The mammalian heart contains a heterogeneous population of resident macrophages that are scattered between cardiomyocytes and display noncanonical organ-specific skills that go beyond canonical phagocytosis (1). In mice, macrophages populate the heart during embryogenesis (2). Once seeded, embryonic-derived macrophages self-maintain through local proliferation (3,4). Monocyte-derived macrophages subsequently replenish embryonic tissue macrophages when depleted because of aging (5) or cardiac damage (6), and are able to self-maintain through local proliferation (4).
Following myocardial infarction (MI) proper healing requires a series of coordinated events in which circulating inflammatory Ly6Chigh monocytes differentiate first into Ly6Chigh (also known as M1 proinflammatory) macrophages and next into Ly6Clow (or M2 reparative) macrophages (7,8). The accumulation of monocyte-derived macrophages largely depends on emergency medullary or extramedullary hematopoiesis (8,9), a response that also occurs in humans (10). Similarly, cardiac pressure overload induces a strong immune response. At an early phase, cardiac resident macrophages proliferate and are regulated by Kruppel-like factor 4 (11). Next, infiltrating Ly6Chigh monocytes (11) differentiate into M1 inflammatory macrophages (or CCR2+ macrophages) that promote adverse left ventricular remodeling (12). On the contrary, subsequent M2 macrophages inhibit CD4 T-cell activation by pressure overload and exhibit anti-inflammatory properties (13).
Considering their abundance and the high plasticity that drives distinct cardioprotective effects following cardiac injury (i.e., ischemia and cardiac overload) (14), the therapeutic manipulation of macrophages in tissue injury and repair is intriguing. Yet, the mechanisms governing cardiac macrophage polarization and the role of macrophage subsets in modulating localized responses from cardiomyocytes and fibroblasts are still poorly understood in vivo.
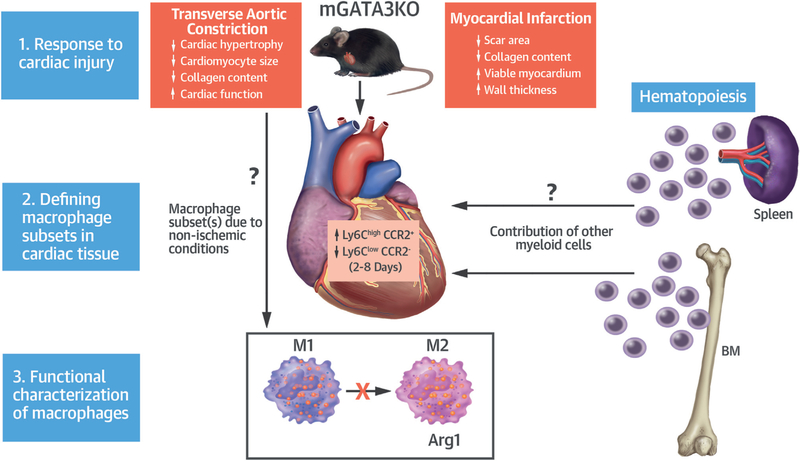
In this issue of the Journal, Yang et al. (15) report new evidence that manipulating macrophages in vivo improves cardiac injury outcomes and thus is an interesting therapeutic target. In this study, the authors show that the deletion of the transcription factor GATA3 in myeloid cells (mGATA3KO) improved cardiac function in mice with cardiac injuries such as MI and cardiac pressure overload (transverse aortic constriction) (Figure 1).
FIGURE 1. Effect of GATA3 Deficiency in Myeloid Cells (mGATA3) on Cardiac Injury and Repair.
Following myocardial infarction, mGATA3 deficiency results in increased circulating Ly6ChighCCR2+ monocytes and macrophages (M1 type), and reduced Ly6ClowCCR2−(M2 type) macrophages in the ischemic heart. mGATA3KO mice displayed cardioprotective attributes, possibly from the inhibition of M2 polarization of cardiac macrophages. Cardioprotective effects were also observed in a transverse aortic constriction model of cardiac pressure overload. BM = bone marrow.
