Abstract
Ablative and chronic stimulation procedures targeting the internal pallidum (GPi) and the subthalamic nucleus (STN) have led to major advancements in the treatment of Parkinson's disease and other movement disorders. Although these procedures have evolved to primarily target the posterior ventrolateral sensorimotor portion of GPi and to less selectively target STN, centrally, the ideal targets within these structures remain to be fully established. In this study, we sought to identify the optimal targeting sites in GPi and STN for reversal of parkinsonian signs through a series of reversible injections of the GABAA agonist muscimol in these nuclei in parkinsonian primates.
Akinesia and bradykinesia were strongly ameliorated by discrete inactivation within the centromedial extent of the sensorimotor territory in GPi and the lateral portion of the sensorimotor territory in STN. This suggests that akinesia and bradykinesia might, in fact, originate from abnormalities in the same, or at least overlapping, motor circuits in the parkinsonian state. Inactivation of areas outside of the motor territories did not improve parkinsonism but induced circling and behavioral abnormalities. The segregation of basal ganglia–thalamocortical circuits appears to be therefore maintained, at least to a large extent, in the parkinsonian state.
These results underscore that inactivation of discrete regions in the central territory of GPi and the lateral portion of STN are sufficient to ameliorate parkinsonian motor signs and that extension of lesions into nonmotor territories may be deleterious. Surgical outcomes might therefore be optimized by placing more discrete lesions and by restricting the extent of chronic stimulation.
Keywords: basal ganglia, globus pallidus, subthalamic nucleus, Parkinson's disease, pallidotomy, deep brain stimulation
The basal ganglia are part of primarily segregated circuits that also involve the cortex and thalamus (Alexander and Crutcher, 1990; Alexander et al., 1990). Increased and abnormally patterned output in motor-related circuits of the basal ganglia to the thalamus may in part be responsible for the motor disturbances in Parkinson's disease (PD) (Albin et al., 1989;Bergman et al., 1990; DeLong, 1990; Wichmann and DeLong, 1996). Based on this premise and from previous experiences with pallidal ablative surgeries in patients with PD (Talairach et al., 1950; Guiot and Brion, 1953; Riechert and Wolff, 1953; Narabayashi and Okuma, 1954; Cooper and Bravo, 1958; Svennilson et al., 1960), the last decade has seen major advances in ablative and chronic stimulation techniques aimed at reducing basal ganglia output through interruption of neuronal activity in the internal pallidum (GPi) or the subthalamic nucleus (STN) (Laitinen et al., 1992; Benabid et al., 1994; Lozano et al., 1995;Krack et al., 1998; Baron et al., 1996, 2000; Alvarez et al., 1999,2000; Fine et al. 2000). Although the mechanisms by which electrical stimulation works in parkinsonian patients remain uncertain, the similarities between the effects of chronic stimulation and ablative lesions suggest that electrical stimulation may also serve to functionally inhibit the targeted nuclei.
Even with the large number of GPi and STN procedures being performed for treatment of PD, the ideal targets within these structures remain to be fully established. Based in large part on the results of early empirical investigations in patients (Svennilson et al., 1960), most of the current pallidal lesioning and chronic stimulation procedures evolved to specifically target the ventrolateral posterior “sensorimotor” portion of GPi (DeLong, 1971; Vitek et al., 1998). Some clinicians, however, have suggested that this strategy should be modified, for example, to include the central nuclear territory of GPi (Gross et al., 1999) or the pallidothalamic fibers (Iacono et al., 1997; Krauss et al., 1997; Patil et al., 1998). Although experiments in parkinsonian monkeys (Wichmann et al., 1994b) suggested that lesioning the dorsolateral sensorimotor territory of the STN could also be a beneficial treatment option for PD; with few exceptions (Alvarez et al., 1999, 2000), lesions have not been placed in this site, mostly because of concerns that permanent hemiballism could be induced (Martin, 1927; Whittier, 1947; Barlas et al., 2001;Guridi and Obeso, 2001). Instead, chronic stimulation in STN has become an increasingly popular mode of treatment for PD. To avoid stimulation of corticospinal fibers in the adjacent internal capsule, stimulator leads are, however, generally targeted toward the center of STN and, therefore, could be predicted to nonselectively effect both motor and nonmotor basal ganglia circuits (Limousin et al., 1998).
On this background, we sought to identify the optimal targeting sites in GPi and STN for reversal of parkinsonian signs through a systematic series of transient inactivation experiments in parkinsonian primates and to correlate these findings with the location of the sensorimotor territories in these nuclei.
MATERIALS AND METHODS
Animals
A total of seven monkeys (Macaca mulatta) were used for these studies. Two animals (Monkeys D and Z) were used for the inactivation studies, four (Monkeys D, I, O, and Z) were used for mapping of neuronal responses to proprioceptive manipulations in GPi, and three (Monkeys T, Q, and U) were used for mapping of neuronal responses in STN. Each monkey was trained for several weeks to sit in a primate chair and to permit passive examination of the extremities, trunk, and orofacial region. All animal experimentation was performed in accordance with the policy on the use of animals in neuroscience research statement revised and approved by the Society for Neuroscience in January 1995 and with the approval of the Animal and Care and Use Committee of Emory University.
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration and surgical procedures
After behavioral conditioning, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP HCl) (Sigma, St. Louis, MO) was administered via a series of systemic injections (Monkeys T and U), intracarotid injections (Monkeys D and I), or via both routes (Monkey O, Q, and Z) to induce moderately severe bilateral parkinsonian signs. Subsequent to documenting by daily observations that the induced conditions were stable for at least 6 weeks, the animals underwent surgical placement of cylindrical stainless steel recording chambers (20 mm diameter) that were affixed over a trephined hole in the monkeys' skulls with dental acrylic. Metal bolts were also imbedded into the dental acrylic cap to permit subsequent stabilization of the animals' heads during recording and injection sessions. In all cases, one chamber was placed opposite the most affected side, targeting the external pallidum (GPe), GPi, and STN with a rostral parasagittal approach, 25° from the vertical. In Monkey D, an additional chamber was placed over the opposite hemisphere, targeting GPe in the coronal plane, 40° from the vertical. All surgeries were performed under 1–2% isoflurane anesthesia, with sterile techniques.
Extracellular recording techniques
Glass-coated platinum–iridium microelectrodes (0.5–1.0 MΩ impedance) were exclusively used for extracellular single-unit recording in STN and for part of the recordings in GPi. The electrodes were lowered through the dura into the brain using a hydraulic microdrive (Narishige, Tokyo, Japan). The subsequent injection experiments were performed using a combination recording–injection system described in detail previously (Hamada and DeLong, 1992). Briefly, this system consists of a 30 ga stainless steel cannula through which a thin Teflon-coated tungsten wire (75 μm coated diameter; 60–80 KΩ impedance) is threaded. This low-impedance system permits isolation of individual cell activity in GPi and reliable distinction of multineuronal activity in STN. Using the microdrive, this system was introduced into the brain through a 24 ga stainless steel guide tube. Drugs were injected using a 10 μl Hamilton syringe connected to the injection cannula via a Teflon tube.
