Abstract
Metabotropic glutamate receptors (mGluRs) act as modulators in the CNS of vertebrates, but their role in motor pattern generation in particular is primarily unknown. The intracellular signaling mechanisms of the group I mGluRs (mGluR1 and mGluR5), and their endogenous role in regulating locomotor pattern generation have been investigated in the spinal cord of the lamprey. Application of the group I mGluR agonist (R,S)-3,5-dihydroxyphenylglycine (DHPG) produced oscillations of the intracellular Ca2+ concentration ([Ca2+]i) in neurons. The oscillations were blocked by the mGluR5 antagonist 2-methyl-6-(phenylethynyl)pyridine (MPEP) but not by the mGluR1 antagonist 7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxylate ethyl ester. These [Ca2+]ioscillations were abolished by a phospholipase C blocker and after depletion of internal Ca2+ stores by thapsigargin but did not involve protein kinase C activation. Furthermore, they were dependent on Ca2+ influx, because no [Ca2+]i oscillations were produced by DHPG in a Ca2+-free solution or after blockade of L-type Ca2+ channels. The mGluR5 is activated by an endogenous release of glutamate during locomotion, and a receptor blockade by MPEP caused an increase in the burst frequency. Thus, our results show that mGluR5 induces [Ca2+]i oscillations and regulates the activity of locomotor networks through endogenous activation.
Keywords: mGluR5, locomotion, spinal cord, modulation, glutamate, lamprey
The generation of coordinated motor patterns involves not only the fast-acting ionotropic receptors but also the relatively slow metabotropic receptors. The role of ionotropic glutamate receptors in spinal locomotor networks has been studied extensively (Cazalets et al., 1992; Grillner et al., 1998; Reith and Sillar, 1998; Kiehn et al., 2000). However, there is limited knowledge about the contribution of metabotropic glutamate receptors (mGluRs) in locomotor pattern generation. mGluRs with pharmacological characteristics corresponding to groups I, II, and III exist in the lamprey, a lower vertebrate experimental model (Krieger et al., 1996,1998; Cochilla and Alford, 1998). Activation of postsynaptic group I mGluRs can increase intracellular calcium concentration ([Ca2+]i), potentiate NMDA-induced responses, and modulate the frequency of the locomotor rhythm (Krieger et al., 2000). These mGluRs appear not to have a major role in the generation of the basic locomotor rhythm, but they play a role in its modulation (Krieger et al., 1998).
This group consists of two receptor subtypes (mGluR1 and mGluR5) that elevate the levels of inositol triphosphate (IP3) through the activation of phospholipase C (PLC) (Pin and Duvoisin, 1995; Anwyl, 1999; Fagni et al., 2000). Although these two subtypes commonly use a similar signal transduction pathway, their activation may result in different patterns of the [Ca2+]i response. In expression systems, mGluR1 elicits a single-peaked nonoscillatory [Ca2+]i response, whereas mGluR5 elicits oscillations (Kawabata et al., 1996; Nakanishi et al., 1998). In contrast, an activation of native mGluR5 in neurons induces different cellular effects. In hippocampus neurons, mGluR5 elicits a single-peaked [Ca2+]i response (Rae et al., 2000), whereas it induces [Ca2+]ioscillations in the neocortex (Flint et al., 1999). In neurons of the subthalamic nucleus, mGluR5 potentiates NMDA receptor currents (Awad et al., 2000).
In this study, we investigated which group I mGluR subtype mediates the [Ca2+]i increase and the underlying intracellular signal transduction pathway. We also compared the pharmacology of this subtype with that inducing the potentiation of NMDA responses. Finally, we examined whether the group I mGluR subtype mediating a [Ca2+]i increase is endogenously activated during locomotion. Our results show that activation of mGluR5 induces [Ca2+]ioscillations that do not require protein kinase C (PKC) activation, but depend on Ca2+ entry through L-type channels. Thus, in lamprey spinal cord neurons the two subtypes of group I mGluRs have different cellular effects; mGluR1 potentiates NMDA receptors and mGluR5 induces [Ca2+]ioscillations. These two receptor subtypes are activated by endogenous release of glutamate during locomotion and mediate opposite effects on the frequency of the locomotor rhythm.
MATERIALS AND METHODS
Cell dissociation. Larval lampreys (Petromyzon marinus) were anesthetized with tricaine methane sulfonate (MS222; 100 mg/l) and the spinal cord was isolated in cooled oxygenated physiological solution. To identify motoneurons (MNs), fluorescein-coupled dextran amine (FDA) was applied before dissociation into muscle tissue along the entire length of the preparation after cutting all dorsal roots to allow the transport of the dye only through the ventral roots, thus retrogradely labeling MNs. The dissociation was performed in Leibovitz's L-15 culture medium (Sigma, St. Louis, MO) supplemented with penicillin–streptomycin (2 μl/ml), with the osmolarity adjusted to 270 mOsm (El Manira and Bussières, 1997). The spinal cord was incubated for 30 min in collagenase (1 mg/ml; Sigma) and then in protease for 45 min (2 mg/ml; Sigma). The tissue was subsequently washed with the culture medium and triturated through a sterilized pipette. The supernatant containing the dissociated cells was distributed in 10–12 Petri dishes (35 mm) and incubated at 10–12°C for 1–4 d.
