Abstract
Repeated THC administration produces motivational and somatic adaptive changes leading to dependence in rodents. To investigate the molecular basis for cannabinoid dependence and its possible relationship with the endogenous opioid system, we explored Δ9-tetrahydrocannabinol (THC) activity in mice lacking μ-, δ- or κ-opioid receptor genes. Acute THC-induced hypothermia, antinociception, and hypolocomotion remained unaffected in these mice, whereas THC tolerance and withdrawal were minimally modified in mutant animals. In contrast, profound phenotypic changes are observed in several place conditioning protocols that reveal both THC rewarding and aversive properties. Absence of μ receptors abolishes THC place preference. Deletion of κ receptors ablates THC place aversion and furthermore unmasks THC place preference. Thus, an opposing activity of μ- and κ-opioid receptors in modulating reward pathways forms the basis for the dual euphoric–dysphoric activity of THC.
Keywords: Δ9-tetrahydrocannabinol, place preference, place aversion, knock-out, tolerance, dependence, reward
Cannabinoids and opioids are the most widely consumed illicit drugs worldwide (Smart and Ogborne, 2000). Both types of compounds mimic endogenous ligands and act through distinct G-protein-coupled receptor families known as cannabinoid (Felder and Glass, 1998) and opioid (Kieffer, 1995) receptors. Pharmacological studies have shown functional interactions between the two systems (Manzanares et al., 1999). Thus, cannabinoid and opioid agonists share several pharmacological properties, including antinociception and hypothermia (Narimatsu et al., 1987; Vivian et al., 1998). Biochemical studies have revealed that repeated THC administration increases opioid peptide gene expression (Corchero et al., 1997a,b). Acute THC also increases extracellular levels of endogenous enkephalins in the nucleus accumbens (Valverde et al., 2001). The existence of cross-tolerance between opioid and cannabinoid agonists has been supported by a variety of studies. Thus, morphine-tolerant animals show decreased THC antinociceptive responses, whereas THC-tolerant rodents show a decrease in morphine antinociception (Hine, 1985; Thorat and Bhargava, 1994). Cross-dependence between opioid and cannabinoid compounds has also been reported. Indeed, the opioid antagonist naloxone precipitated a withdrawal syndrome in THC-tolerant rats (Kaymakcalan et al., 1977), whereas the cannabinoid antagonist SR171416A was able to precipitate abstinence in morphine-dependent rats (Navarro et al., 1998). Besides, the severity of opioid withdrawal was reduced by the administration of THC (Hine et al., 1975; Valverde et al., 2001) or the endogenous cannabinoid agonist anandamide (Vela et al., 1995). This bidirectional cross-dependence has been recently confirmed by using knock-out mice, because opioid dependence was reduced in mice lacking the CB1 cannabinoid receptor (Ledent et al., 1999), whereas cannabinoid dependence was reduced in mice lacking the preproenkephalin gene (Valverde et al., 2000).
Another important aspect of marijuana activity is the complexity of evoked emotional responses and in particular the possibility of dual euphoria–dysphoria effects (Halikas et al., 1985). Electrical brain stimulation (Gardner et al., 1988) and in vivo microdialysis (Chen et al., 1990; Tanda et al., 1997) have suggested that cannabinoids produce their rewarding action by stimulating mesolimbic dopaminergic transmission, a common substrate for the rewarding effects of other substances of abuse (Koob, 1992), and that μ-opioid receptors could be involved (Tanda et al., 1997). The endogenous cannabinoid system participates in the rewarding effects of opioids, because both morphine self-administration (Ledent et al., 1999) and place preference (Martin et al., 2000) are decreased in mice lacking the CB1 receptor. However, the possible involvement of the endogenous opioid system in the different motivational responses induced by cannabinoids remains to be clarified. GABAergic (Onaivi et al., 1990) and corticotropin-releasing factor (Rodriguez de Fonseca et al., 1996) systems have been suggested to be involved in the anxiogenic responses induced by cannabinoids. These anxiogenic effects could have some influence in the dysphoric properties of cannabinoids, but the mechanisms that underlie the potential aversive effects of THC remain unexplored.
To investigate these major aspects of cannabinoid–opioid interactions, we have examined whether the genetic ablation of μ-opioid (Matthes et al., 1996), δ-opioid (Filliol et al., 2000), or κ-opioid (Simonin et al., 1998) receptors in mice has any influence on THC tolerance, physical dependence, and motivational responses.
MATERIALS AND METHODS
Mice. The generation of mice lacking either μ-opioid (MOR −/−), δ-opioid (DOR −/−), or κ-opioid (KOR −/−) receptors has been described previously (Matthes et al., 1996;Simonin et al., 1998; Filliol et al., 2000). Mice weighing 22–24 gm at the start of the study were housed, grouped, and acclimatized to the laboratory conditions (12 hr light/dark cycle, 21 ± 1°C room temperature, 65 ± 10% humidity) 1 week before the experiment with ad libitum access to food and water. All animals were 1:1 hybrids from 129/SV and C57B1/6 mouse strains. Wild-type littermates were used for the control groups in all experiments. Mutants and their wild-type littermates showed comparable spontaneous locomotor activity, except for DOR −/− mice, which displayed significant hyperlocomotion (increase of 161.85 ± 19.58% comparing with wild-type controls,F(1,23) = 6.277, p < 0.05) as previously reported (Filliol et al., 2000). Behavioral tests and animal care were conducted in accordance with the standard ethical guidelines (National Institutes of Health, 1995; Council of Europe, 1996) and approved by the local ethical committee. The observer was blind to the genotype and treatment in all experiments.
Drugs. THC (Sigma, Poole, UK) was dissolved in a solution of 5% ethanol, 5% cremophor El, and 90% distilled water, and injected in a volume of 0.1 ml per 10 gm body weight. The selective CB1 cannabinoid receptor antagonist SR141716A was dissolved in a solution of 10% ethanol, 10% cremophor El, and 80% distilled water, and injected by intraperitoneal route in a volume of 0.2 ml per 10 gm body weight.
Tolerance and withdrawal. Animals were injected intraperitoneally twice daily at 9:00 A.M. and 7:00 P.M. for 5 d with THC (20 mg/kg) or vehicle. On day 6, mice only received the morning injection. Four different responses were measured once a day during the chronic THC treatment: body weight, rectal temperature, antinociception, and locomotor activity. Body weights were recorded for each animal, using an electronic balance (Mettler PM 4800; sensitive to 0.01 gm), once a day before morning injections. Locomotor measurements for each mouse were taken 20 min after morning injections by placing animals in individual actimeters (9 × 20 × 11 cm) (Imetronic, Bordeaux, France) equipped with two lines of six infrared beams for 10 min, and recording both horizontal and vertical activity, under a dim light (<20 lux). Antinociceptive measurements for each mouse were taken 30 min after morning injection by using the tail immersion assay as described previously (Janssen et al., 1963). Antinociceptive responses were also evaluated in the hot plate test (Columbus Instruments, Columbus, OH) on the first day. For the tail immersion, the time to withdraw the tail from the bath was registered (50 ± 0.5°C), with a cutoff latency of 15 sec to prevent tissue damage. For the hot plate (52 ± 0.5°C), two different nociceptive thresholds were measured: paw licking (cutoff latency of 30 sec) and jumping (cutoff latency of 240 sec). Rectal temperature was measured in each mouse using an electronic thermocouple flexible rectal probe (Panlab, Madrid, Spain). The probe was placed 3 cm into the rectum of the mice for 20 sec before the temperature was recorded, and measures were taken 40 min after morning injection.
