Abstract
Migrating axons require the correct presentation of guidance molecules, often at multiple choice points, to find their target. Netrin 1, a bifunctional cue involved in both attracting and repelling axons, is involved in many cell migration and axon pathfinding processes in the CNS. The netrin 1 receptor DCC and itsCaenorhabditis elegans homolog UNC-40 have been implicated in directing the guidance of axons toward netrin sources, whereas the C. elegans UNC-6 receptor, UNC-5 is necessary for migrations away from UNC-6. However, a role of vertebrate UNC-5 homologs in axonal migration has not been demonstrated. We demonstrate that the Unc5h3 gene product, shown previously to regulate cerebellar granule cell migrations, also controls the guidance of the corticospinal tract, the major tract responsible for coordination of limb movements. Furthermore, we show that corticospinal tract fibers respond differently to loss of UNC5H3. In addition, we observe corticospinal tract defects in mice homozygous for a spontaneous mutation that truncates the Dcctranscript. Postnatal day 0 netrin 1 mutant mice also demonstrate corticospinal tract abnormalities. Last, interactions between the Dcc and Unc5h3 mutations were observed in gene dosage experiments. This is the first evidence of an involvement in axon guidance for any member of the vertebrateunc-5 family and confirms that both the cellular and axonal guidance functions of C. elegans unc-5 have been conserved in vertebrates.
Keywords: dorsal funiculus, mouse, spinal cord, pyramidal decussation, rcm, P3 domain
Development of the CNS depends on correct cellular migrations and axonal pathfinding to establish connectivity. Although axons navigate pathways that can be complex and cover large distances, their routes are simplified through intermediate targets, termed choice points (Stoeckli and Landmesser, 1998). As an axon encounters successive choice points during pathfinding, it responds to different sets of molecules. These guidance molecules provide attractive and repulsive signals to specific receptors present on the growth cone. For example, the Caenorhabditis elegansbifunctional cue UNC-6 guides axon migrations by attractive or repulsive mechanisms depending on the response of its receptor (Hedgecock et al., 1990; Wadsworth et al., 1996; Culotti and Merz, 1998). Cells and axons expressing the UNC-6 receptor UNC-5 are repelled by an UNC-6 gradient (Hedgecock et al., 1990; Leung-Hagesteijn et al., 1992; Wadsworth et al., 1996). In contrast, UNC-6 is predominantly attractive for cells and axons expressing the UNC-40 receptor (Hedgecock et al., 1990; Chan et al., 1996).
In vertebrates, similar mechanisms are involved in commissure formation. Spinal cord commissural axons expressing the vertebrateunc-40 homolog Dcc are attracted ventrally toward regions of netrin 1 (Ntn1) expression in the floor plate (Kennedy et al., 1994; Keino-Masu et al., 1996). On reaching the midline, additional cues are necessary for axons to cross the midline, such as axonin-1 (contactin 2) and NrCAM (Stoeckli and Landmesser, 1998). Another receptor molecule, Roundabout (Robo), is postulated to be required on the axons to prevent them from recrossing the midline, presumably by repulsion from Slit expressed at the midline (Li et al., 1999). Thus, the migration route of axons appears broken down into steps, each of which requires a particular set of molecules.
Here we show that in addition to its role in controlling the migrations of cerebellar neurons (Ackerman et al., 1997; Leonardo et al., 1997),Unc5h3 also plays a role in axonal guidance, a role not demonstrated previously for the vertebrate unc-5 genesin vivo. Our results indicate that Unc5h3controls the pathfinding of corticospinal tract (CST) axons at two distinct points, the decussation and the final turn into the dorsal funiculus. Also, we describe CST defects in mice homozygous for a newly identified mutant allele of Dcc,Dcckanga, which, unlike the targeted mutant allele, survives to adulthood. Furthermore, we demonstrate that aberrant axonal projections in Unc5h3rcmmutant mice are modulated by mutations in the Dcc gene. Last, we show that Ntn1 mutant mice have defects in the pyramidal decussation and dorsal funiculus, demonstrating a role for this guidance cue in the control of CST axons.
MATERIALS AND METHODS
Mice. The Unc5h3rcm(Unc5h3rcmTg(Ucp)1.23Kz),Dcctm1Wbg, and Ntn1(Ntn1Gt(pGT1.8TM)629Wcs) mutations were maintained on C57BL/6J × SJL/J segregating, 129×1/SvJ, and C57BL/6J backgrounds, respectively (Serafini et al., 1996; Ackerman et al., 1997; Fazeli et al., 1997). Dcckangamice arose in a C.AKR-Tgncog/+ research mouse colony at The Jackson Laboratory, and the Tgncog mutation has been segregated out of the line. Mutant mice were obtained from heterozygous crosses and were identified by either PCR or their abnormal gait; Ntn1/Ntn1mice were identified by the absence of corpus callosum and anterior commissure in forebrain sections (Serafini et al., 1996).
Immunohistology. Adult mice were anesthetized with tribromoethanol and intracardially perfused with PBS followed by cold 4% paraformaldehyde (PFA) in PBS. After overnight incubation at 4°C, brains and spinal cords were dissected and rinsed in PBS. Brains and spinal cords of newborn mice were immersed in either 4% PFA (anti-DCC and anti-neurofilament) or Carnoy's fixative (60% methanol, 30% chloroform, and 10% acetic; anti-neurofilament only) for 24 hr. After processing in ethanol or sucrose, the tissue was embedded in either OCT (Tissue-Tek, Torrance, CA) at −20°C or paraffin and sectioned at 7–12 μm.
For immunofluorescence studies with mouse monoclonal anti-calcium/calmodulin (CaM) kinase II antibody (Oncogene Research Products, San Diego, CA), antigen retrieval was performed on paraffin sections by microwaving slides in 0.01 m sodium citrate, pH 6, for 8 min. After boiling, the slides were cooled to room temperature in this solution for 30 min, washed in PBS, and incubated in the anti-CaM kinase II antibody (1:100) overnight at 4°C before detection with Cy3-labeled goat anti-mouse secondary antibody (1:100;Chemicon, Temecula, CA). For studies with mouse monoclonal anti-DCC (Novocastra) and anti-neurofilament antibody 2H3 (Developmental Studies Hybridoma Bank, Iowa City, IA), antigen retrieval was performed as described above. Sections were washed in Tris-buffered saline and incubated in anti-DCC (1:30) or anti-neurofilament (1:50) overnight at 4°C before detection with biotin-labeled goat anti-mouse IgG1 secondary antibody (1:500; Southern Biotechnology Associates, Inc., Birmingham, AL) and Alexa Fluor 488-labeled streptavidin (1:500; Molecular Probes, Eugene, OR). For studies with the mouse monoclonal anti-neurofilament antibody 2H3 only, paraffin sections were blocked in PBS, 0.3% Triton X-100, 3% nonfat dried milk, and 5% DMSO (PBS/T-MD) twice for 1 hr each. Primary antibody (1:50 in PBS/T-MD) was applied and incubated for 2 d at 4°C. After washes in PBS and 0.3% Triton X-100 (PBS/T) and PBS/T-MD, Cy3-conjugated goat anti-mouse secondary antibody (1:500 in PBS/T-MD) was applied and incubated overnight at 4°C. Slides were mounted using either Slow Fade Light Anti-Fade media (Molecular Probes) or Fluoromount G (Electron Microscopy Sciences, Ft. Washington, PA). All images were captured from a Leitz (Wetzlar, Germany) DMRXE microscope with the SPOT digital camera (Diagnostic Instruments, Sterling Heights, MI), an Olympus Optical (Tokyo, Japan) BX50 microscope with a Retiga 1300 camera (Optical Analysis Corp., Nashua, NH) or a Leitz TCSNT confocal microscope (Leica, Bannockburn, IL).
Biotin dextran amine tract tracing. All animal procedures were approved by the Animal Care and Use Committee at The Jackson Laboratory.Unc5h3rcm/Unc5h3rcm(n = 5) andDcckanga/Dcckanga(n = 6) animals and their corresponding wild-type controls (n = 6), were anesthetized with tribromoethanol, and 3 holes were made in the skull over the right cerebral hemisphere ∼1–2 mm apart, using a handheld microdrill with a 1 mm dental drill bit (Fine Science Tools, North Vancouver, British Columbia, Canada). One microliter of 15% biotin dextran amine (BDA; Molecular Probes) was injected at a depth of 1 mm using a 32 gauge needle attached to a 5 μl Hamilton (Reno, NV) syringe. After 7–10 d, the animals were anesthetized and intracardially perfused with 4% PFA in PBS. The dissected brains and spinal cords were fixed overnight, rinsed in PBS, and allowed to sink in 20% sucrose in PBS before embedding in OCT medium at –20°C for cryosectioning.
Serial sections (10 μm) were mounted on slides coated with poly-l-lysine (Sigma, St. Louis, MO). Sections were air-dried at room temperature for 1 hr, fixed in acetone for 10 min, and air-dried again for 15 min. Sections were washed in PBS/T and incubated in blocking solution (PBS/T and 2% BSA) for 30 min. Streptavidin-Cy3 (Jackson ImmunoResearch, West Grove, PA) was applied at a concentration of 0.5 μg/ml and incubated overnight at 4°C. To assess the injection quality, the forebrains of injected animals were cryosectioned (10 μm), and every third cryosection was stained with streptavidin-Cy3. Slides were mounted using Slow Fade Light Anti-Fade media, and images were captured as above.
In situ hybridization. Postnatal day 0 (P0) brains were fixed overnight at 4°C in 4% PFA in PBS. The paraffin-embedded tissues were sectioned and mounted on Plus slides (Fisher Scientific, Springfield, NJ). Prehybridization washes, hybridization, and posthybridization washes were performed as described previously using33P-labeled Unc5h3 andNtn1 antisense and sense riboprobes (Przyborski et al., 1998). Sections were counterstained with hematoxylin.
Southern blot analysis and reverse transcription-PCR.Genomic DNA (15 μg) was digested by EcoRI and transferred to a nylon filter by standard methods (Osmonics Inc., Minnetonka, MN). Hybridization was performed at 65°C with a [32P]dCTP-labeled probe corresponding to bp 3149–4266 of the Dcc coding region.
