Abstract
Attenuated behavioral sensitivity to neurosteroids has been reported for mice deficient in the GABAA receptor δ subunit. We therefore investigated potential subunit-specific neurosteroid pharmacology of the following GABAA receptor isoforms in a transient expression system: α1β3γ2L, α1β3δ, α6β3γ2L, and α6β3δ. Potentiation of submaximal GABAA receptor currents by the neurosteroid tetrahydrodeoxycorticosterone (THDOC) was greatest for the α1β3δ isoform. Whole-cell GABA concentration–response curves performed with and without low concentrations (30 nm) of THDOC revealed enhanced peak GABAA receptor currents for isoforms tested without affecting the GABA EC50. α1β3δ currents were enhanced the most (>150%), whereas the other isoform currents were enhanced 15–50%. At a higher concentration (1 μm), THDOC decreased peak α1β3γ2L receptor current amplitude evoked by GABA (1 mm) concentration jumps and prolonged deactivation but had little effect on the rate or extent of apparent desensitization. Thus the polarity of THDOC modulation depended on GABA concentration for α1β3γ2L GABAA receptors. However, the same protocol applied to α1β3δ receptors resulted in peak current enhancement by THDOC of >800% and prolonged deactivation. Interestingly, THDOC induced pronounced desensitization in the minimally desensitizing α1β3δ receptors. Single channel recordings obtained from α1β3δ receptors indicated that THDOC increased the channel opening duration, including the introduction of an additional longer duration open state. Our results suggest that the GABAA receptor δ subunit confers increased sensitivity to neurosteroid modulation and that the intrinsic gating and desensitization kinetics of α1β3δ GABAA receptors are altered by THDOC.
Keywords: GABAA receptor, δ subunit, neurosteroid, desensitization, single channel, gating
Fast synaptic inhibition in the mammalian CNS is mediated mainly by activation of GABAA receptor channels (Macdonald and Olsen, 1994; Whiting et al., 1995). GABAA receptor function is modulated by various clinically important drugs that act on allosteric modulatory sites (Macdonald and Olsen, 1994; Sieghart, 1995). For example, neurosteroids, which represent a class of molecules that are synthesized in the nervous system, have been demonstrated to have anxiolytic, hypnotic, anesthetic, and anticonvulsant effects (Baulieu and Robel, 1990; Paul and Purdy, 1992; Macdonald and Olsen, 1994; Lambert et al., 1995) and may be involved in memory enhancement, behavioral actions, and neuroprotection (Frye, 1995; Green et al., 2000; Yoo et al., 1996).
Several studies have shown that neurosteroids bind to GABAA receptors at sites different from GABA, benzodiazepines, and barbiturates (Gee et al., 1988; Turner et al., 1989) and can act as positive or negative modulators of receptor function (Majewska et al., 1986; Gee et al., 1988; Puia et al., 1990;Gee and Lan, 1991; Park-Chung et al., 1999). Neurosteroid enhancement of submaximal GABAA receptor currents occurs through increases in both channel open frequency and open duration (Puia et al., 1990; Twyman and Macdonald, 1992). At high concentrations, neurosteroids can directly activate GABAA receptor channels (Lambert et al., 1995).
The GABAA receptor is a pentameric structure formed by the coassembly of subunit polypeptides from a large multigene family (McKernan and Whiting, 1996; Barnard et al., 1998) that are differentially expressed both temporally and spatially throughout the brain (Zheng et al., 1993, 1995). This heterogeneous expression confers specific physiological and pharmacological properties of GABAA receptors (Sigel et al., 1990; Mathews et al., 1994). For example, it has been demonstrated that the presence of α and γ subunits can affect neurosteroid modulation. The α subunit subtype was found to influence efficacy, whereas the γ subunit subtype influenced both efficacy and EC50for neurosteroid interaction with GABAA receptors (Gee and Lan, 1991; Lan et al., 1991; Sapp et al., 1992). Also, Zhu et al. (1996) reported that the presence of δ subunits inhibited neurosteroid modulation but not direct activation, of GABAA receptors. However, a recent study (Mihalek et al., 1999) demonstrated that mice lacking the GABAA receptor δ subunit had attenuated behavioral responses to systemic neurosteroid administration. This suggested an important role for the δ subunit either in the neurosteroid modulation of GABAA receptor currents or in the neural circuits relevant to the behavioral effects of neurosteroids. Approximately 30% of cerebellar GABAA receptors are thought to contain the δ subunit. δ mRNA is also found in the hippocampus and thalamus (Benke et al., 1991; Laurie et al., 1992a,b; McKernan and Whiting, 1996).
We used whole-cell and single-channel patch-clamp recordings and applied GABA using an ultra fast application system to investigate neurosteroid allosteric modulation of GABAAreceptor currents in mammalian cells transiently transfected with recombinant GABAA receptors containing α1 or α6 with β3 and γ2L or δ subunits.
MATERIALS AND METHODS
Expression of recombinant GABAAreceptors. The cDNAs encoding rat α1, α6, β3, δ, and γ2L GABAA receptor subunit subtypes were individually subcloned into the plasmid expression vector pCMVNeo. All subunits have been sequenced and are identical to published sequences. Human embryonic kidney cells (HEK293T; a gift from P. Connely, COR Therapeutics, San Francisco, CA) were maintained in DMEM, supplemented with 10% fetal bovine serum, at 37°C in 5% CO2/95% air. Cells were transfected with 4 μg of each subunit plasmid along with 1–2 μg of pHOOK (Invitrogen, Carlsbad, CA) for immunomagnetic bead separation (Greenfield et al., 1997), using a modified calcium phosphate coprecipitation technique as described previously (Angelotti et al., 1993). The next day, cells were replated, and recordings were made 18–30 hr later.
Electrophysiology and drug application. Patch-clamp recordings were performed on transfected fibroblasts bathed in an external solution consisting of (in mm): NaCl 142, KCl 8, MgCl2 6, CaCl21, HEPES 10, glucose 10, pH 7.4, 325 mOsm). Electrodes were formed from soda lime (whole cell), thin-walled borosilicate (whole cell), or thick-walled borosilicate (excised patch) glass (World Precision Instruments, Pittsburgh, PA) with a Flaming Brown electrode puller (Sutter Instrument Co., San Rafael, CA). Electrodes had resistances of 0.8–8.0 MΩ when filled with an internal solution consisting of (in mm): KCl 153, MgCl2 1, MgATP 2, HEPES 10, EGTA 5, pH 7.3, 300 mOsm. Lower resistance electrodes were used for experiments in which cells were lifted from the recording dish (see Fig. 5). Higher resistance electrodes were used for single-channel recordings and were coated with Q-dope. The combination of internal and external solutions produced a chloride equilibrium potential near 0 mV. Unless stated otherwise, cells were voltage clamped at −10 to −75 mV using either an Axon 1D or a 200A amplifier (Axon Instruments, Foster City, CA). No voltage-dependent effects were observed in this range. Tetrahydrodeoxycorticosterone (THDOC) (Sigma, St. Louis, MO) was prepared as a 10 mm stock in dimethylsulfoxide (DMSO). THDOC was dissolved in external solution containing DMSO at a maximal final concentration of 0.1%. For most experiments, drugs were applied using a modified U-tube (Greenfield et al., 1997). For preapplication experiments, drugs were delivered (via gravity) to whole cells using a rapid perfusion system consisting of three-barrel square glass connected to a Warner Perfusion Fast-Step (Warner Instrument Corp., Hamden, CT). The glass was pulled to a final barrel size of ∼250 μm. The solution exchange time was estimated routinely by stepping a dilute external solution across the open electrode tip to measure a liquid junction current. The 10–90% rise times for solution exchange were consistently ≤1–2 msec, although exchange was probably slower around cells. For single-channel experiments, drugs were applied either directly to the bath or via the multibarrel apparatus.