Following MI, mGATA3KO mice showed less left ventricular dilatation, improved contractility, reduced scar area, and increased viable myocardium. This effect was associated with reduced neutrophil infiltration in the myocardium at 2 days and fewer CCR2+ monocytes/macrophages at 2 and 8 days postinjury. Macrophage subset analysis showed an increase in the percentage of Ly6ChighCCR2+ (M1) cells, likely derived from monocytes, and a decreased percentage of Ly6ClowCCR2~ (M2) cells, suggesting that cardiac improvement could possibly be due to having more inflammatory cells and fewer antiinflammatory cells in the infarcted tissue. In fact, parallel alterations in blood were consistent with a relative increase of Ly6Chigh and reduced Ly6Clow monocytes in mGATA3KO versus controls that may indicate a role of GATA3 in the regulation of hematopoiesis and monocyte polarization in blood.
Cardioprotective effects (i.e., reduced cardiac hypertrophy and collagen content) of GATA3 deficiency in myeloid cells were also seen in nonischemic conditions using the transverse aortic constriction model of chronic pressure overload. However, phenotype analysis of myeloid cells in the circulation and cardiac infiltration were not presented, making it impossible to speculate whether the immune alterations observed in the MI model were also relevant in nonischemic conditions.
In vitro studies show that GATA3 expression in macrophages is transient and detected in M2 polarized macrophages only. In fact, a detrimental M2 differentiation through a GATA3-dependent mechanism regulated by micro RNA-720 has been reported in cancer (16,17). Moreover, GATA3KO macrophages did not produce the profibrogenic Arg-1, produced by M2 macrophages, suggesting the possibility that the lack of M2 phenotype in mGATA3KO mice may contribute to reduced cardiac fibrosis and remodeling. Other observed cardioprotective effects, such as the enhanced myocardium viability, will require additional studies that further explore independent mechanisms.
Overall, the results of the present study suggest a pathological role of M2 macrophages in cardiac damage following MI and cardiac pressure overload. In fact, the role of M1 and M2 macrophages is the subject of intense debate. Although such reductionist dichotomy may offer the advantage of standardization, macrophage polarization is more likely a spectrum that reflects the highly adaptive nature of these cells to their environment. Perhaps, the M1/M2 (or Ly6Chigh vs. LyC6Low macrophages) model prevents the discovery of new tissue-specific functions in response to specific insults (i.e., ischemic or pressure overload). The lack of phenotypic resolution in defining the macrophage spectrum of polarization could explain the many discrepancies in the literature on whether M2 macrophages contribute to cardiac damage, like in the present study, rather than being cardioprotective as reported by others (13,18–21).
As a whole, the present study provides further evidence that targeting macrophage polarization to prevent cardiac damage and promote repair is theoretically feasible. However, our current knowledge of cardiac macrophage biology suffers from undeniable limitations, including our insufficient understanding of the microenvironmental cues that drive macrophage polarization in vivo and the lack of selective strategies to target specific macrophage subsets. Another main limitation is that most of the available evidence is derived from mouse models and the data on phenotype and functions of human macrophages in homeostasis and disease are only starting to emerge and challenging the M1/M2 paradigm (22,23).
While continuing exploring the phenotype and function of macrophages in the human heart, there is a tremendous need to identify new strategies to determine the spectrum of macrophage phenotype and functions in human steady state and disease. The recent effort of the human cell atlas initiative is an initial step ahead toward the goal of defining the diversity of all cells in health and disease (24). The inclusion of immune cells from human cardiac tissues in future datasets will provide fundamental cues to help resolve this intricated matter.
Acknowledgments
Dr. Giannarelli is supported by the National Institutes of Health grants UH2TR002067, R21TR001739, K23HL111339, and R03HL135289. Dr. Fernandez is supported by National Institutes of Health grant 5T32HL007824-20.
Footnotes
Editorials published in the Journal of the American College of Cardiology reflect the views of the authors and do not necessarily represent the views of JACC or the American College of Cardiology.