Total or poly(A+) RNA was isolated from adult brain (1 μg), and cDNA was prepared using random hexamers and Superscript reverse transcriptase (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. For PCR, 2 μl of the reverse transcription (RT) reaction was incubated with buffer, dNTPs, 400 nm primers, and ExTaq polymerase (TaKaRa, Tokyo, Japan) according to the manufacturer's directions. Exon structure was based on the human genomic sequence of Dcc (Cho et al., 1994;Cooper et al., 1995). Primer sequences were designed from theDcc coding sequence (GenBank accession number NM_007831(Cooper et al., 1995): exon 26, 5′-GCCATCCCTGTACCAACACTAGAAAG-3′ (bp 3748–3773); exon 27, 5′-CTGGCTGAGACAAAAGCGGT-3′ (bp 4111–4092); exon 28, 5′-TGGCTGGATCCTCTGTGGGCT-3′ (bp 4252–4232); and exon 29, 5′-TTAAAAGGCTGAGCCTGTGATGG-3′ (bp 4344–4322). For genomic PCR, 100 ng of DNA was used in standard PCRs using a 400 nmconcentration of each primer corresponding to the intron sequence on either side of exon 29: forward primer, 5′-AGCCTACCAAGACCTCATCCTGAC-3′; and reverse primer, 5′-GCAGTGACAAAGACCCAGAACTATG-3′.
RESULTS
The CST is absent from the dorsal funiculus of the spinal cord inUnc5h3rcm/Unc5h3rcmmice
The C. elegans UNC-5 and UNC-40 receptors mediate UNC-6-dependent migrations of both axons and cells during development of the nervous system (Hedgecock et al., 1990). Similarly, the vertebrate homologs of unc-6 and unc-40,Ntn1 and Dcc, are necessary for the migration of axons of several commissures and neurons of the pontine nuclei (Serafini et al., 1996; Fazeli et al., 1997). Although theunc-5 homolog Unc5h3 is necessary for proper granule cell migration during cerebellar formation (Ackerman et al., 1997; Przyborski et al., 1998), the role of the vertebrate UNC-5 homologs in the guidance of axons during CNS development has not been established. Thus, we examined several axon tracts known to be under netrin 1 and DCC guidance inUnc5h3rcm/Unc5h3rcmmice.
Examination of the corpus callosum and anterior and hippocampal commissures revealed no apparent defects inUnc5h3rcm/Unc5h3rcmbrains (data not shown), nor were obvious defects in commissural axons observed in transverse sections of spinal cord in mutant mice. However, a dramatic reduction of the size of the dorsal funiculus was seen inUnc5h3rcm mutant mice (Fig.1A,B). The dorsal funiculus is composed of three axonal tracts: the ascending gracilis and cuneatus tracts, which enter the spinal cord from the hindlimbs and forelimbs, respectively, and the descending corticospinal tract, which resides in the ventral-most portion of the dorsal funiculus (Joosten, 1990; Stanfield, 1992). When compared with that of wild-type mice, the dorsal funiculus appeared shorter and thinner at the ventral aspect, suggesting CST defects inUnc5h3rcm/Unc5h3rcmmice.
Fig. 1.
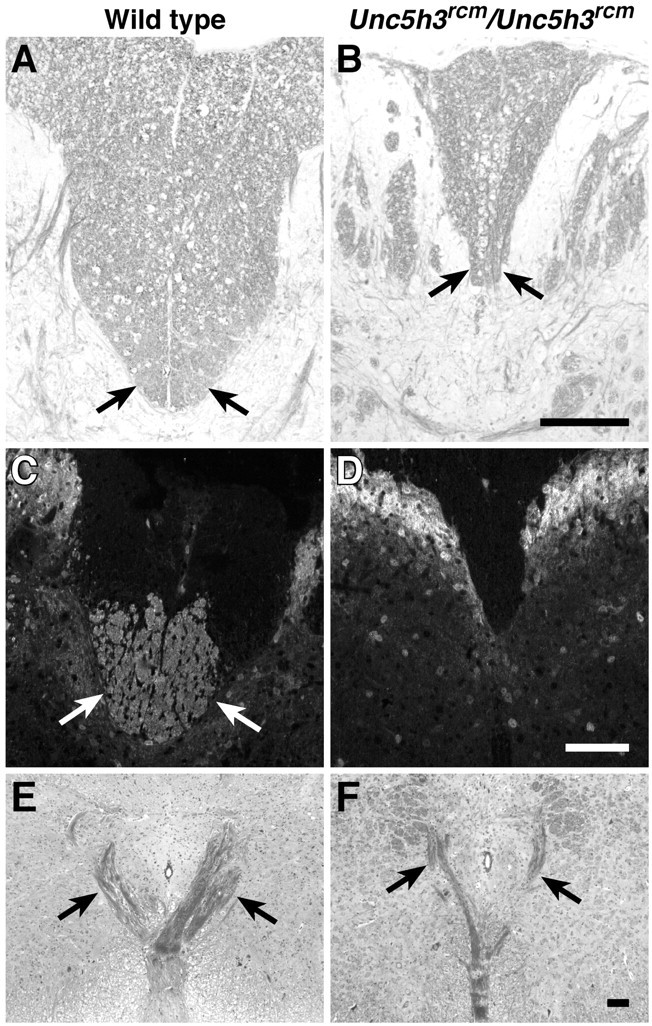
CST abnormalities inUnc5h3rcm/Unc5h3rcmmice. The dorsal funiculus (arrows) is shown in transverse sections of cervical spinal cord from wild-type (A, C) andUnc5h3rcm/Unc5h3rcm(B, D) mice stained with LFB and counterstained with cresyl violet (A, B) or antibody against CaM kinase II (C, D). The ipsilateral ventral pyramidal tract crosses the midline dorsally, forming the pyramidal decussation (E, wild type; F,Unc5h3rcm/Unc5h3rcm). Note the thinner decussation of pyramidal tract fibers (arrows) inUnc5h3rcm/Unc5h3rcmbrain, and the CST is missing from the dorsal funiculus of the mutant spinal cord. Scale bars, 100 μm.
To more closely examine the CST in mutant animals, immunohistochemistry was performed using an antibody against CaM kinase II, which is expressed on the CST but not the ascending tracts, in the dorsal funiculus (Terashima et al., 1994). Compared with the wild type, there were almost no axons expressing CaM kinase II in theUnc5h3rcm/Unc5h3rcmdorsal funiculus, confirming the absence of the CST in these animals (Fig. 1C,D). This defect was also observed in two other mutant alleles of Unc5h3 (data not shown).
CST axons originate from layer V neurons in the cerebral cortex and pass through the internal capsule and over the pontine nuclei in the pons, becoming the distinct ventral ipsilateral bundles constituting the pyramidal tract in the hindbrain (Stanfield, 1992; Gianino et al., 1999). Just before the spinal cord, the ventral CST fibers cross dorsally over the midline, forming the pyramidal decussation, and enter the spinal cord, continuing down the cord as the ventral-most tract in the dorsal funiculus of the spinal cord. The pyramidal tract was examined in adult wild-type andUnc5h3rcm/Unc5h3rcmhindbrains stained with the myelin stain Luxol fast blue (LFB). No apparent differences between wild-type and mutant animals were observed in this tract as it passed through the hindbrain (data not shown). The pyramidal decussation, although thinner than that of the wild type, was also present inUnc5h3rcm/Unc5h3rcmmice (Fig. 1E,F), but the CST axons could not be traced further in LFB-stained sections. Therefore, the CST disruption in these mice appears to be, at least in part, below the level of the pyramidal decussation.
Unc5h3rcm mutant CST axons follow two abnormal trajectories
To visualize the path of CST axons we performed anterograde labeling ofUnc5h3rcm/Unc5h3rcmand wild-type control adult animals. BDA was injected unilaterally into the motor cortex, and 1 week after surgery, brains and spinal cords were dissected and serially sectioned. Examination of coronal sections from BDA-injected mutant and wild-type animals confirmed that the pyramidal tract traversed the hindbrain normally in mutant animals (Fig. 2A,B). However, just before the decussation in the ventral region of the hindbrain ofUnc5h3rcm mutant animals, the pyramidal tract broadened, spreading from medial to lateral (Fig.2C,D). In wild-type controls, broadening of the pyramidal tract was not observed, and at the level of the pyramidal decussation, the labeled CST axons moved dorsally, crossing the midline (Fig.2E). In contrast, at the decussation ofUnc5h3rcm mutant mice, two distinct bundles formed, with one bundle in its normal position close to the midline and another axon bundle located in a slightly lateral position (Fig. 2F).
Fig. 2.
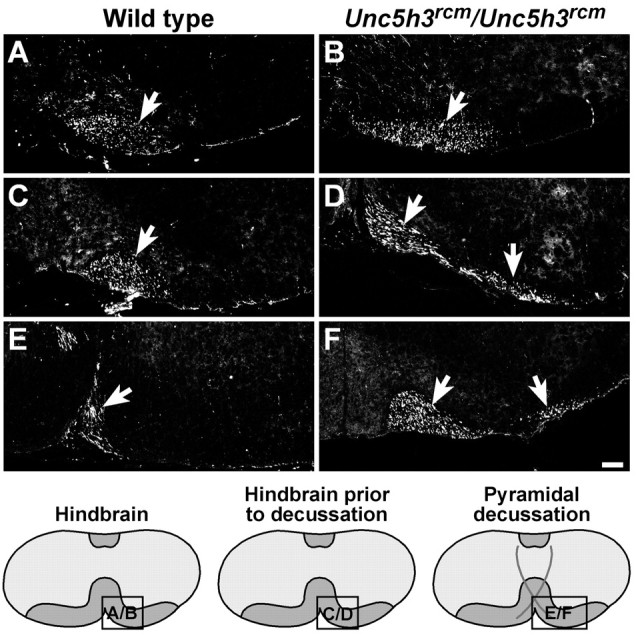
The migration of the CST through theUnc5h3rcm/Unc5h3rcmhindbrain. BDA injected into the motor cortex of wild-type (A, C, E) andUnc5h3rcm/Unc5h3rcm(B, D, F) animals was visualized with streptavidin-Cy3. The trajectories of the ipsilateral pyramidal tract (arrow) appear similar in both wild-type (A) andUnc5h3rcm/Unc5h3rcm(B) mice through the hindbrain before the pyramidal decussation. Just before the pyramidal decussation (C, D), the pyramidal tract broadens (arrows) inUnc5h3rcm/Unc5h3rcmmice, whereas the labeled fibers in the wild-type brain remain bundled (arrow). At the pyramidal decussation, the mutant tract splits (arrows) into medial and lateral fiber bundles (F). The relative level of sections is shown in the accompanying diagrams at the bottom. Scale bars, 100 μm.
Once achieving the dorsal contralateral gray matter, decussated fibers curved into the ventral-most portion of the dorsal funiculus of the wild-type spinal cord (Fig.3A). Tracing of the more medial bundle of axons in theUnc5h3rcm/Unc5h3rcmhindbrain confirmed that, as seen in LFB-stained sections, pyramidal tract axons crossed to the contralateral side (data not shown). However, in contrast to the wild-type CST mice, only a few decussatedUnc5h3rcm/Unc5h3rcmCST axons were funneled correctly into the contralateral dorsal funiculus, as evidenced by a small amount of staining in the ventral region of the dorsal funiculus of the cervical spinal cord (Fig.3B). Therefore, most CST fibers that cross the midline in these mutant animals do not enter the dorsal funiculus.
