Abstract
Central neurons have multiple types of voltage-dependent potassium channels, whose activation during action potentials shapes spike width and whose activation and inactivation at subthreshold voltages modulate firing frequency. We characterized the voltage-dependent potassium currents flowing during the action potentials of hippocampal CA3 pyramidal neurons and examined the susceptibility of the underlying channel types to inactivation at subthreshold voltages. Using acutely dissociated neurons that permitted rapid voltage clamp, action potentials recorded previously were used as the command voltage waveform, and individual components of potassium current were identified by pharmacological sensitivity. The overall voltage-dependent potassium current in the neurons could be split into three major components based on pharmacology and kinetics during step voltage pulses: ID (fast activating, slowly inactivating, and sensitive to 4-aminopyridine at 30 μm),IA (fast activating, fast inactivating, and sensitive to 4-aminopyridine at 3 mm), andIK (slowly activating, noninactivating, and sensitive to external TEA at 3–25 mm). The potassium current during the action potential was composed of approximately equal contributions of ID andIA, with a negligible contribution ofIK. ID andIA had nearly identical trajectories of activation and deactivation during the action potential. BothIA and ID showed steady-state inactivation at subthreshold voltages, but maximal inactivation at such voltages was incomplete for both currents. Because of the major contribution of both IDand IA to spike repolarization, it is likely that modulation or partial inactivation at subthreshold voltages of either current can influence spike timing with minimal effect on spike width.
Keywords: action potential, 4-aminopyridine, IA, ID, IK, pyramidal neuron, spike, potassium current
Although action potentials in squid axons are formed by just two types of voltage-dependent channels (Hodgkin and Huxley, 1952), vertebrate central neurons each express at least a dozen different types of voltage-dependent ion channels (Llinás, 1988; Brown et al., 1990; Hille, 2001). The expression in central neurons of multiple types of potassium channels in particular confers the ability to fire with a variety of patterns over a broad range of frequencies (Connor and Stevens, 1971a,b; Rudy, 1988;Storm, 1990; Hille, 2001; Rudy and McBain, 2001).
How do the many channel types present in a particular neuron work together to determine its firing properties? This issue has been addressed primarily by computer modeling using Hodgkin–Huxley-like equations, extended by the addition of many conductances, with equations for each conductance based on experimental analysis of voltage and time dependence (Connor and Stevens, 1971b; Huguenard and McCormick, 1992, 1994; Johnston and Wu, 1995; Locke and Nerbonne, 1997b). This approach has been powerful and informative, but it has limitations. For central neurons, the connection between experimental measurements and modeling is quite indirect. For example, most voltage-clamp studies of potassium channels in central neurons are based on measurements of kinetics using voltage steps far longer than a typical action potential (0.5–2 msec). Thus, modeling of events during the action potential is typically based on kinetic models whose behavior is based on extrapolations of kinetics that are actually measured on a far slower time scale.
Our goal was to directly measure the potassium current flowing during the action potential of hippocampal CA3 pyramidal neurons and to determine the relative contribution of different channel types to the overall current. We performed voltage-clamp experiments using experimentally recorded action potentials as the command waveform, a procedure that has been used previously in a variety of cell types to examine the flow of various currents during the action potential (Llinás et al., 1982; de Haas and Vogel, 1989; Doerr et al., 1989; Zaza et al., 1997; Raman and Bean, 1999). We then used pharmacology to distinguish various components of the overall potassium current. Our results fit well with previous studies in hippocampal pyramidal neurons (Storm, 1987, 1990; Wu and Barish, 1992, 1999) and other excitatory neurons (Locke and Nerbonne, 1997a,b; Kang et al., 2000), suggesting that the potassium currents known asIA andID each contribute significantly to the repolarization of the action potential. In addition, we examined how the inactivation of IA andID during slow subthreshold depolarizations affects their subsequent activation during action potentials.
MATERIALS AND METHODS
Cell preparation. Long–Evans rats (postnatal day 5–12) were anesthetized with isoflurane and decapitated, and brains were quickly removed and placed in ice-cold, oxygenated dissociation solution containing (in mm): 82 Na2SO4, 30 K2SO4, 5 MgCl2, 10 HEPES, 10 glucose, and 0.001% phenol red, buffered to pH 7.4 with NaOH. Hippocampi were dissected and cut with a McIlwain tissue chopper (Mickle Engineering, Gomshall, UK) into 350-μm-thick slices. The slices were transferred into the dissociation solution with 3 mg/ml protease (Sigma type XXIII; Sigma, St. Louis, MO), incubated at 37°C for 8 min, and then rinsed twice in dissociation solution with added 1 mg/ml trypsin inhibitor and 1 mg/ml bovine serum albumin (at 37°C). After enzyme treatment, the slices were stored in dissociation solution with trypsin inhibitor and bovine serum albumin at 22°C under a pure oxygen atmosphere. Slices were withdrawn as needed, and the CA3 region was dissected and triturated through a fire-polished Pasteur pipette to release single cells. Hippocampal pyramidal cells were identified morphologically by their large pyramidal shaped cell body (12–16 μm by 20–36 μm) with a thick stump of apical dendrite.
Recording pipettes. Electrodes were pulled from borosilicate glass micropipettes (VWR Scientific, West Chester, PA) with a Sachs-Flaming puller (Sutter Instruments, San Rafael, CA) to yield resistances between 1 and 1.5 MΩ. To reduce pipette capacitance (and facilitate optimal series resistance compensation), the shanks of the electrodes were wrapped with thin strips of Parafilm (American National Can, Greenwich, CT) to within several hundreds of microns of the tip.
Current-clamp recordings. Action potentials were recorded with an Axoclamp 2B amplifier (Axon Instruments, Foster City, CA) in bridge mode. Resting membrane potentials typically ranged from −45 to −65 mV. We presume that the less-negative resting potentials may reflect depolarization caused by trauma during the isolation or caused by leak around the seal. To elicit full-blown action potentials, cells were hyperpolarized to potentials between −90 and −60 mV with steady injection of DC. Action potentials were evoked by short (generally 1 msec) current injections so that the period of current injection was over before the action potential. Voltage was filtered at 10 kHz (four pole Bessel filter), sampled at an interval of 10–25 μsec using a Digidata 1200A digital-to-analog (D/A) and analog-to-digital (A/D) converter and Clampex7 software (Axon Instruments), and stored on a computer hard disk.
Whole-cell voltage-clamp recordings. Currents were recorded with an Axopatch 200A amplifier (Axon Instruments), filtered with a corner frequency of 10 kHz (four pole Bessel filter), sampled at an interval of 10–50 μsec using a Digidata 1200A D/A and A/D converter and Clampex7 software (Axon Instruments), and stored on a computer hard disk. In some cases, currents were later digitally filtered with a corner frequency of 1 kHz (boxcar smoothing). Compensation (∼80%) for series resistance (typically ∼2.5 times higher than the pipette resistance) was routinely used. Seal resistances were typically 1–4 GΩ, and cells had input resistances between ∼100 and ∼900 MΩ after establishing the whole-cell configuration.
Solutions. The standard pipette solution for both current-clamp and voltage-clamp experiments was (in mm): 108 K2HPO4, 9 HEPES, 9 EGTA, and 4.5 MgCl2, buffered to pH 7.4 with KOH (Sodickson and Bean, 1996). To promote the stability of the recordings, 14 mm creatine phosphate (Tris salt), 4 mm Mg-ATP, and 0.3 mmTris-GTP were included in the pipette solution. Stocks (10×) of the creatine phosphate, ATP and GTP, were stored at −80°C. Standard external solution was Tyrode's solution containing (in mm): 150 NaCl, 4 KCl, 2 CaCl2, 2 MgCl2, 10 HEPES, and 10 glucose, pH 7.4, with NaOH. Tetrodotoxin (TTX; Calbiochem, La Jolla, CA) was added at 0.3–1 μm to block sodium channels. To focus on currents through voltage-dependent potassium channels, we blocked calcium entry by replacing external Ca2+ with equimolar (2 mm) Co2+. We chose Co2+ replacement rather than blocking calcium entry with Cd2+ or La3+, because these both produce dramatic shifts in the voltage dependence of IA(Klee et al., 1995). However, even Co2+replacement probably produces a smaller shift of the voltage dependence of IA in the depolarizing direction compared with Ca2+ (Numann et al., 1987); thus, the degree of inactivation of IAat subthreshold voltages may be somewhat underestimated.
After establishing the whole-cell configuration, cells were lifted from the bottom of the recording chamber, and extracellular solutions were delivered through an array of gravity-fed quartz capillaries (inner diameter, 145 μm) placed in front of the cell. Stock solutions of TEA (1 m), 4-aminopyridine (4-AP) (1 m), and TTX (0.3 mm) were prepared in deionized water and either stored at 4°C (TEA and 4-AP) or in aliquots at −20°C (TTX). All reagents, unless noted otherwise, were purchased from Sigma.
Leak subtraction. Correction for linear leak currents was done by subtracting a scaled current elicited by a 10 mV hyperpolarizing (or, at a holding potential of −110 mV, depolarizing) prepulse. For experiments using action potential waveforms as voltage commands, leak currents were defined by recording the currents in response to an inverted, scaled-down (by a factor of five) action potential used as command waveform, delivered from a holding potential of −80 or −90 mV.
Analysis. Data were analyzed and displayed using ClampFit6 (Axon Instruments), Microcal (Northampton, MA) Origin 5.0, and Igor Pro 3.12 (WaveMetrics, Lake Oswego, OR). All reported voltages are corrected for a liquid junction potential of −10 mV between the pipette solution and the Tyrode's solution (in which the pipette current is zeroed before sealing onto a cell), measured as described byNeher (1992). Statistics are given as mean ± SEM.
All experiments were performed at room temperature.