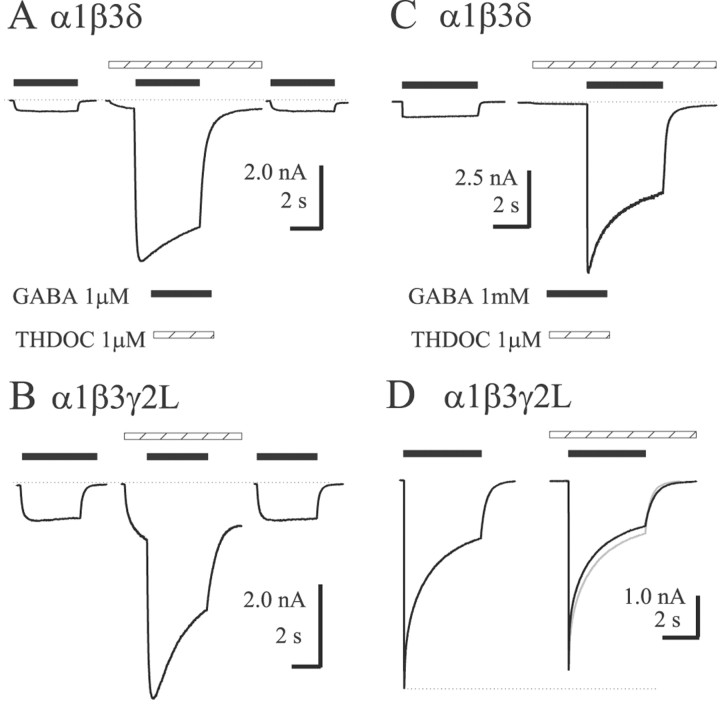
Fig. 5.
Kinetics and polarity of THDOC modulation depend on subunit composition and GABA concentration. THDOC (1 μm) was preapplied with 1 μm GABA (A, B) and 1 mm GABA (C, D) using the concentration jump technique. Hatched bars indicate THDOC application;filled bars indicate GABA application. For the rapidly desensitizing α1β3γ2L receptors, cells were lifted from the recording dish to increase resolution of the peak currents. Direct activation was observed during the preapplication for both isoforms, with greater relative currents evoked from α1β3γ2L. Greater enhancement of 1 μm GABA currents was observed for α1β3δ receptors, although both isoforms showed slightly increased desensitization and prolonged deactivation (A,B). With 1 mm GABA, α1β3δ receptors were enhanced substantially and pronounced desensitization was observed (C), whereas α1β3γ2L receptors were slightly inhibited (D). The control trace in D was normalized and overlaid ingray to show the minimal effect on apparent desensitization. The dashed line emphasizes the decreased peak current in the presence of THDOC.
Analysis of currents. Whole-cell currents were low-pass filtered at 2–5 kHz, digitized at 10 kHz, and analyzed using the pCLAMP8 software suite (Axon Instruments). For concentration–response plots, peak currents evoked by GABA or THDOC at multiple concentrations were fitted to a sigmoidal function using a four-parameter logistic equation (sigmoidal concentration–response) with a variable slope. The equation used to fit the concentration–response relationship was I = I(max)/1 + 10(LogEC50 − Logdrug)∗Hill slope, where I was the peak current at a given GABA concentration, andI(max) was the maximal peak current. The desensitization and deactivation time courses of GABAA receptor currents elicited with the concentration jump technique were fit using the Levenberg-Marquardt least squares method with one or two or three component exponential functions of the form Σanτn, wheren is the best number of exponential components, ais the relative amplitude of the component, and τ is the time constant. Additional components were accepted only if they significantly improved the fit, as determined by an Ftest on the sum of squared residuals. For comparison of deactivation time courses, a weighted summation of the fast and slow decay components (af ∗ τf + as ∗ τs) was used. Single-channel data were digitized at 20 kHz, filtered at 2 kHz via the internal Axon 200A amplifier filter, and stored on VHS videotape for analysis off-line. Stretches of single-channel activity were analyzed using the 50% threshold detection method of Fetchan 6.0 (pClamp 8.0). Overlapped openings and bursts were not included in the analysis. Although overlapping openings, indicating multiple channels, were observed in most patches, they would not affect the open duration histograms. Open duration histograms were generated and fitted using Interval5 software (Dr. Barry S. Pallotta, University of North Carolina, Chapel Hill, NC). The number of exponential functions required to describe the data was determined by a log-likelihood method (additional components were accepted if they significantly improved the fit). Events with durations <150 μsec (1.5 times the system dead time) were shown in the plots but were not considered by the fitting routine. Additional data reduction and filtering were implemented for figure display purposes only. Numerical data were expressed as mean ± SEM. Statistical significance, using Student's t test (two-tailed, paired, or unpaired as appropriate) was taken as p < 0.05.
RESULTS
Direct activation by THDOC depended on subunit composition
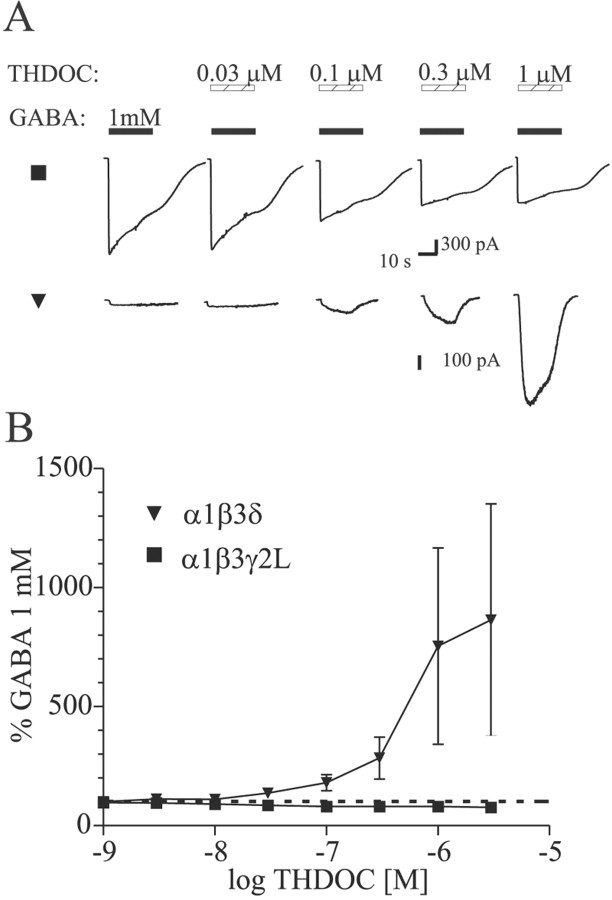
All constructs produced THDOC-sensitive currents in HEK293T cells. Cells were voltage clamped at −65 mV, and whole-cell currents were recorded in response to increasing concentrations of THDOC (Fig.1A,C). The GABAA receptor isoforms exhibited different THDOC sensitivities (EC50 values) (Fig.1C). Although there is little mechanistic information in this analysis, it is necessary to describe direct activation so that appropriate concentrations can be chosen for subsequent modulation experiments (see below). Additionally, we observed a subunit and subtype dependence of the direct effects of THDOC. α6 subtype-containing receptors had lower EC50values for THDOC activation than α1 subtype-containing receptors (p < 0.05), similar to observations for GABA concentration–response curves (Fisher et al., 1997) (Fig.1C, Table 1). The α1β3γ2L isoform was ∼2.5-fold less sensitive to THDOC than the α6β3γ2L isoform. The α1β3δ isoform was at least sixfold less sensitive to THDOC than the α6β3δ isoform. Regarding the α1β3δ receptor complex, a complete concentration–response curve could not be obtained because of high final DMSO concentration (>0.3%) with higher THDOC concentrations (>30 μm). The direct activation was first observed at 30–100 nm THDOC for all receptor isoforms. At higher concentrations of THDOC (>10 μm), a “rebound” current was observed on washout in all constructs. The rebound was more clearly evident under conditions of faster perfusion (Fig. 1B), which may explain why this effect was not reported previously. Maximum currents were significantly different between γ2L and δ subunit-containing receptors (p < 0.05) (Table 1). THDOC elicited larger currents from GABAA receptors containing the γ2L subunit than from receptors containing the δ subunit (Fig.1C, Table 1). α1β3 and α6β3 isoforms showed current amplitudes intermediate between αβδ and αβγ isoforms (Fig.1C). For each isoform, maximum currents were in the same range of peak amplitudes when evoked by THDOC or GABA (data not shown).
Fig. 1.
