Abstract
A key question in evolutionary biology concerns the relative importance of different sources of adaptive genetic variation, such as de novo mutations, standing variation, and introgressive hybridization. A corollary question concerns how allelic variants derived from these different sources may influence the molecular basis of phenotypic adaptation. Here, we use a protein-engineering approach to examine the phenotypic effect of putatively adaptive hemoglobin (Hb) mutations in the high-altitude Tibetan wolf that were selectively introgressed into the Tibetan mastiff, a high-altitude dog breed that is renowned for its hypoxia tolerance. Experiments revealed that the introgressed coding variants confer an increased Hb–O2 affinity in conjunction with an enhanced Bohr effect. We also document that affinity-enhancing mutations in the β-globin gene of Tibetan wolf were originally derived via interparalog gene conversion from a tandemly linked β-globin pseudogene. Thus, affinity-enhancing mutations were introduced into the β-globin gene of Tibetan wolf via one form of intragenomic lateral transfer (ectopic gene conversion) and were subsequently introduced into the Tibetan mastiff genome via a second form of lateral transfer (introgression). Site-directed mutagenesis experiments revealed that the increased Hb–O2 affinity requires a specific two-site combination of amino acid replacements, suggesting that the molecular underpinnings of Hb adaptation in Tibetan mastiff (involving mutations that arose in a nonexpressed gene and which originally fixed in Tibetan wolf) may be qualitatively distinct from functionally similar changes in protein function that could have evolved via sequential fixation of de novo mutations during the breed’s relatively short duration of residency at high altitude.
Keywords: introgression, hemoglobin, hypoxia, high-altitude, Tibetan mastiff, Tibetan wolf
Introduction
A key question in evolutionary biology concerns the relative importance of different sources of adaptive genetic variation in natural populations. In addition to new mutations and standing variation, recent studies of natural animal populations have revealed numerous cases in which putative adaptations are attributable to allelic variants that were introduced via introgressive hybridization (Song et al. 2011; Heliconius Genome Consortium 2012; Pardo-Diaz et al. 2012; Hedrick 2013; Huerta-Sánchez et al. 2014; Natarajan, Projecto-Garcia, et al. 2015; Racimo et al. 2015; Enciso-Romero et al. 2017; Miao et al. 2017; vonHoldt et al. 2017; Jones et al. 2018). In contrast to de novo mutations and low-frequency variants at mutation-drift equilibrium, alleles derived from other species have already been pretested by selection, albeit on a different genetic background. For this reason, selectively introgressed alleles may be especially likely to comprise coadapted combinations of mutations (Hedrick 2013).
In cases where a given species has colonized a new environment, adaptation to newly encountered challenges may be facilitated and expedited by introgressive hybridization with a closely related resident species that has already evolved refined solutions to those same challenges. In this way, the recipient species capitalizes on adaptive solutions that evolved over a longer period of time in the donor species. Just such a scenario has been described in the case of the Tibetan mastiff, an ancient and phenotypically distinct dog breed that was originally bred as a flock guardian at high altitudes in the Himalayas and Tibetan Plateau (Messerschmidt 1983; Li et al. 2008). Tibetan mastiffs do extraordinarily well at high altitude compared with other dog breeds, and their exceptional hypoxia tolerance appears to stem in part from a legacy of introgressive hybridization with the Tibetan wolf (Canis lupus laniger) (Miao et al. 2017; vonHoldt et al. 2017), a high-altitude subspecies of the gray wolf that can be found at elevations >5,000-m above sea level (Sharma et al. 2004). Population genomic studies of both Tibetan mastiff and Tibetan wolf have identified specific genes that exhibit evidence of positive selection, and therefore represent candidate loci for high-altitude adaptation (Wang et al. 2013; Gou et al. 2014; Zhang et al. 2014; Fan et al. 2016). In Tibetan mastiffs, one of the strongest signatures of positive selection is observed for the chromosomal region spanning the β-globin gene cluster (Wang et al. 2013; Gou et al. 2014; Fan et al. 2016), a compact set of tandemly duplicated genes that encode the β-type subunits of hemoglobin (Hb), the red blood cell protein responsible for circulatory O2 transport.
Vertebrate Hb is a heterotetramer that consists of two α-chain and two β-chain subunits (α2β2). Genetically based changes in the oxygenation properties of Hb are known to play a key role in hypoxia adaptation of mammals and other vertebrates (Weber 2007; Storz, Scott, et al. 2010; Storz 2016, 2019). Under severe hypoxia, an increased Hb–O2 affinity can improve tissue O2 delivery if the benefit of safeguarding arterial O2 saturation more than offsets the cost associated with a diminished diffusion gradient for O2 unloading in the systemic circulation (Bencowitz et al. 1982; Willford et al. 1982; Storz 2016).
The adult Hb of Tibetan mastiffs is distinguished from that of all other domestic dog breeds by a pair of amino acid replacements at adjacent residue positions in the β-chain subunit (Gou et al. 2014). Due to evidence for a recent selective sweep in the chromosomal region spanning the adult-expressed β-globin gene, Gou et al. (2014) suggested that one or both of the mastiff-specific substitutions are responsible for an adaptive increase in Hb–O2 affinity. Subsequent genomic analyses revealed that both amino acid replacements—along with the entire β-globin gene cluster—were introgressed from the Tibetan wolf (Miao et al. 2017). Thus, the adult Hbs of Tibetan mastiff and Tibetan wolf are distinguished from those of all other dogs and wolves by the same pair of β-chain amino acid replacements.
The evidence for positive selection on the wolf-derived β-globin variants in Tibetan mastiff suggests that the introgression contributed to hypoxia adaptation at high altitude. However, like most purported examples of adaptive introgression, the phenotypic effects of the introgressed variants have not been experimentally tested (Racimo et al. 2015; Suarez-Gonzalez et al. 2018).
Results and Discussion
Structural Variation
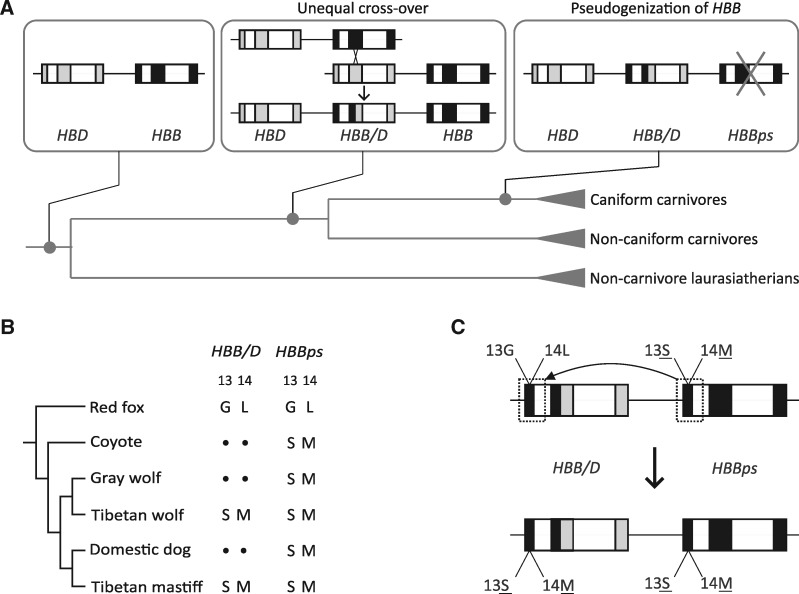
All canids share the same complement of adult Hb genes, including a tandemly linked pair of nearly identical α-type globin genes on Chromosome 6 and a set of triplicated β-type genes on Chromosome 21: δ-globin (HBD), β/δ-globin (HBB/D), and β-globin (HBB). The HBB/D gene is a chimeric fusion gene that originated via unequal crossing-over between the tandemly linked HBD and HBB genes (Gaudry et al. 2014) (fig. 1A). The recombination event that produced the chimeric HBB/D fusion gene occurred in the stem lineage of carnivores and is therefore shared by all extant species in the group (Gaudry et al. 2014). The nonchimeric HBB gene at the 3′ end of the cluster is orthologous to the adult β-globin gene of humans and other mammals (Hoffmann et al. 2008; Opazo et al. 2008a, 2008b, 2009; Janecka et al. 2015). However, in canids and other caniform carnivores (bears, pinnipeds, and members of the superfamily Musteloidea), HBB is a nonexpressed pseudogene (hereafter referred to as HBBps; fig. 1A) (Gaudry et al. 2014; Zaldívar-López et al. 2017). We generated sequence data which revealed that orthologs of the adult-expressed α- and β-type globin genes are almost completely invariant at the amino acid level in all domestic dogs and wolves. The only exceptions are the adult-expressed HBB/D genes of Tibetan mastiffs and Tibetan wolves, which are distinguished from those of all other dogs and wolves by two missense substitutions at adjacent sites: G13S and L14M (fig. 1B). In principle, the sharing of the derived Ser-β13 and Met-β14 amino acid states between Tibetan mastiffs and Tibetan wolves could be attributable to the parallel fixation of de novo mutations that arose independently in both lineages or the parallel fixation of pre-existing variants that were inherited from the common ancestor of wolves and domestic dogs. However, genomic analyses clearly demonstrate that HBB/D and the rest of the β-globin gene cluster of Tibetan mastiffs was derived via introgression from the Tibetan wolf (Miao et al. 2017).
