Abstract
Introduction:
Studies have shown that women with obesity have longer labors. The purpose of this systematic review and meta-analysis is to examine existing evidence regarding labor induction in women with obesity, including processes and outcomes. The primary outcome was cesarean birth following labor induction. Secondary outcomes were the timing and dosage of prostaglandins, the success of mechanical cervical ripening methods, and synthetic oxytocin dose and timing.
Methods:
Searches were performed in PubMed MEDLINE, Embase, CINAHL, EBSCO, the Cochrane Database of Systematic Reviews, the Database of Abstracts of Effects, Google Scholar, and ClinicalTrials.gov. Searches were limited to studies published in English after 1990. Ten studies published between 2009 and 2017 were included in this review. All were observational studies comparing processes and outcomes of induction of labor in relation to maternal body mass index. The primary outcome was cesarean birth following labor induction. We assessed heterogeneity using Cochran’s Q test and tau-squared and I2 statistics. We also calculated fixed-effect models to estimate pooled relative risks and weighted mean differences.
Results:
Ten cohort studies met inclusion criteria; 8 studies had data available for a meta-analysis of the primary outcome. Cesarean birth was more common among women with obesity compared with women of normal weight following labor induction (Mantel-Haenszel fixed-effect odds ratio, 1.82; 95% CI, 1.55–2.12; P < .001). Maternal obesity was associated with a longer time to birth, higher doses of prostaglandins, less frequent success of cervical ripening methods, and higher dose of synthetic oxytocin, as well as a longer time to birth after oxytocin use.
Discussion:
Women with obesity are more likely than women with a normal weight to end labor induction with cesarean birth. Additionally, women with obesity require longer labor inductions involving larger, more frequent applications of both cervical ripening methods and synthetic oxytocin.
Keywords: obesity, induction of labor, oxytocin, prostaglandins, cervical ripening, transcervical catheters, intrapartum
INTRODUCTION
The rising prevalence of obesity in the United States over the past 3 decades has resulted in a much higher incidence of women becoming pregnant with a high body mass index (BMI).1 A recent report of US birth certificate data revealed that more than 50% of women who gave birth in 2014 had a prepregnancy BMI that was classified as either overweight (25.6%) or obese (24.8%).2 The incidence of maternal obesity is even higher among some racial and ethnic minority groups, with nearly half of Hispanic women (45.75%) and more than half of non-Hispanic black women (56.9%) being obese during childbearing years.3
Use of labor induction for all women in the United States has also increased significantly, rising from 9.9% in 1990 to 23% in 2008, and remains at this level today.4,5 Notably, rates of labor induction are highest among women with obesity.6 Use of labor induction increases in a dose-dependent manner with each increase in a woman’s BMI category.6,7 Higher maternal BMI is positively associated with complications such as gestational diabetes, hypertensive disorders of pregnancy, fetal macrosomia, and stillbirth in the third trimester.8 These complications in turn result in increased use of labor induction among women with obesity.8 In addition, women who are obese are less likely than women of normal weight to initiate spontaneous labor before 41 weeks’ gestation, placing them at higher risk for post-term pregnancy and labor induction.6,9
In all women, labor induction is associated with longer labor course, more dysfunctional labor patterns, increased use of interventions (epidural analgesia, invasive fetal monitoring, and instrumental or operative birth), and extended hospital stays.10–12 In addition, there is some evidence that induction of labor is more likely to be unsuccessful in women with an increased BMI.7 In a retrospective population cohort study of 80,887 women, women with a BMI of at least 40 kg/m2 had a 29% risk of an unsuccessful labor induction compared with a 13% risk among women of normal weight (odds ratio [OR], 2.73; 95% CI, 2.53–2.96).7
With several recent meta-analyses and clinical trials reporting a decreased risk of cesarean birth among women who have labor induction compared with expectant management, use of this intervention may increase in the future.13,14 However, we lack evidence regarding the most effective methods of labor induction among women who are obese and the risk of cesarean birth following labor induction in this population.15 The purpose of this systematic review and meta-analysis was to collect and examine existing evidence to estimate the influence of maternal obesity on labor induction processes and outcomes. The primary outcome was cesarean birth following labor induction. Secondary outcomes were prostaglandin dosage and timing, the success of mechanical cervical ripening methods, and synthetic oxytocin dosage and timing.
METHODS
We searched electronic databases including PubMed, MEDLINE, Embase, CINAHL, EBSCO, the Cochrane Database of Systematic Reviews, the Database of Abstracts of Effects, Google Scholar, and ClinicalTrials.gov. Primary Medical Subject Heading (MeSH) terms included obese, obesity, labor induced, labor induction methods, cervical ripening, oxytocin, transcervical catheter and prostaglandins. Also, artificial rupture of membranes and mechanical cervical ripening were searched. Searches took place in September and October of 2016, with a search of ClinicalTrials.gov following in February 2017. All searches were updated in September 2017.
Searches included English-language studies published between 1990 and March 2017. We limited the search to investigations published after 1990 because of the rapid increase in obesity among women and substantive changes in labor induction practices over the past several decades. The exposure for this review was labor induction. Observational studies that provided information about labor induction outcomes and/or cervical ripening outcomes stratified by maternal BMI were included. We included induction of labor with prostaglandins, transcervical catheters, oxytocin, and combinations of these methods. We did not include women undergoing induction of labor who had artificial rupture of membranes alone. The use of other methods for labor induction, such as stripping membranes and laminaria, were also not included. The primary outcome was cesarean birth following a trial of labor after induction. We also report secondary outcomes by the induction agent, including time to birth, the dose and timing of prostaglandins, achievement of active-phase labor, success of the cervical ripening attempt, and total dose and timing of oxytocin required for induction.
The initial search of the literature by the primary author was repeated using the same criteria by a university research librarian. Results of these 2 independent searches were combined (Figure 1).16 EndNote (Clarivate Analytics, Philadelphia, PA) was used to remove duplicates. Titles and abstracts were screened using inclusion criteria; then full-text articles of selected studies were retrieved. Any discrepancies in decisions regarding study inclusion were discussed by the authors until agreement was reached. The Newcastle-Ottawa scale was used to rate the quality of observational studies included in this review.17
Figure 1.
