Abstract
Injury to peripheral nerve initiates a degenerative process that converts the denervated nerve from a suppressive environment to one that promotes axonal regeneration. We investigated the role of matrix metalloproteinases (MMPs) in this degenerative process and whether effective predegenerated nerve grafts could be producedin vitro. Rat peripheral nerve explants were cultured for 1–7 d in various media, and their neurite-promoting activity was assessed by cryoculture assay, in which neurons are grown directly on nerve sections. The neurite-promoting activity of cultured nerves increased rapidly and, compared with uncultured nerve, a maximum increase of 72% resulted by 2 d of culture in the presence of serum. Remarkably, the neurite-promoting activity of short-term cultured nerves was also significantly better than nerves degeneratedin vivo. We examined whether in vitrodegeneration is MMP dependent and found that the MMP inhibitorN-[(2R)-2(hydroxamidocarbonylmethyl)-4-methylpantanoyl]-l-tryptophan methylamide primarily blocked the degenerative increase in neurite-promoting activity. In the absence of hematogenic macrophages, MMP-9 was trivial, whereas elevated MMP-2 expression and activation paralleled the increase in neurite-promoting activity. MMP-2 immunoreactivity localized to Schwann cells and the endoneurium and colocalized with gelatinolytic activity as demonstrated by in situ zymography. Finally,in vitro predegenerated nerves were tested as acellular grafts and, compared with normal acellular nerve grafts, axonal ingressin vivo was approximately doubled. We conclude that Schwann cell expression of MMP-2 plays a principal role in the degenerative process that enhances the regeneration-promoting properties of denervated nerve. Combined with their low immunogenicity, acellular nerve grafts activated by in vitropredegeneration may be a significant advancement for clinical nerve allografting.
Keywords: Wallerian degeneration, acellular nerve graft, MMP-2, Schwann cell, cryoculture, basal lamina, metalloproteinase, chondroitin sulfate proteoglycan, axon regeneration, rat sciatic nerve
Injury to peripheral nerve is followed by Wallerian degeneration in the denervated nerve, which involves the degradation and phagocytosis of axons and myelin sheaths, proliferation by Schwann cells, and the remodeling of endoneurial basal laminas (Stoll and Muller, 1999). The success of nerve regeneration depends on axons accessing the laminin-rich basal lamina scaffolding of the degenerating nerve stump (Salonen et al., 1987). Schwann cells play an important role in nerve degeneration and are essential for sustained regeneration (Fu and Gordon, 1997). Recruited hematogenic macrophages also play a pivotal role in these processes. Although some debate remains about the functional abilities and contributions of Schwann cells and macrophages in nerve regeneration, most evidence indicates that macrophages play a primary role in nerve degeneration and myelin removal, yet some findings suggest they are not essential for the remodeling that supports axonal growth (Perry and Brown, 1992; Reichert et al., 1994; Fernandez-Valle et al., 1995; Dailey et al., 1998).
Nerve degeneration and regeneration after injury involves extensive remodeling of the extracellular matrix (ECM) that depends on the release of proteolytic enzymes by neurons, Schwann cells, and invading macrophages. One important class of enzymes involved are the matrix metalloproteinases (MMPs), a family of proteinases known to degrade ECM molecules including collagen, fibronectin, laminin, and a variety of proteoglycans (Yong et al., 1998). Chondroitin sulfate proteoglycans (CSPGs) are abundant in peripheral nerve and inhibit the neurite-promoting activity of laminin (Zuo et al., 1998a). MMP-2 and MMP-9 can degrade inhibitory CSPGs and deinhibit the neurite-promoting activity of laminin in the endoneurial basal lamina (Zuo et al., 1998b; Ferguson and Muir, 2000). MMP-2 and MMP-9 are upregulated after nerve injury (La Fleur et al., 1996; Kherif et al., 1998; Ferguson and Muir, 2000) and thus may represent a degenerative mechanism that promotes nerve regeneration by the degradation of inhibitory CSPGs.
Understanding the molecular mechanisms that govern nerve degeneration applies directly to the efforts to improve nerve regeneration and to the development of bioactive nerve grafts. Numerous studies have examined the potential of allogenic nerve grafting (Bain, 1998). However, because of concern about host–graft immunorejection, the application of live allogenic nerve grafts is presently viewed as a developing technology. This concern is greatly reduced with acellular nerve grafts, but freeze-killed nerves are less effective in supporting regeneration (Gulati, 1988; Evans et al., 1994, 1998). The absence of viable cells greatly delays the nerve degeneration and remodeling that promotes the regenerative process. This deficit can be overcome by predegeneration of nerves in vivo, which reduces the initial delay of axon penetration and enhances regeneration into acellular nerves (Osawa et al., 1990; Ochi et al., 1994; Danielsen et al., 1995). Nonetheless, in vivo predegeneration of nerve is not feasible for clinical allografting.
In this study, we used nerve explant cultures to examine the role of MMPs in the degenerative process in the absence of hematogenic macrophages. Cultured nerves were also tested for their ability to support nerve regeneration in vivo to determine whether effective predegenerated acellular nerve allografts can be producedin vitro.
MATERIALS AND METHODS
Nerve explant culture. Adult (180–200 gm) female Sprague Dawley rats (Harlan, Indianapolis, IN) were used as nerve donors and graft recipients. This project was reviewed and approved by the Institutional Animal Care and Use Committee. Donor rats were deeply anesthetized with isofluorane and decapitated. Sciatic nerves were exposed through a gluteal muscle-splitting incision and isolated free of underlying fascia. A 15 mm nerve segment was excised rostral to the bifurcation into common peroneal and tibial nerves. The segments were rinsed with sterile Ringer's solution and stabilized by pinning the ends to a thin plastic support. The nerve explants were cultured for 1, 2, 4, and 7 d in DMEM containing N2 supplements (DMEM/N2) or DMEM/N2 supplemented with 2 or 10% fetal bovine serum (FBS) (Atlanta Biologicals, Atlanta, GA). As specified, some explants were cultured in the presence of the MMP inhibitorN-[(2R)-2(hydroxamidocarbonylmethyl)-4-methylpantanoyl]-l-tryptophan methylamide (GM6001) (50 μm) (Galardy et al., 1994). The cultured nerves were washed thoroughly in DMEM and then transferred to sealed tubes. The tubes were immersed in liquid nitrogen for 2 min and then thawed in a 37°C water bath for 5 min. This freeze–thaw cycle was repeated twice, yielding freeze-killed (acellular) nerve segments. Freshly excised nerves (uncultured controls) were freeze-killed using the same procedure. The acellular nerve segments were then (1) embedded for cryosectioning for use in cryoculture assays or (2) stored in liquid nitrogen (for up to 2 weeks) for biochemical analysis and for use as interpositional nerve grafts. Nerve explants prepared for histological examinations were fixed with aldehydes, and freeze-killing was omitted.
Nerve degeneration in vivo was accomplished by a single transection of the sciatic nerve near the pelvis. The proximal stump was displaced and ligated to preclude axonal growth. The leg muscles and skin were closed, and the transected nerve was allowed to degenerate in situ for 2 or 7 d.
Cryoculture bioassay. Cryoculture is a neurite outgrowth assay in which neurons are cultured directly on fresh-frozen nerve sections (Carbonetto et al., 1987). Briefly, nerve segments were sectioned on the longitudinal axis at 20 μm thickness, mounted on 3-aminopropyl triethoxysilane-coated coverslips, and stored at −20°C until use. Purified cultures of dissociated dorsal root ganglionic (DRG) neurons from day 8 chick embryos were seeded directly on the nerve sections in DMEM/N2 containing 1% heat-treated albumin and 10 ng/ml nerve growth factor. Cryoculture assays were terminated after 24 hr of growth by fixation with 100% methanol. DRG neurons were selectively labeled by GAP-43 immunofluorescence (see below). Epifluorescent photomicrographs were acquired using a SPOT digital camera system (Diagnostic Instruments, Sterling Heights, MI) and Axioskop II microscope (Carl Zeiss, Thornwood, NY). Individual neurite lengths were measured directly using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD). Only neurons with neurites longer than one cell body diameter (≈15 μm) were included in the analysis. More than 250 neurons were scored in each condition.
