Abstract
BACKGROUND
The Dietary Approaches to Stop Hypertension (DASH) diet lowers blood pressure (BP) more effectively in blacks compared to other US racial subgroups. Considering chronic kidney disease (CKD) raises BP through complex mechanisms, DASH may affect BP differently among blacks with and without CKD. We compared the association of DASH accordance to BP and prevalent hypertension among blacks with and without CKD.
METHODS
Our study involved 3,135 black Americans enrolled in the Jackson Heart Study (2000–2004) with diet and office BP data. Using linear models adjusted for demographics, health behaviors, and clinical factors, we determined the association of a modified DASH score (excluding sodium intake, ranging from 0 to 8 with increasing DASH accordance) with BP. We performed tests for interaction between DASH score and CKD status.
RESULTS
Among participants (mean age: 55 years; hypertension: 60%; CKD: 19%), the median DASH score was similar among participants with and without CKD (1.0 [interquartile range (IQR): 0.5–2] and 1.0 [IQR: 0.5–1.5]). CKD status modified the association of the DASH score with systolic BP (SBP) and diastolic BP (DBP; P interactions were 0.06 and <0.01). Among participants without CKD, SBP and DBP were not associated with the DASH score (−0.4 [95% confidence interval: −1.0, 0.1] mm Hg and −0.1 [−0.4, 0.2] mm Hg per one unit higher DASH score). Among participants with CKD, one unit higher DASH score was associated with lower SBP by 1.6 (0.5, 2.6) mm Hg and lower DBP by 0.9 (0.3, 1.5) mm Hg.
CONCLUSIONS
Despite low DASH scores overall, better DASH accordance was associated with lower BP among Black Americans with CKD.
Keywords: Black American, blood pressure, chronic kidney disease, diet, hypertension, nutrition
Black Americans with chronic kidney disease (CKD) have high rates of uncontrolled hypertension.1 Uncontrolled hypertension is a major risk factor for the development of CKD, progression to kidney failure, and death from cardiovascular disease (CVD).2,3 CVD is the leading cause of death among blacks with CKD and CVD mortality rates are twice as high in blacks with CKD compared to blacks with normal kidney function.4 Diet is a major disease modifier of both hypertension and CVD. Therefore, identifying dietary patterns that improve hypertension control rates among blacks with CKD could positively impact kidney and CVD outcomes for this patient population.
The Dietary Approaches to Stop Hypertension diet (DASH),5–7 which is high in fruits, vegetables, whole grains, low-fat dairy, nuts, and legumes, and reduced in sweets and saturated fat, is endorsed nationally and abroad8–12 to treat hypertension in adults with normal kidney function. DASH has been demonstrated in randomized controlled trials to lower blood pressure (BP) more effectively in blacks compared to whites.13–15 However, it is unclear whether DASH also benefits blacks with CKD. Several factors contribute to BP elevation as kidney function declines, such as diminished natriuresis and upregulation of the renin–angiotensin–aldosterone system.16 There is evidence that DASH lowers BP by enhancing natriuresis17,18 and modulating of the renin–angiotensin–aldosterone system.19 Therefore, it is plausible that DASH would lower BP more favorably in blacks with CKD compared to blacks with normal kidney function.
Sodium reduction is the only diet modification that is currently endorsed by national and international kidney disease guidelines to lower BP in adults with CKD.20,21 However, we previously published results of a 2-week pre–post pilot feeding study that demonstrated that a sodium-reduced DASH diet improved BP in 7 out of 10 hypertensive black adults with moderate CKD.22 It is not clear whether DASH lowers BP in blacks with CKD without concomitant sodium reduction. In this study, we evaluated the association of DASH accordance (without explicit sodium reduction) with BP and prevalent hypertension among a population-based cohort of black Americans with and without CKD enrolled in the Jackson Heart Study (JHS).23
METHODS
Study population
The JHS is the largest longitudinal, population-based study designed to investigate causes of CVD among black Americans.23,24 From 2000 to 2004 (Exam 1), the JHS enrolled 5,306 adults residing in metropolitan Jackson, MS between 21 and 95 years old. After obtaining written informed consent, trained staff performed baseline assessments to ascertain data on sociodemographic, behavioral, and biological risk factors for CVD. Our study involves JHS participants who completed baseline food frequency questionnaires (FFQ) and measurements of office BP, serum creatinine concentration, and urine creatinine and albumin concentrations. We excluded participants with indeterminant CKD status, invalid FFQ data (defined as daily energy intake ≤600 kcal or ≥4,800 kcal or suspicion of inaccurate reporting), or missing covariate data for education, alcohol intake, smoking status, and body mass index. The protocol was approved by institutional review boards at each participating site and this study was approved by the Duke Health Institutional Review Board.
