Abstract
Several lines of evidence have shown that nerve growth factor (NGF), the progenitor of the neurotrophin family of growth factors, plays a fundamental role in the developmental plasticity of the rat visual cortex. However, the expression of NGF receptors (NGFRs) TrkA and p75NTR and the possible sites of NGF action in the visual cortex remain to be elucidated so far. Using a highly sensitive ECL immunoblot analysis, we have been able to show, in the present study, that the TrkA protein is expressed in the rat visual cortex and that it is developmentally upregulated during the critical period for cortical plasticity. In contrast, the expression level of the low-affinity NGF receptor p75NTR seems to remain nearly constant throughout development. In the analysis of possible pathways involved in the regulation of NGFR expression, we found that neither blockade of the visual input nor NGF administration to the visual cortex resulted in a modulation of NGFR levels of expression. On the other hand, the selective destruction of cholinergic afferents to the visual cortex caused a dramatic, but not complete, reduction of the cortical NGFRs, which suggests that these receptors are located on cholinergic terminals predominantly. At the functional level, we found that, after the elimination of the cholinergic afferents to the visual cortex, the NGF-induced increase of both acetylcholine and glutamate release from cortical synaptosomes was strongly impaired. These results indicate that the cholinergic input is an important mediator of visual cortex responsiveness to NGF action.
Keywords: acetylcholine, glutamic acid, nerve growth factor, neurotransmitter release, p75NTR, synaptosomes, TrkA, visual cortex, Western blot
The neurotrophins of the nerve growth factor (NGF) family, including brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4 (NT-4), are important modulators of the developmental plasticity in the mammalian visual cortex (Thoenen, 1995; Bonhoeffer, 1996; Berardi and Maffei, 1999;McAllister et al., 1999). Neurotrophins act through binding to membrane receptors belonging to the tyrosine kinase family, Trks. NGF binds TrkA, BDNF and NT-4 bind TrkB, and NT-3 binds TrkC. The interaction with Trks induces dimerization, autophosphorylation of the receptors, and successive activation of different signal transduction pathways (Bothwell, 1995; Chao and Hempstead, 1995). Moreover, all neurotrophins bind with a lower-affinity p75NTR, a member of the tumor necrosis factor family of receptors. p75NTR can modify the binding and function of neurotrophins when coexpressed with Trks. In the absence of Trks, NGF activation of p75NTR may induce a cell death program, a distinct property of p75NTR (Carter and Lewin, 1997; Frade and Barde, 1998).
Several lines of evidence have demonstrated the prominent role of NGF in the plasticity of the visual cortex. First, administrating NGF to the visual cortex during the critical period for cortical plasticity [i.e., in the rat between postnatal day 15 (P15) and P45] prevents the effects of monocular deprivation, whereas inactivating the endogenous NGF affects the correct development of the visual cortex (Maffei et al., 1992; Berardi et al., 1994). Second, using an antibody that specifically activates TrkA and another that specifically blocks NGF binding to p75NTR (Clary et al., 1994) allowed Pizzorusso et al. (1999) to demonstrate that the NGF action in the visual cortex is mediated mainly by interaction with TrkA and to a lesser extent with p75NTR (Pizzorusso et al., 1999). Understanding the spatial and temporal expression pattern in the visual cortex of the NGF receptors (NGFRs) TrkA and p75NTR is a central step to identify the possible targets of NGF action. p75NTR is present in the visual cortex with a fiber-like distribution that follows the distribution of the cholinergic afferents (Pioro and Cuello, 1990). Concerning TrkA, controversial results have been obtained for its mRNA expression at the cortical level (Schoups et al., 1995; Cellerino and Maffei, 1996), and also no immuno-positive structures have been detected by immunohistochemical analysis (Sobreviela et al., 1994; Prakash et al., 1996; Mufson et al., 1997).
In this study, we demonstrated by Western blot analysis that the expression of TrkA, but not p75NTR, is upregulated in the visual cortex during development. However, their expression is not modulated by the visual input or by NGF itself. Furthermore, we showed that the elimination of the cholinergic afferents to the visual cortex induces a dramatic decrease of NGFR expression in the visual cortex, suggesting that NGFRs are mainly localized in cholinergic terminals. Finally, we provide evidence that NGF is no longer able to induce a potentiation of neurotransmitter release from the visual cortex when deprived of the cholinergic input, indicating that the cholinergic system is a fundamental element in the modulation of the function of NGF in the plasticity of the visual cortex.
MATERIALS AND METHODS
Animals and surgical procedures. Long–Evans hooded rats (Charles River, Calco, Italy) of different postnatal ages were anesthetized with avertin (1.5 ml/kg, i.p.) and decapitated for Western blot analysis. For immunohistochemistry, animals were anesthetized and transcardially perfused with saline solution, followed by 4% paraformaldehyde in 0.1 m PBS. Brains were removed, cryoprotected overnight in 30% sucrose in PBS, and cut coronally at 40 μm with a freezing microtome. For the study of neurotransmitter release, animals were killed by decapitation, and tissues were rapidly removed. Tetrodotoxin (TTX) (Sigma, St. Louis, MO), an Na+ channel blocker, was administered by intraocular injection with a pulled micropipette connected to a microinjector. The micropipette was inserted at the ora serrata, and the injection volume was slowly released in the vitreous. TTX (1–2 μl of a 3.5 mm solution in 0.05m citrate buffer, pH 4.8) was injected into the right eye. As control, the left eye was injected with the citrate vehicle solution. The pupillary response to illumination was adopted to monitor TTX effect. Two protocols of animal treatments were used. In the chronic protocol, animals were treated with a single TTX injection every 24–36 hr, for 7 d, from P23 to P30. In the short-term protocol, P30 animals were subjected to only one injection and killed 12 hr later. In both cases, visual cortices were explanted after a maximum period of 12 hr and separately analyzed. NGF (purified from mouse submandibular gland; kindly provided by Dr. D. Mercanti, Department of Neurobiology, Consiglio Nazionale delle Ricerche, Rome, Italy) was directly infused into the visual cortex by means of a cannula minipump system. Anesthetized rats were placed in a stereotaxic frame for minipump implantation. Miniature minipumps (pumping rate of 0.5 μl/hr; Alzet 1007D; Alza Scientific Products, Palo Alto, CA) were filled with NGF or cytochrome-C (CYT-C) (1 μg/μl in sterile saline) and connected with polyethylene tubing to 30 gauge stainless steel cannulas. A small hole was made in the skull (1 mm lateral and in correspondence with lambda), and the cannula was lowered into the cortex. The minipump was positioned subcutaneously under the neck, and the cannula was secured to the skull with acrylic cement. After the dental acrylic had hardened, the scalp was sutured. As an additional control, five animals were implanted with two minipumps: one containing NGF and the other containing CYT-C, in the left and right visual cortex, respectively. Seven days later, animals were killed, and left and right visual cortices were separately extracted for Western blot analysis. The correct stereotaxic coordinates for selectively lesioning the two nuclei that project specifically to the visual cortex were established previously by wheat germ agglutinin–horseradish peroxidase transport by Siciliano et al. (1997). Coordinates are as follows: 0.5 mm posterior to bregma, 2.7 mm from the midline, and 7.4 mm from the pial surface for nucleus basalis magnocellularis (NB); 0.8 mm anterior to bregma, 0.8 mm from the midline, and 7.7 mm from the pial surface for the nucleus of the horizontal limb of the diagonal band of Broca (DBBh). Quisqualic acid (0.5–1 μl; Sigma) (0.16m) dissolved in 0.1 m PBS, pH 7.4, was unilaterally injected in both the NB and the DBBh of anesthetized rats using a glass micropipette connected to a microinjector. Each infusion lasted 3 min, and an additional 3 min were allowed for diffusion before the pipette was removed. The brain areas ipsilateral and contralateral to the injected side were separately analyzed. As expected, the strongest effects of the quisqualic acid injection were detected in the ipsilateral side. However, possibly because of diffusion of the drug, a minor effect was found also in the contralateral side. Thus, presented data compare exclusively the effects obtained in the ipsilateral area of quisqualic acid-treated with the ipsilateral area of PBS-treated or control animals.
