Abstract
Mammalian free-running circadian rhythms are entrained to the external light/dark cycle by photic signaling to the suprachiasmatic nuclei via the retinohypothalamic tract (RHT). We investigated the circadian entrainment and clock properties ofmath5−/− mutant mice.math5 is a critical regulator of retinal ganglion cell development; math5−/− mice show severe optic nerve hypoplasia. By anterograde cholera toxin B tracing, we find that math5−/− mice do not develop an identifiable RHT pathway. This appears to be attributable to agenesis or dysgenesis of the majority of RHT-projecting retinal ganglion cells. math5−/− mice display free-running circadian rhythms with a period ∼1 hr longer than B6/129 controls (24.43 ± 0.10 vs 23.62 ± 0.19 hr;p < 0.00001). The free-running period of heterozygote mice is indistinguishable from that of controls.math5−/− mice show no entrainment to light/dark cycles, whereas heterozygote mice show normal entrainment to both 12 hr light/dark cycles and to a 1 hr skeletal photoperiod.math5−/− mice show reduced ability to entrain their rhythms to the nonphotic time cue of restricted running wheel access but demonstrate both free-running behavior and entrained anticipation of wheel unlocking in these conditions, suggesting the presence of a second diurnal oscillatory system inmath5−/− animals. These results demonstrate that retinal ganglion cell input is not necessary for the development of a free-running circadian timekeeping system in the suprachiasmatic nucleus but is important for both photic entrainment and determination of the free-running period.
Keywords: circadian rhythm, math5, photic entrainment, retinohypothalamic tract, nonphotic entrainment, phase shifting
In the absence of external time cues, mammalian behavior remains temporally organized in nearly day-long cycles of activity and rest. These circadian rhythms have a discrete genetic and anatomic basis (Herzog, 2001; Reppert and Weaver, 2001). The suprachiasmatic nuclei (SCN) of the hypothalamus contain the central circadian pacemaker. Lesions of the suprachiasmatic nucleus result in behavioral arrhythmicity, whereas transplantation of fetal SCN tissue can restore rhythmic behavior with the clock period dependent on the period of the donor tissue (Ralph et al., 1990;Weaver, 1998). In the absence of external time cues (i.e., free-running conditions), the period of the circadian clock differs significantly from 24 hr in most mammals. Free-running rhythms thus require continual synchronization with the exogenous 24 hr light/dark cycle.
The eyes are necessary for photic entrainment in most mammals, but the classical photoreceptors (rods and cones) are dispensable (Foster et al., 1991; Lucas and Foster, 1999). The identity of the photopigment underlying circadian entrainment has not been definitively demonstrated, but its localization appears to be in the inner retina (Berson et al., 2002). The retinohypothalamic tract (RHT) connects a small subset of retinal ganglion cells with the SCN (Hendrickson et al., 1972; Moore, 1973, 1995). This tract is thought to play an essential role in photic entrainment. In hamsters, resection of the RHT eliminates photic entrainment (Johnson et al., 1988). Conversely, resection of the optic tract with preservation of the RHT tract in hamsters results in a blind animal still able to entrain to light (Klein and Moore, 1979). Recently, a candidate photopigment, melanopsin, has been found to be expressed in RHT and other non-visual-projecting retinal ganglion cells (Gooley et al., 2001;Hannibal et al., 2002; Hattar et al., 2002; Provencio et al., 2002).
math5, a murine atonal homolog, is a helix-loop-helix proneural gene necessary for retinal ganglion cell differentiation in mice (Brown et al., 2001; Wang et al., 2001). This gene is expressed nearly exclusively in developing retinal ganglion cells (RGCs) (Brown et al., 1998). Homozygousmath5−/− mice lack >80% of retinal ganglion cells, including nearly all Brn3b-positive cells (Brown et al., 2001; Liu et al., 2001; Wang et al., 2001). Heterozygous mice are anatomically normal. The current study was designed to determine whether the RHT-projecting retinal ganglion cells are also dependent on math5 for their development or whether these cells are among the math5-independent RGCs seen inmath5−/− mice. We find that >90% of melanopsin-positive cells fail to develop inmath5−/− mice, leading to loss of an identifiable retinohypothalamic tract. Themath5−/− mouse thus has a genetically deafferented circadian clock. We demonstrate that this deafferented clock cannot entrain to light/dark cycles, has an abnormal free-running circadian period, and has abnormal responses to nonphotic stimuli.
MATERIALS AND METHODS
Animals. math5−/− mice were generated as described previously (Wang et al., 2001). All analysis was performed on a mixed C57BL/6J and 129/SvEv background (hereafter referred to as B6/129). Mice ranged in age from 12 to 16 weeks at the start of the experiment. Each mouse was individually housed in a polycarbonate cage containing a running wheel. All animal work was performed under animal care guidelines from the Association for Research in Vision and Ophthalmology.
Genotyping of mice was performed by PCR of DNA extracted from distal tail biopsy. Primers for wild-type math5 were 5′-TAC GCG AAA GGT CAG AGG TCA C-3′ and 5′-TGA GCC ACG AAC AGA TGA AAG C-3′, and primers for disrupted math5 were 5′-AGG GCC GCA AGA AAA CTA TCC-3′ and 5′-ACT TCG GCA CCT TAC GCT TCT TCT-3′. After 2 min of denaturation at 94°C, 35 cycles of denaturation at 94°C for 30 sec, annealing at 64°C for 30 sec, and extension at 72°C for 50 sec were performed. Products were analyzed by agarose gel electrophoresis with ethidium bromide staining.