Fig. 1.
Evolutionary changes in the β-globin gene cluster of canids. (A) The ancestor of laurasiatherian mammals (the supraordinal group that contains carnivores as well as artiodactyls, perissodactyls, and bats) possessed a tandemly linked pair of HBD and HBB genes. In the stem lineage of carnivores, an unequal cross-over event yielded a chimeric fusion gene (HBB/D) flanked by the parental HBD and HBB genes on the 5′ and 3′ sides, respectively (Gaudry et al. 2014). The HBB gene was subsequently inactivated in the common ancestor of caniform carnivores, so the β-chain subunit of adult Hb is exclusively encoded by HBB/D in all extant species (Gaudry et al. 2014; Zaldívar-López et al. 2017). (B) Amino acid states of residue positions 13 and 14 in the expressed HBB/D gene and the HBBps pseudogene in representative canids. (C) Inferred gene conversion event in Tibetan wolf whereby derived amino acid states at sites 13 and 14 were transferred from the HBBps pseudogene to the tandemly linked HBB/D gene.
Intriguingly, the derived amino acid states in HBB/D that are shared between Tibetan mastiff and Tibetan wolf are present in the tandemly linked HBBps pseudogene of all canids (fig. 1B). This suggests the hypothesis that the pair of missense substitutions in the HBB/D gene were originally derived via gene conversion from the paralogous HBBps pseudogene of Tibetan wolf. To test this hypothesis, we used bioinformatics methods to unravel the history of interparalog gene conversion across the canine β-globin gene cluster. In the β-globin gene clusters of Tibetan mastiff and Tibetan wolf, our results revealed that a recent HBBps → HBB/D conversion event has overwritten the entire 5′ end of the HBB/D coding sequence, extending from 65-bp upstream of the start codon to the middle of the first intron (fig. 1C and supplementary table S1, Supplementary Material online). Thus, the HBBps-derived conversion tract spans all of HBB/D exon 1, which includes the two missense mutations responsible for G13S and S14M.
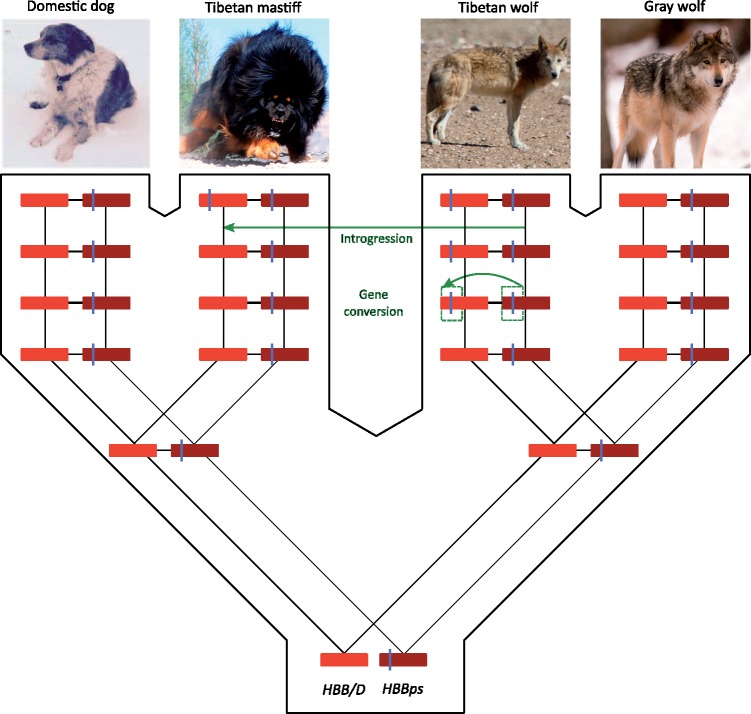
The conversion event that introduced the two missense mutations into the adult-expressed HBB/D gene occurred initially in the Tibetan wolf lineage (fig. 2). Thus, Ser-β13 and Met-β14 were introduced into the HBB/D gene of Tibetan wolf via one form of intragenomic lateral transfer (ectopic gene conversion from a paralogous pseudogene) and were subsequently introduced into the Tibetan mastiff genome via a second form of lateral transfer (introgression). In both Tibetan wolf and Tibetan mastiff, the paired variants were eventually fixed (and, at least in Tibetan mastiff, population genomic data suggest that the variants were fixed via positive directional selection) (Wang et al. 2013; Gou et al. 2014). However, the variants did not arise as de novo point mutations in the HBB/D genes of either the wolf or mastiff. Ancestral state reconstructions suggest that the canid-specific G13S and L14M substitutions in the HBBps gene occurred after the gene had become transcriptionally inactivated (fig. 3). The joint fixation of the Ser-13 and Met-14 variants in both Tibetan wolf and in Tibetan mastiff, in combination with the strong signatures of positive selection in Tibetan mastiff (Wang et al. 2013; Gou et al. 2014; Fan et al. 2016), suggests the hypothesis that the amino acid replacements are responsible for an adaptive increase in Hb–O2 affinity. To test this hypothesis, we first characterized the oxygenation properties of native canid Hbs to measure the net effect of the two substitutions, and we then conducted site-directed mutagenesis experiments involving recombinantly expressed Hbs to measure their independent and joint effects.
Fig. 2.
History of gene conversion between the HBBps pseudogene and the tandemly linked HBB/D gene in Tibetan wolves, and subsequent introgression of the converted HBB/D allele into Tibetan mastiffs. Hash marks denote the paired amino acid replacements, G13S and L14M, which occurred in the dog/wolf ancestor. Image “Tibetan wolf in Changthang” by Stanzin Namgail is licensed under CC BY-SA 4.0 and image “Gray Wolf Standing in the Snow” by Eric Kilby is licensed under CC BY-SA 2.0.
