Abstract
We studied the importance of the hippocampus and subiculum for anterograde and retrograde memory in the rat using social transmission of food preference, a nonspatial memory task. Experiment 1 asked how long an acquired food preference could be remembered. In experiment 2, we determined the anterograde amnesic effects of large lesions of the hippocampus that included the subiculum. In experiment 3, large lesions of the hippocampus that included the subiculum were made 1, 10, or 30 d after learning to determine the nature and extent of retrograde amnesia. Normal rats exhibited memory of the acquired food preference for at least 3 months after learning. Hippocampal lesions that included the subiculum produced marked anterograde amnesia and a 1–30 d temporally graded retrograde amnesia. The results show the importance of the hippocampus and related structures for nonspatial memory and also demonstrate the temporary role of these structures in long-term memory.
Keywords: anterograde, retrograde, temporally graded, hippocampus, subiculum, social transmission, food preference, rat
Temporally graded retrograde amnesia (TGRA) refers to a phenomenon of premorbid memory loss whereby information acquired recently is more impaired than information acquired more remotely. Studies of human amnesia have illuminated this phenomenon (Hodges, 1994; Squire and Alvarez, 1995), but such studies necessarily rely on retrospective methods and imperfect tests. Studies in experimental animals have the advantage that retrograde amnesia can be studied prospectively, the locus and extent of brain lesions can be determined accurately, and the timing and strength of original learning can be precisely controlled.
A number of studies have been reported in which animals have been given equivalent amounts of training at two or more different times before damage to the hippocampal formation or the fornix (Ramos, 1998; Squire et al., 2001). Temporally graded retrograde amnesia is the most common finding. That is, animals with hippocampal damage, entorhinal damage, or fornix section typically have impaired memory for material learned just before surgery, but material learned more remotely is spared (for exceptions, see Sutherland et al., 2001).
This pattern of findings suggests that the hippocampus (and related structures) is necessary for memory storage and retrieval for only a limited time after learning. One account of the phenomenon suggests that memory is stored in the same neocortical structures that were involved in processing the relevant information during learning. Initially, the hippocampus serves to bind these cortical regions and to allow memory to be reactivated for retrieval. Over time and through a process of reorganization, the connections among the cortical regions are progressively strengthened until the cortical memory can be reactivated and retrieved independently of the hippocampus (Squire and Alvarez, 1995).
An alternative suggestion is that memories that are initially hippocampus dependent remain dependent on the hippocampus. In this view, older memories have a more redundant and spatially distributed representation within the hippocampus than recent memories. TGRA occurs because a partial lesion of the hippocampus is more likely to spare a remote memory than a memory acquired recently (Nadel and Moscovitch, 1997). This idea predicts that TGRA will not be observed when hippocampal lesions are complete (e.g., recent and remote memories will be similarly impaired).
These ideas have been explored in rats using a learning paradigm based on the social transmission of a food preference. This task involves a “demonstrator” rat that is fed an odorous food and is then allowed to interact with a “subject” rat. During this social interaction, the subject rat makes an association between the food odor and constituents of the demonstrator's breath (Galef and Wigmore, 1983). Subsequently, when the subject rat is presented with a choice between two odorous foods, the subject rat expresses a memory for this association by choosing the same food odor that was present on the demonstrator's breath.
In studies of retrograde amnesia for a socially acquired food preference, TGRA was observed after lesions of the dorsal hippocampus (Winocur, 1990) and also after larger lesions that damaged virtually all of the dorsal and ventral hippocampus but spared the subiculum (Winocur et al., 2001). In both of these cases, the retrograde amnesia covered a period of ∼1–5 d. Interestingly, studies of anterograde amnesia for the same task suggest that, to impair memory function, hippocampal lesions must be combined with lesions of the subiculum (Alvarez et al., 2001). One implication of this finding for studies of retrograde amnesia is that combined hippocampus and subiculum lesions may be more disruptive than lesions limited to the hippocampus itself. For example, it is possible that combined hippocampus and subiculum lesions would result in a more extensive TGRA covering a longer period than 1–5 d. Another possibility is that TGRA would not occur and that recent and remote memory would be impaired to a similar degree. Indeed, the view that hippocampus-dependent memories remain dependent on the hippocampus could be extended to suppose that whatever medial temporal lobe structures are needed to form a memory will always be needed to support this memory. Accordingly, if memory for a socially acquired food preference depends initially on both the hippocampus and subiculum, then one might expect that a complete lesion of both hippocampus and subiculum made at any time after learning should abolish the memory. That is, TGRA should not be observed.
