Abstract
The functional expression of large-conductance Ca2+-activated K+(KCa) channels in lumbar motoneurons (LMNs) of the developing chick embryo is regulated in part by interactions with striated muscle target tissues. Here we show that the functional expression of KCa channels in LMNs developingin vitro can be stimulated by application of a skeletal muscle extract (MEX) or by coculture with hindlimb myotubes. A similar stimulation of KCa channels in vitro can be produced by the trophic factors glial cell line-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factor but not by neurotrophin (NT)-3 or NT-4. The actions of MEX and hindlimb myotubes are blocked by a GDNF-neutralizing antiserum. Moreover, injection of this same antiserum into the embryonic hindlimb reduced the functional expression of KCa channels in vivo to levels seen in LMNs deprived of interactions with the hindlimb. The effects of GDNF on KCa channel expression in LMNs require 24 hr of continuous exposure to reach maximum and are blocked by the translation inhibitor anisomycin, indicating the need for synthesis of new proteins. GDNF actions are also blocked by the farnesyl transferase inhibitor manumycin, suggesting a role for Ras in the actions of GDNF. Finally, the actions of GDNF are inhibited by PP2, an inhibitor of Src family tyrosine kinases, and by LY29003, an inhibitor of phosphatidylinositol 3 kinases, but not by PD98059, an inhibitor of the Erk signaling cascade. None of these treatments alter expression of voltage-activated Ca2+ channels. Thus, the actions of GDNF on LMN KCa channel expression appear to use a transduction pathway similar to that used for regulation of apoptosis.
Keywords: motoneuron, development, Ca2+-activated K+ channels, trophic factors, GDNF, kinase
Large-conductance Ca2+-activated K+ (KCa) channels play an important role in the regulation of action potential waveform and temporal discharge patterns in many types of neurons (Vergara et al., 1998; Martin-Caraballo and Greer, 2000). We have shown previously that the functional expression of KCa channels in developing chick lumbar spinal motoneurons (LMNs) coincides with the elimination of synapses in target tissues (Phillips and Bennett, 1987a,b; Martin-Caraballo and Dryer, 2002), which in turn is dependent on a specific pattern of repetitive spike discharge (Thompson, 1983). Therefore, it is of interest to understand more about the factors that lead to developmental regulation of motoneuron KCa channels. This process is regulated in part by electrical activity in the motoneurons themselves and also by interactions with target tissues (Martin-Caraballo and Dryer, 2002). Thus, early ablation of the hindlimb primordium significantly reduced the developmental expression of functional KCachannels in LMNs, whereas treatments that cause an increase in LMN sprouting along the surface of the embryonic muscle (Tang and Landmesser, 1993) cause an increase in plasma membrane KCa channels. Finally, macroscopic KCa currents in LMNs developing in vitro was increased by coculture with hindlimb myotubes.
Target-dependent regulation of KCa channel expression in developing autonomic neurons is mediated by trophic factors (Raucher and Dryer, 1995; Subramony et al., 1996; Cameron et al., 1998). Here we have examined whether a similar process occurs in LMNs. The role of neurotrophic factors in developmental regulation of motoneurons has been studied extensively in other contexts, especially with respect to regulation of apoptosis. One growth factor family of special interest includes glial cell-derived neurotrophic factor (GDNF) and closely related molecules known as neurturins (Baloh et al., 2000). These molecules act through a two-component signaling pathway consisting of a family of glycosylphosphatidylinositol (GPI)-linked surface molecules [GDNF family receptor (GFR)α-1–4] and the receptor tyrosine kinase Ret. GDNF is expressed in embryonic muscle (Henderson et al., 1994; Wright and Snider, 1996), and the corresponding receptor proteins GFRα-1 and Ret are expressed in developing LMNs (Yu et al., 1998). Moreover, GDNF supports the survival of motoneurons in vivo and in vitro (Henderson et al., 1994; Soler et al., 1999) and contributes to the formation of neuromuscular synapses (Nguyen et al., 1998; Keller-Peck et al., 2001;Wang et al., 2002), whereas survival of LMNs is greatly reduced in mice deficient in GDNF or GFRα-1 (Cacalano et al., 1998; Garces et al., 2000).
Multiple signaling pathways typically mediate the actions of neurotrophic factors (Segal and Greenberg, 1996; Soler et al., 1998,1999; Wu et al., 1998; Dolcet et al., 1999; Lhuillier and Dryer, 2000,2002). In LMNs, the phosphatidylinositol (PI) 3-kinase/Akt pathway contributes to the anti-apoptotic effects of GDNF. In ciliary ganglion cells, TGFβ1 upregulation of KCa expression is mediated by Erk- and PI3-kinase-dependent pathways (Lhuillier and Dryer, 2000, 2002). Here we demonstrate a role for GDNF in the normalin vivo regulation of KCa channel expression in embryonic LMNs. GDNF-induced KCachannel expression is slow in onset, dependent on protein synthesis, and mediated by a pathway that includes PI3 kinase signaling.
MATERIALS AND METHODS
Embryo treatments and cell dissociation and culture. Labeling, dissociation, and culture of chick LMNs were performed as described by Martin-Caraballo and Dryer (2002). Briefly, chick LMNs were retrogradely labeled in ovo with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI; 1 mg/ml in 20% ethanol and 80% saline). Dye injection into muscles of the thigh and foreleg was performed 24 hr before spinal cord dissociation. In some experiments, DiI was injected into the hindlimb at embryonic day (E) 8 together with colchicine (2 μl of a 0.5 mg/ml solution) or GDNF- or NT-4-neutralizing antibodies (2 μl of a 0.5 mg/ml solution). These reagents were prepared in a physiological saline containing (in mm): 139 NaCl, 3 KCl, 1 MgCl2, 3 CaCl2, and 17 NaHCO3. Control embryos for those experiments were injected with DiI and the saline vehicle. Spinal cords were excised into a Ca2+- and Mg2+-free solution, mildly trypsinized (at E8, 0.2% for 30 min), dissociated by trituration, and plated onto poly-d-lysine-coated glass coverslips. Basal culture medium consisted of Eagle's minimal essential medium (BioWhittaker, Walkersville, MD), supplemented with 10% heat-inactivated horse serum, 2 mm glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin. For experiments involving nerve–muscle cocultures, E11 hindlimb muscles were dissected and cleaned of connective tissue in a Ca2+- and Mg2+-free solution. After incubation for 15 min with 0.05% type II collagenase, tissue was dissociated by trituration through a series of fire-polished Pasteur pipettes. For nerve–muscle cocultures, myotubes were plated and allowed to adhere to poly-d-lysine-coated glass coverslips for 45 min, and an excess of medium was then added. Myotube cultures were maintained for 2 d before adding dissociated LMNs. In other experiments, LMNs were cultured with muscle extract (MEX) prepared as described by Arakawa et al. (1990). Briefly, muscle tissue from E13 embryonic hindlimb was excised, cleared of skin and connective tissue, and homogenized in PBS containing (in mm): 137 NaCl, 2.7 KCl, 8 Na2 PO4, 1.5 KH2PO4, 1 EDTA, 1 EGTA, and 2% protein inhibitor cocktail (Sigma, St. Louis, MO). The homogenate was centrifuged at 100,000 × g at 4°C for 1 hr. Aliquots of the supernatant were stored at −80°C until use. Protein concentration was determined according to the Bradford method using a commercially available protein assay reagent (Bio-Rad, Hercules, CA). In some other experiments, we prepared MEX from hindlimbs excised at E8 and obtained results indistinguishable from those obtained with E13 hindlimb extracts. We used E13 muscle in most experiments because it reduced the number of animals required to perform the experiments. It should be noted that all experiments involving long-term (e.g., 24–72 hr) culture of LMNs require the addition of some type of trophic substance to the culture medium. Without trophic support, 100% of LMNs die within 12 hr. We have shown previously that the neurotrophin (NT)-4 can keep LMNs alive for 72 hrin vitro without evoking stimulation of KCa expression (Martin-Caraballo and Dryer, 2002).
