Abstract
Objective: The purpose of this pilot study was to determine whether 60 mins of intermittent pneumatic compression therapy (IPC) could acutely increase leg blood flow-induced shear stress and enhance vascular endothelial function in persons with spinal cord injury (SCI).
Design: Pretest with multiple posttests, within subject randomized control design.
Setting: University of Southern Mississippi, Spinal Cord Injury Research Program within the School of Kinesiology, recruiting from the local community in Hattiesburg, Jackson, and Gulfport, MS.
Participants: Eight adults with SCI (injury level: T3 and below; ASIA class A-C; age: 41±17 yrs).
Interventions: A 60-min IPC session was performed in one leg (experimental leg; EXP), with the other leg serving as a control (CON).
Outcomes Measures: Posterior-tibial artery shear rate (Doppler-ultrasound) was examined at rest, and at 15 and 45 mins during IPC. Endothelial function was assessed using the flow-mediated dilation (FMD) technique, before and after IPC.
Results: Resting FMD (mm) was similar between legs at rest. A two-way repeated measures ANOVA (leg x time) revealed that during IPC, peak shear rate increased in the EXP leg (215±137 to 285±164 s−1 at 15 mins; +39±29%, P = 0.03), with no change occurring in the CON. In addition, FMD significantly increased in the EXP leg (Pre IPC: 0.36±0.14 vs. Post IPC: 0.47±0.17 mm; P = 0.011, d = 0.66), with no change occurring in the CON leg.
Conclusion: These preliminary findings suggests that IPC therapy may acutely increase leg shear stress within 15 mins, with a resultant moderate-large improvement in vascular endothelial function after 60 mins in people with SCI.
Keywords: Spinal Injury, Endothelial Function, Compression Therapy
Introduction
In persons with spinal cord injury (SCI), atherosclerosis, a putative process leading to cardiovascular disease (CVD),1 occurs at an accelerated rate in paralyzed lower limbs.2–6 This occurrence is mediated largely by extreme inactivity, resulting in rapid skeletal muscle atrophy and inadequate blood flow-induced shear stress through sub-lesional vessels.2–5,7 Shear stress, which represents the frictional force of blood moving across the vascular endothelium, stimulates the production and release of endothelial-derived vasodilators, most notably nitric oxide, which promote smooth muscle relaxation and increased blood flow to the active or stimulated region.8–14 Nitric oxide also performs a myriad of anti-atherogenic functions and is considered the most important molecule governing endothelial health.11 In this regard, vascular endothelial health, at least in able-bodied (AB) individuals, is maintained by regularly elevating shear stress through skeletal muscle contractions.13,14 Persons with SCI may not be able to voluntarily contract skeletal muscle in lower limbs, and therefore, alternative therapies are required to elevate leg shear stress, maintain endothelial health, and decrease CVD risk.
The lower limbs in persons with SCI can be stimulated via electrical simulation, which can be effective in improving leg vascular health.15,16 However, this technology can be expensive and difficult to administer for some, thus, making this treatment not widely available outside of a clinical setting. Alternatively, recent evidence demonstrates that intermittent pneumatic compression therapy (IPC) can acutely increase shear stress in lower limb arteries of AB individuals.17,18 IPC is a non-invasive FDA approved therapy commonly used to treat individuals with symptoms of venous insufficiency and claudication pain in peripheral arterial disease.17–24 This therapy is performed by inflating and deflating pneumatic cuffs, which are secured around the calf and foot region, in regular intervals for a period of time (i.e., 1 hour, 3 inflation cycles/minute) to simulate the mechanical compression of muscle contractions. While the use of IPC in clinical AB populations has been extensively tested, the efficacy of its use for improving vascular health outcomes in people with SCI is currently unknown.
Therefore, this study recruited a paraplegic SCI cohort into a between leg control, pre-post prospective study design. We hypothesized that a single 60 min IPC session would acutely increase leg shear stress, and result in a clinically meaningful improvement in endothelial function (i.e. absolute 1% improvement in FMD).25
Methods
Subjects
Subjects with SCI were recruited from Hattiesburg and Gulfport, MS. Inclusion for participation consisted of the following: ≥2 years post spinal injury; T3 neurological injury level and below; American Spinal Injury Association (ASIA) classification A, B and C; wheelchair reliant; 18+ years of age. Exclusion criteria consisted of pressure wounds on the buttocks or feet, unhealed bone fractures or history of fragility fractures, severe osteoporosis (T-score of -4 or less), uncontrolled autonomic dysreflexia, and unstable cardiovascular or metabolic disease. Informed consent was obtained in accordance with the requirements of the University of Southern Mississippi Institutional Review Board.
Experimental Protocols
Following written consent, each participant completed a medical history questionnaire. A familiarization was then performed to acquaint subjects to the experimental measurements. Subsequently, anthropometric indices were recorded (height and weight), and body composition was assessed using dual-energy x-ray absorptiometry (DEXA). Lastly, the leg chosen to undergo IPC therapy [experimental leg (EXP)] was randomized, with the other serving as the control (CON).