Assessments
DASH scores
Participants’ usual dietary patterns were assessed using the Delta Nutrition Intervention Research Initiative FFQ, a validated 158-item region-specific diet assessment tool.25 Participants’ diet was assessed using the nutrient-based DASH accordance score derived by Mellen et al.26 To determine the DASH score,26 participants were assigned an intermediate point or full point (0.5 or 1 point, respectively) for achieving daily nutrient targets for total fat, saturated fat, protein, cholesterol, fiber, magnesium, calcium, potassium, and sodium (Table 1). In order to evaluate the relationship between DASH accordance and outcomes, independent of dietary sodium intake, we excluded sodium from our composite score and report results based on a one-unit change (i.e., 1 point) in the 8-variable DASH score unless stated otherwise.
Table 1.
Nutrient targets for DASH score
| DASH diet nutrients | Intermediate target (0.5 point) | Full target (1 point) |
|---|---|---|
| Potassium, mg/1,000 kcal | 1,534–2,237 | ≥2,238 |
| Calcium, mg/1,000 kcal | 402–589 | ≥590 |
| Magnesium, g/1,000 kcal | 158–237 | ≥238 |
| Fiber, g/1,000 kcal | 9.5–14.7 | ≥14.8 |
| Protein, % total energy | 16.5–17 | ≥18 |
| Total cholesterol, mg/1,000 kcal | 71.5–107.1 | ≤71.4 |
| Total fat, % total energy | 28–32 | ≤27 |
| Saturated fat, % total energy | 7–11 | ≤6 |
| Sodium, g/1,000 kcala | 1,144–1,286 | ≤1,143 |
Abbreviations: DASH, Dietary Approaches to Stop Hypertension.
aSodium was excluded from the DASH score used in the main analyses but included in the 9-variable DASH score used in the sensitivity analyses.
Blood pressure
BP was obtained after participants rested quietly for 5 minutes in a seated position with their back and arm supported, legs uncrossed, and feet flat on the floor. Two BP measurements were obtained 1 minute apart and averaged. BP was measured using a random-zero sphygmomanometer (Hawksley and Sons, Lancing, United Kingdom)27 at Exam 1 (2000–2004) and a semiautomated oscillometric device (Omron HEM-907XL) at follow-up visits. To improve the generalizability of statistical inferences of longitudinal data, a BP-comparability substudy was performed in 2,115 participants whose BP was measured simultaneously with a random-zero sphygmomanometer and the oscillometric device using a Y-connector at Exam 2 (2005–2008), permitting calibration of random-zero BP measurements to semiautomated measurements using robust regression.28 We used calibrated BP values for the current analyses. Hypertension was defined as mean systolic BP (SBP) ≥140 mm Hg, or mean diastolic BP (DBP) ≥90 mm Hg, or use of antihypertensive medications.
Kidney function
Serum creatinine, urine albumin, and urine creatinine concentrations were measured at baseline. We used the creatinine-based CKD-EPI equation to determine estimated glomerular filtration rate (eGFR).29 We used spot urine samples, or 24-hour urine samples when spot samples were unavailable, to determine urine albumin-to-creatinine ratio (ACR). We defined CKD as eGFR <60 ml/min/1.73 m2 and/or presence of albuminuria. Albuminuria was defined as ACR ≥30 mg/g. CKD status was indeterminant for participants with an eGFR ≥60 ml/min/1.73 m2 and missing ACR, or missing eGFR and ACR <30 mg/g. We classified CKD as stage 1 for eGFR ≥90 ml/min/1.73 m2 and ACR ≥30 mg/g, stage 2 for eGFR 60–89 ml/min/1.73 m2 and ACR ≥30 mg/g, stage 3 for eGFR 30–59 ml/min/1.73 m2, stage 4 for eGFR 15–29 ml/min/1.73 m2, stage 5 for eGFR <15 ml/min/1.73 m2, and unstageable for ACR ≥30 mg/g and missing eGFR.
Covariates
Surveys were administered to collect data on participants’ medical histories, medication use, household income, smoking status, education, alcohol use, and physical activity level. A description of data collection methods for each covariates and definitions for diabetes, body mass index, and use of antihypertensive medications are provided in Supplementary Item 1.
Statistical analysis
We performed descriptive statistics to assess participant characteristics overall and by CKD status. We used linear regression models for continuous BP outcomes (i.e., SBP and DBP) and a modified Poisson regression model with robust standard errors for hypertension in order to estimate the prevalence ratio (PR). Models were adjusted for participants’ age, sex, income, education, smoking status, alcohol use, physical activity, and body mass index. Models estimating the associations between the DASH score and SBP or DBP were also adjusted for participants’ use of antihypertensive medications. We performed tests for interaction between CKD and the DASH score to determine the effect modification of CKD on primary associations. We also examined correlations between the DASH score and daily sodium intake. To determine if our inference was sensitive to dietary sodium, we adjusted our BP models for absolute daily sodium intake and also performed separate analyses using a 9-variable DASH score26 that included a score for dietary sodium. We also conducted separate sensitivity analyses to test for an interaction effect of albuminuria (defined as ACR ≥30 mg/g—an independent marker of kidney dysfunction) within multivariable models quantifying the association between the DASH score and SBP or DBP while adjusting for age, sex, income, education, smoking status, alcohol use, physical activity, body mass index, and eGFR, including a DASH score × albuminuria interaction term. To determine if our inference was sensitive to the moderate amount of missing data related to indeterminant CKD status, we conducted two extreme-case analyses in which we assumed all missing CKD data were not CKD, or alternatively, that all were prevalent CKD. In a post hoc analysis, we evaluated the effect modification of continuous eGFR, instead of CKD, on diet and prevalent hypertension. All hypotheses tests were two sided at the 0.05 level for main effects and 0.10 level for interaction effects. Statistical analyses were performed using R version 3.4.4 (Vienna, Austria) and SAS 9.4 (SAS institute, Cary, NC).
