Abstract
Endophytic fungi have the ability to live inside the host plant tissues without causing neither symptoms of diseases/or harm. Opportunistic infections are accountable for majority of the outbreaks, thereby putting a burden on the health system. To investigate and characterize the bioactive compounds for the control of bacteria of clinical importance, extracts from endophytic fungi were isolated from indigenous South African medicinal plants. Extracts from endophytic fungi were isolated from 133 fungal strains and screened against Gram positive and negative bacteria namely Bacillus cereus, Escherichia coli, Enterococcus faecium, and E. gallinarum using disk diffusion. Furthermore, gas chromatography–mass spectrometry was performed to identify the bioactive compounds. Sixteen out of one hundred and thirty-three (12%) fungi extracts exhibited antibacterial properties against some of the selected bacteria. E. coli was found to be the most susceptible in contrast to E. faecium and E. gallinarum which were the most resistant. The isolate MHE 68, identified as Alternaria sp. displayed the greater spectrum of antibacterial activities by controlling selected clinical bacteria strains including resistant E. faecium and E. gallinarum. The chemical analysis of the extract from MHE 68 indicated that linoleic acid (9,12-octadecadienoic acid (Z,Z)) and cyclodecasiloxane could be accountable for the antibacterial activity. This is the first study conducted on the secondary metabolites produced by endophytic fungal strains isolated from the Pelargonium sidoides DC. possessing antibacterial properties.
Keywords: Endophytic fungi, antibacterial activity, secondary metabolites, bioactive compounds
1. Introduction
Endophytic fungi play an essential part in the physiological and ecological roles [1] including growth promoter, stress tolerance, drought resistance, insect, and herbivores repliers. Antibiotic-producing fungi were the first and continue to be dominating the market [2]. Endophytes are defined as microorganisms that live/colonize within the plant tissues and cause no damage or symptoms of disease. There are regarded as being more beneficial to the plant than detrimental [3]. Current research focuses on using untapped location, medicinal plants and their endophytic fungi to discovery novel, affordable, efficacies pharmaceutical active compounds. This is in the hope of neutralizing the enormous problem of resistance [4].
Despite the knowledge about flora, fauna, and the traditional use of medicinal plant in Southern Africa, South Africa in particular remains an untapped location for host medicinal and aromatic plants with novel microorganisms [5].
The variance of plant to fungal diversity is 1 to 6 [5], increases the probabilities of discovering novel metabolites in the fungal community. From a large number of medicinal plants indigenous to South Africa, Pelargonium sidoides DC. have been reported to be the most traditional use plant for primary health care [6]. Due to the aptitude of this plant to produce secondary metabolites, it can be expected that endophytic fungi possessing some antimicrobial properties can be isolated [7].
For decades, bacteria have emerged as important healthcare-associated pathogens. The rapid spread of enterococci with resistance to vancomycin (VRE) has been of particular concern worldwide [7] when a substantial percentage of the population is immune compromised patients [8]. Although, Enterococcus faecium is the leading bacteria responsible for medical intensive care units’ device-associated infections, other enterococcal species such as E. avium, E. gallinarum, E. casseliflavus, are of clinical concerns. Opportunistic diseases in developing countries are a major cause of human mortality due to inadequate sanitation, a lack of safe drinking water, malnourishment, war, and famine claiming approximately 2 million lives a year [9]. While most coliforms are harmless to human health, the presence of Escherichia coli, can be accountable for outbreaks of infectious diarrhoea and held responsible for a number of death in developing countries.
Manganyi et al. [10] reported in depth the biodiversity and phylogenetic relationship of the endophytic fungi isolated from Pelargonium sidoides DC. The primary objective of the current study was to screen for the antibacterial properties of fungal extracts against seven selected bacteria of clinical interest. And finally to determine the chemical profile of the most abundant bioactive compounds using gas chromatography mass spectrophotometry (GC-MS).
2. Materials and methods
2.1. Endophytic fungi isolated from Pelargonium sidoides DC
One hundred and thirty three (n = 133) endophytic fungi were successfully isolated from healthy leave and roots of Pelargonium sidoides. Morphological and molecular identification were performed using internal transcribe spacer (ITS) region as describe by [10]. The pure cultures were preserved in the Agricultural Research Council (ARC, Mycology) on water, slant, and freeze dry for future use.