Finally, it was reported recently that large hippocampal plus subicular lesions in rats failed to impair anterograde memory for the social transmission of food preference (STFP) task (Burton et al., 2000). This finding is at odds with other reports that hippocampal damage (Winocur, 1990; Winocur et al., 2001) or hippocampal plus subicular damage (Bunsey and Eichenbaum, 1995; Alvarez et al., 2001) is sufficient to produce anterograde amnesia for this task. In view of these different findings, we studied both anterograde and retrograde amnesia for a socially acquired food preference after hippocampal lesions that included the subiculum.
Experiment 1 was designed to determine whether the STFP task is suitable for detecting extensive TGRA. Accordingly, we asked how retention of a learned food preference at 1 week, 1 month, and 3 months after learning was affected by varying the strength of original learning. In experiment 2, we determined the anterograde amnesic effects of large lesions of the hippocampus that included the subiculum. Lesions were made before administering the STFP task, and retention was assessed 48 hr after learning. In experiment 3, large lesions of the hippocampus that included the subiculum were made 1, 10, or 30 d after learning to determine the nature and extent of retrograde amnesia.
MATERIALS AND METHODS
Experiment 1
This experiment was designed to determine in normal animals how long the social transmission of food preference can be retained and whether the strength of retention can be influenced by varying the amount of training.
Subjects. We tested 114 experimentally naive, male Long–Evans rats weighing 300–350 gm at the beginning of the experiment. Six rats served as demonstrator rats, and 108 rats served as subject rats. Rats were housed individually and maintained on a 12 hr light/dark cycle.
Procedure. The task consisted of the following three distinct phases. In phase I, demonstrator rats were first accustomed for 4 d to a routine of 23 hr of food deprivation, followed by 1 hr of feeding. During the 1 hr, 40–50 gm of meal chow was available in a glass jar attached to the floor of the demonstrator's cage. After the feeding session, the food was weighed, and the amount of food eaten was recorded.
In phase II, demonstrator rats were exposed to flavored meal chow during the 1 hr feeding session of each day (1% cinnamon, e.g., 1 gm of cinnamon per 100 gm of meal chow, or 2% cocoa, e.g., 1 gm of coca per 50 gm of meal chow). Each demonstrator was fed only one of the two flavors. After 4 d, when demonstrator rats were always eating at least 5 gm of the flavored food during the daily feeding session, the demonstrator rat was allowed to interact with a subject rat for a variable period of time (see below). Specifically, the demonstrator rat was placed in the home cage of a subject rat but separated from the subject rat by a cylinder-shaped wire screen. The subject rat was food deprived for 1 hr before the interaction, and no food or water was available to either rat during the interaction. The subject and demonstrator rats were always unfamiliar with each other.
In phase III, after a prescribed delay, the subject rat was food deprived for 8 hr and then presented in its home cage with a choice of a novel food and the food that the demonstrator rat had consumed before interacting with the subject rat (familiar food). Two jars were attached to the floor of the subject rat's cage and remained in place for 2 hr. One jar was filled with meal chow flavored with 1% cinnamon, and the other jar was filled with meal chow flavored with 2% cocoa. The familiar flavor (cinnamon or cocoa) was counterbalanced across all conditions, as was the right or left location of the familiar food. At the end of the 2 hr feeding period, the jars were weighed and the amount of food eaten from each jar was recorded.
Experimental design. Nine different groups of rats (n = 12 per group) were tested (three strength of training levels × three delay intervals). Training involved one of the following: (1) a 10 min interaction between the subject rat and demonstrator rat; (2) a 30 min interaction between the subject rat and demonstrator rat; or (3) a 30 min interaction between the subject rat and demonstrator rat on each of 3 consecutive days (a different demonstrator rat was used on each day). After the interaction, retention was tested after 1 week, 1 month, or 3 months.
Experiment 2
This experiment asked whether large lesions of the hippocampus and subiculum produce anterograde amnesia for the social transmission of food preference task. Ibotenic acid (IBO) was used to create the hippocampal lesions because it provides good control of lesion extent and spares fibers (Jarrard, 1989).
Subjects. We used 35 male Long–Evans rats weighing between 300 and 350 gm at the beginning of the experiment. Fifteen rats underwent surgery to damage the hippocampus and subiculum (after surgery, these rats were given an object recognition task as part of another study before being given the STFP task). Sixteen rats served as a control group. Four additional rats served as demonstrator rats. Rats were housed individually and maintained on a 12 hr light/dark cycle.