All testing was performed in a dimly lit, temperature (21–23° Celsius) and humidity (∼50%) controlled laboratory. Participants reported to the laboratory between 6:00–11:00am, following an overnight fast, having refrained from caffeine and alcohol for 12 hours, and any strenuous physical activity for 24 hours. Participants were transferred to the examination table using a mechanical lift (Invacare® Reliant 450), positioned semi-recumbent, and instrumented for heart rate, blood pressure (BP), and vascular measurements. Participants rested quietly for 30 minutes prior to endothelial function testing in both legs. Endothelial function was re-assessed in both legs following 60 mins IPC. The order of baseline vascular testing was randomized, however, the order of post-IPC testing was not and the EXP leg was always assessed immediately following completion of IPC (∼3 mins post-treatment). Shear rate indices were recorded at baseline, and following minutes 15 and 45 of IPC.
Intermittent Pneumatic Compression
IPC was delivered at a rate of three inflation/deflation cycles per minute (4-s inflation/16-s deflation; 120 mmHg). The rationale for this dosage stems from previous work demonstrating this to be effective for increasing blood flow and shear stress in AB individuals.17,18,22,26,27 To do this, one small cuff (SC5; 5 cm bladder) was positioned around the mid-foot, and a larger cuff (CC17; 17 cm bladder) was positioned around the calf (∼1 cm distal to the fibular head). The inflation unit (Hokanson® E20-Rapid Cuff Inflator) inflated the foot-cuff first, followed shortly (∼0.5 second delay) by the calf-cuff.
Experimental Measurements
Vascular Endothelial Function and Blood Velocity
All vascular assessments were performed by the same sonographer. Endothelial function was assessed in both posterior-tibial arteries by measuring flow-mediated dilation (FMD) with a duplex Doppler-ultrasound (Logiq P5; GE® Medical systems, Milwaukee, WI, USA) in accordance with previously published guidelines.6,10,28 The FMD technique is considered the non-invasive, gold standard assessment of vascular endothelial function in humans.10,29 In general, this technique employs duplex Doppler-ultrasound technology to simultaneously examine a peripheral artery and its associated blood velocity signal before, during and after a brief period of circulatory arrest applied proximal or distal to a limb.10,28 Importantly, FMD assessed in peripheral arteries (e.g., brachial) has been shown to strongly correlate with coronary artery endothelial function, the severity of ischemic heart disease, and left ventricular ejection fraction in patient groups30,31, thus, making this assessment an important biomarker of cardiovascular health. At present, the artery was imaged 3–5 cm superior to the calcaneus and ∼1 cm posterior to the medial malleolus on the right and left ankles with an 11-MHz linear array transducer. Blood velocity signals were obtained using the same probe in pulse wave mode, operating at a frequency of 5-MHz and insonation angle of 60°. The velocity cursor was set mid-vessel and a large sample volume was used to encompass the entire vessel lumen without extending beyond it. To ensure consistency and stability in the measurement, probe placement was marked on the skin using a permanent marker, and the transducer was stabilized using a custom-designed clamp. In addition, all probe settings were kept consistent for each limb throughout the study. Using a video capture system (El Gato®; San Francisco, CA) two minutes of baseline artery diameter and blood velocity data were recorded at 30 Hz. Subsequently, a pneumatic cuff (220 mmHg) (Hokanson®) positioned ∼1 cm distal from the fibular head was inflated for 5 mins. Video capture resumed during the last 30 seconds of occlusion, and continued for 4 mins post cuff-release. Diameters and velocities (see below) were analyzed off-line by one operator, separate from the sonographer, using an automated edge-detecting software (FMD Studio®, QUIPU; Pisa, Italy). The sonographer was not blinded to IPC treatment legs.
Cardiovascular Measures
Heart rate (HR) was continuously monitored using a lead-II surface electrocardiography (ECG) (Logiq P5; GE® Medical systems, Milwaukee, WI, USA), and blood pressure (BP) was measured via automated sphygmomanometry (Omron® Intellisense, HEM-907XL) on the left upper-arm.
Body Composition
A DEXA (GE®, Lunar Prodigy Advance) scan was performed to determine total body and regional lean and fat mass, %-body fat, and total body bone mineral density. The participants were transferred from their wheelchair, via the mechanical lift, and positioned supine on the DEXA scanner. Their legs were strapped proximal and distal to the knees to steady the distal femur and proximal tibia, with a slight internal rotation of the hips to position the greater trochanter in the best scanning position.
