Abstract
Background and Aims
Mycorrhizal associations in mycoheterotrophic plants are generally more specialized than in autotrophs. Mycoheterotrophs typically bear small, inconspicuous flowers that often self-pollinate to maximize seed set, although some have structurally complex flowers indicative of xenogamy. A trade-off has previously been proposed between specialization in these above- and below-ground symbioses, although empirical data are lacking.
Methods
We used next-generation DNA sequencing to compare the mycorrhizal communities from the roots of a mycoheterotrophic species, Thismia tentaculata (Thismiaceae), and its neighbouring autotrophs. We furthermore conducted detailed assessments of floral phenology and pollination ecology, and performed artificial pollination experiments to determine the breeding system.
Key Results
Thismia tentaculata maintains a symbiotic association with a single arbuscular mycorrhizal Rhizophagus species. The flowers are pollinated by a single species of fungus gnats (Corynoptera, Sciaridae), which are attracted by the yellow pigments and are temporarily restrained within the perianth chamber before departing via apertures between the anthers. The plants are self-compatible but predominantly xenogamous.
Conclusions
Our findings demonstrate that T. tentaculata maintains highly specialized associations with pollinators and mycorrhizal fungi, both of which are widely distributed. We suggest that specialization in multiple symbiotic interactions is possible in mycoheterotrophs if redundant selective pressures are not exerted to further restrict an already constrained suite of life-history traits.
Keywords: Arbuscular mycorrhizal fungi, Corynoptera fungus gnats, mycoheterotrophy, pollination ecology, Rhizophagus, specificity, symbiosis, Thismia tentaculata, xenogamy
INTRODUCTION
Important drivers of species diversification in plants are known to include abiotic factors such as geological, climatic and ecological processes, and biotic factors such as pollination system and seed dispersal mechanism (Hoorn et al., 2010; Van der Niet and Johnson, 2012; Hughes and Atchison, 2015; Lagomarsino et al., 2016). These factors usually influence genetic variation among populations and generate patterns of genetic differentiation that are spatially and temporally strongly structured (Thompson, 2005; Benton, 2009). In general, when plants rely on highly specific interactions, these interactions limit their geographical distributions and hence influence diversification patterns. Well-known examples include pollination mutualisms (Smith et al., 2008) and parasitic plants (Thorogood et al., 2008, 2009), in which the distributions of the plants are limited by the biotic partners with which they interact.
Plants that maintain highly specific symbioses with mycorrhizal fungi are of particular interest due to their biological dependence. Mycorrhizal interactions play an important role in plant metabolism (Finlay, 2008), with autotrophic plants generally obtaining essential resources (water and minerals) from the fungi, and in exchange the plants transfer photosynthetically fixed carbon to their mycorrhizal partners (Smith and Read, 2008). The host plants are sometimes achlorophyllous, however, and in these cases do not provide carbon, but instead obtain it from fungi that simultaneously maintain a mycorrhizal relationship with neighbouring photosynthetic plants (although some mycoheterotrophs rely on saprotrophic fungi that digest decaying organic matter). In these non-mutualistic ‘mycoheterotrophic’ interactions the achlorophyllous plants can be regarded as ‘cheaters’ (Bidartondo, 2005). Unlike the promiscuous interactions between autotrophic plants and their mycorrhizal fungi (Giovannetti et al., 2004), the interaction of mycoheterotrophic plants with mycorrhizal fungi is often highly specific, involving only a narrow range of fungi (Bidartondo, 2005; Merckx et al., 2012; Gomes et al., 2017). The geographical distribution of mycoheterotrophic plants might theoretically be limited by the distribution of their fungal partners, and host specificity has therefore been suggested as a likely explanation for the narrow distributional ranges of many mycoheterotrophs (Yamato et al., 2011; Merckx, 2013).
Since mycoheterotrophic plants have a limited carbon supply, occupy patchy distributions and are restricted to habitats that are often unfavourable for common pollinators (Waterman et al., 2013), it has often been hypothesized that they might rely heavily on reproductive systems that enable greater assurance of seed production (e.g. Dressler, 1981; Leake, 1994; Molvray et al., 2000; Zhang and Saunders, 2000; Bidartondo, 2005; Waterman and Bidartondo, 2008; Klooster and Culley, 2009; Suetsugu, 2014, 2015). In a review of the biology of mycoheterotrophs, Bidartondo (2005; Waterman and Bidartondo, 2008) proposed a trade-off between specialization and generalization in above- and below-ground symbioses: most plant species are generalists with regard to both their pollinator and mycorrhizal mutualisms, and whilst some plant species have evolved specialist pollination systems, they are typically non-mycorrhizal or generalists towards mycorrhizal fungi. Mycoheterotrophic plants, however, ‘cheat’ their mycorrhizal partners and are more likely to either self-fertilize or be pollinated by generalist or very common pollinators due to the evolutionary instability inherent in maintaining multiple symbiotic interactions. As a consequence, mycoheterotrophs rarely develop metabolically expensive reproductive structures for attracting visitors, instead allocating greater resources to maximizing fruit set.
Unlike most mycoheterotrophic plants, which typically bear small, inconspicuous flowers, there are some that have structurally very complex flowers, casting doubt on assumptions of their reliance on generalist pollinators and/or self-pollination. Thismia species (Thismiaceae), for example, have showy flowers with a conspicuously pigmented corolla, a trap-like perianth tube, long tepal appendages, and nectaries. These specializations are highly variable between species and are clearly not consistent with self-pollination; it is likely that genera such as Thismia are either xenogamous or maintain a mixed selfing–outcrossing reproductive strategy.
Thismia species are highly enigmatic: they remain hidden underground throughout most of their life cycle, only emerging from amongst the leaf litter when they flower and fruit after periods of heavy rain. Little is known of the mycorrhizal fungal interactions of Thismia, although studies have shown that species in temperate Australia and New Zealand are dependent on arbuscular mycorrhizal fungi, with considerable specificity towards a narrow range of Glomeraceae (Merckx et al., 2012; Gomes et al., 2017). No pollination studies have been conducted for any Thismia species to date, although several hypotheses have been proposed, including autogamous self-pollination (Miers, 1866), fly pollination (Li and Bi, 2013; Mar and Saunders, 2015), and even mammal pollination by potoroos (Potorous tridactylus; Roberts et al., 2003).
