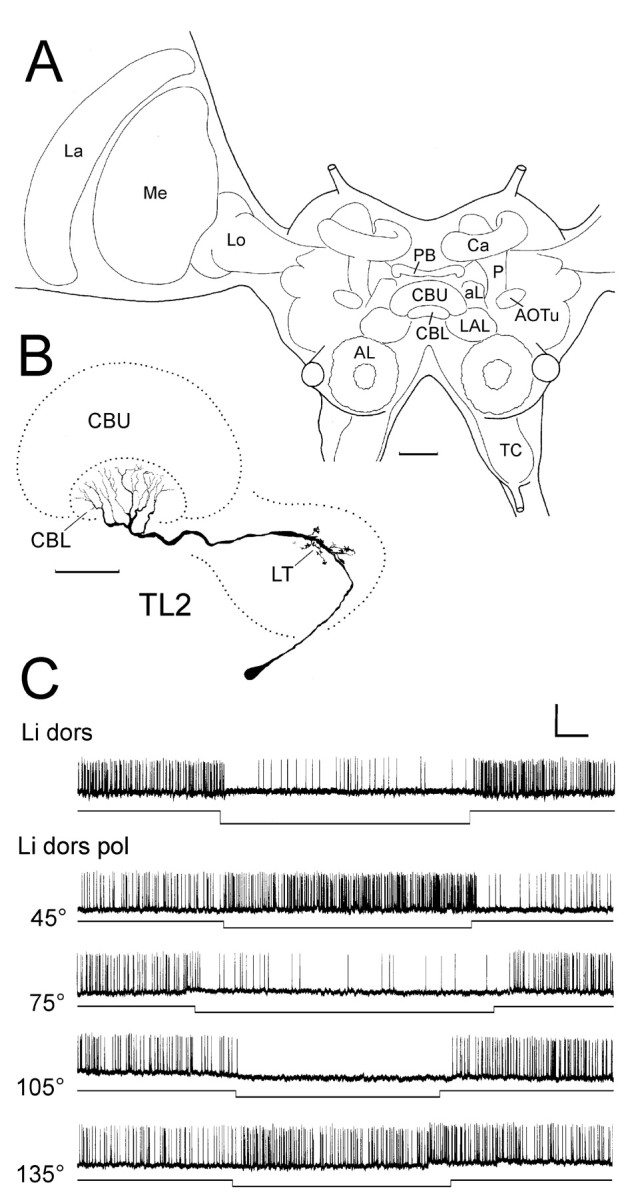
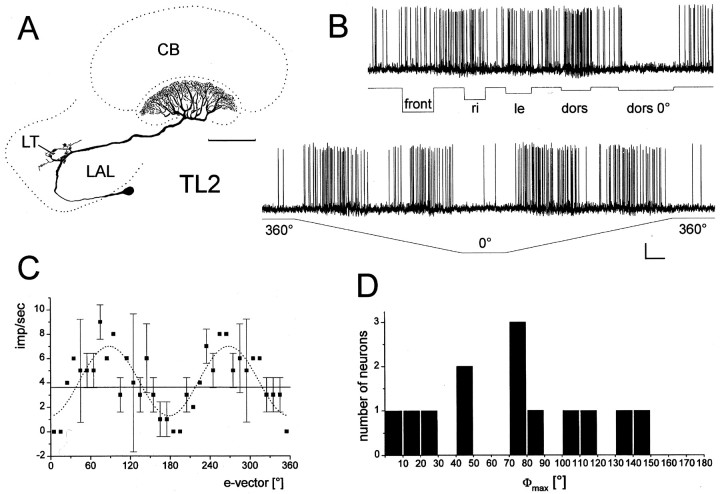
Responses of a TL2 tangential neuron of the lower division of the central body to polarized light. A, Frontal diagram of the locust brain, indicating the position and subdivisions of the central complex in relation to other neuropil structures. AL, Antennal lobe; aL, α-lobe of the mushroom body; AOTu, anterior optic tubercle; Ca, calyces of the mushroom body;CBL, CBU, lower and upper division of the central body; La, lamina; LAL, lateral accessory lobe; Lo, lobula; Me, medulla;P, pedunculus of the mushroom body; PB, protocerebral bridge; TC, tritocerebrum. Scale bar, 200 μm. B, C, Frontal reconstruction (B) and intracellular recording (C) of a Lucifer yellow-injected TL2 neuron of the central body. The neuron has its cell body in the inferior median protocerebrum. It innervates the lateral triangle (LT) of the lateral accessory lobe and layer 2 of the lower division of the central body (CBL). Scale bar, 100 μm. C, The neuron is tonically inhibited by dorsal unpolarized light (Li dors). It shows tonic excitation or inhibition to dorsal polarized light (Li dors pol) depending on e-vector orientation. Calibration: 10 mV, 1 sec.
This study demonstrates a novel sensory aspect of signaling in the central complex. We show that neurons of the locust central complex are sensitive to dorsally presented polarized light and suggest an involvement of this brain structure in sky compass orientation.
Parts of this study have been published in abstract form (Müller and Homberg, 1994; Homberg and Müller, 1995; Vitzthum et al., 1997).
MATERIALS AND METHODS
Preparation. Experiments were performed on adult locusts (Schistocerca gregaria) obtained from a crowded laboratory colony. Animals were anesthetized by cooling and were waxed anterior uppermost to a metal holder. The heads of the locusts were immobilized by a wax–rosin mixture, and their legs were removed. For intracellular recordings from the central protocerebrum, a small window was cut into the head capsule between the two compound eyes. The right antenna and the median ocellus of the animal were removed. After removing fat and some tracheal sacs, the midbrain was supported and slightly lifted by a stainless steel platform that served as the indifferent electrode. A small area of the midbrain was desheathed to facilitate microelectrode penetration. In some preparations, the recording site was stabilized further by a stainless steel ring that was gently pushed onto the brain. In some recordings, especially when hemolymph pumping movements prevented stable recordings, the animals' head was cut off from the thorax. Successful recordings from isolated heads were possible for up to 3 hr by perfusing the preparation regularly with locust saline (Clements and May, 1974).
Electrophysiology. Electrodes (resistance in the tissue, 80–200 MΩ) were drawn from glass capillaries (Hilgenberg, Malsfeld, Germany). They were filled either with 5% Lucifer yellow (Molecular Probes, Eugene, OR) or with 4% Neurobiotin (Vector Laboratories, Burlingame, CA) at the tip. Lucifer-filled electrodes were backed up with 0.1 m LiCl, and electrodes filled with Neurobiotin were backed up with 1 m KCl. Electrodes were aimed at neural processes in the center of the brain close to the stump of the median ocellus at a depth of 150–200 μm from the frontal surface of the brain. Intracellular signals were conventionally amplified, monitored with an oscilloscope (Hameg, Frankfurt/Main, Germany) and stored on digital audio tape (DTR 1802; Biologic, Claix, France) for off-line analysis. After recording, neurons were stained by iontophoretic injection of Lucifer yellow with 0.5–5 nA constant hyperpolarizing current or of Neurobiotin with 1–3 nA depolarizing current for 3–20 min.
Experiments were performed in dim ambient light (irradiance <0.1 μW/cm2). Stationary white light stimuli were produced by 150 W halogen light sources (3200 K; infrared light excluded by heat absorbance filters; cutoff wavelength, 724 nm). Light stimuli were delivered to different parts of the visual field through flexible light guides: (1) to the frontal binocular field of view (visual angle, 14°; irradiance, 3.6 mW/cm2); (2) to the lateral field of view (right or left eye; visual angle, 3°; irradiance, 9 μW/cm2); and (3) to the dorsal field of view (zenith of the animal) through a neutral density filter (visual angle, 2.1°, irradiance, 5 μW/cm2; in some experiments: visual angle, 8°; irradiance, 20 μW/cm2). To test for polarization sensitivity, the neutral density filter in the dorsal light path (3) was replaced by a Polaroid HN38S polarization filter (Schlund, Zürich, Switzerland) with identical light absorbance.e-vector orientations were changed stepwise (stationary stimulation) or continuously (rotatory stimulation) over a range of 180° or 360°. An e-vector orientation parallel to the body axis of the animal was defined as 0°. To test for motion sensitivity, a black and white square (half black, half white; visual angle, 10°), and a black and white grating (32° × 72°; spatial wavelength, 9°) were moved by hand in various parts of the visual field.