Data Analysis
Vascular Endothelial Function
The FMD studio software processes the acquired 30-Hz Doppler-ultrasound data into 1 sec average bins. Following data processing, the vascular endothelial function testing and blood velocity data were then exported to Microsoft Excel (2013)®, from which a 3 sec rolling average was applied to the sec-sec diameter and blood velocity data. Baseline artery diameter was then determined from the 2 min data average prior to cuff-inflation, and the largest diameter value following cuff-release was considered the peak change, and used to calculate FMD in both absolute (mm) and relative (%) terms; i.e., FMD% change=(peak diameter-baseline diameter)/baseline diameter*100. FMD was also normalized to the shear rate stimulus (FMD%/AUC), using the shear rate AUC as described below.32 While leg-specific data is unavailable, previous research suggests the threshold smallest worthwhile clinical change in brachial FMD is 1%.25 For example, Inaba et al. (2010) reported in a meta-analysis that the pooled relative risks of cardiovascular events per 1% increase in FMD for the brachial artery, adjusted for confounding risk factors, was 0.87 (95% CI, 0.83–0.91); suggesting that a 1% increase and 1-SD increase in FMD is associated with 13% and 41% decrease in future cardiovascular related risk, respectively.25
Shear Rate
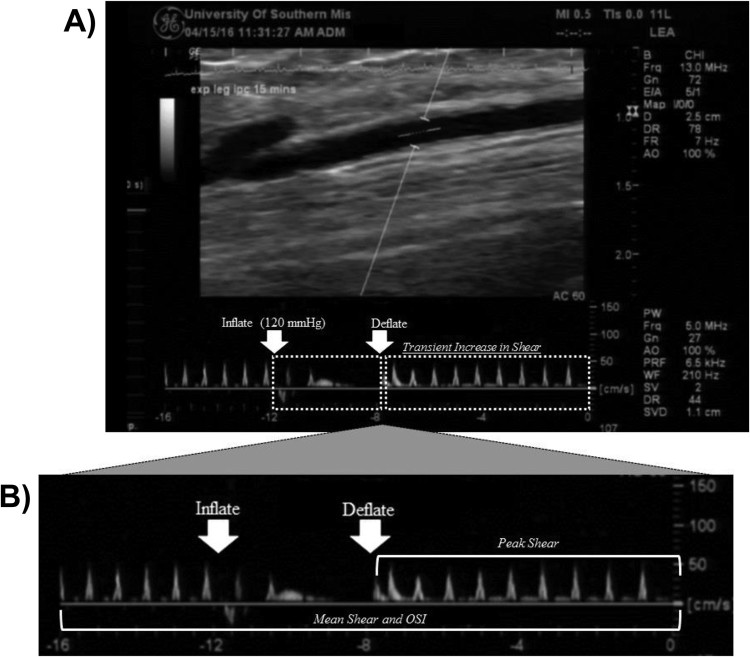
Blood velocity signals were analyzed (FMD Studio®, QUIPU) by obtaining the flow-envelope (area under tracing) for both the antegrade (positive area) and retrograde (negative area) velocity signals. Mean velocity was defined as the difference between antegrade and retrograde velocity signals. Shear rate (s−1), which is a non-invasively measured surrogate for shear stress in humans, was then calculated as 4*mean velocity/diameter,29 and shear rate incremental area-under-the-curve (AUC) up until 40 sec post cuff-release during FMD (representation of the shear stress stimulus promoting vasodilation) was calculated using the trapezoidal method (i.e. change in shear rate/second). The rationale for utilizing a 40 sec AUC, instead of AUC to peak diameter33 stems from previous evidence demonstrating that shear rates integrated over 40 seconds explain the greatest degree of residual variation in vasodilation for the FMD test (∼90%).32 Mean shear rate responses to IPC were analyzed by averaging mean shear rate across 3 compression cycles (i.e. 4 sec inflate, 16 sec deflate) equating to 60 seconds of data. The peak mean shear response was considered the average of 3 maximum shear rate values (i.e., mean shear) within 3 compression cycles, excluding inflation portion of the cycle to avoid movement artifact. The oscillatory shear index (OSI) was also determined at rest, and during 15 and 45 mins of IPC: OSI=retrograde shear rate / (antegrade shear rate + retrograde shear)*100 to provide an index of shear rate pattern17 (See Figure 1 for example image highlighting metrics used in calculating aforementioned shear rate profiles—mean, peak and OSI).
Figure 1.
Doppler-ultrasound image of posterior-tibial artery blood velocity (cm/sec) response to 15 mins of IPC in the EXP leg of a representative subject (Panel A). Note the increase in blood velocity (i.e., increase in shear rate) following IPC cuff deflation. Panel B expands the 16 sec Doppler blood velocity sweep to highlight metrics (i.e., bracketed regions) used for calculating mean, peak, and oscillatory shear index—OSI profiles.
Statistical Analysis
To examine differences between endothelial function, and shear rate indices at rest and during IPC therapy, 2-way (leg x time) repeated measures ANOVA’s were performed. Bonferroni post hoc tests were selected to examine possible interactions between study measurements. Cohen’s d effect sizes were calculated to examine meaningfulness of change in endothelial function between legs, pre to post IPC therapy. Normality was checked, and non-normally distributed data were log transformed. All data are presented as mean ± standard deviation, unless specified otherwise. All statistical analyses were performed using Sigma Plot Analysis software (Version 12.0). Statistical significance was set a priori at P < 0.05, and moderate-large effect sizes were considered clinically meaningful. All data were analyzed with the intention to treat.
Results
The average age and duration of SCI was 41±17 years, and 20±19 years, respectively. Average body mass index and bodyfat% were 30±8 kg/m2 and 39±12%, while resting heart rate and mean arterial pressure were 70±10 bpm and 89±8 mmHg, respectively. Within the cohort, three subjects had diagnosed metabolic disease (type 2 diabetes), and one had a history of cardiovascular disease (coronary artery disease). Importantly, no apparent differences were noted between these participant’s vascular responses pre and post IPC, as compared to the rest of the cohort.