Calcium imaging. Before calcium imaging, the dissociated cells were incubated at room temperature for 1–2 hr with Fluo-4/acetoxymethyl (AM) (5 μm; Molecular Probes, Eugene, OR) added to the medium. The same procedure was used to load FDA-labeled MNs with Fluo-4/AM after identification. After removal of the incubation medium, the cells were perfused with a solution containing (in mm): 124 NaCl, 2 KCl, 1.2 MgCl2, 5 CaCl2, 10 glucose, and 10 HEPES, with pH adjusted to 7.6. The following drugs were tested: (R,S)-3,5-dihydroxyphenylglycine (DHPG), 7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxylate ethyl ester (CPCCOEt), 2-methyl-6-(phenylethynyl)pyridine (MPEP), thapsigargin, 1-(5-isoquinolinesulfonyl)-2-methylpiperazine (H-7), ryanodine, nimodipine, staurosporine, and ω-conotoxin GVIA (ω-CgTx). DHPG, CPCCOEt, and MPEP were purchased from Tocris Cookson (Bristol, UK). Thapsigargin, H-7, ryanodine, and nimodipine were purchased from Research Biochemicals (Natick, MA); staurosporine and U-73122 were supplied by Labkemi (Stockholm, Sweden); and ω-CgTx was obtained from Peptides International (Herts, UK). Unless stated otherwise, DHPG was added to the perfusing solution for 3 min at a concentration of 100 μm. The effect of CPCCOEt, MPEP, thapsigargin, H-7, ryanodine, staurosporine, and nimodipine on the DHPG-induced [Ca2+]i response was tested using two parallel series of cultured neurons from the same dissociation, one representing controls and the other preincubated with the different drugs.
The 488 nm line of an argon laser was used for excitation of Fluo-4/AM with an emission filter passing wavelengths of >515 nm. The loaded cells were visualized using a confocal laser scanner (Odyssey; Noran Instruments, Middleton, WI) with 10× (0.25 NA) or 20× (0.40 NA) objectives (Nikon, Tokyo, Japan) attached to a Nikon Diaphot inverted microscope. Brightness-over-time plots were generated by sampling (7–15 Hz) the averaged intensity within a manually specified region of interest within the cell soma. Analyses of the brightness-over-time data were performed with pClamp (Axon Instruments, Foster City, CA). The imaging experiments were typically done on three to seven cells in each dish, in the same field of view, and on several dishes from the same dissociation. The unidentified neurons included in this study were monopolar and had a small diameter corresponding primarily to MNs and interneurons. The mechanosensory dorsal root ganglion cells were not included. Changes in fluorescence (ΔF), which is a measure of changed intracellular calcium concentration, were normalized to the resting fluorescence levels (Frest) of the cells, and the fluorescence from a region that did not include dye-filled neurons (Fbackground) was subtracted. Thus, the fluorescence data are presented as ΔF/F = ΔF/(Frest −Fbackground). The percentage values reported in the text and figures were calculated as the average of the number of cells with DHPG-induced calcium oscillations per total number of cells in that particular dish. The data are presented as means ± SD; n = the total number of neurons tested.
When two parallel series of cultured cells were used, the difference between the proportion of cells displaying calcium oscillations in control dishes and in preincubated dishes was tested with Fisher's exact test to determine any relationship between the two experimental conditions. The reported p value is double the single-sidedp. Fisher's exact test was performed with an on-line calculator provided by SISA (http://home.clara.net/sisa/binomial.htm). When the same cells were used as controls and test subjects, the statistical significance was tested with McNemar's test to compare paired groups, performed with an on-line calculator provided by GraphPad (GraphPad Software, San Diego, CA).
Electrophysiology. Whole-cell recordings were performed from neurons in culture using an Axopatch 200A patch-clamp amplifier (Axon Instruments). The cells were perfused through a gravity-driven multibarreled microperfusion system placed close to the recorded cell. Neurons had a resting membrane potential between −60 and −55 mV. The effect of DHPG was tested in control solution and in the presence of the mGluR1 antagonist CPCCOEt. The control solution contained (in mm): 124 NaCl, 2 KCl, 1.2 MgCl2, 5 CaCl2, 10 glucose, and 10 HEPES, with pH adjusted to 7.6. For whole-cell recordings, the pipettes were filled with a solution containing (in mm): 113 KCH3SO3, 1.2 MgCl2, 10 glucose, and 10 HEPES, with pH adjusted to 7.6 with KOH. Data acquisition and analysis were performed with pClamp software.
Extracellular measurements of ventral root activity were performed on the isolated spinal cord in vitro. The preparation was mounted in a cooled (8–12°C) homemade Sylgard-lined chamber that was continuously perfused with an extracellular solution of the following composition (in mm): 138 NaCl, 2.1 KCl, 1.8 CaCl2, 1.2 MgCl2, 4 glucose, 2 HEPES, and 0.5 l-glutamine, bubbled with O2, pH adjusted to 7.4. Fictive locomotion was induced by bath application of NMDA (100 μm). The cycle duration was calculated as the time between midpoints of two successive bursts and averaged over 60–120 cycles. In these experiments, n = the number of animals. The analysis of the locomotor rhythm was performed with DATA-PAC (Run Technologies, Laguna Hills, CA).