2.2. Production of secondary metabolites
The fungal isolates were revived by culturing them on Potato Dextrose agar (PDA, Merck, Darmstadt, Germany) and incubated at 25 °C for 10 days. A plug of active mycelia was inoculated into a 250 mL Erlenmeyer flask containing 50 mL of malt extract broth (MEB; Merck, Darmstadt, Germany). The numbers of spores were counted with a hemocytometer (Merck, Johannesburg, South Africa) and adjusted to 1 × 106 conidia/mL. The secondary metabolites were produced by fermentation as described by Premjanu and Jaynthy [11] and each fermentation performed in triplicate. Briefly, fungal cell mass was removed by filtration through a 0.45 µm syringe filter and the resulting filtrate stored in sterile conical flasks at 4 °C, until further use.
2.3. Bacteria strains
The target bacterial strains used were both environmental strains and control strain (American Type Culture Collection, ATCC; Table 1) with potential clinical implications and are well-known to be resistance against modern antibiotics. Bacteria were cultured in nutrient broth (NB; Merck, Darmstadt, Germany) for 24 h at 37 °C to reach a final suspension of 1 × 107 cells/mL.
Table 1.
Target bacteria with their origin and accession number.
| Target bacteria | Accession no. | Origin |
|---|---|---|
| Escherichia coli | ATCC 25922 | ATCC collection |
| Escherichia coli | ID = O177 | Environmental isolate from cattle faeces |
| Bacillus cereus | ATCC 10876 | ATCC collection |
| Enterococcus faecalis | ATCC S1299 | Environmental isolate from ground water |
| Enterococcus faecium | ATCC 700221 | Environmental isolate from ground water |
| Enterococcus gallinarum | ATCC 700425 | ATCC collection |
ID: Identified as….
2.4. Antibacterial properties
One hundred and thirty-three (133) extracts were screened for their antibacterial activities against six targeted bacteria strains. The disk diffusion assay was used as described by Ahmad et al. [12] and the experiment done in triplicate. The zone inhibition as the degree of activity was expressed as diameter (mm).
2.5. Characterization by gas-chromatography mass spectrometry
The most active fungal extracts were selected to undergo secondary metabolites identification using gas-chromatography mass spectrometry GCMS, (GC-MS TQ8050; Shimadzu, Johannesburg, South Africa) equipped with a Multifunctional Autosampler (AOC-6000), a capillary column (RTX-5, 60 m × 0.25 mm × 0.25 µm, New Delhi, India) as described by Sharma et al. [7]. The identities of the compounds were determined by searching known molecules in databases of NIST05; WILEY 8, and FFNSC1.3 libraries.
3. Results and discussion
3.1. Diversity of fungal extracts with antibacterial activity
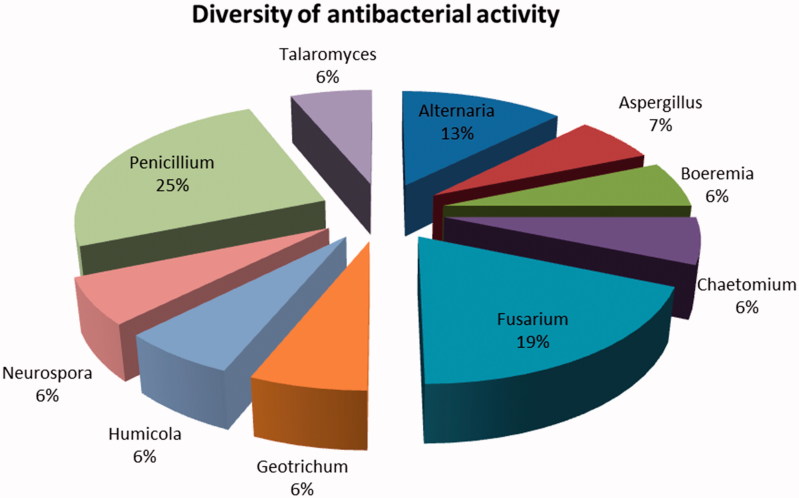
From all the fungal isolates (n = 133) tested; only 16 displayed inhibition activity against the selected bacteria (Table 2). The results (Figure 1) revealed that approximately 25% were Penicillium sp. which was the most dominant genera followed by Fusarium sp (19%), Alternaria sp. (13%), and Aspergillus sp. (7%). These genera belong to the ascomycete’s class, which is reported to be one of the two larger class of endophytes [13].The results of Fusarium sp. being the second most prevalent genera is not surprising as the Fusaria genus is the largest group of filamentous fungi [14]. Geotrichum sp. which falls under the same division (Ascomycota) as fungi, has been isolated from clones cocoa resistant VSD M.05 [15] and has been associated with the growth promoting protection capabilities of the plant hosts from pests and diseases.