Surgery. In 15 rats, hippocampal and subicular lesions were created by ibotenic acid using aseptic procedures. Isoflurane (5%) was delivered in O2 at 1 l/min to induce anesthesia. The animal was positioned in a David Kopf Instruments (Tujunga, CA) stereotaxic instrument, and the incisor bar was adjusted until bregma was level with lambda. Anesthesia was maintained throughout surgery with isoflurane gas (0.8–2.0% isoflurane delivered in O2 at 1 l/min). The bone overlying the target sites was removed using a high-speed drill. Ibotenic acid (Biosearch Technologies, San Rafael, CA) was dissolved in 0.1m PBS to provide a solution with a concentration of 10 mg/ml and a pH of 7.4. IBO was injected with a 10 μl Hamilton syringe mounted on a stereotaxic frame and held with a David Kopf Instruments microinjector (model 5000). The syringe needle was first lowered to the surface of the dura, and a small puncture was made in the dura just below the needle tip. The syringe needle was then lowered to the target and left in place for 1 min before beginning the injection. After the injection, the syringe needle was left in place for 2 min to reduce the spread of IBO up the needle tract. For animals with ibotenic acid lesions of the hippocampus (H-IBO group), IBO was injected into 18 sites on each side of the brain (for stereotaxic coordinate details, see the H-IBO group by Clark et al., 2000).
Neurohistological methods. Rats were administered an overdose of sodium pentobarbital and perfused transcardially with buffered 0.9% NaCl solution, followed by 10% formaldehyde solution (in 0.1 m phosphate buffer). Brains were then removed from the skull and cryoprotected in 20% glycerol–10% formaldehyde solution. Coronal sections (50 μm) were cut with a freezing microtome beginning just anterior to the hippocampus and continuing caudally through the length of the hippocampal region. Every fifth section was mounted and stained with thionin to assess the extent of the lesions.
Procedure. All testing was performed postoperatively. The procedure was the same as in experiment 1, except that all interactions between demonstrator and subject rats were 30 min in duration. Forty-eight hours after learning, rats were tested for food preference.
Experiment 3
Large radiofrequency lesions of the hippocampus that included the subiculum (H-RF) were made 1, 10, or 30 d after learning.
Subjects. We used 87 experimentally naive, male Long–Evans rats weighing 300–350 gm at the beginning of the experiment. Sixteen rats served as demonstrator rats, and 71 rats served as subject rats. Rats were housed individually and maintained on a 12 hr light/dark cycle.
Surgery. In experiment 2, ibotenic acid lesions were used because they allow virtually all of the hippocampus to be removed with little or no extrahippocampal damage. For experiment 3, radiofrequency lesions were used instead of ibotenic acid lesions to study retrograde amnesia because ibotenic acid raises a concern that structures outside of the hippocampus could be adversely affected by the activity of hippocampal neurons as they fire themselves to death. It has been suggested (Anagnostaras et al., 2001) that extrahippocampal memories acquired before surgery might be disrupted by the extensive and prolonged stimulation of hippocampus neurons caused by injection of ibotenic acid during surgery. This concern does not apply to the study of anterograde amnesia because no relevant memories have been established at the time of surgery.
Hippocampal and subicular lesions were created following the same surgical procedure as in experiment 2, except that the lesions were made with a radiofrequency electrode and generator (model RF-4A; Radionics, Burlington, MA). The electrode was first lowered to the surface of the dura, and a small puncture was made in the dura just below the electrode tip. The electrode was then lowered to the target and left in place for 1 min before heating the tissue to 80–90°C (depending of the target site) for a period of 1 min. The current to the electrode was then turned off, and the electrode was removed after the tip temperature fell to 41°C. Lesions were made at 12 sites on each side of the brain and were intended to damage the dorsal and ventral hippocampus (for more details, see the H-RF group byClark et al., 2000). Animals were allowed to recover for 10 d before behavioral testing.
Neurohistological methods. The neurohistological procedures were the same as in experiment 2.
Procedure. The behavioral procedure was the same as in experiment 1, except that all interactions between demonstrator and subject rats were 30 min in duration. The hippocampal lesions were made 1, 10, or 30 d after training (n = 10 per group), and retention was tested 10 d later. Control rats were trained and tested at corresponding times (1 d group, n = 19; 10 d group, n = 12; 30 d group,n = 10).
RESULTS
Experiment 1
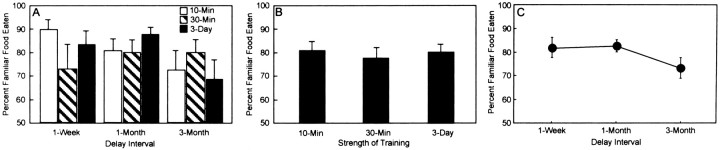
Figure 1Aillustrates the percent preference for the familiar food for each of the strength of training conditions at the 1 week, 1 month, and 3 month delays. Percentage of preference for the familiar food was calculated as follows: [F/(F + N) * 100], where F is the amount of familiar food consumed, andN is the amount of novel food consumed. All groups exhibited a significant preference for the familiar food (score >50%; one-sample t test). An ANOVA (training conditions × delay interval) yielded no effect of training condition (F(2,99) = 0.19; p > 0.1) and no effect of delay (F(2,99) = 1.75; p > 0.1). These results indicate that varying the length of the interaction between the subject rat and the demonstrator rat did not measurably affect retention. Figure1B illustrates the percent preference for the familiar food for each strength of training condition (delays combined). Across all three delays, the 10 min interaction yielded an 80.7% preference for the familiar food, the 30 min interaction yielded a 77.4% preference, and the 30 min interaction for 3 consecutive days yielded a 79.7% preference.