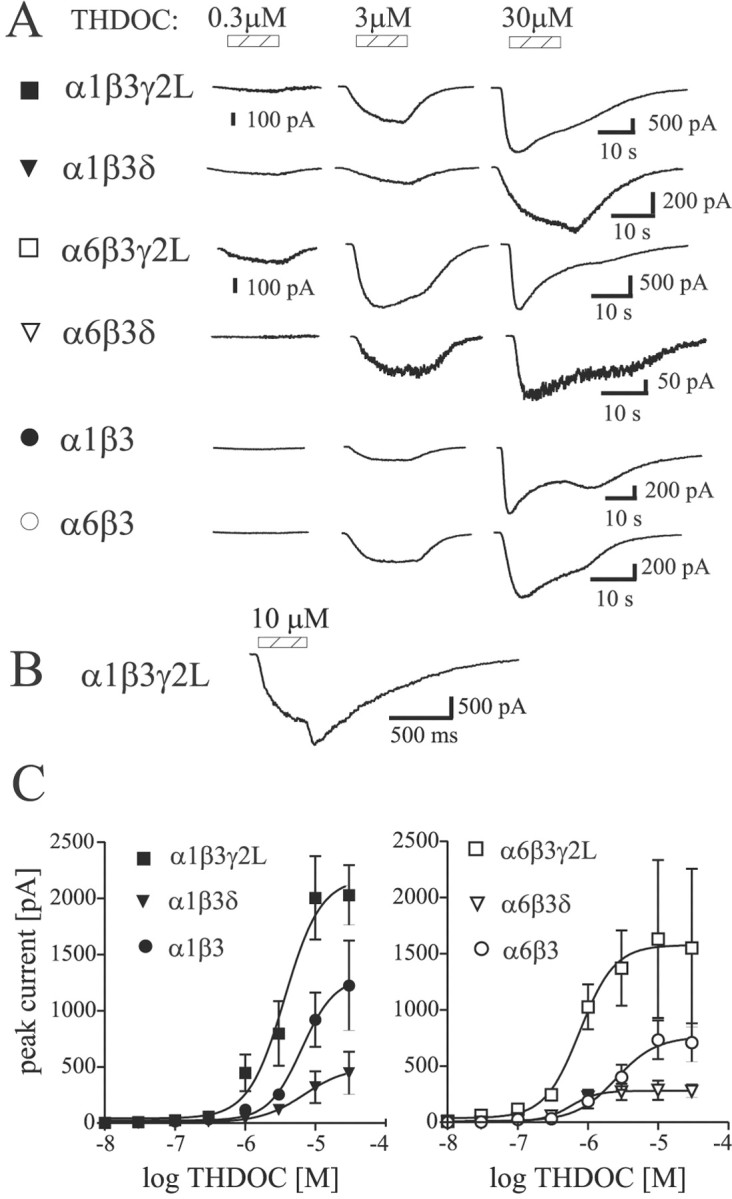
Direct activation of GABAA receptors by THDOC. A, Representative currents evoked by increasing THDOC concentrations for α1β3γ2L (▪), α1β3δ (▾), α6β3γ2L (■), α6β3δ (▿), α1β3 (●), and α6β3 (○) GABAA receptor isoforms. The hatched bars indicate application of various THDOC concentrations. Note the small inflections in the currents after application of 30 μm THDOC. B, Concentration jump using 10 μm alphaxalone shows a rebound current more clearly because of faster solution exchange (see Materials and Methods).C, Concentration–response relations for direct activation by THDOC. Mean ± SEM current amplitudes are shown. Theleft and right panels show α1- and α6-containing isoforms, respectively. Smaller currents were observed for δ subunit-containing isoforms. Symbols are as inA. See Table 1 for fitted parameters.
Table 1.
Pharmacological properties of studied GABAAreceptors
| Isoform | THDOC | GABA | THDOC + GABA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | EC50 | nH | Imax(pA) | n | EC50 | nH | n | EC50 | nH | Max (%) | |
| α1β3γ2L | 3 | 1.9 | 1.4 | 2141 ± 200 | 4 | 2.3 | 1.4 | 6 | 2.8 | 1.5 | 128 ± 10 |
| α1β3δ | 4 | 9.51-a | 0.9 | 498 ± 150 | 4 | 5.8 | 1.0 | 4 | 6.6 | 1.1 | 265 ± 47* |
| α1β3 | 3 | 6.7 | 1.1 | 1302 ± 360 | 3 | 3.0 | 2.0 | 3 | 1.7 | 1.6 | 113 ± 4 |
| α6β3γ2L | 4 | 0.7 | 2.2 | 1588 ± 700 | 5 | 0.6 | 1.3 | 4 | 0.6 | 1.3 | 124 ± 6 |
| α6β3δ | 3 | 1.4 | 1.3 | 285 ± 60 | 4 | 0.6 | 1.1 | 5 | 0.4 | 1.3 | 145 ± 15 |
| α6β3 | 3 | 2.6 | 1.6 | 778 ± 130 | 3 | 0.6 | 1.2 | 3 | 1.2 | 1.1 | 152 ± 26 |
n indicates the number of cells tested. All EC50 values are in micromolar. nH is the hill coefficient. Max (%) indicates the maximum current observed with THDOC + GABA compared with maximum current observed with GABA alone.
Significant difference from all other isoforms (p < 0.05).
Indicates that full concentration–response curve could not be obtained because of high DMSO vehicle concentration.
THDOC produced increased modulation of α1β3δ receptor currents
To evaluate the effect of subunit composition on THDOC modulation of GABAA receptor currents, increasing concentrations of THDOC were coapplied with an EC30 GABA concentration determined for each isoform. Low THDOC concentrations (<300 nm) were considered “modulatory” concentrations, because little or no direct activation was observed in this range in whole-cell recordings. Higher concentrations of THDOC resulted in more substantial direct activation of GABAA receptor currents. Neurosteroid modulation was observed for all isoforms tested (Fig.2). For α1β3δ receptors, the extent of the modulatory effect was more pronounced than for the other isoforms. For α1β3δ and α1β3γ2L receptors, a significant difference in THDOC-induced potentiation was detected for concentrations of 100 nm, 300 nm, and 1 μm (p < 0.05). At concentrations below 100 nm, the modulatory effect of THDOC was not significant different among all isoforms tested. Replacement of δ with γ2L subunits reduced the apparent GABAAreceptor sensitivity to THDOC potentiation. The α6 subtype-containing GABAA receptors were enhanced similarly by THDOC, whether the δ or γ2L subunit was present.
Fig. 2.
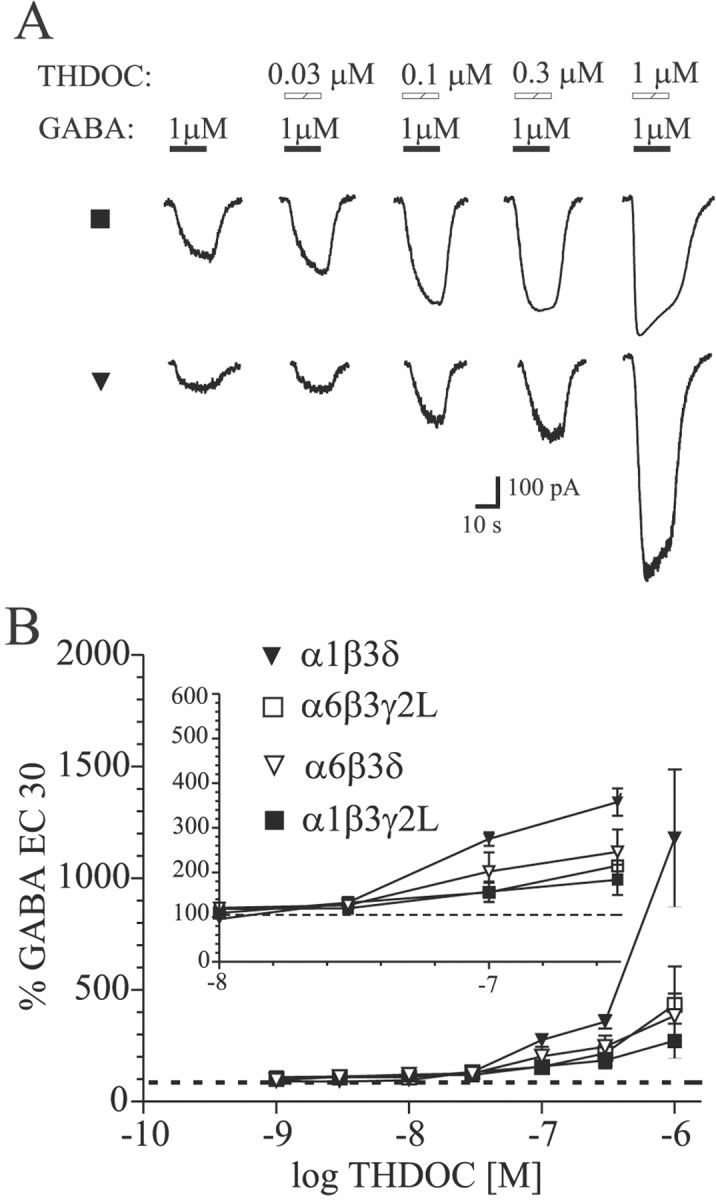
Modulation of submaximal GABA-evoked currents by THDOC coapplication. A, Current traces showing THDOC enhancement of EC30 GABA concentrations for α1β3γ2L (▪) and α1β3δ (▾) GABAA receptors. Hatched bars indicate THDOC application; filled barsindicate GABA application. B, Summary plot of THDOC enhancement of currents evoked by EC30 GABA concentration for each isoform. Data from lower THDOC concentrations are expanded in the inset for clarity. Significant potentiation was observed for THDOC concentrations of 100 nm and higher (p < 0.05).
