Abstract
For the last two decades, the involvement of 5-HT1Areceptors in the regulation of vigilance states has been studied extensively thanks to pharmacological tools, but clear-cut conclusion has not been reached yet. By studying mutant mice that do not express this receptor type (5-HT1A−/−) and their wild-type 129/Sv counterparts, we herein demonstrate that 5-HT1A receptors play key roles in the control of spontaneous sleep–wakefulness cycles, as well as in homeostatic regulation and stress-induced adaptive changes of paradoxical sleep. Both strains of mice exhibited a diurnal sleep–wakefulness rhythm, but 5-HT1A−/− animals expressed higher amounts of paradoxical sleep than wild-type mice during both the light and the dark phases. In wild-type mice, pharmacological blockade of 5-HT1A receptors by WAY 100635 (0.5 mg/kg, i.p.) promoted paradoxical sleep, whereas the 5-HT1A agonist 8-OH-DPAT (0.25–1 mg/kg, s.c.) had an opposite effect. In contrast, none of the 5-HT1A receptor ligands affected sleep significantly in 5-HT1A−/− mice. However, 5-HT1B receptor stimulation by CP 94253 (1–3 mg/kg, i.p.) induced a reduction in paradoxical sleep in both strains, this effect being more pronounced in 5-HT1A−/− mutants. Finally, in contrast to wild-type mice, 5-HT1A−/− mutants did not exhibit any rebound of paradoxical sleep after either a 9 hr instrumental paradoxical sleep deprivation or a 90 min immobilization stress. Altogether, these data indicate that, in the mouse, 5-HT1A receptors participate in the spontaneous and homeostatic regulation, as well as in stress-induced adaptive changes of paradoxical sleep.
Keywords: sleep–wakefulness, 5-HT1A receptors, sleep deprivation, immobilization stress, knock-out mice, paradoxical sleep homeostasis
The implication of serotonin (5-hydroxytryptamine, 5-HT) receptors of the 5-HT1A type in the regulation of vigilance states has been the matter of numerous investigations using appropriate pharmacological tools (Portas et al., 2000; Ursin, 2002). In particular, the inhibitory effect of systemic treatment with various 5-HT1A receptor agonists, notably the prototypical one 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT), on paradoxical sleep (PS) is considered as a key observation in support of the idea that 5-HT1Areceptors play an important role in the regulation of this vigilance state in mammals (Dzoljic et al., 1992; Monti and Jantos, 1992;Quattrochi et al., 1993; Tissier et al., 1993; Driver et al., 1995). These receptors are located on both somas and dendrites of serotonergic neurons in raphe nuclei (somatodendritic autoreceptors) (Vergé et al., 1986; Sotelo et al., 1990) and target neurons receiving serotonergic projections (postsynaptic receptors) (Kia et al., 1996;Riad et al., 2000). The PS inhibition induced by 5-HT1A receptor agonists would result from activation of postsynaptic receptors (Tissier et al., 1993), notably those at pontine level (Sanford et al., 1994; Horner et al., 1997;Thakkar et al., 1998). In contrast, activation of somatodendritic 5-HT1A autoreceptors in anterior raphe nuclei would induce a PS enhancement (Portas et al., 1996; Bjorvatn et al., 1997).
However, the specific involvement of 5-HT1Areceptors in sleep–wakefulness regulations remains questionable because none of the ligands used so far is really selective of 5-HT1A receptors. Indeed, even 8-OH-DPAT, which is classically considered as a selective 5-HT1Aagonist (Hoyer et al., 1994), acts at 5-HT7receptors at relatively low doses (Wood et al., 2000). An alternative strategy to assess the potential role of 5-HT1Areceptors in sleep–wakefulness regulation under baseline conditions, as well as after various behavioral challenges (Sallanon et al., 1983;Houdouin et al., 1991a; Cespuglio et al., 1995; Gonzalez et al., 1996;Gonzalez and Valatx, 1998), is now possible thanks to the availability of knock-out mice that do not express this receptor type (Heisler et al., 1998; Parks et al., 1998; Ramboz et al., 1998). Phenotypical characterization of these mutants showed that they exhibit marked behavioral alterations, in particular increased responses in anxiety-relevant tests, in sharp contrast with the apparent decrease in anxiety-like behaviors in mutant mice that do not express 5-HT1B receptors (Zhuang et al., 1999). Interestingly, the latter 5-HT1B−/− mutants show increased amounts of PS under baseline conditions but no rebound of this sleep stage after its selective deprivation (Boutrel et al., 1999). Whether 5-HT1A−/− mutants also exhibit alterations in PS regulation, possibly opposite to those observed in 5-HT1B−/− mutants, was an interesting question to be addressed with regard to the well established relationships between stress–anxiety-driven behaviors and sleep–wakefulness (Cespuglio et al., 1995; Marinesco et al., 1999; Vazquez-Palacios and Velazquez-Moctezuma, 2000).
All of these considerations led us to investigate the characteristics of sleep–wakefulness regulations in 5-HT1A−/− mice compared with wild-type counterparts, first, under baseline conditions and in response to the administration of 5-HT1A and 5-HT1B receptor ligands, and second, after selective PS deprivation or immobilization stress.
MATERIALS AND METHODS
All of the procedures involving animals and their care were conducted in conformity with the institutional guidelines that are in compliance with national and international laws and policies (Council directive 87-848, October 19, 1987, Ministère de l'Agriculture et de la Forêt, Service vétérinaire de la santéet de la protection animale, permissions 75-116 to M.H. and 0315 to J.A.).
All mice used for these studies were of the 129/Sv strain. Those used for spontaneous sleep–wakefulness analysis, PS deprivation, and immobilization stress were produced by heterozygous breeding, and their genotype was determined according to the method of Ramboz et al. (1998). Other groups of mice, produced from homozygous breeding of knock-out and wild-type strains, were used for pharmacological experiments.
Surgery
Male wild-type (5-HT1A+/+) and mutant (5-HT1A−/−) mice were used at 2–3 months of age (body weight, 22–26 gm). Animals were implanted under sodium pentobarbital anesthesia (70–75 mg/kg, i.p.) with the classical set of electrodes (made of enameled nichrome wire, 150 μm in diameter) for polygraphic sleep monitoring as described previously (Boutrel et al., 1999). In brief, EEG electrodes were inserted through the skull onto the dura over the right cortex (2 mm lateral and 4 mm posterior to the bregma) and over the cerebellum (at midline, 2 mm posterior to lambda), electrooculography electrodes were positioned subcutaneously on each side of the orbit, and EMG electrodes were inserted into the neck muscles. All electrodes were anchored to the skull with Superbond (Limoge-Lendais et al., 1994) and acrylic cement and soldered to a miniconnector also embedded in cement. After completion of surgery, animals were housed in individual cages (20 × 20 × 30 cm) and maintained under standard laboratory conditions: 12 hr light/dark cycle (light on at 7:00 A.M.), 24 ± 1°C ambient temperature, and food and water available ad libitum. The animals were allowed 7–10 d to recover and habituate to the recording conditions.