Cholera toxin subunit B immunohistochemistry. Cholera toxin subunit B (Sigma, St. Louis, MO) was pressure-injected into the posterior chamber of the right eye in adultmath5−/− and B6/129 control mice as described previously (Mikkelsen, 1992; Mikkelsen and Serviere, 1992). Cholera toxin subunit B antiserum raised in goat was purchased from List Biologic (Campbell, CA; product 703, lot 7032G). Mice were anesthetized with ketamine and xylazine and injected with 5 μl (50 μg) of cholera toxin subunit B using a Hamilton (Reno, NV) syringe (gauge 33) into the vitreous humor of the right eye. After 24 hr, the mice were anesthetized, killed, and fixed by perfusion. Animals were perfused initially with 30 ml of PBS containing 15 IU/l heparin for 5 min and then with 400 ml 4% paraformaldehyde dissolved in 0.1 m phosphate buffer, pH 7.4, for 15 min. Brains were removed, immersed in the same fixative for 4 hr at room temperature, cryoprotected in 30% sucrose and PBS for 2 d at 4°C, immersed in optimal cutting temperature compound for 4 hr, frozen, cut into 40-μm-thick sections in a cryostat, and collected in PBS as free-floating sections. Immunohistochemistry was performed using the avidin–biotin procedure as described previously (Hsu et al., 1981).
Melanopsin immunohistochemistry. Adultmath5−/− and B6/129 control mice were anesthetized with ketamine and xylazine, killed, and fixed by transcardiac perfusion with 30 ml of 15 IU/l heparin followed by 30 ml of 4% paraformaldehyde. The cornea and lens were removed. A fiducial mark was made by burning the dorsal aspect of the eye with a heated 21 gauge needle before the eyes were removed. A cut was made from the fiducial mark to the optic nerve to maintain orientation. The eyes were postfixed for 48 hr in 4% paraformaldehyde. Flat-mounted retina preparations were subjected to a 72 hr incubation at 4°C with a 1:2500 dilution of anti-melanopsin antiserum. The primary antiserum was raised in a rabbit against a peptide representing the 15 N-terminal amino acids of mouse melanopsin with an appended C-terminal cysteine to facilitate conjugation to keyhole limpet hemocyanin. Immunopositive cells were visualized by incubating the retina in a 1:500 dilution of a Cy3-conjugated anti-rabbit IgG secondary antibody (1:500;Jackson ImmunoResearch, West Grove, PA) for 1 hr at room temperature. Processed retinas were mounted and coverslipped under Vectashield (Vector Laboratories, Burlingame, CA) and viewed under epifluorescence.
Photic entrainment. Wheel-running activity was recorded through continuous computer sampling of magnetic switches triggered by running wheel rotation (Actimetrics, Evanson, IL). Mice were kept in total darkness (DD) and allowed ad libitum access to the running wheel, food, and water. Free-running periods were calculated by periodogram analysis using ClockLab software (Actimetrics). To assay photic entrainment, mice were placed in a 12 hr light/dark (LD 12:12) lighting cycle with broad-spectrum fluorescent lighting. Luminance was measured as 100 lux at the cage surface. Mice were monitored in LD 12:12 for 14 d, after which the mice were again placed in DD. To test heterozygous mice for subtle defects in photic entrainment,math5 heterozygote and B6/129 control sibling mice were placed into a skeletal photoperiod of 1 hr of light/d (LD 1:23).
Phase response measurement. Sixmath5+/− mice and 4 B6/129 sibling controls were singly housed and placed in LD 12:12 for 7 d to coordinate entrainment among the mice. The mice were then released into DD for 4 d. On the fifth day, the mice were subjected to ∼100 lux of light for 1 hr; this corresponded to early subjective night [mean circadian time (CD) ± SD, 14.9 ± 1.4]. Free-running rhythms were again monitored for 7 d, and phase shift was assayed by determination of wheel-running onsets. For the phase advance portion of the curve, the mice were entrained with 10 d of LD 12:12 before being released into darkness for 4 d to reestablish a free-running rhythm. On the fifth day, the mice received 1 hr of ∼100 lux of light in the late subjective night (mean CT, 22.0 ± 1.1). Phase shift was measured as for the phase delay curve above.
Wheel lock with activity monitor. After 11 d of free-running activity, sevenmath5−/− mice and seven B6/129 sibling controls were kept in constant darkness with restricted access to the running wheel for 2 hr/d (10 A.M. to 12 P.M.). All mice ran vigorously of their own volition; no special measures had to be taken to induce activity. Activity was monitored through the use of a plastic platform placed adjacent to the water bottle spout. When drinking or incidentally stepping on this platform, the mouse triggered a microswitch that sent a signal to the computer recording activity data. Scheduled running wheel access occurred for 46 d, after which mice were allowed unlimited access.
RESULTS
math5−/− mice lack a well developed retinohypothalamic tract
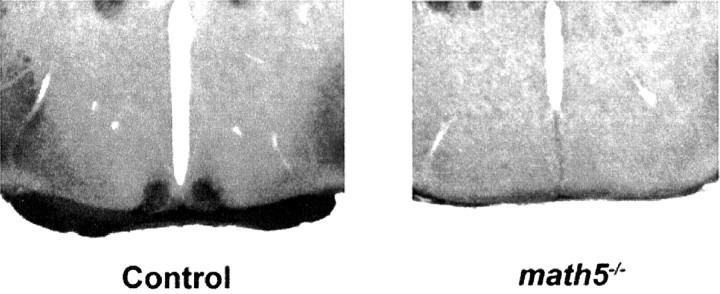
The murine RHT consists of ∼500 retinal ganglion cell axons that project directly to the SCN (Moore et al., 1995). Intravitreal injection of cholera toxin subunit B is an effective method to trace efferent projections from the retina (Mikkelsen, 1992). To determine whether the RHT is spared or lost in homozygousmath5−/− mutant mice, fourmath5−/− mice and sibling B6/129 control mice underwent intravitreal injection with cholera toxin subunit B. Cholera toxin staining confirmed substantial loss of the optic chiasm in math5−/− mice. In all math5−/− mice, no significant staining of the SCN was observed, whereas the SCN of B6/129 mice exhibited robust staining (Fig. 1). Hematoxylin staining of math5−/− mouse brain sections revealed anatomically normal-appearing SCN.
Fig. 1.