RESULTS
Current-clamp recordings
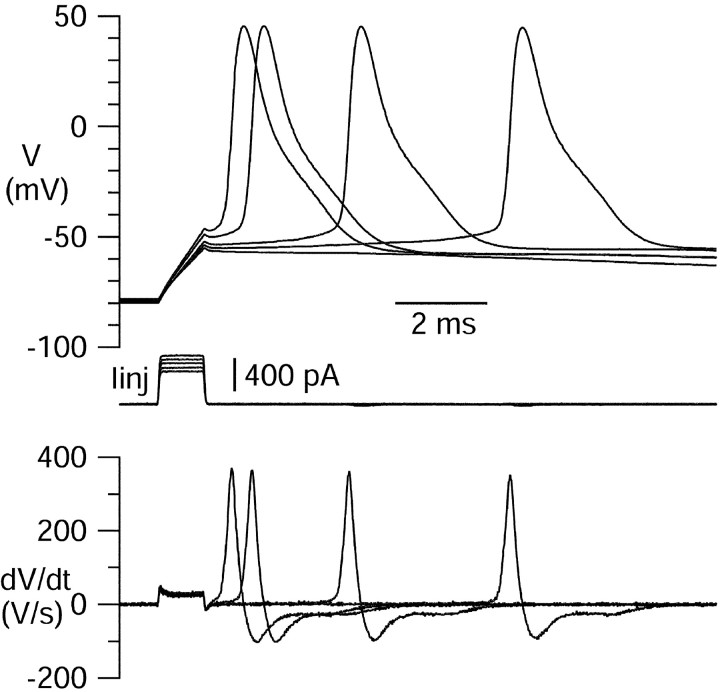
We began by recording action potentials in the dissociated neurons and examining the effects of various potassium channel blockers on action potential shape. Cells were held at resting potentials negative to −60 mV by injecting steady hyperpolarizing current [between 0 and 580 pA, with a mean of 128 (n = 66)], and single action potentials were elicited by 1 msec current injections (Fig.1). The average voltage threshold, measured as the least depolarized voltage (just after the current injection) for which an action potential fired, was −55 ± 1 mV (66 cells). Analysis of 255 action potentials from 17 cells yielded an average overshoot potential of +31 ± 2 mV, a maximal upstroke of 213 ± 18 mV/msec, a maximal downstroke of −64 ± 4 mV/msec, and a spike width of 1.4 ± 0.1 msec at 0 mV. These are similar to parameters reported for intact hippocampal pyramidal cells in brain slices recorded at room temperature (Bergles, 1995).
Fig. 1.
Action potentials in an isolated CA3 neuron. The cell was hyperpolarized by steady injection of DC (−55 pA), and action potentials were triggered by 1 msec injections of current of increasing amplitude. Bottom panel, The first derivative of the action potentials. External solution was normal Tyrode's solution with 2 mm CaCl2.
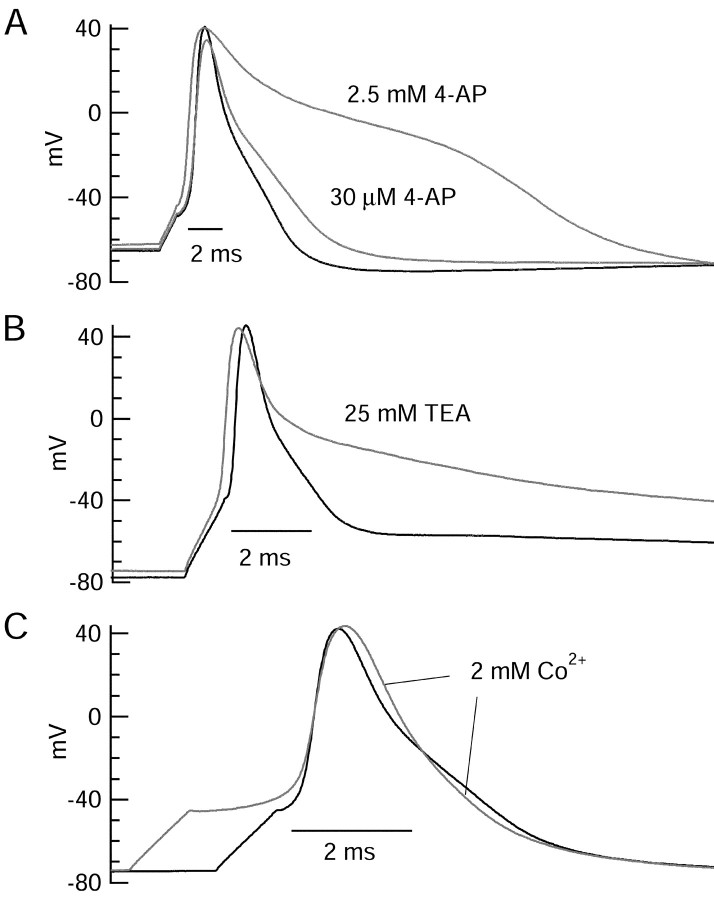
Figure 2 shows the effect of various potassium channel blockers on the action potential. Addition of 4-AP resulted in delayed repolarization of the action potential (Fig.2A). With exposure to 30 μm4-AP, action potentials were 38 ± 10% (n = 7) wider (as measured by the area under the curve). At 2.5 mm, 4-AP had a more dramatic effect, increasing the action potential width by 310 ± 79% (n = 3). Exposure to 25 mm external TEA (Fig.2B) also produced large effects, often leading to a sustained depolarization or plateau near −40 mV after the action potential. TEA had more pronounced effects on the later phases of repolarization than on the early phase (from the peak near +40 mV to ∼0 mV), whereas 2.5 mm 4-AP dramatically affected both phases. Interestingly, the effects on action potential shape of low 4-AP (modest broadening), high 4-AP (dramatic broadening, including early phase of repolarization), and TEA (dramatic broadening, but little effect on early phase) in CA3 neurons were very similar to those observed in callosal-projecting rat visual cortical neurons (Locke and Nerbonne, 1997b).
Fig. 2.
Effect of potassium channel blockers on action potential shape. A, 4-AP at 30 μm and 2.5 mm produced a dose-dependent delay in repolarization. Hyperpolarizing DC was −30 pA, and stimulus current (1 msec) was 490 pA. B, TEA at 25 mm had little effect on the initial phase of repolarization but produced a dramatic slowing of late repolarization. Hyperpolarizing DC was −40 pA, and injected current was 780 pA. C, Replacing external 2 mmcalcium with 2 mm cobalt resulted in a delay of firing and little overall change in action potential shape. Action potentials were aligned at the time of maximal upstroke to allow comparison of time course. Hyperpolarizing DC was −6 pA, and injected current was 850 pA.
In adult hippocampal neurons studied in brain slices, blocking calcium entry or rapidly chelating intracellular calcium significantly slows the decay of the action potential, suggesting a prominent role for calcium-activated potassium current in action potential repolarization (Storm, 1987; Poolos and Johnston, 1999; Shao et al., 1999). In contrast, in the younger CA3 neurons we studied, removing external calcium (substituting cobalt) had relatively small effects on the action potentials (Fig. 2C). Most commonly, the action potential became slightly wider in the initial phase of repolarization, consistent with a block of a small fraction of calcium-activated potassium current that contributes to initial repolarization, and slightly narrower in the later phase of repolarization, consistent with removal of a shoulder attributable to net inward calcium current. Possibly the role of calcium-activated potassium channels would be larger in the absence of the 9 mm EGTA present in the internal solution, although in adult cells, Storm (1987) found that EGTA was ineffective at disrupting repolarization, in contrast to the faster chelator BAPTA. In subsequent voltage-clamp experiments, we focused on purely voltage-activated potassium currents, using Tyrode's solution in which external Ca2+ was replaced by equimolar (2 mm) Co2+.
