Abstract
Objectives: A rapid decline in lean mass (LM), fat-free mass (FFM) and increased intramuscular fat (IMF) predispose persons with spinal cord injury (SCI) to chronic medical conditions including dyslipidemia, insulin resistance, type 2 diabetes mellitus and cardiovascular disease. (1) To determine the relationship between dual energy x ray absorptiometry (DXA) and gold standard magnetic resonance imaging (MRI) LM values; (2) to develop predictive equations based on this relationship for assessing thigh LM in persons with chronic SCI.
Study Design: Cross-sectional predicational design.
Settings: Clinical research medical center.
Participants: Thirty-two men with chronic (>1 y post-injury) motor complete SCI.
Methods: Participants completed total body DXA scans to determine thigh LM and were compared to measurements acquired from trans-axial MRI.
Outcome measures: MRI was used to measure whole muscle mass (MMMRI-WM), absolute muscle mass (MMMRI-ABS) after excluding IMF, and knee extensor muscle mass (MMMRI-KE). DXA was used to measure thigh LM (LMDXA) and (FFMDXA). To predict MMMRI-KE, LMDXA was multiplied by 0.52 and yielded LMDXA-KE.
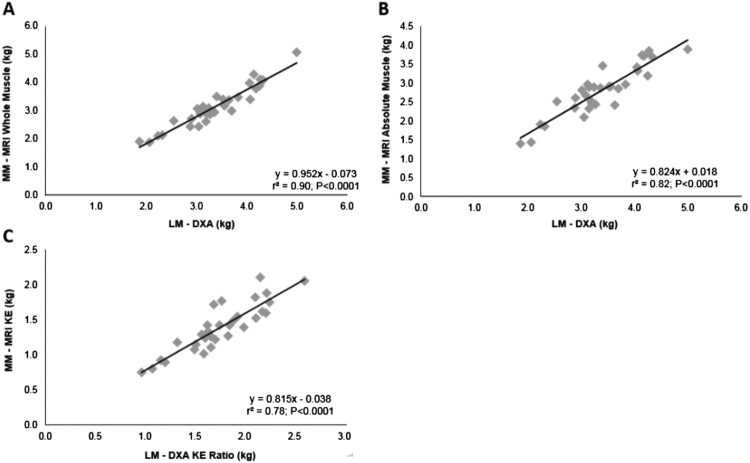
Results: LMDXA predicted MMMRI-WM [r2 = 0.90, standard error of the estimate (SEE) = 0.23 kg, P < 0.0001] and MMMRI-ABS (r2 = 0.82, SEE = 0.28 kg, P < 0.0001). LMDXA-KE predicted MMMRI-KE (r2 = 0.78, SEE = 0.16 kg, P < 0.0001).
Conclusion: DXA measurements revealed an acceptable agreement with the gold standard MRI and may be a viable alternative for assessing thigh skeletal muscle mass after SCI.
Keywords: Spinal cord injury, Dual energy x-ray absorptiometry, Magnetic resonance imaging, Lean mass
Introduction
Rapid decline in lean mass (LM) and increased adiposity are marked adaptations after spinal cord injury (SCI).1–8 Additionally, prolonged immobilization and decline in physical activity may result in a substantial reduction in skeletal muscle size within the first few weeks of injury.1 Intramuscular fat (IMF) has been shown to be three times greater in persons with incomplete SCI compared with abled-bodied controls.5 Changes in muscle quality are tightly associated with several medical conditions including dyslipidemia, insulin resistance, type 2 diabetes mellitus, osteoporosis, and cardiovascular disease;1,4,5,9,10 therefore, it is of a great clinical interest to monitor the progression of muscle atrophy and to assist with implementation of interventions to attenuate these deleterious changes in muscle mass after SCI.
Thigh skeletal muscle cross-sectional area (CSA) has been continuously studied in persons with SCI, because of its relationship with energy balance and metabolic health.11–16 Muscle atrophy has been linked to decline in basal metabolic rate,10,15,16 reduced total energy expenditure,13,14 insulin resistance and impaired glucose tolerance,4,10–12,17 as well as the high prevalence of obesity in the SCI population.18,19 Additionally, ectopic adipose tissue has been associated with chronic inflammation, increased total cholesterol, and reduced strength and mobility.20,21 Moreover, IMF has shown to interfere with insulin signaling and may serve as a source of circulating triglycerides and free-fatty acids, which are both risk factors for cardiovascular disease.20–22
Methods of quantifying skeletal muscle size continue to be refined for the purposes of assessing metabolic health, predict health related risk factors, and evaluating the effectiveness of exercise interventions.5,6,23–25 Common methods have been used to quantify skeletal muscle size in persons with SCI including anthropometrics,25,26 magnetic resonance imaging (MRI),5,23,24,27,28 computed tomography (CT)28,29 and dual-energy x ray absorptiometry (DXA).6,7,30,31 Anthropometrics, including midthigh circumference and skinfold thickness, are available in almost every clinical setting and relatively inexpensive; however, anthropometrics are less accurate when measuring skeletal muscle size in persons with SCI.25,26 Anthropometrics can predict 75 and 78% of the variance in absolute skeletal muscle CSA (excluding IMF) and whole muscle CSA in persons with SCI, respectively.25 The anthropometric-derived equations may be advantageous for clinicians seeking a timely assessment of skeletal muscle size; however, for researchers engaging in exercise intervention, 22–25% error is likely to mask training effects of exercise.
