Abstract
Group A Streptococcus (GAS) is a common and versatile human pathogen causing a variety of diseases. One of the many virulence factors of GAS is the secreted pore-forming cytotoxin streptolysin O (SLO), which has been ascribed multiple properties, including inflammasome activation leading to release of the potent inflammatory cytokine IL-1β from infected macrophages. IL-1β is synthesized as an inactive pro-form, which is activated intracellularly through proteolytic cleavage. Here, we use a macrophage infection model to show that SLO specifically induces ubiquitination and degradation of pro-IL-1β. Ubiquitination was dependent on SLO being released from the infecting bacterium, and pore formation by SLO was required but not sufficient for the induction of ubiquitination. Our data provide evidence for a novel SLO-mediated mechanism of immune regulation, emphasizing the importance of this pore-forming toxin in bacterial virulence and pathogenesis.
Keywords: Group A Streptococcus; Streptolysin O; Ubiquitin, IL-1β
Introduction
A common trait among pathogenic microbes is their ability to circumvent or counteract the protective measures induced by an invaded host, often taking advantage of host factors or processes for their own benefit. One network within host cells frequently exploited or manipulated by pathogens is the ubiquitination system [1]. In this study, we demonstrate that the common human pathogen Group A Streptococcus (GAS or Streptococcus pyogenes) induces ubiquitination and degradation of the inactive pro-form of the inflammatory cytokine IL-1β, an event that is dependent on the bacterial virulence factor streptolysin O (SLO).
Ubiquitin is a small and a highly conserved protein that can be covalently attached to a substrate protein and thereby modify its activity, function or localization, or mark it for secretion or destruction [2]. Ubiquitination occurs in a 3-step enzymatic process and results in either the attachment of a single ubiquitin, or ubiquitin chains that may be linear or branched. Within such chains, ubiquitin proteins can be linked together via any of its 7 lysines (K6, K11, K27, K29, K33, K48, or K63) or via the N-terminal methionine (M1). The nature of the ubiquitin linkage dictates the fate of the substrate protein; K48-linked poly-ubiquitin chains generally target proteins for proteasomal degradation, whereas M1- (linear) or K63-linked chains are mostly associated with, for example, protein-protein interactions, protein trafficking, or degradation of proteins by autophagy [2, 3].
The Nlrp3 inflammasome is an intracellular protein complex that is assembled and activated in response to a wide range of stimuli, mostly pertaining to disruptions in cellular homeostasis [4]. Within an inflammasome, the cysteine protease caspase-1 may cleave the inactive proforms of the inflammatory cytokines IL-1β and IL-18 into their active forms, and also initiate a pro-inflammatory type of cell death known as pyroptosis through processing of the pore-forming protein gasdermin D [5]. The inflammasome plays an important role in innate immunity and is a key mediator of powerful inflammatory signals upon infection as well as sterile tissue damage. The activity and function of these complexes are tightly regulated, both at the level of generation and through post-translational modification of the pro-IL-1β protein per se. Several of these control measures are mediated by ubiquitination and deubiquitination processes [6, 7, 8, 9, 10, 11].
GAS is an extracellular bacterial pathogen responsible for superficial as well as severe and sometimes life-threatening diseases, causing around 500,000 deaths annually [12]. This bacterium produces an impressive set of virulence factors, among them a variety of secreted toxins, proteases and superantigens with prominent roles in pathogenesis. The 2 pore-forming proteins SLO and streptolysin S act through different mechanisms but are both membrane-damaging toxins with the ability to lyse red blood cells [13]. Of particular interest for this study is SLO, which belongs to a family of cholesterol-dependent cytolysins that can insert into the lipid bilayer of host cell membranes and may result in pores up to 50 nm in diameter [14]. SLO has been ascribed multiple functions including the induction of oncosis or apoptosis in macrophages [15, 16], and prevention of phagolysosomal acidification and xenophagic killing in keratinocytes [17, 18]. In addition, SLO is a known activator of the inflammasome and generates release of mature IL-1β from macrophages [19, 20]. The role of IL-1β in GAS infection seems complex both in human disease and in murine experimental settings, and presumably appropriate management of the levels of this very potent proinflammatory cytokine is important to the pathogen as well as the infected host [21, 22, 23, 24, 25].
Here, we describe a previously unknown capacity of GAS to induce SLO-dependent ubiquitination of pro-IL-1β. This ubiquitination event results in increased pro-IL-1β degradation, thus limiting the amount of pro-IL-1β available for inflammasome-mediated maturation and reducing the release of pro-inflammatory IL-1β.
Materials and Methods
Bacterial Strains and Growth Conditions
The wild type (wt) GAS strain 854 is an M type 1 strain isolated from a patient with a retroperitoneal abscess [26]. The isogenic mutant strains used in this study are listed in Table 1. Bacteria were grown in Todd-Hewitt broth supplemented with 0.5% yeast extract (THY) in 5% CO2 at 37°C. Overnight cultures were reinoculated in THY and grown to late exponential phase (optical density of 1.1–1.3 at 600 nm), washed with phosphate-buffered saline, and diluted before use.
Table 1.
Description and references of streptococcal strains used in this study
Mice
Genetically modified mouse strains IL-1β−/− [27], caspase-1/11−/− [28], Asc−/− [29], Nlrp3−/− [30] and receptor-interacting kinase 3 (Rip3)−/− [31] were all on a C57BL/6 (B6) background. B6 mice were bred in-house. Genetically modified mice or cells from such mice were kindly provided by Catharina Svanborg (IL-1β−/−), Bengt Johansson-Lindbom (caspase-1/11−/−) and Russell E. Vance (Asc−/−, Nlrp3−/−). Rip3−/− mice were originally generated by K. Newton and V. Dixit (Genentech), and provided to E. Lien by Drs. Kaiser, Mocarski, Dillon and Green. All animal experiments were conducted in accordance with protocols approved by the Lund/Malmö Animal Ethics Committee.
Generation and Infection of Bone Marrow-Derived Macrophages
Bone marrow was isolated from murine femurs and tibiae, and progenitor cells were differentiated into bone marrow-derived macrophages (BMDMs) for 7 days (37°C, 5% CO2) in RPMI 1,640 (Gibco) supplemented with 10% fetal bovine serum (Sigma), 2.5 mM L-glutamine, and macrophage colony-stimulating factor. Prior to infection, 10 × 106 BMDMs were seeded on 90 mm Petri dishes (unless otherwise stated), primed with 1 µg/mL lipopolysaccharide (LPS; Sigma) for 4 h, and infected at a multiplicity of infection of 20 and incubated for 90 min (unless otherwise stated). For longer incubation times, the bacterial suspension was replaced with fresh medium containing 300 µg/mL gentamicin (Sigma) to kill extracellular bacteria at 90 min post infection. When indicated, BMDMs were stimulated with recombinant SLO (rSLO; Bio-Rad) at the concentration of 10 µg/mL in the presence or absence of ∆slo bacteria. Cell culture medium was supplemented with 50 mM KCl (Sigma) in order to block K+ efflux. Nigericin (Sigma) was used at 10 µM concentration for 60 min. MG-132 (Sigma), 3-Methyladenine (3-MA; Sigma) or Bafilomycin A1 (Sigma) were added to the cell cultures 30 min before infection and were present throughout the whole infection at indicated concentrations.