Pharmacological treatments
Drugs were dissolved in 0.1 ml of saline, except for the 5-HT1B agonist 3-(1,2,5,6-tetrahydro-4-pyridyl)-5-propoxypyrrolo[3,2-b]pyridine (CP 94253),which was dissolved in 0.1 ml of warm distilled water. All injections were performed between 9:30 and 10:00 A.M.; CP 94253 and the 5-HT1A antagonistN-[2-[4-(2-methoxyphenyl)-1-piperazinyl] ethyl]-N-(2-pyridinyl) cyclohexane carboxamide (WAY 100635) were injected intraperitoneally, whereas 8-OH-DPAT was injected subcutaneously. For baseline data, mice were injected intraperitoneally or subcutaneously with the vehicle only, as appropriate. A washout period of at least 2 d for CP 94253 and WAY 100635 and 7 d for 8-OH-DPAT was allowed between two consecutive treatments.
PS deprivation
Mice were placed for 9 hr, starting at 10:00 A.M., on platforms (control conditions, 7.5 cm in diameter, 3 cm high; deprivation conditions, 3.5 cm in diameter, 4 cm high) surrounded by water (2 cm deep) (Boutrel et al., 1999) at an ambient temperature of 24°C, with access to food and water ad libitum. At the end of this period, they were returned to their home cage and allowed to recover for 12 hr (from 7:00 P.M. to 7:00 A.M. the next morning). Each mouse underwent the paired control and deprivation procedures (separated by at least 1 week).
Immobilization stress
At least 10 d after completion of the deprivation procedure, mice were immobilized for 90 min, from 6:30 to 8:00 P.M., by wrapping them inside a plastic grid. At the end of this period, they were returned to their home cage for sleep–wakefulness monitoring. Each mouse underwent, first, the control procedure (the animal remained free in its home cage and was connected a few minutes before being recorded) and, second (2–3 d later), the immobilization procedure.
Polygraphic recordings
For the study of spontaneous sleep–wakefulness cycles, each animal was recorded for 48 hr, beginning at 7:00 P.M., i.e., at the onset of the dark period. For pharmacological studies, sleep–wakefulness parameters were recorded for 8 hr after injections, i.e., from 10:00 A.M. to 6:00 P.M.. For PS deprivation experiments, recordings were performed from the beginning of the deprivation period (at 10:00 A.M.) and continued until 12 hr after the end of deprivation, and, for the stress procedure, recordings were performed for 12 hr after the end of immobilization challenge.
Data analysis and statistics
Polygraphic recordings were scored manually every 15 sec epoch using Somnologica software (Flaga, Reykjavik, Iceland), and the amounts of each state of vigilance [wakefulness (W), slow-wave sleep (SWS), and PS] were calculated per hour.
Spontaneous sleep–waking cycles. For each animal, the amounts of vigilance states for every hour throughout 48 hr were averaged for the light and the dark phases. The mean values (expressed as minutes ± SEM) for each strain of mice were used for calculating the ANOVA for the genotype. In case of significance (p < 0.05), the F test was followed by the Student's t test for mean comparisons.
Pharmacological experiments. The effects of each dose of a given compound on each state of vigilance were analyzed for every 2 hr period after injection and are expressed as minutes ± SEM. For a given treatment, each animal was referred to its own baseline, represented by the data obtained after injection of vehicle. Statistical analyses were performed using ANOVA for factors treatment and strain, and, in case of significance (p < 0.05), the F test was followed by the post hocFisher's test for assessing the effect of each dose of compounds.
PS deprivation and immobilization stress. For each animal, the PS amounts during the small platform condition and the following 12 hr recovery period and those during the 12 hr post-stress period were compared with their respective control values (i.e., PS amounts during the large platform condition and the corresponding control recovery period and those during the same period with no stress, respectively). Paired t tests were performed to assess statistical significance of the data.
Chemicals
The following drugs were used: WAY 100635 (0.5 mg/kg, i.p.; Wyeth Research, Princeton, NJ), 8-OH-DPAT (0.25–1.0 mg/kg, s.c. ; Research Biochemicals, Natick, MA), and CP 94253 (1–3 mg/kg i.p.; Pfizer, Groton, CT).
RESULTS
Spontaneous sleep–wakefulness cycles
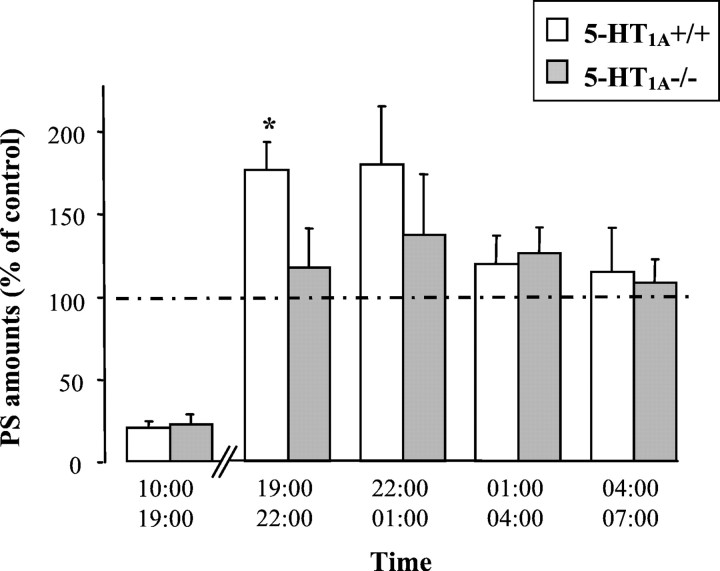
All mice exhibited a clear-cut circadian sleep–wakefulness rhythm, with larger amounts of sleep during the light period than during the dark one (Fig. 1). However, 5-HT1A−/− mutants differed significantly (p < 0.05) from wild-type mice by a greater amount of PS, during both the light (approximately +39%) and the dark (approximately +45%) phase (Table 1). This enhancement was accounted for by an increase in the mean duration of PS episodes during the light phase (1.25 ± 0.04 vs 1.08 ± 0.02 min; mean ± SEM; n = 8 in each group;p < 0.05) and by an increase in the number of PS episodes during the dark phase (35.6 ± 2.5 vs 26.8 ± 2.5;p < 0.05).
Fig. 1.