Shear Rate Response to IPC
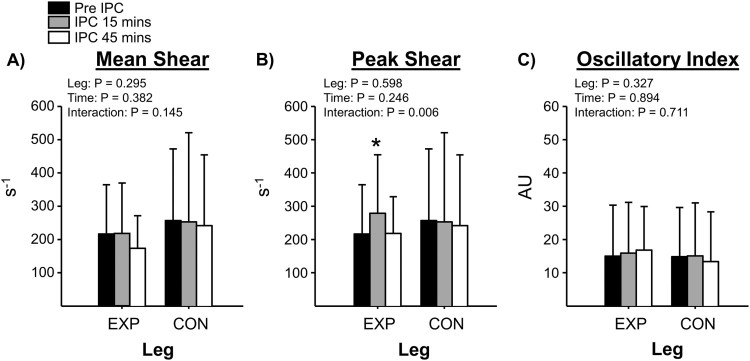
Fig. 2 summarizes the mean, peak and oscillatory (OSI) shear data at baseline, and during 15 and 45 mins of IPC. There was a significant effect (P = 0.006) for peak shear rate to increase at 15 mins during IPC. No differences were noted for mean shear and OSI between study time-points (P > 0.05). To note, CON leg shear rate data were not collected in one subject due to physical limitations (i.e., history of blood clots in that leg). HR, mean arterial pressure, and posterior tibial artery diameters were not significantly altered during the 60-min IPC intervention (P > 0.05).
Figure 2.
Representation of shear rate response to IPC. Panel A depicts mean shear (3 compression cycle average); Panel B depicts peak shear (peak response from 3 compression cycles averaged); Panel C depicts the oscillatory index (ratio of retrograde to mean shear). *Denotes P<0.05 between Pre and Post IPC within EXP leg.
Endothelial Function following IPC
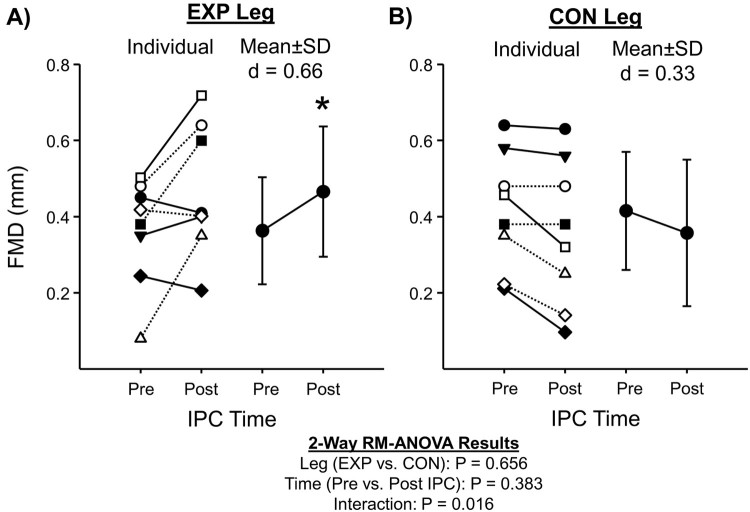
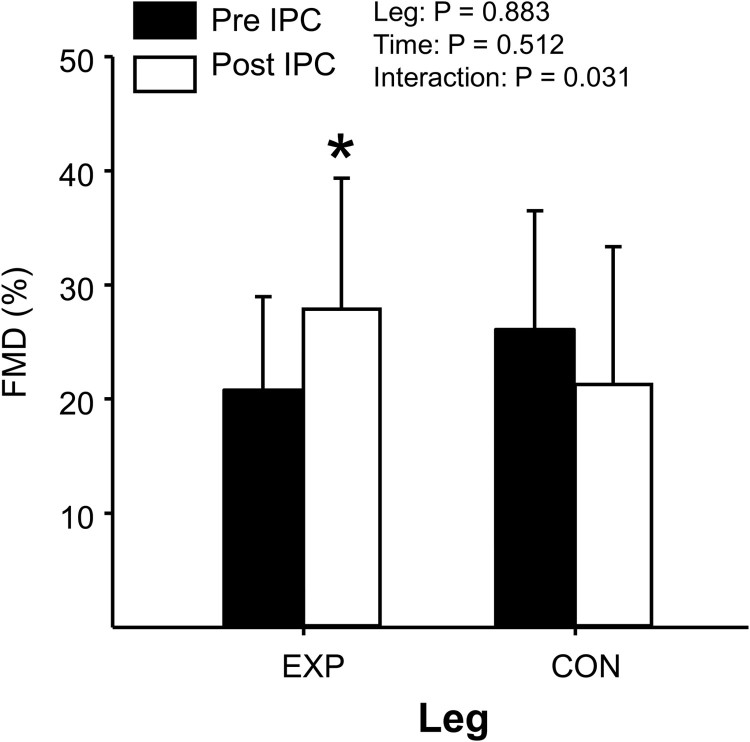
Table 1 summarizes posterior-tibial artery diameter and shear rate AUC responses to FMD testing pre and post IPC in both legs. Fig. 3 summarizes individual data and mean responses for absolute FMD (mm), and Fig. 4 summarizes mean relative FMD (%) pre and post IPC for both legs, respectively. No changes were noted for baseline posterior-tibial artery diameters or shear AUC pre and post IPC (P > 0.05); however, all FMD indices demonstrated a significant increase in the EXP leg following IPC (e.g. absolute, relative, and ratio), with no changes occurring in the CON leg (P > 0.05). To note, FMD testing was not performed on the CON leg in two subjects due to physical limitations, and an inability to obtain optimal image quality for analysis.