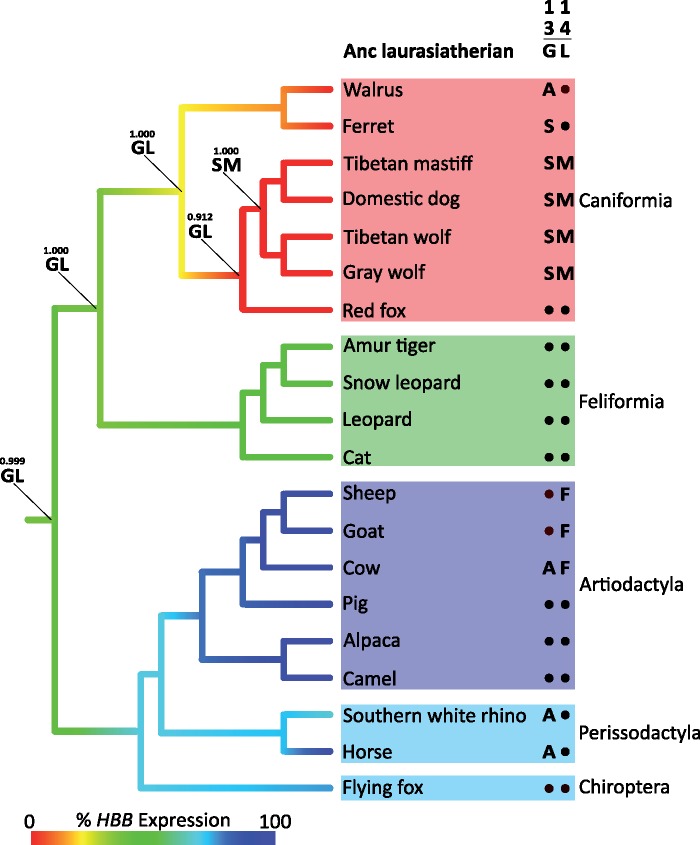
Fig. 3.
Ancestral state estimates reveal that the canid-specific G13S and L14M substitutions in HBB occurred after the gene was pseudogenized in the stem lineage of caniform carnivores. Branches are color coded according to inferred expression levels of Hb isoforms that incorporate the product of HBB relative to isoforms that incorporate the product of HBB/D. For all examined caniform carnivores, % expression of HBB is zero (supplementary table S5, Supplementary Material online). For extant species, amino acid states at β13–14 in the products of HBB/D and HBB are shown to the right of each branch tip, and maximum likelihood estimates of ancestral states are indicated for each internal node in the line of descent of dogs and wolves. Values above the ancestral amino acid estimates represent the posterior probabilities of each two-site combination.
Functional Properties of Dog and Wolf Hbs
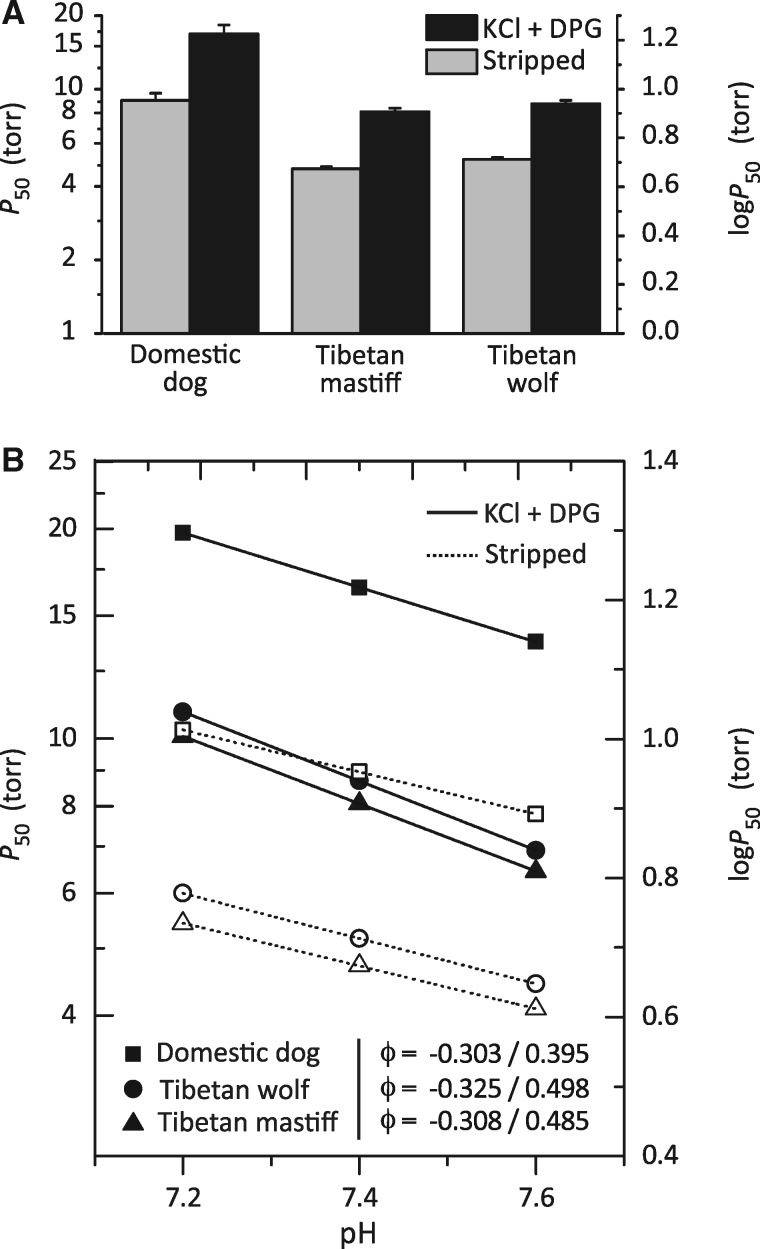
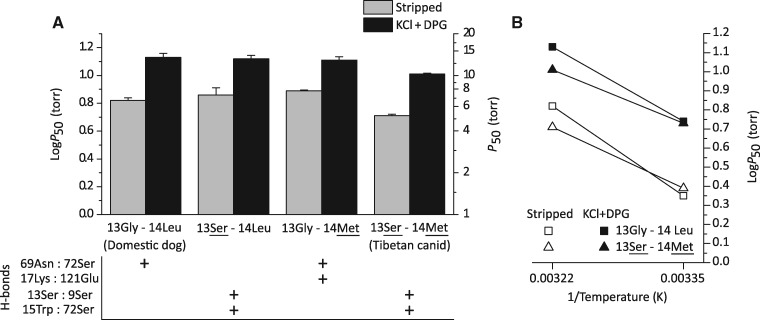
To test the net effects of the β-chain amino acid substitutions G13S and L14M, we measured the oxygenation properties of purified native Hbs from Tibetan wolf, Tibetan mastiff, and multiple breeds of domestic dog (which have Hbs that are structurally identical to that of gray wolf). In the absence of allosteric effectors (stripped), domestic dog Hb has an O2 affinity (measured as P50, the PO2 at which Hb is 50% saturated) of 8.96 ± 1.06 Torr. Under the same experimental conditions, O2 affinities of Tibetan wolf and Tibetan mastiff Hbs are significantly higher (i.e., P50’s are lower: 5.16 ± 1.05 and 4.72 ± 1.15 Torr, respectively) (fig. 4A and supplementary table S2, Supplementary Material online). In each of the examined canids, Hb–O2 affinity was reduced to a similar extent in the presence of Cl− ions and the organic phosphate 2,3-diphosphoglycerate (DPG), the two most important allosteric cofactors in mammalian red cells (fig. 4A and supplementary table S2, Supplementary Material online). These results indicate that the increased Hb–O2 affinity of Tibetan wolf and Tibetan mastiff is attributable to an increase in intrinsic O2 affinity, not to a suppression of sensitivity to allosteric cofactors.