Voltage-dependent potassium currents elicited by step depolarizations
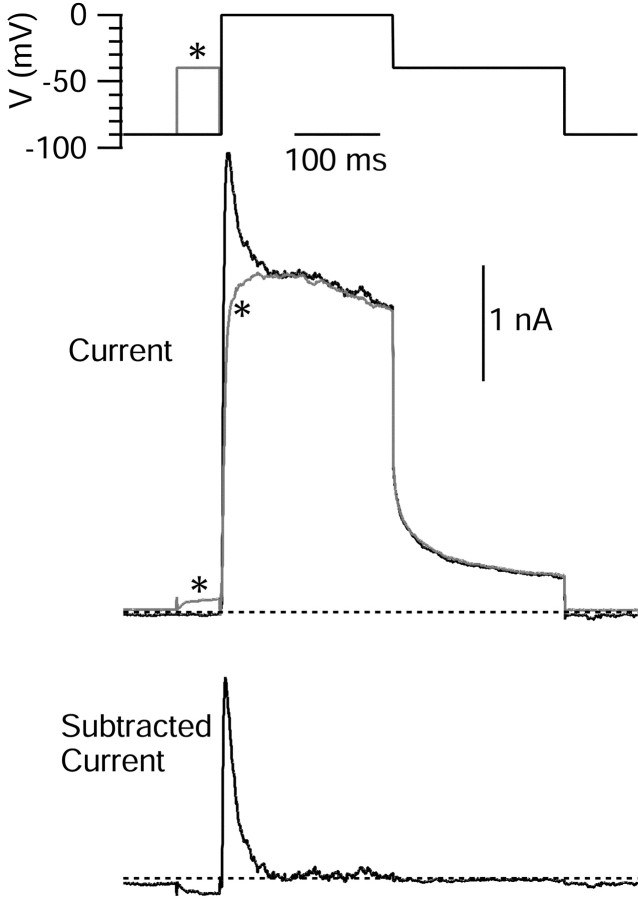
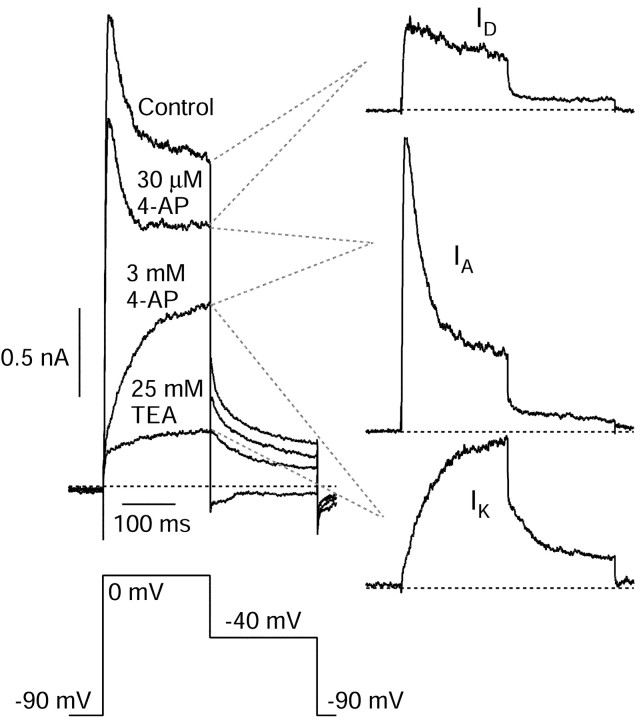
To characterize the components of voltage-dependent potassium currents sensitive to 4-AP and TEA, we began with experiments using voltage-clamp protocols using step depolarizations. Figure3 shows a voltage protocol used for one series of experiments, along with representative currents. Currents were elicited in Tyrode's solution with 1 μm TTX to block voltage-dependent sodium currents and with external calcium replaced by cobalt to block voltage-dependent calcium channels and calcium-activated potassium currents. Voltage steps from −90 mV to 0 mV activated outward current consisting of two kinetic components, an early transient peak, reached within a few milliseconds, and a maintained component with little decay for the remainder of a 200 msec test pulse. When a 50 msec prepulse to −40 mV preceded the test pulse to 0 mV, almost all of the initial transient current was removed, leaving a relatively slowly activating, slowly inactivating current (Connor and Stevens, 1971b; Numann et al., 1987; Klee et al., 1995). Subtraction of the current at 0 mV with and without prepulses yielded a current that activates quickly (time to peak of 5.2 ± 0.2 msec;n = 33 cells) and inactivates completely with a time constant of 15 ± 1 msec (n = 33 cells).
Fig. 3.
Isolation of IA by prepulse inactivation. Cells were held at −90 mV, and the voltage was stepped to 0 mV with or without a prepulse to −45 mV. There was a 2 msec return to −90 mV after the prepulse. Subtraction yields fast-activating and fast-inactivating outward current. External solution was 2 mm cobalt Tyrode's solution to block voltage-dependent calcium channels and calcium-activated potassium channels. TTX (1 μm) was included to block voltage-dependent sodium channels. Asterisks indicate records with prepulse. Dotted lines indicate zero current.
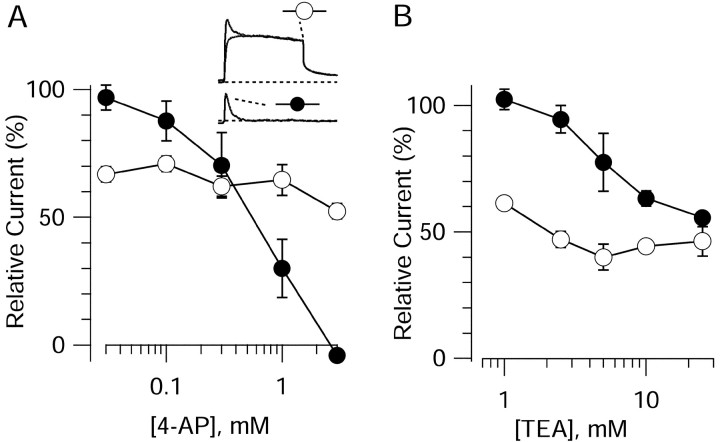
Using this protocol to distinguish between these two kinetic components of current, we found that they had different pharmacology. Figure4A shows the dose–response relationship for inhibition of these components of current by 4-AP. Application of 4-AP at 30 μmhad practically no effect on the transient, prepulse-sensitive component of current, and half-block of this component required between 300 μm and 1 mm 4-AP. Both the kinetic characteristics of the current and the moderate sensitivity to block by 4-AP of the transient current are consistent with identification as A-type potassium current (IA). Effects of 4-AP on the maintained, nonprepulse-sensitive current were very different. At 30 μm, 4-AP reduced the sustained current, measured at the end of a 200 msec test pulse (“late current”) by 33 ± 3% (n = 19), and there was only modest additional block by concentrations ≤3 mm (which blocked by 48 ± 3%; n = 15). This high sensitivity to 4-AP of a slowly inactivating current is consistent with the potassium current now referred to asID described previously in hippocampal pyramidal cells (Storm, 1988; Ficker and Heinemann, 1992; Wu and Barish, 1992; Bossu et al., 1996; Li and McArdle, 1997; Martina et al., 1998) as well as other neurons (McCormick, 1991; Surmeier et al., 1991;Locke and Nerbonne, 1997a; Martina et al., 1998). The additional block of late current at 3 mm 4-AP might reflect the presence of an additional sustained current component with low 4-AP sensitivity, which most likely represents the delayed rectifier potassium current IK (Storm, 1990).
Fig. 4.
Differential pharmacological sensitivity of fast-inactivating and slowly inactivating components of potassium current. Currents were elicited by the protocol in Figure 3 using pairs of test pulses to 0 mV with and without prepulses. Filled circles indicate the fast-inactivating component of current obtained by subtraction. Open circles indicate measurement of the current at the end of the step to 0 mV.Inset, top, Superimposed currents with and without prepulse. Bottom, Fast-inactivating component of current obtained by subtraction of these records. Dotted lines indicate zero current.
External TEA at 1 mm had no effect on the transient, prepulse-sensitive current (Fig. 4B). Increasing the concentration of TEA produced some inhibition, with block of 44 ± 2% at 25 mm TEA (n = 10) of this component of current. The concentration dependence of TEA sensitivity is consistent with approximately half of the transient current being sensitive to TEA inhibition, with half block of this component by 5 mm TEA. The late current was much more sensitive to TEA, with 1 mm TEA producing 39 ± 2% (n = 8) inhibition. Increasing the TEA concentration to 5 mm produced inhibition of 60 ± 5% (n = 6), and there was no additional effect of increasing the concentration to 25 mm (54 ± 6% inhibition; n = 10).
The experiment shown in Figure 5 examined in more detail the kinetics of the components of outward current (activated by a step from −90 to 0 mV) identified by the cumulative application of 30 μm 4-AP, 3 mm 4-AP, and 25 mm TEA. The component of current inhibited by 30 μm 4-AP shows rapid activation kinetics and slow inactivation (∼20–30% decay over 200 msec). Both these kinetic characteristics and the high sensitivity to 4-AP are consistent with the properties of the current named IDdescribed in studies of hippocampal pyramidal cells in brain slices (Storm, 1990). The component of current identified by the further action of 3 mm 4-AP shows rapid activation and rapid inactivation. These characteristics, together with the sensitivity of this component to prepulse inactivation (Fig. 3) and its moderate sensitivity to 4-AP, are consistent with the current component named IA in hippocampal (Storm, 1990) and other neurons (Rogawski, 1985). The current sensitive to inhibition by 25 mm TEA (applied in the presence of 3 mm 4-AP) showed slow activation and no inactivation over 200 msec. These characteristics are consistent with the delayed rectifier current calledIK (Segal and Barker, 1984; Brown et al., 1990; Locke and Nerbonne, 1997). Our functional definition ofIK was based on applying TEA afterIA had been blocked by 3 mm 4-AP, because there was some TEA sensitivity of the inactivating, prepulse-sensitive current (which is probably primarily IA). There was a small component of outward current remaining with 3 mm4-AP and 25 mm TEA; we did not attempt to further characterize this current.
Fig. 5.
Strategy for pharmacological separation ofIA,ID, andIK. Left panel, Sequentially recorded traces obtained in control solution (2 mm cobalt Tyrode's solution with 1 μm TTX) and in the presence of 30 μm 4-AP, 3 mm 4-AP, and 3 mm 4-AP plus 25 mm TEA. Right panel, Subtracted currents sensitive to 30 μm4-AP (ID), 3 mm 4-AP but not 30 μm 4-AP (IA), and 25 mm TEA but not 3 mm 4-AP (IK). Horizontal dotted lines indicate zero current.
Together, these results are consistent with three distinct components of voltage-activated potassium current in isolated CA3 neurons. One current is rapidly inactivating and prepulse sensitive and is weakly sensitive to 4-AP. One is very sensitive to 4-AP, slowly inactivating, and not sensitive to a prepulse. A third is sensitive to external TEA, slowly inactivating, and not prepulse sensitive. These correspond well to the currents named IA,ID, andIK described previously in studies of hippocampal pyramidal cells in brain slices (Storm, 1990) and cultured neurons (Wu and Barish, 1992, 1999). These three major components of voltage-activated potassium current in CA3 neurons seem nearly identical to the components of potassium current distinguished in detailed studies on CA1 pyramidal neurons (Martina et al., 1998) as well as another central excitatory projection neuron, callosal-projecting visual cortical neurons (Locke and Nerbonne, 1997a).