RESULTS
mGluR5 activation causes calcium oscillations in spinal cord neurons
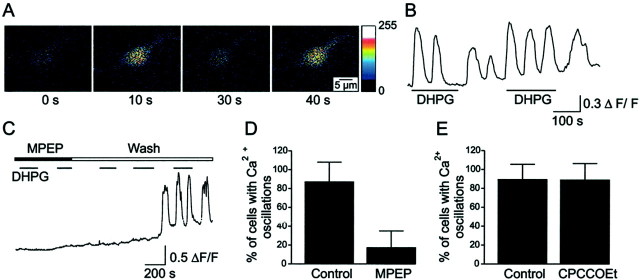
The group I mGluR agonist DHPG (100 μm) was applied to lamprey spinal cord neurons loaded with Fluo-4/AM. On average, 83.2 ± 27.0% of the neurons in a single dish (n= 150; 35 dishes) exhibited oscillations in the intracellular free calcium concentration ([Ca2+]i) (Fig.1). Figure 1A shows the changes in [Ca2+]iin a single neuron loaded with Fluo-4/AM as revealed by the change in fluorescence during the application of DHPG. The different images were acquired at 0, 10, 30, and 40 sec. The fluorescence pattern revealed that DHPG induced [Ca2+]ioscillations (Fig. 1A,B), with the first and third frames showing low [Ca2+]i, whereas the second and fourth frames display high [Ca2+]i. The oscillatory response normally lasted during the entire recording period, occurred in a range from 0.2 to 4 min after the start of DHPG application, and was also elicited by consecutive applications of the agonist (Fig. 1B). The frequency of the DHPG-induced [Ca2+]ioscillatory response showed a large variation between neurons and ranged between 0.005 and 0.033 Hz (n = 150). Identified FDA-labeled MNs also showed [Ca2+]ioscillations in response to DHPG (n = 9 of 11; eight dishes).
Fig. 1.
DHPG-induced [Ca2+]i oscillations in lamprey spinal cord neurons are mediated by MPEP-sensitive, CPCCOEt-insensitive group I mGluRs. A, Images showing an example of [Ca2+]i oscillations induced by DHPG in a Fluo-4/AM-loaded neuron. B, The group I mGluR agonist DHPG (100 μm) produced oscillatory [Ca2+]i responses, which were elicited by consecutive applications of the agonist. C, DHPG did not induce any [Ca2+]i oscillations in a neuron preincubated with MPEP (100 μm), whereas reapplication of DHPG after washout of MPEP produced [Ca2+]i oscillations.D, Almost all neurons displayed [Ca2+]i oscillations in response to DHPG in the control, but fewer responded to DHPG in the presence of MPEP. E, CPCCOEt did not block the oscillatory [Ca2+]i response to DHPG.
The above results show that activation of group I mGluRs produces [Ca2+]ioscillations in lamprey spinal cord neurons. To determine the pharmacological profile of the receptors responsible for these oscillations, the mGluR1-specific antagonist CPCCOEt (Annoura et al., 1996; Casabona et al., 1997) and mGluR5-specific antagonist MPEP (Gasparini et al., 1999) were used. Figure 1C shows a neuron in which application of DHPG in the presence of MPEP did not induce any [Ca2+]i response. However, reapplication of DHPG after washout of MPEP elicited [Ca2+]ioscillations in the same cells. In total, only 17.1 ± 17.8% of the neurons per dish (6 of 37 neurons; seven dishes) responded to DHPG in the presence of MPEP (100 μm) (Fig.1D). In these, cases the DHPG-induced calcium response consisted of a single peak. After washout of MPEP, DHPG induced [Ca2+]ioscillations in 87.0 ± 21.1% of the neurons per dish (31 of 37 cells; seven dishes; p < 0.001 comparing the effect of DHPG alone with that of DHPG in the presence of MPEP). The effect of CPCCOEt on DHPG-evoked [Ca2+]ioscillations was also examined (Fig. 1E). In control dishes, 93.1 ± 13.7% of neurons (25 of 28; nine dishes) showed [Ca2+]ioscillations, compared with 92.4 ± 17.4% of neurons (33 of 35, 11 dishes) in dishes preincubated for 30 min with CPCCOEt (100 μm; p > 0.1). These results indicate that DHPG-induced [Ca2+]ioscillations are attributable to an action on an MPEP-sensitive, CPCCOEt-insensitive group I mGluR (i.e., mGluR5). Application of DHPG in the presence of CPCCOEt did not induce any change in the membrane potential of spinal neurons in culture, recorded using whole-cell patch clamp (data not shown). This is in accordance with the lack of an effect of DHPG on the membrane potential of neurons recorded in the intact spinal cord in the presence of TTX (Krieger et al., 2000).
DHPG induces [Ca2+]i oscillations by a PLC-dependent release from internal stores
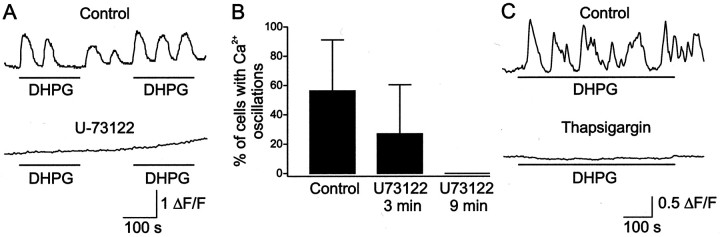
The intracellular pathway underlying [Ca2+]ioscillations was investigated by using a PLC blocker and by depleting the intracellular Ca2+ stores. Figure2A shows a neuron in which DHPG application induced an oscillatory response, which was abolished by applying the PLC blocker U-73122 (0.5–1 μm). DHPG elicited [Ca2+]ioscillations in 56.3 ± 34.7% (23 of 40 cells tested; eight dishes) of the neurons in control dishes. After a 3 min application of U-73122, oscillations occurred only in 27.0 ± 33.4% neurons (10 of 40 cells; eight dishes; p < 0.001), and after 9 min the blocker completely abolished the oscillations in all neurons (Fig. 2B).
