Abstract
The synaptic connections of Aplysia sensory neurons (SNs) undergo dramatic homosynaptic depression (HSD) with only a few low-frequency stimuli. Strong and weak SN synapses, although differing in their probabilities of release, undergo HSD at the same rate; this suggests that the major mechanism underlying HSD in these SNs may not be depletion of the releasable pool of vesicles. In computational models, we evaluated alternative mechanisms of HSD, including vesicle depletion, to determine which mechanisms enable strong and weak synapses to depress with identical time courses. Of five mechanisms tested, only release-independent, stimulus-dependent switching off of release sites resulted in HSD that was independent of initial synaptic strength. This conclusion that HSD is a release-independent phenomenon was supported by empirical results: an increase in Ca2+ influx caused by spike broadening with a K+ channel blocker did not alter HSD. Once induced, HSD persisted during 40 min of rest with no detectable recovery; thus, release does not recover automatically with rest, contrary to what would be expected if HSD represented an exhaustion of the exocytosis mechanism. The hypothesis that short-term HSD involves primarily a stepwise silencing of release sites, rather than vesicle depletion, is consistent with our earlier observation that HSD is accompanied by only a modest decrease in release probability, as indicated by little change in the paired-pulse ratio. In contrast, we found that there was a dramatic decrease in the paired-pulse ratio during serotonin-induced facilitation; this suggests that heterosynaptic facilitation primarily involves an increase in release probability, rather than a change in the number of functional release sites.
Keywords: synaptic depression, vesicle depletion, univesicular release, computer simulations, silencing of release sites, serotonin-induced facilitation
Homosynaptic depression (HSD) is a form of synaptic plasticity that plays a role in a number of behaviorally important neural processes, including habituation (Cohen et al., 1997), discrimination of novel stimuli, and balancing of multiple synaptic inputs that have diverse tonic firing rates (Abbott et al., 1997). Although HSD is an extremely widespread form of synaptic plasticity, the underlying mechanisms have rarely been demonstrated.
Early analyses at the neuromuscular junction suggested that HSD represents exhaustion after repeated activation, perhaps involving depletion of releasable vesicles (Elmqvist and Quastel, 1965; Thies, 1965; Betz, 1970). Other presynaptic changes contribute to short-term HSD, including spike propagation failure (Hatt and Smith, 1976; Brody and Yue, 2000), calcium channel inactivation (Patil et al., 1998), and inhibition of excitation-secretion coupling via presynaptic autoreceptors (Redman and Silinsky, 1994). Alternatively, HSD may be mediated by changes in postsynaptic receptors (Trussell et al., 1993;Narasimhan and Linden, 1996; Kandler et al., 1998; Carroll et al., 1999). Although the most commonly accepted explanation for short-term synaptic depression is still depletion of the readily releasable pool of vesicles, there have been few studies that have critically evaluated the contribution of depletion (Zucker and Bruner, 1977; von Gersdorff et al., 1997; Faber, 1998).
HSD at the synaptic connections of the mechanosensory neurons (SN) inAplysia that trigger the gill, siphon, and tail defensive withdrawal reflexes contributes importantly to some forms of behavioral habituation (Cohen et al., 1997). HSD at these synapses is unusual in that it is effectively induced with small numbers of stimuli at long interstimulus intervals (ISIs) (e.g., 5 min) (Byrne, 1982; Eliot et al., 1994; Armitage and Siegelbaum, 1998). Several lines of evidence indicate that depression at these synapses is a purely presynaptic phenomenon: (1) accompanying HSD, there is a decrease in quantal content (Castellucci and Kandel, 1974). (2) During HSD, there is no detectable change in postsynaptic sensitivity to the putative SN transmitter glutamate. (3) HSD is unaffected by application of a glutamate antagonist to reversibly block postsynaptic receptors during induction (Armitage and Siegelbaum, 1998).
What presynaptic change is responsible for HSD at Aplysia SN synapses? One class of mechanism is a decrease in the probability of release. During HSD there is not a decrease in Ca2+ influx through those channels that mediate release (Armitage and Siegelbaum, 1998). However, there are numerous possible changes in exocytosis-related proteins that could result in decreased release probability (Hsu et al., 1996). Depletion of the readily releasable pool of vesicles (Byrne, 1982) has been widely believed to account for HSD at these synapses because quantitative computational models suggested that this is a plausible mechanism (Gingrich and Byrne, 1985). However, the depletion model has not yet been tested with HSD produced by brief trains of stimuli.
We began this computational study because of two key observations we recently made about HSD in Aplysia SNs that together have important implications for our understanding of this plasticity. First, we observed that paired-pulse facilitation is inversely related to initial synaptic strength, with strong synapses showing little, if any, paired-pulse facilitation (Jiang and Abrams, 1998). This difference in paired-pulse ratios was maintained after synaptic depression. Thus, strong and weak synapses must differ in the properties of individual release sites, rather than simply differing in the number of release sites. The failure of strong synapses to display paired-pulse facilitation suggests that these synapses have a high probability of release during the first paired spike (Stevens and Wang, 1995; Debanne et al., 1996; Murthy et al., 1997). Our second key observation was that, despite this difference in release properties, strong and weak synapses underwent HSD at the same rate (Jiang and Abrams, 1998). As became apparent through our exploration of a wide variety of mechanisms of HSD in simulations, these two observations greatly constrain those mechanisms that can account for HSD at these synapses.
To evaluate possible mechanisms contributing to HSD, we developed Monte Carlo simulations that incorporated a key aspect of CNS synaptic physiology: the stochastic nature of univesicular exocytosis. At most of the several CNS synapses that have been analyzed, individual release sites release either zero or one vesicle during each spike (see Discussion). However, most previous models of HSD represented the entire synaptic connection between a presynaptic and postsynaptic neuron as a single large terminal that releases multiple quanta, ignoring the stochastic and univesicular aspects of release (Gingrich and Byrne, 1985; Yamada and Zucker, 1992; Melkonian, 1993; Canepari and Cherubini, 1998). These two types of models, one large terminal with multivesicular release and multiple terminals with univesicular release, have very different consequences for plasticity because they differ fundamentally in the kinetics of vesicle depletion (Faber, 1998;Matveev and Wang, 2000).
In addition to examining models where multivesicular release was restricted, we asked what synaptic properties would be required if vesicle depletion resulting from unrestricted multivesicular release were to account for HSD in this system. We found that this is an unsatisfactory explanation for HSD at these synapses because of the implausibly high release probabilities that would be required.
MATERIALS AND METHODS
Electrophysiology. Aplysia californica, weighing 70–200 gm (obtained from Alacrity or Marinus, Inc.) were anesthetized by injection with isotonic MgCl2. Abdominal ganglia were removed and the ventral surface of the left hemiganglion was desheathed in a 1:1 mixture of MgCl2 and artificial seawater. Ganglia were superfused at room temperature with high divalent saline (6× normal Ca2+; 1.6× normal Mg2+) (Goldsmith and Abrams, 1991) to reduce spontaneous synaptic activity: (in mm): 328 NaCl, 10 KCl, 66 CaCl2, 88 MgCl2, and 10 Na-HEPES, pH 7.6, supplemented with nutrients [7 mm glucose, MEM essential and nonessential amino acids (0.2× normal concentration; Invitrogen, Carlsbad, CA), and MEM vitamin solution (0.7× normal concentration; Invitrogen)]. This high-divalent saline does not alter synaptic strength (Jiang and Abrams, 1998). 4-aminopyridine (4-AP) and 3,4-diaminopyridine (3,4-DAP) (both from Sigma, St. Louis, MO) were dissolved in saline at their final concentrations. Siphon SNs and LFS motoneurons (MNs) were penetrated with single 10–20 MΩ microelectrodes filled with either 2 m KCl or 2m K-acetate and 0.4 m KCl. During penetration, 0.5–1.0 nA hyperpolarizing current was injected to prevent SN firing. SN action potentials were elicited by injection of 2 msec depolarizing current pulses. The membrane potential of postsynaptic MNs was hyperpolarized at 50 or 60 mV below the resting potential to prevent action potentials. After a synaptic connection was identified, the synapse was rested for a minimum of 10 min before beginning an experimental protocol. During experiments on synaptic depression, action potentials in SNs were elicited at a 15 sec interval. Data were acquired digitally and analyzed using Spike software (Hilal Associates). In paired-pulse measurements, each synapse was activated only once with paired stimulation (at a 50 msec interval) because at these synapses paired-pulse facilitation is extremely labile (Jiang and Abrams, 1998).
Computer simulations. The computational models were representations of a synaptic connection between a single siphon SN and a MN, consisting of 40 release sites. Based on the morphological studies of Bailey and Chen (1983), who found that presynaptic varicosities did not contain multiple release sites (active zones), each release site was functionally independent. Electron microscopic measurements have determined the number of vesicles immediately adjacent to the presynaptic active zones to be ∼15 vesicles in nondepressed SN synapses (Bailey and Chen, 1988); this value represents an upper estimate of the size of the releasable pool, because not all vesicles near the presynaptic membrane may be releasable. In these simulations, initial numbers of readily releasable vesicles per release site were varied from 2 to 20. The simulated HSD protocol consisted of a series of 15 action potentials with a 15 sec ISI. Each action potential produced Ca2+ transients that were equal in magnitude for all release sites. Because Ca2+ imaging analysis demonstrated that Ca2+ transients do not change during HSD (Armitage and Siegelbaum, 1998), we used a simulated transient that was a constant amplitude square pulse, 1 msec in duration. The Ca2+ concentration at release sites increases extremely rapidly in microdomains adjacent to the mouths of open Ca2+ channels, reaching concentrations of a few hundred micromolar, and then falls rapidly once the channels close (Fogelson and Zucker, 1985; Adler et al., 1991;Roberts et al., 1991; Llinas et al., 1992). Therefore in these simulations, we used a square pulse of Ca2+ that reached a concentration of 200 μm. The sampling interval (δt) for each simulation was always a fraction of the duration of the Ca2+ rise; δt was either 0.01, 0.1, or 0.25 msec. Release probability of each site during a single simulation interval (Psite,δt) was calculated as a function of the number of releasable vesicles (n), the probability of release of an individual vesicle (Pves), and the concentration of intracellular Ca2+([Ca2+]i), according to the equation:
| Equation 1 |
Pves,Ca the probability of individual vesicles being released as a function of [Ca2+]i was calculated from the following equation based on the Ca2+ dependence of exocytosis as determined by Heidelberger et al. (1994) as the product ofPves, the maximum possible per vesicle probability, and a fraction that depends on [Ca2+]i:
| Equation 2 |
where Kd1 = 143 μm, Kd2 = 57.2 μm, Kd3 = 22.9 μm, and Kd4 = 9.2 μm (Heidelberger et al., 1994).
Whereas Pves does not vary over time,Pves,Ca is a continuously changing function of free [Ca2+]i. Because of the near saturating intracellular Ca2+concentration during the Ca2+ transient, this fraction equaled 0.95 during the period of elevated [Ca2+]i.
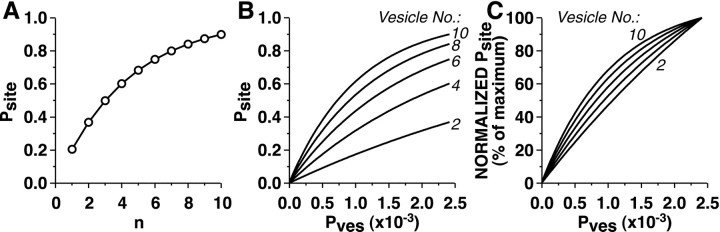
During each sampling interval, for each release site,Psite,δt was compared with a randomly generated number between 0 and 1; a vesicle release event was generated when Psite,δt was greater than the random value. Psite(equivalent to Pr) is the probability that a release event will occur at a single release site during the entire 1 msec Ca2+ transient. For each set of initial synaptic parameters and for the δt used in the simulation,Pves was selected so that Psite was 0.25, 0.75, or 0.9 with one specific number of releasable vesicles (n; usually n= 6, 10, or 20) (Fig. 1). (ThePves values selected depended on the sampling interval, δt; to produce a givenPsite, lower values ofPves were required when δt was smaller). The vesicle number, n, was then varied; the range of initial n values that were used gave initial values forPsite ranging from 0.05 to 0.9.