Gel zymography. Nerve segments were placed in ice-cold extraction buffer (50 mm Tris-HCl, pH 7.6, containing 1% Triton X-100, 200 mm NaCl, and 10 mm CaCl2) and homogenized by probe sonication (15 sec). The samples were agitated for 30 min at 4°C, and the soluble fraction was collected by centrifugation (12,000 × g for 20 min). The total protein content of the soluble fractions was determined using the Bradford Reagent (Bio-Rad Laboratories, Hercules, CA). Bovine serum albumin dissolved in extraction buffer was used as a protein standard. The extracts were solubilized in nonreducing Laemmli sample buffer without heating and electrophoresed at 4°C on 10% SDS-polyacrylamide gels containing 1.5 mg/ml porcine gelatin. The gels were briefly rinsed in water and then washed in 2.5% Triton X-100 three times over 45 min. The Triton X-100 was removed with three 5 min water washes, and the zymographic gels were developed for 21 hr at 37°C in incubation buffer (50 mm Tris-HCl, pH 8.0, 5 mmCaCl2, 0.02% sodium azide). Gels were fixed and stained with 0.05% Coomassie brilliant blue. Protein bands with gelatinolytic activity appeared as a clear lysis zones within the blue background of the gelatin gel. Comigration of gelatinolytic bands was compared with latent and activated forms of recombinant human MMP-2 and MMP-9, as well as prestained molecular weight standards (Bio-Rad). Digital photomicrographs were acquired, and densitometry of gelatinolytic bands was performed using Image-Pro Plus software.
In situ zymography. Cryosections (10 μm) of unfixed normal and cultured nerves were mounted on slides and overlaid with reaction buffer (in mm: 50 Tris-HCl, 150 NaCl, 5 CaCl2, and 0.2 sodium azide, pH 7.6) containing 20 μg/ml intramolecularly quenched, fluorescein-labeled gelatin substrate (Molecular Probes Inc., Eugene, OR) (Oh et al., 1999). After incubation for 24 hr at 37°C, the sections were rinsed with PBS and fixed with 4% paraformaldehyde in phosphate buffer. The sections were rinsed with water and mounted using Citifluor. Fluorescein–gelatin peptides generated by gelatinolytic activity in the tissue sections were observed and photographed by epifluorescence microscopy.
Interpositional nerve grafting. Six rats were given bilateral acellular nerve grafts, one normal (uncultured) and one predegenerated in vitro (cultured for 2 d in 2% serum). Host rats were deeply anesthetized using xylazine (15 mg/kg, i.m.) and ketamine (110 mg/kg, i.p.). The sciatic nerve was exposed and supported by a plastic insert placed between the nerve and underlying tissue. The region of the nerve halfway between the sciatic notch and bifurcation was first coated with fibrin glue. A 2.5 mm segment of the host nerve was excised using serrated scissors. The graft was thawed and freshly trimmed to 10 mm with a scalpel blade. The graft was coapted to the host nerve stumps by epineurial neurorrhaphy using one 9-0 Ethilon suture at each end. Fibrin glue was then applied to stabilize the coaptations that, in combination with the initial fibrin coating applied to the host nerve, reduced protrusion of nerve elements (endoneurial mushrooming) (Menovsky and Bartels, 1999). The muscle was closed with 4-0 sutures, and the skin was closed with wound clips. After recovery from the anesthetic, animals were returned to standard housing. At 8 d after grafting, the host rats were deeply anesthetized and decapitated. The graft and 3 mm of proximal and distal host nerve were removed and immersed in 4% paraformaldehyde in 0.1m phosphate buffer, pH 7.4, overnight at 4°C. The specimens were equilibrated with PBS and immersed in 30% sucrose in phosphate buffer for 2 d at 4°C. The specimens were embedded and cryosectioned on the transverse plane in a recorded measure. Regenerating axons within the grafts were labeled by GAP-43 immunofluorescence (see below). Epifluorescent photomicrographs were acquired, and GAP-43-positive axon profiles (pixel counts) were scored using Image-Pro Plus software.
Immunofluorescent labeling. Fixed tissue sections were treated with 0.5% Triton X-100 in PBS for 10 min. Nonspecific antibody binding was blocked by pretreatment with PBS containing 0.1% Triton X-100 and 10% normal serum (blocking buffer). Primary antibodies were diluted in blocking buffer and applied overnight at 4°C. Bound primary antibodies were labeled with swine anti-rabbit Igs (Dako, Carpinteria, CA) or goat anti-mouse Igs (Sigma, St. Louis, MO) conjugated with fluorescein or rhodamine for 1 hr at room temperature in darkness. The anti-mouse secondary antibody was preadsorbed with rat serum before use. Neurite length (cryoculture) and axonal regeneration (grafting) were assessed by immunolabeling with polyclonal anti-GAP-43 IgG (2 μg/ml) (Ferguson and Muir, 2000) (NB300-143; Novus Biological, Littleton, CO). Other primary antibodies included: polyclonal anti-MMP-2 IgG (4 μg/ml) (MMP-2/475) (Muir, 1995); polyclonal anti-MMP-9 IgG (4 μg/ml) (AB19047; Chemicon, Temecula, CA); polyclonal anti-S-100 antiserum (1:500; Dako); polyclonal OX42 antiserum (1:500; Serotek, Raleigh, NC); and monoclonal anti-neurofilament IgG (4 μg/ml) (NAP4) (Harris et al., 1993). In some instances, epifluorescent photomicrographs were inverted and contrast-enhanced for printing in Photoshop (Adobe Systems, San Jose, CA).
Statistics. Significance was analyzed using Student'st test. Data are presented as means ± SEM.
RESULTS
The neurite-promoting activity of cultured nerve segments
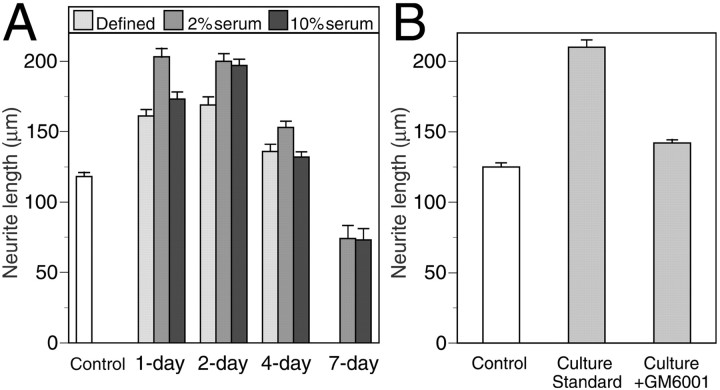
Freshly excised (cellular) rat sciatic nerve segments were cultured for up to 7 d in medium containing 0, 2, and 10% FBS. Control (uncultured) and cultured nerves were cryosectioned, and their neurite-promoting activity was assessed by cryoculture assay. Results are shown in Figure 1A. Embryonic chick DRG neurons grown on sections of control nerves had an average neurite length of 118 μm. Neuritic growth on sections of nerve explants cultured for 1–4 d was significantly greater than that in the control condition (p < 0.001). For nerves cultured in defined medium (0% serum), neurite-promoting activity reached a maximum at 2 d in vitro, representing a 43% increase compared with control nerves. There was more than a 70% increase in the neurite-promoting activity for nerve explants cultured for 1 or 2 d in medium containing 2% serum. Nerve explants cultured in 10% serum reached a similar maximum at 2 d in vitro as well. The neurite-promoting activity of nerve explants declined after longer culture periods and fell below the level of the control condition at 7 d. These data indicate that the neurite-promoting activity of nerve explants increased markedly when cultured for short periods in vitro with and without the addition of serum to the culture medium. Nerve explants were prevented from adhering to the culture vessel, and no cell outgrowth was observed. However, cell viability in all conditions was confirmed in separate experiments in which robust cell migration was observed from nerve explants that were minced and pressed to the culture surface.
