Abstract
Most crustacean muscle fibers receive double excitatory innervation by functionally different motor neurons termed slow and fast. By using specific ω-toxins we show that the terminals of the slow closer excitor (SCE) and the fast closer excitor (FCE) at a crab muscle are endowed with different sets of presynaptic Ca2+ channel types. ω-Agatoxin, a blocker of vertebrate P/Q-type channels, reduced the amplitude of EPSCs by decreasing the mean quantal content of transmitter release in both neurons by 70–85%, depending on the concentration. We provide the first evidence that ω-conotoxin-sensitive channels also participate in transmission at crustacean neuromuscular terminals and are colocalized with ω-agatoxin-sensitive channels in an axon-type-specific distribution. ω-Conotoxin, a blocker of vertebrate N-type channels, inhibited release by 20–25% only at FCE, not at SCE endings. Low concentrations of Ni2+, which block vertebrate R-type channels, inhibited release in endings of the SCE by up to 35%, but had little effects in FCE endings.
We found that two neuropeptides, the FMRFamide-like DF2 and proctolin, which occur in many crustaceans, potentiated evoked transmitter release differentially. Proctolin increased release at SCE and FCE endings, and DF2 increased release only at FCE endings. Selective blocking of Ca2+ channels by different ω-toxins in the presence of peptides revealed that the target of proctolin-mediated modulation is the ω-agatoxin-sensitive channel (P/Q-like), that of DF2 the ω-conotoxin-sensitive channel (N-like). The differential effects of these two peptides allows fine tuning of transmitter release at two functionally different motor neurons innervating the same muscle.
Keywords: P/Q-type Ca2+ channels, N-type Ca2+ channels, R-type Ca2+channels, crustacea, DF2, proctolin, RFamide, axon-type specific peptidergic modulation, ω-agatoxin, ω-conotoxin
Terminals of slow and fast neurons innervating crustacean muscles differ in morphological and physiological parameters such as number of release sites, quantal content, and facilitation or depression of transmitter release (Hoyle and Wiersma, 1958; Bittner, 1968; Rathmayer and Hammelsbeck, 1985;Atwood and Wojtowicz, 1986; King et al., 1996; Bradacs et al., 1997;Nguyen et al., 1997; Lnenicka et al., 1998; Msghina et al., 1998,1999). While studying peptidergic modulation of release by the FMRFamide-like DF2 (DRNFLRFamide) and proctolin, we noted that DF2 affected the slow and fast axons differentially. We investigated whether the differences are linked to the presence of different presynaptic Ca2+ channel types.
In studies of mammalian neurons, six types of voltage-gated Ca2+ channels have been classified by their electrophysiological and pharmacological properties. They are usually referred to as L-, N-, P-, Q-, R-, and T-type Ca2+ channels (Dunlap et al., 1995;Randall, 1998). The high voltage-activated Ca2+ channels are distinguished by their selective sensitivity to peptide toxins (Olivera et al., 1994). N-type channels are blocked by toxins isolated from Conussnails, the ω-conotoxins GVIA and MVIIA (Olivera et al., 1994). P/Q-type channels are insensitive to these two ω-conotoxins, but are blocked by two toxins from the venom of the spiderAgelenopsis aperta, ω-agatoxin IVA and FTX (Olivera et al., 1994; Randall and Tsien, 1995). For R-type channels, no antagonist has yet been found, but they are more sensitive to NiCl2 than the other types (Randall, 1998). The blockers have been successfully used in vertebrates to determine the contribution of Ca2+ channel types to transmitter release (Wu et al., 1998, 1999). With the exception of L- and T-type channels, all others are involved in transmitter release in the mammalian CNS (Meir et al., 1999).
Less is known about Ca2+ channel types in invertebrate neurons. There is evidence for L-, N-, P/Q-, or T-like channels in molluscs (for review, see Kits and Mansvelder, 1996), insects (for review, see Wicher et al., 2001), and crustaceans (Araque et al., 1994; Blundon et al., 1995; Chrachri, 1995; Wright et al., 1996; Hong and Lnenicka, 1997; Hurley and Graubard, 1998;Garcia-Colunga et al., 1999). In crayfish, additional subtypes are present that are pharmacologically different from channels characterized in vertebrate neurons (Richmond et al., 1995, 1996; Hong and Lnenicka, 1997). At crustacean neuromuscular junctions, transmitter release is thought to be mediated through P-type channels, with no contribution by N-, Q-, or L-type (Araque et al., 1994; Blundon et al., 1995; Wright et al., 1996; Hurley and Graubard, 1998).
We show that terminals of a slow and a fast excitatory axon innervating the same muscle are endowed with different sets of colocalized Ca2+ channel types: the slow terminals with ω-agatoxin-sensitive channels pharmacologically resembling vertebrate P/Q-type and Ni-sensitive R-like channels, and the fast terminals with ω-agatoxin-sensitive and ω-conotoxin-sensitive channels, the latter pharmacologically resembling vertebrate N-type. Moreover, we show that modulation of transmitter release by the peptides proctolin and DF2 is axon-type-specific, because proctolin modulates the ω-agatoxin-sensitive channels, and DF2 modulates the ω-conotoxin-sensitive channels.
MATERIALS AND METHODS
Animals and preparation. Crabs (Eriphia spinifrons) were collected in the Bay of Naples (Italy) and kept in artificial seawater at 16°C in Konstanz. Electrophysiological studies were performed exclusively on the identified slow-contracting type I fibers 2 and 3 (rarely 4), and the fast-contracting type IV fibers 7 and 8 (Rathmayer and Maier, 1987) of the closer muscle of the first three pairs of walking legs. The legs were obtained by inducing autotomy. The opener muscle was removed, and the cuticle of the propodite was cut away dorsally leaving a miniature chamber of ∼0.5 ml volume above the ventrally located closer muscle. Eriphiais one of the few crustaceans in which selective stimulation of the slow closer excitor (SCE) or the fast closer excitor (FCE) can be achieved in most preparations. Composition of the muscle of different fiber types, preparation, and methods for isolation and selective stimulation of individual motor axons have been described previously (Rathmayer and Erxleben, 1983).
Solutions and chemicals. The saline had a composition of (in mm): 490 NaCl, 8 KCl, 10 CaCl2, 12 MgCl2, and 10 HEPES at pH 7.4. The toxins and peptides were dissolved in distilled water at 1 mm concentration and stored at −20°C. Stock solution aliquots were diluted in saline before experiments. The solutions were applied to the muscle directly at the recording site through a gravity-fed superfusion system with a flow rate of 1 ml/min. After each change of solutions, intervals of 5 min (peptide containing solutions) and 45–60 min (toxin containing solutions) were allowed for equilibration of the solutions in the small volume bathing the muscle before recording was resumed. During recording, the muscle was again superfused with solution containing either toxins or peptides, or both. All experiments were performed at controlled room temperature of 20°C. The time protocol for the different experiments is given in Results. All toxins were obtained from Alomone Labs (Jerusalem, Israel), the peptide proctolin was purchased from Sigma (Deisenhofen, Germany), and the peptide DNRFLRFamide (also referred to as DF2) from Bachem (Bubendorf, Switzerland).
Postsynaptic currents. EPSCs were recorded focally from individual release boutons using macropatch electrodes (Dudel, 1981) with tip openings of ∼10 μm diameter and a DC resistance of 0.1–0.3 MΩ. The macropatch electrode is specific for current recording within the region of the electrode lumen with an amplifier designed for stimulating and recording from individual release sites (Zeitz Instruments, Augsburg, Germany). When recording EPSCs from the slow-contracting type I fibers, the two excitatory axons (SCE and FCE) supplying the closer muscle and innervating these fibers were individually stimulated through a suction electrode in the meropodite. The type of EPSCs can be easily distinguished because, in this fiber type, those of the SCE show facilitation, and those of the FCE show depression (Rathmayer and Hammelsbeck, 1985) (Fig.1). Focal stimulation of individual release sites by current pulses delivered through the macropatch electrode is not suitable in these fibers because release sites of the slow and the fast axon lie closely adjacent and thus prevent selective stimulation. In addition, release sites of a third, inhibitory axon in the immediate vicinity exert strong presynaptic inhibition in these fibers when costimulated (Rathmayer and Djokaj, 2000). However, in the fast contracting type IV fibers (for details, see Rathmayer and Maier, 1987) that are innervated by a branch of the FCE only, release from individual FCE boutons was stimulated by brief current pulses of 0.05–0.2 msec duration and 1–4 μA amplitude through the macropatch electrode.