Fig. 3.
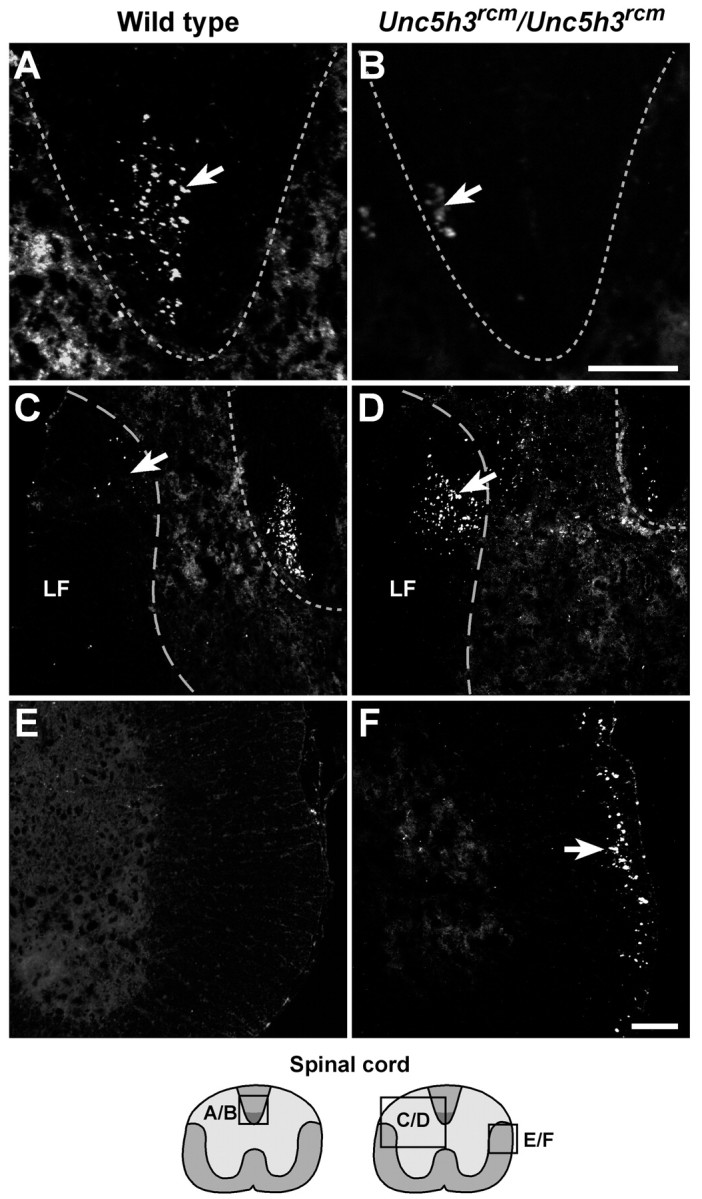
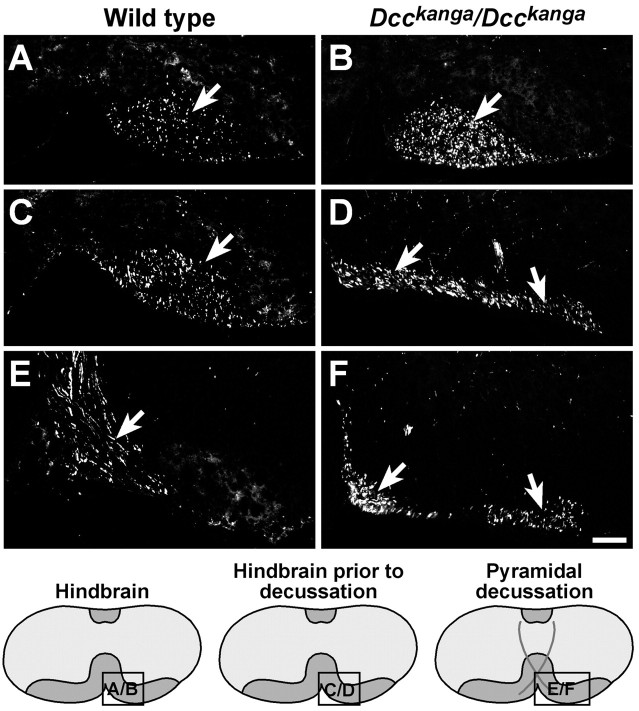
Aberrant CST axon migration inUnc5h3rcm/Unc5h3rcmspinal cord. BDA injected into the motor cortex of wild-type (A, C, E) andUnc5h3rcm/Unc5h3rcm(B, D, F) animals was visualized with streptavidin-Cy3. Labeled contralateral CST fibers (arrow) are present in the wild-type (A) dorsal funiculus (dotted line), whereas only a few labeled fibers are seen in the dorsal funiculus ofUnc5h3rcm/Unc5h3rcmanimals (B, arrow). In contrast to the wild-type spinal cord, many labeled axons are visible in the contralateral lateral funiculus (C, D, elongated dotted line, arrows) and the dorsal gray matter at the thoracic level of the spinal cord ofUnc5h3rcm/Unc5h3rcmmice. The ipsilateral lateral funiculus of the wild-type cervical spinal cord (E) does not contain labeled CST fibers, whereas many labeled ipsilateral CST fibers move from the ventral pyramidal tract into the ipsilateral lateral funiculus of theUnc5h3rcm/Unc5h3rcmspinal cord (F, arrow). The relative level of sections is shown in the accompanying diagrams at thebottom. LF, Contralateral lateral funiculus. Scale bars, 100 μm.
Many decussated labeled axons were observed in the dorsal gray matter adjacent to the dorsal funiculus and just into the white matter of the contralateral lateral funiculus ofUnc5h3rcm/Unc5h3rcmcervical and thoracic spinal cord, whereas only a few labeled fibers were seen in the lateral white matter of cervical and thoracic spinal cord sections of wild-type controls (Fig. 3C,D). These axons in wild-type mice likely comprise a minor proportion of CST fibers (<5%) that normally split from the main bundle after decussating and instead of entering the dorsal funiculus enter the gray matter of upper cervical spinal cord sections directly (Gianino et al., 1999). However, it is not clear whether this normal pathway of a small percentage of fibers is acting as a permissive default substrate for the aberrantUnc5h3rcm mutant fibers.
As mentioned above, inUnc5h3rcm/Unc5h3rcmmice, a significant proportion of labeled CST axons split from the pyramidal tract just before decussating. These fibers continued to follow an ipsilateral trajectory into the cervical spinal cord, whereas in wild-type mice, this split in the pyramidal tract was not seen, and all of the visible fibers crossed the midline (Fig.3E,F). Although the mutant fibers remained ipsilateral, they did not stay ventral but eventually entered the lateral funiculus of the spinal cord, which was not observed in the ipsilateral lateral white matter in wild-type animals (Fig.3E,F). TheseUnc5h3rcm/Unc5h3rcmipsilateral CST axon fibers were positioned in the outermost region of the lateral funiculus of the spinal cord, away from the spinal gray matter.
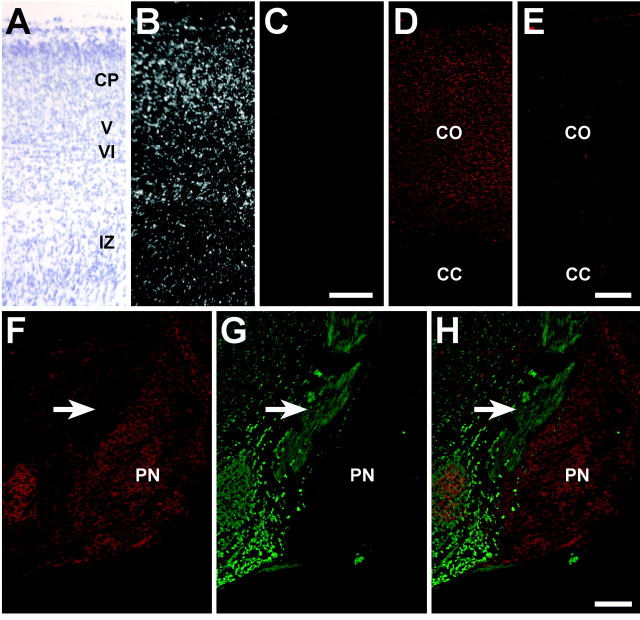
To determine whether Unc5h3 is expressed in cortical neurons that generate CST axons, in situ hybridization was performed on the presumptive motor cortex at embryonic day 17.5 (E17.5) and P0 (a time when leading axons have entered the cervical spinal cord).Unc5h3 mRNA was widely expressed in the wild-type cortex, with highest levels in the cortical plate. Low levels of expression were also noted in other regions of the cortex, including layer V, that were not seen in the sense control (Fig.4A–C; data not shown).
Fig. 4.
Unc5h3 and DCC expression at P0.In situ hybridization was performed withUnc5h3-specific antisense and sense probes on sagittal sections of P0 wild-type forebrain (A–C). At P0, the neurons of the cortical plate and presumptive layers V and VI express the Unc5h3 transcript (B). A corresponding bright-field photograph of the sections is shown inA, and the Unc5h3-specific sense control showing no signal is shown in C. Immunofluorescence with monoclonal anti-DCC antibody and secondary and tertiary antibody control (without primary antibody) on sagittal sections of P0 wild-type forebrain (D, E) shows that DCC is expressed throughout the cortex (CO). DCC immunofluorescence on sagittal sections of P0 wild-type hindbrain (F, H, overlay) revealed very few DCC-positive fibers. The presumptive CST axons (arrow) were detected by immunofluorescence with antibody to the 160 kDa neurofilament on neighboring sections (G, H, overlay). CC, Corpus callosum;CO, cortex; CP, cortical plate;IZ, intermediate zone; PN, pontine nuclei; V, layer V; VI, layer VI. Scale bars, 100 μm.
Expression of another netrin 1 receptor, DCC, was also detected throughout the wild-type E17.5 and P0 cortex by immunofluorescence (Fig. 4D,E; data not shown). However, we were not able to detect DCC-positive CST fibers as they passed through the hindbrain of E17.5 or P0 animals (Fig. 4F–H; data not shown).
Dcckanga: a spontaneous mutant allele of Dcc
Because of the perinatal lethality of the recessiveDcc-targeted mutant allele, it has not been possible to study the role of Dcc in structures that develop postnatally (Fazeli et al., 1997). However, this difficulty has been circumvented by our identification of a spontaneous recessive mutation ofDcc that survives into adulthood.