Immunoblot analysis. Proteins were extracted from brain areas of interest according to Knüsel et al. (1994). Protein content was estimated with the Bradford method (Bio-Rad, Milan, Italy). Samples were boiled in sample buffer, electrophoresed in 10% SDS-PAGE minigels, and transferred to nitro-cellulose (Amersham Biosciences, Bucks, UK). Protein blots were probed overnight at 4°C with anti-rat TrkA antiserum (denoted RTA) (Clary et al., 1994), anti-TrkA #9142 (New England Biolabs, Beverly, MA), anti-p75NTR (Promega, Madison, WI), or anti-β-tubulin (Sigma), 1:1000, in Tris-buffered saline (TBS) with 2% nonfat dry milk (Bio-Rad) and 0.1% Tween 20. Blots were then incubated with horseradish peroxidase-labeled secondary antibody (1:3000; Bio-Rad) for 2 hr at 30°C and analyzed using ECL chemiluminescence system (Amersham Biosciences). To estimate the level of NGFR expression detected in the Western blot experiments, filters were stripped for 30 min at 50°C in 62.5 mmTris, pH 6.8, 2% SDS, and 100 mmβ-mercaptoethanol and then reprobed with anti-β-tubulin antibody.
Immunoprecipitation. Lysates were immunoprecipitated with 2 μg of RTA or anti-TrkA antibody #9142, while rocking on a rotating wheel for 2 hr at 4°C. Immunoprecipitates were collected at 4°C by incubating with protein A-Sepharose beads (30 μl of a 1:1 solution in TBS; CL-4B; Amersham Biosciences) for 2 hr. After several washes with TBS, Sepharose-bound proteins were eluted in loading buffer and processed for SDS-PAGE immunoblot analysis.
Densitometry on Western blots. To assess semiquantitatively the different signals obtained in Western blot analysis, several sheets of x-ray film were exposed to each blot for varying lengths of time between 1 and 15 min. The bands of the developed films were quantified using the MCID image analysis system. This system identifies objects within a user-defined window, measures the brightness of each pixel and the total area of the objects, and then calculates the mean optical density (O.D.) for each sample. A window size was chosen to include one band for each measurement. For each band, an index of the precipitated silver in the emulsion of the film was calculated by multiplying the mean O.D. by the area of the band. Only values within the linear range of the film were used for these calculations. The linear range of the film was determined by loading an increasing amount of both PC12 and visual cortex samples; in each blot, a PC12 sample was loaded as a positive control. To compare the signals obtained from different visual cortices, the same amount of protein for each sample was loaded in each lane (i.e., 200 μg for visual cortex). O.D. of NGFR and β-tubulin bands were calculated as described above. Ratios of NGFR/β-tubulin O.D. values (mean ± SEM from different experiments) were calculated and plotted on a graph.
Immunohistochemistry. After several washes in PBS, sections were incubated 2 hr with agitation at room temperature in a blocking solution (4% normal horse or goat serum and 0.1% Tween 20 in TBS) and then probed with anti-choline acetyltransferase (1:3000; monoclonal antibody mAb305; Chemicon, Temecula, CA) or RTA (1:1000). Sections were washed and incubated with biotinylated secondary antibody for 1 hr at room temperature (horse anti-mouse or goat anti-rabbit; 1:200; Vector Laboratories, Burlingame, CA). After incubation in avidin–biotin complex solution (Elite kit; Vector Laboratories), the staining was developed by the diaminobenzydine–nickel method.
Acetylcholinesterase histochemistry. Brain sections were washed in 0.1 m acetate buffer, pH 5.1, for 5 min and then incubated in the same solution containing 2 mm glycine, 2 mmCuSO4, 80 mmMgCl2, 1 mg/ml acetylcholine iodide, and 0.1 mm ethopropazine for 3 hr at 38°C. After a 10 min wash in 0.1 m phosphate buffer (PB), sections were incubated in 1% ammonium sulfide in 0.1 mPB for 3 min. After a 10 min wash in 0.9% NaCl, sections were incubated in 0.2% AuCl for 7 min. The reaction was stopped with sodium thiosulfate.
Synaptosome preparation. Control and lesioned animals were killed by decapitation, visual cortices were rapidly removed, and the tissue was weighted and primarily processed according to the method of Gray and Whittaker (1962) to obtain crude synaptosomes. Briefly, the tissues were homogenized at 4°C in 40 vol of 0.32 m sucrose, pH 7.4, using a glass Teflon tissue grinder. The homogenates were centrifuged (5 min; 1000 ×g), and synaptosomes were isolated from the supernatant by centrifugation (20 min; 12,000 × g). Protein concentration was determined with the Bio-Rad protein assay kit. The same synaptosomal preparations were used in part for the study of neurotransmitter release, in part for the analysis of lesion efficacy (choline uptake and ChAT activity), and in part for the immunoblot analysis of NGFR expression.
Neurotransmitter release. The synaptosomal pellets were resuspended in physiological medium (in mm): 125 NaCl, 3 KCl, 1.2 MgSO4, 1.2 CaCl2, 1.0 NaH2PO4, 22 NaHCO3, and 10 glucose (the solution was aerated with a 95% O2 and 5% CO2mixture), pH 7.2–7.4. Identical aliquots of the synaptosomal suspension (200 μg of synaptosomal protein content from ∼10 mg of fresh tissue) were layered on microporous filters at the bottom of parallel superfusion chambers maintained at 37°C (Raiteri et al., 1974) and superfused (0.5 ml/min) with physiological medium supplemented with 0.1% dialyzed bovine serum albumin. After 36 min of equilibration, the samples were collected as follows: one 3 min sample (minutes 36–39, basal release; fraction 1), one 6 min sample (minutes 39–45, evoked release; fraction 2), and one 3 min sample (minutes 45–48, basal release; fraction 3). The depolarizing stimulus (15 mm KCl, 90 sec) was applied at t= 39 min. When appropriate, NGF (100 ng/ml) was added att = 30 min and maintained until the end of the experiment. For the acetylcholine release study, we used a previously established protocol (Marchi and Raiteri, 1996). Briefly, synaptosomes were preincubated at 37°C for a saturating period of time (15 min) in the presence of a nonsaturating amount of [3H]choline (0.08 μm) and then layered in the superfusion chambers. With these preincubation conditions, the technique allows detecting modifications in acetylcholine release but does not allow the comparison of basal acetylcholine release in different experimental situations because [3H]acetylcholine levels in the superfusate are not proportional to the amount of cholinergic synaptosomes present in the preparation. Collected fractions were analyzed for tritium content (reflecting [3H]acetylcholine), and results are expressed as fractional rate in percentage (amount of radioactivity in a fraction divided by the amount of radioactivity remaining in the synaptosomal preparation). Also in the glutamate release study, synaptosomes were preincubated at 37°C for 15 min but in the absence of radioactive precursor. Endogenous glutamate was measured by HPLC analysis with fluorometric detection, after precolumn derivatization with o-phtalaldehyde, and expressed as nanomoles of glutamate in the superfusate fraction per milligram of protein of the synaptosomal preparation. Because this technique measures the release of endogenous glutamate (Pende et al., 1993), the amount of glutamate in the collected fractions is proportional to the amount of glutamatergic synaptosomes present in the preparation, and comparisons between basal glutamate release in different experimental conditions are allowed. Data are shown as the mean ± SEM of the values obtained in the evoked release fraction minus those obtained in the averaged basal release fractions.
Choline uptake. The uptake of [3H]choline was studied according to the following procedure: each aliquot of synaptosomal suspension (500 μl) containing ∼3 mg of freshly dissected tissue (∼50 μg of protein) was preincubated in a rotatory thermostated water bath for 10 min at 37°C. [3H]Choline was added to a final concentration of 0.3 μm and incubated for 2 min. After labeling, samples were rapidly collected on glass microfiber filters (GF/B; Whatman, Maidstone UK) using vacuum and washed three times with 5 ml of standard medium. Filters were counted for radioactivity. Blank values were obtained by labeling samples at 4°C.