Voltage-dependent potassium currents elicited by action potential waveforms
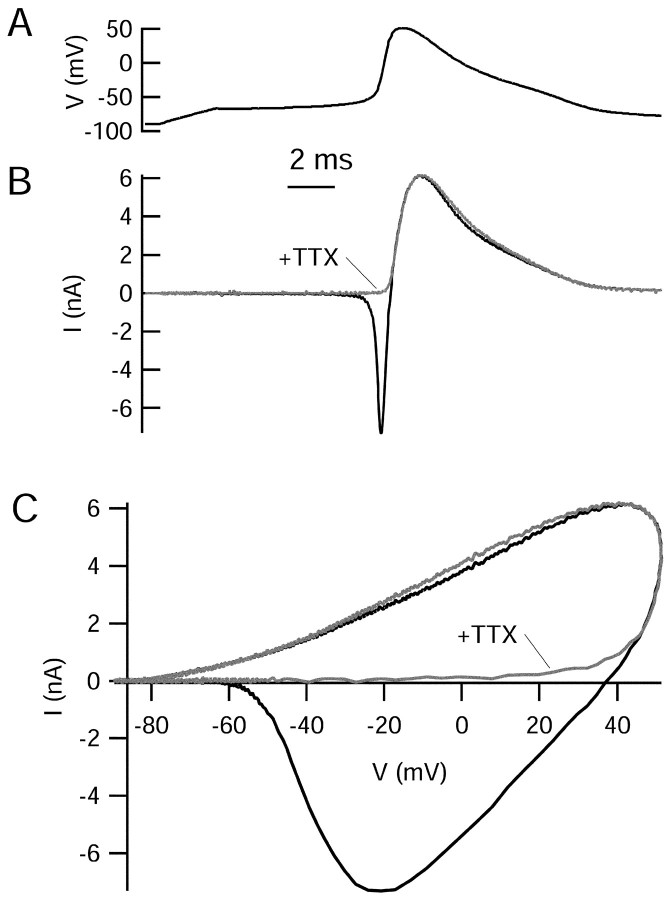
We subsequently examined currents elicited under voltage clamp using an action potential recorded previously as the command voltage. With unmodified Tyrode's solution, the ionic current elicited by the action potential waveform (Fig.6A) consisted of a large inward current during the upstroke of the action potential followed by a large outward current during the repolarization phase of the action potential (Fig. 6B). The relationship between the elicited current and the voltage trajectory during the action potential is seen by plotting the elicited current as a function of the voltage during the action potential (Fig. 6C). The inward current was completely blocked by TTX, suggesting that the upstroke of the action potential is entirely caused by TTX-sensitive sodium current (although calcium channels were not blocked in this experiment). Application of TTX had no effect on the outward current after the peak of the action potential, consistent with inactivation of the sodium current being complete before the falling phase of the action potential. With the outward current isolated after block of sodium current, it can be seen that the activation of the outward current during the action potential is very abrupt. There is almost no current activated during the upstroke of the action potential until a voltage of approximately +30 mV is achieved (0.42 msec after the start of the action potential, counting from the time the voltage crosses the threshold of −55 mV). The outward current increases very rapidly during the time that the action potential is near its peak, increasing from ∼1 to 6 nA in 0.46 msec, during which time the voltage rises from +40 to +55 mV and back to + 40 mV. The outward current then declines smoothly during the falling phase of the action potential, reflecting both the decrease in driving force for potassium ions and also deactivation of potassium channels.
Fig. 6.
Ionic current elicited by action potential waveform clamp. A, Command waveform, consisting of a previously recorded action potential (peak, +51 mV; maximal upstroke, +435 mV/msec; maximal downstroke, −62 mV/msec). B, Ionic currents elicited by the action potential waveform in normal Tyrode's solution (containing 2 mmCaCl2) and after application of 1 μmTTX. C, Ionic currents plotted as a function of voltage during the action potential.
Experiments examining action potential-elicited currents require good voltage control of large currents with rapid kinetics. Evidence for good voltage control comes from the lack of difference in outward currents when the large inward sodium current is blocked. If there were series resistance errors or other loss of voltage control, the voltage seen by the cell would be artifactually depolarized during the flow of sodium current, resulting in larger potassium currents. Such effects were seen if higher resistance pipettes or inadequate series resistance compensation were used.
Sodium-activated potassium currents have been reported in some neurons (Dryer, 1994). The experiment in Figure 6 shows that such a current does not contribute significantly to repolarization of the action potential in CA3 neurons, because total potassium current was unchanged when sodium influx was blocked.
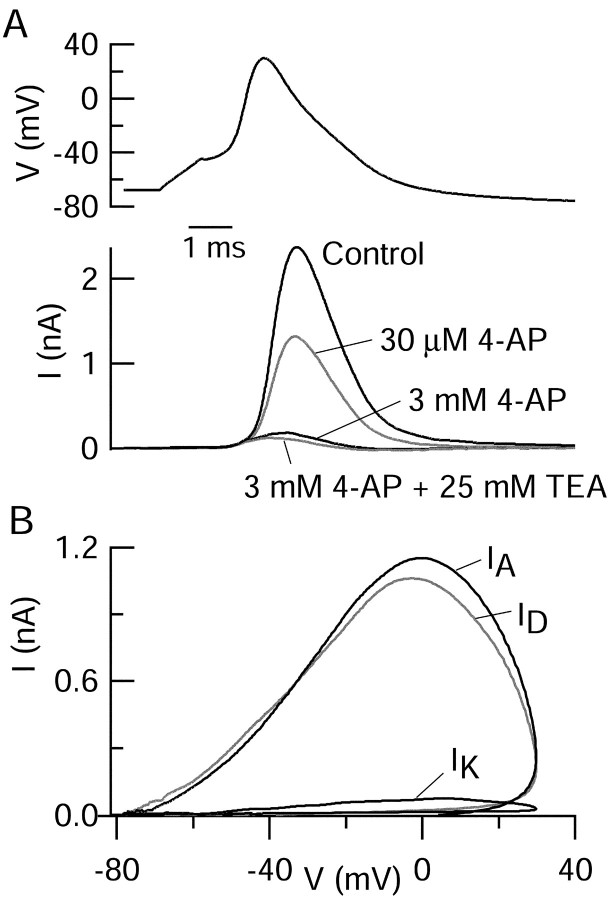
We used the pharmacological manipulations developed with step depolarizations to determine the contribution of various components of potassium current to the overall outward current elicited by the action potential waveform. An example is shown in Figure7. Application of 30 μm4-AP inhibited the peak outward current by 38 ± 2% (n = 22), and application of 3 mm4-AP inhibited the peak current by 84 ± 2% (n = 20). Adding 25 mm TEA (in the continuing presence of 4-AP) had relatively little effect, producing a detectable amount of additional block in 6 of 11 cells (∼2% of the peak control current). An alternative way to quantify the components of outward currents during the action potential is to examine the total charge carried by the various components of current, obtained by integrating the current over the course of the action potential waveform. Based on this analysis, the contributions to the overall charge byID,IA, andIK were 39 ± 2% (n = 22), 49 ± 2% (n = 20), and 2 ± 1% (n = 11), respectively. A total of 11 ± 2% (n = 11) remained unblocked by the combination of 3 mm 4-AP and 25 mm TEA.
Fig. 7.
Pharmacological dissection of potassium current elicited by action potential waveform. A, Current elicited by action potential waveform in Tyrode's solution with calcium replaced by cobalt to eliminate voltage-activated calcium current and with 1 μm TTX to block sodium current. Action potential (peak, +30 mV; maximal upstroke, +135 mV/msec; maximal downstroke, −50 mV/msec) was recorded previously in normal Tyrode's solution. To separate IA,ID, andIK, 4-AP and TEA were applied according to the strategy summarized in Figure 5. B,IA,ID, and IKobtained by subtraction. ID was obtained as the current sensitive to 30 μm 4-AP,IA as the current sensitive to 3 mm 4-AP but not 30 μm 4-AP, andIK as the current sensitive to 25 mm TEA but not 3 mm 4-AP.
Figure 7B plots ID andIA as a function of the voltage during the action potential. The pattern of flow ofID andIA during the action potential waveform was very similar. As for total outward current during the action potential, both showed negligible activation before the peak of the action potential was reached (in this case, near +30 mV) and rapid activation immediately after the peak. The similar kinetics ofID andIA during the action potential is consistent with the rapid activation kinetics of both during step depolarizations. In addition, bothID andIA decayed at approximately the same rate during the falling phase of the action potential. Given the difference in inactivation kinetics of the two channels, this suggests that the decrease in the current can be attributed primarily to deactivation and, to a lesser extent, to a decrease in the driving force on potassium ions (approximately twofold greater at 0 mV than −45 mV).