Fig. 2.
The [Ca2+]ioscillations induced by DHPG are mediated through PLC activation and require a release from intracellular stores. A, DHPG induced [Ca2+]i oscillations in a spinal cord neuron in controls that were abolished by the PLC blocker U-73122 (1 μm). B, Percentage of neurons that displayed [Ca2+]i oscillations in response to DHPG in controls, after 3 min, and after 9 min of application of U-73122. C, DHPG elicited [Ca2+]i oscillations in neurons from control dishes, whereas no oscillatory response was produced in neurons from dishes preincubated with thapsigargin (1 μm).
The source of the calcium underlying the DHPG-induced oscillations was examined by using thapsigargin, which depletes internal Ca2+ stores by blocking the Ca2+ pumps (Thastrup et al., 1990). Parallel series of experiments were performed on neurons from the same dissociation; one served as a control and the other was preincubated for 45 min with thapsigargin (1 μm). In the controls, the application of DHPG induced [Ca2+]ioscillations in all neurons tested (n = 23; five dishes), whereas no oscillations were elicited in neurons preincubated with thapsigargin (n = 12; three dishes) (Fig.2C). This finding suggests that these Ca2+ oscillations are mediated by release from internal stores.
Furthermore, the DHPG-induced [Ca2+]ioscillations were not blocked by ryanodine (100 μm; 45 min preincubation), which blocks the ryanodine receptors at high concentrations. DHPG induced [Ca2+]ioscillations in 100% of the cells in controls (n = 18; four dishes) and in 91.7 ± 14.4% of the cells preincubated for 45 min with ryanodine (100 μm;n = 9; four dishes; p > 0.1; data not shown).
The involvement of PKC in the signaling pathway underlying DHPG-induced [Ca2+]ioscillations was also tested using the PKC blockers H-7 (10 μm; 30 min preincubation) and staurosporine (2 μm; 30 min preincubation). However, the oscillations could not be blocked by either of the two PKC inhibitors. In controls, 55.8 ± 38.0% of the cells showed oscillations (21 of 43 cells tested; nine dishes) in comparison with 57.2 ± 30.3% of the H-7-treated cells (8 of 17 cells tested; four dishes) (p = 0.7). In dishes preincubated with staurosporine, [Ca2+]ioscillations were elicited in 77.6 ± 25.4 of the cells (22 of 27 cells tested; five dishes) compared with 71.4 ± 25.4% in controls (15 of 18 cells tested; four dishes; p > 0.1). Therefore, the oscillatory response induced by mGluR5 activation is not mediated by ryanodine receptors and does not involve PKC activation; thus, it may derive from the mobilization of Ca2+ from internal stores via an activation of IP3 receptors.
Calcium influx through L-type channels is necessary for the production of DHPG-induced [Ca2+]ioscillations
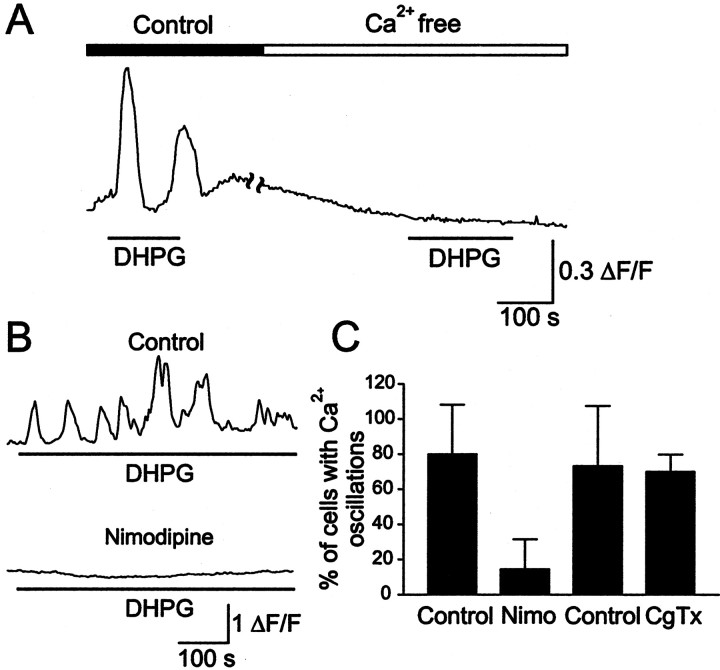
The importance of extracellular Ca2+in the generation of [Ca2+]ioscillations by DHPG was examined using Ca2+-free solution and antagonists of voltage-gated Ca2+ channels. In control dishes, DHPG induced [Ca2+]ioscillations, but after switching to a Ca2+-free solution, reapplication of DHPG did not induce any calcium response (Fig.3A) (n = 15; four dishes). This indicates that extracellular Ca2+ is necessary for the production of [Ca2+]ioscillations in spinal cord neurons. To determine whether the extracellular Ca2+ contributes to the generation of the DHPG response by entering through voltage-gated channels, specific blockers were used. Parallel series of experiments were done; one series served as a control and the other was preincubated with Ca2+ channel antagonists. Blockade of L-type channels by nimodipine (10 μm) abolished the [Ca2+]ioscillations induced by DHPG (Fig. 3B). In control dishes, 80.0 ± 28.3% of the neurons tested (n = 10; two dishes) exhibited oscillatory responses to DHPG application (Fig.3C), whereas only 14.5 ± 17.1% of the neurons preincubated with nimodipine showed [Ca2+]ioscillations (n = 12; four dishes; p = 0.01) (Fig. 3C). Blockade of N-type channels by ω-CgTx (1 μm) did not affect the DHPG-induced [Ca2+]i oscillations. In control dishes, 73.3 ± 34.1% of the neurons tested (n = 13; three dishes) displayed [Ca2+]ioscillations in response to DHPG, compared with 70.0 ± 10.0% of neurons (n = 7) in dishes (n = 3) preincubated with ω-CgTx (p > 0.1) (Fig.3C).