Adult mice homozygous for a spontaneous mutation that results in the mild to severe inability to maintain an upright position were identified in a production colony. These mice often move their hind legs in a concerted manner, resulting in a somewhat hopping gait; thus this mutation was named kanga. Analysis of brains of these mice demonstrated that both the corpus callosum and the anterior commissure were missing (Fig.5A,B). In addition, the pontine nuclei were absent from the hindbrain (data not shown).
Fig. 5.
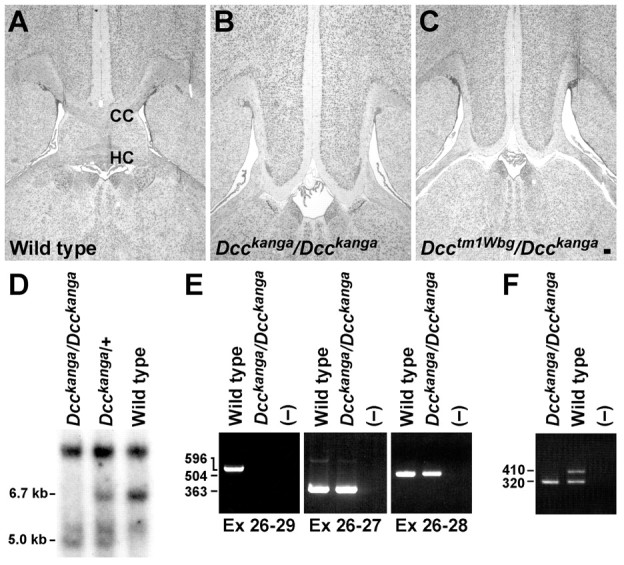
kanga, a spontaneous mutant allele of Dcc. Luxol fast blue- and cresyl violet-stained frontal sections through the forebrain of wild-type (A),Dcckanga/Dcckanga(B), andDcctm1Wbg/Dcckanga(C) mice are shown. Note the absence of the corpus callosum (CC) and hippocampal commissures (HC) in theDcckanga/DcckangaandDcctm1Wbg/Dcckangaforebrains. D, Southern blot ofEcoRI-digested genomic DNA from wild-type,Dcckanga/+, andDcckanga/Dcckangamice probed with cDNA corresponding to a portion of theDcc coding region (bp 3149–4266). Note the 5.0 and 6.7 kb RFLPs between wild-type andDcckanga heterozygotes or homozygotes.E, RT-PCR of Dcc transcripts inDcckanga mutants. DcccDNA between exon 26 through the last exon of coding region (exon 29) does not amplify fromDcckanga/Dcckanga(Ex 26–29). However, primers corresponding to exons 26 and 27 or exons 26 and 28 (Ex 26–27, Ex 26–28, respectively) do amplify products from mutant cDNA. F, PCR analysis of exon 29 using primers from the surrounding introns (410 bp) demonstrates that exon 29 is deleted inDcckanga/Dcckangagenomic DNA. An unrelated fragment from chromosome 3 (320 bp) was amplified as an internal control for the PCR. Scale bar, 100 μm.
A genome scan using polymorphic microsatellite markers was performed on affected F2 animals obtained by intercrossing the F1 progeny from a mapping cross (BALB/cBy.Akr affected × C57BL/6J). Analysis of these results demonstrated linkage of the kanga gene with markers D18Mit9 and D18Mit123 on chromosome 18 near the previously reported position of the Dcc gene (Justice et al., 1992; Stassen et al., 1996). The similarity of brain defects in kanga mutant animals to those reported for the targeted allele of Dcc (Fazeli et al., 1997), combined with the map position of kanga, suggested that kangawas a mutant allele of Dcc. Complementation tests were performed by crossing mice heterozygous for the targeted Dccallele (Dcctm1Wbg) and kangaheterozygotes. Offspring were scored for ataxia at weaning. Ataxic animals were obtained at a frequency of 12% (9 of 75), andkanga was renamed Dcckanga. Histological analysis of affected animals from the complementation test confirmed that like Dcctm1Wbg mutant mice, the corpus callosum and hippocampal commissure were missing in the forebrain (Fig. 5C).
Southern blot analysis of wild-type andDcckanga/Dcckangagenomic DNA revealed restriction fragment length polymorphisms (RFLPs) within the Dcc gene, suggesting a rearrangement in theDcckanga genome (Fig. 5D). To further analyze the mutation in theDcckanga allele, RT-PCR was performed using Dcc coding region-specific primers. No abnormalities in the length or sequence of amplified fragments through exon 26 of theDcc coding sequence were observed in RT-PCR products fromDcckanga/DcckangacDNA (data not shown). However, when reactions were performed using primers corresponding to exons 26 and 29 (the last coding exon), products were not obtained from mutant cDNA (Fig. 5E). In reactions using the exon 26 primer with primers corresponding to exon 27 or 28, amplified products were obtained from mutant cDNA. Combined, these results suggest that Dcc transcripts in these mutants do not contain exon 29 (Fig. 5E). In agreement, PCRs using primers corresponding to intron sequence surrounding exon 29 failed to amplify from mutant DNA, demonstrating that this exon is deleted inDcckanga/Dcckangagenomic DNA (Fig. 5F).
The CST ofDcckanga/Dcckangamice is abnormal at the pyramidal decussation
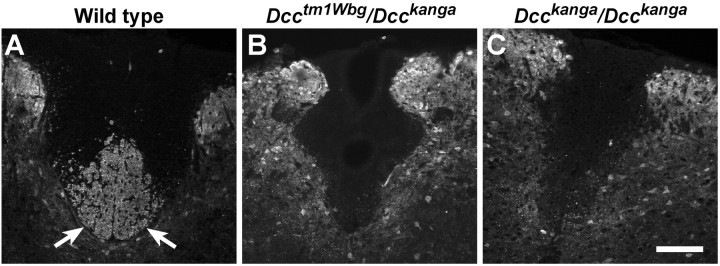
A hopping gait has been reported previously in mice with CST defects (Dottori et al., 1998). Furthermore, DCC is expressed on the CST axons during extension of this tract (Shu et al., 2000). This prompted us to examine the CST ofDcctm1Wbg/Dcckangamice from the complementation test andDcckanga/Dcckangamice. Anti-CaM kinase II immunohistochemical staining of transverse sections of spinal cord from adultDcctm1Wbg/DcckangaandDcckanga/Dcckangamice demonstrated that likeUnc5h3rcm/Unc5h3rcmmice, the CST is absent from the dorsal funiculus of the spinal cord (Fig. 6). However, in contrast toUnc5h3rcm/Unc5h3rcmmice, in which a large number of CST axons crossed the midline, LFB staining did not detect decussating CST fibers in the hindbrains of these mutant mice (data not shown).
Fig. 6.
The CST is absent from the dorsal funiculus of the spinal cord inDcckanga/ Dcckangamice. The dorsal funiculus is shown in transverse sections of cervical spinal cord from wild-type (A),Dcctm1Wbg/Dcckanga(B), andDcckanga/Dcckanga(C) mice stained with antibody against CaM kinase II. CaM kinase II-positive fibers are present in the wild-type CST (arrows) but absent from the dorsal funiculus of mutant mice. Scale bar, 100 μm.
Anterograde tracing of the CST was performed by unilateral injection of BDA, as described above. In the pyramidal tract,Dcckanga/Dcckanga-labeled CST axons resembled the BDA-injected wild-type control mice (Fig.7A,B). However, similar toUnc5h3rcm mutants, the labeled fiber bundle spread medially to laterally just before the level expected of the decussation (Fig. 7C,D), and this broadened tract split into medial and lateral fiber bundles (Fig. 7E,F). However unlikeUnc5h3rcm/Unc5h3rcmmice, neither axon bundle was observed to decussate in theDcckanga mutant brain (Fig.8A,B), confirming our LFB staining results. Furthermore, the medial pyramidal tract fiber bundle extended ipsilaterally in the ventral funiculus until at least the upper thoracic spinal cord, and the more lateral fiber bundle moved into the ventral regions of the ipsilateral lateral funiculus in the spinal cord (Fig. 8C,D). These lateral labeled fibers were slightly more ventral in the lateral funiculus relative to the nondecussating Unc5h3rcm mutant fibers. However, in bothUnc5h3rcm/Unc5h3rcmandDcckanga/Dcckangamice, CST axons separated from the main CST fiber bundle to project ipsilaterally and laterally into the spinal cord. As expected from our CaM kinase II immunofluorescence results, labeled fibers were not observed in the dorsal funiculus ofDcckanga mutant mice (Fig.8E,F). Thus, mutation of the Dccgene disrupts the decussation of all CST axons, whereasUnc5h3 mutations only affect the midline crossing of some CST axons.
Fig. 7.
The CST broadens and splits in the hindbrain ofDcckanga/Dcckangamice. BDA injected into the motor cortex of wild-type (A, C, E) andDcckanga/Dcckanga(B, D, F) animals was visualized with streptavidin-Cy3. No differences in the placement of pyramidal tract fibers (arrow) in the hindbrain before the pyramidal decussation were noted between wild-type and mutant mice (A, B). Just before the expected level of the pyramidal decussation inDcckanga/Dcckangamice, labeled fibers broaden (arrows), whereas the labeled fibers (arrow) in the wild-type hindbrain remain bundled (C, D). At the pyramidal decussation, theDcckanga/Dcckangatract splits (arrows) into medial and lateral fiber bundles (F). The relative level of sections is shown in the diagrams at the bottom. Scale bars, 100 μm.
Fig. 8.