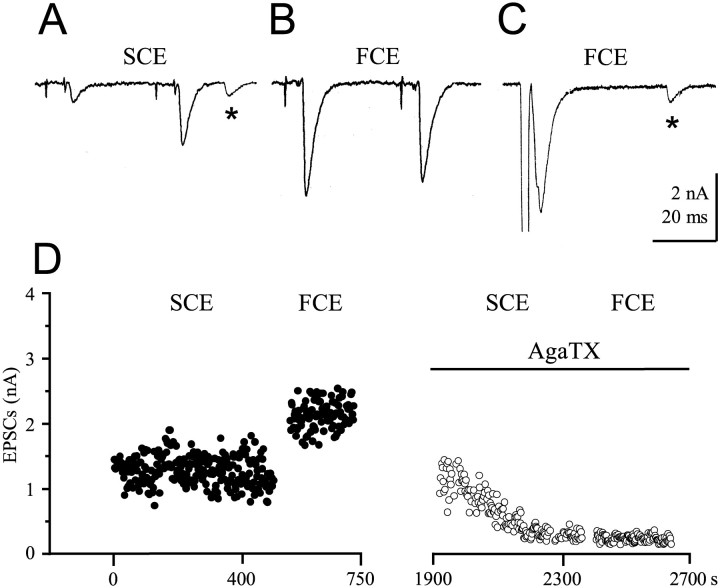
Fig. 1.
EPSCs after stimulation of the SCE and FCE.A, Type I fiber. Twin pulse stimulation of the SCE generating two EPSCs, the first by release of one transmitter quantum, the second of three quanta caused by facilitation.Asterisks in A and C mark spontaneously released single quanta. B, EPSCs after twin pulse stimulation of the FCE. The amplitude of the second EPSC is typically smaller than that of the first in type I fibers because of depression of release. C, Direct stimulation of a release bouton of FCE in a type IV fiber with a single pulse through the macropatch electrode. D, Stimulation and recording paradigm for the SCE and FCE. Both axons were stimulated selectively, as shown in A and B, but the FCE usually for a shorter period than the SCE. Only the EPSC amplitudes generated by the second of the twin pulses are plotted. In the experiment shown, 20 min was allowed for equilibration after 10−8m ω-AgaTX application before resuming stimulation and recording. The SCE was stimulated first. The short equilibration time was chosen to show the gradual development of the toxin effect.
When the SCE or FCE axon was stimulated with a suction electrode, twin pulses at 30 Hz with a repetition rate of 0.5 Hz were delivered. For the analysis of the effects of toxins on the amplitude of EPSCs, the currents generated by the second pulse of the twin stimuli were analyzed. The second EPSC does not show much amplitude fluctuation in the current records. This is particularly true for the facilitated EPSCs of the SCE. Normally, 200–300 samples were taken for each trial with SCE stimulation, and 100 for FCE stimulation. In the type I fibers, sites could be found where single release boutons of both the slow and the fast axon are located closely adjacent and the EPSCs generated by selective stimulation of either one axon could be recorded by the same macropatch electrode. In experiments using type IV fibers, single pulses were used with a repetition rate of 0.5 Hz. Because of the small-amplitude fluctuation of the EPSCs in this fiber type, only 150 samples were taken for analysis for each trial. The patch electrodes were filled with normal saline. Optimal release sites were identified by scanning a fiber with the electrode for sites that produced fast-rising EPSCs and single quanta responses with an amplitude of ∼500 pA. The seal resistance of the macropatch electrode was monitored by applying a test current pulse through the electrode. Only preparations in which seal resistance did not change by >5% over the period of the experiment were used for further analysis. Because the seal is not a tight, high-resistance seal, solutions applied in the immediate vicinity of the macropatch could reach the boutons under the recording electrode. This was obvious from application of 10−6m GABA, which blocked release within minutes.
Statistical significance was determined by using Student'st test. Data are presented as means ± SEM.
Data acquisition and analysis. EPSC recordings were stored on a personal computer using an interface and patch-clamp software ISO-2 (M. Friedrich, Niedernhausen, Germany). Data were analyzed using pClamp (Axon Instruments, Foster City, CA) or ANA-3 in the ISO-2 program. Origin software (Microcal, Northampton, MA) was used for statistics and for the generation of histograms and of the dose–response curves for the two ω-toxins.
Analysis of mean quantal content of release was performed for EPSCs generated by the first of each twin pulse stimulus. Usually, 200–300 trials were analyzed. When quantal content was low, which is the case for the endings of SCE in the type I fibers, the number of quanta released by each impulse could be determined with a high degree of certainty. Mean quantal content (mc) of EPSCs was determined directly by counting the number of zero releases (failures) and, in the case of release, the individual quanta on the basis of averaged single quanta responses (miniatures), and relating them to the number of trials (Cooper et al., 1995). When quantal content was higher (up to 15 quanta per bouton), i.e., in the EPSCs to the second pulse to SCE and in the FCE responses, the mean quantal content (mp) was determined by dividing the peak amplitude of the EPSCs by the average of 40–50 miniature currents generated by spontaneous or late release of single quanta.
RESULTS
ω-Agatoxin-sensitive Ca2+channels are present in terminals of the slow and the fast axon
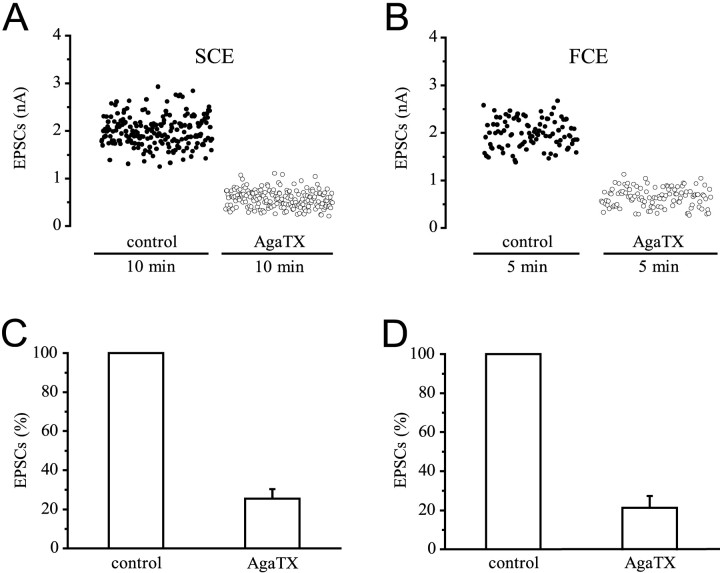
EPSCs of the slow and the fast axon are significantly reduced by ω-agatoxin IVA (ω-AgaTX). EPSCs after twin pulse stimulation of the slow axon SCE (Fig. 1A) and the fast axon FCE (Fig.1B) in a type I fiber were recorded from a site where both axons had a release bouton under the macropatch electrode, and after direct single-pulse stimulation of a release bouton of the FCE on a type IV fiber (Fig. 1C). An example for the conduction of a typical experiment with stimulation first of the SCE, followed by stimulation of the FCE in a type I fiber, is given in Figure1D. The amplitudes of the EPSCs generated by the second pulse are plotted. After 20 min in a solution containing 10−8m ω-AgaTX, stimulation of the SCE was resumed for 7 min, followed by stimulation of the FCE for 5 min in the presence of toxin. Because the full blocking effect on EPSCs of the SCE was obtained only after 35 min, 60 min was allowed for equilibration in all other experiments. Application of ω-AgaTX reduced the EPSC amplitudes of both the SCE and FCE axon. Figure2 quantitatively shows results obtained from a typical experiment and a summary diagram for all ω-AgaTX experiments at a concentration of 10−8m that is close to saturation (Fig.3). When the controls were normalized, the reduction of mean EPSC amplitude was 74.6 ± 5% (p < 0.001; n = 10) at the SCE endings (Fig. 2C) and 78.8 ± 6.1% (p < 0.001;n = 11) at FCE endings (Fig. 2D).
Fig. 2.
Effect of 10−8mω-AgaTX on EPSC amplitudes of the SCE and FCE. A,B, Stimulation and recording as in Figure1D. After establishing the controls, toxin was added and present for 1 hr before stimulation and recording were resumed. ω-AgaTX reduced the EPSCs of both the SCE and FCE from a mean amplitude of 2–0.6 nA. C, Summary of 10 experiments (SCE). D, Summary of 11 experiments (FCE).
