Abstract
Principal neurons in the lateral nucleus of the amygdala (LA) exhibit a continuum of firing properties in response to prolonged current injections ranging from those that accommodate fully to those that fire repetitively. In most cells, trains of action potentials are followed by a slow afterhyperpolarization (AHP) lasting several seconds. Reducing calcium influx either by lowering concentrations of extracellular calcium or by applying nickel abolished the AHP, confirming it is mediated by calcium influx. Blockade of large conductance calcium-activated potassium channel (BK) channels with paxilline, iberiotoxin, or TEA revealed that BK channels are involved in action potential repolarization but only make a small contribution to the fast AHP that follows action potentials. The fast AHP was, however, markedly reduced by low concentrations of 4-aminopyridine and α-dendrotoxin, indicating the involvement of voltage-gated potassium channels in the fast AHP. The medium AHP was blocked by apamin and UCL1848, indicating it was mediated by small conductance calcium-activated potassium channel (SK) channels. Blockade of these channels had no effect on instantaneous firing. However, enhancement of the SK-mediated current by 1-ethyl-2-benzimidazolinone or paxilline increased the early interspike interval, showing that under physiological conditions activation of SK channels is insufficient to control firing frequency. The slow AHP, mediated by non-SK BK channels, was apamin-insensitive but was modulated by carbachol and noradrenaline. Tetanic stimulation of cholinergic afferents to the LA depressed the slow AHP and led to an increase in firing. These results show that BK, SK, and non-BK SK-mediated calcium-activated potassium currents are present in principal LA neurons and play distinct physiological roles.
Keywords: AHP, BK channels, SK channels, apamin, paxilline, adaptation
In many neurons, influx of calcium during action potentials activates a number of potassium channels. Activity of these channels generates currents that contribute to action potential repolarization and the afterhyperpolarization (AHP) that follows them (Lancaster and Pennefather, 1987; Storm, 1987; Sah and McLachlan, 1992). Single-channel studies have identified two types of calcium-activated potassium channel named BK and SK channels. BK channels are large-conductance (200–400 pA), voltage-sensitive channels that are selectively blocked by iberiotoxin (Ibtx) (Galvez et al., 1990), low concentrations of tetraethylammonium (TEA; ≤1 mm), and paxilline (Sanchez and McManus, 1996; Strobaek et al., 1996). SK channels have a smaller single-channel conductance (10–20 pS), are voltage-insensitive and insensitive to low concentrations of TEA, but are potently blocked by the bee toxin apamin (Castle et al., 1989).
Macroscopically, three types of calcium-activated potassium current have been described in central neurons that have been labeledIC,IAHP, and sIAHP.IC contributes to action potential repolarization and the fast AHP that follows single spikes (Lancaster and Adams, 1986; Lancaster and Nicoll, 1987; Storm, 1987; Sah and McLachlan, 1992). IAHP and sIAHP, which mediate the medium and slow AHPs, respectively, follow single and trains of action potentials (Storm, 1990; Sah, 1996). It is now clear that BK channels underlie IC, and activation of SK channels generates IAHP (Sah, 1996). However, although SK channels have also been suggested to underlie sIAHP (Kohler et al., 1996;Marrion and Tavalin, 1998; Bowden et al., 2001), the evidence for this is not compelling. sIAHP is apamin-insensitive, whereas all cloned SK channels are apamin-sensitive when expressed in mammalian cell lines (Kohler et al., 1996; Shah and Haylett, 2000). Furthermore, sIAHP is modulated by a number of neurotransmitters such as noradrenaline (Madison and Nicoll, 1982), 5-hydroxytryptamine (5-HT) (Pedarzani and Storm, 1993), acetylcholine (Benardo and Prince, 1982; Cole and Nicoll, 1984), and histamine (Haas and Konnerth, 1983), whereas such modulation has not been described for the cloned SK channels. These results raise the possibility that the channels underlying sIAHP may represent an as yet unidentified type of calcium-activated potassium channel.
The amygdaloid complex is a structure located within the medial temporal lobe that has been attributed with placing emotional significance to sensory input (LeDoux, 1995). Anatomically the amygdaloid complex can be divided into 13 nuclei, the main ones being the lateral nucleus (LA), the basal nucleus (BLA), and the central nucleus (Price et al., 1987; Pitkänen et al., 1997). Neurons within the LA and BLA have been separated into two main types: principal pyramidal-like cells, which are glutamatergic and form projection neurons and local circuit interneurons, which are GABAergic (McDonald, 1984). We have shown that spiny principal neurons within the LA show a wide range of firing properties that form a continuum ranging from those that show marked spike-frequency adaptation to those that fire repetitively in response to a depolarizing current injection (Faber et al., 2001). We have suggested that a combination of the density and distribution of voltage-dependent and calcium-activated potassium channels determine the firing properties of LA neurons. In the current study we have examined the types of calcium-activated potassium current present in LA principal neurons and their physiological roles.
MATERIALS AND METHODS
All experiments were performed on rat brain slices maintainedin vitro. Wistar rats (unsexed; 17- to 28-d-old) were anesthetized with intraperitoneal phenobarbitone (50 mg/kg) and killed by decapitation. These procedures were in accordance with the guidelines of the Institutional Animal Ethics Committee. Rat brains were rapidly removed and placed in ice-cold artificial CSF (aCSF) containing (mm): NaCl 118, KCl 2.5, NaHCO3, glucose 10, MgCl22.5, CaCl2 2.5, and NaHPO41.2. Coronal slices (400-μm-thick) containing the amygdala were cut using a microslicer DTK-1000 (Dosaka). The slices were allowed to recover in oxygenated (95% O2 and 5% CO2) aCSF at 30°C for 30 min and then kept at room temperature for a further 30 min before experiments were performed. Slices were then transferred to the recording chamber as required. Within the recording chamber slices were held in position using a nylon net stretched over a flattened U-shaped platinum wire and were continuously perfused with oxygenated aCSF maintained at 30°C. In experiments where the effect of a lower concentration of calcium was examined, the composition of the aCSF was modified to contain 0.5 mm CaCl2. When using cadmium to block voltage-gated Ca2+channels, NaHPO4 was removed from the aCSF to prevent cadmium from precipitating out of solution.
Whole-cell recordings were made from neurons in the LA using infrared differential interference contrast techniques. Electrodes (3–6 MΩ) were filled with a pipette solution containing (mm): KMeSO4 135, NaCl 8, HEPES 10, Mg2ATP 2, and Na3GTP 0.3, pH 7.3 with KOH, osmolarity 280–300 mOsm/kg. Signals were recorded using a patch-clamp amplifier (Axopatch 1-D; Axon instruments, Foster City, CA). Responses were filtered at 5 kHz and digitized at 10 kHz (ITC-16; Instrutech, Great Neck, NY). All data were acquired, stored, and analyzed on a Power Macintosh using Axograph (Axon Instruments).
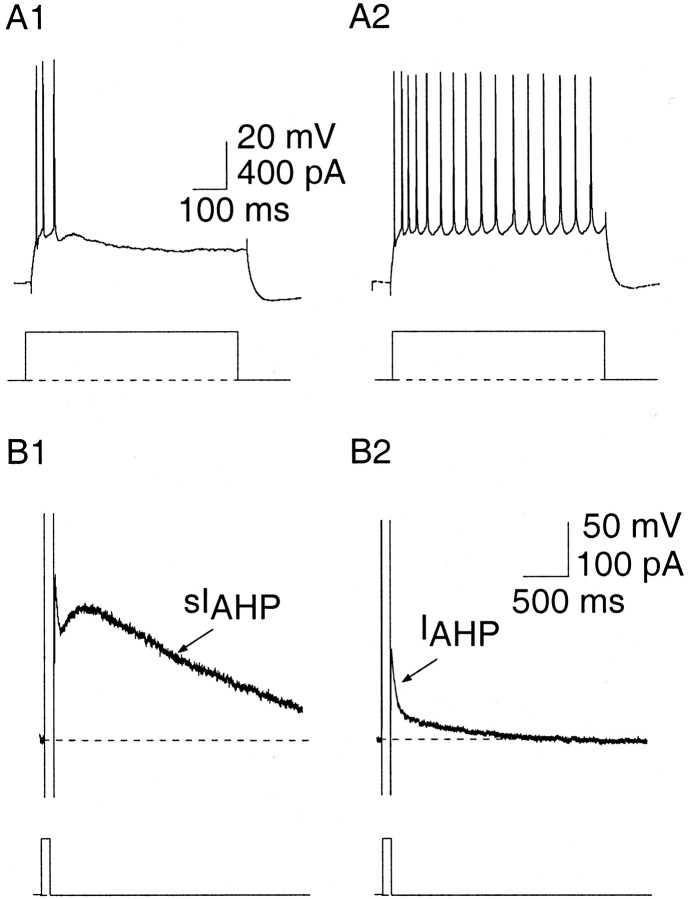
To investigate the firing properties of neurons, six to eight current injection steps (600 msec) were applied from −100 to +400 pA or +600 pA in 100 pA increments. Action potential half-widths were measured using Axograph (Axon instruments) as the spike width at the half-maximal voltage. The fast AHP immediately follows the downstroke of the action potential (Fig. 2) and was measured by subtracting the peak amplitude of the hyperpolarizing deflection after the spike from the threshold for spike initiation. Medium and slow AHPs were evoked in current clamp by a 50 msec, 400 pA current injection from a holding potential of −70 mV. The currents underlying the medium and slow AHPs were investigated in voltage clamp by giving a 100 msec, 50 mV step from a holding potential of −50 mV (Fig.1B). The medium AHP can be distinguished from the slow AHP by its fast time course and sensitivity to apamin: the medium AHP lasts several hundred milliseconds, whereas slow AHP lasts up to 6 sec. The current underlying the medium AHP,IAHP, was blocked using apamin and examined by subtracting currents recorded in the presence of apamin from control currents. For afferent stimulation, a bipolar stimulating electrode was placed in the external capsule. Tetanic stimulation was given as a 30 Hz stimulus lasting 500 msec, followed by a 5 sec delay before giving either a depolarizing step in voltage clamp to evoke the AHP current or a depolarizing current injection in current clamp to examine spike-frequency adaptation. This protocol was repeated four times, twice without afferent stimulation to obtain control responses of the AHP or firing properties and twice with afferent stimulation. The protocol was then repeated again after the application of a drug if appropriate. Drugs were applied by adding them to the superfusate at the appropriate concentration. Complete exchange of solutions was achieved within 2 min. Access resistance was 5–30 MΩ and was monitored throughout the experiment. Only cells with a membrane potential greater than −55 mV were included in this study. Instantaneous frequency was acquired by taking the inverse of the interspike interval. Results are expressed as mean ± SEM. Student's t tests were used for statistical comparisons between groups.