ChAT activity determination. ChAT activity was determined according to the radiochemical method of Fonnum (1975). Briefly, tissue samples were homogenized in 20 vol of 10 mmEDTA, pH 7.4, and 0.2% Triton X-100 at 4°C. The homogenate (2 μl) was added to 5 μl of the incubation medium ([1-14C]acetyl coenzyme A [specific activity, 51 mCi/mmol; Amersham Biosciences] diluted with unlabeled compound [Boehringer Mannheim, Mannheim, Germany] to give finally 16.9 mCi/mmol in 0.6 mm solution, 10 mm choline chloride, 300 mmNaCl, 41 mm sodium phosphate buffer, pH 7.4, and 0.1 mm physostigmine salicylate in 100 mm EDTA and 0.05% Triton X-100) and incubated for 15 min at 37°C. The radioactivity was determined with a liquid scintillation spectrometer. ChAT activity is expressed as nanomoles per milligram of protein per hour. Protein concentration in homogenates was determined as described above.
Statistical analysis. One-way ANOVA with Tukey's post hoc test or Student's two-tailed t test were performed to test the significance of differences between groups, withp < 0.05 as threshold for significant difference.
RESULTS
TrkA protein is expressed in the rat visual cortex
The presence of TrkA protein in the rat visual cortex was analyzed by immunocytochemistry using the RTA antibody. As reported previously (Sobreviela et al., 1994; Prakash et al., 1996; Mufson et al., 1997), no RTA immuno-positive signal was detected with this technique in the visual cortex (data not shown). On the contrary, a positive signal was obtained using RTA for Western blot analysis. As shown in Figure1A, a band of the expected molecular weight of TrkA (140 kDa) was detected in the visual cortex of adult rats by loading 200 μg of protein extracts. Smaller amounts of protein extracts were sufficient to detect a positive signal in the basal forebrain area and in pheochromocytoma cells (positive control), in which TrkA is abundantly expressed. No signal was detected on a negative control cell line (epidermal rat fibroblasts). Another band of ∼110 kDa, possibly corresponding to a partially glycosylated form of TrkA, was detected in all positive samples. As reported previously, the molecular weight of the bands revealed by RTA in CNS areas appeared slightly lower than that observed in PC12 cells (Holtzman et al., 1995).
Fig. 1.
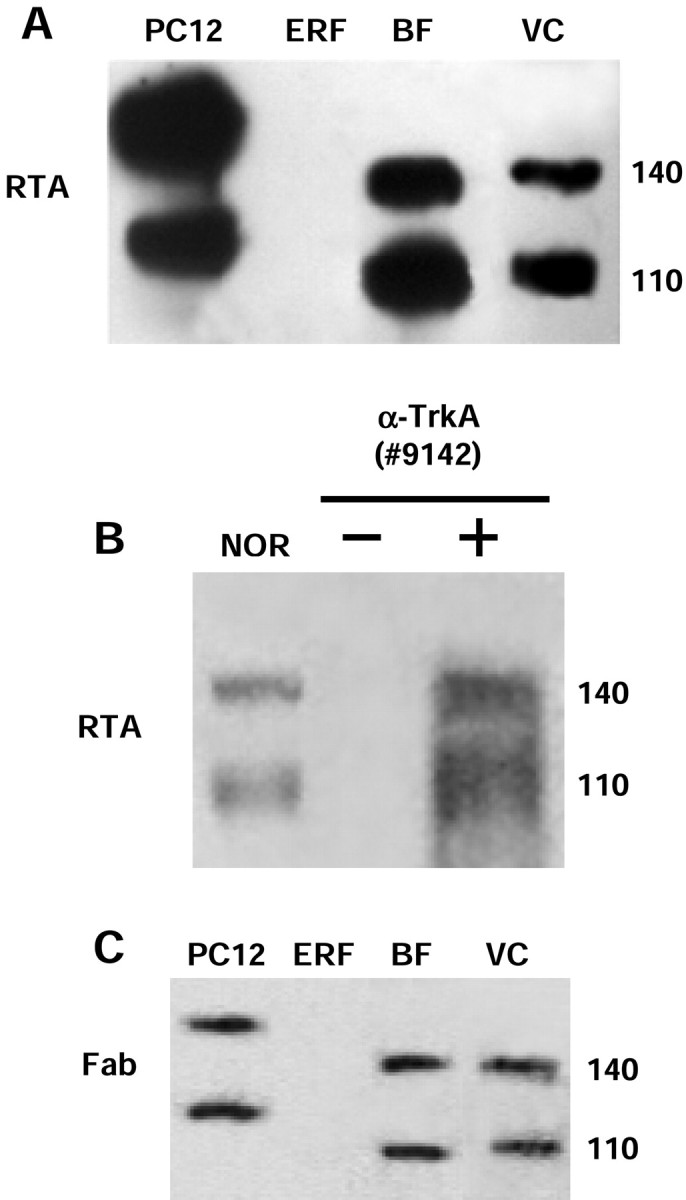
RTA immunoblot analysis for detection of the TrkA receptor and control tests for the specificity of the RTA signal.A, Protein extracts were prepared from pheochromocytoma cells (PC12; positive control; 20 μg/lane), epidermal rat fibroblasts (ERF; negative control; 20 μg/lane), basal forebrain (BF; 50 μg/lane), and visual cortex (VC; 200 μg/lane). The 140 kDa band corresponds to the functional form of TrkA receptor, whereas the 110 kDa band is an underglycosylated form of TrkA. B, Western blot of protein extracts (1 mg) from visual cortex of adult rats, immunoprecipitated with the anti-TrkA antibody (#9142; New England Biolabs) and immunoblotted with the RTA antibody. The immunoprecipitated sample (+ α-TrkA) showed a signal similar to the non-immunoprecipitated one [normal (NOR)]. No signal was detected by omitting anti-TrkA (#9142) from the immunoprecipitation step (− α-TrkA). C, The Fab fragment of the RTA antibody was used as the probe. The signal obtained in all positive samples was similar to that obtained using the RTA antibody (for comparison, see A).
The specificity of the RTA antibody has already been thoroughly verified both in vitro (Clary et al., 1994) and in vivo (Pizzorusso et al., 1999). We further tested the specificity of the signal detected by RTA in our experiments by immunoprecipitation. One milligram of protein extract from adult rat visual cortex was immunoprecipitated with anti-TrkA antibody (#9142) and then probed with RTA in Western blot analysis. As shown in Figure1B, the signal obtained in the immunoprecipitated sample is similar to what was obtained in normal samples. Omission of the anti-TrkA antibody (#9142) in the immunoprecipitation step completely eliminated the signal. The fact that the RTA antibody showed a positive signal on cortical samples immunoprecipitated with the anti-TrkA antibody (#9142), which recognizes a different epitope on the same TrkA antigen, decreases the probability of a nonspecific interaction between RTA and other antigens. A similar result was obtained using RTA as the immunoprecipitating antibody and anti-TrkA (#9142) as the probe (data not shown).
In another set of control experiments, the monovalent Fab fragment of the RTA antibody was used as the primary antibody for Western blot analysis. As shown in Figure 1C, the number and molecular weight of the bands detected by the Fab fragment in all positive samples analyzed were similar to those obtained with the RTA antibody (for comparison, see Fig. 1A). This result suggests that a putative nonspecific interaction between RTA and other antigens, possibly attributable to its bivalent nature, is unlikely.
Developmental expression of TrkA and p75NTR in the rat visual cortex
It is known that the expression of NGF in the visual cortex of the rat increases during the critical period of cortical plasticity, with a peak at P21 (Large et al., 1986). We therefore decided to investigate whether the expression of NGFRs is similarly regulated in the developing visual cortex. Protein extracts were prepared from the visual cortex of rats at various postnatal ages (P5, P10, P15, P20, P30, and adult) and analyzed by Western blotting. As shown in Figure2A, the TrkA protein is already present at P5, and its expression increases from P10 to P20 and reaches a plateau at P30 that is maintained into adulthood. p75NTR is also present in the visual cortex of the rat during the entire postnatal period, but its expression does not change significantly during development. Quantification of the signals obtained by densitometric analysis (Fig.2B) revealed an increase in the level of cortical TrkA expression, ∼150%, between P5 and P30.
Fig. 2.