Anterograde cholera toxin B staining for the retinohypothalamic tract in control (B6/129; left) andmath5−/− (right) mice. Note the minimal optic chiasm inmath5−/−mice. No staining was seen in any math5−/− SCN.
math5−/− retinas show markedly reduced numbers of melanopsin-positive retinal ganglion cells
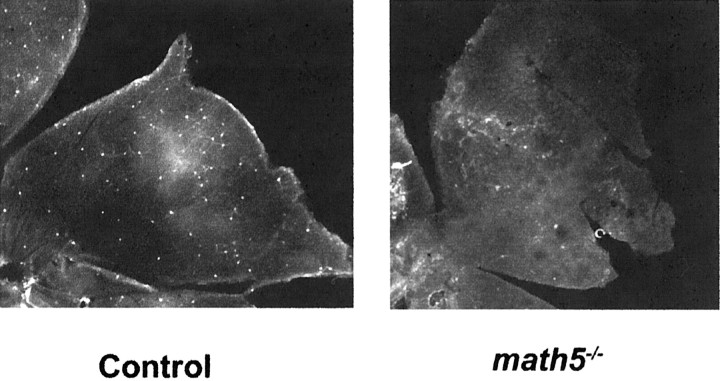
Loss of anterograde cholera toxin staining of the SCN from the retina could be attributable either to loss of the melanopsin-positive, light-sensitive retinal ganglion cells (Gooley et al., 2001; Hannibal et al., 2002; Hattar et al., 2002; Provencio et al., 2002) or to misrouting of the axons of these cells. We stained the retinas ofmath5−/− and B6/129 mice for melanopsin immunoreactivity (Provencio et al., 2002). The number of melanopsin-expressing cells was markedly reduced inmath5−/− retinas, which showed only a few melanopsin-positive cells (Fig.2). Cell counts ofmath5−/− retinas revealed 60.3 ± 10.5 melanopsin-positive cells per retina (mean ± SE; n = 4). This corresponds to ∼6% of the normal complement of melanopsin-positive cells (A. M. Castrucci and I. Provencio, unpublished observations). The few melanopsin-positive cells tended to be found in the peripheral retina and had normal-appearing morphology. Loss of the retinohypothalamic tract is therefore most likely attributable to agenesis or dysgenesis of the majority of RHT-projecting cells rather than misrouting of their axons.
Fig. 2.
Representative whole-mount retinal immunohistochemical specimens for melanopsin in control B6/129 andmath5−/− retinas. One retinal quadrant is shown in each image.
Loss of photic entrainment and lengthened free-running period in math5−/− mice
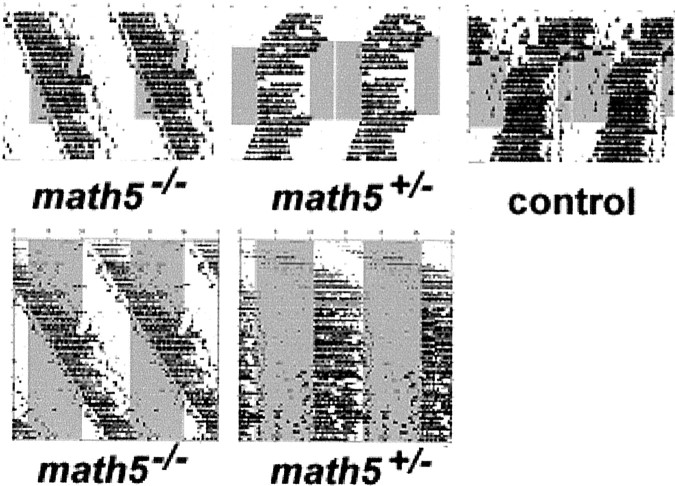
If photic entrainment of circadian activity requires an intact retinohypothalamic tract, one would expect that mice genetically lacking this pathway would be unable to synchronize their circadian rhythms to external lighting cues. Allmath5−/− mice recorded in LD 12:12 conditions failed to entrain their circadian rhythms to external time cues and showed free-running behavior (Fig.3). In contrast, allmath5+/− heterozygotes and control siblings showed normal entrainment to 100 lux light/dark cycles. Unexpectedly, math5−/− mice uniformly exhibited free-running periods significantly >24 hr (24.43 ± 0.10 hr; n = 7; Table1). There was no difference in free-running periods betweenmath5−/− mice kept in a light/dark cycle and those in total darkness, suggesting that the circadian clock of these animals was oblivious to external lighting conditions.math5+/− heterozygote mice showed free-running periods (23.52 ± 0.13 hr) indistinguishable from those of B6/129 control animals (23.62 ± 0.19 hr). The period difference between math5−/− andmath5+/− mice (and control mice) was highly statistically significant (p < 0.00001 by two-tailed pair-wise Student's t test).
Fig. 3.
math5−/− mice do not entrain to a light/dark cycle. Top panels, Mice were allowed to run freely for 1 week and were then subjected to LD 12:12 (lights on represented by gray bars) for 14 d, followed by 1 week of DD conditions. All data are shown as double-plotted wheel-running raster plots. Bottom panel, Comparison of math5−/− andmath5+/− mice for entrainment during 50 d of LD 12:12. No entrainment was seen in any of sevenmath5−/− mice tested.
Table 1.
Free-running circadian periods ofmath5−/−, math5+/−, and control mice
| Value | math5−/− | math5+/− | math5+/+ |
|---|---|---|---|
| Mean τ (hr) | 24.43* | 23.59 | 23.62 |
| SD (hr) | 0.10 | 0.13 | 0.19 |
| n | 7 | 7 | 4 |
Significantly different from heterozygote or control period (p < 0.0001 by two-tailed Student'st test).