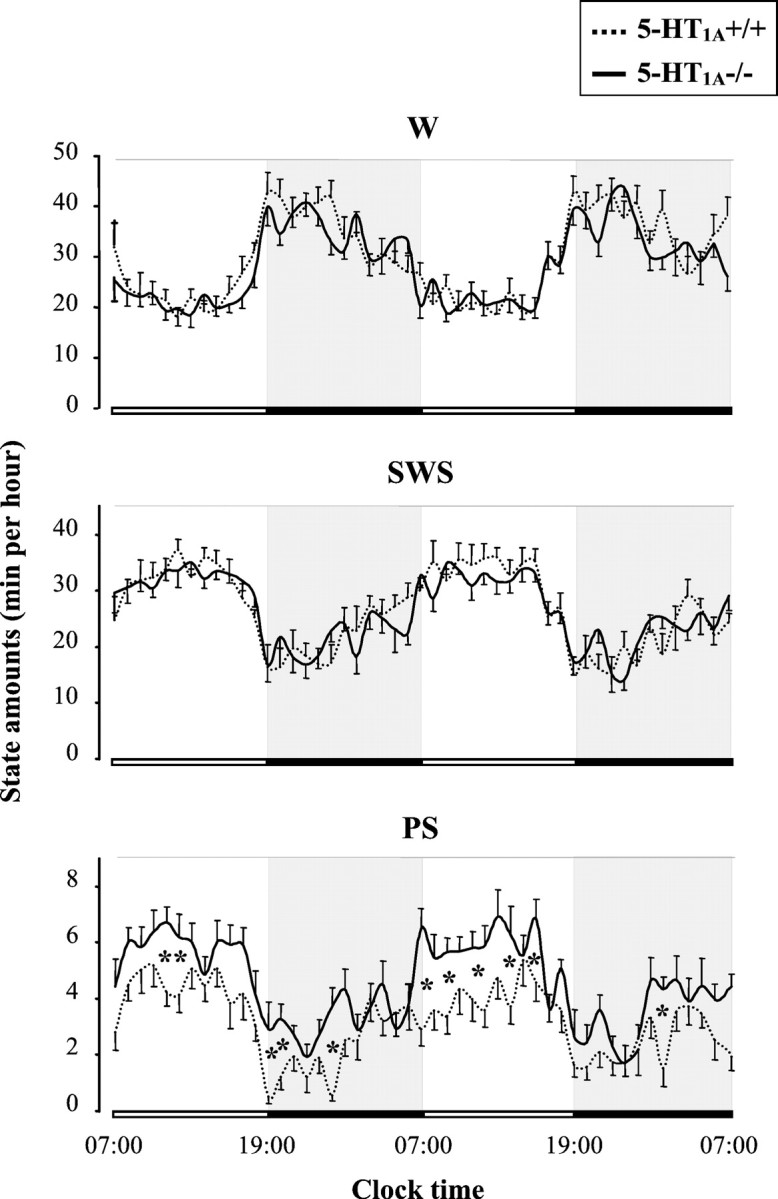
Circadian variations of W, SWS, and PS in 5-HT1A+/+ (dotted line) and 5-HT1A−/− (solid line) mice. Data (mean ± SEM of 9 and 8 animals, respectively) are expressed as minutes per hour during two consecutive light/dark cycles (lights on from 7:00 A.M. to 7:00 P.M.). *p < 0.05, significant difference between groups; Student's ttest.
Table 1.
Amounts of W, SWS, and PS in 5-HT1A+/+ and 5-HT1A−/− mice under normal conditions
| Genotype | Period | W | SWS | PS |
|---|---|---|---|---|
| 5-HT1A+/+ (n = 9) | 24 hr | 720.5 ± 25.0 | 641.9 ± 21.5 | 77.6 ± 5.7 |
| 12 hr light | 282.6 ± 13.3 | 387.8 ± 10.1 | 49.6 ± 4.4 | |
| 12 hr dark | 437.9 ± 13.3 | 254.2 ± 12.5 | 27.9 ± 1.8 | |
| 5-HT1A−/− (n = 8) | 24 hr | 696.5 ± 25.1 | 634.2 ± 25.9 | 109.3 ± 3.6* |
| 12 hr light | 271.0 ± 11.4 | 380.0 ± 11.6 | 68.9 ± 3.2* | |
| 12 hr dark | 425.5 ± 19.2 | 254.2 ± 18.1 | 40.4 ± 2.2* |
Results are expressed as minutes (mean ± SEM ofn animals) during the 12 hr light (7:00 A.M. to 7:00 P.M.) and dark (7:00 P.M. to 7:00 A.M.) periods and during the complete nycthemeral cycle (24 hr).
p < 0.05, significantly different from 5-HT1A+/+ group; Student'st test.
In contrast, the amounts of W and SWS were identical in both groups throughout the entire circadian period (Fig. 1, Table 1).
Pharmacological data
Activation or blockade of 5-HT1A receptors
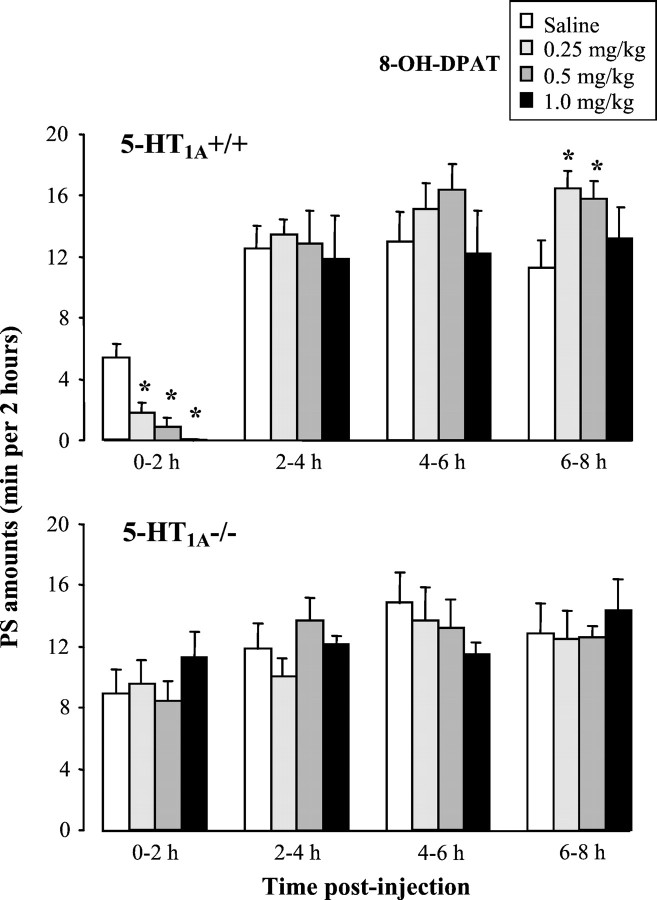
In wild-type mice, 8-OH-DPAT (0.25–1 mg/kg, s.c.) induced, during the first 2 hr period after injection, a dose-related inhibition of PS (ANOVA; F(3,15) = 20.4;p < 0.0001) (Fig. 2) and SWS (ANOVA; F(3,15) = 15.9;p < 0.0001; data not shown), as well as a concomitant increase in W (ANOVA; F(3,15) = 21.0;p < 0.0001; data not shown). These initial modifications of sleep–wakefulness states were significant for all doses of 8-OH-DPAT tested (p < 0.001;post hoc Fisher's test). They were followed by a rebound of PS observed between 6 and 8 hr after the injection, which was significant (p < 0.05; Fisher's test) for the doses of 0.25 and 0.5 mg/kg 8-OH-DPAT (Fig. 2). In contrast, 8-OH-DPAT had no effect on sleep or wakefulness amounts in 5-HT1A−/− mice, except for a nonsignificant PS enhancement (125.3 ± 18.6% of baseline; n = 6;p = 0.17) during the first 2 hr after injection at the dose of 1 mg/kg (Fig. 2). On the whole, the difference between strains during this period was highly significant for the three states of vigilance (ANOVA; F(1,36) = 43.1, 21.5, and 32.0; p < 0.001 for PS, SWS, and W, respectively).