Fig. 4.
Oxygenation properties of purified Hbs from Tibetan wolves, Tibetan mastiffs, and domestic dogs in the absence (“stripped”) and presence of allosteric cofactors (Cl− ions and DPG). (A) P50 values (±SE), the PO2 at 50% saturation, at 37 °C, pH 7.4. The lower the P50, the higher the O2 affinity. (B) Bohr effect of canid Hbs, as indicated by a plot of log-P50 versus pH in the presence and absence of anionic cofactors (filled and open symbols, respectively). Bohr coefficients (φ) are shown in the absence/presence of anionic effectors.
In the presence of both Cl− and DPG, domestic dog Hb has a significantly lower Bohr effect (i.e., a lower sensitivity to pH) than Tibetan mastiff (ANCOVA, F[1, 2] = 4107, P = 0.0002) and the Tibetan wolf (ANCOVA, F[1, 2] = 2646, P = 0.0004; fig. 4B and supplementary table S2, Supplementary Material online). The Bohr effect describes the reduction in Hb–O2 affinity caused by a decline in pH, which allows more complete O2 unloading to acidic tissues during exercise (Bohr et al. 1904). Since an increased Hb–O2 affinity can inhibit O2 unloading in the systemic circulation, the enhanced pH sensitivity of Tibetan canid Hbs should mitigate this potentially negative effect on tissue oxygenation by increasing O2 unloading to metabolizing cells.
Structural Modeling
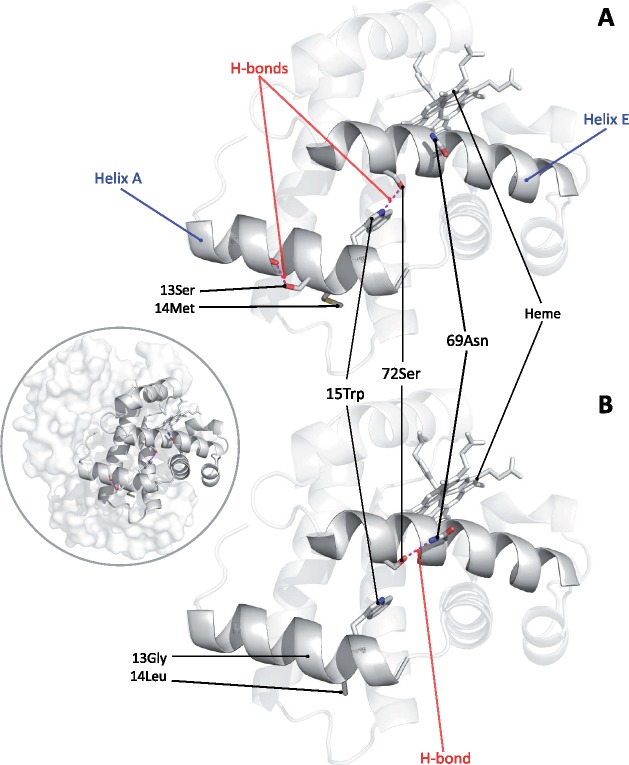
To investigate the structural mechanism responsible for the increased Hb–O2 affinity conferred by the G13S and L14M substitutions, we conducted a molecular modeling analysis using the crystal structure of domestic dog Hb (Bhatt et al. 2011). According to model predictions, the substitution L14M adds flexibility to the A-helix, due to the smaller side chain volume of Met relative to Leu (16.25 Å3 vs. 21.40 Å3). The added flexibility afforded by the L14M substitution creates a new hydrogen bond between Lys-17 and Glu-121 (supplementary fig. S1A, Supplementary Material online). However, this bond is eliminated by the G13S substitution, as reorientation of the A-helix allows Ser-13 to form a hydrogen bond with the main chain oxygen of Ser-9 (fig. 5A and supplementary fig. S1B, Supplementary Material online). The resultant reorientation of the A-helix allows the formation of a new hydrogen bond between Trp-15 in the A-helix and Ser-72 in the E-helix. This interhelical contact is not present in the normal Hb of domestic dog as Ser-72 is stabilized by an intrahelical hydrogen bond with Asn-69 (fig. 5B). In the four individual subunits of the Hb tetramer, the heme group is held in place by a coordination bond between the iron atom and the imidazole side chain of the “proximal histidine” (α87, β92) in the F-helix. On the opposite side of the heme plane, the iron–O2 bond is stabilized by a hydrogen bond with the imidazole side chain of the “distal histidine” (α58, β63) in the E-helix. In the deoxygenated state, the ferrous heme iron, Fe(II), is situated outside the heme plane, away from the imidazole side chain of the distal histidine (Perutz 1970). Upon transition to the oxygenated state, movement of the E- and F-helices shifts Fe(II) into the heme plane, thereby facilitating O2 binding. Consequently, substitutions that reorient the E- or F-helices may alter heme reactivity by shifting the distance of Fe(II) from the heme plane. Although G13S alone is sufficient to create the hydrogen bond between Trp-15 and Ser-72, L14M is required to produce the necessary reorientation of the E-helix, which is predicted to tighten up the connection between the A-, E-, and F-helices and shift Fe(II) closer to heme plane. Thus, the model-based prediction is that both G13S and L14M are required to produce the observed increase in O2 affinity, and that either mutation by itself would be insufficient.
Fig. 5.
Structural model of dog Hb showing the net effect of β-chain substitutions G13S and L14M. (A) β-Chain of Tibetan mastiff/Tibetan wolf Hb, showing that the two substitutions result in the addition of two intra- and inter-helical hydrogen bonds (9-Ser: 13Ser and 15-Trp: 72-Ser, respectively). (B) β-Chain of domestic dog/gray wolf Hb, showing that 72-Ser forms an intrahelical hydrogen bond with 69Asn instead of the interhelical bond with 15Trp. Inset: structural model of tetrameric dog Hb with the β-chain subunit highlighted.
The Hb tetramer undergoes an oxygenation-linked transition in quaternary structure, from the deoxy (tense- or T-state) to the oxy state (relaxed- or R-state). During this transition the α1β1 and α2β2 dimers slide and rotate in relation to one another, resulting in the breakage and formation of numerous intra- and inter-subunit bonds (Perutz 1970). The change in enthalpy caused by the breakage/formation of these bonds (ΔHT→R), combined with the change in enthalpy caused by the oxygenation-linked dissociation of allosterically bound cofactors, contributes to the overall enthalpy of Hb oxygenation (ΔH′; Atha and Ackers 1974; Weber and Campbell 2011). Thus, amino acid substitutions that affect the T → R transition are expected to change ΔH′. As the G13S and L14M substitutions in Tibetan canid Hbs tighten up the connection between the A-, E-, and F-helices, inducing a coordinated shift in quaternary structure that moves the Fe(II) closer to heme plane, the T-state is shifted towards a more R-like conformation. This is predicted to alter ΔHT→R of the protein in addition to increasing Hb–O2 affinity.