Fig. 1.
Dependence of release site probability (Psite) on the number of releasable vesicles and the probability of release of individual vesicles.A,Psite as a function of the number of readily releasable vesicles (n). In this graph, the probability of release of individual vesicles (Pves) was selected to achieve aPsite of 0.9 with 10 releasable vesicles.B,Psite as a function ofPves for different values ofn. C, NormalizedPsite as a function ofPves for different values ofn. Normalized Psite equals 100 times Psite divided by the maximumPsite for each curve (Psite is at a maximum in these curves whenPves equals 0.0024). In bothB and C, n = 2, 4, 6, 8, and 10. Note that the curves for different values ofn are not parallel. In A, the nonlinear curve indicates why, as n decreases with depletion, synapses with relatively large releasable pools are less affected than synapses with relatively small releasable pools. InB and C, the nonparallel curves illustrate why, as Pves decrements (by a given percentage) through repeated synaptic activation, there is a smaller impact on Psite whenPves or n is initially large than when Pves or n is initially small. [The relationship betweenPsite and Pvesdepends on the sampling interval (δt) becausePves is the per vesicle release probability for a single sampling interval; however, the qualitative relationship is independent of δt; in these curves, δt = 0.01 msec].
After a release event occurred at a given site, depending on the model, subsequent release was altered in one of three possible alternative ways: (1) for univesicular release models, no further release events were permitted until the next stimulus. (2) For limited multivesicular release models, the probability of subsequent releases for a site was reduced to a fraction of the normalPsite,δt by multiplyingPsite,δt by a refractoriness factor; this refractoriness factor was 0.33 immediately after an exocytosis event, and it recovered with a time constant of 3 msec. (In addition,Psite decreased because it was a function of the number of docked vesicles that remained after previous exocytosis events.) (3) For unlimited multivesicular release models, the site was capable of additional release events with no restriction (Psite was reduced only because of the reduced size of the releasable pool as a result of depletion).
In these simulations, we compared HSD at strong and weak synapses. Differences in initial synaptic strength were achieved by varying either the number of releasable vesicles (n) or the per vesicle release probability (Pves). (All synaptic connections initially consisted of 40 functionally active release sites.) In each set of simulations, the initial values for either n or Pves were selected to produce an ∼2.8-fold difference inPsite between strong and weak synapses [based on the differences in synaptic strength of the strong and weak synapses for which Jiang and Abrams (1998) characterized paired-pulse ratios].
HSD was produced by either depletion of vesicles, decrement ofPves, or reduction in the number of functionally active release sites (Nsite). In the case of depletion, reduction in the releasable pool was a direct consequence of the release event itself. In the case of decrement ofPves or inactivation of release sites, the synaptic parameter decreased as an exponential function of either the release events at an individual site or the action potentials (independent of release). To obtain these exponential functions, empirical HSD data for the combined populations of strong and weak SN synapses were first fit by a single “empirical” exponential. These computational studies were initiated before the empirical experiments on HSD in this study were completed; therefore, exponentials for simulations were fit to the published data of Jiang and Abrams (1998). The exponential for the average empirical data had a τ of 24 sec (with a 15 sec ISI) and reached an asymptote of 34%. An exponential function that determined the decrement of the synaptic parameter (Nsite orPves) was then developed by successive iterations until the final amount of depression and the rate of depression observed in pilot simulations approximately fit the empirical exponential. In developing these exponential equations, the parameter for the model release sites that varied between strong and weak synapses (n or Pves) was changed to an intermediate value to produce a intermediate strength synapse. These fit exponential parameters were then used in simulations in which synaptic strength was either initially strong or weak.
All simulations were created and run with Stella Research software (High Performance Systems, Inc., Hanover, NH) on either a Macintosh G3 or a Pentium II computer. Because of the stochastic nature of these Monte Carlo simulations, for each model tested, simulations of the series of 15 action potentials were repeated 80 times, and the results were averaged.
Statistical analysis. In both empirical and simulation experiments, the time course of HSD was compared using a repeated measures ANOVA; data were first normalized to the amplitude of the initial EPSP; the analyses were conducted on both arc sine transformed data, and on nontransformed data; because the transformation did not alter the conclusion of these statistical tests, the results presented are from the nontransformed data. Differences with a p< 0.05 were considered to be significant. Effects of serotonin [5-hydroxytryptamine (5-HT)] were analyzed with a paired ttest.
RESULTS
HSD occurs independently of initial synaptic strength
The observation of Jiang and Abrams (1998) that the average rates of HSD were similar among SN-to-MN connections, despite large differences in initial synaptic strength, appeared to restrict which mechanisms could account for HSD. We replicated this analysis on a second set of synapses between SNs and LFS MNs, grouping depression data according to whether the initial strength of connections was <8 or >8 mV. The results were identical to those of our previous study: HSD developed independent of synaptic strength (Fig.2A,B). The primary question that we addressed in the subsequent simulation studies was which mechanisms of HSD would produce a time course of synaptic decrement that was independent of initial synaptic strength. In these simulation studies, we considered three alternative classes of mechanisms of HSD: (1) a decrease in the probability of release of individual vesicles, (2) a decrease in the number of readily releasable vesicles (“vesicle depletion”), and (3) a decrease in the number of functional release sites.
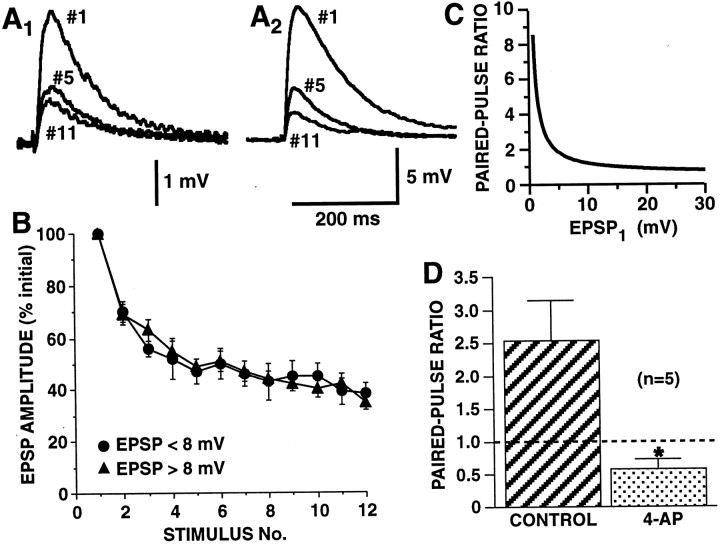
Fig. 2.
Strong and weak SN-to-MN synaptic connections undergo HSD with an identical time course, but differ in their paired-pulse ratios. A, B, Synapses were depressed by stimulating siphon SNs in the abdominal ganglion to fire single action potentials at a 15 sec interstimulus interval (ISI).A, Examples of HSD at a weak synapse (A1, initial amplitude = 3.2 mV) and a strong synapse (A2, initial amplitude = 12.5 mV). EPSPs are shown for the first, fifth, and eleventh stimuli. B, Group data on HSD for strong and weak synapses. Synaptic connections are grouped according to initial EPSP amplitudes as either strong (>8 mV) or weak (<8 mV) (mean EPSP on trial 1, 22.3 ± 2.1,n = 25, for strong synapses and 5.7 ± 0.3 mV,n = 10, for weak synapses). There was no significant difference in the time course of HSD between the two groups of synapses (repeated measure ANOVA testing interaction between initial EPSP amplitude and trial number,F(9,24) = 1.08, p = 0.42). In each experiment, EPSP amplitude is normalized to the amplitude of the EPSP on trial 1. Mean amplitude of all the EPSPs on trial 12 was 36 ± 3% of the initial amplitude. C,The inverse relationship between paired-pulse ratio and initial EPSP amplitude. Curve is hyperbolic function from Jiang and Abrams (1998), which was fit to empirical paired-pulse ratios for nondepressed SN synapses. Note, that, in contrast to initially weak synapses, initially strong synapses show relatively little paired-pulse facilitation, suggesting that these stronger synapses have higher release site probabilities. D, At weak synapses, increasing Ca2+ influx by broadening the SN spike eliminates paired-pulse facilitation. For control and 4-AP-treated synapses, after a weak synaptic connection was identified, ganglia were superfused with high divalent saline, with or without 2 mm 4-AP, for 15 min, and then tested with paired-pulse stimulation. With 4-AP, SN action potentials broadened 2.9 ± 0.23-fold, and initially weak connections (<8 mV EPSPs) increased 2.08 ± 0.31-fold (to >8 mV), and displayed no paired-pulse facilitation; the paired-pulse ratio was significantly different between the two groups (∗p < 0.02). Mean EPSP1 = 4.78 ± 2.17 mV for controls and 10.64 ± 1.53 mV for 4-AP, respectively. C,D, Each synapse was tested with paired-pulse stimulation (ISI, 50 msec) only once because of lability of paired-pulse facilitation at these synapses; thus, paired-pulse ratios for control and 4-AP were from different synapses in the same ganglia (n represents the number of ganglia). (For synapses treated with 4-AP, the strength of the synapse was measured with a single spike before the application of 4-AP; once 4-AP was applied, the synapses were not activated before the paired-pulse test.)
One change that would produce a decrease in the probability of release of individual vesicles is Ca2+ channel inactivation. However, Armitage and Siegelbaum (1998) found that during HSD, the Ca2+ influx through those channels that mediate transmitter release from SNs does not decrease. Therefore, to alter release probability, we variedPves, which represents the release probability of individual vesicles and is dependent on the state of the proteins that mediate release before Ca2+influx (see Materials and Methods).
Properties that determine initial strength of model synaptic connections
There are several possible synaptic properties that could explain the difference in initial synaptic strength between strong and weak synapses. The possibility that strong synapses differ from weak synapses simply by having a greater number of synaptic contacts is inconsistent with the observation of Jiang and Abrams (1998) that strong and weak synapses differ substantially in the release properties of individual sites. They found that paired-pulse facilitation was inversely related to initial synaptic strength (see summary of relationship in Fig. 2C). In the present study, we confirmed the finding that stronger synapses (with EPSP amplitudes initially greater than ∼8 mV) usually showed no paired-pulse facilitation, whereas weaker SN synapses showed reliable paired-pulse facilitation. An inverse relationship between paired-pulse ratios and synaptic strength is likely to be a consequence of the initial probabilities of release at strong synapses being relatively high; ifPsite is already high, there can be little increase in the release probability during the second paired spike (Murthy et al., 1997). However, additional factors other than presynaptic release can also cause the paired-pulse ratio to vary inversely with synaptic strength (Kim and Alger, 2001). To verify that at Aplysia SN-to-MN synapses, the paired-pulse ratio reflects release probability, we tested the effect on initially weak synapses of increasing transmitter release. This increase in transmitter release was achieved by increasing Ca2+ influx with 2 mm 4-AP, which prolongs the presynaptic action potential. Spike broadening produced a 2.08 ± 0.31-fold increase in EPSP amplitude, which was accompanied by a decrease in the paired-pulse ratio from 2.54 ± 0.60 in control synapses to 0.58 ± 0.14 in 4-AP-treated synapses (p < 0.02; two tailed t test) (Fig. 2D). This observation confirms the inverse relationship between paired-pulse facilitation and release probability, consistent with the conclusion that initially strong synapses, which lack paired-pulse facilitation, have high release probabilities. This enhanced release probability at strong synapses must result from an increase in either of two synaptic properties: the number of readily releasable vesicles (n) or the initial probability of exocytosis for individual vesicles (Pves). Therefore for each mechanism of HSD that we explored, we developed two types of models. In one type, strong and weak synapses differed in the initial number of readily releasable vesicles at each release site. In the other type, strong and weak synapses differed in Pves. The initial difference in either vesicle number orPves between simulated strong and weak synapses was adjusted so that initial differences in simulated synaptic strength approximated the empirical 2.8-fold difference between the strong and weak groups of synapses studied by Jiang and Abrams (1998).