Fig. 2.
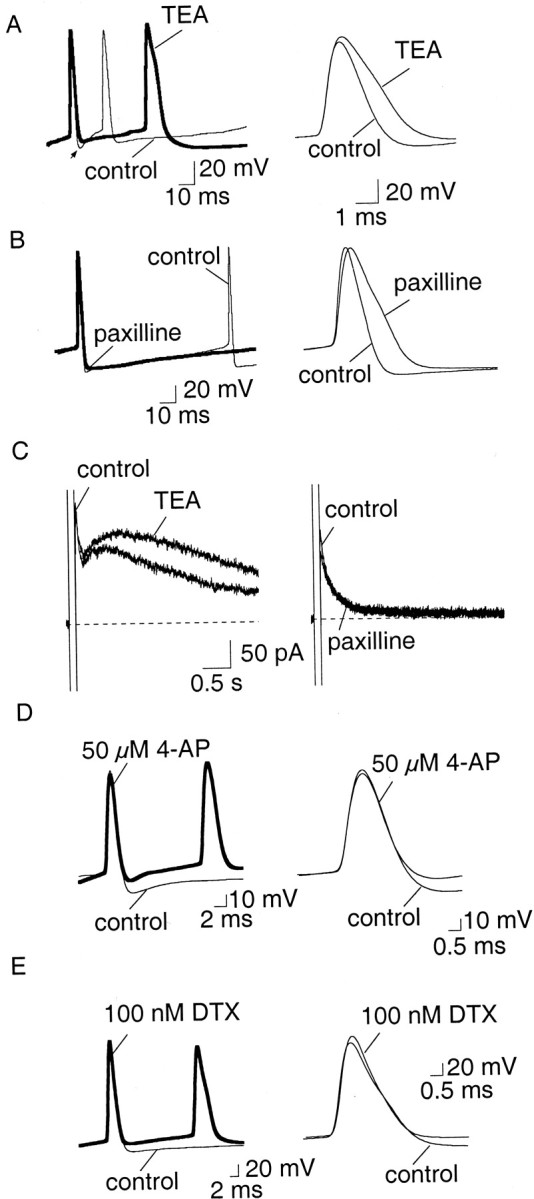
Currents mediated by BK channels are involved in action potential repolarization in LA neurons, but contribute little to the fast AHP. A, B, The effect of BK channel blockers TEA (1 mm, A) and paxilline (10 μm, B) on action potentials. The fast AHP is indicated by the arrow. TEA both broadens the spike (p < 0.001) and reduces the fast AHP immediately after action potentials (p < 0.005). Paxilline has little effect on the fast AHP, but significantly (p < 0.005) increased the spike half-width (B). C, BK channels do not contribute to the slow AHP. Neither TEA (1 mm,left) nor paxilline (10 μm, right) blocked the currents underlying the slow AHP. The increase in the amplitude of sIAHP in the presence of TEA is attributable to the large broadening of action poten tials and consequent increased calcium influx.D, E, Two blockers of voltage-dependent potassium currents 4-AP (C) and α-dendrotoxin (DTX, D) reduce the fast AHP that follows action potentials without slowing action potential repolarization.
Fig. 1.
Electrophysiological properties of principal neurons in the LA. A, Neurons in the LA show a continuum of firing patterns ranging from full accommodation where they fire only one to five spikes (A1) to firing repetitively (A2) in response to a 400 pA, 600 msec current injection. B, The current underlying the AHP is evoked by a 100 msec, 50 mV step and can be separated into two components,IAHP and sIAHP. Cells that accommodate more have a larger current underlying the slow AHP than those that fire repetitively. B1 shows the current evoked in the cell shown in A1, and B2 shows the current evoked in the cell shown in A2.
Drugs and chemicals. Ibtx, α-dendrotoxin (DTX), and apamin were obtained from Alomone Laboratories (Jerusalem, Israel); nickel, cadmium, EGTA, BAPTA, carbachol, isoprenaline, paxilline, tetraethylammonium (TEA), atropine, noradrenaline, dopamine, 5-hydroxytryptamine (5-HT), muscarine, and 4-aminopyridine (4-AP) were obtained from Sigma (St. Louis, MO). 1S,3R-trans-ACPD and 1-ethyl-2-benzimidazolinone (EBIO) were obtained from Tocris Cookson (Bristol, UK). UCL1848 was a kind gift from Dr. Ganellin (University College, London, UK).
RESULTS
Two examples of principal neuron that form a continuum of firing patterns in the LA (Faber et al., 2001) are shown in Figure 1. The firing properties of LA neurons range from those that show spike-frequency adaptation (∼80% of neurons) (Fig.1A1) to those that fire repetitively in response to a 600 msec depolarizing current injection (∼10%) (Fig.1A2). Repetitively firing principal neurons are clearly distinguishable from interneurons by their broader action potential widths (Mahanty and Sah, 1998). Principal neurons also differ from each other in the currents underlying the AHPs evoked by a 100 msec depolarizing step. Cells that accommodate have a significantly (p < 0.05) larger sIAHP than cells that do not (Fig. 1B) (Faber et al., 2001). For ease we shall refer to accommodating cells as AC and cells that fire repetitively as RFC. The other type of principal neuron that we have previously described (Faber et al., 2001), which fire a single spike in response to a 600 msec current injection and comprise ∼10% of LA neurons, will not be included. A total of 261 LA neurons were recorded from with a mean resting potential of −63.7 ± 0.4 mV and a mean input resistance of 146.8 ± 4 MΩ. Because of the relative proportions of AC and RFC, the majority of the experiments were performed on AC: unless otherwise stated, the experiments were performed on AC.
BK-mediated currents
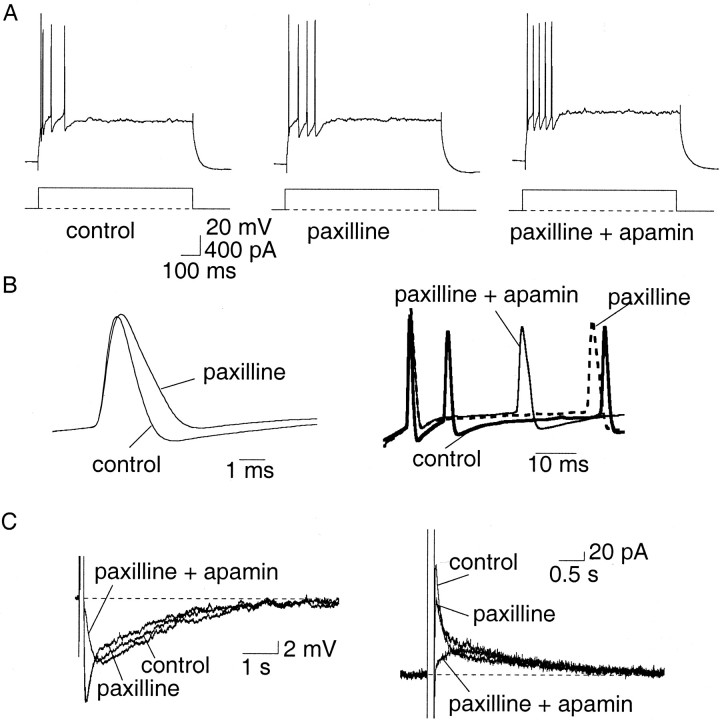
To test the role of BK channels, we first examined the effect of blocking these channels on action potentials. BK channels can be blocked by paxilline (Sanchez and McManus, 1996; Strobaek et al., 1996), iberiotoxin (Galvez et al., 1990), and by low concentrations of TEA. In 14 AC and 5 RFC, TEA (1 mm) caused a dramatic (p < 0.001) broadening of the action potential with the half-width increasing from a mean of 1.3 ± 0.1 to 2.0 ± 0.1 msec (n = 19) (Fig.2A). Lower concentrations of TEA (0.5 mm) had a similar effect (data not shown). Application of TEA reduced the fast AHP by 60% from 8.0 ± 1.5 to 5.5 ± 1.8 mV (p < 0.005; n = 6). However, in addition to blocking BK channels, TEA also blocks a number of voltage-gated potassium channels (Coetzee et al., 1999). Therefore, we next examined the effects of the more selective BK channel blockers paxilline and Ibtx. In 13 AC and two RFC, paxilline (10 μm) significantly (p < 0.001) increased the mean spike half-width from 1.1 ± 0.04 to 1.4 ± 0.04 msec (Fig. 2B) (n = 15). Similarly, iberiotoxin (50 nm) also slowed the repolarization of action potentials and increased the mean half-width from 1.3 ± 0.1 to 1.7 ± 0.1 msec (n = 9; p < 0.005) (six AC and three RFC). In contrast to the actions of TEA, however, the fast AHP after single spikes was little affected by paxilline (Fig.2B) and iberiotoxin (data not shown); in paxilline it was reduced from 9.8 ± 1 to 7.2 ± 1 mV, but this did not reach statistical significance (n = 15;p > 0.05). Blockade of BK channels did not reduce the slow AHP, although in some cells it was slightly enhanced, presumably because of the larger influx of Ca2+allowed by the broader spikes. This effect is not evident with paxilline in Figure 2C because the cell shown in the figure is an RFC, in which the slow AHP is of very small amplitude.