Flow Chart Describing Literature Extraction Process
Abbreviation: OR, odds ratio.
For the primary outcome of cesarean birth, the meta package in R version 4.8.1 (R Foundation, Vienna, Austria) was used to perform a Mantel-Haenszel method fixed-effect meta-analysis of the influence of maternal obesity on cesarean birth among women who were induced to calculate ORs. Fixed-effect analyses were selected over random-effects analyses because of the low number of studies available; statistical analyses are considered descriptive only.18 Presence of heterogeneity between studies was assessed with Cochran’s Q test; heterogeneity magnitude was assessed with tau-squared and I2 statistics. One study author was contacted for additional data for the analysis.19
For secondary outcomes, time to birth, the number of prostaglandin doses required, achievement of active-phase labor, success of cervical ripening attempt, and total dose and timing of the induction agent required for induction of labor were calculated. If an OR was not provided in the reviewed studies, we calculated one from raw data using MedCalc for Windows, version 17.2 (MedCalc Software, Ostend, Belgium).
RESULTS
The initial searches returned a total of 505 articles (Figure 1).16 Once duplicates were removed, 274 unique articles remained. Screening of the titles and abstracts to identify those that met the inclusion criteria of English language articles, articles pertaining to induction of labor, and peer-reviewed published literature from 1990 to September 2017 resulted in the exclusion of 215 articles. Full-text copies of the remaining 59 articles were obtained and analyzed again for eligibility. An additional 49 studies were excluded for the following reasons: not focused on induction of labor (n = 27), BMI not stratified (n = 12), no details for induction of labor protocol (n = 7), included only nulliparous women (n = 2), used nonstandard BMI categorizations (n = 1). Ten studies remained for the analysis.19–28
The final group for the systematic review was 10 studies that included a total of 7881 women. Eight of the 10 studies reporting outcomes of cesarean births were included in the meta-analysis (n = 5450 participants). Five of the included studies were conducted in the United States;21,22,24,26,28 the remaining studies were carried out in Israel,20,25 Ireland,27 France,23 and Canada19 (Table 1). The studies included in this review scored between 7 and 9 stars on the Newcastle-Ottawa scale, indicating high quality.17 Cochran’s Q statistic for the studies included in the meta-analysis indicated minimal heterogeneity between studies (Q [7] = 7.56; P > .05; tau-squared = 0.00; I2 = 7.4% [0%–70.0%]).
Table 1.
Description of Included Studies
| Study Year Country | Sample n | Methodology | Inclusion Criteria | Time of BMI Determination and Classification | Induction Method Used and Dosage Schedule | Labor Induction Protocol | Definition of Onset of Active Labor | Study Outcomes |
|---|---|---|---|---|---|---|---|---|
| Anabusi et al20 2016 Israel | 181 | Cohort study | Singleton, cephalic, intact membranes, term (≥ 37 wk gestation), with an unfavorable cervix, planning induction | Hospital admission | Cook or Foley catheter | Catheter remained in place for up to 12 h | 4 cm | Time from device insertion to birth |
| Prostaglandins (if first attempt was unsuccessful) | Ripening continued to Bishop score increase of 2 points or >3 cm dilation | Successful cervical ripening | ||||||
| Cesarean birth rate | ||||||||
| Maternal and neonatal adverse events | ||||||||
| Beckwith et al22 2017 United States | 709 | Retrospective cohort | Singleton, live birth, nonanomalous fetus/newborn, induced labor | Hospital admission | Misoprostol 25 mcg | Ripening continued to Bishop score >5 | 6 cm | Primary: failure to achieve active labor |
| Foley bulb inflated to 30 mL, accompanied by oxytocin | Secondary: cesarean birth rate, doses of misoprostol used, need for protocol deviation | |||||||
| Gauthier et al23 2012 France | 285 | Retrospective cohort with matching | Singleton, cephalic, live birth, term (>37 wk gestation), one previous cesarean, no contraindications to vaginal birth, Bishop score <6, >18 y of age | First prenatal visit | Dinoprostone 10 mg for 12 h if Bishop score <3 or 1 mg if Bishop score 4–6 | Ripening to Bishop score of >6 | Bishop score >6 | Primary: first cervical ripening attempt unsuccessful |
| Continuing for up to 3 days | ||||||||
| Lassiter et al24 2016 United States | 329 | Retrospective cohort | All women undergoing induction at the research site, gestational age ≥37 wk, Bishop score <5 | Hospital admission | Misoprostol 25 mcg | Ripening continued until favorable cervix; then oxytocin was started | Favorable cervix | Primary: time to birth |
| Oxytocin 1 milliunit per min, increased 1–2 milliunits every 30 min | Secondary: number of doses of misoprostol, duration of oxytocin, cesarean birth | |||||||
| Maeder et al28 2017 United States | 280 | Retrospective cohort | Singleton, cephalic, documented weight and height at initiation of prenatal care and labor admission, labor induction, oxytocin | Hospital admission | Oxytocin at 1–2 milliunits per min, increased every 15–30 min | Cervical ripening to 3–4 cm | Not reported | Primary: total oxytocin |
| Secondary: length of labor, method of birth | ||||||||
| Oxytocin per protocol 1–2 milliunits, maybe increased every 15–30 min | ||||||||
| Melamed et al25 2010 Israel | 488 | Retrospective cohort | Singleton, cephalic, one previous cesarean, no contraindication for vaginal birth | First prenatal visit | Dinoprostone 3 mg | Ripening continued until Bishop score >7 | Bishop score ≥7 | Primary: failure of cervical ripening with prostaglandin |
| Oxytocin 2.5 milliunits per min, increased by 2.5 milliunits per min every 20 min | ||||||||
| Oxytocin if prostaglandin unsuccessful | ||||||||
| O’Dwyer et al27 2013 Ireland | 1927 | Prospective cohort | Singleton pregnancy in the first trimester, Northern European race, >18 y of age, no gestational diabetes mellitus | On enrollment (in the first trimester) | Prostaglandin and oxytocin | Ripening continued to favorable cervix | Favorable cervix | Primary: mode of birth, obstetric outcomes |
| Induction of labor with amniotomy then oxytocin | ||||||||
| Pevzner et al21 2009 United States | 1273 | Cohort study | Singleton pregnancy, ≥36 wk gestation, ≥ 18 y of age, low parity (≤3 previous births) | Hospital admission | Misoprostol 100 or 50 mcg | Ripening protocol continued for 24 h or until active labor | 4 cm | Primary: active labor |
| Secondary: total oxytocin for induction, birth in <24 h, type of birth | ||||||||
| Dinoprostone 10 mcg42 | ||||||||
| Roloff et al26 2015 United States | 413 | Retrospective cohort | Viable pregnancy with a singleton, cephalic at term (37–42 wk gestation) | Hospital admission | Cook or Foley catheter | Cervical ripening agent administered every 4–6 h as needed at the discretion of the attending physician | 6 cm | Primary: cumulative oxytocin needed for vaginal birth |
| Prostaglandins (if first attempt was unsuccessful) | ||||||||
| Vinturache et al19 2014 Canada | 1996 | Retrospective cohort | Term singleton pregnancies, participation in All Our Babies cohort study | On enrollment (before 25 wk gestation) | Misoprostol 25 mcg Foley bulb inflated to 30 mL, accompanied by oxytocin use | No timing of interventions or Bishop score reported | Not reported | Primary: type of birth Secondary: obstetric outcomes |
Abbreviation: BMI, body mass index.
