Abstract
Activity-dependent changes in neuronal excitability and synaptic strength are thought to underlie memory encoding. In hippocampal CA1 neurons, small conductance Ca2+-activated K+ (SK) channels contribute to the afterhyperpolarization, affecting neuronal excitability. In the present study, we examined the effect of apamin-sensitive SK channels on the induction of hippocampal synaptic plasticity in response to a range of stimulation frequencies. In addition, the role of apamin-sensitive SK channels on hippocampal-dependent memory encoding and retention was also tested. The results show that blocking SK channels with apamin increased the excitability of hippocampal neurons and facilitated the induction of synaptic plasticity by shifting the modification threshold to lower frequencies. This facilitation was NMDA receptor (NMDAR) dependent and appeared to be postsynaptic. Mice treated with apamin demonstrated accelerated hippocampal-dependent spatial and nonspatial memory encoding. They required fewer trials to learn the location of a hidden platform in the Morris water maze and less time to encode object memory in an object-recognition task compared with saline-treated mice. Apamin did not influence long-term retention of spatial or nonspatial memory. These data support a role for SK channels in the modulation of hippocampal synaptic plasticity and hippocampal-dependent memory encoding.
Keywords: synaptic plasticity, Ca2+-activated K+ channels, excitability, hippocampus, spatial memory, object memory
In hippocampal pyramidal neurons, action potentials are followed by an afterhyperpolarization (AHP) with three kinetic components. The predominant components, the medium AHP (mAHP) and slow AHP (sAHP), are attributable to the activation of small conductance Ca2+-activated K+ (SK) channels (Blatz and Magleby, 1986;Lancaster and Nicoll, 1987; Storm, 1990; Sah, 1996; Stocker et al., 1999). In addition to their different kinetics, the mAHP and the sAHP can be pharmacologically distinguished because apamin blocks the mAHP but not the sAHP (Kohler et al., 1996; Sah and Clements, 1999; Stocker et al., 1999). Apamin, a peptide derived from bee venom, is a highly selective blocker of SK channels, having no other known targets (Garcia et al., 1991). In CA1 neurons, synaptic activation may induce Ca2+ influx through NMDA receptors (NMDARs) (Alford et al., 1993; Kovalchuk et al., 2000), as well as through voltage-gated Ca2+channels (Magee and Johnston, 1995). Synaptic activation of a Ca2+-dependent K+ current resembling theIsAHP reduces postsynaptic excitability in response to high-frequency synaptic input (Lancaster et al., 2001).
Multiple forms of synaptic plasticity occur at the Schaffer collateral CA1 synapses, including long-term potentiation (LTP) and long-term depression (LTD) (Malenka and Nicoll, 1993). Essential for these processes is the influx of Ca2+ through NMDARs and the consequent rise in cytosolic Ca2+ (Lynch et al., 1983; Brocher et al., 1992; Malenka et al., 1992; Mulkey and Malenka, 1992). The magnitude of the rise in cytosolic Ca2+, as determined by the degree and pattern of NMDAR activation, distinguishes whether a synapse undergoes LTP or LTD. Trains of afferent stimuli capable of inducing synaptic plasticity cause a summation of EPSPs that generate action potentials. The consequent increases in intracellular Ca2+ may activate SK channels; thus SK channels may represent a mechanism for modulating the induction of synaptic plasticity. Using a single stimulus frequency (100 Hz for 1 sec or 5 Hz for 3 min) (Behnisch and Reymann, 1998; Norris et al., 1998; Foster, 1999), the magnitude of LTP induced in the CA1 region was increased by extracellular application of apamin. The present experiments investigated whether SK channels modulate the threshold for synaptic plasticity as defined by the frequency–response function (Bear, 1995) and the mechanism through which such a modulation may occur. By using a wide range of stimulation frequencies, the results show that SK channel activity modulated the threshold for the induction of synaptic plasticity through a postsynaptic mechanism that required NMDAR activation.
SK channel blockade has been shown to (1) facilitate hippocampal-independent learning (Messier et al., 1991; Fournier et al., 2001) and (2) enhance spatial memory in hippocampal-lesioned mice but not in intact mice (Ikonen et al., 1998; Ikonen and Riekkinen, 1999). Differences in behavioral paradigm and the precise memory process addressed complicate the literature concerning the cognitive effects of apamin in rodents. Based on our electrophysiological findings, hippocampal-dependent tests were specifically modified to examine the effects of apamin on the initial stages of memory encoding. The data demonstrate that apamin facilitated spatial and nonspatial memory encoding in C57BL/6 mice.
MATERIALS AND METHODS
Electrophysiology
Hippocampal slices were prepared from 3- to 6-week-old male C57BL/6NHsd mice (Harlan Sprague Dawley, Indianapolis, IN). Animals were anesthetized with halothane and decapitated. The cerebral hemispheres were quickly removed and placed in a partially frozen solution of artificial CSF (ACSF) (in mm): 119 NaCl, 2.5 KCl, 1.2 MgSO4, 2.5 CaCl2, 1 NaHPO4, 26.2 NaHCO3, and 10 glucose and equilibrated with 95% O2 and 5% CO2. Hippocampi were removed, placed on an agar block, and transferred to a slicing chamber containing a similarly partially frozen solution. Transverse hippocampal slices (300–500 μm thick) were cut with a Vibratome tissue slicer, transferred into a humidified holding chamber, and allowed to recover for ≥1 hr before recordings were performed. The following drugs were used: apamin (Calbiochem, La Jolla, CA) and d-2 amino-5-phosphonovaleric acid (d-APV) (Tocris Cookson, Ellisville, MO). Extracellular field potentials were recorded in the stratum radiatum using electrodes (3–6 MΩ) filled with 3m NaCl. For whole-cell recordings, CA1 pyramidal neurons were visualized with a water-immersion objective (40×; Zeiss, Thornwood, NY) using a microscope equipped with infrared/differential interference contrast optics (ZeissAxioskop 2FS) and a CCD camera (Sony, Tokyo, Japan). Whole-cell recording pipettes were fabricated from TW150F-4 thin-wall borosilicate glass (World Precision Instruments, Sarasota, FL) and had resistances of 1.5–3 MΩ. Pipettes were filled with an intracellular solution containing (in mm): 140 KMeSO4, 8 NaCl, 1 MgCl2, 10 HEPES, 2 Mg-ATP, 0.4 Na2-GTP, and 20 μm EGTA, pH 7.3, 290 mOsm. Slices were continuously perfused with ACSF. Whole-cell, patch-clamp currents were recorded with an Axopatch 200A amplifier (Axon Instruments, Foster City, CA), digitized using an ITC-16 analog-to-digital converter (InstruTech, Port Washington, NY), and transferred to a computer using Pulse software (Heka Elektronik, Lambrecht/Pfalz, Germany). CA1 neurons were voltage clamped at −55 mV, and IAHP tail currents were evoked by a depolarizing voltage command to +20 mV for 200 msec followed by a return to −55 mV. Experiments on control slices were interleaved with those on experimental slices. Data were collected and analyzed online (10 kHz sampling rate) using IGOR (WaveTech, Lake Oswego, OR) and a program kindly donated by Dr. Greg Hjelmstad (University of California San Francisco, San Francisco, CA). The maximal initial slope of the field EPSP was measured to monitor the strength of synaptic transmission, minimizing contamination by voltage-dependent events. Summary graphs were obtained by normalizing each experiment according to the average value of all points on the 10 min baseline, aligning the points with respect to the start of the (LTP and LTD) induction protocol, dividing each experiment into 1 min bins, and averaging these across experiments. The amount of potentiation or depression of the synaptic response was measured 40–50 min after conditioning. Data are expressed as mean ± SEM, as a percentage of the baseline. Student's t test and two-factor ANOVA were used to determine significance between groups of data; p < 0.05 was considered significant. Experiments were included in the data analysis only when LTP could be generated at the end of the experimental manipulation, ensuring that the occurrence of short-term potentiation or LTD was attributable to the experimental manipulation.