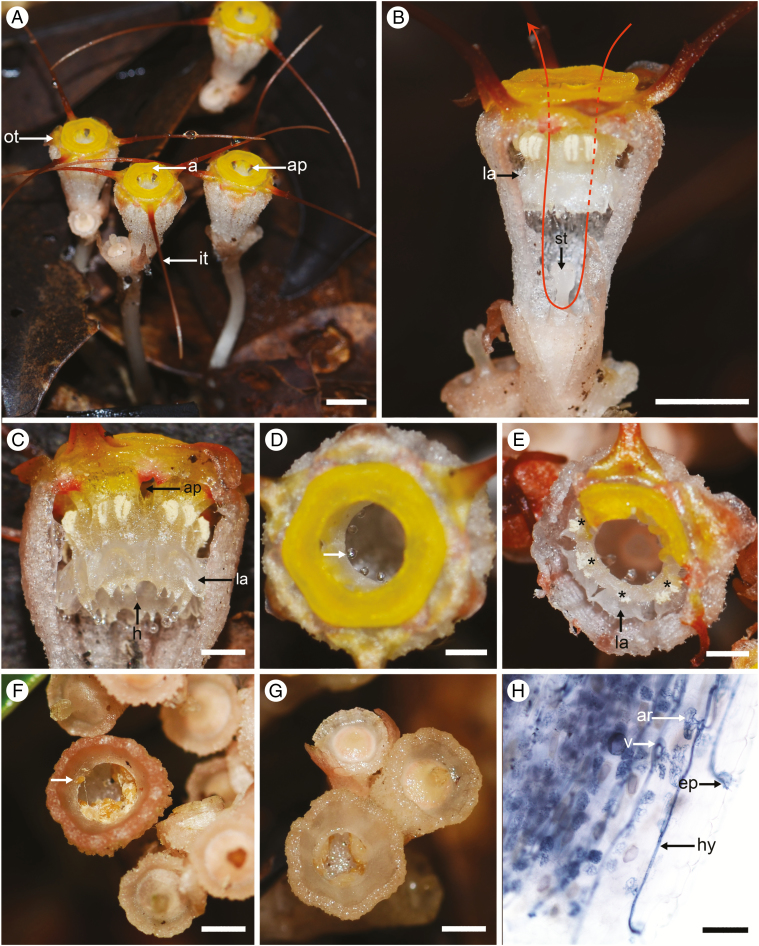
We aim to empirically address the gap in our understanding of the biotic interactions of Thismia, including both the above-ground plant–pollinator associations and the below-ground plant–mycorrhizal associations. Our research focuses on Thismia tentaculata (Fig. 1), a species with a very restricted distribution in Hong Kong and central Vietnam (Larsen and Averyanov, 2007; Ho et al., 2009). In contrast to its highly reduced vegetative structure, T. tentaculata has structurally highly complex and showy flowers (Fig. 1A), with an elaborate perianth chamber capped with a conspicuous yellow annulus surrounding an apical aperture (0.8–1 mm diameter; Fig. 1D), three extended filiform tepal appendages (~17 mm long; Fig. 1A), and six stamens suspended from the inner margin of the annulus (Fig. 1C). The stamens are fused laterally into a ring (Fig. 1C), although with separate filaments and with the gaps between the filaments forming small apertures that are visible on the inside rim of the annulus (ap in Fig. 1A, C). Each pendent stamen has several hairs that descend from the apex of the connective (h in Fig. 1C), and a lateral staminal appendage that extends horizontally from the stamen towards the inner wall of the perianth chamber (la in Fig. 1C, E).
Fig. 1.
Thismia tentaculata. (A) Several flowering individuals. (B) Dissected flower with proximal part of the perianth tube removed. The unidirectional movement of fungus gnat pollinators is shown by the red arrow (dotted line showing the path behind the annulus and stamens). (C) Dissected flower, showing the staminal ring, with apertures between stamen filaments. (D) Top view of the perianth tube, showing bright yellow annulus and the small droplet of exudate (arrowed) forming at the tip of each pendent hair. (E) The perianth tube, partially destroyed by an insect, showing the lateral appendages; stamen positions are marked by asterisks. (F) A fruit-cup with tiny seeds (arrow shows seed deposited on rim of the fruit-cup due to rain-splash). (G) An empty fruit-cup after rain. (H) Mycorrhizal fungal hyphae in root cortical cells of T. tentaculata. Abbreviations: a, annulus; ap, aperture; ar, arbuscule; ep, entry point; h, hair; hy, hyphae; it, inner tepal; la, lateral appendage; ot, outer tepal; st, stigma; v, vesicle. Photographs by X. Guo. Scale bars: (A, B) = 5 mm; (C–G) = 2 mm; (H) = 100 µm.
The mycorrhizal specificity of T. tentaculata was investigated here using next-generation DNA sequencing of the ribosomal 18S small subunit (SSU) in fungal communities associated with the roots of T. tentaculata and co-occurring green plants. We furthermore conducted floral phenological observations, monitored the activities of floral visitors and performed artificial pollination experiments to determine the breeding system of T. tentaculata, enabling a test of the hypothesis that the species maintains a predominantly xenogamous reproductive strategy. We further assessed the balance in the degree of specialization of the symbiotic associations maintained with its arbuscular mycorrhizal fungi and effective pollinators, and inferred the potential influence of these symbioses on the species distribution.
MATERIALS AND METHODS
Study sites
As with many mycoheterotrophic plants, Thismia tentaculata is rare: in Hong Kong, the species is restricted to only seven populations found on Tai Mo Shan in the New Territories. Population sizes are typically limited to only three to five individuals, although two larger populations exist in Hong Kong (comprising ~20 individuals over ~5 m2 and ~40 individuals over ~20 m2, respectively) in secondary deciduous and bamboo mixed forest, at elevations of 800–900 m. These larger populations were selected as study sites, with all field observations performed cumulatively over 34 d during two consecutive flowering seasons in May–August 2016 and 2017.
Mycorrhizal fungi
Three individuals of T. tentaculata from two populations were sampled for morphological examination and molecular identification of mycorrhizal fungi. The roots of each T. tentaculata specimen and its neighbouring autotrophic plants were preserved in 2× CTAB (hexadecyl trimethylammonium bromide) buffer. Neighbouring autotrophs were sampled by selecting up to six root tips (~1 cm) retrieved from the soil clump (10 cm diameter) surrounding the T. tentaculata roots.
For the morphological study, the T. tentaculata root samples were cut into 1-cm fragments and preserved in 70 % alcohol. The root fragments were cleared with 10 % KOH at 60 °C for 2 h, transferred into 5 % acetic acid for 5 min, then stained with 5 % ink (Parker Quink black writing ink; Yang et al., 2010) for 30 min and immersed in tap water for 14 h. The stained root fragments were compressed under a cover glass to observe the morphology of arbuscular mycorrhizal fungi using light microscopy and photographed using a Nikon 80i imaging system.
Fungal DNA was extracted from the CTAB-preserved roots with the Qiagen DNeasy Plant Mini Kit (Qiagen, Hilden, Germany). Fungal SSU rDNA was then amplified via amplicon libraries by PCR using primers AMV4.5NF (5′-AAGCTCGTAGTTGAATTTCG-3′) and AMDGR (5′-CCCAACTATCCCTATTAATCAT-3′) (Sato et al., 2005; Van Geel et al., 2014). Library construction for one sample of neighbouring plants was unsuccessful due to low DNA concentration; amplicon sequencing was therefore performed for a total of five samples (three T. tentaculata root samples and two neighbouring root samples) using the BGISEQ-500 platform.
Raw reads were trimmed by removing barcodes and primer sets, and then assembled using FLASH (Magoc and Salzberg, 2011) to obtain raw tags. At least 100 000 raw tags were obtained from each sample. We used the QIIME pipeline (Caporaso et al., 2010) and the program UCHIME (Edgar et al., 2011) to further filter the raw data and remove chimera. The effective sequences were then clustered into operational taxonomic units (OTUs) at 97 % similarity (Hijri and Sanders, 2005) using the UPARSE pipeline (Edgar, 2013), and the most abundant sequence in each OTU cluster was selected as the representative sequence for further analysis. Unique tags that could not be assigned to any OTU cluster were excluded. The taxonomic annotation of OTUs was performed using the BLAST method in the program QIIME based on the SILVA database (Quast et al., 2013), enabling identification of Glomeromycota fungi. OTUs classified as Glomeromycota were further searched against the MaarjAM database (Öpik et al., 2010) to obtain detailed taxonomic information and to match virtual taxa. Only OTUs belonging to Glomeromycota were retained in the downstream analyses.
In addition to the sequences obtained from root samples, the dataset of 286 virtual taxa generated by Merckx et al. (2012) was added for the phylogenetic analysis, including SSU rDNA data of arbuscular mycorrhizal fungi from 30 fully mycoheterotrophic flowering plant species and the mycoheterotrophic gametophytes of four fern and lycophyte species. A total of 340 sequences were included in the dataset, with 54 OTUs newly obtained in this study. There were several taxon-rich clades (involving 159 taxa) in preliminary analyses that did not contain any newly obtained OTUs. These taxa were therefore excluded from the dataset to improve tree-search efficiency, with representatives of each major group of arbuscular mycorrhizal fungi maintained to allow taxonomic interpretation. A total of 181 sequences were therefore used in the final analyses (GenBank accession numbers are provided in Supplementary Data Table S1). Sequences were aligned automatically using the MAFFT (Katoh et al., 2002) plugin in Geneious Pro v.7.1.9 (Biomatters; http://www.geneious.com) using default settings, and then manually edited and optimized. A phylogenetic tree was reconstructed using maximum likelihood methods with RAxML v.8.2.6 (Stamatakis, 2006) provided by the CIPRES Science Gateway (Miller et al., 2010). The dataset was run under the general time-reversible model with rate heterogeneity modelled by a γ distribution (GTR + Γ). One thousand inferences were run from distinct random stepwise addition sequence maximum parsimony starting trees to achieve the best-scoring maximum likelihood tree.