Vesicle depletion as a mechanism of HSD
In this class of model, we explored the possibility that HSD was a consequence of depletion of the readily releasable pool of vesicles. At most of the CNS synapses that have been analyzed, individual morphological release sites (i.e., active zones) have been found to release either zero or one vesicle during each action potential (Korn et al., 1982; Redman, 1990; Wojtowicz et al., 1994); some other central synapses release a few vesicles, although only during a minority of spikes (Auger et al., 1998). These studies suggest that a mechanism exists to reduce the likelihood that multiple vesicles are released at a single site. We therefore included a restriction in most models, so that once one vesicle fused at a release site, the site became temporarily refractory to further release for several milliseconds. In univesicular release models, this refractoriness was absolute; during an action potential, vesicle fusion was restricted to a single event per release site. In limited multivesicular release models, after an initial fusion event, additional vesicle fusions were permitted, but with a transient reduction in release probability.
To model limited multivesicular release, in which the refractoriness was partial, we used an estimate from cerebellar synapses of >27% for the number of release events in which two quanta were released, instead of one (Auger et al., 1998). In these limited multivesicular release models, immediately after a vesicle fusion event, the calculated total probability for the release site (Psite,δt) was reduced by a refractoriness factor (0.66; i.e., to 33% of the normalPsite,δt in the absence of refractoriness), and this partial refractoriness recovered with a time constant of 3 msec. [This time constant was somewhat shorter than the duration of the very transient refractoriness after a vesicle fusion at hippocampal synapses (Stevens and Wang, 1995; Dobrunz et al., 1997); we accelerated the recovery from refractoriness to further favor additional exocytotic events.]
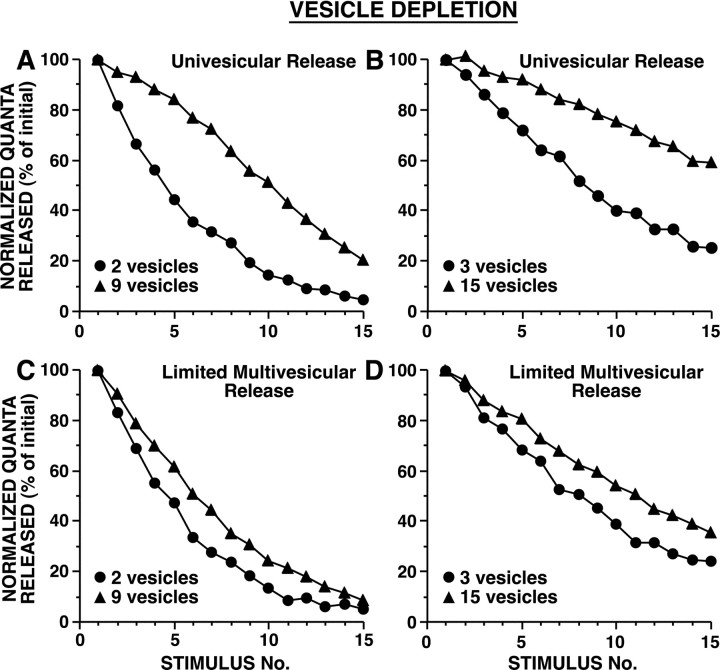
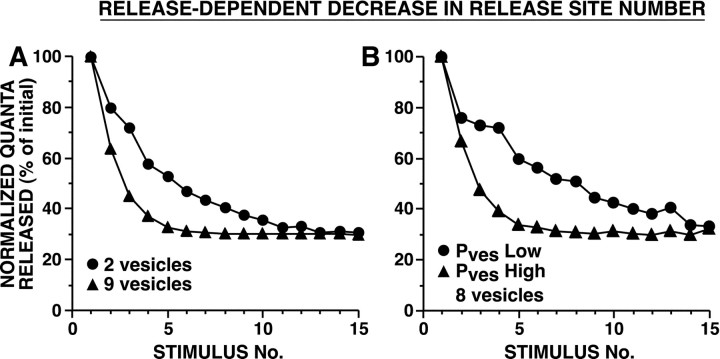
In vesicle depletion models in which strong and weak synapses differed in their initial number of readily releasable vesicles, strong synapses depressed more gradually (Fig. 3). This is because, with release of a given number of vesicles, release sites with a larger pool of readily releasable vesicles underwent smaller proportional changes in n, resulting in relatively smaller changes in Psite, than release sites with fewer releasable vesicles (Fig. 1A). Release sites with a larger n have a higher probability of releasing a vesicle during each action potential, which favors depletion; nevertheless, this higher frequency of release is not sufficient to outweigh the effect of depletion of a given number of vesicles having a more modest impact on Psite when there is a larger releasable pool. In vesicle depletion models in which strong and weak synapses differed inPves, synapses with higher probabilities of release underwent substantially more rapid vesicle depletion (Fig. 4). These differences in the time course of HSD for synapses of different strengths were observed whether there was univesicular or limited multivesicular release (Figs. 3, 4). Although limited multivesicular release increased total vesicle release by ∼30%, there was only a modest effect on the time course of HSD.
Fig. 3.
Simulated HSD as a result of vesicle depletion when strong and weak synapses differ in their initial number of readily releasable vesicles. During each simulation,Pves and Nsiteremained constant. A, B, Univesicular release, in which after an initial vesicle release event at a release site, further release was blocked for 5 msec. C,D, Limited multivesicular release, in which after an initial release event, Psite, δt was reduced by a factor of 0.66 and then recovered exponentially with a time constant of 3 msec.Pves was selected to achieve aPsite of 0.9 with 10 releasable vesicles (A) or with 20 releasable vesicles (B); in C and D,Pves values were the same as inA and B, respectively. Average initial number of quanta released were: for A, 14.8 for 2 vesicles and 35.0 for 9 vesicles; for B, 11.4 for 3 vesicles and 32.6 for 15 vesicles; for C, 15.1 for 2 vesicles and 51.5 for 9 vesicles; and for D, 12.0 for 3 vesicles and 45.9 for 15 vesicles. In other simulation experiments,Pves values were twofold and fourfold smaller; with these lower Pves values, strong and weak synapses still depressed at different rates, although with the smallest Pves values tested, HSD was extremely modest because minimal depletion occurred. Note that strong synapses underwent HSD at a slower rate than weak synapses [repeated measure ANOVA testing interaction between vesicle number and trial number: (A)F(14,145) = 139, p< 0.001; (B)F(14,145) = 68, p< 0.001; (C)F(14,145) = 26, p< 0.001; and (D)F(14,145) = 9.5, p< 0.001]. Sampling interval was 0.01 msec.
Fig. 4.
Simulated HSD as a result of vesicle depletion when strong and weak synapses differ in the release probability of individual vesicles. During each simulation,Pves and Nsiteremained constant. A, Univesicular release.B, Limited multivesicular release (as in Fig.3C,D). Pves inA was selected to achieve aPsite of 0.25 and 0.75 forPves Low and PvesHigh, respectively, with six releasable vesicles; the samePves values were used in B. Average initial number of quanta released were: for A, 11.0 for Pves Low and 29.7 forPves High; and for B, 11.6 for Pves Low and 37.6 forPves High. Strong synapses underwent HSD at a faster rate than weak synapses [repeated measure ANOVA testing interaction between vesicle number and trial number: (A) F(14,145) = 43, p < 0.001; (B)F(14,145) = 54, p< 0.001]. Sampling interval was 0.01 msec.
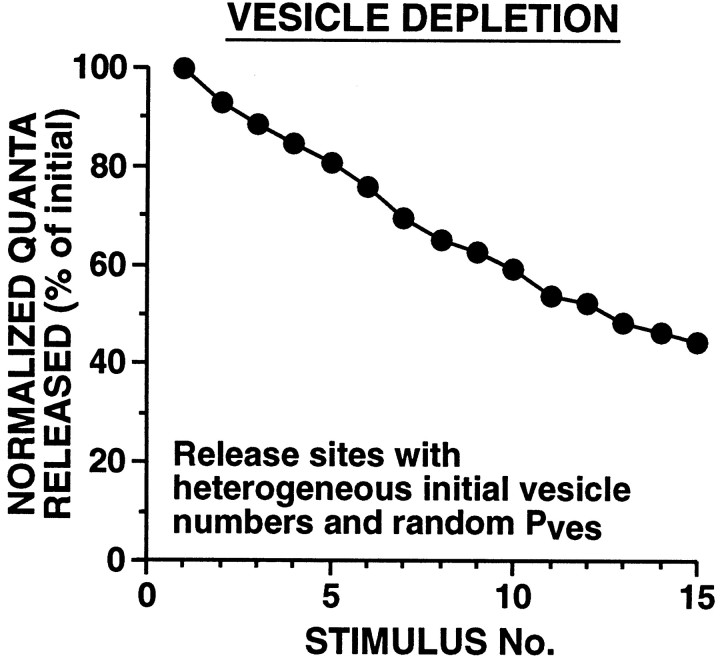
Because only one, or occasionally a few, vesicles were released at each active zone per presynaptic spike, the overall kinetics of HSD resulting from vesicle depletion tended to be linear (until the releasable pool was nearly depleted) (Figs. 3, 4). This was in striking contrast to actual SN synapses where HSD occurs exponentially (Fig. 2). One possibility is that the linear time course of HSD observed with vesicle depletion models was a consequence of the homogeneity of initial parameters among release sites. To test this possibility, we created several models with heterogeneous release sites where these parameters varied widely among sites. In some of these models, a series of initial values for vesicle number and Pveswere distributed in a regular manner among release sites. In another heterogeneous parameter model, each release site had one of four possible initial n values, andPves values were assigned randomly to each release site (Fig. 5). In all of these heterogeneous release site models, the profile of HSD was initially linear, in contrast to the exponential decay of the SN EPSP observed experimentally.
Fig. 5.
Simulated HSD at synapses with heterogeneous release site properties. To assess whether the linear time course of HSD observed with vesicle depletion models was a consequence of the specific parameters chosen, we created models where these parameters varied widely among active zones. During each simulation,Pves and Nsiteremained constant. Each of 10 release sites (of 40 total) had 3, 8, 12, or 20 vesicles initially. Pves was randomly assigned to each release site at the beginning of each simulation;Pves varied within a threefold range up to a maximum value that produced a Psite of 0.9 with nine releasable vesicles. With this and all other vesicle depletion models tested, HSD developed with a nonexponential time course. Sampling interval was 0.25 msec.