RESULTS
Study population
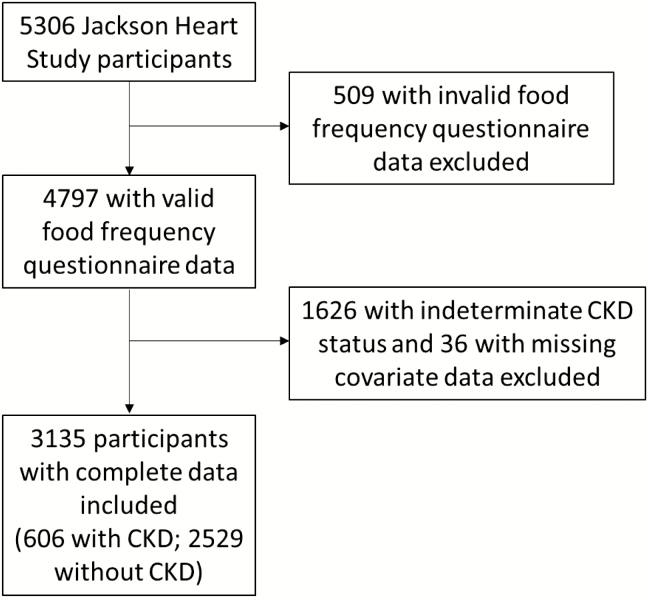
Of 5,306 total JHS participants, 2,171 (41%) were excluded (Figure 1) and their baseline characteristics were similar to participants remaining in our final analytic sample (Supplementary Table 1). Among the 3,135 participants included, mean age was 55 years, mean eGFR was 93 ml/min/1.73 m2, and 60% had hypertension. Compared to participants without CKD, participants with CKD were older, had less education, had lower household income, were less physically active, had higher prevalence of hypertension and diabetes, and had higher mean SBP (Table 2). The prevalence of CKD stage 1 was 32%, stage 2 was 19%, stage 3 was 41%, stage 4 was 4%, stage 5 was 3%, and CKD stage could not be determined for the 1% with known ACR but unknown creatine values.
Figure 1.
Flowchart for Jackson Heart Study participants included and excluded from analysis. CKD, chronic kidney disease.
Table 2.
Baseline characteristics of Jackson Heart Study participants overall and by CKD status
| All | No CKD | CKD | |
|---|---|---|---|
| N (%) | 3,135 | 2,529 (81) | 606 (19) |
| Age in years, mean ± SD | 55.1 ± 12.9 | 53.4 ± 12.4 | 62.0 ± 12.7 |
| Male, n (%) | 1,155 (37) | 949 (38) | 206 (34) |
| Education ≤ high school, n (%) | 1,114 (36) | 803 (32) | 311 (52) |
| Incomea: 0–1.5x poverty, n (%) | 953 (30) | 705 (28) | 248 (41) |
| Current smoker, n (%) | 343 (11) | 279 (11) | 64 (11) |
| Alcohol use in last year, n (%) | 1,375 (44) | 1,179 (47) | 196 (32) |
| Physical activityb | |||
| Poor, n (%) | 1,476 (47) | 1,128 (45) | 348 (57) |
| Intermediate, n (%) | 1,019 (33) | 847 (34) | 172 (28) |
| Ideal, n (%) | 640 (20) | 554 (22) | 86 (14) |
| BMI, kg/m2, mean ± SD | 31.7 ± 7.1 | 31.5 ± 6.9 | 32.7 ± 7.7 |
| eGFR, ml/min/1.73 m2, mean ± SD | 93.4 ± 23.6 | 98.6 ± 17.7 | 71.7 ± 31.8 |
| Hypertension, n (%) | 1,867 (60) | 1,342 (53) | 525 (87) |
| Antihypertensive medication use, n (%) | 1,611 (51) | 1,136 (45) | 475 (78) |
| Diabetesa | 669 (21) | 411 (16) | 258 (43) |
| SBP, mm Hg, mean ± SD | 127.1 ± 16.8 | 125.1 ± 15.3 | 135.3 ± 19.6 |
| DBP, mm Hg, mean ± SD | 76.0 ± 8.7 | 76.0 ± 8.3 | 76.0 ± 10.2 |
Abbreviations: BMI, body mass index; CKD, chronic kidney disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; SD, standard deviation.
aNumber of participants with missing data was 524 for household income and 4 for diabetes status.
bPhysical activity was “poor” if 0 minute/week of moderate and vigorous activity, “intermediate” if 0–150 minutes/week of moderate or 0–75 minutes/week vigorous activity, or “ideal” if >150 minutes/week of moderate or >75 minutes/week of vigorous activity.
