Abstract
Background:
The coexistence of chronic obstructive pulmonary disease (COPD) and obstructive sleep apnea (OSA) is termed overlap syndrome (OS). COPD and OSA both have increased risks of developing cardiovascular diseases. This study aimed to explore if patients with OS exhibited a higher prevalence of cardiovascular complications, and if patients with OS exhibited vascular endothelial dysfunction and abnormalities in the cellular immune function of T lymphocytes.
Methods:
Totally 25 patients with stable COPD (COPD group), 25 patients with OSA (OSA group), 25 patients with OS (OS group), and 20 healthy adults (control group) were enrolled between January 2017 and December 2017 from the Respiratory Department of Tianjin Medical University General Hospital. The clinical characteristics of the four groups were collected and the expression levels of soluble vascular cell adhesion molecule-1 (sVCAM-1), tumor necrosis factor-α (TNF-α), and T-lymphocyte subsets were detected. One-way analysis of variance, χ2 test and Pearson correlation were used to manage the data.
Results:
The prevalence of hypertension and coronary heart disease was significantly higher in the OS group than in the control, OSA, and COPD groups (χ2 = 20.69, P < 0.05 and χ2 = 11.03, P < 0.05, respectively). The levels of sVCAM-1 and TNF-α were significantly higher in the OS group than in other groups (F = 127.40, P < 0.05 and F = 846.77, P < 0.05, respectively). The percentage of CD4+ lymphocytes and CD4+/CD8+ were both significantly lower in the OS group than in any other group (F = 25.40, P < 0.05 and F = 75.08, P < 0.05, respectively). There were significantly negative correlations in the levels of sVCAM-1 and TNF-α with CD4+/CD8+ lymphocytes (r = –0.77, P < 0.05 and r = –0.83, P < 0.05, respectively).
Conclusions:
The prevalence of hypertension and coronary heart disease was higher in patients with OS than in patients with either OSA or COPD alone. Patients with OS exhibited more severe vascular endothelial injury, stronger inflammatory response, and lower cellular immune function.
Keywords: Chronic obstructive pulmonary disease, Obstructive sleep apnea, Overlap syndrome, Endothelium, T-lymphocyte subsets
Introduction
Chronic obstructive pulmonary disease (COPD) and obstructive sleep apnea (OSA) are often observed in clinical practice. The coexistence of COPD and OSA is termed overlap syndrome (OS). The prevalence of OS in the general population has been reported to be 1.0% to 3.6%, whereas its prevalence among patients with OSA and COPD are relatively high at 7.6% to 55.7% and 2.9% to 65.9%, respectively.[1] Because of repeated episodes of hypoxia and re-oxygenation, OSA causes systemic inflammation, oxidative stress, endothelial dysfunction, autonomic dysfunction, and metabolic disorders. All of which have been considered important pathophysiological mechanisms leading to cardiovascular diseases in patients with OSA.[2,3] Similarly, patients with COPD may also have increased risks of developing cardiovascular diseases.[4] Studies have suggested that patients with OS have more cardiovascular complications, with a poorer quality of life and higher mortality rate, than do patients with either OSA or COPD alone.[5] Endothelial dysfunction has long been considered the pathophysiological mechanism underlying cardiovascular diseases.[6] Some studies have also indicated that T lymphocyte abnormalities play important roles in endothelial dysfunction. Hansson et al[7] found that the cytotoxicity of activated CD8+ T lymphocytes can enhance their killing potential toward vascular endothelial cells and thus contributes to the progression of atherosclerosis. So our study included OS patients as the study population to elucidate if patients with OS exhibited a higher prevalence of cardiovascular complications, vascular endothelial dysfunction, and abnormalities in the cellular immune function of T lymphocytes. The relationship between the cellular immune function of T lymphocytes and the vascular endothelial function was also investigated.
Methods
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Tianjin Medical University General Hospital (No. IRB2015-YX-008). Informed written consent was obtained from all patients prior to their enrollment in this study.
Study population
Between January 2017 and December 2017, 25 patients with each condition (stable COPD, OSA, and OS) diagnosed in the Respiratory Department of Tianjin Medical University General Hospital were assigned to the COPD group, OSA group, and OS group, respectively. In addition, 20 healthy volunteers were enrolled to the control group. For these healthy volunteers, pulmonary function test and polysomnography were performed to exclude COPD and OSA, respectively. Diagnostic criteria for COPD: presence of dyspnea, chronic cough or sputum production, a history of exposure to risk factors, and a post-bronchodilator forced expiratory volume in 1 s /forced vital capacity (FEV1/FVC) <70%. Other diseases that caused persistent airflow limitation were excluded. Diagnostic criteria for OSA: apnea-hypopnea index (AHI) ≥5/h. According to the disease severity of COPD and OSA, percentage of FEV1 in predicted value (FEV1% predicted) was in the range of 30% to 80%, and AHI ≥15/h in OS groups.