Pollination ecology
Detailed floral phenological observations were conducted over two consecutive flowering seasons in June–July 2016 and 2017. A total of 30 flower buds from 12 individuals in two populations were tagged and monitored at 4-h intervals each day (1000–1800 h) throughout anthesis until abscission of the perianth tube. Observations focused on the following characteristics: anther dehiscence and release of pollen grains; presence of stigmatic exudate; changes in the colour and orientation of floral organs; the opening and closing of apertures between stamen filaments; and the abscission of the perianth tube.
Stigmatic receptivity was determined by assessing the presence of the peroxidase enzyme using the Peroxtesmo Ko test (Dafni and Maués, 1998). One 15 × 15-mm Peroxtesmo Ko paper (Macherey-Nagel, Düren, Germany) was soaked in 1 mL of distilled water, and a droplet of fresh solution was applied directly onto stigmas of varying ages, ranging from flower buds (1 d prior to the onset of anthesis) to flowers during the final day of anthesis. Pollen grains from newly opened flowers were used in artificial cross- and self-pollination treatments.
Controlled pollination experiments were conducted to determine the breeding system (June 2016 and 2017). Flower buds were enclosed in small nylon bags and then subjected to the following experimental treatments (Dafni, 1992): (1) the control, in which ten flowers from different individuals were bagged but untreated to evaluate the need for pollinators; (2) a test for autogamous self-pollination, in which ten flowers from different individuals were bagged and pollinated using pollen from the same flower; (3) a test for cross-pollination, in which nine flowers from different individuals were bagged and pollinated using pollen from another individual located >10 m away; and (4) a test of natural pollination, in which 38 flowers from 19 individuals were left unbagged, untreated and exposed to pollinators throughout. The fruit set resulting from each treatment was assessed in mid-August.
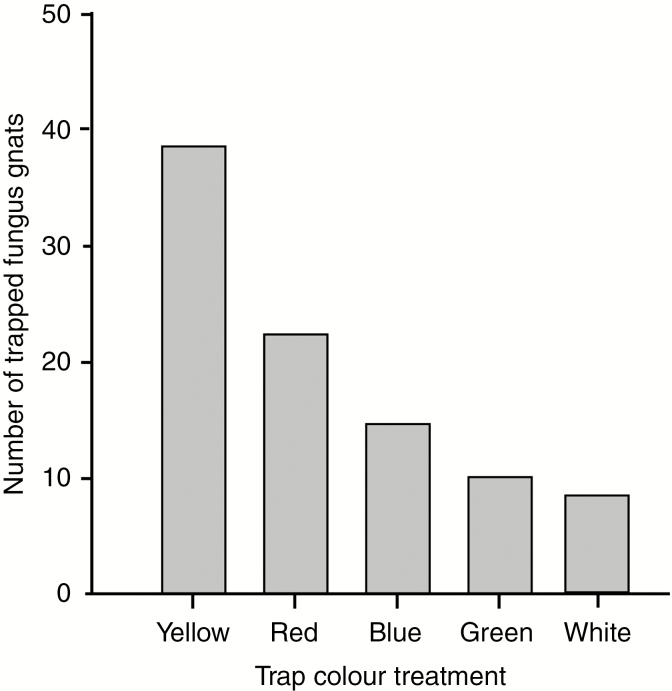
The colour preferences of floral visitors were assessed to determine whether the yellow annulus that surrounds the aperture at the apex of the perianth tube serves as a visual cue for pollinators. This was achieved using sticky traps (CatchMaster 904 Clear Window Fly Trap, China) overlaid on coloured cards (10 × 7 cm) with the following RGB colour values: red 255, 0, 0; yellow 255, 255, 0; blue 0, 0, 255; green 0, 255, 0; and white 255, 255, 255. Three traps were prepared for each colour treatment and mounted within the T. tentaculata populations at soil level. The traps were removed after 1 week, and the trapped insects counted and identified. Replicates were not performed in order to minimize the potential impact on the pollinator population.
Floral visitors were monitored from ~60 individuals in two populations, with the activities of floral visitors observed and recorded whenever possible. In order to determine the presence of pollen grains on floral visitors, the insects were sampled and dried using silica gel; they were then sputter-coated with gold–palladium and examined using a Hitachi S4800 (Tokyo, Japan) scanning electron microscope at 10–15 kV. Pollinators were identified by DNA barcoding of cytochrome c oxidase I, following a standard protocol (Lau et al., 2017). The sexes of pollinators were determined by detecting the reproductive organs under a stereo microscope (undertaken by Dr Junhao Huang, Zhejiang A&F University, China).
Seed dispersal
Field observations of fruiting in T. tentaculata were undertaken from mid-August until late September 2016. The fruits develop very close to the ground, with the seeds fully exposed at maturity in an upright cup-like hypanthium that closely resembles rain-splash dispersal structures in unrelated taxa (Nakanishi, 2002). A laboratory experiment was conducted to test the hypothesis of rain-splash seed dispersal, following methods detailed by Nakanishi (2002). A shoot with an open fruit capsule containing mature seeds was mounted upright on the floor, surrounded by 3 m2 of white paper to enable easier detection of the scattered seeds. Artificial raindrops (~0.1 mL) were dripped into the open capsule from a height of 2 m, and the distance over which the seeds were scattered from the capsule was measured. A total of 25 seeds were scored, although the limited number of plants available precluded further replication.
RESULTS
Mycorrhizal fungi
Intracellular hyphae (hy in Fig. 1H) were observed in the root cortical cells of T. tentaculata, often with arbuscules (ar in Fig. 1H) and vesicles (v in Fig. 1H). These morphological features suggest that the mycorrhizae are Paris-type arbuscular mycorrhizae. Appressoria at the entry point (ep in Fig. 1H) on the root epidermis were also observed, from which hyphae can penetrate into the host cortical parenchyma.
Amplicon sequencing produced 2 037 894 raw reads, of which 986 068 were clean. After assembly and quality control, 460 213 tags were obtained. A total of 251 OTUs were generated by clustering at 97 % similarity, of which 186 belong to the Glomeromycota; of these, six were found in all three T. tentaculata root samples and 54 were found in the root samples of neighbouring autotrophs. The other 132 OTUs were not found in all replicates and hence were excluded from subsequent analyses.
The majority of the sequences obtained from T. tentaculata, with relative abundance of 99.8 %, differed in only one base with two sequences (GenBank LN900906 and LN900647). A BLAST search against the MaarjAM database (Öpik et al., 2010) revealed that all these sequences belong to virtual taxon VTX00092, which corresponds to a species of Rhizophagus and was previously obtained from an unnamed Australian Thismia species (Merckx et al., 2017). The other five OTUs common to all three T. tentaculata root samples have low relative abundance (<0.05 %).