Fig. 1.
Cryoculture assay of nerve explant cultures.A, Freshly excised rat sciatic nerve explants were cultured for 1, 2, 4, and 7 d in DMEM/N2 containing 0, 2, or 10% FBS. B, Nerve explants were cultured for 2 d in DMEM/N2 containing 2% serum (culture standard) with and without the addition of GM6001 (MMP inhibitor). The nerves were then cryosectioned, and embryonic DRG neurons were seeded onto the tissue sections in DMEM/N2 containing NGF. After 24 hr, DRG neurons were immunostained for GAP-43, and neuritic growth was measured by digital photomicroscopy and image analysis. The control condition was normal nerve (0 d in culture). Data represent the mean ± SEM neurite lengths of >250 neurons scored in each condition from at least four separate nerve explant cultures tested in two or more separate experiments.
Comparison of in vitro and in vivo predegeneration
Several laboratories, including our own, have found that peripheral nerves predegenerated in vivo are more capable of supporting neurite growth than normal nerve in cryoculture assays (Bedi et al., 1992; Agius and Cochard, 1998; Ferguson and Muir, 2000). Here, using the cryoculture assay, we compared the neurite-promoting activity of rat sciatic nerves predegenerated in vitro to those predegenerated in vivo. As described above (Fig. 1), neuritic growth of DRG neurons on nerve explants cultured for 2 d in 2% serum (in vitro predegeneration) was 70% greater than control nerves (not predegenerated). Also, nerve explant culture for longer periods (4 and 7 d) resulted in progressively less neurite-promoting activity. Nerves cultured for 7 d had 37% less activity than the control condition. In comparison, the neurite-promoting activity of nerves predegenerated in vivowas much lower that that seen for nerves predegenerated in vitro. Neuritic growth on nerves predegenerated in vivofor 2 d was 35.8 μm, which corresponds to 72% less activity than the control condition (126.5 μm) (p < 0.001). However, this inhibition was reversed over time, and in vivo predegeneration for 7 d resulted in neuritic growth that was 12% greater than the control condition (p = 0.06). These data show that in vitro predegeneration increased the neurite-promoting activity of nerve segments to a greater extent than that achieved by in vivo predegeneration. Although longer time points were not included in the present study, other cryoculture and grafting studies indicate that the maximal positive effects of in vivo predegeneration are achieved by 7–8 d (Bedi et al., 1992; Danielsen et al., 1994; Ochi et al., 1994;Keilhoff et al., 1999).
In vitro degeneration is MMP dependent
Our previous studies indicate a role for MMPs in the degenerative process (Zuo et al., 1998b; Ferguson and Muir, 2000). In the in vitro nerve degeneration model, which excludes the contribution of hematogenic macrophages, we tested the hypothesis that the elevation of neurite-promoting activity observed for cultured nerve explants was dependent on MMP activity. Nerve segments were cultured for 2 d in medium containing 2% serum with and without the addition of the MMP inhibitor GM6001. The neurite-promoting activity of the cultured nerves was assessed by cryoculture assay. Results are shown in Figure1B. Similar to that shown in Figure1A, the mean neurite length of DRG neurons grown on cultured nerves (2 d; 2% serum) was 210 μm, representing a 68% increase over that of (uncultured) control nerves (p < 0.001). However, this increase was reduced to only 14% for nerves cultured in the presence of GM6001 (p < 0.001). Dissociation culture (squash preparations) of the nerve segments in each condition showed profuse cell outgrowth, indicating no loss of cell viability. In addition, treatment of isolated Schwann cell cultures with GM6001 confirmed the very low toxicity of this hydroxamate-based dipeptide (data not shown). We have reported previously that GM6001 is not toxic to DRG neurons (Zuo et al., 1998b). At the concentration used in these experiments (50 μm), GM6001 is a potent inhibitor of the gelatinases (MMP-2 and MMP-9) as well as many other MMPs (Galardy et al., 1994). These results strongly implicate MMP activity in a degenerative process that increases the neurite-promoting activity of cultured nerve explants.
MMP expression in the cultured nerve segments: zymographic gel analysis
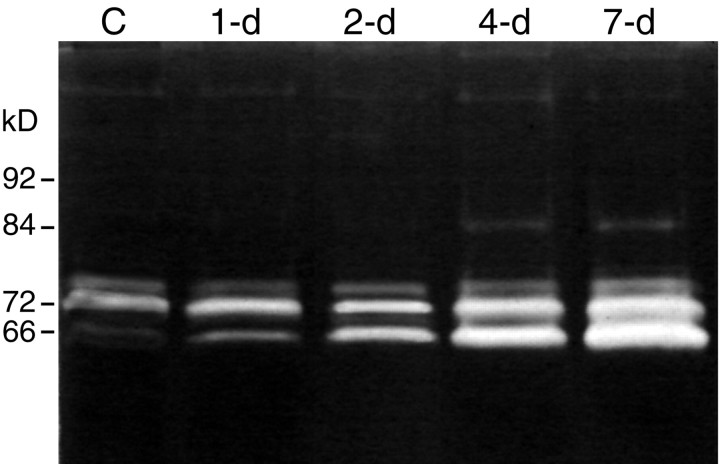
MMP-2 and MMP-9 are the main extracellular proteinases capable of degrading gelatin (cleaved collagen), and their major substrate is collagen type IV of the basal lamina. MMP-2 is constitutively expressed by Schwann cells in vivo and is upregulated after nerve injury in the rat. In contrast, MMP-9 is undetectable in normal nerve and is present after injury in association with invading granulocytes and macrophages (Yamada et al., 1995; La Fleur et al., 1996; Kherif et al., 1998; Ferguson and Muir, 2000). Our examination of in vitro nerve degeneration provides a unique opportunity to determine the role of MMP expression by resident nerve cells with a minimal contribution by hematogenic cells. MMP levels in cultured nerve explants were first examined by gelatin substrate-overlay gel electrophoresis (zymography). Gelatin zymography is very sensitive in the detection of MMP-2 and MMP-9 and has the added advantage of revealing both latent and activated forms. Nerve segments were cultured for 1, 2, 4, and 7 d in the presence of 2% serum. A representative zymographic analysis of extracted nerves is shown in Figure 2. Normal (uncultured control) nerve showed a predominant gelatinolytic band atMr = 72 kDa that comigrated with the proform of human recombinant MMP-2. A trace of activated MMP-2 was observed (Mr = 66 kDa), whereas MMP-9 (Mr = 92 and 84 kDa) was not detected. In the cultured nerves, there was a rapid increase in activated MMP-2 and a substantial increase in total MMP-2 content. MMP-9 was undetectable in nerves cultured for 1 or 2 d, and only trace amounts of activated MMP-9 were found in the 4 and 7 d samples. Similar results were obtained for nerve explants cultured in defined medium (data not shown), confirming that serum did not contribute to the gelatinolytic activity observed in the nerve samples. These findings indicated that MMP-2 is rapidly activated and upregulated in nerve degeneration in vitro. It is notable that gelatin zymography is several-fold more sensitive in detecting MMP-9 than MMP-2 (Ladwig et al., 2002), signifying that the MMP-9 content in the nerve samples was negligible. The identification of gelatinolytic bands as MMP-2 and MMP-9 in nerve was confirmed previously by Western immunoblotting (Ferguson and Muir, 2000).
Fig. 2.
