Abstract
Objective: To evaluate the role of intravesical lidocaine in preventing autonomic dysreflexia (AD) during routine catheter changes in individuals with spinal cord injury (SCI) at T6 or above.
Design: Prospective observational cohort study.
Setting: Outpatient urology clinic.
Participants: Fifty consecutive individuals with SCI at or above T6 and a history of AD having a routine indwelling catheter change.
Interventions: A treatment group of individuals received 10 ml of 2% lidocaine administered into the existing catheter 4–6 minutes prior to catheter change. The control group had the same amount of lidocaine administered into the urethra or suprapubic tract after removing the old catheter and immediately prior to inserting the new catheter (due to the delayed onset of action of the anesthetic, this was assumed to have no initial effect). Systolic blood pressures (SBP) were measured immediately after catheter insertion and then every 30–45 seconds for 5 minutes.
Outcome measures: Incidence and magnitude of AD as determined by SBP following catheter change.
Results: The incidence of AD in the lidocaine treatment group was 14.8% vs 47.8% in the control group (P = .011). Pretreatment with lidocaine also demonstrated a significantly attenuated rise in SBP immediately after the catheter change (9.5 mmHg vs 26.9 mmHg for post-treatment, P = .014) relative to baseline SBP.
Conclusion: In individuals with SCI at risk of AD, pretreatment with intravesical lidocaine prior to catheter change significantly decreased both the incidence and magnitude of AD. This suggests that pretreatment with intravesical lidocaine is helpful in individuals with SCI who are prone to AD.
Keywords: Autonomic dysreflexia, Lidocaine, Catheter, Spinal cord injury
Introduction
Autonomic dysreflexia (AD) is a potentially life-threatening escalation in blood pressures, which occurs in individuals with spinal cord injury (SCI). Individuals with SCI at or above T6 are particularly predisposed to AD, given their lack of regulation of amplified sympathetic signals and the resultant vasoconstriction of the splanchnic vessels that leads to this relative hypertension.1,2 AD is classically caused by a noxious stimulus below the neurological level of injury (NLI), though may be due to other causes.3,4 Because of poor hand function, those with cervical SCI who are at the most risk for AD are also most likely to have indwelling urethral or suprapubic catheters to maintain bladder drainage.5,6 These indwelling catheters are routinely changed every four weeks, though this procedure may be done more frequently in those who have recurrent catheter encrustation or bladder stones. As such, individuals with cervical SCI who may require these frequent procedures are at risk for AD during the catheter change (a noxious bladder intervention that may provoke bladder or sphincter contraction as the catheter passes into the bladder).
Multiple past studies have implicated unmyelinated C-fibers as the cause for involuntary bladder contractions and one of the drivers of AD.7,8 Intravesical lidocaine is thought to work preferentially on these small C-fiber afferents.9 Previously, intravesical lidocaine has been found to be a safe, effective topical agent in uninjured individuals for bladder biopsies, cautery, and possible management in women with interstitial cyctitis.10,11 The SCI consortium guidelines for management of AD recommends use of 2% lidocaine prior to inserting a catheter when patients are having AD (level of evidence: strong expert consensus)12 and it has been our clinical practice to use intravesical lidocaine during catheter changes in those at-risk individuals with SCI to help decrease the potential risk of AD. However, no studies to our knowledge have explored the role of intravesical lidocaine in the prevention of AD. This prospective, observational cohort, quality assurance study was undertaken to determine whether or not intravesical lidocaine was effective at decreasing AD during catheter changes.
Methods
This prospective, IRB approved study contained two primary groups: one group pretreated with intravesical lidocaine instillation through the existing catheter into the bladder prior to a catheter change (treatment group) and one group treated with intravesical lidocaine instillation immediately following catheter removal into the urethra or suprapubic site and prior to catheter insertion (control group). All consecutive SCI patients who presented for routine indwelling catheter change in our Urology clinic and met inclusion criteria were asked to participate in the study. Individuals were included if they had an SCI at or above T6 as well as a history of AD. All individuals had their catheter changed in one of two manners as detailed below. Demographic data including age, sex, and duration of SCI as aligned with the International Spinal Cord Injury Core Data Set13 were collected for each patient (Table 1). American Spinal Injury Association impairment scale (AIS) and NLI14 were included when known by individuals or previously documented in the chart. Baseline blood pressures were recorded several minutes after individuals were positioned (reclined) for catheter change and repeated at least twice to ensure that blood pressures had stabilized at a reclined baseline.
