Abstract
Peripheral 18-oxocortisol (18oxoF) level could contribute to the detection of aldosterone-producing adenoma (APA) in patients with primary aldosteronism. However, peripheral 18oxoF varies among such patients, which is a big drawback concerning its clinical application. We studied 48 cases of APA, 35 harboring KCNJ5 mutation, to clarify the significance of clinical and pathological parameters about peripheral 18oxoF. Peripheral 18oxoF concentration ranged widely from 0.50 to 183.13 ng/dL and correlated positively with intratumoral areas stained positively for steroidogenic enzymes (P<0.0001). The peripheral 18oxoF level also correlated significantly with that of circulating aldosterone (P<0.0001) but not with that of cortisol, a precursor of 18oxoF. However, a significant correlation was detected between peripheral 18oxoF and intratumoral glucocorticoids (P<0.05). In addition, peripheral 18oxoF correlated positively with the number of hybrid cells double positive for 11β-hydroxylase and aldosterone synthase (P<0.0001). Comparing between the cases with and those without KCNJ5 mutation, the KCNJ5-mutated group demonstrated a significantly higher concentration of peripheral 18oxoF (28.4±5.6 versus 3.0±0.9 ng/dL, P<0.0001) and a larger intratumoral environment including the hybrid cells (P<0.001), possibly representing a deviation from normal aldosterone biosynthesis. After multivariate analysis, KCNJ5 mutation status turned out to be the most associated factor involved in 18oxoF synthesis in APA (P<0.0001). Results of our present study first revealed that enhanced 18oxoF synthesis in APA could come from a functional deviation of aldosterone biosynthesis from the normal zona glomerulosa and the utility of peripheral 18oxoF measurement could be influenced by the prevalence of KCNJ5 mutation in an APA.
Keywords: adrenocortical adenoma, 18-oxocortisol, hybrid cells, hyperaldosteronism, KCNJ5 mutation
Primary aldosteronism (PA) is a hypertensive disorder resulting in more severe organ damage than essential hypertension because of both direct and indirect effects of autonomous aldosterone secretion.1,2 PA is also the most common cause of secondary hypertension, and its prevalence is considered to be as high as 5% to 10% of all patients with hypertension.3,4 Early diagnosis and appropriate management for PA are, therefore, clinically required to decrease the elevated risk of developing hypertensive complications, such as cardiovascular and cerebral events.5 However, confirmatory tests of PA and adrenal venous sampling for determining PA subtypes are generally time-consuming, invasive and labor intensive.6,7
18-oxocortisol (18oxoF) has been recently reported as a potential clinical biomarker for differentiating subtypes of PA.8 18oxoF has been also termed hybrid steroid because of its structural features of both glucocorticoid and mineralocorticoid.9 In healthy human subjects, 18oxoF is converted from circulating cortisol by aldosterone synthase, CYP11B2,9 and its clinical significance is scarce because of its extremely low concentration in plasma. However, it is also true that patients with PA have higher plasma and urinary concentrations of 18oxoF than patients with essential hypertension.10,11 In addition, we have previously demonstrated using segmental adrenal venous sampling that aldosterone-producing adenoma (APA) secretes more 18oxoF into adrenal veins than the contralateral adrenal gland and in case of bilateral hyperaldosteronism (BHA).12 Therefore, as peripheral plasma concentration of 18oxoF (p18oxoF) is significantly elevated in patients with APA than in those with BHA, this parameter could be useful to differentiate APA from BHA among patients with PA.8 Although not totally accurate, measurement of p18oxoF is expected to be a useful and low-invasive biomarker for clinical subtyping of PA.
However, the status of 18oxoF synthesis in APA, as well as its clinical and biological significance, has remained largely unknown. For instance, the concentration of p18oxoF enormously differs among APA cases. In particular, one-fourth of patients with APA had concentrations of p18oxoF as low as those found in patients with BHA.8 The concentration of p18oxoF has been known to be influenced by the intratumoral status of CYP11B2, a rate-limiting enzyme of 18oxoF. In addition to that, 11β-hydroxylase (CYP11B1) is also a candidate influential factor because of converts 11-deoxycortisol to cortisol and the status of glucocorticoid synthesis in APA.13,14 In familial hyperaldosteronism (FH) type 1, a chimeric gene of CYP11B1 and CYP11B2 has been well known as a pivotal factor for enhanced 18oxoF synthesis.15 Both CYP11B1 and CYP11B2 double-positive hybrid cells (B1+B2 cells) have been also reported to exist in APAs,16 which could contribute to the high concentration of p18oxoF.
In addition, the utility of p18oxoF concentration level in determining subtypes of PA appears to differ between whites and Asian patients.8,17 This difference could be possibly because of the status of a somatic gene mutation in APA,18 especially KCNJ5 mutation, because the prevalence of KCNJ5 mutation in APAs among Europeans is significantly lower than that among Japanese.19,20 The influence of KCNJ5 mutation on 18oxoF synthesis has remained unclear but results of several previously reported studies demonstrated that KCNJ5 mutation-induced adrenocortical cells and APA had a potential to produce abundant 18oxoF.18,21
We, therefore, hypothesized that 18oxoF synthesis in APA could be influenced by the intratumoral status of the precursor steroids synthesis and CYP11B1 and CYP11B2 double-positive hybrid tumor cells. Thus, in this study, we examined the potential impacts of these pathological factors on 18oxoF synthesis in APA and evaluated how KCNJ5 mutation could influence 18oxoF synthesis.
Methods
The present study was approved by the ethics committee of Tohoku University School of Medicine (numbers: 2011–236, 2014–1-404, and 2015–1-278). We obtained informed consents from all PA cases examined. The data that support the findings of this study are available from the corresponding author on reasonable request.