Quantification of LMN survival. Motoneuron survival was quantified by counting the number of bright, DiI-labeled motoneurons across two diameters of the 10 mm coverslip. Cells were counted 3 hr after plating (before the onset of significant apoptosis) and again 24 hr after plating. Survival was calculated from the ratio of these values, which in the absence of trophic factors is 0% but which ranges from 80 to 100% in the presence of the trophic factors or target tissue extracts used in this study.
Electrophysiology. LMNs were identified during patch-clamp recordings using an inverted-stage microscope equipped with epifluorescent optics and rhodamine filters. All LMNs selected for recording showed a punctate fluorescent staining pattern because of retrograde transport of DiI from its site of injection in the hindlimb. Recordings were performed at room temperature (22–24°C). All external recording solutions contained 600 nmtetrodotoxin (TTX) to block voltage-evoked inward Na+ currents during whole-cell recording. Recording electrodes were made from thin wall borosilicate glass (3–4 MΩ) and filled with a solution consisting of (in mm): 120 KCl, 2 MgCl2, 10 HEPES-KOH, and 10 EGTA, pH 7.4, except in measurements of Ca2+ currents, in which KCl was replaced with CsCl. Normal external salines for measurements of KCa contained (in mm): 145 NaCl, 5.4 KCl, 0.8 MgCl2, 5.4 CaCl2, 5 glucose, and 13 HEPES-NaOH, pH 7.4. For recordings of voltage-activated Ca2+ currents, the external solutions contained (in mm): 145 tetraethylammonium chloride, 10 CaCl2, 5 glucose, and 10 HEPES, pH 7.4 (with tetraethylammonium hydroxide). The corresponding Ca2+-free solutions were the same except that the CaCl2 was replaced with MgCl2. To measure KCa or voltage-activated Ca2+ currents, a 25 msec depolarizing step to +30 mV was applied from a holding potential of −40 mV in normal external saline and after a 3 min incubation in Ca2+-free external saline, and net current amplitude was obtained by digital subtraction (control − Ca2+-free). Voltage commands and data acquisition and analysis were performed with an AxoPatch 1D amplifier and Pclamp software (Axon Instruments, Foster City, CA). For quantitative analyses, we normalized for cell size by dividing current amplitudes by cell capacitance, determined by integration of the current transient evoked by a 10 mV voltage step from a holding potential of −60 mV. Throughout, all data values are presented as mean ± SEM. Statistical analyses consisted of Student's unpairedt test when single comparisons were made and one-way ANOVA followed by post hoc analysis using Tukey's honest significant difference test for unequal n for the more typical experimental designs that entailed comparisons between multiple groups (Statistica software; StatSoft, Tulsa, OK). Throughout,p < 0.05 was regarded as significant. In every experiment, data are collected from a minimum of two platings of LMNs (i.e., from multiple cultures). In most experiments, each bar on a graph represents data from three to four platings.
Chemicals and drugs. Anisomycin, LY 29400, manumycin, NT-3, NT-4, tetrodotoxin, trypsin, and collagenase were from Sigma. BDNF, NT-4-neutralizing antiserum, GDNF-neutralizing antiserum, and the trophic factors BDNF and GDNF were obtained from R & D Systems (Minneapolis, MN). PD98059 was obtained from RBI (St. Louis, MO); colchicine, K252a, PP3, PP2, KN92, and KN93 were obtained from Calbiochem (San Diego, CA); and culture supplements and serum were from BioWhittaker.
RESULTS
Target-derived GDNF regulated the functional expression of LMN KCa channels
The largest changes in the functional expression of LMN KCa channels occur between E8 and E11, and this process is partly dependent on interactions with target tissues (Martin-Caraballo and Dryer, 2002). To determine whether a similar effect can be evoked by a soluble target-derived trophic factor, we incubated E8 LMNs for 72 hr in the presence or absence of target MEX (Fig. 1A). A 72 hr exposure to MEX (50 μg/ml protein concentration) evoked a significant (p < 0.05) increase in macroscopic KCa current density compared with acutely dissociated motoneurons (Fig. 1B). It bears noting that identical effects were observed with MEX prepared from either E13 or E8 hindlimb (data not shown), and subsequent analyses were performed with E13 MEX. The effect of MEX is mimicked by GDNF (Fig.1B). The neurotrophin BDNF also produced a significant increase in the functional expression of KCa channels (Fig. 1B), but this effect was somewhat less robust than that of GDNF. Several other trophic factors that were tested, including the neurotrophins NT-3 and NT-4, did not cause a significant increase in the functional expression of KCa channels in LMNs. Therefore, the changes in KCa expression are not a simple consequence of time in culture. It should be noted that we were unable to culture E8 LMNs for >12 hr in normal culture media in the absence of trophic factors or MEX because of ongoing apoptotic cell death that has long been known to occur in spinal motoneurons developing in vitro (O'Brien and Fischbach, 1986). Because GDNF produced the largest effect on the functional expression of KCa channels, and because it is expressed in hindlimb target tissues (Henderson et al., 1994; Wright and Snider, 1996), we have chosen to focus on the actions of this neurotrophic factor.
Fig. 1.