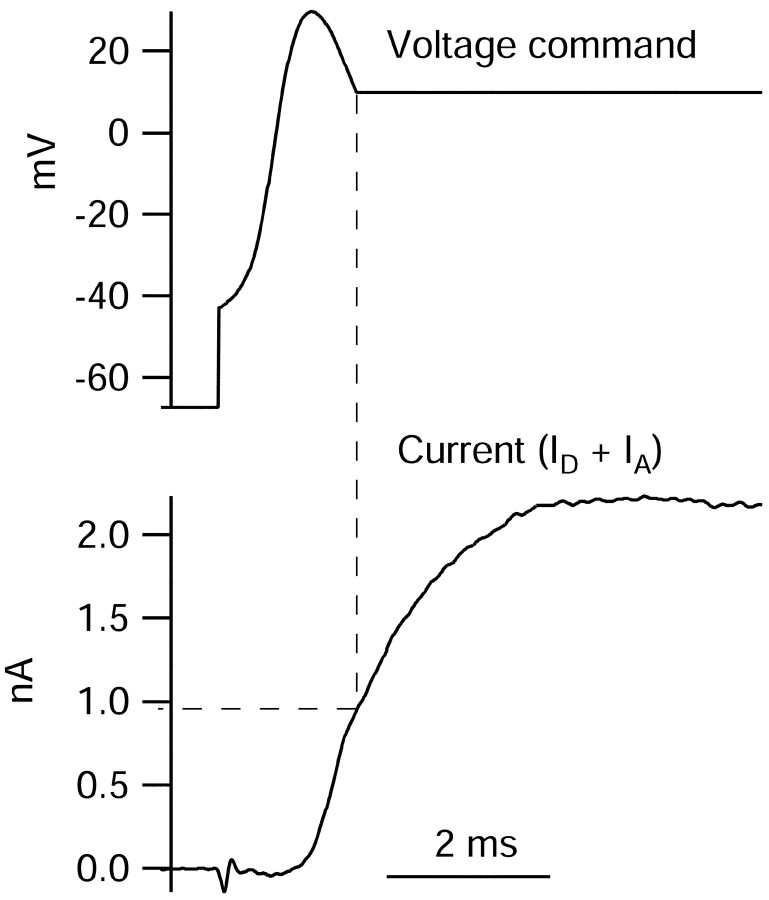
How complete is activation of potassium channels during the action potential? Does activation reach completion before deactivation begins? We approached this question by testing whether maintaining the depolarization of an action potential produced larger currents (Fig.8). The activation ofID andIA is particularly interesting in this context, so we isolated total ID andIA by block with 3 mm 4-AP. The voltage command consisted of a partial action potential, interrupted and extended at a voltage of +10 mV on the falling phase. This is the point on the action potential at which maximal outward current is normally reached. Extending the action potential resulted in a substantial increase inID andIA. Peak current was reached after another ∼3 msec at +10 mV and was more than twice that reached on initially reaching +10 mV on the falling phase of the action potential. Thus, in aggregate, IA andID are <50% fully activated during the action potential. In four experiments using this protocol, activation of IA andID during the action potential was 46 ± 9% of that achieved with extended step to +10 mV.
Fig. 8.
Assay of extent of activation ofIA and ID during an action potential. Total IA andID were isolated as current sensitive to 3 mm 4-AP. Voltage command consisted of a partial action potential interrupted and extended at +10 mV on the falling phase. Current reached at +10 mV during the falling phase was 0.95 nA, compared with a peak of 2.23 nA reached ∼3 msec later at the same voltage. The vertical dashed line indicates time at which falling phase of action potential reaches +10 mV; the horizontal dashed line indicates outward current at this time.
The experiment in Figure 8 also suggests that there is little or no inactivation of ID andIA during a single action potential, because decay of the combined current is not detectable until the action potential has been extended for ∼3 msec at +10 mV, approximately three times its normal duration.
Activation of IA andID preceding a spike
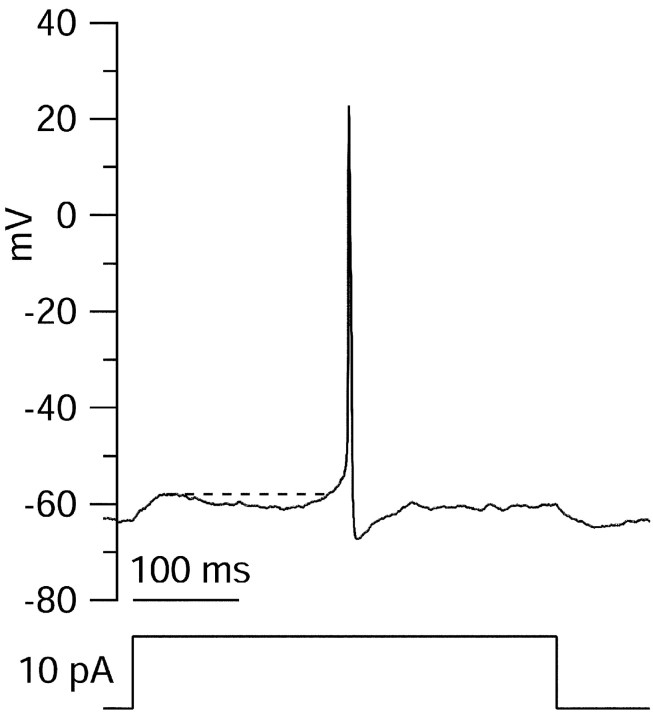
Both IA andID are believed to be activated at subthreshold potentials and to contribute to delayed firing of action potentials during slow approaches to threshold (Connor and Stevens, 1971a; Segal et al., 1984; Storm, 1988). Because of the slow inactivation of ID, this effect can last ≤10 sec (Storm, 1988). As in studies on more intact cells, we observed delayed firing when threshold was approached slowly in isolated CA3 neurons. Figure 9 shows an example in which action potentials were evoked by long (400 msec) injections of current. The delay of the first spike was determined for the least depolarizing current injection that caused action potential firing. In 42 experiments from 31 cells, the time to peak for the first spike ranged from 40 to 350 msec with a mean value of 49 msec. This is considerably longer than the average membrane time constant of 12 ± 11 msec (mean ± SD), suggesting that the delay reflects more than the time required to reach threshold. In 20% of the experiments, the time to peak for the first spike was >86 msec. This cutoff represents three times the mean membrane time constant plus 50 msec (approximately the time for IA to activate and inactivate). In the example in Figure 9, the membrane was charged to a subthreshold voltage of −58 mV after current injection. An ∼200 msec delay followed, during which the voltage first hyperpolarized and then depolarized until threshold was reached, and an action potential was fired. This sequence is consistent with a sequence of activation and inactivation of potassium current, presumably some combination of IA andID, at subthreshold voltages.
Fig. 9.
Delayed firing of an action potential near threshold. An action potential elicited near threshold with stimulation by 400 msec steps of current is shown. Note relaxation from initial depolarization, followed by a second phase of depolarization and then spike firing. Spike occurs 203 msec after start of current step. Thedashed line indicates voltage reached during initial depolarizing phase (−58 mV).
The experiments like that shown in Figure 9 fit well with previous results in recordings from hippocampal pyramidal neurons in brain slices, suggesting that delayed firing of action potentials can result from activation and inactivation of IAand ID at subthreshold voltages. How complete is this inactivation? This is an especially interesting question in light of the fact that IAand ID are also the major currents contributing to the repolarization of the action potential. If inactivation were complete during slow approach to threshold, the repolarization of the resulting action potentials might be greatly altered. Therefore, we examined the time course of inactivation ofIA andID at relevant voltages. We used voltage-clamp protocols in which the action potential command waveform was preceded by a prepulse of increasing duration. 4-AP and TEA were used to pharmacologically separate and quantitatively assessIA andID. As shown in Figure10, there is substantial but incomplete suppression of IA andID by rectangular prepulses to −45 mV (the threshold potential) of ≤3 sec duration. As expected from their kinetics, IA is affected more rapidly and to a greater extent than ID.
Fig. 10.
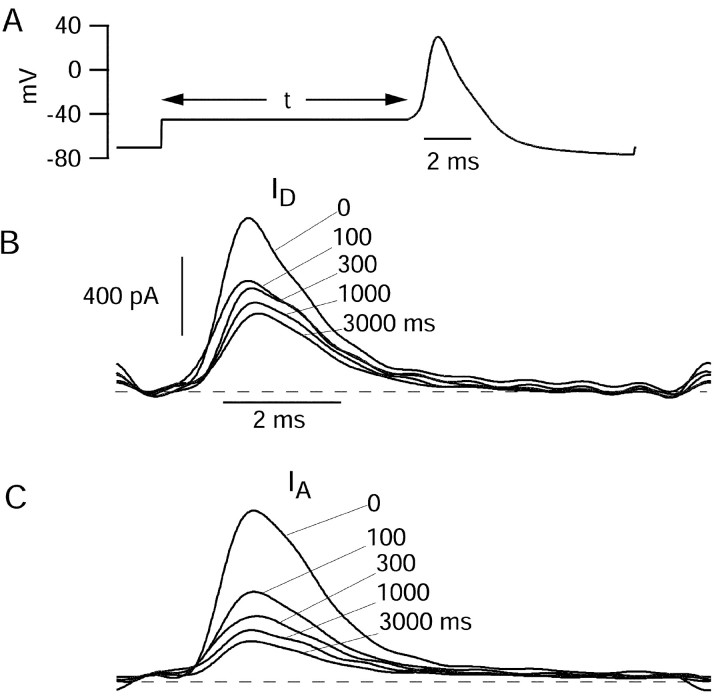
Partial inactivation ofIA and ID by prepulses to −45 mV. A, Currents were elicited by an action potential waveform preceded by a variable length prepulse to −45 mV, from a steady holding potential of −70 mV.Arrows indicate variable length of prepulse (0-3000 msec).B, ID (obtained as fraction of current sensitive to 30 μm 4-AP) elicited by the action potential after prepulses of indicated lengths.C, IA (obtained as fraction of current sensitive to 3 mm 4-AP but not 30 μm 4-AP) elicited by the action potential after prepulses of indicated lengths. External solution was 2 mmcobalt Tyrode's containing 1 μm TTX.Dashed lines indicate zero current.