The difference in the actions of TEA and paxilline on the fast AHP suggests that a voltage-dependent current, other than that mediated by BK channels, is the main contributor to the fast AHP. To test this possibility we examined two other known blockers of voltage-gated potassium channels. Application of 4-AP (30–50 μm) significantly reduced the fast AHP to 53% of control values, from 11.4 ± 1.7 to 6.8 ± 2.1 mV (n = 7;p < 0.005) (Fig. 2D). 4-AP (30–50 μm) had no effect on the spike half-width, showing that it was not exerting its action through BK channels. The mean half-width was 1.2 ± 0.1 msec both in control conditions and in the presence of 4-AP (n = 7; p > 0.05) (Fig. 2D). Low concentrations of 4-AP blocks potassium channels that are also blocked by the more selective potassium channel blocker DTX (Coetzee et al., 1999). DTX (100 nm) also blocked the fast AHP by 60% (p < 0.005) from 9.2 ± 1.2 to 6.2 ± 1.3 mV (n = 10) without increasing the spike half-width (Fig. 2E). The mean spike half-width was 1.0 ± 0.1 msec in control compared with 1.1 ± 0.1 msec in DTX (n = 13; p > 0.05). Together, these findings indicate that in LA neurons the current mediating the fast AHP is largely caused by activation of voltage-activated potassium channels that are sensitive to 4-AP and DTX, whereas BK channels make a minimal contribution.
In hippocampal pyramidal neurons, trains of action potentials show a frequency-dependent spike broadening, which has been attributed to fast inactivation of BK channels (Shao et al., 1999). Principal neurons in the LA also show a similar broadening of action potentials (Faber et al., 2001). However, unlike in the hippocampus, this broadening was not affected by TEA, paxilline, or iberiotoxin (data not shown), indicating that it is unlikely to be attributable to inactivation of BK channels.
Currents underlying the medium and slow afterhyperpolarization
Our first aim was to ascertain whether the currents underlying the AHP evoked by a depolarizing step (shown in Fig. 1) are calcium-activated currents. We first tested the effect of reducing calcium influx by either lowering extracellular Ca2+ or by blocking voltage-activated calcium currents with cadmium or nickel. Extracellular calcium was reduced by replacing aCSF containing 2.5 mmCa2+ with 0.5 mmCa2+ containing aCSF. Lowering the extracellular calcium concentration was associated with a dramatic reduction of the AHP (Fig. 3A, left) (n = 6). Under voltage-clamp, the current underlying the AHP (evoked by a 100 msec depolarization to 0 mV from a holding potential of −50 mV) could be separated into two components. One peaked shortly after the depolarizing voltage step and had a rapid decay (τ, 86 ± 14 msec; n = 10; see below), whereas the other was much slower to reach a peak and decayed with a time constant of 1.4 ± 0.1 sec (n = 30). These two components are similar toIAHP and sIAHP described in other cells (Fig. 1) (Sah, 1996). Both of these currents were abolished by reducing extracellular calcium (Fig. 3A, right panel). Blocking voltage-dependent calcium channels with cadmium (250 μm; n = 8) or nickel (5 mm; n = 6) (Fig. 3B) also blocked the AHP and the underlying current. As a final test, loading cells with the calcium chelator EGTA (10 mm) or BAPTA (10 mm) also fully blocked the AHP and the underlying currents (Fig. 3C) (n = 12). These results show that both components of the AHP result from activation of calcium-activated potassium currents secondary to calcium influx via voltage-gated calcium channels.
Fig. 3.
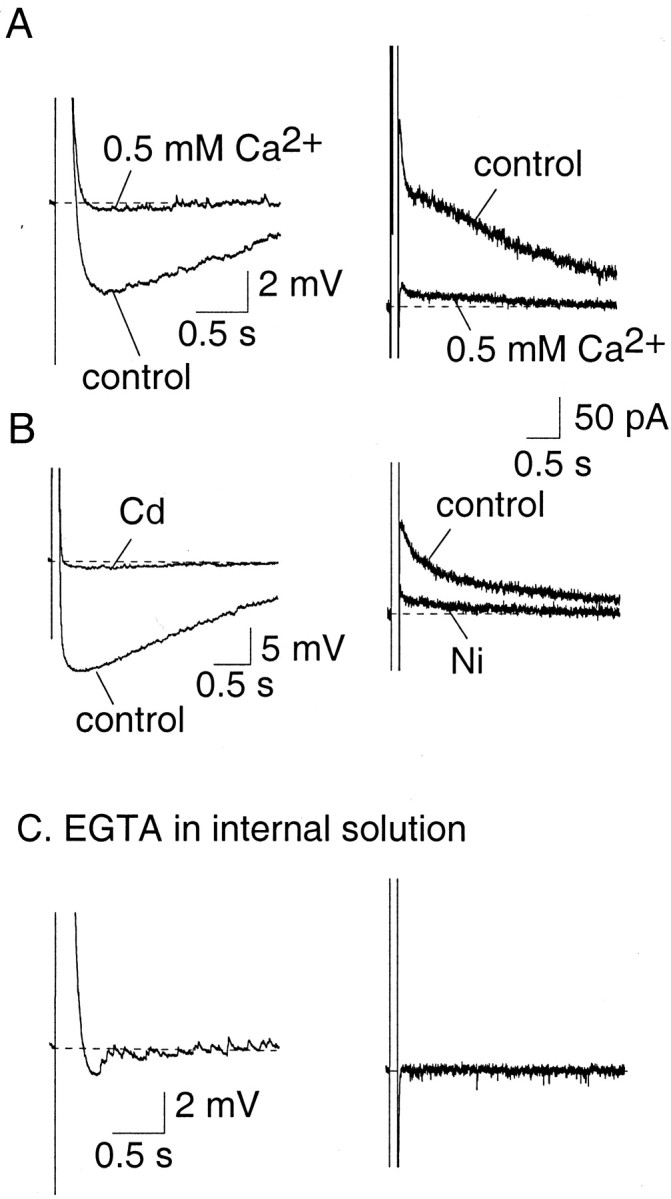
Activation of the AHP current requires calcium influx and a rise in cytosolic calcium. A, The AHP recorded under current clamp (left) and the underlying current recorded in voltage clamp (right) were blocked by perfusing slices with aCSF containing 0.5 mmCa2+. B, Blocking voltage-gated calcium channels blocks the AHP (left traces, cadmium 0.25 mm) and the underlying current (right traces, nickel 5 mm). C, Inclusion of high concentrations of the calcium buffer EGTA (10 mm) in the pipette solution abolished the AHP.