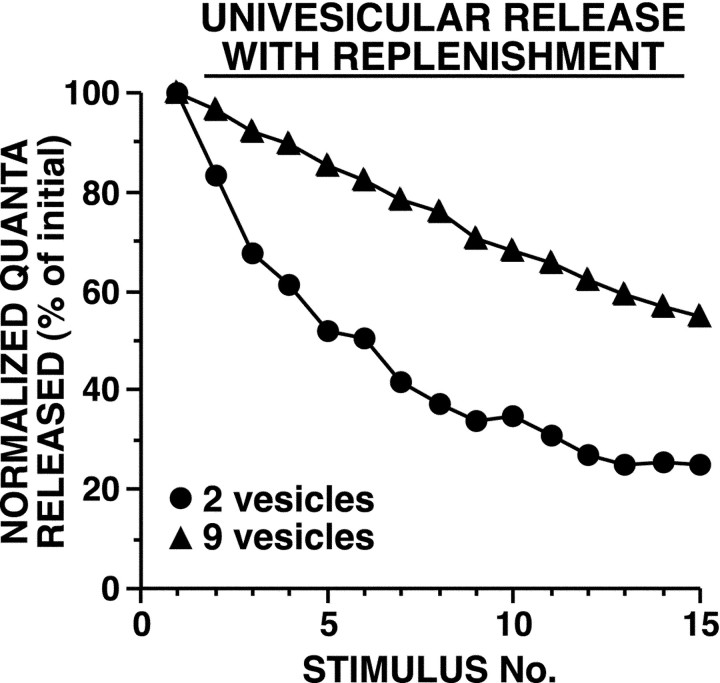
In some vesicle depletion models, a mechanism for replenishing the readily releasable pool was included. We used several approaches to estimate a time constant for recovery from HSD; the shortest time constant, ∼150 sec, came from analyzing the increase in depressed EPSPs after a rest of 100 sec (see below). Adding replenishment at this rate to the model had minimal effect on the development of HSD or its dependence on initial synaptic strength (Fig.6). This is because within the period in which the first six stimuli occurred, which equaled only half of the time constant for recovery, near maximal HSD had already developed.
Fig. 6.
Simulated HSD as a result of vesicle depletion with replenishment, when strong and weak synapses differ in their initial number of readily releasable vesicles. Vesicle replenishment occurred with a time constant of 150 sec [this time constant was calculated based on the apparent “recovery” of the depressed EPSP by ∼48% after 100 sec of rest (Fig. 13C)]. During each simulation, Pves andNsite remained constant.Pves was selected to achieve aPsite of 0.9 with 10 releasable vesicles; release was univesicular. Average initial number of quanta released were 15.2 for two vesicles and 35.4 for nine vesicles. Strong synapses underwent HSD at a slower rate than weak synapses [repeated measure ANOVA testing interaction between vesicle number and trial number:F(14,145) = 129, p< 0.001]. Sampling interval was 0.01 msec.
With unrestricted multivesicular release, vesicle depletion can produce strength-independent HSD, but only if there are very high probabilities of release
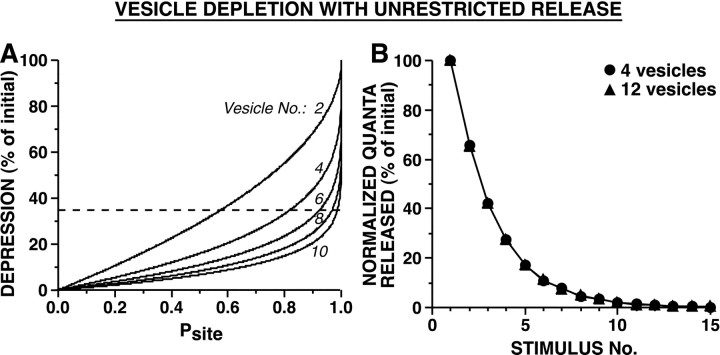
These depletion models involved either univesicular or limited multivesicular release because evidence suggests that at central synapses, after release of a single vesicle, the release site is refractory for some milliseconds (see introductory remarks and Discussion). We also asked whether depletion could account for the observed depression at these synapses if there were no refractoriness after an initial vesicle fused. Unrestricted multivesicular release, in which release of each vesicle is a completely independent event, resulted in HSD that was independent of the initial size of the releasable pool (when Pves was constant) (Fig. 7B). In contrast, when strong and weak synapses differed inPves, the synapses with the smallerPves underwent HSD more gradually (synapses had the same number of readily releasable vesicles; data not shown). We then determined what release probabilities would be necessary if a depletion mechanism were responsible for the substantial HSD after the first spike. We adjustedPves to achieve an average simulated synaptic decrement of 35% after the first stimulus, as observed byJiang and Abrams (1998). With a very small releasable pool of four vesicles, an average Psite of 82% was required to produce 35% reduction in release after a single stimulus; higher Psite values were required with larger releasable pools (Fig. 7A). Thus, even if release sites exhibited unrestricted multivesicular release (as in the model of Fig. 7B), vesicle depletion could account for the initial dramatic HSD only if release site probabilities were atypically high. At these SN synapses, Psite must frequently be <0.5 because paired-pulse ratios are frequently >2 (Fig. 2D).
Fig. 7.
Simulated HSD as a result of vesicle depletion with unrestricted multivesicular release when strong and weak synapses differ in the initial number of readily releasable vesicles. In these models, release of each vesicle is an independent event, so that subsequent release at a release site is unaffected by previous vesicle release events. A, Relationship between amount of depression after the first stimulus andPsite for release sites with different numbers of releasable vesicles (n = 2, 4, 6, 8, and 10). Broken line corresponds to 35% HSD, the approximate amount of depression observed by Jiang and Abrams (1998)after the first stimulus, to indicate thePsite required, for a givenn, to achieve this amount of HSD. Note that to produce depression of 35% with the first stimulus,Psite must be 0.82 for release sites with 4 vesicles and >0.9 for release sites with ≥6 vesicles.B, Strong and weak synapses undergo HSD at identical rates when there is unrestricted multivesicular release.Pves was selected so that the first stimulus released 35% of the releasable pool, producing an initial HSD of 35% (with unrestricted release, althoughPsite varies as a function ofn, for a given Pves the percentage of vesicles released is constant).Psite was 0.82 and 0.99 with 4 and 12 releasable vesicles, respectively. Average initial number of quanta released were: 56.1 for 4 vesicles and 168.3 for 12 vesicles. There was no significant difference in the time course of HSD between strong and weak synapses (repeated measure ANOVA testing interaction between vesicle number and trial number,F(14,145) = 0.62, p= 0.84). Sampling interval was 0.01 msec.
Decrease in the probability of release of individual vesicles (Pves) as a mechanism of HSD
We next explored two classes of models in which the probability of release of individual vesicles (Pves) decreased. In the vesicle depletion models described above, HSD resulted from reduction in the releasable pool, which was a direct consequence of the release event itself; in contrast, in the other HSD models, one synaptic parameter (eitherPves orNsite) was decremented during repetitive stimulation according to an exponential equation. In the case of decreases in Pves, in one class of model, the decrement of Pvesoccurred with each release event; in another class of model,Pves decreased with each stimulus. To produce HSD that resembled the empirical exponential, in adjusting the exponential parameters that determined the decrease inPves, we used a simulated synaptic strength intermediate between that of the strong and weak synapses.
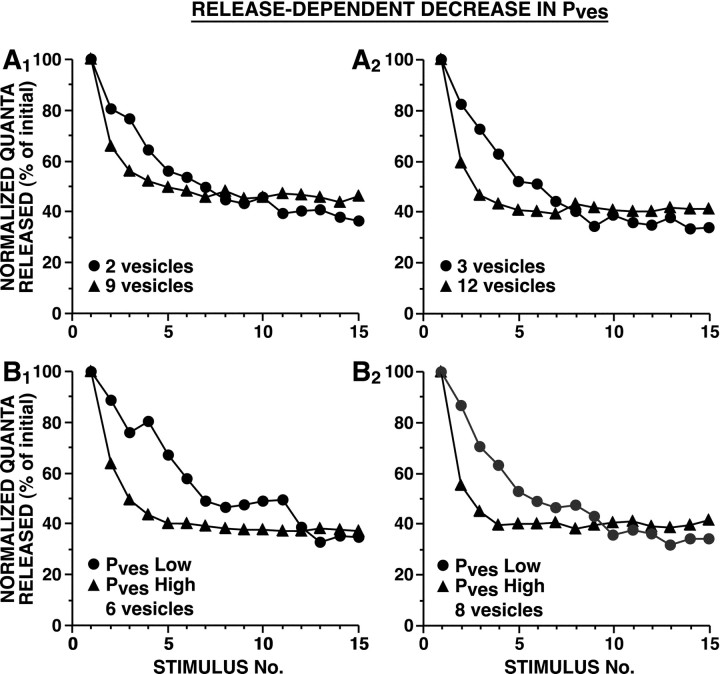
In the case of release-dependent decrement inPves, strong and weak synapses underwent HSD with substantially different time courses. Both for models in which synaptic strength was determined by differences in the number of releasable vesicles (Fig.8A) and for models in which strength was determined by differences in initialPves (Fig. 8B), HSD developed more rapidly at stronger synapses. Stronger synapses, which had a greater Psite, underwent more rapid depression because the rate of change inPves depended on the frequency of vesicle release. The final plateau reached tended to be slightly lower for weak synapses; this is because for a given percentage decrease inPves,Psite decreases more steeply when initial total release probability is small (Fig.1B,C).
Fig. 8.
Simulated HSD as a result of release-dependent decrement of Pves. In these two models, Pves at each release site decremented exponentially each time there was a release event at that site. During each simulation, n andNsite remained constant. InA, strong and weak synapses differed in the number of releasable vesicles. In B, strong and weak synapses differed in the initial Pves. InA, Pves was selected to achieve a Psite of 0.9 with 10 (A1) or 20 (A2) releasable vesicles. InB, Pves was selected to achieve a Psite of 0.25 and 0.75 forPves Low and Pves High, respectively, with six (B1) or eight (B2) releasable vesicles; in this figure and Figures 9-11, we chose the exponential parameters for an intermediate strength synapse to approximately match the empirical HSD; nevertheless, the simulated HSD curves for strong and weak synapses differed from one another, and from the expected exponential because the decrement inPves occurred as a function of synaptic strength. [Because these studies were initiated before the analysis of the data shown in Figure 1, all simulations were fit to the earlier published data of Jiang and Abrams (1998).] Average initial number of quanta released were: for A1, 12.4 for 2 vesicles and 35.0 for 9 vesicles; for A2, 9.6 for 3 vesicles and 27.5 for 12 vesicles; for B1, 6.6 forPves Low and 22.7 forPves High; and for B2, 10.0 for Pves Low and 30.3 forPves High. The time course of HSD was significantly different between strong and weak synapses [repeated measure ANOVA testing interaction between vesicle number and trial number: (A1) F(14,145) = 16, p < 0.001; (A2)F(14,145) = 13, p< 0.001; repeated measure ANOVA testing interaction betweenPves and trial number: (B1)F(14,145) = 11, p< 0.001; and (B2)F(14,145) = 16, p< 0.001]. Sampling interval was 0.25 msec.
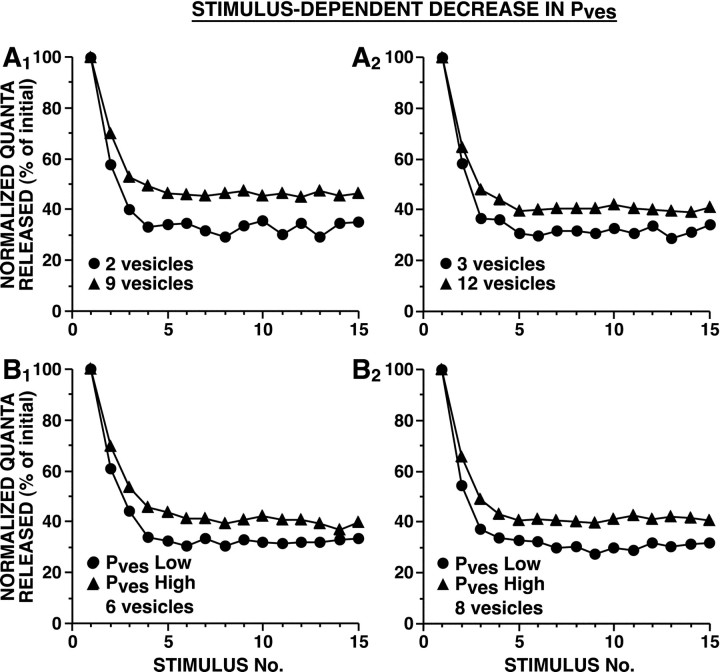
In the case of stimulus-dependent decrement inPves, weak synapses depressed more rapidly and to a lower plateau, whether initial synaptic strength was determined by differences in Pves or in the size of the releasable pool of vesicles (Fig.9). When strong and weak synapses differ in vesicle number, the shape of the curves forPsite as a function ofPves varies with the number of docked vesicles. As Pves decreases, synapses with a larger number of docked vesicles (and a largerPsite) experience a relatively smaller decrease in Psite than synapses with fewer docked vesicles (Fig. 1B,C). When strong and weak synapses differ in their initialPves, the relative change in total probability depends on the initialPves caused by the nonlinear shape of the Psite versusPves relationship; therefore depression is greater for synapses with a lower initialPves (Fig.1B,C).