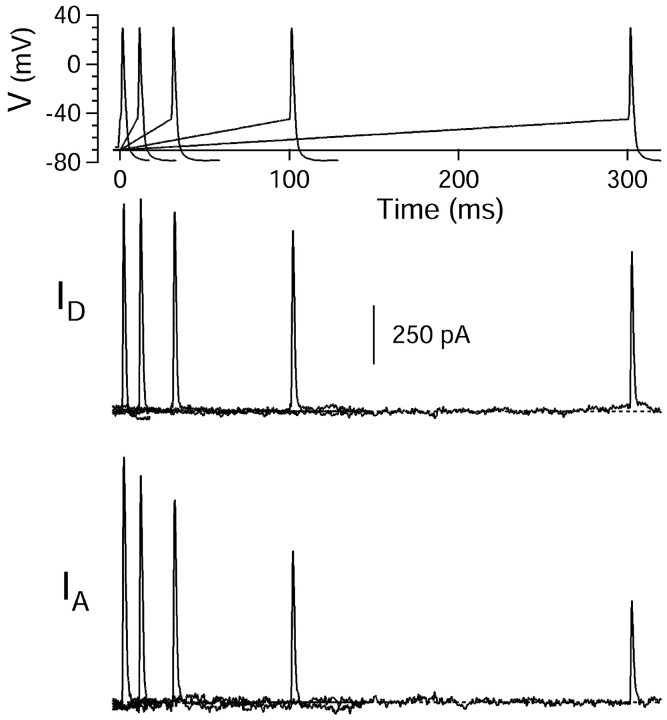
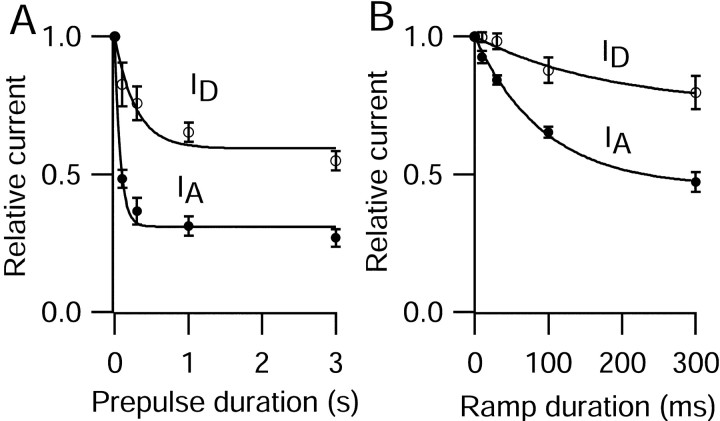
Some depolarizing voltage trajectories that were observed to occur preceding a spike do not resemble a rectangular voltage step but gradually lead up to threshold. Therefore, we also tested the extent of inactivation of IA andID with a depolarizing ramp of increasing duration, up to a threshold voltage of −45 mV, and coupled to the action potential command waveform (Fig.11). When preceded by a depolarizing ramp to threshold, IA andID flowing during a subsequent action potential were both reduced, with considerably more effect onIA thanID. These data are summarized in Figure 12. Both prepulse protocols induced substantial inhibition of either current, with the square pulse inducing greater attenuation. In both cases, the time dependence was faster for IA than forID, and inactivation of both potassium currents was incomplete. A steady state ofIA peak current amplitude was reached at 31% (square prepulse) and 45% (ramp) of control. Conversely, inactivation of ID saturated at 59% (square prepulse) and 75% (ramp) of control. These findings show that even sustained depolarizations to potentials close to threshold produce only partial inactivation of ID andIA, leaving a substantial fraction of these rapid rectifiers available for repolarization of the ensuing action potential.
Fig. 11.
Partial inactivation ofIA and ID by ramps preceding the action potential. Currents were elicited by an action potential waveform preceded by ramps (of variable lengths from 0 to 300 msec) from −70 mV to −45 mV (top panel).ID (middle panel) was obtained as the fraction of current sensitive to 30 μm4-AP elicited by the waveforms. IA was obtained as the fraction of current sensitive to 3 mm 4-AP but not 30 μm 4-AP. External solution was 2 mm cobalt Tyrode's containing 1 μmTTX.
Fig. 12.
Time course of reduction ofID and IA by prepulses or ramps to threshold potentials. A, Normalized peak current of ID (○;n = 3) or IA (●;n = 4) elicited by the action potential waveform is plotted against the duration of a step depolarization to −45 mV. Error bars show mean ± SEM. Fittedcurves are single exponential functions.ID, Time constant of 282 msec; steady-state, 59%. IA, Time constant of 75 msec; steady-state, 31%. B, Normalized peak current of ID (○; n = 7) or IA (●; n = 9) elicited by the action potential waveform is plotted against the duration of a depolarizing ramp as in Figure 11.ID, Time constant of 183 msec; steady-state, 75%. IA, Time constant of 94 msec; steady-state, 45%.
DISCUSSION
Previous voltage-clamp studies using brain slices, cultured neurons, and acutely isolated neurons have identified three principal voltage-activated potassium currents in hippocampal pyramidal neurons:IA,ID, andIK (Gustafsson et al., 1982; Segal and Barker, 1984; Zbicz and Weight, 1985; Lancaster and Adams, 1986; Numann et al., 1987; Sah et al., 1988; Storm, 1988; Lancaster et al., 1991;Klee et al., 1995; Bossu et al., 1996). Our results fit well with these previous studies in finding that most of the voltage-activated potassium current can be assigned to these three components, each identified with a particular pharmacological and kinetic profile. The component identified as ID is highly sensitive to 4-AP, fast activating, and slowly inactivating.IA is weakly sensitive to 4-AP, fast activating, and fast inactivating. IKis sensitive to external TEA, slowly activating, and virtually noninactivating.
Our results suggest that essentially all of the potassium current underlying repolarization of single action potentials in hippocampal CA3 neurons comes from ID andIA, with approximately equal contributions of each. The participation ofID in action potential repolarization agrees with previous results showing effects of low concentrations of 4-AP on action potential width in hippocampal pyramidal neurons (Storm, 1988; Wu and Barish, 1992). Besides contributing nearly equal amounts of current during the action potential,ID andIA seem interchangeable (at least during the spike itself) in that they follow nearly identical trajectories of voltage- and time-dependent activation and deactivation during the action potential (Fig. 7). In both cases, the decline of current during the later phases of the action potential appears to be primarily attributable to deactivation (along with the decreased driving force) rather than inactivation, because there is very little inactivation of either current even if the action potential is artificially prolonged several-fold (Fig. 8). The lack of substantial inactivation of IA during a single spike is consistent with the observation of Keros and McBain (1997)that arachidonic acid speeds inactivation ofIA but does not affect spike width. It is also interesting that there is a significant surplus capability of potassium current from ID andIA during a single spike in that the combined current reaches only ∼40% of maximal activation (Fig. 8). However, our experiments were performed at room temperature, and it is possible that at physiological temperature, the degree of activation of both ID andIA during a spike might be higher. Both the participation of two different channel types and the reserve capability can be considered safety factors tending to ensure rapid repolarization.
It is possible to make working hypotheses for the molecular basis of the potassium channel subunits making up the various components of potassium current in hippocampal pyramidal neurons. Kv2.1 is a likely candidate for IK, because Murakoshi and Trimmer (1999) found that delayed rectifier current could be inhibited by intracellular application of antibodies to Kv2.1, and Du et al. (2000) showed that treatment of CA1 neurons with antisense oligonucleotides directed against Kv2.1 reduced a delayed rectifier similar to the IK component in dissociated neurons. The lack of effect on single spike width of antisense treatment (Du et al., 2000) fits well with our finding of negligible activation of IK during an action potential. In agreement with the pharmacology ofIK, Kv2.1 channels expressed heterologously in mammalian cells are blocked only weakly by 4-AP, with a half-blocking concentration of 3 mm (Shi et al., 1994). The kinetic and pharmacological properties ofIK as we recorded it in acutely isolated CA3 neurons match very well with those of a corresponding component of potassium current in nucleated patches from CA1 pyramidal neurons in brain slice (Martina et al., 1998) proposed to correspond to Kv2 channels. Because CA1 pyramidal neurons express Kv2.2 as well as Kv2.1 subunits (Martina et al., 1998), it is very possible that the channels underlying IK might be heteromultimers (Du et al., 2000).
Kv4 family subunits are likely candidates for channels contributing toIA. CA3 pyramidal neurons express both Kv4.2 and Kv4.3 subunits (Serodio and Rudy, 1998). In several types of central neurons that have been examined, the amplitude ofIA is correlated with the level of expression of mRNA for Kv4.2 subunits (Song et al., 1998; Tkatch et al., 2000), and in both sympathetic neurons (Malin and Nerbonne, 2000) and cerebellar granule neurons (Shibata et al., 2000),IA is eliminated or greatly reduced by transfection with dominant negative Kv4.3 subunits, expected to eliminate currents from all Kv4 family channels. In CA1 neurons, which, unlike CA3 neurons, do not express significant levels of Kv4.3 (Serodio and Rudy, 1998), both expression of Kv4.2 immunoreactive protein andIA are dramatically reduced in regions of heterotopia induced by prenatal injections of methylazoxymethanol, consistent with a major role for Kv4.2 subunits in the generation ofIA (Castro et al., 2001). As in a subset of sympathetic neurons (Malin and Nerbonne, 2001), a component of IA in CA3 neurons might also originate from Kv1 family subunits, which, together with β1 subunits, can produce an IA-like current (Rettig et al., 1994); this might account for the component ofIA blocked weakly by TEA.
The molecular identification of ID in hippocampal pyramidal neurons is less certain. This current has kinetic properties of fast activation and slow inactivation and is blocked by 30 μm 4-AP. These properties would fit with channels made by Kv3.1 subunits (Grissmer et al., 1994; Martina et al., 1998), but few hippocampal pyramidal neurons appear to express detectable levels of Kv3.1 subunits (Weiser et al., 1995; Martina et al., 1998; Du et al., 2000). Channels formed by Kv1.5 subunits are also candidates for ID in hippocampal pyramidal neurons. Kv1.5 polypeptides are expressed in the cell bodies of CA3 neurons (Maletic-Savatic et al., 1995) and can underlie rapidly activating, slowly inactivating currents with high sensitivity to 4-AP (London et al., 2001).