Morris water maze
To assess hippocampal-dependent spatial learning and memory, naive male C57BL/6NHsd mice (4–6 weeks of age) were trained in a Morris water maze (Silva et al., 1998; Cho et al., 1999). Before the start of behavioral testing, mice were habituated over a 3 d period to daily handling and intraperitoneal injection. Over the following 2 d, all mice received nonspatial habituation trials (one trial per day). During these trials, a clear Plexiglas platform (13 cm diameter) was placed in the center of a white polyethylene pool (60 cm high, 109 cm diameter), and floor-to-ceiling curtains were drawn around the pool to block the animals' use of extra-maze cues. The platform was 1 cm below the surface of the water, and the water was rendered opaque by the addition of nontoxic white Tempra paint. Each mouse was placed on the platform for 60 sec and then released into the pool at four locations adjacent to the platform and allowed to swim and climb onto the platform.
Spatial training. After nonspatial habituation, mice were trained on the spatial (hippocampal-dependent) version of the water maze task. Training comprised 24 trials (four trials per day) during which the platform remained submerged 1 cm below the water surface in a fixed position in the center of one quadrant of the pool. During a given trial, the mouse was introduced into the pool at one of four possible start points (north, south, west, and east) and allowed 60 sec to swim to the platform. The order of start points varied in a pseudorandom manner for each mouse every day. After remaining on the platform for 30 sec, the mouse was placed into a holding cage for a 45 sec intertrial interval. Throughout water maze testing, the water temperature was maintained at 22–23°C. Each mouse received intraperitoneal apamin (0.4 mg/kg, 10 ml/kg; Calbiochem) or 0.9% saline (10 ml/kg) 30 min before the first training trial of each day. This dose of apamin was defined in pilot studies conducted to determine a dose that was behaviorally effective but that induced no motor or convulsive effects.
Spatial memory testing. After the fourth, 12th, and 20th training trials, a probe test was conducted in which each mouse received a 30 sec free swim in the pool with the platform removed. Twenty-four hours after the final training trial (24th trial), each mouse received a 60 sec probe test of long-term retention. The behavior of the mice during training and probe tests was recorded with a computerized video tracking system (EthoVision 2.2; Noldus, Leesburg, VA) and analyzed to determine the amount of time spent in each of the four quadrants of the water maze.
Object recognition
To assess the effects of apamin on nonspatial hippocampal-dependent memory, naive male C57BL/6NHsd mice (4–6 weeks of age) were tested in an object-recognition memory task (Vnek and Rothblat, 1996; Clark et al., 2000). Before object recognition testing, all mice were habituated to intraperitoneal injection and to the open-field arena (38 × 38 × 64 cm high) for 5 min each day for 3 d. During a subsequent sample session, two identical novel objects [Duplo or Lego blocks (Lego Company, Billund, Denmark), toys, etc.] were placed into opposite corners (southwest and northeast) of the open-field arena, and the mouse was allowed to explore the objects. Pilot studies revealed that C57BL/6 mice averaged 38 sec of exploration of either sample object during a 5 min sample session. For the present study, the object recognition task was modified to explicitly examine the influence of apamin on object memory encoding. To manipulate encoding, mice were allowed to explore the sample objects until either 19 sec (minimal training) or 38 sec (extensive training) of object exploration had been accumulated. Twenty-four hours after the sample session, a test session was conducted during which each mouse was placed back into the arena containing one of the familiar objects and a novel object for 5 min. The spatial position of the novel object was counterbalanced so that one-half of the mice experienced the novel object in the southwest corner of the open field, whereas the other half of the mice experienced the novel object in the northeast corner. After each session, all objects were cleaned with 10% ethanol to reduce the possibility that mice were imparting some odor cue to the objects that would influence object exploratory behavior during a subsequent test session. Pilot studies were conducted to select objects that elicited equivalent degrees of exploration in mice. This is necessary to verify that naive mice exhibited no inherent preference for one object over the other.
The behavior of each mouse was recorded using the EthoVision system and scored to determine the amount of time spent exploring each of the objects during each session. Object exploration was defined as any time that the mouse's head was oriented toward the object, was within 2–3 cm of the object, and its vibrissas were moving. Object recognition memory was quantified by measuring the difference in exploration times between the novel and familiar object. A novel object preference index, a ratio of the amount of time spent exploring the novel object over the total time spent exploring both objects, was used to measure recognition memory. A novel object preference ratio of >0.5 indicates that the mouse spent more time exploring the novel object than the familiar one.
RESULTS
Apamin blocks SK channels underlying the mAHP and increases excitability
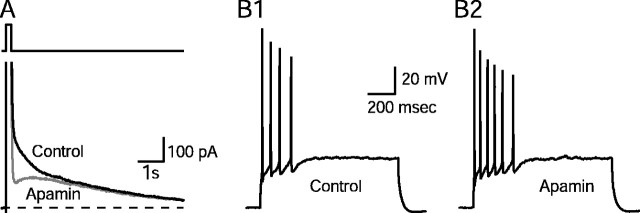
CA1 neurons express an apamin-sensitiveImAHP (Fig.1A) thought to be mediated by apamin-sensitive SK channels (Kohler et al., 1996; Stocker et al., 1999). Action potentials recorded in response to current injections showed that apamin (100 nm) increased the number of action potentials discharged in CA1 neurons (Fig.1B). Control cells fired an average ± SEM of 4.7 ± 1.2 action potentials per depolarizing pulse (Fig.1B1), which was increased to 6.7 ± 1.7 in the presence of apamin (Fig. 1B2) (n = 5;p = 0.04; paired Student's t test). This result indicates that blockade of apamin-sensitive SK channels increases excitability. Such changes in excitability may influence the threshold for the induction of synaptic plasticity.
Fig. 1.
Blockade of the apamin-sensitive afterhyperpolarization (mAHP) increases excitability.A, IAHPs were evoked in the whole-cell configuration by a 200 msec depolarizing pulse to +20 mV followed by a return to the −55 mV holding potential.IAHPs were obtained in the presence and absence of apamin (100 nm). After application of apamin, the medium-duration component (ImAHP) of the tail current was selectively inhibited. Dashed lineindicates zero current. B, Apamin increased the number of action potentials. B1, Response of a pyramidal neuron to a 1 sec depolarizing current pulse. B2, Response of the same neuron to the same depolarizing current pulse in the presence of apamin (control cells fired an average ± SEM of 4.7 ± 1.2 action potentials/depolarizing pulse, which increased to 6.7 ± 1.7 with apamin; n = 5; p = 0.04; paired Student's t test).