Fig. 2.
Effects of the 5-HT1A agonist 8-OH-DPAT on paradoxical sleep in 5-HT1A+/+ (top) and 5-HT1A−/− (bottom) mice during four successive 2 hr periods after injection. Data (mean ± SEM of 5 and 6 animals, respectively) are expressed as minutes per 2 hr after subcutaneous injection of saline (open bars) or 8-OH-DPAT at various doses (filled bars). *p < 0.05, significantly different from saline; post hoc Fisher's test.
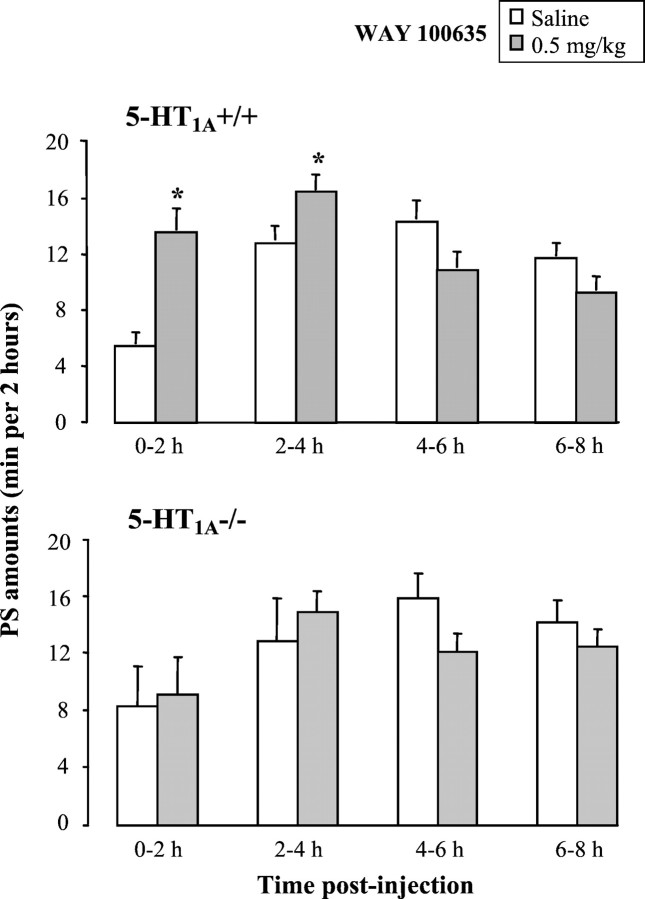
Blockade of 5-HT1A receptors by WAY 100635 (0.5 mg/kg, i.p.) induced in 5-HT1A+/+ mice, but not in 5-HT1A−/− mutants, a significant increase in PS amounts (p = 0.001; paired Student'st test) during the first 4 hr after the injection (Fig.3) and no modifications in W and SWS in any strain (data not shown).
Fig. 3.
Effects of blockade of 5-HT1Areceptors on paradoxical sleep in 5-HT1A+/+ (top) and 5-HT1A−/− (bottom) mice during four successive 2 hr periods after treatment. Data (mean ± SEM of 6 and 4 animals, respectively) are expressed as minutes per 2 hr after injection of saline (white bars) or the 5-HT1A antagonist WAY 100635 at the dose of 0.5 mg/kg intraperitoneally (gray bars). *p < 0.05, significantly different from saline; paired Student's t test.
Activation of 5-HT1B receptors
5-HT1B receptor activation by CP 94253 (1–3 mg/kg, i.p.) induced, during the first 4 hr after injection, a significant reduction in PS amounts in wild-type mice (ANOVA;F(3,23) =3.6; p = 0.028), as well as in mutants (ANOVA;F(3.23) = 9.6; p < 0.001). This reduction reached significance for the doses of 2 and 3 mg/kg in wild-type (p = 0.023 and 0.007, respectively; post hoc Fisher's test) and mutant (p < 0.001 for both doses) mice and was more pronounced in 5-HT1A−/− animals (ANOVA;F(1,46) = 9.63; p = 0.003) (Fig. 4). In contrast, neither W nor SWS were significantly affected by CP 94253 in both groups of mice (data not shown).
Fig. 4.
Effects of the 5-HT1B agonist CP 94253 at various doses on paradoxical sleep in 5-HT1A+/+ (dotted line) and 5-HT1A−/− (solid line) mice during the first 4 hr period after injection. Data (mean ± SEM of 7 animals in each group) are expressed as percentages of PS amounts in saline-treated mice (0 onabscissa). *p < 0.05, significant difference between groups; Fisher's test.
Paradoxical sleep deprivation
During PS deprivation (small platform), mice of both groups exhibited nearly the same amounts of SWS (data not shown) but only ∼20% of PS compared with those observed under control conditions (large platform) (Fig. 5). During the recovery period, PS amounts were significantly enhanced in 5-HT1A+/+ mice compared with those under control conditions (Fig. 5, Table 2), notably during the first 6 hr (+76.4 ± 22.3%; n = 6;p < 0.05; paired Student's t test). This increase was accounted for by an increase in the number of PS episodes (Table 2) with no modification of their mean duration (1.21 ± 0.11 vs 1.11 ± 0.10 min; NS; paired Student's ttest). In contrast, in 5-HT1A−/− mice, only a slight but not significant increase in PS amounts was observed after PS deprivation compared with those under control conditions (Fig. 5, Table2) (+20.6 ± 16.9% during the first 6 hr of the recovery period;n = 6; NS; paired Student's t test), and neither the number (Table 2) nor the mean duration (1.22 ± 0.10 vs 1.16 ± 0.05 sec; NS; paired Student's t test) of PS episodes were altered during the recovery period in PS-deprived compared with nondeprived mutants. In addition, no modifications in W and SWS amounts were observed after PS deprivation in wild-type and 5-HT1A−/− mice (data not shown).
Fig. 5.
Paradoxical sleep amounts observed during 9 hr of PS deprivation and 12 hr thereafter, in 5-HT1A+/+ (white bars) and 5-HT1A−/− (gray bars) mice. Data (mean ± SEM of 6 animals in each group) are expressed as percentage of paired values obtained under control conditions (large platform). *p < 0.05, significant difference between groups; Student's t test.
Table 2.