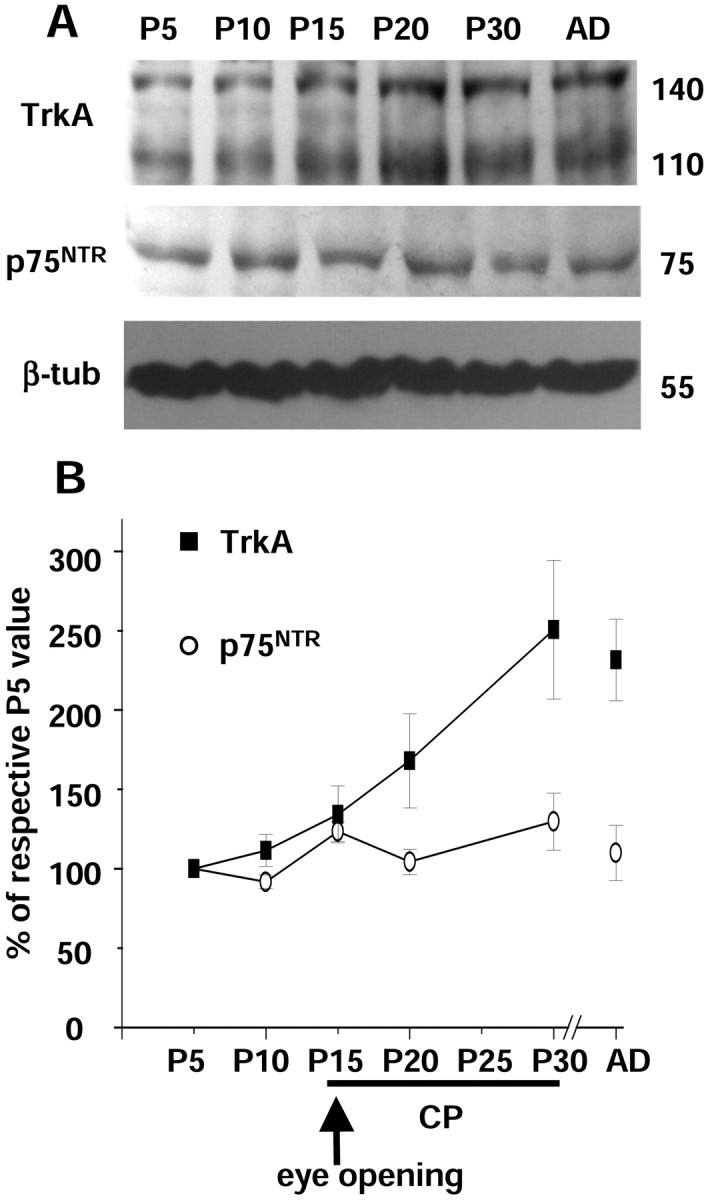
Analysis of NGFR expression in the rat visual cortex at different postnatal ages [P5, P10, P15, P20, P30, and adult (AD)]. A, Immunoblots of protein extracts from the visual cortex with RTA, anti-p75NTR, or anti-β-tubulin (β-tub) antibodies. B, The ratios between optical density values of TrkA (filled squares) or p75NTR (open circles) and β-tubulin signals are expressed as percentage of the ratios obtained at P5 and plotted as a function of age. Eachpoint represents the mean ± SEM of 12 experiments. The TrkA signal obtained at P20 is statistically different from that obtained at P5 and P30 (one-way ANOVA and post hocTukey's test; p < 0.05). CP, Critical period. The arrow indicates the time of eye opening in the rat (P15).
Modulation of cortical NGFR expression
Next, we decided to investigate possible mediators of the developmental upregulation of cortical TrkA by analyzing animals that have been subjected, during the critical period, to manipulations that are known to interfere with the developmental plasticity of the visual cortex: (1) blockade of the visual input and (2) administration of NGF to the visual cortex.
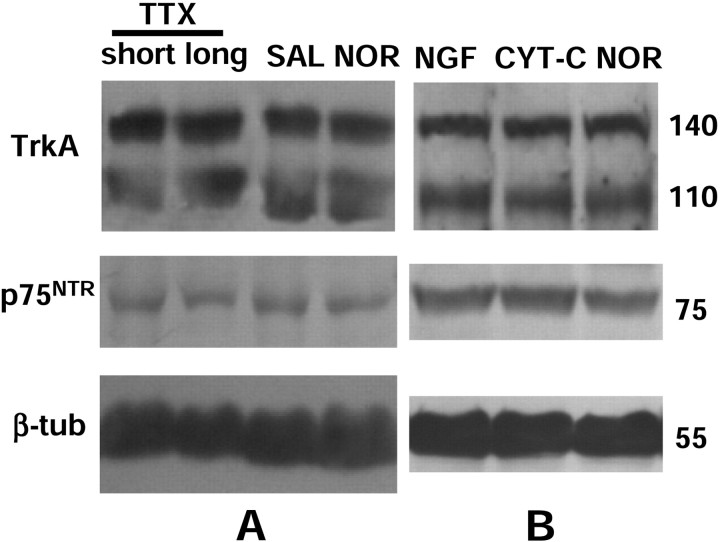
The visual input was blocked by monocular injections of TTX (3.5 mm, 1–2 μl). Both a short-term treatment (a single injection at P30) and a chronic treatment (from P23 to P30, covering the peak of the critical period for cortical plasticity) were used. Left and right visual cortices were separately analyzed by Western blotting. The obtained results clearly show that the expression of NGFRs in the visual cortex is not modulated after blockade of the visual input by neither the short-term nor the chronic TTX treatment (Fig. 3A).
Fig. 3.
Analysis of NGFR expression in the rat visual cortex after blocking the visual input (A) or administrating NGF to the visual cortex (B) at the peak of the critical period. A, Animals received intraocular injection of TTX (1–2 μl of a 3.5 mmsolution in 0.05 m citrate buffer, pH 4.8) in the right eye and a saline solution in the left eye. Short-term treatment (short): single injection at P30. Chronic treatment (long): one injection every 24–36 hr for 7 d, from P23 to P30. Left and right visual cortices were separately analyzed 12 hr after the last injection. Both short and chronic treatments induced no significant modulation in cortical NGFR expression compared with control [saline (SAL)] and untreated animals [normal (NOR)]. B, Osmotic minipumps (rate of 0.5 μl/hr) were loaded with NGF or CYT-C (1 μg/μl) and implanted in the left or right visual cortex of young animals (P23), respectively. Animals were analyzed 7 d later. Left and right visual cortices were separately analyzed. Cortical NGFR expression was not significantly modulated by this treatment. The signal was similar to that observed in CYT-C-treated cortex and in the untreated one (NOR). Anti-β-tubulin antibody was used as internal control for quantification of the results (data not shown). β-tub, β-Tubulin.
The administration of NGF to the visual cortex was performed by surgically implanting osmotic minipumps loaded with NGF (1 μg/μl) into the left visual cortex of P23 rats; control animals were implanted with a CYT-C-containing minipump. As shown in Figure 3B, the expression of NGFRs in the NGF-treated cortex was not significantly different from that in the CYT-C-treated one.
Effects of the lesion of basal forebrain cholinergic neurons
Because no immunocytochemical signal has been detected for TrkA on tissue sections at the cortical level, its cellular localization is unknown. Possible candidate cell structures are the cortical projections arising from basal forebrain cholinergic nuclei (Seiler and Schwab, 1984; Domenici et al., 1994; Pizzorusso et al., 1999). We tested this hypothesis using a lesion approach: the expression of NGFRs was analyzed by Western blot in the visual cortex of animals subjected to selective elimination of basal forebrain nuclei that specifically project to the visual cortex (DBBh and NB). At P16, animals were unilaterally injected with the excitotoxic drug quisqualic acid (0.16m in PBS, 0.5–1 μl per injection site) and analyzed 15 d later to allow the complete degeneration of neuronal somata and projecting fibers. This drug has been widely used by several groups and is considered to be the most potent excitotoxin in damaging cortical cholinergic terminals (Dunnett et al., 1991).
We first verified the efficacy of the lesion by analyzing different cholinergic markers in both the basal forebrain and the visual cortex. After the lesion, we found a striking decrease of ChAT immunoreactivity in the injected basal forebrain area and an almost complete absence of the cortical AChE histochemical staining in lesioned animals compared with control ones (data not shown).