Zymographic analysis of nerve explant cultures. Nerve explants were cultured for 0 (C, control), 1, 2, 4, and 7 d in DMEM/N2 containing 2% serum. The nerves were then extracted and analyzed by gelatin-overlay electrophoresis. Zymography reveals both proform and activated gelatinases that appear as clear bands within the stained gel. Control nerve contained predominantly pro-MMP-2 and trace amounts of activated MMP-2. There was a progressive increase in MMP-2 content and a rapid conversion to the activated form within the nerve explants cultured for ≥2 d. MMP-9 was negligible in the control and early explants, whereas a trace amount was detected at 4 and 7 d. The molecular masses indicate the positions of recombinant human pro-MMP-9 (92 kDa), activated MMP-9 (84 kDa), pro-MMP-2 (72 kDa), and activated MMP-2 (66 kDa).
MMP activity in the cultured nerve segments: in situ zymography
The activity of MMPs is regulated by gene transcription, by proenzyme activation, and by the action of tissue inhibitors of metalloproteinases (TIMPs). We examined the net gelatinolytic activity in nerve segments by in situ zymography. Tissue sections were overlaid with quenched, fluorescein–gelatin, which is converted to fluorescent peptides by gelatinolytic activity within tissues. Constitutive gelatinolytic activity was detected in normal nerve primarily associated with Schwann cells aligned along the endoneurial basal lamina (Fig. 3A,B). In cultured nerves, there was a widespread increase in gelatinolytic activity that was diffuse within the endoneurium, and Schwann cells were labeled more intensively (Fig. 3C,D). We also examined the gelatinolytic activity in the nerve explants cultured in the presence of GM6001. As described above, GM6001 blocked the increases in the neurite-promoting activity achieved by in vitrodegeneration. Gelatinolytic activity in GM6001-treated nerve explants was greatly reduced (Fig. 3E,F). Together these findings indicate that gelatinolytic activity was markedly increased by nerve explant culture, and that GM6001 effectively blocked de novo MMP activity during in vitro degeneration.
Fig. 3.
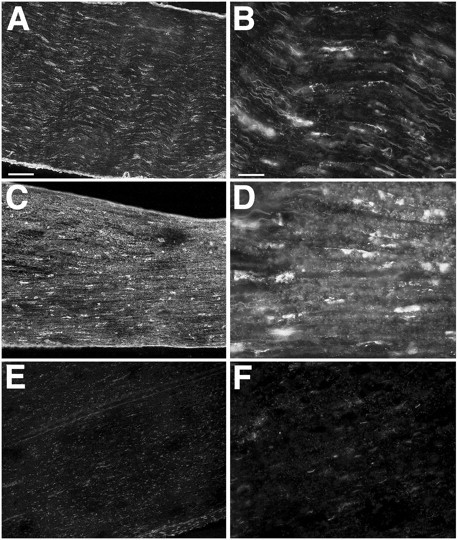
Localization of net gelatinolytic activity in nerve segments by in situ zymography. Tissue sections of control nerve (A, B) and cultured nerve explants (2 d; 2% serum) (C, D) were overlaid with quenched, fluorescein-labeled gelatin, which is converted to fluorescent peptides by gelatinolytic activity within tissues. Constitutive gelatinolytic activity was detected in normal nerve, (A) which, at higher magnification (B), was associated with Schwann cells. Gelatinolytic activity was more intense and diffuse throughout the endoneurium in the cultured nerves (C, D). Gelatinolytic activity in nerves cultured in the presence of GM6001 was markedly decreased (E, F). All epifluorescent images were obtained using the same exposure parameters, and image enhancements were applied equally. Scale bars: (inA) A, C, E, 100 μm; (inB) B, D, F, 25 μm.
MMP localization in the cultured nerve segments: immunofluorescent labeling
We confirmed previous findings by Kherif et al. (1998) that immunolabeling for MMP-2 in normal nerve is localized in Schwann cells, whereas MMP-9 is undetectable (data not shown). We also examined the distributions of MMP-2 and MMP-9 in nerve explants cultured for 2 d by immunofluorescence microscopy. MMP-2 immunolabeling of culture nerves was intense within Schwann cells and the surrounding basal laminas (Fig. 4A). Schwann cell staining with S-100 indicated that the most intense MMP-2 immunolabeling was associated with migrating Schwann cells (Fig.4B; and see below). Also, MMP-2 immunoexpression was very similar to the pattern of gelatinolytic activity obtained byin situ zymography. In contrast, MMP-9 immunolabeling was virtually absent within the nerve fascicles, except for rare cellular profiles. Some cellular immunoexpression of MMP-9 was seen in the surrounding epineurium (Fig. 4C). OX42 labeling was used to identify macrophages that were scattered throughout the epineurium and rarely within the nerve fascicles of cultured nerves (Fig.4D). The compartmental distributions of MMP-9 and OX42 labeling suggested that macrophages were the main source of MMP-9. In addition, Schwann cells, and perhaps perineurial fibroblasts, expressed MMP-2, and MMP-2 immunoreactivity was also observed diffusely in the surrounding ECM.
Fig. 4.
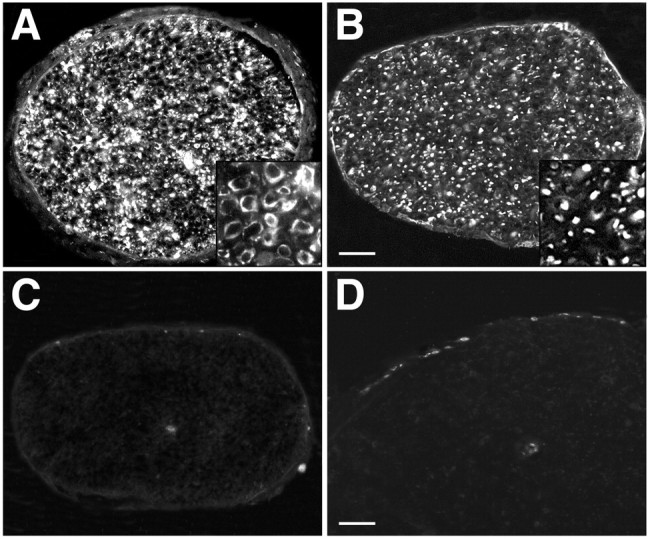
Immunoexpression of MMP-2 and MMP-9 in cultured nerve explants. A, MMP-2 immunolabeling of culture nerves (2 d; 2% serum) was intense within Schwann cells and the surrounding basal laminas (inset). B, S-100 immunolabeling shows the repositioning of an expanded population of Schwann cells within the nerve. C, MMP-9 immunolabeling was virtually absent within the nerve fascicles, except for a rare cellular profile. Some cells in the surrounding epineurium were labeled for MMP-9. D, OX42 labeling shows macrophages scattered throughout the epineurium and rarely within the nerve fascicles of cultured nerves. Scale bars: (in B)A–C, 100 μm; D, 50 μm.Insets in A and B are magnified 4×.
Cell distributions and axonal degeneration in the cultured nerve segments
After nerve injury, Schwann cells become activated, dissociate their myelin, and migrate extensively. S-100 immunolabeling of the cultured nerve explants showed that many Schwann cells had lost their elongated morphology and close association with axons, typical of the injury response (Fig. 4B). As expected when disconnected from the circulatory system, the number of macrophages in the nerve explants was much lower than that observed in nerve degeneration in vivo. Moreover, very few macrophages were found within the nerve fascicles, and nearly all OX42-labeled cells were confined to the epineurium (Fig. 4D). It was clear that the macrophages present in the epineurial compartment at the time of nerve excision did not invade the inner nerve compartments during culture. Accordingly, the nerve explants in vitrorepresent a model of nerve degeneration in which the contribution of Schwann cells may be assessed independently from those of invading macrophages.