In addition to their role in action potential repolarization, it is believed that both IA andID can undergo a sequence of partial activation and inactivation at subthreshold voltages and thus tend to produce a delay in spike firing (Connor and Stevens, 1971a; Segal et al., 1984; Storm, 1988; Luthi et al., 1996). Indeed, in our experiments on isolated neurons, such a delay of action potential firing was observed when just enough sustained current was injected to depolarize the membrane to threshold (Fig. 9). Although the roles ofIA andID in spike repolarization appear to be interchangeable, this is unlikely to be true of their roles at subthreshold voltages, where differences in inactivation rates and completeness are expected to have more significant functional effects. At subthreshold voltages, we found that inactivation ofIA was both faster and more complete than that of ID. We did not attempt to resolve activation of IA andID during the lead-up to action potentials. In principle, such currents might be small if channels can inactivate at subthreshold voltages without first activating. This is true for cloned Kv4.2 channels (Bähring et al., 2001), but this important issue has apparently not yet been explored for nativeIA andID in central neurons. The nonmonotonic change in voltage during the lead up to the action potential with just-threshold current injections suggests that one or both currents undergo partial activation followed by inactivation, but the currents needed to produce the changes in subthreshold voltage are probably just a few picoamperes.
One consequence of having two different channel types contributing equally to repolarization is to minimize effects on repolarization rate when either current is reduced. This may be especially important because both ID andIA also play roles in subthreshold phenomena. Thus, one current can undergo a cycle of partial activation and inactivation leading up to the spike without resulting in elimination of the current available for repolarizing the spike. Because IA andID contribute approximately equally to the current underlying repolarization, we can observe the consequences of reducing this current by half by examining the effects of 30 μm 4-AP, which blocksID but notIA. This resulted in a relatively modest broadening of the action potential. In adult pyramidal neurons, calcium-activated potassium current through BK channels also contributes significantly to repolarization (Storm, 1987; Poolos and Johnston, 1999; Shao et al., 1999), which would further minimize the effects on spike repolarization of reducing eitherID andIA alone.
Both ID andIA are known to be modulated by transmitters and second messenger systems (Nakajima et al., 1986;Deadwyler et al., 1995; Keros and McBain, 1997; Hoffman and Johnston, 1998; Colbert and Pan, 1999; Wu and Barish, 1999; Mu et al., 2000; Lien et al., 2002). The redundancy of their roles in spike repolarization means that modulation of either one may be able to produce significant functional effects at subthreshold voltages without compromising rapid spike repolarization.
Footnotes
This work was supported by National Institutes of Health Grants NS36855, NS38312, and HL35034 and by Fonds zur Förderung der wissenschaftlichen Forschung Grant J1853-MED. We thank Dr. Marco Martina for comments on this manuscript.
Correspondence should be addressed to Bruce P. Bean, Department of Neurobiology, Harvard Medical School, 220 Longwood Avenue, Boston, MA 02115. E-mail: Bruce_Bean@hms.harvard.edu.
REFERENCES
- 1.Bähring R, Boland LM, Varghese A, Gebauer M, Pongs O. Kinetic analysis of open- and closed-state inactivation transitions in human Kv4.2 A-type potassium channels. J Physiol (Lond) 2001;535:65–81. doi: 10.1111/j.1469-7793.2001.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergles DA. PhD thesis. Stanford University; 1995. The actions of norepinephrine on hippocampal interneurons. [Google Scholar]
- 3.Bossu JL, Capogna M, Debanne D, McKinney RA, Gahwiler BH. Somatic voltage-gated potassium currents of rat hippocampal pyramidal cells in organotypic slice cultures. J Physiol (Lond) 1996;495:367–381. doi: 10.1113/jphysiol.1996.sp021600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown DA, Gahwiler BH, Griffith WH, Halliwell JV. Membrane currents in hippocampal neurons. Prog Brain Res. 1990;83:141–160. doi: 10.1016/s0079-6123(08)61247-9. [DOI] [PubMed] [Google Scholar]
- 5.Castro PA, Cooper EC, Lowenstein DH, Baraban SC. Hippocampal heterotopia lack functional Kv4.2 potassium channels in the methylazoxymethanol model of cortical malformations and epilepsy. J Neurosci. 2001;21:6626–6634. doi: 10.1523/JNEUROSCI.21-17-06626.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colbert CM, Pan E. Arachidonic acid reciprocally alters the availability of transient and sustained dendritic K+ channels in hippocampal CA1 pyramidal neurons. J Neurosci. 1999;19:8163–8171. doi: 10.1523/JNEUROSCI.19-19-08163.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connor JA, Stevens CF. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol (Lond) 1971a;213:21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connor JA, Stevens CF. Prediction of repetitive firing behaviour from voltage clamp data on an isolated neurone soma. J Physiol (Lond) 1971b;213:31–53. doi: 10.1113/jphysiol.1971.sp009366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deadwyler SA, Hampson RE, Mu J, Whyte A, Childers S. Cannabinoids modulate voltage sensitive potassium A-current in hippocampal neurons via a cAMP-dependent process. J Pharmacol Exp Ther. 1995;273:734–743. [PubMed] [Google Scholar]
- 10.de Haas V, Vogel W. Sodium and potassium currents recorded during an action potential. Eur Biophys J. 1989;17:49–51. doi: 10.1007/BF00257145. [DOI] [PubMed] [Google Scholar]
- 11.Doerr T, Denger R, Trautwein W. Calcium currents in single SA nodal cells of the rabbit heart studied with action potential clamp. Pflügers Arch. 1989;413:599–603. doi: 10.1007/BF00581808. [DOI] [PubMed] [Google Scholar]
- 12.Dryer SE. Na+-activated K+ channels: a new family of large-conductance ion channels. Trends Neurosci. 1994;17:155–160. doi: 10.1016/0166-2236(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 13.Du J, Haak LL, Phillips-Tansey E, Russell JT, McBain CJ. Frequency-dependent regulation of rat hippocampal somato-dendritic excitability by the K+ channel subunit Kv2.1. J Physiol (Lond) 2000;522:19–31. doi: 10.1111/j.1469-7793.2000.t01-2-00019.xm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ficker E, Heinemann U. Slow and fast transient potassium currents in cultured rat hippocampal cells. J Physiol (Lond) 1992;445:431–455. doi: 10.1113/jphysiol.1992.sp018932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grissmer S, Nguyen AN, Aiyar J, Hanson DC, Mather RJ, Gutman GA, Karmilowicz MJ, Auperin DD, Chandy KG. Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1.1, 1.2, 1.3, 1.5, and 3.1, stably expressed in mammalian cell lines. Mol Pharmacol. 1994;45:1227–1234. [PubMed] [Google Scholar]
- 16.Gustafsson B, Galvan M, Grafe P, Wigstrom H. A transient outward current in a mammalian central neurone blocked by 4-aminopyridine. Nature. 1982;299:252–254. doi: 10.1038/299252a0. [DOI] [PubMed] [Google Scholar]
- 17.Hille B. Ion channels of excitable membranes, Ed 3. Sinauer; Sunderland, MA: 2001. [Google Scholar]
- 18.Hodgkin AL, Huxley AF. A quantitative description of membrane currents and its application to conduction and excitation in nerve. J Physiol (Lond) 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffman DA, Johnston D. Downregulation of transient K+ channels in dendrites of hippocampal CA1 pyramidal neurons by activation of PKA and PKC. J Neurosci. 1998;18:3521–3528. doi: 10.1523/JNEUROSCI.18-10-03521.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huguenard JR, McCormick DA. Simulation of the currents involved in rhythmic oscillations in thalamocortical relay neurons. J Neurophysiol. 1992;68:1373–1383. doi: 10.1152/jn.1992.68.4.1373. [DOI] [PubMed] [Google Scholar]
- 21.Huguenard JR, McCormick DA. Electrophysiology of the neuron. Oxford UP; New York: 1994. [Google Scholar]
- 22.Johnston D, Wu SM. Foundations of cellular neurophysiology. MIT; Cambridge, MA: 1995. [Google Scholar]
- 23.Kang J, Huguenard JR, Prince DA. Voltage-gated potassium channels activated during action potentials in layer V neocortical pyramidal neurons. J Neurophysiol. 2000;83:70–80. doi: 10.1152/jn.2000.83.1.70. [DOI] [PubMed] [Google Scholar]
- 24.Keros S, McBain CJ. Arachidonic acid inhibits transient potassium currents and broadens action potentials during electrographic seizures in hippocampal pyramidal and inhibitory interneurons. J Neurosci. 1997;17:3476–3487. doi: 10.1523/JNEUROSCI.17-10-03476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klee R, Ficker E, Heinemann U. Comparison of voltage-dependent potassium currents in rat pyramidal neurons acutely isolated from hippocampal regions CA1 and CA3. J Neurophysiol. 1995;74:1982–1995. doi: 10.1152/jn.1995.74.5.1982. [DOI] [PubMed] [Google Scholar]
- 26.Lancaster B, Adams PR. Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol. 1986;55:1268–1282. doi: 10.1152/jn.1986.55.6.1268. [DOI] [PubMed] [Google Scholar]
- 27.Lancaster B, Nicoll RA, Perkel DJ. Calcium activates two types of potassium channels in rat hippocampal neurons in culture. J Neurosci. 1991;11:23–30. doi: 10.1523/JNEUROSCI.11-01-00023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li XY, McArdle JJ. Novel transient outward K+ current of mature murine hippocampal neurones. Pflügers Arch. 1997;434:195–202. doi: 10.1007/s004240050383. [DOI] [PubMed] [Google Scholar]