SK channel-mediated currents
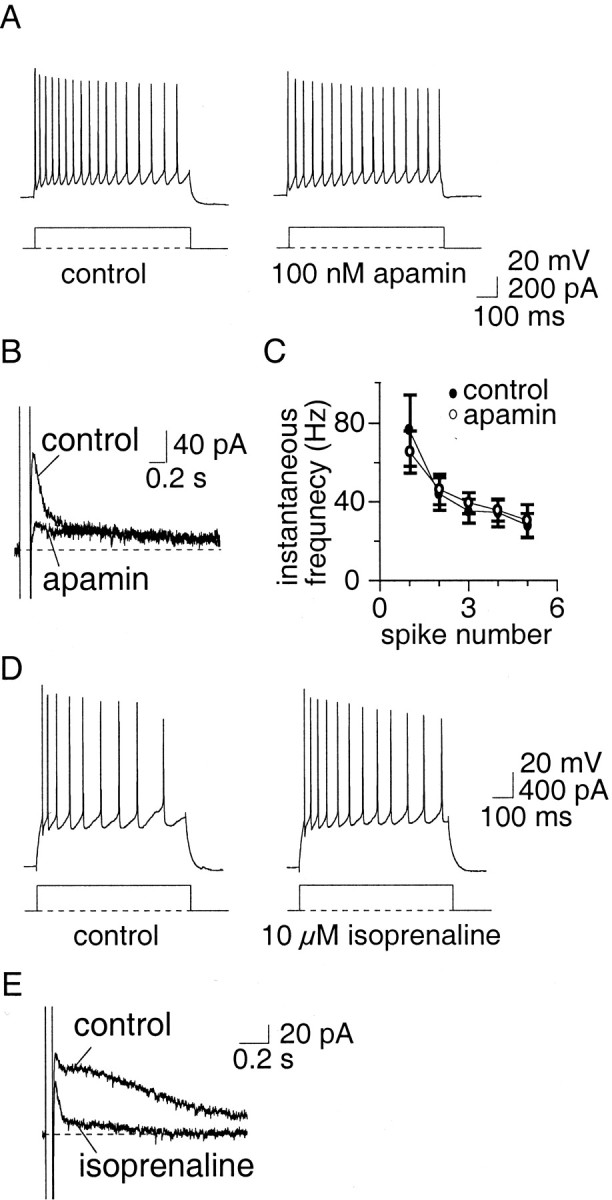
The SK channel blocker apamin (100 nm) (Castle et al., 1989) had no effect on action potential width (Fig.4A), but selectively and irreversibly blocked the medium AHP (Fig. 4B) andIAHP (Fig. 4C) in 10 of 11 AC. The slow AHP and sIAHP were unaffected by apamin (Fig. 4B,C) (n = 15). Similarly to apamin, UCL1848 (100 nm), another selective SK channel blocker (Chen et al., 2000; Shah and Haylett, 2000), had no effect on action potentials (data not shown) but selectively blocked the medium AHP and IAHP (Fig.4C, traces on right) (n = 4). The currents mediated by SK channels were extracted by subtraction of the current before and after application of apamin or UCL1848 and are shown in the insets of Figure 4C. This current has a rapid time to peak and decays with a time constant of 86 ± 14 msec (n = 10). Bicuculline methiodide, which has been reported to block SK channels (Johnson and Seutin, 1997) also selectively blocked the medium AHP andIAHP (n = 2; data not shown). Blockade of SK channels had no significant effect (p > 0.05) on accommodation in AC (Fig.4D,E). The number of spikes fired (Fig.4D) and the instantaneous firing frequency (Fig.4E) were unaffected both at threshold and twice threshold, with the mean number of spikes fired at twice threshold being 4.2 ± 0.7 in control compared with 5.3 ± 1.1 in apamin (n = 6; p > 0.05). UCL1848 also had no significant effect on spike-frequency adaptation in terms of either the number of spikes fired or the firing frequency (p > 0.05; data not shown).
Fig. 4.
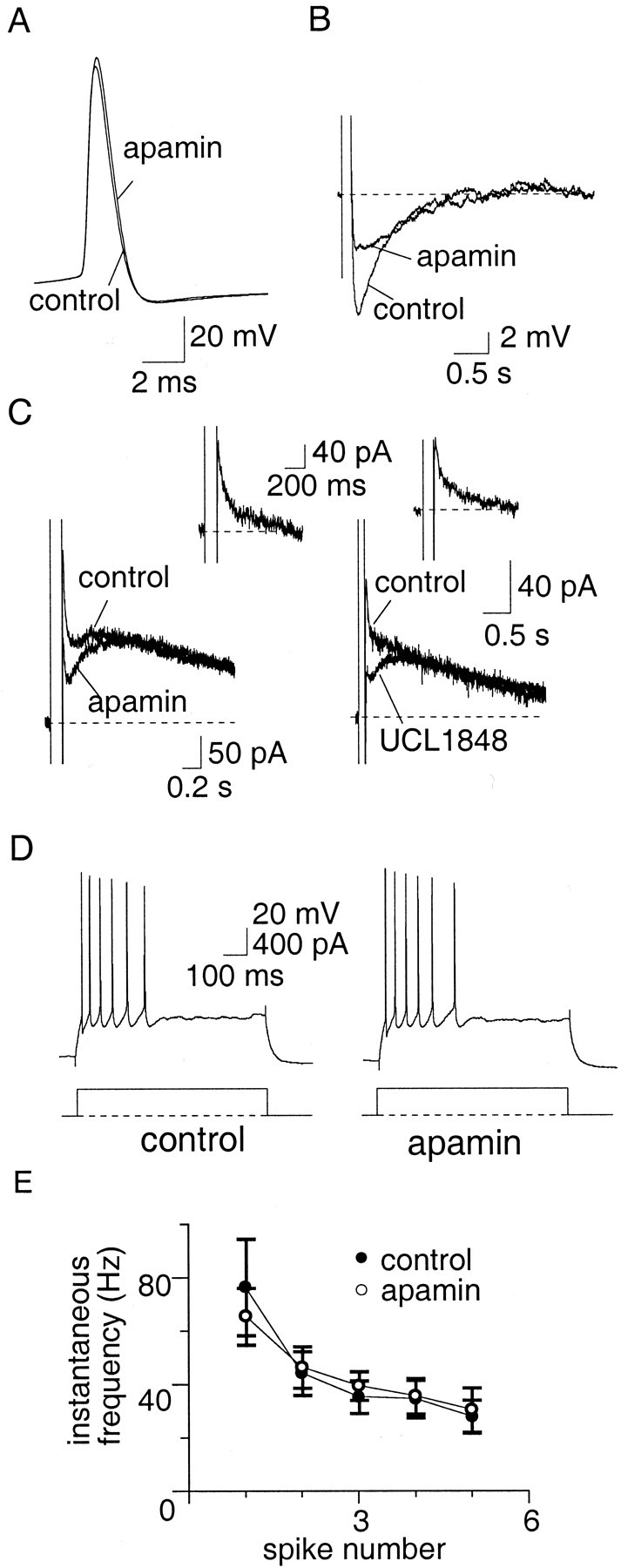
Apamin-sensitive channels mediate the medium AHP but play no role in spike-frequency adaptation. A,Action potentials recorded in control aCSF and in the presence of apamin (100 nm). Apamin had no effect (p > 0.05) on the spike half-width.B, The AHP evoked by a 100 msec depolarizing current injection. In the presence of apamin, the medium AHP is blocked.C, Left, Apamin blocksIAHP but has no effect on the sIAHP. C, Right, UCL1848 also blocks theIAHP. The SK-mediatedIAHP obtained by subtraction is shown in the insets. D, Train of action potentials evoked by a 600 msec, 400 pA current injection. Apamin had no effect on spike-frequency adaptation. The instantaneous firing frequency during the spike train, and the lack of effect by apamin, is shown in E.
These results indicate that although apamin-sensitive SK channels are present on LA neurons, they are not significantly activated by single action potentials. One possible explanation for this is that the calcium influx during action potentials does not raise calcium concentrations near SK channels enough to significantly activate them. As blockade of BK channels broadens the action potential, it is likely that calcium influx would be potentiated (Jackson et al., 1991) and thus might increase the activity of SK channels. In support of this, application of paxilline increased the early interspike interval (between spikes 1 and 2) by activating apamin-sensitive channels (Fig.5A,B). Similar increases in the early interspike interval between the first few spikes were also observed with TEA (data not shown). However, this change in the early interspike interval was not large enough to change the mean number of spikes evoked in AC at twice threshold current injections: 3.6 ± 0.4 in control versus 3.9 ± 0.9 in paxilline (n = 13; p > 0.05); 2.9 ± 0.5 in control versus 2.1 ± 0.4 in TEA (n = 14;p > 0.05); and 4.6 ± 0.8 versus 5.4 ± 1.2 in Ibtx (n = 7; p > 0.05).
Fig. 5.
Enhancement of the apamin-sensitive potassium current increases the early interspike interval. Paxilline (10 μm) had no effect on spike-frequency adaptation (A) but increased the early interspike interval (B, right) by increasing the spike half-width (B, left traces), mediated by enhanced calcium influx during the spike. This effect on the interspike interval was reversed by apamin (A, B, right traces).
The benzimidazolinone EBIO has recently been reported to enhance the calcium sensitivity of SK channels and to slow their decay after activation by calcium (Pedarzani et al., 2001). Therefore we also examined the effect of EBIO to confirm that the lack of effect of SK channel blockers on firing was attributable to an inadequate activation of SK channels in LA neurons. In agreement with Pedarzani et al. (2001), loading cells with EBIO by including it in the internal pipette solution (2 mm) (Fig.6A), or adding EBIO (0.5 mm) to the superfusate (data not shown), slowed the decay of the apamin-sensitive current. The mean time constant of decay in the presence of EBIO was significantly (p < 0.001) enhanced from a mean of 86 ± 14 msec (n = 10) under control conditions to 227 ± 26 msec (n = 9) in EBIO. Application of EBIO also enhanced the early interspike interval, an effect that was reversed by apamin (Fig. 6B,C).
Fig. 6.