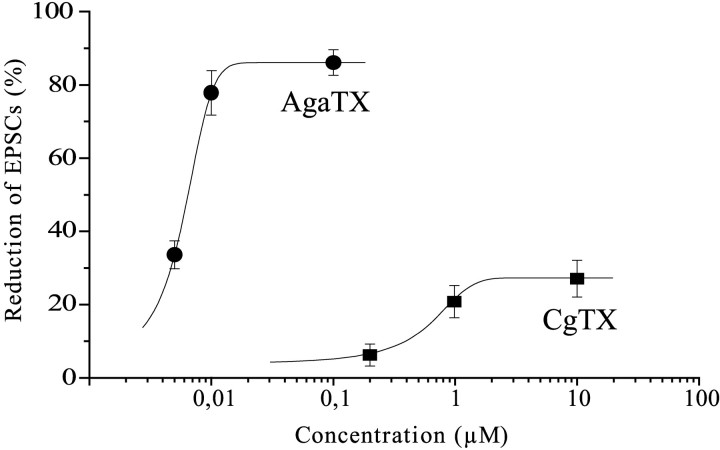
Fig. 3.
Dose–response curves for ω-agatoxin and ω-conotoxin determined for EPSCs elicited by the FCE. The curves were fit to data with the equation y = A1+ (A2 − A1)/(1 + 10∧(logX0 − X) ∗ p).
Similar results were also obtained with type N muscle fibers 7 and 8, which are innervated only by the FCE, both with ω-AgaTX and another toxin blocker of P/Q-type channels, FTX 3.3 (10−7m). Supporting results were obtained when mean quantal content of the EPSCs in type I fibers was analyzed. In the SCE, where two methods were used for analysis (see Materials and Methods), ω-AgaTX (10−8m) significantly reducedmc by 81.4 ± 3.2% andmp by 82.5 ± 3.2%, in the FCEmp was reduced by 73.1 ± 8.8% (p < 0.001; n = 8) (Table1).
Table 1.
Distribution of P/Q-, N-, and R-like Ca2+channels and participation in transmitter release (%) at neuromuscular junctions of the SCE and the FCE in the crab Eriphia, deduced from effects of 10−8m ω-AgaTX, 10−6m ω-CgTX, and Ni2+ on mean quantal content of transmitter released and on amplitude of EPSCs (I)
| Parameter | SCE | FCE | ||||
|---|---|---|---|---|---|---|
| P/Q-like | N-like | R-like | P/Q-like | N-like | R-like | |
| mc (%) | 81.4 ± 3.2 | 4.5 ± 1.8 | 36.1 ± 8.7 | |||
| (n = 8) | (n = 7) | (n = 4) | ||||
| mp (%) | 82.5 ± 3.2 | 5.6 ± 1.8 | 37.1 ± 8.2 | 73.1 ± 8.8 | 29.7 ± 7.9 | 15.0 ± 6.4 |
| (n = 8) | (n = 7) | (n = 6) | (n = 8) | (n = 11) | (n = 5) | |
| I (nA) (%) | 73.5 ± 4.9 | 2.3 ± 1.5 | 35.0 ± 3.9 | 77.8 ± 6.1 | 20.6 ± 4.4 | 13.0 ± 3.8 |
| (n = 10) | (n = 7) | (n = 6) | (n = 11) | (n = 10) | (n = 6) | |
Quantal content was determined by counting quanta (mc) and by analysis of peak of EPSCs (mp). Data show mean ± SEM. Statistically insignificant data (p > 0.05) are shown in italics. Uncertainty regarding the specificity of the blockers, particularly Ni2+ (see Results), could explain the deviation from 100%.
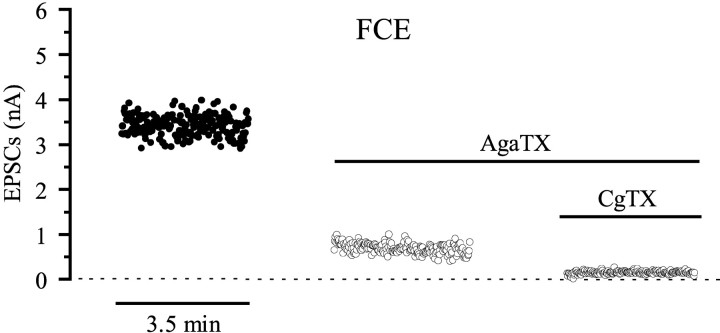
The data show an almost equal and prominent contribution of ω-AgaTX-sensitive channels on transmitter release from the two types of axons. A dose–response curve for ω-AgaTX was obtained at three different concentrations by determining the amplitude reduction of EPSCs elicited by the FCE. The reduction measured 33.6 ± 3.8% (n = 3) with 5 × 10−9m, 77.8 ± 6.1% (n = 11) with 10−8m, and saturated at 86.1 ± 3.5% (n = 6) with 10−7m ω-AgaTX (Fig. 3). Even at saturating toxin concentration, on average 14% of the release remained unaffected, suggesting that it is mediated by channels insensitive to ω-AgaTX. The calculated EC50 value was 5.6 nm. Figure 4 shows qualitatively that the fraction of release that is unblocked at the saturating concentration of 10−7m ω-AgaTX is almost completely abolished by adding ω-CgTX in the presence of ω-AgaTX.
Fig. 4.
Additive effects two ω-toxins. Stimulation and recording as in Figure 1D. Blocking P/Q-like channels with a saturating dose of 10−7m ω-AgaTX reduced the mean amplitude of EPSCs of the FCE from 3.4 to 0.7 nA. Blocking additionally N-like channels by ω-CgTX (10−6m) further decreased the mean amplitudes to almost zero (0.15 nA).
ω-Conotoxin-sensitive Ca2+ channels are present in the terminals of the fast, but not of the slow axon
In experiments identical to that shown in Figure 2, with both an SCE and FCE bouton under the same macropatch electrode and selective stimulation of either the SCE or the FCE, application of ω-conotoxin GVIA (ω-CgTX, usually 10−6m, equilibration time usually 45 min) resulted in small or no effects on the EPSC amplitudes of the SCE, but in a clear reduction of those of the FCE. The absence of significant effects on the SCE was also seen at saturating toxin concentration of 10−5m. A dose–response curve for ω-CgTX was obtained for three concentrations (Fig. 3). The amplitude reduction of EPSCs elicited by the FCE amounted to 6.1 ± 3% (n = 3) for 2 × 10−7m, 20.6 ± 4.4% (n = 10) for 10−6m, and 27.1 ± 5% (n = 3) for 10−5m ω-CgTX, giving an EC50 value of 0.5 μm.
An example of a typical experiment is given in Figure5, A and B. Pooling the data from seven experiments (Fig. 5C,D) showed that the effect of ω-CgTX on the SCE was always very small and statistically not significant (reduction by 2.3 ± 1.5%; p > 0.05), whereas the reduction of the mean EPSC amplitudes of the FCE was statistically significant (p < 0.001). A similar result was obtained for FCE endings on the type IV fibers. ω-CgTX (10−6m) reduced EPSC amplitudes in these fibers by 24.9 ± 6% (p < 0.001; n = 3; data not shown).
Fig. 5.
Effect of 10−6mω-CgTX on EPSC amplitudes of the SCE and FCE. A,B, Stimulation and recording as in Figure1D. Toxin was present for 45 min before stimulation and recording were resumed. ω-CgTX affected the amplitude of the EPSCs of the SCE insignificantly (mean amplitude 3.1 nA in both samples), but reduced the EPSCs of the FCE from a mean of 6.1 nA in the control to 4.6 nA. C, Summary of seven experiments for the SCE. D, Summary of 10 experiments for the FCE.
Analysis of the mean quantal content (mp) of EPSCs showed a clear effect of ω-CgTX in the FCE (reduction by 29.7 ± 7.9%; p< 0.001; n = 11), whereas neithermc normp values for the SCE were significantly affected (Table 1).