Diet accordance
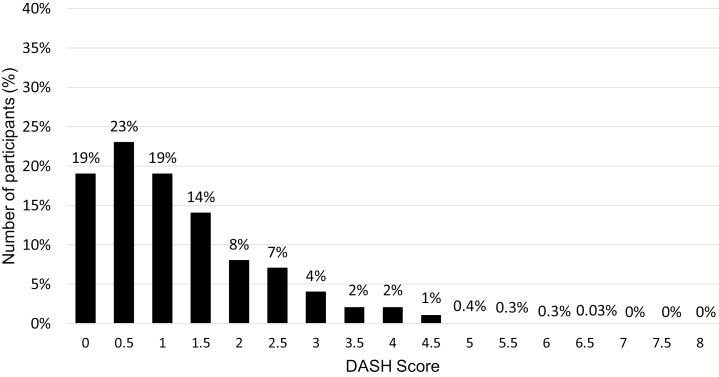
DASH scores ranged from 0 to 6.5 out of 8 (Figure 2) with an overall median score of 1.0 (interquartile range [IQR]: 0.5–2.0). DASH scores were similar among participants with and without CKD (1.0 [IQR: 0.5–2] vs. 1.0 [IQR: 0.5–1.5], respectively). Regarding accordance to individual nutrient targets, restricting saturated fat was the most frequently met target, and achieving daily potassium recommendations was the least frequently met target (Supplementary Table 2).
Figure 2.
Frequency distribution for the DASH score among Jackson Heart Study participants. DASH, Dietary Approaches to Stop Hypertension.
Relation between DASH accordance and BP
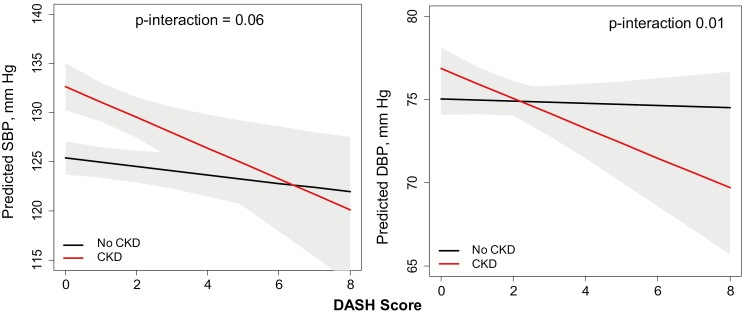
CKD status modified the relation of the DASH score to both SBP and DBP (P interactions = 0.06 and 0.01, respectively; Figure 3). Among participants without CKD, the DASH score was not associated with SBP or DBP (−0.4 [−1.0, 0.1] mm Hg and −0.1 [−0.4, 0.2] mm Hg per one unit higher DASH score, respectively). However, among participants with CKD, there was a statistically significant inverse association between the DASH score and BP; one unit higher DASH score was associated with lower SBP by 1.6 (0.5, 2.6) mm Hg and lower DBP by 0.9 (0.3, 1.5) mm Hg (Figure 3) on average.
Figure 3.
Effect modification of CKD status on DASH score and blood pressure among Jackson Heart Study participants. Models were adjusted for age, sex, income, education, smoking status, alcohol use, physical activity, body mass index, use of antihypertensive medication, diet, CKD status (yes/no), and diet × CKD interaction term. BP, blood pressure; CKD, chronic kidney disease; DASH, Dietary Approaches to Stop Hypertension; DBP, diastolic BP; SBP, systolic BP.
Relation between DASH accordance and prevalent hypertension
In cross-sectional analyses, CKD modified the association between the DASH score and prevalent hypertension (P interaction = 0.01). Among those without CKD, one unit higher DASH score was associated with higher prevalence of hypertension by 4% (PR: 1.04 [1.01, 1.07]). However, among those with CKD, this association was not present (PR: 0.99 [0.96, 1.02] per one unit higher DASH score).
Sensitivity analyses
Controlling for dietary sodium intake
Consistent with our main findings, when a variable for dietary sodium was included in the DASH score, CKD modified the relationship between the 9-variable DASH score and both SBP (P interaction <0.05) and DBP (P interaction =0.01). Among participants without CKD, the 9-variable DASH score was not associated with SBP or DBP (−0.3 [−0.8, 0.2] mm Hg and −0.03 [−0.3, 0.2] per one unit higher DASH score, respectively). However, among participants with CKD, one unit higher 9-variable DASH score was associated with a lower SBP by 1.5 (0.5, 2.5) mm Hg and a lower DBP by 0.8 (0.3, 1.4) mm Hg on average. Inferences were the same when BP models were adjusted for absolute dietary sodium intake as a continuous covariate.