MRI and CT are considered gold standards for measuring thigh muscle CSA based on high accuracy and reproducibility.30–33 MRI and CT provide detailed trans-axial images of soft tissues; however, their use in clinical practice is challenged by multiple factors. MRI and CT are expensive and require extensive operator training and post-processing time.23,25,27 Moreover, image acquisition and analysis may be further complicated in persons SCI due to mobility issues, difficulty lying supine for extended time, involuntary muscle spasms and movement artifact.23,27 Contrarily, CT exposes individuals to risk of ionization due to elevated levels of radiation.34 DXA provides an efficient and viable alternative and has been used to assess total and regional body composition in persons with SCI.6,7,34–36 The short and long-term precision of DXA have recently reported for persons with chronic SCI. The root mean square-coefficients of variation were 2.7 and 3.0% for short and long-term assessments of the lower extremity, respectively.36 DXA scans are cost-effective and require only 5–10 min, low amounts of radiation, and minimal post-processing time.36 Reviews of DXA indicate robust theoretical and empirical validity in estimating whole body and regional body composition in abled-bodied and SCI populations;6,36–39 however, DXA has not been used to specifically quantify regional thigh LM in persons with SCI. Considering the extensive infiltration of IMF following injury,4,5 determining the viability of accurately quantifying thigh LM via DXA after SCI is an important consideration in mitigating SCI-specific challenges and may provide clinical and research alternatives to MRI and CT.
The purpose of the current study was: (1) to determine the relationship between DXA and MRI; (2) to develop predictive equations based on this relationship for assessing thigh LM in persons with SCI with and without accounting for IMF. The derived DXA equations have the potential to reduce the time, costs, and operator expertise needed to assess thigh skeletal muscle mass in persons with SCI. We hypothesized that DXA will provide an alternative assessment tool to accurately predict thigh LM in persons with SCI.
Materials and methods
Participants
Thirty-two participants with chronic SCI (>1-yr post-injury) participated in two clinical trials (registered with clinicaltrials.gov: NCT01652040 and NCT02660073). The physical characteristics of the cohort are summarized in Table 1. Detailed protocols for both clinical trials, inclusion and exclusion criteria were recently published.40,41 For the purpose of the current study, the inclusion criteria were modified to include men and women, age up to 65 years, BMI greater than 30 kg/m2 and those with an ASIA impairment scale (AIS) C. Participants provided informed consent that was approved by local ethics committee.
Table 1. Participant characteristics.
| Characteristics | Tetraplegic (n = 10) | Paraplegic (n = 22) | Total participants (n = 32) |
|---|---|---|---|
| Age (yr) Sex |
35 ± 13 Male (n = 10) Female (n = 0) |
38 ± 10 Male (n = 21) Female (n = 1) |
37 ± 11 Male (n = 31) Female (n = 1) |
| Ethnicity | African American (n = 3) Caucasian (n = 7) |
African American (n = 9) Caucasian (n = 13) |
African American (n = 12) Caucasian (n = 20) |
| Weight (kg) | 76 ± 12 | 78 ± 13 | 77 ± 14 |
| Height (m) | 1.8 ± 0.04 | 1.78 ± 0.08 | 1.8 ± 0.07 |
| BMI (kg/m2) | 23 ± 5 | 25 ± 4 | 24 ± 4 |
| Level of injury | C5-C7 | T3-L1 | C5-L1 |
| Time since injury (yr) | 8 ± 9 | 11 ± 10 | 10 ± 10 |
| AIS classification | A (n = 5) B (n = 4) C (n = 1) |
A (n = 14) B (n = 8) C (n = 0) |
A (n = 19) B (n = 12) C (n = 1) |
Values are presented as mean ± standard deviation (SD).
AIS, American Spinal Injury Association Impairment Scale; BMI, body mass index; n, number.
Assessment of body weight and height
Participants were weighed using a wheelchair scale. The wheelchair was weighed empty and subtracted from the total weight. Height was measured in a supine position by placing a board at the soles of the feet to ensure dorsiflexion and using a Harpenden Stadiometer to the nearest 0.1 cm.6,25,26
MRI
MRI scans were performed using a total body GE Signa 1.5-T MRI within the McGuire VAMC Radiology Department. MRI acquisition details were previously published.23,25–27 Participants lied in a supine position with legs strapped to avoid involuntary movements. 12–15 trans-axial images (slice thickness, 0.8 cm; interslice space, 1.6 cm) were captured from hip to knee joints of the right lower extremity. A localized GE body array flex coil was used to ensure an adequate signal-to-noise ratio and higher image resolution. Scan times were approximately 3.5 min.
Thigh compartments were manually traced using X-Vessel (Version 2.011) by a single blinded research investigator. A detailed description of the tracing technique was published elsewhere.5,23,25 CSAs for three thigh regions were isolated: (1) whole muscle refers to the total muscle CSA of the thigh excluding bone, (2) absolute muscle refers to the muscle CSA of the thigh, after accounting for IMF and (3) knee extensor refers to the CSA of the quadriceps muscle group. Briefly, signal intensity was used to separate between muscle and IMF pixels. After plotting a histogram, a signal intensity mid-point was determined based on the average between muscle and fat peaks. A detailed explanation was recently published on how MRI was used to quantify IMF in persons with SCI.23
After calculating CSA, volume was determined for each MRI by multiplying the corresponding CSA (cm2) by 2.4 cm (slice thickness + interslice space). Each volume was summed and multiplied by the assumed muscle density (1.1 g/cm3) to determine muscle mass (kg). Three regions of interest (ROIs) were identified: (1) whole thigh muscle mass (MMMRI-WM), or the entire muscle mass of the thigh excluding bone CSA, (2) absolute thigh muscle mass (MMMRI-ABS), the entire lean portion of the thigh excluding IMF and (3) knee extensor muscle mass (MMMRI-KE). Modlesky et al. showed that the lean portion (20%) of adipose tissue accounted for more than half of the variance when comparing MRI muscle mass and fat free soft tissue mass from DXA.24 Therefore, when calculating MMMRI-ABS it was important to include 20% of IMF mass to account for the lean portion of adipose tissue and ensure an accurate estimation of thigh LM in persons with SCI.27,28,30,42
Using the known image slice thickness and interslice space, the total length of the MRI region of interest (ROI) was calculated and used to match to the DXA ROI using the following equation:
DXA
DXA scans were performed using a Lunar iDXA bone densitometer at the Richmond VA Medical Center. Quality control was assessed monthly per GE instructions. The detailed clinical DXA procedures for persons with SCI were previously outlined.6,7,36 Total body scans were captured to assess thigh LM (LMDXA: water, protein, carbohydrate and soft tissue minerals) and thigh fat free mass (FFMDXA: lean mass, bone mass and other connective tissues).38,42 Total body scans were performed for a total of 5–10 min and analyzed by a DXA trained researcher using Lunar enCORE software version 16.