Biochemical techniques were used to quantify the extent of the lesion. Quisqualic acid injection induced a strong decrease in both cortical ChAT activity and choline uptake (−69.2 ± 1.9 and −41.4 ± 7.6%, respectively, when compared with untreated animals; one-way ANOVA and post hoc Tukey's test; p < 0.05), whereas PBS injection had no significant effect (+5.4 ± 5.4 and +7.4 ± 4.9%). All of the results obtained in this analysis are strictly consistent with data already obtained by other investigators (Arendash et al., 1987; Dunnett et al., 1991; Siciliano et al., 1997).
Finally, the presence of cortical NGFRs was analyzed in lesioned animals. As shown in Figure4A, the quisqualic acid-induced lesions provoked a marked, but not complete, decrease in both TrkA and p75NTR protein expression at the cortical level. Densitometric analysis of the data demonstrated that the expression of both receptors is decreased ∼70% in the visual cortex of lesioned animals compared with control animals (Fig.4B). PBS injections did not significantly change cortical NGFR expression. These results indicate that the main part of the NGFR signal detected by immunoblot analysis in the visual cortex of the rat is attributable to the presence of cortical projections arising from the lesioned area.
Fig. 4.
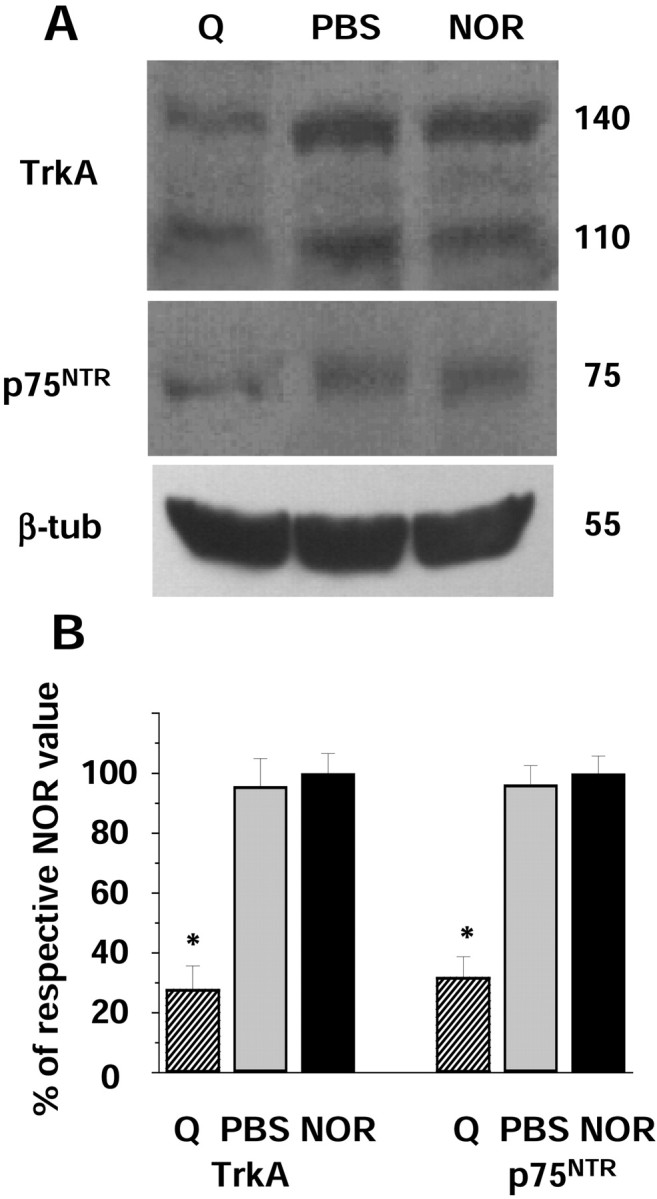
Effects of basal forebrain cholinergic neuron lesions on NGFR expression in the visual cortex. A, Immunoblots of visual cortex of quisqualic acid-treated (Q), PBS-treated (PBS), or normal (NOR) rats probed with RTA, anti-p75NTR, or anti-β-tubulin antibodies.B, The graph reports the ratios between cortical TrkA or p75NTR–β-tubulin optical density values (mean ± SEM of 12 experiments) for the visual cortex of quisqualic acid- or PBS-injected animals normalized to the values obtained in untreated animals. Both TrkA and p75NTRvalues obtained in lesioned animals are statistically different from those obtained in PBS-treated and normal ones (one-way ANOVA andpost hoc Tukey's test; *p < 0.05). β-tub, β-Tubulin.
NGF-potentiating effects on K+-evoked neurotransmitter release from visual cortical synaptosomes of basal forebrain cholinergic neuron-lesioned rats
In a previous paper, we showed that neurotrophins induce a potentiation of neurotransmitter release from visual cortical synaptosomes during the critical period for plasticity (Sala et al., 1998). Concerning NGF, we found that it potentiates the K+-evoked release of acetylcholine and glutamate, but it does not influence the spontaneous basal release. To investigate possible effects of the cholinergic input on cortical NGF responsiveness, we decided to study the release of acetylcholine and glutamate in visual cortical synaptosomes obtained from basal forebrain cholinergic neuron (BFCN)-lesioned animals.
First, we verified by Western blot that NGFRs are expressed also in the synaptosomal preparation obtained from the rat visual cortex and that, after the BFCN lesion, their level of expression decreases dramatically (∼70% when compared with normal and PBS-injected animals). In visual cortical synaptosomes obtained from lesioned animals, ChAT activity and choline uptake decreased strongly (−71.1 ± 2.2 and −41.4 ± 7.6%, respectively). Thus, the extent of the decrease in NGFR expression and cholinergic markers detected in the synaptosomal preparation from the visual cortex of lesioned animals is strictly comparable with what obtained on total protein extracts.
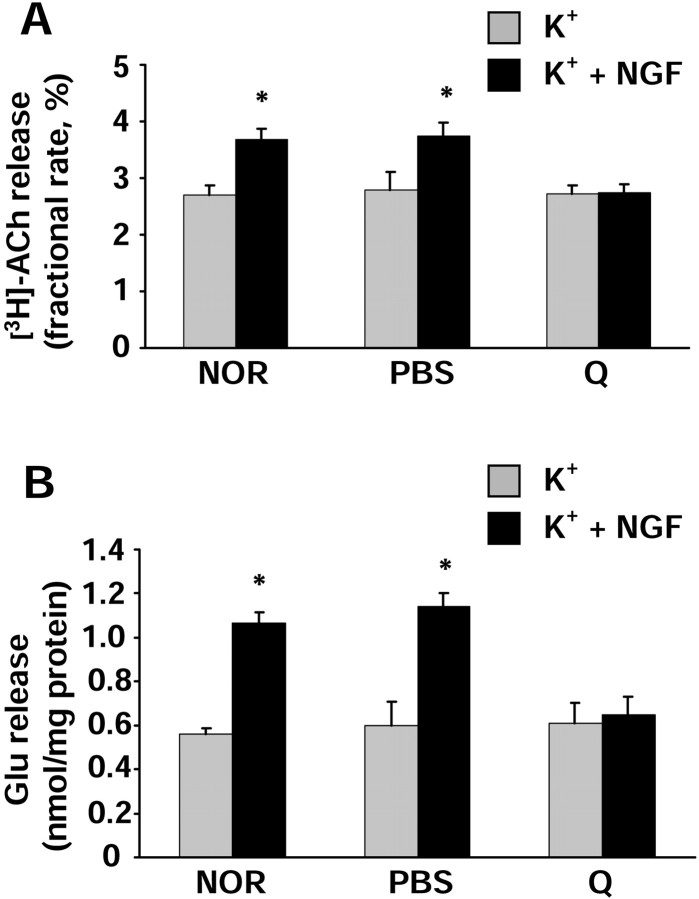
Finally, we analyzed the neurotransmitter release on the synaptosomal preparation from the rat visual cortex. As shown previously (Sala et al., 1998), we found that, in normal animals, NGF potentiated the K+-evoked [3H]acetylcholine release by ∼35% when compared with the K+-evoked release obtained in the absence of NGF (Fig.5A). A similar result was obtained in animals subjected to injection of PBS in the BFCN. On the contrary, animals injected with quisqualic acid were no longer responsive to the NGF-potentiating effect. Although the synaptosomal preparation obtained from quisqualic acid-lesioned animals contains less cholinergic synaptosomes than the preparation obtained from normal or PBS-injected animals, as demonstrated by the strong decrease in ChAT activity and choline uptake, no difference was found in the basal [3H]acetylcholine release between the different groups. This is not surprising, because the technique used in this study to measure [3H]acetylcholine release does not allow the comparison of basal release in different experimental conditions (see Material and Methods). In conclusion, although cholinergic synaptosomes in the preparation from lesioned animals are in a smaller amount, they are still able to respond to a K+-induced depolarizing stimulus but not to NGF application.