Table 1. Posterior-tibial Artery Diameter and Shear AUC during FMD.
| EXP pre | EXP post | CON pre | CON post | |
|---|---|---|---|---|
| Base Diam (mm) | 1.79±0.29 | 1.70±0.28 | 1.68±0.44 | 1.73±0.43 |
| Peak Diam (mm) | 2.15±0.32 | 2.17±0.34 | 2.09±0.48 | 2.09±0.50 |
| Shear AUC (AU) | 16285±4805 | 15164±5011 | 16381±9461 | 16166±8463 |
| Time to Peak Diam (sec) | 62±41 | 56±30 | 66±19 | 71±25 |
Diam (Diameter), AUC (Area under Curve)
Figure 3.
Individual (left side panel) and mean summary data (right side panel) for absolute FMD (mm) at baseline (pre) and 60-minutes post-IPC (post) for the EXP (Panel A) and CON leg (Panel B), respectively. Dashed lines represent subjects with documented cardio-metabolic disease (type-2 diabetes, obesity, and coronary artery disease). *Denotes P<0.05 between Pre and Post IPC within EXP leg.
Figure 4.
Summary of relative FMD (%) between EXP and CON legs following 60 mins IPC. *Denotes P<0.05 between Pre and Post IPC within EXP leg.
Discussion
The major novel finding of this pilot study was that 60 mins of IPC acutely increased leg endothelial function in a majority of people with SCI. This acute improvement in endothelial function may be related to IPC’s ability to increase leg shear rate—a known potent stimulator of the vascular endothelium—as occurred within 15 mins of treatment. Collectively, these preliminary findings suggest that IPC therapy may be an effective strategy for improving endothelial function in the paralyzed legs in people living with SCI.
Shear Rate Response to IPC
In persons with SCI, the paralyzed lower extremities are at increased risk for developing atherosclerosis.2–5 The underlying mechanism(s) for this response are likely multi-factorial, but a reduction in blood flow induced-shear stress due to a lack of physical activity is a plausible mediator.34 Importantly, previous research has demonstrated that IPC can transiently increase blood flow and shear rate when applied to the lower legs of AB individuals.17,18 In other studies, IPC significantly increased popliteal artery inflow in patients with peripheral vascular disease,18 as well as popiteal venous outflow in tetraplegic SCI subjects.23 For the first time, we demonstrate that IPC may be a viable option to acutely increase posterior-tibial artery shear rate in people with SCI.
Two important shear rate observations were made: 1) while the mean shear rate (during and following the compression cycle) was similar between rest and IPC, the peak shear rate (maximum following compression cycles only) response did increase on average by 39±29%; and 2) the peak shear rate response to IPC was biphasic, with an initial increase occurring at 15 mins, followed to a return to baseline at 45 mins. The cause of this biphasic response is unclear; however, previous work demonstrates that 60 mins of higher-frequency IPC (i.e. 12 compressions/minute) produces a similar mean shear rate pattern in the popliteal artery of AB individuals.17 The authors of this previous work also found there is increased oscillatory shear (i.e. increased retrograde shear, and subsequent, lower mean shear) with the higher-frequency IPC. In the current study we utilized a lower-frequency IPC (3 compression/minute), and indeed, no changes were noted for OSI throughout the study intervention. Another potential explanation for the diminished peak shear rate response from 15 to 45 mins of IPC in the EXP leg could be related to alterations in shear pattern during the phases of each compression cycle. For example, peak shear rate increases during the deflation phase of each cycle; however, mean shear rate—when averaged across inflation and deflation phases—and OSI do not significantly differ from baseline (pre-IPC). This would imply that oscillatory shear and or retrograde shear rate were indeed elevated during the 4 s-sec IPC inflation phase. However, vascular endothelial function improved following the 60 min IPC session, thus, transient increases in OSI during each 4-sec cuff inflation phase do not appear to impact leg vascular health in people with SCI. Nonetheless, the underlying mechanisms relating to the reduction in peak shear rate during prolonged IPC in people with SCI remains unknown and will require further investigation.
Endothelial Function following IPC
Repetitive increases in shear stress35,36 have been shown to transiently modulate endothelial function, thus, producing an anti-atherogenic vascular endothelial phenotype.9,12,37 Given this evidence, we posited that IPC could serve as a means to enhance endothelial function in people with SCI. In agreement with our hypothesis, IPC therapy acutely increased posterior-tibial artery endothelial function in people with SCI. Although, previous work in AB demonstrates no effect of a 60 min IPC session on popliteal artery endothelial function17, it is possible this therapy may confer greater benefits to people with SCI.
The underlying mechanisms contributing to the observed improvement in vascular endothelial function following 60 mins of IPC are unclear; however, two potential factors may have contributed: 1) a muscle pump-like effect, and 2) enhanced production of endothelial-derived vasodilators. First, following application of an external pressure (e.g. 120 mmHg), the resulting vascular compression would, in theory, displace venous volume back to the heart,23 widen the arterial-venous pressure gradient, and in turn, increase arterial inflow within the stimulated region.38,39 Second, animal studies have demonstrated that repeated external compressions can stimulate the production and release of endothelial-derived vasodilators. For example, IPC performed bilaterally on the legs in rats has been shown to increase the expression of endothelial derived nitric oxide synthase—the enzyme catalyzing the reaction to produce nitric oxide—as well as, stimulate nitric oxide-mediated vasodilation, with subsequent hyperemia within the microvasculature of cremaster muscles.40,41 Mechanical compressions have also been shown to stimulate vasodilation in soleus muscle feed arteries of rats, mediated by both endothelial-dependent and –independent signaling pathways.42 Furthermore, recent work in humans has also demonstrated that 60 mins of bilateral leg IPC performed in healthy AB subjects result in a reduction in circulating markers of nitric oxide transport (e.g. plasma nitrite, and red blood cell nitric oxide), which may reflect some systemic beneficial effects on distant vascular beds.43 Whether or not these mechanisms contribute to enhanced vascular endothelial function in people with SCI remains unknown.