Ni2+-sensitive Ca2[supi]+ channels are prominent in terminals of the slow axon, but less distinct in the fast axon
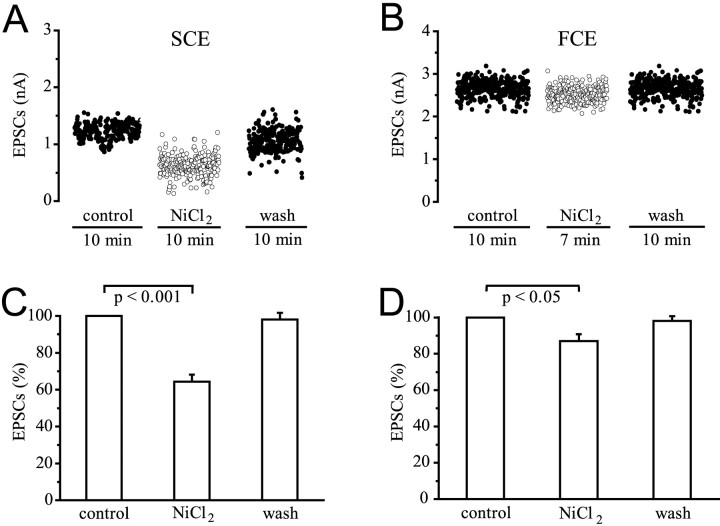
NiCl2 in low concentrations is a selective blocker of R-type Ca2+ channels in mammalian neurons. At higher concentrations, it blocks all types of Ca2+ channels. In our experiments, Ni2+ (2 × 10−6 to 6 × 10−4m) always had effects on the EPSC amplitudes of the SCE starting 5 min after application, but the concentrations required varied. In all experiments, the reduction of the EPSC amplitudes by Ni2+ was statistically highly significant in the SCE (35.7 ± 3.9%;p < 0.001; n = 6). A small reduction of mean EPSC amplitudes (13 ± 3.8%; p < 0.05;n = 6) was obtained for the FCE too, but it was inconsistent and statistically less significant. Similar results were obtained by determining mean quantal contentmp from the peak of EPSCs of the FCE and SCE, or, in the case of the SCE,mc by counting single quanta (Table1). In each individual experiment, the effects on the FCE were always much smaller than on the SCE. The differences between SCE and FCE values are statistically significant (p < 0.05). Figure 6, A andB, shows results from one particular experiment where low concentrations of Ni2+ had no effect at all, but a concentration as high as 10−3m significantly affected only the EPSC amplitudes of the SCE. Figure 6, C and D, gives a summary of six experiments using lower concentrations, with the amplitude of the control EPSCs normalized. The effect of Ni2+ was largely reversible after 20 min of washing with saline.
Fig. 6.
Effect of NiCl2 on EPSC amplitudes of the SCE and FCE. A, B, Stimulation and recording as in Figure 1D. 10−3m Ni2+ reduced the EPSCs of the SCE from a mean of 1.2–0.6 nA, and those of the FCE insignificantly from a mean of 2.6–2.5 nA. Washing for 20 min reversed the effect of Ni2+, although recovery was not complete during the period of recording. C,D, Summary of six experiments for the SCE (C) and FCE (D).
The modulation of transmitter release by the peptide DF2 involves ω-conotoxin-sensitive Ca2+ channels
As many as 12 LRFamide-like peptides have been identified in crustaceans (Weimann et al., 1993; Sithigorngul et al., 1998,2001; Mercier et al., 2001), of which four have been shown to modulate transmitter release from neuromuscular endings in crayfish and lobster (Kravitz et al., 1980; Mercier et al., 1990; Skerrett et al., 1995;Worden et al., 1995; Jorge-Rivera and Marder, 1996; Friedrich et al., 1998). Among them is DRNFLRFamide, also referred to as DF2, which enhances junction potential amplitudes by increasing the number of transmitter quanta released (Skerrett et al., 1995). DF2 was used in the present study.
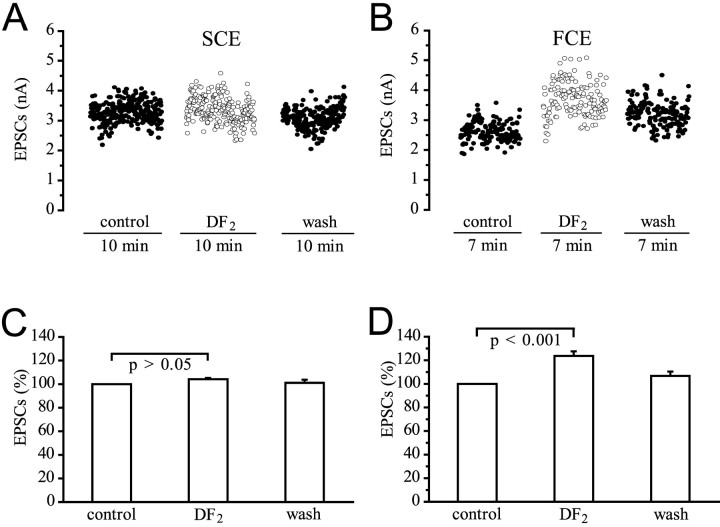
DF2 (5 × 10−7to 10−6m) always significantly potentiated release at endings of the FCE, but surprisingly had no statistically significant effect on EPSCs of the SCE. Figure 7, A andB, shows an example of a typical experiment with selective stimulation of either the SCE or the FCE when their EPSCs were recorded through a macropatch electrode from the same site. In this experiment, the average amplitude of the FCE remained higher after washing than in the controls. Figure 7, C and D, gives a summary of all experiments. DF2 affected the EPSC amplitudes of the SCE insignificantly. The amplitude increase was only 4.2 ± 1.1% (p > 0.05; n= 7), but the EPSC amplitudes of the FCE were increased significantly by 23.8 ± 3.9% (p < 0.001;n = 8). The different effect of DF2 on SCE and FCE endings was also reflected in an analysis of the mean quantal content of the EPSCs of the SCE and FCE. In the SCE, mc was not significantly different from the controls (p > 0.05; n = 7), but in the FCE,mp was increased by 19.3 ± 4.2% (p < 0.01; n = 8; data not shown).
Fig. 7.
Effect of the peptide DF2(10−6m) on EPSC amplitudes of the SCE and FCE. A, B, Stimulation and recording as in Figure 1D. DF2 had little effect on mean amplitude of EPSCs of the SCE (3.3 nA in the control, 3.4 nA in the presence of DF2, and 3.1 nA after washing), but increased the EPSCs of the FCE from a mean of 2.6–3.7 nA. C, Summary of seven experiments for the SCE.D, Summary of eight experiments for the FCE.
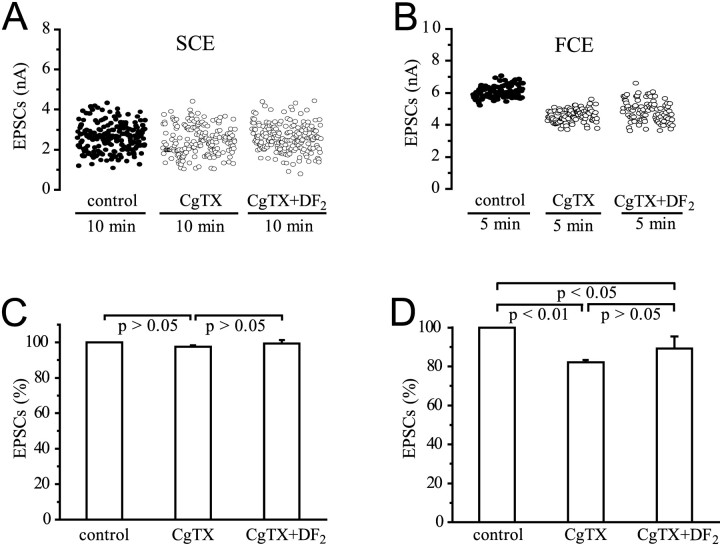
In the SCE, because of the absence of ω-CgTX-sensitive channels (see above), neither ω-CgTX by itself nor DF2 plus toxin had a significant effect on EPSC amplitudes (Fig.8A,C) (p > 0.05; n = 5). The absence of effects of DF2 on release from the SCE terminals suggests that either these terminals lack the receptor for this peptide or the peptide is effective only at terminals endowed with ω-CgTX-sensitive Ca2+ channels. At the FCE terminals, ω-CgTX reduced mean EPSC amplitudes by 17.9 ± 1.3% (p < 0.01; n = 5) (Fig.8D), and ω-CgTX and DF2together by 10.8 ± 6.2% (p < 0.05;n = 5). When amplitudes of EPSCs mediated by ω-CgTX-resistant release were normalized to the value before exposure to DF2, no significant increase was seen (p > 0.05; n = 5). Thus, the potentiation of EPSC amplitudes of the FCE by DF2(on average, ∼24%) (Fig. 7D) when ω-CgTX-sensitive channels were available was abolished by blocking these channels. The insignificant small potentiation occasionally observed could be attributable to the small fraction of N-like channels not being blocked at the concentration of 10−6m CgTX used in these experiments (see dose–response curve in Fig. 3).
Fig. 8.