Body Mass Index Categories
The timing of BMI measurement differed between studies (Table 1), including prepregnancy,22 the first prenatal visit,23,25 prenatal enrollment in the first trimester,27 perinatal enrollment before 25 weeks’ gestation,19 and the time of labor admission.19–22,24,26 Beckwith et al22 were the only investigators who analyzed outcomes using both the prepregnancy BMI and the time of hospital admission BMI with the rationale that a difference in effect could be due to a difference in volume distribution at the time of hospital admission. In addition, obesity was defined differently in several of the included studies (Tables 2, 3, and 4). Most of the studies used standard BMI categories defined by the Institute of Medicine29 or the World Health Organization,30 but 3 studies20,22,26 included only obese and nonobese categories, using a BMI of 30 kg/m2 to separate the categories. In one of the included studies, the normal and overweight BMI categories were described but used differently in the analysis.27
Table 2.
Primary Outcome-Cesarean Birth: Odds of Having a Cesarean Birth Among Women with Obesity Undergoing Labor Induction
| Study, Year | Indication for Cesarean Birth | BMI Ranges, kg/m2 | Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Anabusi et al, 201620 | Not reported | ≤30 | 1 | |
| >30 | 1.58 (0.77–3.24)a | .21 | ||
| Beckwith et al, 201722 | Failure to reach active labor prior to 5 cm dilatation | <30 | 1 | |
| >30 | 1.4(1.01–1.94) | .04 | ||
| Gauthier et al, 201223 | Not reported | 20–25 | 1 | |
| ≥30 | 2.16 (1.25–3.73) | .005 | ||
| Lassiter et al, 201624 | Not reported | ≤30 | 1 | |
| 30–39.9 | 2.78 (1.52–5.10)a | <.001 | ||
| ≥40 | 3.14 (1.58–6.25)a | .001 | ||
| O’Dwyer et al, 201327 | Emergency cesarean | 20–25 | 1 | |
| >30 | 3.03 (1.89–4.86)a | <.001 | ||
| Pevzner et al, 200921 | Arrest disorders | <30 | 1 | |
| Failure to progress | ||||
| Failed labor induction | 30–39 | 1.73 (1.28–2.35)b | .002 | |
| Nonreassuring FHR pattern | >40 | 2.32 (1.58–3.42)b | <.001 | |
| Malpresentation | ||||
| Roloff et al, 201526 | Failure to progress | <30 | 1 | |
| Category 2–3 FHR tracing | ||||
| Hemorrhage | >30 | 1.54 (0.55–4.31)a | .407 | |
| Vinturache et al, 201419 | Emergency cesarean | 18.5–24.9 | 1 | |
| >30 | 2.2 (1.2–4.1)c | .011 |
Abbreviations: BMI, body mass index; FHR, fetal heart rate.
Odds ratios and significance values calculated using MedCalc; unadjusted odds ratios reported.
Adjusted for parity, race, and treatment group.
Adjusted for maternal age, parity, preexisting health conditions (diabetes mellitus, hypertension, chronic heart disease, chronic renal diseases), pregnancy complications (gestational diabetes, preeclampsia, eclampsia, placental abruption, placenta previa, prolonged rupture of membrane, intrauterine growth restriction), fertility treatments, previous cesarean birth.
Table 3.