Fig. 9.
Simulated HSD as a result of stimulus-dependent decrement of Pves. In these models, Pves at each release site decremented exponentially each time there was a presynaptic action potential. During each simulation, n andNsite remained constant. InA, strong and weak synapses differed in the number of releasable vesicles. In B, strong and weak synapses differed in the initial Pves. InA, Pves was selected to achieve a Psite of 0.9 with 10 (A1) or 20 (A2) releasable vesicles; inB, Pves was selected to achieve a Psite of 0.25 and 0.75 forPves Low and PvesHigh, respectively, with 6 (B1) or 8 (B2) releasable vesicles. Average initial number of quanta released were: for A1, 12.1 for 2 vesicles and 34.5 for 9 vesicles; forA2, 9.5 for 3 vesicles and 28.1 for 12 vesicles; forB1, 10.1 for Pves Low and 30.4 for Pves High; and forB2, 10.5 for Pves Low and 30.0 for Pves High. The time course of HSD was significantly different between strong and weak synapses [repeated measure ANOVA testing interaction between vesicle number and trial number: (A1) F(14,145) = 9.6, p < 0.001; (A2)F(14,145) = 2.0, p= 0.021; repeated measure ANOVA testing interaction betweenPves and trial number: (B1)F(14,145) = 3.3, p< 0.001; and (B2)F(14,145) = 5.2, p< 0.001]. Sampling interval was 0.25 msec.
It was initially surprising that despite our efforts to approximate the empirical HSD curves by selecting an appropriate time constant for the decrement of Pves, the simulated curves for strong and weak synapses consistently diverged from one another, whether this decrement was a consequence of the release event or the presynaptic spike. This failure to obtain similar HSD for strong and weak synapses, even when fitting the decrementing parameter to the empirical data, led to the conclusion that decrement ofPves cannot account for synaptic depression at these SN synapses.
Stimulus-dependent decrease in the number of functionally active release sites provides a synaptic strength-independent mechanism of HSD
In these models, we explored the possibility that inactivation of the exocytosis mechanism at release sites could underlie HSD. Inactivation of individual release sites was triggered either by release events or by action potentials according to an exponential function that approximately matched the empirical exponential for HSD.
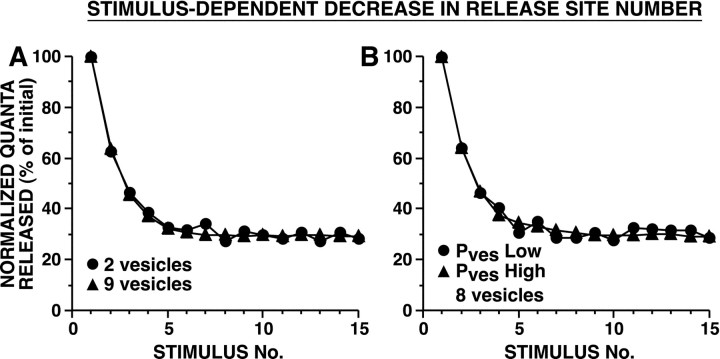
When release site inactivation was driven by release events, strong and weak synapses decremented with different time courses, whether the difference in initial synaptic strength was explained by differences inPves or in n (Fig.10). This was because strong synapses, which had a greater Psite, underwent release-dependent inactivation of release sites more frequently than weaker synapses. In contrast, when inactivation of release sites occurred in a stimulus dependent manner, the HSD process was completely independent of synaptic strength; strong and weak synapses decremented with the identical time course (Fig.11).
Fig. 10.
Simulated HSD as a result of release-dependent decrement in release site number. In these models, release sites had a fixed probability of switching to an inactive state after a release event. During each simulation, n andPves remained constant. In A, strong and weak synapses differed in the number of releasable vesicles. In B, strong and weak synapses differed in the initialPves. In A,Pves was selected to achieve aPsite of 0.9 with 10 releasable vesicles; inB, Pves was selected to achieve a Psite of 0.25 and 0.75 forPves Low and PvesHigh, respectively, with 8 releasable vesicles. Average initial number of quanta released were: for A, 18.0 for two vesicles and 39.0 for nine vesicles; for B, 10.1 forPves Low and 29.4 forPves High. The time course of HSD was significantly different between strong and weak synapses; [(A) repeated measure ANOVA testing interaction between vesicle number and trial number:F(14,145) = 18, p< 0.001; (B) repeated measure ANOVA testing interaction between Pves and trial number:F(14,145) = 12, p< 0.001]. Sampling interval was 0.25 msec.
Fig. 11.
Simulated HSD as a result of stimulus-dependent decrement in release site number. In these models,Nsite decremented exponentially with each presynaptic action potential. During each simulation, nand Pves remained constant. InA, strong and weak synapses differed in the number of releasable vesicles. In B, strong and weak synapses differed in the initial Pves. InA, Pves was selected to achieve a Psite of 0.9 with 10 releasable vesicles; in B, Pves was selected to achieve a Psite of 0.25 and 0.75 for Pves Low andPves High, respectively, with 8 releasable vesicles. Average initial number of quanta released were: forA, 11.8 for two vesicles and 34.2 for nine vesicles; forB, 9.6 for Pves Low and 29.2 for Pves High. There was no significant difference in the time course of HSD between strong and weak synapses; [(A) repeated measure ANOVA testing interaction between vesicle number and trial number:F(14,145) = 1.5, p= 0.13. (B) repeated measure ANOVA testing interaction between Pves and trial number:F(14,145) = 1.3, p= 0.19]. Sampling interval was 0.25 msec.
The development of HSD at SN-to-MN synapses in the ganglion is also unaffected by increasing transmitter release
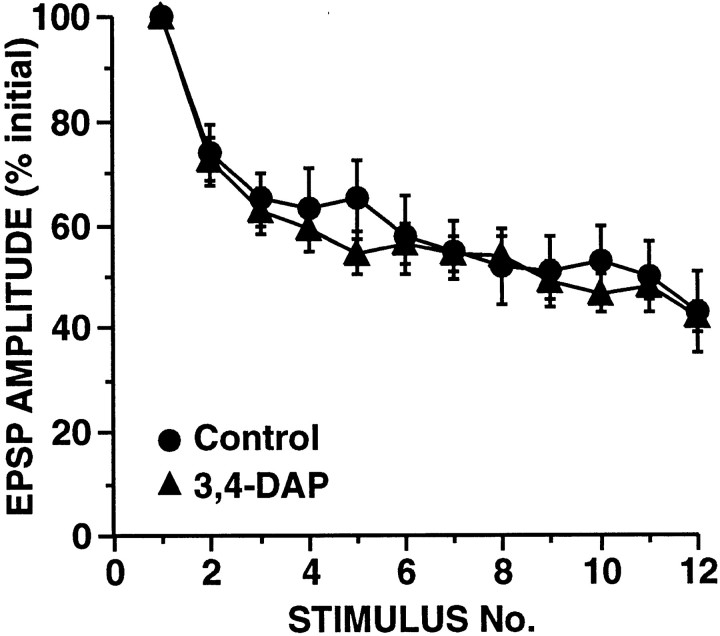
A major conclusion of these simulations was that HSD that was independent of synaptic strength could not result from any alteration in synaptic properties that was initiated by vesicle release. This implies that processes underlying synaptic strength-independent HSD at these SN synapses must be activated by the presynaptic action potentials directly rather than by release events. To directly test the prediction that the development of HSD is unaffected by the magnitude of release, we asked whether the rate of HSD would be increased when Ca2+ influx was augmented by broadening the presynaptic action potential. A moderate increase in the duration of the normal action potential produces a moderate increase in the SN-to-MN EPSP as demonstrated by studies using K+ channel blockers (Hochner et al., 1986a; Sugita et al., 1997). We used a low concentration of the K+ channel blocker 3,4-DAP (5 μm) to broaden the SN action potential approximately threefold. No comparisons were made within SNs; given the fivefold to sixfold variability in SN-to-MN synaptic connections, it is therefore not possible to accurately assess the increase in synaptic strength that occurred with this amount of spike broadening. On average, across preparations, EPSP amplitude increased ∼50%; this is probably an underestimate because Sugita et al. (1997) observed a similar increase in EPSP amplitude with twofold broadening of the SN spike produced by 4-AP. As predicted, in our experiments with 3,4-DAP, the broadened spikes and increased transmitter release were not accompanied by a detectable change in the rate of HSD (Fig.12).
Fig. 12.
Increasing release by broadening the SN action potential with the K+ channel blocker 3,4-DAP does not affect the rate of HSD. Superfusing abdominal ganglia with 5 μm 3,4-DAP before and during experiments resulted in an ∼2.95-fold increase in the duration of the SN action potential. Although no comparisons were made within preparations, on average, EPSP amplitude increased ∼50% in 3,4-DAP-treated ganglia (n = 17) as compared with in control ganglia (n = 12). [This is a smaller increase than expected (Sugita et al., 1997); however, given the wide (more than sixfold) range of initial EPSP amplitudes, it is not possible to obtain an accurate measure of the effect of spike broadening without within-cell comparisons.] Note that with broadened SN spikes, there was no significant difference in the rate of HSD (repeated measure ANOVA testing interaction between 3,4-DAP and trial number:F(9,11) = 1.1, p = 0.45).
Repetitive stimulation of SNs at low frequencies results in long-term synaptic depression that does not recover appreciably with rest
Both the simulation studies and these spike-broadening experiments clearly demonstrated that HSD does not develop as a consequence of the exocytosis event itself, and is not caused by vesicle depletion. If HSD at these SN synapses represents a inactivation of release sites rather than an exhaustion of the exocytosis mechanism, does synaptic strength gradually recover after repetitive stimulation? In other words, if release sites are “turned off,” must they be actually “turned back on,” or does transmitter release automatically recover with rest?
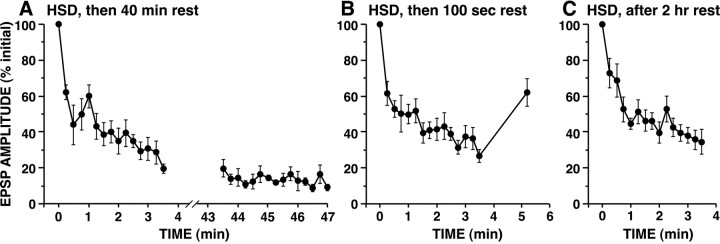
It is generally believed that SN synapses recover from HSD when rested because it has been consistently observed that depressed EPSP amplitudes recover partially several minutes after cessation of repetitive stimulation (Byrne, 1982). We reexamined this issue, investigating whether depressed SN synapses would fully recover after substantially longer rest periods (as would be predicted if depression were simply an exhaustion phenomenon). In preliminary experiments, we observed that a rest period of 10 min did not result in substantial recovery of the depressed EPSP. Similarly, data of Armitage and Siegelbaum (1998) on SN-to-MN synapses in culture revealed no significant recovery from HSD after 10 min of rest. We therefore conducted experiments with a substantially longer rest period, waiting 40 min after a series of 15 stimuli. Surprisingly, when tested after the 40 min rest, synapses still showed no detectable recovery (Fig.13A). The decreased amplitude of the SN EPSPs after 40 min did not result from a general deterioration of the recording. If recording conditions deteriorated, causing EPSPs to appear smaller, but HSD recovered, we would expect to induce normal HSD after a rest; however, because additional HSD could not be induced, we conclude that the synapses remained in their fully depressed state. Moreover, control synapses that were allowed to rest for a longer period of time were capable of undergoing normal HSD, indicating that the absence of HSD in these ganglia after 40 min in culture medium, is not caused by deterioration of the ability of synapses to undergo plasticity (Fig. 13C). These observations suggest that the increase in EPSP amplitude typically observed after a short rest (Fig. 13B) reflects either a form of transient facilitation or transient recovery (which is not maintained), initiated by the repetitive stimulation during the HSD protocol.