Experimental Considerations
Our subjects varied in age (27–77 years), neurological injury level, SCI duration, and classification, all of which could have confounding influences on study results. Given the inherent difficulty in recruiting people with SCI, we had to expand our inclusion criteria to accept of wide range of individuals to achieve a sufficient sample number for comparison. Still, albeit the limited sample and heterogeneous SCI group, our data demonstrated significance and moderate-large effects for IPC to increase peak shear rate and improve endothelial function in the EXP leg of a majority of the subjects (over 60%), a response that was not at all present in the CON leg. The rationale for using a 120 mmHg IPC is based on evidence demonstrating this dosage to be optimal for treating peripheral vascular complications in AB individuals.18,19,21,22,27,38 Although positive effects were noted, whether this pressure and duration (60 mins) is most optimal for providing peripheral vascular benefits in people with SCI will require future research. Uniquely, our study also investigated the posterior-tibial artery which permitted the examination of vascular changes within the region of compression, as opposed to a more proximal up-stream vessel. Future research will be needed to determine whether a more proximal vessel, such as the popliteal artery or femoral artery, responds differently to acute or chronic IPC in people with SCI.
It is now generally accepted that people with SCI have increased atherosclerotic burden, particularly within the paralyzed legs.34,44 Although we do not provide comparative data to AB individuals to denote any apparent endothelial dysfunction and/or increased vascular stiffening, our data demonstrate that the posterior-tibial arteries of the current SCI cohort appeared to exhibit inward remodeling (i.e. reduced diameter) and low rates of blood flow at rest, which is characteristic to people with SCI, as compared to previously published data in AB individuals.4,45 For example, one report demonstrates that posterior-tibial artery diameters average around 2.5 mm in AB subjects,40 as compared to 1.75 mm in the present SCI cohort (∼50% smaller internal diameter). In another study,4 a similar cohort of people with SCI exhibited lower leg arterial function as compared to AB controls. Future research will be needed to determine whether people with SCI and multiple other cardiovascular risk factors can attain similar or added benefits from partaking in IPC treatment.
Perspectives
A majority of people with SCI are subjected to prolonged periods on inactivity. From a clinical standpoint, our findings have important implications as people with SCI are more prone to develop CVD, which may be attributable to atherosclerosis in the lower limbs.34,44 The underlying link between alterations in lower limb endothelial function and systemic risk for CVD development in people with SCI is unclear; however, research conducted in AB patients (i.e., peripheral artery disease) demonstrate that populations prone to atherosclerosis in legs appear to also have an overall heightened oxidative stress and pro-inflammatory status.46 Therapies which increase blood flow and shear stress through an artery can provide beneficial effects on the peripheral vasculature.9,15,35–37,47,48 Therefore, targeting the paralyzed lower limbs with an efficacious and economically viable therapy, like IPC, may provide a means to not only improve lower limb endothelial function, but also combat CVD progression in SCI. Indeed, future research should examine whether IPC can serve as an effective chronic therapy, and determine whether it is as suitable adjuvant to more traditional treatments for improving both regional and systemic markers of cardio-metabolic health.
Conclusion
In summary, these preliminary findings suggest that that 60 mins of IPC therapy can result in a moderate-large improvement in endothelial function in the stimulated region, an effect which may be related to increases in leg shear stress early during IPC application. Future studies should investigate the underlying mechanisms mediating this response, determine the optimal IPC protocol and prescription for improving endothelial function, and evaluate the chronic effects of IPC therapy on endothelial function and other cardio-metabolic health outcomes in people with SCI.
Acknowledgements
The authors would like to thank the time and effort put in by all volunteer subjects.