Blocking SK channels facilitates the induction of synaptic plasticity
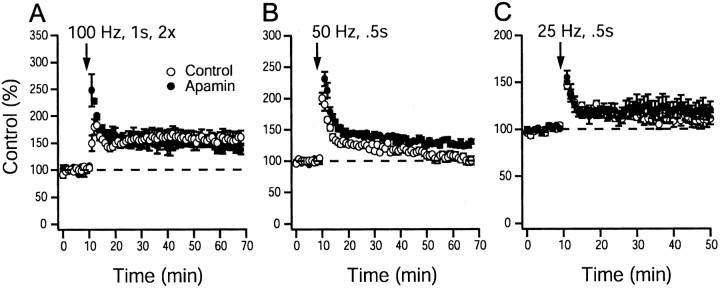
To investigate the role of SK channels on the induction of synaptic plasticity at CA1 synapses, stimulation protocols that evoke LTP or LTD were delivered to mouse hippocampal brain slices in the presence or absence of apamin. Figure2A shows the effect of apamin (100 nm) application on the ability of high-frequency stimulation (100 Hz applied twice for 1 sec, separated by 10 sec) to generate LTP. Equal extents of LTP were observed in control (164 ± 7%; n = 9 slices per 6 animals) and apamin-treated (165 ± 6%; n = 10 slices per 6 animals) slices, showing that apamin does not alter the ability of high-frequency stimulation to induce robust LTP (p > 0.05; unpaired Student's ttest). After a 50 Hz, 0.5 sec stimulus, significantly more LTP was induced in the presence of apamin (125 ± 3%, n = 13 slices per 8 animals for apamin-treated slices; 106 ± 4%,n = 12 slices per 8 animals for control slices;p < 0.05; unpaired Student's t test) (Fig.2B). Using a 25 Hz, 0.5 sec stimulus, LTP was not different in control and apamin-treated slices (120 ± 6%,n = 8 slices per 6 animals for apamin-treated slices; 109 ± 9%, n = 8 slices per 6 animals for control slices; p > 0.05; unpaired Student's ttest) (Fig. 2C).
Fig. 2.
Apamin block of SK channel activity enhances plasticity induced by high-frequency stimulation.A, A 100 Hz, 1 sec tetanus in control and apamin (100 nm)-treated slices (164 ± 7%,n = 9 slices per 6 animals for controls; 165 ± 6%, n = 10 slices per 6 animals for apamin;p > 0.05; unpaired Student's ttest). B, A 50 Hz, 0.5 sec stimulation protocol in control and apamin (100 nm)-treated slices (106 ± 4%, n = 12 slices per 8 animals for control slices, 125 ± 3%, n = 13 slices per 8 animals for apamin-treated slices; p < 0.05; unpaired Student's t test). C, A 25 Hz, 0.5 sec stimulation protocol in control and apamin (100 nm)-treated slices (109 ± 9%, n= 8 slices per 6 animals for controls; 120 ± 6%,n = 8 slices per 6 animals for apamin;p > 0.05; unpaired Student's ttest). Control and apamin-treated slices were interleaved. Synaptic strength was measured as the initial slope of the recorded field EPSP. Dashed line indicates baseline response inA–C.
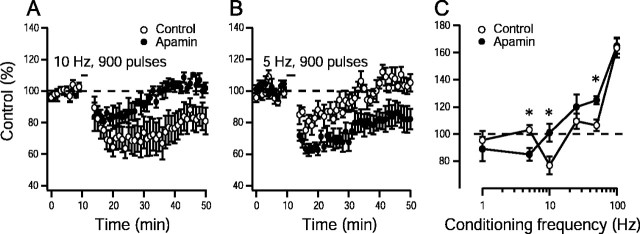
To determine whether apamin affects the threshold for induction of synaptic plasticity, its effects on lower stimulation frequencies were examined. A 10 Hz stimulation for 900 pulses resulted in LTD in control slices (77 ± 6%; n = 10 slices per 6 animals), whereas apamin-treated slices did not show changes in synaptic strength (101 ± 7%; n = 9 slices per 5 animals;p < 0.05; unpaired Student's t test) (Fig.3A). In addition, 5-Hz stimulation for 900 pulses resulted in LTD in apamin-treated slices (85 ± 5%; n = 9 slices per 5 animals) but did not affect long-lasting changes in synaptic strength in control slices (103 ± 4%; n = 10 slices per 6 animals;p < 0.05; unpaired Student's t test) (Fig.3B). These results suggest that apamin alters the frequency–response relationship (Bear, 1995) for the induction of synaptic plasticity. The modification threshold is the level of postsynaptic response at which the sign of the synaptic modification reverses from LTD to LTP (Bienenstock et al., 1982). The smooth transition from LTD to LTP may be demonstrated by systematically varying the frequency of conditioning stimulation for a given number of pulses. The frequency–response relationships for control and apamin-treated slices are presented in Figure 3C and demonstrate that blockade of SK channels with apamin shifts the frequency–response function to the lower frequencies, facilitating the induction of synaptic plasticity.
Fig. 3.
Apamin block of SK channel activity shifts the synaptic modification threshold to lower frequencies. Induction of synaptic plasticity by 10 Hz, 900 pulse stimulation in control slices (77 ± 6%; n = 10 slices per 6 animals) and apamin (100 nm)-treated slices (101 ± 7%; n = 9 slices per 5 animals;p < 0.05; unpaired Student's ttest) (A) and 5 Hz, 900 pulse stimulation protocol in control slices (103 ± 4%; n = 8 slices per 5 animals) and apamin (100 nm)-treated slices (85 ± 5%; n = 9 slices per 5 animals;p < 0.05; unpaired Student's ttest) (B). Dashed line indicates baseline response. C, Frequency–response relationship for the induction of LTP and LTD in controls and experiments from slices in which apamin (100 nm) was applied. The mean effect of 900 pulses of conditioning stimulation delivered at various frequencies to the Shaffer collaterals on the synaptic response measured 40–50 min after conditioning is shown. ∗p < 0.05 versus respective control data point; Student's t test. Dashed line indicates the transition between LTD and LTP.
Blocking SK channels does not affect neurotransmitter release
To investigate whether the apamin-induced shift in the frequency–response function at CA1 synapses involves presynaptic or postsynaptic changes, the effects of apamin on paired-pulse facilitation, post-tetanic potentiation, and short-term depression were investigated.
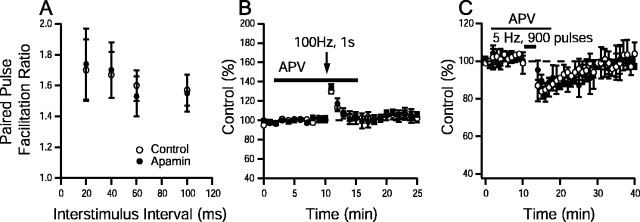
Paired-pulse facilitation, an increased second response to two stimuli applied in rapid succession, is thought to reflect an increase in the probability of neurotransmitter release (Katz and Miledi, 1968). Paired-pulse facilitation was tested at interstimulus intervals ranging from 20 to 100 msec and was not significantly altered by application of apamin (p > 0.05; paired Student'st test; n > 8 for all interstimulus intervals) (Fig. 4A), suggesting that apamin does not alter neurotransmitter release.
Fig. 4.
SK channels do not have presynaptic effects in CA1. A, Paired-pulse facilitation (PPF), measured as the ratio of the second response to the first, was plotted as a function of interstimulus interval for controls and in the presence of apamin (n > 8 for all interstimulus intervals). No significant differences were detected (p > 0.05; paired Student's t test). B, Time course of post-tetanic potentiation elicited by 100 Hz, 1 sec tetanus in control and apamin-treated slices. Post-tetanic potentiation (peak enhancement in controls, 132 ± 6% of baseline,n = 9 slices per 4 animals; peak enhancement in apamin, 134 ± 5% of baseline, n = 9 slices per 4 animals) was not different between groups (p > 0.05; unpaired Student'st test). C, Time course of short-term depression elicited by 5 Hz, 900 pulse stimulation in control and apamin-treated slices (80 ± 7% of baseline,n = 6 slices per 3 animals; peak depression in apamin, 82 ± 5% of baseline, n = 6 slices per 3 animals). No significant differences were detected between groups (p > 0.05; paired Student'st test). Synaptic strength was measured as the initial slope of the recorded field EPSP. Solid line in Band C indicates the duration of d-APV application.