Functional Testing of Putatively Adaptive Mutations
To test predictions of our modeling analysis, we synthesized and experimentally tested four recombinant Hb (rHb) mutants representing each possible two-site combination of ancestral/derived amino acid states at β13 and β14. Consistent with model-based predictions, experimental measurements on the rHb mutants revealed that G13S and L14M only produce a significant increase in Hb–O2 affinity in combination (fig. 6A). Our model predicts that the joint effect of G13S and L14M (also revealed in the comparison between the native Hbs of domestic dog/gray wolf vs. Tibetan mastiff/Tibetan wolf) reduce the overall movement of the E-helix during the transition between deoxy- and oxygenated states, thereby altering ΔHT→R. To test this, we measured O2 equilibrium curves on domestic dog and Tibetan canid rHbs at two separate temperatures, 25 °C and 37 °C, to quantify the enthalpy and entropy of oxygenation (ΔH and ΔS, respectively). We then calculated the Gibbs free energy of oxygenation (ΔG) according to the relation ΔG = ΔH – TΔS. The Hbs of Tibetan mastiff and Tibetan wolf exhibit lower values of both ΔH and ΔS (estimated as the slope and Y-intercept of the van’t Hoff plot, respectively; fig. 6B and supplementary table S3, Supplementary Material online). The negative slopes displayed in figure 6B reflect the endothermic nature of heme deoxygenation (Roughton et al. 1936; Atha and Ackers 1974), which dictates a negative relationship between Hb–O2 affinity and temperature (Weber and Campbell 2011). As predicted by our modeling results, the difference in the enthalpy of oxygenation between the alternative rHb mutants (ΔΔH) indicates that the 13Ser-14Met genotype (characteristic of Tibetan canids) has a (numerically) lower ΔH relative to the 13Gly-14Leu genotype (characteristic of domestic dogs and gray wolves), in both the presence and absence of allosteric effectors. As differential binding of allosteric effectors can alter the overall ΔH (Weber and Campbell 2011), the observed difference in ΔH between the rHb mutants in the absence of allosteric effectors (fig. 6B and supplementary table S3, Supplementary Material online) suggests this difference is due to changes in ΔHT→R.
Fig. 6.
Functional effects of amino acid substitutions in Tibetan mastiff and Tibetan wolf Hb. (A) O2-affinities of rHbs representing the (ancestral) wildtype genotype of domestic dog/gray wolf (13Gly-14Leu), the derived, double-mutant genotype of Tibetan mastiff/Tibetan wolf (13Ser-14Met), and each of the possible single-mutant genotypes (13Ser-14Leu and 13Gly-14Met). P50 values (±SE) are shown in the absence (“stripped”) and presence of anionic effectors (“KCl + DPG”) at 37 °C. The lower the P50, the higher the O2 affinity. P50 values for the double mutant (13Ser-14Met) were significantly higher than those of the other three genotypes for both treatments (stripped and KCl + DPG). For each genotype, the panel below denotes the presence or absence of key hydrogen bonds in the β-chain subunit. (B) van’t Hoff plot of rHbs representing wildtype genotypes of domestic dog/gray wolf (13Gly-14Leu) and Tibetan mastiff/Tibetan wolf (13Ser-14Met) derived from measures of O2 affinity at 25 and 37 °C.
Adaptive Significance of Causative Amino Acid Substitutions
Numerous statistical approaches have been developed to detect introgression using genomic polymorphism data, and such analyses can be integrated with population genetic tests for evidence of positive selection or associations with phenotype (Racimo et al. 2015). However, conclusive evidence that introgressed alleles have contributed to adaptive phenotypic evolution in the recipient species requires experimental measurements of phenotypic effects. Specifically, experimental evidence is required to document that the introgressed allele increases fitness on the genetic background of the recipient species, or that it contributes to a change in phenotype in the direction that is predicted to be adaptive (Natarajan, Projecto-Garcia, et al. 2015; Suarez-Gonzalez et al. 2018). Our experimental results demonstrate that the derived amino acid changes in Tibetan mastiff and Tibetan wolf reduce Hb P50 (i.e., increase Hb–O2 affinity) by ∼50%, relative to the wildtype dog Hb. This change in Hb–O2 affinity is in the direction that is expected to enhance arterial O2 saturation under hypoxic conditions and is therefore consistent with population genetic evidence for positive selection in relation to altitude (Wang et al. 2013; Gou et al. 2014; Fan et al. 2016).
In addition to the genomic evidence for adaptive introgression in Tibetan mastiffs (Miao et al. 2017), our results indicate that the causative G13S and L14M mutations in the donor species, Tibetan wolf, initially arose and were fixed in a nonexpressed pseudogene (HBBps). Missense mutations introduced by gene conversion from paralogous pseudogenes have been documented to have seemingly neutral or deleterious phenotypic effects in other species (Storz, Runck, et al. 2010; Casola et al. 2012; Natarajan, Hoffmann, et al. 2015). The Hb mutations in Tibetan wolf Hb appear to represent a rare case in which such mutations have been favored by selection and have contributed to an adaptive change in phenotype.
The evidence for positive selection on the introgressed missense mutations in Tibetan mastiff (Wang et al. 2013; Gou et al. 2014; Fan et al. 2016) suggests that they conferred an adaptive benefit in the high-altitude environment shared by both Tibetan mastiff (a recent arrival) and Tibetan wolf (the long-term resident). The βG13S and βL14M mutations in HBBps must have been neutral when they first occurred in the common ancestor of canids because all available evidence indicates that this gene was a nonexpressed pseudogene, as it is not transcribed in any extant caniform carnivores (fig. 3) (Gaudry et al. 2014; Zaldívar-López et al. 2017). This represents a possible example of the Dykhuizen–Hartl effect (Kimura 1983) whereby an initially neutral mutation later becomes beneficial upon a change in the external environment and/or genetic background. In the case of the Tibetan wolf, the β13/14 mutations could have experienced two separate changes in fitness effects. With regard to changes in genetic background, the initially neutral β13/14 mutations were transferred from the HBBps pseudogene to the expressed HBB/D gene due to gene conversion, so the latent phenotypic effects of the mutations were suddenly manifest in the context of Hb–O2 transport. With regard to changes in the external environment, there are good reasons to expect that mutations that alter Hb–O2 affinity would have different physiological effects at low- and high-altitude because differences in the partial pressure of O2 (PO2) of inspired air require Hb to load and unload O2 at different blood PO2’s. Under normoxic conditions at sea level, an increased Hb–O2 affinity can be deleterious because it reduces the O2 diffusion gradient between capillary blood and the cells of metabolizing tissues, thereby compromising tissue oxygenation. However, under severe environmental hypoxia, an increased Hb–O2 affinity can help safeguard arterial O2 saturation at low PO2, thereby minimizing the reduction in O2 offloading by Hb for a given arterio-venous difference in PO2 (Turek, Kreuzer, and Ringnalda 1978; Turek, Kreuzer, Turek-Maischeider, et al. 1978; Bencowitz et al. 1982; Willford et al. 1982; Storz, Scott, et al. 2010; Storz 2016, 2019). Thus, an affinity-enhancing mutation that is deleterious at low altitude could have beneficial effects on tissue O2 delivery at extremely high altitudes, a trade-off that has been documented in other mammals (Eaton et al. 1974; Turek, Kreuzer, and Ringnalda 1978; Turek, Kreuzer, Turek-Maischeider, et al. 1978; Chappell and Snyder 1984). Due to elevational differences in optimal blood P50, affinity-altering Hb mutations can be expected to have different selection coefficients in highland dogs and wolves than in their lowland conspecifics.