Effect modification of albuminuria on diet and BP
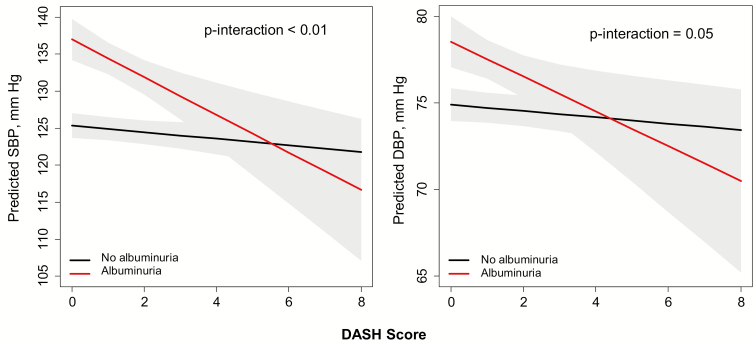
In multivariable models adjusted for our prespecified covariates and eGFR, albuminuria modified the relation of the DASH score to both SBP and DBP (P interactions were <0.01 and 0.05, respectively). Among participants without albuminuria, the DASH score was not associated with SBP or DBP (−0.4 [−1.0, 0.2] mm Hg and −0.2 [−0.5, 0.1] mm Hg per one unit higher DASH score, respectively). However, among participants with albuminuria, one unit higher DASH score was associated with a lower SBP by 2.1 (0.6, 3.6) mm Hg and a lower DBP by 1.0 (0.2, 1.8) mm Hg on average (Figure 4).
Figure 4.
Effect modification of albuminuria on DASH score and blood pressure among Jackson Heart Study participants. Models were adjusted for age, sex, income, education, smoking status, alcohol use, physical activity, body mass index, use of antihypertensive medication, estimated glomerular filtration rate, albuminuria (yes/no), and diet × albuminuria interaction term. Albuminuria is defined as urine albumin-to-creatinine ratio ≥30 mg/g. BP, blood pressure; DASH, Dietary Approaches to Stop Hypertension; DBP, diastolic BP; SBP, systolic BP.
Extreme cases analyses of missing CKD data
When the BP main analyses were repeated under the two extreme assumptions, the interaction effects were strengthened when all missing data were assumed as non-CKD, and attenuated when all missing data were assumed to be CKD. In the latter case, though no longer statistically significant, the direction of the effects did not change. The statistical inference for prevalent hypertension was not sensitive to either extreme assumption.
Post hoc analysis
When we substituted CKD status with eGFR in our multivariable models for prevalent hypertension adjusting for participants’ age, sex, income, education, smoking status, alcohol use, physical activity, body mass index, and continuous eGFR, the association between DASH score and prevalent hypertension was statistically significant (PR: 1.02 [1.00, 1.04] per one unit higher DASH score).
DISCUSSION
Among 3,135 participants enrolled in the JHS, greater accordance to DASH was associated with lower SBP and DBP levels. Despite DASH scores being low overall, the association between DASH accordance and BP was more favorable among participants with CKD compared to those without CKD. Results from our pilot feeding study previously demonstrated that DASH improves BP among black adults (N = 10) with CKD.30 Our current study expands those findings to a larger sample of black Americans and provides additional evidence that the relationship between DASH accordance and BP is modified by markers of kidney function.31
The degree to which JHS participants adhered to DASH is consistent with dietary patterns in other US-based populations. For example, using a 9-point scale, median DASH scores were 1.5 among an urban population-based cohort of 1,534 middle-aged black and white adults32 and 2.9 among a random population sample of 2007–2012 National Health and Nutrition Examination Survey participants (N = 5,848),33 compared to a median of 1 on an 8-point scale in our final analytic sample. In both studies, no participants met full DASH accordance and black race was associated with lower DASH scores.32,33 Our data in an exclusively black sample suggest that black adults with CKD may benefit from DASH even when accordance is suboptimal. If confirmed, it is important to note that sodium reduction is the only diet modification that is currently endorsed by national and international kidney disease guidelines to lower BP in adults with CKD.20,21 Our study provides important evidence that non-sodium-based diet modifications may also lower BP in blacks with CKD.
Although randomized controlled trials have consistently demonstrated that DASH lowers BP in adults with normal kidney function,5–7,18,34,35 we only observed a statistically significant inverse association between DASH accordance and BP among JHS participants with CKD. It is possible that the degree of DASH accordance among our sample was not high enough for us to observe an association between DASH score and BP overall. However, our findings are important because they raise the possibility that DASH may benefit BP in adults with CKD at lower degrees of accordance than what is needed to benefit adults with normal kidney function.