THDOC enhanced GABAA receptor currents without changing the GABA EC50
Although concentration–response curves are empirical descriptions and do not specify any particular mechanism, the effect of modulators on the GABA concentration–response relation can provide insight into modulator mechanism(s) of action. Thus, complete GABA concentration–response curves with and without 30 nm THDOC were obtained for each isoform (Fig. 3). A THDOC concentration of 30 nm was chosen as a modulatory concentration because it was less than EC5for THDOC direct activation for each isoform (Fig. 1), thus minimizing the effect of direct activation. Furthermore, in each cell, 30 nm THDOC was applied alone to verify the minimal activation of current compared with GABA-evoked currents and to confirm that such currents, when present, were not significantly affecting the analysis. For all GABAA receptor isoforms tested, THDOC clearly enhanced the GABA-activated currents. Although the GABA EC50 values remained unchanged, the maximum currents were significantly enhanced for each isoform (p < 0.05) (Table 1). The extent of potentiation by THDOC was significantly greater for the α1β3δ isoform (Fig. 3C) than for the α1β3γ2L isoform (Fig.3A) and the α1β3δ isoform (Fig. 3E) (p < 0.05). No significant difference in THDOC-induced potentiation was detected among α6β3γ2L, α6β3δ, and α6β3 receptors (Fig.3B,D,F). Focusing on α subunit subtype dependence, the extent of potentiation by THDOC was significantly higher for α1β3δ isoforms than for α6β3δ isoforms (p < 0.05) and higher for α6β3 than for α1β3 isoforms (p < 0.05), whereas no significant difference was found between GABAA receptors containing α1β3γ2L and α6β3γ2L subunits.
Fig. 3.
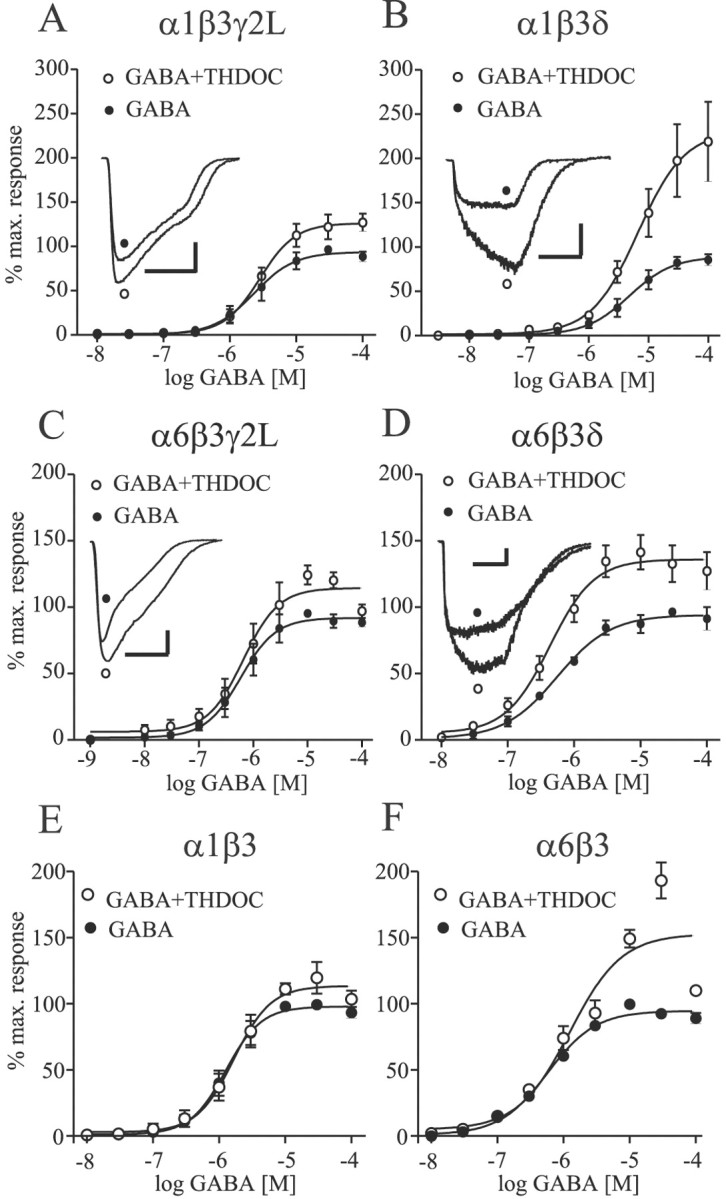
THDOC enhanced the maximal GABAAreceptor currents without changing the GABA EC50.A–D, GABA concentration–response curves were obtained in the absence (●) and presence (○) of 30 nm THDOC for α1β3γ2L (A), α1β3δ (B), α6β3γ2L (C), α6β3δ (D), α1β3 (E), and α6β3 (F) isoforms. Representative maximal GABA currents without (●) and with (○) THDOC coapplication are shown in the inset of A–D. For the GABA plus THDOC curves, the currents were normalized to the amplitude of a 100 μm GABA test pulse obtained from the same cell. Fitted parameters are given in Table 1.
Neurosteroid modulation changed polarity at high GABA concentration
To investigate further the differences in neurosteroid modulation of α1β3γ2L and α1β3δ isoforms at high GABA concentration likely to occur at synapses, we coapplied increasing concentrations of THDOC with 1 mm GABA (Fig.4A). We chose these two GABAA receptors because of the significant difference in their THDOC-induced modulation described earlier. Consistent with previous data from cerebellar granule neurons (Zhu and Vicini, 1997), at a saturating concentration of GABA, the polarity of THDOC modulation of α1β3γ2L receptors reversed (Fig.4B): peak currents were inhibited by THDOC in a concentration-dependent manner. In contrast, α1β3δ receptor currents were increasingly potentiated by THDOC concentrations (up to 30 μm) (Fig. 4B). Although direct activation of GABAA receptor currents occurred at high THDOC concentrations, the strong potentiation observed for α1β3δ receptors could not be accounted for simply by increased direct activation (Fig. 5).
Fig. 4.
Modulation of maximal GABA-evoked currents by THDOC coapplication. A, Increasing concentrations of THDOC were coapplied with 1 mm GABA to α1β3γ2L (▪) and α1β3δ (▾) GABAA receptors. Representative traces are shown for each isoform. Hatched bars indicate THDOC application; filled bars indicate GABA application. B, Summary of THDOC modulation, showing increasing enhancement for α1β3δ receptors and inhibition for α1β3γ2L receptors. The dashed line indicates 100% of control (1 mm GABA alone) current amplitude.Symbols indicate the mean ± SEM responses of four cells for each isoform.