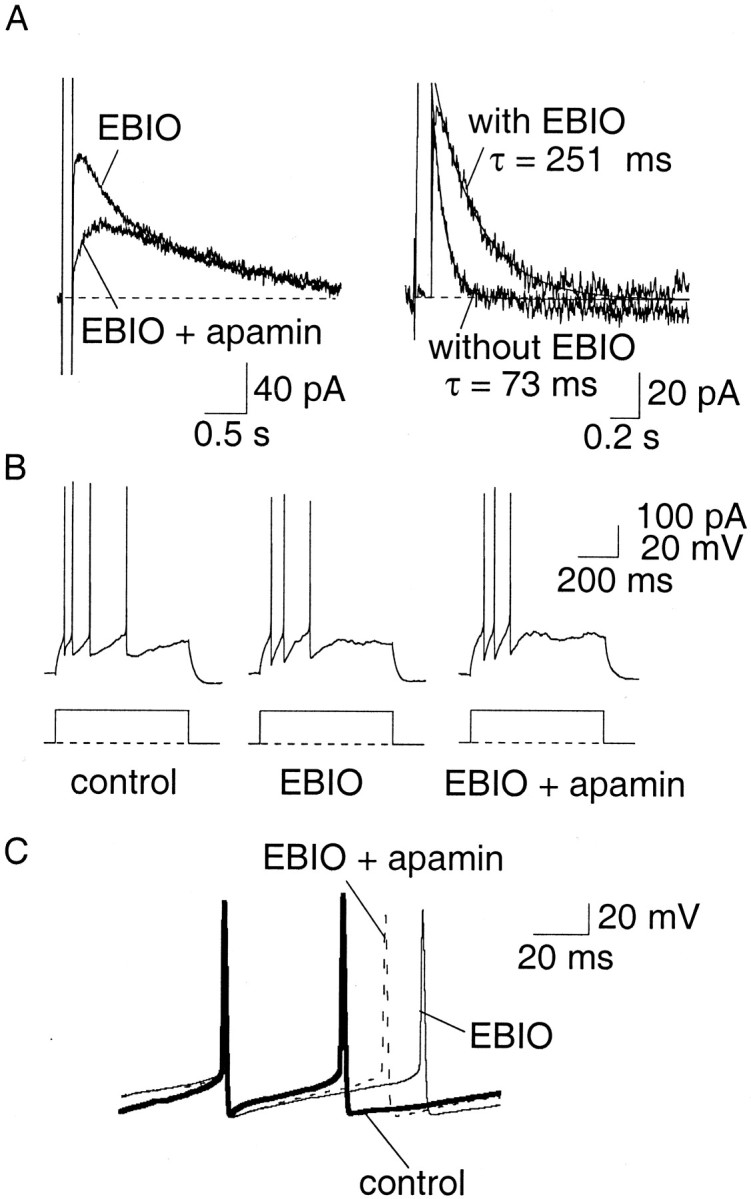
EBIO slows the decay ofIAHP and increases the early interspike interval. A, Inclusion of EBIO (2 mm) in the internal pipette solution slowed the decay of the SK-mediated current. Currents underlying the AHP recorded in the presence of EBIO and after application of 100 nm apamin (left). The time courses of the apamin-sensitive current, with and without EBIO (in different cells), have been superimposed. B, C, Perfusion of EBIO (0.5 mm) slows the interspike interval in an apamin-sensitive manner.
The slow AHP
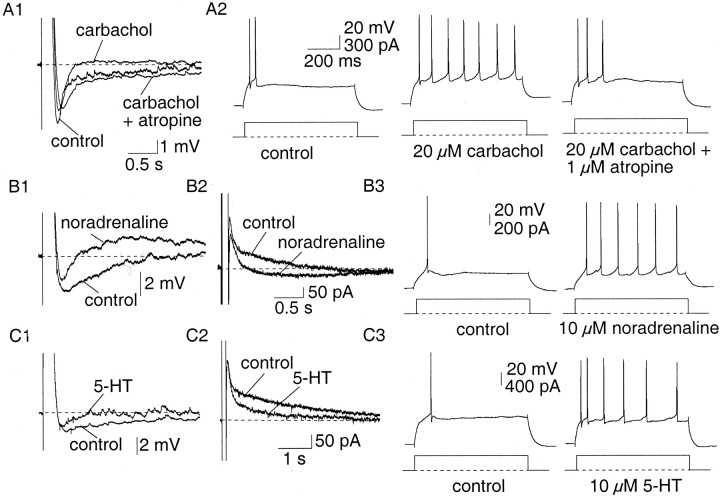
In agreement with what has been reported in many other cells types, the slow AHP that follows trains of action potentials was insensitive to blockers of SK and BK channels (as shown above). One distinguishing feature of the slow AHP is its modulation by a number of neurotransmitters (Sah, 1996). In agreement with this, the slow AHP and the underlying current were reduced by carbachol (20 μm;n = 16), isoprenaline (10 μm;n = 7), noradrenaline (10 μm;n = 7), 5-HT (10 μm;n = 7) and 1S,3R-trans-ACPD (100 μm;n = 2). The actions of carbachol and noradrenaline are illustrated in Figure7. In 8 of 16 cells inhibition of the slow AHP by carbachol was associated with the generation of an afterdepolarization (ADP). The effects of carbachol could be reversed by the muscarinic antagonist atropine (1 μm;n = 6) (Fig. 7A). Similarly, in two of seven cells, blockade of the slow AHP by noradrenaline was also associated with the generation of an ADP.
Fig. 7.
Neurotransmitters modulate the slow AHP and reduce spike-frequency adaptation. A1, Application of carbachol (20 μm) depressed the slow AHP but not the medium AHP in an atropine-sensitive manner. A2, This was accompanied by a reduction in spike-frequency adaptation, which was reversed by atropine (1 μm). B1, In the presence of noradrenaline (10 μm), the slow AHP was blocked and was replaced with a slow afterdepolarization. B2, In voltage clamp, noradrenaline selectively blocked the sIAHP, evoking an inward current. B3, This caused a concurrent reduction in accommodation. C, Similarly, 5-HT (10 μm) selectively blocked the slow AHP (C1) and sIAHP (C2), which caused a decrease in spike-frequency adaptation (C3).
Selective depression of the slow AHP by the neurotransmitters was associated with a reduction in spike-frequency adaptation. In AC, carbachol (20 μm) significantly reduced accommodation, increasing the number of spikes fired at the threshold depolarizing current injection from a mean of 2.6 ± 0.3 to 5.9 ± 0.8 (n = 16; p < 0.001) (Fig.7A). These effects were reversed by atropine (n = 6). Similarly, isoprenaline (10 μm) also reduced (n = 7) spike-frequency adaptation. In AC, the mean number of spikes evoked increased from 2.9 ± 0.9 to 6.3 ± 0.9 after application of isoprenaline (n = 7; p < 0.05). These effects were reversible on washout. 5-HT (10 μm) also reversibly reduced accommodation in five of seven neurons, increasing the number of evoked action potentials from a mean of 1.7 ± 0.3 to 4.0 ± 0.9 at threshold (n = 7; p < 0.05) (Fig.7C). Similar blockade of the slow AHP and associated increases in firing were seen with noradrenaline (10 μm;n = 7) (Fig.7B), muscarine (10 μm;n = 2) and 1S,3R-trans-ACPD (100 μm;n = 2).
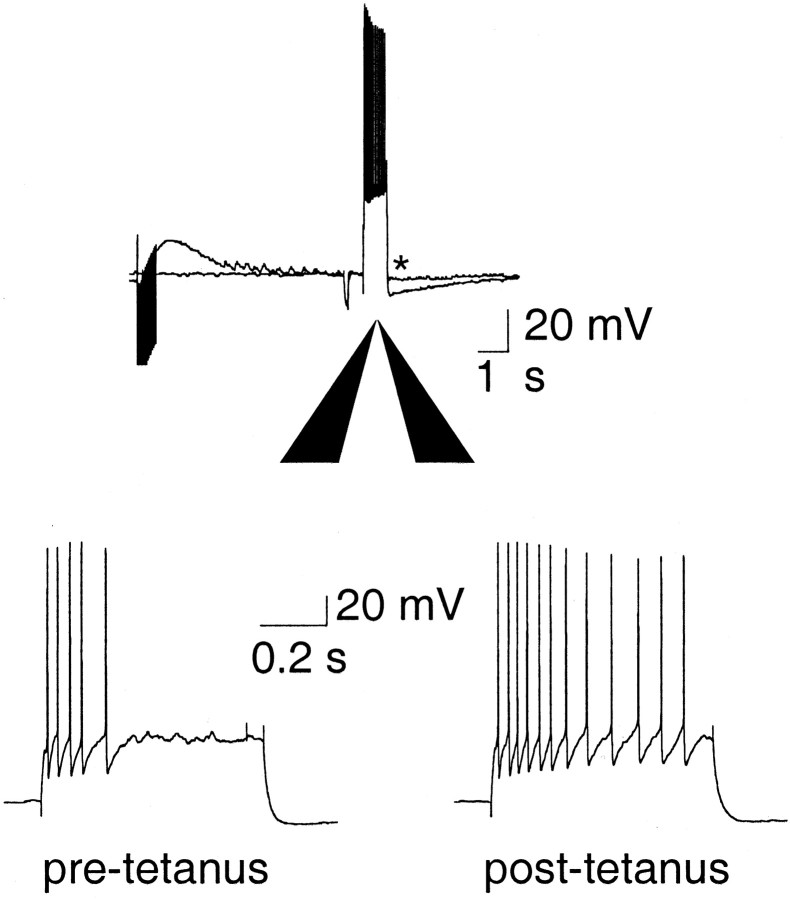
Finally, to ascertain whether modulation of AHPs by neurotransmitters and the concurrent changes in firing rates are physiologically relevant, we examined the effect of afferent stimulation on AHPs and firing properties. The amygdala has a significant innervation by the cholinergic system, the afferents of which travel in the external capsule and can therefore be stimulated by placing a bipolar electrode in the external capsule (Washburn and Moises, 1992). A train of stimuli (30 Hz, 500 msec) to the external capsule caused a depression of the slow AHP in all 11 neurons tested (Fig.8A). This depression was either reduced (n = 6) or prevented (n = 1) by the application of muscarinic antagonist atropine (1 μm) (Fig. 8B), showing that the main neurotransmitter mediating the depression of the AHP is acetylcholine acting at muscarinic receptors. As expected, the reduction in the slow AHP was associated with a marked decrease in spike-frequency adaptation (Fig. 9) (n = 11).