- 29.Lien CC, Martina M, Schultz JH, Ehmke H, Jonas P. Gating, modulation and subunit composition of voltage-gated K(+) channels in dendritic inhibitory interneurones of rat hippocampus. J Physiol (Lond) 2002;538:405–419. doi: 10.1113/jphysiol.2001.013066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Llinás RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988;242:1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- 31.Llinás RR, Sugimori M, Simon SM. Transmission by presynaptic spike-like depolarization in the squid giant synapse. Proc Natl Acad Sci USA. 1982;79:2415–2419. doi: 10.1073/pnas.79.7.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Locke RE, Nerbonne JM. Three kinetically distinct Ca2+-inde-pendent depolarization-activated K+ currents in callosal-projecting rat visual cortical neurons. J Neurophysiol. 1997a;78:2309–2320. doi: 10.1152/jn.1997.78.5.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Locke RE, Nerbonne JM. Role of voltage-gated K+ currents in mediating the regular-spiking phenotype of callosal-projecting rat visual cortical neurons. J Neurophysiol. 1997b;78:2321–2335. doi: 10.1152/jn.1997.78.5.2321. [DOI] [PubMed] [Google Scholar]
- 34.London B, Guo W, Pan XH, Lee JS, Shusterman V, Rocco CJ, Logothetis DA, Nerbonne JA, Hill JA. Targeted replacement of KV1.5 in the mouse leads to loss of the 4-aminopyridine-sensitive component of I (K, slow) and resistance to drug-induced qt prolongation. Circ Res. 2001;88:940–946. doi: 10.1161/hh0901.090929. [DOI] [PubMed] [Google Scholar]
- 35.Luthi A, Gahwiler BH, Gerber U. A slowly inactivating potassium current in CA3 pyramidal cells of rat hippocampus in vitro. J Neurosci. 1996;16:586–594. doi: 10.1523/JNEUROSCI.16-02-00586.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maletic-Savatic M, Lenn NJ, Trimmer JS. Differential spatiotemporal expression of K+ channel polypeptides in rat hippocampal neurons developing in situ and in vitro. J Neurosci. 1995;15:3840–3851. doi: 10.1523/JNEUROSCI.15-05-03840.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malin SA, Nerbonne JM. Elimination of the fast transient in superior cervical ganglion neurons with expression of KV4.2W362F: molecular dissection of IA. J Neurosci. 2000;20:5191–5199. doi: 10.1523/JNEUROSCI.20-14-05191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malin SA, Nerbonne JM. Molecular heterogeneity of the voltage-gated fast transient outward K+ current, IAf, in mammalian neurons. J Neurosci. 2001;21:8004–8014. doi: 10.1523/JNEUROSCI.21-20-08004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martina M, Schultz JH, Ehmke H, Monyer H, Jonas P. Functional and molecular differences between voltage-gated K+ channels of fast-spiking interneurons and pyramidal neurons of rat hippocampus. J Neurosci. 1998;18:8111–8125. doi: 10.1523/JNEUROSCI.18-20-08111.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCormick DA. Functional properties of a slowly inactivating potassium current in guinea pig dorsal lateral geniculate relay neurons. J Neurophysiol. 1991;66:1176–1189. doi: 10.1152/jn.1991.66.4.1176. [DOI] [PubMed] [Google Scholar]
- 41.Mu J, Zhuang SY, Hampson RE, Deadwyler SA. Protein kinase-dependent phosphorylation and cannabinoid receptor modulation of potassium A current (IA) in cultured rat hippocampal neurons. Pflügers Arch. 2000;439:541–546. doi: 10.1007/s004249900231. [DOI] [PubMed] [Google Scholar]
- 42.Murakoshi H, Trimmer JS. Identification of the kv2.1 K+ channel as a major component of the delayed rectifier K+ current in rat hippocampal neurons. J Neurosci. 1999;19:1728–1735. doi: 10.1523/JNEUROSCI.19-05-01728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakajima Y, Nakajima S, Leonard RJ, Yamaguchi K. Acetylcholine raises excitability by inhibiting the fast transient potassium current in cultured hippocampal neurons. Proc Natl Acad Sci USA. 1986;83:3022–3026. doi: 10.1073/pnas.83.9.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- 45.Numann RE, Wadman WJ, Wong RK. Outward currents of single hippocampal cells obtained from the adult guinea-pig. J Physiol (Lond) 1987;393:331–353. doi: 10.1113/jphysiol.1987.sp016826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poolos NP, Johnston D. Calcium-activated potassium conductances contribute to action potential repolarization at the soma but not the dendrites of hippocampal CA1 pyramidal neurons. J Neurosci. 1999;19:5205–5212. doi: 10.1523/JNEUROSCI.19-13-05205.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raman IM, Bean BP. Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. J Neurosci. 1999;19:1663–1674. doi: 10.1523/JNEUROSCI.19-05-01663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rettig J, Heinemann SH, Wunder F, Lorra C, Parcej DN, Dolly JO, Pongs O. Inactivation properties of voltage-gated K+ channels altered by presence of beta-subunit. Nature. 1994;369:289–294. doi: 10.1038/369289a0. [DOI] [PubMed] [Google Scholar]
- 49.Rogawski MA. The A-current: how ubiquitous a feature of excitable cells is it? Trends Neurosci. 1985;8:214–219. [Google Scholar]
- 50.Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988;25:729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- 51.Rudy B, McBain CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 2001;24:517–526. doi: 10.1016/s0166-2236(00)01892-0. [DOI] [PubMed] [Google Scholar]
- 52.Sah P, Gibb AJ, Gage PW. Potassium current activated by depolarization of dissociated neurons from adult guinea pig hippocampus. J Gen Physiol. 1988;92:263–278. doi: 10.1085/jgp.92.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Segal M, Barker JL. Rat hippocampal neurons in culture: potassium conductances. J Neurophysiol. 1984;51:1409–1433. doi: 10.1152/jn.1984.51.6.1409. [DOI] [PubMed] [Google Scholar]
- 54.Segal M, Rogawski MA, Barker JL. A transient potassium conductance regulates the excitability of cultured hippocampal and spinal neurons. J Neurosci. 1984;4:604–609. doi: 10.1523/JNEUROSCI.04-02-00604.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Serodio P, Rudy B. Differential expression of Kv4 K+ channel subunits mediating subthreshold transient K+ (A-type) currents in rat brain. J Neurophysiol. 1998;79:1081–1091. doi: 10.1152/jn.1998.79.2.1081. [DOI] [PubMed] [Google Scholar]
- 56.Shao LR, Halvorsrud R, Borg-Graham L, Storm JF. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J Physiol (Lond) 1999;521:135–146. doi: 10.1111/j.1469-7793.1999.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi G, Kleinklaus AK, Marrion NV, Trimmer JS. Properties of Kv2.1 K+ channels expressed in transfected mammalian cells. J Biol Chem. 1994;269:23204–23211. [PubMed] [Google Scholar]
- 58.Shibata R, Nakahira K, Shibasaki K, Wakazono Y, Imoto K, Ikenaka K. A-type K+ current mediated by the Kv4 channel regulates the generation of action potential in developing cerebellar granule cells. J Neurosci. 2000;20:4145–4155. doi: 10.1523/JNEUROSCI.20-11-04145.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sodickson DL, Bean BP. GABAB receptor-activated inwardly rectifying potassium current in dissociated hippocampal CA3 neurons. J Neurosci. 1996;16:6374–6385. doi: 10.1523/JNEUROSCI.16-20-06374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song WJ, Tkatch T, Baranauskas G, Ichinohe N, Kitai ST, Surmeier DJ. Somatodendritic depolarization-activated potassium currents in rat neostriatal cholinergic interneurons are predominantly of the A type and attributable to coexpression of Kv4.2 and Kv4.1 subunits. J Neurosci. 1998;18:3124–3137. doi: 10.1523/JNEUROSCI.18-09-03124.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Storm JF. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol (Lond) 1987;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Storm JF. Temporal integration by a slowly inactivating K+ current in hippocampal neurons. Nature. 1988;336:379–381. doi: 10.1038/336379a0. [DOI] [PubMed] [Google Scholar]
- 63.Storm JF. Potassium currents in hippocampal pyramidal cells. Prog Brain Res. 1990;83:161–187. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- 64.Surmeier DJ, Stefani A, Foehring RC, Kitai ST. Developmental regulation of a slowly-inactivating potassium conductance in rat neostriatal neurons. Neurosci Lett. 1991;122:41–46. doi: 10.1016/0304-3940(91)90188-y. [DOI] [PubMed] [Google Scholar]
- 65.Tkatch T, Baranauskas G, Surmeier DJ. Kv4.2 mRNA abundance and A-type K+ current amplitude are linearly related in basal ganglia and basal forebrain neurons. J Neurosci. 2000;20:579–588. doi: 10.1523/JNEUROSCI.20-02-00579.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weiser M, Bueno E, Sekirnjak C, Martone ME, Baker H, Hillman D, Chen S, Thornhill W, Ellisman M, Rudy B. The potassium channel subunit KV3.1b is localized to somatic and axonal membranes of specific populations of CNS neurons. J Neurosci. 1995;15:4298–4314. doi: 10.1523/JNEUROSCI.15-06-04298.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu RL, Barish ME. Two pharmacologically and kinetically distinct transient potassium currents in cultured embryonic mouse hippocampal neurons. J Neurosci. 1992;12:2235–2246. doi: 10.1523/JNEUROSCI.12-06-02235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu RL, Barish ME. Modulation of a slowly inactivating potassium current, ID, by metabotropic glutamate receptor activation in cultured hippocampal pyramidal neurons. J Neurosci. 1999;19:6825–6837. doi: 10.1523/JNEUROSCI.19-16-06825.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zaza A, Micheletti M, Brioschi A, Rocchetti M. Ionic currents during sustained pacemaker activity in rabbit sino-atrial myocytes. J Physiol (Lond) 1997;505:677–688. doi: 10.1111/j.1469-7793.1997.677ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zbicz KL, Weight FF. Transient voltage and calcium-dependent outward currents in hippocampal CA3 pyramidal neurons. J Neurophysiol. 1985;53:1038–1058. doi: 10.1152/jn.1985.53.4.1038. [DOI] [PubMed] [Google Scholar]