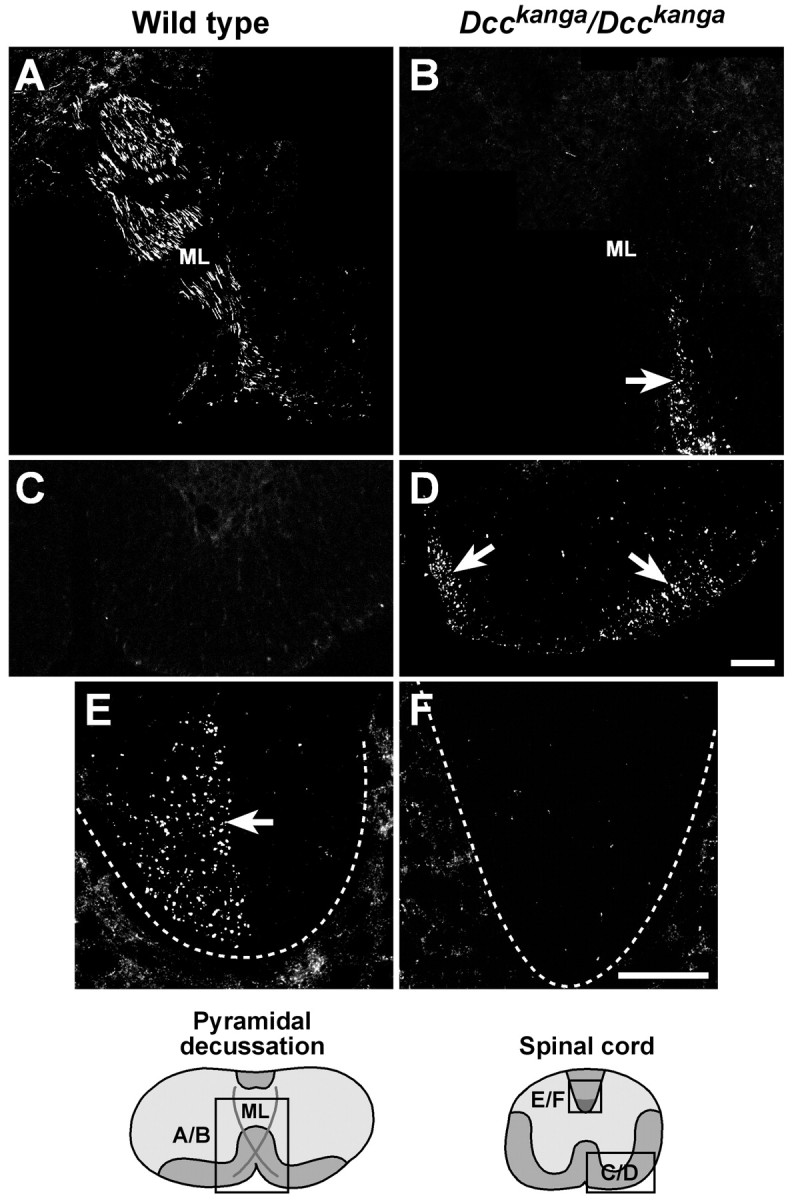
The CST migrates incorrectly in the spinal cord ofDcckanga/Dcckangamice. BDA injected into the motor cortex of wild-type (A, C, E) andDcckanga/Dcckanga(B, D, F) animals was visualized with streptavidin-Cy3. Labeled fibers cross the midline (ML) dorsally in the wild-type pyramidal decussation (A) but do not decussate inDcckanga/Dcckangamice (B, arrow). Two bundles of ipsilateral fibers were observed in theDcckanga/Dcckangaventral cervical spinal cord (arrows) but not the wild-type spinal cord (C, D). The dorsal funiculus (dotted lines) in the wild type (arrow) but not mutant spinal cord contains labeled contralateral CST axons (E, F). The relative level of sections is shown in the diagrams at the bottom. Scale bars, 100 μm.
Dcc modulates the position of nondecussating CST fibers in theUnc5h3rcm/Unc5h3rcmspinal cord
The presence of nondecussating CST fibers in bothUnc5h3rcm andDcckanga mutant mice suggested that these genes might cooperate in guidance of CST fibers. Gene dosage experiments were performed to further investigate this possibility. Mice with a combination of Unc5h3rcm andDcctm1Wbg mutations were generated, and the position of CST fibers was determined by immunofluorescence analysis with antibodies to CaM kinase II.
In mice homozygous for the Unc5h3rcmmutation but wild type at the Dcc locus, CST fibers that crossed the midline were found in the contralateral lateral funiculus, whereas those that failed to cross were found in the ipsilateral lateral funiculus but not the ventral funiculus (Figs. 3D,F,9A). In contrast, only nondecussating fibers were observed inDcckanga/Dcckangamice in the ventral and the lateral funiculus as two separate bundles (Figs. 8D, 9B). Mice homozygous for theUnc5h3rcm mutation and heterozygous for the Dcctm1Wbg mutation had CaM kinase II-positive fibers in both the lateral and ventral funiculus (Fig.9C; data not shown). A few CaM kinase II-positive axons were also visible in the ventral funiculus of the cervical spinal cord ofUnc5h3rcm/+;Dcctm1Wbg/+ mice (Fig. 9D); however, labeled fibers were not seen in mice heterozygous for the Dcctm1Wbgmutation (Fig. 9E). Although aberrant CST fibers were found in the ventral funiculus of the cervical spinal cord, they did not continue into the thoracic spinal cord (data not shown). Also, as demonstrated by LFB staining, some CST fibers still crossed the midline at the pyramidal decussation inUnc5h3rcm/Unc5h3rcm;Dcctm1Wbg/+ mice (Fig. 9F). These results demonstrate that UNC5H3 and DCC act synergistically in the guidance of a portion of CST axons.
Fig. 9.
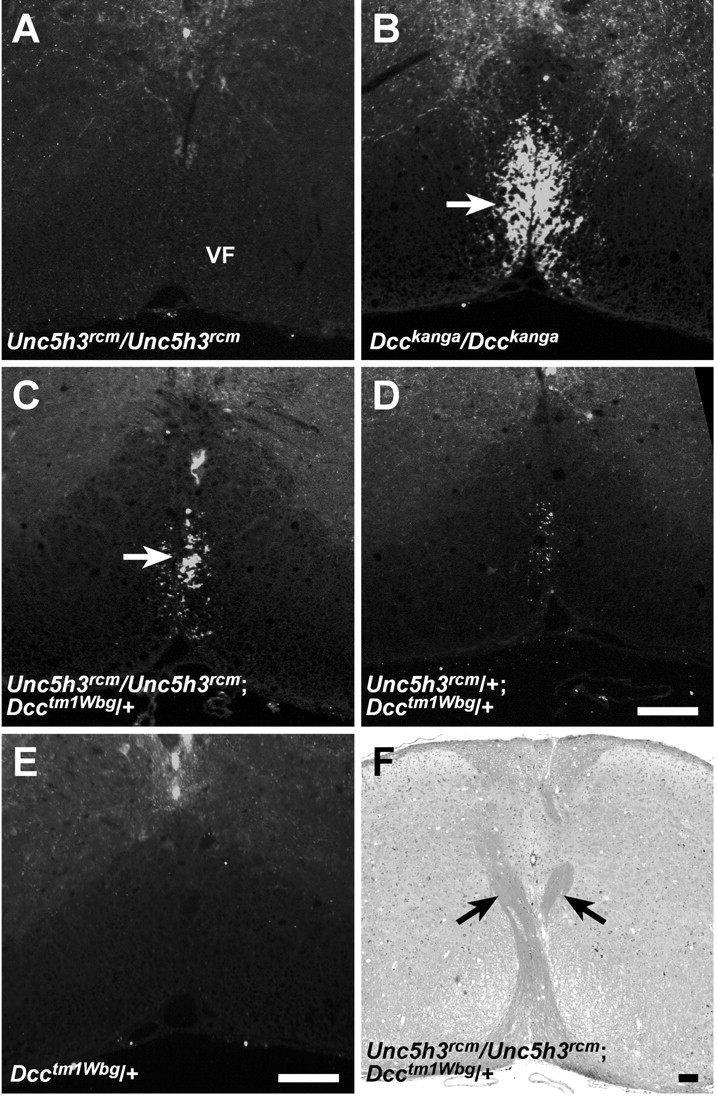
CST fibers are present in the ventral funiculus ofUnc5h3rcm/Unc5h3rcm;Dcctm1Wbg/+ adult mice. Transverse sections of adult cervical spinal cord fromUnc5h3rcm/Unc5h3rcm(A),Dcckanga/Dcckanga(B),Unc5h3rcm/Unc5h3rcm;Dcctm1Wbg/+ (C),Unc5h3rcm/+;Dcctm1Wbg/+ (D), andDcctm1Wbg/+ (E) mice were stained with anti-CaM kinase II antibody. Note that immunopositive fibers are present in the ventral funiculus (VF; arrow) ofDcckanga/Dcckanga,Unc5h3rcm/Unc5h3rcm;Dcctm1Wbg/+, andUnc5h3rcm/+;Dcctm1Wbg/+ but notUnc5h3rcm/Unc5h3rcmand Dcctm1Wbg/+ mice. Decussating CST fibers (arrows) are still present inUnc5h3rcm/Unc5h3rcm;Dcctm1Wbg/+ mice (F). Scale bars, 100 μm.
Dorsal funiculus defects in Ntn1/Ntn1 mice
Ligand-binding and other assays suggest that both DCC and UNC5H3 are receptors for the guidance molecule netrin 1 (Keino-Masu et al., 1996; Serafini et al., 1996; Leonardo et al., 1997; Przyborski et al., 1998; Stein et al., 2001). Furthermore, Dcc andNtn1 homozygous mutant mice have similar commissural defects (Serafini et al., 1996; Fazeli et al., 1997). Thus, we investigated whether netrin 1 also mediates CST axon guidance.
In situ hybridization using an Ntn1-specific probe was performed on sections at the level of the pyramidal decussation, which was visualized on an adjacent section with antibody to neurofilament (Fig. 10A).Ntn1 was highly expressed at the midline just ventral to the central canal and adjacent to the point at which the decussating CST axons crossed the midline (Fig. 10B).
Fig. 10.
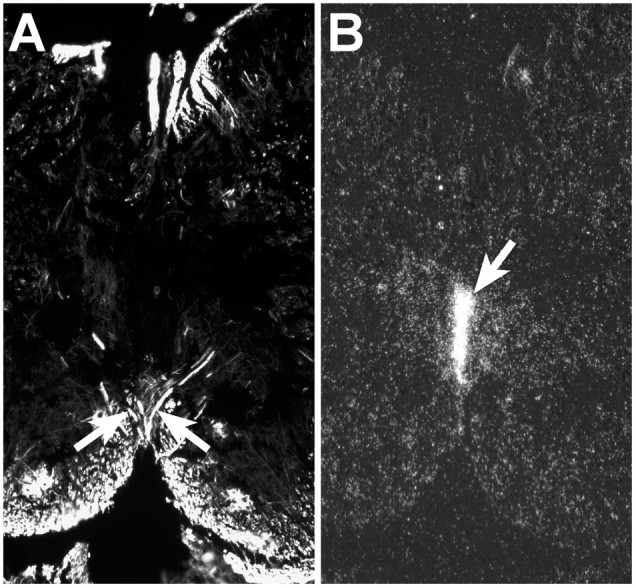
Ntn1 is expressed adjacent to the midline crossover point of CST fibers at the pyramidal decussation in P0 mice. The pyramidal decussation of wild-type mice at P0 was visualized by anti-2H3 (160 kDa neurofilament isoform) antibody (A, arrows). In situ hybridization with an Ntn1-specific probe is shown on an adjacent section (B). At P0, Ntn1 expression is observed at the midline immediately below the central canal extending ventrally (arrow) toward the pyramidal decussation. Scale bar, 100 μm.