Effect of the peptide DF2(10−6m) on EPSC amplitudes of the SCE and FCE in the presence of ω-CgTX (10−6m). Equilibration time for the toxin was 45 min.A, B, Stimulation and recording as in Figure 1D. In the SCE, neither the toxin nor DF2 had an effect (mean amplitudes of EPSCs 2.5 nA in the control, 2.4 in the presence of toxin, and 2.5 when toxin and peptide were present together). Mean amplitude of EPSCs in the FCE was reduced by the toxin from 6.1 nA in the control to 4.6 nA. Addition of DF2 increased the amplitude to 4.9 nA. C,D, Summary of five experiments for the SCE (C) and FCE (D).
Blocking the P/Q-like channels with ω-AgaTX reduced EPSC amplitudes elicited by the SCE (Fig. 2). In the experiments shown in Figure9, the average reduction was 57 ± 10.3% (p < 0.001; n = 3). In the presence of toxin, DF2 had no potentiating effect on the EPSCs elicited by the SCE (Fig. 9A,C). The reduction of EPSC amplitudes was again 57 ± 11.3% (n = 3), a value identical to that without DF2. In the FCE terminals, application of DF2 to a preparation with the ω-AgaTX-sensitive channels blocked, resulted in a significant potentiation of the EPSCs (Fig. 9B,D). When the ω-AgaTX-resistant release was normalized and compared with ω-AgaTX-resistant release in the presence of DF2, the increase in the EPSC amplitudes by the peptide was 24.4 ± 7.7% (p < 0.05; n = 3) in the FCE. Taken together, the results indicate that the targets of DF2 signaling are the ω-CgTX-sensitive N-like channels and that ω-AgaTX-sensitive P/Q-like channels remain unaffected.
Fig. 9.
Effect of the peptide DF2(10−6m) on EPSC amplitudes of the SCE and FCE in the presence of ω-AgaTX (10−8m). Equilibration time for the toxin was 60 min.A, B, Stimulation and recording as in Figure 1D. In the SCE, the toxin reduced mean amplitude of EPSCs from 1.6 to 0.5 nA. DF2 had no potentiating effect. In the FCE, the toxin reduced the mean amplitude of EPSCs from 2.1 in the control to 0.6 nA. Application of DF2 in the presence of ω-AgaTX still led to potentiation of release, with doubling the mean EPSC amplitude to 1.2 nA.C, D, Summary of three experiments for the SCE (C) and the FCE (D).
The modulation of transmitter release by the peptide proctolin depends on ω-agatoxin-sensitive Ca2+ channels and does not involve ω-conotoxin-sensitive channels
The pentapeptide proctolin (amino acid sequence RYLPT) is widely distributed in the nervous system of crustaceans. Besides its well known postsynaptic effects, including modulation of the sarcolemmal L-type Ca2+ channels (Rathmayer et al., 2001), proctolin also enhances transmitter output at neuromuscular terminals in crustaceans (Pasztor and Golas, 1993; Jorge-Rivera et al., 1998; Rathmayer et al., 2001).
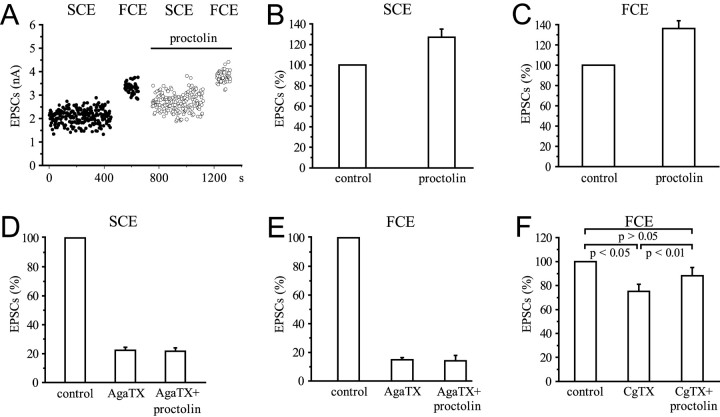
In our study, proctolin (10−6m) significantly (p < 0.001) increased the amplitudes of EPSCs generated by both the SCE and FCE. The EPSC amplitudes of the SCE were increased by 27 ± 7.9% (n = 6), and those of the FCE were increased by 36.3 ± 7.5% (n = 6) (Fig.10A–C). The absence of any effect on the amplitude of single quanta and the increase in mean quantal content mc of EPSCs in the SCE by 27.2 ± 7.9% (n = 3; data not shown) show that this effect is presynaptic. Blocking the ω-AgaTX-sensitive channels prevented the potentiation of release by proctolin in both SCE and FCE endings (Fig. 10D,E). ω-AgaTX reduced the amplitude of EPSCs of the SCE by 77.6 ± 2% (n = 3), of the FCE by 85.3 ± 1.6% (n = 3). Application of proctolin in the presence of the toxin did not change this reduction significantly: the amplitude of the EPSCs of the SCE remained reduced by 78.3 ± 2.3% (n = 3), and those of the FCE remained reduced by 86 ± 3.7% (n = 3). However, blocking the N-like channels with ω-CgTX, which reduced the EPSC amplitudes of the FCE significantly by 24.9 ± 6% (p < 0.05; n = 3) (Fig. 10F), still permitted a potentiation of release by proctolin. When amplitudes of proctolin-potentiated EPSCs mediated by ω-CgTX-resistant channels (P/Q-like channels) were normalized to the value before application of the peptide, the resulting increase by 17.6 ± 1.1% (p < 0.01; n = 3) (Fig. 10F) was significant. This shows that at terminals with the N-like channel blocked, proctolin can still enhance release by its action on the P/Q-like channels, whereas blocking of the P/Q-like channels prevents modulation of release by this peptide. This leads to the conclusion that the potentiating effect of proctolin depends on the availability of ω-AgaTX-sensitive Ca2+ channels.
Fig. 10.
Effect of proctolin (10−6m) on EPSC amplitudes in the presence of ω-AgaTX and ω-CgTX. A, Stimulation and recording as in Figure1D. B, C, Effect of proctolin on the SCE and FCE. Summary of six experiments.D, E, No effects of proctolin after blocking P/Q-like channels with ω-AgaTX (10−8m) in the SCE and FCE. Summary of three experiments.F, Blocking N-like channels by ω-CgTX (10−6m) in the FCE does not prevent the potentiating effect of proctolin. Summary of three experiments.
DISCUSSION
The specific blocking by ω-toxins is an important and well established criterion for characterizing different Ca2+ channel subtypes in mammalian nervous systems (Olivera et al., 1994). The ω-toxins have also been widely used for the classification of invertebrate channels, including those of crustaceans. However, because no Ca2+channel has yet been sequenced in crustaceans, and the molecular and electrophysiological correspondence to the vertebrate subtype profiles is not established (for review, see Kits and Mansvelder, 1996; Skeer et al., 1996; Jeziorski et al., 2000), one should be cautious in applying the mammalian channel classification. Invertebrate Ca2+ channels, defined only by pharmacological criteria derived from mammalian studies, may be reclassified when differences in their peptide sequence become apparent. We chose to term the subtypes involved in release at crustacean neuromuscular junctions according to their specific sensitivity to blockers ω-agatoxin-sensitive or ω-conotoxin-sensitive channels, which, pharmacologically, resemble vertebrate P/Q- or N-types. We also refer to P/Q- or N-like channels for what is called P/Q-or N-type in vertebrate studies.
Our finding that two functionally different types of motor axons innervating the same muscle in the crab Eriphia are endowed with different sets of Ca2+ channel types and that the observed differential effects of two peptides could be based on these differences are not affected by this general uncertainty.
Different Ca2+ channel types are differentially colocalized at SCE and FCE terminals
The predominant role of P/Q-like channels in transmitter release observed in our study is in accord with results from crayfish and crab (Araque et al., 1994; Blundon et al., 1995; Wright et al., 1996; Hong and Lnenicka, 1997; Hurley and Graubard, 1998) and mammals, but in the rat, motor terminals at some muscles also contain a small fraction of N-type channels (Westenbroek et al., 1998). In frog and lizard neuromuscular synapses, N- or L-type channels mediate transmission (Lindgren and Moore, 1989; Katz et al., 1995; Arenson and Gill, 1996).