Action potentials were amplified, filtered, displayed on an oscilloscope and played over an audio monitor. Based on the characteristic neuronal discharge frequencies and patterns of recorded cells (DeLong, 1971; DeLong et al., 1985), the boundaries of the encountered nuclei were identified and plotted on graph paper. For all well isolated units in GPi and STN, an assessment for audible changes in neuronal discharge activity in response to passive manipulation of the contralateral forelimb and hindlimb and, in most cases, the trunk and orofacial regions was performed.
Pharmacological injections
A pilot series of injections of the GABAA agonist muscimol hydrobromide (Sigma, St. Louis, MO) at different concentrations (ranging from 0.1 to 4.0 μg/μl; dissolved in saline; 1.0 μl volume) was performed in GPi and STN in one monkey (Monkey D) to identify threshold concentrations that resulted in clear clinical benefits. Based on the effects seen with these concentrations, the subsequent injection experiments were performed with a concentration of 1.0 μg/μl in GPi and GPe and 0.1 μg/μl in the more tightly cellular compacted STN. Only one injection was performed per day.
In all experiments, muscimol was injected in 0.1 μl boluses, in 30 sec intervals, to a total volume of 1.0 μl. After each injection, the cannula was left at the injection site for an additional period of 5 min to prevent backflow along the injection track.
GPi. Muscimol injections were performed in Monkeys D and Z in three parasagittal planes, separated by 2 mm, with one to three injections per plane, separated by at least 1 mm. These injections were completed over 22 d in Monkey D (n = 6 sites) and over 14 d in Monkey Z (n = 8 sites).
GPe. Muscimol injections were performed in both monkeys in the same parasagittal planes used for injections into GPi (Monkey D,n = 6 sites; Monkey Z, n = 3 sites). Also, in Monkey D, additional injections were placed in GPe more laterally (n = 3 sites) and in the opposite hemisphere (n = 13 sites).
STN. Injections were performed in both monkeys over parasagittal planes separated by 1 mm, with one to two injections performed per plane, separated by at least 1 mm. These injections were completed over 20 d in Monkey D (n = 5 sites) and over 18 d in Monkey Z (n = 6 sites).
Controls. Injections of normal saline (1.0 μl) were performed at multiple locations throughout the targeted nuclei. To test whether drug diffusion into the zona incerta could have accounted for behavioral effects from dorsal injections into STN, an additional injection of muscimol (0.1 μg/μl) was performed in the zona incerta in Monkey D.
Acquisition of behavioral data
Before the injections, the monkeys were placed into an observation cage and videotaped for a 20 min baseline period. Twenty minutes after each injection, the animals were again videotaped in the cage for another 20 min period. The delay after the injections was needed to remove the recording–injection system and to place the animal in the cage.
Data analysis
After completion of the experiments, an investigator blinded to the treatments and the locations of the injections reviewed the videotapes. Because the muscimol injections into individual nuclei were completed in clusters and because both Monkeys D and Z showed partial clinical recovery between these series of injections (see Results), the baseline assessments were grouped separately across individual nuclei.
The observation periods were scored for the following parameters: (1) contralateral forelimb akinesia and (2) generalized akinesia, both rated from 0 to 5 (0, none; 1, mild; 2, moderate; 3, moderately severe, with only three to four movements over the 20 min observation period; 4, severe, with one to two movements; and 5, extreme, with no movement); (3) generalized bradykinesia, rated from 0 to 4 (0, none; 1, slight; 2, mild to moderate; 3, moderately severe; 4, severe); (4) circling, rated 0 to 4 (0, none; 1, mild; 2, moderate; 3, severe; 4, extreme); (5) “atypical” behavior (for description, see Results), rated from 0 to 3 (0, none; 1, mild; 2, moderate; 3, severe); and (6) dyskinesias, rated from 0 to 3 (0, none; 1, mild; 2, moderate; 3, severe). Muscimol injection effects were considered significant if the postinjection scores differed from all of the corresponding baseline scores. The magnitudes of effects (see Figs.2, 3) were graded by subtracting the effective postinjection scores from the corresponding mean baseline scores. Additional parkinsonian features, such as tremor, gait abnormality, and rigidity, were either absent or could not be objectively rated. Eye movement abnormalities were occasionally induced by the injections, but because the monkeys' eyes were not always adequately visualized on the videotapes, this parameter could not be systematically assessed.
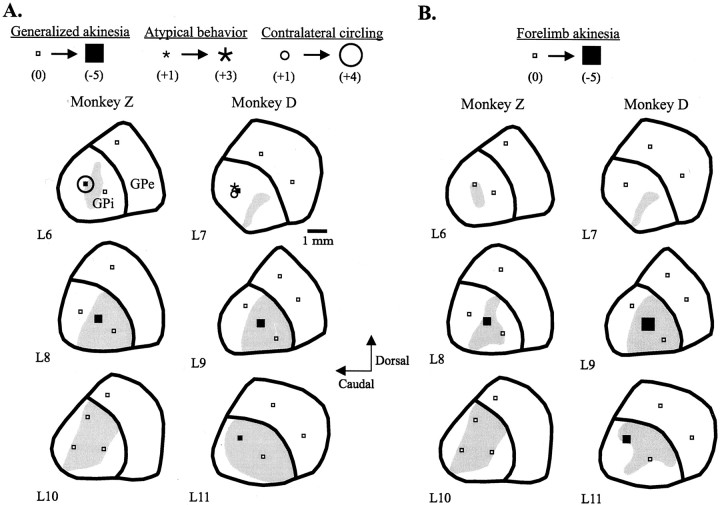
Fig. 2.
Parasagittal reconstructions (lateral 6–11) illustrating the sites of focal injections of muscimol in the GPi and the resultant improvements in generalized (A) and contralateral forelimb (B) akinesia in two parkinsonian monkeys. The sites producing contralateral circling and atypical behavior are also indicated (A), as are sites of injections in the GPe. Open small squares indicate sites with no motor effects, and filled squares, asterisks, and open circles indicate the levels of changes in postinjection scores from mean baseline scores for akinesia, atypical behavior, and contralateral circling, respectively. The gray shaded regions represent general (A) and specifically, forelimb (B) sensorimotor territories across four monkeys (detailed in Fig. 1A).
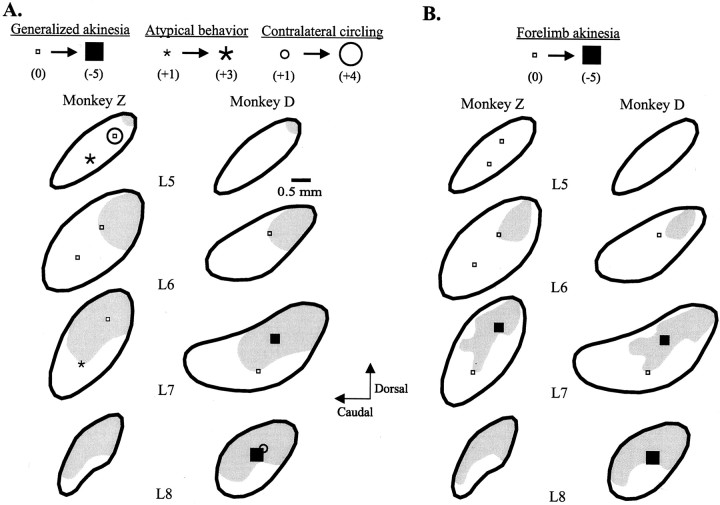
Fig. 3.