The degradation of axons was examined in cultured nerve explants by immunolabeling of neurofilaments. Results are shown in Figure5. Unlike the contiguous neurofilament staining observed in normal nerve (Fig. 5A), the neurofilament profiles in nerve segments cultured for 2 d were fragmented and irregular (Fig. 5B). Similar to axonal degeneration in vivo, the cultured nerves contained both annular and condensed neurofilament profiles, indicative of cytoskeleton disintegration and axonal degeneration. The degeneration of axons was especially obvious in semithin sections stained with toluidine blue that showed a void or a dense pellet within the residual myelin sheaths (Fig. 5D). The degenerative changes observed in the nerves cultured for 2 d were reminiscent of the initial phase of Wallerian degeneration seen in vivo (for review, see Stoll and Muller, 1999). The main features of the secondary phase of Wallerian degeneration were also observed in cultured nerves, including morphologic changes in the myelin sheath and myelin extrusion by Schwann cells, as well as Schwann cell proliferation (Fig.5D). However, the degenerative processes resulting in additional myelin degeneration (collapse and condensation) and phagocytotic removal did not occur in the 2 d nerve explant cultures. Despite the substantial degenerative alterations, the basal lamina scaffold remained structurally intact, and remodeling was indicated by the high level of laminin expression by Schwann cells (Fig. 5C).
Fig. 5.
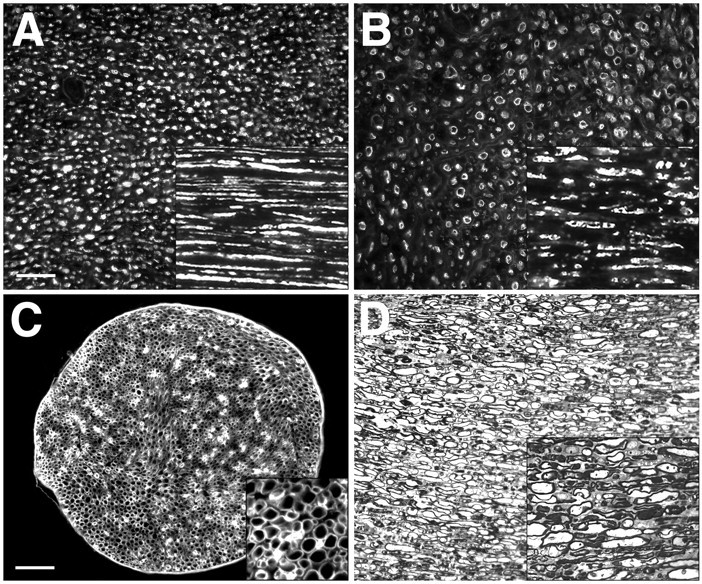
Wallerian degeneration in cultured nerve explants. The degenerative changes observed in the nerve segments cultured for 2 d were reminiscent of the initial phases of Wallerian degeneration seen in vivo. A, Neurofilament immunolabeling shows the compact and contiguous formation of axons in normal nerve compared with the annular and fragmented axons found in cultured nerve explants (2 d; 2% serum) (B). Insets in Aand B are longitudinal sections (same scale).C, Immunolabeling for laminin in cultured nerve (2 d; 2% serum) indicates that basal laminas are structurally intact and that laminin expression is upregulated in Schwann cells (inset). D, The degeneration of axons and the extrusion of myelin by Schwann cells was especially evident in semithin sections stained with toluidine blue. Degenerative processes resulting in additional myelin degeneration (collapse and condensation) and phagocytotic removal were not observed in the 2 d cultured nerve segments (D, inset). Scale bars: (in A) A, B, D, 25 μm;C, 100 μm. Insets in Cand D are magnified 4×.
Cultured nerve as acellular interpositional grafts
Previous studies show that peripheral nerves predegeneratedin vivo are better acellular nerve grafts than are normal nerves. We tested the hypothesis that predegeneration in vitro improves nerve regeneration through acellular nerve allografts. Host rats received bilateral, acellular nerve grafts, one control (not predegenerated) and one predegenerated in vitro(cultured for 2 d in 2% serum). Axonal regeneration was assessed after 8 d by scoring GAP-43-immunopositive profiles (total pixel count) in transverse sections. Axonal growth was observed in all grafts and was centrally distributed, indicating good alignment and coaptation of proximal host nerve and graft (Fig.6). In six of six animals, the number of axons (inferred by GAP-43-immunopositive pixels) that crossed the proximal nerve–graft coaptation and entered the graft was greater in the in vitro predegenerated graft than in the contralateral control graft. On average the score of axons within the in vitro predegenerated grafts was twofold greater, and there was a significant difference in the mean axon scores at each distance within the grafts (p < 0.05) (Fig.6B). This indicates that in vitropredegenerated grafts improved regeneration by decreasing the initial delay of axonal growth. Moreover, the greatest difference was found in the number of axons observed in the initial 1–2 mm of the grafts (p < 0.01), suggesting that the relative rate of axonal ingress into the in vitro predegenerated grafts continued to increase throughout the 8 d period. In both graft conditions, axonal growth occurred within basal lamina tubes and was accompanied by host derived Schwann cells. These findings show that axonal regeneration into acellular nerve grafts is enhanced and accelerated by in vitro predegeneration.
Fig. 6.
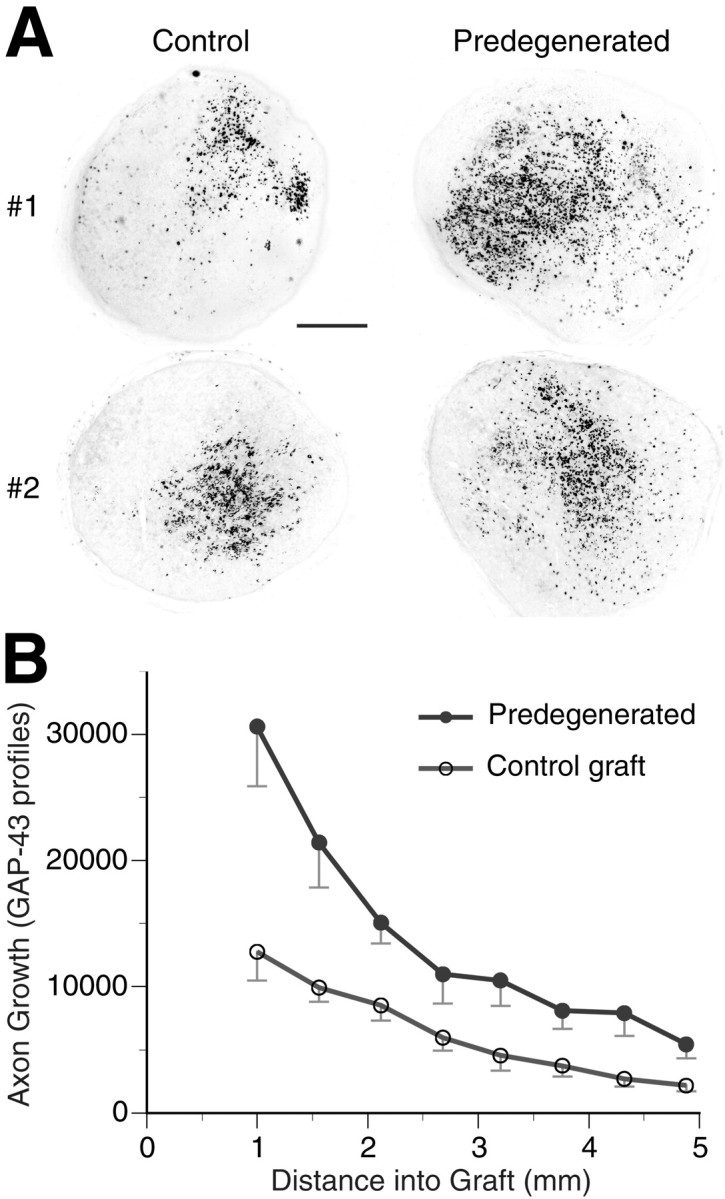
Axonal regeneration within acellular nerve grafts predegenerated in vitro. Normal and cultured (2 d, 2% serum) nerve grafts were freeze-killed, trimmed to 10 mm in length, and used as interpositional grafts for the repair of transected sciatic nerves. Host rats received bilateral grafts, one normal (uncultured) and one predegenerated (cultured). Axonal regeneration was assessed after 8 d by scoring GAP-43-immunopositive profiles (expressed as total pixel count) in transverse sections. A, Representative sections of control and predegenerated grafts from two animals are shown. Sections show the axonal regeneration at 1.5 mm into the grafts. Pixel values of the immunofluorescent images were inverted.B, Quantitative analysis was performed at measured distances within the grafts. Data represent means ± SEM of six nerves in each condition. Scale bar, 200 μm.