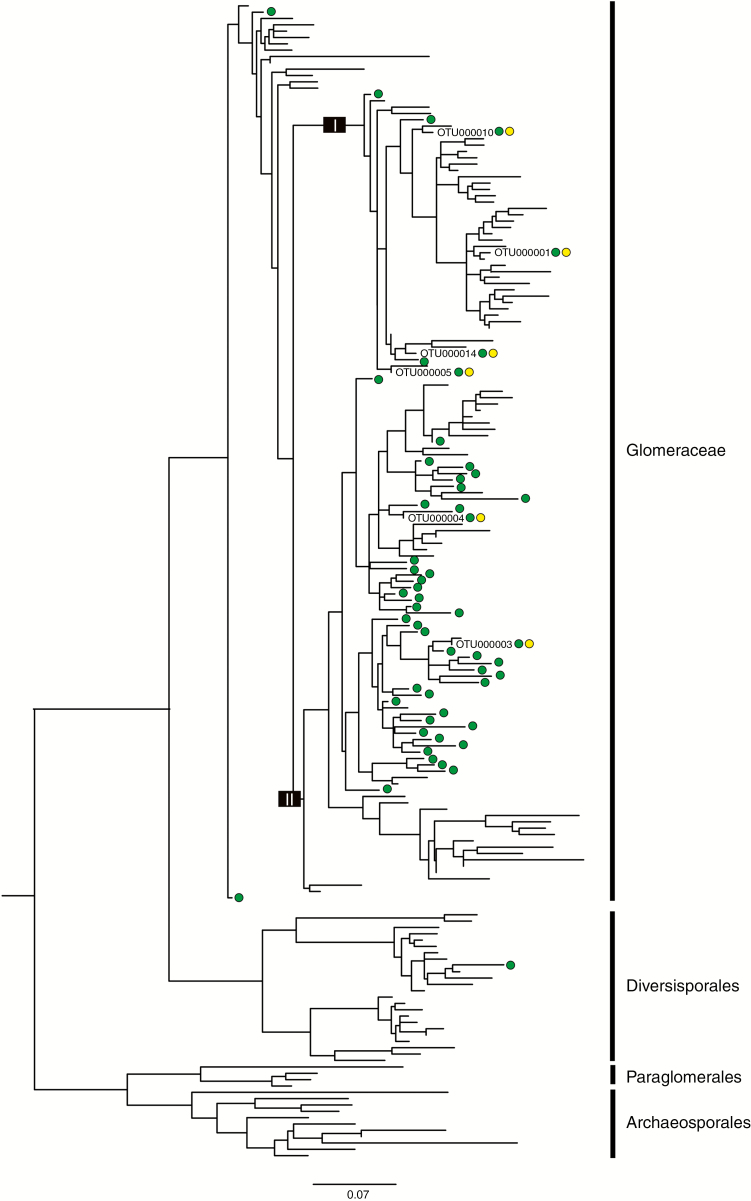
The best-scoring maximum likelihood tree of the fungal community associated with T. tentaculata and neighbouring autotrophs is presented as Fig. 2. The fungal OTUs were assigned to Glomeraceae clades I and II; amongst the autotrophic plants, the same fungal clades were retrieved with the addition of two OTUs located at the Glomeraceae basal grade and one OTU from the Diversisporales.
Fig. 2.
The best-scoring maximum likelihood tree of the fungal community associated with Thismia tentaculata and neighbouring autotrophs, showing phylogenetic relationships among the Glomeromycota OTUs found in all the samples, including reference sequences. Green dots indicate fungal OTUs present in neighbouring autotrophic plants; yellow dots are those found in T. tentaculata. The scale bar shows the number of substitutions per site.
Pollination ecology
Each T. tentaculata individual has up to five flowers that develop successively, although with only one flower per individual reaching anthesis concurrently. The species has a prolonged anthesis over 16 (rarely 17) d (the temporal change in floral structures of different anthesis stages is shown in Supplementary Data Fig. S1). A close floral synchrony was observed among individuals, with the majority of flowers within a population entering anthesis on the same day. The stigmas and stamens are functional concurrently throughout anthesis. An exudate was observed on the tip of each downwardly projecting stamen connective (arrowed in Fig. 1D).
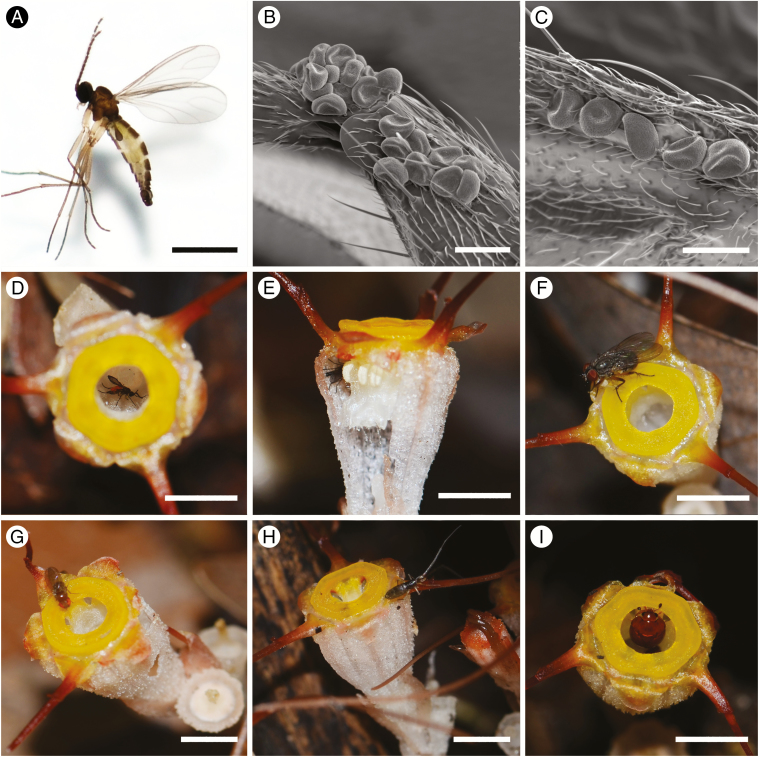
Floral visitors observed included fungus gnats (Fig. 3A, D, E), muscid flies (Fig. 3F), fruit flies (Fig. 3G), springtails (Fig. 3H) and nitidulid beetles (Fig. 3I). Larger beetles and fruit flies were observed to enter and escape from the perianth chamber via the annulus; they therefore failed to make contact with the thecae and therefore cannot be effective pollinators. Among the observed floral visitors, only fungus gnats (Fig. 3A) are potential pollinators since individuals were retrieved from between adjacent theca, and were observed to leave the perianth chamber via small apertures between the adjacent staminal filaments (Fig. 3E). Pollen grains of T. tentaculata were furthermore observed on the bodies of fungus gnats (Fig. 3B, C) that were collected before entering the perianth chamber; no pollen was found on the other floral visitors. A total of 16 fungus gnats were collected, including both sexes (five males and one female among six individuals assessed). All specimens belong to a single small, dark-winged fungus gnat species identified using DNA barcoding as belonging to the genus Corynoptera (Sciaridae).
Fig. 3.
Floral visitors to Thismia tentaculata. (A) Fungus gnat (Corynoptera sp.). (B, C) Scanning electron micrographs of pollen grains of T. tentaculata attached to the leg and wing of a Corynoptera fungus gnat. (D) A Corynoptera fungus gnat constrained within the perianth tube. (E) A fungus gnat escaping from the floral chamber via small apertures between the staminal filaments. (F) House fly. (G) Fruit fly. (H) Springtail. (I) Nitidulid beetle. Photographs by X. Guo. Scale bars: (A) = 0.5 mm; (B, C) = 20 µm; (D–I) = 5 mm.
The assessment of the colour preferences of the floral visitors revealed that fungus gnats were particularly attracted by the yellow traps mounted immediately above the soil level (Fig. 4).
Fig. 4.
Results of the colour trap experiments designed to assess the colour preference of the Corynoptera fungus gnats (see text for details of the experimental design).
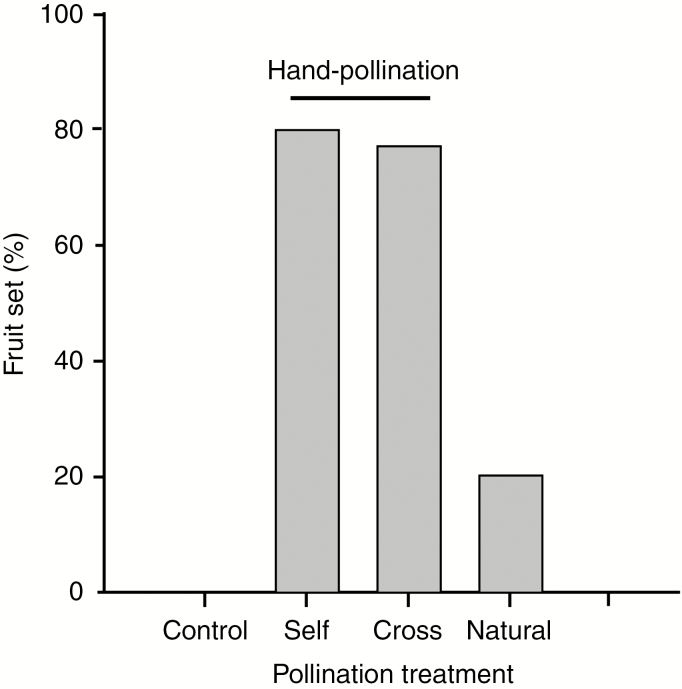
The hand-pollination experiments (Fig. 5) revealed an absence of fruit set in the bagged controls, indicating that autogamous fertilization does not occur. Thismia tentaculata is self-compatible, with 80 and 78 % fruit set resulting from the self- and cross-pollination treatments, respectively. Individuals that were left to be pollinated naturally yielded 21 % fruit set.