Characteristics of paradoxical sleep in 5-HT1A+/+ and 5-HT1A−/− mice during 12 hr after a 9 hr PS deprivation or a 90 min immobilization stress
| Control conditions | Experimental conditions | ||||
|---|---|---|---|---|---|
| Amounts (min) | Number | Amounts (min) | Number | ||
| PS deprivation | |||||
| 5-HT1A+/+ (n = 6) | 0–6 hr | 12.5 ± 1.5 | 11.3 ± 1.0 | 20.8 ± 1.6* | 17.2 ± 1.6* |
| 6–12 hr | 18.3 ± 1.1 | 16.2 ± 1.5 | 20.1 ± 1.7 | 18.0 ± 2.6 | |
| 5-HT1A−/− (n = 6) | 0–6 hr | 20.2 ± 2.92-160 | 16.5 ± 1.82-160 | 23.1 ± 2.7 | 19.7 ± 2.2 |
| 6–12 hr | 25.8 ± 1.92-160 | 21.2 ± 2.2 | 28.3 ± 2.22-160 | 24.7 ± 1.8 | |
| Immobilization stress | |||||
| 5-HT1A+/+ (n = 9) | 0–6 hr | 12.2 ± 1.1 | 10.3 ± 1.1 | 15.9 ± 2.2 | 15.0 ± 2.0* |
| 6–12 hr | 19.1 ± 1.1 | 16.1 ± 1.2 | 23.4 ± 1.5* | 22.1 ± 1.5* | |
| 5-HT1A−/− (n = 7) | 0–6 hr | 19.2 ± 2.12-160 | 16.6 ± 1.82-160 | 17.0 ± 1.8 | 15.1 ± 1.1 |
| 6–12 hr | 24.7 ± 3.0 | 21.4 ± 2.22-160 | 25.1 ± 1.5 | 23.1 ± 1.6 | |
The data (mean ± SEM of n animals) represent the amounts of PS expressed as minutes and the number of PS episodes for the 0–6 and 6–12 hr periods of recovery after control (large platform) and PS deprivation (small platform) procedure (top) and control and immobilization stress (bottom).
p < 0.05, significantly different from control conditions; paired Student's t test.
F2-160: p < 0.05, significantly different from 5-HT1A+/+ group; unpaired Student's ttest.
Immobilization stress
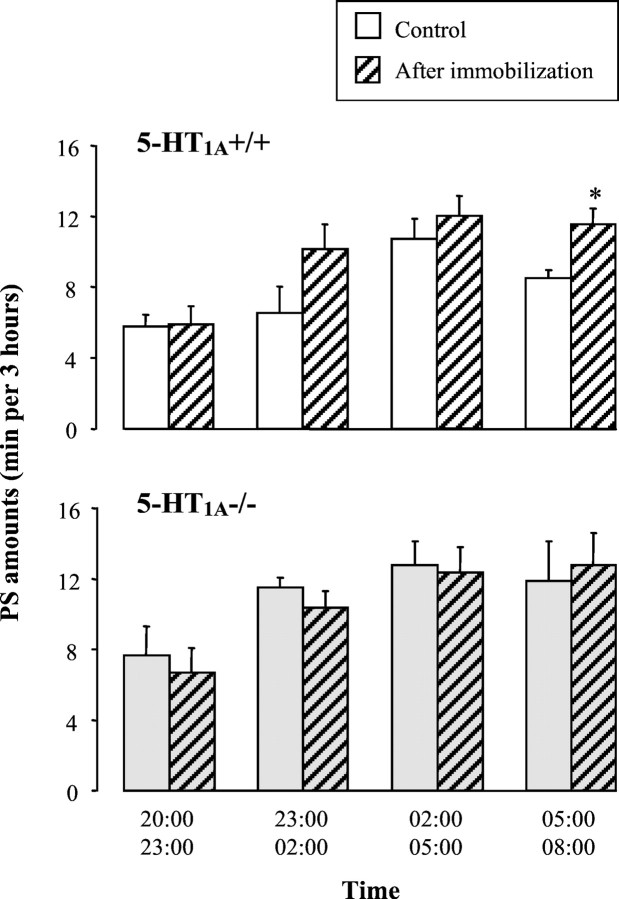
After 90 min of immobilization, wild-type mice, but not 5-HT1A−/− mutants, exhibited a significant increase in PS amounts during the 12 hr recovery period compared with the control conditions [respectively, +27.5 ± 10.6% (n = 9; p < 0.05) and −0.2 ± 7.0% (n = 7; NS); paired Student's ttest]. This PS rebound in wild-type mice was mainly observed during the second half of the night (Table 2), especially for the last 3 hr (Fig. 6), and was accounted for by an increase in the number of PS episodes (Table 2), with no change of their mean duration (1.20 ± 0.05 vs 1.06 ± 0.07 min; NS; paired Student's t test). In contrast, no significant modifications in the amounts of W and SWS after the immobilization procedure were observed in wild-type and 5-HT1A−/− mice (data not shown).
Fig. 6.
Paradoxical sleep amounts observed during 12 hr after 90 min of immobilization in 5-HT1A+/+ (top) and 5-HT1A−/− (bottom) mice. Data (mean ± SEM of 9 and 7 animals, respectively) are expressed as minutes per 3 hr under control conditions (white and gray solid bars) and after immobilization stress (hatched bars). *p < 0.05, significantly different from respective control value; paired Student's t test.
DISCUSSION
Regulation of sleep–wakefulness cycles under baseline conditions
In the present work, we found that knock-out mice, which do not express the 5-HT1A receptor, and their wild-type counterparts exhibit similar circadian sleep–wakefulness cycles with predominance of wakefulness during the dark period and sleep during the light one. These data are comparable with those generally obtained in mice (Tobler et al., 1997) and notably in the 129/Sv strain (Boutrel et al., 1999), which shares the same genetic background as the present ones. However, 5-HT1A−/− mutants differed from 5-HT1A+/+ wild-type mice by higher amounts of PS during the entire circadian period, whereas wakefulness and SWS amounts were similar in both groups. Because we used in this part of the study only mice derived from heterozygous breeding, it is most probable that the difference in PS amounts is accounted for by the 5-HT1A gene disruption rather than by some heterogeneity in the genetic background between both groups of mice (Gerlai, 1996). This is further supported by the fact that pharmacological blockade of 5-HT1A receptors with WAY 100635 induced in wild-type mice an increase in PS amounts, thereby mimicking the change that occurred spontaneously in mutants lacking 5-HT1A receptors. Accordingly, both the larger amounts of spontaneous PS in 5-HT1A−/− mutants and the PS enhancement after 5-HT1A receptor blockade in wild-type mice indicate that these receptors mediate a tonic inhibitory influence on PS in this species.
The lack of significant effect of 8-OH-DPAT on sleep–wakefulness cycle in 5-HT1A−/− mutants would suggest that 5-HT7 receptors at which this ligand acts as a partial agonist (Wood et al., 2000) are not involved in the regulation of vigilance states, at least in the mouse. This conclusion is in line with previous results showing that the effects of 8-OH-DPAT on sleep and wakefulness in the mouse were completely prevented by the selective 5-HT1A receptor antagonist WAY 100635 (Boutrel et al., 1999). In any case, additional investigations are needed to assess the possible implication of 5-HT7 receptors in sleep–wakefulness regulation because Hagan et al. (2000) reported recently a decrease in PS after administration of a 5-HT7 receptor antagonist in rats. Indeed, we did find a tendency to an increase in PS amounts in 5-HT1A−/− mice treated with 8-OH-DPAT (Fig. 2), which would fit with the idea of 5-HT7 receptor stimulation exerting a facilitatory influence on PS expression in rodents.