DISCUSSION
Sciatic nerve explant cultures are a valuable model to examine cellular and molecular aspects of Wallerian degeneration in the absence of hematogenic macrophages. Our observations confirm previous reports that significant degenerative changes occur rapidly in cultured nerve explants that lead to Schwann cell proliferation, fragmentation and liberation of myelin debris, and axonal disintegration (Crang and Blakemore, 1986; Perry and Brown, 1992; Reichert et al., 1994;Brück et al., 1995; Shen et al., 1999).
Peripheral nerve degeneration in vivo results in an increased turnover of several ECM molecules that depends on the release and activation of proteolytic enzymes by neurons, Schwann cells, and invading macrophages. Modulation of MMP activities after injury implicates MMP-2 and MMP-9 in remodeling of the ECM during nerve degeneration and regeneration (La Fleur et al., 1996; Kherif et al., 1998; Ferguson and Muir, 2000). MMP-9 is expressed in the peripheral nerve immediately after injury and primarily at the site of injury. MMP-9 expression correlates with the breakdown of the blood–nerve barrier, the accumulation of granulocytes, and the invasion of macrophages (Shubayev and Myers, 2000; Siebert et al., 2001). Most evidence suggests that hematogenic cells contribute significantly to the elevation of MMP-9 activity (Taskinen and Röytta, 1997). In contrast, MMP-2 is expressed constitutively by Schwann cells in normal peripheral nerve (Yamada et al., 1995). Several days after injury, MMP-2 expression is upregulated and latent enzyme is substantially converted to its active form (Ferguson and Muir, 2000). In the present study of peripheral nerve degeneration in vitro, we find that MMP-9 is present in trace amounts primarily associated with a minor population of cells restricted to the epineurial sheath. Immunolabeling for MMP-9 is essentially absent in the endoneurial compartment of cultured nerves. In contrast, MMP-2, particularly the activated form, rapidly increases within the endoneurium in cultured nerves. Taken together with immunolocalization and in situzymography data, we conclude that MMP-2 is expressed by Schwann cells and that active enzyme is released into the surrounding endoneurium during in vitro nerve degeneration. MMP-2 is activated at the cell surface through a unique multistep pathway involving membrane-type MMPs (MT-MMPs) and the tissue inhibitor of MMPs, TIMP-2 (Sternlicht and Werb, 2001). Although little is known about MT-MMP expression in peripheral nerve, activated Schwann cells upregulate MMP-2 and TIMP-2 and appear capable of autonomous activation of MMP-2, which occurs in isolated Schwann cell culture (Muir, 1995; Huang et al., 2000).
In vitro degeneration results in a substantial increase in the neurite-promoting activity of nerve explants. This increase is blocked by the addition of MMP inhibitor, as is the coincidental increase in net gelatinolytic activity (demonstrated by in situ zymography). The rise in neurite-promoting activity occurs rapidly in the cultured nerve explants and in parallel with the upregulation and activation of MMP-2. In contrast, the initial effect of in vivo degeneration only suppresses the already low neurite-promoting activity of normal nerve, during which time there is no change in MMP-2 expression or activation in vivo. However, the neurite-promoting activity of transected nerve does increase over time in vivo, and this coincides with a burst of MMP-2 expression and activation (Ferguson and Muir, 2000; Shubayev and Myers, 2000). In a previous cryoculture study, we found that the neurite-promoting activity of normal rat nerve was increased significantly by exogenous treatment with MMP-2. However, MMP-2 treatment had little effect on nerves already predegenerated in vivo, suggesting that these nerves already had been modified by endogenous MMP-2 activity (Ferguson and Muir, 2000). Neurite-inhibiting CSPGs are abundant in peripheral nerve and inhibit the neurite-promoting activity of laminin (Zuo et al., 1998a). MMP-2 can degrade inhibitory CSPGs and deinhibit the neurite-promoting activity of laminin in the endoneurial basal lamina (Zuo et al., 1998b, Ferguson and Muir, 2000). Combined with our present observations of nerve explants, we conclude that MMP-2 represents a sufficient, if not principal, degenerative mechanism for the enhancement of the growth-promoting properties of denervated nerve (and predegenerated nerve grafts).
Axonal regeneration occurs in response to the Schwann cell basal lamina, which is normally preserved after injury. After an early activation phase, the neurite-promoting activity of nerve explants declined and fell below the level of normal (not degenerated) nerves after 7 d in culture. Axonal growth in the cryoculture assay (on nerve tissue sections) is exquisitely sensitive to disruption of the basal lamina. In addition to MMPs, TIMPs are also induced in the distal segment of nerve after injury. La Fleur et al. (1996) found that TIMP-1 inhibitor activity was present in excess of proteinase activity in extracts of injured nerve and proposed that TIMP-1 protected basement membrane from uncontrolled degradation after nerve injury. Like MMP-9, TIMP-1 is expressed by infiltrating macrophages, and cytokines expressed by macrophages may participate in the regulation of TIMP levels during nerve repair (La Fleur et al., 1996). Although the expression of other TIMPs in nerve is poorly documented, these findings raise the possibility that TIMP levels may be extraordinarily low in nerve explants devoid of infiltrating macrophages. Accordingly, MMP activities in nerve explants might remain unchecked, and elevated levels of MMP-2 could result in uncontrolled modification of the basal lamina and loss of neurite-promoting activity over time.
In vitro assays indicate that nerve segments predegeneratedin vivo have greater neurite-promoting activity than normal segments of nerve (Bedi et al., 1992; Agius and Cochard, 1998; Ferguson and Muir, 2000). However, in vivo studies testing predegenerated nerve grafts have produced conflicting results, especially when using cellular (live) nerve grafts (Gordon et al., 1979; Danielsen et al., 1994; Hasan et al., 1996). Nonetheless, predegeneration appears to be particularly advantageous for the enhancement of regeneration into acellular grafts (Ochi et al., 1994;Danielsen et al., 1995). This indicates that, in degeneration, cellular and molecular mechanisms act to enhance the growth-promoting properties of the basal lamina, which then retains the ability to stimulate nerve regeneration after the cellular elements have been killed. In vitro predegeneration results in a substantial increase in the growth-promoting ability of acellular nerve grafts that was readily demonstrated in our cryoculture and grafting models. Acellular nerve grafting is associated with a substantial latency in the onset of axonal regeneration (Danielsen et al., 1995). Importantly, in vitro predegeneration markedly accelerates the ingress of axons into acellular nerve grafts and thus overcomes a major shortcoming associated with freeze-killed nerve grafts. Moreover, our evidence indicates that the neurite growth-promoting effects achieved byin vitro predegeneration are superior to those resulting from predegeneration in vivo. Also, because in vivo predegeneration of human donor nerve is impractical, in vitro predegeneration may greatly expand the clinical potential for acellular grafts. Additional study is required to determine whetherin vitro predegeneration alone or in combination with other enhancement strategies can overcome other shortcomings of acellular grafts such as limits on graft length.