Increased effectiveness of DASH in CKD, compared to non-CKD, is plausible because several pathologic mechanisms that contribute to hypertension in CKD, such as reduced natriuresis, upregulated renin–angiotensin–aldosterone system activity,16 increased sympathetic nervous system activity,36 and impaired nitric-oxide-induced endothelium-mediated vasodilatation,37 are all potential mechanisms proposed to be mitigated by DASH.17,18 We previously reported evidence that kidney function markers modulate the relationship between BP and diet in a post hoc analysis of the DASH trial.31 Our results demonstrated that subclinical kidney dysfunction (defined as daily urine albumin excretion ≥7 mg/day) was associated with enhanced BP response to DASH. Our current study expands those findings by showing that urine albumin excretion at concentrations of 30 mg/g or more modifies the association between DASH accordance and BP in a larger cohort.
The safety of DASH must be established in CKD considering DASH is higher in potassium, phosphorus, and protein than what is currently recommended by National Kidney Foundation Kidney Disease Outcomes Quality Initiative guidelines for adults with CKD stages 3 or higher.21 Our pilot feeding study demonstrated that adults with moderate CKD can consume DASH for a short term without developing incident hyperkalemia, hyperphosphatemia, or metabolic acidosis.22 However, longer term, randomized controlled studies are needed to confirm those findings. Evidence from other studies also suggests a DASH dietary pattern may be safe in CKD. For example, serum potassium concentrations did not increase in adults with hypertensive CKD stage 4 who consumed a high fruit and vegetable diet for 1 year.38 The absence of hyperkalemia and observation of improved metabolic acidosis, markers of kidney injury, and BP among participants in that study38 suggest that DASH may be safe and provide additional health benefits in CKD. Furthermore, greater accordance to DASH has been associated with lower risk for incident CKD39 and kidney function decline40 in US population-based studies. Our observation that there was a strong association between diet and BP among participants with CKD despite participants having an overall low accordance rate to daily targets for potassium, calcium, magnesium, and protein consumption raises the potential for a DASH-style dietary pattern to be beneficial even if the content of these nutrients was reduced to meet kidney-related diet safety concerns.
The cross-sectional association between greater DASH accordance with higher prevalence of hypertension among JHS participants without CKD was unexpected. This finding may reflect participants with hypertension having a greater likelihood of being advised to follow healthful diets like DASH. As observations were cross-sectional, we cannot draw inferences regarding any causal associations.
Strengths of our study include its large sample size of black Americans, prevalent CKD, and assessment of diet using a regionally validated FFQ. Limitations of our study include its cross-sectional design that precludes determination of causal relationships; single timepoint measurements of serum creatinine and urine albumin, which may result in misclassification of CKD; intrinsic limitations of FFQ data involving recall bias and reporting error; potential selection bias with exclusion of 41% of the JHS participants; and defining hypertension by participant self-report, mean BP values obtained at single clinic visits, and use of antihypertensive agents, which may result in misclassification of hypertension. Although we adjusted for several demographic and health behaviors that influence diet and BP, our results could have also been impacted by unmeasured confounders. The generalizability of our results to non-black racial groups and individuals with CKD stages 4 and 5 (which only comprised 7% of our CKD population) is also limited.
In conclusion, our findings suggest that CKD may be a compelling indication to recommend DASH to black patients due to its favorable association with BP, even at low levels of DASH accordance. Randomized controlled studies are needed to confirm the efficacy and test the safety of DASH on BP in black adults with moderate and severe CKD.
Supplementary Material
ACKNOWLEDGMENTS
The Jackson Heart Study is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, and HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. C.C.T is supported by a supplemental training award from the National Heart, Lung, Blood Institute (R01HL122836). C.A.D. is partially supported by the Duke Clinical and Translational Science Award (UL1TR001117). C.M.R. is supported by a mentored research scientist development award from the National Institute of Diabetes and Digestive and Kidney Diseases (K01DK107782).
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1. Muntner P, Anderson A, Charleston J, Chen Z, Ford V, Makos G, O’Connor A, Perumal K, Rahman M, Steigerwalt S, Teal V, Townsend R, Weir M, WrightJT, Jr.. Chronic Renal Insufficiency Cohort Study I: hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 2010; 55:441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C. Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med 2005; 165:923–928. [DOI] [PubMed] [Google Scholar]