Table 1. Individual demographics.
| Demographic characteristics of the Treatment and Control groups | ||||
|---|---|---|---|---|
| Treatment group | Control group | P value | Aggregate | |
| Sex | ||||
| Male | 24 (89%) | 19 (83%) | 0.534 | 43 (86%) |
| Female | 3 (11%) | 4 (17%) | 7 (14%) | |
| AIS* | ||||
| A | 16 (61%) | 10 (48%) | 0.451 | 26 (55%) |
| B | 5 (19%) | 6 (29%) | 0.421 | 11 (23%) |
| C | 2 (8%) | 3 (14%) | 0.450 | 5 (11%) |
| D | 3 (12%) | 2 (9%) | 0.862 | 5 (11%) |
| Bladder Management | ||||
| Foley | 5 (19%) | 5 (22%) | 0.782 | 10 (20%) |
| SPC | 22 (81%) | 18 (78%) | 40 (80%) | |
| Neurological Level of Injury | ||||
| C1–C4 | 8 (30%) | 11 (48%) | 0.194 | 19 (38%) |
| C5–C8 | 13 (48%) | 11 (48%) | 0.982 | 24 (48%) |
| T1–T6 | 6 (22%) | 1 (4%) | 0.072 | 7 (14%) |
|
Mean Age (years) (min, max, SD) |
43.5 (21,67,12.5) | 47.9 (31,76,12.7) | 0.576 | 45.6 (21,76,12.8) |
| Years Since Injury | ||||
| 0–2 Years | 6 (22%) | 6 (26%) | 0.756 | 12 (24%) |
| 3–10 Years | 5 (19%) | 3 (13%) | 0.607 | 8 (16%) |
| >10 years | 16 (59%) | 14 (61%) | 0.910 | 30 (60%) |
| Baseline Blood Pressure (mmHg) | 113/70 | 105/63 | 0.140 | 109/68 |
AIS, ASIA impairment scale; SPC, suprapubic catheter; SD, standard deviation. *n = 47 (1 missing AIS in treatment group and 2 missing AIS in control group).
In the treatment group, the indwelling catheter was disconnected from the storage bag and 10 ml of 2% lidocaine hydrochloride gel (Uro-jet, Amphastar Pharmaceuticals Inc., Rancho Cucamonga, CA, USA) was instilled through the catheter. To ensure an adequate amount of the lidocaine bolus reached the bladder and was not retained within the catheter, a 5 ml air flush was then injected into the catheter to force the lidocaine into the bladder. Serial blood pressures were then recorded at approximately 30–60 second intervals, with the individual already positioned for the procedure, over the next four to six minutes with an automated wrist blood pressure cuff (Omron Healthcare Inc. Lake Forest, IL, USA) until a baseline had been established (when repeated systolic blood pressure (SBP) was no longer decreasing). This amount of time of observation for lidocaine to take effect, while differing from the product package insert, was chosen to fit with our clinical experience and reasoning that if blood pressure declines and then stabilizes at some decreased value following administration, the lidocaine has acted sufficiently on the C-fibers within the bladder to decrease their sympathetic input and thereby reached the point of anesthesia. An MD observer recorded these blood pressures and timestamps to prevent any interruption in the sterile procedure by nursing and to ensure the protocol was correctly followed. No penile clamp was applied to males in an effort to minimize potential external painful stimuli that might contribute to AD. The catheter was then changed per normal sterile protocol and immediate blood pressure was taken within the first 30 seconds after new catheter insertion to assess for AD. Subsequent blood pressures were taken in 30–45 second intervals to ensure no AD had occurred with catheter change. As per the most recent standards, an episode of AD was defined as SBP increase of >20 mmHg from supine baseline.4 Serial blood pressures were taken over the five minutes following catheter change to monitor for clinical safety and resolution of any potential AD episodes.