Data analysis. Physiological data were analyzed off-line using a CED 1401 plus interface and Spike2 software (Cambridge Electronic Design, Cambridge, UK). Parts of the recordings were printed with a laser printer. Background activities were determined as mean spike rates measured over 2–5 sec before experimental stimulation. To determine e-vector response curves from rotatorye-vector stimulations, means (±SD) of spike frequencies were determined in consecutive 10° bins from two or four rotations (equal numbers of clockwise and counterclockwise rotations) and plotted as a function of e-vector orientation. e-vectors eliciting maximal inhibition and excitation (Φmin and Φmax) were determined by fitting these plots to sin2functions. The fitting procedure was made by a least square fit that followed the Levenberg–Marquardt algorithm (Origin 4.1 software; Microcal, Northampton, MA).
Histology. After physiological characterization and dye injection, brains were dissected out of the head capsule and immersed in fixative for at least 1 hr. Lucifer yellow-injected brains were fixated in 4% paraformaldehyde in 0.1 mphosphate buffer at pH 7.4, whereas Neurobiotin required a fixative containing 4% paraformaldehyde, 0.25% glutaraldehyde, and 0.2% saturated picric acid in 0.1 m phosphate buffer at pH 7.4. Lucifer yellow-injected brains were dehydrated through an ethanol series, cleared in methyl salicylate, and examined with a fluorescence microscope (Zeiss). For detailed anatomical examination, the brains were subsequently processed for Lucifer yellow immunocytochemistry by means of the indirect peroxidase antiperoxidase (PAP) technique (Sternberger, 1986), as described by Homberg and Würden (1997). The Neurobiotin-injected brains were rinsed in PBS (0.01 m phosphate buffer; 0.45m NaCl) with 0.1% Triton X-100 (Sigma, Deisenhofen, Germany), embedded in gelatin–albumin, and sectioned at 30 μm with a Vibratome (Technical Products, St. Louis, MO). The free-floating sections were incubated for at least 18 hr with Streptavidin conjugated to a polymere of horseradish peroxidase (Sigma), diluted at 1:2000 or with Streptavidin conjugated to horseradish peroxidase (Amersham Buchler, Braunschweig, Germany) at 1:200 in PBS with 0.5% Triton X-100. The sections were subsequently treated for 10–20 min with a solution of 3,3′-diaminobenzidine tetrahydrochloride (0.3 mg/ml) in 0.05 mTris–HCl buffer, pH 7.4, with 0.3% nickel ammonium sulfate and H2O2 (0.015%). The sections were mounted and cleared like the Lucifer yellow-injected brains (Homberg and Würden, 1997). All neurons were reconstructed from serial frontal sections by using a Zeiss microscope with camera lucida attachment. The terminology for brain structures largely follows the nomenclature of Strausfeld (1976) and, for central complex subdivisions, Williams (1975), Homberg (1991), and Müller et al. (1997). Positional information is given with respect to the body axis of the animal.
RESULTS
This study is based on the characterization of 41 polarization-sensitive interneurons (POL neurons) of 140 neurons recorded and dye-injected in the median protocerebrum of the locust brain. Seventy-one percent of the recorded neurons either did not respond to any of the visual stimuli or, if they responded to light, did not show e-vector-specific responses. Those neurons, furthermore, differed in morphology from the POL neurons, indicating that only specific morphological types of neurons in the median protocerebrum are polarization-sensitive. Thirty-eight of the 41 POL neurons had arborizations in the central complex. The three additional POL neurons innervated the lateral accessory lobes and/or the anterior optic tubercles of the protocerebrum, which are closely associated with the central complex. Nine recordings from POL neurons were obtained from isolated head preparations (indicated in the figure legends), and 32 recordings were from intact animals with legs removed. No differences in response characteristics were observed between the two groups of animals. None of the POL neurons was sensitive to motion stimuli.
Three types of tangential neuron of the central body are polarization-sensitive
The upper and lower divisions of the locust central body are organized into distinct layers, which are primarily formed by the arborization domains of tangential neurons (Müller et al., 1997;Homberg et al., 1999). Although the upper division of the central body is composed of three major layers (Homberg, 1991), a detailed study of the lower division showed that at least five distinct types of tangential neuron innervate single or several of a total of six layers (Müller et al., 1997). Three types of tangential neuron showed polarization sensitivity (see Figs. 1-6). All of these neurons had arborizations in the lower division of the central body and could be identified as TL1, TL2, and TL3 neurons (Müller et al., 1997), whereas a forth type, TL5 neurons was not polarization-sensitive.
Fig. 2.
Responses of TL2 neurons to unpolarized and polarized light. A–C, Frontal reconstruction (A), intracellular recording (B), and e-vector response plot of a TL2 neuron, studied in an isolated head preparation.A, The neuron arborizes in the lateral triangle (LT) of the lateral accessory lobe (LAL) and in layer 2 of the lower division of the central body (CB). Scale bar, 100 μm.B, The neuron does not respond to illumination of the animal from frontal (front), right (re), or left (le) but is tonically excited by dorsal unpolarized light (dors). The neuron is completely inhibited by dorsal polarized light withe-vector orientation at 0° (dors 0°). Rotation of the polarizer through 360° results in alternating excitations and inhibitions, independent of turning direction. Calibration: 5 mV, 2 sec. C, e-vector response plot (means ± SD; n = 2, one clockwise and one counterclockwise rotation). Solid lineindicates background activity. Fitting the data to a sin2-function (dotted line) reveals a Φmax of 88.4°. D, Distribution of Φmax from 13 recorded TL2 neurons.
Fig. 3.
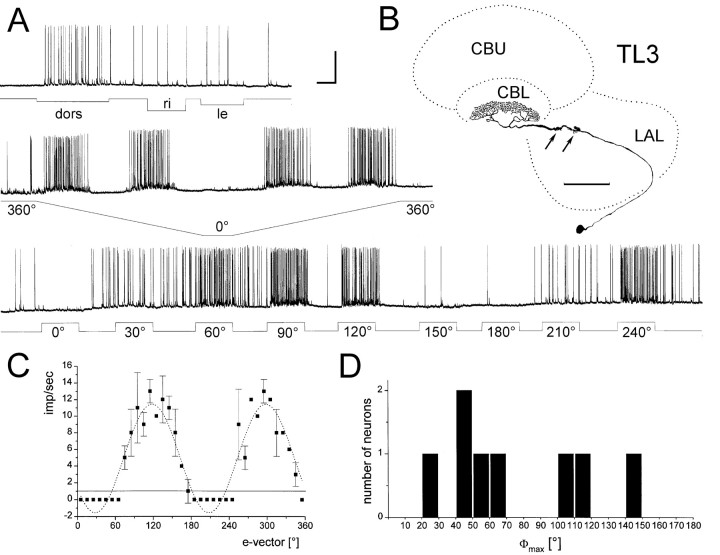
Polarization-sensitive TL3 neurons.A, Intracellular recording from a TL3 neuron (shown inB). The neuron is tonically excited by unpolarized dorsal light (dors) but does not respond to lateral stimulation from right or left (ri, le) eye. The neuron shows polarization opponency when the animal is stimulated dorsally through a rotating polarizer (trace 2) or with stationary polarized light (trace 3). Calibration: 30 mV, 2 sec. B, The neuron has minute processes in the median olive (arrows) of the lateral accessory lobe (LAL) and invades layer 5 of the lower division of the central body (CBL). Scale bar, 100 μm.C, e-vector response plot (means ± SD; n = 2). Background activity (solid line) is one spike per second. Sin2-fitting (dotted line) reveals a Φmax of 117.7°.D, Distribution of Φmax from eight TL3 neurons.
Fig. 4.