Table 2.
Antimicrobial activity of extracts produced by endophytic fungal isolated from Pelargonium sidoides.
| Sample. No | Sample ID | Probable ID | Zone of inhibition (mm) |
||||||
|---|---|---|---|---|---|---|---|---|---|
|
E. coli ATCC 25922 |
E. coli ATCC 0177 |
B. cereus ATCC 10876 |
E. faecalis ATCC S1299 |
E. faecium ATCC 700221 |
E. gallinarum ATCC 700425 |
||||
| 1 | RNK 001 | Talaromyces | sp. | + (9) | – | – | – | + (6) | – |
| 2 | RNK 004 | Penicillium | glabrum | ++ (11) | – | – | – | – | – |
| 3 | RNK 016 | Alternaria | tenuissima | – | – | + (9) | – | – | – |
| 4 | PG 9 | Chaetomium | subaffine | – | + (9) | + (6) | – | – | – |
| 5 | PG 10 | Humicola | sp. | – | – | + (6) | – | – | – |
| 6 | END 015 | Boeremia | exigua var. pseudolilacis | – | – | – | ++ (11) | – | – |
| 7 | END 017,1 | Penicillium | sp. | + (9) | – | – | – | – | – |
| 8 | END 021 | Penicillium | commune | + (10) | – | – | – | – | – |
| 9 | MHE 001 | Fusarium | solani | – | ++ (11) | – | – | – | – |
| 10 | MHE 010 | Neurospora | crassa | + (9) | + (8) | – | – | – | – |
| 11 | MHE 011 | Penicillium | sp. | + (9) | – | – | – | – | – |
| 12 | MHE 033 | Aspergillus | sp. | + (2) | + (9) | – | – | – | – |
| 13 | MHE 055 | Fusarium | solani | – | ++ (12) | – | – | – | – |
| 14 | MHE 056 | Fusarium | sp. | – | – | + (8) | – | ++ (11) | – |
| 15 | MHE 059 | Geotrichum | candidum | + (9) | – | – | – | – | – |
| 16 | MHE 068 | Alternaria | sp. | – | – | + (8) | – | ++ (11) | ++ (12) |
Figure 1.
Diversity of fungal extracts displaying antibacterial activity.
3.2. Antibacterial activity against selected bacteria
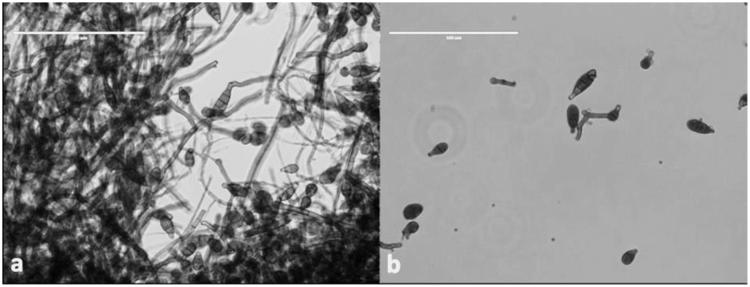
Furthermore, the results showed that most endophytic fungal extracts from medicinal plants have limited antibacterial activity. None of the isolates tested were able to control all six pathogenic bacteria. Only MHE 068 isolate, identified as Alternaria sp. (Figure 2) displayed signaficant antibacterial activity against three bacterial strains (B. cereus, E. faecium (ATCC 700221) and E. gallinarum). These findings are supported by [2], who reported similar results about Alternaria sp. exhibiting antibacterial activities against Bacillus sp., Staphylococcus aureus, E. faecalis, and E. coli. Despite of the overall 25% activity of Penicillium genera, the results show that Penicillium sp. could only inhibit E. coli (ATCC 25922) and nothing else. The discovery of antibiotics started with Penicillium strain producing bioactive compounds with significant biological properties which revolutionized medicine and pharmaceutical products. The Penicillium extracts in this study exhibited narrow spectrum of activity.
Figure 2.