We show that application of ω-AgaTX resulted in up to 85% inhibition of release in both SCE and FCE terminals. The EC50 value of 5.6 nm calculated from the dose–response curve for ω-AgaTX is lower than reported for stomatogastric neurons of a crab (Hurley and Graubard, 1998), but similar to those for P/Q-type channels in rat cerebellar neurons (Randall and Tsien, 1995) and cockroach neurons (Benquet et al., 1999). This proves the eminent role of the ω-AgaTX-sensitive channel, resembling vertebrate P/Q-type Ca2+channels, at both neurons, and the involvement of additional, ω-AgaTX-insensitive channels, in release, although to a lesser extent. Our study is the first demonstration that two neurons innervating the same muscle coexpress several Ca2+ channel types differentially. We show that, in addition to ω-AgaTX, ω-CgTX, a blocker of vertebrate N-type channels, also inhibits release at endings of the FCE, but not of the SCE. The existence of N-type channels was reported for a motor neuron innervating abdominal muscles in lobster (Grossman et al., 1991). Although its physiological type was not stated, it is likely a fast-type neuron because of its high output terminals. Effects of ω-CgTX were not observed in recent studies of motor neurons in crustaceans (Araque et al., 1994; Wright et al., 1996; Hurley and Graubard, 1998). This led to the conclusion that N-like channels are not involved in neuromuscular transmission in crustaceans. However, two of the studies were performed on the opener muscle of crayfish, which receives excitatory innervation through a single motor neuron. Perhaps this neuron functionally resembles a slow rather than a fast type with consequences for the type of presynaptic Ca2+ channels expressed.
In endings of the SCE of Eriphia, another type of Ca2+ channel is colocalized with the ω-AgaTX-sensitive channel. This channel is insensitive to ω-CgTX. Because it is blocked by low concentrations of Ni2+, it fits the classification of vertebrate R-type channels. There is no other explicit report on the occurrence of R-like channels at crustacean neuromuscular junctions, but one paper mentions a small reduction of EPSC amplitudes at lobster neuromuscular junctions at micromolar Ni2+ concentrations (Grossman et al., 1991). In Eriphia, minute effects of Ni2+ were sometimes also observed on release from the FCE. In all experiments, the inhibition by Ni2+ was much stronger in terminals of the SCE than in the FCE. We could not determine if the effect on EPSCs of the FCE was attributable to a blocking of channels other than R-like because the concentration of Ni2+ might not have been low enough for a selective effect. A small population of R-like channels present in the FCE endings cannot be ruled out.
Although ω-toxins can be used to identify the existence of different Ca2+ channel types and to investigate their contribution to transmitter release, the percentage of inhibition exerted by different blockers does not truly reflect the fraction of various channel types involved in the release. The efficacy of channels depends on their location in the terminal. Channels in the immediate vicinity of release sites have a higher effectiveness than channels more distant, such as R- and probably also N-type channels (Wu et al., 1999; Qian and Noebels, 2001). In addition, at least in crayfish slow and fast neuromuscular terminals, the Ca2+sensitivity of the release seems to differ (Msghina et al., 1999).
Peptidergic modulation of transmitter release is axon type-specific and involves different types of Ca2+ channels
FMRFamides enhance transmitter release at crustacean neuromuscular junctions (Kravitz et al., 1980; Mercier et al., 1990; Skerrett et al., 1995; Worden et al., 1995; Jorge-Rivera and Marder, 1996; Friedrich et al., 1998). Our finding that one of the FMRFamides, DF2, is effective in modulating release in the fast but not in the slow neuron innervating the same muscle, is new, and makes generalized statements on the role of modulators precarious. In previous studies, the physiological type of the neuron investigated was not considered.
The potentiating effect of proctolin on release at neuromuscular junctions of Eriphia is in accord with previous findings in crustaceans (Pasztor and Golas, 1993; Jorge-Rivera et al., 1998;Rathmayer et al., 2001). We show that the presynaptic targets of this modulation are ω-AgaTX-sensitive Ca2+channels resembling the P/Q-type. They are present in both types of axons, which explains why proctolin is effective on both axon types.
Modulation of Ca2+ channels by peptides occurs mainly through phosphorylation downstream of the activation of G-protein-dependent or -independent cascades (for review, see Dolphin, 1995; Kits and Mansvelder, 1996; Meir et al., 1999) or direct gating of channels (Cottrell, 1997). Generally, the major target for the modulation in invertebrates and vertebrates are neuronal N-type, in some cases also P/Q-type, but not T-type channels (Kits and Mansvelder, 1996; Wu and Saggau, 1997; Sun and Dale, 1999). In crustacean muscle fibers, L- type Ca2+ channels are one target of postsynaptic peptidergic modulation.
At neuromuscular junctions of Eriphia, the peptide DF2 potentiates release only at the terminals of the FCE axon. This could be attributable to the fact that only FCE endings are endowed with a receptor for this peptide or that modulation is targeted to ω-CgTX-sensitive channels. A selective modulation of N-type channels by FMRFamide has been reported for a neuroneuronal synapse of Aplysia (Fossier et al., 1994). Unlike DF2, the peptide proctolin increases transmitter release in Eripha by modulating the ω-AgaTX-sensitive channel resembling vertebrate P/Q-type, whereas the N-like channel is insensitive to it. In addition to these presynaptic effects, proctolin postsynaptically modulates the sarcolemmal L-type Ca2+ channels (Rathmayer et al., 2001) and non-voltage-dependent K+ channels (Erxleben et al., 1995). It also modulates the degree of phosphorylation of an actin filament-associated protein (Brüstle et al., 2001).
Functional significance of differential peptidergic modulation
Neuropeptides permit a large variety of modes to modulate properties of neurons and other target cells, e.g., by altering the strength of synaptic transmission and thus influencing intercellular communication. In nervous systems, this ensures plasticity of neuronal discharge patterns and the configuration and selection of circuits that enable specific motor behaviors (for literature on crustaceans, seeHarris-Warrick and Marder, 1991; Marder and Calabrese, 1996). These central effects of modulators are often enhanced by additional effects of the same peptides in the periphery, e.g., at the heart or at neuromuscular targets, where they can effectively alter the efficacy of motor patterns.
One strategy of achieving specificity in this modulation is the colocalization of peptides with classic transmitters and the release of distinct cotransmitter complements (Blitz et al., 1999; Wood et al., 2000) (for review, see Nusbaum et al., 2001). Another strategy of achieving specificity in peptidergic actions is the axon type-specific modulation of the efficacy of discharge patterns of motor neurons at the target cells. The release of proctolin should result in widespread modulation because it is effective at the terminals of both slow and fast motor neurons, whereas the release of DF2will enhance the efficacy of transmission only at endings of fast neurons. The molecular basis for this differential effect could be the modulation of different types of Ca2+channels in the terminals of these two types of motor neurons.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (Grants Ra 113/8-3 and 9-2). We gratefully acknowledge the support of the German Academic Exchange Service to A.G. We thank Dr. C. Erxleben for helpful comments on this manuscript, M. A. Cahill for correcting the English, and Prof. A. deSantis and C. Zazo of the fishermen crew of the Stazione Zoologica Naples for help in obtaining the animals.
Correspondence should be addressed to Prof. Dr. Werner Rathmayer, University of Konstanz, Faculty of Biology, Fach M 623, D-78457 Konstanz, Germany. E-mail: werner.rathmayer@uni-konstanz.de.
Dr. Gaydukov is on leave from Moscow State University, Faculty of Biology, Moscow 119 899, Russia.