In the control group, individuals presenting for routine catheter change similarly had baseline BP taken. The catheter was removed and 10 ml of 2% lidocaine was injected into the urethra or suprapubic catheter tract prior to immediate placement of the new sterile catheter. Earlier pilot studies we had completed on the effects of intravesical lidocaine indicated that the onset of action was greater than this approximate 10 seconds to insert a new catheter. Therefore, the anesthetic effects of intravesical lidocaine were assumed not to act in this group, effectively allowing this group to serve as a control. Automated serial blood pressures were then taken at the conclusion of the catheter change as above for at least five minutes to monitor for clinical safety.
Statistics
Individuals were semi-randomly assigned to a given group based upon the clinical availability of two sets of nurses, each performing the sterile catheter changes per one of the protocols. Groups were analyzed for significant differences between their baseline characteristics. Comparing the lidocaine treatment group with the control group, all data were analyzed to identify the rate of AD for each group and a χ2 statistical measure was calculated, with a P value of <.05 deemed statistically significant. Using the incidences of AD for each group, a number needed to treat (NNT) was also calculated. This statistical concept (NNT) provides an objective measurement of the impact of a treatment, in this study calculating how many patients would need to be treated in order to prevent one episode of AD with routine catheter changes in at-risk individuals with SCI.15
Results
Fifty consecutive individuals participated in the study, with 27 in the treatment group given intravesical lidocaine 4–6 minutes prior to catheter change and 23 in the control group. Demographics appear in Table 1. No significant differences were found in initial demographic characteristics between groups (P values .07–.98). There were no interruptions between catheter changes in either group, therefore ensuring that the amount of time for lidocaine to take effect was similar between individuals within each group.
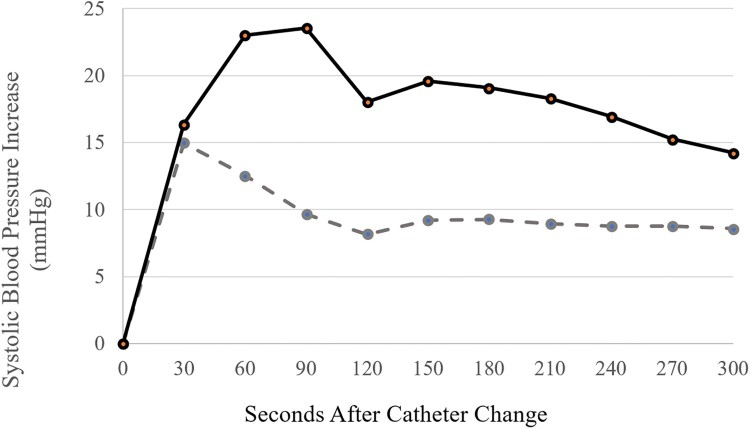
In the treatment group, four individuals out of 27 (14.8%) experienced AD with the catheter change when lidocaine was allowed time to act on the bladder (details in Table 2). In the control group, the rate of AD when lidocaine was administered following catheter removal and prior to new catheter placement was 47.8%. This demonstrates a significant decrease in AD incidence following pretreatment with lidocaine (χ2 = 6.46, P = .011). Based upon the incidences of AD in the lidocaine treatment and control groups, the NNT to prevent one episode of AD in an individual with SCI at risk was 3.0. The mean increase in SBP following catheter change from baseline for the treatment group was 9.5 mmHg. This was significantly less than the increase experienced by individuals in the control group (26.9 mmHg, P = .014). Notably, after receiving intravesical lidocaine, 14 individuals in the treatment group experienced a decreased SBP when comparing baseline to SBP at the time of catheter change (mean SBP prior to catheter change of 110 mmHg for treatment group). Utilizing this last pre-catheter change SBP as a unique starting point, Figure 1 depicts an attenuated SBP increase over five minutes of monitoring directly following new catheter insertion for the treatment relative to the control group.