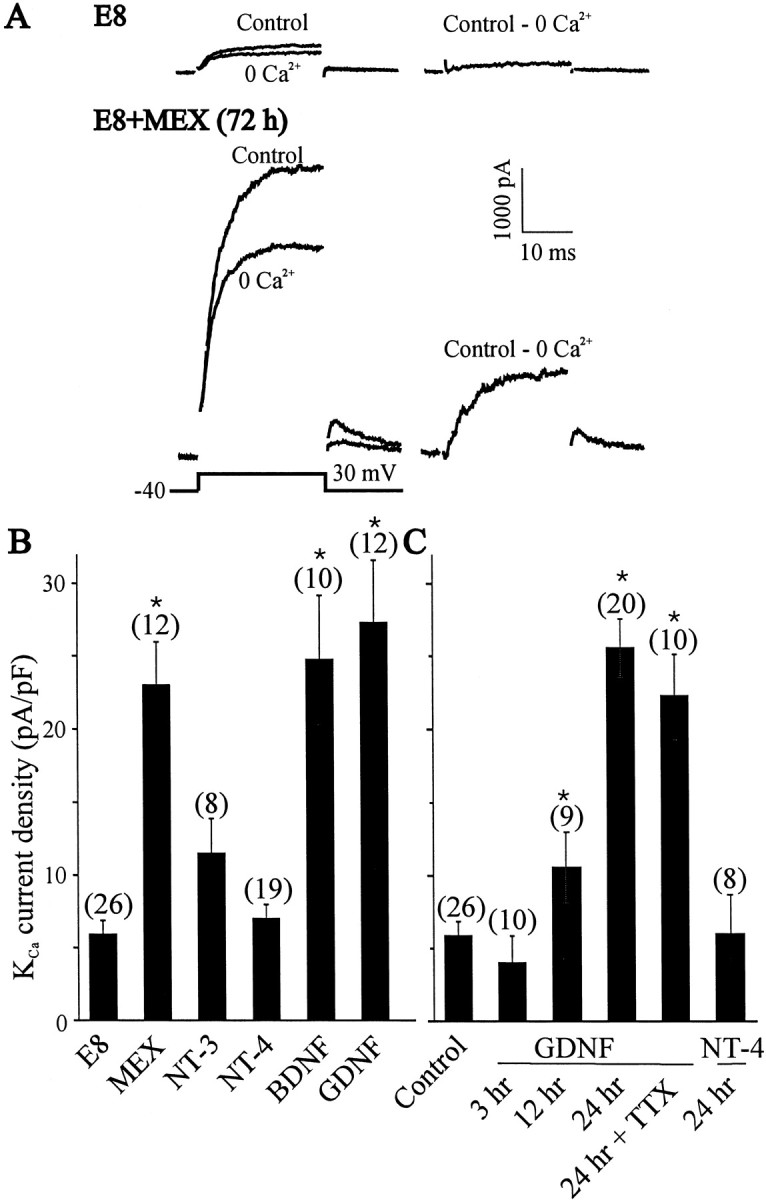
Effect of MEX and growth factors on the expression of macroscopic KCa current in LMNs in vitro. A, Representative traces of outward currents evoked in E8 LMNs and after 72 hr in the presence of MEX (50 μg/ml). Outward currents were evoked in control and Ca2+-free salines (left traces) by 25 msec depolarizing pulses to +30 mV from a holding potential of −40 mV (shown at bottomleft). Net macroscopic KCa was obtained after digital subtraction of raw traces (right trace). B, Summary of the effects of MEX and trophic factors on the functional expression of KCa in cultured LMNs. Motoneurons were dissociated on E8 and maintained in culture for 72 hr in the presence of 50 μg/ml MEX or 10 ng/ml of the trophic factors BDNF, NT-3, NT-4, and GDNF. All of these factors are capable of promoting LMN survival in vitro. E8 represents control neurons examined 3 hr after dissociation. Note robust stimulation of KCa by MEX, GDNF, and BDNF. C, Time course of GDNF stimulation of KCa channel expression. Maximal expression of KCa channel occurred after 24 hr exposure to GDNF (10 ng/ml). The effect of GDNF on KCa channel expression is independent of electrical activity because TTX did not prevent the functional expression of KCa channels. Note that 24 hr incubation with NT-4 fails to increase KCa channel expression. In these experiments, LMNs were dissociated on E8 and maintained in culture for 3, 12, or 24 hr in the presence of GDNF as indicated. Control represents E8 LMNs examined 3 hr after dissociation. In this and subsequent figures, error bars indicate SEM, the number of cells recorded is given above each bar, andasterisks denote p < 0.05 from control as determined by one-way ANOVA followed by Tukey's honest significant difference test for unequal n.
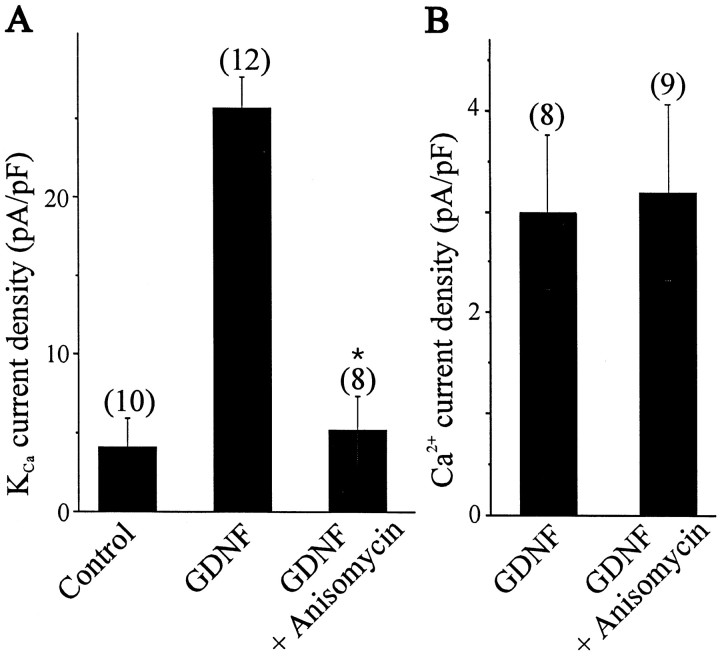
The effects of GDNF on KCa channel expression are relatively slow in onset (Fig. 1C). Thus, functional expression of KCa channels after 3 hr treatment with GDNF was low when compared with cells maintained in vitro for 3 hr without trophic factors. However, a statistically significant increase in KCa current occurred after 12 hr exposure to GDNF, and a 24 hr treatment with GDNF induced maximal increases in KCa current density. In contrast, treatment with NT-4 was ineffective in stimulating KCa channel expression, even after 24 hr of continuous exposure. There was no significant change in LMN cell capacitance after 24 hr treatment with GDNF or NT-4 (33.8 ± 1.3 pF, n = 20 vs 33.9 ± 1.5 pF, n = 8; p > 0.05, respectively). Moreover, the different effects of NT-4 and GDNF on KCa channel expression after 24 hr in vitro cannot be attributed to survival of different populations of LMNs. Thus, after 24 hr in culture, 95 ± 7% (n = 12) of DiI-labeled motoneurons survived in the presence of GDNF, whereas culture of LMNs in the presence of NT-4 resulted in a survival rate of 81 ± 9% (n = 11) of DiI-labeled cells. Moreover, these treatments have no effect on the expression of voltage-activated Ca2+ currents (data not shown). We have shown previously that the addition of CNTF to culture media allowed LMNs to express functional macroscopic KCacurrents, but this effect appeared to be an indirect one because it was completely abolished by a blockade of ongoing electrical activity (Martin-Caraballo and Dryer, 2002). The GDNF-induced stimulation of KCa channel expression appears to be a more direct effect or at least different in the sense that GDNF actions do not require electrical activity. Thus, adding 60 nm TTX to the culture media did not alter responses to GDNF (Fig. 1C).
As described previously (Martin-Caraballo and Dryer, 2002), coculture of E8 LMNs with hindlimb myotubes produced a robust increase in KCa channel density after 24 hr in culture compared with LMNs examined shortly after plating (Fig.2A). This effect of myotubes is significantly reduced when GDNF-neutralizing antisera are added to the culture media (Fig. 2A). There was no significant difference in the cell capacitance of LMNs cultured with hindlimb myotures with or without GDNF-neutralizing antisera in the culture media (36.9 ± 2 pF, n = 12 vs 37.9 ± 4.2 pF, n = 20; p > 0.05). In contrast, incubation with a NT-4-neutralizing antiserum had no effect on the actions of myotubes (Fig. 2A), consistent with the observation that NT-4 fails to simulate KCachannels in LMNs (Fig. 1B,C). Activation of KCa channels requires Ca2+ entry via voltage-activated Ca2+ channels. However, we found that the GDNF-neutralizing antiserum had no effect on the density of Ca2+ currents in LMNs (Fig.2B). These in vitro data support the hypothesis that GDNF is a target-derived factor involved in the developmental regulation of LMN KCa channels. To further test the role of GDNF as a target-derived factor, MEX was treated overnight with different neutralizing antisera specific for either GDNF or NT-4. LMNs cultured for 24 hr in the presence of MEX express a robust macroscopic KCa (Fig.2C). The expression of KCa channels was reduced significantly when the MEX was treated overnight with GDNF-neutralizing antiserum (Fig. 2C). In contrast, pretreatment of MEX with a NT-4-neutralizing antiserum had no effect on MEX-induced KCa channel expression. These data suggest that GDNF or a closely related soluble factor from hindlimb muscle contributes to regulation of the electrophysiological differentiation of LMNs.
Fig. 2.