math5+/− mice show normal entrainment to the skeletal photoperiod and normal phase response characteristics to light
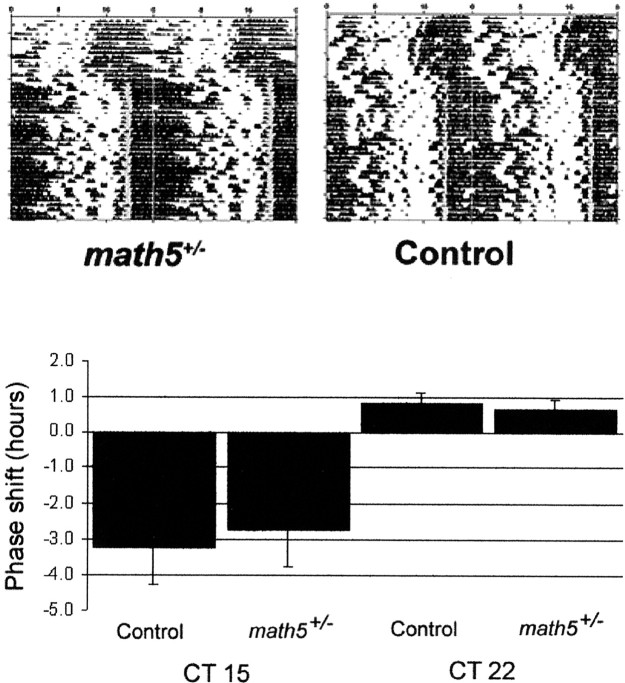
The prolonged free-running period ofmath5−/− mice could be attributable to either loss of an influence of the retinohypothalamic-projecting ganglion cells on the free-running period of SCN pacemaking cells or to a direct effect ofmath5 within the circadian clock mechanism. All murine clock mechanism genes studied to date (including mPer1,mPer2, Bmal, Clock, mCry1, and mCry2) have shown at least subtle free-running or entrained circadian abnormalities in heterozygotes and behave as semidominant alleles (Antoch et al., 1997; Thresher et al., 1998; van der Horst et al., 1999; Vitaterna et al., 1999; Bunger, 2000; Bae et al., 2001; Zheng et al., 2001). We therefore further analyzed the circadian entrainment of heterozygousmath5+/− mice. We evaluated these mice for a subtle entrainment phenotype by testing the ability of heterozygous and control mice to entrain to a minimal (skeleton) photoperiod of LD 1:23 (n = 4 of each genotype). No difference in the ability to entrain to a skeletal photoperiod was seen between math5+/− and control B6/129 animals (Fig. 4, top panel). All heterozygotes and control mice were able to entrain to the skeletal photoperiod with no qualitative difference in entrainment angle or stability.
Fig. 4.
math5+/− mice entrain to a skeletal photoperiod and show normal photic phase shifting responses. Top, Representative double-plotted wheel running actograms for math5+/−(left) and control (right) mice kept in LD 1:23 (lights on represented by gray bars).Bottom, Phase responses for photic phase shifting inmath5+/− and control B6/129 mice. Phase responses were calculated on the basis of the change in the free-running wheel activity phase after a 1 hr 100 lux light stimulus given at the indicated phase. n = 7 formath5+/− mice; n= 4 for control mice. Differences between genotypes were not statistically significant (p > 0.4 for each).
We additionally asked whether differences in the phase-shifting response of the free-running clock to a brief light pulse could be observed between the math5+/− and B6/129 mice. Free-running mice were subjected to light pulses in the early and late subjective night (CT, 15 and 22), and subsequent phase shifts were measured. As shown in Figure 4, bottom panel, there was no qualitative difference in the photic phase response ofmath5+/− mice in either the advance or delay portion of the phase–response curve.
math5−/− mice do not entrain activity to running wheel availability
Regularly scheduled exercise can entrain free-running circadian rhythms in mice (Edgar et al., 1991). To determine whether themath5−/− mouse's circadian clock could entrain to this nonphotic stimulus, we subjectedmath5−/− and B6/129 control mice to scheduled wheel-running availability. Mice were allowed access to their running wheels for 2 hr/d. During this window of activity, mice spontaneously ran vigorously on their wheels (Fig.5). At all other times, the wheels were mechanically locked. Drinking activity was monitored to assess behavioral rhythmicity. Six of seven control mice demonstrated entrainment to running wheel availability within 46 d of wheel lock (Fig. 5). The single mouse that failed to entrain did not run vigorously during times of wheel availability. Only one of sevenmath5−/− mice showed entrainment to wheel availability after 46 d of wheel lock. It is unlikely that this failure to entrain to an external stimulus reflects the wheel availability falling in the “dead zone” of the phase–response curve, because the long duration of wheel locking ensured that wheel availability was presented at all circadian phases.
Fig. 5.

math5−/− mice fail to synchronize activity to limited wheel availability. Double-plotted drinking activity actograms are shown for threemath5+/− (left) and three control (right) mice. Mice were housed in DD conditions. Wheel access was limited to 2 hr/d (gray bars) for 46 d.
Free-running math5−/− mice anticipate a 24 hr wheel availability rhythm
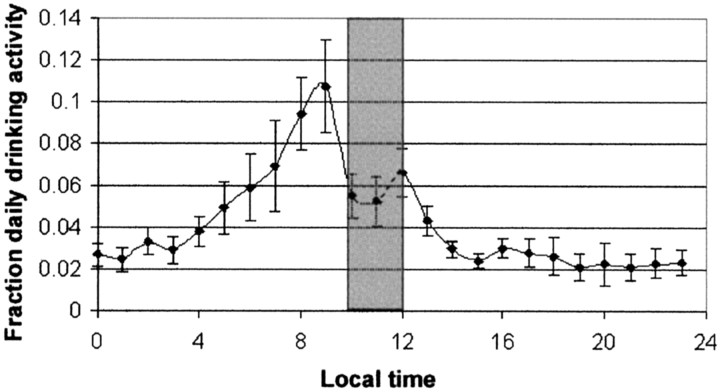
We analyzed the daily drinking activity of themath5−/− mice during activity restriction by plotting this activity summated for each 1 hr of the 24 hr day for the six nonentrainingmath5−/− mice and 46 d of recording (total, 276 recording days) (Fig.6). These data demonstrated that themath5−/− mice anticipated the release of wheel lock by at least ∼2 hr despite not having overall activity entrained by the limited wheel availability.
Fig. 6.
math5−/− mice show 24 hr anticipatory behavior during limited wheel availability. Mean drinking monitor activity per hour was summated over the six nonentraining math5−/− mice during 46 d of limited wheel availability (n = 276 d for each point). Data are shown as mean ± SE. Thegray bar represents the period of wheel availability.Points represent succeeding hours (i.e., thepoint at 0 hr represents time 0–1).
DISCUSSION
In this study, we have demonstrated thatmath5−/− mutant mice do not develop a detectable retinohypothalamic tract, resulting in a genetically deafferented circadian clock. This clock cannot entrain to external light/dark stimuli. Two properties of this “blind” circadian clock are unexpectedly altered: the free-running period of activity is increased by ∼1 hr, and this clock is not readily entrained by timed running wheel availability.