Endophytic fungi Alternaria (a) Conidia structure wrapped in mycelia (b) individual conidia structure (scale bars: 100 µm).
In addition, it can be noted that the three Fusarium isolates displaying some antibacterial activities against B. cereus, E. coil (ATCC 0177), and E. faecium (ATCC 700221). As reported by [16], the endophytic Fusarium sp. is primarily known to exhibit good antibacterial activities against E. coli.
3.3. Characterization of bioactive compounds by GC-MS analysis
As previously stated, only the most effective extract (MHE 68, Alternaria sp.) was further analysed by GC-MS. Out of twenty compounds, separated and preliminary identified, the fatty acid, 9,12-octadecadienoic acid (Z,Z) (34%) commonly known as linoleic acid, was detected as dominant compound followed by several peaks initially identified as a cyclic volatile, eicosamethyl-cyclodecasiloxane oligomers (Table 3). Like several endophytic fungi, Alternaria sp. has been reported to exhibit significant level antibacterial activity against Gram positive and negative bacteria [17]. The antibacterial activity can be attributed to the high level of linoleic acid which has been reported to inhibit the binding of E. coli heat-labile enterotoxin (LT) to the receptor ganglioside GM1 in rabbit [18].
Table 3.
Main compounds identified in fungal extracts (Sample MHE 68).
| Name | Retention Time (min) | Height | Area | |
|---|---|---|---|---|
| 1 | Tetradecamethyl hexasiloxane | 21.9 | 49846 | 107935 |
| 2 | Tetradecamethyl hexasiloxane | 24.1 | 70746 | 134156 |
| 3 | Group of octadecadienoic acid | 25.9 | 84122 | 146379 |
| 4 | Group of octadecadienoic acid | 26.6 | 178016 | 271510 |
| 5 | Group of octadecadienoic acid | 26.7 | 304562 | 593966 |
| 6 | Group of octadecadienoic acid | 26.7 | 172773 | 340337 |
| 7 | Group of octadecadienoic acid | 26.9 | 96415 | 287016 |
| 8 | Group of octadecadienoic acid | 27.1 | 779997 | 1597988 |
| 9 | Eicosamethyl cyclodecasiloxane | 27.2 | 114397 | 250984 |
| 10 | 1H-Purin-6-amine, N-((3-fluorophenyl)methyl)-6-(3-fluorobenzylamino)purine | 27.6 | 22586 | 68648 |
| 11 | Eicosamethyl cyclodecasiloxane | 28.4 | 108477 | 292869 |
| 12 | Tetradecamethyl hexasiloxane | 29.6 | 99113 | 272854 |
| 13 | 1H-Purin-6-amine, N-((3-fluorophenyl)methyl)-6-(3-fluorobenzylamino)purine | 29.9 | 13195 | 78786 |
| 14 | Eicosamethyl cyclodecasiloxane | 30.6 | 102788 | 244851 |
| 15 | 1H-Purin-6-amine, N-((3-fluorophenyl)methyl)-6-(3-fluorobenzylamino)purine | 30.8 | 23772 | 61412 |
| 16 | Eicosamethyl cyclodecasiloxane | 31.6 | 50978 | 107960 |
| 17 | Propanoic acid | 32.6 | 26327 | 64355 |
| 18 | Methyl 2,3,4-tri-O-acetyl-6-deoxy-6-iodo-α-D-glucopyranoside | 33.2 | 19795 | 84775 |
| 19 | 1,2-Benzenediol | 33.7 | 25879 | 94106 |
| 20 | 6-Decylsulfonylhexane-1,2,3,4,5-pentol | 34.6 | 25345 | 107673 |
Furthermore, the antibacterial activity of plant volatile oils including cyclic volatiles have been demonstrated its activity against 25 different genera of bacteria such as E. coli and E. faecalis (NCTC 775) [19]. In conclusion, the study confirmed the potential use of endophytes from untapped indigenous medicinal plant for the control of opportunistic pathogens responsible for the mortality rate in developing countries. The extract from the Alternaria strain clearly confirmed the presence of linoleic acid. However, further studies on the optimization of the fermentation process and purification of the compounds are needed. As well as further bio-guided fractionation by nuclear magnetic resonance (NMR) and MS spectroscopy is necessary in future studies. This does not omit that the data obtained is critical in the investigation of novel bioactive compounds against bacterial strains of clinical importance. This is the first report on endophytic fungi isolated from Pelargonium sidoides DC. which were screened for their antibacterial activities.