Fig. 5.
Percentage fruit set in T. tentaculata resulting from different controlled pollination experiments (see text for explanation of treatments).
Seed dispersal
Fruit capsules that were observed in situ to contain numerous seeds prior to the onset of rain were subsequently observed to be empty (Fig. 1F, G), suggesting that raindrops had resulted in seed dispersal. This was confirmed by direct observation of raindrops hitting the fruit capsules. The laboratory experiment with artificial raindrops indicated rain-splash dispersal over distances of 9.3–78 cm, with a mean of 28.2 cm (x = 25).
DISCUSSION
Specialization towards a narrow range of mycorrhizal fungi
Phylogenetic analysis of the arbuscular mycorrhizal fungi indicates that T. tentaculata is associated with relatively few fungal OTUs, all of which belong to a narrow lineage within Glomeraceae (Fig. 2). Among the six arbuscular mycorrhizal fungi associated with T. tentaculata, OTU000001 (virtual taxon VTX00092) has 59 666 effective sequences and the highest relative abundance (99.8 %, Fig. 2). This overwhelming abundance suggests that VTX00092 is the dominant host fungus of T. tentaculata. In contrast, the other five fungal OTUs have considerably lower relative abundance (<0.05 %). Only one tag was obtained for OTU000004, OTU000010 and OTU000014 in at least one T. tentaculata sample, and 3–19 tags were obtained for OTU000003 and OTU000005. Given the very small number of tags, it is likely that these sequences may originate from the soil or surrounding roots and they can therefore be considered unimportant components. A single virtual taxon, VTX00092 (identified as Rhizophagus by Merckx et al., 2017), is involved in the obligate three-way symbiosis between T. tentaculata and its autotrophic hosts: T. tentaculata therefore maintains a highly specialized fungal association.
The Rhizophagus species VTX00092 obtained from T. tentaculata has also been recorded from an unnamed Thismia species from Australia (Merckx et al., 2017) and the sister genus Afrothismia from West Africa (Merckx and Bidartondo, 2008), suggesting that this fungal species is geographically widely distributed. It is therefore clear that the occurrence of the fungi does not limit the dispersal potential of the mycoheterotrophs. Highly specific and phylogenetically conserved arbuscular mycorrhizal interactions have been documented in previous studies of the Thismiaceae (Merckx and Bidartondo, 2008; Merckx et al., 2012, 2017): in some Thismia species from Australia, mycorrhizal interactions are strictly bound to a Rhizophagus fungal lineage (Gomes et al., 2017; Merckx et al., 2017) and Afrothismia species are dependent on a subclade of Glomus fungi (Merckx and Bidartondo, 2008; Merckx et al., 2017). Such a high degree of specialization is thought to constrain plant distribution, at least limiting it to a narrower geographical range than that of the fungus. It is hypothesized that species with highly specific ecological interactions are likely to be more vulnerable to anthropogenic change (Kiers et al., 2010; Ellers et al., 2012) and have lower diversification potential than generalists (Poisot et al., 2011). Thismia is the most species-rich genus in Thismiaceae, however, with ~70 species (Kumar et al., 2017), and is considerably more diverse than its closest extant autotrophic relative, Tacca (13 species: Govaerts et al., 2007), suggesting increased speciation and/or reduced extinction rates. A recent biogeographical study (Merckx et al., 2017) suggests that Thismia has diversified and radiated recently due to the broad geographical distribution of the host fungi. A high degree of mycorrhizal specificity therefore does not necessarily limit the distributional range of the plants and hence is not a barrier to further diversification.
Associations with narrow lineages of arbuscular mycorrhizal fungi have also been found in mycoheterotrophic species in the Burmanniaceae, Corsiaceae, Gentianaceae and Triuridaceae of Africa and South America (Merckx et al., 2012). It should be noted that not all mycoheterotrophs show specialization in their fungal associations. Some mycoheterotrophs are also able to associate with a wide range of fungal lineages (Merckx et al., 2012): Sciaphila ledermannii (Triuridaceae), for example, is associated with 13 virtual fungal taxa, including members of the Acaulosporaceae, Gigasporaceae and Glomeraceae; and Campylosiphon congestus (Burmanniaceae) is associated with at least eight virtual fungal taxa in the Acaulosporaceae and Glomeraceae. Strict mycorrhizal loyalty towards a narrow range of arbuscular mycorrhizal fungi is therefore not a requirement for mycoheterotrophs; indeed, it may be less common than previously assumed, with additional sampling likely to reveal further examples of broad fungal host ranges.
Despite the strict mycorrhizal specificity shown by some mycoheterotrophs, different species are often observed to occur sympatrically. In our study site, T. tentaculata co-occurs with its congener T. hongkongensis, as well as Sciaphila ramosa, Sciaphila secundiflora and Burmannia championii. An unnamed Australian Thismia species was similarly found to co-occur with its closely related congener, T. clavarioides (Merckx et al., 2017). As previously mentioned, these sympatric species have been shown to associate with distinct fungi: this partitioning of fungi between sympatric mycoheterotrophs may be a mechanism to avoid nutritional competition and thus facilitate co-occurrence, as proposed for some specialized photosynthetic orchids (Waterman et al., 2011; Jacquemyn et al., 2014).
Specialist fungus gnat pollination
Thismia tentaculata has an extended anthesis period over 16 (rarely 17) d, although individual plants flower over considerably longer periods since they possess up to five flowers that enter anthesis consecutively. Controlled pollination experiments reveal that the species is self-compatible, with fruit set following artificial self-pollination (80 %) similar to that arising from cross-pollination (78 %) (Fig. 5). The lower fruit set rate (21 %) in natural conditions may suggest pollinator limitation. Flowers bagged prior to anthesis did not produce fruit, indicating that spontaneous autogamy does not occur; this may be due, at least in part, to the lateral appendages (la in Fig. 1C, E) that possibly prevent pollen from falling onto the stigmas. Contrary to earlier predictions based on dissected flowers of T. hongkongensis (Mar and Saunders, 2015), there is no evidence of protandry in T. tentaculata.
Although various insects, including fungus gnats, fruit flies, beetles and springtails, were observed to visit T. tentaculata flowers, only Corynoptera fungus gnats (Sciaridae; Fig. 3A, D, E) were identified as effective pollinators based on the presence of pollen on their body (head, thorax, abdomen, wings and legs: Fig. 3B, C) and evidence of inter-floral movement. These observations are consistent with earlier inferences of the presumed pollinators of T. hongkongensis, based on the retrieval of dead fungus gnats (Mycetophilidae/Sciaridae) and scuttle flies (Phoridae) from the floral chamber (Mar and Saunders, 2015).
Fungus gnats (Mycetophilidae and Sciaridae) are small, short-lived and weak-flying insects common in moist forests, with larvae that feed on mushrooms and fungal hyphae in decaying plant materials. Pollination by fungus gnats is uncommon, but is nevertheless known to occur in 11 angiosperm families: Orchidaceae, Liliaceae, Asparagaceae, Araceae, Aristolochiaceae, Polygonaceae, Apocynaceae, Saxifragaceae, Garryaceae, Celastraceae and Hamamelidaceae (Mochizuki and Kawakita, 2017 and references therein). Mechanisms used by these plants to attract fungus gnats are diverse, including mimicry of fungal oviposition sites (Sugawara, 1988; Scanlen, 2006), sexual deception (Blanco and Barboza, 2005; Phillips et al., 2014) and nectar reward (Goldblatt et al., 2004; Okuyama et al., 2004).