REFERENCES
- 1.Hulsmans M, Clauss S, Xiao L, et al. Macrophages facilitate electrical conduction in the heart. Cell 2017;169:510–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Epelman S, Lavine KJ, Randolph GJ. Origin and fun ctio ns of tissue macrophages. Immunity 2014; 41:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epelman S, Lavine KJ, Beaudin AE, et al. Embryonic and adult-derive d resident cardiac macrophages are maintained through distinct mechanisms at steady state and during in fla mmation. Immunity 2014;40:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sager HB, Hulsmans M, Lavine KJ, et al. Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circ Res 2016;119:853–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molawi K, Wolf Y, Kandalla PK, et al. Progressive replacement of embryo-derived cardiac macrophages with age. J Exp Med 2014;211:2151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heidt T, Courties G, Dutta P, et al. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction.Circ Res 2014;115:284–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hilgendorf I, Gerhardt LM, Tan TC, et al. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res 2014;114:1611–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dutta P, Sager HB, Stengel KR, et al. Myocardial infarction activates CCR2(+) hem atopoietic stem and progenitorceLLs. CeLL Stem CeLL 2015;16:477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutta P, Courties G, Wei Y, et al. Myocardial infarction acceLerates atheroscLerosis. Nature 2012;487:325–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim EJ, Kim S, Kang DO, Seo HS. Metabolic activity of the spleen and bone marrow in patients with acute myocardiaL infarction evaLuated by 18f-fluo - rodeoxygLucose positron emission tom ographic im aging. Circ Cardiovasc Imaging 2014;7:454–60. [DOI] [PubMed] [Google Scholar]
- 11.Liao X, Shen Y, Zhang R, et al. Distinct roles of resident and nonresident macrophages in nonischemic cardiomyopathy. Proc Natl Acad Sci U S A 2018;115:E4661–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel B, Ismahil MA, Hamid T, Bansal SS, Prabhu SD. M ononuclear phagocytesare dispensable fo r cardiac remodeLing in estabLished pressure-overload heart failure. PLoS One 2017;12:e0170781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujiu K, Wang J, Nagai R. Cardioprotective function of cardiac macrophages. Cardiovasc Res 2014;102:232–9. [DOI] [PubMed] [Google Scholar]
- 14.Hulsmans M, Sam F, Nahrendorf M. Monocyte and macrophage contributions to cardiac remodeling. J Mol Cell Cardiol 2016;93:149–55.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang M, Song L, Wang L, et al. Deficiency of GATA3-positive macrophages improves cardiac function following myocardial infarction or pressure overload hypertrophy. J Am Coll Cardiol 2018;72:885–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W, Wang Z, Luo Y, Zhong D, Luo Y, Zhou D. GATA3 expression correlates with poor prognosis and tumor-associated macrophage infiltration in peripheral T cell lymphoma. Oncotarget 2016;7:65284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong Y, Yi C. MicroRNA-720 suppresses M2 macrophage polarization by targeting GATA3. Biosci Rep 2016;36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z, Huang S, Sheng Y, et al. Topiramate modulates post-infarction inflammation primarily by targeting monocytes or macrophages. Cardiovasc Res 2017;113:475–87. [DOI] [PubMed] [Google Scholar]
- 19.Kimbrough D, Wang SH, Wright LH, et al. HDAC inhibition helps post-MI healing by modulating macrophage polarization. J Mol Cell Cardiol 2018;119:51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Y, Halade GV, Zhang J, et al. Matrix metalloproteinase-28 deletion exacerbates cardiac dysfunction and rupture after myocardial infarction in mice by inhibiting M2 macrophage activation. Circ Res 2013;112:675–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlson S, Helterline D, Asbe L, et al. Cardiac macrophages adopt profibrotic/M2 phenotype in infarcted hearts: role of urokinase plasminogen activator. J Mol Cell Cardiol 2017;108:42–9. [DOI] [PubMed] [Google Scholar]
- 22.Xue J, Schmidt SV, Sander J, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014;40:274–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bajpai G, Schneider C, Wong N, et al. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat Med 2018. June 11 [E-pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Regev A, Teichmann SA, Lander ES, et al. Human Cell Atlas Meeting Participants: Science Forum: The Human Cell Atlas. eLife 2017. December 5 PMID: 29206104. [Google Scholar]