On the sixth day, 4 hr after the last THC or vehicle injection, mice were placed in a circular clear plastic observation area (30 cm diameter, 80 cm height) for a 15 min period of observation. Body weight and rectal temperature were consecutively measured, and animals received administration of SR141716A (10 mg/kg, i.p.). Mice were then replaced in the observation area and observed for 45 min. Measurement of somatic signs before and after SR 141716A challenge were divided in 5 min time intervals, as previously described (Hutcheson et al., 1998). The number of bouts of sniffing, writhing, wet dog shakes, and front paw tremor were counted. Penile licking or erection, ataxia, hunched posture, tremor, ptosis, and piloerection were scored 1 for appearance and 0 for nonappearance within each 5 min time period. Scores for the level of activity were made by giving in each 5 min period a value of 0 = low activity (less than five complete crossings of the observation area), 1 = normal activity (between five and twenty complete crossings of the observation area), or 2 = increased activity (more than twenty complete crossings of the observation area). A quantitative value was calculated in each animal for the different checked signs by adding the scores obtained in each 5 min time period. A global withdrawal score, ranging from 0 to 100, was calculated for each animal by giving to each individual sign a relative weight, as previously described (Koob et al., 1992): 0.9 point for the appearance of each checked sign in each 5 min time period; 0.4 point for each bout of counted sign.
Statistics. Acute effects and global withdrawal scores were compared by using two-way ANOVA (genotype and treatment) between subjects followed by one-way ANOVA for individual differences. Values of tolerance studies were compared by using three-way ANOVA (genotype and treatment as between groups factors and day as within group factor), followed by corresponding two-way and one-way ANOVAs andpost hoc comparisons when applied.
Place conditioning. An unbiased place conditioning procedure was used to evaluate both rewarding and aversive properties of THC (Valjent and Maldonado, 2000) in animals lacking each opioid receptor. The apparatus consisted of two main square conditioning compartments (15 × 15 × 15 cm) separated by a triangular central division (Maldonado et al., 1997). The light intensity within the conditioning chambers was 50 ± 5 lux. The movement and location of the mice were recorded by computerized monitoring software (Videotrack; View Point, Lyon, France) with images relayed from a camera placed above the apparatus. During the preconditioning phase, drug-naive mice were placed in the middle of the central division and had ad libitum access to both compartments (striped and dotted compartment) of the conditioning apparatus for 20 min, with the time spent in each compartment being recorded. The conditioning phase consisted of five pairings with THC and five pairings with vehicle for a 45 min conditioning time in all experiments. Mice were injected with vehicle or THC (1 and 5 mg/kg, i.p.) and then immediately confined to the conditioning compartment. Treatments were counterbalanced as closely as possible between compartments. Control animals received vehicle every day. The test phase was conducted exactly as the preconditioning phase, i.e., ad libitum access to each compartment for 20 min. Mice conditioned with the dose of 1 mg/kg of THC received a single THC (1 mg/kg, i.p.) injection in the home cage 24 hr before starting the conditioning procedures, to avoid the dysphoric effects of the first drug exposure (Valjent and Maldonado, 2000). As previously described (Stinus et al., 1990; Valverde et al., 1996;Maldonado et al., 1997), the time in central area was proportionally shared and added to the time value of each conditioned compartment, as follows: the time spent in each compartment is multiplied by the total time (1200) and divided by the total time minus the time spent in the central area:
The total amount of time spent in the central area was similar in all groups of the different experiments (see Statistics). A place conditioning score was calculated for each animal as the difference between time spent in the drug-paired compartment during the test and preconditioning phases.
Statistics. Raw time score values were used for statistical analysis in all the experiments. These values were compared by using two-way ANOVA between subjects (genotype and treatment) followed by one-way ANOVA for individual differences. Time spent in the drug-paired compartment during preconditioning in the different groups was compared by a one-way ANOVA (experimental group as between subjects factor) to ensure use of an unbiased procedure (experiment with MOR −/− mice:F(5,75) = 0.414, p = 0.837; experiment with DOR −/− mice:F(5,64) = 0.753, p = 0.587; experiment with KOR −/− mice:F(5,60) = 0.420, p = 0.833; experiment with KOR −/− mice without priming:F(3,37) = 0.05, p = 0.985). To verify any possible influence of the mutation in mouse spontaneous behavior in the place conditioning paradigm, time spent in the central compartment during preconditioning in the different groups was also compared by two-way ANOVA between subjects (genotype and treatment). No significant effect of genotype (experiment with MOR −/− mice: F(1,81) = 0.017,p = 0.897; experiment with DOR −/− mice:F(1,70) = 1.136, p = 0.290; experiment with KOR −/− mice:F(1,68) = 1.377, p = 0.260), treatment (experiment with MOR −/− mice:F(2,81) = 1.757, p = 0.180; experiment with DOR −/− mice:F(2,70) = 0.372, p = 0.372; experiment with KOR −/− mice:F(2,68) = 3.905, p = 0.053) nor interaction between these two factors (experiment with MOR −/− mice: F(2,81) = 0.504,p = 0.606; experiment with DOR −/− mice:F(2,70) = 0.657, p = 0.522; experiment with KOR −/− mice:F(2,68) = 2.060, p = 0.136) was observed in any experiment. Individual comparisons of time spent in the drug-paired compartment during preconditioning and test phases were made with paired two-tailed Student's ttest.
RESULTS
THC tolerance and withdrawal in MOR −/−, DOR −/−, and KOR −/− mice
To induce tolerance and dependence, we chronically injected a high dose of THC (20 mg/kg). The first THC injection produced similar antinociception in wild-type, MOR −/−, DOR −/−, and KOR −/− mice, in both the hot plate (paw licking: mean value, 79.3 ± 3.26% of analgesia, F(5,68) = 0.788,p = 0.562; jump: mean value, 95.9 ± 1.22% of analgesia, F(5,68) = 0.913,p = 0.478) and the tail immersion (mean value, 47.8 ± 5.14% of analgesia;F(5,68) = 1.386; p = 0.24). This first THC injection also produced hypolocomotion (mean decrease, 66.2 ± 2.04%; F(5,67)= 0.867; p = 0.508) and hypothermia (mean decrease, 3.63 ± 0.34°C; F(5,67) = 2.091; p = 0.077) that did not differ between genotypes.
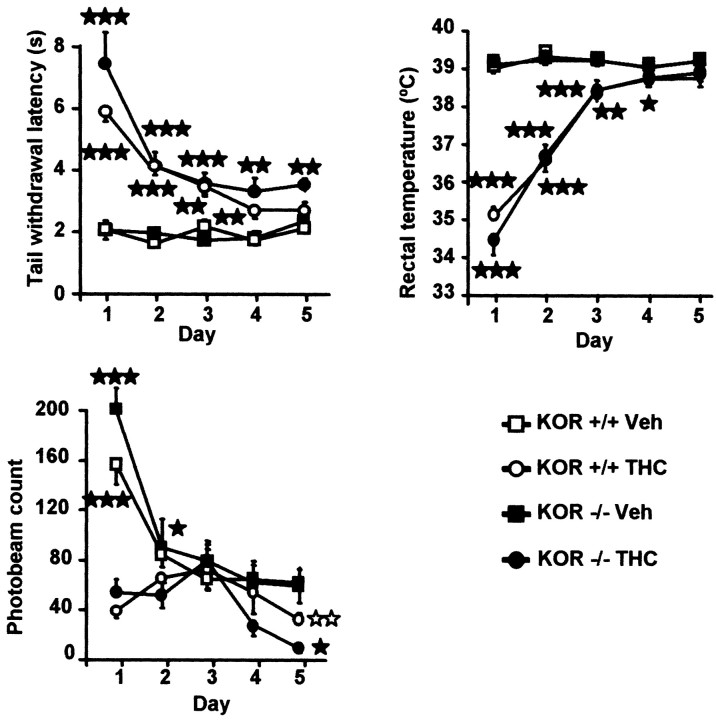
During repeated THC administration, a progressive decrease in the antinociceptive, hypolocomotor, and hypothermic activity of the drug was observed. This tolerance developed similarly in wild-type, MOR −/−, and DOR −/− mice, whereas minor changes were observed in KOR −/− mice (Table 1, Fig.1). Tolerance for locomotor responses was developed on the second day of THC treatment in all groups. However, habituation in vehicle-treated mice during the first 3 d of testing was observed. This habituation can also be important for reducing initial differences between THC and vehicle-treated groups. Noticeable was the significant lower locomotion in THC-treated KOR −/− mice compared with THC-treated wild-type controls on day 5 (F(1,21) = 13.426; p< 0.005). Tolerance to the hypothermic effects developed similarly in all mouse genotypes. Tolerance to antinociceptive effects of THC was also similar in all genotypes (Table1, Fig. 1).