Parasagittal reconstructions (lateral 5–8) illustrating the sites of focal injections of muscimol in the STN and the resultant improvements in generalized (A) and contralateral forelimb (B) akinesia in two parkinsonian monkeys. The sites producing contralateral circling and atypical behavior are also indicated in Figure A.Open small squares indicate sites with no motor effects, and filled squares, asterisks, andopen circles indicate the levels of changes in postinjection scores from mean baseline scores for akinesia, atypical behavior, and contralateral circling, respectively. The gray shaded regions represent general (A) and specifically, forelimb (B) sensorimotor territories across three monkeys (detailed in Fig. 1B).
Histology
At the completion of the experiments, the monkeys were killed with an overdose of pentobarbital (100 mg/kg) and perfused transcardially with normal saline, followed by 10% neutral formalin. The brains were then blocked, frozen, and sectioned in 50 μm sections in the parasagittal plane. Every second section was stained with cresyl violet for visualization of injection cannula tracks. The location of neurons that were examined for sensorimotor passive responses and the injection sites were determined by comparing the histological data with the depth and coordinate readings during the physiological recording experiments.
RESULTS
Neuronal discharge was typically silenced within 1 to 3 min from the beginning of the injection. The onset of clinical effects generally occurred within 3–5 min after the completion of the muscimol injections and persisted for 90 min to 2 hr. The clinical scoring results from muscimol injections in GPi and STN are summarized in Table1.
Table 1.
Clinical ratings at baseline and after focal muscimol injections in GPi and STN in two parkinsonian monkeys
| Nucleus | Monkey | Injection | Site | Generalized akinesia (0–5) | Generalized bradykinesia (0–4) | Contralateral forelimb akinesia (0–5) | Contralateral circling (0–4) | Atypical behavior (0–3) | Dyskinesias (0–3) |
|---|---|---|---|---|---|---|---|---|---|
| GPi | D | Baseline1-a | 3.2 | 2.8 | 4.9 | 0 | 0 | 0 | |
| Muscimol | L7caudal | 2* | 3 | 5 | 1* | 2* | 0 | ||
| L9dorsal | 3 | 3 | 4 | 0 | 0 | 0 | |||
| L9central | 1* | 1* | 1* | 0 | 0 | 0 | |||
| L9rostral | 3 | 3 | 5 | 0 | 0 | 0 | |||
| L11dorsal | 2* | 3 | 3* | 0 | 0 | 0 | |||
| L11central | 3 | 3 | 5 | 0 | 0 | 0 | |||
| Z | Baseline | 3.4 | 3.3 | 4.9 | 1.2 | 0 | 0 | ||
| Muscimol | L6caudal | 2* | 2* | 4 | 4* | 0 | 0 | ||
| L9central | 3 | 3 | 4 | 3 | 0 | 0 | |||
| L8caudal | 3 | 3 | 5 | 3 | 0 | 0 | |||
| L8central | 1* | 2* | 3* | 2 | 0 | 0 | |||
| L8rostral | 3 | 2* | 4 | 0 | 0 | 0 | |||
| L10dorsal | 3 | 3 | 4 | 1 | 0 | 0 | |||
| L10caudal | 3 | 3 | 4 | 2 | 0 | 0 | |||
| L10rostral | 3 | 3 | 5 | 3 | 0 | 0 | |||
| STN | D | Baseline | 2.8 | 1.0 | 4.8 | 0 | 0 | 0 | |
| Muscimol | L6dorsal | 2 | 1 | 5 | 0 | 0 | 0 | ||
| L7dorsal | 1* | 1 | 3* | 0 | 0 | 0 | |||
| L7ventral | 2 | 1 | 5 | 0 | 0 | 0 | |||
| L8central | 0* | 1 | 2* | 1* | 0 | 1* | |||
| Z | Baseline | 1.9 | 2.4 | 4.7 | 2.3 | 0 | 0 | ||
| Muscimol | L5dorsal | 2 | 3 | 5 | 4* | 0 | 0 | ||
| L5ventral | 2 | 2 | 5 | 2 | 2* | 0 | |||
| L6dorsal | 2 | 2 | 5 | 3 | 0 | 0 | |||
| L6ventral | 2 | 3 | 5 | 2 | 0 | 0 | |||
| L7dorsal | 2 | 2 | 3* | 2 | 0 | 1* | |||
| L7ventral | 3 | 2 | 5 | 2 | 1* | 0 |
Mean scores across multiple baseline observations (minimum number of observations;n = 6).
Indicates postinjection scores that differ from all corresponding baseline scores.
GPe
None of the muscimol injections in GPe (n = 25) in Monkeys D and Z produced clinically evident effects (see Fig.2).
GPi
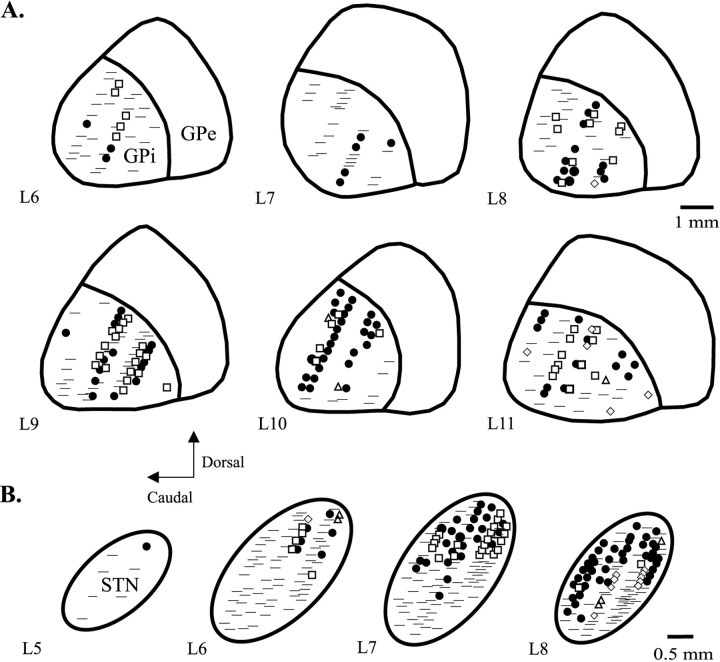
The somatotopic organization of neuronal responses to proprioceptive manipulations was similar between animals, and these data were therefore pooled across monkeys for GPi (n = 4) and STN (n = 3). Proprioceptive responses were found in 52% (132 of 252) of examined cells in GPi, including 70% (64 of 91) in the lateral third (L10–L11), 54% (55 of 101) in the central third (L8–L9), and 22% (13 of 60) in the medial third (L6–L7) (Fig. 1A). Forelimb responses were elicited in 31% (76 of 252) of cells, with the greatest percentage of responses present laterally (46% laterally, 28% centrally, and 13% medially). Hindlimb responses were elicited in 18% (46 of 252) of cells, with the greatest percentage of responses present centrally (18% laterally, 26% centrally, and 8% medially). The limb responses showed a strong proximal-to-distal gradient, with the majority of responses occurring at the shoulders and hips and infrequent responses elicited in the digits. Limb-related neurons responded in 39% (40 of 102) of cases to movements at more than one joint but rarely responded (5 of 102) to movements of both the contralateral forelimb and hindlimb.