Compared with de novo mutations and segregating allelic variants that contribute to adaptive phenotypic change, introgressed alleles that were previously fixed in a different species have been pretested by selection. For this reason, it has been suggested that selectively introgressed alleles may be especially likely to combine multiple mutations (e.g., epistatic modifiers) that interact to fine-tune a selected phenotypic effect and/or mitigate deleterious pleiotropic effects (Hedrick 2013). This conjecture has not been previously tested because it requires experimental measurements of the phenotypic effects of individual mutations. The epistatic interaction that we observed between the two β-globin mutations in Tibetan wolf and Tibetan mastiff indicates that neither mutation alone would have conferred an adaptive advantage at high altitude, as the increased Hb–O2 affinity required both changes in tandem. This suggests that the molecular underpinnings of the putative Hb adaptation in Tibetan mastiff (involving mutations that arose in a nonexpressed gene and which originally fixed in Tibetan wolf) may be qualitatively distinct from functionally similar changes in protein function that could have evolved via the sequential fixation of de novo mutations during the breed’s relatively short duration of residency at high altitude.
Materials and Methods
Genomic Data Collection
The β-globin gene cluster of the domestic dog was mined from genome build CanFam3.1 (chr21: 28,060,000–28,239,000). The annotated domestic dog β-globin gene cluster was used as a reference sequence for the assembly of Tibetan mastiff and gray wolf β-globin clusters using previously published SRA files (Tibetan mastiff: SRX445517, SRX445523, and SRX445529; gray wolf: SRX655650, SRX3424047, and SRX3424050). Reads were mapped to the reference sequence using Geneious 7.1.9 (Biomatters) under high stringency (maximum 5% mismatches and gaps allowed). Single nucleotide polymorphism tables built previously for the Tibetan wolf (Zhang et al. 2014) were used to verify single nucleotide polymorphisms in the coding sequences of β-type globin genes.
Tests of Gene Conversion
Discrete genomic regions containing adult-type β-globin genes (HBD, HBB/D, and HBBps; from the 5′ mRNA cap to the 3′ poly-A signal) were aligned using MUSCLE (Edgar 2004) and tests of gene conversion were performed using GENECONV (Sawyer 1989) under default parameters.
Ancestral State Reconstruction
Ancestral amino acid states were estimated from an alignment of HBB orthologs from 34 mammalian species (supplementary table S4, Supplementary Material online). Using an input phylogeny based on results of previous studies (KoepfLi et al. 2008; Li et al. 2008; Meredith et al. 2011; Fan et al. 2016), reconstructed sequences were estimated using baseml, as implemented in the software package PAML 4.7 (Yang 2007). Reconstructed sequences were estimated under the GTR + G substitution model, which was determined by MODELTEST (Posada and Crandall 1998) to provide the best fit to the data. The same phylogeny, along with experimental measures of Hb isoform expression (supplementary table S5, Supplementary Material online), was also used to estimate ancestral HBB expression using the Phytools package in R (Revell 2012).
Sample Isolation and cDNA Sequencing
We collected blood from Tibetan wolves at an altitude of 4,300 m in the KeKe XiLi area of the Qinghai-Tibetan Plateau, and from Tibetan mastiffs and domestic dogs at an altitude of 2,300 m in Xining, China. Animals were handled and blood samples were collected in accordance with regulations of the Animal Experimental and Medical Ethics Committee of the Qinghai University Medical College, Qinghai University. RNA was extracted from ∼200 μl of flash frozen whole blood from domestic dogs (n = 2), Tibetan mastiffs (n = 3), and Tibetan wolves (n = 2) using an RNeasy Universal Plus Mini Kit (Qiagen). cDNA was synthesized from freshly prepared RNA using Superscript IV Reverse transcriptase (Invitrogen). Gene specific primers were used to amplify the α- and β-type globin transcripts. Polymerase chain reactions were conducted using 1 μl of cDNA template in 0.2-ml tubes containing 25 µl of reaction mixture (0.5 µl of each dNTP [2.5 mM], 2.5 µl of 10× Reaction Buffer [Invitrogen], 0.75 µl of 50-mM MgCl2, 1.25 µl of each primer [10 pmol/µl], 1 µl of Taq polymerase [Invitrogen], and 16.75 µl of ddH2O), using an Eppendorf Mastercycler Gradient thermocycler. Following a 5-min denaturation period at 94 °C, the desired products were amplified using cycling profile of 94 °C for 30 s, 48–53 °C for 30 s, and 72 °C for 60 s for 30 cycles followed by a final extension period of 5 min at 72 °C. Amplified products were run on a 1.5% agarose gel where bands of the correct size were excised and purified using Zymoclean Gel DNA recovery columns (Zymo Research) and sequenced by Sanger sequencing with gene specific primers.
Functional Analyses of Hbs
Blood samples (∼200 μl) were added to a 5× volume of ice cold water and incubated on ice for 30 min to lyse the red blood cells. Samples were centrifuged at 20,000 × g for 10 min to remove cell debris. Buffer was added to the supernatants to a final concentration of 0.01-M HEPES/0.2-M NaCl (pH 7.4) and passed through a PD-10 desalting column (GE Healthcare) equilibrated with 25 ml of 0.01-M HEPES (pH 7.4). Hb proteins eluted from the PD-10 column were concentrated using Amicon Ultra-4 Centrifugal Filter Units (Millipore). O2–equilibrium curves for Hb solutions (0.1-mM Hb in 0.1-M HEPES/0.05-M ethylenediaminetetraacetic acid buffer) were measured at 37 °C using a Blood Oxygen Binding System (Loligo Systems). O2–equilibrium curves were measured in the absence (stripped) and presence of chloride ions (0.1-M KCl) and organic phosphates (0.2-mM DPG). Each Hb solution was sequentially equilibrated with three to five different oxygen tensions (PO2) at saturation levels between 30% and 70% while the absorbance was continually monitored at 430 nm (deoxy peak) and 421 nm (oxy/deoxy isosbestic point). Hill plots (log[fractional saturation/[1 − fractional saturation]] vs. logPO2) constructed from these measurements were used to determine the PO2 at half saturation (P50) and the cooperativity coefficient (n50) from the χ-intercept and slope of these plots, respectively. P50 values were measured at three different pH levels, where the pH of working solutions were adjusted with NaOH to as near 7.2, 7.4, or 7.6 as possible, then precisely measured with a pH-1 Micro pH meter and a needle-type pH microsensor (PreSens Precision Sensing GmbH). Individual measurements were pooled according to species/breed and a linear regression was fit to plots of log P50 versus pH. The resulting equation was used to estimate P50 values at pH 7.40 (±SE). O2–equilibrium curves were measured for rHbs as described above, but at both 37 and 25 °C. Van’t Hoff plots were created from these measurements (log P50 vs. 1/temperature) and a linear regression was fit to each plot. The Gibbs free energy of oxygenation (ΔG) was calculated from these regressions according to the relation ΔG = ΔH – TΔS, where ΔH (enthalpy of oxygenation) is derived from the slope of the regression, multiplied by –R (the universal gas constant), and ΔS (entropy of oxygenation) is derived from the Y-intercept, multiplied by R.
Structural Modeling
Modeling of canid Hbs was performed using Modeller 9.19 (Webb and Sali 2014). Oxy and deoxy structures of human Hb (PDB ID, 1hho and 2hhb, respectively) were used as templates. The root-mean-square deviations between the template and model for domestic dog and Tibetan wolf Hbs were 0.12 and 0.15 Å in both oxy and deoxy formats. Graphics were prepared using the PyMOL Molecular Graphics System, Version v1.7.6.3 (Schrödinger, LLC, New York City, NY). Hydrogen bonds and subunit contacts were estimated using Hydrogen Bond Calculation at Center for Informational Biology, Ochanomizu University, Japan (http://cib.cf.ocha.ac.jp/bitool/HBOND/; last accessed April 26, 2019) and by “Proteins, interfaces, structures and assemblies” service PISA at the European Bioinformatics Institute. (http://www.ebi.ac.uk/pdbe/prot_int/pistart.html; last accessed April 26, 2019), respectively.
