Abstract
Introduction: Hospitalized patients with urinary tract infections (UTIs) often present with comorbid illnesses and are subsequently prescribed multiple medications, which increases the likelihood of drug-drug interactions. Therefore, this study aimed to explore the prevalence, levels, risk factors, and clinical relevance of potential drug-drug interactions (pDDIs) in hospitalized patients with UTIs. Secondly, we aimed to develop management guidelines and identify monitoring parameters for the most frequent interactions.
Methods: A retrospective cross-sectional study was conducted in internal medicine wards of two tertiary care hospitals in Peshawar, Khyber Pakhtunkhwa, Pakistan. The clinical profiles of 422 patients with UTIs were reviewed for pDDIs using the Micromedex Drug-Reax®. Logistic regression was applied to assess the association of pDDIs with various risk factors. The clinical relevance of frequent pDDIs was identified by assessing the potential adverse outcomes of pDDIs including patients’ signs, symptoms, and abnormal laboratory findings.
Results: Of 422 patients, at least one pDDI was identified in 62.3% patients, while 40% patients had at least one major pDDI. A total of 1,086 pDDIs were identified, of which 53.4% and 39.3% were of moderate and major severity, respectively. Patients with most frequent pDDIs were presented with hypoglycemia, hepatotoxicity, nephrotoxicity, hypertension, and decreased therapeutic response. These adverse events were more prevalent in patients taking higher doses of interacting drugs. Multivariate regression analysis revealed significant association of pDDIs with six or more medicines (p < 0.001), diabetes mellitus (p < 0.001), ischemic heart disease (p = 0.02), and congestive cardiac failure (p = 0.04).
Conclusions: Patients with UTIs present with a considerable number of clinically important pDDIs. Polypharmacy, diabetes mellitus, ischemic heart disease, and congestive cardiac failure increase the risk of pDDIs. Knowledge about the most frequent pDDIs will enable healthcare professionals to implement optimized monitoring and management strategies regarding associated adverse consequences in order to ensure patient safety. Most of the interactions can be managed by considering alternative therapy and dose reduction.
Keywords: patient safety, potential drug-drug interactions, urinary tract infections, clinical relevance, polypharmacy, adverse drug effects
Introduction
Urinary tract infections (UTIs) are among the major health problems that affect millions of people (Foxman, 2010; Blondal et al., 2016). Each year, in the United States UTIs account for nearly seven million clinic visits, one million emergency visits, and 100,000 hospital admissions (Schappert, 1999).
Patients with UTIs are hospitalized due to the severe nature of the disease, comorbid illnesses, and associated complications (Briongos-Figuero et al., 2012). Such patients are usually prescribed with antipyretics and antibiotics including cephalosporins, aminoglycosides, and quinolones (Dhodi et al., 2014; Panayappan et al., 2017). Apart from the use of these drugs, a large number of other drugs are also prescribed in order to treat the associated symptoms and comorbid illnesses (Dhodi et al., 2014). The simultaneous use of such large number of drugs increases the risk of drug-drug interactions (DDIs) by altering the pharmacokinetic parameters or the pharmacodynamic profile of drugs (Zwart-van-Rijkom et al., 2009; Juurlink et al., 2013). DDIs may lead to a number of undesirable consequences such as decreased or abolished clinical effectiveness, adverse effects, hospitalization, and prolongation of hospital stay (Ray et al., 2004; Juurlink et al., 2013; Khan et al., 2017). DDIs account for 20–30% of adverse effects, of which 70% require clinical intervention and 1–2% are life-threatening (Kohler et al., 2000). Hence, proper consideration of DDIs and their timely management is essential for the safe and effective use of medicines among patients with UTIs.
Studies have addressed the issue of potential DDIs (pDDIs) in hospitalized patients (Zwart-van-Rijkom et al., 2009) as well as in specific clinical specialties such as oncology (Van-Leeuwen et al., 2013), cardiology (Murtaza et al., 2016; Khan et al., 2017), psychiatry (Hahn et al., 2013), and internal medicine (Vonbach et al., 2007; Ismail et al., 2013). Moreover, some studies have investigated pDDIs among patients with specific infectious diseases such as hepatitis C and acquired immune deficiency syndrome (Patel et al., 2011; Kondili et al., 2017; Langness et al., 2017) as well as pDDIs in patients with chronic diseases such as liver cirrhosis (Franz et al., 2012), heart failure (Straubhaar et al., 2006), hypertension (Subramanian et al., 2018), stroke (Caratozzolo et al., 2016), and hematopoietic stem cell transplantation (Trevisan et al., 2015). However, this issue remains unaddressed among patients with UTIs, despite being a frequent cause of hospitalization (Ismail et al., 2013). Additionally, the irrational use of drugs and scarcity of literature is common in developing countries. Therefore, studies are needed regarding various aspects of pDDIs and their clinical relevance among hospitalized patients with UTIs. Consequently, such studies will improve patients’ safety, achieve positive clinical outcomes, and help healthcare professionals to manage pDDIs and reduce their associated problems.
This study aimed to explore the prevalence, levels, risk factors, and clinical relevance of pDDIs in hospitalized patients with UTIs. Secondly, the study aimed to develop management guidelines and identify monitoring parameters for the most frequent interactions.
Methods
Study Settings and Design
This was a retrospective cross-sectional study, conducted in the internal medicine wards of two tertiary care hospitals in Peshawar, Khyber Pakhtunkhwa, Pakistan: Hayatabad Medical Complex and Khyber Teaching Hospital. Clinical pharmacy services and computerized drug interaction screening programs do not exist in both hospitals. Patients’ profiles are maintained in handwritten format using predefined charts.
Inclusion and Exclusion Criteria
The inclusion criteria were the following:
Patients diagnosed with UTIs and admitted to internal medicine wards.
Age ≥18 years.
Both male and female patients.
Profiles were excluded if they were incomplete with respect to relevant data required for this study.
Data Source
Based on the above criteria, we included 422 patients’ profiles. Administrative permission from both hospitals was obtained in order to access patients’ clinical records. Data regarding hospital admissions, patients’ demographics, diagnoses, comorbidities/complications, medication therapy, signs/symptoms, and laboratory tests were collected.
Screening of Medication Profiles for pDDIs
All medications prescribed during hospitalization (from the time of admission till discharge) were evaluated for pDDIs using the Micromedex Drug-Reax® (Micromedex Drug-Reax, 2017). This software classifies drug interactions on the basis of severity and documentation levels as follows (Micromedex Drug-Reax, 2017):
Severity Levels:
Contraindicated: Concurrent use of the interacting pair is contraindicated.
Major: The interacting pair may result in permanent damage/death; medical intervention is needed to prevent or minimize the adverse outcome.
Moderate: The combination may worsen the patient’s condition and/or require an alteration in therapy.
Minor: There are limited clinical effects of the interaction. These may include an increase in the severity or frequency of adverse effects, and major alteration of therapy is not required.
Documentation Levels (Scientific Evidence):
Excellent: Controlled studies have demonstrated the existence of interaction.
Good: Well-controlled studies are lacking, but documentation strongly suggests the existence of interaction.
Fair: Existing documentation is less, but physicians suspect the presence of interaction on the basis of pharmacological considerations, or evidences are good for interactions involving pharmacologically similar drug.
The overall prevalence of pDDIs and prevalence based on the severity levels (contraindicated, major, moderate, and minor) have been reported. Levels (severity and documentation) of pDDIs were also identified.