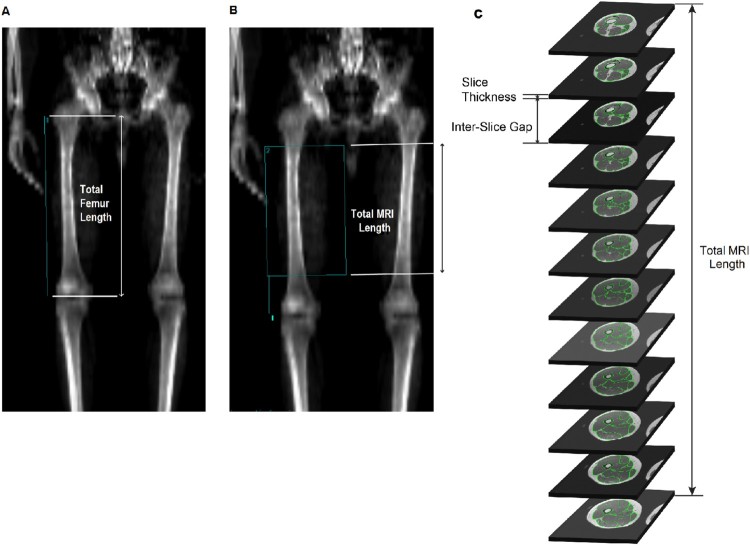
To match DXA and MRI ROIs, the calculated MRI length was applied to the femur displayed using Lunar enCORE. The MRI length was determined based on the slice thickness (0.8 cm) and the interslice space (1.6 cm) starting distally with the first slice above the femoral condyles and proximally toward the right hip joint. The number of slices included was based on anatomical distinction of the four heads of the knee extensor muscle group. On the DXA captured scan, a line was drawn along the right femur from the greater trochanter to the tip of lateral femoral epicondyle to determine femur length (Fig. 1(A)). Using the Shields Model for DXA Knee Measurement43 the lower boundary of the ROI was set at 20% of the femur length, measured from the terminus of the lateral femoral condyle (Fig. 1(B)). The length of the DXA ROI was equal to that of the MRI length and the width was set to include all muscle tissues, while excluding any tissues from the left thigh or from the genito-pelvic region (Fig. 1(B)). After ROI selection, thigh LMDXA and FFMDXA were then calculated.
Figure 1.
(A) The line drawn along the length of the right femur from the greater trochanter to the terminus of the lateral femoral condyle, which was used to determine total femur length. (B) Line 1 representing 20% of the femur length, extending from the terminus of the lateral femoral condyle and marking the distal boundary of the DXA ROI. The ROI length was set to that of the calculated MRI length. A 0.8 cm slice thickness and 1.6 cm inter-slice gap were used to calculate total MRI length. The width was expanded to include all muscle tissues while excluding any tissues from the left leg or genito-pelvic region. (C) The corresponding sequence of trans-axial MRIs.
Predicted MMMRI-KE from DXA
Using a recently published ratio26 indicated that the knee extensor muscle group, measured by MRI, accounts for 52% of the thigh absolute muscle CSA (excluding IMF). MMMRI-KE was estimated as 52% of thigh LMDXA and referred to as LMDXA-KE. This ratio was developed based of subsample (n = 11) from the entire sample of the current study.26
Statistical analysis
Statistical analyses were performed using SPSS, version 24. All data was normally distributed and no outliers were identified. Linear regression was used to identify relationships between LMDXA and MMMRI-WM, MMMRI-ABS, and MMMRI-KE. SEE was calculated to quantify the accuracy of DXA measurements compared to MRI. Bland–Altman analyses were conducted to assess the level of agreement between DXA and MRI measurements. Two-tailed paired t-tests were performed to assess statistical significance between DXA and MRI outcome variables. Finally, bootstrapping analysis approach was used to validate the developed prediction models based on a random generating sample of 1000. All values are presented as means ± SD and an α of 0.05 was chosen for each analysis.
Results
Participant characteristics
Participant demographics are presented in Table 1. Participants ranged in age from 18–61 years with BMI ranging from 15.4–31.1 kg/m2. No descriptive data were significantly different between Caucasians and African Americans or between paraplegic and tetraplegic participants.
Regression analysis between DXA and MRI
Mean ± SD for DXA and MRI measurements are presented in Table 2. LMDXA predicted MMMRI-WM (r2 = 0.90, SEE = 0.23 kg, P < 0.0001; Fig. 2(A); 95% CI:0.83–1.07) and MMMRI-ABS (r2 = 0.82, SEE = 0.28 kg, P < 0.0001; Fig. 2(B); 95% CI:0.68–0.97). Bootstrapping for LMDXA vs MMMRI-WM was 0.059 kg (SEE = 0.059; P = 0.001; 95% CI: 0.82–1.06) and for LMDXA vs MMMRI-ABS was 0.062 kg (SEE = 0.062; P = 0.001; 95% CI: 0.70–0.93).