Fig. 13.
Limited recovery of depressed SN synaptic connections with rest. A, SN synaptic connections that were rested 40 min after induction of HSD. SNs were activated 15 times. After the 15th stimulus, synapses were rested 40 min and again stimulated repetitively. Note, there was no apparent recovery of depressed SN connections. Note also that no additional HSD was induced by the subsequent stimulation after rest. B,After HSD develops at SN synapses, EPSPs show partial recovery after a 100 sec period of rest. After the 15th stimulus, synapses were rested 100 sec and then SNs were stimulated once more. C,Prolonged incubation in culture medium does not interfere with the expression of HSD at SN synapses. Abdominal ganglia were superfused for 2 hr in culture medium before SN synapses were stimulated 15 times. In all three protocols during the series of 15 stimuli, the ISI was 15 sec. [Although the increase in EPSP amplitude after 100 sec of rest inB does not represent actual recovery of the synapse to the initial naive state, we used this percentage of recovery as an estimate of the time constant of replenishment for models that included vesicle recycling (Fig. 6).]
Observations of Byrne (1982) that very short-term recovery from HSD at these SN synapses after <2 min of rest was more dramatic when HSD was produced by higher frequencies of stimulation suggested that Ca2+ influx may accelerate recovery from depression. Consistent with this possibility, Eliot et al. (1994) found that SNs in culture that were extracellularly stimulated exhibited somewhat more profound HSD than impaled SNs, which presumably had elevated intracellular Ca2+ levels. At cerebellar synapses, recovery from HSD is also stimulated by activity and intracellular Ca2+ (Dittman and Regehr, 1998). With longer ISIs, residual free Ca2+ in varicosities completely decays before the next action potential (Armitage and Siegelbaum,1998, their Fig. 5); indeed Gingrich and Byrne (1985) found that activity-dependent recovery was negligible at these SN synapses with an ISI of 30 sec. Because we used a relatively long ISI (15 sec), we omitted this Ca2+-dependent recovery process from the simulations. However, the lack of recovery observed in the experiments of Figure 13 after rest periods of >10 min suggests that the previously reported activity-dependent “recovery” at these synapses may actually represent transient, short-term facilitation, rather than true reversal of HSD.
Serotonin-induced facilitation is mediated primarily by an increase in Psite rather than a change inNsite
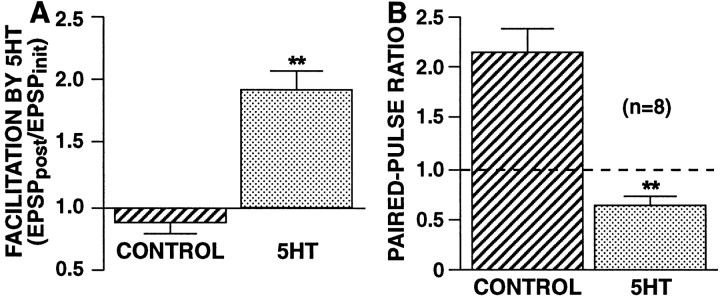
Having obtained evidence that HSD involves a decrease inNsite, which presumably involves the switching of release sites to a functionally inactive state, we asked whether facilitation by 5-HT of SN synapses is also primarily mediated by a change in Nsite or whether it involves an increase in Psite. Specifically, we investigated whether there was a change inPsite by examining the paired-pulse ratio during 5-HT-induced synaptic facilitation. We focused on facilitation of nondepressed synapses, because facilitation of previously depressed synapses involves multiple processes, including reversal of synaptic depression (Hochner et al., 1986b; Byrne and Kandel, 1996) with a possible increase inNsite. With exposure to 5-HT, SN synapses showed a more than threefold decrease in their paired-pulse ratio (PPR = 0.63 ± 0.08 in 5-HT vs 2.13 ± 0.26 in control synapses; p < 0.002; two-tailed ttest) (Fig. 14), suggesting a large increase in the release probability with the first paired spike. Because the facilitation observed in these experiments was 92 ± 16%, this apparent increase in Psiteshould be sufficient to account for the observed increase in synaptic strength.
Fig. 14.
Facilitation by 5-HT is accompanied by a large decrease in the paired-pulse ratio. Within each ganglion (n = 8), paired-pulse ratios were measured at SN-to-MN synapses in control saline and in the presence of 20 μm 5-HT. Because the paired-pulse ratio decrements sharply with testing (Jiang and Abrams, 1998), the paired-pulse ratio was tested only once per synapse; comparisons were made between separate synapses recorded either in control saline or in 5-HT saline within each ganglion. Control measurements were made before measurements in 5-HT. Each synapse was first tested with a single stimulus and then tested 15 min later with paired stimuli. For 5-HT-induced facilitation, superfusion with 20 μm 5-HT was begun 2.5 min before the paired test. A,Facilitation by 5-HT. The ratio of the EPSP produced by the first of the two paired stimuli (in either 5-HT or control saline) (EPSPpost) to the initial EPSP recorded 15 min before the paired test (EPSPinit). B,Paired-pulse ratios measured in control saline or 5-HT saline. Paired stimuli were at a 50 msec ISI. Initial EPSP amplitudes (recorded in control saline) were 8.53 ± 1.6 mV for synapses that were subsequently tested in control saline and 9.74 ± 2.7 mV for synapses that were subsequently tested in 5-HT (difference not significant). Both 5-HT-induced facilitation and the decrease in the paired-pulse ratio with 5-HT exposure were highly significant (**p < 0.002; two-tailed paired ttest).
DISCUSSION
Activity-dependent switching off of release sites can explain strength-independent HSD
Although HSD at Aplysia SN synapses was first demonstrated to be a presynaptic phenomenon >25 years ago (Castellucci and Kandel, 1974), the underlying mechanism is still not understood. Recently at these SN synapses, Jiang and Abrams (1998) found that, whereas synaptic strength was positively correlated with release probability, as assessed by the paired-pulse ratio, development of HSD was independent of synaptic strength. This observation suggested that HSD does not primarily result from changes in the size of the releasable pool; if, on the contrary, HSD were to involve vesicle depletion, we predicted that synapses with larger pools of readily releasable vesicles would be less affected by depletion of a given number of vesicles than synapses with smaller releasable pools.
We tested this prediction in the present study using computer simulations of Aplysia SN synapses; we also used this approach to evaluate a variety of other possible mechanisms of HSD, including changes in the probability of exocytosis of individual vesicles (Pves) and changes in the number of functionally active release sites (Nsite). We assessed whether each possible mechanism of HSD would produce synaptic decrement with kinetics that are consistent with those observed at actual SN-to-MN connections. The specific feature of empirical HSD against which we compared the results of each model was that the time course of HSD is independent of initial synaptic strength. Of a total of five classes of possible mechanisms of HSD, we found that only one mechanism, stimulus-dependent inactivation of individual release sites, could produce strength-independent HSD (Table1, column E). In considering the various classes of possible mechanisms for HSD, we first discuss the evidence against vesicle depletion.
Table 1.
Alternative mechanisms of HSD
| HSD is consequence of exocytosis event | HSD is consequence of presynaptic spike (independent of release) | ||||
|---|---|---|---|---|---|
| HSD results from decrease in: | HSD results from decrease in: | ||||
| Initially strong and weak synapses differ in: | Number of readily releasable vesicles (n) | Release probability of individual vesicles (Pves) | Number of functional release sites (Nsite) | Release probability of individual vesicles (Pves) | Number of functional release sites (Nsite) |
| Number of readily releasable vesicles (n) | HSD is strength dependent1-a | HSD is strength dependent | HSD is strength dependent | HSD is strength dependent | HSD is strength independent1-b |
| Release probability of individual vesicles (Pves) | HSD is strength dependent | HSD is strength dependent | HSD is strength dependent | HSD is strength dependent | HSD is strength independent |
| Number of active zones (Nsite)1-c | Nonphysiological | Nonphysiological | Nonphysiological | Nonphysiological | Nonphysiological |
Indicates that HSD depends on initial strength of synaptic connections.
Indicates that HSD develops independently of initial strength of synaptic connections.
Not tested in simulations because paired-pulse ratio data indicate that strong and weak synapses differ in the properties of their individual release sites (not only in number of release sites).
Univesicular release dramatically alters kinetics of HSD resulting from vesicle depletion
An underlying assumption of most of the models tested was that immediately after fusion of a vesicle at a release site, subsequent exocytotic events at this site are restricted. Studies of several different central synapses suggest that during a single action potential, the number of vesicles released at each active zone is either zero or one (Korn et al., 1982, 1994; Redman, 1990). Although multivesicular release could be difficult to detect because of receptor saturation, several studies suggest that postsynaptic sites are not fully saturated (Faber et al., 1992; Kruk et al., 1997; Liu et al., 1999). In contrast, in a study of synapses between inhibitory interneurons in the cerebellum, Auger et al. (1998) demonstrated that during each presynaptic spike, more than one vesicle can be released at a synaptic terminal; however, at most of these cerebellar synapses, release of two vesicles was estimated to occur only during a minority of release events. Considered together these studies suggest that a mechanism exists by which fusion of one vesicle inhibits fusion of nearby vesicles within the same release site for several milliseconds, as proposed by Dobrunz et al. (1997) and Geppert et al. (1997), although this inhibition may not be absolutely effective.
In all vesicle depletion models with univesicular release, HSD varied depending on initial synaptic strength. We also created a family of models with limited multivesicular release, in which subsequent exocytosis events could occur after an initial release event, although with reduced probability. With limited multivesicular release, we observed that a vesicle depletion mechanism of HSD was also inconsistent with the kinetics of HSD being independent of initial synaptic strength. Thus, with both univesicular release and limited multivesicular release, vesicle depletion resulted in HSD with kinetics that depend on initial release probability.
Vesicle depletion is inadequate to account for the substantial HSD observed after only a single stimulus
As expected, in models in which there was unrestricted multivesicular release, we observed that depletion-mediated HSD was independent of the initial number of releasable vesicles and therefore independent of initial synaptic strength. We therefore asked whether depletion could account for the observed magnitude of HSD if there were no restrictions on multiple vesicle fusion events at a release site. Synaptic depression at Aplysia SN synapses after a single stimulus is typically between 25 and 40% for ISIs of 10–20 sec (Figs. 2, 12), (Byrne, 1982; Eliot et al., 1994; Armitage and Siegelbaum, 1998; Jiang and Abrams, 1998). To achieve a synaptic decrement of 36% after the first action potential (the average initial depression observed by Jiang and Abrams, 1998),Psite for release sites with six releasable vesicles would need to average >0.9 (Fig. 7A). Thus, if release probabilities were sufficiently high that several vesicles were released with each action potential, release sites would rarely fail to release at least one vesicle. In contrast, at synapses where the release probabilities of individual sites have been analyzed,Psite (commonly calledPr) varies greatly among connections within a study, ranging fourfold to sixfold, with low values ≤0.2 (Korn et al., 1984; Hessler et al., 1993; Rosenmund et al., 1993;Murthy et al., 1997) Low-probability release sites must predominate at those SN synapses that show greater than twofold paired-pulse facilitation; (i.e., at there must be a low probability of release with the first paired stimulus) (e.g., controls in Figs.2D and 14) (Jiang and Abrams, 1998). With much smaller releasable pools (e.g., two vesicles) lower probabilities of release would effectively produce rapid HSD; however, such a small number of releasable vesicles is inconsistent with estimates based on electron microscopy (Bailey and Chen, 1988). Thus, even if there were unrestricted multivesicular release, vesicle depletion is unlikely to be able to account for the large initial synaptic decrement displayed by the SN synapses. The substantial decrease in synaptic strength that occurs at a number of mammalian cortical synapses after only the first stimulus in a train also suggests that depletion may not explain the early components of depression; this conclusion was reached by Matveev and Wang (2000), who analyzed cortical synapses that show synaptic decrement >60% with single stimuli (Varela et al., 1997).