Influences of Maternal Obesity on Secondary Outcomes by Labor Inducing Agent
| Study | Secondary Outcome | BMI Ranges, kg/m2 | Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Prostaglandins | ||||
| Beckwith et al, 201722 | Failure to attain an active phase of labora | Prepregnancy, <30 | 1 | |
| Prepregnancy, >30 | 1.56b (1.18–2.05) | <.05 | ||
| Admission, <30 | 1 | |||
| Admission, >30 | 3.23b (1.15–9.09) | <.05 | ||
| Gauthier et al, 201223 | First cervical ripening attempt failure | 20–25 | 1 | |
| ≥30 | 2.32 (1.37–4.00) | .0019 | ||
| Melamed et al, 201025 | Prostaglandin ripening failure | 21–25 | 1 | |
| 26–30 | 5.75c (1.92–23.27) | .006 | ||
| >30 | 6.22c (3.73–41.03) | .004 | ||
| Pevzner et al, 200921 | Failed to reach active labor | <30 | 1 | |
| 30–39 | 2.31d (1.28–4.16) | .0054 | ||
| >40 | 2.50d (1.24–5.08) | .01 | ||
| Transcervical catheters | ||||
| Anabusi et al, 201620 | Cervical ripening failuree | ≤30 | 1 | |
| >30 | 2.72d (0.48–15.24) | .255 | ||
| Beckwith et al, 201722 | Failure to attain an active phase of labore | Prepregnancy, <30 | 1 | |
| Prepregnancy, >30 | 1.20b (0.74–1.96) | >.05 | ||
| Admission, <30 | 1 | |||
| Admission, >30 | 1.61b (0.70–3.69) | >.05 | ||
| Oxytocin | ||||
| O’Dwyer et al, 201327 | Oxytocin required for IOL | 20–25 | 1 | |
| Overweight | 0.88d (0.69–1.13) | .35 | ||
| >30 | 1.56d (1.18–2.06) | .0016 | ||
| Pevzner et al, 200921 | Oxytocin required for IOL | <30 | 1 | |
| 30–39 | 1.51f (1.15–1.97) | <.05 | ||
| >40 | 2.24f (1.57–3.18) | <.05 | ||
| Roloff et al, 201526 | Oxytocin required for IOL | <30 | 1 | |
| >30 | 5.33d (1.03–27.5) | .04 | ||
| Vinturache et al, 201419 | Oxytocin required for IOL | 18.5–24.9 | 1 | |
| 25–29.9 | 1.69d (1.33–2.14) | <.0001 | ||
| ≥30 | 2.36d (1.74–3.21) | <.0001 |
Abbreviations: BMI, body mass index; IOL, induction of labor.
Failure to achieve an active phase of labor was defined as a cesarean birth performed at a cervical dilatation of 5 cm or less.
Adjusted for primiparity, gestational age at birth, cervix dilatation and effacement at induction initiation, fetal birth weight, and presence of hypertensive disorders of pregnancy.
Adjusted for gestational age, maternal age, BMI, parity, and cervical effacement.
Odds ratio and significance values were calculated using MedCalc; raw odds data presented.
Cervical ripening failure calculation is based on the numbers for cervical ripening success, defined as second Bishop score increased by 2 or more points or cervical dilatation of 3 cm or more.
Adjusted for parity, race, and treatment group.
Table 4.
Influences of Maternal Obesity on Time to Birth and Medication Dosage by Labor Inducing Agent
| Study | BMI Ranges kg/m2 | Time to Birth h | P Value | Medication Dosage in Labor | P Value |
|---|---|---|---|---|---|
| Prostaglandins | |||||
| Gauthier et al, 201223 | 20–25 | 21.20 (17.18)a,b | 1.2 (0.3)c | ||
| ≥30 | 26.25 (19.22)a,b | .0258 | 1.4(0.6)c | .0126 | |
| Lassiter et al, 201624 | <30 | 17.72 (7.3)a,b | 1.59d | ||
| 30–0 | 20.01 (8.3)a,b | 2.05d | |||
| ≥40 | 22.90 (11.6)a,b | .0001 | 2.32d | .003 | |
| O’Dwyer et al, 201327 | 20–29 | 6.10a,e | 2.5f | ||
| Overweight | 6.00a,e | 2.7f | |||
| >30 | 6.60a,e | NS | 2.8f | NS | |
| Transcervical catheters | |||||
| Anabusi et al, 201620 | ≤30 | 16.00g | Not applicable | ||
| >30 | 16.95g | .092 | |||
| Oxytocin | |||||
| Lassiter et al, 201624 | <30 | 17.72 (7.3)a,b | 7.17a,h | ||
| 30–40 | 20.01 (8.3)a,b | 8.54a,h | |||
| >40 | 22.90 (11.6)a,b | .0001 | 10.39a,h | .023 | |
| Maeder et al, 201728 | <25 | 13.96 (8.10)a,b | 6.37 (7.06)a,h | ||
| 25–29.9 | 16.00 (7.54)a,b | 5.98 (5.00)a,h | |||
| ≥30 | 18.30 (8.65)a,b | .018 | 7.50 (6.49)a,h | .252 | |
| O’Dwyer et al, 201327 | 20–25 | 6.10a,e | Not reported | ||
| Overweight | 6.00a,e | ||||
| >30 | 6.60a,e | NS | |||
| Pevzner et al, 200921 | <30 | 22.70 (11.4–39.6)b,g | 2.6 (0.4–13.5)g,h | ||
| 30–39 | 24.90 (12.2–43.0)b,g | 3.5 (0.3–15.8)g,h | |||
| >40 | 27.00 (12.9–50.4)b,g | <.001 | 5.0 (0.4–20.9)g,h | <.001 |
Abbreviations: BMI, body mass index; NS, not significant.
Mean (SD, if reported).
Time from labor induction to birth.
Mean (SD) number of prostaglandin doses.
Mean number of doses of misoprostol.
Time in first stage of labor for nulliparous women.
Mean prostaglandin dose for nulliparous women.
Median (10%−90% range, if reported).
Total units of oxytocin.
Labor Induction Protocols
There were also variations among included studies in labor induction protocols. For example, successful cervical ripening was variously defined as a Bishop score from 3 to greater than 7 (Table 1).20,25 Dosage of prostaglandins, route of administration, and timing of induction agents also varied between studies included in this review (Table 1). Two different doses were reported by the investigators of studies involving dinoprostone (Cervidil: 3 mg or 10 mg vaginally), and both dose and route varied for studies involving misoprostol (Cytotec; 25–100 mcg administered orally or vaginally). Among studies reporting results of labor inductions involving transcervical catheters, both involved Foley catheters,20,22 with one comparing Cook catheters with Foley catheters.20 We identified no studies that included a combination of prostaglandins and transcervical catheters.