Fig. 1.
A, The influence of strength of training on percent preference for the familiar food across delays.White bars indicate performance of groups given a 10 min interaction with demonstrator rats, striped barsindicate performance of groups given a 30 min interaction with demonstrator rats, and black bars indicate performance of groups given 30 min interactions with different demonstrator rats on 3 consecutive days. B, The influence of strength of training when the delays were combined. C, Percentage of preference for the familiar food across delays (strength of training combined at each delay). Error bars show SEM.
Retention of the socially acquired food preference was evident even 3 months after training. Averaging across the three training conditions, animals exhibited an 81.8% preference for the familiar food after 1 week, an 82.5% preference after 1 month, and a 73.4% preference after 3 months (Fig. 1C). Forgetting was quite limited across the first 3 months after learning (1 week vs 3 months,t(70) = 1.39, p = 0.17; 1 month vs 3 months, t(70) = 1.79, p < 0.08).
Experiment 2
Histological findings
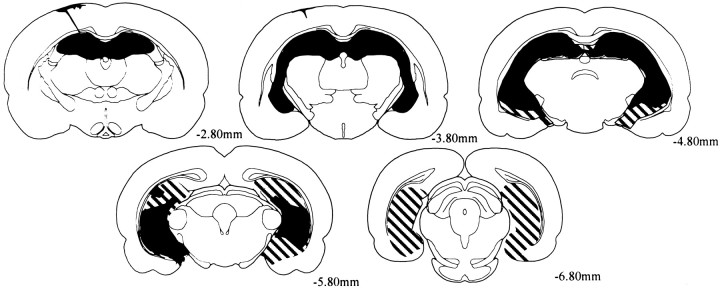
Figure 2 illustrates the extent of the largest and smallest lesion.
Fig. 2.
Reconstructions of coronal sections showing the largest (striped) and smallest (black) lesions in rats with ibotenic acid lesions. Each series of sections progresses (left to right) from anterior to posterior levels. Numbers represent the distance in millimeters posterior to bregma.
Hippocampal damage
All rats sustained extensive bilateral damage to all the cell fields of the hippocampus and dentate gyrus. The average percent damage to the hippocampus was 93.0% (range of 75.2–100.0%). In instances when the lesion was not complete, minor sparing was evident in the ventromedial portion of the ventral hippocampus, the most medial region of the dorsal hippocampus, and the dorsal hippocampus at the most posterior levels. In all but three rats, the posterior margin of the hippocampal lesion encroached on the entorhinal cortex, but this damage was generally very minor. All rats sustained some damage to the cortical regions that overlie the dorsal hippocampus. In most cases, this cortical damage was very minor. There was also minor damage to the fimbria that was associated with the placement of the Hamilton syringe during surgery. There was no damage to either the amygdala or the perirhinal cortex.
Subiculum damage
All animals sustained some damage to the subiculum. The total amount of direct damage to the subiculum was variable. The average percent damage to the subiculum was 55.6% (range of 4.8–98.9%).
Behavioral findings
The two groups consumed a similar amount of food during the test phase (control, 6.5 gm; H-IBO, 8.1 gm;t(29) = 1.44; p > 0.1).
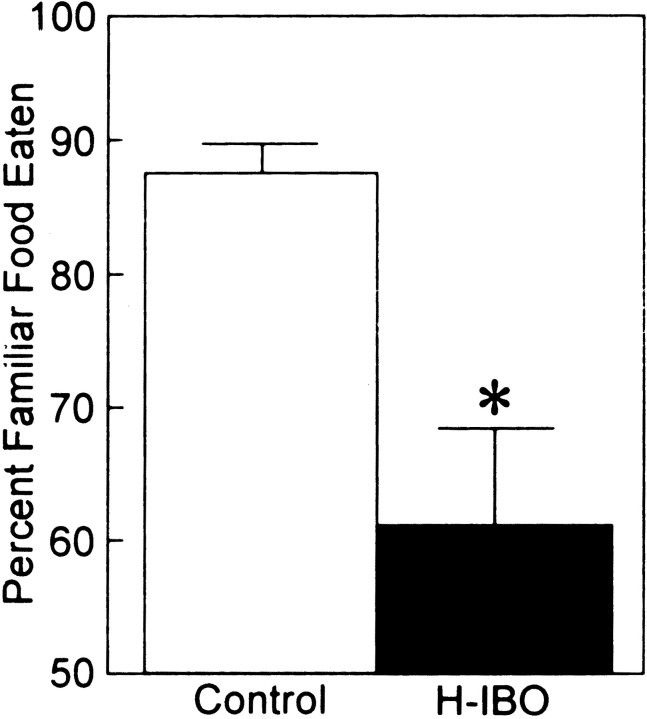
Figure 3 shows the percent preference for the familiar food for the H-IBO group and the control group 48 hr after learning. The control group exhibited an 87.4% preference for the familiar food. This score was well above the chance score of 50% (t(14) = 16.31; p < 0.0001) and higher than the preference score of the H-IBO group (t(29) = 3.63; p < 0.01). The H-IBO group exhibited only a 61.1% preference for the familiar food, which was not greater than chance (t(14) = 1.56; p > 0.1).