Much of the research on nerve explant culture and nerve graft preservation has focused on the cold storage of nerve segments. Cold-storage methods aim to preserve the nerve structure using minimal and ischemic conditions that suppress cellular and proteolytic activities. Cold storage greatly decreases the viability of antigen-presenting cells and therefore reduces the concerns of allograft immunorejection (Levi et al., 1994). Immunorejection of cellular allografts negates their regenerative potential. For this reason, prolonged cold storage of allografts results in better regeneration than fresh allografts (Evans et al., 1998, 1999). However,Lassner et al. (1995) reported that culture medium (DMEM rather than Cold Storage Solution) has a positive effect on maintaining Schwann cell viability and sustaining Wallerian degeneration and improves the regenerative potential of nerve grafts stored in cold, ischemic conditions. However, the concerns of immunorejection increase with more cellular allografts. More than cold preservation, the complete destruction of antigen-presenting cells in nerve grafts by freeze-killing virtually eliminates the concerns of graft immunorejection (Evans et al., 1998). As stated above, predegeneration enhances the growth-promoting properties of nerve grafts. Importantly, the combination of in vivo predegeneration and freeze-killing is more effective for obtaining a growth-promoting allograft not hampered by cellular immunorejection (Osawa et al., 1990). We find that culture of nerve grafts using conditions to support cell viability and cell-mediated degeneration significantly enhances the regenerative potential of nerve allografts. Once optimal degeneration/remodeling in vitro is achieved, the nerve explants are then freeze-killed and stored frozen for later use as interpositional nerve grafts. These findings support the assertion that predegenerated acellular nerve grafts have a greater potential for clinical applications than do cellular nerve grafts in allografting without immunosuppression.
In conclusion, it is evident that degeneration/remodeling of denervated nerve plays an important role in the regenerative capacity of peripheral nerves. All nervous tissues contain a preponderance of growth-inhibiting signals, but it is a robust degenerative competence that enables the regenerative capacity of the peripheral nervous system. We provide strong evidence that MMP-2 plays a principal role in establishing the growth-promoting properties of denervated peripheral nerve. Moreover, we describe in vitro conditions to optimize the degenerative competence and enhance the growth-promoting potential of peripheral nerve. This process has been applied to the production of predegenerated acellular nerve grafts that may have considerable potential for clinical allogenic nerve grafting. Long-term neurological studies are required to assess the full potential of this graft preparation to improve recovery of function.
Footnotes
This work was supported by grants awarded to D.M. from the National Institutes of Health (NS37901) and the Florida State Brain and Spinal Cord Injury Rehabilitation Trust Fund.
Correspondence should be addressed to Dr. David Muir, Pediatric Neurology, Box 100296, University of Florida College of Medicine, Gainesville, FL 32610. E-mail: muir@ufbi.ufl.edu.
REFERENCES
- 1.Agius E, Cochard P. Comparison of neurite outgrowth induced by intact and injured sciatic nerves: a confocal and functional analysis. J Neurosci. 1998;18:328–338. doi: 10.1523/JNEUROSCI.18-01-00328.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bain JR. Peripheral nerve allografting: review of the literature with relevance to composite tissue transplantation. Transplant Proc. 1998;30:2762–2767. doi: 10.1016/s0041-1345(98)00804-5. [DOI] [PubMed] [Google Scholar]
- 3.Bedi KS, Winter J, Berry M, Cohen J. Adult rat dorsal root ganglion neurons extend neurites on predegenerated but not on normal peripheral nerves in vitro. Eur J Neurosci. 1992;4:193–200. doi: 10.1111/j.1460-9568.1992.tb00867.x. [DOI] [PubMed] [Google Scholar]
- 4.Brück W, Brück Y, Maruschak B, Friede RL. Mechanisms of macrophage recruitment in Wallerian degeneration. Acta Neuropathol (Berl) 1995;89:363–367. doi: 10.1007/BF00309630. [DOI] [PubMed] [Google Scholar]
- 5.Carbonetto S, Evans D, Cochard P. Nerve fiber growth in culture on tissue substrata from central and peripheral nervous systems. J Neurosci. 1987;7:610–620. doi: 10.1523/JNEUROSCI.07-02-00610.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crang AJ, Blakemore WF. Observations on Wallerian degeneration in explant cultures of cat sciatic nerve. J Neurocytol. 1986;15:471–482. doi: 10.1007/BF01611730. [DOI] [PubMed] [Google Scholar]
- 7.Dailey AT, Avellino AM, Benthem L, Silver J, Kliot M. Complement depletion reduces macrophage infiltration and activation during Wallerian degeneration and axonal regeneration. J Neurosci. 1998;18:6713–6722. doi: 10.1523/JNEUROSCI.18-17-06713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danielsen N, Kerns JM, Holmquist B, Zhao Q, Lundborg G, Kanje M. Pre-degenerated nerve grafts enhance regeneration by shortening the initial delay period. Brain Res. 1994;666:250–254. doi: 10.1016/0006-8993(94)90779-x. [DOI] [PubMed] [Google Scholar]
- 9.Danielsen N, Kerns JM, Holmquist B, Zhao Q, Lundborg G, Kanje M. Predegeneration enhances regeneration into acellular nerve grafts. Brain Res. 1995;681:105–108. doi: 10.1016/0006-8993(95)00300-f. [DOI] [PubMed] [Google Scholar]
- 10.Evans PJ, Midha R, Mackinnon SE. The peripheral nerve allograft: a comprehensive review of regeneration and neuroimmunology. Prog Neurobiol. 1994;43:187–233. doi: 10.1016/0301-0082(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 11.Evans PJ, Mackinnon SE, Levi AD, Wade JA, Hunter DA, Nakao Y, Midha R. Cold preserved nerve allografts: changes in basement membrane, viability, immunogenicity, and regeneration. Muscle Nerve. 1998;21:1507–1522. doi: 10.1002/(sici)1097-4598(199811)21:11<1507::aid-mus21>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 12.Evans PJ, MacKinnon SE, Midha R, Wade JA, Hunter DA, Nakao Y, Hare GM. Regeneration across cold preserved peripheral nerve allografts. Microsurgery. 1999;19:115–127. doi: 10.1002/(sici)1098-2752(1999)19:3<115::aid-micr1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson TA, Muir D. MMP-2 and MMP-9 increase the neurite-promoting potential of Schwann cell basal laminae and are upregulated in degenerated nerve. Mol Cell Neurosci. 2000;16:157–167. doi: 10.1006/mcne.2000.0859. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Valle C, Bunge RP, Bunge MB. Schwann cells degrade myelin and proliferate in the absence of macrophages: evidence from in vitro studies of Wallerian degeneration. J Neurocytol. 1995;24:667–679. doi: 10.1007/BF01179817. [DOI] [PubMed] [Google Scholar]
- 15.Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- 16.Galardy RE, Cassabonne ME, Giese C, Gilbert JH, Lapierre F, Lopez H, Schaefer ME, Stack R, Sullivan M, Summers B, Tressler R, Terrel D, Wee J, Allen SD, Castellot JJ, Barletta JP, Schultz GS, Fernandez LA, Fisher S, Cui T-Y. Low molecular weight inhibitors in corneal ulceration. Ann NY Acad Sci. 1994;732:315–323. doi: 10.1111/j.1749-6632.1994.tb24746.x. [DOI] [PubMed] [Google Scholar]