Diagnosis of PA and Eligibility of APA
The APA cases were retrospectively retrieved from a cohort of the PA Sendai Study in which individual patients were registered once they provided their informed consent in writing. All the cases were diagnosed with PA and underwent unilateral adrenalectomy in Tohoku University Hospital (Sendai, Japan), from 2011 after the Great Earthquake that affected our area, until 2015. The diagnosis of PA was confirmed by the captopril challenge test (50 mg of captopril), and the laterality of hyperaldosteronism was determined by cosyntropin-stimulation adrenal venous sampling as previously reported.22 In this procedure, we used commercially available kits to measure plasma renin concentration and plasma aldosterone concentration; namely, Renin Riabead Kit (Dainabot, Tokyo, Japan) and SPAC-S Aldosterone Radioimmunoassay Kit (TFB, Inc, Tokyo), respectively.22 Blood pressure was measured using Omron Hem 907 (Omron Healthcare Co Ltd, Kyoto, Japan) as shown in our previous study.8 After unilateral adrenalectomy, the removed adrenal glands were analyzed and confirmed as APA by histopathologic examination and based on postoperative biochemical data. Exclusion criteria were as follows: the patients with other concurrent adrenal tumor(s) revealed by computed tomography; with a cortisol concentration of >3.0 μg/dL after an overnight 1-mg dexamethasone suppression test23; with multiple APA; and those showing intratumoral heterogeneity of CYP11B2 expression.24 In total, 48 cases were examined in this study.
Measurement of p18oxoF and Intratumoral Steroid Hormones
Using fusaric acid, p18oxoF was measured by liquid chromatography-tandem mass spectrometry as previously reported.8 Peripheral blood samples for p18oxoF measurement were obtained in the morning after a 30-minute rest. In this study, the lower limit of quantification for 18oxoF was 0.50 ng/dL because of the amount of plasma sample used. Besides, we measured tissue concentrations of 18oxoF, aldosterone, corticosterone, cortisol, and 11-deoxycortisol by liquid chromatography-tandem mass spectrometry. Sample preparation for the measurement of each steroid hormone by liquid chromatography-tandem mass spectrometry is described in the online-only Data Supplement. The steroid concentration in frozen tissue was adjusted by estimated tumor volume based on the length, width, and height of the removed APA.
Histopathologic Analysis for Expression of Steroidogenic Enzymes
All resected adrenal specimens were embedded in paraffin for immunohistochemical staining of CYP11B1, CYP11B2, and C17 (17α-hydroxylase), and for immunofluorescence staining of B1+B2 cells.16,25,26 The immunohistochemical staining and immunofluorescence staining methods are described in the online-only Data Supplement. All immunohistochemical staining sections were digitally scanned and captured by Image Scope AT2 (Leica, Wetzlar, Germany) for digital image analysis. We measured the cross-sectional area (CSA) of APA, and the immunoreactivity of each of these enzymes above was quantitated using HALO digital image software Area Quantification version 1.0 (Indica Laboratories, Corrales, NM) as previously described.25 The status of individual steroidogenic enzymes was evaluated by the following 2 quantitative approaches. One was the ratio of positive area to CSA of APA referred as immu-noreactivity, and the other the area of positive staining in APA referred as positive area.
As for the manual counting of the number of B1+B2 cells, the immunofluorescence images were obtained as square pictures of 1024 pixels (512 μm) using a confocal laser-scanning microscope system (A1R, Nikon, Tokyo). The number of B1+B2 cells was calculated as the average of the counts obtained from 3 pictures from other lesions in each APA cases and adjusted for CSA. As controls, 7 cases of non-functional adrenocortical adenoma were analyzed.
Detection of KCNJ5 Mutation in APA
We prepared 5 other tissue sections of those adrenal specimens at 10 μm thickness for the detection of KCNJ5 mutation in APA and micro-dissected APA regions in the sections.25 Genomic DNA was isolated from those tissues using the Qiagen AllPrep DNA/RNA FFPE kit and submitted it to polymerase chain reaction using a KCNJ5 primer (forward 5’-CGA CCA AGA GTG GAT TCC TT-’3 and reverse 5’-AGG GTC TCC GCT CTC TTC TT-’3) as already described by our group.27 Detection of KCNJ5 mutation was performed with the Abi Prism 310 genetic analyzer (Applied Biosystems, Foster City, CA). In this study, we did not evaluate other somatic mutations such as CACNA1D and ATPase because of its extremely lower frequency compared with that of KCNJ5 mutation or wild type in Japanese patients with APA.20
Statistical Analysis
In terms of p18oxoF and plasma renin activity, the values below lower limits of detection were assigned to analytical sensitivity values for subsequent statistical comparison. All data were presented as the mean±SD when in normal distribution and as the median (interquartile range) when in log-normal distribution. For analysis, data on p18oxoF, plasma aldosterone concentration, presence of intratumoral steroid, CSA, expression of enzymes, and number of B1+B2 cells were log-transformed because of their distributions. Spearman correlation analysis and Mann-Whitney U test were used for measuring the strength of the relationship between 2 variables and comparison between different groups, respectively. To investigate independent correlates, we also performed multivariate linear regression analysis with the backward stepwise procedure. Statistical significance was set at P≤0.05, and all analyses were performed with Stat Flex version 6.0 (Artech Co Ltd, Osaka, Japan).
Results
Baseline Parameters of 48 APA Cases
Baseline characteristics of all APA cases (n=48) were summarized in Table 1. The great majority of the cases examined harbored computed tomography-detectable adrenal tumors which were pathologically diagnosed as APA, while only 3 cases had no computed tomography-detectable adrenal tumors. These 3 cases were confirmed as aldosterone-producing microad-enomas. The concentration of p18oxoF ranged from 0.50 to 183.13 ng/dL with a median of 11.92 ng/dL (Figure S1 in the online-only Data Supplement). Spearman correlation analysis revealed that the concentration of p18oxoF was significantly correlated with age (Spearman r=−0.471, P=0.0007) and duration of hypertension (Spearman r=−0.328, P=0.02).
Table 1.