Photic entrainment is the primary process by which most animals synchronize their circadian rhythms with the external environment (Lowrey and Takahashi, 2000). Previous experiments in genetically blind mice, including rd/rd (Foster et al., 1991),rds/rds (Argamaso et al., 1995), and rd/rd; cl(Freedman et al., 1999), have demonstrated that outer retinal photoreceptors (rods and cones) are not necessary for photic entrainment. These mice all entrain normally to external light/dark cycles. However, enucleated mice do not entrain their rhythms to light (Halberg et al., 1954; Freedman et al., 1999), demonstrating that the circadian photoreceptive system in rodents is ocular. Inmath5−/− mice, differentiation of retinal ganglion cells is disrupted, and <20% of the normal complement of retinal ganglion cells develop (Brown et al., 2001; Wang et al., 2001). The retinas ofmath5−/− mice maintain their inner laminar structure, and no loss of photoreceptor cells in the outer nuclear layer is observed. The inability ofmath5−/− mice to entrain to light thus demonstrates the necessity of retinal ganglion cells for circadian entrainment. Recent work has demonstrated that the subset of RHT-projecting, melanopsin-expressing retinal ganglion cells is directly photoresponsive (Berson et al., 2002; Hattar et al., 2002). In the present work, we demonstrate that math5 is necessary for the development of most melanopsin-expressing retinal ganglion cells and the retinohypothalamic tract. The loss of photic entrainment in these mice demonstrates that retinal ganglion cells are essential for this process and is consistent with the hypothesis that the retinohypothalamic tract is necessary for photic entrainment (Moore, 1995).
To our knowledge, the math5−/−mouse is the second genetically blind strain to show the absence of photic circadian entrainment. The more severe anophthalmic mouse (ZRDCT-AN) carries a mutation that results in abortive embryonic eye development (Tucker et al., 2001). Several of the features of circadian rhythmicity found in the math5−/−mice were also seen in anophthalmic mice. Anophthalmic mice fail to entrain to light/dark cycles (Silver, 1977; Faradji et al., 1980;Laemle and Ottenweller, 1998). Free-running periods of anophthalmic mice are also consistently >24 hr (although they are ∼15 min shorter than observed in math5−/− mice;Laemle and Ottenweller, 1998). However, unlikemath5−/− mutants, anophthalmic mice show significant variability in the presence, stability, and nonphotic entrainment of their rhythms (Laemle and Ottenweller, 1998). Anophthalmic mice also show variable morphologic defects in the SCN (Silver, 1977), which were not observed in our studies of themath5−/− mice. The nature of the anophthalmic mutation in mice is not definitively known, but related anophthalmic mutants in hamsters show pleiotropic phenotypes with both ocular and hypothalamic dysgenesis (Asher, 1981). It is likely that the phenotype of the anophthalmic mouse is an amalgam of an ocular developmental phenotype and associated (and variable) ventral hypothalamic dysmorphogenesis.
The cause of the lengthened free-running period inmath5−/− mice is unclear. It is doubtful that math5 plays a direct role in the inner clock mechanism, because it is not expressed in the brains of adult animals (Brown et al., 1998; Wang et al., 2001). Furthermore, math5heterozygotes show no abnormalities in the free-running period, entrainment to the skeletal photoperiod, or phase-shifting response to light as has been observed for heterozygous animals of most central oscillator genes, including both cryptochromes (Thresher et al., 1998; van der Horst et al., 1999; Vitaterna et al., 1999),period 1 and 2 (Zheng et al., 1999; Albrecht et al., 2001; Bae et al., 2001; Zheng et al., 2001), Clock(Vitaterna et al., 1994), and Bmal/MOP3 (Bunger, 2000). The apparent absence of a retinohypothalamic tract may result in a developmental abnormality in the SCN; a decrease in the number of GFAP-positive astrocytes has been noted in rats enucleated at birth, for example (Munekawa et al., 2000). Early enucleation similarly causes changes in the anatomy and location of the SCN (Holtzman et al., 1989; Nagai et al., 1992). Alternatively, the RHT could provide a tonic input that in part determines the period in the mouse. Input from the RHT can clearly affect the free-running period, as seen in the period lengthening observed in mice in constant lighting conditions (i.e., Aschoff's rule; for review, see Daan, 2000). However, no consistent lengthening of the free-running period has been reported in mice enucleated as adults, so it is unlikely that the RHT provides a “dark current” of tonic input to the SCN that helps determine the free-running period.
Six of seven tested math5−/− mice did not entrain to a nonphotic stimulus. A similar failure to entrain to wheel lock conditions has been noted in a subset of anophthalmic mice (Laemle and Ottenweller, 1999). The mechanism of nonphotic phase shifting is not as well understood as that of photic phase shifts. Although serotonergic pathways have been implicated (Penev et al., 1995), depletion of serotonin in hamsters does not affect phase shifts in a novelty wheel-running paradigm (Bobrzynska et al., 1996). However, ablations of the intergeniculate leaflet do attenuate the phase-shifting effects of nonphotic stimuli (Janik and Mrosovsky, 1994). It is possible that the failure of retinogeniculate connections to form in the math5−/− mutant similarly disrupts the IGL pathway and thereby affects nonphotic as well as photic phase shifting. Alternatively, the failure of these mice to entrain to a 24 hr period of wheel lock may reflect the altered relationship between a ∼24.5 hr free-running circadian clock and the nonphotic phase–response curve. It is possible that these mice might entrain more easily to a non-24 hr nonphotic stimulus (i.e., wheel locking on a 25 hr time cycle).
The wheel-anticipatory behavior ofmath5−/− animals reveals the presence of a second circadian timekeeping system in these animals. This oscillator is desynchronized from the general behavioral oscillator. A similar second oscillator has been seen with experiments on food availability (the so-called food-entrainable oscillator;Stephan et al., 1979a,b; Rosenwasser et al., 1984; Abe et al., 1989). This oscillator is not dependent on the suprachiasmatic nucleus (Stephan et al., 1979b; Stephan, 1983) but is attenuated by hindbrain lesions in the parabrachial region (Davidson et al., 2000). It remains to be seen whether the wheel-anticipatory oscillator seen in themath5−/− mice is the same as the described food-entrainable oscillator. Themath5−/−mouse may be a very useful model for studying secondary oscillators, because these animals spontaneously display both anticipatory and free-running rhythm in internal desynchronization.