- 17.Gordon L, Buncke H, Jewett DL, Muldowney B, Buncke G. Predegenerated nerve autografts as compared with fresh nerve autografts in freshly cut and precut motor nerve defects in the rat. J Hand Surg [Am] 1979;4:42–47. doi: 10.1016/s0363-5023(79)80103-3. [DOI] [PubMed] [Google Scholar]
- 18.Gulati AK. Evaluation of acellular and cellular nerve grafts in repair of rat peripheral nerve. J Neurosurg. 1988;68:117–123. doi: 10.3171/jns.1988.68.1.0117. [DOI] [PubMed] [Google Scholar]
- 19.Harris J, Moreno S, Shaw G, Mugnaini E. Unusual neurofilament composition in cerebellar unipolar brush neurons. J Neurocytol. 1993;22:1039–1059. doi: 10.1007/BF01235748. [DOI] [PubMed] [Google Scholar]
- 20.Hasan NA, Neumann MM, de Souky MA, So KF, Bedi KS. The influence of predegenerated nerve grafts on axonal regeneration from prelesioned peripheral nerves. J Anat. 1996;189:293–302. [PMC free article] [PubMed] [Google Scholar]
- 21.Huang D, Rutkowski JL, Brodeur GM, Chou PM, Kwiatkowski JL, Babbo A, Cohn SL. Schwann cell-conditioned medium inhibits angiogenesis. Cancer Res. 2000;60:5966–5971. [PubMed] [Google Scholar]
- 22.Keilhoff G, Fansa H, Schneider W, Wolf G. In vivo predegeneration of peripheral nerves: an effective technique to obtain activated Schwann cells for nerve conduits. J Neurosci Methods. 1999;89:17–24. doi: 10.1016/s0165-0270(99)00034-5. [DOI] [PubMed] [Google Scholar]
- 23.Kherif S, Dehaupas M, Lafuma C, Fardeau M, Alameddine HS. Matrix metalloproteinases MMP-2 and MMP-9 in denervated muscle and injured nerve. Neuropathol Appl Neurobiol. 1998;24:309–319. doi: 10.1046/j.1365-2990.1998.00118.x. [DOI] [PubMed] [Google Scholar]
- 24.Ladwig GP, Robson MC, Liu R, Kuhn MA, Muir D, Schultz GS. Ratios of activated matrix metalloproteinase-9 to tissue inhibitor of matrix metalloproteinase-1 in wound fluids are inversely correlated with healing of pressure ulcers. Wound Repair Regen. 2002;10:26–37. doi: 10.1046/j.1524-475x.2002.10903.x. [DOI] [PubMed] [Google Scholar]
- 25.La Fleur M, Underwood JL, Rappolee DA, Werb Z. Basement membrane and repair of injury to peripheral nerve: defining a potential role for macrophages, matrix metalloproteinases, and tissue inhibitor of metalloproteinases-1. J Exp Med. 1996;184:2311–2326. doi: 10.1084/jem.184.6.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lassner F, Becker M, Fansa H, Walter GF, Berger A. Preservation of peripheral nerve grafts: a comparison of normal saline, HTK organ preservation solution, and DMEM Schwann cell culture medium. J Reconstr Microsurg. 1995;11:447–453. doi: 10.1055/s-2007-1006559. [DOI] [PubMed] [Google Scholar]
- 27.Levi A, Evans PJ, Mackinnon SE, Bunge RP. Cold storage of peripheral nerves: an in vitro assay of cell viability and function. Glia. 1994;10:121–131. doi: 10.1002/glia.440100206. [DOI] [PubMed] [Google Scholar]
- 28.Menovsky T, Bartels RH. Stabilization and accurate trimming of nerve ends: practical use of fibrin glue: technical note. Neurosurgery. 1999;44:224–226. doi: 10.1097/00006123-199901000-00142. [DOI] [PubMed] [Google Scholar]
- 29.Muir D. Differences in proliferation and invasion by normal, transformed and NF1 Schwann cell cultures are influenced by matrix metalloproteinase expression. Clin Exp Metastasis. 1995;13:303–314. doi: 10.1007/BF00133486. [DOI] [PubMed] [Google Scholar]
- 30.Ochi M, Wakasa M, Ikuta Y, Kwong WH. Nerve regeneration in predegenerated basal lamina graft: the effect of duration of predegeneration on axonal extension. Exp Neurol. 1994;128:216–225. doi: 10.1006/exnr.1994.1130. [DOI] [PubMed] [Google Scholar]
- 31.Oh LY, Larsen PH, Krekoski CA, Edwards DR, Donovan F, Werb Z, Yong VW. Matrix metalloproteinase-9/gelatinase B is required for process outgrowth by oligodendrocytes. J Neurosci. 1999;19:8464–8475. doi: 10.1523/JNEUROSCI.19-19-08464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osawa T, Tohyama K, Ide C. Allogeneic nerve grafts in the rat, with special reference to the role of Schwann cell basal laminae in nerve regeneration. J Neurocytol. 1990;19:833–849. doi: 10.1007/BF01186814. [DOI] [PubMed] [Google Scholar]
- 33.Perry VH, Brown MC. Macrophages and nerve regeneration. Curr Opin Neurobiol. 1992;2:679–682. doi: 10.1016/0959-4388(92)90038-m. [DOI] [PubMed] [Google Scholar]
- 34.Reichert F, Saada A, Rotshenker S. Peripheral nerve injury induces Schwann cells to express two macrophage phenotypes: phagocytosis and the galactose-specific lectin MAC-2. J Neurosci. 1994;14:3231–3245. doi: 10.1523/JNEUROSCI.14-05-03231.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salonen V, Peltonen J, Röytta M, Virtanen I. Laminin in traumatized peripheral nerve: basement membrane changes during degeneration and regeneration. J Neurocytol. 1987;16:713–720. doi: 10.1007/BF01637662. [DOI] [PubMed] [Google Scholar]
- 36.Shen ZL, Lassner F, Becker M, Walter GF, Bader A, Berger A. Viability of cultured nerve grafts: an assessment of proliferation of Schwann cells and fibroblasts. Microsurgery. 1999;19:356–363. doi: 10.1002/(sici)1098-2752(1999)19:8<356::aid-micr2>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 37.Shubayev VI, Myers RR. Upregulation and interaction of TNFα and gelatinases A and B in painful peripheral nerve injury. Brain Res. 2000;855:83–89. doi: 10.1016/s0006-8993(99)02321-5. [DOI] [PubMed] [Google Scholar]
- 38.Siebert H, Dippel N, Mader M, Weber F, Bruck W. Matrix metalloproteinase expression and inhibition after sciatic nerve axotomy. J Neuropathol Exp Neurol. 2001;60:85–93. doi: 10.1093/jnen/60.1.85. [DOI] [PubMed] [Google Scholar]
- 39.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stoll G, Muller HW. Nerve injury, axonal degeneration and neural regeneration: basic insights. Brain Pathol. 1999;9:313–325. doi: 10.1111/j.1750-3639.1999.tb00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taskinen HS, Röytta M. The dynamics of macrophage recruitment after nerve transection. Acta Neuropathol (Berl) 1997;93:252–259. doi: 10.1007/s004010050611. [DOI] [PubMed] [Google Scholar]
- 42.Yamada T, Miyazaki K, Koshikawa N, Takahashi M, Akatsu H, Yamamoto T. Selective localization of gelatinase A, an enzyme degrading β-amyloid protein, in white matter microglia and in Schwann cells. Acta Neuropathol (Berl) 1995;89:199–203. doi: 10.1007/BF00309334. [DOI] [PubMed] [Google Scholar]
- 43.Yong VW, Krekoski CA, Forsyth PA, Bell R, Edwards DR. Matrix metalloproteinases and diseases of the CNS. Trends Neurosci. 1998;21:75–80. doi: 10.1016/s0166-2236(97)01169-7. [DOI] [PubMed] [Google Scholar]
- 44.Zuo J, Hernandez YJ, Muir D. Chondroitin sulfate proteoglycan with neurite-inhibiting activity is up-regulated following peripheral nerve injury. J Neurobiol. 1998a;34:41–54. [PubMed] [Google Scholar]
- 45.Zuo J, Ferguson TA, Hernandez YJ, Stetler-Stevenson WG, Muir D. Neuronal matrix metalloproteinase-2 degrades and inactivates a neurite-inhibiting chondroitin sulfate proteoglycan. J Neurosci. 1998b;18:5203–5211. doi: 10.1523/JNEUROSCI.18-14-05203.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]