Table 1.
Three-way ANOVA of hypothermia, hypolocomotion, and antinociception induced during chronic THC treatment in mice lacking μ-, δ-, or κ-opioid receptor
| MOR | DOR | KOR | ||||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Temperature | ||||||
| Day (D) | F(5,45) = 31.32 | p < 0.001 | F(5,33) = 25.09 | p < 0.001 | F(5,36) = 53.27 | p < 0.001 |
| Treatment (T) | F(1,49) = 152.8 | p < 0.001 | F(1,37) = 97.07 | p < 0.001 | F(1,40) = 172.3 | p < 0.001 |
| Genotype (G) | F(1,49) = 0.685 | p = 0.412 | F(1,37) = 0.017 | p = 0.895 | F(1,40) = 1.132 | p = 0.294 |
| D × T | F(5,45) = 25.92 | p < 0.001 | F(5,33) = 22.72 | p < 0.001 | F(5,36) = 57.60 | p < 0.001 |
| D × G | F(5,45) = 1.713 | p = 0.151 | F(5,33) = 0.631 | p = 0.677 | F(5,36) = 1.142 | p = 0.356 |
| T × G | F(1,49) = 0.434 | p = 0.513 | F(1,37) = 0.124 | p = 0.727 | F(1,40) = 1.134 | p = 0.293 |
| D × T × G | F(5,45) = 0.881 | p = 0.502 | F(5,33) = 0.547 | p = 0.739 | F(5,36) = 1.265 | p = 0.300 |
| Locomotor activity | ||||||
| Day (D) | F(5,32) = 13.82 | p < 0.001 | F(5,22) = 7.223 | p < 0.001 | F(5,34) = 25.11 | p < 0.001 |
| Treatment (T) | F(1,36) = 16.84 | p < 0.001 | F(1,26) = 5.325 | p < 0.05 | F(1,38) = 20.26 | p < 0.001 |
| Genotype (G) | F(1,36) = 7.216 | p < 0.05 | F(1,26) = 3.287 | p = 0.081 | F(1,38) = 0.185 | p = 0.669 |
| D × T | F(5,32) = 6.845 | p < 0.001 | F(5,22) = 4.833 | p < 0.01 | F(5,34) = 9.164 | p < 0.001 |
| D × G | F(5,32) = 2.014 | p = 0.103 | F(5,22) = 0.851 | p = 0.529 | F(5,34) = 1.288 | p < 0.05 |
| T × G | F(1,36) = 3.473 | p = 0.071 | F(1,26) = 0.072 | p = 0.727 | F(1,38) = 0.700 | p = 0.408 |
| D × T × G | F(5,32) = 0.509 | p = 0.768 | F(5,22) = 0.905 | p = 0.495 | F(5,34) = 0.830 | p = 0.882 |
| Tail immersion | ||||||
| Day (D) | F(5,43) = 9.615 | p < 0.001 | F(5,35) = 3.987 | p < 0.01 | F(5,37) = 12.15 | p < 0.001 |
| Treatment (T) | F(1,47) = 71.10 | p < 0.001 | F(1,39) = 65.76 | p < 0.001 | F(1,41) = 85.28 | p < 0.001 |
| Genotype (G) | F(1,47) = 0.372 | p = 545 | F(1,39) = 0.178 | p = 0.675 | F(1,41) = 3.397 | p = 0.073 |
| D × T | F(5,43) = 14.99 | p < 0.01 | F(5,35) = 6.987 | p < 0.001 | F(5,37) = 13.428 | p < 0.001 |
| D × G | F(5,43) = 1.150 | p = 0.349 | F(5,35) = 1.823 | p = 0.134 | F(5,37) = 1.587 | p = 0.188 |
| T × G | F(1,47) = 0.261 | p = 0.612 | F(1,39) = 1.081 | p = 0.305 | F(1,41) = 1.825 | p = 0.184 |
| D × T × G | F(5,43) = 1.195 | p = 0.328 | F(5,35) = 0.823 | p = 0.542 | F(5,37) = 0.659 | p = 0.657 |
Three-way ANOVA repeated measures with treatment and genotype as between-subjects factors and day as within-subjects factor. See Materials and Methods for details. Overall significant effects of day and treatment and interactions between these two factors are observed in the three strains of mice. Main significant effect of genotype is only observed for locomotor activity in the MOR strain, and significant interaction between day and genotype is only observed for locomotor activity of the KOR strain.
Fig. 1.
Adaptive responses to chronic THC in KOR −/− mice. Development of tolerance to the antinociceptive, hypothermic, and hypolocomotor effects of chronic THC (20 mg/kg, i.p., twice daily) is minimally affected in KOR −/− mice. Number of mice per group from 10 to 14. Values are expressed as means ± SEM. ★p < 0.05; ★★p < 0.01; ★★★p < 0.001; comparison between treatments (one-way ANOVA); ⋆⋆p < 0.01, comparison between genotypes (one-way ANOVA).
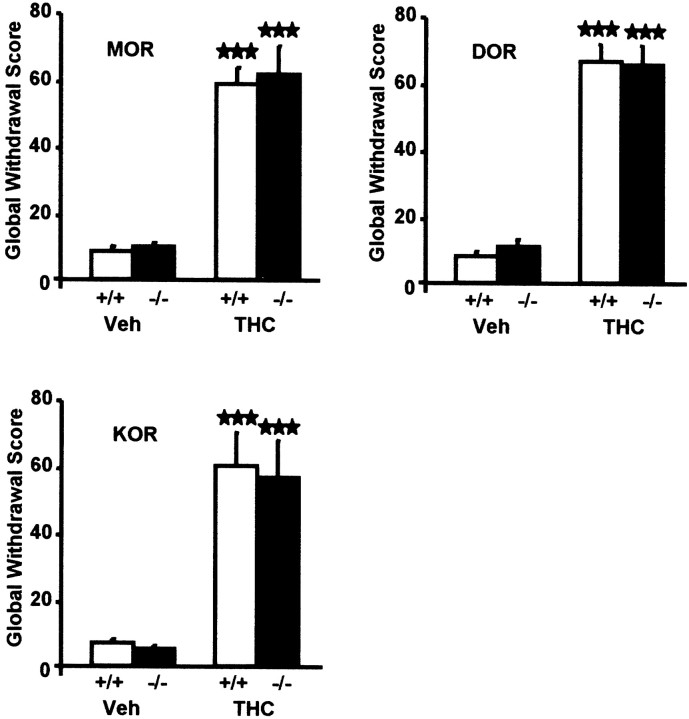
We then administered the cannabinoid antagonist SR141716A (10 mg/kg) in chronically THC-treated mice. A significant expression of several signs of withdrawal, including wet dog shakes, paw tremor, ptosis, piloerection, mastication, and sniffing, was detected in wild-type, MOR −/−, DOR −/−, and KOR −/− mice. Expression of these signs was comparable in mutant animals and their respective wild-type controls, except for paw tremor that was significantly reduced in THC-treated DOR −/− mice (vehicle-treated DOR +/+ = 2.46 ± 1.39; vehicle-treated DOR −/− = 3.25 ± 1.13; THC-treated DOR +/+ = 81.6 ± 9.78; THC-treated DOR −/− = 64.4 ± 13.4; two-way ANOVA, genotype:F(1,38) = 76.97, p < 0.001; treatment: F(1,38) = 5.173,p < 0.05, genotype × treatment:F(1,38) = 5.556, p < 0.05; one-way ANOVA for genotype comparison:F(1,14) = 4.894, p < 0.05). Other signs of THC withdrawal such as body tremor, writhing, hunched posture, and penile licking were less obvious, and no difference was detectable in any of these signs when comparing mutant and wild-type groups. We determined global withdrawal scores for each MOR −/−, DOR −/−, and KOR −/− genotype and their wild-type controls. Total withdrawal score comparisons revealed no significant alteration of THC withdrawal in any group of mutant mice (Fig.2). Indeed, two-way ANOVA revealed a significant effect of THC treatment (experiment with MOR −/− mice:F(1,41) = 123.2, p < 0.001; experiment with DOR −/− mice:F(1,38) = 241.3, p < 0.001; experiment with KOR −/− mice:F(1,36) = 44.31, p < 0.001), but not effect of genotype (experiment with MOR −/− mice:F(1,41) = 0.122, p = 0.873; experiment with DOR −/− mice:F(1,38) = 0.135, p = 0.715; experiment with KOR −/− mice:F(1,36) = 0.092, p = 0.736) nor interaction between treatment and genotype (experiment with MOR −/− mice: F(1,41) = 0.058,p = 0.810; experiment with DOR −/− mice:F(1,38) = 0.525, p = 0.525; experiment with KOR −/− mice:F(1,36) = 0.019, p = 0.891) in the three experiments.