Fig. 1.
Parasagittal reconstructions (lateral 5–11; according to the atlas of Winters et al., 1969) illustrating pooled proprioceptive responses of neurons to passive manipulations of the contralateral forelimb (•) and hindlimb (□), trunk (▵), and orofacial (
 ) regions in the GPi in four parkinsonian monkeys (A) and the STN in three parkinsonian monkeys (B).
) regions in the GPi in four parkinsonian monkeys (A) and the STN in three parkinsonian monkeys (B).
Inactivation studies
Generalized akinesia markedly improved after muscimol injections in the central portion of GPi in both Monkeys D and Z (L8central and L9central, respectively) and to a lesser extent after a dorsolateral injection (L11dorsal) in Monkey D (Fig.2). Although more medial injections in both monkeys (L6caudal and L7caudal, respectively) were also associated with improved generalized akinesia scores, this activity was dominated by contralateral turning (see below). Generalized bradykinesia also improved after the centralmost injections in both monkeys, as well as after injections at L8rostral and L6caudal in Monkey Z. Injection effects on contralateral forelimb akinesia closely paralleled the improvements in generalized akinesia, with consistent benefits induced by the centralmost injections in both monkeys and additional effects produced by the dorsolateral injection in Monkey D. Contralateral circling, associated with contraversive neck and trunk twisting and contraversive gaze, was strongly induced by an especially medial, and caudal, injection (at L6) in Monkey Z and, to a lesser extent, by the most medial (and caudal) injection (at L7) in Monkey D. Atypical behavior, characterized by marked hypervigilance, and stereotypic hopping and skin picking was also induced by the medial injection in Monkey D.
STN
Across monkeys (n = 3), neurons with proprioceptive responses were concentrated in the dorsolateral portion of STN, with the sensorimotor territory extending progressively more ventrally toward the lateral region of STN (Fig. 1B). Proprioceptive responses were elicited in 39% (112 of 294) of cells. Of 112 responsive cells, 65% (73) responded to the examination of the forelimb, 25% (28) to the hindlimb, 4% (5) to the trunk, and 9% (10) to the orofacial region. Forelimb-related responses were concentrated laterally: 39% (41 of 103) of cells responded to forelimb manipulation at L8, 22% (25 of 109) at L7, 8% (6 of 72) at L6, and 14% (1 of 7) at L5. Hindlimb-related responses were concentrated centrolaterally: 2% (2 of 104) of cells responded at L8, 18% (20 of 109) at L7, 7% (5 of 75) at L6, and 0 of 7 at L5. Similar to the pattern in GPi, the limb responses showed a strong proximal-to-distal gradient, with only infrequent responses elicited in the digits. Also similar to GPi, proprioceptive responses were commonly elicited at multiple joints [39% (40 of 101) of limb-responsive cells] and infrequently in both the contralateral forelimb and hindlimb [4% (4 of 101)].
Inactivation studies
General akinesia improved markedly after the most laterally placed injection (L8central) and moderately after a dorsal centrolateral (L7dorsal) injection of muscimol in Monkey D (Fig. 3). The injections in STN in Monkey Z (overall more medial than the injections in Monkey D) had no clear effects on generalized akinesia or generalized bradykinesia. Because of spontaneous resolution of generalized bradykinesia (subsequent to the GPi studies), the effects of STN injections on bradykinesia could not be evaluated in Monkey D. Contralateral forelimb akinesia improved greatly after the most laterally placed injection in Monkey D and, to a lesser extent, after dorsal centrolateral injections (L7dorsal) in both animals. Contralateral circling, often accompanied by truncal and head turning, as well as contralateral gaze deviation, and horizontal nystagmus was strongly induced by the overall most medial and dorsal placed injection (L5dorsal, Monkey Z) and, to a modest extent, by the lateral injection in Monkey D. Atypical behavior, characterized chiefly by hypervigilance, was strongly induced by a ventral injection in the same medialmost injected plane (L5ventral) and, to a lesser degree, by a ventral centrolateral injection (L7ventral) in Monkey Z. Mild, mainly action-induced choreac dyskinesias were induced in the contralateral hindlimb by the most lateral, and dorsal, injections in each animal (L8central in Monkey D and L7dorsal in Monkey Z).
Control injections
Scores did not change from baseline values after injections of physiological saline in GPi and STN and muscimol in the zona incerta (at L6.5 in Monkey D).
DISCUSSION
The results showed that inactivation of small portions of the sensorimotor territories of GPi and STN strongly ameliorates motor disturbances in parkinsonian primates. Inactivation of areas outside of the motor territories did not improve parkinsonism but induced circling and behavioral abnormalities. Injections into GPe produced no discernable effects.
Effects of focal inactivation on parkinsonian motor disabilities
Based on autoradiographic studies of the spread of muscimol after intracerebral injections in rats (Martin 1991), the effects of even our largest injections should have been restricted to a spherical area of no more than 1 mm in diameter. Akinesia and bradykinesia were strongly ameliorated in parkinsonian monkeys by inactivation of discrete areas within the centromedial extent of the sensorimotor territory in GPi and the lateral portion of the sensorimotor territory in STN. This suggests that the principle differentiation of the basal ganglia into motor and nonmotor areas remains intact in the parkinsonian state and that parkinsonian akinesia and bradykinesia are primarily the result of abnormal discharge in the motor circuit of the basal ganglia.
The effects of inactivation of STN and GPi differed in some aspects. Notably, far smaller absolute amounts of muscimol in STN had essentially the same anti-parkinsonian effects as larger doses in GPi, and STN inactivation resulted in prominent dyskinesias, whereas GPi inactivation did not. A more spatially concentrated organization of the motor and other circuits in STN relative to GPi probably accounted for the similar anti-parkinsonian responses to different doses of muscimol, whereas differences in the positions of STN and GPi within the basal ganglia circuitry may have, in large part, resulted in the contrasting dyskinesia effects. Inactivation of STN also affects GPe activity, which is thought to result in altered inhibition of GPi via the monosynaptic GPe–GPi projection (Shink et al., 1996). Furthermore, the STN likely contains portions of the motor circuit that traverse the substantia nigra pars reticulata (SNr) and are therefore not reached at all by GPi injections. Finally, in contrast to GPi inactivation, STN inactivation produces only a reduction here (Wichmann et al., 1994b) rather than an abolishment of GPi output and potentially causes important changes in the discharge pattern of GPi neurons, as well. Thus, GPi and STN inactivation produce different effects on GPi output that may be significant in their own right and may also, importantly, influence compensatory mechanisms in downstream portions of the motor circuit, such as thalamus, cortex, and brainstem structures.