The fungus gnats associated with T. tentaculata are likely to be attracted to the flowers by visual cues (and possibly also olfactory cues, although these are not assessed here). The movements of the fungus gnats as they arrive at the flower suggest that the orange-red tepal appendages (Fig. 1A) may function as footholds, as is observed in the branched petals of the fungus gnat-pollinated Mitella pauciflora (Katsuhara et al., 2017). The elongated tepal appendages of neighbouring individuals often overlap, possibly providing a pathway for pollinators between flowers. In general, fungus gnats are attracted to bright colours such as yellow, which is used to trap sciarid flies in greenhouses (Cloyd and Dickinson, 2005). The bright yellow annulus (Fig. 1D) may act as a visual cue for pollinators, directing their entry into the perianth chamber. This is supported by the results of the colour test: the yellow insect traps mounted at ground level attracted considerably more fungus gnats (2-fold) than the other coloured traps (Fig. 4). Although T. tentaculata flowers emit little human-detectable scent during anthesis, it would be fascinating to know whether the floral scent mimics the fruiting bodies of mushrooms to attract gnats that deposit their eggs in the flowers, highlighting the utility of floral scent analyses using gas chromatography–mass spectrometry.
The fungus gnats follow a tightly controlled unidirectional route (arrowed in Fig. 1B) as they enter and leave the flowers, entering through the apical aperture within the yellow annulus and then leaving via one of the six small apertures located between the filaments of the pendent stamens (ap in Fig. 1A, C) that are suspended on the inside of the perianth chamber from the annulus. Hairs are attached to the apex of the pendent staminal connectives (h in Fig. 1C); similar hairs have been reported for other Thismia species (e.g. T. hongkongensis; Mar and Saunders, 2015), although their function has never been elucidated before. We observed small droplets of slightly viscous exudate (arrowed in Fig. 1D) forming at the tip of each pendent hair. Although the volume of exudate was too small to enable chemical analysis, we observed springtails (although not fungus gnats) apparently consuming this exudate, suggesting that it may function as a nutritive reward. Having entered the floral chamber, the fungus gnats are temporarily restrained within the perianth chamber (Supplementary Data Video S1) by the lateral appendages and cannot easily escape through the apical aperture. The fungus gnats are likely to make contact with the stigmas, which are located at the base of the chamber (st in Fig. 1B). We observed that many fungus gnats were temporarily attached to the sticky stigmatic exudate, and often had to struggle to release themselves (Supplementary Data Video S2); this prolonged activity inevitably increases the contact time between the fungus gnat and the stigma, thereby enhancing opportunities for pollen deposition and successful pollination.
We observed fungus gnats escaping from the floral chamber via small apertures between the staminal filaments (Figs 1C and 3E), having climbed the ribbed inner wall of the perianth chamber and passing between the lateral appendages attached to the stamens (la in Fig. 1C, E). As the gnats depart the flower they are likely to make contact with the dehiscent anthers, collecting pollen.
Seed dispersal
Seeds of T. tentaculata are dispersed by rain splash over short distances of <1 m, although secondary dispersal, possibly with the seeds carried further afield by temporary rain channels, may increase dispersal range. It is notable that T. tentaculata produces numerous tiny seeds. This could be a reproductive strategy to maximize the likelihood of at least a few offspring locating a suitable fungal host (Waterman et al., 2013). The large-scale fertilization of numerous ovules required for the production of such vast quantities of seed would be dependent on a very efficient pollination system. Our field observations suggest that populations of fungus gnats are generally very extensive, and that pollinator visits to the flowers are likely to be frequent.
Symbiotic balancing of specialization towards fungi and pollinators
Highly specialized symbiosis has often been regarded as a potential evolutionary dead end (Cope, 1896; Gould, 1970; Futuyma and Moreno, 1988), with generalists having greater potential to adapt to new environments and hence providing more opportunities for speciation and lowering their risk of extinction (Zayed et al., 2005). Specialists, in contrast, require numerous adaptations to enable reliance on a particular resource and this may lead lineages to become increasingly committed to that niche, making evolutionary reversal or a shift to a more generalist system unlikely (Futuyma and Moreno, 1988). For mycoheterotrophic plants, it has been hypothesized that specialization involving multiple symbiotic interactions might be an evolutionarily unstable strategy (Bidartondo, 2005); mycoheterotrophs that already engage in highly specialized symbiotic interactions with a narrow range of arbuscular mycorrhizal fungi would therefore be unlikely to develop a specialized pollination dependence. It has also been hypothesized that a restriction to habitats with few pollinators may lead to reliance on self-pollination among mycoheterotrophs (Dressler, 1981; Leake, 1994). Interestingly, our pollination study indicates that T. tentaculata is largely xenogamous and relies on highly specialized pollen dispersal agents (Corynoptera fungus gnats).
The observed specialization towards both mycorrhizae and pollinators provides support for the hypothesis proposed by Waterman et al. (2013): the maintenance of dual specializations would be possible if symbiotic associates do not exert redundant selective pressures that further restrict an already constrained suite of life history traits. Despite its pollinator specificity, Thismia species appear to rely on a common and abundant pollen dispersal agent across their distribution ranges; fungus gnats are common and important pollinators in moist habitats such as understorey forests, streamsides or subalpine meadows (Mochizuki and Kawakita, 2017). The distribution ranges of Thismia species, which are possibly already restricted due to their reliance on a narrow range of arbuscular mycorrhizal fungi, are therefore unlikely to be further limited by their symbiosis with fungus gnats. Additional examples of mycoheterotrophs with specialized pollination systems include the mycoheterotrophic species Monotropa uniflora (Monotropaceae), pollinated by Bombus bees (Klooster and Culley, 2009), and Cryptostylis hunteriana (Orchidaceae), pollinated by a species of ichneumon wasp (Lissopimpla excelsa) via pseudo-copulation (Nicholls, 1938). It is notable that in these examples both the Lissopimpla wasps and Bombus bees have wide distributions; as with the Corynoptera fungus gnats in the present study, a common or widely distributed partner may promote specialization. More thorough investigations into specializations towards both fungi and pollinators will help reveal whether ‘double specialists’ are common among mycoheterotrophs.
While the specialization of mycoheterotrophs above and below ground can be promoted by the wide distribution of arbuscular mycorrhizal fungi and pollinators, long-term evolutionary instability, as suggested by Bidartondo (2005), is also possible. The evolution of a trait might be unstable in the long term, but is nevertheless possible if the trait provides a short-term benefit, which specialization often does. The specialized pollination system in T. tentaculata might be beneficial in the short term if the comparatively large quantity of pollen transferred by fungus gnats ensures extensive seed set. Future research could include an investigation of whether specialization in both arbuscular mycorrhizal fungi and pollinators can persist over long periods in evolutionary history.
Specificity in arbuscular mycorrhizal fungi and pollinators may drive species diversity in Thismia indirectly by affecting their patterns of distribution. Despite the vast number of tiny seeds produced, T. tentaculata seeds are only dispersed over short distances by rain splash, and long-range dispersal is likely to be rare. In accordance with the drift–selection model (sensuTremblay et al., 2005), patchy fungal distributions, together with high mycorrhizal specificity, could contribute to small population sizes and reduced gene flow. This combination might lead to rapid genetic drift and strong selection, and ultimately cause differentiation between subdivided populations and incipient speciation. Molecular phylogenetic studies of mycoheterotrophs are particularly challenging, not least because of the scarcity of study materials. As new data on mycoheterotrophs and their autotrophic relatives gradually become available, however, it may be possible to compare diversification rates, and detect changes in speciation or extinction rates associated with shifts in symbiotic specializations.