Clinical Relevance
The clinical relevance of the 10 most frequent pDDIs was identified by assessing the potential adverse outcomes of pDDIs including patients’ signs, symptoms, and abnormal laboratory findings. The clinical features have been stratified based on dosage variations of the interacting drugs. The following cutoff points were used for defining higher daily doses: aspirin: ≥150 mg; nitroglycerin: ≥5.2 mg; ramipril: ≥10 mg; bisoprolol: ≥10 mg; furosemide: ≥60 mg; isoniazid: ≥150 mg; rifampin: ≥300 mg; and pyrazinamide: ≥500 mg. In this study, adverse drug events were defined as follows: hypoglycemia: random blood sugar <80 mg/dl or fasting blood sugar <70 mg/dl; hypertension: systolic blood pressure (BP) >130 mmHg or diastolic BP >80 mmHg; hypotension: systolic BP <90 mmHg or diastolic BP <60 mmHg; tachycardia: heart rate >100 beats/ min; bradycardia: heart rate <60 beats/min; hypokalemia: serum potassium <3.5 mmol/L; hyponatremia: serum sodium <135 mmol/L; hypernatremia: serum sodium >145 mmol/L; hypochloremia: serum chloride <95 mmol/L; hyperchloremia: serum chloride >105 mmol/L; leukocytosis: total leukocyte count >11,000/μl; neutrophilia: neutrophil count >76%; decreased platelets counts: <150,000/μl; increased alkaline phosphatase: >126 U/L; increased serum bilirubin: >1 mg/dl; increased alanine aminotransferase: >59 U/L (male), >36 U/L (female). Monitoring parameters and management guidelines were described for the most frequent interactions. A list of the clinically important pDDIs (based on severity levels) was developed along with their potential adverse outcomes.
Statistical Analysis
Descriptive statistics were used for presenting data in the form of frequencies and percentages with median and interquartile range (IQR), where appropriate. Logistic regression analysis was applied in order to identify association for one or more pDDIs with patients’ characteristics. Moreover, association for major pDDIs with patients’ characteristics was also identified. Dependent variables in the model were exposure to all types- or major pDDIs, while patients’ characteristics were independent variables. For each independent variable, odds ratios (OR) and 95% confidence intervals (CI) were determined. Initially, the univariate logistic regression analysis was carried out. Then, for variables with p values of <0.15, multivariate analyses were performed. We considered, p value of <0.05 as statistically significant. SPSS-v23 was used for statistical analyses of the data.
Results
Patients’ General Characteristics
Patient demographics and comorbidities are shown in Table 1 . Of a total of 422 patients, 284 (67.3%) were female. The median age was 55 years (IQR = 41–65), median prescribed drugs were 9 (7–12), and median hospital stay was 5 days (3–7). Majority of patients were aged 18–60 years (72%). Most of the patients were prescribed with 6–10 medicines (47.6%). Diabetes mellitus (DM) (n = 212; 50.2%), hypertension (191; 45.3%), hepatitis (45; 10.7%), chronic kidney disease (CKD) (42; 10%), and ischemic heart disease (IHD) (41; 9.7%) were the most frequent comorbidities.
Table 1.
General characteristics of study subjects (n = 422).
| Characteristic | Patients: N (%) |
|---|---|
| Gender | |
| Male | 138 (32.7) |
| Female | 284 (67.3) |
| Age (years) | |
| 18–60 | 304 (72) |
| >60 | 118 (28) |
| Median (IQR) | 55 (41–65) |
| Drugs prescribed per patient | |
| ≤5 | 63 (14.9) |
| 6–10 | 201 (47.6) |
| >10 | 158 (37.4) |
| Median (IQR) | 9 (7–12) |
| Hospital stay (days) | |
| ≤3 | 105 (24.9) |
| 4–6 | 196 (46.4) |
| >6 | 121 (28.7) |
| Median (IQR) | 5 (3–7) |
| Comorbidities | |
| Diabetes mellitus | 212 (50.2) |
| Hypertension | 191 (45.3) |
| Hepatitis | 45 (10.7) |
| Chronic kidney disease | 42 (10) |
| Ischemic heart disease | 41 (9.7) |
| Stroke | 30 (7.1) |
| Respiratory tract infection | 23 (5.5) |
| Congestive cardiac failure | 21 (5) |
| Decompensated chronic liver disease | 20 (4.7) |
| Anemia | 16 (3.8) |
| Acute gastroenteritis | 15 (3.6) |
| Pneumonia | 14 (3.3) |
| Rheumatic arthritis | 12 (2.8) |
| Malaria | 11 (2.6) |
| Tuberculosis | 11 (2.6) |
| Cholelithiasis | 9 (2.1) |
| Chronic obstructive pulmonary disease | 7 (1.7) |
| Hepatic encephalopathy | 7 (1.7) |
| Meningitis | 7 (1.7) |
| Myeloma | 6 (1.4) |
| Miscellaneous | 145 (34.4) |
IQR, interquartile range.
Prevalence of pDDIs
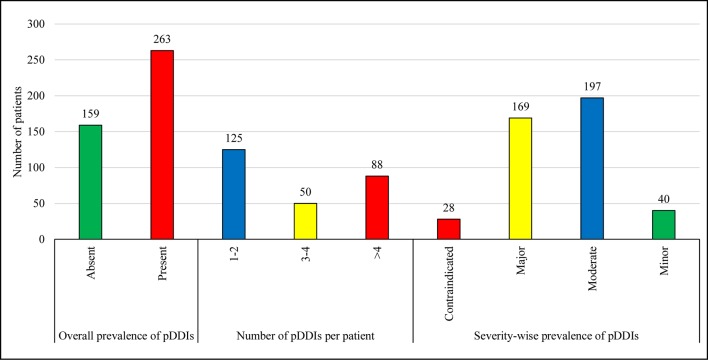
Figure 1 illustrates the prevalence of pDDIs. Of a total of 422 patients, 62.3% were exposed to at least one pDDI. Based on severity-wise prevalence, 46.7% and 40% patients were presented with pDDIs of moderate and major severity, respectively, while the prevalence of contraindicated and minor severity were observed less frequently.
Figure 1.
Overall-prevalence is the occurrence of at least one pDDI irrespective of severity type. The total number of UTI patients was 422. Therefore, the overall prevalence of pDDIs is 62.3% (263 out of 422). Data are presented in the form of frequencies. The prevalence of pDDIs has also been reported on the basis of severity levels. pDDIs, potential drug-drug interactions.
Levels of Potential Drug-Drug Interactions
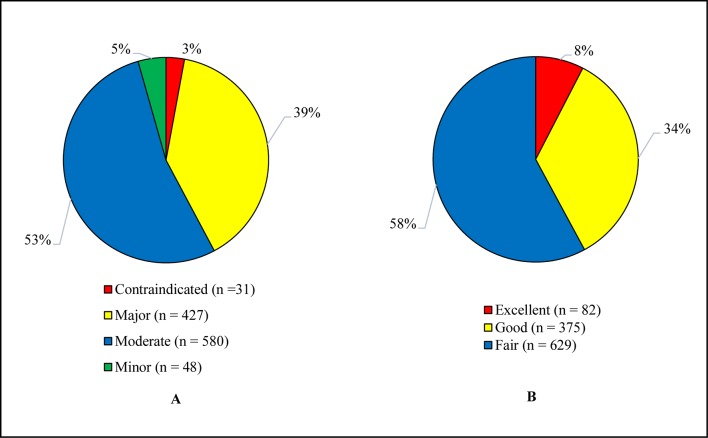
Figure 2 illustrates the levels of pDDIs. The recorded pDDIs were categorized on the basis of severity and documentation levels. A total of 1,086 interactions were identified, of which 53.4% were of moderate and 39.3% major severity, whereas 57.9% and 34.5% were about fair and good scientific evidence, respectively.