Neither synaptic changes that are initiated by release events nor stimulus-dependent decrement of Pves can account for HSD that occurs independently of initial synaptic strength
All three release-dependent changes that we studied in simulations (i.e., changes in n, Pves, or Nsite) resulted in rates of HSD that varied with synaptic strength. Any change in a synaptic parameter that is a consequence of exocytosis will of necessity occur at a rate that is proportional to the probability of vesicle release. Thus, we observed that release-dependent decrement either inPves or inNsite produced a greater rate of HSD at strong than weak synapses. In the case of vesicle depletion, when strong and weak synapses differ in initial numbers of vesicles, the situation is more complex; increased vesicle release at high probability release sites is outweighed by the restriction on multivesicular release in combination with the more modest effect that loss of a given number of vesicles has on sites with larger releasable pools (Fig. 1A). In the case of stimulus-dependent changes in release probability caused by decrement ofPves, rates of HSD depend on initial synaptic strength because the slope of the relationship betweenPsite andPves increases asPves decreases (Fig.1B,C).
Alteration of Ca2+ influx confirms that HSD at SN synapses is release-independent
Several independent empirical results support the conclusion that HSD does not occur as a consequence of release. In our experiments in which threefold broadening of the presynaptic action potential by 3,4-DAP was used to produce an increase in release, the rate of HSD was unaffected (Fig. 12). In contrast, HSD caused by vesicle depletion or any other release-dependent change should be substantially altered when Ca2+ influx is increased. Our conclusion that HSD is release-independent is also supported by results of earlier experiments that decreased transmitter release by reducing the rise in intracellular free Ca2+, without producing a substantial effect on HSD (Castellucci and Kandel, 1974; Armitage and Siegelbaum, 1998). Interestingly, Zucker and Bruner (1977) evaluated HSD at a crayfish neuromuscular junction and also concluded that the properties of HSD were incompatible with the depletion hypothesis, based in part on the minimal sensitivity to changes in Ca2+ influx. The observation that it is possible to vary Ca2+ influx and alter release without significantly affecting the development of HSD does not mean that HSD is initiated independently of Ca2+ influx. Our recent preliminary experiments indicate that when Ca2+ influx is completely eliminated, a series of presynaptic action potentials no longer results in the development of HSD; this suggests that HSD is initiated by Ca2+ influx, but that HSD has a much higher sensitivity to intracellular Ca2+ than does the exocytosis process itself.
All-or-none persistent inactivation of release sites
Examination of SN synapses rested for tens of minutes after induction of HSD revealed that once inactivated, release sites do not automatically recover to a functional state. This observation that there is no automatic recovery from HSD would explain the observation that appreciable HSD occurs at these synapses even with intervals as long as 15 min (Lin and Glanzman, 1996). The hypothesis that HSD is primarily mediated by silencing of release sites, although novel, is supported by two other recent observations. Based on the lack of substantial increase in the paired-pulse ratio, Jiang and Abrams (1998)suggested that depression must result primarily from a reduction in the number of release sites. Recently, Royer et al. (2000) monitored release probability during HSD and concluded it did not change substantially, confirming the conclusion of Jiang and Abrams (1998). This inactivation of release sites may actually be a consequence of large decreases in the release probability of a subset of release sites that occurs with each presynaptic spike, such that total probability of release for those terminals that are affected becomes negligible. If release sites are switched off, why during HSD does the EPSP decay to a plateau level, rather than the EPSP disappearing because all sites are eventually inactivated? Most probably, some release sites are unaffected and do not turn off, although the percentage of inactivation-resistant sites may vary among synapses. Indeed among synapses, the final amount of depression is actually quite variable; in some cases, there is no detectable release after HSD.
In recent studies of long-term potentiation and long-term depression in the CA1 region of hippocampus of mammals, evidence has accumulated that suggests that during plasticity the sensitivity to transmitter of each postsynaptic site is rapidly turned on or off in a step-like manner as a single unit (Isaac et al., 1995; Liao et al., 1995; Carroll et al., 1999). Our results suggest that an analogous turning off of presynaptic release sites occurs during HSD at Aplysia SN synapses. One surprising aspect of the proposed mechanism is that a release site (a morphologically discrete region ∼0.5 μm in diameter) must be functionally turned off completely as a single unit, rather than undergoing a gradual reduction in release probability. What mechanism could produce such a site-wide change in exocytosis? It is possible that a second messenger cascade exhibits a threshold for activation, producing either no change or a near maximal change. Alternatively, it is conceivable that an array of cytoskeletal or plasma membrane proteins involved in vesicle docking or priming are somehow coupled across each release site so that these proteins undergo a change in configuration as a single unit.
Contributions of other mechanisms of HSD
Although our evidence suggests that inactivation of release sites is the major mechanism responsible for HSD that occurs with relatively small numbers of stimuli, it is likely other mechanisms also contribute. The modest increase in paired-pulse facilitation that occurs with HSD, which in particular is evident at strong synapses (Jiang and Abrams, 1998), suggests there may also be a decrease in total probability of release. With somewhat longer stimulation protocols than used here (≥35 stimuli), vesicle depletion has been demonstrated at these same SN synapses by electron microscopy (Bailey and Chen, 1988). Eliot et al. (1994) observed that spontaneous release frequency was not affected after a few low-frequency stimuli, but was moderately reduced after 2 min of 1 Hz stimulation; this decrease in spontaneous release may reflect vesicle depletion. It is worth noting that with the stimulus protocol used in the present study, most of the observed decrease in EPSP amplitude occurs within the first few stimuli (Fig. 2). Depletion may make a relatively greater contribution to the later phase of HSD produced by substantially more extensive or higher frequency stimulation.
Heterosynaptic facilitation involves a distinct mechanism: modulation of release probability
In this study of SN synapses, we observed that paired-pulse facilitation is completely eliminated during 5-HT-induced heterosynaptic facilitation of nondepressed synapses. This suggests that heterosynaptic facilitation involves an increase in the total probability of release (Psite). Thus, facilitation appears to operate by a different type of mechanism than does HSD. Increasing release probability by increasing Ca2+ influx with a K+ blocker similarly eliminated paired-pulse facilitation. Our conclusion concerning 5-HT-induced facilitation contrasts with the conclusion of Royer et al. (2000) that 5-HT-induced facilitation primarily involves an increase in the number of active release sites. A possible explanation for this difference is that Royer et al. (2000) studied the action of 5-HT only at previously depressed SN synapses. Our evidence that 5-HT-induced facilitation involves an increase in Psite is consistent with the observation that 5-HT increases Ca2+ influx (Eliot et al., 1993) by increasing the duration of the presynaptic action potential (Hochner et al., 1986a; Baxter and Byrne, 1990; Goldsmith and Abrams, 1992) and that mimicking this spike broadening with K+ channel blockers reproduces a substantial component of the 5-HT-induced facilitation of nondepressed synapses (Sugita et al., 1997).
Footnotes
This work was supported by National Institutes of Health (NIH) Grant MH 55880 (T.W.A.), and NIH training Grant GM 08181 (T.D.G.). Robert Zucker made critical comments about the initial model, and Dan Ruchkin provided helpful suggestions about stochastic models. We thank Scott Thompson and Darrin Brager for thoughtfully critiquing the manuscript.
Correspondence should be addressed to Dr. Thomas W. Abrams, Department of Pharmacology, University of Maryland School of Medicine, BRB 4-002 655 West Baltimore Street, Baltimore, MD 21201-1559. E-mail:tabrams@umaryland.edu.
REFERENCES
- 1.Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–224. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- 2.Adler EM, Augustine GJ, Duffy SN, Charlton MP. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991;11:1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armitage BA, Siegelbaum SA. Presynaptic induction and expression of homosynaptic depression at Aplysia sensorimotor neuron synapses. J Neurosci. 1998;18:8770–8779. doi: 10.1523/JNEUROSCI.18-21-08770.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auger C, Kondo S, Marty A. Multivesicular release at single functional synaptic sites in cerebellar stellate and basket cells. J Neurosci. 1998;18:4532–4547. doi: 10.1523/JNEUROSCI.18-12-04532.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey CH, Chen M. Morphological basis of long-term habituation and sensitization in Aplysia. Science. 1983;220:91–93. doi: 10.1126/science.6828885. [DOI] [PubMed] [Google Scholar]
- 6.Bailey CH, Chen M. Morphological basis of short-term habituation in Aplysia. J Neurosci. 1988;8:2452–2459. doi: 10.1523/JNEUROSCI.08-07-02452.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baxter DA, Byrne JH. Differential effects of cAMP and serotonin on membrane current, action-potential duration, and excitability in somata of pleural sensory neurons of Aplysia. J Neurophysiol. 1990;64:978–990. doi: 10.1152/jn.1990.64.3.978. [DOI] [PubMed] [Google Scholar]
- 8.Betz WJ. Depression of transmitter release at the neuromuscular junction of the frog. J Physiol (Lond) 1970;206:629–644. doi: 10.1113/jphysiol.1970.sp009034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brody DL, Yue DT. Release-independent short-term synaptic depression in cultured hippocampal neurons. J Neurosci. 2000;20:2480–2494. doi: 10.1523/JNEUROSCI.20-07-02480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrne JH. Analysis of synaptic depression contributing to habituation of gill-withdrawal reflex in Aplysia californica. J Neurophysiol. 1982;48:431–438. doi: 10.1152/jn.1982.48.2.431. [DOI] [PubMed] [Google Scholar]
- 11.Byrne JH, Kandel ER. Presynaptic facilitation revisited: state and time dependence. J Neurosci. 1996;16:425–435. doi: 10.1523/JNEUROSCI.16-02-00425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canepari M, Cherubini E. Dynamics of excitatory transmitter release: analysis of synaptic responses in CA3 hippocampal neurons after repetitive stimulation of afferent fibers. J Neurophysiol. 1998;79:1977–1988. doi: 10.1152/jn.1998.79.4.1977. [DOI] [PubMed] [Google Scholar]
- 13.Carroll RC, Lissin DV, von Zastrow M, Nicoll RA, Malenka RC. Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nat Neurosci. 1999;2:454–460. doi: 10.1038/8123. [DOI] [PubMed] [Google Scholar]