CONCLUSIONS
This study addresses a fundamental gap in our knowledge of specialization of mycoheterotrophs with regard to arbuscular mycorrhizal fungi and pollinators. The results of our mycorrhizal community analyses demonstrate that the mycoheterotroph T. tentaculata maintains a symbiotic association with a single arbuscular mycorrhizal Rhizophagus species, on which it is dependent for its carbon supply. Pollination ecology observations suggest that the flowers are predominantly xenogamous and pollinated by a single species of fungus gnats (Corynoptera, Sciaridae), which are attracted by the yellow pigments and are temporarily restrained within the perianth chamber before departing via apertures between the anthers. The maintenance of dual specializations in T. tentaculata, which has previously been hypothesized as an evolutionarily unstable strategy, is possible in this case due to the wide distribution of both the Rhizophagus fungi and Corynoptera fungus gnats.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: GenBank accession numbers of samples used in the phylogenetic analyses. Figure S1: Thismia tentaculata, showing flowers at different phenological stages. Video S1: a Corynoptera fungus gnat circling within the floral tube of Thismia tentaculata. Video S2: a Corynoptera fungus gnat temporarily stuck on the stigma of Thismia tentaculata.
FUNDING
This work was financially supported by the Environment and Conservation Fund of the Hong Kong Government (grant number ECF 2015–41), awarded to R.M.K.S.
ACKNOWLEDGEMENTS
We gratefully acknowledge the assistance of Dr Junhao Huang (Zhejiang A&F University, China) and Dr Ming Bai (Institute of Zoology, China) for their help in identifying the floral visitors. We are also very grateful to Anping Chai for assistance with the field study, Zongxin Ren and Ziyin Yang for useful discussion, and Laura Wong for general technical support.
LITERATURE CITED
- Benton MJ. 2009. The Red Queen and the Court Jester: species diversity and the role of biotic and abiotic factors through time. Science 323: 728–732. [DOI] [PubMed] [Google Scholar]
- Bidartondo MI. 2005. The evolutionary ecology of mycoheterotrophy. New Phytologist 167: 335–352. [DOI] [PubMed] [Google Scholar]
- Blanco MA, Barboza G. 2005. Pseudocopulatory pollination in Lepanthes (Orchidaceae: Pleurothallidinae) by fungus gnats. Annals of Botany 95: 763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, et al. . 2010. QIIME allows analysis of high-throughput community sequencing data. Nature Methods 7: 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloyd RA, Dickinson A. 2005. Effects of growing media containing diatomaceous earth on the fungus gnat Bradysia sp. nr. coprophila (Litner) (Diptera: Sciaridae). Hortscience 40: 1806–1809. [Google Scholar]
- Cope ED. 1896. The primary factors of organic evolution. Chicago: Open Court Publishing. [Google Scholar]
- Dafni A. 1992. Pollination ecology: a practical approach. Oxford: Oxford University Press. [Google Scholar]
- Dafni A, Maués MM. 1998. A rapid and simple procedure to determine stigma receptivity. Sexual Plant Reproduction 11: 177–180. [Google Scholar]
- Dressler RL. 1981. The orchids – natural history and classification. Cambridge, UK: Harvard University Press. [Google Scholar]
- Edgar RC. 2013. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nature Methods 10: 996–998. [DOI] [PubMed] [Google Scholar]
- Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. 2011. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27: 2194–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellers J, Kiers ET, Currie CR, McDonald BM, Visser B. 2012. Ecological interactions drive evolutionary loss of traits. Ecology Letters 15: 1071–1082. [DOI] [PubMed] [Google Scholar]
- Finlay RD. 2008. Ecological aspects of mycorrhizal symbiosis: with special emphasis on the functional diversity of interactions involving the extraradical mycelium. Journal of Experimental Botany 59: 1115–1126. [DOI] [PubMed] [Google Scholar]
- Futuyma DJ, Moreno G. 1988. The evolution of ecological specialization. Annual Review of Ecology and Systematics 19: 207–233. [Google Scholar]
- Van Geel M, Busschaert P, Honnay O, Lievens B. 2014. Evaluation of six primer pairs targeting the nuclear rRNA operon for characterization of arbuscular mycorrhizal fungal (AMF) communities using 454 pyrosequencing. Journal of Microbiological Methods 106: 93–100. [DOI] [PubMed] [Google Scholar]
- Giovannetti M, Sbrana C, Avio L, Strani P. 2004. Patterns of below-ground plant interconnections established by means of arbuscular mycorrhizal networks. New Phytologist 164: 175–181. [DOI] [PubMed] [Google Scholar]
- Goldblatt P, Bernhardt P, Vogan P, Manning JC. 2004. Pollination by fungus gnats (Diptera: Mycetophilidae) and self-recognition sites in Tolmiea menziesii (Saxifragaceae). Plant Systematics and Evolution 244: 55–67. [Google Scholar]
- Gomes SIF, Aguirre Gutierrez J, Bidartondo MI, Merckx VSFT. 2017. Arbuscular mycorrhizal interactions of Thismia are more specialized than autotrophic plants. New Phytologist 213: 1418–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould SJ. 1970. Dollo on Dollo’s law: irreversibility and the status of evolutionary laws. Journal of the History of Biology 3: 189–212. [DOI] [PubMed] [Google Scholar]
- Govaerts R, Wilkin P, Saunders RMK. 2007. World checklist of Dioscoreales: yams and their allies. London: Kew Publishing. [Google Scholar]
- Hijri M, Sanders IR. 2005. Low gene copy number shows that arbuscular mycorrhizal fungi inherit genetically different nuclei. Nature 433: 160–163. [DOI] [PubMed] [Google Scholar]
- Ho GWC, Mar SS, Saunders RMK. 2009. Thismia tentaculata (Burmanniaceae tribe Thismieae) from Hong Kong: first record of the genus and tribe from continental China. Journal of Systematics and Evolution 47: 605–607. [Google Scholar]
- Hoorn C, Wesselingh FP, ter S teege H, et al. . 2010. Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science 330: 927–931. [DOI] [PubMed] [Google Scholar]
- Hughes CE, Atchison GW. 2015. The ubiquity of alpine plant radiations: from the Andes to the Hengduan Mountains. New Phytologist 207: 275–282. [DOI] [PubMed] [Google Scholar]
- Jacquemyn H, Brys R, Merckx VSFT, Waud M, Lievens B, Wiegand T. 2014. Co-existing orchid species have distinct mycorrhizal communities and display strong spatial segregation. New Phytologist 202: 616–627. [DOI] [PubMed] [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30: 3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuhara KR, Kitamura S, Ushimaru A. 2017. Functional significance of petals as landing sites in fungus-gnat pollinated flowers of Mitella pauciflora (Saxifragaceae). Functional Ecology 31: 1193–1200. [Google Scholar]
- Kiers ET, Palmer TM, Ives AR, Bruno JF, Bronstein JL. 2010. Mutualisms in a changing world: an evolutionary perspective. Ecology Letters 13: 1459–1474. [DOI] [PubMed] [Google Scholar]
- Klooster MR, Culley TM. 2009. Comparative analysis of the reproductive ecology of Monotropa and Monotropsis: two mycoheterotrophic genera in the Monotropoideae (Ericaceae). American Journal of Botany 96: 1337–1347. [DOI] [PubMed] [Google Scholar]
- Kumar P, Gale SW, Li J-H, Bouamanivong S, Fischer GA. 2017. Thismia nigricoronata, a new species of Burmanniaceae (Thismieae, Dioscoreales) from Vang Vieng, Vientiane Province, Laos, and a key to subgeneric classification. Phytotaxa 319: 225–240. [Google Scholar]
- Lagomarsino LP, Condamine FL, Antonelli A, Mulch A, Davis CC. 2016. The abiotic and biotic drivers of rapid diversification in Andean bellflowers (Campanulaceae). New Phytologist 210: 1430–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen K, Averyanov LV. 2007. Thismia annamensis and Thismia tentaculata, two new species of Thismiaceae from central Vietnam. Rheedea 17: 13–19. [Google Scholar]