Cesarean Birth
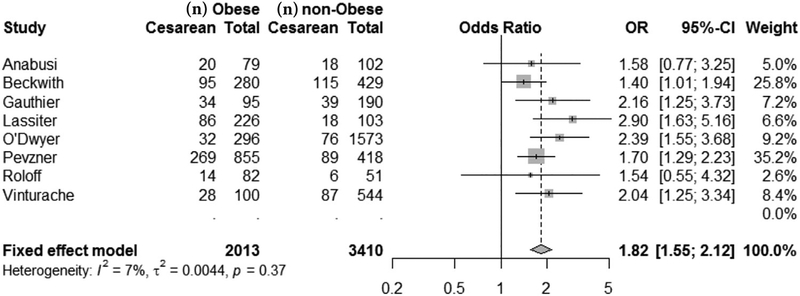
A fixed-effect meta-analysis of 8 studies with usable data indicated that cesarean birth following labor induction was more common among women with obesity compared with women of normal weight (OR, 1.82; 95% CI, 1.55–2.12; P < .001; Figure 2). Moreover, an increasing degree of maternal obesity was associated with a higher risk of cesarean birth following labor induction in a dose-dependent manner in both of the 2 studies that reported more than one category of maternal obesity (Table 2).21,24 As in the other studies, Beckwith et al22 found that the risk of a cesarean birth following labor induction was significantly higher among women with obesity compared with women of normal weight (OR, 1.5; 95% CI, 1.03–2.20). However, these investigators did not find a significant association between labor induction and cesarean birth among the subgroup of women who used transcervical catheters (OR, 1.14; 95% CI, 0.59–2.19).22
Figure 2.
Forest Plot of Mantel-Haenszel Fixed-Effect Meta-Analysis Displaying Odds of Cesarean Birth
Abbreviations: BMI, body mass index; IOL, induction of labor.
Secondary Outcomes
In addition to the primary outcome of cesarean birth following labor induction, associations between maternal obesity and secondary outcomes of timing, dosage, success of cervical ripening methods, and synthetic oxytocin dosage and timing were examined (Tables 3 and 4) in those studies that included the secondary outcomes of interest. For these secondary outcomes, we analyzed the results by method of labor induction (eg, prostaglandins, transcervical catheters, oxytocin).
Prostaglandins
Prostaglandin use was evaluated for failure to achieve active labor and for the type of prostaglandin and dose. Investigators of 7 studies21–25,27,31 reported outcomes of labor inductions involving vaginal and oral prostaglandins stratified by maternal BMI. In all of these studies, women with obesity were significantly less likely than women of normal weight to successfully complete cervical ripening and/or achieve active-phase labor (Table 3). Similar to the findings for cesarean birth following labor induction, in studies including subcategories of women with different degrees of obesity, higher BMI was associated in a dose-dependent manner with a higher likelihood that cervical ripening was not successful.25 Likewise, using normal weight women as a reference group, maternal BMI at the time of hospital admission was associated with higher odds that cervical ripening would be unsuccessful (OR, 3.23; 95% CI, 1.37–4.0) compared with women with prepregnancy obesity (OR, 1.56; 95% CI, 1.18–2.05).22
Three of the 6 studies reporting use of prostaglandins (Table 3) for labor induction provided information on the mean number of doses required for cervical ripening in women of different BMI ranges.23–25 Among women using dinoprostone, there were no differences in the number of doses required for cervical ripening related to maternal BMI.23,27 In contrast, in the study by Lassiter et al, there was an increase in the mean number of vaginal misoprostol doses required in women who had higher BMIs compared with women with lower BMIs (1.59 doses for women with a BMI <30 kg/m2; 2.05 and 2.32 doses required for women with BMI 30–40 kg/m2 and >40 kg/m2, respectively; P = .003).24
Transcervical Catheters
Investigators of 2 studies20,22 included in this review examined the use of transcervical catheters among women with different BMI categories (Tables 3 and 4). Anabusi et al20 found that the median time to birth following labor induction involving a transcervical catheter was similar for women with obesity and women of normal weight (16 hours for normal-weight and overweight women verses 16 hours and 57 minutes for women with obesity; P = .092), and women with obesity were not significantly more likely to have unsuccessful cervical ripening than were women of normal weight following transcervical catheter use (OR, 2.72; 95% CI, 0.48–15.24). Similarly, Beckwith and colleagues22 did not find significant differences in the abilities of women with obesity versus women of normal weight to achieve active-phase labor following transcervical catheter cervical ripening (OR, 1.14; 95% CI, 0.59–2.19).
Oxytocin
Use of synthetic oxytocin (Pitocin) for labor induction was the focus of 5 studies19,21,24,26,27 (Tables 3 and 4). Investigators of all 5 studies found that women with obesity who used oxytocin for labor induction either had a longer labor duration or required higher doses of oxytocin compared with women of normal weight. As with other outcomes in this review, the odds of requiring oxytocin for induction of labor were higher in women who were overweight or obese and with each increase in BMI category.
DISCUSSION
This systematic review and meta-analysis examined the influence of maternal obesity on labor induction processes and outcomes. In this meta-analysis, women with obesity were nearly 2 times more likely than women of normal weight to end labor with cesarean birth following labor induction.
We found evidence in this review that labor induction appears to take more time as maternal BMI increases, and it requires both increased number of doses and higher doses of induction agents. More women required synthetic oxytocin for induction of labor with each increase in BMI classification, which likely added time to their labor progress compared with women who went into labor spontaneously following cervical ripening. It is known that labor progress is altered in women with obesity, who are less likely to go into labor spontaneously at term and who have longer durations of spontaneous labor compared with women of normal weight.10 These differences in labor initiation and labor progress may be caused by myriad endocrine and inflammatory alterations present in women with obesity.15 Taken together, the known lower likelihoods of initiating spontaneous labor and achieving normal labor progress seen in women with obesity corroborate our findings that labor induction can be longer and may be more difficult for women with obesity.
Higher doses of prostaglandins and oxytocin were required for women with obesity compared with women of normal weight in all studies included in this review except for one in which dinoprostone was used.27 O’Dwyer et al27 reported a shorter labor duration for all women than the other oxytocin studies included in this review. This discrepancy is likely due to the investigators’ labor management protocol, which included straight-line partograms to define active-phase labor, a method that was more conservative than other included studies’ definitions of labor. Perhaps the more frequent administration schedule of misoprostol and oxytocin compared with 12-hour dinoprostone allowed us to better observe the influence of maternal BMI on medication requirements.