Soluble IL-1β Measurement
Supernatants from infected BMDMs were cleared from debris by centrifugation (300 g, 5 min) and analyzed using IL-1β ELISA kits (BD Biosciences or R&D Systems) according to the manufacturer's instructions.
Immunoprecipitation and Deubiquitinase Treatment
Cells used for infection were lysed with NP-40 cell lysis buffer (150 mM sodium chloride, 1% NP-40 [Sigma], 50 mM Tris, complete protease inhibitor cocktail [Roche]). Lysates were incubated with 0.3 µg antibody (IL-1β: AF-401 [R&D Systems], IL-18: 5180R [BioVision])/106 cells at 4°C overnight and precipitated using protein G Dynabeads (Thermo Scientific) incubated at 4°C for 1 h. When indicated, precipitated samples were digested with deubiquitinase enzymes (DUB) using the UbiCREST DUB Set (Boston Biochem) according to the manufacturer's instructions. Precipitated proteins were eluted by incubation at 70°C for 10 min using elution buffer containing lithium dodecyl sulfate (Thermo Scientific) under reducing conditions.
Protein Separation and Immunoblot Assays
Following infection of 5 × 105 BMDMs as described above, cell lysates were prepared by using NP-40 lysis buffer followed by the addition of lithium dodecyl sulfate sample buffer and sample reducing agent (Life Technologies). Whole cell lysates or precipitated samples were separated by SDS-PAGE using NuPAGE 12% bis-Tris gels (Life Technologies) under reducing conditions and transferred to hybond polyvinylidene difluoride. For the analysis presented in Figure 5, whole cell lysates were run on a 4–15% TGX Stain Free Gel (Bio-Rad), and transferred to polyvinylidene difluoride membranes using the Trans-Blot Turbo Transfer System (Bio-Rad). This system allows UV-based detection of total protein content in each lane after transfer to the membrane. For detection, the following primary and horseradish peroxidase-conjugated secondary antibodies were used: anti-IL-1β (AF-401; R&D Systems), anti-IL-18 (5180R; BioVision), anti-caspase-1 p20 (AG-20B-0042; AdipoGen), anti-caspase-8 (ALX 804-448; Enzo), anti-Rip1 (clone D94C12; Cell Signaling), anti-Rip3 (2283; ProSci), anti-A20 (56309; Cell Signaling), anti-pan-ubiquitin (clone P4D4; Santa Cruz Biotechnology), anti-GAPDH (G9545; Sigma), rabbit anti-goat IgG (61-1620; Thermo Scientific or 705-035-003; Jackson Immuno Research), donkey anti-mouse IgG (715-036-151; Jackson Immuno Research), donkey anti-rat IgG (712-036-153; Jackson), and goat anti-rabbit IgG (111-035-144; Jackson). Stained membranes were incubated in Clarity Western ECL blotting substrate for chemiluminescence (Bio-Rad) and developed. Blots were documented and quantified using ChemiDoc imaging system (Bio-Rad), Quantity One and Image Lab software.
Fig. 5.
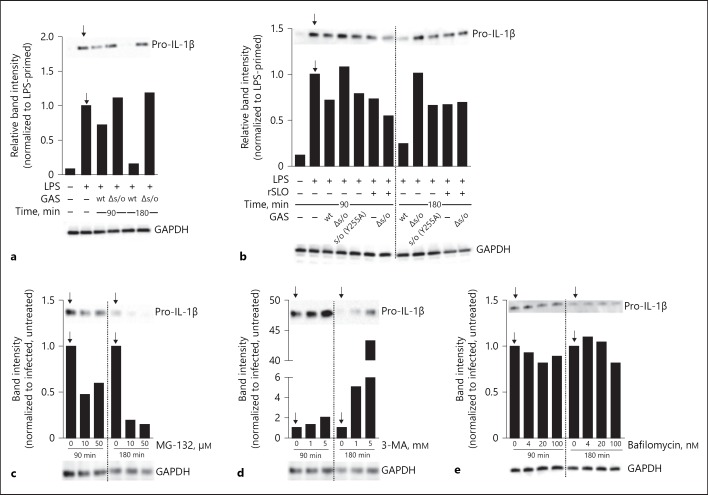
Pro-IL-1β is degraded upon streptolysin O (SLO) proficient Group A Streptococcus (GAS) infection. a Macrophages deficient for the inflammasome linker protein ASC were left untreated, primed or primed, and infected with wild type (wt) or Δslo Group A Streptococcus (GAS) for the designated time points, as indicated, and (pro-)IL-1β levels in whole cell lysates were evaluated by immunoblot. b Macrophages deficient for ASC were left untreated, primed or primed, and infected with wt, Δslo, or slo(Y255A) GAS in the presence or absence of 10 µg/mL recombinant SLO (rSLO) for the designated time points, as indicated, and pro-IL-1β levels in whole cell lysates were evaluated by immunoblot. Lipopolysaccharide (LPS)-primed ASC deficient macrophages were infected with wt GAS for the indicated times in the presence of specific inhibitors as follows; c MG-132 (proteasome), d 3-MA (autophagy, PI3K inhibitor) or e Bafilomycin (autophagy, V-ATPase inhibitor). Band intensities were quantified based on whole protein content of cell lysates loaded onto gels followed by normalization to infected cells (without inhibitor) at each time point (indicated by arrows). The housekeeping protein GAPDH was used as a visual loading control but not as a basis for quantification. Figure shows representative blots of at least 3 independent experiments.
Statistical Analysis
Statistical calculations were performed using Prism version 7.0 (GraphPad software). Data were analyzed using 1-way ANOVA (3 or more groups with 1 categorical variable) or 2-way ANOVA (3 or more groups with 2 categorical variables): ns, not significant, *** p < 0.001.