Table 2. Mean ± SD and percentage difference of MRI and DXA measurements in 32 persons with SCI.
| Measurement | Mean ± SD | Range | P-values | Percentage Difference |
|---|---|---|---|---|
| MMMRI-WM | 3.13 ± 0.72 kg | 1.9–5.1 kg | <0.0001 | 7.3% |
| LMDXA | 3.36 ± 0.72 kg | 1.9–5.0 kg | ||
| MMMRI-ABS | 2.79 ± 0.65 kg | 1.4–3.9 kg | <0.0001 | 20.4% |
| LMDXA | 3.36 ± 0.72 kg | 1.9–5.0 kg | ||
| MMMRI-WM/FFMDXA | 89.26 ± 6.47% | 76.7–100% | <0.0001 | 6.9% |
| LMDXA/FFMDXA | 95.86 ± 0.97% | 93.9–97.7% | ||
| MMMRI-ABS/FFMDXA | 79.01 ± 8.05% | 63.8–96.4% | <0.0001 | 16.4% |
| LMDXA/FFMDXA | 95.86 ± 0.97% | 93.9–97.7% | ||
| MMMRI-KE | 1.39 ± 0.34 kg | 0.75–2.11 kg | <0.0001 | 26.0% |
| LMDXA-KE | 1.75 ± 0.37 kg | 0.97–2.60 kg |
MM, muscle mass; LM, lean mass; MMMRI-WM, MM measured by MRI using thigh whole muscle CSA; LMDXA, LM measured by DXA; MMMRI-ABS, MM measured by MRI using whole thigh muscle CSA after subtracting intramuscular fat; LMDXA-KE, predicted knee extensor LM using ratio; MMMRI-KE, knee extensor muscle mass measured by MRI.
Figure 2.
(A) Linear regression analyses showing the relationships between LMDXA and MMMRI-WM. (B) LMDXA and MMMRI-ABS excluding IMF. (C) LMDXA-KE [LMDXA in conjunction with a ratio predicting knee extensor size (*0.52)] and MMMRI-KE.
After accounting for FFM, a statistically significant difference was noted between MMMRI-WM/FFMDXA and LMDXA/FFMDXA (P < 0.0001), as well as MMMRI-ABS/FFMDXA and LMDXA/FFMDXA (P < 0.0001). Additionally, LMDXA-KE predicted MMMRI-KE (r2 = 0.78, SEE = 0.16 kg, P < 0.0001; Fig. 2(C); 95% CI:0.82–1.22). Bootstrapping for LMDXA-KE vs MMMRI-KE was 0.076 kg (SEE = 0.076; P = 0.001; 95% CI: 0.88–1.2).
Agreement between DXA and MRI
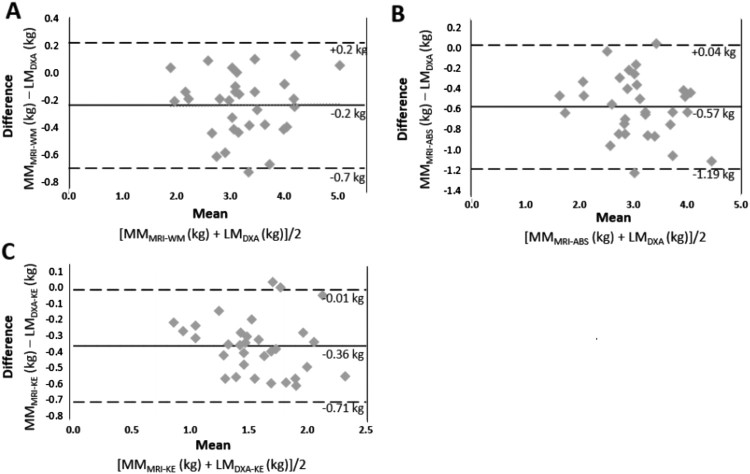
Bland–Altman plots in Figure 3 show a high level of agreement between DXA and MRI measurements, with DXA overestimating thigh LM compared to MMMRI-WM (mean bias = −0.2 kg; Fig. 3(A)), MMMRI-ABS (mean bias = −0.5 kg; Fig. 3(B)) and MMKE-MRI (mean bias = −0.4 kg; Fig. 3(C)).
Figure 3.
(A) Bland-Altman analysis between LMDXA and MMMRI-WM. In each plot, the difference between the values obtained from DXA and MRI is plotted along the y-axis and the average of the measurements is plotted along the x-axis. The solid line represents the mean difference between measurements and dashed lines represent 2 SD above and below the mean difference. (B) Agreement between LMDXA and MMMRI-ABS after excluding IMF. (C) Bland Altman plots between LMDXA-KE and MMMRI-KE.
Derived equations
A field equation was developed to predict MRI whole thigh muscle mass from DXA LM: MMMRI-WM = 0.952 (LMDXA) – 0.073 (Fig. 2(A)). A second equation was developed to predict the MRI absolute thigh lean mass, after accounting for IMF and bone mass from DXA LM: MMMRI-ABS = 0.824 (LMDXA) + 0.018 (Fig. 2(B)). Finally, knee extensor muscle mass was predicted from DXA LM using the following equation: MMMRI-KE = 0.815 (0.52 × LMDXA) – 0.038 (Fig. 2(C)).
Discussion
The current study demonstrated that DXA may be an effective tool to predict thigh skeletal muscle mass after SCI. DXA predicted 90% of the variance in whole muscle mass, 82% of the variance in absolute muscle mass, and 78% of the variance in knee extensor mass as determined by gold standard MRI. The derived SCI-specific equations estimated total and absolute thigh muscle mass as well as the mass of the knee extensor muscle group. Bootstrapping analysis approach ensured the validation of the predicted models in a random generated sample with reasonably accepted SEE.