Clinical and Pathological Baseline Parameters of APA
| Parameters | All | KCNJ5 Positive | KCNJ5 Negative | P Value |
|---|---|---|---|---|
| N | 48 | 35 | 13 | … |
| Age, y* | 51.7±11.8 | 49.0±11.5 | 59.0±9.5 | <0.01 |
| Sex, M and F* | 26, 22 | 14, 21 | 12, 1 | <0.01 |
| Body mass index, kg/m2 | 24.7±3.4 | 24.0±2.8 | 26.5±4.1 | NS |
| PAC, ng/dL† | 40.8 [31.7–57.6] | 48.6 [35.2–62.2] | 32.6 [24.8–41.3] | <0.05 |
| PRA, ng/mL per hour | 0.20 [0.10–0.45] | 0.30 [0.10–0.50] | 0.20 [0.10–0.23] | NS |
| ARR, ng/dL per ng/mL per hour | 200.4 [95.4–404.8] | 198.3 [95.5–409.6] | 202.5 [92.1–348.3] | NS |
| 18-oxocortisol, ng/dL‡ | 11.9 [3.7–28.7] | 17.3 [8.9–36.2] | 1.9 [0.92–3.6] | <0.0001 |
| Duration of hypertension, y† | 9.0 [2.0–16.0] | 5.0 [2.0–11.8] | 17.0 [8.8–23.5] | <0.05 |
| Blood pressure, mm Hg | 151.0±21.2/95.3±15.3 | 151.4±22.8/94.9±22.8 | 149.9±16.8/96.4±13.0 | NS |
| The number of AHT, n† | 2.7±1.7 | 2.3±1.4 | 3.5±2.0 | <0.05 |
| Decrease in AHT after surgery, y† | 1.7±1.5 | 1.5±1.5 | 2.2±1.1 | NS |
| Serum potassium, mmol/L | 3.84±0.53 | 3.85±0.54 | 3.83±0.53 | NS |
| Usage of potassium replacement, % | 81.3 | 82.9 | 76.9 | NS |
| MD of APA, mm§ | 14.0±6.3 | 15.6±5.3 | 9.6±7.0 | <0.001 |
| CSA of APA, mm2‡ | 46.0 [25.8–86.4] | 66.6 [38.3–113.4] | 20.0 [11.8–26.2] | <0.0001 |
| Immunoreactivitiy, % | ||||
| CYP11B1† | 13.3 [7.9–24.0] | 15.3 [8.4–25.0] | 7.8 [5.4–15.8] | <0.05 |
| CYP11B2* | 27.7±12.2 | 24.2±10.8 | 37.3±11.0 | <0.01 |
| C17 | 15.2±8.8 | 15.6±8.6 | 14.3±9.8 | NS |
| Positive area, mm2 | ||||
| CYP11B1§ | 6 8 [2 5–15 3] | 10 2 [4 7–22 6] | 1.3 [0.6–4.6] | <0.001 |
| CYP11B2§ | 12.4 [6.7–18.7] | 14.1 [10.5–19.7] | 6.2 [3.9–10.2] | <0.001 |
| C17§ | 5.6 [2.9–14.1] | 9.1 [4.0–17.0] | 1.8 [0.7–5.2] | <0.001 |
| B1+B2 cells, n/mm2§ | 48.8 [21.7–101.7] | 67.5 [33.4–126.5] | 21.7 [3.5–33.5] | <0.001 |
| CSA-adjusted B1+B2 cells, n§ | 3402.7 [578.0–7836.7] | 4559.7 [1928.0–10797.2] | 278.3 [72.8–602.7] | <0.001 |
| KCNJ5 mutation, % | 72.9 | 100 | 0 | … |
Data are described as mean±SD in normal distribution and median [interquartile range] in log-normal distribution. P values are calculated based on comparisons between KCNJ5 mutation positive and negative APA cases. AHT indicates antihypertensive agent; APA, aldosterone-producing adenoma; ARR, aldosterone-to-renin ratio; B1+B2 cells, the number of CYP11B1 and CYP11B2 coexpressed cells; C17, 17α-hydroxylase; CSA, cross-sectional area; CYP11B1, 11β-hydroxylase; CYP11B2, aldosterone synthase; C17, 17α-hydroxylase; MD, maximum diameter; NS, not significant; PAC, plasma aldosterone concentration; and PRA, plasma renin activity.
P<0.01.
P<0.05.
P<0.0001.
P<0.001.
The associations between p18oxoF and other steroid hormones in plasma and urine were then evaluated (Figure 1). The concentration of p18oxoF was significantly correlated with plasma aldosterone concentration, possibly because of their common rate-limiting enzyme CYP11B2.9 However, the concentration of p18oxoF was by no means correlated with that of circulating cortisol, a precursor of 18oxoF. Similar trends were detected between p18oxoF and urinary concentration of aldosterone and cortisol. 18oxoF is a derivative of cortisol, but p18oxoF did not demonstrate any significant correlation with the concentration of either circulating or urinary cortisol.
Figure 1.
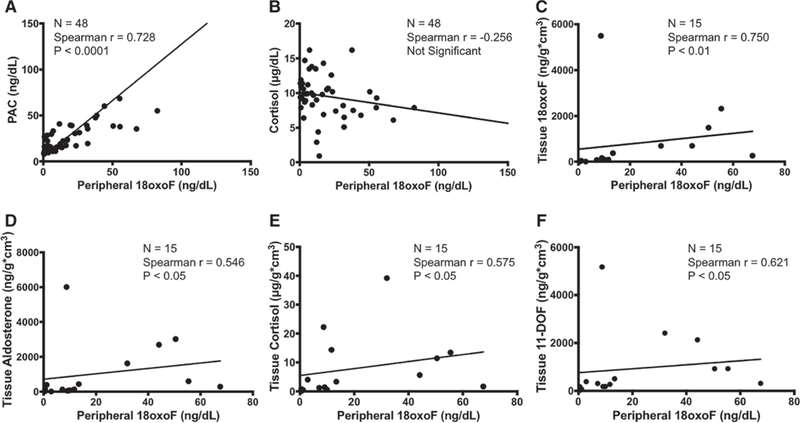
Correlations between peripheral 18-oxocortisol (18oxoF) and circulating (A and B) or intratumoral steroid (C–F). Peripheral 18oxoF was significantly correlated with plasma aldosterone concentration (PAC) but not with circulating cortisol, a precursor of 18oxoF. However, intratumoral steroid evaluation revealed a significant correlation between peripheral 18oxoF and intratumoral glucocorticoids, cortisol, and 11-deoxycortisol (11-DOF).