Post-tetanic potentiation, a slow decay of the postsynaptic responses after repetitive stimulation has been terminated, presumably reflects the slow decay of elevated presynaptic Ca2+ levels induced by the tetanic stimulus (Zucker, 1989). The effects of apamin on post-tetanic potentiation were examined using a 100 Hz, 1 sec tetanus delivered in the presence of d-APV (100 μm), an NMDA receptor antagonist. The time course of post-tetanic potentiation was not different between control and apamin-treated slices, nor were differences detected in the peak enhancement achieved in the presence or absence of apamin (control, 132 ± 6%, n = 9 slices per 4 animals; apamin treated, 134 ± 5%,n = 9 slices per 5 animals; p > 0.05; unpaired Student's t test) (Fig. 4B).
Short-term depression was also examined using a 5 Hz, 900 stimuli tetanus delivered in the presence of d-APV (100 mm). This same protocol had revealed differences between control and apamin-treated slices when performed in the absence ofd-APV (Fig. 3B). In the presence ofd-APV (Fig. 4C), the magnitude and time course of depression were not different between control slices (peak depression, 80 ± 7%, n = 9 slices per 4 animals) and apamin-treated slices (peak depression, 82 ± 5%,n = 9 slices per 5 animals; p > 0.05; unpaired Student's t test). The lack of effect of apamin on paired-pulse facilitation, post-tetanic potentiation, and short-term depression suggests that apamin does not affect presynaptic events but rather alters NMDAR-dependent postsynaptic events to shift the threshold for the induction of synaptic plasticity in CA1 synapses.
Blocking SK channels accelerates hippocampal-dependent spatial memory encoding
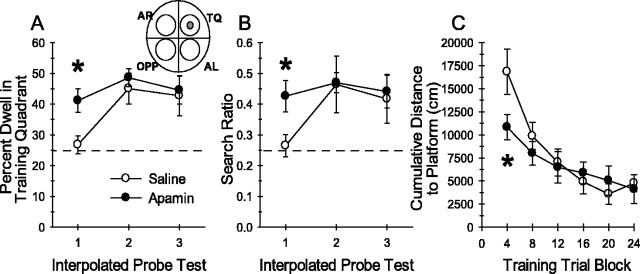
The results presented above indicate that apamin facilitates the induction of synaptic plasticity in the CA1 region of the hippocampus. It was hypothesized that apamin might also alter hippocampal-dependent memory assessed in the Morris water maze, a task considered to require the activation of NMDARs and synaptic plasticity in the hippocampus (Morris et al., 1982; Tsien et al., 1996). Considering the finding that apamin shifted the threshold for the induction of synaptic plasticity (Fig. 3C), it was predicted that apamin would exert its greatest influence during the initial stages of spatial memory encoding. Specifically, the effects of systemic apamin were examined using a version of the Morris water-maze task modified to explicitly assess the encoding of spatial memory. The rationale being that if synaptic plasticity is more easily induced in the presence of apamin, fewer trials may be required to encode spatial memory in apamin-treated mice. Naive mice received apamin (0.4 mg/kg, i.p.; n = 10) or 0.9% saline (n = 9) 30 min before daily training for 6 d (four trials per day) in the water maze task. The platform location remained fixed throughout all training trials. Immediately after the fourth, 12th, and 20th training trials, each mouse received a 30 sec probe test. These interpolated probe tests assess the development of a spatial bias for the training quadrant of the pool at an early (probe 1), intermediate (probe 2), and late (probe 3) stage of spatial memory encoding. Apamin treatment accelerated the development of a spatial bias for the training quadrant during the first interpolated probe test (probe 1), as shown in Figure5A. Planned comparisons analyses revealed that apamin-treated mice spent significantly more time in the training quadrant than saline-treated mice (mean ± SEM; apamin, 41.1 ± 3.8%; saline, 26.8 ± 2.9%;t(17) = −2.94; p = 0.009). In addition, apamin-treated mice exhibited more accurate search behavior as indicated by search ratio (Fig. 5B), computed as the number of crossings into a circular zone encompassing the platform divided by the total number of crossings into all four zones (Fig.5B, inset diagram) (apamin, 0.43 ± 0.05; saline, 0.26 ± 0.04; t(17) = −2.52; p = 0.02). Saline-treated mice required 12 training trials to develop this degree of preference (probe 2). Thus, after minimal training (just four trials), apamin-treated mice exhibited significant spatial memory of the training quadrant, whereas control mice exhibited a chance level of performance. There were no additional differences in performance on probes 2 and 3 between apamin- and saline-treated mice, indicating that after 12 training trials, the saline-treated mice had acquired the memory for platform location and were performing as accurately as apamin-treated mice. Spatial memory encoded by apamin-treated mice was stable throughout the training session, because there was no difference in training-quadrant preference across the three interpolated probe tests. Two-factor, repeated-measures (treatment × four trial block) ANOVA on cumulative distance to platform measures revealed a significant treatment × four trial block interaction (F(4,68) = 2.53; p < 0.05) and a significant effect of four trial block (F(4,68) = 15.75; p < 0.001). Tukey multiple comparisons tests revealed a significant difference between apamin- and saline-treated mice in cumulative distance to platform on the first four trial block (Fig.5C). The cumulative distance to platform is a score of the proximity of the mouse to the platform during training and is a more sensitive measure of spatial behavior than escape latency (Gallagher et al., 1993). The difference in cumulative distance measures between apamin- and saline-treated mice reflects more accurate platform search behavior by the apamin-treated mice, a finding that is consistent with the observed differences in spatial search behavior during probe 1. An identical analysis of escape latency data found a significant effect of four trial block (F(4,68) = 8.50;p < 0.001) but no treatment × four trial block interaction (F(4,68) = 0.44;p > 0.5) and no significant effect of treatment (F(4,68) = 0.54; p > 0.5). Apamin treatment did not cause any overt influence on swimming, and swim speeds were not different between groups (F(1,17) = 0.29; p > 0.5). Analyses restricted to the data from the first four training trials also indicated no significant differences in escape latencies (t(17) = 0.33; p > 0.05) or swim speed (t(17) = 0.04;p > 0.05). Collectively, these results suggest that apamin-mediated blockade of SK channels facilitated the encoding of hippocampal-dependent spatial memory.
Fig. 5.
Apamin block of SK channels facilitates the encoding of spatial memory. A, A modified Morris water maze task was used to examine the effects of apamin on encoding of spatial memory. Mice were trained for four trials per day for 6 d, and 30 sec probe tests were presented immediately after the fourth, 12th, and 20th trial. Mean ± SEM percentage of time spent dwelling (Percent Dwell) in the training quadrant during the interpolated probe tests revealed that mice treated with 0.4 mg/kg apamin (n = 10) spent significantly more time in the training quadrant during the first probe test than saline-treated (n = 9) control mice (∗p < 0.009 vs saline-treated mice on probe test 1; planned comparison Student's t test). Thedashed line at 25% represents chance performance.AL, Adjacent left; AR, adjacent right; OPP, opposite; TQ, training quadrant. B, Mean ± SEM search ratio reflects the accuracy with which mice search in the correct location within the training quadrant of the pool. Search ratio is computed as the number of times the animal crosses into the zone (see circular regions of inset diagram) encompassing the platform (shaded zone) divided by the total number of crossings into all four zones. The dashed line at 0.25 represents chance performance during the probe tests or the lack of spatial bias for any particular pool location. Apamin-treated mice exhibited a significantly higher search ratio than saline-treated mice during the first probe test (∗p < 0.02 vs saline-treated mice on probe test 1; planned comparison Student'st test). Measures of the percentage of time spent dwelling in the training quadrant or search ratio from the second or third probe tests were equivalent between the two groups, indicating that there were no group differences in platform search behavior after more training. C, Mean ± SEM cumulative distance to platform measures of saline- and apamin-treated mice plotted in blocks of four training trials. This measure indicates the proximity of the mice to the platform during each training trial. Consistent with the data from probe test 1, apamin-treated mice swam in closer proximity to the platform during the first four trial block of training than saline-treated mice (∗p < 0.04; post hoc Tukey multiple comparisons test).