Results
GAS Expressing SLO Induces Ubiquitination of Pro-IL-1β in Macrophages
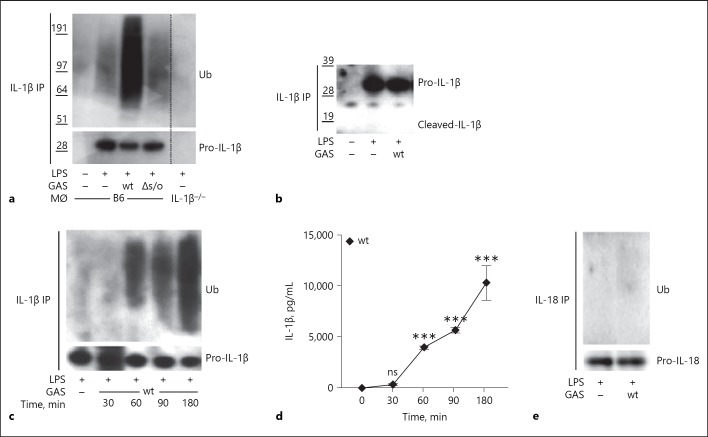
Nlrp3 inflammasome activation and maturation of IL-1β requires 2 sequential signals [32], the first one (referred to as “priming”) mediates the upregulation of the Nlrp3 and pro-IL-1β proteins in addition to multiple post-translational modifications of proteins involved in the inflammasome complex. Priming is commonly a response to a microbial ligand or endogenous danger signal, and can be achieved through receptors activating the transcription factors NF-κB and AP-1 [32]. In vivo, priming is likely to occur through multiple receptors, in vitro however priming is often mediated by LPS through TLR4. The second signal (“activation”) leads to the assembly of the inflammasome complex, caspase-1 activation and cleavage and secretion of mature IL-1β, and can be mediated by a wide array of stimuli, including SLO [19, 20]. As ubiquitination has previously been reported to influence levels of secreted IL-1β [7, 9, 10], we set out to investigate the potential role for ubiquitination in the regulation of mature IL-1β release during GAS infection. We used immunoprecipitation to isolate pro-IL-1β from LPS-primed, GAS-infected murine BMDMs and revealed possible ubiquitination of the precipitate by western blotting. In agreement with existing data [9, 10], LPS priming induced a low level of pro-IL-1β ubiquitination (Fig. 1a). This post-translational modification has previously been shown to support more efficient maturation and secretion of IL-1β [9]. Upon infection with wt GAS, pro-IL-1β ubiquitination was markedly increased, while this was not observed in cells infected with an isogenic bacterial mutant lacking SLO expression (Δslo; Fig. 1a). As expected, no ubiquitinated protein could be detected after IL-1β immunoprecipitation of primed IL-1β−/− BMDMs (Fig. 1a), confirming the specificity of the IL-1β antibody. Of note, this antibody recognizes pro- and mature IL-1β alike, implying that both forms may be precipitated in our assays. However, cleaved IL-1β is rapidly released from the cell and consequently mature IL-1β is not readily detectable in lysates of cells where the inflammasome has been activated. Indeed, we did not observe any cleaved IL-1β in the precipitated samples from cells infected with wt GAS (Fig. 1b), suggesting that the detected ubiquitination pattern mostly reveals post-transcriptional modifications of the pro-form (Fig. 1a). This does not exclude the possibility that released mature IL-1β may also be ubiquitinated, although ubiquitin is commonly detached from target proteins before secretion as part of the maintenance of ubiquitin homeostasis in the cell [33]. The ubiquitination of pro-IL-1β was visible after 60 min and increased up to 180 min of infection (Fig. 1c), demonstrating kinetics similar to that of inflammasome-dependent IL-1β release after GAS infection of macrophages (Fig. 1d). Nlrp3 inflammasome activation may also lead to the maturation and secretion of IL-18 [34], the pro-form of this cytokine, however, does not seem to be ubiquitinated upon GAS infection of BMDMs (Fig. 1e).
Fig. 1.
Group A Streptococcus (GAS) expressing streptolysin O (SLO) induces ubiquitination of pro-IL-1β in macrophages. a B6 macrophages were primed with lipopolysaccharide (LPS) or primed and infected with GAS as indicated and cell lysates were subjected to IL-1β immunoprecipitation (IP) and immunoblot analysis for ubiquitin (Ub) and (pro-)IL-1β. Primed IL-1β−/− macrophages were used as a control for specificity. b IL-1β IP and immunoblot analysis of lysates from LPS-primed or primed and infected macrophages, as indicated. c Macrophages were primed with LPS or primed and infected with wild type (wt) GAS for the indicated times. IL-1β was precipitated from cell lysates and analyzed for Ub and (pro-)IL-1β by immunoblot. d LPS-primed macrophages were infected by wt GAS and levels of released IL-1β were assessed by ELISA. e IL-18 was precipitated from cell lysates of LPS-primed or primed and infected macrophages, as indicated, followed by immunoblot analysis for Ub and (pro-)IL-18. Blots show 1 representative of 2 or 3 independent experiments. Graph shows means plus SD for triplicate samples and is representative of 2 independent experiments.
Taken together, these data suggest that GAS infection of macrophages rapidly induces increased ubiquitination of pro-IL-1β, but not pro-IL-18, and that this process is dependent on the cytolysin SLO.
Pro-IL-1β Ubiquitination Induced by SLO Requires Pore Formation
SLO is secreted from the bacteria as monomers, which oligomerize into a prepore on the host cell membrane. The prepore complex then inserts fully into the membrane to form a pore that can reach as much as 50 nm in diameter [14, 35]. Complete pore formation is required for the induction of cell lysis and inflammasome activation [35], which seems to be the case also for similar toxins found in other bacterial pathogens, such as the Listeria monocytogenes listeriolysin O [36] and pneumolysin secreted by S. pneumoniae [37].
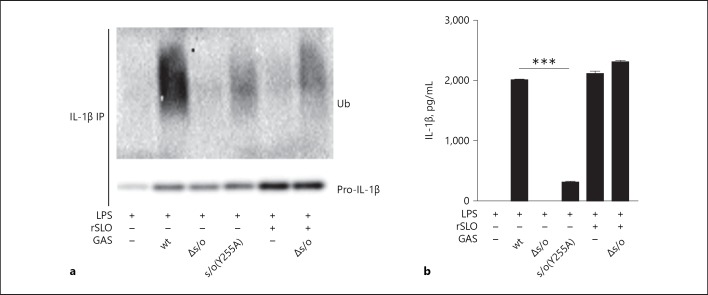
Through the introduction of point mutations into SLO, pore formation can be blocked at different steps of the oligomerization-insertion process [38]. To study the effect of the pore forming capacity of SLO on pro-IL-1β ubiquitination, we took advantage of an isogenic bacterial strain expressing a mutated form of SLO that is locked in the prepore stage (slo[Y255A]), thus secreting SLO monomers that oligomerize on the host cell membrane but are not able to fully insert and form pores [17]. We observed decreased pro-IL-1β ubiquitination (Fig. 2a), and as expected also reduced IL-1β release (Fig. 2b), in BMDMs infected with the mutant strain compared to wt GAS, suggesting that pore-formation is important not only for inflammasome activation by SLO but also for SLO-mediated ubiquitination of pro-IL-1β.
Fig. 2.