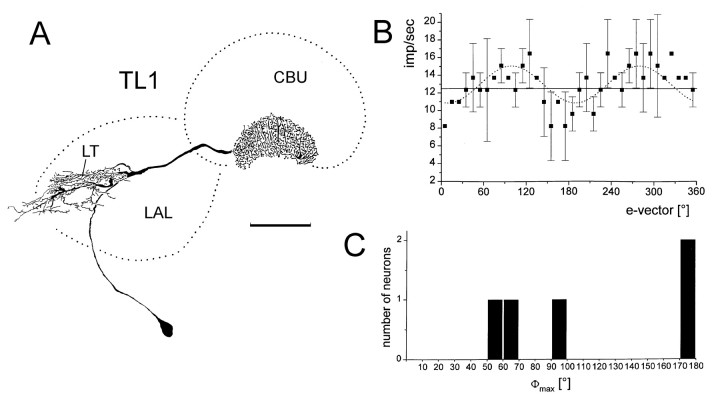
Polarization-sensitive TL1 neurons.A, B, Frontal reconstruction (A) and e-vector-response plot (B) of a TL1 neuron. The neuron has its cell body in the ventromedian protocerebrum. Arborizations extend throughout the lateral triangle (LT) of the lateral accessory lobe and invade the lower division of the central body.CBU, Upper division of the central body. Scale bar, 100 μm. B, e-vector-response plot (means ± SD; n = 2). The neuron has a background activity of 12.6 impulses per second (solid line). Sin2-fitting (dotted line) reveals a Φmax of 99.4°.C, Distribution of Φmax from five TL1 neurons.
Fig. 5.
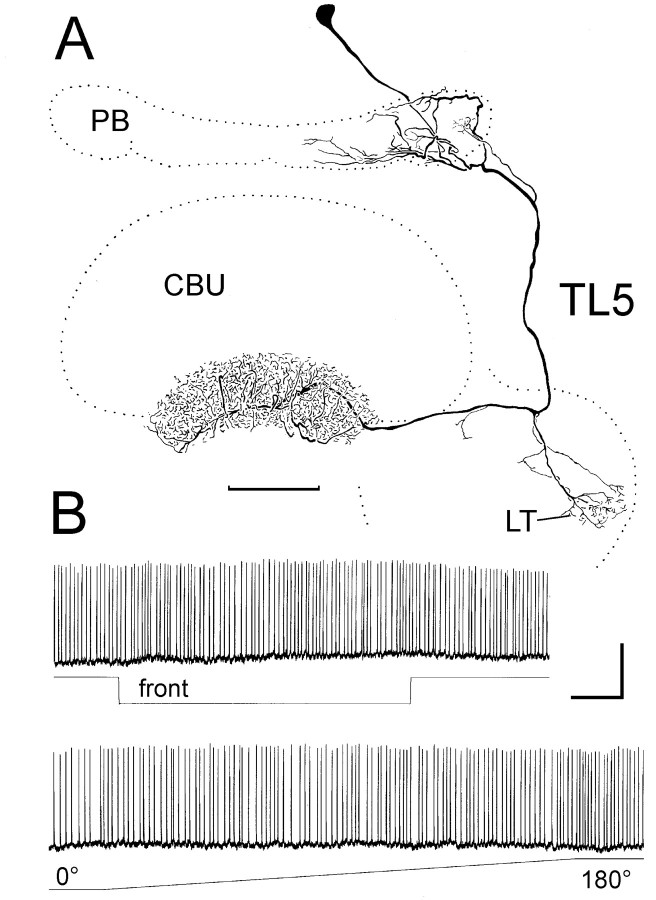
TL5 tangential neurons of the lower division of the central body are not sensitive to polarized light.A, Frontal reconstruction of a TL5 neuron. The neuron has its cell body in the pars intercerebralis. It innervates the ipsilateral hemisphere of the protocerebral bridge (PB), the lateral triangle of the lateral accessory lobe (LT), and all layers of the lower division of the central body. CBU, Upper division of the central body.B, The neuron shows regular background spiking activity and no response to frontal light (front) or polarized light with e-vector orientation rotating from 0° to 180°. Calibration: 5 mV, 1 sec.
Fig. 6.
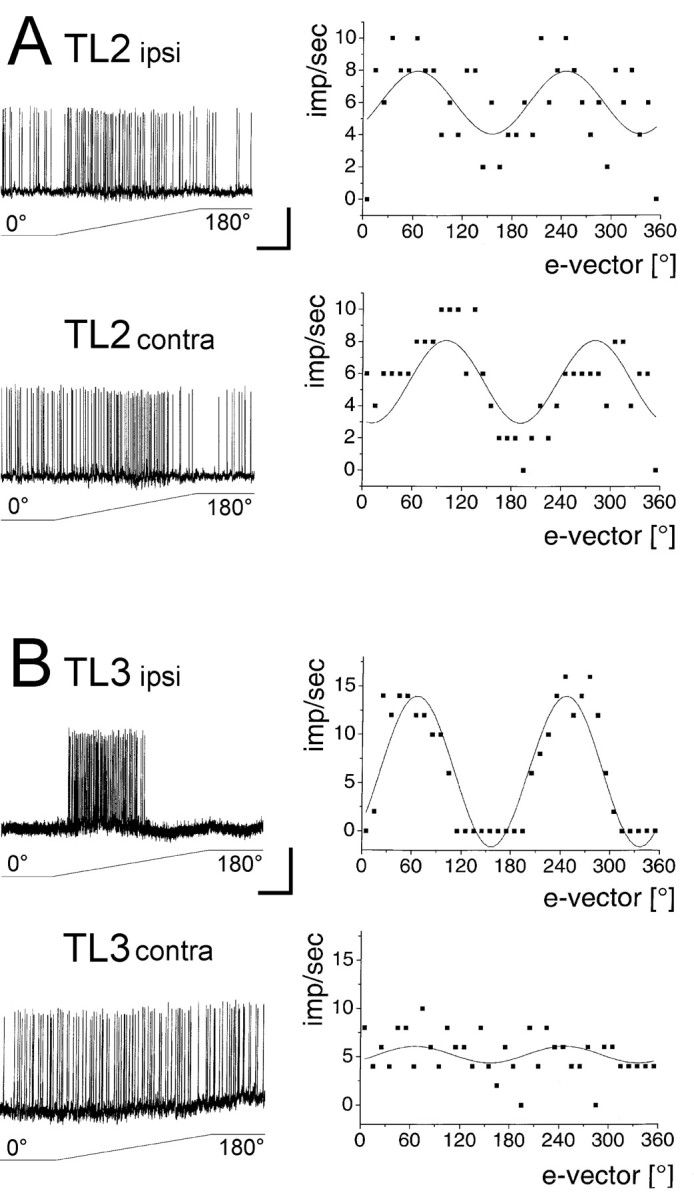
Ocular dominance for polarized-light responses in TL2 and TL3 neurons. A, Responses of a TL2 neuron recorded in an isolated head preparation. Intracellular recording (left) and e-vector response plots (right) for stimulation of the ipsilateral and contralateral eye show that the neuron receives input from both compound eyes. Data from two 180° rotations of the polarizer are shown in the e-vector response plots. Solid lines: sin2-fit. Note considerable difference in Φmax for stimulation of the ipsilateral (66.0°) versus the contralateral (101.1°) eye. Stimulation of both eyes reveals an intermediate Φmax of 88.6° (data not shown). Calibration: 10 mV, 2 sec. B, Responses of a TL3 neuron recorded in an isolated head preparation. The neuron receives input almost exclusively from the ipsilateral eye. Φmax for ipsilateral and contralateral stimulation is nearly identical (66.6° and 66.1°) and matches Φmax for binocular stimulation (66.4°). Calibration: 5 mV, 2 sec.