Peripheral 18oxoF and Immunoreactivities of Steroidogenic Enzymes in Tumor Tissues
The mean maximum diameter (MD) and CSA of APAs were 13.96 mm and 63.93 mm2, respectively. p18oxoF was significantly correlated with MD and CSA of APAs (Figure 2). The ratio of CYP11B1 correlated negatively with that of MD, whereas the ratio of CYP11B2 was positively correlated with that of MD; however, no significant correlation was observed with the ratio of C17 (Figure S2). However, all areas that stained positively for the enzymes examined were positively and significantly correlated with MD. In addition, p18oxoF was significantly correlated with the areas showing positive staining for steroidogenic enzymes in these cases. The results indicated that APA cases with a high concentration of p18oxoF possibly expressed substantial amounts of steroidogenic enzymes to produce glucocorticoids as well as aldosterone.
Figure 2.
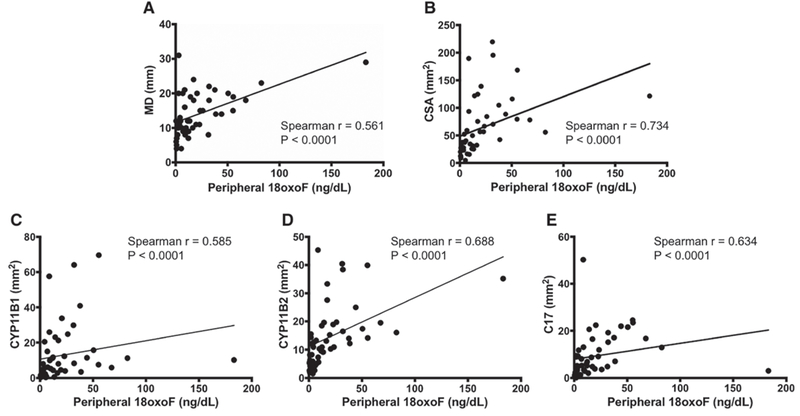
Comparison between peripheral 18-oxocortisol (18oxoF) and pathologic parameters. A and B, Peripheral 18-oxocortisol (18oxoF) was significantly correlated with the tumor size. C–E, Similarly, peripheral 18oxoF showed positive correlations with the expression of all the steroidogenic enzymes examined. C17 indicates 17α-hydroxylase; CSA, cross-sectional area; CYP11B1, 11β-hydroxylase; CYP11B2, aldosterone synthase; and MD, maximum diameter.
Tissue Concentration of 18oxoF and Other Steroid Hormones
We then measured 18oxoF and other steroid hormones in frozen tissue of 15 available cases of APA to identify possible influences of intratumoral synthesis of its precursors on the concentration of 18oxoF. Both p18oxoF and tissue concentration of 18oxoF were significantly and positively correlated (Figure 1). Tissue concentrations of 18oxoF and aldosterone were also significantly correlated with each other (Spearman r=0.843, P<0.01). Of particular interest, tissue concentration of 18oxoF and that of cortisol were also positively correlated in contrast to their concentrations in peripheral blood (Spearman r=0.675, P<0.01). 18oxoF was also significantly correlated with the concentration of 11-deoxycortisol, a precursor of cortisol (Spearman r=0.871, P<0.01) but not with that of corticosterone, a precursor of aldosterone in the tissues examined. p18oxoF was also correlated with the tissue concentration of aldosterone, cortisol, and 11-deoxycortisol, indicating that not circulating cortisol but an intratumoral synthesis of its precursors significantly regulated 18oxoF synthesis in APA.
Association of p18oxoF and CYP11B1 and CYP11B2 Double-Positive Hybrid Cells in APA
We then evaluated B1+B2 cells in APA (Figure S3). The number of these hybrid cells was 48.8 (21.7–101.7)/mm2 in APA and significantly larger than that in nonfunctional adrenocortical adenoma (3.8 [1.8–6.1]/mm2, P=0.0007). This tendency remained the same before and after adjustment with CSA. B1+B2 cells in APA were also positively and significantly correlated with MD (Spearman r=0.308, P=0.03) and CSA (Spearman r=0.536, P=0.0001), while in nonfunctional adrenocortical adenoma no correlation was observed with any clinicopathological factor. In addition, the status of B1+B2 cells in APA was significantly correlated with p18oxoF (Spearman r=0.617, P<0.0001) and plasma aldosterone concentration (Spearman r=0.312, P=0.03) after adjustment for CSA (Figure S3). Serum cortisol concentration, however, did not correlate with the status of B1+B2 cells in APA. These results as a whole indicated that a large number of B1+B2 cells also could lead to enhanced 18oxoF synthesis in APA, as reported in FH type I.
Influence of KCNJ5 Mutation Status on Intratumoral Synthesis of Steroid Hormones in APA
We subsequently examined the association between clinico-pathological factors and KCNJ5 mutation status about their influence on p18oxoF synthesis in APA. Thirty-five of the 48 APA cases (72.9%, including 1 case of microadenoma) harbored the KCNJ5 mutation (Table 1). The concentration of p18oxoF in KCNJ5-mutated APA was significantly higher than that in APA without this mutation (Table 1). Between 2 major KCNJ5 mutations, G151R and L168R, the L168R group showed a significantly higher concentration of p18oxoF than the G151R group (P=0.03, Tables S1 and S2). In addition, interestingly, the areas that stained positive for steroidogenic enzymes and B1+B2 cells were significantly more abundant in KCNJ5-mutated APA than in APA without the KCNJ5 mutation. The KCNJ5 mutation status could account for the differences in p18oxoF concentration among patients of different age. KCNJ5 mutation could be a key driver which accelerates 18oxoF synthesis by promoting synthesis of its precursors and direct synthesis from B1+B2 cells.