Table 2. Details of episodes of AD where lidocaine was administered prior to catheter change.
| Details of Subset of Pretreated Individuals Who Experienced AD | |||||
|---|---|---|---|---|---|
| NLI/AIS | Bladder management | Pre-lidocaine BP (mmHg) | Nadir BP following lidocaine (mmHg) | Duration lidocaine in bladder prior to catheter change associated AD (sec) | Peak BP (mmHg) |
| C5/B | SPC | 89/50 | 82/46 | 725 | 153/108 |
| C5/A | SPC | 85/49 | 85/49 | 953 | 123/77 |
| C7/A | SPC | 128/83 | 118/74 | 699 | 150/89 |
| C6/B | Foley | 119/64 | 92/55 | 610 | 147/86 |
NLI, neurological level of injury; AIS, ASIA impairment scale;11 BP, blood pressure; AD, autonomic dysreflexia.
Figure 1.
Systolic blood pressure* increase over time originating directly following catheter change for both treatment group (dashed line) and control group (solid line). *Given decrease in systolic blood pressure for select individuals following pretreatment with lidocaine, systolic blood pressure directly prior to catheter change was utilized as baseline (instead of reclined, pre-lidocaine baseline).
Discussion
Given its lubricating properties, viscous lidocaine is at times used during routine catheter change in individuals with SCI at risk for AD. Previous research comparing lidocaine gel to nonanesthetic lubricants in uninjured individuals have shown moderate16 to no effect17,18 to adding the anesthetic. However, in individuals with SCI, where a noxious stimulus may lead to dangerous AD, its function had yet to be fully explored. The purpose of this study was to evaluate the role of intravesical lidocaine in preventing AD in individuals with SCI undergoing routine catheter change. Here we demonstrate the novel finding that pretreatment with intravesical lidocaine, given four to six minutes prior to the catheter change, significantly decreased the incidence and magnitude of AD.
The NNT of 3.0 means that for every three patients pretreated with lidocaine, one would have experienced AD without it. This NNT value for lidocaine to prevent AD episodes with routine catheter change is much lower than other accepted preventative treatment options in other fields of medicine (for comparison, the NNT for daily use of aspirin to prevent cardiovascular problems is 1667).19 The decreased rate of AD following pretreatment with lidocaine is clinically significant and supported by the this low NNT of 3.0, making decreasing AD well within the capabilities of providers for this common procedure.
A statistically significant attenuation of the SBP increase also occurred following catheter change in individuals pretreated with intravesical lidocaine. Emerging evidence in individuals with SCI has associated blood pressure fluctuations, like AD, with persistent cognitive deficits,20,21 immunosuppression,22 and early cardiovascular disease,23 in addition to classic acute risks of stroke and retinal hemorrhage associated with AD.24 To mitigate these risks, the use of intravesical lidocaine is recommended for catheter changes in individuals with SCI at risk for AD.
The findings of this study, lend support to the consortium guidelines for AD management,12 demonstrating for the first time evidence for intravesical lidocaine assisting in AD management. It should be noted that this study was not designed to evaluate the impact of intravesical lidocaine on AD within the control group. To properly assess this, an additional group of individuals with no lidocaine gel used for catheter change would be needed. In this way, the control group used for this study may be a conservative estimate of the rate of AD with routine catheter change in those at risk. Within those patients pretreated with lidocaine who still experienced AD with catheter change (Table 2), injuries tended to be motor complete. This detail cedes that while lidocaine pretreatment decreased the magnitude of SBP increase, the AD threshold was still reached in some high-risk patients. As such, vigilant monitoring for AD during such procedures continues to be appropriate. Further research may be beneficial to determine the optimal time after instilling lidocaine prior to the catheter change to prevent AD.