Fig. 8.
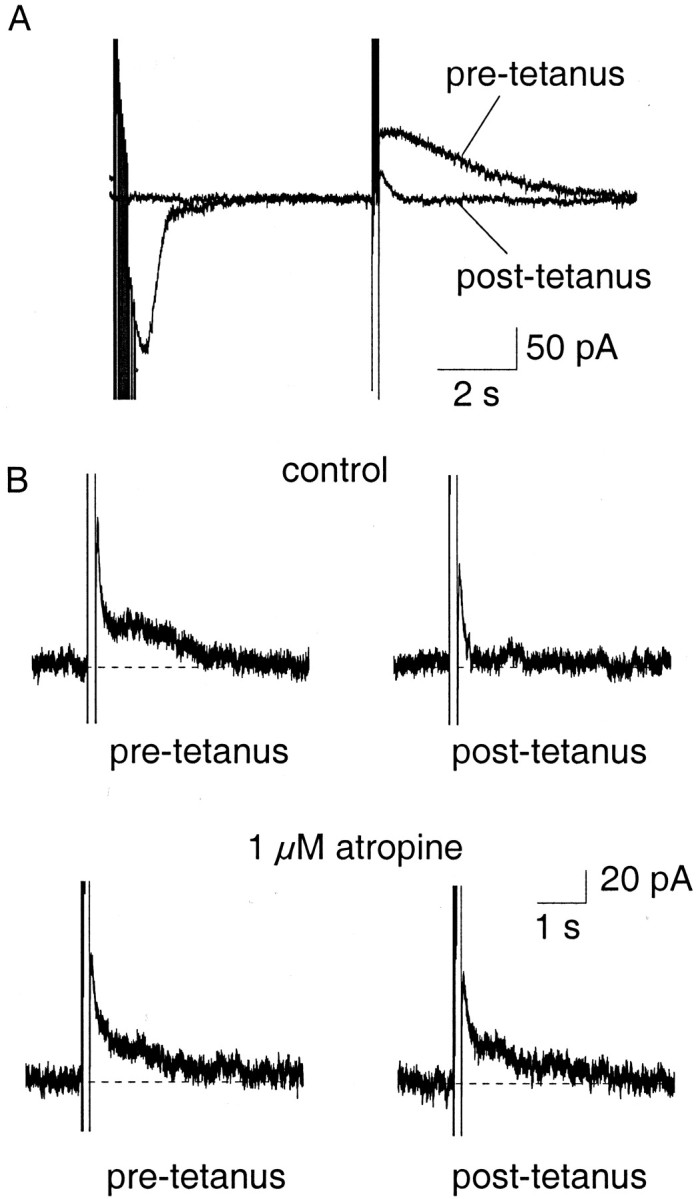
Synaptic stimulation of cholinergic afferents selectively depresses the slow IAHP.A, Cholinergic afferents were stimulated at 30 Hz for 500 msec and followed by a 3 sec delay before evoking theIAHP with a 100 msec voltage step. After the tetanus, the slow IAHP was depressed. B, Depression of the slowIAHP by tetanic stimulation (top traces) was reversed by atropine (1 μm;bottom traces), showing that the reduction is caused by activation of muscarinic receptors.
Fig. 9.
Tetanic stimulation evoked depression of the AHP is accompanied by a reduction in spike-frequency adaptation. Current-clamp recordings show that the AHP was depressed after tetanic stimulation (indicated by an asterisk, top trace), and this was accompanied by a reduction in spike-frequency adaptation in response to a 600 msec, 400 pA current injection (bottom traces).
The above results show that in cells that accommodate fully, both apamin-sensitive and apamin-insensitive calcium-activated potassium channels are present. Spike-frequency adaptation is in the large part determined by activation of the apamin-insensitive current. The apamin-sensitive current contributes to a component of the AHP-evoked during spike trains, but does not appear to play a major role in setting the early interspike interval under physiological conditions. We have shown previously that cells that show little spike-frequency adaptation have a much smaller slow AHP (Faber et al., 2001). This result suggests that the apamin-sensitive conductance may play a larger role in controlling the early interspike interval in RFC. The effects of blocking the apamin-sensitive and apamin-insensitive current in RFC are shown in Figure 10. In four of four cells, apamin blocked IAHP with little effect on sIAHP (Fig.10B). However, application of apamin had little effect on the number of action potentials evoked by a twice threshold 200 pA, 600 msec current injection (Fig. 10A) and, as with AC, had no effect on the instantaneous firing frequency (Fig.10C). The mean number of spikes evoked by a threshold current injection was and 4.0 ± 1.8 both under control and in the presence of apamin (n = 4). Similar to AC, isoprenaline (10 μm) selectively depressed the slow AHP and slow IAHP in RFC (Fig.10E). This led to an increase in the firing rate at threshold from 2.5 ± 0.6 to 4.2 ± 0.5 spikes (n = 4; p > 0.05). However this increase was not significant, which is not surprising in view of the relatively smaller amplitude slow AHP present in RFC.
Fig. 10.
Apamin-sensitive currents do not affect spike-frequency adaptation in repetitively firing neurons.A, Current-clamp recordings from a cell that showed little spike-frequency adaptation. Apamin (100 nm) blockedIAHP (B) but had no significant effect (p > 0.05) on the number of spikes evoked (A) or on the instantaneous firing frequency (average data from four neurons;C). D, Similarly to AC, isoprenaline (10 μm) increased the firing rate of RFC through blockade of the slow IAHP(E).
DISCUSSION
We have shown that three types of calcium-activated potassium channel are present in principal LA neurons. These are BK channels, apamin-sensitive SK channels, and apamin-insensitive calcium-activated potassium channels. In neurons, these channels have been shown to underlie three different calcium-activated potassium currents with distinct functional roles. We find that in principal neurons of the LA these channels also fulfill different physiological roles.
Activation of BK-type calcium-activated potassium channels underlies the current IC which, in a number of cell types, is well known to be involved in action potential repolarization and the fast AHP that immediately follows it (Lancaster and Nicoll, 1987; Storm, 1987; Sah and McLachlan, 1992). In LA principal neurons, we found that BK channels also contribute to action potential repolarization but have no effect on the slower AHP. Surprisingly, however, blockade of BK channels had very little effect on the fast AHP. In contrast, 4-AP and α-dendrotoxin significantly attenuated the fast AHP without broadening the action potential. We have previously shown that LA neurons express a large dendrotoxin and 4-AP-sensitive current that shows rapid activation kinetics (Faber and Sah, 2000). Our results indicate that this current is activated during the action potential and contributes to the fast AHP. Dendrotoxin is a selective voltage-gated potassium channel blocker that acts only on channels that contain Kv.1, Kv1.2, or Kv1.6 subunits (Coetzee et al., 1999). Low concentrations of 4-AP also block channels containing these subunits (Coetzee et al., 1999). Thus, the channels that mediate the fast AHP are likely to contain one or more of these subunits.
In adrenal chromaffin cells, blockade of BK channels and consequent broadening of the action potential leads to a reduction in repetitive activity (Solaro et al., 1995; Lovell and McCobb, 2001). This effect has been attributed to a reduction in the entry of sodium and calcium channels into desensitized states when BK channels are active and the resulting hyperpolarization after the action potential. Similarly, in amygdalar neurons we also noted that the early interspike interval was longer when BK channels are blocked (Fig. 5). However, because the slow AHP appears to be the major determinant of repetitive activity in these cells, there was no change in the number of action potentials evoked by current injections. In hippocampal pyramidal neurons the sodium current has been shown to undergo prolonged inactivation during action potentials. However, the effects of this inactivation are only seen in the dendrites where sodium channel density is lower (Jung et al., 1997). Thus, it is possible that recordings in dendrites of LA pyramidal cells would also show more dramatic changes during blockade of BK channels.
Similar to some other cell types, there is clear spike broadening during action potential trains in LA neurons (Faber et al., 2001). In hippocampal CA1 pyramidal neurons this phenomenon has been attributed to rapid inactivation of BK channels during action potentials (Shao et al., 1999). In contrast, we find that in LA neurons spike broadening is unaffected after blockade of BK channels, indicating that it is likely to be caused by a different mechanism (data not shown). BK channels are composed of an α subunit in heterologous combination β subunits. Four β subunits, β1 (KCNMB1), β2(KCNMB2), β3(KCNMB3), and β4 (KCNMB4) have so far been cloned (Dworetzky et al., 1994;Knaus et al., 1994; Tseng-Crank et al., 1996; Brenner et al., 2000;Meera et al., 2000). The presence of the β subunit significantly modifies the pharmacology, voltage dependence, and kinetics of the assembled protein (McManus et al., 1995; Dworetsky et al., 1996;Nimigean and Magleby, 1999; Wallner et al., 1999; Xia et al., 1999,2000; Brenner et al., 2000). Channels containing the α subunit in isolation or in combination with the β1 or β4 subunits produce sustained currents that do not inactivate. In contrast, the presence of β2 and β3 subunits gives rise to channels that show rapid inactivation (Wallner et al., 1999; Xia et al., 1999, 2000; Brenner et al., 2000; Uebele et al., 2000). Because BK channels involved in action potential repolarization do not appear to inactivate between action potentials, our results suggest that BK channels involved in spike repolarization in LA neurons are likely have a different subunit composition to those found in CA1 pyramidal neurons.