All the factors related to the enhanced 18oxoF synthesis in APA correlated with tumor size. By incorporating age, sex, the status of immunoreactivity of each steroidogenic enzyme, MD, number of B1+B2 cells and KCNJ5 mutation status, multivariate linear regression analysis with the backward stepwise procedure revealed independent associated factors for 18oxoF synthesis in APA (Table 2). Immunoreactivity of CYP11B2, MD, and KCNJ5 mutation status turned out as an independent associated factor. Finally, we confirmed that KCNJ5 mutation status was the most significantly associated factor about enhanced 18oxoF synthesis in APA.
Table 2.
Factors Associated With 18oxoF Synthesis in APA
| Variable | β | SE | Standardized β | 95% CI | P Value |
|---|---|---|---|---|---|
| Immunoreactivity of CYP11B2, aldosterone synthase, %* | 0.029 | 0.012 | 0.25 | 0.040–0.46 | 0.02 |
| Maximum diameter of APA, mm* | 0.057 | 0.023 | 0.26 | 0.047–0.47 | 0.02 |
| KCNJ5 mutation (+, -)† | 1.93 | 0.37 | 0.62 | 0.381–0.86 | <0.0001 |
Adjusted R2=0.619, F=16.32, and P<0.0001. 18oxoF indicates 18-oxocortisol; and APA, aldosterone-producing adenoma.
P<0.05.
P<0.0001.
Discussion
The results of the present study demonstrated that 18oxoF synthesis in APA was regulated by both the status of intratumoral synthesis of its precursors and the number of B1+B2 cells. This is the first study which clearly demonstrated an intratumoral unique environment where rate-limiting enzymes of both of aldosterone and cortisol was crucial for the synthesis of p18oxoF. It was also notable that the environment could be reflected in the tumor size and that the presence of KCNJ5 mutation in APA was an independent associated factor for enhanced 18oxoF synthesis mediated by both of the factors above.
In normal adrenals, 18oxoF is mainly converted from circulating cortisol by CYP11B2 in the zona glomerulosa because of centripetal blood flow.9,28 18oxoF is, therefore, recognized as a by-product of CYP11B2, in general. Our previous studies confirmed that not only normal adrenal tissue but also adrenal hyperplasia showed preserved zonation of the adrenal cortex and did not express both CYP11B1 and CYP11B2 in the same cortical cells as FH.16,29,30 In contrast with those previous findings, our present study showed that APA harbors an environment of mixture of several steroidogenic enzymes. The environment could lead to 18oxoF synthesis by cell to cell interaction because of easier contact between CYP11B2 and the precursors of 18oxoF because of expression of CYP11B1 and C17. Contrary to circulating cortisol, the amount of intra-tumoral glucocorticoids demonstrated a significant correlation with p18oxoF, indicating that those steroids were mainly converted to 18oxoF in APA. However, we could by no means rule out the possibility of the influence of circulating cortisol on 18oxoF synthesis because of the influence of several related factors, such as its binding protein and different half-life.
Additionally, our study revealed that B1+B2 cells in APA could be a cause of direct 18oxoF synthesis. In FH type 1, the expression of the chimeric gene of CYP11B1 and CYP11B2 in adrenocortical cells was reported to cause 18oxoF overproduction.31 An in vitro study also confirmed that adrenocortical cells expressing both cortisol and aldosterone synthetases could produce 18oxoF directly.32 Therefore, the existence of these hybrid cells in APA could be thought as an associated factor for 18oxoF synthesis. Moreover, the acquisition of these hybrid cells in APA might represent the sequence of neo-plastic or abnormal development. The correlation of p18oxoF with tumor size was considered plausible because the size of a tumor is generally considered to be in parallel with the degree of deviation of normal cells in which tumor arise. These findings were also consistent with the fact that p18oxoF could clinically detect larger APAs, not microadenomas, with more accuracy.8
Moreover, the present results also revealed that the presence of the KCNJ5 somatic mutation was also significantly correlated with 18oxoF synthesis in APA via an intratumoral unique environment. The mutated KCNJ5 gene increases sodium influx and chronic depolarization in aldosterone-producing cells, resulting in autonomous overproduction of aldosterone by increased CYP11B2 expression.33
Results of previously reported meta-analysis study clearly demonstrated clinical significance of KCNJ5 mutation in APA on severe hyperaldosteronism.34 However, HAC15 cells harboring increased expression of the KCNJ5 mutation gene were also reported to increase mRNA expression of CYP11B1 as well as of CYP11B2 in vitro.35 Therefore, the production of cortisol and 18oxoF was also more pronounced in HAC cells harboring the KCNJ5 mutation than in those with KCNJ5 wild-type gene in vitro.21 In addition, results of previously reported study did demonstrate the impacts of somatic mutations of APA on the plasma concentration of 18oxoF.18 Williams et al18 reported that KCNJ5-mutated APA group had significantly higher levels of 18oxoF and 18-hydroxycortisol than other genotypes, such as CACNA1D and ATPase, although the cause of these difference has remained unclear. Results of our present study did reveal that the difference could be derived from intratumoral tissue microenvironment, that is, the degree of expression of steroidogenic enzymes per cells and the hybrid cells. However, the association between these hybrid cells and KCNJ5 mutation in the human body has remained unclear in a mechanistic sense, but in FH type 3 which is caused by genetic mutation KCNJ5, adrenal glands harbors abundant hybrid cells.36 In our study, KCNJ5 mutation status was similarly correlated with not only the area that stained positive for CYP11B2 but also for both aldosterone and p18oxoF, which also indicated that these changes in KCNJ5-mutated cells of APA could occur via the same mechanisms. However, serum cortisol concentration was by no means correlated with 18oxoF or KCNJ5 mutation status in our cohort. This is because circulating cortisol in APA cases largely consist of cortisol secretion from adjacent and contralateral adrenal glands. The presence of somatic KCNJ5 mutations might reflect the biological deviation from normal aldosterone-producing adrenocortical cells, but further investigations are required for clarification.