Fig. 3.
Percent preference for the familiar food for control (n = 16) animals and H-IBO (n = 15) animals. Training occurred after the lesions were made, and retention was tested 48 hr later. The control group performed above chance and scored higher than the H-IBO group. The H-IBO group did not perform above chance. *p < 0.05, significant difference between the two groups. Error bars show SEM.
We next determined whether there was a relationship between the extent of hippocampal damage, the extent of subicular damage, or the extent of hippocampal plus subicular damage and the preference score. None of the correlation values approached significance (hippocampus,r = −0.167; subiculum, r = 0.059; hippocampus plus subiculum, r = 0.014; all pvalues > 0.1), perhaps because the lesions were large and did not extend across a sufficient range for a correlation to emerge. We also found no difference in preference between the eight animals with the least extensive hippocampal damage (range of 75.2–95.7%; percent preference score, 62.7%) and the seven animals with the most extensive hippocampal damage (range of 96.4–100%; percentage of preference score, 59.3%; t(13) = 0.23;p > 0.1). Similarly, the seven animals with the least extensive subicular damage (range of 4.7–49.0%; percent preference score, 58.6%) were not different from the eight animals with the most extensive subicular damage (range of 65.9–98.8%; percent preference score, 63.2%; t(13) = 0.32;p > 0.1).
Experiment 3
Histological findings
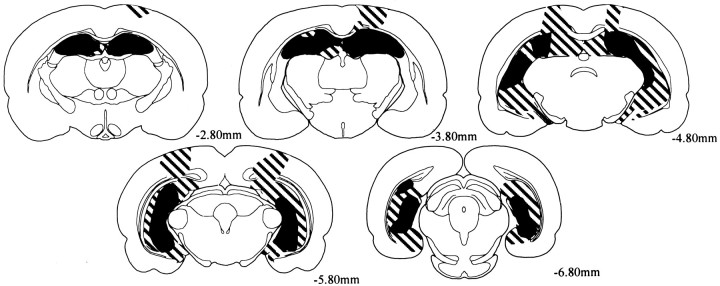
Figure 4 illustrates the extent of the largest and smallest lesion.
Fig. 4.
Reconstructions of coronal sections showing the largest (striped) and smallest (black) lesion in rats with radiofrequency lesions. Each series of sections progresses (left to right) from anterior to posterior levels. Numbers represent the distance in millimeters posterior to bregma.
Hippocampal damage
All animals sustained extensive bilateral damage to all the cell fields of the hippocampus, including the dentate gyrus. The average percent damage to the hippocampus was 87.3% (range of 58.0–100%). The spared hippocampal tissue involved mainly the most anterior portion of the dorsal hippocampus and the most ventromedial portion of the ventral hippocampus. All animals had extensive damage to the alveus as it passed over the dorsal edge of the dorsal hippocampus. Additionally most animals had direct damage to the fimbria on the lateral edge of the dorsal hippocampus.
Subiculum damage
All animals sustained damage to the subiculum. The total amount of direct damage to the subiculum was variable (mean of 48.8%; range of 8.0–100%). The bulk of the spared subicular tissue was located in the most posterior aspects of the ventral subiculum. In cases in which the dorsal subiculum was spared from the direct damage of the RF lesion, heavy gliosis was apparent in the dorsal subiculum. Because the subiculum is the major contributor of fibers that make up the fornix (Swanson and Cowan, 1975; Witter and Groenewegen, 1990), it is likely that this indirect damage was attributable to retrograde degeneration caused by the extensive damage to the alveus (subicular axons contribute to the fornix by way of the fimbria and alveus). This type of degeneration was not observed in the ventral subiculum.
Other damage
In six of the animals that sustained the largest amount of subicular damage, there was also minor damage to the entorhinal cortex that amounted to <20% of total entorhinal volume. The entorhinal cortex was entirely spared in all other animals. In most cases, there was also some damage to the cortical regions directly dorsal to the dorsal hippocampus. No animal had any damage to the perirhinal cortex or the amygdala.
Behavioral findings
The two groups consumed a similar amount of food during the test phase (control, 4.5 gm; H-IBO, 4.2 gm;t(69) = 0.57; p > 0.1).