Figure 2.
(A) Severity levels of pDDIs. (B) Documentation levels of pDDIs. The total identified pDDIs were categorized based on the severity and documentation levels. pDDIs, potential drug-drug interactions.
Risk Factors of Potential Drug-Drug Interactions
Multivariate logistic regression analysis showed significant association of all types of pDDIs with six to eight prescribed medicines (OR = 7; p < 0.001), eight or more prescribed medicines (OR = 32; p < 0.001), DM (OR = 2.8; p < 0.001), and IHD (OR = 4.3; p = 0.02). Similarly, there was a significant association of major pDDIs with six to eight prescribed medicines (OR = 7.7; p = 0.009), eight or more prescribed medicines (OR = 40; p < 0.001), IHD (OR = 3; p = 0.01), and congestive cardiac failure (CCF) (OR = 3.3; p = 0.04) as presented in Table 2 .
Table 2.
Logistic regression analysis based on exposure to all types and major interactions.
| Variables | All types of interactions | Major interactions | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Gender | ||||||||
| Male | Reference | Reference | Reference | – | ||||
| Female | 1.6 (1-2.4) | 0.03 | 0.9 (0.6-1.7) | 1 | 1.3 (0.9-2) | 0.2 | – | – |
| Age (Years) | ||||||||
| 18-60 | Reference | – | Reference | – | ||||
| >60 | 1.2 (0.8-1.9) | 0.4 | – | – | 1.2 (0.8-1.8) | 0.4 | – | – |
| Drugs prescribed | ||||||||
| ≤5 | Reference | Reference | Reference | Reference | ||||
| 6-8 | 5.7 (2.5-12.9) | <0.001 | 7 (2.8-17.2) | <0.001 | 6.4 (1.4-28.4) | 0.01 | 7.7 (1.7-35.2) | 0.009 |
| >8 | 34 (15.1-76.8) | <0.001 | 32 (13-81.5) | <0.001 | 46.2 (11-193) | <0.001 | 40 (9-174) | < 0.001 |
| Hospital stay (days) | ||||||||
| ≤3 | Reference | Reference | Reference | Reference | ||||
| 4-6 | 2.3 (1.4-3.7) | 0.001 | 1.1 (0.6-2.1) | 0.7 | 1.7 (1-2.9) | 0.04 | 0.8 (0.4-1.5) | 0.4 |
| >6 | 2.6 (1.5-4.5) | 0.001 | 0.9 (0.5-2) | 0.9 | 3.3 (1.9-5.8) | <0.001 | 1.3 (0.7-2.7) | 0.4 |
| Comorbidities | ||||||||
| Diabetes mellitus | 3.8 (2.5-5.8) | <0.001 | 2.8 (1.6-4.7) | <0.001 | 2.3 (1.6-3.5) | <0.001 | 1.4 (0.8-2.3) | 0.2 |
| Hypertension | 2.5 (1.7-3.8) | <0.001 | 1 (0.6-1.9) | 0.9 | 2.4 (1.6-3.5) | <0.001 | 1.3 (0.8-2.2) | 0.3 |
| Hepatitis | 0.9 (0.5-1.9) | 0.9 | – | – | 0.9 (0.5-1.7) | 0.7 | – | – |
| Chronic kidney disease | 1.8 (0.9-3.7) | 0.10 | 0.8 (0.3-1.8) | 0.6 | 1.4 (0.7-2.7) | 0.3 | – | – |
| Ischemic heart disease | 4.9 (1.9-12.7) | 0.001 | 4.3 (1.3-15) | 0.02 | 4.2 (2.1-8.4) | <0.001 | 3 (1.3-7) | 0.01 |
| Stroke | 2.6 (1-6.4) | 0.04 | 1.7 (0.6-5.3) | 0.3 | 1.8 (0.8-3.8) | 0.13 | 1.1 (0.4-2.7) | 0.9 |
| Respiratory tract infection | 2.3 (0.8-6.2) | 0.11 | 1.7 (0.5-5.5) | 0.4 | 1.7 (0.7-3.9) | 0.2 | – | – |
| Congestive cardiac failure | 3.8 (1.1-13.1) | 0.03 | 1.4 (0.4-5.5) | 0.6 | 6.9 (2.3-21) | 0.001 | 3.3 (1-10.6) | 0.04 |
| Decompensated chronic liver disease | 1.4 (0.5-3.8) | 0.5 | – | – | 1.5 (0.6-3.8) | 0.4 | – | – |
| Anemia | 0.5 (0.2-1.2) | 0.13 | 0.4 (0.1-1.3) | 0.1 | 0.7 (0.2-1.9) | 0.5 | – | – |
CI, confidence interval; OR, odds ratio.
Clinical Relevance of Potential Drug-Drug Interactions
The prescribed dosage of the interacting drugs is shown in Table 3 . The drugs were given in a variety of doses and administration frequencies. However, most of the patients received low doses of the following interacting drugs: aspirin, ramipril, bisoprolol, furosemide, ceftriaxone, isoniazid, rifampin, and pyrazinamide. Higher doses of the interacting drugs were comparatively less frequent for the following drugs: insulin, metformin, nitroglycerin, ceftriaxone, isoniazid, rifampin, and pyrazinamide. See Supplementary Table 1 for the most frequent interactions. Most frequently prescribed antimicrobial agents (AMAs) and drugs besides AMAs are listed in Supplementary Table 2 and Table 3 , respectively.
Table 3.
Prescribed dose regimen of the interacting drugs.
| Interacting pair | Dose categoriesa | Prescribed dose regimen | Number of patients |
|---|---|---|---|
| Aspirin + Insulin | Low + High | 75mg OD + 24-80 units per day | 29 |
| Low + Low | 75mg OD + 8-20 units per day | 7 | |
| High + High | 150mg OD + 24-50 units per day | 3 | |
| High + High | 300mg OD + 30 units per day | 3 | |
| Insulin + Metformin | High + High | 21-80 units per day + ≥850mg OD/BD | 13 |
| High + Low | 21-50 units per day + 500mg OD | 8 | |
| High + High | 21-72 units per day + 500mg BD | 8 | |
| Low + High | ≤20 units per day + 500mg BD | 2 | |
| Low + Low | ≤20 units per day + ≤500mg OD | 1 | |
| Low + High | ≤20 units per day + ≥850mg OD | 1 | |
| Insulin + Ramipril | High + Low | 21-50 units per day + 5mg OD | 10 |
| High + Low | 21-50 units per day + 2.5mg OD | 8 | |
| High + High | 21-60 units per day + 10mg OD | 4 | |
| Low + Low | ≤20 units per day + 5mg OD | 2 | |
| Aspirin + Nitroglycerin | Low + High | 75mg OD + 2.6mg BD | 13 |
| High + High | 300mg OD + 2.6mg BD | 5 | |
| Low + Low | 75mg OD + 0.5mg OD | 1 | |
| High + Low | 150mg OD + 0.5mg OD | 1 | |
| High + High | 150mg OD + 2.6mg BD | 1 | |
| Aspirin + Ramipril | Low + Low | 75mg OD + 5mg OD | 7 |
| Low + Low | 75mg OD + 2.5mg OD | 6 | |
| Low + High | 75mg OD + 10mg OD | 4 | |
| High + Low | 300mg OD + 2.5mg OD | 2 | |
| High + Low | 300mg OD + 5mg OD | 1 | |
| Aspirin + Bisoprolol | Low + Low | 75mg OD + 5mg OD | 7 |
| Low + Low | 75mg OD + 2.5mg OD | 5 | |
| High + Low | 300mg OD + 2.5mg OD | 3 | |
| High + Low | 150mg OD + 2.5mg OD | 2 | |
| High + Low | 150mg OD + 5mg OD | 1 | |
| Low + High | 75mg OD + 10mg OD | 1 | |
| High + Low | 300mg OD + 5mg OD | 1 | |
| Aspirin + Furosemide | Low + Low | 75mg OD + 40mg OD | 8 |
| Low + Low | 75mg OD + 20mg OD | 4 | |
| Low + High | 75mg OD + 40mg BD | 4 | |
| Low + High | 75mg OD + 60mg OD | 1 | |
| Low + High | 75mg OD + 60mg BD | 1 | |
| Low + High | 75mg OD + 80mg OD | 1 | |
| High + Low | 300mg OD + 40mg OD | 1 | |
| Calcium containing products + Ceftriaxone | Low + Low | 200mg/L BD + 1gm BD ATD | 6 |
| Low + Low | 200mg/L BD + 2gm OD ATD | 5 | |
| Low + High | 200mg/L BD + 3gm OD ATD | 3 | |
| Low + High | 200mg/L BD + 2gm BD ATD | 3 | |
| High + High | 1gm + 2gm BD ATD | 1 | |
| Low + High | 169mg OD + 2gm BD ATD | 1 | |
| Isoniazid + Rifampin | Low + Low | 75mg OD + 150mg OD | 15 |
| High + High | 150mg OD + 300mg OD | 4 | |
| Pyrazinamide + Rifampin | Low + Low | 400mg OD + 150mg OD | 15 |
| High + High | 500mg OD + 300mg OD | 4 |
ATD, alternate day; BD, twice a day; OD, once a day.