Fig. 2.
Somatic expression of withdrawal from THC after SR 141716A administration (10 mg/kg, i.p.) is similar in MOR −/−, DOR −/−, and KOR −/− mice and in their respective wild-type controls. Mice received a chronic administration of vehicle or THC (20 mg/kg, i.p., twice daily) for 6 d. Values are expressed as mean ± SEM of global withdrawal scores calculated by giving to each individual sign a proportional weight. Number of mice per group from 10 to 14. ★★★p < 0.001, comparison between treatments (one-way ANOVA).
THC-induced place conditioning
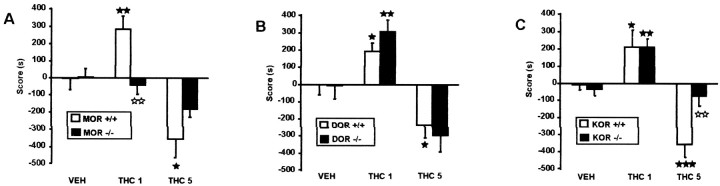
We explored motivational responses to THC in MOR −/−, DOR −/−, and KOR −/− mice, using several place conditioning protocols. We first investigated the rewarding properties of THC, in a place conditioning protocol that minimizes the aversive activity of THC. Mice received a single injection of a low dose of THC (1 mg/kg) in their home cage and were subsequently conditioned to the same dose of the drug. Time spent by all the mice in the two conditioning compartments of the apparatus was similar during the preconditioning phase in the different experiments (mean in striped compartment: 615.8 sec; mean in dotted compartment: 582.6 sec). No initial place preference or aversion was observed in any of the experiments. Under those conditions, all the groups of wild-type mice showed a strong place preference, as previously reported (Valjent and Maldonado, 2000). This was revealed by significant increases in the time spent in the drug-paired compartment from the preconditioning to the test phase in MOR +/+, DOR +/+, and KOR +/+ mice (see Table 4), as well as by significant scores when comparing THC-treated mice with the respective vehicle controls (Tables2, 3, Fig.3). A similar conditioned place preference to THC (1 mg/kg) was observed in DOR −/− and KOR −/− mutants receiving the previous THC single injection (Tables2-4, Fig. 3). In contrast, this response was completely absent in MOR −/− mice (Tables 2-4, Fig. 3), demonstrating that μ-opioid receptors are essential for the rewarding effects of THC.
Table 4.
Paired Student's t test comparisons of the time spent in the drug-paired compartment during the preconditioning and test phases, in mice lacking μ-, δ-, or κ-opioid receptor
| MOR | |||||
|---|---|---|---|---|---|
| pre-cond | test | t | p | ||
| +/+ | VEHICLE | 677 ± 63 | 672 ± 92 | t(1,13) = 0.075 | p = 0.942 |
| THC1 | 589 ± 64 | 874 ± 71 | t(1,12) = −6.958 | p < 0.01 | |
| THC5 | 651 ± 58 | 296 ± 76 | t(1,12) = 2.932 | p < 0.05 | |
| −/− | VEHICLE | 607 ± 65 | 611 ± 79 | t(1,13) = 0.076 | p = 0.941 |
| THC1 | 615 ± 65 | 573 ± 71 | t(1,13) = 0.477 | p = 0.641 | |
| THC5 | 574 ± 51 | 394 ± 77 | t(1,12) = 1.091 | p = 0.297 | |
| DOR | |||||
|---|---|---|---|---|---|
| pre-cond | test | t | p | ||
| +/+ | VEHICLE | 734 ± 66 | 733 ± 75 | t(1,8) = 0.035 | p = 0.973 |
| THC1 | 590 ± 62 | 780 ± 63 | t(1,12) = −3.739 | p < 0.01 | |
| THC5 | 589 ± 45 | 359 ± 71 | t(1,12) = 3.253 | p < 0.01 | |
| −/− | VEHICLE | 582 ± 91 | 576 ± 107 | t(1,9) = 0.070 | p = 0.945 |
| THC1 | 578 ± 61 | 879 ± 53 | t(1,11) = −4.443 | p < 0.001 | |
| THC5 | 620 ± 54 | 330 ± 79 | t(1,12) = 3.31 | p < 0.01 | |
| KOR | |||||
|---|---|---|---|---|---|
| pre-cond | test | t | p | ||
| +/+ | VEHICLE | 612 ± 57 | 606 ± 51 | t(1,11) = 1.013 | p = 0.333 |
| THC1 | 531 ± 87 | 745 ± 86 | t(1,10) = −2.971 | p < 0.05 | |
| THC5 | 663 ± 69 | 308 ± 72 | t(1,11) = 4.142 | p < 0.01 | |
| −/− | VEHICLE | 604 ± 106 | 572 ± 89 | t(1,9) = 0.022 | p = 0.983 |
| THC1 | 573 ± 66 | 788 ± 90 | t(1,11) = −3.670 | p < 0.01 | |
| THC5 | 563 ± 45 | 493 ± 67 | t(1,10) = 0.343 | p = 0.739 | |
Values of time spent in the drug-paired compartment during the preconditioning and test phases are expressed in seconds. Paired Student's t test was used to compare the time spent in the drug-paired side during preconditioning and test phases within each group of mice. See Materials and Methods for details. The significant differences between time spent in the drug-paired compartment during the preconditioning and test phases observed in MOR +/+ mice with 1 and 5 mg/kg THC were abolished in MOR −/− mice. The significant difference between time spent in the drug-paired compartment during the preconditioning and test phases observed in KOR +/+ mice with 5 mg/kg THC was abolished in KOR −/− mice.
Table 2.
Two-way ANOVA of place conditioning scores to THC of mice lacking μ-, δ-, or κ-opioid receptor
| MOR | DOR | KOR | ||||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Genotype (G) | F(1,81) = 0.503 | p = 0.480 | F(1,70) = 0.02 | p = 0.887 | F(1,68) = 2.879 | p = 0.095 |
| Treatment (T) | F(1,81) = 10.04 | p < 0.001 | F(2,70) = 30.66 | p < 0.001 | F(2,68) = 21.261 | p < 0.001 |
| G × T | F(2,81) = 6.516 | p < 0.01 | F(2,70) = 0.351 | p = 0.266 | F(2,68) = 3.391 | p < 0.05 |
Two-way ANOVA with treatment and genotype as between-subjects factors. See Materials and Methods for details. Overall significant effects of treatment are observed in the three strains of mice. A significant interaction between genotype and treatment is observed in MOR and KOR strains.
Table 3.