Fig. 3.
DHPG-induced [Ca2+]i oscillations require an influx of extracellular Ca2+ through L-type Ca2+ channels. A, Removal of Ca2+ from the perfusing solution abolished the [Ca2+]i oscillations induced by DHPG. The illustrated neuron displayed [Ca2+]i oscillations in response to DHPG, but after switching to Ca2+-free solution DHPG was unable to produce any [Ca2+]ioscillations. B, In controls, the application of DHPG produced [Ca2+]i oscillations, whereas no oscillatory [Ca2+]i response was observed in neurons preincubated with the L-type Ca2+ channel blocker nimodipine (10 μm). C, The percentage of neurons per dish that produced [Ca2+]i oscillations in response to DHPG in controls and in dishes preincubated with nimodipine and with ω-CgTx.
Endogenous activation of mGluR5 during locomotor network activity
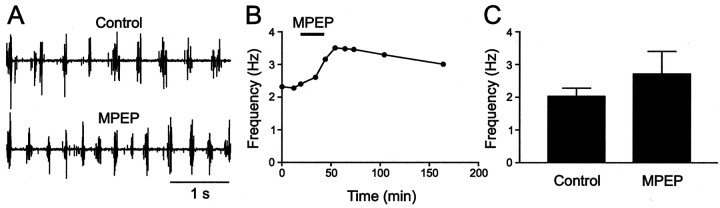
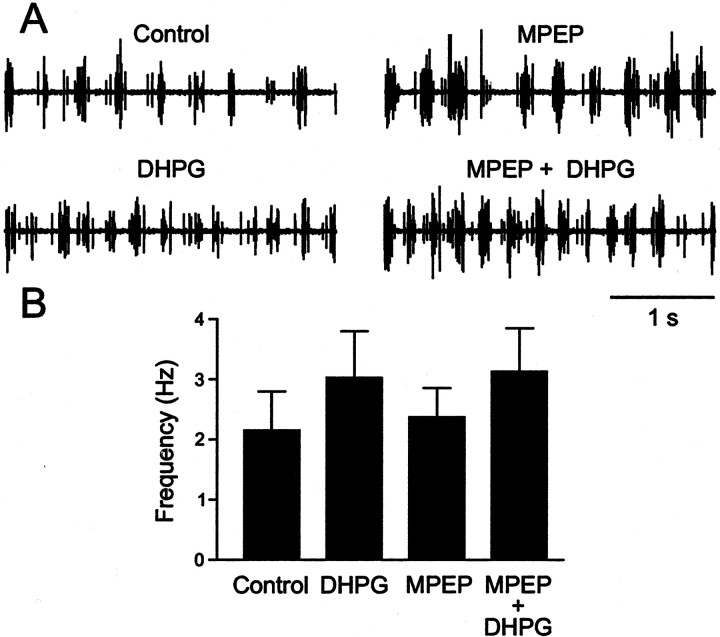
To test whether endogenously released glutamate activates this receptor subtype during locomotion, the mGluR5 antagonist MPEP was used. Locomotor rhythm was induced in all experiments in a spinal cord preparation by NMDA (100 μm). An application of MPEP (50–100 μm) (Fig.4A) reversibly increased the frequency of the locomotor rhythm from 2.0 ± 0.2 Hz to 2.7 ± 0.6 Hz (Fig. 4B,C) (n= 7; p < 0.05). We subsequently examined whether MPEP affects the increase in locomotor frequency induced by the mGluR1 and mGluR5 agonist DHPG (Krieger et al., 1998). Figure5 shows an experiment in which application of DHPG increased the locomotor frequency that persisted in the presence of MPEP. This indicates that the increase in frequency is not mediated by mGluR5 but rather by mGluR1. This mGluR subtype is activated by endogenously released glutamate, because application of the mGluR1 antagonist CPCCOEt alone decreases the frequency of the locomotor rhythm (Krieger et al., 1998). The DHPG-increased locomotor frequency was not significantly (p > 0.1) different in the controls (from 2.1 ± 0.6 Hz to 3.0 ± 0.7 Hz) and with MPEP (from 2.4 ± 0.4 Hz to 3.1 ± 0.7 Hz) (n = 7) (Fig. 5B). Thus, these results show that mGluR5 is activated during locomotion and that the two subtypes of group I mGluRs produce opposite effects on the frequency of the locomotor rhythm.
Fig. 4.
mGluR5 activation by endogenously released glutamate during locomotor network activity. A, The mGluR5 antagonist MPEP reversibly increased the frequency of the locomotor rhythm induced by NMDA. B, C, The increase in locomotor frequency during MPEP application.
Fig. 5.