Pro-IL-1β ubiquitination is dependent on bacterial secretion of and pore formation by streptolysin O (SLO). LPS-primed B6 macrophages were infected with wild type (wt), Δslo or slo(Y255A) Group A Streptococcus (GAS) or stimulated with 10 µg/mL recombinant SLO (rSLO) for 90 min in the presence or absence of Δslo bacteria, as indicated. a IL-1β precipitates from cell lysates were assayed for ubiquitin (Ub) and (pro-)IL-1β by immunoblot. b Supernatants were analyzed for IL-1β by ELISA. Blot shows 1 representative of at least 3 independent experiments. Graph shows means plus SD for triplicate samples and is representative of 3 independent experiments.
Pore-formation by SLO induces multiple processes in the affected cell [13]. To investigate whether ubiquitination of pro-IL-1β was a response induced primarily by the membrane damage caused by SLO, or by the streptococcal infection, we treated LPS-primed BMDMs with rSLO in the absence of bacteria. While rSLO efficiently induced the release of mature IL-1β (Fig. 2b), it was not able to promote ubiquitination of the IL-1β proform (Fig. 2a). Similarly, the addition of rSLO during an infection with the Δslo bacterial strain did not generate pro-IL-1β ubiquitination (Fig. 2a) but as expected resulted in efficient IL-1β release (Fig. 2b). These observations allow us to conclude that SLO needs to be expressed and secreted from the bacterium during infection in order to support pro-IL-1β ubiquitination and that pore-formation by SLO is required, but not sufficient, for the ubiquitination process. Further, in contrast to the baseline ubiquitination of pro-IL-1β that is induced by LPS priming [9], the additional ubiquitination of pro-IL-1β induced by SLO-proficient streptococcal infection does not seem to be required to facilitate subsequent release of mature IL-1β.
Streptococcus-Induced Ubiquitination of Pro-IL-1β Occurs Independently of SLO-Mediated Inflammasome Signaling
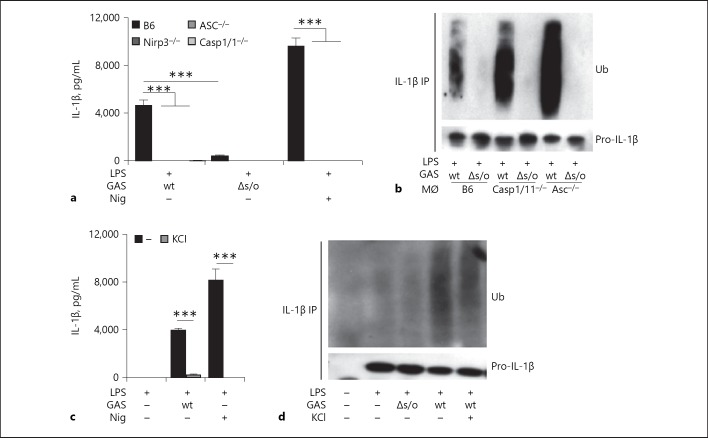
Inflammasome activation and IL-1β release induced by GAS is known to be mostly due to pore-formation by SLO and to be dependent on the inflammasome sensor protein Nlrp3, the linker protein ASC and the cysteine protease caspase-1 [19, 20]. Because we found a requirement for SLO in pro-IL-1β ubiquitination, we hypothesized that ubiquitination may occur as a response to SLO-mediated inflammasome activation. Thus, we infected B6 (wt) BMDMs or BMDMs deficient for the components required for inflammasome activation by GAS (Nlrp3, ASC and caspase-1, knock-outs on B6 background) and analyzed them for IL-1β release and pro-IL-1β ubiquitination. As expected, Nlrp3, ASC, and caspase-1 were all required for release of mature IL-1β upon GAS infection (Fig. 3a). However, pro-IL-1β was ubiquitinated also in their absence (Fig. 3b), suggesting that this ubiquitination event is not a process mediated by inflammasome activation per se. Although the amounts of pro-IL-1β were similar in cell lysates of different genotype BMDMs, the ubiquitination pattern appeared stronger in caspase-1-, and particularly ASC-deficient as compared to B6 macrophages (Fig. 3b), suggesting that these well-known inflammasome components may affect the regulation of the ubiquitination process.
Fig. 3.
Streptococcus-induced ubiquitination of pro-IL-1β occurs independently of SLO-mediated inflammasome signaling. a Primed macrophages of different genotypes were infected with wild type (wt) or Δslo Group A Streptococcus (GAS), as indicated. The Nlrp3 inflammasome-activating toxin Nigericin (Nig) was used as a control. Supernatants were assessed for IL-1β by ELISA. b Different genotype macrophages were primed and infected with wt or Δslo GAS. IL-1β was precipitated from cell lysates and ubiquitin (Ub) and (pro-)IL-1β were assessed by immunoblot. c B6 macrophages were primed or primed and infected or treated with Nig, as indicated, in the presence or absence of KCl and IL-1β in the supernatant was assessed by ELISA. d B6 macrophages were primed or primed and infected, as indicated, in the presence or absence of KCl and IL-1β was precipitated from cell lysates and ubiquitin (Ub) and (pro-)IL-1β were assessed by immunoblot. Graphs show means plus SD for triplicate samples and are representatives of at least 3 independent experiments. Blots show 1 representative of 3 independent experiments.
The Nlrp3 inflammasome can be activated by a number of bacterial toxins [39], and although the actual molecular mechanisms governing its activation are still largely unknown, in most cases, it can be blocked by the inhibition of K+ efflux from the cell, suggesting the disruption of intracellular ion homeostasis may be an activating trigger [4]. To explore whether the ion flux conduits affecting inflammasome activation may be involved in pro-IL-1β ubiquitination, BMDMs supplemented with extracellular K+ to block efflux were infected and analyzed as described above. While K+ efflux was essential for SLO-mediated inflammasome activation and release of mature IL-1β (Fig. 3c), it was dispensable for ubiquitination of the pro-form (Fig. 3d), which is in line with our data suggesting that Nlrp3 inflammasome activation is not required for pro-IL-1β ubiquitination.
Pro-IL-1β Ubiquitination after Streptococcal Infection Is of Heterogeneous Linkage Specificity
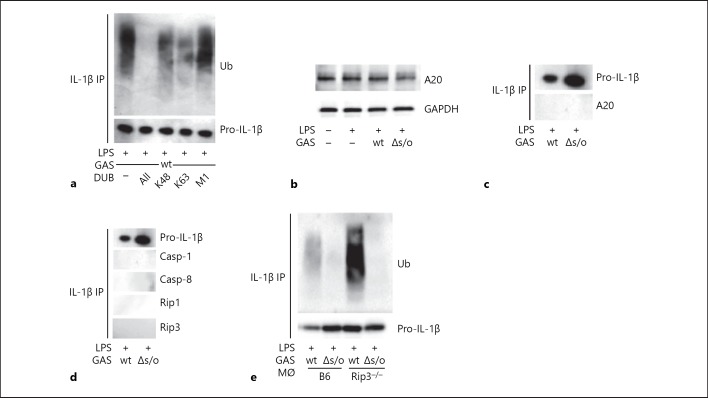
Ubiquitin monomers can be linked together through any of their 7 internal lysines or their N-terminal methionine residues, and the nature of this linkage decides the fate of the ubiquitinated target [2]. To investigate which mode of ubiquitination is present on pro-IL-1β after GAS infection, we took advantage of the so-called DUBs. The DUBs are a family of enzymes that can specifically cleave 1 or more type of intra-ubiquitin bonds, for example, all linkages or K48 or K63 linkages only [2]. Thus, we pulled down pro-IL-1β from BMDMs infected with wt GAS and treated the precipitate with different DUB enzymes. While the non-specific linkage enzyme USP2 as expected detached all ubiquitin moieties on pro-IL-1β, the K48- and K63-specific DUBs, OTUB1, and AMSH, both only partially reduced associated ubiquitin signals, suggesting the presence of both types of linkages. In contrast, the M1 linkage-specific enzyme Otulin did not appear to change the level of ubiquitination, implying ubiquitin is not attached to pro-IL-1β by M1 linkage (Fig. 4a).