TL2 neurons
Polarization-sensitive TL2 neurons were encountered most frequently (13 recordings). The neurons had cell bodies in the inferior median protocerebrum. Dendritic arborizations were in a small area within the lateral accessory lobe of the brain termed the lateral triangle (Figs. 1A,B,2A). Axonal projections were confined to specific layers within the lower division of the central body. Twelve of the thirteen stained neurons had ramifications in the second upper layer (layer 2) of the lower division of the central body (Figs. 1,2) and only one neuron had arborizations in the lower layers 4/5 (data not shown). When giving flashes of unpolarized white light in the dorsal visual field, eight TL2 neurons (61%) showed tonic inhibition of spiking activity (Fig. 1C), one neuron (8%) was tonically excited (Fig. 2B), and four neurons (31%) showed no clear response. Frontal or lateral light flashes were less effective than dorsal stimulation (Fig.2B). Polarized light presented dorsally elicited tonic excitations, tonic inhibitions, or no change in spiking activity depending on the orientation of the polarizer (Fig. 1C). Short rebound reactions often occurred after excitatory or inhibitory responses (Fig. 1C). The responses showed polarization opponency, i.e., e-vectors eliciting maximal excitation (Φmax) were perpendicular toe-vectors eliciting maximal inhibition (Φmin) (Figs. 1C,2B,C). When stimulating with a rotating polarizer, maximal spike activity occurred with a period of 180°, intersected by periods of minimal spike activity, indicating again polarization opponency (Fig. 2B). The response was independent of turning direction, and e-vector response plots revealed differences in Φmax of <10°, when comparing stationary and rotatory stimulation (data not shown). Φmax values differed widely within the 13 recorded neurons, and no classification was apparent (Fig.2D). Considering the observation that all but one neuron (which had Φmax at 102°) had ramifications in layer 2 of the lower division of the central body, there is no evidence for an e-vector map represented by the layering of the lower division.
TL3 neurons
TL3 tangential neurons (eight recordings) of the lower division of the central body had cell bodies intermingled with TL2 neurons in the inferior median protocerebrum (Fig.3B). Dendritic ramification were confined to a second small area within the lateral accessory lobe, the median olive, and consisted of tiny dense knots of fine processes typical of TL3 neurons (Fig. 3B). Arborizations of most TL3 neurons in the lower division of the central body were confined to single layers. In seven preparations (88%), neurons had projections in layer 5 of the lower division (Fig. 3B). In one preparation, two TL3 neurons were simultaneously stained with arborizations in layers 2, 4, and 5 (data not shown). TL3 neurons had low background spiking activity and, in contrast to most TL2 neurons, responded with tonic excitation to unpolarized dorsal light (Fig. 3A). Lateral visual stimulation was without effect (Fig. 3A). Like TL2 neurons, TL3 neurons showed tonic responses to polarized light, often with complete inhibition of spiking activity at Φmin (Fig. 3A). The responses of all neurons showed polarization opponency (Fig. 3A,C). Φmax-values varied greatly among the neurons, which indicates that no particular e-vector preference is represented in layer 5 of the lower division of the central body (Fig.3D). The recording corresponding to the double-impaled TL3 neurons had Φmax at 146.9°.
TL1 neurons
Five injections of polarization-sensitive neurons revealed TL1 tangential neurons of the lower division of the central body (Müller et al., 1997). The neurons had cell bodies in the ventromedian protocerebrum. They invaded the lateral triangle of the lateral accessory lobe with fine arborizations and had beaded terminals throughout layers 2–6 of the lower division of the central body (Fig.4A). Most neurons showed high background activity of 10–15 impulses/sec. The neurons either did not respond to unpolarized light or showed only weak excitations (data not shown). Dorsal stimulation with polarized light revealed, as for TL2 and TL3 neurons polarization opponency (Fig.4B). Φmax values were ∼60° (56.4°, 60.5°), 100° (99.4°), and 170° (171.8°; 171.6°).
Other tangential neurons
Recordings from a variety of tangential neurons of the upper division of the central body did not reveal selective responses to polarized light. Recordings from TL5 neurons of the lower division of the central body (n = 2), likewise, showed no evidence for polarization sensitivity (Fig. 5). TL5 neurons had cell bodies in the pars intercerebralis. Dendritic ramifications invaded the ipsilateral hemisphere of the protocerebral bridge and the lateral triangle of the lateral accessory lobe. Beaded terminals extended throughout the lower division of the central body (Fig. 5A). In both recordings, the neurons had high regular background activity of ∼10 impulses/sec. One neuron showed a weak on-excitation to frontal illumination (data not shown). The second TL5 neuron did not respond to the visual stimuli including polarized light (Fig. 5B).
TL2 and TL3 neurons differ in ocular dominance
Ocular dominance was tested in two TL2 and in three TL3 neurons. One compound eye was stimulated dorsally while the other eye was shielded from the light path through a piece of black cardboard. The TL2 neurons showed polarization sensitivity irrespective of whether the ipsilateral or the contralateral eye was stimulated (Fig.6A). One of the two neurons (Fig. 6A) showed a considerable difference in Φmax for stimulation of the ipsilateral and contralateral eye (Φmax-ipsi = 66.0°; Φmax-contra = 101.1°) and an intermediate value for binocular stimulation (Φmax = 88.6°). In contrast, all TL3 neurons showed marked responses only when the ipsilateral eye was stimulated but no or only weak polarization sensitivity after stimulation of the contralateral eye (Fig.6B).
Three types of POL neurons are columnar neurons of the central complex
In addition to a layered organization, the protocerebral bridge and the central body are subdivided from right to left into linear rows of 16 columns, 8 columns in each hemisphere. These columns are innervated individually by columnar neurons. Most types of columnar neuron described so far connect columns of the bridge to columns of the central body in a topographic manner, and, in addition, send axonal fibers to the lateral accessory lobes (Williams, 1975; Müller et al., 1997; Vitzthum and Homberg, 1998). Columnar neurons were penetrated less frequently than tangential cells, probably caused by their small fiber diameter. In total, six columnar neurons comprising three distinct cell types were found to be polarization-sensitive. Each type of neuron was recorded and stained twice, and none of the neurons have been described before.
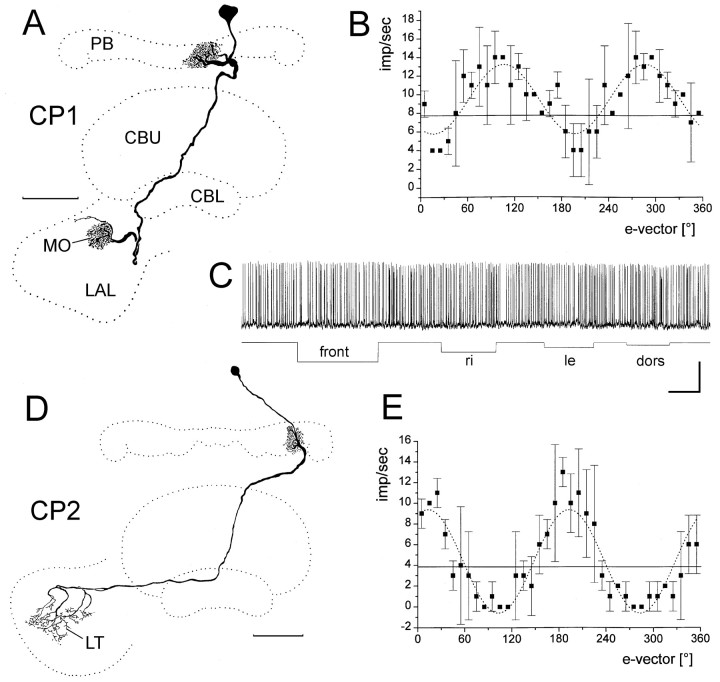
One type of columnar POL neuron, termed columnar neuron of the protocerebral bridge type 1 (CP1) connected single columns of the protocerebral bridge to the contralateral median olive of the lateral accessory lobe (Fig. 7A). The second type of neuron, termed CP2, connected single columns of the protocerebral bridge to the lateral triangle of the contralateral accessory lobe (Fig. 7D). None of these neurons had arborizations in the central body. One CP1 neuron was weakly excited by unpolarized light (data not shown), and the others did not respond (Fig. 7C). All neurons showed tonic responses to polarized light with clear polarization opponency (Fig. 7B,E).
Fig. 7.