These results also demonstrated that the clinical utility of measuring p18oxoF for subclassification of PA could depend on the prevalence of the KCNJ5 mutation in APA. In this study, 29 out of 35 APAs harboring the KCNJ5 mutation (82.9%) had significantly high concentrations of p18oxoF of >6.1 ng/dL, which was significantly high and corresponded to the maximum value found in BHA in our previous study.8 However, only 2 out of 13 cases (15.4%) with KCNJ5 wild type had a concentration of p18oxoF higher than this particular value. In addition, there were 6 cases (12.5%) with a p18oxoF concentration lower than 1.2 ng/dL corresponding to the minimum value found in BHA.8 In all of these 6 cases, the tumors turned out to harbor the wild-type KCNJ5 gene and were smaller than 10 mm in their greatest dimension. As it is well known, the prevalence of the KCNJ5 mutation in APA is markedly different among different races.34 Therefore, measurement of p18oxoF could have potential for subtyping the patients with PA in a cohort with a known high prevalence of KCNJ5-mutated APA, such as Asian patients.20,34,37, 38 However, further studies are needed to determine the detailed methods of measuring p18oxoF and the cutoff value of p18oxoF for PA subtyping.
It is also entirely true that our present study had some limitations. First, we excluded APA cases with nonfunctional adenomas or cortisol cosecretion to exclude their possible influences. Second, the analysis of intratumoral steroid concentration was performed only in 15 APA cases because of tissue availability. Third, we could not evaluate the difference of p18oxoF between KCNJ5 mutation and other reported relatively rare somatic mutations of APA because of the rarity of these mutated APA among Japanese. Therefore, further investigations are required to clarify these points.
Perspectives
We first revealed that KCNJ5 mutation resulted in more enhanced 18oxoF synthesis in APA by increasing the status of intratumoral glucocorticoid synthesis and because of the high number of CYP11B1 and CYP11B2 double-positive hybrid cells compared with APAs harboring the wild-type KCNJ5 gene. This result indicated that the presence of KCNJ5 mutation, which is rarely detected in normal and hyperplastic adrenocortical cells, might reflect the degree of functional deviation from normal aldosterone-producing cells. Results of our previous study demonstrated that the measurement of p18oxoF could contribute to the differentiation of patients with PA but its utility was indeed influenced by the prevalence of KCNJ5 mutations among patients with APA. This could be considered one reason why measurement of p18oxoF was less effective among European patients with PA.17 Therefore, among the cohort of APA patients with high prevalence of the KCNJ5 mutation, measurement of p18oxoF is considered clinically useful by selecting the patients requiring adrenal venous sampling and possibly shortening the time from initial diagnosis to proper management.
Supplementary Material
Novelty and Significance.
What Is New?
We revealed that intratumoral environment of aldosterone-producing adenoma could cause the differences in peripheral 18-oxocortisol concentration in aldosterone-producing adenoma and the significance of KCNJ5 mutation.
What Is Relevant?
Peripheral 18-oxocortisol concentration is an expected biomarker for easier and less invasive differentiation of aldosterone-producing adenoma among patients with primary aldosteronism but its utility could depend on the prevalence of the KCNJ5 mutation in aldosterone-producing adenoma.
Summary
Our present study first revealed that enhanced 18-oxocortisol synthesis in aldosterone-producing adenoma could come from intratumoral glucocorticoid synthesis and, 11β-hydroxylase and aldosterone synthase double-positive hybrid cells, which could reflect the functional deviation of aldosterone biosynthesis from the normal zona glomerulosa. The KCNJ5 somatic mutation status is the most associated factor involved in 18-oxocortisol synthesis.
Acknowledgments
We sincerely appreciate the following health care professionals and investigators who contributed with their expertise in the present study: Kei Omata, MD; Yoshikiyo Ono, MD; and Yasuhiro Igarashi, MD, from Tohoku University Hospital. We also thank Akane Sugawara, Mika Ainoya, and Hiroko Kato for secretarial assistance, and Kazue Ise, Yasuko Tsukada, and Kumi Kikuchi for technical assistance.
Sources of Funding
This study was partially supported by JSPS KAKENHI Grant Number JP18K08500 and Health Labour Sciences Research Grant Number H29-Nanji-Ippan-046. C.E. Gomez-Sanchez is supported by National Heart, Lung and Blood Institute grant R01 HL27255 and the National Institute of General Medical Sciences grant U54 GM115428.
Footnotes
Disclosures
None.
The online-only Data Supplement is available with this article at https://www.ahajoumals.org/doi/suppl/10.1161/HYPERTENSIONAHA.118.12064.