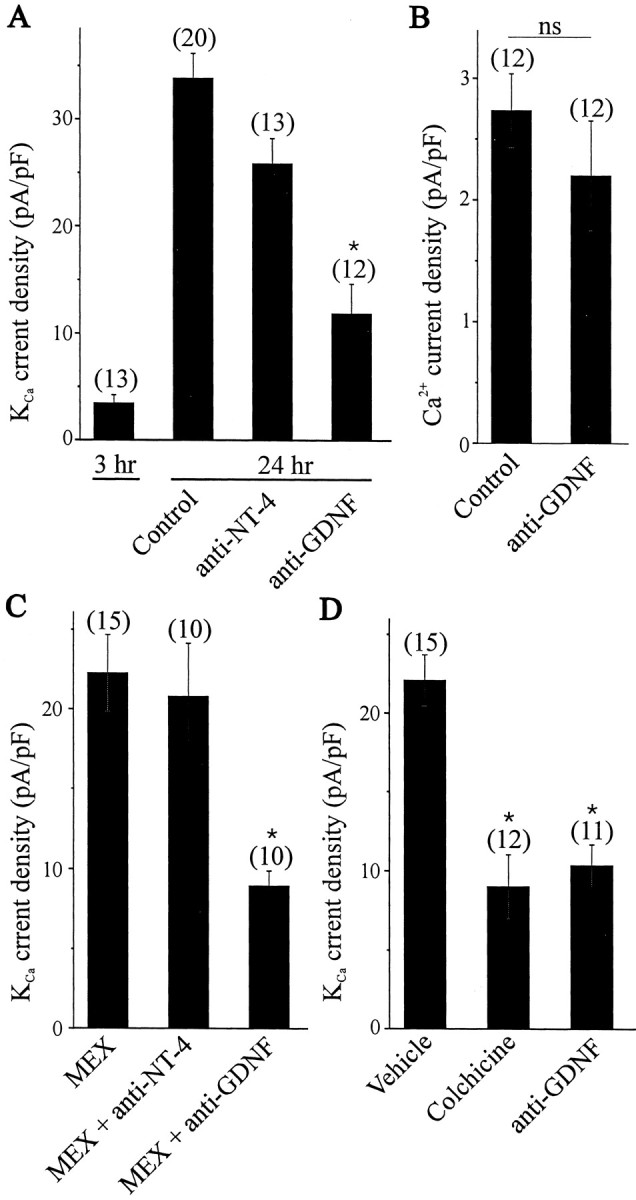
Effect of trophic factor-neutralizing antisera on KCa channel expression in developing LMNs.A, Coculture of E8 LMNs with hindlimb myotubes for 24 hr allowed for a significant developmental increase in the functional expression of KCa channels. The stimulatory effect of hindlimb myotubes on KCa channel expression was reduced by incubation with neutralizing antisera specific for GDNF but not NT-4.B, GDNF-neutralizing antiserum did not affect the density of Ca2+ currents in LMNs cocultured for 24 hr with hindlimb myotubes. C, The stimulatory effect of 24 hr treatment with MEX on KCa channel expression in E8 LMNs was also reduced by overnight incubation of MEX with neutralizing antisera specific for GDNF but not NT-4. Neutralizing antisera were used at 10 μg/ml. D, Reduction in the functional expression of KCa channels in LMNs developing in ovo after hindlimb injections of the microtubule inhibitor colchicine or GDNF-neutralizing antiserum. Both of these treatments evoked a comparable reduction in KCa expression.
Additional evidence in support of this hypothesis was obtained by several experiments performed in vivo. If soluble target-derived trophic factors are required for the functional expression of KCa channels, then inhibition of retrograde axonal transport should reduce functional expression of this current. To test this hypothesis, we injected the microtubule inhibitor colchicine into E8 embryonic hindlimb. Colchicine inhibits retrograde transport along the axon by blocking microtubule assembly (Alonso and Assenmacher, 1983). Control embryos were injected with vehicle, and functional expression of KCa channels was assayed in E11 LMNs. We observed that colchicine injection into embryonic hindlimbs caused a robust decrease in LMN KCacurrent density compared with controls (Fig. 2D), a result consistent with our earlier studies showing that target interactions are one of the important factors regulating motoneuron KCa because target ablation produced a comparable decrease in KCa current density (Martin-Caraballo and Dryer, 2002). More importantly, we observed that injection of GDNF-neutralizing antiserum directly into embryonic hindlimb also produced a significant decrease in KCa current density (Fig. 2D). This inhibition, although not complete, is comparable with that produced by ablation of the hindlimb (Martin-Caraballo and Dryer, 2002). These findings strongly suggest that target-derived GDNF plays an important role in the regulation of KCa channel expression in LMNs developingin vivo. It should be noted that our earlier studies showed that ongoing LMN electrical activity also played a role in regulation of KCa channels (Martin-Caraballo and Dryer, 2002), and this probably was the reason why treatments affecting target-dependent regulation produced only a partial inhibition of the developmental expression of KCa.
The effects of GDNF on LMN KCa channels require protein synthesis and PI3 kinase signaling
The effects of GDNF on the functional expression of KCa channels could entail a modulatory effect on preexisting plasma membrane channel proteins. GDNF could also act by stimulating insertion of a pool of intracellular KCa channels, as occurs in chick ciliary ganglion neurons in response to TGFβ1 (Lhuillier and Dryer, 2002). Finally, the effects of GDNF could be a sustained effect requiring synthesis and membrane insertion of new channel subunits or associated proteins. The effect of GDNF on LMN KCa channels is much slower in onset than the effects of TGFβ1 that we described in ciliary neurons and that are primarily dependent on insertion of preexisting channel proteins (Lhuillier and Dryer, 2000). Instead, the time course of GDNF-induced stimulation of KCa channel expression favors the hypothesis that GDNF-induced stimulation of KCa channel expression requires synthesis of new channel molecules and/or associated proteins, in which case inhibition of protein synthesis would be expected to alter the response to GDNF.
To test this hypothesis, GDNF-treated LMNs were exposed to the protein synthesis inhibitor anisomycin (Fig.3A). Anisomycin treatment abolished the effects of GDNF on the functional expression of KCa channels as assessed by measurements of current density. It bears noting that cell size was also significantly reduced after anisomycin treatment, which was readily apparent on visual inspection but was also reflected in measurements of cell capacitance (18 ± 1.6 pF, n = 8 vs control 35.1 ± 1.5 pF, n = 20). Importantly, anisomycin did not produce significant effects on the density of voltage-activated Ca2+ currents (Fig. 3B).
Fig. 3.
Effect of protein synthesis inhibition on the GDNF-induced expression of KCa channels. A, Inhibition of protein synthesis with anisomysin (25 μm) blocks GDNF stimulation of KCa channels. B, Protein synthesis inhibition does not change the expression of voltage-dependent Ca2+ currents. LMNs were dissociated on E8 and maintained in culture for 24 hr in the presence of GDNF with or without anisomycin.