THDOC altered the macroscopic desensitization of α1β3δ GABAA receptor currents
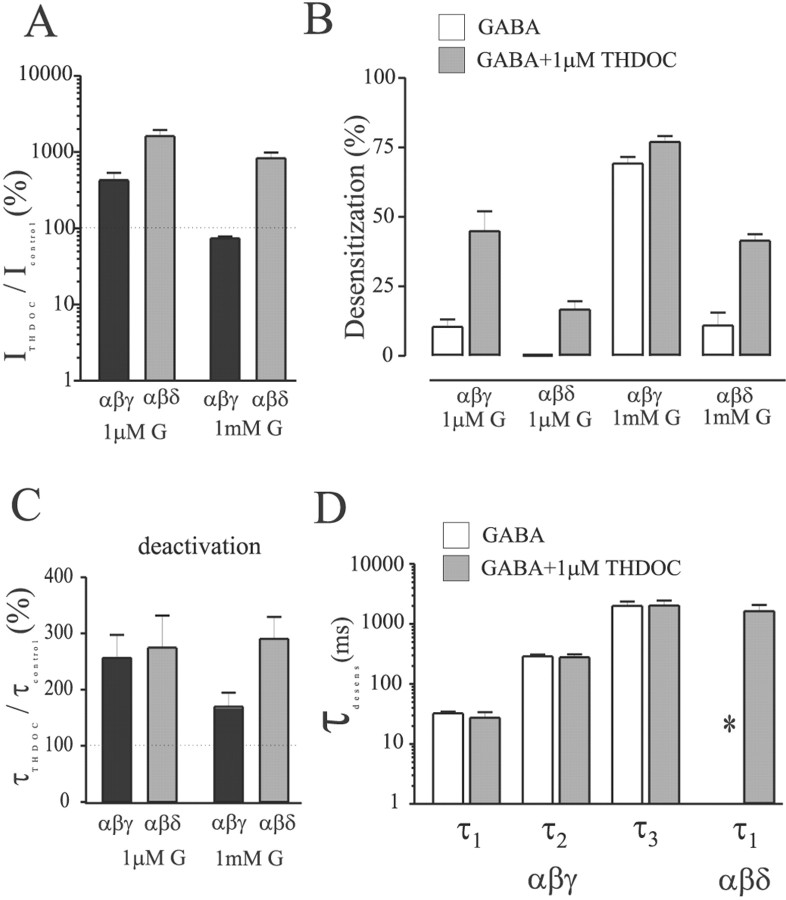
Having established a clear difference between modulation of α1β3γ2L and α1β3δ currents by THDOC, we used the concentration jump technique to determine the effects on macroscopic kinetics using preapplication of THDOC (Figs. 5,6). After obtaining a control response to GABA, cells were jumped from control solution to THDOC (1 μm) alone for preincubation of at least 1.5 sec, then to THDOC (1 μm) plus GABA (1 mm) for 4 sec, and then allowed to deactivate in the presence of THDOC (1 μm) alone. This allowed us to separate the effects of direct activation from modulation of the currents (because the THDOC was preapplied), and the rapid solution changes allowed better resolution of peak currents, desensitization, and deactivation. Also, the preapplication protocol ensured that the receptors were equilibrated with THDOC at the time of GABA application. THDOC (1 μm) reversibly potentiated both α1β3δ (Fig.5A) and α1β3γ2L (Fig. 5B) GABAA receptor currents elicited by low (∼EC30) concentrations of GABA. The mean enhancement was fourfold larger for α1β3δ GABAA receptors (∼1600%; n = 6) than for α1β3γ2L receptors (∼400%; n = 6) (p < 0.05) (Fig. 6A,left pair of bars). Although the potentiated currents desensitized to a greater extent (measured as the percentage of current “lost” relative to peak current) than control currents for both isoforms, this effect was more pronounced for α1β3γ2L receptors. The left half of Figure 6B shows the subunit-dependent differences in desensitization extent in the presence of low concentration GABA with or without THDOC (compareopen and shaded bars for each isoform). After washout of 1 μm GABA, the deactivation rate of THDOC-modulated currents was prolonged similarly for both isoforms (∼250%) (Fig. 5A,B). The left pair of bars in Figure 6C shows THDOC-induced changes in the time constant of deactivation after washout of low concentration GABA.
Fig. 6.
Summary of THDOC effects on peak currents, desensitization, and deactivation. A, Effect of THDOC (1 μm) on currents evoked by 1 μm and 1 mm GABA for α1β3δ (gray bars) and α1β3γ2L (black bars) GABAAreceptors. Values are expressed as a percentage of current evoked by GABA alone for each cell. Note the logarithmic axis. The dashed line indicates 100% of control current amplitude.B, Percentage increase in the weighted time constant of deactivation for the same conditions as in A. Thedashed line indicates 100% of control deactivation.C, Extent of desensitization observed with 1 mm GABA alone (white bars) or 1 mm GABA + 1 μm THDOC (gray bars) for α1β3δ and α1β3γ2L GABAAreceptors. Data are expressed as the percentage of peak current lost during a 4 sec application of GABA or GABA + THDOC. D, The rates of desensitization during a 4 sec pulse of 1 mmGABA for α1β3δ and α1β3γ2L GABAA receptors in the absence (white bars) and presence of preapplied 1 μm THDOC. α1β3γ2L GABAA receptor responses were fitted best by three exponentials with similar time constants and relative areas (data not shown) whether or not THDOC was present. Although the small amount of desensitization observed for α1β3δ in the presence of GABA alone was not well fitted (∗, the time constant was longer than the pulse duration), the pronounced desensitization observed in the presence of THDOC had a weighted time constant ∼2 sec. The data are from four to eight cells per condition.
The polarity of THDOC modulation of currents evoked by coapplication of a saturating (1 mm) concentration of GABA depended on subunit composition (Fig. 4). Similar results were observed during concentration jump experiments in which THDOC was preapplied. After a control response to GABA alone (1 mm) was obtained, cells were jumped into THDOC alone (1 μm) for a 1.5 sec preincubation followed by a 4 sec pulse of GABA + THDOC and then allowed to deactivate in the presence of THDOC. α1β3δ GABAA receptors were enhanced by ∼800% (n = 8) (Fig. 5C), whereas α1β3γ2L GABAA receptors were inhibited ∼20% (n = 4) (Fig. 5D). The right half of Figure6A summarizes this difference in THDOC modulation of peak current using saturating GABA concentration. The concentration jump experiments allowed resolution of subunit-dependent differences in the macroscopic kinetics of THDOC modulation as well. Specifically, THDOC (1 μm) substantially increased the rate and extent of desensitization of α1β3δ currents (Figs.5C, 6B). α1β3δ receptors normally exhibit minimal desensitization, even in the presence of saturating (1 mm) GABA concentrations (Fig.6B) (Saxena and Macdonald, 1994; Haas and Macdonald, 1999). In contrast, peak currents were inhibited, and the extent of macroscopic desensitization was unaltered by THDOC for α1β3γ2L receptor currents (Fig. 5D). Figure 6B(right half) summarizes the changes in extent of desensitization when 1 mm GABA was applied with or without THDOC (1 μm). Furthermore, the time course of desensitization of α1β3γ2L receptor currents evoked by 1 mm GABA was fitted best with the sum of three exponential functions with similar rate constants in the presence or absence of THDOC (Fib. 6D, left portion, compare open and shaded bars). The rate of desensitization could not be measured accurately for α1β3δ receptors during 4 sec pulses (because the time constant was much longer than the application duration). However, in the presence of THDOC, the weighted desensitization time constant decreased to ∼2 sec (Fig. 6D). Although current deactivation after washout of 1 mm GABA was prolonged by THDOC (1 μm) for both isoforms, the relative increase was smaller for α1β3γ2L GABAA receptors (p < 0.05) (Fig. 6C, right pair of bars).
THDOC introduced a third, longer open state for α1β3δ GABAA receptors
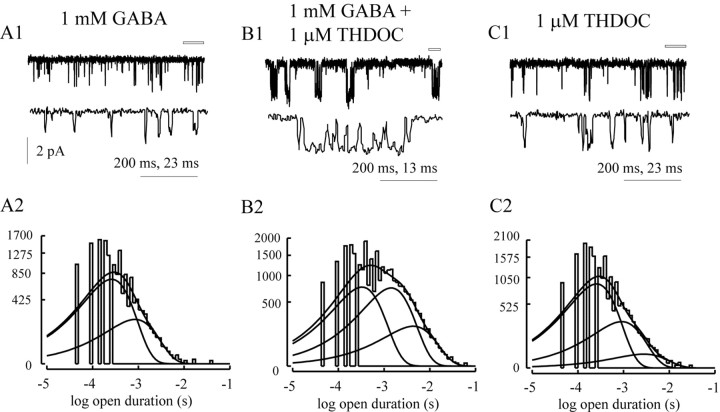
Single-channel recordings were obtained from α1β3δ receptors to investigate the basis for the large change in efficacy produced by THDOC. Consistent with our previous reports (Fisher and Macdonald, 1997; Haas and Macdonald, 1999), α1β3δ GABAA receptor single-channel openings evoked during steady-state application of 1 mm GABA were brief (Fig. 7A1), with a mean open duration of 0.445 ± 0.026 msec (Table2). The distribution of open durations was best described by the sum of two exponential functions with time constants of ∼300 μsec and ∼1 msec (Fig. 7A2). Coapplication of 1 μm THDOC increased the mean channel opening duration. The distribution of open durations required a third exponential function to account for the longer openings, with a time constant of 5.94 ± 0.98 msec and relative area of 9.8 ± 2.9%. Although the shortest exponential function had a similar time constant, the second time constant and its relative area were increased significantly compared with the openings evoked by GABA alone (p < 0.05) (Table 2). Because THDOC (1 μm) can directly activate GABAA receptor currents, we also measured single-channel currents from α1β3δ GABAAreceptors in the presence of THDOC (1 μm) alone. The mean open duration was not different from that observed with GABA alone, and the first two open durations were also unchanged in terms of time constant and relative area (Table 2). However, a third open state with small relative area (4.0 ± 1.6%) was required to fit the distribution. The longer open state in the presence of THDOC alone accounted for 12.4% of the charge passed, whereas the longer open state in the presence of both drugs accounted for 39.0% of the charge passed. Although neurosteroids have been reported to increase the frequency of channel openings, we did not consider changes in open frequency because of the confounding appearance of desensitization in the presence of THDOC macroscopically (Fig. 5). At the single-channel level, quiescent periods rarely observed with GABA alone would decrease overall opening frequency in the presence of GABA and THDOC for α1β3δ GABAA receptor channels.