CP1 and CP2 neurons are polarization-sensitive.A–C, Frontal reconstruction (A),e-vector response plot (B), and responses to unpolarized light stimuli of a CP1 neuron.A, The CP1 neuron has its soma in the pars intercerebralis. It innervates the innermost column L8 in the left hemisphere of the protocerebral bridge (PB) and has arborizations throughout the median olive (MO) of the lateral accessory lobe (LAL). B,e-vector-response plot (means ± SD;n = 2). Solid line, Background activity. Sin2-fitting (stippled line) reveals a Φmax of 106.2°.C, The neuron shows no response to unpolarized light stimuli. Calibration: 20 mV, 2 sec. D, E, Reconstruction (D) and e-vector response plot (E) of a CP2 neuron.D, The neuron innervates column L4 in the left hemisphere of the protocerebral bridge and has arborizations throughout the lateral triangle of the lateral accessory lobe (LT). E, e-vector response plot (means ± SD; n = 2).Solid line, Background activity. Sin2-fitting (stippled line) reveals a Φmax of 13.0°.
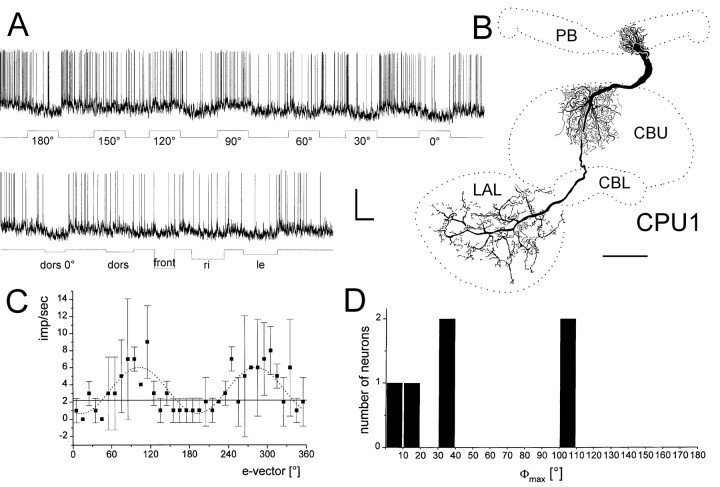
Two columnar POL neurons, termed columnar neuron of the protocerebral bridge/upper division of the central body, type 1 (CPU1) had arborizations in the upper division of the central body. The neurons had dense arborizations in single columns of the protocerebral bridge. A fiber projected through the posterior chiasm to the central body and had a second tree of ramifications in layer I of the upper division of the central body. An axonal process gave rise to beaded arborizations throughout the lateral accessory lobe, but spared the lateral triangle and the median olive (Fig.8B). Both CPU1 neurons were weakly inhibited by ipsilateral and frontal light pulses (Fig.8A). Polarized light presented dorsally resulted in tonic changes in spiking activity (excitatory or inhibitory) with pronounced rebounds after lights off (Fig. 8A).e-vector response plots showed polarization opponency as in all other neurons described so far (Fig. 8C). The distribution of Φmax for the six columnar neurons is shown in Figure 8D. All neurons invaded columns in the left hemisphere of the protocerebral bridge, including the innermost column L8 (Φmax = 106.2°) (Fig.7A,B), column L6 (Φmax = 102.2°) (Fig. 8), column L4 (Φmax = 39.4°; 13°) (Fig. 7D,E), column L2 (Φmax = 5.6°), and a double impalement of two CP2 neurons arborizing in columns L2 and L4 (Φmax = 37.6°).
Fig. 8.
Response properties of a CPU1 columnar neuron.A, Intracellular recording, showing tonic excitation (at 120°, 90°) or inhibition (at 30°, 0°, 180°) to dorsal polarized light. Note pronounced rebound reactions after lights off. Weak inhibitory responses also occur to illumination from frontal (front) and from the left (le) but not to light from dorsal and from the right (dors, ri). Calibration: 20 mV, 2 sec. B, Frontal reconstruction of the neuron. It innervates column L6 in the lateral hemisphere of the protocerebral bridge (PB), the innermost contralateral column in layer 1 of the upper division of the central body (CBU), and large areas in the contralateral accessory lobe (LAL). Scale bar, 100 μm.C, e-vector response plot (means ± SD; n = 2). Solid line, Background activity. Sin2-fitting (stippled line) reveals a Φmax of 102.2°.D, Distribution of Φmax from six columnar neurons of the central complex.
The anterior optic tubercles are part of the polarization-sensitive pathways to the central complex
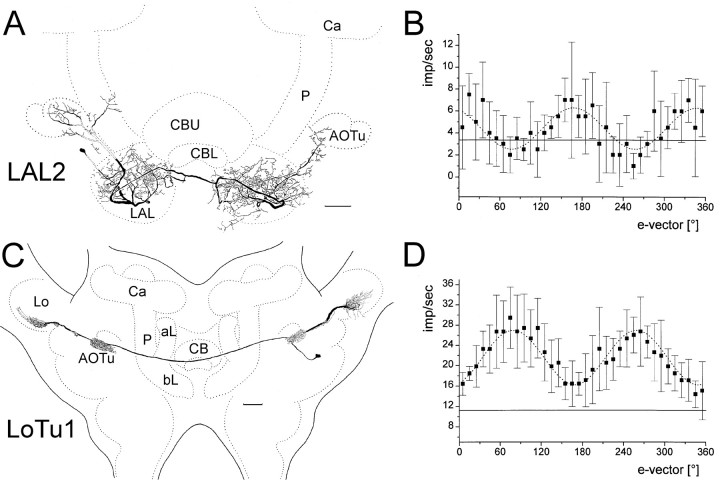
Three recordings from POL neurons revealed neurons that innervated the lateral accessory lobes and/or the anterior optic tubercles but not the central complex. One neuron, named LAL1, connected the right and left lateral accessory lobes. Fine processes in one lobe were associated with the lateral triangle, and varicose arborizations on the other side were distributed throughout the lateral accessory lobe (data not shown). The second neuron (LAL2) (Fig.9A) had its cell body in the inferior lateral protocerebrum close to the anterior face of the brain. Dendritic ramifications were in the upper division of the anterior optic tubercle and in posterior parts of the ipsilateral accessory lobe, including the median olive and lateral triangle. An axonal fiber crossed the midline of the brain below the central body and innervated the contralateral accessory lobe with processes extending to the median base of the contralateral optic tubercle (Fig. 9A). The third neuron (termed LoTu1) had nearly symmetrical arborizations in both brain hemispheres (Fig. 9C). The cell body was in the inferior lateral protocerebrum. Dense fine arborizations were confined to the lower division of the anterior optic tubercle and via the anterior optic tract in a ventral layer of the anterior lobe of the lobula complex. An axonal fiber crossed the midline via the intertubercle tract and invaded symmetric areas in the contralateral hemisphere (Fig. 9C).
Fig. 9.
Morphology and e-vector responses of POL neurons without arborizations in the central complex.A, Polarization-sensitive LAL2 neuron with arborizations in the upper division of the ipsilateral anterior optic tubercle (AOTu), wide arborizations in both lateral accessory lobes (LAL), and processes extending to the base of the contralateral anterior optic tubercle. Scale bar, 100 μm.B, Sin2-fitting (stippled line) of the e-vector response plot of the neuron (means ± SD; n = 4) revealed a Φmax of 167.4°. Solid line, Background activity. C, LoTu1 neuron with arborizations in the lower division of the anterior optic tubercle and in a ventral shell in the anterior lobe of the lobula complex of both brain hemispheres.aL, bL, Ca,P, α-lobe, β-lobe, pedunculus, and calyces of the mushroom body. Scale bar, 100 μm. D, The neuron is excited by all e-vector orientations but the response magnitude depends on e-vector orientation. Sin2-fitting (dotted line) of thee-vector-response plot of the neuron (means ± SD;n = 4) reveals a Φmax of 79.0°.