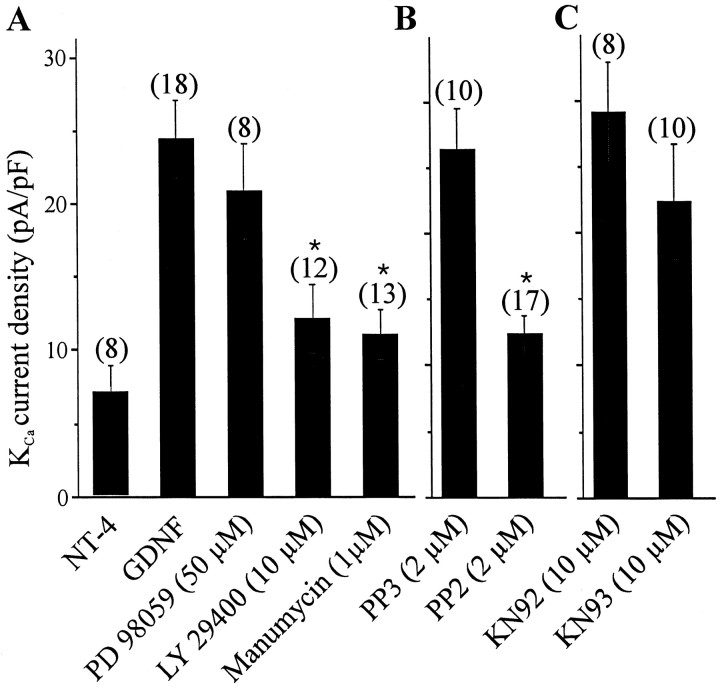
These data indicate that GDNF stimulation of KCachannel expression requires de novo protein synthesis, and it was of interest to determine whether the intracellular signaling pathways that contribute to the regulation of KCachannel expression are similar to those known to regulate LMN survival. To test this, we have used selective inhibitors of protein kinases involved in intracellular signaling cascades implicated in other trophic factor actions (Fig. 4). These compounds have been shown to be effective in perturbing the normal pattern of cell survival and differentiation in developing neurons (Soler et al., 1998; Wu et al., 1998; Lhuillier and Dryer, 2000;Encinas et al., 2001). In these experiments, GDNF-treated E8 motoneurons were maintained for 24 hr in the presence or absence of specific protein kinase inhibitors. Throughout these experiments, the neurotrophic factor NT-4 was kept in the culture medium to promote cell survival (Becker et al., 1998). It bears noting that NT-4 is able to promote nearly full survival of cultured LMNs even when PI3 kinase is inhibited. However, NT-4 does not affect KCachannel expression despite being a potent survival factor for LMNs.
Fig. 4.
Effect of transduction cascade inhibitors on GDNF stimulation of KCa channels. In these experiments, LMNs were dissociated on E8 and maintained in culture for 24 hr in the presence of GDNF (10 ng/ml). NT-4 (10 ng/ml) was also included in the cultured medium to prevent motoneuron cell death. Control neurons were cultured for 24 hr in the presence of GDNF and NT-4 and in the absence of inhibitors. A, Role of Erk, PI3 kinase, and Ras in the stimulation of KCa channels by GDNF. Incubation of LMNs in the presence of LY 29400 and manumycin, but not PD98059, significantly reduced the GDNF-induced expression of KCacurrents. B, Role of src-family kinases in GDNF actions. GDNF actions were inhibited by the active src-kinase inhibitor PP2 but not by the inactive structural congener PP3. C, No role for CaM kinase-II in GDNF actions. Stimulatory effects of GDNF were not different in cultures containing the CaM kinase-II inhibitor KN93 and its inactive congener KN92.
In chick ciliary ganglion neurons, the MAP kinase Erk is required for TGFβ1-evoked stimulation of KCa channels (Lhuillier and Dryer, 2000). However, this does not appear to be the case for GDNF actions in LMNs. Thus, inhibition of MAP kinase signaling with the MAP kinase kinase (MEK1) inhibitor PD98059 did not produce any significant change in GDNF-evoked expression of KCa (Fig. 4A). In contrast, application of the PI3-kinase inhibitor LY29400 (10 μm) caused a significant reduction in the functional expression of KCa channels in response to GDNF (Fig. 4A). It is important to note that this pattern of regulation, sensitivity to inhibitors of PI3 kinase and resistance to inhibitors of Erk signaling, is also observed in the chick motoneuron survival responses to GDNF (Soler et al., 1999).
Studies in other model systems suggest that GDNF can cause activation of a PI3 kinase cascade by recruitment of src-family tyrosine kinases to the receptor, leading to activation of small GTPases, such as Ras, which then lead to PI3 kinase activation. We performed two lines of experiments to test whether similar cascades are used for GDNF-evoked stimulation of KCa channels in LMNs. In one set of experiments, we examined the effects of the src-family kinase inhibitor PP2, as well as its inactive enatiomer PP3 (Encinas et al., 2001). PP2 caused a significant reduction in GDNF-induced stimulation of macroscopic KCa currents, whereas the inactive enantiomer PP3 had no effect (Fig. 4B). PP2 had no effect on cell size. It bears noting that a comparable inhibition of GDNF action was also produced by K252a, a broad-spectrum inhibitor of protein tyrosine kinases, including src-family kinases (Oberstar et al., 1997) (data not shown), but this drug, unlike the others used in this study, also caused a reduction in LMN size as determined by capacitance measurements and is likely to evoke many changes in cellular physiology.
Signaling through PI3 kinase pathways in some cases requires farnesylation of the small GTPase Ras (Klesse et al., 1998; Besset et al., 2000). Therefore, we examined the effects of the farnesyltransferase inhibitor manumycin A, which is widely used to inhibit signaling through Ras and closely related small GTPases (Hara and Han, 1995). Manumycin A treatment also caused inhibition of GDNF actions (Fig. 4A). Finally, because a calmodulin (CaM)-dependent kinase pathway is involved in the survival of LMNs and the functional expression of potassium channels in developing hippocampal neurons (Soler et al., 1998; Wu et al., 1998), we tested whether similar pathways contribute to GDNF-induced expression of KCa channels. However, KN93, a specific inhibitor of CaM-dependent kinase II, and KN92, an inactive enantiomer, had no effect on GDNF stimulation of KCa channel expression (Fig. 4C). These data suggest that many of the features of the transduction cascade underlying GDNF stimulation of KCa channels are similar to those used to enhance LMN survival. Moreover, they are different in at least one major respect (i.e., the apparent noninvolvement of Erk cascades) from neurotrophic regulation of KCa channels in chick autonomic neurons (Lhuillier and Dryer, 2000).
DISCUSSION
In this study, we have examined neurotrophic regulation of the functional expression of KCa channels in embryonic chick LMNs. Three main conclusions can be drawn from these experiments. First, soluble trophic factors derived from muscle cells can enhance the functional expression of KCachannels in LMNs. Second, target-derived GDNF contributes to the normal developmental regulation of these channels. Third, the GDNF-induced stimulation of KCa channels is relatively slow in onset, dependent on protein synthesis, and appears to involve activation of a GTPase- and PI3 kinase-dependent signaling pathway, a feature similar to the pathways used for regulation of cell survival.
Regulation of KCa channels by target-derived trophic factors in LMNs
The functional expression of KCa channels in developing LMNs is regulated by interactions with hindlimb muscles as well as by ongoing electrical activity within the LMNs themselves (Martin-Caraballo and Dryer, 2002). In the present study, we have focused on the role of target tissues and have shown that a soluble extract of hindlimb muscle can stimulate the functional expression of KCa channels in LMNs developing in vitro. Moreover, at least two different trophic factors, GDNF and BDNF, are able to increase macroscopic KCacurrent density without producing corresponding effects on voltage-activated Ca2+ current. Both of these trophic factors are also able to regulate apoptosis of developing spinal motoneurons (Koliatsos et al., 1993; Becker et al., 1998; Dolcet et al., 1999; Soler et al., 1999). Several related neurotrophic factors, including NT-3 and NT-4, did not have any significant effect on KCa channel expression in LMNs developingin vitro, although these factors also promote spinal motoneuron survival in vivo and in vitro (Qin-Wei et al., 1994; Becker et al., 1998; Caldero et al., 1998). Thus, it is clear that although some trophic factors can regulate both apoptosis and electrophysiological differentiation of developing motoneurons, these two processes are not inextricably linked.