Vector Construction and Site-Directed Mutagenesis
The canid globin sequences were synthesized by Invitrogen GeneArt Gene Synthesis (Carlsbad, CA) after optimizing the nucleotide sequences in accordance with Escherichia coli codon preferences. The synthesized globin gene cassette was cloned into a custom pGM vector system along with the methionine aminopeptidase (MAP) gene, as described previously (Natarajan et al. 2011, 2013). We engineered each of the β-chain codon substitutions by whole plasmid amplification using mutagenic primers and Phusion High-Fidelity DNA Polymerase (New England BioLabs, Ipswitch, MA), phosphorylation with T4 Polynucleotide Kinase (New England BioLabs), and circularization with an NEB Quick Ligation Kit (New England BioLabs). Each engineered codon change was verified by DNA sequencing.
Expression and Purification of rHbs
rHb expression was carried out in the Escherichia coli JM109 (DE3) strain as described previously (Natarajan et al. 2011, 2013). Bacterial cell lysates were then loaded onto a HiTrap Q HP anion exchange column (GE Healthcare) equilibrated with 20-mM Tris/0.5-mM ethylenediaminetetraacetic acid (pH 8.3) and eluted with a linear gradient of 0–0.25-M NaCl. Hb-containing fractions were then loaded on to a SP HP cation exchange column (GE Healthcare) and eluted with a linear pH gradient (pH 6.8–8.4). Eluted Hb factions were concentrated using Amicon Ultra-4 Centrifugal Filter Units (Millipore) and oxygenation properties were measured as described above.
Supplementary Material
Supplementary data are available at Molecular Biology and Evolution online.
Supplementary Material
Acknowledgments
We thank K. Campbell, J. Hite, S. Mohammadi, O. Matoo, C. Natarajan, and two anonymous reviewers for constructive comments on the manuscript and M. Tift for technical assistance. This work was supported by grants from the National Institutes of Health (Grant No. HL087216 to J.F.S.), the National Science Foundation (Grant Nos. MCB-1517636 and RII Track-2 FEC-1736249 to J.F.S.), the National Basic Research Program of China (Grant No. 2012CB518200 to R.-L.G.), the Program of International S&T Cooperation of China (Grant No. 0S2012GR0195 to R.-L.G.), and the National Natural Science Foundation of China (Grant No. 31571231 to R.-L.G.).
Sequence data generated in this study are deposited at GenBank accession numbers MK720822--MK720826
References
- Atha DH, Ackers GK.. 1974. Calorimetric determination of the heat of oxygenation of human hemoglobin as a function of pH and the extent of reaction. Biochemistry 1311:2376–2382. [DOI] [PubMed] [Google Scholar]
- Bencowitz HZ, Wagner PD, West JB.. 1982. Effect of change in P50 on exercise tolerance at high altitude: a theoretical study. J Appl Physiol Respir Environ Exerc Physiol. 536:1487–1495. [DOI] [PubMed] [Google Scholar]
- Bhatt VS, Zaldívar-López S, Harris DR, Couto CG, Wang PG, Palmer AF.. 2011. Structure of greyhound hemoglobin: origin of high oxygen affinity. Acta Crystallogr D Biol Crystallogr. 67(Pt 5):395–402. [DOI] [PubMed] [Google Scholar]
- Bohr C, Hasselbalch K, Krogh A.. 1904. Ueber einen in biologischer Beziehung wichtigen Einfluss, den die Kohlensäurespannung des Blutes auf dessen Sauerstoffbindung übt1. Acta Physiol. 16:402–412. [Google Scholar]
- Casola C, Zekonyte U, Phillips AD, Cooper DN, Hahn MW.. 2012. Interlocus gene conversion events introduce deleterious mutations into at least 1% of human genes associated with inherited disease. Genome Res. 223:429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell MA, Snyder LR.. 1984. Biochemical and physiological correlates of deer mouse alpha-chain hemoglobin polymorphisms. Proc Natl Acad Sci U S A. 8117:5484–5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton JW, Skelton TD, Berger E.. 1974. Survival at extreme altitude: protective effect of increased hemoglobin-oxygen affinity. Science 1834126:743–744. [DOI] [PubMed] [Google Scholar]
- Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 325:1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enciso-Romero J, Pardo-Diaz C, Martin SH, Arias CF, Linares M, McMillan WO, Jiggins CD, Salazar C.. 2017. Evolution of novel mimicry rings facilitated by adaptive introgression in tropical butterflies. Mol Ecol. 2619:5160–5172. [DOI] [PubMed] [Google Scholar]
- Fan Z, Silva P, Gronau I, Wang S, Armero AS, Schweizer RM, Ramirez O, Pollinger J, Galaverni M, Ortega-Del Vecchyo D, et al. 2016. Worldwide patterns of genomic variation and admixture in gray wolves. Genome Res. 262:163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudry MJ, Storz JF, Butts GT, Campbell KL, Hoffmann FG.. 2014. Repeated evolution of chimeric fusion genes in the β-globin gene family of laurasiatherian mammals. Genome Biol Evol. 65:1219–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou X, Wang Z, Li N, Qiu F, Xu Z, Yan D, Yang S, Jia J, Kong X, Wei Z, et al. 2014. Whole-genome sequencing of six dog breeds from continuous altitudes reveals adaptation to high-altitude hypoxia. Genome Res. 248:1308–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick PW. 2013. Adaptive introgression in animals: examples and comparison to new mutation and standing variation as sources of adaptive variation. Mol Ecol. 2218:4606–4618. [DOI] [PubMed] [Google Scholar]
- Heliconius Genome Consortium. 2012. Butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature 487:94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann FG, Opazo JC, Storz JF.. 2008. New genes originated via multiple recombinational pathways in the beta-globin gene family of rodents. Mol Biol Evol. 2512:2589–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta-Sánchez E, Jin X, Asan, Bianba Z, Peter BM, Vinckenbosch N, Liang Y, Yi X, He M, Somel M, et al. 2014. Altitude adaptation in Tibetans caused by introgression of Denisovan-like DNA. Nature 512:194–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janecka JE, Nielsen SS, Andersen SD, Hoffmann FG, Weber RE, Anderson T, Storz JF, Fago A.. 2015. Genetically based low oxygen affinities of felid hemoglobins: lack of biochemical adaptation to high-altitude hypoxia in the snow leopard. J Exp Biol. 21815:2402–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MR, Mills LS, Alves PC, Callahan CM, Alves JM, Lafferty DJR, Jiggins FM, Jensen JD, Melo-Ferreira J, Good JM.. 2018. Adaptive introgression underlies polymorphic seasonal camouflage in snowshoe hares. Science 3606395:1355–1358. [DOI] [PubMed] [Google Scholar]
- Kimura M. 1983. The neutral theory of molecular evolution. Cambridge, UK:Cambridge University Press. [Google Scholar]
- Koepfli K-P, Deere KA, Slater GJ, Begg C, Begg K, Grassman L, Lucherini M, Veron G, Wayne RK.. 2008. Multigene phylogeny of the Mustelidae: resolving relationships, tempo and biogeographic history of a mammalian adaptive radiation. BMC Biol. 6:10.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Liu Z, Li Y, Zhao X, Dong L, Pan Z, Sun Y, Li N, Xu Y, Xie Z.. 2008. Origin and phylogenetic analysis of Tibetan Mastiff based on the mitochondrial DNA sequence. J Genet Genomics. 35:335–340. [DOI] [PubMed] [Google Scholar]