The following cut off points were used for defining higher daily doses, aspirin: ≥150mg; nitroglycerin: ≥5.2mg; ramipril: ≥10mg; bisoprolol: ≥10mg; furosemide: ≥60mg; isoniazid: ≥150mg; rifampin: ≥300mg; and pyrazinamide: ≥500mg.
Relevant clinical findings in low- and high-dose groups for the 10 most frequent pDDIs are presented in Table 4 . Patients with the interactions aspirin + insulin, insulin + metformin, and insulin + ramipril were presented with signs/symptoms and abnormal laboratory findings indicating hypoglycemia. Signs/symptoms of hypoglycemia were highly prevalent in the high-dose groups. Nitroglycerin toxicity and decreased antiplatelet response were more frequent in patients taking high doses of aspirin + nitroglycerin. Signs/symptoms suggesting poor response and electrolyte abnormalities were more frequent in the high-dose groups of the following interacting drugs: aspirin + ramipril, aspirin + furosemide, and calcium-containing products + ceftriaxone. Similarly, signs/symptoms of hypertension were highly prevalent among high-dose groups of aspirin + bisoprolol. In patients with interactions, isoniazid + rifampin and pyrazinamide + rifampin, signs/symptoms of hepatotoxicity such as anorexia, paleness, weight loss, abdominal pain, weakness, hepatomegaly, fatigue, and myalgia were observed. Similarly, abnormal laboratory findings such as increased alanine aminotransferase, alkaline phosphatase, and serum bilirubin were reported. These signs/symptoms were more prevalent among low-dose groups of isoniazid, rifampin, and pyrazinamide. Management guidelines and monitoring parameters have also been provided in Table 4 . Adverse consequences for the most frequent pDDIs were reduced therapeutic efficacy, electrolyte abnormalities, hypoglycemia, hypertension, hepatotoxicity, bleeding, and hypotension.
Table 4.
Clinical relevance of ten most frequent potential drug-drug interactions.
| Interactions (N) | Dose categories (N) | Signs/symptoms and laboratory investigations | Patients: N (%a) | Management guidelines or monitoring parameters |
|---|---|---|---|---|
| Aspirin – Insulin (42) | Low + Low (7) | Drowsiness | 2 (28.6) | Monitor the patient’s blood glucose, clinical signs of hypoglycemia and adjust the dose of insulin if necessary |
| Tachycardia | 2 (28.6) | |||
| Pale | 2 (28.6) | |||
| Irregular heart rate | 1 (14.3) | |||
| Dehydration | 1 (14.3) | |||
| Headache | 1 (14.3) | |||
| Confusion | 1 (14.3) | |||
| Low + High (29) | Drowsiness | 7 (24.1) | ||
| Tachycardia | 5 (17.2) | |||
| Pale | 5 (17.2) | |||
| Confusion | 5 (17.2) | |||
| Fatigue | 4 (13.8) | |||
| Irregular heart rate | 4 (13.8) | |||
| Headache | 3 (10.3) | |||
| Dehydration | 3 (10.3) | |||
| Weakness | 3 (10.3) | |||
| Palpitations | 1 (3.4) | |||
| Loss of consciousness | 1 (3.4) | |||
| Blurred vision | 1 (3.4) | |||
| Shakiness | 1 (3.4) | |||
| High + High (6) | Drowsiness | 2 (33.3) | ||
| Tachycardia | 2 (33.3) | |||
| Pale | 2 (33.3) | |||
| Fatigue | 2 (33.3) | |||
| Weakness | 2 (33.3) | |||
| Headache | 2 (33.3) | |||
| Palpitations | 1 (16.6) | |||
| Depressive | 1 (16.6) | |||
| Loss of consciousness | 1 (16.6) | |||
| Insulin – Metformin (33) | Low + Low (1) | Tachycardia | 1 (100) | Monitor the patient’s blood glucose, clinical signs of hypoglycemia and adjust the dose of insulin if necessary |
| Headache | 1 (100) | |||
| High + Low (8) | Dehydration | 2 (25) | ||
| Pale | 2 (25) | |||
| Weakness | 1 (12.5) | |||
| Loss of consciousness | 1 (12.5) | |||
| Shakiness | 1 (12.5) | |||
| Tachycardia | 1 (12.5) | |||
| Low + High (3) | Drowsiness | 1 (33.3) | ||
| High + High (21) | Tachycardia | 5 (23.8) | ||
| Drowsiness | 4 (19) | |||
| Weakness | 4 (19) | |||
| Headache | 3 (14.3) | |||
| Blurred vision | 1 (4.8) | |||
| Confusion | 1 (4.8) | |||
| Loss of consciousness | 1 (4.8) | |||
| Fatigue | 1 (4.8) | |||
| Decreased RBS | 1 (4.8) | |||
| Insulin – Ramipril (24) | Low + Low (2) | Pale | 1 (50) | Monitor the patient’s blood glucose, clinical signs of hypoglycemia and adjust the dose of insulin if necessary |
| Decreased FBS | 1 (50) | |||
| High + High (4) | Pale | 3 (75) | ||
| Drowsiness | 1 (25) | |||
| Fatigue | 1 (25) | |||
| Tachycardia | 1 (25) | |||
| Irregular heart rate | 1 (25) | |||
| Blurred vision | 1 (25) | |||
| Dehydration | 1 (25) | |||
| Palpitations | 1 (25) | |||
| High + Low (18) | Tachycardia | 7 (38.9) | ||
| Drowsiness | 4 (22.2) | |||
| Dehydration | 4 (22.2) | |||
| Headache | 3 (16.6) | |||
| Weakness | 2 (11.1) | |||
| Fatigue | 2 (11.1) | |||
| Pale | 2 (11.1) | |||
| Shakiness | 1 (5.6) | |||
| Confusion | 1 (5.6) | |||
| Irregular heart rate | 1 (5.6) | |||
| Aspirin – Nitroglycerin (21) | Low + High (13) | Hypotension | 5 (38.5) | Analgesic doses of aspirin increase signs of nitroglycerin toxicity, patients should be monitored for hypotension, headache, and for signs of bleeding in patients with long term antiplatelet use of aspirin along with nitroglycerin |
| Tachycardia | 3 (23.1) | |||
| Pale | 2 (15.4) | |||
| Drowsiness | 2 (15.4) | |||
| Vertigo | 1 (7.7) | |||
| Weakness | 1 (7.7) | |||
| Headache | 1 (7.7) | |||
| Fatigue | 1 (7.7) | |||
| Decreased platelets | 1 (7.7) | |||
| High + High (6) | Hypotension | 4 (66.7) | ||
| Drowsiness | 2 (33.3) | |||
| Tachycardia | 2 (33.3) | |||
| Weakness | 2 (33.3) | |||
| Pale | 2 (33.3) | |||
| Fatigue | 1 (16.7) | |||
| Depressed | 1 (16.7) | |||
| Headache | 1 (16.7) | |||
| Palpitations | 1 (16.7) | |||
| Loss of consciousness | 1 (16.7) | |||
| Dehydration | 1 (16.7) | |||
| Decreased platelets | 1 (16.7) | |||
| Low + Low (1) | Hypotension | 1 (100) | ||
| High + Low (1) | Headache | 1 (100) | ||
| Bradycardia | 1 (100) | |||
| Hypertension | 1 (100) | |||
| Aspirin – Ramipril (20) | Low + Low (13) | Hypertension | 9 (45) | Patients’ blood pressure, hemodynamic parameters, and renal function should be monitored. If an adverse effect is noted, the following options may be considered: (a) aspirin dosage less than 100 mg per day (b) an alternative non-aspirin antiplatelet agent (c) replacing ACE inhibitors with angiotensin receptor blockers |