The normal regulation of KCa channels in LMNs appears to require retrograde axonal transport from the periphery, because KCa expression is markedly reduced byin vivo disruption of microtubules caused by injections of colchicine into the hindlimb. These data provide additional evidence of an essential role for trophic interactions between LMNs and cells in the periphery. In this regard, previous work has demonstrated synthesis of GDNF in muscle tissue and Schwann cells but not in the spinal cord during embryonic development (Henderson et al., 1994; Wright and Snider, 1996). In contrast, BDNF is expressed by central neurons within the spinal cord and is not expressed in hindlimb muscles of chick embryos at these developmental stages (Henderson et al., 1993; Sedel et al., 1999). The ability of peripheral injections of GDNF-neutralizing antisera to reduce KCa channel expression in LMNs provides strong evidence of a role for this family of factors in the functional electrophysiological differentiation of motoneurons, and this result is mimicked in two different in vitro models used in this study.
Mechanisms of GDNF-induced channel expression
Maximal GDNF-dependent stimulation of macroscopic KCa currents is slow in onset and occurs after ∼24 hr of continuous exposure to this trophic factor. Moreover, GDNF stimulation of KCa channels requires protein synthesis, because it can be blocked by the ribosomal inhibitor anisomycin. The inhibitory effect of anisomycin on macroscopic KCa currents is not associated with inhibition of voltage-activated Ca2+ currents, because these are maintained in the face of 24 hr of protein synthesis inhibition. This pattern contrasts with the effects of target-derived factors on KCa channel expression observed in developing ciliary neurons of the chick ciliary ganglion (Subramony et al., 1996; Lhuillier and Dryer, 2000). In those cells, KCa regulation is mediated by an avian ortholog of TGFβ1 secreted from striated muscle target cells in the iris (Cameron et al., 1998). The actions of TGFβ1 in ciliary cells are composed of an acute post-translational effect that entails insertion of preexisting channels into the plasma membrane, as well as a more sustained effect that requires transcription and protein synthesis (Subramony et al., 1996; Lhuillier and Dryer, 2000, 2002). The pattern in LMNs also differs from that observed in choroid cells of the ciliary ganglion, where the developmental expression of KCa channels appears to be cell autonomous and does not require interactions with target tissues (Cameron and Dryer, 2000). It bears noting that the kinetic properties of the large-conductance KCa channels in these three cell types (ciliary neurons, choroid neurons, and LMNs) are markedly different based on analyses of single-channel gating, macroscopic deactivation kinetics, and macroscopic current fluctuations (Cameron and Dryer, 2000; Martin-Caraballo and Dryer, 2002). It is tempting to speculate that the kinetic and developmental differences of KCa channels in these cell types share a common molecular basis, e.g., differences in the developmental expression of auxiliary subunits, differences in the processing of α-subunit splice variants, etc. There is considerable precedent in the K+ channel literature of a role for auxiliary subunits in regulation of channel gating and trafficking (Xia et al., 1998; Manganas and Trimmer, 2000).
GDNF signal transduction entails interaction with a multicomponent complex composed of Ret tyrosine kinase and the GPI-anchored co-receptors GFRα1–α4 (Jing et al., 1996; Worby et al., 1996;Saarma, 2000) or in some cases via Ret-independent pathways (Poteryaev et al., 1999; Trupp et al., 1999). In either case, receptor stimulation causes activation of Src-homology 2 domains in cytoplasmic adapter proteins, which can then activate various intracellular signaling cascades, including the Ras–Erk and Ras–PI3 kinase pathways (Poteryaev et al., 1999; Hayashi et al., 2000; Encinas et al., 2001), depending on the cell type. Therefore, we examined whether similar cascades underlie GDNF stimulation of KCa in LMNs. Consistent with this general outline, we observed that PP2, an inhibitor of Src family tyrosine kinases (Encinas et al., 2001), caused a significant inhibition of GDNF effects on KCacurrent density in LMNs. Similarly, we observed that manumycin, an inhibitor of a subset of small GTPases, including Ras (Hara and Han, 1995), also blocked the effects of GDNF.
Previous studies have shown that GDNF and related factors inhibit apoptosis in chicken motoneurons via a pathway that is dependent on PI3 kinase but independent of Erk signaling (Soler et al., 1999). A similar pattern is observed with BDNF (Dolcet et al., 1999). Regulation of KCa channels by GDNF appears to entail similar pathways, because the PI3 kinase inhibitor LY294002 reduced GDNF stimulation of KCa currents, whereas PD98059, which is an inhibitor of MEK1 and thus of Erk pathway signaling, had no effect. Despite these previous studies on LMN survival, we were somewhat surprised by this later result, because both the acute and sustained neurotrophic regulation of KCa in chick ciliary neurons requires activation of Erk signaling cascades (Lhuillier and Dryer, 2000). Again, the difference in the intercellular cascades required for stimulation of macroscopic KCa channels may be related to the fact that different channel complexes are expressed in different cell types. A role for PI3 kinase in ion channel regulation has been established in many other systems (Wu et al., 1998; Melnikova and Gardner, 2001;Lhuillier and Dryer, 2002). The phosphorylated products of these enzymes, including PtdIns[3,4,5]P3 or PtdIns[3,4]P2, can cause direct or indirect activation of a wide variety of intracellular signaling enzymes, many of which contain pleckstrin-homology and FYVE-finger domains that can bind PtdIns[3,4,5]P3 or PtdIns[3,4]P2. Several of these enzymes, including small GTPases, guaninine nucleotide exchange factors, and ADP-ribosylation factors, are involved in the processing and targeting of membrane proteins (Corvera and Czech 1998; Rameh and Cantley 1999). Another cellular target, the Akt/PKB family of protein kinases, plays a role in transcriptional regulation, among other processes (Alessi and Cohen, 1998; Kops and Burgering, 1999). Therefore, it is likely that PI3 kinase activation regulates the functional expression of macroscopic KCa channels at several levels.
In summary, we have demonstrated that the effect of target tissues on the functional expression of the large-conductance KCa channels of chick LMNs entails secretion of soluble trophic factors of the GDNF family from peripheral target tissues, and that the effect of this trophic factor requires synthesis of new proteins via a cascade that entails activation of small GTPases and PI3 kinase.
Footnotes
This work was supported by a Muscular Dystrophy Association Research Grant (S.E.D.), National Institutes of Health Grant NS32748 (S.E.D.), and an Alberta Heritage Foundation for Medical Research Postdoctoral Fellowship (M.M.-C.). We thank Hannah Nguyen for technical assistance.
Correspondence should be addressed to Dr. Stuart E. Dryer, University of Houston, Department of Biology and Biochemistry, Houston, TX 77204-5513. E-mail: sdryer@uh.edu.