Fig. 7.
THDOC enhanced single-channel open duration in α1β3δ GABAA receptor single channels. Representative α1β3δ GABAA receptor single-channel currents evoked by 1 mm GABA alone (A1), 1 mmGABA + 1 m THDOC (B1), and 1 mTHDOC alone (C1). A portion of the top trace in each panel is expanded in the trace directly beneath it (indicated by the open bar). Thetraces in A1 and B1 are from the same patch. The larger scale factor applies to the top traces. A2, B2, andC2 are the open duration histograms for all patches obtained for each condition (n = 3, 5, and 3 respectively). Superimposed lines are the fitted exponential functions describing the distributions.
Table 2.
Single-channel opening properties for α1β3δ GABAA receptors
| Drug | τ1 | A1 | τ2 | A2 | τ3 | A3 | Mean | n |
|---|---|---|---|---|---|---|---|---|
| 1 mm GABA | 0.31 ± 0.02 | 0.81 ± 0.06 | 1.02 ± 0.09 | 0.19 ± 0.06 | 0.444 ± 0.026 | 3 | ||
| 1 mm GABA + 1 μm THDOC | 0.357 ± 0.02 | 0.43 ± 0.04 | 1.822 ± 0.20 | 4.47 ± 0.03 | 5.94 ± 0.98 | 0.10 ± 0.03 | 1.52 ± 0.15 | 5 |
| 1 μm THDOC | 0.29 ± 0.02 | 0.72 ± 0.02 | 1.00 ± 0.23 | 0.24 ± 0.01 | 3.94 ± 1.70 | 0.04 ± 0.02 | 0.535 ± 0.054 | 3 |
The open duration histograms were fitted with multiple exponential functions, where τ is the time constant and A is the relative area.
DISCUSSION
GABAA receptors are targets for CNS actions of neurosteroids. Our results demonstrated a novel subunit dependence of neurosteroid action. Specifically, receptors containing the δ subunit were preferentially enhanced by the neurosteroid THDOC. THDOC affected both the single-channel gating kinetics and the macroscopic desensitization of α1β3δ GABAA receptor channels. These findings may be the basis for the attenuated neurosteroid sensitivity in mice lacking the δ subunit (Mihalek et al., 1999). Additionally, our results suggest the importance of GABA concentration for THDOC modulation of receptors containing the γ2 subunit.
It is believed that neurosteroids, like barbiturates, exert their action on GABAA receptors via two distinct binding sites (Majewska et al., 1986; Gee et al., 1988; Turner et al., 1989; Lambert et al., 1995, 1996; Zorumski et al., 1998; Park-Chung et al., 1999). Low (nanomolar) concentrations of neurosteroids allosterically enhance GABA-mediated currents, whereas higher (micromolar) concentrations directly activate GABAA receptors. We observed a rebound current on washout of THDOC for concentrations ≥10 μm in all tested GABAA receptor isoforms. This phenomenon may indicate a third binding site, presumably within the channel pore, that produces a low-affinity open-channel block similar to that observed for barbiturates.
Although no clear subunit specificity has been demonstrated for neurosteroid modulation of GABAA receptor currents as there has been for benzodiazepine modulation of GABAA receptor currents (Lambert et al., 1995), the α and γ subunits in GABAA receptors have some influence on the EC50 and efficacy of modulation by neurosteroids such as THDOC that act as positive modulators (Puia et al., 1993). Puia et al. (1990) and Zhu et al. (1996) reported no subunit-dependent differences in the sensitivity to THDOC activation or amplitude of THDOC-evoked currents among different GABAA receptor isoforms. However, our results indicate that the α1 and α6 subtypes conferred different EC50 values for THDOC, similar to the difference reported for GABA (Fisher et al., 1997). THDOC and GABA were equally effective as agonists for all studied GABAAreceptors, as indicated by peak currents obtained in whole-cell recordings. However, the maximum current amplitudes produced by THDOC and GABA were larger for γ than for δ subunit-containing receptors, which might be related to differences in expression efficiency or intrinsic gating efficacy. This difference has been reported for currents evoked by GABA as well (Fisher and Macdonald, 1997; Haas and Macdonald, 1999). However, the basis for this difference remains unclear.
Zhu et al. (1996) reported that incorporation of the δ subunit inhibited the modulatory (but not the direct) effect of THDOC on GABAA receptor currents. In contrast, we found a significant neurosteroid potentiation in all tested receptors, and α1β3δ receptors were potentiated more than α1β3γ2L and α1β3 receptors. Replacement of GABAA receptor α1 with α6 subtypes in αβδ receptors decreased the extent of THDOC potentiation, although THDOC enhanced both α1β3γ2L and α6β3γ2L GABAA receptor currents to similar extents. The latter finding contrasts with previous reports showing a decrease in steroid sensitivity with α6 compared with α1 in αβγ combinations (Puia et al., 1993; Zhu et al., 1996). Neurosteroid modulation of α1β3δ receptor currents was greater than that of α6β3δ and α1β3γ2L GABAAreceptor currents. However, dentate granule cells become less sensitive to THDOC with developmental progression (Cooper et al., 1999), despite an increase in δ subunit expression in this brain region (Laurie et al., 1992a). This may be related to variable steroid modulation when δ subunits coassemble with α subtypes other than α1, or when γ and δ subunits are present in the same receptor. Additionally, it is possible that a neuronal environment alters the sensitivity of αβδ GABAA receptors. In fact, by combining electrophysiological recordings and single-cell PCR techniques, Zhu and Vicini (1996) observed an inverse relation between the presence of δ subunit mRNA and neurosteroid potentiation in cultured cerebellar granule cells. Also, there is evidence for a phosphorylation dependence of allopregnanalone modulation of GABAergic IPSCs in the hypothalamus (Fancsik et al., 2000). Such post-translational receptor modifications may contribute to potential differences between neuronal preparations and recombinant systems, among other cellular processes. Nevertheless, our results indicated that δ subunit-containing GABAA receptors are clearly enhanced by neurosteroids, particularly in combination with the α1 subunit subtype, suggesting a critical role for the δ subunit in the assembly of neurosteroid-sensitive GABAA receptors. This observation is strengthened by the small degree of potentiation observed in α1β3 GABAA receptors. The enhanced THDOC sensitivity of αβδ isoforms was in agreement with a recent report by Mihalek et al. (1999), who found that the absence of the δ subunit resulted in a significant decrease in the sensitivity to neurosteroids. However, the precise mechanism by which the δ subunit knock-out attenuated neurosteroid effects awaits further study. It should be noted that GABAA receptors containing both γ2 and δ subunits may be present in vivo. Whether the presence of a δ subunit would have a “dominant” effect on neurosteroid modulation remains unknown. Our data also support a recent study showing preferential steroid enhancement of α4β3δ over α4β3γ2 GABAA receptors (Adkins et al., 2001).