Fig. 4.
Pro-IL-1β ubiquitination after Group A Streptococcus (GAS) infection is of heterogeneous linkage specificity. a lipopolysaccharide (LPS)-primed macrophages were infected with wild type (wt) GAS followed by precipitation of IL-1β. Precipitates were subjected to deubiquitinase (DUB) proteolysis removing ubiquitin (Ub) of all linkages (“All”) or specifically K48, K63 or M1 linkages, as indicated, and ubiquitin and (pro-)IL-1β were assessed by immunoblot. b Immunoblot for A20 in lysates of untreated, primed or primed, and infected (wt or Δslo GAS) macrophages, as indicated. c Immunoblot for A20 in IL-1β precipitates from macrophages primed and infected with wt or Δslo GAS. d IL-1β was precipitated from whole cell lysates from macrophages after priming and infection with wt or Δslo GAS and analyzed for co-precipitation of the indicated proteins. e B6 and receptor-interacting kinase 3 (Rip3)−/− macrophages were primed and infected with wt or Δslo GAS. Precipitates of IL-1β from cell lysates were assayed for ubiquitin (Ub) and (pro-)IL-1β. Blots show 1 representative of 2 or 3 independent experiments.
The enzyme A20 was first described as an inhibitor of the NF-κB activation and has as such a prominent role in immune homeostasis [40]. A20 has both E3 ligase and deubiquitinase activity and has subsequently been implicated in multiple immune signaling pathways, for example, regulating ubiquitination of pro-IL-1β. More specifically, A20 seems to restrict the ubiquitination of pro-IL-1β through a mechanism dependent on Rip3, thereby limiting secretion of mature IL-1β [9]. Therefore, we investigated the possibility that GAS infection might reduce A20 protein levels, which in turn could allow for increased pro-IL-1β ubiquitination. However, LPS-primed, wt- or Δslo-infected BMDMs all expressed comparable levels of A20, excluding this possibility (Fig. 4b). We further found, contrary to previous observations [9], that pro-IL-1β did not co-precipitate with A20, caspases or Rip kinases (Fig. 4c, d). In addition, our data indicate that pro-IL-1β ubiquitination was not abolished, but rather increased, by the absence of Rip3 (Fig. 4e). These findings agree with the observation that GAS-induced ubiquitination, contrary to the LPS-mediated, A20-restricted ubiquitination of pro-IL-1β, does not seem to be required for the maturation and secretion of this cytokine, and suggests that the infection-driven mechanism governing the ubiquitination of pro-IL-1β described here differs from mechanisms previously reported.
Pro-IL-1β is Degraded upon Infection with SLO-Proficient GAS
Previous studies have proposed that ubiquitinated pro-IL-1β protein may be degraded in the proteasome [7, 10] or by autophagy [41]. Thus, we set out to investigate whether GAS infection induces pro-IL-1β degradation, a possible outcome downstream of ubiquitination. In order to avoid the inflammasome-dependent processing of pro-IL-1β, we infected BMDMs lacking the integral inflammasome component ASC and observed a decrease in pro-IL-1β levels upon infection with wt bacteria as compared to the strain lacking SLO, or uninfected cells, and this decrease became more pronounced with time (Fig. 5a). In contrast, in macrophages that were infected with the SLO prepore-locked mutant slo (Y225A), treated with rSLO or infected with ∆slo bacteria in the presence of rSLO (all stimuli that induce reduced or no ubiquitination of pro-IL-1β [Fig. 2a]) degradation was significantly reduced (Fig. 5b). As this result supports the hypothesis that SLO-dependent pro-IL-1β ubiquitination increases protein degradation, we carried out infections in the presence of well-established inhibitors of the proteasome or autophagy, expecting that inhibition of the responsible degradative pathway would hamper the reduction of pro-IL-1β. The proteasome inhibitor MG-132 was not able to inhibit, but rather increased, the degradation of pro-IL-1β (Fig. 5c). However, 3-MA, which blocks autophagy by inhibiting phosphatidylinositol 3 kinases, did reduce pro-IL-1β degradation in wt-infected cells (Fig. 5d), suggesting that the ubiquitination induced by SLO-proficient GAS infection may promote increased autophagy-mediated degradation of pro-IL-1β. Because 3-MA is a broad inhibitor potentially disturbing multiple cellular processes, we performed an additional set of experiments using the V-ATPase pump inhibitor Bafilomycin, which prevents the acidification of lysosomes leading to a blockage in autophagosome-lysosome fusion and inhibition of autophagic flux. Surprisingly, under the conditions tested, Bafilomycin had minimal or no effect on the process (Fig. 5e), suggesting either that autophagy may not be responsible for the breakdown of pro-IL-1β and that 3-MA is blocking another degradative process, or that the inhibitors are differentially able to inhibit autophagic processes under the conditions tested. Neither of the inhibitors induced cell death as determined by LDH release (data not shown).
Discussion
During the mid-80s, invasive and life-threatening GAS disease increased significantly and it was found that these infections were mainly caused by a single clone of serotype M1 that was rapidly disseminated worldwide [42]. More recently, a similar propagation of an M89 clone has been observed [43]. Interestingly, both these clonal expansions were preceded by the acquisition of a genetic region that in these strains conferred increased expression of the pore-forming protein SLO and its co-toxin NADase [43, 44, 45], indicating that SLO and NADase make important contributions to virulence. In the current report, we describe a property of SLO that is likely to play an important role in virulence; the ability of SLO to drive ubiquitination and degradation of pro-IL-1β in infected macrophages, thus limiting the pool of pro-IL-1β available for inflammasome-mediated maturation and release. This previously unknown function for SLO may limit the innate immune response evoked by the infection and may at least partly explain why increased SLO expression levels correlate with enhanced fitness. It is interesting to note that while our data indicate that the ubiquitination triggered by SLO limits the release of processed IL-1β, SLO also induces secretion of mature IL-1β from macrophages [19, 20, 35]. This may reflect the activation and simultaneous control of IL-1β release, resulting in optimal circumstances for successful infection [22].