REFERENCES
- 1.Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8:55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 2.Alonso G, Assenmacher Retrograde axoplasmic transport of neurosecretory material. An immunocytochemical and electron-microscopic study of transected axons in normal and colchicine-treated rats. Cell Tissue Res. 1983;233:183–196. doi: 10.1007/BF00222242. [DOI] [PubMed] [Google Scholar]
- 3.Arakawa Y, Sendtner M, Thoenen H. Survival effect of ciliary neurotrophic factor (CNTF) on chick embryonic motoneurons in culture: comparison with other neurotrophic factors and cytokines. J Neurosci. 1990;10:3507–3515. doi: 10.1523/JNEUROSCI.10-11-03507.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baloh RH, Enomoto H, Johnson EM, Jr, Milbrandt J. The GDNF family ligands and receptors–implications for neural development. Curr Opin Neurobiol. 2000;10:103–110. doi: 10.1016/s0959-4388(99)00048-3. [DOI] [PubMed] [Google Scholar]
- 5.Becker E, Soler RM, Yuste VJ, Gine E, Sanz-Rodriguez C, Egea J, Martin-Zanca D, Comella JX. Development of survival responsiveness to brain-derived neurotrophic factor, neurotrophin 3 and neurotrophin 4/5, but not to nerve growth factor, in cultured motoneurons from chick embryo spinal cord. J Neurosci. 1998;18:7903–7911. doi: 10.1523/JNEUROSCI.18-19-07903.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Besset V, Scott RP, Ibanez CF. Signaling complexes and protein-protein interactions involved in the activation of the Ras and phosphatidylinositol 3-kinase pathways by the c-Ret receptor tyrosine kinase. J Biol Chem. 2000;275:39159–39166. doi: 10.1074/jbc.M006908200. [DOI] [PubMed] [Google Scholar]
- 7.Cacalano G, Farinas I, Wang LC, Hagler K, Forgie A, Moore M, Armanini M, Phillips H, Ryan AM, Reichardt LF, Hynes M, Davies A, Rosenthal A. GFRα1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron. 1998;21:53–62. doi: 10.1016/s0896-6273(00)80514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caldero J, Prevette D, Mei X, Oakley RA, Li L, Milligan C, Houenou L, Burek M, Oppenheim RW. Peripheral target regulation of the development and survival of spinal sensory and motor neurons in chick embryo. J Neurosci. 1998;18:356–370. doi: 10.1523/JNEUROSCI.18-01-00356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cameron J, Dryer SE. BK-Type KCa channels in two parasympathetic cell types: differences in kinetic properties and developmental expression. J Neurophysiol. 2000;84:2767–2776. doi: 10.1152/jn.2000.84.6.2767. [DOI] [PubMed] [Google Scholar]
- 10.Cameron J, Lhuillier L, Subramony P, Dryer SE. Developmental regulation of neuronal K+ channels by target-derived TGFβ in vivo and in vitro. Neuron. 1998;21:1045–1053. doi: 10.1016/s0896-6273(00)80622-4. [DOI] [PubMed] [Google Scholar]
- 11.Corvera S, Czech MP. Direct targets of phosphoinositide 3-kinase products in membrane traffic and signal transduction. Trends Cell Biol. 1998;8:442–446. doi: 10.1016/s0962-8924(98)01366-x. [DOI] [PubMed] [Google Scholar]
- 12.Dolcet X, Egea J, Soler RM, Martin-Zanca D, Comella JX. Activation of phosphatidylinositol 3-kinase, but not extracellular-regulated kinases, is necessary to mediate brain-derived neurotrophic factor-induced motoneuron survival. J Neurochem. 1999;73:521–531. doi: 10.1046/j.1471-4159.1999.0730521.x. [DOI] [PubMed] [Google Scholar]
- 13.Encinas M, Tansey MG, Tsui-Pierchala BA, Comella JX, Milbrandt J, Johnson EM., Jr c-Src is required for glial cell line-derived neurotrophic factor (GDNF) family ligand-mediated neuronal survival via a phosphatidylinositol-3 kinase (PI-3K)-dependent pathway. J Neurosci. 2001;21:1464–1472. doi: 10.1523/JNEUROSCI.21-05-01464.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garces A, Haase G, Airaksinen MS, Livet J, Filippi P, deLapeyriere O. GFRalpha 1 is required for development of distinct subpopulations of motoneuron. J Neurosci. 2000;20:4992–5000. doi: 10.1523/JNEUROSCI.20-13-04992.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hara M, Han M. Ras farnesyltransferase inhibitors suppress the phenotype resulting from an activated Ras mutation in Caenorhabditis elegans. Proc Natl Acad Sci USA. 1995;92:3333–3337. doi: 10.1073/pnas.92.8.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi H, Ichihara M, Iwashita T, Murakami H, Shimono Y, Kawai K, Kurokawa K, Murakumo Y, Imai T, Funahashi H, Nakao A, Takahashi M. Characterization of intracellular signals via tyrosine 1062 in RET activated by glial cell line-derived neurotrophic factor. Oncogene. 2000;19:4469–4475. doi: 10.1038/sj.onc.1203799. [DOI] [PubMed] [Google Scholar]
- 17.Henderson CE, Camu W, Mettling C, Gouin A, Poulsen K, Karihaloo M, Rullamas J, Evans T, McMahon SB, Armanini MP. Neurotrophins promote motor neuron survival and are present in embryonic limb bud. Nature. 1993;363:266–270. doi: 10.1038/363266a0. [DOI] [PubMed] [Google Scholar]
- 18.Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simmons L, Moffet B, Vandlen RA, Simpson LC. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266:1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- 19.Jing S, Wen D, Yu Y, Holst PL, Luo Y, Fang M, Tamir R, Antonio L, Hu Z, Cupples R, Louis JC, Hu S, Altrock BW, Fox GM. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell. 1996;85:1113–1124. doi: 10.1016/s0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- 20.Keller-Peck CR, Feng G, Sanes JR, Yan Q, Lichtman JW, Snider WD. Glial cell line-derived neurotrophic factor administration in postnatal life results in motor unit enlargement and continuous synaptic remodeling at the neuromuscular junction. J Neurosci. 2001;21:6136–6146. doi: 10.1523/JNEUROSCI.21-16-06136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klesse LJ, Parada LF. p21 ras and phosphatidylinositol-3 kinase are required for survival of wild-type and NF1 mutant sensory neurons. J Neurosci. 1998;18:10420–10428. doi: 10.1523/JNEUROSCI.18-24-10420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koliatsos VE, Clatterbuck RE, Winslow JW, Cayouette MH, Price DL. Evidence that brain-derived neurotrophic factor is a trophic factor for motor neurons in vivo. Neuron. 1993;10:359–367. doi: 10.1016/0896-6273(93)90326-m. [DOI] [PubMed] [Google Scholar]
- 23.Kops GJ, Burgering BM. Forkhead transcription factors: new insights into protein kinase B (c-akt) signaling. J Mol Med. 1999;77:656–665. doi: 10.1007/s001099900050. [DOI] [PubMed] [Google Scholar]
- 24.Lhuillier L, Dryer SE. Developmental regulation of neuronal KCa channels by TGFβ 1: transcriptional and post-transcriptional effects mediated by Erk MAP kinase. J Neurosci. 2000;20:5616–5622. doi: 10.1523/JNEUROSCI.20-15-05616.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lhuillier L, Dryer SE. Developmental regulation of neuronal KCa channels by TGFβ1: an essential role for PI3 kinase signaling and membrane insertion. J Neurophysiol. 2002;88:954–964. doi: 10.1152/jn.2002.88.2.954. [DOI] [PubMed] [Google Scholar]
- 26.Manganas LN, Trimmer JS. Subunit composition determines Kv1 potassium channel surface expression. J Biol Chem. 2000;275:29685–29693. doi: 10.1074/jbc.M005010200. [DOI] [PubMed] [Google Scholar]