The findings in the present study may have clinical ramifications for the relationship between human eye disease and circadian synchronization. It is known that a subset of blind humans can still entrain their rhythms to light, whereas others cannot (Sack et al., 1992); similarly, some blind human subjects show suppression of their melatonin levels by light, whereas others do not (Czeisler et al., 1995). By analogy to the circadian entrainment phenotypes shown by the rodless and coneless rd/rd; cl mice (which show normal circadian entrainment; Freedman et al., 1999; Lucas et al., 1999) and the math5−/− mice described in the present study, one might expect that human patients with outer retinal disease (such as retinitis pigmentosa) would show normal entrainment to external light/dark cycles, but individuals with inner retinal disease (i.e., end-stage glaucoma or optic nerve hypoplasia) would have difficulty. This might be manifested by an increased incidence of sleep disorders. In one study of the self-reported sleep problems in blind individuals (Tabandeh et al., 1998), patients with glaucoma and inner retinal disease showed a higher incidence of self-reported poor sleep compared with patients with outer retinal dysfunction. If the inability to entrain to nonphotic stimuli is also a feature of severe or developmental inner retinal disease, this would compound poor photic phase entrainment and might explain why social cues fail to entrain subsets of blind patients (Klerman et al., 1998).
Together, math5−/− mice and outer retinal degenerate mice form a complementary model demonstrating that the phototransduction pathway required for vision is mechanistically distinct from the phototransduction pathway required for resetting the circadian clock. Both types of mice are behaviorally blind, yet mice that lack classical photoreceptors maintain photic entrainment, whereas mice that lack retinal ganglion cells lose this ability. Results from this study support the model of separate pathways for vision and circadian entrainment, implicating the retinal ganglion cell as a necessary component for signaling of photic information to the circadian clock.
Footnotes
R.W. was supported by a Doris Duke Fellowship. I.P. was supported by National Institutes of Health (NIH) Grant R01MH062405. L.G. was supported by NIH Grant EY013426. R.N.V.G. was supported by a career development award from Research to Prevent Blindness (RPB), the Becker/Association of University Professors of Ophthalmology/RPB clinician-scientist award, and NIH Grant K08EY00403.
Correspondence should be addressed to Dr. Russell N. Van Gelder, Department of Ophthalmology and Visual Sciences, Campus Box 8096, Washington University School of Medicine, 660 South Euclid Avenue, St. Louis, MO 63110. E-mail: vangelder@vision.wustl.edu.
REFERENCES
- 1.Abe H, Kida M, Tsuji K, Mano T. Feeding cycles entrain circadian rhythms of locomotor activity in CS mice but not in C57BL/6J mice. Physiol Behav. 1989;45:397–401. doi: 10.1016/0031-9384(89)90146-7. [DOI] [PubMed] [Google Scholar]
- 2.Albrecht U, Zheng B, Larkin D, Sun ZS, Lee CC. MPer1 and mper2 are essential for normal resetting of the circadian clock. J Biol Rhythms. 2001;16:100–104. doi: 10.1177/074873001129001791. [DOI] [PubMed] [Google Scholar]
- 3.Antoch MP, Song EJ, Chang AM, Vitaterna MH, Zhao Y, Wilsbacher LD, Sangoram AM, King DP, Pinto LH, Takahashi JS. Functional identification of the mouse circadian Clock gene by transgenic BAC rescue. Cell. 1997;89:655–667. doi: 10.1016/s0092-8674(00)80246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Argamaso SM, Froehlich AC, McCall MA, Nevo E, Provencio I, Foster RG. Photopigments and circadian systems of vertebrates. Biophys Chem. 1995;56:3–11. doi: 10.1016/0301-4622(95)00009-m. [DOI] [PubMed] [Google Scholar]
- 5.Asher JH., Jr Concerning the primary defect leading to the pleiotropic effects caused by anophthalmic white (Wh) in the Syrian hamster Mesocricetus auratus. J Exp Zool. 1981;217:159–169. doi: 10.1002/jez.1402170203. [DOI] [PubMed] [Google Scholar]
- 6.Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- 7.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 8.Bobrzynska KJ, Vrang N, Mrosovsky N. Persistence of nonphotic phase shifts in hamsters after serotonin depletion in the suprachiasmatic nucleus. Brain Res. 1996;741:205–214. doi: 10.1016/s0006-8993(96)00913-4. [DOI] [PubMed] [Google Scholar]
- 9.Brown NL, Kanekar S, Vetter ML, Tucker PK, Gemza DL, Glaser T. Math5 encodes a murine basic helix-loop-helix transcription factor expressed during early stages of retinal neurogenesis. Development [Suppl] 1998;125:4821–4833. doi: 10.1242/dev.125.23.4821. [DOI] [PubMed] [Google Scholar]
- 10.Brown NL, Patel S, Brzezinski J, Glaser T. Math5 is required for retinal ganglion cell and optic nerve formation. Development [Suppl] 2001;128:2497–2508. doi: 10.1242/dev.128.13.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bunger MK. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czeisler CA, Shanahan TL, Klerman EB, Martens H, Brotman DJ, Emens JS, Klein T, Rizzo JF. Suppression of melatonin secretion in some blind patients by exposure to bright light. N Engl J Med. 1995;332:6–11. doi: 10.1056/NEJM199501053320102. [DOI] [PubMed] [Google Scholar]
- 13.Daan S. The Colin S. Pittendrigh Lecture: Colin Pittendrigh, Jurgen Aschoff, and the natural entrainment of circadian systems. J Biol Rhythms. 2000;15:195–207. doi: 10.1177/074873040001500301. [DOI] [PubMed] [Google Scholar]