| Drowsiness | 5 (25) | |||
| Tachycardia | 5 (25) | |||
| Increased BUN | 5 (25) | |||
| Increased serum creatinine | 4 (20) | |||
| Hyponatremia | 3 (15) | |||
| Fatigue | 2 (10) | |||
| Headache | 2 (10) | |||
| Irregular heart rate | 1 (5) | |||
| Nausea | 1 (5) | |||
| Chest pain | 1 (5) | |||
| Hypernatremia | 1 (5) | |||
| Hypokalemia | 1 (5) | |||
| Confusion | 1 (5) | |||
| High + Low (3) | Increased BUN | 3 (100) | ||
| Increased serum creatinine | 3 (100) | |||
| Irregular heart rate | 1 (33.3) | |||
| Loss of consciousness | 1 (33.3) | |||
| Headache | 1 (33.3) | |||
| Chest pain | 1 (33.3) | |||
| Tachycardia | 1 (33.3) | |||
| Hyponatremia | 1 (33.3) | |||
| Hypokalemia | 1 (33.3) | |||
| Low + High (4) | Hypertension | 4 (100) | ||
| Increased BUN | 3 (75) | |||
| Hyponatremia | 3 (75) | |||
| Increased serum creatinine | 2 (50) | |||
| Nausea | 2 (50) | |||
| Palpitations | 1 (25) | |||
| Irregular heart rate | 1 (25) | |||
| Tachycardia | 1 (25) | |||
| Fatigue | 1 (25) | |||
| Drowsiness | 1 (25) | |||
| Blurred vision | 1 (25) | |||
| Urinary retention | 1 (25) | |||
| Aspirin – Bisoprolol (20) | Low + Low (12) | Hypertension | 8 (66.7) | Patients’ blood pressure and hemodynamic parameters should be monitored |
| Nausea | 3 (25) | |||
| Irregular heart rate | 3 (25) | |||
| Drowsiness | 3 (25) | |||
| Tachycardia | 2 (16.7) | |||
| Palpitations | 2 (16.7) | |||
| Headache | 1 (8.3) | |||
| Confusion | 1 (8.3) | |||
| Loss of consciousness | 1 (8.3) | |||
| High + Low (7) | Hypertension | 3 (42.9) | ||
| Loss of consciousness | 3 (42.9) | |||
| Drowsiness | 2 (28.6) | |||
| Nausea | 2 (28.6) | |||
| Chest pain | 2 (28.6) | |||
| Tachycardia | 1 (14.3) | |||
| Headache | 1 (14.3) | |||
| Fatigue | 1 (14.3) | |||
| Palpitations | 1 (14.3) | |||
| Irregular heart rate | 1 (14.3) | |||
| Low + High (1) | Irregular heart rate | 1 (100) | ||
| Headache | 1 (100) | |||
| Tachycardia | 1 (100) | |||
| Aspirin – Furosemide (20) | Low + Low (12) | Increased BUN | 9 (75) | Patients should be monitored for signs of renal toxicity and salicylate toxicity. Diuretic effectiveness should be assured including its effects on blood pressure. Avoid high dose of salicylates in those taking loop diuretics, an alternative analgesic should be given |
| Hypertension | 6 (12) | |||
| Increased serum creatinine | 5 (41.7) | |||
| Hyponatremia | 4 (33.3) | |||
| Pedal edema | 3 (25) | |||
| Drowsiness | 3 (25) | |||
| Fatigue | 2 (16.7) | |||
| Chest pain | 2 (16.7) | |||
| Confusion | 2 (16.7) | |||
| Nausea | 2 (16.7) | |||
| Hypokalemia | 2 (16.7) | |||
| Hyperchloremia | 1 (8.3) | |||
| Hypochloremia | 1 (8.3) | |||
| Low + High (7) | Pedal edema | 4 (57.1) | ||
| Increased BUN | 3 (43) | |||
| Hyperchloremia | 3 (43) | |||
| Hyponatremia | 3 (43) | |||
| Hypertension | 2 (29) | |||
| Hypokalemia | 2 (29) | |||
| Drowsiness | 2 (29) | |||
| Headache | 2 (29) | |||
| Fatigue | 2 (29) | |||
| Increased serum creatinine | 2 (29) | |||
| Hypernatremia | 1 (14.3) | |||
| Urinary retention | 1 (14.3) | |||
| Ascites | 1 (14.3) | |||
| Confusion | 1 (14.3) | |||
| High + Low (1) | Chest pain | 1 (100) | ||
| Increased BUN | 1 (100) | |||
| Increased serum creatinine | 1 (100) | |||
| Calcium containing products – Ceftriaxone (19) |
Low + Low (11) | Increased BUN | 5 (45.4) | Do not mix or administer ceftriaxone concurrently with calcium-containing IV solutions in the same IV administration line, including continuous calcium-containing infusions such as parenteral nutrition via a Y-site. Monitor patient for signs of nephrotoxicity or decreased ceftriaxone effectiveness |
| Leukocytosis | 4 (36.3) | |||
| Increased serum creatinine | 3 (27.3) | |||
| Neutrophilia | 2 (18.2) | |||
| Fever | 2 (18.2) | |||
| Low + High (7) | Fever | 4 (57.1) | ||
| Increased serum creatinine | 3 (43) | |||
| Leukocytosis | 2 (29) | |||
| Neutrophilia | 2 (29) | |||
| Increased BUN | 2 (29) | |||
| Chest pain | 1 (14.3) | |||
| Nephrolithiasis | 1 (14.3) | |||
| High + High (1) | Increased BUN | 1 (100) | ||
| Increased serum creatinine | 1 (100) | |||
| Leukocytosis | 1 (100) | |||
| Isoniazid – Rifampin (19) | Low + Low (15) | Anorexia | 4 (26.7) | Patients should be monitored for signs and symptoms of liver toxicity including fever, anorexia, vomiting and jaundice. Baseline and periodic LFTs monitoring is suggested |
| Abdominal pain | 4 (26.7) | |||
| Fever | 3 (20) | |||
| Pale | 3 (20) | |||
| Nausea | 3 (20) | |||
| Weakness | 3 (20) | |||
| Increased ALP | 3 (20) | |||
| Myalgia | 2 (13.3) | |||
| Weight loss | 2 (13.3) | |||
| Increased serum bilirubin | 1 (6.7) | |||
| Fatigue | 1 (6.7) | |||
| Increased ALT | 1 (6.7) | |||
| Hepatomegaly | 1 (6.7) | |||
| High + High (4) | Anorexia | 3 (75) | ||
| Increased ALP | 3 (75) | |||
| Increased ALT | 2 (50) | |||
| Increased serum bilirubin | 1 (25) | |||
| Abdominal pain | 1 (25) | |||
| Ascites | 1 (25) | |||
| Hepatomegaly | 1 (25) | |||
| Pyrazinamide – Rifampin (19) | Low + Low (15) | Anorexia | 4 (26.7) | Monitoring of LFTs at baseline and at 2, 4, 6, and 8 weeks of treatment. Patient education about reporting symptoms of liver injury |
| Abdominal pain | 4 (26.7) | |||
| Fever | 3 (20) | |||
| Pale | 3 (20) | |||
| Nausea | 3 (20) | |||
| Weakness | 3 (20) | |||
| Increased ALP | 3 (20) | |||
| Myalgia | 2 (13.3) | |||
| Weight loss | 2 (13.3) | |||
| Increased serum bilirubin | 1 (6.7) | |||
| Fatigue | 1 (6.7) | |||
| Increased ALT | 1 (6.7) | |||
| Hepatomegaly | 1 (6.7) | |||
| High + High (4) | Anorexia | 3 (75) | ||
| Increased ALP | 3 (75) | |||
| Increased ALT | 2 (50) | |||
| Increased serum bilirubin | 1 (25) | |||
| Abdominal pain | 1 (25) | |||
| Ascites | 1 (25) | |||
| Hepatomegaly | 1 (25) |
ACE inhibitors, angiotensin converting enzyme inhibitors; ALP, alkaline phosphatase; ALT, alanine aminotransferase; BUN, blood urea nitrogen; FBS, fasting blood sugar; HbA1c, glycated hemoglobin (A1c); LFTs, liver function tests; RBS, random blood sugar.