Based on the models, inactivation of GPe should enhance discharge activity in STN and GPi and thereby produce disturbances in motor function. None of the muscimol injections placed throughout GPe, however, worsened motor function. Primarily consistent with our findings, Kato and Kimura (1992) observed only modest flexion posturing abnormalities during pharmacological blockade in GPe in normal primates. However, in contrast to these results, Blanchet et al. (1994)reported that large excitotoxic lesions in GPe produced a worsening of parkinsonian signs and levodopa-induced dyskinesias in parkinsonian primates. The reason for this discrepancy may be the fact that these excitotoxic lesions were far larger than the areas inactivated in our study. In addition, there may be additional pharmacologic differences between injections of muscimol and excitotoxins such as ibotenic acid.
The basal ganglia motor circuit has been considered to be primarily organized in a somatotopic manner in normal animals (DeLong, 1971; DeLong et al., 1985; Wichmann et al., 1994a). This arrangement probably undergoes significant reorganization in parkinsonism that may go beyond the alterations in discharge of individual neurons. Although the extent and distribution of limb representations in GPi and STN probably remain relatively unchanged in parkinsonian monkeys and humans (Vitek et al., 1998), individual basal ganglia neurons show a prominent loss in specificity of proprioceptive responses in the parkinsonian state (Filion et al., 1988; Vitek et al., 1998). This loss of specificity may partially account for the findings that both forelimb and generalized akinesia were ameliorated by inactivation of single sites in GPi and STN. However, the effects of inactivation may not be entirely reflected by the somatotopy of the injected nuclei because, for example, the distribution of dyskinesias also do not seem to correspond to the nuclear somatotopy, even in the normal state (Whittier and Mettler, 1949; Carpenter et al., 1950; Hamada and DeLong, 1992) (but see Wichmann et al., 1994b). Therefore, as further supported by the demonstration of multiple overlapping homunculi in the STN in normal primates (Nambu et al., 1996), the somatotopic representations within basal ganglia nuclei appear to be organized in a highly complex manner.
The division of the motor circuit into subcircuits (Monakow et al., 1978; Hoover and Strick, 1993; Yoshida et al., 1993; Nambu et al., 1996; Takada et al., 1998) may also be to some extent disordered in the parkinsonian state. Bradykinesia, a motor execution problem, has been suggested to result from abnormalities in the motor subcircuit originating in the primary motor cortex (Hallett, 1990), whereas higher-order motor control problems, such as akinesia (Benecke et al., 1987; Bloxham et al., 1987; Pullman et al., 1988; Hallett, 1990;Jahanshahi et al., 1992; Pascual-Leone et al., 1994), have been linked to abnormalities in the supplementary motor area-based motor subcircuit (Dick et al., 1989; Hallett, 1990; Playford et al., 1992). However, in line with the contentions of Gross and et al. (1999), our experiments suggest that both akinesia and bradykinesia may result from abnormal discharge activity in the rostral portion of the sensorimotor territory of GPi. Akinesia and bradykinesia could therefore originate from abnormalities in the same or at least overlapping circuits in the parkinsonian state.
Circling and other behavioral disturbances
Circling is a common feature in animals with an imbalance between the basal ganglia outputs in the two hemispheres (Oberlander et al., 1977; Olpe et al., 1977, Scheel-Kruger et al., 1977; Hikosaka and Wurtz, 1983a–c; Sirinathsinghji, 1985; Bankiewicz et al., 1986). For instance, localized inactivation of the medial SNr induces contraversive turning, which has been attributed to abnormalities of the nigro-collicular projection (Hikosaka and Wurtz, 1983a–c; Burbaud et al., 1998; Lestienne and Thullier, 1998; Wichmann et al., 2001). In the present experiments, contraversive circling, as well as truncal and head turning, gaze deviation, and horizontal nystagmus, were induced by muscimol injections placed into the rostral “associative” portions of GPi and STN and could be readily dissociated from sites producing anti-akinetic and anti-bradykinetic effects. In this case, circling behavior may have resulted principally through disinhibition of thalamic circuits, perhaps inducing abnormal activity in cortical oculomotor areas.
Patients with PD often show behavioral abnormalities, including increased irritability, loss of interest in daily activities, depression, and cognitive deficits. After treatment with MPTP, the monkeys often displayed comparable features, such as aversion to physical contact by the investigators, reduced grooming, and diminished interest in their environment. Moreover, distinct idiosyncratic behaviors, including stereotypic hopping and picking of the skin, were induced by discrete inactivation in the medial portion of GPi and in the ventral medial region of STN. Because these injection sites were distinct from those that induced strong contraversive circling, these results support the concept that the nonmotor territory of the basal ganglia is likely further segregated into distinct subcircuits (Yeterian and Pandya, 1991; Haber et al., 1995) and changes in these circuits probably contribute to the behavioral deficits in PD.
As postulated for the motor abnormalities in PD, analogous abnormal neuronal discharge in nonmotor portions of the basal ganglia may account for associated behavioral disturbances. However, the inactivation results suggest that behavioral abnormalities should not be treated equivalently to the motor disturbances in PD, i.e., by extending surgical lesions into the nonmotor territory of the basal ganglia. This impression is further supported by reports of untoward cognitive and behavioral side effects, including manic episodes, in human patients with lesions or chronic stimulation involving these circuits (Lombardi et al., 2000; Miyawaki et al., 2000; Saint-Cyr et al., 2000).
The reason why motor and nonmotor abnormalities appear to respond differently to basal ganglia inactivation is unclear. Conceivably, the compensatory mechanisms that come into play after lesioning are different between these circuits or perhaps many of the behavioral abnormalities in parkinsonism result from other mechanisms, such as cortical neuronal degeneration (Vermersch et al., 1993) or nondopaminergic neurotransmitter deficits (Bedard et al., 1998, 1999), which are only further aggravated by subcortical lesioning. Regardless of the mechanisms involved, the more compressed anatomical architecture of the STN, relative to GPi, could impart a higher associated risk for cognitive side effects from stimulation or inadvertent extension of lesions into nonmotor regions. Ongoing studies in patients with PD should help to further clarify these issues.
Surgical strategies in humans
The present studies underscore that inactivation of discrete regions in the central territory of GPi and the lateral portion of STN are sufficient to ameliorate parkinsonian motor signs (at least bradykinesia and akinesia) and that larger lesions that extend into nonmotor territories may, in fact, be deleterious. These results suggest that surgical results might be optimized by placing more discrete lesions and restricting the extent of chronic stimulation to minimize the risk of involvement of nearby structures, such as the associative areas in the basal ganglia, the internal capsule, or the optic tract. Moreover, the demonstration here of strong anti-parkinsonian effects with strictly fiber-sparing inactivation methods suggests that strategies to destroy pallidal outflow tracts also may not be warranted. Although no effects were produced by muscimol-induced inactivation in GPe, the findings of Blanchet et al. (1994) suggest that GPe should be carefully avoided during GPi-targeted surgeries. Clinical–magnetic resonance imaging correlation studies in parkinsonian patients assessing for clinical outcome correlations of inadvertent extension of lesions into GPe could be undertaken to further address this issue.
Footnotes
This work was supported by National Institutes of Health Grant 5 K08 NS01818, as well as by generous support from the Tomlin family.