In addition, we examined the CST of Ntn1/Ntn1 mice. Because these mice die soon after birth (Serafini et al., 1996), it was not possible to trace the CST using BDA. Furthermore, CaM kinase II was not expressed in the dorsal funiculus at P0, making immunohistochemistry with this antibody uninformative. Thus, to visualize the pyramidal decussation and the dorsal funiculus of the cervical spinal cord, we performed immunofluorescence staining with a monoclonal antibody to the 160 kDa isoform of neurofilament. This neurofilament isoform is expressed on descending CST fibers, which at birth have reached the midcervical level of the spinal cord (Stanfield, 1992; Gianino et al., 1999), as well as the two ascending tracts of the dorsal funiculus (Fig. 11A).
Fig. 11.
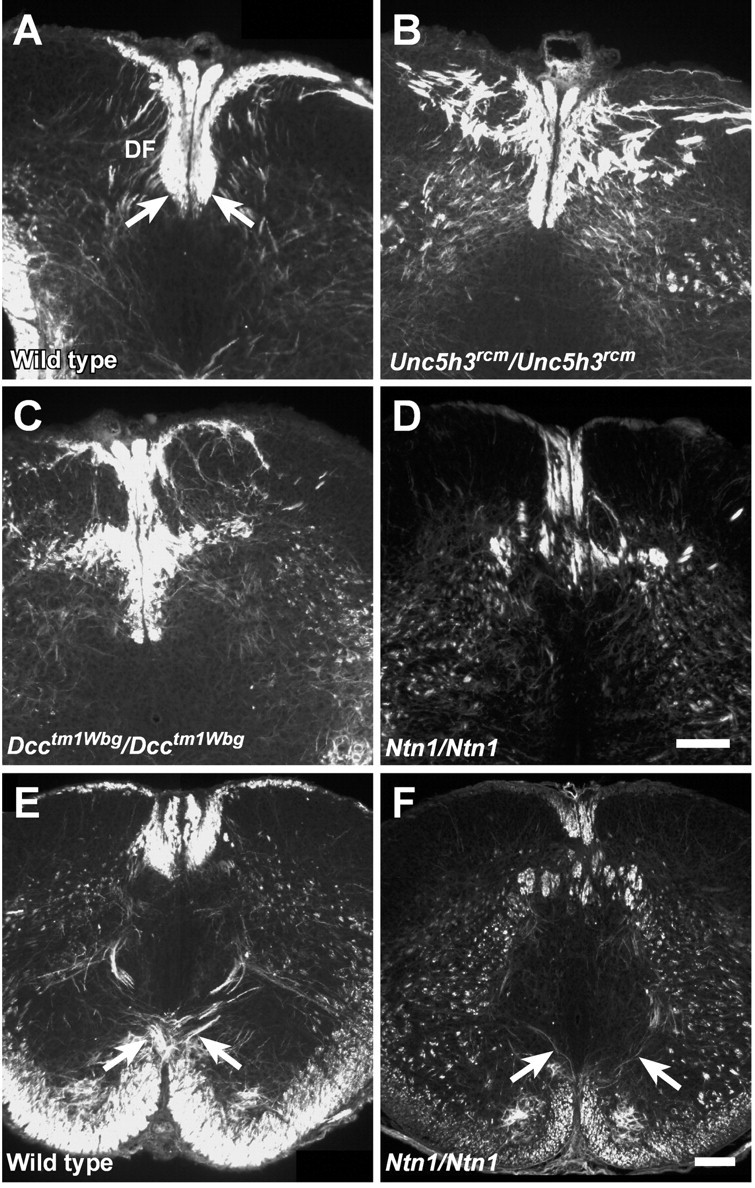
The dorsal funiculus is abnormal inUnc5h3rcm/Unc5h3rcm,Dcctm1Wbg/Dcctm1Wbg, and Ntn1/Ntn1 newborn mice. Transverse sections of P0 upper cervical spinal cord from wild type (A),Unc5h3rcm/Unc5h3rcm(B),Dcctm1Wbg/Dcctm1Wbg(C), and Ntn1/Ntn1(D) mice were immunostained with anti-2H3 antibody. Note the abnormal shape of the ventral portion of the dorsal funiculus (df; A, arrows) is accompanied by many neurofilament-positive misplaced axons throughout the dorsal funicular region of the mutant spinal cords. The pyramidal decussation (arrows) was visualized with anti-2H3 antibody in wild-type mice (E) but was much reduced in Ntn1/Ntn1 newborn mice (F). Scale bars, 100 μm.
Analysis of hindbrain and spinal cord sections of P0Unc5h3rcm/Unc5h3rcmmice confirmed that pyramidal tract axons decussated, but the dorsal funiculus in the cervical spinal cord appeared abnormal even in P0 animals (Fig. 11B). P0Dcctm1Wbg/Dcctm1Wbganimals also exhibited abnormalities in the dorsal funiculus, with the expected absence of the pyramidal decussation (Fig. 11C; data not shown).
Similar analysis of the upper cervical spinal cord of P0Ntn1/Ntn1 mice demonstrated that the dorsal funiculus of these animals was clearly abnormal when compared with the wild-type controls (Fig. 11D). Furthermore, the examination of serial sections of the hindbrain demonstrated that the pyramidal decussation was much reduced in these mice (Fig.11E,F). Because neurofilament is widely expressed in the hindbrain and spinal cord white matter, it is not clear whether these mice have the aberrant ipsilateral fibers seen in the tracing studies ofUnc5h3rcm/Unc5h3rcmandDcckanga/Dcckangaadult mice. However, these results demonstrate that netrin 1 also regulates CST development and may act, at least in part, via the UNC5H3 and DCC receptors.
DISCUSSION
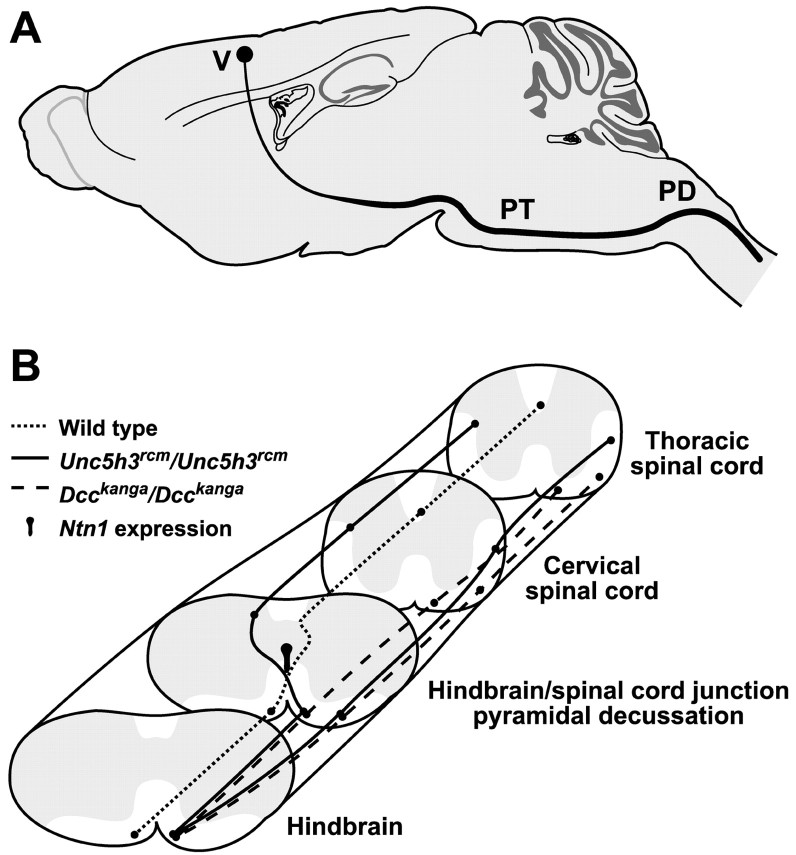
As axons move through complicated migration pathways, they arrive at intermediate targets that act as choice points to guide their path. Our results demonstrate that netrin 1 and two of its receptors, UNC5H3 and DCC, are necessary at multiple points along the migration of the longest mammalian axonal tract, the corticospinal tract (Fig.12). In addition to demonstrating that these molecules are necessary for CST axon guidance, our results reveal the existence of at least two groups of CST axons under different genetic regulation at the pyramidal decussation.
Fig. 12.
CST pathways inUnc5h3rcm/Unc5h3rcmandDcckanga/Dcckangamice. A, Normal path of the CST through the brain. Axons from layer V neurons (V) in the cerebral cortex migrate through the internal capsule to the ventral aspect of the brain, where they proceed as parallel ipsilateral fiber bundles on either side of midline. In the hindbrain, these fibers form the pyramidal tract (PT), which crosses midline at the junction of the hindbrain and spinal cord (PD) before entering the dorsal spinal cord. B, CST abnormalities inUnc5h3rcm/Unc5h3rcmandDcckanga/Dcckangamice. Normally, the pyramidal tract decussates at the hindbrain–spinal cord junction and continues down the spinal cord in the ventral-most portion of the contralateral dorsal funiculus (dotted line). InUnc5h3rcm/Unc5h3rcmmice, some CST axons in the ipsilateral pyramidal tract separate from the main fiber bundle (solid lines). Although the more medial fiber bundle decussates, it does not move into the dorsal funiculus but instead enters and continues in the contralateral lateral funiculus and dorsal gray matter of the spinal cord. The lateral fiber bundle moves further laterally into the ipsilateral lateral funiculus of the spinal cord. Many CST axons in the ipsilateral pyramidal tract ofDcckanga/Dcckangamice also separate with neither fiber bundle decussating (elongated dotted lines). The medial fiber bundle continues in the ipsilateral ventral funiculus of the spinal cord. As seen inUnc5h3rcm/Unc5h3rcmmice, the lateral fiber bundle moves further laterally into the ipsilateral lateral funiculus of the spinal cord. Ntn1expression is present dorsal to the crossover point of the pyramidal decussation.