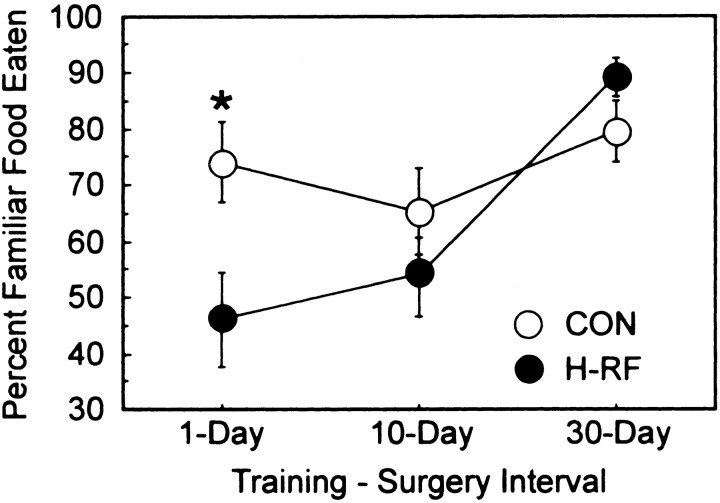
Figure 5 shows the percent preference for the familiar food for the H-RF groups and the control groups across the three training–surgery intervals of 1, 10, and 30 d. The data were first submitted to an ANOVA (two groups × three training–surgery intervals), which revealed a significant effect of training–surgery interval (F(2,65) = 6.77; p < 0.01) and a significant interaction of group by training–surgery interval (F(2,65)= 3.26; p < 0.05). The effect of group was not significant (F(1,65) = 2.60;p > 0.1). The interaction reflects the fact that hippocampal lesions affected performance differently as a function of the training–surgery interval. When lesions were made 1 d after training, the operated animals performed at chance (46.2%) and poorer than control animals (74.2%) (t(27) = 2.46; p < 0.05). When hippocampal lesions were made 10 d after training, the operated animals performed numerically worse than the control animals (54.4 vs 65.5%), but this difference was not significant (t(20) = 1.0;p > 0.1). Furthermore, operated animals did not perform above chance (t(9) = 0.55;p > 0.1), whereas the control group was marginally above chance (t(11) = 2.01;p = 0.070). Finally, at the 30 d training–surgery interval, the H-RF and control groups performed similarly (89.3 vs 79.6%, respectively), and both groups performed well above chance (t values > 5.4; p values < 0.001 ). The 30 d lesion group also performed better than either the 1 d lesion group (t(18) = 4.57; p< 0.001) or the 10 d lesion group (t(18) = 4.02; p < 0.001). The finding that longer training–surgery intervals improved the performance of the lesion groups provides evidence of temporally graded retrograde amnesia. This was confirmed with a linear trend analysis for the three lesion groups (1, 10, and 30 d;F(2,27) = 20.36; p < 0.001).
Fig. 5.
Percent preference for the familiar food for control animals (CON) and H-RF animals across three training–surgical intervals. For the 1 d condition, the control group performed significantly better than the H-RF group. The H-RF groups did not perform above chance in the 1 and 10 d conditions. In the 30 d condition, the two groups performed similarly, and both groups performed well above chance. *p < 0.05, significant difference between the control and H-RF groups. Error bars show SEM.
In six animals, the entorhinal cortex sustained minor damage. To determine whether this damage influenced the results, we compared the performance of those animals with entorhinal damage and those without entorhinal damage. Five of the six animals with entorhinal damage were in the 30 d lesion group. There was no difference between the preference scores of the five animals with entorhinal damage (89.8% preference) and the five animals without entorhinal damage (89.0% preference; t(8) = 0.11;p > 0.1).
DISCUSSION
Experiment 1
The results of experiment 1 show that the socially acquired food preference provides a suitable task for studying retrograde amnesia across a relatively long time period. It is perhaps surprising that so little forgetting was evident across the 3 month period covered by our study. It is well established that biological constraints influence the formation of associations (Garcia and Koelling, 1966). Rats may be strongly prepared to form an association between a food odor and the odor of the demonstrator rat. If so, even a brief learning episode may be sufficient to produce a strong and persistent memory for the trained odor. Accordingly, to observe pronounced forgetting after a 10 min learning episode (the “weakest” training condition in our study), it may be necessary to test at much longer retention intervals than 3 months. In addition, if pronounced forgetting is to be observed at 3 months after learning, the learning episode may need to be shorter than 10 min in duration. Additional study will be needed to clarify these parametric issues.