- 3. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, MohlerER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015; 131:e29–322. [DOI] [PubMed] [Google Scholar]
- 4. Saran R, Robinson B, Abbott KC, Agodoa LYC, Bragg-Gresham J, Balkrishnan R, Dietrich X, Eckard A, Eggers PW, Gaipov A, Gillen D, Gipson D, Hailpern SM, Hall YN, Han Y, He K, Herman W, Heung M, Hirth RA, Hutton D, Jacobsen SJ, Jin Y, Kalantar-Zadeh K, Kapke A, Kovesdy CP, Lavallee D, Leslie J, McCullough K, Modi Z, Molnar MZ, Montez-Rath M, Moradi H, Morgenstern H, Mukhopadhyay P, Nallamothu B, Nguyen DV, Norris KC, O’Hare AM, Obi Y, Park C, Pearson J, Pisoni R, Potukuchi PK, Rao P, Repeck K, Rhee CM, Schrager J, Schaubel DE, Selewski DT, Shaw SF, Shi JXM, Shieu M, Sim JJ, Soohoo M, Steffick D, Streja E, Sumida K, Tamura MK, Tilea A, Tong L, Wang DY, Wang M, Woodside KJ, Xin X, Yin MG, You AS, Zhou H, Shahinian V. US renal data system 2017 annual data report epidemiology of kidney disease in the United States. Am J Kidney Dis 2018; 71:S1–S676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. DASH collaborative research group. N Engl J Med 1997; 336:1117–1124. [DOI] [PubMed] [Google Scholar]
- 6. Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER 3rd, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM, Charleston J, McCarron P, Bishop LM; OmniHeart Collaborative Research Group . Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA 2005; 294:2455–2464. [DOI] [PubMed] [Google Scholar]
- 7. Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER 3rd, Simons-Morton DG, Karanja N, Lin PH; DASH-Sodium Collaborative Research Group . Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. DASH-Sodium collaborative research group. N Engl J Med 2001; 344:3–10. [DOI] [PubMed] [Google Scholar]
- 8. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure ; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003; 289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 9. James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520. [DOI] [PubMed] [Google Scholar]
- 10. Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, SmithSC, Jr, Svetkey LP, Wadden TA, Yanovski SZ, Kendall KA, Morgan LC, Trisolini MG, Velasco G, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, SmithSC, Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice G . 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129:S76–99. [DOI] [PubMed] [Google Scholar]
- 11. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F; Task Force for the Management of Arterial Hypertension of the European Society of Hypertension and the European Society of Cardiology . 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press 2014; 23:3–16. [DOI] [PubMed] [Google Scholar]
- 12. Leung AA, Daskalopoulou SS, Dasgupta K, McBrien K, Butalia S, Zarnke KB, Nerenberg K, Harris KC, Nakhla M, Cloutier L, Gelfer M, Lamarre-Cliche M, Milot A, Bolli P, Tremblay G, McLean D, Tran KC, Tobe SW, Ruzicka M, Burns KD, Vallée M, Prasad GVR, Gryn SE, Feldman RD, Selby P, Pipe A, Schiffrin EL, McFarlane PA, Oh P, Hegele RA, Khara M, Wilson TW, Penner SB, Burgess E, Sivapalan P, Herman RJ, Bacon SL, Rabkin SW, Gilbert RE, Campbell TS, Grover S, Honos G, Lindsay P, Hill MD, Coutts SB, Gubitz G, Campbell NRC, Moe GW, Howlett JG, Boulanger JM, Prebtani A, Kline G, Leiter LA, Jones C, Côté AM, Woo V, Kaczorowski J, Trudeau L, Tsuyuki RT, Hiremath S, Drouin D, Lavoie KL, Hamet P, Grégoire JC, Lewanczuk R, Dresser GK, Sharma M, Reid D, Lear SA, Moullec G, Gupta M, Magee LA, Logan AG, Dionne J, Fournier A, Benoit G, Feber J, Poirier L, Padwal RS, Rabi DM; Hypertension Canada . Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol 2017; 33:557–576. [DOI] [PubMed] [Google Scholar]
- 13. Svetkey LP, Simons-Morton D, Vollmer WM, Appel LJ, Conlin PR, Ryan DH, Ard J, Kennedy BM. Effects of dietary patterns on blood pressure: subgroup analysis of the Dietary Approaches to Stop Hypertension (DASH) randomized clinical trial. Arch Intern Med 1999; 159:285–293. [DOI] [PubMed] [Google Scholar]
- 14. Vollmer WM, Sacks FM, Ard J, Appel LJ, Bray GA, Simons-Morton DG, Conlin PR, Svetkey LP, Erlinger TP, Moore TJ, Karanja N; DASH-Sodium Trial Collaborative Research Group . Effects of diet and sodium intake on blood pressure: subgroup analysis of the DASH-sodium trial. Ann Intern Med 2001; 135:1019–1028. [DOI] [PubMed] [Google Scholar]
- 15. Bray GA, Vollmer WM, Sacks FM, Obarzanek E, Svetkey LP, Appel LJ; DASH Collaborative Research Group . A further subgroup analysis of the effects of the DASH diet and three dietary sodium levels on blood pressure: results of the DASH-Sodium Trial. Am J Cardiol 2004; 94:222–227. [DOI] [PubMed] [Google Scholar]
- 16. Weidmann P, Maxwell MH, Lupu AN, Lewin AJ, Massry SG. Plasma renin activity and blood pressure in terminal renal failure. N Engl J Med 1971; 285:757–762. [DOI] [PubMed] [Google Scholar]