One-way ANOVA of place conditioning scores to THC of mice lacking μ-, δ-, or κ-opioid receptor
| Group | Comparison | MOR | DOR | KOR | |||
|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | ||
| Vehicle | +/+ vs −/− | F(1,27) = 0.003 | p = 0.955 | F(1,18) = 0.473 | p = 0.501 | F(1,19) = 0.017 | p = 0.898 |
| THC1 | +/+ vs −/− | F(1,26) = 12.76 | p < 0.001 | F(1,24) = 2.705 | p = 0.114 | F(1,22) = 0.046 | p = 0.833 |
| THC5 | +/+ vs −/− | F(1,25) = 2.292 | p = 0.143 | F(1,25) = 0.131 | p = 0.721 | F(1,21) = 9.995 | p < 0.01 |
| +/+ | THC1 vs VEHICLE | F(1,26) = 8.953 | p < 0.01 | F(1,21) = 4.088 | p < 0.05 | F(1,22) = 8.041 | p < 0.05 |
| +/+ | THC5 vs VEHICLE | F(1,26) = 5.752 | p < 0.05 | F(1,21) = 7.785 | p < 0.05 | F(1,23) = 9.704 | p < 0.01 |
| −/− | THC1 vs VEHICLE | F(1,27) = 0.124 | p = 0.727 | F(1,21) = 14.437 | p < 0.001 | F(1,21) = 7.165 | p = 0.05 |
| −/− | THC5 vs VEHICLE | F(1,26) = 0.914 | p = 0.348 | F(1,22) = 1.952 | p = 0.177 | F(1,20) = 0.13 | p = 0.910 |
One-way ANOVA with treatment or genotype as between-subjects factors. See Materials and Methods for details. Significant effects of genotype are observed in the MOR strain at 1 mg/kg THC and in the KOR strain at 5 mg/kg THC. The significant effect of treatment observed in MOR +/+ mice is absent in MOR −/− mice with 1 mg/kg THC. The significant effect of treatment observed in KOR +/+ mice is absent in KOR −/− mice with 5 mg/kg THC.
Fig. 3.
THC place preference and aversion. A, Place preference to THC is abolished in MOR −/− mice, whereas place aversion is diminished (number of mice per group from 13 to 14);B, DOR −/− mice show similar place conditioning to THC than their wild-type controls (number of mice per group from 10 to 13); and C, Place aversion to THC is abolished in KOR −/− mice (number of mice per group from 11 to 12) mice. THC1 (1 mg/kg, i.p.); THC5 (5 mg/kg, i.p.). Mice conditioned to the dose of 1 mg/kg THC received a previous THC injection (1 mg/kg, i.p.) in their home cage, 24 hr before the start of the conditioning phase. In wild-type mice, THC1 conditions reveal THC place preference, whereas THC5 conditions show THC place aversion. Values are expressed as mean ± SEM. Scores were calculated as the difference between test and preconditioning time spent in the drug-paired compartment. ★p < 0.05, ★★p < 0.01, comparison between treatments; ⋆⋆p < 0.01, comparison between genotypes (one-way ANOVAs).
We then used another place conditioning protocol where animals received no injection of THC before conditioning and were subjected to a high dose of THC (5 mg/kg). Under those conditions only THC dysphoria is detectable. In agreement with previous studies (Valjent and Maldonado, 2000), wild-type mice showed marked conditioned place aversion, as revealed by significant decrease in time spent in the drug-paired compartment in MOR +/+, DOR +/+, and KOR +/+ (Table 4), and in the score values (Tables 2, 3, Fig. 3). In DOR −/− mice, conditioned place aversion to THC (5 mg/kg) was comparable with wild-type (Tables 2-4, Fig. 3). In MOR −/− mutants, the aversive effects of THC were diminished but not abolished (Tables 2-4, Fig. 3). In KOR −/− animals, THC place aversion was completely absent (Tables 2-4, Fig. 3). The latter finding demonstrates that κ receptors are critically implicated in the dysphoric aspect of THC activity.
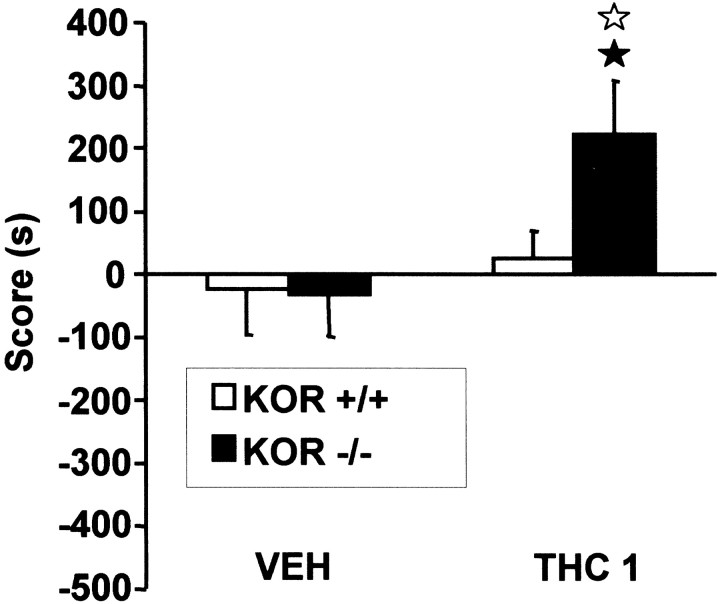
Because this is a first evidence for an involvement of κ receptors in THC aversive properties, we further explored this finding using a third place conditioning protocol. Mice were conditioned to 1 mg/kg of THC without receiving any injection of the drug before the conditioning period. We have previously shown that this protocol does not reveal any motivational effect of THC in wild-type mice, most probably because THC reward and dysphoria equally oppose each other (Valjent and Maldonado, 2000). Accordingly, wild-type mice showed no place conditioning, as shown by comparable time spent in the drug-paired compartment between preconditioning and test (t(1,9) = −0.680; p = 0.514) and comparable score values between animals conditioned to THC or vehicle (F(1,19) = 0.348; p = 0.562) (Fig. 4). In contrast, KOR −/− mice conditioned to 1 mg/kg of THC showed under these experimental conditions a significant place preference as seen by an increase in the time spent in the drug-paired compartment (t(1,9) = −2.770; p< 0.05) and a significantly different conditioning score from vehicle controls (F(1,18) = 5.587;p < 0.05). Also, although vehicle groups from both genotypes showed similar scores (F(1,18) = 0.006; p = 0.938), KOR −/− animals conditioned to THC displayed significantly different scores from wild-type controls (F(1,19) = 4.877; p < 0.05). Therefore, the lack of κ receptors in mutant mice reveals THC place preference, suggesting that, under those conditions, κ receptor activity hinders the rewarding properties of THC in wild-type mice.
Fig. 4.
KOR −/− mice show place preference to THC (1 mg/kg, i.p.), without priming exposure, whereas their wild-type controls do not. Values are expressed as mean ± SEM. Number of mice per group from 9 to 10. Scores calculated as the difference between test and preconditioning time spent in the drug-paired compartment. ★p < 0.05, comparison between treatments; ⋆p < 0.05, comparison between genotypes (one-way ANOVAs).
DISCUSSION
Bidirectional interactions between the opioid and cannabinoid systems have been reported (Manzanares et al., 1999). Here we show that disruption of μ-, δ-, or κ-opioid receptor gene does not modify acute THC responses or the expression of THC withdrawal, and that the development of THC tolerance is only slightly altered in KOR −/− mice. Both μ- and κ-opioid ligands have been previously reported to modulate cannabinoid antinociception (Manzanares et al., 1999). Thus, THC antinociception was blocked in mice by the κ-selective opioid antagonist norbinaltorphimine and by high doses of the nonselective opioid antagonist naloxone (Welch, 1993; Smith et al., 1998). The synergistic effects of morphine and THC on antinociception were also blocked by both norbinaltorphimine and β-funaltrexamine, a μ selective opioid antagonist (Reche et al., 1996). The absence of changes in THC antinociception in this study is unlikely attributable to a ceiling effect, because a maximal response was observed in one response (jump in the hot plate) over three nociceptive thresholds evaluated. Rather, we may suggest that the ablation of one opioid receptor only is not sufficient to reveal significant changes, and indeed, high doses of opioid antagonists are usually required to block THC antinociception (Manzanares et al., 1999). THC antinociception in the tail immersion test was reduced in knock-out mice lacking the preproenkephalin gene (Valverde et al., 2000), but derivatives from proenkephalin are not selective agonists of any opioid receptor.