We found that SLO, pure or added to an infection with SLO-deficient bacteria, did not increase the level of pro-IL-1β ubiquitination, suggesting that SLO has to be secreted from the infecting bacterium and that pore-formation as such is not sufficient, although it is required, for the ubiquitination of pro-IL-1β. It is interesting to note the resemblance between this observation and the ones that have previously been made for SLO-mediated translocation of NADase, that is, both toxins have to be expressed and secreted by the same bacterium for translocation to occur, and translocation cannot be rescued in trans by co-infection with 2 bacterial mutants deficient in either toxin [46]. In contrast to the pro-IL-1β ubiquitination observed here however, translocation is independent of SLO pore formation [38]. Clearly, the potential involvement of NADase in pro-IL-1β ubiquitination is compelling and requires investigation. However, this problem is complicated by the fact that SLO functionality is reduced in the absence of NADase and by the finding that the SLO:NADase interaction is partly dependent on a NADase protein domain that is removed in translocation mutants [47, 48]. In addition, although NADase is currently the only known substrate for SLO-mediated translocation, it remains possible that there are other, yet unknown substrates translocated that are involved in the ubiquitination process.
Our analysis of GAS-infected caspase-1- or ASC-deficient macrophages show that pro-IL-1β ubiquitination proceeds independently of these inflammasome components, and thus does not require inflammasome formation and activation. Instead, the knockout BMDMs, in particular the ASC-deficient cells, exhibited significantly increased levels of ubiquitinated pro-IL-1β. This observation could be explained by an increase in the amount of ubiquitin conjugated to each molecule of pro-IL-1β and/or an increased ratio of ubiquitinated to non-ubiquitinated pro-IL-1β in these cells. These options require further investigation, and presently we can only speculate that our data may propose a role for caspase-1 and especially ASC in the regulation of the ubiquitination-degradation process. Interestingly, there are several reports describing novel roles for the components of the Nlrp3 inflammasome, for example, in transcription or regulation of phagocytosis and metabolic pathways [49, 50, 51], providing precedence for non-inflammasome-related functions of these proteins.
Ubiquitination of target proteins was initially believed to result exclusively in cellular signaling or proteasomal degradation as a result of K63 or K48 linkages respectively. However, as the field expanded it became obvious that the ubiquitin system is complex and dynamic. For example, it has been shown that non-degradative ubiquitin signals can be modified by the addition of further ubiquitin chains leading to the degradation of the target protein [52], and it is possible that our observation described here relates to this finding. Indeed, we found that LPS induced a basal level of ubiquitination of pro-IL-1β, which has previously been described to facilitate cytokine maturation and secretion, and that subsequent infection with SLO-proficient streptococci significantly increases pro-IL-1β ubiquitination. The ubiquitin chain(s) linked to pro-IL-1β as a result of GAS infection contains a heterogeneous mixture of K48 and K63 linkages, and although our current analyses do not allow us to draw conclusions about relative levels of these 2, or presence of other lysine linkage types, our data indicate that the final ubiquitin composition is a signature for cytokine degradation rather than maturation and secretion. Indeed, rSLO efficiently activates the inflammasome and generates the release of mature IL-1β, but does not induce increased ubiquitination of the pro-form, suggesting that the GAS-mediated ubiquitination of the cytokine it not essential for its secretion.
We find that infection with GAS expressing SLO leads to the degradation of pro-IL-1β, which can be blocked using the autophagy inhibitor 3-MA. Of note, 3-MA is a broad phosphatidylinositol 3 kinases inhibitor known to affect multiple cellular processes. As Bafilomycin, which is also commonly used to block autophagy, does not prevent the decay of pro-IL-1β, we believe it is still an open question whether the degradation truly ensues through autophagy or by another yet to be determined pathway. Interestingly, the inhibition of the proteasome during infection increased the degradation of pro-IL-1β, which may imply that the proteasome normally degrades a yet unknown regulating factor of pro-IL-1β stability or turn-over.
It has previously been reported that several pathogens exploit the ubiquitination to interfere with host immune responses. For example, human papilloma virus induces ubiquitination and proteasome-dependent degradation of pro-IL-1β [53], enteropathogenic E. coli downregulates the inflammasome activity by preventing Nlrp3 deubiquitination [54], and Shigella translocates a bacterial E3 ubiquitin ligase into the host cell and promotes the dissemination of the pathogen by inducing cell death [55]. Our results identify another mechanism by which pathogenic bacteria may exploit ubiquitin pathways of the host in order to dampen inflammation. The nature of the E3 ligase mediating this ubiquitination event and whether it is derived from the host or the bacterium remain to be explored. Based on our observations we propose that GAS-induced pro-IL-1β ubiquitination and degradation may represent a previously unrecognized immune evasion strategy, designed to directly dampen the innate immune response evoked by the invading bacterium.
Statement of Ethics
Animals were housed at the BMC facility at Lund University, and all experiments were performed in accordance with national regulations and the approval of the Lund/Malmö local Ethics Committee.
Disclosure Statement
The authors declare that they have no conflicts of interest to disclose.
Funding Sources
This work was supported by grants from the Swedish Foundation for Strategic Research, the Emil and Wera Cornell Foundation, the Crafoord Foundation, the Royal Physiographic Society of Lund, the Gyllenstierna Krapperup's Foundation, HRH Crown Princess Lovisa's Pediatrics Association, by the foundations of Anna and Edwin Berger, Magnus Bergvall, Golje-Lundström, Thelma Zoega, Alfred Österlund, U.S. Public Health Service grants AI070926 and AI029952 (both to M.R.W.), AI07538 and AI129527 (both to E.L.), the Norwegian Cancer Society grant and B05035/001 (to E.L.) and the Research Council of Norway Center of Excellence Funding Scheme project 223255/F50 (to P.O. and E.L.).
Authors Contribution
D.H., E.W., and J.J.P. conceived and designed the experiments and analyzed the data; D.H., E.W., C.V., and G.M.A. performed the experiments; B.B.-S., P.O., E.L., and M.R.W. generated and contributed essential materials; D.H. and J.J.P. wrote the paper; all authors critically reviewed the study.
Acknowledgment
We are grateful to our colleagues at the section for Immunology at Lund University for scientific discussion.