Data from the probe test given 24 hr after the final training trial were examined to test whether memory encoded under SK channel blockade would be differentially retained. Both groups of mice exhibited a spatial bias for searching in the training quadrant during the 24 hr retention probe test. There were no differences between saline- and apamin-treated mice during the final probe trial with regard to the percentage of time spent dwelling in the training quadrant (mean ± SEM; saline, 41.6 ± 4.6; apamin, 38.1 ± 4.3;t(17) = 0.57; p > 0.6) or with regard to the search ratio (saline, 0.43 ± 0.05; apamin 0.38 ± 0.05; t(17) = 0.64; p > 0.5). Together with the data of Figure5A–C, it appears that apamin-treated mice encoded the spatial memory of the platform location with less training than the saline-treated mice. However, once encoded, there was no difference in retention of the spatial memory between apamin- and saline-treated mice.
Blocking SK channels accelerates hippocampal-dependent nonspatial memory encoding.
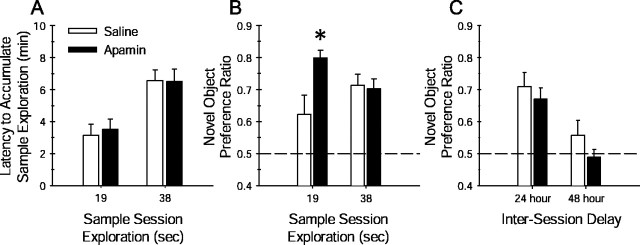
To further examine the role of SK channels in hippocampal memory, the effects of apamin on a nonspatial object-recognition task were examined. This task assesses the encoding and retention of memory for an object and is sensitive to lesions of the hippocampus (Vnek and Rothblat, 1996; Clark et al., 2000) and to manipulation of hippocampal NMDARs (Tang et al., 1999). Given that apamin-treated mice developed a significant spatial memory for the platform location after minimal water-maze training, it was hypothesized that apamin would influence the encoding of object memory in a similar manner. Pilot studies indicated that during a 5 min sample session, C57BL/6J mice typically spend an average of 38 sec exploring each sample object. To examine the influence of apamin on object memory encoding, the amount of sample object exploration was manipulated. Mice were allowed to explore the sample objects until they had accumulated object exploration times of either 19 sec (minimal training) or 38 sec (extensive training). The results obtained for spatial memory encoding led to our prediction that apamin would facilitate object memory retention in mice limited to 19 sec of sample object exploration compared with respective saline-treated mice.
Naive C57BL/6NHsd mice received apamin (0.4 mg/kg, i.p.) or 0.9% saline 30 min before the sample session. Each mouse was placed into the arena containing two identical novel objects. Depending on group assignment, the mouse was removed from the arena after exploring either sample object for 19 sec (minimal training) or 38 sec (extensive training). During the sample session, there was no significant difference between saline- and apamin-treated mice with regard to the time required to reach the respective 19 or 38 sec sample object exploration limit (t(17) = −1.31 and 0.04, respectively; p values of >0.05) (Fig.6A), indicating that all mice exhibited the same curiosity and motivation. Object memory retention was assessed during a test session 24 hr later, in which each mouse was allowed to explore the arena containing one of the familiar objects from the sample session and a novel object. Saline-treated mice limited to 19 sec of sample object exploration exhibited a weaker preference for the novel object during the test session compared with mice permitted 38 sec of sample object exploration (Fig.6B). Planned comparisons analysis revealed that apamin-treated mice limited to 19 sec of sample object exploration exhibited a stronger preference for the novel object compared with the respective saline-treated mice (t(17)= −2.17; p = 0.04) (Fig. 6B). These data suggest that apamin is capable of facilitating object memory encoding. There was no difference in novel object preference ratio between saline- and apamin-treated mice permitted 38 sec of sample object exploration (t(17) = −0.11;p > 0.05), indicating that both groups exhibited equivalent object memory retention. In accordance with our findings of apamin-treated mice in the Morris water-maze task, these findings suggest that apamin block of SK channels facilitates the encoding of nonspatial memory, perhaps by reducing the threshold for memory formation.
Fig. 6.
Apamin block of SK channel activity facilitates the encoding of nonspatial object memory but does not influence the retention of object memory. Object-recognition memory was quantified by computing the novel object preference ratio, the amount of time spent exploring the novel object during the test session divided by the total time spent exploring both the familiar and novel object. A, The object recognition task was modified to test the influence of apamin on object memory encoding. As described in Materials and Methods, during the sample session, saline- and apamin-treated mice were restricted to either 19 sec (minimal training) or 38 sec (extensive training) of sample object exploration. The amount of time required to accumulate either 19 or 38 sec of sample object exploration did not differ between apamin- and saline-treated mice (p values >0.05; unpaired Student's t test). B, Restricting the amount of object exploration during the sample session to 19 sec weakens the degree of preference exhibited by the mouse during a test session 24 hr later. This is illustrated by the lower novel object preference ratio of the saline-treated mice (n = 9) limited to 19 sec of sample object exploration. However, apamin (0.4 mg/kg)-treated mice (n = 10) that were limited to only 19 sec of sample object exploration exhibited a significantly greater novel object preference during the 24 hr test session (∗p < 0.04 vs saline-treated mice permitted 19 sec of object exploration; planned comparison Student'st test). When apamin (0.4 mg/kg)-treated (n = 10) and saline-treated (n= 9) mice were permitted 38 sec of sample object exploration, there was no difference in novel object preference ratio during the 24 hr test session. Each dashed line at 0.5 represents chance performance or a lack of discrimination between the novel and familiar object. C, Object memory retention decays over a similar time course in apamin- and saline-treated mice. Both apamin- and saline-treated mice exhibited similar strong preference for the novel object during a 24 hr retention test; mean ± SEM novel object preference ratios were not significantly different. Four days later, the same mice received a second sample session with two new objects. When tested for retention 48 hr later, both apamin- and saline-treated mice failed to show a strong preference for the novel object over the familiar object. These data indicate that apamin does not affect the retention of object memory.
The retention of object memory decays faster in hippocampal-lesioned rodents (Vnek and Rothblat, 1996; Clark et al., 2000) and is sensitive to genetic manipulation of the hippocampal NMDAR (Tang et al., 1999). Hippocampal-lesioned rats fail to retain object memory over a 24 hr delay (Clark et al., 2000) but are able to retain object memory over a 5 min delay (Mumby et al., 2002). The influence of systemic apamin on the rate of decay of object memory retention was examined in a second cohort of C57BL/6NHsd mice. During a sample session, apamin- (0.4 mg/kg, i.p.) and saline-treated mice were exposed to two identical sample objects for 5 min. Both groups exhibited a similar preference for the novel object during the 24 hr test session, as shown in Figure6C, indicating that apamin did not influence memory retention at 24 hr, consistent with the 38 sec data of Figure6B. Four days later, the same groups were exposed to a second set of sample objects and then tested for retention 48 hr later. As depicted in Figure 6C, neither group exhibited a significant preference for the novel object at the 48 hr test session, suggesting that object memory decayed over the same rate between the two groups. These data indicate that apamin did not influence object memory retention.
DISCUSSION
The present study demonstrates that blockade of synaptically activated SK channels increases excitability and decreases the threshold for the induction of hippocampal synaptic plasticity via a postsynaptic mechanism that requires the activation of NMDARs. The reduced threshold for induction of synaptic plasticity is associated with facilitated memory encoding. This enhancement is correlated with changes in the induction of synaptic plasticity but is not necessarily attributable to these changes, because there is no way to rule out the effects of apamin on other brain structures that can influence hippocampal function.