Correspondence should be addressed to Dr. Mark S. Baron, Department of Neurology, Emory University School of Medicine, 6000 Woodruff Memorial Research Building, Atlanta, GA 30322. E-mail:msbaron@emory.edu.
REFERENCES
- 1.Albin RL, Young AB, Penney BJ. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 2.Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- 3.Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- 4.Alvarez GL, Marcias R, Guridi J, Lopez G, Maragoto C, Teijeiro J. Lesion of the subthalamic nucleus in Parkinson's disease: long term follow-up. Ann Neurol. 1999;46:492–493. [Google Scholar]
- 5.Alvarez GL, Marcias R, Lopez G, Alvarez E, Martres MP, Teijeiro J. Bilateral subthalamotomy in Parkinson's disease. Mov Disord. 2000;15 [Suppl 3]:65. [Google Scholar]
- 6.Bankiewicz KS, Oldfield EH, Chiueh CC, Doppman JL, Jacobowitz DM, Kopin IJ. Hemiparkinsonism in monkeys after unilateral internal carotid artery infusion of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Life Sci. 1986;39:7–16. doi: 10.1016/0024-3205(86)90431-5. [DOI] [PubMed] [Google Scholar]
- 7.Barlas O, Hanagasi HA, Imer M, Sahin HA, Sencer S, Emre M. Do unilateral ablative lesions of the subthalamic nucleus in parkinsonian patients lead to hemiballism? Mov Disord. 2001;16:306–310. doi: 10.1002/mds.1051. [DOI] [PubMed] [Google Scholar]
- 8.Baron MS, Vitek JL, Bakay RA, Green J, Kaneoke Y, Hashimoto T, Turner RS, Woodard JL, Cole SA, McDonald WM, DeLong MR. Treatment of advanced Parkinson's disease by posterior GPi pallidotomy: 1-year results of a pilot study. Ann Neurol. 1996;40:355–366. doi: 10.1002/ana.410400305. [DOI] [PubMed] [Google Scholar]
- 9.Baron MS, Vitek JL, Bakay RAE, Green J, McDonald WM, Cole SA, DeLong MR. Treatment of advanced Parkinson's disease by unilateral posterior GPi pallidotomy: 4-year results of a pilot study. Mov Disord. 2000;15:230–237. doi: 10.1002/1531-8257(200003)15:2<230::aid-mds1005>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 10.Bedard MA, el Massioui F, Malapani C, Dubois B, Pillon B, Renault B, Agid Y. Attentional deficits in Parkinson's disease: partial reversibility with naphtoxazine (SDZ NVI-085), a selective noradrenergic alpha 1 agonist. Clin Neuropharmicol. 1998;21:108–117. [PubMed] [Google Scholar]
- 11.Bedard MA, Pillon B, Dubois B, Duchesne N, Masson H, Agid Y. Acute and long-term administration of anticholinergics in Parkinson's disease: specific effects on the subcortico-frontal syndrome. Brain Cogn. 1999;40:289–313. doi: 10.1006/brcg.1999.1083. [DOI] [PubMed] [Google Scholar]
- 12.Benabid AL, Pollak P, Gross C, Hoffmann D, Benazzouz A, Gao DM, Laurent A, Gentil M, Perret J. Acute and long-term effects of subthalamic nucleus stimulation in Parkinson's disease. Stereotact Funct Neurosurg. 1994;62:76–84. doi: 10.1159/000098600. [DOI] [PubMed] [Google Scholar]
- 13.Benecke R, Rothwell JC, Dick JP, Day BL, Marsden CD. Simple and complex movements off and on treatment in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 1987;50:296–303. doi: 10.1136/jnnp.50.3.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- 15.Blanchet PJ, Boucher R, Bédard PJ. Excitotoxic lateral pallidotomy does not relieve l-DOPA-induced dyskinesia in MPTP parkinsonian monkeys. Brain. 1994;650:32–39. doi: 10.1016/0006-8993(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 16.Bloxham CA, Dick DJ, Moore M. Reaction times and attention in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1987;50:1178–1183. doi: 10.1136/jnnp.50.9.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burbaud P, Bonnet B, Guehl D, Lagueny A, Biolac B. Movement disorders induced by gamma-aminobutyric agonist and antagonist injections into the internal globus pallidus and substantia nigra pars reticulata of the monkey. Brain Res. 1998;780:102–107. doi: 10.1016/s0006-8993(97)01158-x. [DOI] [PubMed] [Google Scholar]
- 18.Carpenter MB, Whittier JR, Mettler FA. Analysis of choreoid hyperkinesia in the rhesus monkey: surgical and pharmacological analysis of hyperkinesia resulting from lesions in the subthalamic nucleus of Luys. J Comp Neurol. 1950;92:292–332. doi: 10.1002/cne.900920303. [DOI] [PubMed] [Google Scholar]
- 19.Cooper IS, Bravo G. Chemopallidectomy and chemothalamectomy. J Neurosurg. 1958;3:244–250. doi: 10.3171/jns.1958.15.3.0244. [DOI] [PubMed] [Google Scholar]
- 20.DeLong MR. Activity of pallidal neurons during movement. J Neurophysiol. 1971;34:414–427. doi: 10.1152/jn.1971.34.3.414. [DOI] [PubMed] [Google Scholar]
- 21.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 22.DeLong MR, Crutcher MD, Georgopoulos AP. Primate globus pallidus and subthalamic nucleus: functional organization. J Neurophysiol. 1985;53:530–543. doi: 10.1152/jn.1985.53.2.530. [DOI] [PubMed] [Google Scholar]
- 23.Dick JP, Rothwell JC, Day BL, Cantello R, Buruma O, Gioux M, Benecke R, Berardelli A, Thompson PD, Marsden CD. The Bereitschafts potential is abnormal in Parkinson's disease. Brain. 1989;112:233–244. doi: 10.1093/brain/112.1.233. [DOI] [PubMed] [Google Scholar]
- 24.Filion M, Tremblay L, Bedard PJ. Abnormal influences of passive limb movement on the activity of globus pallidus neurons in parkinsonian monkeys. Brain Res. 1988;444:165–176. doi: 10.1016/0006-8993(88)90924-9. [DOI] [PubMed] [Google Scholar]
- 25.Fine J, Duff J, Chen R, Chir B, Hutchison W, Lozano AM, Lang AE. Long-term follow-up of unilateral pallidotomy in advanced Parkinson's disease. N Engl J Med. 2000;342:1708–1714. doi: 10.1056/NEJM200006083422304. [DOI] [PubMed] [Google Scholar]
- 26.Gross RE, Lombardi WJ, Lang AE, Duff J, Hutchison WD, Saint-Cyr JA, Tasker RR, Lozano AM. Relationship of lesion location to clinical outcome following microelectrode-guided pallidotomy for Parkinson's disease. Brain. 1999;122:405–416. doi: 10.1093/brain/122.3.405. [DOI] [PubMed] [Google Scholar]
- 27.Guiot G, Brion S. Traitement des mouvements anormaux par la coagulation pallidale. Technique et resultats. Rev Neurol. 1953;89:578–580. [PubMed] [Google Scholar]