- 17. Akita S, Sacks FM, Svetkey LP, Conlin PR, Kimura G; DASH-Sodium Trial Collaborative Research Group . Effects of the Dietary Approaches to Stop Hypertension (DASH) diet on the pressure-natriuresis relationship. Hypertension 2003; 42:8–13. [DOI] [PubMed] [Google Scholar]
- 18. Lin PH, Allen JD, Li YJ, Yu M, Lien LF, Svetkey LP. Blood pressure-lowering mechanisms of the DASH dietary pattern. J Nutr Metab 2012; 2012:472396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Svetkey LP, Moore TJ, Simons-Morton DG, Appel LJ, Bray GA, Sacks FM, Ard JD, Mortensen RM, Mitchell SR, Conlin PR, Kesari M; Group Dcr . Angiotensinogen genotype and blood pressure response in the Dietary Approaches to Stop Hypertension (DASH) study. J Hypertens 2001; 19:1949–1956. [DOI] [PubMed] [Google Scholar]
- 20. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int Suppl 2012; 2:337–414. [Google Scholar]
- 21. Kidney Disease Outcomes Quality Initiative. K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43:S1–290. [PubMed] [Google Scholar]
- 22. Tyson CC, Lin PH, Corsino L, Batch BC, Allen J, Sapp S, Barnhart H, Nwankwo C, Burroughs J, Svetkey LP. Short-term effects of the DASH diet in adults with moderate chronic kidney disease: a pilot feeding study. Clin Kidney J 2016; 9:592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Taylor HA Jr, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, Nelson C, Wyatt SB. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis 2005; 15:S6–S4. [PubMed] [Google Scholar]
- 24. Carpenter MA, Crow R, Steffes M, Rock W, Heilbraun J, Evans G, Skelton T, Jensen R, Sarpong D. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci 2004; 328:131–144. [DOI] [PubMed] [Google Scholar]
- 25. Carithers T, Dubbert PM, Crook E, Davy B, Wyatt SB, Bogle ML, Taylor HA Jr, Tucker KL. Dietary assessment in African Americans: methods used in the Jackson Heart Study. Ethn Dis 2005; 15:S6–49. [PubMed] [Google Scholar]
- 26. Mellen PB, Gao SK, Vitolins MZ, GoffDC, Jr.. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988–1994 and 1999–2004. Arch Intern Med 2008; 168:308–314. [DOI] [PubMed] [Google Scholar]
- 27. Wright BM, Dore CF. A random-zero sphygmomanometer. Lancet 1970; 1:337–338. [DOI] [PubMed] [Google Scholar]
- 28. Abdalla M, Booth JN 3rd, Seals SR, Spruill TM, Viera AJ, Diaz KM, Sims M, Muntner P, Shimbo D. Masked hypertension and incident clinic hypertension among blacks in the Jackson heart study. Hypertension 2016; 68:220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tyson CC LP, Corsino L, Batch BC, Allen J, Sapp S, Barnhart H, Nwankwo C, Burroughs J, Svetkey LP. Short-term effects of the DASH diet in adults with moderate chronic kidney disease: a pilot feeding study. Clin Kidey J 2009; e-pub ahead of print 5 June 2016. doi: 10.1093/ckj/sfw046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tyson CC, Barnhart H, Sapp S, Poon V, Lin PH, Svetkey LP. Ambulatory blood pressure in the dash diet trial: effects of race and albuminuria. J Clin Hypertens (Greenwich) 2018; 20:308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Crews DC, Kuczmarski MF, MillerER, 3rd, Zonderman AB, Evans MK, Powe NR. Dietary habits, poverty, and chronic kidney disease in an urban population. J Ren Nutr 2015; 25:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim H, Andrade FC. Diagnostic status of hypertension on the adherence to the dietary approaches to stop hypertension (DASH) diet. Prev Med Rep 2016; 4:525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, Stevens VJ, Vollmer WM, Lin PH, Svetkey LP, Stedman SW, Young DR; Writing Group of the PCRG . Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA 2003; 289:2083–2093. [DOI] [PubMed] [Google Scholar]
- 35. Blumenthal JA, Babyak MA, Hinderliter A, Watkins LL, Craighead L, Lin PH, Caccia C, Johnson J, Waugh R, Sherwood A. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Arch Intern Med 2010; 170:126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Neumann J, Ligtenberg G, Klein II, Koomans HA, Blankestijn PJ. Sympathetic hyperactivity in chronic kidney disease: pathogenesis, clinical relevance, and treatment. Kidney Int 2004; 65: 1568–1576. [DOI] [PubMed] [Google Scholar]
- 37. Passauer J, Pistrosch F, Büssemaker E, Lässig G, Herbrig K, Gross P. Reduced agonist-induced endothelium-dependent vasodilation in uremia is attributable to an impairment of vascular nitric oxide. J Am Soc Nephrol 2005; 16:959–965. [DOI] [PubMed] [Google Scholar]
- 38. Goraya N, Simoni J, Jo CH, Wesson DE. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin J Am Soc Nephrol 2013; 8:371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rebholz CM, Crews DC, Grams ME, Steffen LM, Levey AS, Miller ER 3rd, Appel LJ, Coresh J. DASH (dietary approaches to stop hypertension) diet and risk of subsequent kidney disease. Am J Kidney Dis 2016; 68:853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu Y, Kuczmarski MF, Miller ER 3rd, Nava MB, Zonderman AB, Evans MK, Powe NR, Crews DC. Dietary habits and risk of kidney function decline in an urban population. J Ren Nutr 2017; 27:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.