Besides, the deletion of μ- or δ-opioid receptors has no measurable consequence on the development of THC tolerance, whereas tolerance was reduced in KOR −/− mice on the last day of treatment. Although these modifications were subtle, the data suggest that κ receptors could contribute to the development of adaptive responses to chronic THC, in agreement with the demonstration of cross-tolerance between THC and κ-opioid agonists (Smith et al., 1994). Besides, THC-induced antinociceptive tolerance can be modified by antisense oligodeoxynucleotides to the κ receptor (Rowen et al., 1998), and mice tolerant to THC also displayed tolerance to the effects of κ selective agonists (U-50,488 and CI-977), but not of μ or δ selective agonists (Smith et al., 1994).
The lack of alteration of THC withdrawal was unexpected, because clear interactions between opioid and cannabinoid dependence have been reported. Thus, naloxone, the prototypic nonselective opioid antagonist, precipitates an opioid-like withdrawal syndrome in cannabinoid-dependent rodents (Kaymakcalan et al., 1977; Navarro et al., 1998) and conversely, the CB1 cannabinoid receptor antagonist SR 141716A induces withdrawal in morphine-dependent rats (Navarro et al., 1998). This suggests that simultaneous activation of the two endogenous systems could participate to both opioid and cannabinoid dependence. Consistent with this notion, gene-targeting experiments have shown attenuated naloxone-precipitated opioid withdrawal in morphine-dependent CB1 knock-out mice (Ledent et al., 1999) and attenuated SR141716A-precipitated cannabinoid withdrawal in THC-dependent mice lacking preproenkephalin gene (Valverde et al., 2000). Pretreatment with THC (Hine et al., 1975; Valverde et al., 2001) or anandamide (Vela et al., 1995), have been also shown to decrease morphine withdrawal. Our finding that the disruption of a single opioid receptor gene has no major consequences on the somatic expression of THC withdrawal may indicate that concomitant changes in the activity of several opioid receptors are implicated in the expression of THC withdrawal. Possibly the analysis of combinatorial double or triple opioid receptor-deleted mice may reveal a concerted implication of several components of the opioid system in the development of THC physical dependence.
The suppression of THC rewarding effects in MOR −/− mice provides a clear genetic evidence to clarify the crucial role of μ receptors in THC motivational responses. Gene targeting experiments have recently demonstrated the central role of μ-opioid receptors in mediating rewarding properties of several drugs of abuse, including morphine (Matthes et al., 1996), ethanol (Roberts et al., 2000), and now THC (this study). μ receptors, therefore, seem to represent a convergent substrate for positive reinforcement. A possible mechanism to explain the involvement of μ receptors in cannabinoid rewarding effects could be caused by their effects on the mesolimbic dopaminergic system. Thus, THC-induced increase in mesolimbic dopaminergic activity (Navarro et al., 1993) was reversed by naloxone (Chen et al., 1990), or naloxonazine, a μ1 antagonist (Tanda et al., 1997). However, another study showed no effect of naloxone on THC excitatory activity on dopamine neurons from the ventral tegmental area (French, 1997). Therefore, other mechanisms could also mediate the implication of μ-opioid receptors in cannabinoid motivational properties. Interestingly, morphine-induced rewarding effects were suppressed in mice deficient in CB1 cannabinoid receptors (Ledent et al., 1999), suggesting a bidirectional influence of μ-opioid and CB1 cannabinoid receptors on reward processes.
δ-opioid receptors do not seem to be involved in the motivational responses to THC. The differential location of CB1 cannabinoid and δ opioid receptors may be important for this result. Although both receptors are localized in the nucleus accumbens (Mansour et al., 1995;Robbe et al., 2001), they might not be colocalized in other brain regions implicated in the rewarding effects of cannabinoids. The present results demonstrate a crucial involvement of κ-opioid receptors in the aversive properties of THC. Thus, THC-induced conditioned place aversion was abolished in KOR −/− mice. In addition, the lack of κ-opioid receptors reveals THC place preference in mutant mice, suggesting that, under those conditions, κ receptor activity hinders the rewarding properties of THC in wild-type mice. These observations indicate that an endogenous κ receptor tone underlies THC aversion, a hypothesis that has not been explored by pharmacological studies. Several peripheral and somatic effects of THC can participate in its aversive properties, as it has been previously reported with κ agonists (Bechara et al., 1987). Indeed, THC produces hypothermia and hypolocomotion that can induce a discomfort state in the mouse, leading to the aversive response. The exact involvement of these somatic responses remains to be clarified.
A possible limitation in the interpretation of these results is the required use of ethanol to dissolve THC and SR 141716A. Indeed, ethanol is also a psychoactive compound sharing some common pharmacological properties with cannabinoids and opioids. The pharmacological responses induced by these vehicle solutions of cannabinoid agents have been reported to be exclusively caused by the activation of CB1 cannabinoid receptors because both acute and chronic THC effects were abolished in knock-out animals deficient in these CB1 receptors (Ledent et al., 1999). However, THC behavioral effects can be modulated by the presence of ethanol, and this modulation may be different in μ-, δ-, or κ-opioid receptor knock-out mice. Therefore, a possible contribution of ethanol in the behavioral responses observed in these knock-out mice cannot be excluded. Ethanol was also present in the SR 141716A solution used to precipitate THC withdrawal. The behavioral signs of cannabinoid withdrawal observed when using this procedure are caused by the blockade of CB-1 cannabinoid receptors because they were abolished in CB-1 knockout mice (Ledent et al., 1999), and these behavioral signs were not elicited by the ethanol-containing vehicle alone (Cook et al., 1998). However, the presence of ethanol could attenuate the behavioral expression of cannabinoid withdrawal, considering the common pharmacological effects of both compounds. This observation must be considered for the interpretation of the cannabinoid withdrawal results. Besides, the possibility of developmental compensations after the deletion of the different opioid receptors and/or influences of the hybrid genetic background (Kelly et al., 1998) cannot be discarded. However, no homologous compensatory changes have been reported in these knock-out mice. Indeed, the abolition of μ-opioid (Matthes et al., 1996), δ-opioid (Filliol et al., 2000), or κ-opioid (Simonin et al., 1998) receptors has no influence on the binding properties and distribution of the other opioid receptors, and did not modify the expression of the different opioid peptide precursors.
The present novel finding highlights the involvement of homeostatic opioid mechanisms in cannabinoid motivational responses. We propose that opposing μ-opioid and κ-opioid receptor activities mediate the dual euphoric–dysphoric effects of THC. This hypothesis is consistent with the proposed opposite role of the two opioid receptors in modulating mesolimbic dopaminergic activity (Di Chiara, 1995) and suggests that THC influences both players within the reward circuitry. A possible mechanism could be that cannabinoid receptor activation modifies endogenous opioid peptide levels in mesolimbic areas that would, in turn, modulate dopaminergic activity. This is supported by findings of increased opioid peptides levels in the hypothalamus (Corchero et al., 1997a,b), or preproenkephalin mRNA levels in the striatum and nucleus accumbens (Manzanares et al., 1998) after cannabinoid treatment. Cannabinoids and opioids might also interact at the level of their signaling activity. Both receptor types are coupled to similar intracellular effectors via Gi/Go-proteins, modulating cAMP levels, K+ and Ca2+ channel activities, and MAP kinase phosphorylation (Bouaboula et al., 1995; Fukuda et al., 1996;Manzanares et al., 1999), and cross-talk could occur downstream of receptors coexpressed in specific mesocorticolimbic neurons (Mansour et al., 1995; Breivogel and Childers, 1998).