Percentage was calculated based on dose categories.
Discussion
DDIs remain one of the important therapeutic challenges in hospitalized patients (Zwart-van-Rijkom et al., 2009). The overall prevalence of pDDIs in our study was higher (62.3%) as compared to the prevalence among patients with other diseases such as HIV (52.2%)(Santos et al., 2016), liver cirrhosis (21.5%) (Franz et al., 2012), and hypertension (48%) (Subramanian et al., 2018). Moreover, our prevalence was lower in comparison with studies in patients with hemodialysis (89.1%) (Al-ramahi et al., 2016), hematopoietic stem cell transplantation (82.5%) (Trevisan et al., 2015), and CKD (95.9%) (Olumuyiwa et al., 2017). Furthermore, the prevalence of major pDDIs in the present study was higher (40%) as compared to a study in patients with liver cirrhosis (21.4%) (Franz et al., 2012). A similar prevalence has been reported in patients with hepatitis C (30–44%) (Kondili et al., 2017), whereas a higher prevalence has been reported in patients with stroke (61%) (Caratozzolo et al., 2016). The prevalence of contraindicated pDDIs in the present study was lower (6.6%) as compared to the prevalence reported by another study among patients with hepatitis C (16.7%) (Patel et al., 2015). These inconsistencies may be attributed to variability in the study design, study population, drug-prescribing patterns, considering types of pDDIs, and drug interaction screening software. Regardless of all these variations, our findings indicated a higher prevalence of pDDIs. Taking into consideration the findings of this study, patients with UTIs are at higher risk for DDIs. In addition, the Pakistani population is more exposed to pDDIs because of irrational use of medicines and non-availability of clinical pharmacy services as well as DDI-screening systems in hospitals (Ismail et al., 2017). Therefore, DDIs may result in a variety of negative clinical consequences such as decreased clinical effectiveness, adverse effects, hospitalization, and prolongation of hospital stay (Ray et al., 2004; Juurlink et al., 2013; Khan et al., 2017). In order to reduce/manage DDIs in hospitals, some evidence-based strategies have been suggested such as screening of medication profiles for pDDIs (Moura et al., 2012), clinical pharmacist involvement in evaluating patient medication profiles for pDDIs (Vonbach et al., 2007; Hahn et al., 2013), procedure for structured assessment of pDDIs (van-Roon et al., 2005), and appraisal of pertinent laboratory investigations (Geerts et al., 2009; Zwart-van-Rijkom et al., 2009).
Healthcare professionals could focus on DDIs with severe adverse outcomes based on their severity and documentation levels. Our findings about a high prevalence of pDDIs with moderate severity and fair documentation are consistent with the findings of other studies among hospitalized patients (Ismail et al., 2013; Murtaza et al., 2016). Therefore, it is essential for healthcare providers to properly identify the type of pDDIs, as it is vital for clinical management of pDDIs, designing prophylactic measures for their prevention, and reducing their associated risks.
Polypharmacy has become a serious concern among hospitalized patients with UTIs. These patients receive multiple therapies for treating comorbidities or associated complications (Dhodi et al., 2014; Panayappan et al., 2017). In literature, a positive relationship has been reported between pDDIs and polypharmacy, which is also indicated by our findings (Van-Leeuwen et al., 2013; Murtaza et al., 2016; Ismail et al., 2017). In our study, a significant association of pDDIs with various comorbidities such as DM, IHD, and CCF are mainly due to the prescription of drugs having high potential for DDI (Straubhaar et al., 2006; Mateti et al., 2011; Amin and Suksomboon, 2014). Salicylates, i.e., aspirin displaces sulfonylureas from protein binding sites, increasing the pharmacological effect and hypoglycemic risk of sulfonylureas (Kubacka et al., 1996). Drugs such as loop diuretics, non-steroidal anti-inflammatory drugs, angiotensin converting enzyme inhibitors (ACEIs), cyclosporine, or aminoglycosides deteriorate the renal status of the patient or compete for renal excretion of metformin, thus altering metformin concentrations in the body, which may change pharmacological response or cause adverse events (Graham et al., 2011). Similarly, some of the most common drug classes involved in DDIs in patients with cardiac diseases are anti-platelets, anticoagulants, anti-hypertensive, ACEIs, and diuretics, while the most common interacting drug pairs in patients with cardiac diseases are heparin-aspirin, aspirin-furosemide, aspirin-clopidogrel, aspirin-captopril, clopidogrel-heparin, clopidogrel-torsemide, and heparin-warfarin (Mateti et al., 2011). Healthcare professionals should have knowledge about possible risk factors for pDDIs, so that patients at risk should be carefully individualized in order to optimize therapy and prevent or minimize the incidence of DDIs.
The clinical relevance of DDIs emphasizes the significance of medications profile screening for DDIs, which is also supported by published studies (Vonbach et al., 2007; Geerts et al., 2009; Ismail et al., 2017). Potential adverse effects of DDIs have been related to clinical manifestations of the patients in the present study. Such methods are helpful for healthcare professionals to monitor the associated adverse outcomes of interactions. Our findings indicate that adverse events were more prevalent among patients taking higher doses of prescribed drugs. Adverse events associated with interactions can be minimized by monitoring patients’ signs/symptoms and lab tests. Therefore, proper consideration should be awarded to this aspect of therapy. Another considerable strength of our study is the provision of a list of most frequent DDIs, including their management strategies and monitoring parameters. Not all identified pDDIs are clinically important. Therefore, there is an immense need to develop a list of clinically important interactions that are observed in hospitalized patients with UTIs. This list will be used by healthcare professionals for selective identification and management of pDDIs in order to develop clinical guidelines and to prevent adverse consequences related to DDIs. As guardians of patient safety and health, healthcare professionals are responsible for identifying and preventing adverse consequences associated with DDIs (Harrington et al., 2011). A clinician’s knowledge about DDIs can decrease the occurrence of adverse drug effects, provide good quality care, and prevent associated medico-legal issues. Most of these interactions are preventable and can be managed by considering alternative therapy and dose reduction.