Limitations
This was a prospective observational cohort study, and as such, it is possible that some individuals in one group may have been more predisposed to AD. Available clinical testing to date has not been able to fully characterize the predisposition an individual may have to vary degrees of AD, so this likely represents a limitation of current clinical practice. Further, our study determined group allocation based on nurse availability, and individuals were not selected based on AD risk. Differences in the diameter of each individual’s catheter further may have led to slightly variable amounts of lidocaine reaching the bladder mucosa. However, to minimize this potential confounder and assure an adequate amount of lidocaine reached the bladder, a 5 ml air flush was delivered through the catheter following the lidocaine instillation. Regardless of potential differences in lidocaine concentration in the bladder, as a test of real-world applicability, a sufficient amount appeared to reach the bladder, limiting the potential implications of this confounder. Further research may be needed to better identify possible outliers of those who demonstrate a response to intravesical lidocaine in atypical manners.
Conclusion
In individuals with SCI at risk for AD, pretreatment with intravesical lidocaine prior to routine catheter change significantly reduces the incidence of AD and magnitude of systolic blood pressure rise with this procedure. These findings support consideration of pretreatment with intravesical lidocaine prior to catheter changes and potentially other urologic procedures in individuals with SCI at risk for AD.
Acknowledgements
The authors would like to especially thank Francia Edquilag MSN-NP for her help with catheter change procedures and her role as a research coordinator.
Disclaimer statements
Funding None.
Conflict of interest Authors have no conflict of interests to declare.
ORCID
Ryan Solinsky http://orcid.org/0000-0002-7121-8678
References
- 1.Guttmann L, Whitteridge D.. Effects of bladder distension on autonomic mechanisms after spinal cord injuries. Brain 1947;70(4):361–404. doi: 10.1093/brain/70.4.361 [DOI] [PubMed] [Google Scholar]
- 2.Liu N, Fougere R, Zhou MW, Nigro MK, Krassioukov AV.. Autonomic dysreflexia severity during urodynamics and cystoscopy in individuals with spinal cord injury. Spinal Cord 2013;51(11):863–7. doi: 10.1038/sc.2013.113 [DOI] [PubMed] [Google Scholar]
- 3.Teasell RW, Arnold JM, Krassioukov A, Delaney GA.. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil 2000;81:506–16. doi: 10.1053/mr.2000.3848 [DOI] [PubMed] [Google Scholar]
- 4.Krassioukov A, Biering-Sørensen F, Donovan W, Kennelly M, Kirshblum S, Krogh K, et al International standards to document remaining autonomic function after spinal cord injury. J Spinal Cord Med 2012;35(4):201–10. doi: 10.1179/1079026812Z.00000000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKinley WO, Jackson AB, Cardenas DD, DeVivo MJ.. Long-term medical complications after traumatic spinal cord injury: a regional model systems analysis. Arch Phys Med Rehabil 1999;80(11):1402–10. doi: 10.1016/S0003-9993(99)90251-4 [DOI] [PubMed] [Google Scholar]
- 6.Huang YH, Bih LI, Chen GD, Lin CC, Chen SL, Chen WW.. Autonomic dysreflexia during urodynamic examinations in patients with suprasacral spinal cord injury. Arch Phys Med Rehabil 2011;92(9):1450-4. doi: 10.1016/j.apmr.2011.03.024 [DOI] [PubMed] [Google Scholar]
- 7.De Groat WC. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Spinal Cord 1995;33(9):493–505. doi: 10.1038/sc.1995.109 [DOI] [PubMed] [Google Scholar]
- 8.Zhou Y, Wang Y, Abdelhady M, Mourad MS, Hassouna MM.. Change of vanilloid receptor 1 following neuromodulation in rats with spinal cord injury. J Surg Res 2002;107:140–4. doi: 10.1016/S0022-4804(02)96481-4 [DOI] [PubMed] [Google Scholar]
- 9.Yokoyama O, Ishiura Y, Nakamura Y, Kunimi K, Mita E, Namiki M.. Urodynamic effects of intravesical instillation of lidocaine in patients With overactive detrusor. J Urol 1997;157:1826–30. doi: 10.1016/S0022-5347(01)64870-5 [DOI] [PubMed] [Google Scholar]
- 10.Pode D, Zylber-Katz E, Shapiro A.. Intravesical lidocaine: topical anesthesia for bladder mucosal biopsies. J Urol 1992;148(3):795–6. doi: 10.1016/S0022-5347(17)36722-8 [DOI] [PubMed] [Google Scholar]
- 11.Amano T, Ohkawa M, Kunimi K, Oshinoya Y, Uchibayashi T.. Topical anaesthesia for bladder biopsies and cautery: intravesical lidocaine versus caudal anaesthesia. Int Urol Nephrol 1995;27:533–7. doi: 10.1007/BF02564737 [DOI] [PubMed] [Google Scholar]
- 12.Linsenmeyer TA, Baker ER, Cardenas DD, Mobley T, Perkash I, Vogel LC, et al. Acute management of autonomic dysreflexia: Individuals with spinal cord injury presenting to health-care facilities. Consortium for spinal cord medicine clinical practice guidelines. 2nd ed. Clinical Practice Guidelines: Spinal Cord Medicine 2001: Consortium for Spinal Cord Medicine.