References
- 1.Lin YH, Machner MP. Exploitation of the host cell ubiquitin machinery by microbial effector proteins. J Cell Sci. 2017 Jun;130((12)):1985–96. doi: 10.1242/jcs.188482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malynn BA, Ma A. Ubiquitin makes its mark on immune regulation. Immunity. 2010 Dec;33((6)):843–52. doi: 10.1016/j.immuni.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaid S, Brandts CH, Serve H, Dikic I. Ubiquitination and selective autophagy. Cell Death Differ. 2013 Jan;20((1)):21–30. doi: 10.1038/cdd.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hafner-Bratkovič I, Pelegrín P. Ion homeostasis and ion channels in NLRP3 inflammasome activation and regulation. Curr Opin Immunol. 2018 Jun;52:8–17. doi: 10.1016/j.coi.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015 Oct;526((7575)):660–5. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 6.Shi CS, Shenderov K, Huang NN, Kabat J, Abu-Asab M, Fitzgerald KA, et al. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol. 2012 Jan;13((3)):255–63. doi: 10.1038/ni.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ainscough JS, Frank Gerberick G, Zahedi-Nejad M, Lopez-Castejón G, Brough D, Kimber I, et al. Dendritic cell IL-1α and IL-1β are polyubiquitinated and degraded by the proteasome. J Biol Chem. 2014 Dec;289((51)):35582–92. doi: 10.1074/jbc.M114.595686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodgers MA, Bowman JW, Fujita H, Orazio N, Shi M, Liang Q, et al. The linear ubiquitin assembly complex (LUBAC) is essential for NLRP3 inflammasome activation. J Exp Med. 2014 Jun;211((7)):1333–47. doi: 10.1084/jem.20132486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duong BH, Onizawa M, Oses-Prieto JA, Advincula R, Burlingame A, Malynn BA, et al. A20 restricts ubiquitination of pro-interleukin-1β protein complexes and suppresses NLRP3 inflammasome activity. Immunity. 2015 Jan;42((1)):55–67. doi: 10.1016/j.immuni.2014.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eldridge MJ, Sanchez-Garrido J, Hoben GF, Goddard PJ, Shenoy AR. The Atypical Ubiquitin E2 Conjugase UBE2L3 Is an Indirect Caspase-1 Target and Controls IL-1β Secretion by Inflammasomes. Cell Reports. 2017 Jan;18((5)):1285–97. doi: 10.1016/j.celrep.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JY, Seo D, You J, Chung S, Park JS, Lee JH, et al. The deubiquitinating enzyme, ubiquitin-specific peptidase 50, regulates inflammasome activation by targeting the ASC adaptor protein. FEBS Lett. 2017 Feb;591((3)):479–90. doi: 10.1002/1873-3468.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005 Nov;5((11)):685–94. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 13.Barnett TC, Cole JN, Rivera-Hernandez T, Henningham A, Paton JC, Nizet V, et al. Streptococcal toxins: role in pathogenesis and disease. Cell Microbiol. 2015 Dec;17((12)):1721–41. doi: 10.1111/cmi.12531. [DOI] [PubMed] [Google Scholar]
- 14.Tweten RK. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect Immun. 2005 Oct;73((10)):6199–209. doi: 10.1128/IAI.73.10.6199-6209.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldmann O, Sastalla I, Wos-Oxley M, Rohde M, Medina E. Streptococcus pyogenes induces oncosis in macrophages through the activation of an inflammatory programmed cell death pathway. Cell Microbiol. 2009 Jan;11((1)):138–55. doi: 10.1111/j.1462-5822.2008.01245.x. [DOI] [PubMed] [Google Scholar]
- 16.Timmer AM, Timmer JC, Pence MA, Hsu LC, Ghochani M, Frey TG, et al. Streptolysin O promotes group A Streptococcus immune evasion by accelerated macrophage apoptosis. J Biol Chem. 2009 Jan;284((2)):862–71. doi: 10.1074/jbc.M804632200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bastiat-Sempe B, Love JF, Lomayesva N, Wessels MR. Streptolysin O and NAD-glycohydrolase prevent phagolysosome acidification and promote group A Streptococcus survival in macrophages. MBio. 2014 Sep;5((5)):e01690–14. doi: 10.1128/mBio.01690-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Seaghdha M, Wessels MR. Streptolysin O and its co-toxin NAD-glycohydrolase protect group A Streptococcus from Xenophagic killing. PLoS Pathog. 2013;9((6)):e1003394. doi: 10.1371/journal.ppat.1003394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harder J, Franchi L, Muñoz-Planillo R, Park JH, Reimer T, Núñez G. Activation of the Nlrp3 inflammasome by Streptococcus pyogenes requires streptolysin O and NF-kappa B activation but proceeds independently of TLR signaling and P2X7 receptor. J Immunol. 2009 Nov;183((9)):5823–9. doi: 10.4049/jimmunol.0900444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hancz D, Westerlund E, Bastiat-Sempe B, Sharma O, Valfridsson C, Meyer L, et al. Inhibition of Inflammasome-Dependent Interleukin 1β Production by Streptococcal NAD+-Glycohydrolase: Evidence for Extracellular Activity. MBio. 2017 Jul;8((4)):e00756–17. doi: 10.1128/mBio.00756-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu LC, Enzler T, Seita J, Timmer AM, Lee CY, Lai TY, et al. IL-1β-driven neutrophilia preserves antibacterial defense in the absence of the kinase IKKβ. Nat Immunol. 2011 Feb;12((2)):144–50. doi: 10.1038/ni.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chella Krishnan K, Mukundan S, Alagarsamy J, Hur J, Nookala S, Siemens N, et al. Genetic Architecture of Group A Streptococcal Necrotizing Soft Tissue Infections in the Mouse. PLoS Pathog. 2016 Jul;12((7)):e1005732. doi: 10.1371/journal.ppat.1005732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castiglia V, Piersigilli A, Ebner F, Janos M, Goldmann O, Damböck U, et al. Type I Interferon Signaling Prevents IL-1β-Driven Lethal Systemic Hyperinflammation during Invasive Bacterial Infection of Soft Tissue. Cell Host Microbe. 2016 Mar;19((3)):375–87. doi: 10.1016/j.chom.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Flaherty RA, Donahue DL, Carothers KE, Ross JN, Ploplis VA, Castellino FJ, et al. Neutralization of Streptolysin S-Dependent and Independent Inflammatory Cytokine IL-1β Activity Reduces Pathology During Early Group A Streptococcal Skin Infection. Front Cell Infect Microbiol. 2018 Jul;8:211. doi: 10.3389/fcimb.2018.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LaRock CN, Todd J, LaRock DL, Olson J, O'Donoghue AJ, Robertson AAB, et al. IL-1β is an innate immune sensor of microbial proteolysis. Science Immunology. 2016 Aug 19;1:eaah3539–eaah3539. doi: 10.1126/sciimmunol.aah3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gryllos I, Tran-Winkler HJ, Cheng MF, Chung H, Bolcome R, 3rd, Lu W, et al. Induction of group A Streptococcus virulence by a human antimicrobial peptide. Proc Natl Acad Sci USA. 2008 Oct;105((43)):16755–60. doi: 10.1073/pnas.0803815105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, et al. Production of mice deficient in genes for interleukin (IL)-1alpha, IL-1beta, IL-1alpha/beta, and IL-1 receptor antagonist shows that IL-1beta is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med. 1998 May;187((9)):1463–75. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995 Feb;80((3)):401–11. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 29.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004 Jul;430((6996)):213–8. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 30.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006 Mar;440((7081)):228–32. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 31.Newton K, Sun X, Dixit VM. Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol. 2004 Feb;24((4)):1464–9. doi: 10.1128/MCB.24.4.1464-1469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci. 2014 Jun;1319((1)):82–95. doi: 10.1111/nyas.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimura Y, Tanaka K. Regulatory mechanisms involved in the control of ubiquitin homeostasis. J Biochem. 2010 Jun;147((6)):793–8. doi: 10.1093/jb/mvq044. [DOI] [PubMed] [Google Scholar]
- 34.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27((1)):229–65. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 35.Keyel PA, Roth R, Yokoyama WM, Heuser JE, Salter RD. Reduction of streptolysin O (SLO) pore-forming activity enhances inflammasome activation. Toxins (Basel) 2013 Jun;5((6)):1105–18. doi: 10.3390/toxins5061105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meixenberger K, Pache F, Eitel J, Schmeck B, Hippenstiel S, Slevogt H, et al. Listeria monocytogenes-infected human peripheral blood mononuclear cells produce IL-1beta, depending on listeriolysin O and NLRP3. J Immunol. 2010 Jan;184((2)):922–30. doi: 10.4049/jimmunol.0901346. [DOI] [PubMed] [Google Scholar]
- 37.Harvey RM, Hughes CE, Paton AW, Trappetti C, Tweten RK, Paton JC. The impact of pneumolysin on the macrophage response to Streptococcus pneumoniae is strain-dependent. PLoS One. 2014 Aug;9((8)):e103625. doi: 10.1371/journal.pone.0103625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magassa N, Chandrasekaran S, Caparon MG. Streptococcus pyogenes cytolysin-mediated translocation does not require pore formation by streptolysin O. EMBO Rep. 2010 May;11((5)):400–5. doi: 10.1038/embor.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, Núñez G. K⁺ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013 Jun;38((6)):1142–53. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coornaert B, Carpentier I, Beyaert R. A20: central gatekeeper in inflammation and immunity. J Biol Chem. 2009 Mar;284((13)):8217–21. doi: 10.1074/jbc.R800032200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris J, Hartman M, Roche C, Zeng SG, O'Shea A, Sharp FA, et al. Autophagy controls IL-1beta secretion by targeting pro-IL-1beta for degradation. J Biol Chem. 2011 Mar;286((11)):9587–97. doi: 10.1074/jbc.M110.202911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nasser W, Beres SB, Olsen RJ, Dean MA, Rice KA, Long SW, et al. Evolutionary pathway to increased virulence and epidemic group A Streptococcus disease derived from 3,615 genome sequences. Proc Natl Acad Sci USA. 2014 Apr;111((17)):E1768–76. doi: 10.1073/pnas.1403138111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu L, Olsen RJ, Nasser W, de la Riva Morales I, Musser JM. Trading Capsule for Increased Cytotoxin Production: Contribution to Virulence of a Newly Emerged Clade of emm89 Streptococcus pyogenes. MBio. 2015 Oct;6((5)):e01378–15. doi: 10.1128/mBio.01378-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sumby P, Porcella SF, Madrigal AG, Barbian KD, Virtaneva K, Ricklefs SM, et al. Evolutionary origin and emergence of a highly successful clone of serotype M1 group a Streptococcus involved multiple horizontal gene transfer events. J Infect Dis. 2005 Sep;192((5)):771–82. doi: 10.1086/432514. [DOI] [PubMed] [Google Scholar]
- 45.Zhu L, Olsen RJ, Lee JD, Porter AR, DeLeo FR, Musser JM. Contribution of Secreted NADase and Streptolysin O to the Pathogenesis of Epidemic Serotype M1 Streptococcus pyogenes Infections. Am J Pathol. 2016 Dec;••• doi: 10.1016/j.ajpath.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madden JC, Ruiz N, Caparon M. Cytolysin-mediated translocation (CMT): a functional equivalent of type III secretion in gram-positive bacteria. Cell. 2001 Jan;104((1)):143–52. doi: 10.1016/s0092-8674(01)00198-2. [DOI] [PubMed] [Google Scholar]
- 47.Ghosh J, Caparon MG. Specificity of Streptococcus pyogenes NAD(+) glycohydrolase in cytolysin-mediated translocation. Mol Microbiol. 2006 Nov;62((4)):1203–14. doi: 10.1111/j.1365-2958.2006.05430.x. [DOI] [PubMed] [Google Scholar]
- 48.Velarde JJ, O'Seaghdha M, Baddal B, Bastiat-Sempe B, Wessels MR. Binding of NAD+-Glycohydrolase to Streptolysin O Stabilizes Both Toxins and Promotes Virulence of Group A Streptococcus. MBio. 2017 Sep;8((5)):e01382–17. doi: 10.1128/mBio.01382-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shao W, Yeretssian G, Doiron K, Hussain SN, Saleh M. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J Biol Chem. 2007 Dec;282((50)):36321–9. doi: 10.1074/jbc.M708182200. [DOI] [PubMed] [Google Scholar]
- 50.Sokolovska A, Becker CE, Ip WK, Rathinam VA, Brudner M, Paquette N, et al. Activation of caspase-1 by the NLRP3 inflammasome regulates the NADPH oxidase NOX2 to control phagosome function. Nat Immunol. 2013 Jun;14((6)):543–53. doi: 10.1038/ni.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bruchard M, Rebé C, Derangère V, Togbé D, Ryffel B, Boidot R, et al. The receptor NLRP3 is a transcriptional regulator of TH2 differentiation. Nat Immunol. 2015 Aug;16((8)):859–70. doi: 10.1038/ni.3202. [DOI] [PubMed] [Google Scholar]
- 52.Swatek KN, Komander D. Ubiquitin modifications. Cell Res. 2016 Apr;26((4)):399–422. doi: 10.1038/cr.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niebler M, Qian X, Höfler D, Kogosov V, Kaewprag J, Kaufmann AM, et al. Post-translational control of IL-1β via the human papillomavirus type 16 E6 oncoprotein: a novel mechanism of innate immune escape mediated by the E3-ubiquitin ligase E6-AP and p53. PLoS Pathog. 2013;9((8)):e1003536. doi: 10.1371/journal.ppat.1003536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yen H, Sugimoto N, Tobe T. Enteropathogenic Escherichia coli Uses NleA to Inhibit NLRP3 Inflammasome Activation. PLoS Pathog. 2015 Sep;11((9)):e1005121. doi: 10.1371/journal.ppat.1005121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suzuki S, Mimuro H, Kim M, Ogawa M, Ashida H, Toyotome T, et al. Shigella IpaH7.8 E3 ubiquitin ligase targets glomulin and activates inflammasomes to demolish macrophages. Proc Natl Acad Sci USA. 2014 Oct;111((40)):E4254–63. doi: 10.1073/pnas.1324021111. [DOI] [PMC free article] [PubMed] [Google Scholar]