In conclusion, we found that single disruption of μ-opioid, δ-opioid, or κ-opioid receptors has minimal influence on somatic adaptations to prolonged cannabinoid exposure. In contrast, we provide strong evidence for the involvement of μ-opioid and κ-opioid receptors in motivational responses to THC. These data shed a new light on mechanisms underlying the possible addictive potential of marijuana, highlighting a major implication of opioid receptor-mediated mechanisms essentially in THC euphoria–dysphoria.
Footnotes
This work was supported by the European Commission (Biomed-2 Grant 98-2227, to R.M.), Dr. Esteve S. A. Laboratories (R.M.), Generalitat de Catalunya (Research Distinction, to R.M.), the Spanish Ministry of Health (Fondo de Investigación Sanitaria Grant 99/0624, to R.M.), the Mission Interministerielle de Lutte contre la Drogue et la Toxicomanie (B.K.), and the Centre National de la Recherche Scientifique (B.K.). We thank J. F. Poirier and N. Scallon for animal care.
Correspondence should be addressed to Rafael Maldonado, Laboratori de Neurofarmacologia, Facultat de Ciéncies de la Salut i de la Vida, Universitat Pompeu Fabra, c/o Dr Aiguader 80, 08003 Barcelona, Spain. E-mail: rafael.maldonado@cexs.upf.es.
REFERENCES
- 1.Bechara A, van der Kooy D. κ receptors mediate the peripheral aversive effects of opiates. Pharmacol Biochem Behav. 1987;28:227–233. doi: 10.1016/0091-3057(87)90219-x. [DOI] [PubMed] [Google Scholar]
- 2.Bouaboula M, Poinot-Chazel C, Bourrie B, Canat X, Calandra B, Rinaldi-Carmona M, Le Fur G, Casellas P. Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem J. 1995;312:637–641. doi: 10.1042/bj3120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breivogel CS, Childers SR. The functional neuroanatomy of brain cannabinoid receptors. Neurobiol Dis. 1998;5:417–431. doi: 10.1006/nbdi.1998.0229. [DOI] [PubMed] [Google Scholar]
- 4.Chen JP, Paredes W, Li J, Smith D, Lowinson J, Gardner EL. Δ9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology. 1990;102:156–162. doi: 10.1007/BF02245916. [DOI] [PubMed] [Google Scholar]
- 5.Cook SA, Lowe JA, Martin BR. CB1 receptor antagonist precipitates withdrawal in mice exposed to Δ9-tetrahydrocannabinol. J Pharmacol Exp Ther. 1998;285:1150–1156. [PubMed] [Google Scholar]
- 6.Corchero J, Avila MA, Fuentes JA, Manzanares J. Δ-9-tetrahydrocannabinol increases prodynorphin and proenkephalin gene expression in the spinal cord of the rat. Life Sci. 1997a;61:39–43. doi: 10.1016/s0024-3205(97)00405-0. [DOI] [PubMed] [Google Scholar]
- 7.Corchero J, Fuentes JA, Manzanares J. Δ9-tetrahydrocannabinol increases proopiomelanocortin gene expression in the arcuate nucleus of the rat hypothalamus. Eur J Pharmacol. 1997b;323:193–195. doi: 10.1016/s0014-2999(97)00144-1. [DOI] [PubMed] [Google Scholar]
- 8.Di Chiara G. The role of dopamine in drug abuse viewed from the perspective of its role in motivation. Drug Alcohol Depend. 1995;38:95–137. doi: 10.1016/0376-8716(95)01118-i. [DOI] [PubMed] [Google Scholar]
- 9.Felder CC, Glass M. Cannabinoid receptors and their endogenous agonists. Annu Rev Pharmacol Toxicol. 1998;38:179–200. doi: 10.1146/annurev.pharmtox.38.1.179. [DOI] [PubMed] [Google Scholar]
- 10.Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for δ- and μ-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- 11.French ED. Δ9-tetrahydrocannabinol excites rat VTA dopamine neurons through activation of cannabinoid CB1 but not opioid receptors. Neurosci Lett. 1997;226:159–162. doi: 10.1016/s0304-3940(97)00278-4. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda K, Kato S, Morikawa H, Shoda T, Mori K. Functional coupling of the δ-, μ-, and κ-opioid receptors to mitogen-activated protein kinase and arachidonate release in Chinese hamster ovary cells. J Neurochem. 1996;67:1309–1316. doi: 10.1046/j.1471-4159.1996.67031309.x. [DOI] [PubMed] [Google Scholar]
- 13.Gardner EL, Paredes W, Smith D, Donner A, Milling C, Cohen D, Morrison D. Facilitation of brain stimulation reward by Δ9-tetrahydrocannabinol. Psychopharmacology. 1988;96:142–144. doi: 10.1007/BF02431546. [DOI] [PubMed] [Google Scholar]
- 14.Halikas JA, Weller RA, Morse CL, Hoffmann RG. A longitudinal study of marijuana effects. Int J Addict. 1985;20:701–711. doi: 10.3109/10826088509044290. [DOI] [PubMed] [Google Scholar]
- 15.Hine B. Morphine and Δ9-tetrahydrocannabinol: two-way cross tolerance for antinociceptive and heart-rate responses in the rat. Psychopharmacology. 1985;87:34–38. doi: 10.1007/BF00431774. [DOI] [PubMed] [Google Scholar]
- 16.Hine B, Torrelio M, Gershon S. Interactions between cannabidiol and Δ9-THC during abstinence in morphine-dependent rats. Life Sci. 1975;17:851–857. doi: 10.1016/0024-3205(75)90435-x. [DOI] [PubMed] [Google Scholar]
- 17.Hutcheson DM, Tzavara ET, Smadja C, Valjent E, Roques BP, Hanoune J, Maldonado R. Behavioural and biochemical evidence for signs of abstinence in mice chronically treated with Δ-9-tetrahydrocannabinol. Br J Pharmacol. 1998;125:1567–1577. doi: 10.1038/sj.bjp.0702228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janssen P, Niemegeers CJ, Dony JG. The inhibitory effect of fentanyl and other morphine-like analgesics on the warm induced tail withdrawal reflex in rat. Arzneimittel-Forsh. 1963;13:502–507. [PubMed] [Google Scholar]
- 19.Kaymakcalan S, Ayhan IH, Tulunay FC. Naloxone-induced or postwithdrawal abstinence signs in Δ9-tetrahydrocannabinol-tolerant rats. Psychopharmacology. 1977;55:243–249. doi: 10.1007/BF00497855. [DOI] [PubMed] [Google Scholar]
- 20.Kelly MA, Rubinstein M, Phillips TJ, Lessov CN, Burkhart-Kasch S, Zhang G, Bunzow JR, Fang Y, Gerhardt GA, Grandy DK, Low MJ. Locomotor activity in D2 dopamine receptor-deficient mice is determined by gene dosage, genetic background, and developmental adaptations. J Neurosci. 1998;18:3470–3479. doi: 10.1523/JNEUROSCI.18-09-03470.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kieffer BL. Recent advances in molecular recognition and signal transduction of active peptides: receptors for opioid peptides. Cell Mol Neurobiol. 1995;15:615–635. doi: 10.1007/BF02071128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- 23.Koob GF, Maldonado R, Stinus L. Neural substrates of opiate withdrawal. Trends Neurosci. 1992;15:186–191. doi: 10.1016/0166-2236(92)90171-4. [DOI] [PubMed] [Google Scholar]
- 24.Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Bohme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- 25.Maldonado R, Saiardi A, Valverde O, Samad TA, Roques BP, Borrelli E. Absence of opiate rewarding effects in mice lacking dopamine D2 receptors. Nature. 1997;388:586–589. doi: 10.1038/41567. [DOI] [PubMed] [Google Scholar]