Disclosure statement
No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- 1.Ross R, Faggiotto A, Bowen-Pope D, Raines E.. The role of endothelial injury and platelet and macrophage interactions in atherosclerosis. Circulation. 1984;70(5 Pt 2):III77–82. [PubMed] [Google Scholar]
- 2.Orakzai SH, Orakzai RH, Ahmadi N, Agrawal N, Bauman WA, Yee F, et al Measurement of coronary artery calcification by electron beam computerized tomography in persons with chronic spinal cord injury: evidence for increased atherosclerotic burden. Spinal cord. 2007;45(12):775–9. [DOI] [PubMed] [Google Scholar]
- 3.Wecht JM, Weir JP, DeMeersman RE, Spungen AM, Bauman WA.. Arterial stiffness in persons with paraplegia. J Spinal Cord Med. 2004;27(3):255–9. [DOI] [PubMed] [Google Scholar]
- 4.Stoner L, Sabatier M, VanhHiel L, Groves D, Ripley D, Palardy G, et al Upper vs lower extremity arterial function after spinal cord injury. J Spinal Cord Med. 2006;29(2):138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thijssen DH, De Groot PC, van den Bogerd A, Veltmeijer M, Cable NT, Green DJ, et al Time course of arterial remodelling in diameter and wall thickness above and below the lesion after a spinal cord injury. Eur J Appl Physiol. 2012;112(12):4103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoner L, Credeur D, Dolbow DR, Gater DR.. Vascular health toolbox for spinal cord injury: Recommendations for clinical practice. Atherosclerosis. 2015;243(2):373–82. [DOI] [PubMed] [Google Scholar]
- 7.Venturelli M, Amann M, Layec G, McDaniel J, Trinity JD, Fjeldstad AS, et al Passive leg movement-induced hyperaemia with a spinal cord lesion: evidence of preserved vascular function. Acta Physiol (Oxf). 2014;210(2):429–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thijssen DH, Dawson EA, Tinken TM, Cable NT, Green DJ.. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension. 2009;53(6):986–92. [DOI] [PubMed] [Google Scholar]
- 9.Naylor LH, Carter H, FitzSimons MG, Cable NT, Thijssen DH, Green DJ.. Repeated increases in blood flow, independent of exercise, enhance conduit artery vasodilator function in humans. Amer J Physiol Heart Circ Physiol. 2011;300(2):H664–9. [DOI] [PubMed] [Google Scholar]
- 10.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, et al Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Amer J Physiol Heart Circ Physiol. 2011;300(1):H2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G.. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension. 2011;57(3):363–9. [DOI] [PubMed] [Google Scholar]
- 12.Laughlin MH, Newcomer SC, Bender SB.. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J Appl Physiol. 2008;104(3):588–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green DJ, O'Driscoll G, Blanksby BA, Taylor RR.. Control of skeletal muscle blood flow during dynamic exercise: contribution of endothelium-derived nitric oxide. Sports Med. 1996;21(2):119–46. [DOI] [PubMed] [Google Scholar]
- 14.Jasperse JL, Laughlin MH.. Flow-induced dilation of rat soleus feed arteries. Am J Physiol. 1997;273(5 Pt 2):H2423–7. [DOI] [PubMed] [Google Scholar]
- 15.Stoner L, Sabatier MJ, Mahoney ET, Dudley GA, McCully KK.. Electrical stimulation-evoked resistance exercise therapy improves arterial health after chronic spinal cord injury. Spinal cord. 2007;45(1):49–56. [DOI] [PubMed] [Google Scholar]
- 16.Thijssen DH, Heesterbeek P, van Kuppevelt DJ, Duysens J, Hopman MT.. Local vascular adaptations after hybrid training in spinal cord-injured subjects. Med Sci Sports Exer. 2005;37(7):1112–8. [DOI] [PubMed] [Google Scholar]
- 17.Sheldon RD, Roseguini BT, Thyfault JP, Crist BD, Laughlin MH, Newcomer SC.. Acute impact of intermittent pneumatic leg compression frequency on limb hemodynamics, vascular function, and skeletal muscle gene expression in humans. J Appl Physiol. 2012;112(12):2099–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delis KT, Labropoulos N, Nicolaides AN, Glenville B, Stansby G.. Effect of intermittent pneumatic foot compression on popliteal artery haemodynamics. Eur J Vasc Endovasc Surg. 2000;19(3):270–7. [DOI] [PubMed] [Google Scholar]
- 19.Delis KT, Nicolaides AN, Wolfe JH, Stansby G.. Improving walking ability and ankle brachial pressure indices in symptomatic peripheral vascular disease with intermittent pneumatic foot compression: a prospective controlled study with one-year follow-up. J Vasc Surg. 2000;31(4):650–61. [DOI] [PubMed] [Google Scholar]
- 20.Delis KT, Nicolaides AN, Labropoulos N, Stansby G.. The acute effects of intermittent pneumatic foot versus calf versus simultaneous foot and calf compression on popliteal artery hemodynamics: a comparative study. J Vasc Surg. 2000;32(2):284–92. [DOI] [PubMed] [Google Scholar]
- 21.Delis KT, Nicolaides AN.. Effect of intermittent pneumatic compression of foot and calf on walking distance, hemodynamics, and quality of life in patients with arterial claudication: a prospective randomized controlled study with 1-year follow-up. Ann Surg. 2005;241(3):431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delis KT, Azizi ZA, Stevens RJ, Wolfe JH, Nicolaides AN.. Optimum intermittent pneumatic compression stimulus for lower-limb venous emptying. Eur J Vasc Endovasc Surg. 2000;19(3):261–9. [DOI] [PubMed] [Google Scholar]
- 23.Nash MS, Mintz CD, Montalvo BM, Jacobs PL.. A randomized blinded comparison of two methods used for venous antistasis in tetraplegia. J Spinal Cord Med. 2000;23(4):221–7. [DOI] [PubMed] [Google Scholar]