Despite the strong relationships between DXA and MRI values, DXA consistently overestimated LM. A slight overestimation (0.23 kg) of LMWM-MRI was observed when comparing DXA to MRI measurements. DXA overestimated LMABS-MRI and LMKE-MRI by 0.57 and 0.36 kg, respectively. Overestimations were most likely due to the inability of DXA to effectively isolate IMF from the surrounding muscle tissues. The findings are consistent with previous studies comparing LM estimates from DXA and gold standard techniques. Modlesky et al. used MRI and DXA to assess skeletal muscle mass in men with SCI. On average DXA overestimated muscle mass by 0.34 and 0.29 kg in SCI and abled-bodied subjects, respectively.24 Similarly, a previous study investigated the relationships between DXA and CT LM values at baseline and after 10 weeks of leg extension training in men and women.42 An increase in muscle mass was observed after training using CT (3.97%) and DXA (2.97%); however, a substantial absolute difference between the two methods was noted at baseline, with DXA overestimating LM by ∼2 kg.42 Another study showed that thigh LM using DXA had a 6 and 9% lesser degree of sarcopenia in both young and older men and women, respectively, compared to MRI.44 The authors suggested that adjustments be made to the software algorithms to better account for the lean content of bone and adipose tissue. The latter two studies showed strong correlations between DXA and gold standard techniques (r = 0.88 and r2 = 0.88); however, systematic errors in DXA collection led to substantial overestimation.42,44
Elder et al. determined that failure to correct for IMF resulted in underestimation of muscle atrophy by ∼12% in individuals with SCI.4 In the current study, a tighter association was observed when thigh muscle mass was uncorrected for IMF, suggesting that DXA does not entirely distinguish between muscle and IMF. Wade and Gorgey showed that failure to account for IMF resulted in a 3.5 and 21.5% overestimation in whole thigh muscle CSA and absolute muscle CSA, respectively, when using anthropometrics.25 When utilizing the predictive equation including a correction factor for IMF, the relationship between anthropometrics and MRI improved and a minimal (< 1%) overestimation was noted.25
An overestimation of 0.36 kg was observed in MMMRI-KE, this was expected since knee extensor LM was estimated using DXA in conjunction with a predetermined ratio.26 The derived equation was capable of predicting 78% of the variance in knee extensor mass. These findings are of clinical significance as the knee extensor muscle group accounts for ∼52% of the entire absolute muscle CSA of the thigh in persons with SCI and has been shown to undergo extensive atrophy following injury.1,5 Due to its large contribution to total thigh LM, the knee extensor muscle group has a notable metabolic contribution and is robustly linked to metabolic insufficiencies after SCI.34,45,46 A previous study determined the effects of 12 weeks of progressive neuromuscular electrical stimulation-resistance training after complete SCI.34 Knee extensor CSA increased by 35% and was associated with increased circulating insulin growth factors,34 an upstream regulator of the hypertrophy signaling pathway. Additionally, O’Brien et al. revealed a relationship between knee extensor size and adiponectin,45 which is associated with increased energy expenditure, improved insulin sensitivity and may play a role in the prevention of type-II diabetes and cardiovascular disease.45–47 Lastly, in 22 men with SCI, knee extensor size was positively correlated with citrate synthase and complex III activity responsible for mitochondrial respiration.48 Due to the clinical implications of knee extensor size for persons with SCI, quantification of individual muscles using DXA may provide greater insights into preventing disturbance in energy balance and the onset of secondary metabolic dysfunction after SCI.
Using DXA, analysis time was greatly reduced compared to standard MRI and required ∼15–20 min for a subject. In comparison, MRI acquisition may require up to 20 min to scan lower extremities and an additional 15–20 min to analyze muscle CSA per image (12–15 images). DXA may also reduce the acquisition costs associated with using MRI scanners and offer greater comfort for patients during scanning. Anecdotal evidence suggests that participants prefer the padded and open table of the DXA rather than the closed MRI system, which may cause feelings of claustrophobia. Moreover, DXA uses an average effective radiation dose of 0.001 mSv (range of 0.00–0.035 mSv) compared to CT scans, with an average effective radiation dose of 2–16 mSv depending on the scanning location (range of 0.9–32 mSv).49 Therefore, DXA may provide several benefits to clinicians, researchers and patients as evidence by reduced acquisition time, reduced costs, greater patient comfort, and minimal radiation exposure.
Limitations
Creating an equal distribution of men and women within a sample with can be quite challenging. With the limited number of women in the current sample, the results may be less generalizable to women; however, because the protocol involved only image analysis and DXA scanning (both of which have been validated in women and men with SCI) the results are less likely affected by the limited number of women. Similarly, because the current sample was limited to those with levels of injury C5-L1 and AIS classification A, B, or C, the predictive equations may be less accurate to predict skeletal muscle mass in persons with higher levels of injury or those with incomplete AIS D SCI.
Utilizing the Shields’ model,43 the lower boundary of the DXA ROI was set at 20% of the femur length, measured from the terminus of the lateral femoral condyle (Fig. 1(B)). This starting point was used to match the DXA to the MRI ROI. Future trials comparing the two techniques may elect to use MRI and DXA-specific markers to mark the exact starting and ending locations of the thigh ROI. This may ensure a more precise matching between the two techniques. Lastly, to ensure the predictive equations provide accurate estimations of thigh muscle mass for persons with SCI, we performed bootstrapping analysis approach that facilitated validation of the predicted models. However, further cross-validation is required using another independent cohort with different physical characteristics. We have recently adopted similar strategy to cross-validate anthropometrically developed equation of thigh muscle CSA in persons with motor complete SCI.26 We should also point that the use of DXA may offer advantages as far as scan and processing time of measuring muscle CSA; however, DXA may still expose persons with SCI to relatively low dose of ionizing radiation compared to MRI.
Conclusions
The current study demonstrates that DXA may effectively predict thigh skeletal muscle mass in persons with SCI. DXA predicted 90 and 82% of the variance in whole thigh and absolute thigh muscle mass, respectively. Moreover, applying a previously published ratio allowed DXA to account for 78% of the variance in knee extensor muscle mass. The current findings suggest that DXA may not be sensitive enough to fully isolate IMF; however, the derived equations offset for such limitation by providing an accurate estimate of thigh muscle mass. The described technique has the potential to dramatically reduce acquisition time, costs, patient discomfort and radiation exposure. Further validation of the proposed equations has the potential to alleviate SCI-specific barriers and improve body composition assessment in persons with SCI.