- 26.Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- 27.Manzanares J, Corchero J, Romero J, Fernandez-Ruiz JJ, Ramos JA, Fuentes JA. Chronic administration of cannabinoids regulates proenkephalin mRNA levels in selected regions of the rat brain. Brain Res Mol Brain Res. 1998;55:126–132. doi: 10.1016/s0169-328x(97)00371-9. [DOI] [PubMed] [Google Scholar]
- 28.Manzanares J, Corchero J, Romero J, Fernandez-Ruiz JJ, Ramos JA, Fuentes JA. Pharmacological and biochemical interactions between opioids and cannabinoids. Trends Pharmacol Sci. 1999;20:287–294. doi: 10.1016/s0165-6147(99)01339-5. [DOI] [PubMed] [Google Scholar]
- 29.Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Cocaine, but not morphine, induces conditioned place preference, sensitization to locomotor responses in CB1 knockout mice. Eur J Neurosci. 2000;12:4038–4046. doi: 10.1046/j.1460-9568.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- 30.Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the μ-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 31.Narimatsu S, Yamamoto I, Watanabe K, Yoshimura H. Change in hypothermia and catalepsy induced by cannabinoids or morphine in mice tolerant to these substances. Eur J Pharmacol. 1987;141:437–443. doi: 10.1016/0014-2999(87)90562-0. [DOI] [PubMed] [Google Scholar]
- 32.Navarro M, Fernandez-Ruiz JJ, de Miguel R, Hernandez ML, Cebeira M, Ramos JA. An acute dose of Δ9-tetrahydrocannabinol affects behavioral and neurochemical indices of mesolimbic dopaminergic activity. Behav Brain Res. 1993;57:37–46. doi: 10.1016/0166-4328(93)90059-y. [DOI] [PubMed] [Google Scholar]
- 33.Navarro M, Chowen J, Rocio A, Carrera M, del Arco I, Villanua MA, Martin Y, Roberts AJ, Koob GF, de Fonseca FR. CB1 cannabinoid receptor antagonist-induced opiate withdrawal in morphine-dependent rats. NeuroReport. 1998;9:3397–3402. doi: 10.1097/00001756-199810260-00012. [DOI] [PubMed] [Google Scholar]
- 34.Onaivi ES, Green MR, Martin BR. Pharmacological characterization of cannabinoids in the elevated plus maze. J Pharmacol Exp Ther. 1990;253:1002–1009. [PubMed] [Google Scholar]
- 35.Reche I, Fuentes JA, Ruiz-Gayo M. Potentiation of Δ9-tetrahydrocannabinol-induced analgesia by morphine in mice: involvement of μ- and κ-opioid receptors. Eur J Pharmacol. 1996;318:11–16. doi: 10.1016/s0014-2999(96)00752-2. [DOI] [PubMed] [Google Scholar]
- 36.Robbe D, Alonso G, Duchamp F, Bockaert J, Manzoni OJ. Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbens. J Neurosci. 2001;21:109–116. doi: 10.1523/JNEUROSCI.21-01-00109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts AJ, McDonald JS, Heyser CJ, Kieffer BL, Matthes HW, Koob GF, Gold LH. μ-Opioid receptor knockout mice do not self-administer alcohol. J Pharmacol Exp Ther. 2000;293:1002–1008. [PubMed] [Google Scholar]
- 38.Rodriguez de Fonseca F, Rubio P, Menzaghi F, Merlo-Pich E, Rivier J, Koob GF, Navarro M. Corticotropin-releasing factor (CRF) antagonist [d-Phe12,Nle21,38,C alpha MeLeu37]CRF attenuates the acute actions of the highly potent cannabinoid receptor agonist HU-210 on defensive-withdrawal behavior in rats. J Pharmacol Exp Ther. 1996;276:56–64. [PubMed] [Google Scholar]
- 39.Rowen DW, Embrey JP, Moore CH, Welch SP. Antisense oligodeoxynucleotides to the kappa 1 receptor enhance Δ9-tetrahydrocannabinol-induced antinociceptive tolerance. Pharmacol Biochem Behav. 1998;59:399–404. doi: 10.1016/s0091-3057(97)00485-1. [DOI] [PubMed] [Google Scholar]
- 40.Simonin F, Valverde O, Smadja C, Slowe S, Kitchen I, Dierich A, Le Meur M, Roques BP, Maldonado R, Kieffer BL. Disruption of the κ-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective κ-agonist U-50,488H and attenuates morphine withdrawal. EMBO J. 1998;17:886–897. doi: 10.1093/emboj/17.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smart RG, Ogborne AC. Drug use and drinking among students in 36 countries. Addict Behav. 2000;25:455–460. doi: 10.1016/s0306-4603(99)00013-1. [DOI] [PubMed] [Google Scholar]
- 42.Smith FL, Fujimori K, Lowe J, Welch SP. Characterization of Δ9-tetrahydrocannabinol and anandamide antinociception in nonarthritic and arthritic rats. Pharmacol Biochem Behav. 1998;60:183–191. doi: 10.1016/s0091-3057(97)00583-2. [DOI] [PubMed] [Google Scholar]
- 43.Smith PB, Welch SP, Martin BR. Interactions between Δ 9-tetrahydrocannabinol and κ opioids in mice. J Pharmacol Exp Ther. 1994;268:1381–1387. [PubMed] [Google Scholar]
- 44.Stinus L, Le Moal M, Koob GF. Nucleus accumbens and amygdala are possible substrates for the aversive stimulus effects of opiate withdrawal. Neuroscience. 1990;37:767–773. doi: 10.1016/0306-4522(90)90106-e. [DOI] [PubMed] [Google Scholar]
- 45.Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common μ1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- 46.Thorat SN, Bhargava HN. Evidence for a bi-directional cross-tolerance between morphine and Δ9-tetrahydrocannabinol in mice. Eur J Pharmacol. 1994;260:5–13. doi: 10.1016/0014-2999(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 47.Valjent E, Maldonado R. A behavioural model to reveal place preference to Δ9-tetrahydrocannabinol in mice. Psychopharmacology. 2000;147:436–438. doi: 10.1007/s002130050013. [DOI] [PubMed] [Google Scholar]
- 48.Valverde O, Fournie-Zaluski MC, Roques BP, Maldonado R. The CCKB antagonist PD-134,308 facilitates rewarding effects of endogenous enkephalins but does not induce place preference in rats. Psychopharmacology. 1996;123:119–126. doi: 10.1007/BF02246168. [DOI] [PubMed] [Google Scholar]
- 49.Valverde O, Maldonado R, Valjent E, Zimmer AM, Zimmer A. Cannabinoid withdrawal syndrome is reduced in pre-proenkephalin knock-out mice. J Neurosci. 2000;20:9284–9289. doi: 10.1523/JNEUROSCI.20-24-09284.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valverde O, Noble F, Beslot F, Dauge V, Fournie-Zaluski MC, Roques BP. Δ9-tetrahydrocannabinol releases, facilitates the effects of endogenous enkephalins: reduction in morphine withdrawal syndrome without change in rewarding effect. Eur J Neurosci. 2001;13:1816–1824. doi: 10.1046/j.0953-816x.2001.01558.x. [DOI] [PubMed] [Google Scholar]
- 51.Vela G, Ruiz-Gayo M, Fuentes JA. Anandamide decreases naloxone-precipitated withdrawal signs in mice chronically treated with morphine. Neuropharmacology. 1995;34:665–668. doi: 10.1016/0028-3908(95)00032-2. [DOI] [PubMed] [Google Scholar]
- 52.Vivian JA, Kishioka S, Butelman ER, Broadbear J, Lee KO, Woods JH. Analgesic, respiratory and heart rate effects of cannabinoid and opioid agonists in rhesus monkeys: antagonist effects of SR 141716A. J Pharmacol Exp Ther. 1998;286:697–703. [PubMed] [Google Scholar]
- 53.Welch SP. Blockade of cannabinoid-induced antinociception by norbinaltorphimine, but not N,N-diallyl-tyrosine-Aib-phenylalanine-leucine, ICI 174,864 or naloxone in mice. J Pharmacol Exp Ther. 1993;265:633–640. [PubMed] [Google Scholar]