- 13.Devivo M, Biering-Sørensen F, Charlifue S, Noonan V, Post M, Stripling T, et al International spinal cord injury core data set. Spinal Cord 2006;44(9):535–40. doi: 10.1038/sj.sc.3101958 [DOI] [PubMed] [Google Scholar]
- 14.American Spinal Injury Association International standards for neurological classification of spinal cord injury. Revised 2011, updated 2015. Atlanta GA.
- 15.Cook RJ, Sackett DL.. The number needed to treat: a clinically useful measure of treatment effect. Br Med J 1995;310(6977):452–4. doi: 10.1136/bmj.310.6977.452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siderias J, Guadio F, Singer AJ.. Comparison of topical anesthetics and lubricants prior to urethral catheterization in males: a randomized controlled trial. Acad Emerg Med 2004;11(6):703–6. doi: 10.1197/j.aem.2003.12.025 [DOI] [PubMed] [Google Scholar]
- 17.Birch BRP, Ratan P, Morley R, Cumming J, Smart CJ, Jenkins JD.. Flexible cystoscopy in men: is topical anaesthesia with lignocaine gel worthwhile? Br J Urol 1994;73:155–9. doi: 10.1111/j.1464-410X.1994.tb07484.x [DOI] [PubMed] [Google Scholar]
- 18.McFarlane N, Denstedt J, Ganapathy S, Razvi H.. Randomized trial of 10 ml and 20 ml of 2% intraurethral lidocaine gel and placebo in men undergoing flexible cystoscopy. J Endourol 2001;15(5):541–4. doi: 10.1089/089277901750299366 [DOI] [PubMed] [Google Scholar]
- 19.Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, et al Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373(9678):1849–60. doi: 10.1016/S0140-6736(09)60503-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duschek S, Weisz N, Schandry R.. Reduced cognitive performance and prolonged reaction time accompany moderate hypotension. Clin Auton Res 2003;13(6):427–32. [DOI] [PubMed] [Google Scholar]
- 21.Phillips AA, Krassioukov AV, Ainslie PN, Cote AT, Warburton DE.. Increased central arterial stiffness explains baroreflex dysfunction in spinal cord injury. J Neurotrauma 2014;31(12):1122–8. doi: 10.1089/neu.2013.3280 [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Guan Z, Reader B, Shawler T, Mandrekar-Colucci S, Huang K, et al Autonomic dysreflexia causes chronic immune suppression after spinal cord injury. J Neurosci 2013;33(32):12970–81. doi: 10.1523/JNEUROSCI.1974-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dance DL, Chopra A, Campbell K, Ditor DS, Hassouna M, Craven BC.. Exploring daily blood pressure fluctuations and cardiovascular risk among individuals with motor complete spinal cord injury: a pilot study. J Spinal Cord Med 2017;40(4):405–14. doi: 10.1080/10790268.2016.1236161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson B, Thomason R, Pallares V, Sadove MS.. Autonomic hyperreflexia: a review. Military Med 1975;140:345–9. doi: 10.1093/milmed/140.5.345 [DOI] [PubMed] [Google Scholar]