- 14.Davidson AJ, Cappendijk SL, Stephan FK. Feeding-entrained circadian rhythms are attenuated by lesions of the parabrachial region in rats. Am J Physiol. 2000;278:R1296–R1304. doi: 10.1152/ajpregu.2000.278.5.R1296. [DOI] [PubMed] [Google Scholar]
- 15.Edgar DM, Kilduff TS, Martin CE, Dement WC. Influence of running wheel activity on free-running sleep/wake and drinking circadian rhythms in mice. Physiol Behav. 1991;50:373–378. doi: 10.1016/0031-9384(91)90080-8. [DOI] [PubMed] [Google Scholar]
- 16.Faradji H, Cespuglio R, Rondot G, Paut L, Jouvet M. Absence of light-dark entrainment on the sleep-waking cycle in mice with intact visual perception. Brain Res. 1980;202:41–49. [PubMed] [Google Scholar]
- 17.Foster RG, Provencio I, Hudson D, Fiske S, De Grip W, Menaker M. Circadian photoreception in the retinally degenerate mouse (rd/rd). J Comp Physiol [A] 1991;169:39–50. doi: 10.1007/BF00198171. [DOI] [PubMed] [Google Scholar]
- 18.Freedman MS, Lucas RJ, Soni B, von Schantz M, Munoz M, David-Gray Z, Foster R. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:502–504. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- 19.Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4:1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- 20.Halberg F, Visscher MB, Bittner JJ. Relation of visual factors to eosinophil rhythm in mice. Am J Physiol. 1954;179:229–235. doi: 10.1152/ajplegacy.1954.179.2.229. [DOI] [PubMed] [Google Scholar]
- 21. Hannibal J, Hindersson P, Knudsen SM, Georg B, Fahrenkrug J. The photopigment melanopsin is exclusively present in pituitary adenylate cyclase-activating polypeptide-containing retinal ganglion cells of the retinohypothalamic tract. J Neurosci 22 2002. RC191(1–7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hendrickson AE, Wagoner N, Cowan WM. An autoradiographic and electron microscopic study of retino-hypothalamic connections. Z Zellforsch Mikrosk Anat. 1972;135:1–26. doi: 10.1007/BF00307084. [DOI] [PubMed] [Google Scholar]
- 24.Herzog ED. The mammalian circadian clock shop. Semin Cell Dev Biol. 2001;12:295–303. doi: 10.1006/scdb.2001.0257. [DOI] [PubMed] [Google Scholar]
- 25.Holtzman RL, Malach R, Gozes I. Disruption of the optic pathway during development affects vasoactive intestinal peptide mRNA expression. New Biol. 1989;1:215–221. [PubMed] [Google Scholar]
- 26.Hsu SM, Raine L, Fanger H. The use of antiavidin antibody and avidin-biotin-peroxidase complex in immunoperoxidase technics. Am J Clin Pathol. 1981;75:816–821. doi: 10.1093/ajcp/75.6.816. [DOI] [PubMed] [Google Scholar]
- 27.Janik D, Mrosovsky N. Intergeniculate leaflet lesions and behaviorally-induced shifts of circadian rhythms. Brain Res. 1994;651:174–182. doi: 10.1016/0006-8993(94)90695-5. [DOI] [PubMed] [Google Scholar]
- 28.Johnson RF, Moore RY, Morin LP. Loss of entrainment and anatomical plasticity after lesions of the hamster retinohypothalamic tract. Brain Res. 1988;460:297–313. doi: 10.1016/0006-8993(88)90374-5. [DOI] [PubMed] [Google Scholar]
- 29.Klein DC, Moore RY. Pineal N-acetyltransferase and hydroxyindole-O-methyltransferase: control by the retinohypothalamic tract and the suprachiasmatic nucleus. Brain Res. 1979;174:245–262. doi: 10.1016/0006-8993(79)90848-5. [DOI] [PubMed] [Google Scholar]
- 30.Klerman EB, Rimmer DW, Dijk DJ, Kronauer RE, Rizzo JF, III, Czeisler CA. Nonphotic entrainment of the human circadian pacemaker. Am J Physiol. 1998;274:R991–R996. doi: 10.1152/ajpregu.1998.274.4.r991. [DOI] [PubMed] [Google Scholar]
- 31.Laemle LK, Ottenweller JE. Daily patterns of running wheel activity in male anophthalmic mice. Physiol Behav. 1998;64:165–171. doi: 10.1016/s0031-9384(98)00045-6. [DOI] [PubMed] [Google Scholar]
- 32.Laemle LK, Ottenweller JE. Nonphotic entrainment of activity and temperature rhythms in anophthalmic mice. Physiol Behav. 1999;66:461–471. doi: 10.1016/s0031-9384(98)00318-7. [DOI] [PubMed] [Google Scholar]
- 33.Liu W, Mo Z, Xiang M. The Ath5 proneural genes function upstream of Brn3 POU domain transcription factor genes to promote retinal ganglion cell development. Proc Natl Acad Sci USA. 2001;98:1649–1654. doi: 10.1073/pnas.98.4.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowrey PL, Takahashi JS. Genetics of the mammalian circadian system: photic entrainment, circadian pacemaker mechanisms, and posttranslational regulation. Annu Rev Genet. 2000;34:533–562. doi: 10.1146/annurev.genet.34.1.533. [DOI] [PubMed] [Google Scholar]
- 35.Lucas RJ, Foster RG. Neither functional rod photoreceptors nor rod or cone outer segments are required for the photic inhibition of pineal melatonin. Endocrinology. 1999;140:1520–1524. doi: 10.1210/endo.140.4.6672. [DOI] [PubMed] [Google Scholar]
- 36.Lucas RJ, Freedman MS, Munoz M, Garcia-Fernandez JM, Foster RG. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:505–507. doi: 10.1126/science.284.5413.505. [DOI] [PubMed] [Google Scholar]
- 37.Mikkelsen JD. Visualization of efferent retinal projections by immunohistochemical identification of cholera toxin subunit B. Brain Res Bull. 1992;28:619–623. doi: 10.1016/0361-9230(92)90112-b. [DOI] [PubMed] [Google Scholar]
- 38.Mikkelsen JD, Serviere J. Demonstration of a direct projection from the retina to the hypothalamic supraoptic nucleus of the hamster. Neurosci Lett. 1992;139:149–152. doi: 10.1016/0304-3940(92)90539-j. [DOI] [PubMed] [Google Scholar]