Abbreviations
AIS ASIA Impairment Scale
CSA cross-sectional area
CT computed tomography
DXA dual-energy x ray absorptiometry
FFMDXA thigh fat free mass by DXA
IMF intramuscular fat
LM lean mass
LMDXA thigh lean mass by DXA
LMDXA-KE knee extensor lean mass by DXA
MMMRI-ABS absolute thigh muscle mass by MRI
MMMRI-KE knee extensor muscle mass by MRI
MMMRI-WM whole thigh muscle mass by MRI
MRI magnetic resonance imaging
ROI region of interest
SCI spinal cord injury
Acknowledgements
We would like to thank the participants who devoted their time and effort to participate in the current study. We would like to thank Hunter Holmes McGuire Research Institute and Spinal Cord Injury Services and Disorders for providing the environment to conduct clinical human research trials. Ashraf S. Gorgey is currently supported by the Department of Veteran Affairs, Veteran Health Administration, Rehabilitation Research and Development Service (B7867-W) and DoD-CDRMP (W81XWH-14-SCIRP-CTA).
Suppliers
Wheelchair scale (Tanita, Arlington Heights, IL)
Signa 1.5-T MRI (GE Healthcare, Chicago, IL)
X-Vessel software version 2.011 (written by Ronald Meyer at Michigan State University, Lansing, MI).
Lunar iDXA bone densitometer (GE Healthcare, Chicago, IL)
Lunar enCORE software version 16 (GE Healthcare, Chicago, IL)
SPSS, version 24 (IBM, Armonk, New York)
Disclaimer statements
Contributors None.
Funding Ashraf S. Gorgey is currently supported by the Department of Veteran Affairs, Veteran Health Administration, Rehabilitation Research and Development Service (B7867-W) and DoD-CDRMP (W81XWH-14-SCIRP-CTA).
Conflicts of interest Authors have no conflict of interests to declare.
ORCID
Ashraf S. Gorgey http://orcid.org/0000-0002-9157-6034
References
- 1.Castro MJ, Apple DF Jr, Hillegass EA, Dudley GA.. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol 1999;80: 373–378. doi: 10.1007/s004210050606 doi: 10.1007/s004210050606 [DOI] [PubMed] [Google Scholar]
- 2.Dolbow DR, Gorgey AS, Ketchum JM, Moore JR, Hackett LA, Gater DR.. Exercise adherence during home-based functional electrical stimulation cycling by individuals with spinal cord injury. Am J Phys Med Rehabil 2012;91: 922–930. doi: 10.1097/PHM.0b013e318269d89f doi: 10.1097/PHM.0b013e318269d89f [DOI] [PubMed] [Google Scholar]
- 3.Dolbow DR, Gorgey AS, Khalil RK, Gater DR.. Effects of a fifty-six month electrical stimulation cycling program after tetraplegia: case report. J Spinal Cord Med 2017;40(4): 485–488. doi: 10.1080/10790268.2016.1234750 doi: 10.1080/10790268.2016.1234750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elder CP, Apple DF, Bickel CS, Meyer RA, Dudley GA.. Intramuscular fat and glucose tolerance after spinal cord injury: a cross-sectional study. Spinal Cord 2004;42: 711–716. doi: 10.1038/sj.sc.3101652 doi: 10.1038/sj.sc.3101652 [DOI] [PubMed] [Google Scholar]
- 5.Gorgey AS, Dudley GA.. Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord 2007;45: 304–309. doi: 10.1038/sj.sc.3101968 doi: 10.1038/sj.sc.3101968 [DOI] [PubMed] [Google Scholar]
- 6.Gorgey AS, Dolbow DR, Gater DR Jr. A model of prediction and cross-validation of fat-free mass in men with motor complete spinal cord injury. Arch Phys Med Rehabil. 2012;93(7):1240–1245. [DOI] [PubMed] [Google Scholar]
- 7.Spungen AM, Adkins RH, Stewart CA, Wang J, Pierson RN, Waters RL, et al. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol 2003;95: 2398–2407. doi: 10.1152/japplphysiol.00729.2002 doi: 10.1152/japplphysiol.00729.2002 [DOI] [PubMed] [Google Scholar]
- 8.Spungen AM, Wang J, Pierson RN Jr, Bauman WA.. Soft tissue body composition differences in monozygotic twins discordant for spinal cord injury. J Appl Physiol 2000;88: 1310–1315. doi: 10.1152/jappl.2000.88.4.1310 doi: 10.1152/jappl.2000.88.4.1310 [DOI] [PubMed] [Google Scholar]
- 9.Goodpaster BH. Mitochondrial deficiency is associated with insulin resistance. Diabetes 2013;62:1032–1035. doi: 10.2337/db12-1612 doi: 10.2337/db12-1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorgey AS, Dolbow DR, Dolbow JD, Khalil RK, Castillo C, Gater DR.. Effects of spinal cord injury on body composition and metabolic profile: Part I. J Spinal Cord Med 2014;37: 693–702. doi: 10.1179/2045772314Y.0000000245 doi: 10.1179/2045772314Y.0000000245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauman WA, Spungen AM.. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism 1994;43(6):749–756. doi: 10.1016/0026-0495(94)90126-0 [DOI] [PubMed] [Google Scholar]
- 12.Bauman WA, Spungen AM.. Invited review carbohydrate and lipid metabolism In chronic spinal cord injury. J Spinal Cord Med 2001;24(4):266–277. doi: 10.1080/10790268.2001.11753584 [DOI] [PubMed] [Google Scholar]
- 13.Buchholz AC, Pencharz PB.. Energy expenditure in chronic spinal cord injury. Curr Opin Clin Nutr Metab Care 2004;7(6):635–639. doi: 10.1097/00075197-200411000-00008 [DOI] [PubMed] [Google Scholar]