Apamin-sensitive channels in LA neurons are responsible for the rapidly activating IAHP. As in other cells, this current had no effect on action potential shape but contributed to the medium AHP evoked by trains of action potentials in cells at both ends of the firing pattern continuum. In other neurons that express the apamin-sensitiveIAHP but have a very small or absent apamin-insensitive slow AHP, depolarizing current injection causes tonic firing of action potentials, withIAHP being an important determinant of the frequency of action potentials (Sah, 1996; Wolfart et al., 2001). In some neurons where both currents are present (e.g., hippocampal CA1 pyramidal neurons), activation ofIAHP has been suggested to play an important role in early spike-frequency adaptation, because the predominant effect of SK channel blockers was to decrease the early interspike interval (Stocker et al., 1999). In contrast, in LA principal neurons apamin and UCL1848, which clearly blockedIAHP, had no effect on either the number of spikes fired or on the early interspike interval. However, enhancement of the SK channel mediated current by either broadening the action potential with BK channel blockers or by altering the calcium sensitivity of the channels with EBIO slowed the early interspike interval as shown in hippocampal pyramidal neurons (Pedarzani et al., 2001). This result suggests that the concentration of calcium attained at SK channels during action potentials in the LA is significantly lower than that seen by channels in the hippocampus. This may be attributable to differences in localization of calcium channels and SK channels in these neurons. Thus, apamin-sensitive currents, although present in amygdalar principal neurons, do not appear to play a role in controlling spike frequency under normal conditions, and the exact physiological role of apamin-sensitive SK channels in the LA remains elusive.
As in many other neurons a third calcium-activated current, sIAHP, is also present in LA principal neurons. This current has slow kinetics, is insensitive to apamin, but is modulated by a number of neurotransmitters. As in hippocampal (Madison and Nicoll, 1982) and BLA neurons (Womble and Moises, 1993), this current is important in spike-frequency adaptation in the LA. Control of excitability by the slow AHP is achieved by hyperpolarization of the membrane, and thus prevention of the membrane potential from reaching threshold for further spiking. As in CA1 hippocampal pyramidal neurons and in BLA neurons, the slow AHP is greatly reduced after activation of cholinergic afferents. This blockade of the slow AHP leads to a large reduction in spike-frequency adaptation. Thus neurotransmitter modulation of this conductance represents an important mechanism for modulating the output of LA neurons.
Although four types of SK channels have so far been cloned, SK1, SK2, SK3, and SK4, only SK1–SK3 are found within the CNS (Stocker and Pedarzani, 2000). It had been suggested that because SK1 is much less apamin-sensitive than SK2 and SK3, the slow AHP may be mediated by SK1 channels (Vegara et al., 1998). SK1 transcripts do not appear to be present in the LA (Stocker and Pedarzani, 2000), whereas the apamin-insensitive slow AHP is seen in the vast majority (∼90%) of principal cells. Furthermore, the apamin insensitivity of SK1 has been found to vary between expression systems, and these channels are blocked by apamin when expressed in mammalian cells (Shah and Haylett, 2000; Strobaek et al., 2000). For these reasons we feel it is unlikely that SK1 channels underlie the sIAHP (Sah and Clements, 1999). Unlike SK1, however, both SK2 and SK3 are present in the LA (Stocker and Pedarzani, 2000), consistent with the presence of apamin-sensitive currents in all principal cells from which we have recorded.
In conclusion, these findings show that BK, SK, and non-SK/BK-mediated currents that comprise the AHP are present in almost all principal neurons in the LA, ranging from those that show full spike-frequency adaptation to those that do not. Although BK channels play an important role in repolarizing the membrane after spike initiation, the slow AHP plays a role in controlling the firing properties of these cells. The role of the medium AHP is currently elusive. However, it is clear that although the non-SK/BK-mediated calcium-activated potassium currents control spike-frequency adaptation in LA neurons, this is not the only factor that dictates the firing properties because blockade of the AHP often does not lead to a complete inhibition of accommodation. This is indicated by the inability of neurotransmitters to completely prevent spike-frequency adaptation despite abolishing the slow AHP. The mechanisms underlying this spike-frequency adaptation remain to be elucidated in the future.
Footnotes
This work was supported by the John Curtin School of Medical Research. We thank John Power, Mikel de Armentia, and Dennis Haylett for comments on this manuscript.
Correspondence should be addressed to Dr. Pankaj Sah, The Division of Neuroscience, The John Curtin School of Medical Research, GPO Box 334, Canberra, ACT 2601 Australia. E-mail: pankaj.sah@anu.edu.au.
REFERENCES
- 1.Benardo LS, Prince DA. Ionic mechanisms of cholinergic excitation in mammalian hippocampal pyramidal cells. Brain Res. 1982;249:333–344. doi: 10.1016/0006-8993(82)90067-1. [DOI] [PubMed] [Google Scholar]
- 2. Bowden SEH, Fletcher S, Loane DJ, Marrion NV. Somatic co-localization of rat SKI and D class (Cav 1.2) L-type calcium channels in rat CA1 hippocampal pyramidal neurons. J Neurosci 21 2001. RC175:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. Cloning and functional characterisation of novel large conductance calcium-activated potassium channel β subunits, hKCNMB3 and hKCNMB4. J Biol Chem. 2000;275:6453–6461. doi: 10.1074/jbc.275.9.6453. [DOI] [PubMed] [Google Scholar]
- 4.Castle NA, Haylett DG, Jenkinson DH. Toxins in the characterization of potassium channels. Trends Neurosci. 1989;12:59–65. doi: 10.1016/0166-2236(89)90137-9. [DOI] [PubMed] [Google Scholar]
- 5.Chen J-Q, Galanakis D, Ganellin CR, Dunn PM, Jenkinson DH. bis-Quinolinium cyclophanes: 8,14-diaza-1,7(1,4)-diquinolinacyclotetradecaphane (UCL 1848), a highly potent, selective, nonpeptidic blocker of the apamin-sensitive Ca2+-activated K+ channel. J Med Chem. 2000;43:3478–3481. doi: 10.1021/jm000904v. [DOI] [PubMed] [Google Scholar]
- 6.Coetzee WA, Amarillo YC, J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B. Molecular diversity of K+ channels. Ann NY Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- 7.Cole AE, Nicoll RA. Characterization of a slow cholinergic postsynaptic potential recorded in vitro from rat hippocampal cells. J Physiol (Lond) 1984;352:173–188. doi: 10.1113/jphysiol.1984.sp015285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dworetzky SI, Trojnacki JT, Gribkoff VK. Cloning and expression of a human large-conductance calcium-activated potassium channel. Brain Res Mol Brain Res. 1994;27:189–193. doi: 10.1016/0169-328x(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 9.Dworetsky SI, Boissard CG, Lum-Ragan M, McKay C, Post-Munson DJ, Tronjnacki JT, Chang C-P, Gribkoff VK. Phenotypic alteration of a human BK (hslo) channels by hslo-beta subunit coexpression: changes in blocker sensitivity, activation/relaxation and inactivation kinetics, and protein kinase A modulation. J Neurosci. 1996;16:4543–4550. doi: 10.1523/JNEUROSCI.16-15-04543.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faber ESL, Sah P. The effect of a 4-AP sensitive potassium current on neurones of the rat lateral amygdala. Soc Neurosci Abstr. 2000;26:897. [Google Scholar]
- 11.Faber ESL, Callister RJ, Sah P. Morphological and electrophysiological properties of principal neurons in the rat lateral amygdala in vitro. J Neurophysiol. 2001;85:714–723. doi: 10.1152/jn.2001.85.2.714. [DOI] [PubMed] [Google Scholar]
- 12.Galvez A, Gimenez-Gallego G, Reuben J, Roy-Contancin L, Feigenbaum P, Kaczorowski G, Garcia M. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem. 1990;265:11083–11090. [PubMed] [Google Scholar]
- 13.Haas HL, Konnerth A. Histamine and noradrenaline decrease calcium-activated potassium conductance in hippocampal pyramidal cells. Nature. 1983;302:432–434. doi: 10.1038/302432a0. [DOI] [PubMed] [Google Scholar]
- 14.Jackson MB, Konnerth A, Augustine GJ. Action potential broadening and frequency-dependent facilitation of calcium signals in pituitary nerve terminals. Proc Natl Acad Sci USA. 1991;888:380–384. doi: 10.1073/pnas.88.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson SW, Seutin V. Bicuculline methiodide potentiates NMDA-dependent burst firing in rat dopamine neurons by blocking apamin-sensitive Ca2+-activated K+ currents. Neurosci Lett. 1997;231:13–16. doi: 10.1016/s0304-3940(97)00508-9. [DOI] [PubMed] [Google Scholar]