We have shown previously that incorporation of the δ subunit abolishes fast desensitization and reduces the overall rate and extent of desensitization (Saxena and Macdonald, 1994; Haas and Macdonald, 1999; Bianchi et al., 2001). Even at saturating (1 mm) GABA concentrations, minimal desensitization and relatively fast deactivation are observed. However, in this study we found pronounced desensitization in α1β3δ GABAA receptors in the presence of 1 μm THDOC. This was probably not caused by open channel block because single-channel open durations were longer, not shorter as would be expected with such a mechanism. The apparent desensitization was accompanied by substantially prolonged deactivation. Although this finding is consistent with the suggested role of desensitized states in the duration of current deactivation (Jones and Westbrook, 1995, 1996), an increase in open frequency and duration could also prolong deactivation. In fact, single-channel recordings revealed that THDOC enhanced current through α1β3δ GABAA receptors, at least through an increase in mean open duration. Steroids were reported previously to increase open duration (Mistry and Cottrell, 1990) and increase frequency as well as duration (Twyman and Macdonald, 1992) in native GABAA receptor single channels obtained from mouse spinal neurons, although it is unlikely that these channels contained the δ subunit. We did not analyze open frequency because of the introduction of desensitized states in α1β3δ GABAA receptors. Periods of desensitization, however, would not confound the analysis of open durations. Although saturating GABA concentrations evoked single-channel openings best described by two exponential functions, a third longer duration open state was observed in the presence of THDOC. We propose that THDOC alters the intrinsic gating behavior of α1β3δ GABAA receptors in at least two ways: (1) by allowing entry into otherwise unavailable desensitized states and (2) by increasing the gating efficacy via changes in opening duration. This change was attributable to an increase in the time constant of the second open state (1.8 compared with 1.0 msec; p < 0.05), a change in the relative proportion of the first and second open states, and the introduction of an additional longer open state (Table2). Note that the modulation of single-channel gating was measured at steady state, so no direct comparisons (in terms of the magnitude of THDOC modulation) can be made with the transient applications performed on whole cells. Although the binding of GABA alone appears insufficient to allow entry into the longer open state, the concomitant binding of THDOC favors transitions to the longer state. Interestingly, THDOC alone (1 μm) activated single-channel events that were well described by three exponential functions, suggesting that neurosteroid binding alone may be sufficient to favor longer openings, although these longer openings were shorter and less frequent than the longer openings observed in the presence of both drugs. Thus it is unlikely that receptors bound only by THDOC contributed to the distinct gating behavior observed in the presence of both drugs. Additionally, the concentration of GABA (1 mm) was more than two orders of magnitude above the functional EC50 value for the α1β3δ combination, resulting in near-saturating occupancy of the GABA binding sites at steady state. It remains unclear whether this effect is unique to the α1β3δ isoform. However, given the weakly inhibited amplitude and similar desensitization time course in the presence of THDOC, it is unlikely that such dramatic effects on single-channel gating would be observed for α1β3γ2L GABAA receptor single channels.
The action of THDOC on channel gating kinetics may be analogous to the effects of barbiturates, which have been shown to prolong native (likely αβγ) GABAA receptor single-channel mean open time by shifting the relative distribution of existing open durations, and similar effects were observed for neurosteroids (Twyman et al., 1989; Twyman and Macdonald, 1992). However, because the longer open state is not significant in the presence of GABA alone, we cannot explicitly demonstrate that this state can be accessed “naturally” by receptors bound by GABA alone. It is unknown whether barbiturates or other modulators can alter the gating behavior of αβδ GABAA receptors in a manner similar to THDOC.
For α1β3γ2L GABAA receptors, modulation of maximal currents by 1 μm THDOC was similar to that reported for cerebellar granule neurons (Zhu and Vicini, 1997) in that peak currents were depressed and deactivation was prolonged, despite no change in the time course of desensitization. This effect contrasts with neurosteroid potentiation of currents activated by low GABA concentration in cerebellar granule neurons (Zhu and Vicini, 1997) and α1β3γ2L GABAA receptors (this study). Although nucleated patches obtained from cerebellar granule neurons in that study were likely to contain extrasynaptic receptors [whichNusser et al. (1998) reported to contain the δ subunit], our data are consistent with γ2 subunit- but not δ subunit-containing isoforms being predominant in that preparation.
In the cerebellum, δ subunit-containing receptors are thought to be extrasynaptic, whereas synaptic receptors are thought to preferentially contain the γ2 subunit (Nusser et al., 1998). However, Mihalek et al. (1999) suggested δ subunit involvement in normal synaptic transmission in the dentate gyrus. Although the synaptic concentration of GABA remains controversial, our results and those of others (Harrison et al., 1987; Zhu and Vicini, 1997) suggest that neurosteroids may modulate IPSCs in at least two ways: (1) by changing the peak current (either positively or negatively, depending on the concentration of both GABA and the neurosteroid) and (2) by prolonging the duration of the IPSC (by slowing deactivation) independent of the GABA concentration. For extrasynaptic αβδ isoforms, neurosteroids may increase basal levels of inhibition by increasing the response to ambient GABA levels. It is also possible that modulation of neuronal circuits necessary for neurosteroid effects depend (either directly or indirectly) on GABAA receptors containing the δ subunit.
In summary, our results showed that the subunit composition of GABAA receptors is an important determinant of the neurosteroid modulation of GABAA receptor activity. Although the precise contribution of αβδ GABAA receptor combinations toward the in vivo effects of neurosteroids remains to be elucidated, the enhanced potentiation of α1β3δ GABAAreceptors may indicate a critical role for this isoform.
Footnotes
This work was supported by National Institutes of Health Grant R01-NS33300 (R.L.M.), the Deutsche Forschungsgemeinschaft WO 770/1-1 (K.M.W.), and a National Institute on Drug Abuse training fellowship T32-DA07281-03 (M.T.B.).
Correspondence should be addressed to Dr. Robert L. Macdonald, Department of Neurology, Vanderbilt University, 2100 Pierce Avenue, Nashville, TN 37212. E-mail:Robert.Macdonald@mcmail.vanderbilt.edu.
REFERENCES
- 1.Adkins CE, Pillai GV, Kerby J, Bonnert TP, Haldon C, McKernan RM, Gonzalez JE, Oades K, Whiting PJ, Simpson PB. α4β3δ GABAA receptors characterized by fluorescence resonance energy transfer-derived measurements of membrane potential. J Biol Chem. 2001;276:38934–38939. doi: 10.1074/jbc.M104318200. [DOI] [PubMed] [Google Scholar]
- 2.Angelotti TP, Uhler MD, Macdonald RL. Assembly of GABAA receptor subunits: analysis of transient single-cell expression utilizing a fluorescent substrate/marker gene technique. J Neurosci. 1993;13:1418–1428. doi: 10.1523/JNEUROSCI.13-04-01418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnard EA, Skolnick P, Olson RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acid A receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- 4.Baulieu E-E, Robel P. Neurosteroids: a new brain function? J Steroid Biochem Mol Biol. 1990;37:395–403. doi: 10.1016/0960-0760(90)90490-c. [DOI] [PubMed] [Google Scholar]
- 5.Benke D, Meterns S, Trzeciak A, Gillesen D, Mohler H. Identification and immunohistochemical mapping of GABAA receptor subtypes containing the δ subunit in rat brain. FEBS Lett. 1991;283:145–149. doi: 10.1016/0014-5793(91)80573-l. [DOI] [PubMed] [Google Scholar]