- 14.Monroe MB, Tataranni PA, Pratley R, Manore MM, Skinner JS, Ravussin E.. Lower daily energy expenditure as measured by respiratory chamber in subjects with spinal cord injury compared with control subjects. Am J Clin Nutr 1998;68(6):1223–1227. doi: 10.1093/ajcn/68.6.1223 doi: 10.1093/ajcn/68.6.1223 [DOI] [PubMed] [Google Scholar]
- 15.Moore CD, Craven BC, Thabane L, Laing AC, Frank-Wilson AW, Kontulainen SA, et al. Lower-extremity muscle atrophy and fat infiltration after chronic spinal cord injury. J Musculoskelet Neuronal Interact 2015;15(1):32–41. [PMC free article] [PubMed] [Google Scholar]
- 16.Nightingale TE, Gorgey AS.. Predicting basal metabolic rate in men with motor complete spinal cord injury. Med Sci Sports Exerc 2018. doi: 10.1249/MSS.0000000000001548. [DOI] [PubMed] [Google Scholar]
- 17.Duckworth WC, Solomon SS, Jallepalli P, Heckemeyer C, Finnern J, Powers A.. Glucose intolerance due to insulin resistance in patients with spinal cord injuries. Diabetes 1980;29(11):906–910. doi: 10.2337/diab.29.11.906 [DOI] [PubMed] [Google Scholar]
- 18.Gater DR. Obesity after spinal cord injury. Phys Med Rehabil Clin N Am 2007;18(2):333–351. doi: 10.1016/j.pmr.2007.03.004 doi: 10.1016/j.pmr.2007.03.004 [DOI] [PubMed] [Google Scholar]
- 19.Gorgey AS, Gater DR.. Prevalence of obesity after spinal cord injury. Top Spinal Cord Inj Rehabil 2007;12(4):1–7. doi: 10.1310/sci1204-1 doi: 10.1310/sci1204-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Addison O, Marcus RL, Lastayo PC, Ryan AS.. Intermuscular fat: a review of the consequences and causes. Int J Endocrinol 2014. doi: 10.1155/2014/309570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kershaw EE, Flier JS.. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004;89:2548–2556. doi: 10.1210/jc.2004-0395 doi: 10.1210/jc.2004-0395 [DOI] [PubMed] [Google Scholar]
- 22.Manns PJ, McCubbin JA, Williams DP.. Fitness, inflammation, and the metabolic syndrome in men with paraplegia. Arch Phys Med Rehabil 2005;86:1176–1181. doi: 10.1016/j.apmr.2004.11.020 doi: 10.1016/j.apmr.2004.11.020 [DOI] [PubMed] [Google Scholar]
- 23.Ogawa M, Lester R, Akima H, Gorgey AS.. Quantification of intermuscular and intramuscular adipose tissue using magnetic resonance imaging after neurodegenerative disorders. Neural Regen Res. 2017;12(12):2100–2105. doi: 10.4103/1673-5374.221170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Modlesky CM, Bickel CS, Slade RA, Meyer Cureton KJ, Dudley GA.. Assessment of skeletal muscle mass in men with spinal cord injury using dual-energy X-ray absorptiometry and magnetic resonance imaging. J Appl Physiol 2004;96: 561–565. doi: 10.1152/japplphysiol.00207.2003 doi: 10.1152/japplphysiol.00207.2003 [DOI] [PubMed] [Google Scholar]
- 25.Wade RC, Gorgey AS.. Anthropometric prediction of skeletal muscle cross-sectional area in persons with spinal cord injury. J Appl Physiol 2017;122(5):1255–1261. doi: 10.1152/japplphysiol.01042.2016 doi: 10.1152/japplphysiol.01042.2016 [DOI] [PubMed] [Google Scholar]
- 26.Wade RC, Lester RM, Gorgey AS.. Validation of anthropometric muscle cross-sectional area equation after spinal cord injury. Int J Sports Med 2018;39(5):366–373. doi: 10.1055/s-0044-102133 doi: 10.1055/s-0044-102133 [DOI] [PubMed] [Google Scholar]
- 27.Lester RM, Johnson K, Khalil RE, Khan R, Gorgey AS.. MRI analysis and clinical significance of lower extremity muscle cross-sectional area after spinal cord injury. Neural Regen Res 2017;12:714–722. doi: 10.4103/1673-5374.206634 doi: 10.4103/1673-5374.206634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R.. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol 1998;85:115–122. doi: 10.1152/jappl.1998.85.1.115 doi: 10.1152/jappl.1998.85.1.115 [DOI] [PubMed] [Google Scholar]
- 29.Gibbs JC, Craven BC, Moore C, Thabane L, Adachi JD, Giangregorio LM.. Muscle density and bone quality of the distal lower extremity among individuals with chronic spinal cord injury. Top Spinal Cord Inj Rehabil 2015;21(4):282–293. doi: 10.1310/sci2104-282 doi: 10.1310/sci2104-282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee SY, Gallagher D.. Assessment methods in human body composition. Curr Opin Clin Nutr Metab Care 2008;11: 566–572. doi: 10.1097/MCO.0b013e32830b5f23 doi: 10.1097/MCO.0b013e32830b5f23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross R. Advances in the application of imaging methods in applied and clinical physiology. Acta Diabetol 2003;40(Suppl 1): S45–S50. doi: 10.1007/s00592-003-0025-y doi: 10.1007/s00592-003-0025-y [DOI] [PubMed] [Google Scholar]
- 32.Manini TM, Clark BC, Nalls MA, Goodpaster BH, Ploutz-Snyder LL, Harris TB.. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr 2007;85:377–384. doi: 10.1093/ajcn/85.2.377 doi: 10.1093/ajcn/85.2.377 [DOI] [PubMed] [Google Scholar]