We also found evidence that certain cervical ripening agents may be better than others for women women with obesity.31 Misoprostol for cervical ripening may also be more effective in women with obesity because of physiologic changes in prostaglandin expression that may decrease the response to dinoprostone in some women.31,32 Alternatively, there may be some pharmacokinetic or pharmacodynamic differences in how these prostaglandins function in women with obesity. More investigations comparing these methodologies in women with obesity are needed to further elucidate the relationship between maternal obesity and labor induction success using different doses and choice of cervical ripening agents.
Transcervical catheters for labor induction were not included in most of the studies of this review, and those that did include this method had small sample sizes. However, transcervical catheters appeared to be more successful than misoprostol for helping women with obesity complete cervical ripening, attain an active phase of labor, and end induction with vaginal birth.22 More research is needed on the use transcervical catheters for induction of labor among women with obesity. Future studies including the use of a combination of transcervical catheters and misoprostol in women with obesity are also needed, as this combination has shown success in mixed-weight groups of women33 but has not been studied in women with obesity.
Additionally, we found evidence that gestational weight gain sufficient to move a woman from one BMI category to the next is also problematic.22 BMI at the time of labor and birth was the most common timing used to measure BMI in the studies examined in this review. The Beckwith et al study used both a prepregnancy BMI and a BMI at the time of birth. These authors found a greater correlation between BMI and unsuccessful cervical ripening among women with obesity at the time of birth compared with women of normal weight. This may be due to a greater distribution volume at the time of birth compared with prepregnancy BMI. Given these findings, we suggest using the BMI at the time of hospital admission for labor management decisions.
Changes in practice during the period of studies included in this review may have influenced outcomes for women with obesity. In the Safe Prevention of the Primary Cesarean Delivery,34 the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine recommended that clinicians use 6 cm of cervical dilatation as the start of active-phase labor, in contrast to previous definitions of active phase starting at 4 cm of cervical dilitation.35 It is possible that the significant differences we observed in this metaanalysis between maternal BMI and cesarean birth following labor induction would not be seen, or would not be as strong, since the Safe Prevention guidelines were published. Women with obesity are known to show slowest labor progress between 4 and 6 cm dilatation compared with women with normal range BMIs.31 Therefore, the new recommendation in the Safe Prevention guidelines could be particularly important for future clinical outcomes in women with obesity.
This systematic review is limited by the studies available on this subject in the published literature. We found considerable differences between studies with regard to the way maternal obesity was categorized and the standardization of labor induction processes. Also, some studies included in this review adjusted for a range of maternal factors when calculating odds of cesarean birth and other outcomes following labor induction, whereas others presented unadjusted odds or raw numbers only. Despite these differences in statistical adjustment, the overall ORs for cesarean birth following labor induction by maternal BMI were similar across the studies included in this review. This similarity suggests maternal BMI may have a clinically important influence on labor induction processes and outcomes.
The influences of maternal BMI were calculated using ORs rather than risk ratios, as ORs were reported in the original studies. The OR may overinflate the odds of an occurrence when the occurrence is common in a cohort.36 Future studies examining risks associated with maternal BMI may consider reporting risk ratios instead.
Another limitation is our decision to use a fixed-effect method for the meta-analysis.18 Fixed-effect meta-analyses presume that studies fundamentally share a common effect and therefore only include within-study variance in the model, with larger studies given greater weight. Random-effects meta-analyses do not presume this and include both within-study and between-study variance, with studies weighted more equally (ie, smaller studies weighted upward and larger studies weighted downward) in the model. At the core, choosing between fixed-effect and random-effects meta-analyses is a determination of whether selected studies vary substantially in their methodologies, populations, or in other ways that affect outcomes. Typically, a random-effects analysis is justified, as most studies vary substantially in meaningful ways. Borenstein et al18 recommend, however, that when there are few studies available, such as in our analysis, a fixed-effect analysis is appropriate with a caveat: The fixed-effect meta-analysis is for descriptive purposes only; generalizing to studies beyond those described is not possible.
These results gathered from a range of well-conducted studies performed over the past few decades in a variety of countries lend credence to the observation that maternal obesity increases a woman’s risk for cesarean birth following labor induction. That said, our inclusion of only observational studies (2 of which were a secondary analysis of a trial20,21 and one of which was a secondary analysis of an observational study19) is another limitation of this review, as observational studies do not include the same level of control for bias as randomized trials. Our decision to only include observational studies in this review if they scored high in methodologic quality offsets this limitation.
This review is also limited by our inability to present separate estimates of the influence of maternal obesity on labor induction outcomes and processes by parity. Labor progress and outcomes appear to be most altered in women who are obese and nulliparous.37,38 However, a sufficient number of investigations in the existing literature that allowed presentation of results for both nulliparous and multiparous women separately could not be found. Finally, this analysis was not able to compare longer-term outcomes for women or their neonates following labor induction.
Despite these limitations, this study has a number of strengths. First, study authors conducted a thorough search of existing literature, using duplicate searches by the primary author and a trained university librarian. Selected studies met a strict set of inclusion and exclusion criteria, including high standards for methodologic quality using established tools. Additionally, by including women of mixed parity, we were able to better isolate the influence of maternal obesity on labor induction processes and outcomes.10 This review focused on contemporary labor induction practices by limiting the inclusion years of chosen studies. Our use of meta-analysis demonstrating minimal heterogeneity between studies is a final strength of this investigation. More information is needed, but this first meta-analysis on this subject is an important step toward guiding the format of future investigations.
Clinical Implications
Clinicians should consider maternal BMI as they undertake labor induction. As shown in this review, women with obesity are more likely than women of normal weight to have a cesarean birth following induction of labor. Therefore, clinicians should carefully discuss the risks and benefits of labor induction in women with obesity, along with maternal or fetal indications for expedited birth.