Experiment 2
The results of experiment 2 show that large lesions of the hippocampus and subiculum impair anterograde memory for the STFP task. The control group scored higher than the H-IBO group, and the H-IBO group failed to perform above chance. Although the lesion group was unmistakably impaired, it is not clear from this study what the relative importance might be of the hippocampus and the subiculum. All of the animals had at least some damage to both structures. Furthermore, no relationship was apparent between the extent of hippocampal damage or the extent of subicular damage and performance.
The present study confirms previous findings with the STFP task (Winocur, 1990; Bunsey and Eichenbaum, 1995; Alvarez et al., 2001;Winocur et al., 2001). Possible reasons why this finding has not always been obtained (Burton et al., 2000) are considered in “General discussion” below.
Experiment 3
The present results provide a clear demonstration of TGRA after large lesions of the hippocampus that include the subiculum. When the lesions were made 1 or 10 d after the learning episode, animals did not perform above chance. Additionally, the 1 d lesion group was impaired relative to its control group. The 10 d control group performed marginally above chance (p = 0.07). Nevertheless, the two groups were not significantly different. In contrast, the 30 d lesion group performed well above chance and performed similarly to its control group. Furthermore, the 30 d lesion group performed significantly better than either the 1 or 10 d lesion groups. These data suggest that, with sufficient time, the acquired food preference becomes independent of the hippocampus.
General discussion
Impaired new learning of the food preference task (e.g., anterograde amnesia) has been reported for rats with dorsal hippocampal lesions (Winocur, 1990), dorsal plus ventral hippocampal lesions (Winocur et al., 2001), and hippocampus plus subicular lesions (Bunsey and Eichenbaum, 1995; Alvarez et al., 2001). The results from experiment 2 confirm that hippocampal damage plus subicular damage is sufficient to produce anterograde memory impairment of the STFP task in rats. Additionally, genetically altered mice with hippocampal dysfunction also exhibit anterograde amnesia for the food preference task (Mayeux-Portas et al., 2000; Rampon et al., 2000).
In contrast to the findings of these studies and the finding of experiment 2 of the present study, Burton et al. (2000) reported that large hippocampal lesions that included the subiculum failed to impair new learning of the STFP task. As discussed previously (Alvarez et al., 2001), this study differed in potentially important ways from the studies that found an impairment. One possibility is that a preference for familiar food can sometimes reflect a nonassociative, hippocampus-independent phenomenon, such as habituation of food neophobia, rather than an acquired association between food odor and the demonstrator's breath. In the present study, the average preference score of control animals in experiment 1 (collapsed across training conditions and delay intervals) was 79.3%. The preference score of the control group from experiment 2 was 87.4%. The preference score of the control animals in the four other studies that found new learning to be affected by hippocampal lesions (Winocur, 1990; Bunsey and Eichenbaum, 1995; Alvarez et al., 2001; Winocur et al., 2001) ranged from ∼72 to ∼91%. In contrast, in the study by Burton et al. (2000), control animals exhibited only a weak preference of 61%, even when the odorant concentration was approximately four times greater than was used in the other studies.
The present study found a 1–30 d TGRA after large hippocampal lesions that included the subiculum. Winocur et al. (2001) reported a 1–5 d gradient of retrograde amnesia after large hippocampal lesions that spared the subiculum. That is, animals with lesions performed as well as controls after only a 5 d training–surgery interval. In contrast, in the present study, even a 10 d training–surgery interval failed to spare the performance of the lesion group. The most likely explanation for this difference between the two studies is the locus and extent of the lesions. In the Winocur et al. (2001) study, 18 of 20 animals had lesions involving at least 80% of the hippocampus (e.g., the dentate gyrus and CA fields). Although these were large lesions, the subiculum was spared (one animal was reported to have some unilateral ventral subiculum damage). Furthermore, the lesions were made by the neurotoxin NMDA, which spares fibers of passage and consequently should not have damaged efferent and afferent subicular fibers in the alveus and fornix. In the present study, all subjects had some direct subicular damage. Additionally, our lesions were made by radiofrequency, which damages both cell bodies and fibers and consequently interrupted fibers to and from the subiculum.
The present results show that, even when both the hippocampus and subiculum are damaged, TGRA is still observed. Thus, the structures important for forming memory for a food odor are involved in memory storage only temporarily. By 30 d after learning, memory for this task becomes independent of the hippocampus and subiculum. These findings appear to count against the proposal that the structures important in initial learning remain important for storage and retrieval (Nadel and Moscovitch, 1997). If that were the case, removal of the hippocampus and subiculum should have impaired the retention of both recently and remotely acquired memories. The present study indicates that memories that are initially hippocampus dependent become hippocampus independent by 30 d after learning on the STFP task. This result adds to what now is a substantial list of studies, using a variety of behavioral paradigms, that report temporally graded retrograde amnesia after lesions of the hippocampus, fornix, or entorhinal cortex (for review, see Squire et al., 2001). The pattern of retrograde amnesia that is observed is presumably determined by location and extent of damage, the strength of initial training, and the type of information being learned.