Funding Statement
We would like to express our sincere gratitude to North West University, the National Research Foundation (NRF) and the Food Security and Safety for the financial support.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Thatoi H, Behera BC, Mishra RR. Ecological role and biotechnological potential of mangrove fungi. Mycology. 2013;4:54–71. [Google Scholar]
- 2.Smith RA, M'ikanatha NM, Read AF. Antibiotic resistance: a primer and call to action. Health Commun. 2015;30:309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khorasani M. Cylindrocarpon species in Pacific Northwest Douglas-fir Nurseries, diversity and effects of temperature and fungicides on mycelial growth [MSc dissertation]. Seattle, WA: University of Washington; 2013. [Google Scholar]
- 4.Malhadas C, Malheiro R, Pereira JA. Antimicrobial activity of endophytic fungi from olive tree leaves. World J Microbiol Biotechnol. 2017;33:46. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee A, Chowdhury R. Bile and unsaturated fatty acids inhibit the binding of cholera toxin and Escherichia coli heat-labile enterotoxin to GM1 receptor. Antimicrob Agents Chemother. 2008;52:220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorman HJD, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 2000;88:308–316. [DOI] [PubMed] [Google Scholar]
- 7.Sharma A, Kumar V, Kanwar MK, et al. . Phytochemical profiling of the leaves of Brassica juncea L. using GC-MS. Int Food Res J. 2017;24:547–551. [Google Scholar]
- 8.Nelson PE, Dignani MC, Anaissie EJ. Taxonomy, biology, and clinical aspects of Fusarium species. Clin Microbiol Rev. 1994;7:479–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar CG, Mongolla P, Joseph J, et al. . Chemical. Antimicrobial activity from the extracts of fungal isolates of soil and dung samples from Kaziranga National Park, Assam. J Mycol Med. 2010;20:283–289. [Google Scholar]
- 10.Manganyi MC, Regnier T, Kumar A. Biodiversity and antibacterial screening of endophytic fungi isolated from Pelargonium sidoides. S Afr J Bot. 2018;116:192–199. [Google Scholar]
- 11.Sabol K, Patterson JE, Lewis JII, et al. . Emergence of daptomycin resistance in Enterococcus faecium during daptomycin therapy. Antimicrob Agents. 2005;49:1664–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmad S, Khan MA, Ayaz S, et al. . Antibacterial and antifungal studies of the crude extract and solvent fractions of Onosma khyberianum. Pharmacologia. 2013;4:525–528. [Google Scholar]
- 13.Hénock BNY, Dovie DB. Diarrheal diseases in the history of public health. Arch Med Res. 2007;38:159–163. [DOI] [PubMed] [Google Scholar]
- 14.Ratnaweera PB, De Silva ED, Williams DE, et al. . Antimicrobial activities of endophytic fungi obtained from the arid zone invasive plant Opuntia dillenii and the isolation of equisetin, from endophytic Fusarium sp. BMC Complement Altern Med. 2015;15:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amin N, Salam M, Junaid M, et al. . Isolation and identification of endophytic fungi from cocoa plant resistante VSD M.05 and cocoa plant Susceptible VSD M.01 in South Sulawesi. Indonesia Int J Curr Microbiol App Sci. 2014;3:459–467. [Google Scholar]
- 16.Sadrati N, Daoud H, Zerroug A. Screening of antimicrobial and antioxidant secondary metabolites from endophytic fungi isolated from wheat (Triticum Durum). J Plant Prot Res. 2013;53:1–9. [Google Scholar]
- 17.Premjanu N, Jaynthy C. Identification and characterization of antimicrobial metabolite from an endophytic fungus, Colletotrichum gloeosporioides isolated from Lannea corammendalica. Int J Chem Tech Res. 2015;07:369–374. [Google Scholar]
- 18.Department of Agriculture , Forestry and Fisherie. Medicinal Plants of South Africa. 2013; [cited 2017 May 29]. Available from: http://www.daff.gov.za/Daffweb3/Portals/0/Brochures%20and%20Production%20guidelines/Brochure%20Medical%20Plants%20Of%20South%20Africa.pdf
- 19.Gouda S, Das G, Sen SK, et al. . Treasure house of bioactive compounds of medicinal importance. Front Microbiol. 2016;7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]