We describe a new mutant allele of the Dcc gene,Dcckanga, which we hypothesize is a hypomorphic allele. In contrast to the perinatal death of mice homozygous for the null Dcctm1Wbg-targeted allele (Fazeli et al., 1997), Dcckangahomozygotes survive into adulthood. We have demonstrated that the last coding exon of DCC is deleted in theDcckanga mutant genome. Interestingly, this exon encodes a region known as the P3 domain that has been shown by in vitro axon turning assays to be required for both homodimerization of DCC and netrin 1-dependent attraction of axons (Kolodziej et al., 1996; Stein et al., 2001). In addition, results from similar assays demonstrate that the DCC P3 domain is also the target region for binding of the Slit receptor, Robo, which silences the netrin 1 response of DCC-expressing neurons (Stein and Tessier-Lavigne, 2001). After crossing the midline, it is hypothesized that axons upregulate Robo, which in turn binds to the P3 domain of DCC. This binding prevents homodimerization of DCC, thus converting the netrin 1-attractive response of these neurons to one of repulsion from the Slit ligand. CST axons do not cross the midline inDcckanga mutant mice, providing in vivo confirmation of the requirement of the P3 DCC domain in netrin 1-mediated attraction to the midline. However, the absence of decussating CST axons in Dcckanga mutant mice precludes the analysis of Robo–DCC interactions in CST development.
The viability of the Dcckanga mutant mice allows analysis of the role of Dcc in CST development, which our results show separates into two bundles just before the pyramidal decussation in these mice (Fig. 12). Although both of these bundles move ipsilaterally into the spinal cord, the more medially located bundle approaches but does not cross the midline, whereas the lateral bundle maintains its lateral position. This failure of midline crossing is seen in other commissural axons under netrin 1 regulation in the developing brain (Serafini et al., 1996; Fazeli et al., 1997). In agreement, the CST pyramidal decussation is much reduced inNtn1/Ntn1 animals. Combined, these data suggest thatDcc controls CST formation, in part via the attraction of DCC-expressing axons toward netrin 1 expressed at the midline in the developing brain and spinal cord (Keino-Masu et al., 1996).
We show that DCC is expressed throughout the cortex, andUnc5h3 is expressed in many layers of the cortex at a time when CST axons are extending. These results are consistent with the cell-autonomous phenotypes seen in mutants of the C. eleganshomologs unc-5 and unc-40, suggesting that the effect of mutation of Unc5h3 or Dcc on the CST is a primary defect of the axon pathfinding of CST axons. However, we were unable to detect DCC on neurofilament-positive axons likely to be the CST over the pontine nucleus in the hindbrain. This is consistent with published results showing low levels of DCC expression on CST axons in the forebrain region but not in caudal regions of the brain (Shu et al., 2000). This may indicate that the DCC on these axons is below the levels of detection, as seen previously for EphA4 (Dottori et al., 1998; Leighton et al., 2001). Alternatively, DCC may be expressed only on pioneering axons, which represent only a small minority of the axons in the CST once later-arriving axons have fasciculated. Last, it is possible that this tract, unlike others affected by mutation ofDcc, uses DCC in a non-cell-autonomous manner.
Our results further indicate that netrin 1 is a possible ligand for these receptors by its expression pattern adjacent to the pyramidal decussation and by its abnormal phenotype at the decussation and the dorsal funiculus. The extensive disruption of the dorsal funiculus in all of the mutants examined at P0 suggests that the gracilis and cuneatus tracts, in addition to the CST, may also be abnormal, a possibility requiring further study.
UNC5H3 is necessary for correct guidance of CST axons at two choice points: the pyramidal decussation and the turn into the dorsal funiculus (Fig. 12). Like CST axons in theDcckanga mutant mice, the CST inUnc5h3rcm/Unc5h3rcmmice splits into two fiber bundles at the pyramidal decussation. The lateral bundle continues ipsilaterally into the spinal cord. In contrast, the medial bundle decussates, possibly because of netrin 1-mediated attraction of DCC-expressing axons to the midline, but fails to turn into the dorsal funiculus, suggesting that DCC is not sufficient for the dorsal choice point. The final turn of CST axons into the dorsal funiculus takes place from a position lateral and slightly ventral with respect to the dorsal funiculus, suggesting that a repulsive guidance cue for turning axons would be expressed lateral to turning axons. The expression of Ntn1 transcripts at the ventral midline of the P0 pyramidal decussation makes it likely that UNC5H3-expressing axons would not be repulsed from netrin 1 to turn into the dorsal funiculus and instead use another, as yet unknown, repulsive ligand, which is expressed in the region lateral to migrating axons.
The existence of axons that are independently controlled at two separate choice points, the pyramidal decussation and the turn into the dorsal funiculus, may be indicative of two distinct sets of pioneering axons, perhaps arising from particular regions of the cortex. In agreement, mutations in the C. elegans unc-40 andunc-5 genes disrupt the navigation of pioneering axons (Hedgecock et al., 1990; Leung-Hagesteijn et al., 1992). Alternatively, the broadening and eventual bifurcation of the CST inDcckanga andUnc5h3rcm mutant mice at the expected level of the decussation suggests a role for DCC and UNC5H3 in fasciculation. This possibility is supported by work in C. elegans, which demonstrated that UNC-6 acts as a short-range cue for fasciculating PVP axons (Wadsworth et al., 1996; Culotti and Merz, 1998). Netrin 1 has also been implicated in short-range axonal guidance, directing DCC-expressing retinal ganglion cell axons as they exit the optic disk into the optic nerve (Deiner et al., 1997; de la Torre et al., 1997; Kennedy, 2000). In Drosophila, UNC5 can also demonstrate a short-range function by preventing axons from crossing the midline when expressed ectopically (Keleman and Dickson, 2001). Furthermore, UNC5H3 and DCC have immunoglobulin-like domains in the extracellular regions, and DCC also has extracellular fibronectin type III repeats, domains commonly found in cell adhesion molecules such as L1 and neural cell adhesion molecule (Van Vactor, 1998). Thus, in Unc5h3rcm mutant andDcckanga mutant mice, it may be that later-arriving axons are selectively defasciculating and making pathfinding errors at the choice point of the pyramidal decussation. In addition, Unc5h3rcm mutant axons that do cross the midline may defasciculate from pioneering axons at the turn into the dorsal funiculus. Consistent with a role ofUnc5h3 in fasciculation, a few labeled axons that may represent pioneering axons were found in the contralateral dorsal funiculus inUnc5h3rcm/Unc5h3rcmmice.
Nonallelic noncomplementation was observed between theUnc5h3rcm andDcctm1Wbg mutations. Although it is not clear whether the fibers in the ventral funiculus ofUnc5h3rcm/Unc5h3rcm;Dcctm1Wbg/+ mice are altered from a normally attractive or repulsive response, a repulsion mechanism is supported by a previous study (Hong et al., 1999). Because both mutations are null alleles, this phenotype is likely attributable to the simultaneous reduction of UNC5H3 and DCC protein levels, not the presence of an altered protein product that binds to and impedes the function of another (Regan and Fuller, 1988;Regan and Fuller, 1990). Although physical interactions of UNC5H3 and DCC have been shown to mediate repulsion when ectopically expressed inXenopus embryos (Hong et al., 1999), and additional results in C. elegans and Drosophila also suggest interactions (Hamelin et al., 1993; Chan et al., 1996; Colavita and Culotti, 1998; Keleman and Dickson, 2001), the fact that CST axons are not observed in the ventral funiculus of theUnc5h3rcm mutant mouse makes it unlikely that Dcctm1Wbg modulation of theUnc5h3rcm mutant phenotype is attributable to direct interactions of these protein products. Rather, this result suggests that these molecules may have partially redundant roles in mediating CST guidance at the midline. This suggestion is further strengthened by recent C. elegans studies demonstrating thatunc-40 functions in both unc-5-independent and -dependent UNC-6-repulsed distal tip cell migrations (Merz et al., 2001).
The CST has a complicated migration path, likely controlled by many genes in addition to those genes in the netrin 1 signaling pathway, some of which have been identified (Cohen et al., 1998;Dottori et al., 1998; Kullander et al., 2001). Our results indicate that, even within the CST, some axons are apparently under the control of separate guidance mechanisms. Netrin 1, which is expressed in the internal capsule, has been shown to stimulate axonal outgrowth from cultured rat cortical explants (Richards et al., 1997). Interestingly, our analysis of Unc5h3rcm mutant andDcckanga mutant mice did not reveal defects in the CST as it traversed the hindbrain until just before the decussation. These results suggest that these two gene products are involved only in guidance of CST fibers into the spinal cord, or that absence of these genes is functionally compensated by other family members during the initial outgrowth stages of the CST.
Footnotes
This work was supported by National Institutes of Health (NIH) Grant NS35900 (S.L.A.), NIH Postdoctoral Fellowship NS10757 (J.H.F.), NIH Resource Grant RR01183, and institutional National Cancer Institute core Grant CA34196 (The Jackson Laboratory). We thank Greg Martin and Jennifer Smith for assistance with images, Dr. Terrie Cunliffe-Beamer for surgical training, Chantal Longo-Guess for technical assistance, the Histology Department of The Jackson Laboratory for expertise and training, and Dr. Tom Gridley and Dr. Robert Burgess for critical reading of this manuscript. Dr. Robert Weinberg, Dr. Amin Fazeli, and Dr. Marc Tessier-Lavigne kindly providedDcctm1Wbg and Ntn1 mice and the Ntn1 in situ probe. The 2H3 monoclonal antibody, developed by Drs. T. M. Jessell and J. Dodd, was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa, Department of Biological Sciences (Iowa City, IA).
Correspondence should be addressed to Susan L. Ackerman, The Jackson Laboratory, 600 Main Street, Bar Harbor, ME 04609. E-mail:sla@jax.org.
Dr. Przyborski's present address: School of Biological and Biomedical Sciences, University of Durham, South Road, Durham DH1 3LE, UK.