Neural circuits derive flexibility from activity-driven bidirectional modification of synaptic strength (Sejnowski, 1977; Bienenstock et al., 1982). An important characteristic of this process is the threshold for synaptic modification (Bear, 1995), which is defined by the frequency–response function for the induction of synaptic plasticity. As postsynaptic activity increases, the threshold for LTD is reached first, and an additional increase leads to a transition from LTD to LTP. This transition represents the synaptic modification threshold (Bear, 1995). A prominent model for the regulation of the synaptic modification threshold proposes that the direction of altered synaptic efficacy, potentiation, or depression is determined by the level of postsynaptic Ca2+ during neural activity (Lisman, 1989; Artola and Singer, 1993; Malenka and Nicoll, 1993). The rise in Ca2+ within the dendritic spine is the critical trigger for synaptic plasticity. Stronger depolarization allows more Ca2+ to enter and leads to synaptic potentiation (Lisman, 1989; Artola and Singer, 1993; Bliss and Collingridge, 1993; Cummings et al., 1996; Malenka and Nicoll, 1999), whereas weaker depolarization leads to less Ca2+ influx and synaptic depression (Mulkey and Malenka, 1992; Dudek and Bear, 1993). Therefore, any manipulation that influences the magnitude or dynamics of Ca2+ increase within dendritic spines may profoundly influence the form of the resulting synaptic plasticity.
Synaptic activation of the channels underlying theIsAHP (Lancaster et al., 2001; Martin et al., 2001) regulates synaptic efficacy and may influence the threshold for synaptic plasticity, as hypothesized by previous studies (Sah and Bekkers, 1996). Our results showed that application of apamin caused a shift of the synaptic modification threshold to lower frequencies, an effect that is consistent with facilitated induction of synaptic plasticity. Apamin-sensitive SK channels underlie the mAHP in CA1 neurons, which peaks ∼200 msec after the action potential (Sah and Clements, 1999; Stocker et al., 1999), a time course that may enable the mAHP to influence neuronal discharge activity, and the integration of synaptic events as the rate of afferent stimulation increases toward the threshold for synaptic plasticity (5–20 Hz). These are precisely the stimulation frequencies around which apamin exerted its significant effects on the induction of synaptic plasticity.
Our results suggest that SK channel activity modulates the induction of synaptic plasticity that requires postsynaptic depolarization and NMDAR activation. Postsynaptic depolarization induced by repetitive synaptic stimulation raises intracellular Ca2+levels through voltage-gated Ca2+ channels or NMDARs, permitting the activation of SK channels. By hyperpolarizing the postsynaptic membrane, SK channels decrease excitability and modulate the activation of NMDARs, which involves voltage-dependent removal of the Mg2+ block (Mayer et al., 1987). By affecting the degree of NMDAR activation and the subsequent Ca2+ entry, SK channels may modulate the induction of synaptic plasticity. Our experiments suggest that SK channels are dendritically localized. Although direct evidence for the distribution of SK channels in the dendrites is currently unavailable, it has been suggested that apamin-sensitive SK channels are located predominantly in proximal and distal dendrites of motor neurons (Cangiano et al., 2002). In addition, Ca2+-activated K+ channels have been reported in the dendrites of mammalian neurons (Andreasen and Lambert, 1995; Sah and Bekkers, 1996; Schwindt and Crill, 1997).
Synaptic plasticity is believed to represent, at least in part, the cellular mechanisms responsible for learning and memory. It is generally accepted that some form of an increase in synaptic efficacy in the hippocampus is necessary for encoding spatial memory in the water maze task (Moser et al., 1998). Whether such memory formation in the hippocampus is dependent on LTP or LTD has been difficult to establish (Holscher, 1997; Jeffery, 1997; Shors and Matzel, 1997). In the present study, blockade of SK channels increases excitability, reduces the threshold for hippocampal synaptic plasticity, and facilitates hippocampal memory encoding. Systemically administered apamin crosses the blood–brain barrier (Habermann, 1984), and high densities of apamin-sensitive SK channels are present in limbic regions, including the hippocampus (Mourre et al., 1987; Gehlert and Gackenheimer, 1993; Stocker and Pedarzani, 2000). Apamin-treated mice acquired a spatial memory for the water maze platform location after just four training trials (minimal training), whereas saline-treated mice required as many as 12 trials to demonstrate spatial memory acquisition. In the object recognition task, apamin-treated mice exhibited a significantly stronger test session preference for the novel object than saline-treated mice when limited to 19 sec of sample object exploration (minimal training). The parallel between the reduction of the threshold for synaptic plasticity and the improved memory encoding after minimal spatial or nonspatial training suggests a correlation between the facilitation of the induction of synaptic plasticity and memory encoding. The amount of induced plasticity cannot be equated with the rate of learning, because control and apamin-treated slices showed the same amount of LTP and LTD. However, the rate of learning seems to be dependent on the threshold for the induction of synaptic plasticity.
Previous studies indicate that apamin enhances spatial memory in mice with lesions of the hippocampal formation but have failed to detect an influence of apamin on memory retention in intact mice after extensive training (Ikonen et al., 1998; Ikonen and Riekkinen, 1999). Moreover, it was proposed recently that there are differences in apamin sensitivity between mice and rats, with rats being relatively insensitive to the cognitive effects of apamin (van der Staay et al., 1999). However, this claim is not substantiated by recent findings. Independent laboratories have demonstrated that in rats, apamin enhances the induction of synaptic plasticity (Behnisch and Reymann, 1998; Norris et al., 1998) and facilitates nonspatial memory (Deschaux et al., 1997; Fournier et al., 2001). Our data suggest that apamin exerts its influence on an early stage of memory encoding, an effect that may not have been detected given the approaches used previously. This indicates that apamin facilitated memory after minimal spatial or nonspatial training. Apamin did not have a significant effect on memory retention in mice after extensive spatial training, consistent with previous reports of the effects of apamin in mice and rats (Ikonen et al., 1998; Ikonen and Riekkinen, 1999; van der Staay et al., 1999).
From our behavioral data, no distinction can be made between an effect of apamin that leads to enhanced memory formation and that of an enhanced processing of the sensory input that precedes the formation of memory. However, the results suggest that it is unlikely that the enhancing effects of apamin are a consequence of sensory, motor, or attentional influences. If apamin was to influence sensory or attentional mechanisms, then apamin treatment would have enhanced object memory retention in both groups of mice, those limited to 19 sec of sample exploration as well as those allowed 38 sec of sample exploration. The beneficial effect of apamin on spatial memory encoding in the Morris water maze cannot be attributed to enhanced motor function, because no differences in swim speed between apamin- and saline-treated mice were observed.
Collectively, the data from electrophysiological and behavioral studies indicate that blockade of SK channels by apamin increases excitability, shifts the threshold for the induction of synaptic plasticity, and facilitates hippocampal-dependent memory. The behavioral significance of this apamin-induced increase in excitability is to facilitate the processing of to-be-remembered information. The behavioral studies do not indicate whether the apamin-mediated enhancement in memory is caused by a facilitation of the induction of LTP or LTD. However, the shift in the threshold for synaptic plasticity produced by apamin could represent a mechanism for ensuring that there is coincident stimulation of hippocampal NMDARs leading to an enhancement of synaptic efficacy during the initial stages of learning. Learning-induced reduction of the AHP has been shown to underlie learning and memory in other behavioral paradigms. A potentiation of EPSPs and a reduction in the mAHP and sAHP currents is associated with classical conditioning of the eye-blink response in rabbits (Disterhoft et al., 1988; LoTurco et al., 1988; Coulter et al., 1989) and with olfactory operant conditioning in rats (Saar et al., 1998). Single-unit recording studies of hippocampal neurons from behaving rabbits during eye-blink conditioning trials have revealed increases in neuronal firing rates that are specific to learning (Berger et al., 1983; McEchron and Disterhoft, 1999). Therefore, the AHP is a negative regulator of learning, and reduction of the AHP by apamin appears to facilitate learning and memory. Together with the results presented here, it appears that apamin-sensitive SK channels represent a neural mechanism capable of regulating hippocampal-dependent memory.