- Meredith RW, Janecka JE, Gatesy J, Ryder OA, Fisher CA, Teeling EC, Goodbla A, Eizirik E, Simao TLL, Stadler T, et al. 2011. Impacts of the Cretaceous terrestrial revolution and KPg extinction on mammal diversification. Science 3346055:521–524. [DOI] [PubMed] [Google Scholar]
- Messerschmidt DMR. 1983. The Tibetan Mastiff: canine sentinels of the range. Rangelands 5:172–174. [Google Scholar]
- Miao B, Wang Z, Li Y.. 2017. Genomic analysis reveals hypoxia adaptation in the Tibetan mastiff by introgression of the gray wolf from the Tibetan Plateau. Mol Biol Evol. 34:734–743. [DOI] [PubMed] [Google Scholar]
- Natarajan C, Hoffmann FG, Lanier HC, Wolf CJ, Cheviron ZA, Spangler ML, Weber RE, Fago A, Storz JF.. 2015. Intraspecific polymorphism, interspecific divergence, and the origins of function-altering mutations in deer mouse hemoglobin. Mol Biol Evol. 324:978–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C, Inoguchi N, Weber RE, Fago A, Moriyama H, Storz JF.. 2013. Epistasis among adaptive mutations in deer mouse hemoglobin. Science 3406138:1324–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C, Jiang X, Fago A, Weber RE, Moriyama H, Storz JF.. 2011. Expression and purification of recombinant hemoglobin in Escherichia coli. PLoS One 65:e20176.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C, Projecto-Garcia J, Moriyama H, Weber RE, Muñoz-Fuentes V, Green AJ, Kopuchian C, Tubaro PL, Alza L, Bulgarella M, et al. 2015. Convergent evolution of hemoglobin function in high-altitude Andean waterfowl involves limited parallelism at the molecular sequence level. PLoS Genet. 1112:e1005681.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo JC, Hoffmann FG, Storz JF.. 2008a. Differential loss of embryonic globin genes during the radiation of placental mammals. Proc Natl Acad Sci U S A. 10535:12950–12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo JC, Hoffmann FG, Storz JF.. 2008b. Genomic evidence for independent origins of β-like globin genes in monotremes and therian mammals. Proc Natl Acad Sci U S A. 1055:1590–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo JC, Sloan AM, Campbell KL, Storz JF.. 2009. Origin and ascendancy of a chimeric fusion gene: the β/δ-globin gene of paenungulate mammals. Mol Biol Evol. 267:1469–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo-Diaz C, Salazar C, Baxter SW, Merot C, Figueiredo-Ready W, Joron M, McMillan WO, Jiggins CD.. 2012. Adaptive introgression across species boundaries in Heliconius butterflies. PLoS Genet. 86:e1002752.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz MF. 1970. Stereochemistry of cooperative effects in haemoglobin. Nature 2285273:726–739. [DOI] [PubMed] [Google Scholar]
- Posada D, Crandall KA.. 1998. MODELTEST: testing the model of DNA substitution. Bioinformatics 149:817–818. [DOI] [PubMed] [Google Scholar]
- Racimo F, Sankararaman S, Nielsen R, Huerta-Sánchez E.. 2015. Evidence for archaic adaptive introgression in humans. Nat Rev Genet. 16:359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Met Ecol Evol. 32:217–223. [Google Scholar]
- Roughton FJ, Adair GS, Barcroft J, Goldschmidt G, Herkel W, Hill RM, Keys AB, Ray GB.. 1936. The thermochemistry of the oxygen-haemoglobin reaction: comparison of the heat as measured directly on purified haemoglobin with that calculated indirectly by the Van’t Hoff Isochore. Biochem J. 3011:2117–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer S. 1989. Statistical tests for detecting gene conversion. Mol Biol Evol. 65:526–538. [DOI] [PubMed] [Google Scholar]
- Sharma DK, Maldonado JE, Jhala YV, Fleischer RC.. 2004. Ancient wolf lineages in India. Proc Biol Sci. 271(Suppl 3):S1–S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Endepols S, Klemann N, Richter D, Matuschka F-R, Shih C-H, Nachman MW, Kohn MH.. 2011. Adaptive introgression of anticoagulant rodent poison resistance by hybridization between old world mice. Curr Biol. 2115:1296–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF. 2016. Hemoglobin-oxygen affinity in high-altitude vertebrates: is there evidence for an adaptive trend? J Exp Biol. 21920:3190–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF. 2019. Hemoglobin: insights into protein structure, function, and evolution. Oxford, UK:Oxford University Press. [Google Scholar]
- Storz JF, Runck AM, Moriyama H, Weber RE, Fago A.. 2010. Genetic differences in hemoglobin function between highland and lowland deer mice. J Exp Biol. 21315:2565–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Scott GR, Cheviron ZA.. 2010. Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. J Exp Biol. 21324:4125–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Gonzalez A, Lexer C, Cronk QCB.. 2018. Adaptive introgression: a plant perspective. Biol Lett. 14. 20170688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek Z, Kreuzer F, Ringnalda BE.. 1978. Blood gases at several levels of oxygenation in rats with a left-shifted blood oxygen dissociation curve. Pflugers Arch. 3761:7–13. [DOI] [PubMed] [Google Scholar]
- Turek Z, Kreuzer F, Turek-Maischeider M, Ringnalda BE.. 1978. Blood O2 content, cardiac output, and flow to organs at several levels of oxygenation in rats with a left-shifted blood oxygen dissociation curve. Pflugers Arch. 3763:201–207. [DOI] [PubMed] [Google Scholar]
- vonHoldt B, Fan Z, Ortega-Del Vecchyo D, Wayne RK.. 2017. EPAS1 variants in high altitude Tibetan wolves were selectively introgressed into highland dogs. PeerJ. 5:e3522.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G-D, Zhai W, Yang H-C, Fan R-X, Cao X, Zhong L, Wang L, Liu F, Wu H, Cheng L-G, et al. 2013. The genomics of selection in dogs and the parallel evolution between dogs and humans. Nat Commun. 4:1860.. [DOI] [PubMed] [Google Scholar]
- Webb B, Sali A.. 2014. Comparative protein structure modeling using MODELLER. Curr Protoc Bioinformatics 471:5.6.1–6.32. [DOI] [PubMed] [Google Scholar]
- Weber RE. 2007. High-altitude adaptations in vertebrate hemoglobins. Respir Physiol Neurobiol. 158(2-3):132–142. [DOI] [PubMed] [Google Scholar]
- Weber RE, Campbell KL.. 2011. Temperature dependence of haemoglobin-oxygen affinity in heterothermic vertebrates: mechanisms and biological significance. Acta Phys. 2023:549–562. [DOI] [PubMed] [Google Scholar]
- Willford DC, Hill EP, Moores WY.. 1982. Theoretical analysis of optimal P50. J Appl Physiol Respir Environ Exerc Physiol. 524:1043–1048. [DOI] [PubMed] [Google Scholar]
- Yang Z. 2007. PAML 4: Phylogenetic Analysis by Maximum Likelihood. Mol Biol Evol. 248:1586–1591. [DOI] [PubMed] [Google Scholar]
- Zaldívar-López S, Rowell JL, Fiala EM, Zapata I, Couto CG, Alvarez CE.. 2017. Comparative genomics of canine hemoglobin genes reveals primacy of beta subunit delta in adult carnivores. BMC Genomics. 181:141.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Fan Z, Han E, Hou R, Zhang L, Galaverni M, Huang J, Liu H, Silva P, Li P, et al. 2014. Hypoxia adaptations in the grey wolf (Canis lupus chanco) from Qinghai-Tibet Plateau. PLoS Genet. 107:e1004466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.