- 14.Castellucci VF, Kandel ER. A quantal analysis of the synaptic depression underlying habituation of the gill-withdrawal reflex in Aplysia. Proc Natl Acad Sci USA. 1974;71:5004–5008. doi: 10.1073/pnas.71.12.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen TE, Kaplan SW, Kandel ER, Hawkins RD. A simplified preparation for relating cellular events to behavior: mechanisms contributing to habituation, dishabituation, and sensitization of the Aplysia gill-withdrawal reflex. J Neurosci. 1997;17:2886–2899. doi: 10.1523/JNEUROSCI.17-08-02886.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Debanne D, Guerineau NC, Gahwiler BH, Thompson SM. Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. J Physiol (Lond) 1996;491:163–176. doi: 10.1113/jphysiol.1996.sp021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dittman JS, Regehr WG. Calcium dependence and recovery kinetics of presynaptic depression at the climbing fiber to Purkinje cell synapse. J Neurosci. 1998;18:6147–6162. doi: 10.1523/JNEUROSCI.18-16-06147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobrunz LE, Huang EP, Stevens CF. Very short-term plasticity in hippocampal synapses. Proc Natl Acad Sci USA. 1997;94:14843–14847. doi: 10.1073/pnas.94.26.14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eliot LS, Kandel ER, Siegelbaum SA, Blumenfeld H. Imaging terminals of Aplysia sensory neurons demonstrates role of enhanced Ca2+ influx in presynaptic facilitation. Nature. 1993;361:634–637. doi: 10.1038/361634a0. [DOI] [PubMed] [Google Scholar]
- 20.Eliot LS, Kandel ER, Hawkins RD. Modulation of spontaneous transmitter release during depression and posttetanic potentiation of Aplysia sensory-motor neuron synapses isolated in culture. J Neurosci. 1994;14:3280–3292. doi: 10.1523/JNEUROSCI.14-05-03280.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elmqvist D, Quastel DM. A quantitative study of end-plate potentials in isolated human muscle. J Physiol (Lond) 1965;178:505–529. doi: 10.1113/jphysiol.1965.sp007639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faber DS. Synaptic depression at an identified central synapse: evidence for different constraints on evoked and spontaneous quanta. In: Faber DS, Korn H, Redman SJ, Thompson SM, Altman JS, editors. Central synapses: quantal mechanisms and plasticity. Human Frontier Science Program; Strasbourg: 1998. pp. 158–168. [Google Scholar]
- 23.Faber DS, Young WS, Legendre P, Korn H. Intrinsic quantal variability due to stochastic properties of receptor-transmitter interactions. Science. 1992;258:1494–1498. doi: 10.1126/science.1279813. [DOI] [PubMed] [Google Scholar]
- 24.Fogelson AL, Zucker RS. Presynaptic calcium diffusion from various arrays of single channels. Implications for transmitter release and synaptic facilitation. Biophys J. 1985;48:1003–1017. doi: 10.1016/S0006-3495(85)83863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geppert M, Goda Y, Stevens CF, Sudhof TC. The small GTP-binding protein Rab3A regulates a late step in synaptic vesicle fusion. Nature. 1997;387:810–814. doi: 10.1038/42954. [DOI] [PubMed] [Google Scholar]
- 26.Gingrich KJ, Byrne JH. Simulation of synaptic depression, posttetanic potentiation, and presynaptic facilitation of synaptic potentials from sensory neurons mediating gill-withdrawal reflex in Aplysia. J Neurophysiol. 1985;53:652–669. doi: 10.1152/jn.1985.53.3.652. [DOI] [PubMed] [Google Scholar]
- 27.Goldsmith BA, Abrams TW. Reversal of synaptic depression by serotonin at Aplysia sensory neuron synapses involves activation of adenylyl cyclase. Proc Natl Acad Sci USA. 1991;88:9021–9025. doi: 10.1073/pnas.88.20.9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldsmith BA, Abrams TW. cAMP modulates multiple K+ currents, increasing spike duration and excitability in Aplysia sensory neurons. Proc Natl Acad Sci USA. 1992;89:11481–11485. doi: 10.1073/pnas.89.23.11481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatt H, Smith DO. Synaptic depression related to presynaptic axon conduction block. J Physiol (Lond) 1976;259:367–393. doi: 10.1113/jphysiol.1976.sp011471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heidelberger R, Heinemann C, Neher E, Matthews G. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature. 1994;371:513–515. doi: 10.1038/371513a0. [DOI] [PubMed] [Google Scholar]
- 31.Hessler NA, Shirke AM, Malinow R. The probability of transmitter release at a mammalian central synapse. Nature. 1993;366:569–572. doi: 10.1038/366569a0. [DOI] [PubMed] [Google Scholar]
- 32.Hochner B, Klein M, Schacher S, Kandel ER. Action potential duration and the modulation of transmitter release from the sensory neurons of Aplysia in presynaptic facilitation and behavioral sensitization. Proc Natl Acad Sci USA. 1986a;83:8410–8414. doi: 10.1073/pnas.83.21.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hochner B, Klein M, Schacher S, Kandel ER. Additional component in the cellular mechanism of presynaptic facilitation contributes to behavioral dishabituation in Aplysia. Proc Natl Acad Sci USA. 1986b;83:8794–8798. doi: 10.1073/pnas.83.22.8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu SF, Augustine GJ, Jackson MB. Adaptation of Ca(2+)-triggered exocytosis in presynaptic terminals. Neuron. 1996;17:501–512. doi: 10.1016/s0896-6273(00)80182-8. [DOI] [PubMed] [Google Scholar]
- 35.Isaac JT, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 36.Jiang XY, Abrams TW. Use-dependent decline of paired-pulse facilitation at Aplysia sensory neuron synapses suggests a distinct vesicle pool or release mechanism. J Neurosci. 1998;18:10310–10319. doi: 10.1523/JNEUROSCI.18-24-10310.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kandler K, Katz LC, Kauer JA. Focal photolysis of caged glutamate produces long-term depression of hippocampal glutamate receptors. Nat Neurosci. 1998;1:119–123. doi: 10.1038/368. [DOI] [PubMed] [Google Scholar]
- 38.Kim J, Alger BE. Random response fluctuations lead to spurious paired-pulse facilitation. J Neurosci. 2001;21:9608–9618. doi: 10.1523/JNEUROSCI.21-24-09608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korn H, Mallet A, Triller A, Faber DS. Transmission at a central inhibitory synapse. II. Quantal description of release, with a physical correlate for binomial n. J Neurophysiol. 1982;48:679–707. doi: 10.1152/jn.1982.48.3.679. [DOI] [PubMed] [Google Scholar]
- 40.Korn H, Faber DS, Burnod Y, Triller A. Regulation of efficacy at central synapses. J Neurosci. 1984;4:125–130. doi: 10.1523/JNEUROSCI.04-01-00125.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korn H, Sur C, Charpier S, Legendre P, Faber DS. The one-vesicle hypothesis and multivesicular release. Adv Second Messenger Phosphoprotein Res. 1994;29:301–322. doi: 10.1016/s1040-7952(06)80022-4. [DOI] [PubMed] [Google Scholar]
- 42.Kruk PJ, Korn H, Faber DS. The effects of geometrical parameters on synaptic transmission: a Monte Carlo simulation study. Biophys J. 1997;73:2874–2890. doi: 10.1016/S0006-3495(97)78316-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- 44.Lin XY, Glanzman DL. Long-term depression of Aplysia sensorimotor synapses in cell culture: inductive role of a rise in postsynaptic calcium. J Neurophysiol. 1996;76:2111–2114. doi: 10.1152/jn.1996.76.3.2111. [DOI] [PubMed] [Google Scholar]
- 45.Liu G, Choi S, Tsien RW. Variability of neurotransmitter concentration and nonsaturation of postsynaptic AMPA receptors at synapses in hippocampal cultures and slices. Neuron. 1999;22:395–409. doi: 10.1016/s0896-6273(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 46.Llinas R, Sugimori M, Silver RB. Microdomains of high calcium concentration in a presynaptic terminal. Science. 1992;256:677–679. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- 47.Matveev V, Wang XJ. Implications of all-or-none synaptic transmission and short-term depression beyond vesicle depletion: a computational study. J Neurosci. 2000;20:1575–1588. doi: 10.1523/JNEUROSCI.20-04-01575.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melkonian DS. Transient analysis of a chemical synaptic transmission. Biol Cybern. 1993;68:341–350. doi: 10.1007/BF00201859. [DOI] [PubMed] [Google Scholar]
- 49.Murthy VN, Sejnowski TJ, Stevens CF. Heterogeneous release properties of visualized individual hippocampal synapses. Neuron. 1997;18:599–612. doi: 10.1016/s0896-6273(00)80301-3. [DOI] [PubMed] [Google Scholar]
- 50.Narasimhan K, Linden DJ. Defining a minimal computational unit for cerebellar long-term depression. Neuron. 1996;17:333–341. doi: 10.1016/s0896-6273(00)80164-6. [DOI] [PubMed] [Google Scholar]
- 51.Patil PG, Brody DL, Yue DT. Preferential closed-state inactivation of neuronal calcium channels. Neuron. 1998;20:1027–1038. doi: 10.1016/s0896-6273(00)80483-3. [DOI] [PubMed] [Google Scholar]
- 52.Redman RS, Silinsky EM. ATP released together with acetylcholine as the mediator of neuromuscular depression at frog motor nerve endings. J Physiol (Lond) 1994;477:117–127. doi: 10.1113/jphysiol.1994.sp020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Redman S. Quantal analysis of synaptic potentials in neurons of the central nervous system. Physiol Rev. 1990;70:165–198. doi: 10.1152/physrev.1990.70.1.165. [DOI] [PubMed] [Google Scholar]
- 54.Roberts WM, Jacobs RA, Hudspeth AJ. The hair cell as a presynaptic terminal. Ann NY Acad Sci. 1991;635:221–233. doi: 10.1111/j.1749-6632.1991.tb36494.x. [DOI] [PubMed] [Google Scholar]
- 55.Rosenmund C, Clements JD, Westbrook GL. Nonuniform probability of glutamate release at a hippocampal synapse. Science. 1993;262:754–757. doi: 10.1126/science.7901909. [DOI] [PubMed] [Google Scholar]
- 56.Royer S, Coulson RL, Klein M. Switching off and on of synaptic sites at Aplysia sensorimotor synapses. J Neurosci. 2000;20:626–638. doi: 10.1523/JNEUROSCI.20-02-00626.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stevens CF, Wang Y. Facilitation and depression at single central synapses. Neuron. 1995;14:795–802. doi: 10.1016/0896-6273(95)90223-6. [DOI] [PubMed] [Google Scholar]
- 58.Sugita S, Baxter DA, Byrne JH. Differential effects of 4-aminopyridine, serotonin, and phorbol esters on facilitation of sensorimotor connections in Aplysia. J Neurophysiol. 1997;77:177–185. doi: 10.1152/jn.1997.77.1.177. [DOI] [PubMed] [Google Scholar]
- 59.Thies RE. Neuromuscular depression and the apparent depletion of transmitter in mammalian muscle. J Neurophysiol. 1965;28:427–442. doi: 10.1152/jn.1965.28.3.427. [DOI] [PubMed] [Google Scholar]
- 60.Trussell LO, Zhang S, Raman IM. Desensitization of AMPA receptors upon multiquantal neurotransmitter release. Neuron. 1993;10:1185–1196. doi: 10.1016/0896-6273(93)90066-z. [DOI] [PubMed] [Google Scholar]
- 61.Varela JA, Sen K, Gibson J, Fost J, Abbott LF, Nelson SB. A quantitative description of short-term plasticity at excitatory synapses in layer 2/3 of rat primary visual cortex. J Neurosci. 1997;17:7926–7940. doi: 10.1523/JNEUROSCI.17-20-07926.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.von Gersdorff H, Schneggenburger R, Weis S, Neher E. Presynaptic depression at a calyx synapse: the small contribution of metabotropic glutamate receptors. J Neurosci. 1997;17:8137–8146. doi: 10.1523/JNEUROSCI.17-21-08137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wojtowicz JM, Marin L, Atwood HL. Activity-induced changes in synaptic release sites at the crayfish neuromuscular junction. J Neurosci. 1994;14:3688–3703. doi: 10.1523/JNEUROSCI.14-06-03688.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamada WM, Zucker RS. Time course of transmitter release calculated from simulations of a calcium diffusion model. Biophys J. 1992;61:671–682. doi: 10.1016/S0006-3495(92)81872-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zucker RS, Bruner J. Long-lasting depression and the depletion hypothesis at crayfish neuromuscular junctions. J Comp Physiol. 1977;121:223–240. [Google Scholar]