The DHPG-induced increase in locomotor frequency is not blocked by the mGluR5 antagonist MPEP. A, DHPG increased locomotor frequency in control conditions and in the presence of the mGluR5 antagonist MPEP. B, The percentage of increase in locomotor frequency by DHPG in controls and with MPEP application.
DISCUSSION
We have shown that activation of group I mGluRs by DHPG induces [Ca2+]ioscillations in lamprey spinal cord neurons. These oscillations were blocked by the mGluR5 antagonist MPEP but not by the mGluR1 antagonist CPCCOEt. In addition to producing [Ca2+]ioscillations, DHPG potentiates NMDA responses and increases the frequency of the locomotor rhythm induced by NMDA in the intact spinal cord of the lamprey (Krieger et al., 2000). The latter two effects are blocked by CPCCOEt, which when applied alone decreases the frequency of the locomotor rhythm. In contrast, blockade of mGluR5 by MPEP increases the frequency (Fig. 4). Because CPCCOEt at the concentration used in this study has been shown to block mGluR1 (Annoura et al., 1996;Casabona et al., 1997; Litschig et al., 1999), whereas MPEP antagonizes mGluR5 (Gasparini et al., 1999), our results show that mGluR5 induces [Ca2+]ioscillations and decreases locomotor frequency, whereas mGluR1 interacts with NMDA receptors and increases the frequency of the locomotor rhythm. The mGluR5 had only a nonsignificant effect on locomotor frequency in the presence of DHPG (Fig. 5), whereas it significantly increased the frequency by blocking the effect of the endogenously released glutamate (Fig. 4). Thus, MPEP appears to be more potent in blocking the activation of mGluR5 by endogenous glutamate than by DHPG on locomotor frequency. This suggests that the DHPG-induced modulation of the locomotor rhythm is mediated primarily by activation of mGluR1 or that the potency of MPEP during fictive locomotion depends on the agonist used to activate mGluR5. Such an agonist-dependent antagonism has been shown using different antagonists on mGluR5 in expression systems (Brabet et al., 1995; Doherty et al., 1999).
mGluR5-mediated [Ca2+]ioscillations have been reported in astrocytes (Nakahara et al., 1997) and in developing neocortical neurons (Flint et al., 1999). The underlying mechanisms have been examined previously only in transfected cells (Kawabata et al., 1996). The mGluR5 possesses a regulatory PKC phosphorylation site and is also coupled to PLC to produce both DAG and IP3 on its activation. IP3increases [Ca2+]iby release of Ca2+ from internal stores, whereas DAG leads to PKC activation that can phosphorylate mGluR5 and thus uncouple the receptor from the intracellular transduction pathway, leading to a decrease in [Ca2+]i levels. The uncoupling of the mGluR5 from PLC decreases the production of DAG and thereby the activation of PKC and the phosphorylation of the receptor. This enables the receptor to couple to its signal transduction pathway again, and a new cycle of [Ca2+]ioscillations starts. In transfected cells, the oscillations are blocked by PKC inhibitors and persist in the presence of a Ca2+-free solution, indicating that they are not dependent on extracellular Ca2+(Kawabata et al., 1996).
Our results show that DHPG induces [Ca2+]ioscillations through signaling mechanisms different from those described above. In lamprey spinal cord neurons the oscillations were abolished in Ca2+-free solution and by a blockade of L-type Ca2+channels but persisted after blockade of PKC and ryanodine receptors. L-type channels in lamprey spinal cord neurons start activating at negative voltages (El Manira and Bussières, 1997). The only channels that are activated at negative voltages among the cloned L-type channels are those expressed by α1D (Cav1.3) subunit. These channels start to be activated at membrane potentials of approximately −60 mV (Svirskis and Hounsgaard, 1997; Platzer et al., 2000; Koschak et al., 2001), which correspond to the resting membrane potential of lamprey spinal cord neurons. In imaging experiments, application of the L-type Ca2+ channel agonist BayK increased the basal Ca2+level (data not shown), suggesting that in lamprey spinal cord neurons these channels are open at the resting membrane potential and allow a continuous small influx of Ca2+. The role of the Ca2+ influx through L-type channels could be to trigger Ca2+ release from internal stores via Ca2+-induced Ca2+ release and/or to reload the stores after depletion. The DHPG-induced [Ca2+]ioscillations are likely to be mediated by release from internal stores and not through a direct action on L-type Ca2+ channels, because DHPG did not induce any change in the membrane potential at rest. Furthermore, the [Ca2+]ioscillations were blocked by a PLC inhibitor and thapsigargin, which depletes intracellular stores by blocking the ATPase that mediates Ca2+ reuptake into the internal stores. These results indicate that the [Ca2+]ioscillations induced by DHPG in these neurons are attributable to release of Ca2+ from internal stores through PLC- and Ca2+-dependent mechanisms, presumably by acting on IP3-gated intracellular Ca2+ stores. The fact that the mGluR1-specific antagonist CPCCOEt did not block the DHPG-induced oscillations suggests that this receptor does not mediate [Ca2+]ioscillations in lamprey spinal cord neurons. Because both mGluR1 and mGluR5 are commonly coupled to the IP3/[Ca2+]isignal transduction pathway, they are both expected to release Ca2+ from internal stores. However, this appears not to be the case in these neurons. A possible explanation could be that the strength of hydrolysis of polyphosphoinositide (PPI) is different for the MPEP-sensitive and CPCCOEt-sensitive group I mGluRs found in lamprey spinal cord neurons. Such a difference has been shown in the rat brain, in which mGluR1 couples less efficiently than mGluR5 to PPI hydrolysis (Casabona et al., 1997). Thus, our results show the existence of Ca2+-dependent [Ca2+]ioscillations induced by a group I mGluR, with a pharmacological profile similar to mGluR5.