Two neurons showed polarization opponency with inhibitory and excitatory responses depending on the orientation of the polarizer (Φmax = 12.1; 167.4) (Fig. 9B). The LAL1 neuron was inhibited by dorsal unpolarized light, but excited by frontal light (data not shown). The LoTu1 neuron, in contrast, was excited by all e-vector orientations. The response magnitude of LoTu1, however, clearly depended on e-vector orientation (Fig. 9D), and the e-vector (Φmax) eliciting maximal spiking activity was again perpendicular to the e-vector eliciting minimal excitation (Φmin), like in polarization-opponent interneurons.
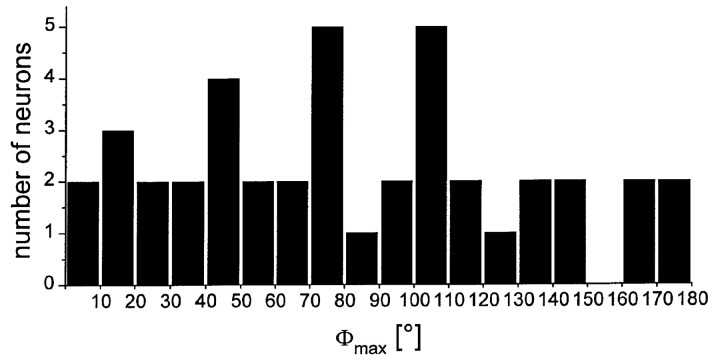
POL neurons in the locust midbrain are not organized into defined classes of Φmax
POL neurons in the optic lobe of the cricket, studied by Labhart (1988) and Labhart and Petzold (1993) fell into three clearly defined Φmax classes. In contrast, the distribution of Φmax from POL neurons in the midbrain of the locust (n = 41), including neurons that could not be stained or that showed multiple dye-filled neurons show widely differing Φmax values without recognizable classes (Fig. 10).
Fig. 10.
Distribution of Φmax orientations from 41 recorded POL neurons in the median protocerebrum of the locust.
DISCUSSION
In search for central mechanisms of sky compass orientation in insects, this study was aimed at identifying and characterizing higher brain areas in the locust involved in polarization vision. We show that a subset of neurons in the central complex is sensitive to dorsally presented polarized light. The neurons have, in part, overlapping branching patterns, and, therefore, may be synaptically connected. Staining of POL neurons without arborizations in the central complex, furthermore, suggests that the anterior optic tubercles are involved in transmitting polarized-light signals from the optic lobe to the central complex.
Polarization vision in insects
Behavioral evidence for the detection of the plane of polarized light has been presented for a number of insect species, most notably honeybees (for review, see Rossel and Wehner, 1986; Rossel, 1993), ants (for review, see Wehner, 1997), crickets (Brunner and Labhart, 1987;Beugnon and Campan, 1989; Herz-mann and Labhart, 1993), and backswimmers (Schwind, 1984). Polarization vision in these insects is used either for sky compass navigation or, in case of the backswimmer and other aquatic insects, for the detection of water surfaces. Accordingly, photoreceptors specialized for the detection of polarized light are usually grouped in a ventral eye region (for the detection of water surfaces) or, more often, in a dorsal eye region, the dorsal rim area (for skylight navigation). Specialized dorsal rim areas have been found in a large number of insect species, indicating that polarized skylight detection is widespread in insects (Labhart and Meyer, 1999). Ommatidia in the dorsal rim area show prominent adaptations for polarized light detection, including changes in rhabdom shape, lack of retinal screening pigment, homochromacy, and orthogonally arranged polarization analyzers (Nilsson et al., 1987; Labhart and Meyer, 1999).
Only a brief report has demonstrated polarization vision in locusts (Eggers and Weber, 1993). Locust larvae walking on a Kramer sphere (Weber et al., 1981) showed menotactic orientation with respect toe-vector orientation. Selective occlusion of various parts of the eyes and ocelli showed that this behavior is mediated through photoreceptors of the dorsal rim area. The dorsal rim area of the desert locust differs markedly from adjacent areas of the compound eye (Eggers and Gewecke, 1993; Paech et al., 1997). Photoreceptors show high polarization sensitivity, are homochromatic blue-sensitive and, within each ommatidium, have perpendicularly aligned microvilli. These features strongly suggest that locusts, like ants and bees, use polarization vision for skylight navigation, perhaps during long migratory flights.
Processing of polarized light information in the brain
Little is known about the central processing of polarized light information in the insect brain. Polarization-sensitive interneurons have been reported in the optic lobe of the cockroaches Periplaneta americana and Leucophaea maderae(Kelly and Mote, 1990; Loesel and Homberg, 2001), the locust S. gregaria (Homberg and Würden, 1997), and the desert antCataglyphis bicolor (Labhart, 2000), but an in-depth analysis only exists for the cricket Gryllus campestris(Labhart, 1988, 1996; Labhart and Petzold, 1993; Labhart et al., 2001;Petzold, 2001). As in the locust central complex, POL neurons in the optic lobe of the cricket, cockroach, locust, and ant showed polarization opponency. In the cricket, three classes of Φmax were found with orientations ∼10°, 60°, and 130° to the longitudinal axis of the animal. POL neurons in the cricket optic lobe were indifferent to unpolarized light and had large visual fields (Labhart, 1988; Labhart and Petzold, 1993). Anatomically, most recordings in the cricket were from one type of commissural interneuron with dendritic arborizations in the dorsal medulla and an axonal projection to the contralateral medulla, but other, less well characterized types of neurons were also encountered (Petzold et al., 1995).
POL neurons in the locust central complex
Our study presents first evidence that the central complex, a major area in the median protocerebrum, is involved in the processing of polarized light. The morphological features of the recorded POL neurons suggest that a particular network of interconnected neurons within the central complex is involved in polarized light signaling. Common projection areas of the recorded POL neurons include the protocerebral bridge, the lower division of the central body, and the median olive and lateral triangle of the lateral accessory lobe. In contrast, only one type of POL neuron (CPU1 neurons) had processes in the upper division of the central body. Tracer injections showed that the median olive and the lateral triangle receive input from fibers originating in the anterior optic tubercle (Hofer and Homberg, 2001). This connection and polarization sensitivity encountered in neurons with ramifications in the anterior optic tubercles strongly suggest that these brain areas are part of the polarization-vision pathway to the central complex.
The recorded POL neurons of the central complex showed no preferences for certain Φmax orientations. This contrasts with the three classes of Φmax orientations found in POL neurons in the cricket (Labhart, 1988; Labhart and Petzold, 1993). Furthermore, we did not find a correlation between Φmax and neuronal branching patterns. The occurrence of widely differing Φmaxorientations in TL2 and TL3 neurons innervating the same layer in the lower division of the central body argues against ane-vector map encoded in the layering of the central body. The results for columnar neurons are less clear because of the small number of recordings. The functional significance of the 16-fold columnar organization of the central complex, present in all insect species, is still unclear (Homberg, 1987). The robust visual responses of columnar neurons, shown here for the first time, should now allow to analyze whether this organization reflects a map fore-vector orientations or for other parameters of the response.
An interesting aspect of signal processing in a neuropil like the central complex, which spans the midline of the brain, is the integration of bilateral inputs. TL2 and TL3 neurons differed considerably in this respect. TL2 neurons received binocular input and apparently pooled the signals from both eyes, whereas TL3 neurons only responded to ipsilateral stimulation.
In contrast to POL neurons in the cricket optic lobe (Labhart and Petzold, 1993), many locust neurons showed, in addition toe-vector-specific responses, reactions to unpolarized light. This could mean that inhibitory and excitatory inputs from orthogonal polarization analyzers are less well balanced than in the cricket. In an extreme case, excitatory light responses might merely be modulated by inhibitory inputs at Φmin, as observed in the LoTu1 neuron. Alternatively, the neurons might receive specific input from nonpolarization-sensitive photoreceptors. In view of a possible function of the central complex in sky compass orientation (see below), responses to unpolarized light in these neurons might signal the position of the sun or the spectral composition of the sky (Wehner, 1984, 1997), and thus could integrate additional features for sky compass navigation in their response.