Footnotes
This work was supported by the Medical Research Service of the Department of Veterans Affairs, National Institute of Mental Health Grant MH 24600, the National Alliance for Research on Schizophrenia and Depression, National Institute on Aging Grant AG05131, and the Metropolitan Life Foundation. We thank Steve Burkhalter and Laura Entwistle for assistance.
Correspondence should be addressed to Dr. Robert E. Clark, Department of Psychiatry, Box 0603, University of California, San Diego, La Jolla, CA 92093. E-mail: reclark@ucsd.edu.
S. Zola's present address: Yerkes Primate Research Center, Emory University, Atlanta, GA 30329.
REFERENCES
- 1.Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez P, Lipton PA, Melrose R, Eichenbaum H. Differential effects of damage within the hippocampal region on memory for a natural, nonspatial odor-odor association. Learn Mem. 2001;8:79–86. doi: 10.1101/lm.38201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunsey M, Eichenbaum H. Selective damage to the hippocampal region blocks long-term retention of a natural and nonspatial stimulus-stimulus association. Hippocampus. 1995;5:546–556. doi: 10.1002/hipo.450050606. [DOI] [PubMed] [Google Scholar]
- 4.Burton S, Murphy D, Qureshi U, Sutton P, O'Keefe J. Combined lesions of hippocampus and subiculum do not produce deficits in a nonspatial social olfactory memory task. J Neurosci. 2000;20:5468–5475. doi: 10.1523/JNEUROSCI.20-14-05468.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galef BG, Wigmore SR. Transfer of information concerning distant foods: A laboratory investigation of the “information-centre” hypothesis. Anim Behav. 1983;31:748–758. [Google Scholar]
- 7.Garcia J, Koelling RA. Relation of cue to consequence in avoidance learning. Psychon Sci. 1966;4:123–124. [Google Scholar]
- 8.Hodges JR. Retrograde amnesia. In: Baddeley A, Wilson BA, Watts F, editors. Handbook of memory disorders. Wiley; New York: 1994. pp. 81–107. [Google Scholar]
- 9.Jarrard LE. On the use of ibotenic acid to lesion selectively different components of the hippocampal formation. J Neurosci Methods. 1989;29:251–259. doi: 10.1016/0165-0270(89)90149-0. [DOI] [PubMed] [Google Scholar]
- 10.Mayeux-Portas V, File SE, Stewart C, Morris RJ. Mice lacking the cell adhesion molecule Thy-1 fail to use socially transmitted cues to direct their choice of food. Curr Biol. 2000;10:68–75. doi: 10.1016/s0960-9822(99)00278-x. [DOI] [PubMed] [Google Scholar]
- 11.Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- 12.Ramos JMJ. Retrograde amnesia for spatial information: dissociation between intra and extramaze cues following hippocampus lesions in rats. Eur J Neurosci. 1998;10:3295–3301. doi: 10.1046/j.1460-9568.1998.00388.x. [DOI] [PubMed] [Google Scholar]
- 13.Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat Neurosci. 2000;3:238–244. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- 14.Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr Opin Neurobiol. 1995;5:169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- 15.Squire LR, Clark RE, Knowlton BJ. Retrograde amnesia. Hippocampus. 2001;11:50–55. doi: 10.1002/1098-1063(2001)11:1<50::AID-HIPO1019>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 16.Sutherland RJ, Weisend MP, Mumby D, Astur RS, Hanlon FM, Koerner A, Thomas MJ, Wu Y, Moses SN, Cole C, Hamilton DA, Hoesing JM. Retrograde amnesia after hippocampal damage: recent vs. remote memories in two tasks. Hippocampus. 2001;11:27–42. doi: 10.1002/1098-1063(2001)11:1<27::AID-HIPO1017>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Swanson LW, Cowan WM. Hippocampo-hypothalamic connections: origin in subicular cortex, not ammon's horn. Science. 1975;189:303–304. doi: 10.1126/science.49928. [DOI] [PubMed] [Google Scholar]
- 18.Winocur G. Anterograde and retrograde amnesia in rats with dorsal hippocampal or dorsomedial thalamic lesions. Behav Brain Res. 1990;38:145–154. doi: 10.1016/0166-4328(90)90012-4. [DOI] [PubMed] [Google Scholar]
- 19.Winocur G, McDonald RM, Moscovitch M. Anterograde and retrograde amnesia in rats with large hippocampal lesions. Hippocampus. 2001;11:18–26. doi: 10.1002/1098-1063(2001)11:1<18::AID-HIPO1016>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Witter MP, Groenewegen HJ. The subiculum: cytoarchitectonically a simple structure, but hodologically complex. Prog Brain Res. 1990;83:47–58. doi: 10.1016/s0079-6123(08)61240-6. [DOI] [PubMed] [Google Scholar]