- 27.Martin-Caraballo M, Dryer SE. Activity- and target-dependent regulation of large-conductance Ca2+-activated K+ channels in developing chick lumbar motoneurons. J Neurosci. 2002;22:73–81. doi: 10.1523/JNEUROSCI.22-01-00073.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin-Caraballo M, Greer JJ. Development of potassium conductances in perinatal rat phrenic motoneurons. J Neurophysiol. 2000;83:3497–3508. doi: 10.1152/jn.2000.83.6.3497. [DOI] [PubMed] [Google Scholar]
- 29.Melnikova IN, Gardner PD. The signal transduction pathway underlying ion channel gene regulation by SP1-C-Jun interactions. J Biol Chem. 2001;276:19040–19045. doi: 10.1074/jbc.M010735200. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen QT, Parsadanian AS, Snider WD, Lichtman JW. Hyperinnervation of neuromuscular junctions caused by GDNF overexpression in muscle. Science. 1998;279:1725–1729. doi: 10.1126/science.279.5357.1725. [DOI] [PubMed] [Google Scholar]
- 31.Oberstar JV, Challacombe JF, Roche FK, Letourneau PC. Concentration-dependent stimulation and inhibition of growth cone behavior and neurite elongation by protein kinase inhibitors KT5926 and K-252a. J Neurobiol. 1997;33:161–171. doi: 10.1002/(sici)1097-4695(199708)33:2<161::aid-neu5>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 32.O'Brien RJ, Fischbach GD. Isolation of embryonic chick motoneurons and their survival in vitro. J Neurosci. 1986;6:3265–3274. doi: 10.1523/JNEUROSCI.06-11-03265.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips WD, Bennett MR. Elimination of distributed acetylcholine receptor clusters from developing fast-twitch fibres in an avian muscle. J Neurocytol. 1987a;16:1–10. doi: 10.1007/BF02456693. [DOI] [PubMed] [Google Scholar]
- 34.Phillips WD, Bennett MR. Elimination of distributed synaptic acetylycholine receptor clusters on developing avian fast-twitch muscle fibres accompanies loss of polyneuronal innervation. J Neurocytol. 1987b;16:785–797. doi: 10.1007/BF01611986. [DOI] [PubMed] [Google Scholar]
- 35.Poteryaev D, Titievsky A, Sun YF, Thomas-Crusells J, Lindahl M, Billaud M, Arumae U, Saarma M. GDNF triggers a novel ret-independent Src kinase family-coupled signaling via a GPI-linked GDNF receptor alpha1. FEBS Lett. 1999;463:63–66. doi: 10.1016/s0014-5793(99)01590-2. [DOI] [PubMed] [Google Scholar]
- 36.Qin-Wei Y, Johnson J, Prevette D, Oppenheim RW. Cell death of spinal motoneurons in the chick embryo following deafferentation: rescue effects of target tissue extracts, soluble proteins, and trophic factors. J Neurosci. 1994;14:7629–7640. doi: 10.1523/JNEUROSCI.14-12-07629.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rameh LE, Cantley LC. The role of phosphoinositide 3-kinase lipid products in cell function. J Biol Chem. 1999;274:8347–8350. doi: 10.1074/jbc.274.13.8347. [DOI] [PubMed] [Google Scholar]
- 38.Raucher S, Dryer SE. Target-derived factors regulate the expression of Ca2+-activated K+ currents in developing chick sympathetic neurons. J Physiol (Lond) 1995;486:605–614. doi: 10.1113/jphysiol.1995.sp020838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saarma M. GDNF–a stranger in the TGF-beta superfamily? Eur J Biochem. 2000;267:6968–6971. doi: 10.1046/j.1432-1327.2000.01826.x. [DOI] [PubMed] [Google Scholar]
- 40.Sedel F, Bechade C, Triller A. Nerve growth factor (NGF) induces motoneuron apoptosis in rat embryonic spinal cord in vitro. Eur J Neurosci. 1999;11:3904–3912. doi: 10.1046/j.1460-9568.1999.00814.x. [DOI] [PubMed] [Google Scholar]
- 41.Segal RA, Greenberg ME. Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci. 1996;19:463–489. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- 42.Soler RM, Egea J, Mintenig GM, Sanz-Rodriguez C, Iglesias M, Comella JX. Calmodulin is involved in membrane depolarization-mediated survival of motoneurons by phosphatidylinositol-3 kinase- and MAPK-independent pathways. J Neurosci. 1998;18:1230–1239. doi: 10.1523/JNEUROSCI.18-04-01230.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soler RM, Dolcet X, Encinas M, Egea J, Bayascas JR, Comella JX. Receptors of the glial cell line-derived neurotrophic factor family of neurotrophic factors signal cell survival through the phosphatidylinositol 3-kinase pathway in spinal cord motoneurons. J Neurosci. 1999;19:9160–9169. doi: 10.1523/JNEUROSCI.19-21-09160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Subramony P, Raucher S, Dryer L, Dryer SE. Posttranslational regulation of Ca2+-activated K+ currents by a target-derived factor in developing parasympathetic neurons. Neuron. 1996;17:115–124. doi: 10.1016/s0896-6273(00)80285-8. [DOI] [PubMed] [Google Scholar]
- 45.Tang J, Landmesser LT. Reduction of intramuscular nerve branching and synaptogenesis is correlated with decreased motoneuron survival. J Neurosci. 1993;13:3095–3103. doi: 10.1523/JNEUROSCI.13-07-03095.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson W. Synapse elimination in neonatal rat muscle is sensitive to pattern of muscle use. Nature. 1983;302:614–616. doi: 10.1038/302614a0. [DOI] [PubMed] [Google Scholar]
- 47.Trupp M, Scott R, Whittemore SR, Ibanez CF. Ret-dependent and -independent mechanisms of glial cell line-derived neurotrophic factor signaling in neuronal cells. J Biol Chem. 1999;274:20885–20894. doi: 10.1074/jbc.274.30.20885. [DOI] [PubMed] [Google Scholar]
- 48.Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Curr Opin Neurobiol. 1998;8:321–329. doi: 10.1016/s0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- 49.Wang LJ, Lu YY, Muramatsu S, Ikeguchi K, Fujimoto K, Okada T, Mizukami H, Matsushita T, Hanazono Y, Kume A, Nagatsu T, Ozawa K, Nakano I. Neuroprotective effects of glial cell line-derived neurotrophic factor mediated by an adeno-associated virus vector in a transgenic animal model of amyotrophic lateral sclerosis. J Neurosci. 2002;22:6920–6928. doi: 10.1523/JNEUROSCI.22-16-06920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Worby CA, Vega QC, Zhao Y, Chao HH, Seasholtz AF, Dixon JE. Glial cell line-derived neurotrophic factor signals through the RET receptor and activates mitogen-activated protein kinase. J Biol Chem. 1996;271:23619–23622. doi: 10.1074/jbc.271.39.23619. [DOI] [PubMed] [Google Scholar]
- 51.Wright DE, Snider WD. Focal expression of glial cell line-derived neurotrophic factor in developing mouse limb bud. Cell Tissue Res. 1996;286:209–217. doi: 10.1007/s004410050689. [DOI] [PubMed] [Google Scholar]
- 52.Wu R-L, Butler DM, Barish ME. Potassium current development and its linkage to membrane expansion during growth of cultured embryonic mouse hippocampal neurons: sensitivity to inhibitors of phosphatidylinositol 3-kinase and other protein kinases. J Neurosci. 1998;18:6261–6278. doi: 10.1523/JNEUROSCI.18-16-06261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xia X, Hirschberg B, Smolik S, Forte M, Adelman JP. dSlo-interacting Protein 1, a novel protein that interacts with large-conductance calcium-activated potassium channels. J Neurosci. 1998;18:2360–2369. doi: 10.1523/JNEUROSCI.18-07-02360.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu T, Scully S, Yu Y, Fox GM, Jing S, Zhou R. Expression of GDNF family receptor components during development: implications in the mechanisms of interaction. J Neurosci. 1998;18:4684–4696. doi: 10.1523/JNEUROSCI.18-12-04684.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]