According to the reciprocal interaction model for PS regulation (McCarley and Massaquoi, 1992), 5-HT exerts an inhibitory influence on mesopontine cholinergic “PS-on” neurons (Honda and Semba, 1994), notably through postsynaptic 5-HT1A receptors (Sanford et al., 1994; Horner et al., 1997). On the contrary, 5-HT might have a facilitatory influence on PS (Portas et al., 1996;Bjorvatn et al., 1997) or SWS (Sakai and Crochet, 2001) through the activation of somatodendritic 5-HT1Aautoreceptors in anterior raphe nuclei. In the present work, the increase of PS amounts observed after both pharmacological and genetic inactivation of 5-HT1A receptors, together with the unchanged levels of SWS, support the view that mainly postsynaptic 5-HT1A receptors are involved in the physiological regulation of PS (Tissier et al., 1993).
Compensation at 5-HT1B receptors in 5-HT1A−/− mutants
Interestingly, we found that 5-HT1B receptor activation by CP 94253 caused a more pronounced reduction of PS amounts in 5-HT1A−/− mutants than in wild-type mice, thereby suggesting that 5-HT1B receptors are supersensitive in these mutants. A similar adaptation of 5-HT1A receptors was apparent in 5-HT1B knock-out mice in which 8-OH-DPAT was found to be more potent than in paired wild-type mice to inhibit PS expression (Boutrel et al., 1999). Because both 5-HT1A and 5-HT1B receptors appear to be involved in a 5-HT-mediated inhibitory control of PS in mice (Boutrel et al., 1999; present study), these data would support the idea that a compensatory increase in the functioning of one receptor occurs after inactivation of the other. Such compensatory changes in 5-HT1A−/− and 5-HT1B−/− mice have been reported previously but with variations from one brain area to another (Bouwknecht et al., 2001; Knobelman et al., 2001), illustrating the complexity of adaptive processes affecting these receptors. With regard to sleep–wakefulness mechanisms, whether 5-HT1B receptors in pontine nuclei possibly controlling PS expression (Boutrel et al., 1999) are supersensitive or upregulated in 5-HT1A−/− mutants is an important question to be addressed in future investigations.
PS rebound after selective PS deprivation or immobilization stress
In agreement with previous reports, wild-type mice were presently found to exhibit a PS rebound after either selective PS deprivation (Sallanon et al., 1983; Adrien and Dugovic, 1984; Gonzalez et al., 1996; Boutrel et al., 1999) or immobilization stress (Cespuglio et al., 1995; Gonzalez et al., 1995; Gonzalez and Valatx, 1998; Meerlo et al., 2001). Interestingly, the rebound after deprivation started immediately at the beginning of the recovery period, whereas that after immobilization stress was delayed by 3–6 hr. Such differences in the time courses of rebound in the two experimental procedures, which have also been observed in rats (Adrien and Dugovic, 1984; Houdouin et al., 1991a), probably reflect two different mechanisms. Indeed, PS deprivation (small platform), in contrast to immobilization stress, does not enhance plasma corticosterone levels (compared with paired control conditions, large platform) (Porkka-Heiskanen et al., 1995). Accordingly, immediate PS rebound would not be attributable to the stress inherent to the platform technique but would rather reflect sleep homeostatic properties (Barbato and Wehr, 1998). On the other hand, the delay in PS rebound after immobilization stress may be accounted for by increased levels of corticosterone just after the stress, because this hormone exerts inhibitory influence on PS (Bradbury et al., 1998; Marinesco et al., 1999). Indeed, under our conditions, preliminary data showed that plasma corticosterone levels increased markedly just after immobilization and returned to baseline only 4–6 hr later (our unpublished observations), i.e., at the time of PS rebound onset.
In contrast to wild-type animals, 5-HT1A−/− mutants did not exhibit any PS rebound after PS deprivation or immobilization stress. This has been found previously with 5-HT1B−/− mice after selective PS deprivation (Boutrel et al., 1999) and immobilization stress (our unpublished data). Accordingly, it can be inferred that both 5-HT1A and 5-HT1B receptors play key roles in the homeostatic regulation of PS and in the PS adaptive response to acute stress. Interestingly, previous studies also showed an absence of sleep rebound when 5-HT neurotransmission had been impaired (Sallanon et al., 1983; Houdouin et al., 1991a,b). Altogether, these data support the idea that the serotonergic system, in addition to the corticotropin-releasing hormone– noradrenergic one (Gonzalez et al., 1995, 1996; Gonzalez and Valatx, 1998), underlie the PS rebound in response to both conditions. Such a lack of PS rebound after acute stress in 5-HT1A−/− mice might be linked to a prolactin deficit (Meerlo et al., 2001) and/or increased corticosterone levels. Indeed, in 5-HT1A (and 5-HT1B) knock-out mice, but not in their wild-type counterparts, plasma corticosterone levels had not returned to baseline, even 6 hr after the end of immobilization (our unpublished observations), thereby accounting for a sustained prevention of PS rebound in these mutants.
Because PS amounts for the recovery period after deprivation or immobilization stress in wild-type mice were similar to those previously observed at baseline in 5-HT1A−/− mutants, it can also be proposed that the latter have reached a maximum level of PS production as a result of the absence of 5-HT-mediated inhibitory control. In this scheme, the 5-HT system, notably through 5-HT1A and 5-HT1B (Boutrel et al., 1999) receptors, would play a predominant role in all adaptive mechanisms involving PS. In any case, the absence of PS rebound after PS deprivation, as well as after immobilization stress, raises the question of the existence of common mechanisms underlying the homeostatic regulations of PS and the PS-mediated adaptations to stress, notably those involving 5-HT1A and 5-HT1Breceptors.
It is interesting to note that both 5-HT1A−/− and 5-HT1B−/− (Boutrel et al., 1999) mutants exhibit, on the one hand, a spontaneous increase in PS amounts and a lack of PS adaptation to PS deprivation or a stressful condition, and, on the other hand, abnormal behaviors (anxiety-like for the 5-HT1A−/− and aggressiveness for the 5-HT1B−/− mice). The present results indicate that these abnormal behaviors are associated with sleep alterations, a situation that is also often described in humans suffering from mood disorders (Gillin, 1998; Van Praag, 1998) and that an altered 5-HT neurotransmission could be, at least, a common factor in these disorders. In this respect, it has to be emphasized that depression is associated with both an increased pressure of paradoxical sleep (Kupfer, 1976; Gillin, 1998) and a general decrease in postsynaptic 5-HT1A receptor density in various brain regions (Drevets et al., 2000; Sargent et al., 2000) in humans. The increased expression of PS observed herein in 5-HT1A−/− mice would suggest that such a sleep anomaly in depressed patients is underlain, at least in part, by the reported downregulation of 5-HT1A receptors in these patients.
Footnotes
This research was supported by grants from Institut National de la Santé et de la Recherche Médicale and the Bristol-Myers Squibb Foundation (unrestricted biomedical research grant program). We are grateful to pharmaceutical companies (Wyeth Research and Pfizer) for generous gifts of drugs. During performance of these studies, B.B. was supported by a Ministére de l'Education Nationale de la Recherche et de la Technologie fellowship and a grant from La Fondation pour la Recherche Médicale.