Footnotes
This work was supported by a grant from the Medical Research Foundation of Oregon (T.T.) and by grants from the National Institutes of Health (J.M., J.P.A.). We thank Drs. Craig Jahr, Laurence Trussell, and members of their laboratories for helpful discussions. We also thank Sophie Davis (Oregon Health and Science University/Portland State University Saturday Academy 2001 summer high school science apprenticeship program) for help with behavioral testing and Laura Tull, Dr. Anastassios Tzingounis, and Dr. Jacques Wadiche for helpful comments on this manuscript.
Correspondence should be addressed to Dr. Thanos Tzounopoulos, Auditory Neuroscience, L-335A, Oregon Hearing Research Center, Oregon Health and Science University, 3181 Southwest Sam Jackson Park Road, Portland, OR 97239-3098. E-mail: tzounopo@ohsu.edu.
REFERENCES
- 1.Alford S, Frenguelli BG, Schofield JG, Collingridge GL. Characterization of Ca2+ signals induced in hippocampal CA1 neurones by the synaptic activation of NMDA receptors. J Physiol (Lond) 1993;469:693–716. doi: 10.1113/jphysiol.1993.sp019838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreasen M, Lambert JD. The excitability of CA1 pyramidal cell dendrites is modulated by a local Ca2+-dependent K+-conductance. Brain Res. 1995;698:193–203. doi: 10.1016/0006-8993(95)00910-i. [DOI] [PubMed] [Google Scholar]
- 3.Artola A, Singer W. Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci. 1993;16:480–487. doi: 10.1016/0166-2236(93)90081-v. [DOI] [PubMed] [Google Scholar]
- 4.Bear MF. Mechanism for a sliding synaptic modification threshold. Neuron. 1995;15:1–4. doi: 10.1016/0896-6273(95)90056-x. [DOI] [PubMed] [Google Scholar]
- 5.Behnisch T, Reymann KG. Inhibition of apamin-sensitive calcium dependent potassium channels facilitate the induction of long-term potentiation in the CA1 region of rat hippocampus in vitro. Neurosci Lett. 1998;253:91–94. doi: 10.1016/s0304-3940(98)00612-0. [DOI] [PubMed] [Google Scholar]
- 6.Berger TW, Rinaldi PC, Weisz DJ, Thompson RF. Single-unit analysis of different hippocampal cell types during classical conditioning of rabbit nictitating membrane response. J Neurophysiol. 1983;50:1197–1219. doi: 10.1152/jn.1983.50.5.1197. [DOI] [PubMed] [Google Scholar]
- 7.Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blatz AL, Magleby KL. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature. 1986;323:718–720. doi: 10.1038/323718a0. [DOI] [PubMed] [Google Scholar]
- 9.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 10.Brocher S, Artola A, Singer W. Intracellular injection of Ca2+ chelators blocks induction of long-term depression in rat visual cortex. Proc Natl Acad Sci USA. 1992;89:123–127. doi: 10.1073/pnas.89.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cangiano L, Wallen P, Grillner S. Role of apamin-sensitive KCa channels for reticulospinal synaptic transmission to motoneuron and for the afterhyperpolarization. J Neurophysiol. 2002;88:289–299. doi: 10.1152/jn.2002.88.1.289. [DOI] [PubMed] [Google Scholar]
- 12.Cho YH, Friedman E, Silva AJ. Ibotenate lesions of the hippocampus impair spatial learning but not contextual fear conditioning in mice. Behav Brain Res. 1999;98:77–87. doi: 10.1016/s0166-4328(98)00054-0. [DOI] [PubMed] [Google Scholar]
- 13.Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coulter DA, LoTurco JJ, Kubota M, Disterhoft JF, Moore JW, Alkon DL. Classical conditioning reduces amplitude and duration of calcium-dependent afterhyperpolarization in rabbit hippocampal pyramidal cells. J Neurophysiol. 1989;61:971–981. doi: 10.1152/jn.1989.61.5.971. [DOI] [PubMed] [Google Scholar]
- 15.Cummings JA, Mulkey RM, Nicoll RA, Malenka RC. Ca2+ signaling requirements for long-term depression in the hippocampus. Neuron. 1996;16:825–833. doi: 10.1016/s0896-6273(00)80102-6. [DOI] [PubMed] [Google Scholar]
- 16.Deschaux O, Bizot JC, Goyffon M. Apamin improves learning in an object recognition task in rats. Neurosci Lett. 1997;222:159–162. doi: 10.1016/s0304-3940(97)13367-5. [DOI] [PubMed] [Google Scholar]
- 17.Disterhoft JF, Golden DT, Read HL, Coulter DA, Alkon DL. AHP reductions in rabbit hippocampal neurons during conditioning correlate with acquisition of the learned response. Brain Res. 1988;462:118–125. doi: 10.1016/0006-8993(88)90593-8. [DOI] [PubMed] [Google Scholar]
- 18.Dudek SM, Bear MF. Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. J Neurosci. 1993;13:2910–2918. doi: 10.1523/JNEUROSCI.13-07-02910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster TC. Involvement of hippocampal synaptic plasticity in age-related memory decline. Brain Res Brain Res Rev. 1999;30:236–249. doi: 10.1016/s0165-0173(99)00017-x. [DOI] [PubMed] [Google Scholar]
- 20.Fournier C, Kourrich S, Soumireu-Mourat B, Mourre C. Apamin improves reference memory but not procedural memory in rats by blocking small conductance Ca2+-activated K+ channels in an olfactory discrimination task. Behav Brain Res. 2001;121:81–93. doi: 10.1016/s0166-4328(00)00387-9. [DOI] [PubMed] [Google Scholar]
- 21.Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- 22.Garcia ML, Galvez A, Garcia-Calvo M, King VF, Vazquez J, Kaczorowski GJ. Use of toxins to study potassium channels. J Bioenerg Biomembr. 1991;23:615–646. doi: 10.1007/BF00785814. [DOI] [PubMed] [Google Scholar]
- 23.Gehlert DR, Gackenheimer SL. Comparison of the distribution of binding sites for the potassium channel ligands [125I]apamin, [125I]charybdotoxin and [125I]iodoglyburide in the rat brain. Neuroscience. 1993;52:191–205. doi: 10.1016/0306-4522(93)90192-i. [DOI] [PubMed] [Google Scholar]
- 24.Habermann E. Apamin. Pharmacol Ther. 1984;25:255–270. doi: 10.1016/0163-7258(84)90046-9. [DOI] [PubMed] [Google Scholar]
- 25.Holscher C. Long-term potentiation: a good model for learning and memory? Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:47–68. doi: 10.1016/s0278-5846(96)00159-5. [DOI] [PubMed] [Google Scholar]
- 26.Ikonen S, Riekkinen P., Jr Effects of apamin on memory processing of hippocampal-lesioned mice. Eur J Pharmacol. 1999;382:151–156. doi: 10.1016/s0014-2999(99)00616-0. [DOI] [PubMed] [Google Scholar]
- 27.Ikonen S, Schmidt B, Riekkinen P., Jr Apamin improves spatial navigation in medial septal-lesioned mice. Eur J Pharmacol. 1998;347:13–21. doi: 10.1016/s0014-2999(98)00075-2. [DOI] [PubMed] [Google Scholar]