- 33.Smith AC, Parrish TB, Abbott R, Hoggarth MA, Mendoza K, Chen YF, et al. Muscle-fat MRI: 1.5 and 3.0 Tesla versus histology. Muscle Nerve 2014;50:170–176. doi: 10.1002/mus.24255 doi: 10.1002/mus.24255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorgey AS, Mather KJ, Cupp HR, Gater DR.. Effects of resistance training on adiposity and metabolism after spinal cord injury. Med Sci Sports Exerc 2012;44(1):165–174. doi: 10.1249/MSS.0b013e31822672aa doi: 10.1249/MSS.0b013e31822672aa [DOI] [PubMed] [Google Scholar]
- 35.Gorgey AS, Chiodo AE, Zemper ED, Hornyak JE, Rodriguez GM, Gater DR.. Relationship of spasticity to soft tissue body composition and the metabolic profile in persons with chronic motor complete spinal cord injury. J Spinal Cord Med 2010;33(1):6–15. doi: 10.1080/10790268.2010.11689669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorgey AS, Cirnigliaro CM, Bauman WA, Adler RA.. Estimates of the precision of regional and whole body composition by dual-energy x-ray absorptiometry in persons with chronic spinal cord injury. Spinal Cord 2018. doi: 10.1038/s41393-018-0079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Genton L, Hans D, Kyle UG, Pichard C MD.. Dual-Energy X-ray absorptiometry and body composition: differences between devices and comparison with reference methods. Nutrition 2002;18(1):66–70. doi: 10.1016/S0899-9007(01)00700-6 doi: 10.1016/S0899-9007(01)00700-6 [DOI] [PubMed] [Google Scholar]
- 38.Kohrt WM. Preliminary evidence that DEXA provides an accurate assessment of body composition. J Appl Physiol 1998;84(1): 372–377. doi: 10.1152/jappl.1998.84.1.372 doi: 10.1152/jappl.1998.84.1.372 [DOI] [PubMed] [Google Scholar]
- 39.Pietrobelli A, Formica C, Wang S, Heymsfield SB.. Dual-energy X-ray absorptiometry body composition model: review of physical concepts. Am J Physiol 1996;271(6 Pt 1):941–951. doi: 10.1152/ajpendo.1996.271.6.E941 [DOI] [PubMed] [Google Scholar]
- 40.Gorgey AS, Khalil RE, Gill R, O'Brien LC, Lavis T, Castillo T, et al. Effects of testosterone and evoked resistance exercise after spinal cord injury (TEREX-SCI): study protocol for a randomised controlled trial. BMJ Open 2017;7(4):e014125. doi: 10.1136/bmjopen-2016-014125 doi: 10.1136/bmjopen-2016-014125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorgey AS, Khalil RE, Lester RM, Dudley GA, Gater DR.. Paradigms of lower extremity electrical stimulation training after spinal cord injury. J Vis Exp 2018;1:132. doi: 10.3791/57000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delmonico MJ, Kostek MC, Johns J, Hurley BF, Conway JM.. Can dual energy X-ray absorptiometry provide a valid assessment of changes in thigh muscle mass with strength training in older adults? Eur J Clin Nutr 2008;62(12):1372–1378. doi: 10.1038/sj.ejcn.1602880 doi: 10.1038/sj.ejcn.1602880 [DOI] [PubMed] [Google Scholar]
- 43.Shields RK, Schlechte J, Dudley-Javoroski S, Zwart BD, Clark SD, Grant SA, et al. Bone mineral density after spinal cord injury: a reliable method for knee measurement. Arch Phys Med Rehabil 2005;86(10): 1969–1973. doi: 10.1016/j.apmr.2005.06.001 doi: 10.1016/j.apmr.2005.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maden-Wilkinson TM, Degens H, Jones DA, McPhee JS.. Comparison of MRI and DXA to measure muscle size and age-related atrophy in thigh muscles. J Musculoskelet Neuronal Interact 2013;13(3):320–328. [PubMed] [Google Scholar]
- 45.O'Brien LC, Graham ZA, Chen Q, Lesnefsky EJ, Cardozo C, Gorgey AS.. Plasma adiponectin levels are correlated with body composition, metabolic profiles, and mitochondrial markers in individuals with chronic spinal cord injury. Spinal Cord 2018;56(9):863–872. doi: 10.1038/s41393-018-0089-8 doi: 10.1038/s41393-018-0089-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Brien LC, Chen Q, Savas J, Lesnefsky EJ, Gorgey AS.. Skeletal muscle mitochondrial mass is linked to lipid and metabolic profile in individuals with spinal cord injury. Eur J Appl Physiol 2017;117(11):2137–2147. doi: 10.1007/s00421-017-3687-9 [DOI] [PubMed] [Google Scholar]
- 47.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 2000;20(6):1595–1599. doi: 10.1161/01.ATV.20.6.1595 doi: 10.1161/01.ATV.20.6.1595 [DOI] [PubMed] [Google Scholar]
- 48.O’Brien LC, Wade RC, Segal L, Chen Q, Savas J, Lesnefsky EJ, et al. Mitochondrial mass and activity as a function of body composition in individuals with spinal cord injury. Physiol Rep 2017;5(3). 10.14814/phy2.1308 doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mettler FA, Huda W, Yoshizumi TT, Mahesh M.. Effective doses in radiology and diagnostic nuclear medicine: a Catalog. Radiology 2008;248(1):254–263. doi: 10.1148/radiol.2481071451 doi: 10.1148/radiol.2481071451 [DOI] [PubMed] [Google Scholar]
- 50.Butler AA, LeRoith D.. Control of growth by the somatropic axis: growth hormone and the insulin-like growth factors have related and independent roles. Annu Rev Physiol 2001;63:141–164. doi: 10.1146/annurev.physiol.63.1.141 doi: 10.1146/annurev.physiol.63.1.141 [DOI] [PubMed] [Google Scholar]