- 28.Guridi J, Obeso JA. The subthalamic nucleus, hemiballismus and Parkinson's disease: reappraisal of a neurosurgical dogma. Brain. 2001;124:5–19. doi: 10.1093/brain/124.1.5. [DOI] [PubMed] [Google Scholar]
- 29.Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosurg. 1995;15:4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hallett M. Clinical neurophysiology of akinesia. Rev Neurol. 1990;146:585–590. [PubMed] [Google Scholar]
- 31.Hamada I, DeLong MR. Excitotoxic acid lesions of the primate subthalamic nucleus result in transient dyskinesias of the contralateral limbs. J Neurophysiol. 1992;68:1850–1858. doi: 10.1152/jn.1992.68.5.1850. [DOI] [PubMed] [Google Scholar]
- 32.Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. I. Relation of visual and auditory responses to saccades. J Neurophysiol. 1983a;49:1230–1253. doi: 10.1152/jn.1983.49.5.1230. [DOI] [PubMed] [Google Scholar]
- 33.Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. II. Visual responses related to fixation of gaze. J Neurophysiol. 1983b;49:1254–1267. doi: 10.1152/jn.1983.49.5.1254. [DOI] [PubMed] [Google Scholar]
- 34.Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. III. Memory-contingent visual and saccade responses. J Neurophysiol. 1983c;49:1268–1284. doi: 10.1152/jn.1983.49.5.1268. [DOI] [PubMed] [Google Scholar]
- 35.Hoover JE, Strick PL. Multiple output channels in the basal ganglia. Science. 1993;259:819–821. doi: 10.1126/science.7679223. [DOI] [PubMed] [Google Scholar]
- 36.Iacono RP, Carlson JD, Kuniyoshi SM, Li YJ, Mohamed AS, Maeda G. Electrophysiologic target localization in posteroventral pallidotomy. Acta Neurochir. 1997;139:433–441. doi: 10.1007/BF01808880. [DOI] [PubMed] [Google Scholar]
- 37.Jahanshahi M, Brown RG, Marsden CD. Simple and choice reaction time and the use of advance information for motor preparation in Parkinson's disease. Brain. 1992;115:539–564. doi: 10.1093/brain/115.2.539. [DOI] [PubMed] [Google Scholar]
- 38.Kato M, Kimura M. Effects of reversible blockade of basal ganglia on a voluntary arm movement. J Neurophysiol. 1992;68:1516–1534. doi: 10.1152/jn.1992.68.5.1516. [DOI] [PubMed] [Google Scholar]
- 39.Krack P, Pollak P, Limousin P, Hoffmann D, Xie J, Benazzouz A, Benabid AL. Subthalamic nucleus or internal pallidal stimulation in young onset Parkinson's disease. Brain. 1998;121:451–457. doi: 10.1093/brain/121.3.451. [DOI] [PubMed] [Google Scholar]
- 40.Krauss JK, Desaloms JM, Lai EC, King DE, Jankovic J, Grossman RG. Microelectrode-guided posteroventral pallidotomy for treatment of Parkinson's disease: postoperative magnetic resonance imaging analysis. J Neurosurg. 1997;87:358–367. doi: 10.3171/jns.1997.87.3.0358. [DOI] [PubMed] [Google Scholar]
- 41.Laitinen LV, Bergenheim AT, Hariz MI. Leksell's posteroventral pallidotomy in the treatment of Parkinson's disease. J Neurosurg. 1992;76:53–61. doi: 10.3171/jns.1992.76.1.0053. [DOI] [PubMed] [Google Scholar]
- 42.Lestienne FG, Thullier F. Performance of visually triggered wrist movements task in monkey: an application of information theory to evaluate deficits following unilateral substantia nigra pars reticulata lesion. Neurosci Lett. 1998;251:177–180. doi: 10.1016/s0304-3940(98)00522-9. [DOI] [PubMed] [Google Scholar]
- 43.Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, Benabid AL. Electrical stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 1998;339:1105–1111. doi: 10.1056/NEJM199810153391603. [DOI] [PubMed] [Google Scholar]
- 44.Lombardi WJ, Gross RE, Trepanier LL, Lang AE, Lozano AM, Saint-Cyr JA. Relationship of lesion location to cognitive outcome following microelectrode-guided pallidotomy for Parkinson's disease: support for the existence of cognitive circuits in the human pallidum. Brain. 2000;123:746–758. doi: 10.1093/brain/123.4.746. [DOI] [PubMed] [Google Scholar]
- 45.Lozano AM, Lang AE, Galvez-Jimenez N, Miyasaki J, Duff J, Hutchinson WD, Dostrovsky JO. Effect of GPi pallidotomy on motor function in Parkinson's disease. Lancet. 1995;346:1383–1387. doi: 10.1016/s0140-6736(95)92404-3. [DOI] [PubMed] [Google Scholar]
- 46.Martin JH. Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neurosci Lett. 1991;127:160–164. doi: 10.1016/0304-3940(91)90784-q. [DOI] [PubMed] [Google Scholar]
- 47.Martin JP. Hemichorea resulting from a local lesion of the brain. (The syndrome of the body of Luys.) Brain. 1927;50:637–651. [Google Scholar]
- 48.Miyawaki E, Perlmutter JS, Tröster AI, Videen TO, Koller WC. The behavioral complications of pallidal stimulation: a case report. Brain Cogn. 2000;42:417–434. doi: 10.1006/brcg.1999.1113. [DOI] [PubMed] [Google Scholar]
- 49.Monakow KH, Akert K, Kunzle H. Projections of the precentral motor cortex and other cortical areas of the frontal lobe to the subthalamic nucleus in the monkey. Exp Brain Res. 1978;33:395–403. doi: 10.1007/BF00235561. [DOI] [PubMed] [Google Scholar]
- 50.Nambu A, Takada M, Inase M, Tokuno H. Dual somatotopical representations in the primate subthalamic nucleus: evidence for ordered but reversed body-map transformations from the primary motor cortex and the supplementary motor area. J Neurosci. 1996;16:2671–2683. doi: 10.1523/JNEUROSCI.16-08-02671.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Narabayashi H, Okuma T. Procaine oil blocking of the globus pallidus for the treatment of rigidity and tremor of parkinsonism. Psychiat Neurol Japonica. 1954;56:471–495. doi: 10.1001/archneurpsyc.1956.02330190052003. [DOI] [PubMed] [Google Scholar]
- 52.Oberlander C, Dumont C, Boissier JR. Rotational behaviour after unilateral intranigral injection of muscimol in rats. Eur J Pharmacol. 1977;43:389–390. doi: 10.1016/0014-2999(77)90047-4. [DOI] [PubMed] [Google Scholar]
- 53.Olpe HR, Schellenberg H, Koella WP. Rotational behavior induced in rats by intranigral application of GABA-related drugs and GABA antagonists. Eur J Pharmacol. 1977;45:291–294. doi: 10.1016/0014-2999(77)90012-7. [DOI] [PubMed] [Google Scholar]