Correspondence should be addressed to Benjamin Boutrel, Institut National de la Santé et de la Recherche Médicale U288, 91 Boulevard de l'Hôpital, 75634 Paris Cedex 13, France. E-mail: boutrel@hotmail.com.
REFERENCES
- 1.Adrien J, Dugovic C. Presence of a paradoxical sleep (PS) inducing factor in the cerebrospinal fluid of PS-deprived rats. Eur J Pharmacol. 1984;100:223–226. doi: 10.1016/0014-2999(84)90227-9. [DOI] [PubMed] [Google Scholar]
- 2.Barbato G, Wehr TA. Homeostatic regulation of REM sleep in humans during extended sleep. Sleep. 1998;21:267–276. doi: 10.1093/sleep/21.3.267. [DOI] [PubMed] [Google Scholar]
- 3.Bjorvatn B, Fagerland S, Eid T, Ursin R. Sleep/waking effects of a selective 5-HT1A receptor agonist given systemically as well as perfused in the dorsal raphe nucleus in rats. Brain Res. 1997;770:81–88. doi: 10.1016/s0006-8993(97)00758-0. [DOI] [PubMed] [Google Scholar]
- 4.Boutrel B, Franc B, Hen R, Hamon M, Adrien J. Key role of 5-HT1B receptors in the regulation of paradoxical sleep as evidenced in 5-HT1B knock-out mice. J Neurosci. 1999;19:3204–3212. doi: 10.1523/JNEUROSCI.19-08-03204.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouwknecht JA, Van der Gugten J, Hijzen TH, Maes RAA, Hen R, Olivier B. Corticosterone responses in 5-HT1B receptor knockout mice to stress or 5-HT1A receptor activation are normal. Psychopharmacology. 2001;153:484–490. doi: 10.1007/s002130000598. [DOI] [PubMed] [Google Scholar]
- 6.Bradbury MJ, Dement WC, Edgar DM. Effects of adrenalectomy and subsequent corticosterone replacement on rat sleep state and EEG power spectra. Am J Physiol. 1998;275:R555–R565. doi: 10.1152/ajpregu.1998.275.2.R555. [DOI] [PubMed] [Google Scholar]
- 7.Cespuglio R, Marinesco S, Baubet V, Bonnet C, El Kafi B. Evidence for a sleep-promoting influence of stress. Adv Neuroimmunol. 1995;5:145–154. doi: 10.1016/0960-5428(95)00005-m. [DOI] [PubMed] [Google Scholar]
- 8.Drevets WC, Frank E, Price JC, Kupfer DJ, Greer PJ, Mathis C. Serotonin type-1A receptor imaging in depression. Nucl Med Biol. 2000;27:499–507. doi: 10.1016/s0969-8051(00)00119-0. [DOI] [PubMed] [Google Scholar]
- 9.Driver HS, Flanigan MJ, Bentley AJ, Luus HG, Shapiro CM, Mitchell D. The influence of ipsapirone, a 5-HT1A agonist, on sleep patterns of healthy subjects. Psychopharmacology. 1995;7:186–192. doi: 10.1007/BF02245186. [DOI] [PubMed] [Google Scholar]
- 10.Dzoljic MR, Ukponmwan O, Saxena PR. 5-HT1-like receptor agonists enhance wakefulness. Neuropharmacology. 1992;31:623–633. doi: 10.1016/0028-3908(92)90140-k. [DOI] [PubMed] [Google Scholar]
- 11.Gerlai R. Gene-targeting studies of mammalian behavior: is it the mutation or the background genotype? Trends Neurosci. 1996;19:177–181. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- 12.Gillin JC. Are sleep disturbances risk factors for anxiety, depressive and addictive disorders? Acta Psychiatr Scand [Suppl] 1998;393:39–43. doi: 10.1111/j.1600-0447.1998.tb05965.x. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez MM, Valatx JL. Involvement of stress in the sleep rebound mechanism induced by sleep deprivation in the rat: use of alpha-helical CRH (9–41). Behav Pharmacol. 1998;9:655–662. doi: 10.1097/00008877-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez MM, Debilly G, Valatx JL, Jouvet M. Sleep increase after immobilization stress: role of the noradrenergic locus coeruleus system in the rat. Neurosci Lett. 1995;202:5–8. doi: 10.1016/0304-3940(95)12209-5. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez MM, Valatx JL, Debilly G. Role of the locus coeruleus in the sleep rebound following two different sleep deprivation methods in the rat. Brain Res. 1996;740:215–226. doi: 10.1016/s0006-8993(96)00871-2. [DOI] [PubMed] [Google Scholar]
- 16.Hagan JJ, Price GW, Jeffrey P, Deeks NJ, Stean T, Piper D, Smith MI, Upton N, Medhurst AD, Middlemiss DN, Riley GJ, Lovell PJ, Bromidge SM, Thomas DR. Characterization of SB-269970-A, a selective 5-HT7 receptor antagonist. Br J Pharmacol. 2000;130:539–548. doi: 10.1038/sj.bjp.0703357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci USA. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honda T, Semba K. Serotonergic synaptic input to cholinergic neurons in the rat mesopontine tegmentum. Brain Res. 1994;647:299–306. doi: 10.1016/0006-8993(94)91329-3. [DOI] [PubMed] [Google Scholar]
- 19.Horner RL, Sanford LD, Annis D, Pack AI, Morrison AR. Serotonin at the laterodorsal tegmental nucleus suppresses rapid-eye-movement sleep in freely behaving rats. J Neurosci. 1997;17:7541–7552. doi: 10.1523/JNEUROSCI.17-19-07541.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houdouin F, Cespuglio R, Gharib A, Sarda N, Jouvet M. Detection of the release of 5-hydroxyindole compounds in the hypothalamus and the n. raphe dorsalis throughout the sleep-waking cycle and during stressful situations in the rat: a polygraphic and voltammetric approach. Exp Brain Res. 1991a;85:153–162. doi: 10.1007/BF00229997. [DOI] [PubMed] [Google Scholar]
- 21.Houdouin F, Cespuglio R, Jouvet M. Effects induced by the electrical stimulation of the nucleus raphe dorsalis upon hypothalamic release of 5-hydroxyindole compounds and sleep parameters in the rat. Brain Res. 1991b;565:48–56. doi: 10.1016/0006-8993(91)91735-j. [DOI] [PubMed] [Google Scholar]
- 22.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PPA. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 23.Kia HK, Miquel MC, Brisorgueil MJ, Daval G, Riad M, El Mestikawy S, Hamon M, Vergé D. Immunocytochemical localization of serotonin 5-HT1A receptors in the rat central nervous system. J Comp Neurol. 1996;365:289–305. doi: 10.1002/(SICI)1096-9861(19960205)365:2<289::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 24.Knobelman DA, Hen R, Blendy JA, Lucki I. Regional patterns of compensation following genetic deletion of either 5-hydroxytryptamine1A or 5-hydroxytryptamine1B receptor in the mouse. J Pharmacol Exp Ther. 2001;298:1092–1100. [PubMed] [Google Scholar]
- 25.Kupfer DJ. REM latency: a psychobiologic marker for primary depressive disease. Biol Psychiatry. 1976;11:159–174. [PubMed] [Google Scholar]