[Ca2+]ioscillations can be transduced into specific signals that may regulate the activity of spinal cord neurons. Ca2+can activate a wide range of Ca2+-sensitive enzymes such as calmodulin kinase II (De Koninck and Schulman, 1998), gene regulation (Greenberg et al., 1992), or Ca2+-dependent ion channels (e.g.,KCa) (Fiorillo and Williams, 1998). It has been shown recently in ventral midbrain dopamine neurons that the mGluR1-induced increase in [Ca2+]i activates an apamin-sensitive KCaconductance, leading to a slow IPSP (Fiorillo and Williams, 1998). Activation of mGluR5 by DHPG in the presence of the mGluR1 antagonist CPCCOEt did not induce any changes in the membrane potential of spinal cord neurons in culture. Furthermore, we have shown previously that application of DHPG in the presence of TTX had no effect on the membrane potential of neurons in the intact spinal cord (Krieger et al., 2000). Together these results suggest that the [Ca2+]i increase induced by mGluR5 is not coupled to the activation of Ca2+-dependent channels.
An important finding of this study is that mGluR5 does not serve similar roles as mGluR1 in the lamprey locomotor network. Regardless of whether the increase in the burst frequency can be accounted for by the interaction between mGluR1 and NMDA receptors (Krieger et al., 2000), the mechanisms by which mGluR5 decreases the frequency are not fully determined. However, because [Ca2+]ioscillation is the only cellular response induced by mGluR5 activation in lamprey spinal cord neurons, these oscillations might regulate the frequency of the locomotor rhythm by activating intracellular messenger pathways.
Footnotes
This work was supported by the Swedish Research Council (project 11562), the Swedish Foundation for Strategic Research, the A. Eriksson Foundation, the Å. Wiberg Foundation, the Swedish Society for Medicine, and the Karolinska Institutet funds. P. Krieger received a fellowship from the Knut and Alice Wallenberg Foundation. D.H. received a fellowship from the Deutsche Forschungsgemeinschaft, Germany. We thank Drs. S. Grillner, D. Parker, and P. Wallén for their comments on this manuscript. We are also grateful to H. Axegren and M. Bredmyr for excellent technical assistance.
Correspondence should be addressed to A. El Manira, Nobel Institute for Neurophysiology, Department of Neuroscience, Karolinska Institutet, S-171 77 Stockholm, Sweden. E-mail: abdel.elmanira@neuro.ki.se.
REFERENCES
- 1.Annoura H, Fukunaga A, Uesugi M, Tatsuoka T, Horikawa Y. A novel class of antagonists for metabotropic glutamate receptors, 7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxylates. Bioorg Med Chem Lett. 1996;6:763–766. [Google Scholar]
- 2.Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- 3.Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ. Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J Neurosci. 2000;20:7871–7879. doi: 10.1523/JNEUROSCI.20-21-07871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brabet I, Mary S, Bockaert J, Pin JP. Phenylglycine derivatives discriminate between mGluR1- and mGluR5-mediated responses. Neuropharmacology. 1995;34:895–903. doi: 10.1016/0028-3908(95)00079-l. [DOI] [PubMed] [Google Scholar]
- 5.Casabona G, Knopfel T, Kuhn R, Gasparini F, Baumann P, Sortino MA, Copani A, Nicoletti F. Expression and coupling to polyphosphoinositide hydrolysis of group I metabotropic glutamate receptors in early postnatal and adult rat brain. Eur J Neurosci. 1997;9:12–17. doi: 10.1111/j.1460-9568.1997.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 6.Cazalets JR, Sqalli-Houssaini Y, Clarac F. Activation of the central pattern generators for locomotion by serotonin and excitatory amino acids in neonatal rat. J Physiol (Lond) 1992;455:187–204. doi: 10.1113/jphysiol.1992.sp019296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cochilla AJ, Alford S. Metabotropic glutamate receptor-mediated control of neurotransmitter release. Neuron. 1998;20:1007–1016. doi: 10.1016/s0896-6273(00)80481-x. [DOI] [PubMed] [Google Scholar]
- 8.De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- 9.Doherty AJ, Collingridge GL, Jane DE. Antagonist activity of alpha-substituted 4-carboxyphenylglycine analogues at group I metabotropic glutamate receptors expressed in CHO cells. Br J Pharmacol. 1999;126:205–210. doi: 10.1038/sj.bjp.0702297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Manira A, Bussières N. Calcium channel subtypes in lamprey sensory and motor neurons. J Neurophysiol. 1997;78:1334–1340. doi: 10.1152/jn.1997.78.3.1334. [DOI] [PubMed] [Google Scholar]
- 11.Fagni L, Chavis P, Ango F, Bockaert J. Complex interactions between mGluRs, intracellular Ca2+ stores and ion channels in neurons. Trends Neurosci. 2000;23:80–88. doi: 10.1016/s0166-2236(99)01492-7. [DOI] [PubMed] [Google Scholar]