- Lau JYY, Guo X, Pang CC, Tang CC, Thomas DC, Saunders RMK. 2017. Time-dependent trapping of pollinators driven by the alignment of floral phenology with insect circadian rhythms. Frontiers in Plant Science 8: 1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake JR. 1994. The biology of myco-heterotrophic (‘saprophytic’) plants. New Phytologist 127: 171–216. [DOI] [PubMed] [Google Scholar]
- Li H-Q, Bi Y-K. 2013. A new species of Thismia (Thismiaceae) from Yunnan, China. Phytotaxa 105: 25–28. [Google Scholar]
- Magoc T, Salzberg SL. 2011. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27: 2957–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar SS, Saunders RMK. 2015. Thismia hongkongensis (Thismiaceae): a new mycoheterotrophic species from Hong Kong, China, with observations on floral visitors and seed dispersal. PhytoKeys 46: 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merckx VSFT. 2013. Mycoheterotrophy. New York: Springer. [Google Scholar]
- Merckx V, Bidartondo MI. 2008. Breakdown and delayed cospeciation in the arbuscular mycorrhizal mutualism. Proceedings of the Royal Society B: Biological Sciences 275: 1029–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merckx VSFT, Janssens SB, Hynson NA, Specht CD, Bruns TD, Smets EF. 2012. Mycoheterotrophic interactions are not limited to a narrow phylogenetic range of arbuscular mycorrhizal fungi. Molecular Ecology 21: 1524–1532. [DOI] [PubMed] [Google Scholar]
- Merckx VSFT, Gomes SIF, Wapstra M, et al. . 2017. The biogeographical history of the interaction between mycoheterotrophic Thismia (Thismiaceae) plants and mycorrhizal Rhizophagus (Glomeraceae) fungi. Journal of Biogeography 44: 1869–1879. [Google Scholar]
- Miers J. 1866. On Myostoma, a new genus of the Burmanniaceae. Transactions of the Linnean Society of London 25: 461–476. [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE), 14 November 2010,New Orleans, LA, 1–8. [Google Scholar]
- Mochizuki K, Kawakita A. 2017. Pollination by fungus gnats and associated floral characteristics in five families of the Japanese flora. Annals of Botany 121: 651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molvray M, Kores PJ, Chase MW. 2000. Polyphyly of mycoheterotrophic orchids and functional influences on floral and molecular characters. In: Wilson KL, Morrison DA, eds. Monocots: systematics and evolution. Melbourne: CSIRO, 441–447. [Google Scholar]
- Nakanishi H. 2002. Splash seed dispersal by raindrops. Ecological Research 17: 663–671. [Google Scholar]
- Nicholls WH. 1938. A new species of the genus Cryptostylis R. Br. Victorian Naturalist 54: 182–183. [Google Scholar]
- Van der Niet T, Johnson SD. 2012. Phylogenetic evidence for pollinator-driven diversification of angiosperms. Trends in Ecology and Evolution 27: 353–361. [DOI] [PubMed] [Google Scholar]
- Okuyama Y, Kato M, Murakami N. 2004. Pollination by fungus gnats in four species of the genus Mitella (Saxifragaceae). Botanical Journal of the Linnean Society 144: 449–460. [Google Scholar]
- Öpik M, Vanatoa A, Vanatoa E, et al. . 2010. The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytologist 188: 223–241. [DOI] [PubMed] [Google Scholar]
- Poisot T, Bever JD, Nemri A, Thrall PH, Hochberg ME. 2011. A conceptual framework for the evolution of ecological specialization. Ecology Letters 14: 841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RD, Scaccabarozzi D, Retter BA, et al. . 2014. Caught in the act: pollination of sexually deceptive trap-flowers by fungus gnats in Pterostylis (Orchidaceae). Annals of Botany 113: 629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast C, Pruesse E, Yilmaz P, et al. . 2013. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Research 41: D590–D596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts N, Wapstra M, Duncan F, Woolley A, Morley J, Fitzgerald N. 2003. Shedding some light on Thismia rodwayi F. Muell. (fairy lanterns) in Tasmania: distribution, habitat and conservation status. Papers and Proceedings of the Royal Society of Tasmania 137: 55–66. [Google Scholar]
- Sato K, Suyama Y, Saito M, Sugawara K. 2005. A new primer for discrimination of arbuscular mycorrhizal fungi with polymerase chain reaction-denature gradient gel electrophoresis. Grassland Science 51: 179–81. [Google Scholar]
- Scanlen E. 2006. Flies’ eggs in Nematoceras triloba agg. New Zealand Native Orchid Group Journal 98: 34. [Google Scholar]
- Smith SE, Read DJ. 2008. Mycorrhizal symbiosis, 3rd edn. Cambridge: Academic Press. [Google Scholar]
- Smith CI, Godsoe WKW, Tank S, Yoder JB, Pellmyr O. 2008. Distinguishing coevolution from covicariance in an obligate pollination mutualism: asynchronous divergence in Joshua tree and its pollinators. Evolution 62: 2676–2687. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. 2006. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- Suetsugu K. 2014. Gastrodia flexistyloides (Orchidaceae), a new mycoheterotrophic plant with complete cleistogamy from Japan. Phytotaxa 175: 270–274. [Google Scholar]
- Suetsugu K. 2015. Autonomous self-pollination and insect visitors in partially and fully mycoheterotrophic species of Cymbidium (Orchidaceae). Journal of Plant Research 128: 115–125. [DOI] [PubMed] [Google Scholar]
- Sugawara T. 1988. Floral biology of Heterotropa tamaensis (Aristolochiaceae) in Japan. Plant Species Biology 3: 7–12. [Google Scholar]
- Thompson JN. 2005. The geographic mosaic of coevolution. Chicago: University of Chicago Press. [Google Scholar]
- Thorogood CJ, Rumsey FJ, Harris SA, Hiscock SJ. 2008. Host-driven divergence in the parasitic plant Orobanche minor Sm. (Orobanchaceae). Molecular Ecology 17: 4289–4303. [DOI] [PubMed] [Google Scholar]
- Thorogood CJ, Rumsey FJ, Hiscock SJ. 2009. Host-specific races in the holoparasitic angiosperm Orobanche minor: implications for speciation in parasitic plants. Annals of Botany 103: 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay RL, Ackerman JD, Zimmerman JK, Calvo RN. 2005. Variation in sexual reproduction in orchids and its evolutionary consequences: a spasmodic journey to diversification. Biological Journal of the Linnean Society 84: 1–54. [Google Scholar]
- Waterman RJ, Bidartondo MI. 2008. Deception above, deception below: linking pollination and mycorrhizal biology of orchids. Journal of Experimental Botany 59: 1085–1096. [DOI] [PubMed] [Google Scholar]
- Waterman RJ, Bidartondo MI, Stofberg J, et al. . 2011. The effects of above- and belowground mutualism on orchid speciation and coexistence. American Naturalist 177: E54–E68. [DOI] [PubMed] [Google Scholar]
- Waterman RJ, Klooster MR, Hentrich H, Bidartondo MI. 2013. Species interactions of mycoheterotrophic plants: specialization and its potential consequences. In: Merckx V, ed. Mycoheterotrophy. New York: Springer, 267–296. [Google Scholar]
- Yamato M, Yagame T, Shimomura N, et al. . 2011. Specific arbuscular mycorrhizal fungi associated with non-photosynthetic Petrosavia sakuraii (Petrosaviaceae). Mycorrhiza 21: 631–639. [DOI] [PubMed] [Google Scholar]
- Yang YN, Ba L, Bai XN, Zhang LC, Wang DL. 2010. An improved method to stain arbuscular mycorrhizal fungi in plant roots. Acta Ecologica Sinica 30: 774–779. [Google Scholar]
- Zayed A, Packer L, Grixti JC, Ruz L, Owen RE, Toro H. 2005. Increased genetic differentiation in a specialist versus a generalist bee: implications for conservation. Conservation Genetics 6: 1017–1026. [Google Scholar]
- Zhang DX, Saunders RMK. 2000. Reproductive biology of a mycoheterotrophic species, Burmannia wallichii (Burmanniaceae). Botanical Journal of the Linnean Society 132: 359–367. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.