- 39.Moore RY. Retinohypothalamic projection in mammals: a comparative study. Brain Res. 1973;49:403–409. doi: 10.1016/0006-8993(73)90431-9. [DOI] [PubMed] [Google Scholar]
- 40.Moore RY. Organization of the mammalian circadian system. Ciba Found Symp. 1995;183:88–106. [PubMed] [Google Scholar]
- 41.Moore RY, Speh JC, Card JP. The retinohypothalamic tract originates from a distinct subset of retinal ganglion cells. J Comp Neurol. 1995;352:351–366. doi: 10.1002/cne.903520304. [DOI] [PubMed] [Google Scholar]
- 42.Munekawa K, Tamada Y, Iijima N, Hayashi S, Ishihara A, Inoue K, Tanaka M, Ibata Y. Development of astroglial elements in the suprachiasmatic nucleus of the rat: with special reference to the involvement of the optic nerve. Exp Neurol. 2000;166:44–51. doi: 10.1006/exnr.2000.7490. [DOI] [PubMed] [Google Scholar]
- 43.Nagai N, Nagai K, Nakagawa H. Effect of orbital enucleation on glucose homeostasis and morphology of the suprachiasmatic nucleus. Brain Res. 1992;589:243–252. doi: 10.1016/0006-8993(92)91283-k. [DOI] [PubMed] [Google Scholar]
- 44.Penev PD, Turek FW, Zee PC. A serotonin neurotoxin attenuates the phase-shifting effects of triazolam on the circadian clock in hamsters. Brain Res. 1995;669:207–216. doi: 10.1016/0006-8993(94)01237-c. [DOI] [PubMed] [Google Scholar]
- 45.Provencio I, Rollag MD, Castrucci AM. Photoreceptive net in the mammalian retina: this mesh of cells may explain how some blind mice can still tell day from night. Nature. 2002;415:493. doi: 10.1038/415493a. [DOI] [PubMed] [Google Scholar]
- 46.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 47.Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- 48.Rosenwasser AM, Pelchat RJ, Adler NT. Memory for feeding time: possible dependence on coupled circadian oscillators. Physiol Behav. 1984;32:25–30. doi: 10.1016/0031-9384(84)90064-7. [DOI] [PubMed] [Google Scholar]
- 49.Sack RL, Lewy AJ, Blood ML, Keith LD, Nakagawa H. Circadian rhythm abnormalities in totally blind people: incidence and clinical significance. J Clin Endocrinol Metab. 1992;75:127–134. doi: 10.1210/jcem.75.1.1619000. [DOI] [PubMed] [Google Scholar]
- 50.Silver J. Abnormal development of the suprachiasmatic nuclei of the hypothalamus in a strain of genetically anophthalmic mice. J Comp Neurol. 1977;176:589–606. doi: 10.1002/cne.901760409. [DOI] [PubMed] [Google Scholar]
- 51.Stephan FK. Circadian rhythm dissociation induced by periodic feeding in rats with suprachiasmatic lesions. Behav Brain Res. 1983;7:81–98. doi: 10.1016/0166-4328(83)90006-2. [DOI] [PubMed] [Google Scholar]
- 52.Stephan FK, Swann JM, Sisk CL. Anticipation of 24-hr feeding schedules in rats with lesions of the suprachiasmatic nucleus. Behav Neural Biol. 1979a;25:346–363. doi: 10.1016/s0163-1047(79)90415-1. [DOI] [PubMed] [Google Scholar]
- 53.Stephan FK, Swann JM, Sisk CL. Entrainment of circadian rhythms by feeding schedules in rats with suprachiasmatic lesions. Behav Neural Biol. 1979b;25:545–554. doi: 10.1016/s0163-1047(79)90332-7. [DOI] [PubMed] [Google Scholar]
- 54.Tabandeh H, Lockley S, Buttery R, Skene D, Defrance R, Arendt J, Bird A. Disturbance of sleep in blindness. Am J Ophthalmol. 1998;126:707–712. doi: 10.1016/s0002-9394(98)00133-0. [DOI] [PubMed] [Google Scholar]
- 55.Thresher RJ, Vitaterna MH, Miyamoto Y, Kazantsev A, Hsu DS, Petit C, Selby CP, Dawut L, Smithies O, Takahashi JS, Sancar A. Role of mouse cryptochrome blue-light photoreceptor in circadian photoresponses. Science. 1998;282:1490–1494. doi: 10.1126/science.282.5393.1490. [DOI] [PubMed] [Google Scholar]
- 56.Tucker P, Laemle L, Munson A, Kanekar S, Oliver ER, Brown N, Schlecht H, Vetter M, Glaser T. The eyeless mouse mutation (ey1) removes an alternative start codon from the Rx/rax homeobox gene. Genesis. 2001;31:43–53. doi: 10.1002/gene.10003. [DOI] [PubMed] [Google Scholar]
- 57.van der Horst GT, Muijtjens M, Kobayashi K, Takano R, Kanno S, Takao M, de Wit J, Verkerk A, Eker AP, van Leenen D, Buijs R, Bootsma D, Hoeijmakers JH, Yasui A. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- 58.Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, Takahashi JS. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994;264:719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vitaterna MH, Selby CP, Todo T, Niwa H, Thompson C, Fruechte EM, Hitomi K, Thresher RJ, Ishikawa T, Miyazaki J, Takahashi JS, Sancar A. Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc Natl Acad Sci USA. 1999;96:12114–12119. doi: 10.1073/pnas.96.21.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang SW, Kim BS, Ding K, Wang H, Sun D, Johnson RL, Klein WH, Gan L. Requirement for math5 in the development of retinal ganglion cells. Genes Dev. 2001;15:24–29. doi: 10.1101/gad.855301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weaver DR. The suprachiasmatic nucleus: a 25-year retrospective. J Biol Rhythms. 1998;13:100–112. doi: 10.1177/074873098128999952. [DOI] [PubMed] [Google Scholar]
- 62.Zheng B, Larkin DW, Albrecht U, Sun ZS, Sage M, Eichele G, Lee CC, Bradley A. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400:169–173. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
- 63.Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sun ZS, Eichele G, Bradley A, Lee CC. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105:683–694. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]