Functional role of the central complex
The central complex is one of the most prominent neuropil structures in the insect brain, yet its functional role has long been a mystery (Homberg, 1987). Accumulating evidence from brain stimulation and lesion experiments mainly in crickets (for review, see Homberg, 1987) single-cell recordings in the locust (Homberg, 1994), behavior-dependent activity labeling (Bausenwein et al., 1994), and behavioral analysis of central-complex defects in the fly (Strauss and Heisenberg, 1993; Martin et al., 1999) suggest a role in the control of locomotor activity, particularly flight and walking. Mutations affecting the proper organization of the ellipsoid body inDrosophila (equivalent to the lower division of the central body in the locust) and/or the protocerebral bridge, as well as targeted expression of tetanus toxin in neurons of these neuropils result in specific locomotor deficiencies. These include reduced locomotor activity, impairments in straightness of walking, walking speed, and leg coordination during turns and start-stops (Leng and Strauss, 1998; Martin et al., 1999).
Our present findings shed new light on the functional role of the central complex and, together with the evidence for a function in motor control, point to a cardinal role in spatial navigation and direction coding. The responses to polarized light, presented in the dorsal field of view, strongly suggest that the sky polarization pattern is the biologically relevant stimulus for these neurons. Whereas polarized skylight provides directional information from the external environment (allothetic cues), information about self-movement (ideothetic cues) might be processed in the central complex as well. Single-cell recordings, in fact, provide clear evidence for self-movement-generated mechanosensory input to the locust central complex (Homberg, 1994). The balance of right–left output from the central complex network might then control steering commands to thoracic motor centers. The role of descending neurons from the lateral accessory lobes in right–left maneuvering has, in fact, been demonstrated clearly for the zig–zag walking path of the silkmothBombyx mori toward a female (Olberg, 1993; Kanzaki et al., 1994; Kanzaki and Mishima, 1996; Kanzaki, 1998; Mishima and Kanzaki, 1998). Taken the evidence together, we propose that the central complex is a center for direction perception and spatial navigation and, therefore, is likely to exploit all information available for that task including, in particular, the sky polarization pattern.
Footnotes
This work was supported by Grants Ho 950/4 and Ho 950/13 from the Deutsche Forschungsgemeinschaft. We thank Dr. Monika Stengl for insightful discussions and suggestions on this manuscript.
Correspondence should be addressed to Dr. Uwe Homberg, Fachbereich Biologie, Tierphysiologie, Universität Marburg, D-35032 Marburg, Germany. E-mail: homberg@mailer.uni-marburg.de.
REFERENCES
- 1.Bausenwein B, Müller NR, Heisenberg M. Behavior-dependent activity labeling in the central complex of Drosophila during controlled visual stimulation. J Comp Neurol. 1994;340:255–268. doi: 10.1002/cne.903400210. [DOI] [PubMed] [Google Scholar]
- 2.Beugnon G, Campan R. Homing in the field cricket, Gryllus campestris. J Insect Behav. 1989;2:187–198. [Google Scholar]
- 3.Brunner D, Labhart T. Behavioural evidence for polarization vision in crickets. Physiol Entomol. 1987;12:1–10. [Google Scholar]
- 4.Clements AN, May TE. Studies on locust neuromuscular physiology in relation to glutamic acid. J Exp Biol. 1974;60:673–705. doi: 10.1242/jeb.60.3.673. [DOI] [PubMed] [Google Scholar]
- 5.Eggers A, Gewecke M. The dorsal rim area of the compound eye and polarization vision in the desert locust (Schistocerca gregaria). In: Wiese K, Gribakin FG, Popov AV, Renninger G, editors. Sensory systems of arthropods. Birkhäuser; Basel: 1993. pp. 101–109. [Google Scholar]
- 6.Eggers A, Weber T. Behavioral evidence for polarization vision in locusts. In: Elsner N, Heisenberg M, editors. Gene–brain–behaviour. Thieme; Stuttgart: 1993. p. 336. [Google Scholar]
- 7.Hanesch U, Fischbach KF, Heisenberg M. Neuronal architecture of the central complex in Drosophila melanogaster. Cell Tissue Res. 1989;257:343–366. [Google Scholar]
- 8.Herzmann D, Labhart T. Spectral sensitivity and absolute threshold of polarization vision in crickets: a behavioral study. J Comp Physiol [A] 1993;165:315–319. [Google Scholar]
- 9.Hofer S, Homberg U. Anatomical organization of the anterior optic tubercle in the brain of the locust Schistocerca gregaria. In: Elsner N, Kreutzberg GW, editors. Göttingen neurobiology report 2001. Thieme; Stuttgart: 2001. p. 521. [Google Scholar]
- 10.Homberg U. Interneurons of the central complex in the bee brain (Apis mellifera, L.). J Insect Physiol. 1985;31:251–264. [Google Scholar]
- 11.Homberg U. Structure and functions of the central complex in insects. In: Gupta AP, editor. Arthropod brain: its evolution, development, structure, and functions. Wiley; New York: 1987. pp. 347–367. [Google Scholar]
- 12.Homberg U. Neuroarchitecture of the central complex in the brain of the locust Schistocerca gregaria and S. americana as revealed by serotonin immunocytochemistry. J Comp Neurol. 1991;303:245–254. doi: 10.1002/cne.903030207. [DOI] [PubMed] [Google Scholar]
- 13.Homberg U. Flight-correlated activity changes in neurons of the lateral accessory lobes in the brain of the locust Schistocerca gregaria. J Comp Physiol [A] 1994;175:597–610. [Google Scholar]
- 14.Homberg U, Müller M. Neurons of the central complex in the locust brain are sensitive to polarized light. In: Burrows M, Matheson T, Newland PC, Schuppe H, editors. Nervous systems and behaviour. Thieme; Stuttgart: 1995. p. 279. [Google Scholar]
- 15.Homberg U, Würden S. Movement-sensitive, polarization-sensitive, and light-sensitive neurons of the medulla and accessory medulla of the locust, Schistocerca gregaria. J Comp Neurol. 1997;386:329–346. [PubMed] [Google Scholar]
- 16.Homberg U, Vitzthum H, Müller M, Binkle U. Immunocytochemistry of GABA in the central complex of the locust Schistocerca gregaria: identification of immunoreactive neurons and colocalization with neuropeptides. J Comp Neurol. 1999;409:495–507. doi: 10.1002/(sici)1096-9861(19990705)409:3<495::aid-cne12>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 17.Ilius M, Wolf R, Heisenberg M. The central complex of Drosophila melanogaster is involved in flight control: studies on mutants and mosaics of the gene ellipsoid body open. J Neurogenet. 1994;9:189–206. doi: 10.3109/01677069409167279. [DOI] [PubMed] [Google Scholar]
- 18.Kanzaki R. Coordination of wing motion and walking suggests common control of zigzag motor program in a male silkworm moth. J Comp Physiol [A] 1998;182:267–276. [Google Scholar]
- 19.Kanzaki R, Mishima T. Pheromone-triggered “flipflopping” neural signals correlated with activities of neck motor neurons of a male moth, Bombyx mori. Zool Sci. 1996;13:79–87. [Google Scholar]
- 20.Kanzaki R, Arbas EA, Hildebrand JG. Physiology and morphology of descending neurons in pheromone-processing olfactory pathways in the male moth Manduca sexta. J Comp Physiol [A] 1991;169:1–14. doi: 10.1007/BF00198168. [DOI] [PubMed] [Google Scholar]
- 21.Kanzaki R, Ikeda A, Shibuya T. Morphological and physiological properties of pheromone-triggered flipflopping descending interneurons of the male silkworm moth, Bombyx mori. J Comp Physiol [A] 1994;175:1–14. [Google Scholar]