- 26.Limoge-Lendais I, Robert C, Degrange M, Goldberg M, Stinus L, Limoge A. Study on Superbond adhesion to the skull for chronic electrode implantation in the rat. Neurosci Protocol. 1994;70:1–11. [Google Scholar]
- 27.Marinesco S, Bonnet C, Cespuglio R. Influence of stress duration on the sleep rebound induced by immobilization in the rat: a possible role for corticosterone. Neuroscience. 1999;92:921–933. doi: 10.1016/s0306-4522(99)00045-7. [DOI] [PubMed] [Google Scholar]
- 28.McCarley RW, Massaquoi SG. Neurobiological structure of the revised limit cycle reciprocal interaction model of REM cycle control. J Sleep Res. 1992;1:132–137. doi: 10.1111/j.1365-2869.1992.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 29.Meerlo P, Easton A, Bergmann BM, Turek FW. Restraint increases prolactin and REM sleep in C57BL/6J but not in BALB/cJ mice. Am J Physiol. 2001;281:R846–R854. doi: 10.1152/ajpregu.2001.281.3.R846. [DOI] [PubMed] [Google Scholar]
- 30.Monti JM, Jantos H. Dose-dependent effects of the 5-HT1A receptor agonist 8-OH-DPAT on sleep and wakefulness in the rat. J Sleep Res. 1992;1:169–175. doi: 10.1111/j.1365-2869.1992.tb00033.x. [DOI] [PubMed] [Google Scholar]
- 31.Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci USA. 1998;95:10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porkka-Heiskanen T, Smith SE, Taira T, Urban JH, Levine JE, Turek FW, Stenberg D. Noradrenergic activity in rat brain during rapid eye movement sleep deprivation and rebound sleep. Am J Physiol. 1995;268:R1456–R1463. doi: 10.1152/ajpregu.1995.268.6.R1456. [DOI] [PubMed] [Google Scholar]
- 33.Portas CM, Thakkar M, Rainnie D, McCarley RW. Microdialysis perfusion of 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) in the dorsal raphe nucleus decreases serotonin release and increases rapid eye movement sleep in the freely moving cat. J Neurosci. 1996;16:2820–2828. doi: 10.1523/JNEUROSCI.16-08-02820.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Portas CM, Bjorvatn B, Ursin R. Serotonin and the sleep/wake cycle: special emphasis on microdialysis studies. Prog Neurobiol. 2000;60:13–35. doi: 10.1016/s0301-0082(98)00097-5. [DOI] [PubMed] [Google Scholar]
- 35.Quattrochi JJ, Mamelak AN, Binder D, Williams J, Hobson JA. Dose-related suppression of REM sleep and PGO waves by the serotonin-1 agonist eltoprazine. Neuropsychopharmacology. 1993;8:7–13. doi: 10.1038/npp.1993.2. [DOI] [PubMed] [Google Scholar]
- 36.Ramboz S, Oosting R, Aït Amara D, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci USA. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, El Mestikawy S, Hamon M, Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B receptors in adult rat brain. J Comp Neurol. 2000;417:181–194. [PubMed] [Google Scholar]
- 38.Sakai K, Crochet S. Role of dorsal raphe in paradoxical sleep generation in the cat: no evidence for serotonergic mechanism. Eur J Neurosci. 2001;13:103–112. [PubMed] [Google Scholar]
- 39.Sallanon M, Janin M, Buda C, Jouvet M. Serotoninergic mechanisms and sleep rebound. Brain Res. 1983;268:95–104. doi: 10.1016/0006-8993(83)90393-1. [DOI] [PubMed] [Google Scholar]
- 40.Sanford LD, Ross RJ, Seggos AE, Morrison AR, Ball WA, Mann GL. Central administration of two 5-HT receptor agonists: effect on REM sleep initiation and PGO waves. Pharmacol Biochem Behav. 1994;49:93–100. doi: 10.1016/0091-3057(94)90461-8. [DOI] [PubMed] [Google Scholar]
- 41.Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, Gunn RN, Grasby PM, Cowen PJ. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY- 100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57:174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- 42.Sotelo C, Cholley B, El Mestikawy S, Gozlan H, Hamon M. Direct immunohistochemical evidence for the existence of 5-HT1A autoreceptors on serotoninergic neurons in the midbrain raphe nuclei. Eur J Neurosci. 1990;2:1144–1154. doi: 10.1111/j.1460-9568.1990.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 43.Thakkar MM, Strecker RE, McCarley RW. Behavioral state control through differential serotonergic inhibition in the mesopontine cholinergic nuclei: a simultaneous unit recording and microdialysis study. J Neurosci. 1998;18:5490–5497. doi: 10.1523/JNEUROSCI.18-14-05490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tissier M, Lainey E, Fattaccini C, Hamon M, Adrien J. Effects of ipsapirone, a 5-HT1A agonist, on sleep/wakefulness cycles: probable post-synaptic action. J Sleep Res. 1993;2:103–109. doi: 10.1111/j.1365-2869.1993.tb00070.x. [DOI] [PubMed] [Google Scholar]
- 45.Tobler I, Deboer T, Fisher M. Sleep and sleep regulation in normal and prion protein-deficient mice. J Neurosci. 1997;17:1869–1879. doi: 10.1523/JNEUROSCI.17-05-01869.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ursin R. Serotonin and sleep. Sleep Med Rev. 2002;6:55–67. doi: 10.1053/smrv.2001.0174. [DOI] [PubMed] [Google Scholar]
- 47.Van Praag HM. Anxiety and increased aggression as pacemakers of depression. Acta Psychiatr Scand [Suppl] 1998;393:81–88. doi: 10.1111/j.1600-0447.1998.tb05971.x. [DOI] [PubMed] [Google Scholar]
- 48.Vazquez-Palacios G, Velazquez-Moctezuma J. Effect of electric foot shocks, immobilization, and corticosterone administration on the sleep-wake pattern in the rat. Physiol Behav. 2000;71:23–28. doi: 10.1016/s0031-9384(00)00285-7. [DOI] [PubMed] [Google Scholar]
- 49.Vergé D, Daval G, Marcinkiewicz M, Patey A, El Mestikawy S, Gozlan H, Hamon M. Quantitative autoradiography of multiple 5-HT1 receptor subtypes in the brain of control or 5,7-dihydroxytryptamine-treated rats. J Neurosci. 1986;6:3474–3482. doi: 10.1523/JNEUROSCI.06-12-03474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wood M, Chaubey M, Atkinson P, Thomas DR. Antagonist activity of meta-chlorophenypiperazine and partial agonist activity of 8-OH-DPAT at the 5-HT7 receptor. Br J Pharmacol. 2000;396:1–8. doi: 10.1016/s0014-2999(00)00213-2. [DOI] [PubMed] [Google Scholar]
- 51.Zhuang X, Gross C, Santarelli L, Compan V, Trillat AC, Hen R. Altered emotional states in knockout mice lacking 5-HT1A or 5-HT1B receptors. Neuropsychopharmacology. 1999;21:52S–60S. doi: 10.1016/S0893-133X(99)00047-0. [DOI] [PubMed] [Google Scholar]