REFERENCES
- 1.Ackerman SL, Kozak LP, Przyborski SA, Rund LA, Boyer BB, Knowles BB. The mouse rostral cerebellar malformation gene encodes an UNC-5-like protein. Nature. 1997;386:838–842. doi: 10.1038/386838a0. [DOI] [PubMed] [Google Scholar]
- 2.Chan SS, Zheng H, Su MW, Wilk R, Killeen MT, Hedgecock EM, Culotti JG. UNC-40, a C. elegans homolog of DCC (deleted in colorectal cancer), is required in motile cells responding to UNC-6 netrin cues. Cell. 1996;87:187–195. doi: 10.1016/s0092-8674(00)81337-9. [DOI] [PubMed] [Google Scholar]
- 3.Cho KR, Oliner JD, Simons JW, Hedrick L, Fearon ER, Preisinger AC, Hedge P, Silverman GA, Vogelstein B. The DCC gene: structural analysis and mutations in colorectal carcinomas. Genomics. 1994;19:525–531. doi: 10.1006/geno.1994.1102. [DOI] [PubMed] [Google Scholar]
- 4.Cohen NR, Taylor JS, Scott LB, Guillery RW, Soriano P, Furley AJ. Errors in corticospinal axon guidance in mice lacking the neural cell adhesion molecule L1. Curr Biol. 1998;8:26–33. doi: 10.1016/s0960-9822(98)70017-x. [DOI] [PubMed] [Google Scholar]
- 5.Colavita A, Culotti JG. Suppressors of ectopic UNC-5 growth cone steering identify eight genes involved in axon guidance in Caenorhabditis elegans. Dev Biol. 1998;194:72–85. doi: 10.1006/dbio.1997.8790. [DOI] [PubMed] [Google Scholar]
- 6.Cooper HM, Armes P, Britto J, Gad J, Wilks AF. Cloning of the mouse homologue of the deleted in colorectal cancer gene (mDCC) and its expression in the developing mouse embryo. Oncogene. 1995;11:2243–2254. [PubMed] [Google Scholar]
- 7.Culotti JG, Merz DC. DCC and netrins. Curr Opin Cell Biol. 1998;10:609–613. doi: 10.1016/s0955-0674(98)80036-7. [DOI] [PubMed] [Google Scholar]
- 8.Deiner MS, Kennedy TE, Fazeli A, Serafini T, Tessier-Lavigne M, Sretavan DW. Netrin-1 and DCC mediate axon guidance locally at the optic disc: loss of function leads to optic nerve hypoplasia. Neuron. 1997;19:575–589. doi: 10.1016/s0896-6273(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 9.de la Torre JR, Hopker VH, Ming GL, Poo MM, Tessier-Lavigne M, Hemmati-Brivanlou A, Holt CE. Turning of retinal growth cones in a netrin-1 gradient mediated by the netrin receptor DCC. Neuron. 1997;19:1211–1224. doi: 10.1016/s0896-6273(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 10.Dottori M, Hartley L, Galea M, Paxinos G, Polizzotto M, Kilpatrick T, Bartlett PF, Murphy M, Kontgen F, Boyd AW. EphA4 (Sek1) receptor tyrosine kinase is required for the development of the corticospinal tract. Proc Natl Acad Sci USA. 1998;95:13248–13253. doi: 10.1073/pnas.95.22.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fazeli A, Dickinson SL, Hermiston ML, Tighe RV, Steen RG, Small CG, Stoeckli ET, Keino-Masu K, Masu M, Rayburn H, Simons J, Bronson RT, Gordon JI, Tessier-Lavigne M, Weinberg RA. Phenotype of mice lacking functional deleted in colorectal cancer (Dcc) gene. Nature. 1997;386:796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- 12.Gianino S, Stein SA, Li H, Lu X, Biesiada E, Ulas J, Xu XM. Postnatal growth of corticospinal axons in the spinal cord of developing mice. Brain Res Dev Brain Res. 1999;112:189–204. doi: 10.1016/s0165-3806(98)00168-0. [DOI] [PubMed] [Google Scholar]
- 13.Hamelin M, Zhou Y, Su MW, Scott IM, Culotti JG. Expression of the UNC-5 guidance receptor in the touch neurons of C. elegans steers their axons dorsally. Nature. 1993;364:327–330. doi: 10.1038/364327a0. [DOI] [PubMed] [Google Scholar]
- 14.Hedgecock EM, Culotti JG, Hall DH. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron. 1990;4:61–85. doi: 10.1016/0896-6273(90)90444-k. [DOI] [PubMed] [Google Scholar]
- 15.Hong K, Hinck L, Nishiyama M, Poo MM, Tessier-Lavigne M, Stein E. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell. 1999;97:927–941. doi: 10.1016/s0092-8674(00)80804-1. [DOI] [PubMed] [Google Scholar]
- 16.Joosten EA. An ultrastructural double-labelling method: immunohistochemical localization of cell adhesion molecule L1 on HRP-labelled developing corticospinal tract axons in the rat. Histochemistry. 1990;94:645–651. doi: 10.1007/BF00271992. [DOI] [PubMed] [Google Scholar]
- 17.Justice MJ, Gilbert DJ, Kinzler KW, Vogelstein B, Buchberg AM, Ceci JD, Matsuda Y, Chapman VM, Patriotis C, Makris A, Tsichlis PN, Jenkins NA, Copeland NG. A molecular genetic linkage map of mouse chromosome 18 reveals extensive linkage conservation with human chromosomes 5 and 18. Genomics. 1992;13:1281–1288. doi: 10.1016/0888-7543(92)90047-v. [DOI] [PubMed] [Google Scholar]
- 18.Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS, Culotti JG, Tessier-Lavigne M. Deleted in colorectal cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- 19.Keleman K, Dickson BJ. Short- and long-range repulsion by the Drosophila unc5 netrin receptor. Neuron. 2001;32:605–617. doi: 10.1016/s0896-6273(01)00505-0. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy TE. Cellular mechanisms of netrin function: long-range and short-range actions. Biochem Cell Biol. 2000;78:569–575. [PubMed] [Google Scholar]
- 21.Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- 22.Kolodziej PA, Timpe LC, Mitchell KJ, Fried SR, Goodman CS, Jan LY, Jan YN. frazzled encodes a Drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell. 1996;87:197–204. doi: 10.1016/s0092-8674(00)81338-0. [DOI] [PubMed] [Google Scholar]
- 23.Kullander K, Croll SD, Zimmer M, Pan L, McClain J, Hughes V, Zabski S, DeChiara TM, Klein R, Yancopoulos GD, Gale NW. Ephrin-B3 is the midline barrier that prevents corticospinal tract axons from recrossing, allowing for unilateral motor control. Genes Dev. 2001;15:877–888. doi: 10.1101/gad.868901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leighton PA, Mitchell KJ, Goodrich LV, Lu X, Pinson K, Scherz P, Skarnes WC, Tessier-Lavigne M. Defining brain wiring patterns and mechanisms through gene trapping in mice. Nature. 2001;410:174–179. doi: 10.1038/35065539. [DOI] [PubMed] [Google Scholar]
- 25.Leonardo ED, Hinck L, Masu M, Keino-Masu K, Ackerman SL, Tessier-Lavigne M. Vertebrate homologues of C. elegans UNC-5 are candidate netrin receptors. Nature. 1997;386:833–838. doi: 10.1038/386833a0. [DOI] [PubMed] [Google Scholar]
- 26.Leung-Hagesteijn C, Spence AM, Stern BD, Zhou Y, Su MW, Hedgecock EM, Culotti JG. UNC-5, a transmembrane protein with immunoglobulin and thrombospondin type 1 domains, guides cell and pioneer axon migrations in C. elegans. Cell. 1992;71:289–299. doi: 10.1016/0092-8674(92)90357-i. [DOI] [PubMed] [Google Scholar]
- 27.Li HS, Chen JH, Wu W, Fagaly T, Zhou L, Yuan W, Dupuis S, Jiang ZH, Nash W, Gick C, Ornitz DM, Wu JY, Rao Y. Vertebrate slit, a secreted ligand for the transmembrane protein roundabout, is a repellent for olfactory bulb axons. Cell. 1999;96:807–818. doi: 10.1016/s0092-8674(00)80591-7. [DOI] [PubMed] [Google Scholar]
- 28.Merz DC, Zheng H, Killeen MT, Krizus A, Culotti JG. Multiple signaling mechanisms of the UNC-6/netrin receptors UNC-5 and UNC-40/DCC in vivo. Genetics. 2001;158:1071–1080. doi: 10.1093/genetics/158.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Przyborski SA, Knowles BB, Ackerman SL. Embryonic phenotype of Unc5h3 mutant mice suggests chemorepulsion during the formation of the rostral cerebellar boundary. Development. 1998;125:41–50. doi: 10.1242/dev.125.1.41. [DOI] [PubMed] [Google Scholar]
- 30.Regan CL, Fuller MT. Interacting genes that affect microtubule function: the nc2 allele of the haywire locus fails to complement mutations in the testis-specific beta-tubulin gene of Drosophila. Genes Dev. 1988;2:82–92. doi: 10.1101/gad.2.1.82. [DOI] [PubMed] [Google Scholar]
- 31.Regan CL, Fuller MT. Interacting genes that affect microtubule function in Drosophila melanogaster: two classes of mutation revert the failure to complement between haync2 and mutations in tubulin genes. Genetics. 1990;125:77–90. doi: 10.1093/genetics/125.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richards LJ, Koester SE, Tuttle R, O'Leary DD. Directed growth of early cortical axons is influenced by a chemoattractant released from an intermediate target. J Neurosci. 1997;17:2445–2458. doi: 10.1523/JNEUROSCI.17-07-02445.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 34.Shu T, Valentino KM, Seaman C, Cooper HM, Richards LJ. Expression of the netrin-1 receptor, deleted in colorectal cancer (DCC), is largely confined to projecting neurons in the developing forebrain. J Comp Neurol. 2000;416:201–212. doi: 10.1002/(sici)1096-9861(20000110)416:2<201::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 35.Stanfield BB. The development of the corticospinal projection. Prog Neurobiol. 1992;38:169–202. doi: 10.1016/0301-0082(92)90039-h. [DOI] [PubMed] [Google Scholar]
- 36.Stassen AP, Groot PC, Eppig JT, Demant P. Genetic composition of the recombinant congenic strains. Mamm Genome. 1996;7:55–58. doi: 10.1007/s003359900013. [DOI] [PubMed] [Google Scholar]
- 37.Stein E, Tessier-Lavigne M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science. 2001;291:1928–1938. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- 38.Stein E, Zou Y, Poo M, Tessier-Lavigne M. Binding of DCC by netrin-1 to mediate axon guidance independent of adenosine A2B receptor activation. Science. 2001;291:1976–1982. doi: 10.1126/science.1059391. [DOI] [PubMed] [Google Scholar]
- 39.Stoeckli ET, Landmesser LT. Axon guidance at choice points. Curr Opin Neurobiol. 1998;8:73–79. doi: 10.1016/s0959-4388(98)80010-x. [DOI] [PubMed] [Google Scholar]
- 40.Terashima T, Ochiishi T, Yamauchi T. Immunohistochemical detection of calcium/calmodulin-dependent protein kinase II in the spinal cord of the rat and monkey with special reference to the corticospinal tract. J Comp Neurol. 1994;340:469–479. doi: 10.1002/cne.903400403. [DOI] [PubMed] [Google Scholar]
- 41.Van Vactor D. Adhesion and signaling in axonal fasciculation. Curr Opin Neurobiol. 1998;8:80–86. doi: 10.1016/s0959-4388(98)80011-1. [DOI] [PubMed] [Google Scholar]
- 42.Wadsworth WG, Bhatt H, Hedgecock EM. Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron. 1996;16:35–46. doi: 10.1016/s0896-6273(00)80021-5. [DOI] [PubMed] [Google Scholar]