References
- 1.Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF Jr. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–1916. doi: 10.1210/jc.2015-4061 [DOI] [PubMed] [Google Scholar]
- 2.Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–1248. doi: 10.1016/j.jacc.2005.01.015 [DOI] [PubMed] [Google Scholar]
- 3.Mulatero P, Stowasser M, Loh KC, Fardella CE, Gordon RD, Mosso L, Gomez-Sanchez CE, Veglio F, Young WF Jr. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89:1045–1050. doi: 10.1210/jc.2003-031337 [DOI] [PubMed] [Google Scholar]
- 4.Stowasser M, Gordon RD. Primary aldosteronism–careful investigation is essential and rewarding. Mol Cell Endocrinol. 2004;217:33–39. doi: 10.1016/j.mce.2003.10.006 [DOI] [PubMed] [Google Scholar]
- 5.Monticone S, D’Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, Mulatero P. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6:41–50. doi: 10.1016/S2213-8587(17)30319-4 [DOI] [PubMed] [Google Scholar]
- 6.Brown MJ. Rapid diagnosis of primary aldosteronism: oxymoron or one small step? Hypertension. 2017;70:247–249. doi: 10.1161/HYPERTENSI0NAHA.117.09199 [DOI] [PubMed] [Google Scholar]
- 7.Monticone S, Satoh F, Dietz AS, Goupil R, Lang K, Pizzolo F, Gordon RD, Morimoto R, Reincke M, Stowasser M, Mulatero P. Clinical management and outcomes of adrenal hemorrhage following adrenal vein sampling in primary aldosteronism. Hypertension. 2016;67:146–152. doi: 10.1161/HYPERTENSI0NAHA.115.06305 [DOI] [PubMed] [Google Scholar]
- 8.Satoh F, Morimoto R, Ono Y, et al. Measurement of peripheral plasma 18-oxocortisol can discriminate unilateral adenoma from bilateral diseases in patients with primary aldosteronism. Hypertension. 2015;65:1096–102. doi: 10.1161/HYPERTENSI0NAHA.114.04453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freel EM, Shakerdi LA, Friel EC, Wallace AM, Davies E, Fraser R, Connell JM. Studies on the origin of circulating 18-hydroxycortisol and 18-oxocortisol in normal human subjects. J Clin Endocrinol Metab. 2004;89:4628–4633. doi: 10.1210/jc.2004-0379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morra di Cella S, Veglio F, Mulatero P, Christensen V, Aycock K, Zhu Z, Gomez-Sanchez EP, Gomez-Sanchez CE. A time-resolved fluoroimmunoassay for 18-oxocortisol and 18-hydroxycortisol. Development of a monoclonal antibody to 18-oxocortisol. J Steroid Biochem Mol Biol. 2002;82:83–88. [DOI] [PubMed] [Google Scholar]
- 11.Mulatero P, di Cella SM, Monticone S, Schiavone D, Manzo M, Mengozzi G, Rabbia F, Terzolo M, Gomez-Sanchez EP, Gomez-Sanchez CE, Veglio F. 18-hydroxycorticosterone, 18-hydroxycortisol, and 18-oxocortisol in the diagnosis of primary aldosteronism and its subtypes. J Clin Endocrinol Metab. 2012;97:881–889. doi: 10.1210/jc.2011-2384 [DOI] [PubMed] [Google Scholar]
- 12.Nakamura Y, Satoh F, Morimoto R, Kudo M, Takase K, Gomez-Sanchez CE, Honma S, Okuyama M, Yamashita K, Rainey WE, Sasano H, Ito S. 18-oxocortisol measurement in adrenal vein sampling as a biomarker for subclassifying primary aldosteronism. J Clin Endocrinol Metab. 2011;96:E1272–E1278. doi: 10.1210/jc.2010-2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arlt W, Lang K, Sitch AJ, et al. Steroid metabolome analysis reveals prevalent glucocorticoid excess in primary aldosteronism. JCI Insight. 2017;2:e93136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue K, Yamazaki Y, Tsurutani Y, Suematsu S, Sugisawa C, Saito J, Omura M, Sasano H, Nishikawa T. Evaluation of cortisol production in aldosterone-producing adenoma. Horm Metab Res. 2017;49:847–853. doi: 10.1055/s-0043-119878 [DOI] [PubMed] [Google Scholar]
- 15.Stowasser M, Bachmann AW, Tunny TJ, Gordon RD. Production of 18-oxo-cortisol in subtypes of primary aldosteronism. Clin Exp Pharmacol Physiol. 1996;23:591–593. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura Y, Kitada M, Satoh F, Maekawa T, Morimoto R, Yamazaki Y, Ise K, Gomez-Sanchez CE, Ito S, Arai Y, Dezawa M, Sasano H. Intratumoral heterogeneity of steroidogenesis in aldosterone-producing adenoma revealed by intensive double-and triple-immunostaining for CYP11B2/B1 and CYP17. Mol Cell Endocrinol 2016;422:57–63. doi: 10.1016/j.mce.2015.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenhofer G, Dekkers T, Peitzsch M, Dietz AS, Bidlingmaier M, Treitl M, Williams TA, Bornstein SR, Haase M, Rump LC, Willenberg HS, Beuschlein F, Deinum J, Lenders JW, Reincke M. Mass spectrometry-based adrenal and peripheral venous steroid profiling for subtyping primary aldosteronism. Clin Chem. 2016;62:514–524. doi: 10.1373/clinchem.2015.251199 [DOI] [PubMed] [Google Scholar]
- 18.Williams TA, Peitzsch M, Dietz AS, Dekkers T, Bidlingmaier M, Riester A, Treitl M, Rhayem Y, Beuschlein F, Lenders JW, Deinum J, Eisenhofer G, Reincke M. Genotype-specific steroid profiles associated with aldosterone-producing adenomas. Hypertension. 2016;67:139–145. doi: 10.1161/HYPERTENSI0NAHA.115.06186 [DOI] [PubMed] [Google Scholar]
- 19.Fernandes-Rosa FL, Williams TA, Riester A, et al. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension. 2014;64:354–361. doi: 10.1161/HYPERTENSI0NAHA.114.03419 [DOI] [PubMed] [Google Scholar]
- 20.Kitamoto T, Suematsu S, Yamazaki Y, Nakamura Y, Sasano H, Matsuzawa Y, Saito J, Omura M, Nishikawa T. Clinical and steroidogenic characteristics of aldosterone-producing adenomas with ATPase or CACNA1D gene mutations. J Clin Endocrinol Metab. 2016;101:494–503. doi: 10.1210/jc.2015-3284 [DOI] [PubMed] [Google Scholar]