- 28.Jeffery KJ. LTP and spatial learning–where to next? Hippocampus. 1997;7:95–110. doi: 10.1002/(SICI)1098-1063(1997)7:1<95::AID-HIPO10>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 29.Katz B, Miledi R. The role of calcium in neuromuscular facilitation. J Physiol (Lond) 1968;195:481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 1996;273:1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- 31.Kovalchuk Y, Eilers J, Lisman J, Konnerth A. NMDA receptor-mediated subthreshold Ca2+ signals in spines of hippocampal neurons. J Neurosci. 2000;20:1791–1799. doi: 10.1523/JNEUROSCI.20-05-01791.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lancaster B, Nicoll RA. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol (Lond) 1987;389:187–203. doi: 10.1113/jphysiol.1987.sp016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lancaster B, Hu H, Ramakers GM, Storm JF. Interaction between synaptic excitation and slow afterhyperpolarization current in rat hippocampal pyramidal cells. J Physiol (Lond) 2001;536:809–823. doi: 10.1111/j.1469-7793.2001.00809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lisman J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc Natl Acad Sci USA. 1989;86:9574–9578. doi: 10.1073/pnas.86.23.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LoTurco JL, Coulter DA, Alkon DL. Enhancement of synaptic potentials in rabbit CA1 pyramidal neurons following classical conditioning. Proc Natl Acad Sci USA. 1988;85:1672–1676. doi: 10.1073/pnas.85.5.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lynch G, Larson J, Kelso S, Barrionuevo G, Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983;305:719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- 37.Magee JC, Johnston D. Synaptic activation of voltage-gated channels in the dendrites of hippocampal pyramidal neurons. Science. 1995;268:301–304. doi: 10.1126/science.7716525. [DOI] [PubMed] [Google Scholar]
- 38.Malenka RC, Nicoll RA. NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms. Trends Neurosci. 1993;16:521–527. doi: 10.1016/0166-2236(93)90197-t. [DOI] [PubMed] [Google Scholar]
- 39.Malenka RC, Nicoll RA. Long-term potentiation–a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 40.Malenka RC, Lancaster B, Zucker RS. Temporal limits on the rise in postsynaptic calcium required for the induction of long-term potentiation. Neuron. 1992;9:121–128. doi: 10.1016/0896-6273(92)90227-5. [DOI] [PubMed] [Google Scholar]
- 41.Martin ED, Araque A, Buno W. Synaptic regulation of the slow Ca2+-activated K+ current in hippocampal CA1 pyramidal neurons: implication in epileptogenesis. J Neurophysiol. 2001;86:2878–2886. doi: 10.1152/jn.2001.86.6.2878. [DOI] [PubMed] [Google Scholar]
- 42.Mayer ML, MacDermott AB, Westbrook GL, Smith SJ, Barker JL. Agonist- and voltage-gated calcium entry in cultured mouse spinal cord neurons under voltage clamp measured using arsenazo III. J Neurosci. 1987;7:3230–3244. doi: 10.1523/JNEUROSCI.07-10-03230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McEchron MD, Disterhoft JF. Hippocampal encoding of non-spatial trace conditioning. Hippocampus. 1999;9:385–396. doi: 10.1002/(SICI)1098-1063(1999)9:4<385::AID-HIPO5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 44.Messier C, Mourre C, Bontempi B, Sif J, Lazdunski M, Destrade C. Effect of apamin, a toxin that inhibits Ca2+-dependent K+ channels, on learning and memory processes. Brain Res. 1991;551:322–326. doi: 10.1016/0006-8993(91)90950-z. [DOI] [PubMed] [Google Scholar]
- 45.Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 46.Moser EI, Krobert KA, Moser MB, Morris RG. Impaired spatial learning after saturation of long-term potentiation. Science. 1998;281:2038–2042. doi: 10.1126/science.281.5385.2038. [DOI] [PubMed] [Google Scholar]
- 47.Mourre C, Cervera P, Lazdunski M. Autoradiographic analysis in rat brain of the postnatal ontogeny of voltage-dependent Na+ channels, Ca2+-dependent K+ channels and slow Ca2+ channels identified as receptors for tetrodotoxin, apamin and (−)-desmethoxyverapamil. Brain Res. 1987;417:21–32. doi: 10.1016/0006-8993(87)90175-2. [DOI] [PubMed] [Google Scholar]
- 48.Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- 49.Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Norris CM, Halpain S, Foster TC. Reversal of age-related alterations in synaptic plasticity by blockade of L-type Ca2+ channels. J Neurosci. 1998;18:3171–3179. doi: 10.1523/JNEUROSCI.18-09-03171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saar D, Grossman Y, Barkai E. Reduced after-hyperpolarization in rat piriform cortex pyramidal neurons is associated with increased learning capability during operant conditioning. Eur J Neurosci. 1998;10:1518–1523. doi: 10.1046/j.1460-9568.1998.00149.x. [DOI] [PubMed] [Google Scholar]
- 52.Sah P. Ca2+-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 1996;19:150–154. doi: 10.1016/s0166-2236(96)80026-9. [DOI] [PubMed] [Google Scholar]
- 53.Sah P, Bekkers JM. Apical dendritic location of slow afterhyperpolarization current in hippocampal pyramidal neurons: implications for the integration of long-term potentiation. J Neurosci. 1996;16:4537–4542. doi: 10.1523/JNEUROSCI.16-15-04537.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sah P, Clements JD. Photolytic manipulation of [Ca2+]i reveals slow kinetics of potassium channels underlying the afterhyperpolarization in hippocampal pyramidal neurons. J Neurosci. 1999;19:3657–3664. doi: 10.1523/JNEUROSCI.19-10-03657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwindt PC, Crill WE. Modification of current transmitted from apical dendrite to soma by blockade of voltage- and Ca2+-dependent conductances in rat neocortical pyramidal neurons. J Neurophysiol. 1997;78:187–198. doi: 10.1152/jn.1997.78.1.187. [DOI] [PubMed] [Google Scholar]
- 56.Sejnowski TJ. Storing covariance with nonlinearly interacting neurons. J Math Biol. 1977;4:303–321. doi: 10.1007/BF00275079. [DOI] [PubMed] [Google Scholar]
- 57.Shors TJ, Matzel LD. Long-term potentiation: what's learning got to do with it? Behav Brain Sci. 1997;20:597–655. doi: 10.1017/s0140525x97001593. [DOI] [PubMed] [Google Scholar]
- 58.Silva AJ, Giese KP, Federov NB, Frankland PW, Kogan JH. Molecular, cellular, and neuroanatomical substrates of place learning. Neurobiol Learn Mem. 1998;70:44–61. doi: 10.1006/nlme.1998.3837. [DOI] [PubMed] [Google Scholar]
- 59.Stocker M, Pedarzani P. Differential distribution of three Ca2+-activated K+ channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol Cell Neurosci. 2000;15:476–493. doi: 10.1006/mcne.2000.0842. [DOI] [PubMed] [Google Scholar]
- 60.Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc Natl Acad Sci USA. 1999;96:4662–4667. doi: 10.1073/pnas.96.8.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Storm JF. Potassium currents in hippocampal pyramidal cells. Prog Brain Res. 1990;83:161–187. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- 62.Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- 63.Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- 64.van der Staay FJ, Fanelli RJ, Blokland A, Schmidt BH. Behavioral effects of apamin, a selective inhibitor of the SKCa-channel, in mice and rats. Neurosci Biobehav Rev. 1999;23:1087–1110. doi: 10.1016/s0149-7634(99)00043-3. [DOI] [PubMed] [Google Scholar]
- 65.Vnek N, Rothblat LA. The hippocampus and long-term object memory in the rat. J Neurosci. 1996;16:2780–2787. doi: 10.1523/JNEUROSCI.16-08-02780.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]