Fig. 5.
Analysis of neurotransmitter release in synaptosomes isolated from untreated [normal (NOR)], PBS-injected (PBS), or quisqualic acid-injected (Q) P30 rat visual cortex. NGF was used at a concentration of 100 ng/ml; KCl was 15 mm. Data are expressed as the mean ± SEM values (n = 12 experiments run in triplicate; evoked release fraction minus averaged basal release fractions). Gray columns represent experiments in which the 90 sec K+-depolarizing stimulus was given alone.Black columns represent experiments in which the K+-depolarizing stimulus was given in the presence of NGF. For each animal group, the values obtained in the presence of NGF have been compared with those obtained in the absence of NGF. Statistical calculations were performed using Student's two-tailedt test; *p < 0.01.A, [3H]Acetylcholine release is expressed as fractional rate (percentage; amount of radioactivity in a fraction divided by the remaining content). Note that the NGF-potentiating effect is almost completely abolished in quisqualic acid-injected animals. B, Endogenous glutamate release is expressed as nanomoles present in the superfusate fraction per milligram of protein of the synaptosomal preparation. The spontaneous basal release did not vary between groups (0.226 ± 0.008 nmol/mg protein). Note the abolition of the NGF effect in BFCN-lesioned animals. For both neurotransmitters, no statistical difference between different groups was detected for the values obtained in the presence of K+ alone (gray columns). NGF had no effect on the spontaneous basal release of both acetylcholine and glutamate in the absence of the K+-depolarizing stimulus (data not shown) (Sala et al., 1998).
Similar results were obtained also in the study of glutamate release. As shown in Figure 5B, we confirmed that, in normal animals, NGF is able to potentiate the K+-evoked release of endogenous glutamate by ∼90% when compared with the K+-evoked glutamate release obtained in the absence of NGF. PBS injection in the BFCN did not modify the NGF-potentiating effect, whereas quisqualic acid injection almost abolished the NGF effect on glutamate release. Because the technique used (HPLC) measures the endogenous glutamate release, the fact that the spontaneous basal release was the same between different groups (0.226 ± 0.008 nmol/mg protein) indicates that the synaptosomal preparation from the visual cortex of lesioned animals contains the same amount of glutamatergic terminals than the other preparations. Also, the K+-evoked glutamate release was the same in the different groups, indicating that the lesion does not damage glutamatergic terminals at the functional level.
DISCUSSION
NGFR expression in the developing rat visual cortex
Although many functional data have suggested the presence of NGFRs in the visual cortex, no direct evidence has been obtained to date. Many laboratories have failed to detect a TrkA signal in the visual cortex using immunohistochemical techniques (our results) (Sobreviela et al., 1994; Prakash et al., 1996; Mufson et al., 1997), and, possibly because of its low level of expression, controversial data have been obtained in mRNA studies (Schoups et al., 1995; Cellerino and Maffei, 1996).
In this paper, we used a highly sensitive ECL immunoblot analysis with the specific anti-TrkA antibody RTA, which enabled us to detect the characteristic signal for TrkA in the visual cortex of the rat. The number and the molecular weight of the bands detected in the present study correspond to previously published data. The 140 kDa band is the mature form of TrkA that serves as the functional NGF receptor, whereas the 110 kDa form is an underglycosylated immature precursor of TrkA (Kaplan et al., 1991; Klein et al., 1991; Meakin and Shooter, 1991;Hempstead et al., 1992). The results obtained in the immunoprecipitation tests and by the monovalent Fab fragment of the RTA antibody, together with several controls performed by Clary et al. (1994), argue strongly in favor of a specific interaction between the RTA antibody and the endogenous TrkA.
The developmental analysis of NGFR expression in the visual cortex showed a clear upregulation of TrkA, but not of p75NTR, during the critical period for plasticity of the rat visual cortex. This supports the notion of the important role played by NGF in the developmental plasticity of the visual cortex. The other members of the Trk family of receptors, TrkB and TrkC, are regulated in a similar way during the development of the visual cortex (Allendoerfer et al., 1994). It must be mentioned that, although full-length forms of TrkB and TrkC are predominant during early development, the relative proportion of full-length and truncated receptors is reversed during the critical period. The level of cortical TrkA expression detected in this study is very low. Nevertheless, it is plausible that, because of the lack of truncated forms of TrkA, a low level of expression is sufficient to allow a correct interaction with the endogenous ligand. Although it is not yet clear whether p75NTR and TrkA are concomitantly needed for the formation of a functional receptor, the result of a differentially regulated expression of the two receptors during development of the visual cortex confirms the recent theory that TrkA and p75NTR do not simply “go together.” Indeed, recent studies have proven that the two receptors can be involved in different functions. In particular, p75NTR can regulate neuronal features through its own signaling cascade independent of the presence of TrkA (Carter and Lewin, 1997; Frade and Barde, 1998).
Neurotrophins and their corresponding receptors are expressed in the rat visual cortex, and their levels of expression vary during development. It has been shown that the expression of BDNF is clearly regulated by the visual input, whereas results on the activity-dependent expression of its specific receptor TrkB are controversial (Castrén et al., 1992; Bozzi et al., 1995; Rossi et al., 1999; Lein and Shatz, 2000). The very low amount of NGF in the visual cortex has not allowed investigators to determine whether its expression is dependent on the visual input (Large et al., 1986) (but see Castrén et al., 1992). As for NGFRs, we found in this study that their expression does not vary after blockade of the visual input at the peak of cortical plasticity. This result implies that the cortical NGFR expression is not dependent on the visual input and that the observed TrkA upregulation is not attributable to the increase of electrical input that takes place at eye opening. In conclusion, it is likely that the visual input does not modulate the expression of NGF or its receptors. However, other possibilities have not yet been investigated. For instance, it is not known whether the visual input can modulate the release–uptake of NGF or the activity of NGFRs in the visual cortex.
In the present study, the administration of NGF to the rat visual cortex did not modulate the cortical expression of TrkA or p75NTR protein. In line with these results, it has also been found that, in the basal forebrain (the brain area with the highest levels of NGFRs), the observed NGF-induced increase of TrkA mRNA is considered to be too small to be detected at the protein level (Li et al., 1995).
Possible sites of cortical NGFR expression
Several lines of evidence have suggested that the cholinergic afferents to the visual cortex might express NGFRs. The p75NTR immunoreactive signal in the cortex coincides with the laminar distribution of cholinergic afferents (Pioro and Cuello, 1990). Unfortunately, a colocalization study of TrkA expression and cholinergic structures in the visual cortex is not possible because no immunocytochemical signal has been detected for TrkA to date (our results) (Sobreviela et al., 1994; Prakash et al., 1996; Mufson et al., 1997). However, applications of NGF or RTA (that are internalized after TrkA binding) to the visual cortex result in retrograde labeling of BFCNs (Seiler and Schwab, 1984; Domenici et al., 1994; Pizzorusso et al., 1999). The approach used in the present study was to analyze the NGFR immunoblot signal in animals subjected to the drug-induced elimination of the cortical cholinergic afferents. We found that, in animals subjected to BFCN lesion, the cortical expression of both TrkA and p75NTR is dramatically reduced. This result adds a new piece of evidence to the hypothesis that cortical NGFRs are located on cholinergic terminals originating from BFCNs and that these terminals are responsible for the majority of NGFR expression detected in the visual cortex. The presence of NGFRs on cholinergic terminals can also partially explain the developmental increase of TrkA described in this study. The development of BFCNs and their projections to the visual cortex is in fact concomitant with the period of cortical TrkA upregulation. It is therefore likely that, during the first weeks of postnatal development, BFCNs increase the synthesis and/or translocation of TrkA from the cell bodies to the nerve terminals, which has been suggested previously for the hippocampal area that is innervated by septal cholinergic neurons (Li et al., 1995). Possible explanations for the different developmental regulation of the cortical p75NTR need to be investigated further.