- 21.Hattangady NG, Karashima S, Yuan L, Ponce-Balbuena D, Jalife J, Gomez-Sanchez CE, Auchus RJ, Rainey WE, Else T. Mutated KCNJ5 activates the acute and chronic regulatory steps in aldosterone production. J Mol Endocrinol. 2016;57:1–11. doi: 10.1530/JME-15-0324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishikawa T, Omura M, Satoh F, Shibata H, Takahashi K, Tamura N, Tanabe A; Task Force Committee on Primary Aldosteronism, The Japan Endocrine Society. Guidelines for the diagnosis and treatment of primary aldosteronism–the Japan Endocrine Society 2009. Endocr J. 2011;58:711–721. [DOI] [PubMed] [Google Scholar]
- 23.Reincke M Subclinical Cushing’s syndrome. Endocrinol Metab Clin North Am. 2000;29:43–56. [DOI] [PubMed] [Google Scholar]
- 24.Nanba K, Chen AX, Omata K, Vinco M, Giordano TJ, Else T, Hammer GD, Tomlins SA, Rainey WE. Molecular heterogeneity in aldosterone-producing adenomas. J Clin Endocrinol Metab. 2016;101:999–1007. doi: 10.1210/jc.2015-3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omata K, Anand SK, Hovelson DH, Liu CJ, Yamazaki Y, Nakamura Y, Ito S, Satoh F, Sasano H, Rainey WE, Tomlins SA. Aldosterone-producing cell clusters frequently harbor somatic mutations and accumulate with age in normal adrenals. J Endocr Soc. 2017;1:787–799. doi: 10.1210/js.2017-00134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubota-Nakayama F, Nakamura Y, Konosu-Fukaya S, Azmahani A, Ise K, Yamazaki Y, Kitawaki Y, Felizola SJ, Ono Y, Omata K, Morimoto R, Iwama N, Satoh F, Sasano H. Expression of steroidogenic enzymes and their transcription factors in cortisol-producing adrenocortical adenomas: immunohistochemical analysis and quantitative real-time polymerase chain reaction studies. Hum Pathol. 2016;54:165–173. doi: 10.1016/j.humpath.2016.03.016 [DOI] [PubMed] [Google Scholar]
- 27.Felizola SJ, Maekawa T, Nakamura Y, Satoh F, Ono Y, Kikuchi K, Aritomi S, Ikeda K, Yoshimura M, Tojo K, Sasano H. Voltage-gated calcium channels in the human adrenal and primary aldosteronism. J Steroid Biochem Mol Biol. 2014;144 (pt B):410–416. doi: 10.1016/j.jsbmb.2014.08.012 [DOI] [PubMed] [Google Scholar]
- 28.Gomez-Sanchez CE. Regulation of adrenal arterial tone by adrenocorticotropin: the plot thickens. Endocrinology. 2007;148:3566–3568. doi: 10.1210/en.2007-0560 [DOI] [PubMed] [Google Scholar]
- 29.Nakamura Y, Maekawa T, Felizola SJ, Satoh F, Qi X, Velarde-Miranda C, Plonczynski MW, Ise K, Kikuchi K, Rainey WE, Gomez-Sanchez EP, Gomez-Sanchez CE, Sasano H. Adrenal CYP11B½ expression in primary aldosteronism: immunohistochemical analysis using novel monoclonal antibodies. Mol Cell Endocrinol. 2014;392:73–79. doi: 10.1016/j.mce.2014.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamazaki Y, Nakamura Y, Omata K, Ise K, Tezuka Y, Ono Y, Morimoto R, Nozawa Y, Gomez-Sanchez CE, Tomlins SA, Rainey WE, Ito S, Satoh F, Sasano H. Histopathological classification of cross-sectional image-negative hyperaldosteronism. J Clin Endocrinol Metab. 2017;102:1182–1192. doi: 10.1210/jc.2016-2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mulatero P, Veglio F, Pilon C, Rabbia F, Zocchi C, Limone P, Boscaro M, Sonino N, Fallo F. Diagnosis of glucocorticoid-remediable aldosteronism in primary aldosteronism: aldosterone response to dexamethasone and long polymerase chain reaction for chimeric gene. J Clin Endocrinol Metab. 1998;83:2573–2575. doi: 10.1210/jcem.83.7.4946 [DOI] [PubMed] [Google Scholar]
- 32.Mulatero P, Curnow KM,Aupetit-Faisant B, Foekling M, Gomez-Sanchez C, Veglio F, Jeunemaitre X, Corvol P, Pascoe L. Recombinant CYP11B genes encode enzymes that can catalyze conversion of 11-deoxycortisol to cortisol, 18-hydroxycortisol, and 18-oxocortisol. J Clin Endocrinol Metab. 1998;83:3996–4001. doi: 10.1210/jcem.83.11.5237 [DOI] [PubMed] [Google Scholar]
- 33.Choi M, Scholl UI, Yue P, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331:768–772. doi: 10.1126/science.1198785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lenzini L, Rossitto G, Maiolino G, Letizia C, Funder JW, Rossi GP. A meta-analysis of somatic KCNJ5 K(+) channel mutations in 1636 patients with an aldosterone-producing adenoma. J Clin Endocrinol Metab. 2015;100:E1089–E1095. doi: 10.1210/jc.2015-2149 [DOI] [PubMed] [Google Scholar]
- 35.Monticone S, Hattangady NG, Nishimoto K, et al. Effect of KCNJ5 mutations on gene expression in aldosterone-producing adenomas and adrenocortical cells. J Clin Endocrinol Metab. 2012;97:E1567–E1572. doi: 10.1210/jc.2011-3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomez-Sanchez CE, Qi X, Gomez-Sanchez EP, Sasano H, Bohlen MO, Wisgerhof M. Disordered zonal and cellular CYP11B2 enzyme expression in familial hyperaldosteronism type 3. Mol Cell Endocrinol. 2017;439:74–80. doi: 10.1016/j.mce.2016.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taguchi R, Yamada M, Nakajima Y, Satoh T, Hashimoto K, Shibusawa N, Ozawa A, Okada S, Rokutanda N, Takata D, Koibuchi Y, Horiguchi J, Oyama T, Takeyoshi I, Mori M. Expression and mutations of KCNJ5 mRNA in Japanese patients with aldosterone-producing adenomas. J Clin Endocrinol Metab. 2012;97:1311–1319. doi: 10.1210/jc.2011-2885 [DOI] [PubMed] [Google Scholar]
- 38.Wang B, Li X, Zhang X, Ma X, Chen L, Zhang Y, Lyu X, Tang Y, Huang Q, Gao Y, Fan Y, Ouyang J. Prevalence and characterization of somatic mutations in Chinese aldosterone-producing adenoma patients. Medicine (Baltimore). 2015;94:e708. doi: 10.1097/MD.0000000000000708 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