- 24.Delis KT, Husmann MJ, Nicolaides AN, Wolfe JH, Cheshire NJ.. Enhancing foot skin blood flux in peripheral vascular disease using intermittent pneumatic compression: controlled study on claudicants and grafted arteriopaths. World J Surg. 2002;26(7):861–6. [DOI] [PubMed] [Google Scholar]
- 25.Inaba Y, Chen JA, Bergmann SR.. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging. 2010;26(6):631–40. [DOI] [PubMed] [Google Scholar]
- 26.Delis KT, Husmann MJ, Cheshire NJ, Nicolaides AN.. Effects of intermittent pneumatic compression of the calf and thigh on arterial calf inflow: a study of normals, claudicants, and grafted arteriopaths. Surgery. 2001;129(2):188–95. [DOI] [PubMed] [Google Scholar]
- 27.Kavros SJ, Delis KT, Turner NS, Voll AE, Liedl DA, Gloviczki P, et al Improving limb salvage in critical ischemia with intermittent pneumatic compression: a controlled study with 18-month follow-up. J Vasc Surg. 2008;47(3):543–9. [DOI] [PubMed] [Google Scholar]
- 28.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Amer Coll Cardiol. 2002;39(2):257–65. [DOI] [PubMed] [Google Scholar]
- 29.Wu B, Credeur D, Fryer S, Stoner L.. The use of shear rate-diameter dose-response curves as an alternative to the flow-mediated dilation test. Med Hypotheses. 2015;84(2):85–90. [DOI] [PubMed] [Google Scholar]
- 30.Kaku B, Mizuno S, Ohsato K, Murakami T, Moriuchi I, Arai Y, et al The correlation between coronary stenosis index and flow-mediated dilation of the brachial artery. Jpn Circ J. 1998;62(6):425–30. [DOI] [PubMed] [Google Scholar]
- 31.Vittorio TJ, Zolty R, Garg PK, Sarswat N, Tseng CH, Jorde UP, et al Interdependence of cardiac and endothelial function in patients with symptomatic chronic heart failure of nonischemic etiology. Echocardiography. 2009;26(8):916–21. [DOI] [PubMed] [Google Scholar]
- 32.Stoner L, McCully KK.. Peak and time-integrated shear rates independently predict flow-mediated dilation. J Clin Ultrasound. 2012;40(6):341–51. [DOI] [PubMed] [Google Scholar]
- 33.Padilla J, Johnson BD, Newcomer SC, Wilhite DP, Mickleborough TD, Fly AD, et al Adjusting flow-mediated dilation for shear stress stimulus allows demonstration of endothelial dysfunction in a population with moderate cardiovascular risk. J Vasc Res. 2009;46(6):592–600. [DOI] [PubMed] [Google Scholar]
- 34.West CR, Alyahya A, Laher I, Krassioukov A.. Peripheral vascular function in spinal cord injury: a systematic review. Spinal cord. 2013;51(1):10–19. [DOI] [PubMed] [Google Scholar]
- 35.Martin JS, Borges AR, Beck DT.. Peripheral conduit and resistance artery function are improved following a single, 1-h bout of peristaltic pulse external pneumatic compression. Eur J Apppl Physiol. 2015;115(9):2019–29. [DOI] [PubMed] [Google Scholar]
- 36.Padilla J, Harris RA, Wallace JP.. Can the measurement of brachial artery flow-mediated dilation be applied to the acute exercise model? Cardiovasc Ultrasound. 2007;5:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Green DJ, Maiorana A, O'Driscoll G, Taylor R.. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561(Pt 1):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delis KT, Slimani G, Hafez HM, Nicolaides AN.. Enhancing venous outflow in the lower limb with intermittent pneumatic compression. A comparative haemodynamic analysis on the effect of foot vs. calf vs. foot and calf compression. Eur J Vasc Endovasc Surg. 2000;19(3):250–60. [DOI] [PubMed] [Google Scholar]
- 39.Laughlin M. The muscle pump, what question do we want to answer? J Appl Physiol. 2005. [DOI] [PubMed] [Google Scholar]
- 40.Chen LE, Liu K, Qi WN, Joneschild E, Tan X, Seaber AV, et al Role of nitric oxide in vasodilation in upstream muscle during intermittent pneumatic compression. J Appl Physiol. 2002;92(2):559–66. [DOI] [PubMed] [Google Scholar]
- 41.Liu K, Chen LE, Seaber AV, Johnson GW, Urbaniak JR.. Intermittent pneumatic compression of legs increases microcirculation in distant skeletal muscle. J Orthop Res. 1999;17(1):88–95. [DOI] [PubMed] [Google Scholar]
- 42.Clifford PS, Kluess HA, Hamann JJ, Buckwalter JB, Jasperse JL.. Mechanical compression elicits vasodilatation in rat skeletal muscle feed arteries. J Physiol. 2006;572(Pt 2):561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rifkind JM, Nagababu E, Dobrosielski DA, Salgado MT, Lima M, Ouyang P, et al The effect of intermittent pneumatic compression of legs on the levels of nitric oxide related species in blood and on arterial function in the arm. Nitric oxide. 2014;40:117–22. [DOI] [PubMed] [Google Scholar]
- 44.Bell JW, Chen D, Bahls M, Newcomer SC.. Evidence for greater burden of peripheral arterial disease in lower extremity arteries of spinal cord-injured individuals. Am J Physiol Heart Circ Physiol. 2011;301(3):H766–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Black CD, Vickerson B, McCully KK.. Noninvasive assessment of vascular function in the posterior tibial artery of healthy humans. Dyn Med. 2003;2(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gardner AW, Parker DE, Montgomery PS, Sosnowska D, Casanegra AI, Ungvari Z, et al Gender and racial differences in endothelial oxidative stress and inflammation in patients with symptomatic peripheral artery disease. J Vasc Surg. 2015;61(5):1249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Credeur DP, Hollis BC, Welsch MA.. Effects of handgrip training with venous restriction on brachial artery vasodilation. Med Sci Sports Exer. 2010;42(7):1296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Credeur DP, Mariappan N, Francis J, Thomas D, Moraes D, Welsch MA.. Vasoreactivity before and after handgrip training in chronic heart failure patients. Atherosclerosis. 2012;225(1):154–9. [DOI] [PubMed] [Google Scholar]