The expression of NGFRs in the visual cortex was dramatically but not totally reduced after lesion. The residual signal might be attributable to the presence of cholinergic terminals that were not targeted by the drug. Another possibility is that the signal originates from cortical neurons. Indeed, some laboratories have identified the mRNA for TrkA in the rat visual cortex (Miranda et al., 1993; Valenzuela et al., 1993;Cellerino and Maffei, 1996), and a direct NGF action on cortical pyramidal neurons has been reported in organotypic cultures of developing ferret visual cortex (McAllister et al., 1995).
Cholinergic system lesion downregulates cortical NGF responsiveness
It has been reported that an increase in cholinergic and glutamatergic input to the visual cortex facilitates depolarizing responses in visual cortical cells (Sillito and Kemp, 1983; Sato et al., 1987; Carmignoto et al., 1997). The NGF-induced potentiation of excitatory neurotransmitter (acetylcholine and glutamate) release has been suggested as a possible mechanism through which NGF can modulate the developmental plasticity in the visual cortex (Akaneya et al., 1997; Carmignoto et al., 1997; Sala et al., 1998). The release of acetylcholine in the visual cortex is most likely attributable to the presence of NGF-responsive cholinergic terminals originating from BFCNs. In fact, we found that cholinergic terminals expressed NGFRs and that, after BFCN lesion, NGF-induced acetylcholine release was dramatically impaired. A similar decrease in NGF-induced response was also found for glutamate release. Although the mechanism through which the lesion induces such effects is unknown, our results clearly indicate that elimination of the cholinergic system, one of the most important neuromodulatory inputs to the visual cortex, during the period that is critical for the correct development of the visual cortex, modulates the total responsiveness of cortical neurons to NGF applications.
The results in the present paper confirm the importance of the cross talk between NGF and the cholinergic input in shaping cortical connections and suggest that the cholinergic system is a putative positive mediator of the NGF action on cortical plasticity.
Footnotes
This work was supported by Ministero dell'Università e della Ricerca Scientifica e Tecnologica, Cofinanziamento 2000/2001, Consiglio Nazionale delle Ricerche (CNR)-targeted project in Biotechnology SP-5, Progetto Strategico Neuroscienze, and Progetto Telethon 934. We are grateful to L. F. Reichardt for kindly providing the RTA antibody, M. Raiteri and G. Bonanno for stimulating discussion, M. Sunesen, M. Usdin, and M. Zoli for critically reading this manuscript, and the staff of Istituto di Neurofisiologia del CNR for technical assistance.
Correspondence should be addressed to Dr. Francesco Mattia Rossi at his present address: Laboratoire de Neurobiologie Moléculaire, Centre National de la Recherche Scientifique Unité de Recherche Associée 2182 “Récepteurs et Cognition,” Institut Pasteur, 28 Rue du Dr. Roux, 75724 Paris Cédex 15, France. E-mail: frossi@pasteur.fr.
REFERENCES
- 1.Akaneya Y, Tsumoto T, Kinoshita S, Hatanaka H. Brain-derived neurotrophic factor enhances long-term potentiation in rat visual cortex. J Neurosci. 1997;17:6707–6716. doi: 10.1523/JNEUROSCI.17-17-06707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allendoerfer KL, Cabelli RJ, Escandòn E, Kaplan DR, Nikolics K, Shatz CJ. Regulation of neurotrophin receptors during the maturation of the mammalian visual system. J Neurosci. 1994;14:1795–1811. doi: 10.1523/JNEUROSCI.14-03-01795.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arendash GW, Millard WJ, Dunn AJ, Meyer EM. Long-term neuropathological and neurochemical effects of nucleus basalis lesions in the rat. Science. 1987;238:952–956. doi: 10.1126/science.2890210. [DOI] [PubMed] [Google Scholar]
- 4.Berardi N, Maffei L. From visual experience to visual function: roles of neurotrophins. J Neurobiol. 1999;41:119–126. [PubMed] [Google Scholar]
- 5.Berardi N, Cellerino A, Domenici L, Fagiolini M, Pizzorusso T, Cattaneo A, Maffei L. Monoclonal antibodies to nerve growth factor affect the postnatal development of the visual system. Proc Natl Acad Sci USA. 1994;91:684–688. doi: 10.1073/pnas.91.2.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonhoeffer T. Neurotrophins and activity-dependent development of the neocortex. Curr Opin Neurobiol. 1996;6:119–126. doi: 10.1016/s0959-4388(96)80017-1. [DOI] [PubMed] [Google Scholar]
- 7.Bothwell M. Functional interactions of neurotrophins and neurotrophin receptors. Annu Rev Neurosci. 1995;18:221–253. doi: 10.1146/annurev.ne.18.030195.001255. [DOI] [PubMed] [Google Scholar]
- 8.Bozzi Y, Pizzorusso T, Cremisi F, Rossi FM, Barsacchi G, Maffei L. Monocular deprivation decreases the expression of messenger RNA for brain-derived neurotrophic factor in the rat visual cortex. Neuroscience. 1995;69:1133–1144. doi: 10.1016/0306-4522(95)00321-9. [DOI] [PubMed] [Google Scholar]
- 9.Carmignoto G, Pizzorusso T, Tia S, Vicini S. Brain-derived neurotrophic factor and nerve growth factor potentiate excitatory synaptic transmission in the rat visual cortex. J Physiol (Lond) 1997;498:153–164. doi: 10.1113/jphysiol.1997.sp021848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter BD, Lewin GR. Neurotrophins live or let die: does p75NTR decide? Neuron. 1997;18:187–190. doi: 10.1016/s0896-6273(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 11.Castrén E, Zafra F, Thoenen H, Lindholm D. Light regulates expression of brain-derived neurotrophic factor mRNA in the rat visual cortex. Proc Natl Acad Sci USA. 1992;89:9444–9448. doi: 10.1073/pnas.89.20.9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cellerino A, Maffei L. The action of neurotrophins in the development and plasticity of the visual cortex. Prog Neurobiol. 1996;49:53–71. doi: 10.1016/0301-0082(96)00008-1. [DOI] [PubMed] [Google Scholar]
- 13.Chao MV, Hempstead BL. p75 and Trk: a two-receptor system. Trends Neurosci. 1995;18:321–326. [PubMed] [Google Scholar]
- 14.Clary DO, Weskamp G, Austin LR, Reichardt LF. TrkA cross-linking mimics neuronal responses to nerve growth factor. Mol Biol Cell. 1994;5:549–563. doi: 10.1091/mbc.5.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Domenici L, Fontanesi G, Cattaneo A, Bagnoli P, Maffei L. Nerve growth factor (NGF) uptake and transport following injection in the developing rat visual cortex. Vis Neurosci. 1994;11:1093–1102. doi: 10.1017/s095252380000691x. [DOI] [PubMed] [Google Scholar]
- 16.Dunnett SB, Everitt BJ, Robbins TW. The basal forebrain cortical cholinergic system: interpreting the functional consequences of excitotoxic lesions. Trends Neurosci. 1991;14:494–501. doi: 10.1016/0166-2236(91)90061-x. [DOI] [PubMed] [Google Scholar]
- 17.Fonnum FA. A rapid radiochemical method for the determination of choline acetyltransferase. J Neurochem. 1975;24:407–409. doi: 10.1111/j.1471-4159.1975.tb11895.x. [DOI] [PubMed] [Google Scholar]
- 18.Frade JM, Barde YA. Nerve growth factor: two receptors, multiple functions. BioEssays. 1998;20:137–145. doi: 10.1002/(SICI)1521-1878(199802)20:2<137::AID-BIES6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 19.Gray EG, Whittaker VP. The isolation of nerve endings from brain: an electron microscopic study of cell fragments derived by homogenisation and centrifugation. J Anat. 1962;96:79–90. [PMC free article] [PubMed] [Google Scholar]