REFERENCES
- 1.Araque A, Clarac F, Buno W. P-type Ca2+ channels mediate excitatory and inhibitory synaptic transmitter release in crayfish muscle. Proc Natl Acad Sci USA. 1994;91:4224–4228. doi: 10.1073/pnas.91.10.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arenson MS, Gill DS. Differential effects of an L-type Ca2+ channel antagonist on activity- and phosphorylation-enhanced release of acetylcholine at the neuromuscular junction of the frog in vitro. Eur J Neurosci. 1996;8:437–445. doi: 10.1111/j.1460-9568.1996.tb01227.x. [DOI] [PubMed] [Google Scholar]
- 3.Atwood HL, Wojtowicz JM. Short-term and long-term plasticity and physiological differentiation of crustacean motor synapses. J Neurobiol. 1986;28:275–362. doi: 10.1016/s0074-7742(08)60111-7. [DOI] [PubMed] [Google Scholar]
- 4.Benquet P, Le Guen J, Dayanithi G, Pichon Y, Tiaho F. ω-AgaIVA-sensitive (P/Q-type) and -resistant (R-type) high-voltage-activated Ba2+ currents in embryonic cockroach brain neurons. J Neurophysiol. 1999;82:2284–2293. doi: 10.1152/jn.1999.82.5.2284. [DOI] [PubMed] [Google Scholar]
- 5.Bittner GD. Differentiation of nerve terminals in the crayfish opener muscle and its functional significance. J Gen Physiol. 1968;51:731–758. doi: 10.1085/jgp.51.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blitz DM, Christie AE, Coleman MJ, Norris BJ, Marder E, Nusbaum MP. Different proctolin neurons elicit distinct motor patterns from a multifunctional neuronal network. J Neurosci. 1999;19:5449–5463. doi: 10.1523/JNEUROSCI.19-13-05449.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blundon JA, Wright SN, Brodwick MS, Bittner GD. Presynaptic calcium-activated potassium channels and calcium channels at a crayfish neuromuscular junction. J Neurophysiol. 1995;73:178–189. doi: 10.1152/jn.1995.73.1.178. [DOI] [PubMed] [Google Scholar]
- 8.Bradacs H, Cooper RL, Msghina M, Atwood HL. Differential physiology and morphology of phasic and tonic motor axons in a crayfish limb extensor muscle. J Exp Biol. 1997;200:677–691. doi: 10.1242/jeb.200.4.677. [DOI] [PubMed] [Google Scholar]
- 9.Brüstle B, Kreissl S, Mykles DL, Rathmayer W. The neuropeptide proctolin induces phosphorylation of a 30 kDa protein associated with the thin filament in crustacean muscle. J Exp Biol. 2001;204:2427–2436. doi: 10.1242/jeb.204.15.2627. [DOI] [PubMed] [Google Scholar]
- 10.Chrachri A. Ionic currents in identified swimmeret motor neurones of the crayfish Pacifastacus leniusculus. J Exp Biol. 1995;198:1483–1492. doi: 10.1242/jeb.198.7.1483. [DOI] [PubMed] [Google Scholar]
- 11.Cooper RL, Stewart BA, Wojtowicz JM, Wang S, Atwood HL. Quantal measurement and analysis methods compared for crayfish and Drosophila neuromuscular junctions, and rat hippocampus. J Neurosci Methods. 1995;61:67–78. doi: 10.1016/0165-0270(95)00024-o. [DOI] [PubMed] [Google Scholar]
- 12.Cottrell GA. The first peptide-gated ion channel. J Exp Biol. 1997;200:2377–2386. doi: 10.1242/jeb.200.18.2377. [DOI] [PubMed] [Google Scholar]
- 13.Dolphin AC. Voltage-dependent calcium channels and their modulation by neurotransmitters and G proteins. Exp Physiol. 1995;80:1–36. doi: 10.1113/expphysiol.1995.sp003825. [DOI] [PubMed] [Google Scholar]
- 14.Dudel J. The effect of reduced calcium on quantal unit current and release at the crayfish neuromuscular junction. Pflügers Arch. 1981;391:35–40. doi: 10.1007/BF00580691. [DOI] [PubMed] [Google Scholar]
- 15.Dunlap K, Luebke JI, Turner TJ. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 1995;18:89–98. [PubMed] [Google Scholar]
- 16.Erxleben CFJ, DeSantis A, Rathmayer W. Effects of proctolin on contractions, membrane resistance, and non-voltage-dependent sarcolemmal ion channels in crustacean muscle fibers. J Neurosci. 1995;15:4356–4369. doi: 10.1523/JNEUROSCI.15-06-04356.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fossier P, Baux G, Tauc L. N- and P-type Ca2+ channels are involved in acetylcholine release at a neuroneuronal synapse: only the N-type channel is the target of neuromodulators. Proc Natl Acad Sci USA. 1994;91:4771–4775. doi: 10.1073/pnas.91.11.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedrich RW, Molnar GF, Schiebe M, Mercier AJ. Protein kinase C is required for long-lasting synaptic enhancement by the neuropeptide DRNFLRFamide in crayfish. J Neurophysiol. 1998;79:1127–1131. doi: 10.1152/jn.1998.79.2.1127. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Colunga J, Valdiosera R, Garcia U. P-type Ca2+ current in crayfish peptidergic neurones. J Exp Biol. 1999;202:429–440. doi: 10.1242/jeb.202.4.429. [DOI] [PubMed] [Google Scholar]
- 20.Grossman Y, Colton JS, Gilman SC. Interaction of Ca-channel blockers and high pressure at the crustacean neuromuscular junction. Neurosci Lett. 1991;125:53–56. doi: 10.1016/0304-3940(91)90129-h. [DOI] [PubMed] [Google Scholar]
- 21.Harris-Warrick RM, Marder E. Modulation of neural networks for behavior. Annu Rev Neurosci. 1991;14:39–57. doi: 10.1146/annurev.ne.14.030191.000351. [DOI] [PubMed] [Google Scholar]
- 22.Hong SJ, Lnenicka GA. Characterization of a P-type calcium current in a crayfish motoneuron and its selective modulation by impulse activity. J Neurophysiol. 1997;77:76–85. doi: 10.1152/jn.1997.77.1.76. [DOI] [PubMed] [Google Scholar]
- 23.Hoyle G, Wiersma CAG. Excitation at neuromuscular junctions in Crustacea. J Physiol (Lond) 1958;143:403–425. doi: 10.1113/jphysiol.1958.sp006068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurley LM, Graubard K. Pharmacologically and functionally distinct calcium currents of stomatogastric neurons. J Neurophysiol. 1998;79:2070–2081. doi: 10.1152/jn.1998.79.4.2070. [DOI] [PubMed] [Google Scholar]
- 25.Jeziorski MC, Greenberg RM, Anderson PAV. The molecular biology of invertebrate voltage-gated Ca2+ channels. J Exp Biol. 2000;203:841–856. doi: 10.1242/jeb.203.5.841. [DOI] [PubMed] [Google Scholar]
- 26.Jorge-Rivera JC, Marder E. TNRNFLRFamide and SDRNFLRFamide modulate muscles of the stomatogastric system of the crab Cancer borealis. J Comp Physiol [A] 1996;179:741–751. doi: 10.1007/BF00207353. [DOI] [PubMed] [Google Scholar]
- 27.Jorge-Rivera JC, Sen K, Birmingham JT, Abbott LF, Marder E. Temporal dynamics of convergent modulation at a crustacean neuromuscular junction. J Neurophysiol. 1998;80:2559–2570. doi: 10.1152/jn.1998.80.5.2559. [DOI] [PubMed] [Google Scholar]
- 28.Katz E, Ferro PA, Cherksey BD, Sugimori M, Llinas R, Uchitel OD. Effects of Ca2+ blockers on transmitter release and presynaptic currents at the frog neuromuscular junction. J Physiol (Lond) 1995;486:695–706. doi: 10.1113/jphysiol.1995.sp020845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King MJR, Atwood HL, Govind CK. Structural features of crayfish phasic and tonic neuromuscular terminals. J Comp Neurol. 1996;372:618–626. doi: 10.1002/(SICI)1096-9861(19960902)372:4<618::AID-CNE9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 30.Kits KS, Mansvelder HD. Voltage gated calcium channels in molluscs: classification, Ca2+ dependent inactivation, modulation and functional roles. Invertebr Neurosci. 1996;2:9–34. doi: 10.1007/BF02336657. [DOI] [PubMed] [Google Scholar]
- 31.Kravitz EA, Glusman S, Harris-Warrick RM, Livingstone MS, Schwarz T, Goy MF. Amines and a peptide as neurohormones in lobsters: actions on neuromuscular preparations and preliminary behavioural studies. J Exp Biol. 1980;89:159–175. doi: 10.1242/jeb.89.1.159. [DOI] [PubMed] [Google Scholar]
- 32.Lindgren CA, Moore JW. Identification of ionic currents at presynaptic nerve endings of the lizard. J Physiol (Lond) 1989;414:201–222. doi: 10.1113/jphysiol.1989.sp017684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lnenicka GA, Arcaro KF, Calabro JM. Activity-dependent development of calcium regulation in growing motor axons. J Neurosci. 1998;18:4966–4972. doi: 10.1523/JNEUROSCI.18-13-04966.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. Physiol Rev. 1996;76:687–717. doi: 10.1152/physrev.1996.76.3.687. [DOI] [PubMed] [Google Scholar]