Following are the potential limitations of this study. This study was conducted at tertiary care hospitals, where patients with UTIs are mainly admitted for the management of complications associated with UTIs and comorbid disease. The pDDIs that we have identified are mainly related to the use of medicines for the management of such problems. Our results may not be generalizable to outpatient settings where the disease and the drug interaction pattern may be different. Furthermore, we have used the term pDDIs, as we could not confirm the causal linkage. We only correlated the potential adverse consequences of interactions with patients’ clinical features. While data concerning negative clinical consequences caused by DDIs are scarce, some retrospective studies are available in the published literature, highlighting the importance of clinical relevance of interactions (Ray et al., 2004; Juurlink et al., 2013).
Conclusions
PDDIs are highly prevalent among patients with UTIs. Software-based screening of pDDIs is recommended in order to identify, prevent/reduce, and manage pDDIs in UTI patients. Knowledge about the most frequent pDDIs could help healthcare professionals to prevent DDIs and their associated adverse outcomes. In patients with UTIs, polypharmacy, DM, IHD, and CCF increase the risk of interactions. The prevalence of adverse events is greater among patients taking higher doses of interacting drugs. Careful monitoring for adverse events associated with pDDIs will contribute to patient safety. Most of the interactions can be managed by considering alternative therapy and dose reduction.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on request.
Ethics Statement
Ethical approval was obtained from Institutional Research and Ethics Board of the Postgraduate Medical Institute, Peshawar. As this was a retrospective study based on the clinical data maintained in the record room, therefore informed consent was not applicable, according to our institutional guidelines.
Author Contributions
SN and MI contributed to the design of the study. SN and FK collected data, organized the data, and contributed to data analysis. SN and MI wrote the first draft of this manuscript. SN, MI, and FK contributed to manuscript drafting and revision. All authors approved the submitted version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ACEIs, angiotensin converting enzymes inhibitors; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AMAs, antimicrobial agents; ATC, anatomical therapeutic chemical classification; ATD, alternate day; BD, twice a day; BP, blood pressure; BUN, blood urea nitrogen; CCF, congestive cardiac failure; CI, confidence interval; CKD, chronic kidney disease; DDIs, drug-drug interactions; DM, diabetes mellitus; FBS, fasting blood sugar; HbA1c, glycated hemoglobin (A1c); IHD, ischemic heart disease; IQR, interquartile range; LFTs, liver function tests; OD, once a day; OR, odds ratio; pDDIs, potential DDIs; RBS, random blood sugar; UTIs, urinary tract infections.
Acknowledgments
The authors are very thankful for the cooperation of staff and administration of the hospitals.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.01032/full#supplementary-material
References
- Al-ramahi R., Raddad A. R., Rashed A. O., Bsharat A., Abu-Ghazaleh D., Yasin E., et al. (2016). Evaluation of potential drug-drug interactions among Palestinian hemodialysis patients. BMC Nephrol. 17 (1), 96–201. 10.1186/s12882-016-0317-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin M., Suksomboon N. (2014). Pharmacotherapy of type 2 diabetes mellitus: an update on drug–drug interactions. Drug Saf. 37 (11), 903–919. 10.1007/s40264-014-0223-2 [DOI] [PubMed] [Google Scholar]
- Blondal K., Ingadottir B., Einarsdottir H., Bergs D., Steingrimsdottir I., Steindorsdottir S., et al. (2016). The effect of a short educational intervention on the use of urinary catheters: a prospective cohort study. Int. J. Qual. Health Care 28 (6), 742–748. 10.1093/intqhc/mzw108 [DOI] [PubMed] [Google Scholar]
- Briongos-Figuero L. S., Gomez-Traveso T., Bachiller-Luque P., Domínguez-Gil-Gonzalez M., Gomez-Nieto A., Palacios-Martín T., et al. (2012). Epidemiology, risk factors and comorbidity for urinary tract infections caused by extended-spectrum beta-lactamase (ESBL)-producing enterobacteria. Int. J. Clin. Pract. 66 (9), 891–896. 10.1111/j.1742-1241.2012.02991.x [DOI] [PubMed] [Google Scholar]
- Caratozzolo S., Gipponi S., Marengoni A., Pari E., Scalvini A., Pasina L., et al. (2016). Potentially serious drug-drug interactions in older patients hospitalized for acute ischemic and hemorrhagic stroke. Eur. Neurol. 76, 161–166. 10.1159/000449376 [DOI] [PubMed] [Google Scholar]
- Dhodi D. K., Jaiswar S., Bhagat S. B., Gambre R. S. (2014). A study to evaluate prescribing pattern of antibiotics among patients of urinary tract infection with preexisting renal disorders in a tertiary care hospital. Int. J. Basic Clin. Pharmacol. 3 (4), 687–691. 10.5455/2319-2003.ijbcp20140825 [DOI] [Google Scholar]
- Foxman B. (2010). The epidemiology of urinary tract infection. Nat. Rev. Urol. 7 (12), 653–660. 10.1038/nrurol.2010.190 [DOI] [PubMed] [Google Scholar]
- Franz C. C., Egger S., Born C., Bravo A. E. R., Krahenbuhl S. (2012). Potential drug-drug interactions and adverse drug reactions in patients with liver cirrhosis. Eur. J. Clin. Pharmacol. 68, 179–188. 10.1007/s00228-011-1105-5 [DOI] [PubMed] [Google Scholar]
- Geerts A. F., De-Koning F. H., De-Smet P. A., Van-Solinge W. W., Egberts T. C. (2009). Laboratory tests in the clinical risk management of potential drug-drug interactions: a cross-sectional study using drug-dispensing data from 100 Dutch community pharmacies. Drug Saf. 32 (12), 1189–1197. 10.2165/11316700-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Graham G. G., Punt J., Arora M., Day R. O., Doogue M. P., Duong J., et al. (2011). Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 50 (2), 81–98. 10.2165/11534750-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Hahn M., Reiff J., Hiemke C., Braus D. F. (2013). Drug-drug-interactions in psychiatry. Psychiatr. Prax. 40 (3), 154–158. 10.1055/s-0032-1332831 [DOI] [PubMed] [Google Scholar]
- Harrington A. R., Warholak T. L., Hines L. E., Taylor A. M., Sherrill D., Malone D. C. (2011). Healthcare professional students’ knowledge of drug-drug interactions. Am. J. Pharm. Educ. 75 (10), 199. 10.5688/ajpe7510199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail M., Aziz S., Noor S., Haider I., Shams F., Haq I., et al. (2017). Potential drug-drug interactions in pediatric patients admitted to intensive care unit of Khyber Teaching Hospital, Peshawar, Pakistan: a cross-sectional study. J. Crit. Care 40 (2017), 243–250. 10.1016/j.jcrc.2017.04.028 [DOI] [PubMed] [Google Scholar]