- 6.Bianchi MT, Haas KF, Macdonald RL. Structural determinants of fast desensitization and desensitization-deactivation coupling in GABAA receptors. J Neurosci. 2001;21:1127–1136. doi: 10.1523/JNEUROSCI.21-04-01127.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper EJ, Johnston GAR, Edwards FA. Effects of naturally occurring neurosteroid on GABAA IPSCs during development in rat hippocampal or cerebellar slices. J Physiol (Lond) 1999;521.2:437–449. doi: 10.1111/j.1469-7793.1999.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fancsik A, Linn DM, Tasker JG. Neurosteroid modulation of GABA IPSCs is phosphorylation dependent. J Neurosci. 2000;20:3067–3075. doi: 10.1523/JNEUROSCI.20-09-03067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher JL, Macdonald RL. Single channel properties of GABAA receptors containing γ2 or δ subtypes expressed with α1 and β3 subtypes in L929 cells. J Physiol (Lond) 1997;505:283–297. doi: 10.1111/j.1469-7793.1997.283bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher JL, Zhang J, Macdonald RL. The role of α1 and α6 subtype amino-terminal domains in allosteric regulation of γ-aminobutyric acida receptor. Mol Pharmacol. 1997;52:714–724. doi: 10.1124/mol.52.4.714. [DOI] [PubMed] [Google Scholar]
- 11.Frye CA. The neurosteroid 3 alpha, 5 alpha-THP has antiseizure and possible neuroprotective effects in an animal model of epilepsy. Brain Res. 1995;696:113–120. doi: 10.1016/0006-8993(95)00793-p. [DOI] [PubMed] [Google Scholar]
- 12.Gee KW, Lan NC. γ-Aminobutyric acidA receptor complexes in rat cortex and spinal cord show differential responses to steroid modulation. Mol Pharmacol. 1991;40:995–999. [PubMed] [Google Scholar]
- 13.Gee KW, Bolger MB, Brinton RE, Coirini H, McEwen BS. Steroid modulation of the chloride ionophore in rat brain: structure-activity requirements, regional dependence and mechanism of action. J Pharmacol Exp Ther. 1988;246:803–812. [PubMed] [Google Scholar]
- 14.Green AR, Hainsworth AH, Jackson DM. GABA potentiation: a logical pharmacological approach for the treatment of acute ischaemic stroke. Neuropharmacology. 2000;39:1483–1494. doi: 10.1016/s0028-3908(99)00233-6. [DOI] [PubMed] [Google Scholar]
- 15.Greenfield LJ, Jr, Sun F, Neelands TR, Burgard EC, Donnelly IL, Macdonald RL. Expression of functional GABAA receptors in transfected L929 cells isolated by immunomagnetic bead separation. Neuropharmacology. 1997;36:63–73. doi: 10.1016/s0028-3908(96)00150-5. [DOI] [PubMed] [Google Scholar]
- 16.Haas KF, Macdonald RL. GABAA receptor subunit γ2 and δ subtypes confer unique kinetic properties on recombinant GABAA receptor currents in mouse fibroblasts. J Physiol (Lond) 1999;514:27–45. doi: 10.1111/j.1469-7793.1999.027af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison NL, Vicini S, Barker JL. A steroid anesthetic prolongs inhibitory postsynaptic currents in cultured rat hippocampal neurons. J Neurosci. 1987;7:604–609. doi: 10.1523/JNEUROSCI.07-02-00604.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones MV, Westbrook GL. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995;15:181–191. doi: 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 19.Jones MV, Westbrook GL. The impact of receptor desensitization on fast synaptic transmission. Trends Neurosci. 1996;19:96–101. doi: 10.1016/s0166-2236(96)80037-3. [DOI] [PubMed] [Google Scholar]
- 20.Lambert JJ, Belelli D, Hill-Venning C, Peters JA. Neurosteroids and GABAA receptor function. Trends Pharmacol Sci. 1995;16:295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- 21.Lambert JJ, Belelli D, Hill-Venning C, Peters JA. Neurosteroid modulation of native and recombinant GABAA receptors. Cell Mol Neurobiol. 1996;16:155–174. doi: 10.1007/BF02088174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lan NC, Gee KW, Bolger MB, Chen JS. Differential responses of expressed recombinant human γ-aminobutyric acidA receptors to neurosteroids. J Neurochem. 1991;57:1818–1821. doi: 10.1111/j.1471-4159.1991.tb06388.x. [DOI] [PubMed] [Google Scholar]
- 23.Laurie DJ, Seeburg PH, Wisden W. The distribution of thirteen GABA-A receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci. 1992a;12:1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laurie DJ, Seeburg PH, Wisden W. The distribution of thirteen GABA-A receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992b;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 26.Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 27.Mathews GC, Bolos-Sy AM, Holland KD, Isenberg KE, Covey DF, Ferrendelli JA, Rothman SM. Developmental alteration in GABAA receptor structure and physiological properties in cultured cerebellar granule neurons. Neuron. 1994;13:149–158. doi: 10.1016/0896-6273(94)90465-0. [DOI] [PubMed] [Google Scholar]
- 28.McKernan RM, Whiting PJ. Which GABAA receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- 29.Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi Z-P, Lagenaur C, Tretter V, Sieghart W, Anagnostaras G, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor delta subunit knock-out mice. Proc Natl Acad Sci USA. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mistry DK, Cottrell GA. Actions of steroids and bemegride on the GABAA receptor of mouse spinal neurones in culture. Exp Physiology. 1990;75:199–209. doi: 10.1113/expphysiol.1990.sp003394. [DOI] [PubMed] [Google Scholar]
- 31.Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park-Chung M, Malayev A, Purdy RH, Gibbs TT, Farb DH. Sulfated and unsulfated steroids modulate γ-aminobutyric acidA receptor function through distinct sites. Brain Res. 1999;830:72–87. doi: 10.1016/s0006-8993(99)01381-5. [DOI] [PubMed] [Google Scholar]
- 33.Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- 34.Puia G, Santi MR, Vicini S, Pritchett DB, Purdy RH, Paul SM, Seeburg PH, Costa E. Neurosteroids act on recombinant human GABAA receptor. Neuron. 1990;4:759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- 35.Puia G, Ducic I, Vicini S, Costa E. Does neurosteroid modulatory efficacy depend on GABAA receptor subunit composition? Receptors Channels. 1993;1:135–142. [PubMed] [Google Scholar]
- 36.Sapp DW, Witte U, Turner DM, Longoni B, Kokka N, Olsen RW. Regional variation in steroid anesthetic modulation of [35S]TBPS binding to γ-aminobutyric acidA receptors in rat brain. J Pharmacol Exp Ther. 1992;262:801–806. [PubMed] [Google Scholar]
- 37.Saxena NC, Macdonald RL. Assembly of GABAA receptor subunit: role of the δ subunit. J Neurosci. 1994;14:7077–7086. doi: 10.1523/JNEUROSCI.14-11-07077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sieghart W. Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 39.Sigel E, Baur R, Trube G, Möhler H, Malherbe P. The effect of subunit composition of rat brain GABAA receptors on channel function. Neuron. 1990;5:703–711. doi: 10.1016/0896-6273(90)90224-4. [DOI] [PubMed] [Google Scholar]
- 40.Turner DM, Ransom RW, Yang JS-J, Olsen EW. Steroid anesthetics and naturally occurring analogs modulate the γ-aminobutyric acid receptor complex at a site distinct from barbiturates. J Pharmacol Exp Ther. 1989;248:960–966. [PubMed] [Google Scholar]
- 41.Twyman RE, Macdonald RL. Neurosteroid regulation of GABAA receptor single-channel kinetic properties of mouse spinal cord neurons in culture. J Physiol (Lond) 1992;456:215–245. doi: 10.1113/jphysiol.1992.sp019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Twyman RE, Rogers CJ, Macdonald RL. Pentobarbital and picrotoxin have reciprocal actions on single GABAA receptor channels. Neurosci Lett. 1989;96:89–95. doi: 10.1016/0304-3940(89)90248-6. [DOI] [PubMed] [Google Scholar]
- 43.Whiting PJ, McKernan RM, Wafford KA. Structure and pharmacology of vertebrate GABAA receptor subtypes. Int Rev Neurobiol. 1995;38:95–138. doi: 10.1016/s0074-7742(08)60525-5. [DOI] [PubMed] [Google Scholar]
- 44.Yoo A, Harris J, Dubrovsky B. Dose-response study of dehydroepiandrosterone sulfate on dentate gyrus long-term potentiation. Exp Neurol. 1996;137:151–156. doi: 10.1006/exnr.1996.0015. [DOI] [PubMed] [Google Scholar]
- 45.Zheng T, Santi MR, Bovolin P, Marlier LNJ-L, Grayson DR. Developmental expression of the α6 GABAA receptor occurs only after cerebellar granule cell migration. Dev Brain Res. 1993;75:91–103. doi: 10.1016/0165-3806(93)90068-l. [DOI] [PubMed] [Google Scholar]
- 46.Zheng T, Zhu WJ, Puia G, Vicini S, Grayson DR, Costa E, Caruncho HJ. Changes in γ-aminobutyrate type A receptor subunits mRNAs, translation product expression, and receptor function during neuronal maturation in vitro. Proc Natl Acad Sci USA. 1995;91:10952–10956. doi: 10.1073/pnas.91.23.10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu WJ, Vicini S. Neurosteroid prolongs GABAA channel deactivation by altering kinetics of desensitized states. J Neurosci. 1997;17:4022–4031. doi: 10.1523/JNEUROSCI.17-11-04022.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu WJ, Wang JF, Krueger KE, Vicini S. δ subunit inhibits neurosteroid modulation of GABAA receptors. J Neurosci. 1996;16:6648–6656. doi: 10.1523/JNEUROSCI.16-21-06648.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zorumski CF, Mennerick S, Covey DF. Enantioselective modulation of GABAergic synaptic transmission by steroids and benz[e]indenes in hippocampal microcultures. Synapse. 1998;29:162–171. doi: 10.1002/(SICI)1098-2396(199806)29:2<162::AID-SYN7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]