- 54.Pascual-Leone A, Valls-Sole J, Brasil-Neto JP, Cohen LG, Hallett M. Akinesia in Parkinson's disease. I. Shortening of simple reaction time with focal, single-pulse transcranial magnetic stimulation. Neurology. 1994;44:884–891. doi: 10.1212/wnl.44.5.884. [DOI] [PubMed] [Google Scholar]
- 55.Patil AA, Hahn F, Sierra-Rodriguez J, Traverse J, Wang S. Anatomical structures in the Leksell pallidotomy target. Stereotact Funct Neurosurg. 1998;70:32–37. doi: 10.1159/000029595. [DOI] [PubMed] [Google Scholar]
- 56.Playford ED, Jenkins IH, Passingham RE, Nutt J, Frackowiak RS, Brooks DJ. Impaired mesial frontal and putamen activation in Parkinson's disease: a positron emission tomography study. Ann Neurol. 1992;32:151–161. doi: 10.1002/ana.410320206. [DOI] [PubMed] [Google Scholar]
- 57.Pullman SL, Watts RL, Juncos JL, Chase TN, Sanes JN. Dopaminergic effects on simple and choice reaction time performance in Parkinson's disease. Neurology. 1988;38:249–254. doi: 10.1212/wnl.38.2.249. [DOI] [PubMed] [Google Scholar]
- 58.Riechert T, Wolff M. Die technishe durchfuhrung von gezielten hirnoperationen. Arch F Psych u Ztschr F Neurol. 1953;190:297–316. doi: 10.1007/BF01225299. [DOI] [PubMed] [Google Scholar]
- 59.Saint-Cyr JA, Trepanier LL, Kumar R, Lozano AM, Lang AE. Neuropsychological consequences of chronic bilateral stimulation of the subthalamic nucleus in Parkinson's disease. Brain. 2000;123:2091–2108. doi: 10.1093/brain/123.10.2091. [DOI] [PubMed] [Google Scholar]
- 60.Scheel-Kruger J, Arnt J, Magelund G. Behavioral stimulation induced by muscimol and other GABA agonists injected into the substantia nigra. Neurosci Lett. 1977;4:351–356. doi: 10.1016/0304-3940(77)90183-5. [DOI] [PubMed] [Google Scholar]
- 61.Shink E, Bevan MD, Bolam JP, Smith Y. The subthalamic nucleus and the external pallidum: two tightly interconnected structures that control the output of the basal ganglia in the monkey. Neuroscience. 1996;73:335–357. doi: 10.1016/0306-4522(96)00022-x. [DOI] [PubMed] [Google Scholar]
- 62.Sirinathsinghji DJ. Behavioural effects in the rat after acute unilateral intranigral infusions of N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Brain Res. 1985;339:366–370. doi: 10.1016/0006-8993(85)90106-4. [DOI] [PubMed] [Google Scholar]
- 63.Svennilson E, Torvik A, Lowe R, Leksell L. Treatment of parkinsonism by stereotctic thermo lesions in the pallidal region. A clinical evaluation of 81 cases. Acta Psychiat Neurol Scand. 1960;35:358–377. doi: 10.1111/j.1600-0447.1960.tb07606.x. [DOI] [PubMed] [Google Scholar]
- 64.Takada M, Tokuno H, Nambu A, Inase M. Corticostriatal projections from the somatic motor areas of the frontal cortex in the macaque monkey: segregation versus overlap of input zones from the primary motor cortex, the supplementary motor area, and the premotor cortex. Exp Brain Res. 1998;120:114–128. doi: 10.1007/s002210050384. [DOI] [PubMed] [Google Scholar]
- 65.Talairach F, Paillas JE, David M. Dyskinesie de type hemiballique traitee par cortectomie frontale limitee, puis par coagulation de l'anse lenticulaire et de la portion interne du globus palli dus: amelioration importante dupuis un an. Rev Neurol. 1950;53:440–451. [PubMed] [Google Scholar]
- 66.Vermersch P, Delacourte A, Javoy-Agid F, Hauw JJ, Agid Y. Dementia in Parkinson's disease: biochemical evidence for cortical involvement using the immunodetection of abnormal Tau proteins. Ann Neurol. 1993;33:445–450. doi: 10.1002/ana.410330506. [DOI] [PubMed] [Google Scholar]
- 67.Vitek JL, Bakay RAE, Hashimoto T, Kaneoke Y, Mewes K, Zhang JY, Rye D, Starr P, Baron M, Turner R, DeLong MR. Microelectrode-guided pallidotomy: technical approach and its application for medically intractable Parkinson's disease. J Neurosurg. 1998;88:1027–1043. doi: 10.3171/jns.1998.88.6.1027. [DOI] [PubMed] [Google Scholar]
- 68.Whittier JR. Ballism and subthalamic nucleus (nucleus hypothalamicus, corpus Luys). Arch Neurol Psychiatry. 1947;58:672–692. doi: 10.1001/archneurpsyc.1947.02300350022002. [DOI] [PubMed] [Google Scholar]
- 69.Whittier JR, Mettler FA. Studies of the subthalamus of the rhesus monkey. II. Hyperkinesia and other physiologic effects of subthalamic lesions, with special reference to the subthalamic nucleus of Luys. J Comp Neurol. 1949;90:319–372. doi: 10.1002/cne.900900304. [DOI] [PubMed] [Google Scholar]
- 70.Wichmann T, DeLong MR. Functional and pathophysiological models of the basal ganglia. Curr Opin Neurobiol. 1996;6:751–758. doi: 10.1016/s0959-4388(96)80024-9. [DOI] [PubMed] [Google Scholar]
- 71.Wichmann T, Bergman H, DeLong MR. The primate subthalamic nucleus. I. Functional properties in intact animals. J Neurophysiol. 1994a;72:494–506. doi: 10.1152/jn.1994.72.2.494. [DOI] [PubMed] [Google Scholar]
- 72.Wichmann T, Bergman H, DeLong MR. The primate subthalamic nucleus. III. Changes in motor behavior and neuronal activity in the internal pallidum induced by subthalamic inactivation in the MPTP model of parkinsonism. J Neurophysiol. 1994b;72:521–529. doi: 10.1152/jn.1994.72.2.521. [DOI] [PubMed] [Google Scholar]
- 73.Wichmann T, Kliem MA, DeLong MR. Antiparkinsonian and behavioral effects of inactivation of the substantia nigra pars reticulata in hemiparkinsonian primates. Exp Neurol. 2001;167:410–424. doi: 10.1006/exnr.2000.7572. [DOI] [PubMed] [Google Scholar]
- 74.Winters W, Kado W, Adey W. A stereotaxic brain atlas for Macaca nemestrina. University of California; Berkeley, CA: 1969. [Google Scholar]
- 75.Yeterian EH, Pandya DN. Prefrontostriatal connections in relation to cortical architectonic organization in rhesus monkeys. J Comp Neurol. 1991;312:43–67. doi: 10.1002/cne.903120105. [DOI] [PubMed] [Google Scholar]
- 76.Yoshida S, Nambu A, Jinnai K. The distribution of the globus pallidus neurons with input from various cortical areas in the monkeys. Brain Res. 1993;611:170–174. doi: 10.1016/0006-8993(93)91791-p. [DOI] [PubMed] [Google Scholar]