- 35.Meir A, Ginsburg S, Butkevich A, Kachalsky SG, Kaiserman I, Ahdut R, Demirgoren S, Rahamimoff R. Ion channels in presynaptic nerve terminals and control of transmitter release. Physiol Rev. 1999;79:1019–1088. doi: 10.1152/physrev.1999.79.3.1019. [DOI] [PubMed] [Google Scholar]
- 36.Mercier AJ, Schiebe M, Atwood HL. Pericardial peptides enhance synaptic transmission and tension in phasic extensor muscles of crayfish. Neurosci Lett. 1990;111:92–98. doi: 10.1016/0304-3940(90)90350-i. [DOI] [PubMed] [Google Scholar]
- 37.Mercier AJ, Badhwar A, Weston AD, Klose M. Intracellular signals that mediate synaptic modulation by a FMRFamide-like neuropeptide in crayfish. In: Wiese K, editor. Crustacean nervous system. Springer; New York: 2001. pp. 49–62. [Google Scholar]
- 38.Msghina M, Govind CK, Atwood HL. Synaptic structure and transmitter release in crustacean phasic and tonic motor neurons. J Neurosci. 1998;18:1374–1382. doi: 10.1523/JNEUROSCI.18-04-01374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Msghina M, Millar AG, Charlton MP, Govind CK, Atwood HL. Calcium entry related to active zones and differences in transmitter release at phasic and tonic synapses. J Neurosci. 1999;19:8419–8434. doi: 10.1523/JNEUROSCI.19-19-08419.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen PV, Marin L, Atwood HL. Synaptic physiology and mitochondrial function in crayfish tonic and phasic motor neurons. J Neurophysiol. 1997;78:281–294. doi: 10.1152/jn.1997.78.1.281. [DOI] [PubMed] [Google Scholar]
- 41.Nusbaum MP, Blitz DM, Swensen AM, Wood D, Marder E. The roles of co-transmission in neural network modulation. Trends Neurosci. 2001;24:146–154. doi: 10.1016/s0166-2236(00)01723-9. [DOI] [PubMed] [Google Scholar]
- 42.Olivera BM, Miljanich GP, Ramachandran J, Adams ME. Calcium channel diversity and neurotransmitter release: The omega-conotoxins and omega-agatoxins. Annu Rev Biochem. 1994;63:823–867. doi: 10.1146/annurev.bi.63.070194.004135. [DOI] [PubMed] [Google Scholar]
- 43.Pasztor VM, Golas LB. The modulatory effects of serotonin, neuropeptide F1 and proctolin on the receptor muscles of the lobster abdominal stretch receptor and their exoskeletal muscle homologues. J Exp Biol. 1993;174:363–374. [Google Scholar]
- 44.Qian J, Noebels JL. Presynaptic Ca2+ channels, neurotransmitter release at the terminal of a mouse cortical neuron. J Neurosci. 2001;21:3721–3728. doi: 10.1523/JNEUROSCI.21-11-03721.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Randall AD. The molecular basis of voltage-gated Ca2+ channel diversity: is it time for T? J Membr Biol. 1998;161:207–213. doi: 10.1007/s002329900327. [DOI] [PubMed] [Google Scholar]
- 46.Randall AD, Tsien RW. Pharmacological dissection of multiple types of Ca2+ channel currents in rat cerebellar granule neurons. J Neurosci. 1995;15:2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rathmayer W, Djokaj S. Presynaptic inhibition and the participation of GABAB receptors at neuromuscular junctions of the crab Eriphia spinifrons. J Comp Physiol [A] 2000;186:287–298. doi: 10.1007/s003590050429. [DOI] [PubMed] [Google Scholar]
- 48.Rathmayer W, Erxleben C. Identified muscle fibers in a crab. I. Characteristics of excitatory and inhibitory neuromuscular transmission. J Comp Physiol. 1983;152:411–420. [Google Scholar]
- 49.Rathmayer W, Hammelsbeck M. Identified muscle fibers in a crab. II. Differences in facilitation properties. J Exp Biol. 1985;116:291–300. [Google Scholar]
- 50.Rathmayer W, Maier L. Muscle fiber types in crabs: studies on single identified muscle fibers. Am Zool. 1987;27:1067–1077. [Google Scholar]
- 51.Rathmayer W, Erxleben C, Djokaj S, Gaydukov A, Kreissl S, Weiss T. Antagonistic modulation of neuromuscular parameters in crustaceans by the peptides proctolin and allatostatin, contained in identified motor neurons. In: Wiese K, editor. Crustacean nervous system. Springer; New York: 2001. pp. 2–19. [Google Scholar]
- 52.Richmond JE, Sher E, Cooke IM. Characterization of the Ca2+ current in freshly dissociated crustacean peptidergic neuronal somata. J Neurophysiol. 1995;73:2357–2368. doi: 10.1152/jn.1995.73.6.2357. [DOI] [PubMed] [Google Scholar]
- 53.Richmond JE, Penner R, Keller R, Cook IM. Characterization of the Ca2+ current in isolated terminals of crustacean peptidergic neurons. J Exp Biol. 1996;199:2053–2059. doi: 10.1242/jeb.199.9.2053. [DOI] [PubMed] [Google Scholar]
- 54.Sithigorngul P, Saraithongkum W, Jaideechoey S, Longyant S, Sithigorngul W. Novel FMRFamide-like neuropeptides from the eyestalk of the giant freshwater prawn Macrobrachium rosenbergii. Comp Biochem Physiol [B] 1998;120:587–595. [Google Scholar]
- 55.Sithigorngul P, Saraithongkum W, Longyant S, Panchan N, Sithigorngul W, Petsom A. Three more novel FMRFamide-like neuropeptide sequences from the eyestalk of the giant freshwater prawn Macrobrachium rosenbergii. Peptides. 2001;22:191–197. doi: 10.1016/s0196-9781(00)00382-x. [DOI] [PubMed] [Google Scholar]
- 56.Skeer JM, Norman RI, Sattelle DB. Invertebrate voltage-gated calcium channel subtypes. Biol Rev. 1996;71:137–154. [Google Scholar]
- 57.Skerrett M, Peaire A, Quigley P, Mercier AJ. Physiological effects of two FMRFamide-related peptides from the crayfish Procambarus clarkii. J Exp Biol. 1995;198:109–116. doi: 10.1242/jeb.198.1.109. [DOI] [PubMed] [Google Scholar]
- 58.Sun QQ, Dale N. G-proteins are involved in 5-HT receptor-mediated modulation of N- and P/Q- but not T-type Ca2+ channels. J Neurosci. 1999;19:890–899. doi: 10.1523/JNEUROSCI.19-03-00890.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weimann JM, Marder E, Evans B, Calabrese RL. The effects of SDRNFLRFamide and TNRNFLRFamide on the motor patterns of the stomatogastric ganglion of the crab Cancer borealis. J Exp Biol. 1993;181:1–26. doi: 10.1242/jeb.181.1.1. [DOI] [PubMed] [Google Scholar]
- 60.Westenbroek RE, Hoskin L, Catterall WA. Localization of Ca2+ channel subtypes on rat spinal motor neurons, interneurons, and nerve terminals. J Neurosci. 1998;18:6319–6330. doi: 10.1523/JNEUROSCI.18-16-06319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wicher D, Walther C, Wicher C. Non-synaptic ion channels in insects. Prog Neurobiol. 2001;64:431–525. doi: 10.1016/s0301-0082(00)00066-6. [DOI] [PubMed] [Google Scholar]
- 62.Wood DE, Stein W, Nusbaum MP. Projection neurons with shared cotransmitters elicit different motor pattern from the same neural circuit. J Neurosci. 2000;20:8943–8953. doi: 10.1523/JNEUROSCI.20-23-08943.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Worden MK, Kravitz EA, Goy MF. Peptide F1, an N-terminally extended analog of FMRFamide, enhances contractile activity in multiple target tissues in lobster. J Exp Biol. 1995;198:97–108. doi: 10.1242/jeb.198.1.97. [DOI] [PubMed] [Google Scholar]
- 64.Wright SN, Brodwick MS, Bittner GD. Presynaptic calcium currents at voltage-clamped excitor and inhibitor nerve terminals of crayfish. J Physiol (Lond) 1996;496:347–361. doi: 10.1113/jphysiol.1996.sp021690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu LG, Saggau P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci. 1997;20:204–212. doi: 10.1016/s0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]
- 66.Wu L-G, Borst JGG, Sakmann B. R-type Ca2+ currents evoke transmitter release at a rat central synapse. Proc Natl Acad Sci USA. 1998;95:4720–4725. doi: 10.1073/pnas.95.8.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu LG, Westenbroek RE, Borst JGG, Catterall WA, Sakmann B. Calcium channel types with distinct presynaptic localization couple differentially to transmitter release in single calyx-type synapses. J Neurosci. 1999;19:726–736. doi: 10.1523/JNEUROSCI.19-02-00726.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]