Maternal BMI near the time of labor should be calculated and used to determine the best agents for cervical ripening. Hospital admission BMI, not prepregnancy BMI, was used by most investigators considering the influence of maternal BMI on labor processes and outcomes.22,39–41 Although prepregnancy BMI may be helpful for guiding antenatal care, maternal BMI at the end of pregnancy may better reflect a woman’s metabolic condition near the time of labor. In an investigation reporting cesarean rates by maternal BMI category, Kominiarek and colleagues found that women’s risk of cesarean increased by 30% with each increase in hospital admission BMI category.41 Although having a BMI in obese ranges at the time of labor admission may be normal in many women achieving full-term pregnancy, it is nevertheless important that clinicians use the most accurate measurement of BMI to guide their decisions on optimal labor induction strategies for individual women.
This review suggests that transcervical catheters may be more effective than prostaglandin agents at achieving cervical ripening in women who are obese. Clinicians should anticipate that women with obesity are more likely than women of normal weight to require repeated doses of misoprostol and/or longer administration, as well as higher doses of synthetic oxytocin, to attain active-phase labor. Clinicians should also expect that induction of labor in a woman with obesity may take longer than in a woman of normal weight and should prepare the clinical team, the labor support person, and the woman for this possibility.
This review supports the need for research to better describe optimal labor induction practices for women with obesity. Prospective investigations using standard BMI categories, standard labor induction regimens, and standard definitions of active labor onset will provide more accurate and precise information for use by clinicians when inducing the labor of women who are obese. Further research should focus on the effectiveness of individual induction agents for women with obesity, as well as that of agents used in combination. Also, researchers in this area should strive to include women with wide variations in BMI as participants, thereby better elucidating changes in labor induction success with different degrees of maternal obesity.
CONCLUSION
Maternal obesity and labor induction are now normal in contemporary clinical practice. Until clinicians have better information on the risks of labor induction and techniques to optimally implement labor induction in women who are obese, this population could see increases in rates of cesarean birth and other poor outcomes because of unsuccessful or prolonged labor induction. Our review supports the need for new labor induction protocols that are individualized by the degree of maternal obesity in both the timing and choice of induction agents. With patience and time, many more women with obesity might achieve normal labor outcomes following a safe and effective labor induction.
Quick Points.
Women with obesity are more likely to end labor induction with cesarean birth.
Women with obesity need higher doses and a longer duration of exposure to prostaglandins to complete labor initiation and birth compared with women of normal weight.
Women with obesity need higher doses of synthetic oxytocin to complete labor initiation and birth compared with women of normal weight.
ACKNOWLEDGMENTS
This study was funded by the National Institutes of Health, award 1K01NR016984.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
Contributor Information
Jessica A. Ellis, Jessica Ellis, CNM, MSN, is a PhD candidate at Georgia State University, a clinical assistant professor at the University of Utah and a clinician with Birthcare Healthcare in Salt Lake City UT..
Carolyn M. Brown, Carolyn Brown, MLS, AHIP, is a health sciences librarian at Emory University in Atlanta, GA..
Brian Barger, Brian Barger, PhD, is an Assistant Professor of Epidemiology and Biostatistics in the School of Public Health at Georgia State University..
Nicole S. Carlson, Nicole Carlson, CNM, PhD, is an assistant professor at Emory University and a practicing clinician at Grady Hospital in Atlanta, GA. Dr. Carlson is a content expert in labor management for women with obesity..
REFERENCES
- 1.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Branum AM, Kirmeyer SE, Gregory ECW. Prepregnancy body mass index by maternal characteristics and state: data from the birth certificate, 2014. Natl Vital Stat Rep. 2016;65(6):1–10. http://www.cdc.gov/nchs/data/nvsr/nvsr65/nvsr65_06.pdf. Accessed September 6, 2016. [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief. 2015;(219):1–8. https://www.cdc.gov/nchs/data/databriefs/db219.pdf. Accessed September 13, 2016. [PubMed] [Google Scholar]
- 4.Osterman MJ, Martin JA. Recent declines in induction of labor by gestational age. NCHS Data Brief. 2014;(155):1–8. [PubMed] [Google Scholar]
- 5.Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64(1):1–65. [PubMed] [Google Scholar]
- 6.Arrowsmith S, Wray S, Quenby S. Maternal obesity and labour complications following induction of labour in prolonged pregnancy. BJOG. 2011;118(5):578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolfe KB, Rossi RA, Warshak CR. The effect of maternal obesity on the rate of failed induction of labor. Am J Obstet Gynecol. 2011;205(2):128.e1–128.e7. [DOI] [PubMed] [Google Scholar]
- 8.Mission JF, Marshall NE, Caughey AB. Obesity in pregnancy: a big problem and getting bigger. Obstet Gynecol Surv. 2013;68(5):389–399. [DOI] [PubMed] [Google Scholar]
- 9.Fisher SC, Kim SY, Sharma AJ, Rochat R, Morrow B. Is obesity still increasing among pregnant women? Prepregnancy obesity trends in 20 states, 2003–2009. Prev Med. 2013;56(6):372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harper LM, Caughey AB, Odibo AO, Roehl KA, Zhao Q, Cahill AG. Normal progress of induced labor. Obstet Gynecol. 2012;119(6):1113–1118. [DOI] [PubMed] [Google Scholar]
- 11.Glantz JC. Elective induction vs. spontaneous labor associations and outcomes. J Reprod Med. 2005;50(4):235–240. [PubMed] [Google Scholar]