- 20.Hempstead BL, Rabin SJ, Kaplan L, Reid S, Parada LF, Kaplan DR. Overexpression of the trk tyrosine kinase rapidly accelerates nerve growth factor-induced differentiation. Neuron. 1992;9:883–896. doi: 10.1016/0896-6273(92)90241-5. [DOI] [PubMed] [Google Scholar]
- 21.Holtzman DM, Kilbridge J, Li Y, Cunningham ET, Jr, Lenn NJ, Clary DO, Reichardt LF, Mobley WC. TrkA expression in the CNS: evidence for the existence of several novel NGF-responsive CNS neurons. J Neurosci. 1995;15:1567–1576. doi: 10.1523/JNEUROSCI.15-02-01567.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan DR, Martin-Zanca D, Parada LF. Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature. 1991;350:158–160. doi: 10.1038/350158a0. [DOI] [PubMed] [Google Scholar]
- 23.Klein R, Jing S, Nanduri V, O'Rourke E, Barbacid M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 1991;65:189–197. doi: 10.1016/0092-8674(91)90419-y. [DOI] [PubMed] [Google Scholar]
- 24.Knüsel B, Rabin SJ, Hefti F, Kaplan DR. Regulated neurotrophin responsiveness during neuronal migration and early differentiation. J Neurosci. 1994;14:1542–1554. doi: 10.1523/JNEUROSCI.14-03-01542.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Large TH, Bodary SC, Clegg DO, Weskamp G, Otten U, Reichardt LF. Nerve growth factor gene expression in the developing brain. Science. 1986;234:352–355. doi: 10.1126/science.3764415. [DOI] [PubMed] [Google Scholar]
- 26.Lein ES, Shatz CJ. Rapid regulation of brain-derived neurotrophic factor mRNA within eye-specific circuits during ocular dominance column formation. J Neurosci. 2000;20:1470–1483. doi: 10.1523/JNEUROSCI.20-04-01470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Holtzman DM, Kromer LF, Kaplan DR, Chua-Couzens J, Clary DO, Knusel B, Mobley WC. Regulation of TrkA and ChAT expression in developing rat basal forebrain: evidence that both exogenous and endogenous NGF regulate differentiation of cholinergic neurons. J Neurosci. 1995;15:2888–2905. doi: 10.1523/JNEUROSCI.15-04-02888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maffei L, Berardi N, Domenici L, Parisi V, Pizzorusso T. Nerve growth factor (NGF) prevents the shift in ocular dominance distribution of visual cortical neurons in monocularly deprived rats. J Neurosci. 1992;12:4651–4662. doi: 10.1523/JNEUROSCI.12-12-04651.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marchi M, Raiteri M. Nicotinic autoreceptors mediating enhancement of acetylcholine release become operative in conditions of impaired presynaptic function. J Neurochem. 1996;67:1974–1981. doi: 10.1046/j.1471-4159.1996.67051974.x. [DOI] [PubMed] [Google Scholar]
- 30.McAllister AK, Katz LC, Lo DC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 31.McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;18:2108–2117. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- 32.Meakin SO, Shooter EM. Molecular investigations on the high-affinity nerve growth factor receptor. Neuron. 1991;6:153–163. doi: 10.1016/0896-6273(91)90130-r. [DOI] [PubMed] [Google Scholar]
- 33.Miranda RC, Sohrabji F, Toran-Allerand CD. Neuronal colocalization of mRNAs for neurotrophins and their receptors in the developing central nervous system suggests a potential for autocrine interactions. Proc Natl Acad Sci USA. 1993;90:6439–6443. doi: 10.1073/pnas.90.14.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mufson EJ, Lavine N, Jaffar S, Kordower JH, Quirion R, Saragovi HU. Reduction in p140-TrkA receptor protein within the nucleus basalis and cortex in Alzheimer's disease. Exp Neurol. 1997;146:91–103. doi: 10.1006/exnr.1997.6504. [DOI] [PubMed] [Google Scholar]
- 35.Pende M, Lanza M, Bonanno G, Raiteri M. Release of endogenous glutamic and aspartic acids from cerebrocortex synaptosomes and its modulation through activation of a gamma-aminobutyric acidB (GABAB) receptor subtype. Brain Res. 1993;604:325–330. doi: 10.1016/0006-8993(93)90384-y. [DOI] [PubMed] [Google Scholar]
- 36.Pioro EP, Cuello AC. Distribution of nerve growth factor receptor like immunoreactivity in the adult rat central nervous system. Effect of colchicine and correlation with the cholinergic system. I. Forebrain. Neuroscience. 1990;34:57–87. doi: 10.1016/0306-4522(90)90304-m. [DOI] [PubMed] [Google Scholar]
- 37.Pizzorusso T, Berardi N, Rossi FM, Viegi A, Venstrom K, Reichardt LF, Maffei L. TrkA activation in the rat visual cortex by antirat trkA IgG prevents the effect of monocular deprivation. Eur J Neurosci. 1999;11:204–212. doi: 10.1046/j.1460-9568.1999.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prakash N, Cohen-Cory S, Frostig RD. Rapid and opposite effects of BDNF and NGF on the functional organization of the adult cortex in vivo. Nature. 1996;381:702–706. doi: 10.1038/381702a0. [DOI] [PubMed] [Google Scholar]
- 39.Raiteri M, Angelini F, Levi G. A simple apparatus for studying the release of neurotransmitters from synaptosomes. Eur J Pharmacol. 1974;25:411–416. doi: 10.1016/0014-2999(74)90272-6. [DOI] [PubMed] [Google Scholar]
- 40.Rossi FM, Bozzi Y, Pizzorusso T, Maffei L. Monocular deprivation decreases brain-derived neurotrophic factor immunoreactivity in the rat visual cortex. Neuroscience. 1999;90:363–368. doi: 10.1016/s0306-4522(98)00463-1. [DOI] [PubMed] [Google Scholar]
- 41.Sala R, Viegi A, Rossi FM, Pizzorusso T, Bonanno G, Raiteri M, Maffei L. NGF and BDNF increase transmitter release in the rat visual cortex. Eur J Neurosci. 1998;10:2185–2191. doi: 10.1046/j.1460-9568.1998.00227.x. [DOI] [PubMed] [Google Scholar]
- 42.Sato H, Hata Y, Masui H, Tsumoto T. A functional role of cholinergic innervation to neurons in the cat visual cortex. J Neurophysiol. 1987;58:765–780. doi: 10.1152/jn.1987.58.4.765. [DOI] [PubMed] [Google Scholar]
- 43.Schoups AA, Elliott RC, Friedman WJ, Black IB. NGF and BDNF are differentially modulated by visual experience in the developing geniculocortical pathway. Dev Brain Res. 1995;86:326–334. doi: 10.1016/0165-3806(95)00043-d. [DOI] [PubMed] [Google Scholar]
- 44.Seiler M, Schwab ME. Specific retrograde transport of nerve growth factor (NGF) from neocortex to nucleus basalis in the rat. Brain Res. 1984;300:33–39. doi: 10.1016/0006-8993(84)91338-6. [DOI] [PubMed] [Google Scholar]
- 45.Siciliano R, Fontanesi G, Casamenti F, Berardi N, Bagnoli P, Domenici L. Postnatal development of functional properties of visual cortical cells in rats with excitotoxic lesions of basal forebrain cholinergic neurons. Vis Neurosci. 1997;14:111–123. doi: 10.1017/s0952523800008816. [DOI] [PubMed] [Google Scholar]
- 46.Sillito AM, Kemp JA. Cholinergic modulation of the functional organization of the cat visual cortex. Brain Res. 1983;289:143–155. doi: 10.1016/0006-8993(83)90015-x. [DOI] [PubMed] [Google Scholar]
- 47.Sobreviela T, Clary DO, Reichardt LF, Brandabur MM, Kordower JH, Mufson EJ. TrkA-immunoreactive profiles in the central nervous system: colocalization with neurons containing p75 nerve growth factor receptor, choline acetyltransferase, and serotonin. J Comp Neurol. 1994;350:587–611. doi: 10.1002/cne.903500407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 49.Valenzuela DM, Maisonpierre PC, Glass DJ, Rojas E, Nuñez L, Kong Y, Gies DR, Stitt TN, Ip NY, Yancopoulos GD. Alternative forms of rat TrkC with different functional capabilities. Neuron. 1993;10:963–974. doi: 10.1016/0896-6273(93)90211-9. [DOI] [PubMed] [Google Scholar]