- Ismail M., Iqbal Z., Khattak M. B., Khan M. I., Arsalan H., Javaid A., et al. (2013). Potential drug-drug interactions in internal medicine wards in hospital setting in Pakistan. Int. J. Clin. Pharm. 35 (3), 455–462. 10.1007/s11096-013-9764-1 [DOI] [PubMed] [Google Scholar]
- Juurlink D. N., Mamdani M., Kopp A., Laupacis A., Redelmeier D. A. (2013). Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA 289 (13), 1652–1658. 10.1001/jama.289.13.1652 [DOI] [PubMed] [Google Scholar]
- Khan Q., Ismail M., Haider I., Haq I., Noor S. (2017). QT interval prolongation in hospitalized patients on cardiology wards: a prospective observational study. Eur. J. Clin. Pharmacol. 73 (11), 1511–1518. 10.1007/s00228-017-2321-4 [DOI] [PubMed] [Google Scholar]
- Kohler G. I., Bode-Boger S. M., Busse R., Hoopmann M., Welte T., Boger R. H. (2000). Drug-drug interactions in medical patients: effects of in-hospital treatment and relation to multiple drug use. Int. J. Clin. Pharmacol. Ther. 38 (11), 504–513. 10.5414/CPP38504 [DOI] [PubMed] [Google Scholar]
- Kondili L. A., Gaeta G. B., Ieluzzi D., Zignego A. L., Monti M., Gori A., et al. (2017). Real-life data on potential drug-drug interactions in patients with chronic hepatitis C viral infection undergoing antiviral therapy with interferon-free DAAs in the PITER Cohort Study. PLoS One 12 (2), e0172159. 10.1371/journal.pone.0172159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubacka R. T., Antal E. J., Juhl R. P., Welshman I. R. (1996). Effects of aspirin and ibuprofen on the pharmacokinetics and pharmacodynamics of glyburide in healthy subjects. Ann. Pharmacother. 30 (1), 20–26. 10.1177/106002809603000103 [DOI] [PubMed] [Google Scholar]
- Langness J. A., Nguyen M., Wieland A., Everson G. T., Kiser J. J. (2017). Optimizing hepatitis C virus treatment through pharmacist interventions: identification and management of drug-drug interactions. World J. Gastroenterol. 23 (9), 1618–1626. 10.3748/wjg.v23.i9.1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateti U. V., Rajakannan T., Nekkanti H., Rajesh V., Mallaysamy S. R., Ramachandran P. (2011). Drug–drug interactions in hospitalized cardiac patients. J. Young Pharm. 3 (4), 329–333. 10.4103/0975-1483.90246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micromedex Drug-Reax (2017). IBM Watson Health, Greenwood Village, Colorado, USA. Available at: https://www.micromedexsolutions.com/ Accessed on October 20, 2017.
- Moura C. S., Prado N. M., Belo N. O., Acurcio F. A. (2012). Evaluation of drug-drug interaction screening software combined with pharmacist intervention. Int. J. Clin. Pharm. 34 (4), 547–552. 10.1007/s11096-012-9642-2 [DOI] [PubMed] [Google Scholar]
- Murtaza G., Khan M. Y., Azhar S., Khan S. A., Khan T. M. (2016). Assessment of potential drug-drug interactions and its associated factors in the hospitalized cardiac patients. Saudi Pharm. J. 24 (2), 220–225. 10.1016/j.jsps.2015.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olumuyiwa J. F., Akinwumi A. A., Ademola O. A., Oluwole B. A., Ibiene E. O. (2017). Prevalence and pattern of potential drug-drug interactions among chronic kidney disease patients in south-western Nigeria. Niger. Postgrad. Med. J. 24 (2), 88–92. 10.4103/npmj.npmj_64_17 [DOI] [PubMed] [Google Scholar]
- Panayappan L., Babu A. S., Davis D., Joseph N., Joshy N., Krishnakumar K. (2017). Urinary tract infection: prescribing pattern of antibiotics at a tertiary care hospital. Asian J. Pharm. Clin. Res. 10 (5), 255–257. 10.22159/ajpcr.2017.v10i5.17287 [DOI] [Google Scholar]
- Patel N., Abdelsayed S., Veve M., Miller C. D. (2011). Predictors of clinically significant drug-drug interactions among patients treated with nonnucleoside reverse transcriptase inhibitor-, protease inhibitor-, and raltegravir-based antiretroviral regimens. Ann. Pharmacother. 45, 317–324. 10.1345/aph.1P576 [DOI] [PubMed] [Google Scholar]
- Patel N., Nasiri M., Koroglu A., Bliss S., Davis M., McNutt L. A., et al. (2015). A cross-sectional study comparing the frequency of drug interactions after adding simeprevir-or sofosbuvir-containing therapy to medication profiles of hepatitis C monoinfected patients. Infect. Dis. Ther. 4 (1), 67–78. 10.1007/s40121-015-0058-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray W. A., Murray K. T., Meredith S., Narasimhulu S. S., Hall K., Stein C. M. (2004). Oral erythromycin and the risk of sudden death from cardiac causes. N. Engl. J. Med. 351 (11), 1089–1096. 10.1056/NEJMoa040582 [DOI] [PubMed] [Google Scholar]
- Santos W. M., Secoli S. R., Padoin S. M. (2016). Potential drug interactions in patients given antiretroviral therapy. Rev Latino-Am. Enfermagem 24, e2832. 10.1590/1518-8345.1193.2832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schappert S. M. (1999). Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments. United States, 1997. Vital Health Stat. 13 143 (13), 1–39. [PubMed] [Google Scholar]
- Straubhaar B., Krahenbuhl S., Schlienger R. G. (2006). The prevalence of potential drug-drug interactions in patients with heart failure at hospital discharge. Drug Saf. 29 (1), 79–90. 10.2165/00002018-200629010-00006 [DOI] [PubMed] [Google Scholar]
- Subramanian A., Adhimoolam M., Kannan S. (2018). Study of drug–drug interactions among the hypertensive patients in a tertiary care teaching hospital. Perspect. Clin. Res. 9 (1), 9–14. 10.4103/picr.PICR_145_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisan D. D., Silva J. B., Oliveira H. C., Secoli S. R., Lima M. H. M. (2015). Prevalence and clinical significance of potential drug–drug interaction in hematopoietic stem cell transplantation. Cancer Chemother. Pharmacol. 75 (2), 393–400. 10.1007/s00280-014-2657-8 [DOI] [PubMed] [Google Scholar]
- Van-Leeuwen R. W., Brundel D. H., Neef C., van-Gelder T., Mathijssen R. H., Burger D. M., et al. (2013). Prevalence of potential drug-drug interactions in cancer patients treated with oral anticancer drugs. Br. J. Cancer 108 (5), 1071–1078. 10.1038/bjc.2013.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van-Roon E. N., Flikweert S., Comte M., Langendijk P. N., Kwee-Zuiderwijk W. J., Smits P., et al. (2005). Clinical relevance of drug-drug interactions: a structured assessment procedure. Drug Saf. 28 (12), 1131–1139. 10.2165/00002018-200528120-00007 [DOI] [PubMed] [Google Scholar]
- Vonbach P., Dubied A., Beer J. H., Krahenbuhl S. (2007). Recognition and management of potential drug-drug interactions in patients on internal medicine wards. Eur. J. Clin. Pharmacol. 63 (11), 1075–1083. 10.1007/s00228-007-0359-4 [DOI] [PubMed] [Google Scholar]
- Zwart-van-Rijkom J. E., Uijtendaal E. V., Ten-Berg M. J., Van-Solinge W. W., Egberts A. C. (2009). Frequency and nature of drug-drug interactions in a Dutch university hospital. Br. J. Clin. Pharmacol. 68 (2), 187–193. 10.1111/j.1365-2125.2009.03443.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on request.