- 12.Grobman WA. Predictors of induction success. Semin Perinatol. 2012;36(5):344–347. [DOI] [PubMed] [Google Scholar]
- 13.Gibson KS, Waters TP, Bailit JL. A risk of waiting: the weekly incidence of hypertensive disorders and associated maternal and neonatal morbidity in low-risk term pregnancies. Am J Obstet Gynecol. 2016;214(3):389.e1–389.e12. [DOI] [PubMed] [Google Scholar]
- 14.Lee VR, Darney BG, Snowden JM, et al. Term elective induction of labour and perinatal outcomes in obese women: retrospective cohort study. BJOG. 2016;123(2):271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlson NS, Hernandez TL, Hurt KJ. Parturition dysfunction in obesity: time to target the pathobiology. Reprod Biol Endocrinol. 2015;13:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in metaanalyses. Ottawa Hospital Research Institute website. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.Accessed September 13, 2016. [Google Scholar]
- 18.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111. [DOI] [PubMed] [Google Scholar]
- 19.Vinturache A, Moledina N, McDonald S, Slater D, Tough S. Prepregnancy body mass index (BMI) and delivery outcomes in a Canadian population. BMC Pregnancy Childbirth. 2014;14:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anabusi S, Mei-Dan E, Hallak M, Walfisch A. Mechanical labor induction in the obese population: a secondary analysis of a prospective randomized trial. Arch Gynecol Obstet. 2016;293(1):75–80. [DOI] [PubMed] [Google Scholar]
- 21.Pevzner L, Powers BL, Rayburn WF, Rumney P, Wing DA. Effects of maternal obesity on duration and outcomes of prostaglandin cervical ripening and labor induction. Obstet Gynecol. 2009;114(6): 1315–1321. [DOI] [PubMed] [Google Scholar]
- 22.Beckwith L, Magner K, Kritzer S, Warshak CR. Prostaglandin versus mechanical dilation and the effect of maternal obesity on failure to achieve active labor: a cohort study. J Matern Fetal Neonatal Med. 2017;30(13):1621–1626. [DOI] [PubMed] [Google Scholar]
- 23.Gauthier T, Mazeau S, Dalmay F, et al. Obesity and cervical ripening failure risk. J Matern Fetal Neonatal Med. 2012;25(3):304–307. [DOI] [PubMed] [Google Scholar]
- 24.Lassiter JR, Holliday N, Lewis DF, Mulekar M, Abshire J, Brocato B. Induction of labor with an unfavorable cervix: how does BMI affect success? J Matern Fetal Neonatal Med. 2016;29(18):3000–3002. [DOI] [PubMed] [Google Scholar]
- 25.Melamed N, Ben-Haroush A, Kremer S, Hod M, Yogev Y. Failure of cervical ripening with prostaglandin-E2: can it be predicted? J Matern Fetal Neonatal Med. 2010;23(6):536–540. [DOI] [PubMed] [Google Scholar]
- 26.Roloff K, Peng S, Sanchez-Ramos L, Valenzuela GJ. Cumulative oxytocin dose during induction of labor according to maternal body mass index. Int J Gynaecol Obstet. 2015;131(1):54–58. [DOI] [PubMed] [Google Scholar]
- 27.O’Dwyer V, O’Kelly S, Monaghan B, Rowan A, Farah N, Turner MJ. Maternal obesity and induction of labor. Acta Obstet Gynecol Scand. 2013;92(12):1414–1418. [DOI] [PubMed] [Google Scholar]
- 28.Maeder AB, Vonderheid SC, Park CG, et al. Titration of intravenous oxytocin infusion for postdates induction of labor across body mass index groups. J Obstet Gynecol Neonatal Nurs. 2017;46(4):494–507. [DOI] [PubMed] [Google Scholar]
- 29.American College of Obstetricins and Gynecologists. ACOG Committee Opinion No. 548: Weight gain during pregnancy. Obstet Gynecol. 2013;121(1):210–212. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. What is overweight and obesity? World Health Organization website https://www.who.int/dietphysicalactivity/childhood_what/en/ Published n.d. November 16, 2018. Accessed November 16, 2018. [Google Scholar]
- 31.Suidan RS, Rondon KC, Apuzzio JJ, Williams SF. Labor outcomes of obese patients undergoing induction of labor with misoprostol compared to dinoprostone. Am J Perinatol. 2015;30(2):187–192. [DOI] [PubMed] [Google Scholar]
- 32.Martínez ME, Heddens D, Earnest DL, et al. Physical activity, body mass index, and prostaglandin E2 levels in rectal mucosa. J Natl Cancer Inst. 1999;91(11):950–953. [DOI] [PubMed] [Google Scholar]
- 33.Levine LD, Downes KL, Elovitz MA, Parry S, Sammel MD, Srinivas SK. Mechanical and pharmacologic methods of labor induction: a randomized controlled trial. Obstet Gynecol. 2016;128(6):1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caughey AB. The safe prevention of the primary cesarean. Clin Obstet Gynecol. 2015;58(2):207–210. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Troendle JF, Yancey MK. Reassessing the labor curve in nulliparous women. Am J Obstet Gynecol. 2002;187(4):824–828. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690–1691. [DOI] [PubMed] [Google Scholar]
- 37.Vahratian A, Siega-Riz AM, Savitz DA, Zhang J. Maternal prepregnancy overweight and obesity and the risk of cesarean delivery in nulliparous women. Ann Epidemiol. 2005;15(7):467–474. [DOI] [PubMed] [Google Scholar]
- 38.Subramaniam A, Jauk VC, Goss AR, Alvarez MD, Reese C, Edwards RK. Mode of delivery in women with class III obesity: planned cesarean compared with induction of labor. Am J Obstet Gynecol. 2014;211(6):700.e1–700.e9. [DOI] [PubMed] [Google Scholar]
- 39.Kominiarek MA, Zhang J, Vanveldhuisen P, Troendle J, Beaver J, Hibbard JU. Contemporary labor patterns: the impact of maternal body mass index. Am J Obstet Gynecol. 2011;205(3):244.e1–244.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norman SM, Tuuli MG, Odibo AO, Caughey AB, Roehl KA, Cahill AG. The effects of obesity on the first stage of labor. Obstet Gynecol. 2012;120(1):130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kominiarek MA, Vanveldhuisen P, Hibbard J, et al. ; Consortium on Safe Labor. The maternal body mass index: a strong association with delivery route. Am J Obstet Gynecol. 2010;203(3):264.e1–264.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wing DA; Misoprostol Vaginal Insert Consortium. Misoprostol vaginal insert compared with dinoprostone vaginal insert: a randomized controlled trial. Obstet Gynecol. 2008;112(4):801–812. [DOI] [PubMed] [Google Scholar]