- 16.Jung HY, Mickus T, Spruston N. Prolonged sodium channel inactivation contributes to dendritic action potential attenuation in hippocampal pyramidal neurons. J Neurosci. 1997;17:6639–6646. doi: 10.1523/JNEUROSCI.17-17-06639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knaus HG, Folander M, Garcia-Calvo ML, Garcia GJ, Kaczorowski GJ, Smith M, Swanson R. Primary sequence and immunological characterisation of β subunit of high conductance Ca2+-activated K+ channel from smooth muscle. J Biol Chem. 1994;269:17274–17278. [PubMed] [Google Scholar]
- 18.Kohler M, Hischberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 1996;273:1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- 19.Lancaster B, Adams PR. Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol. 1986;55:1268–1282. doi: 10.1152/jn.1986.55.6.1268. [DOI] [PubMed] [Google Scholar]
- 20.Lancaster B, Nicoll RA. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol (Lond) 1987;389:187–204. doi: 10.1113/jphysiol.1987.sp016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lancaster B, Pennefather PS. Potassium currents evoked by brief depolarizations in bull-frog sympathetic ganglion cells. J Physiol (Lond) 1987;387:519–548. doi: 10.1113/jphysiol.1987.sp016587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LeDoux JE. Emotion: clues from the brain. Annu Rev Psychol. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- 23.Lovell PV, McCobb DP. Pituitary control of BK potassium channel function and intrinsic firing properties of adrenal chromaffin cells. J Neurosci. 2001;21:3429–3442. doi: 10.1523/JNEUROSCI.21-10-03429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madison DV, Nicoll RA. Noradrenaline blocks accommodation of pyramidal cell discharge in the hippocampus. Nature. 1982;299:636–638. doi: 10.1038/299636a0. [DOI] [PubMed] [Google Scholar]
- 25.Mahanty NK, Sah P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature. 1998;394:683–687. doi: 10.1038/29312. [DOI] [PubMed] [Google Scholar]
- 26.Marrion NV, Tavalin SJ. Selective activation of Ca2+-activated K+ channels by co-localised Ca2+ channels in hippocampal neurons. Nature. 1998;395:900–905. doi: 10.1038/27674. [DOI] [PubMed] [Google Scholar]
- 27.McDonald AJ. Neuronal organisation of the lateral and basolateral amygdaloid nuclei of the rat. J Comp Neurol. 1984;222:589–606. doi: 10.1002/cne.902220410. [DOI] [PubMed] [Google Scholar]
- 28.McManus OB, Helms LM, Pallanck L, Ganetzky B, Swanson R, Leonard RJ. Functional role of the β subunit of high conductance calcium-activated potassium channels. Neuron. 1995;14:645–650. doi: 10.1016/0896-6273(95)90321-6. [DOI] [PubMed] [Google Scholar]
- 29.Meera P, Wallner M, Toro L. A neuronal β subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci USA. 2000;97:5562–5567. doi: 10.1073/pnas.100118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nimigean CM, Magleby KL. The β subunit increases the Ca2+ sensitivity of large conductance Ca2+-activated potassium channels by retaining the gating the bursting states. J Gen Physiol. 1999;113:425–439. doi: 10.1085/jgp.113.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pedarzani P, Storm JF. PKA mediates the effects of monoamine transmitters on the K+ current underlying the slow spike-frequency adaptation in hippocampal neurons. Neuron. 1993;11:1023–1035. doi: 10.1016/0896-6273(93)90216-e. [DOI] [PubMed] [Google Scholar]
- 32.Pedarzani P, Mosbacher J, Rivard A, Cingolani LA, Oliver D, Stocker M, Adelman JP, Fakler B. Control of electrical activity in central neurons by modulating the gating of small conductance Ca2+-activated K+ channels. J Biol Chem. 2001;276:9762–9769. doi: 10.1074/jbc.M010001200. [DOI] [PubMed] [Google Scholar]
- 33.Pitkänen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- 34.Price JL, Russchen FT, Amaral DG. The limbic region. II: The amygdaloid complex. Elsevier; Amsterdam: 1987. [Google Scholar]
- 35.Sah P. Ca2+-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 1996;19:150–154. doi: 10.1016/s0166-2236(96)80026-9. [DOI] [PubMed] [Google Scholar]
- 36.Sah P, Clements JD. Photolytic manipulation of [Ca2+]i reveals slow kinetics of potassium channels underlying the afterhyperpolarization in hippocampal pyramidal neurons. J Neurosci. 1999;19:3657–3664. doi: 10.1523/JNEUROSCI.19-10-03657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sah P, McLachlan EM. Potassium currents contributing to action potential repolarization and the afterhyperpolarization in rat vagal motoneurons. J Neurophysiol. 1992;68:1834–1841. doi: 10.1152/jn.1992.68.5.1834. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez M, McManus OB. Paxilline inhibition of the α subunit of the high conductance calcium-activated potassium channel. Neuropharmacology. 1996;35:963–968. doi: 10.1016/0028-3908(96)00137-2. [DOI] [PubMed] [Google Scholar]
- 39.Shah M, Haylett DG. The pharmacology of hSK1 Ca2+-activated K+ channels expressed in mammalian cell lines. Br J Pharmacol. 2000;129:627–630. doi: 10.1038/sj.bjp.0703111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shao LR, Halvorsrud R, Borg-Graham L, Storm JF. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J Physiol (Lond) 1999;521:135–146. doi: 10.1111/j.1469-7793.1999.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stocker M, Pedarzani P. Differential distributions of three Ca2+-activated K+ channel subunits, SK1, SK2 and SK3 in the adult rat central nervous system. Mol Cell Neurosci. 2000;15:476–493. doi: 10.1006/mcne.2000.0842. [DOI] [PubMed] [Google Scholar]
- 42.Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc Natl Acad Sci USA. 1999;96:4662–4667. doi: 10.1073/pnas.96.8.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Storm JF. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol (Lond) 1987;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Storm JF. Potassium currents in hippocampal pyramidal cells. Prog Brain Res. 1990;83:161–187. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- 45.Strobaek D, Christophersen P, Holm NR, Moldt P, Ahring PK, Johansen TE, Olesen SP. Modulation of the Ca2+-dependent channel, hslo, by the substituted diphnylurea NS 1608, paxilline and internal Ca2+. Neuropharmacology. 1996;35:903–914. doi: 10.1016/0028-3908(96)00096-2. [DOI] [PubMed] [Google Scholar]
- 46.Strobaek D, Jorgensen TD, Christophersen P, Ahring PK, Olesen SP. Pharmacological characterization of small-conductance Ca2+-activated K+ channels stably expressed in HEK 293 cells. Br J Pharmacol. 2000;129:991–999. doi: 10.1038/sj.bjp.0703120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Solaro CR, Prakirya M, Ding JP, Lingle CJ. Inactivating an noninactivating Ca2+ and voltage dependent K+ current in rat adrenal chromaffin cells. J Neurosci. 1995;15:6110–123. doi: 10.1523/JNEUROSCI.15-09-06110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tseng-Crank J, Godinot N, Johansen TE, Ahring PK, Strobaek D, Mertz R, Foster CD, Olesen SP, Reinhart PH. Cloning, expression, and distribution of a Ca2+-activated K+ channel β subunit from human brain. Proc Natl Acad Sci USA. 1996;93:9200–9205. doi: 10.1073/pnas.93.17.9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uebele VN, Lagrutta A, Wade T, Figeuroa DJ, Liu Y, McKenna E, Austin CP, Bennett PB, Swanson R. Cloning and functional expression of two families of β subunits of the large conductance calcium-activation K+ channel. J Biol Chem. 2000;275:23211–23218. doi: 10.1074/jbc.M910187199. [DOI] [PubMed] [Google Scholar]
- 50.Vegara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Curr Opin Neurobiol. 1998;8:321–329. doi: 10.1016/s0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- 51.Wallner M, Meera P, Toro L. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: a transmembrane β-subunit homolog. Proc Natl Acad Sci USA. 1999;96:4137–4142. doi: 10.1073/pnas.96.7.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Washburn MS, Moises HC. Muscarinic responses of rat basolateral amygadaloid neurons recorded in vitro. J Physiol (Lond) 1992;449:121–154. doi: 10.1113/jphysiol.1992.sp019078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolfart J, Neuhoff H, Franz O, Roeper J. Differential expression of the small-conductance, calcium-activated potassium channel SK3 Is critical for pacemaker control in dopaminergic midbrain neurons. J Neurosci. 2001;21:3443–3456. doi: 10.1523/JNEUROSCI.21-10-03443.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Womble MD, Moises HC. Muscarinic modulation of conductances underlying the afterhyperpolarisation in neurons of the rat basolateral amygdala. Brain Res. 1993;621:87–96. doi: 10.1016/0006-8993(93)90301-3. [DOI] [PubMed] [Google Scholar]
- 55.Xia X-M, Ding JP, Lingle CJ. Molecular basis for the inactivation of Ca2+- and voltage-dependent BK channels in adrenal chromaffin cells and rat insulinoma tumor cells. J Neurosci. 1999;19:5255–5264. doi: 10.1523/JNEUROSCI.19-13-05255.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xia X-M, Ding JP, Zeng XH, Duan KL, Lingle CJ. Rectification and rapid activation at low Ca2+ of Ca2+-activated voltage-dependent BK currents: consequences of rapid inactivation by a novel β subunit. J Neurosci. 2000;20:4890–4903. doi: 10.1523/JNEUROSCI.20-13-04890.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]