- 12.Fiorillo CD, Williams JT. Glutamate mediates an inhibitory postsynaptic potential in dopamine neurons. Nature. 1998;394:78–82. doi: 10.1038/27919. [DOI] [PubMed] [Google Scholar]
- 13.Flint AC, Dammerman RS, Kriegstein AR. Endogenous activation of metabotropic glutamate receptors in neocortical development causes neuronal calcium oscillations. Proc Natl Acad Sci USA. 1999;96:12144–12149. doi: 10.1073/pnas.96.21.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gasparini F, Lingenhohl K, Stoehr N, Flor PJ, Heinrich M, Vranesic I, Biollaz M, Allgeier H, Heckendorn R, Urwyler S, Varney MA, Johnson EC, Hess SD, Rao SP, Sacaan AI, Santori EM, Velicelebi G, Kuhn R. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology. 1999;38:1493–1503. doi: 10.1016/s0028-3908(99)00082-9. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg ME, Thompson MA, Sheng M. Calcium regulation of immediate early gene transcription. J Physiol (Paris) 1992;86:99–108. doi: 10.1016/s0928-4257(05)80013-0. [DOI] [PubMed] [Google Scholar]
- 16.Grillner S, Ekeberg Ö, El Manira A, Lansner A, Parker D, Tegnér J, Wallén P. Intrinsic function of a neuronal network: a vertebrate central pattern generator. Brain Res Brain Res Rev. 1998;26:184–197. doi: 10.1016/s0165-0173(98)00002-2. [DOI] [PubMed] [Google Scholar]
- 17.Kawabata S, Tsutsumi R, Kohara A, Yamaguchi T, Nakanishi S, Okada M. Control of calcium oscillations by phosphorylation of metabotropic glutamate receptors. Nature. 1996;383:89–92. doi: 10.1038/383089a0. [DOI] [PubMed] [Google Scholar]
- 18.Kiehn O, Kjaerulff O, Tresch MC, Harris-Warrick RM. Contributions of intrinsic motor neuron properties to the production of rhythmic motor output in the mammalian spinal cord. Brain Res Bull. 2000;53:649–659. doi: 10.1016/s0361-9230(00)00398-1. [DOI] [PubMed] [Google Scholar]
- 19.Koschak A, Reimer D, Huber I, Grabner M, Glossmann H, Engel J, Striessnig J. α1D (Cav1.3) subunits can form L-type Ca2+ channels activating at negative voltages. J Biol Chem. 2001;276:22100–22106. doi: 10.1074/jbc.M101469200. [DOI] [PubMed] [Google Scholar]
- 20.Krieger P, El Manira A, Grillner S. Activation of pharmacologically distinct metabotropic glutamate receptors depresses reticulospinal-evoked monosynaptic EPSPs in the lamprey spinal cord. J Neurophysiol. 1996;76:3834–3841. doi: 10.1152/jn.1996.76.6.3834. [DOI] [PubMed] [Google Scholar]
- 21.Krieger P, Grillner S, El Manira A. Endogenous activation of metabotropic glutamate receptors contributes to burst frequency regulation in the lamprey locomotor network. Eur J Neurosci. 1998;10:3333–3342. doi: 10.1046/j.1460-9568.1998.00337.x. [DOI] [PubMed] [Google Scholar]
- 22.Krieger P, Hellgren-Kotaleski J, Kettunen P, El Manira A. Interaction between metabotropic and ionotropic glutamate receptors regulates neuronal network activity. J Neurosci. 2000;20:5382–5391. doi: 10.1523/JNEUROSCI.20-14-05382.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Litschig S, Gasparini F, Rueegg D, Stoehr N, Flor PJ, Vranesic I, Prezeau L, Pin JP, Thomsen C, Kuhn R. CPCCOEt, a noncompetitive metabotropic glutamate receptor 1 antagonist, inhibits receptor signaling without affecting glutamate binding. Mol Pharmacol. 1999;55:453–461. [PubMed] [Google Scholar]
- 24.Nakahara K, Okada M, Nakanishi S. The metabotropic glutamate receptor mGluR5 induces calcium oscillations in cultured astrocytes via protein kinase C phosphorylation. J Neurochem. 1997;69:1467–1475. doi: 10.1046/j.1471-4159.1997.69041467.x. [DOI] [PubMed] [Google Scholar]
- 25.Nakanishi S, Nakajima Y, Masu M, Ueda Y, Nakahara K, Watanabe D, Yamaguchi S, Kawabata S, Okada M. Glutamate receptors: brain function and signal transduction. Brain Res Brain Res Rev. 1998;26:230–235. doi: 10.1016/s0165-0173(97)00033-7. [DOI] [PubMed] [Google Scholar]
- 26.Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- 27.Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 28.Rae MG, Martin DJ, Collingridge GL, Irving AJ. Role of Ca2+ stores in metabotropic L-glutamate receptor-mediated supralinear Ca2+ signaling in rat hippocampal neurons. J Neurosci. 2000;20:8628–8636. doi: 10.1523/JNEUROSCI.20-23-08628.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reith CA, Sillar KT. A role for slow NMDA receptor-mediated, intrinsic neuronal oscillations in the control of fast fictive swimming in Xenopus laevis larvae. Eur J Neurosci. 1998;10:1329–1340. doi: 10.1046/j.1460-9568.1998.00144.x. [DOI] [PubMed] [Google Scholar]
- 30.Svirskis G, Hounsgaard J. Depolarization-induced facilitation of a plateau-generating current in ventral horn neurons in the turtle spinal cord. J Neurophysiol. 1997;78:1740–1742. doi: 10.1152/jn.1997.78.3.1740. [DOI] [PubMed] [Google Scholar]
- 31.Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+–ATPase. Proc Natl Acad Sci USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]