- 22.Kelly KM, Mote MI. Electrophysiology and anatomy of medulla interneurons in the optic lobe of the cockroach, Periplaneta americana. J Comp Physiol [A] 1990;167:745–756. doi: 10.1007/BF00189765. [DOI] [PubMed] [Google Scholar]
- 23.Labhart T. Polarization-opponent interneurones in the insect visual system. Nature. 1988;331:435–437. [Google Scholar]
- 24.Labhart T. How polarization-sensitive interneurones of crickets perform at low degrees of polarization. J Exp Biol. 1996;199:1467–1475. doi: 10.1242/jeb.199.7.1467. [DOI] [PubMed] [Google Scholar]
- 25.Labhart T. Polarization-sensitive interneurons in the optic lobe of the desert ant Cataglyphis bicolor. Naturwissenschaften. 2000;87:133–136. doi: 10.1007/s001140050691. [DOI] [PubMed] [Google Scholar]
- 26.Labhart T, Meyer EP. Detectors for polarized skylight in insects: a survey of ommatidial specializations in the dorsal rim area of the compound eye. Microsc Res Tech. 1999;47:368–379. doi: 10.1002/(SICI)1097-0029(19991215)47:6<368::AID-JEMT2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 27.Labhart T, Petzold J. Processing of polarized light information in the visual system of crickets. In: Wiese K, Gribakin F, Popov AV, Renninger G, editors. Sensory system of arthropods. Birkhäuser; Basel: 1993. pp. 158–168. [Google Scholar]
- 28.Labhart T, Petzold J, Helbling H. Spatial integration in polarization-sensitive interneurones of crickets: a survey of evidence, mechanisms and benefits. J Exp Biol. 2001;204:2423–2430. doi: 10.1242/jeb.204.14.2423. [DOI] [PubMed] [Google Scholar]
- 29.Leng S, Strauss R. Ethograms of three Drosophila mutant strains with structural defects in the protocerebral bridge. In: Elsner N, Wehner R, editors. Göttingen neurobiology report 1998. Thieme; Stuttgart: 1998. p. 259. [Google Scholar]
- 30.Loesel R, Homberg U. Anatomy and physiology of neurons with processes in the accessory medulla of the cockroach Leucophaea maderae. J Comp Neurol. 2001;439:193–207. doi: 10.1002/cne.1342. [DOI] [PubMed] [Google Scholar]
- 31.Martin J-R, Raabe T, Heisenberg M. Central complex substructures are required for the maintenance of locomotor activity in Drosophila melanogaster. J Comp Physiol [A] 1999;185:277–288. doi: 10.1007/s003590050387. [DOI] [PubMed] [Google Scholar]
- 32.Mishima T, Kanzaki R. Coordination of flipflopping neural signals and head turning during pheromone-mediated walking in a male silkworm moth Bombyx mori. J Comp Physiol [A] 1998;183:273–282. [Google Scholar]
- 33.Müller M, Homberg U. Influence of visual stimuli on the activity of neurons in the central complex of the locust Schistocerca gregaria. In: Elsner N, Breer H, editors. Göttingen neurobiology report 1994. Thieme; Stuttgart: 1994. p. 462. [Google Scholar]
- 34.Müller M, Homberg U, Kühn A. Neuroarchitecture of the lower division of the central body in the brain of the locust Schistocerca gregaria. Cell Tissue Res. 1997;288:159–176. doi: 10.1007/s004410050803. [DOI] [PubMed] [Google Scholar]
- 35.Nilsson D-E, Labhart T, Meyer E. Photoreceptor design and optical properties affecting polarization sensitivity in ants and crickets. J Comp Physiol [A] 1987;161:645–658. [Google Scholar]
- 36.Olberg RM. Pheromone-triggered flip-flopping interneurons in the ventral nerve cord of the silkworm moth, Bombyx mori. J Comp Physiol [A] 1993;152:297–307. [Google Scholar]
- 37.Paech A, Müller M, Homberg U. Ultrastructure and orientation of ommatidia in the dorsal rim area (DRA) of the locust Schistocerca gregaria. In: Elsner N, Wässle H, editors. Göttingen neurobiology report 1997. Thieme; Stuttgart: 1997. p. 471. [Google Scholar]
- 38.Petzold J. PhD thesis. University of Zurich; 2001. Polarisationsempfindliche Neuronen im Sehsystem der Feldgrille, Gryllus campestris: Elektrophysiologie, Anatomie und Modellrechnungen. [Google Scholar]
- 39.Petzold J, Helbling H, Labhart T. Anatomy and physiology of four new types of polarization sensitive interneuron in the cricket Gryllus campestris. In: Elsner N, Menzel R, editors. Göttingen neurobiology report 1995. Thieme; Stuttgart: 1995. p. 415. [Google Scholar]
- 40.Rossel S. Navigation by bees using polarized skylight. Comp Biochem Physiol [A] 1993;104:695–708. [Google Scholar]
- 41.Rossel S, Wehner R. Polarization vision in bees. Nature. 1986;323:128–131. [Google Scholar]
- 42.Schwind R. Evidence for true polarization vision based on a two-channel analyzer system in the eye of the water bug Notonecta glauca. J Comp Physiol [A] 1984;154:53–57. [Google Scholar]
- 43.Sternberger LA. Immunocytochemistry. Wiley; New York: 1986. [Google Scholar]
- 44.Stockhammer K. Die Orientierung nach der Schwingungsrichtung linear polarisierten Lichtes und ihre sinnesphysiologischen Grundlagen. Ergebn Biol. 1959;21:23–56. [Google Scholar]
- 45.Strausfeld N. Atlas of an insect brain. Springer; Heidelberg: 1976. [Google Scholar]
- 46.Strauss R, Heisenberg M. A higher control center of locomotor behavior in the Drosophila brain. J Neurosci. 1993;13:1852–1861. doi: 10.1523/JNEUROSCI.13-05-01852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vitzthum H, Homberg U. Locustatachykinin I/II-immunoreactive neurons in the central complex of the locust brain. J Comp Neurol. 1998;390:455–469. doi: 10.1002/(sici)1096-9861(19980126)390:4<455::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 48.Vitzthum H, Homberg U, Agricola H. Distribution of Dip-allatostatin I-like immunoreactivity in the brain of the locust Schistocerca gregaria with detailed analysis of immunostaining in the central complex. J Comp Neurol. 1996;369:419–437. doi: 10.1002/(SICI)1096-9861(19960603)369:3<419::AID-CNE7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 49.Vitzthum H, Müller M, Homberg U. Polarization-sensitive interneurons in the central complex of the locust Schistocerca gregaria. In: Elsner N, Wässle H, editors. Göttingen neurobiology report 1997. Thieme; Stuttgart: 1997. p. 470. [Google Scholar]
- 50.Waterman TH. Polarization sensitivity. In: Autrum H, editor. Handbook of sensory physiology, Vol VII/6B. Springer; Berlin: 1981. pp. 281–469. [Google Scholar]
- 51.Weber T, Thorson J, Huber F. Auditory behavior of the cricket. I. Dynamics of compensated walking and discrimination paradigms on the Kramer treadmill. J Comp Physiol. 1981;141:215–232. [Google Scholar]
- 52.Wendt B, Homberg U. Immunocytochemistry of dopamine in the brain of the locust Schistocerca gregaria. J Comp Neurol. 1992;321:387–403. doi: 10.1002/cne.903210307. [DOI] [PubMed] [Google Scholar]
- 53.Wehner R. Astronavigation in insects. Annu Rev Entomol. 1984;29:277–298. [Google Scholar]
- 54.Wehner R. Arthropods. In: Papi F, editor. Animal homing. Chapman and Hall; London: 1992. pp. 45–144. [Google Scholar]
- 55.Wehner R. The polarization-vision project: championing organismic biology. In: Schildberger K, Elsner N, editors. Neural basis of behavioural adaptations. Gustav Fischer; Stuttgart: 1994. pp. 103–143. [Google Scholar]
- 56.Wehner R. The ant's celestial compass system: spectral and polarization channels. In: Lehrer M, editor. Orientation and communication in arthropods. Birkhäuser; Basel: 1997. pp. 145–185. [Google Scholar]
- 57.Williams JLD. Anatomical studies of the insect central nervous system: a ground-plan of the midbrain and an introduction to the central complex in the locust, Schistocerca gregaria (Orthoptera). J Zool (Lond) 1975;76:67–86. [Google Scholar]