Measurement of soluble vascular cell adhesion molecule-1, tumor necrosis factor-α, and T-lymphocyte subsets
Five-milliliter peripheral blood was collected from all the enrolled patients, and 2 mL of the blood sample was placed at 4°C for 1 h and then centrifuged at 3000 × g for 15 min. The serum was stored at –80°C until analyzed. The concentrations of soluble vascular cell adhesion molecule-1 (sVCAM-1) and tumor necrosis factor-α (TNF-α) were detected using enzyme-linked immunosorbent assay kits (Invitrogen, Carlsbad, California, USA). The remaining 3 mL of the blood sample was used to detect T-lymphocyte subsets. They were analyzed using a flow cytometer (Beckman, Brea, California, USA).
Statistical analysis
SPSS 20.0 (SPSS Inc., Chicago, IL, USA) was used for data analyses. Measurement data were expressed as mean ± standard deviation, one-way analysis of variance (ANOVA) was used for intergroup comparisons, and the least significant difference test was used for comparisons between two groups. Enumeration data were expressed as the number of cases and were compared with χ2 test. Pearson correlation coefficient was used to measure the relationships between variables. P values < 0.05 were considered statistically significant.
Results
Characteristics of study subjects
There were no significant differences in sex, age, and smoking rate across the four groups (χ2 = 1.28, P > 0.05; F = 0.93, P > 0.05; and χ2 = 0.45, P > 0.05, respectively). The AHI was higher in the OS and OSA groups than in the control and COPD groups (F = 381.49, P < 0.05). FEV1% predicted and FEV1/FVC were lower in the OS and COPD groups than in the control and OSA groups (F = 209.94, P < 0.05 and F = 121.59, P < 0.05, respectively). The differences were statistically significant. The body mass index (BMI) of the OS, OSA, and COPD groups were significantly higher compared with the control group (F = 23.15, P < 0.05). The highest BMI was observed in the OSA group. There was no significant difference in BMI between the OS group and the COPD group. Examinations of hypertension, coronary heart disease (CHD), and stroke complications revealed that the number of hypertension and CHD cases were higher in the OS group than in the OSA and COPD groups (χ2 = 20.69, P < 0.05 and χ2 = 11.03, P < 0.05, respectively). The absolute number of stroke was the highest in the OS group, but the difference was not statistically significant when compared with the OSA and COPD groups (χ2 = 4.96, P > 0.05) [Table 1].
Table 1.
Clinical characteristics of the study subjects in four groups.
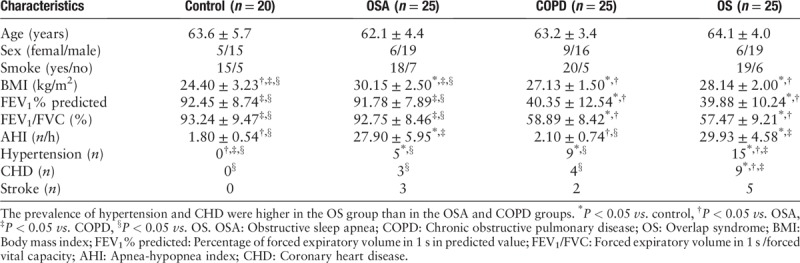
Expression levels of sVCAM-1 and TNF-α
There were significant differences in the levels of sVCAM-1 and TNF-α in the four groups, of which the levels in the OS group were significantly higher than the OSA and COPD groups (F = 127.40, P < 0.05 and F = 846.77, P < 0.05, respectively), as shown in Figure 1.
Figure 1.
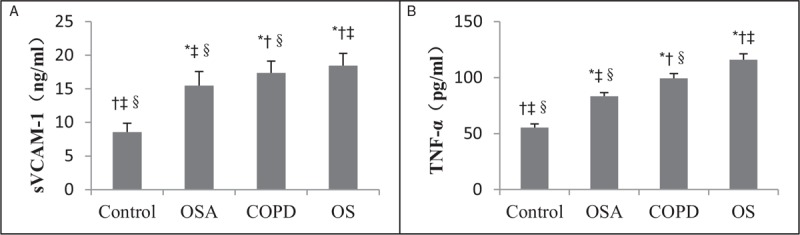
Concentrations of sVCAM-1 (A) and TNF-α (B) in control, OSA, COPD, and OS groups. The concentrations of sVCAM-1 and TNF-α in the OS group were significantly higher than the OSA and COPD groups. ∗P < 0.05 vs. control, †P < 0.05 vs. OSA, ‡P < 0.05 vs. COPD, §P < 0.05 vs. OS. COPD: Chronic obstructive pulmonary disease; OS: Overlap syndrome; OSA: Obstructive sleep apnea; sVCAM-1: Soluble vascular cell adhesion molecule-1; TNF-α: Tumor necrosis factor-α.
Analysis of T-lymphocyte subsets
There was no significant difference in the percentage of CD3+ lymphocytes (CD3+%) in the peripheral blood of the four groups (F = 0.27, P > 0.05). The percentage of CD4+ lymphocytes (CD4+%) and CD4+/CD8+ lymphocytes were both lower in the OS group than in the other three groups (F = 25.40, P < 0.05 and F = 75.08, P < 0.05, respectively), whereas the percentage of CD8+ lymphocytes (CD8+%) in the OS group was higher than that in the other three groups. The differences were statistically significant (F = 18.53, P < 0.05), as shown in Figure 2.
Figure 2.
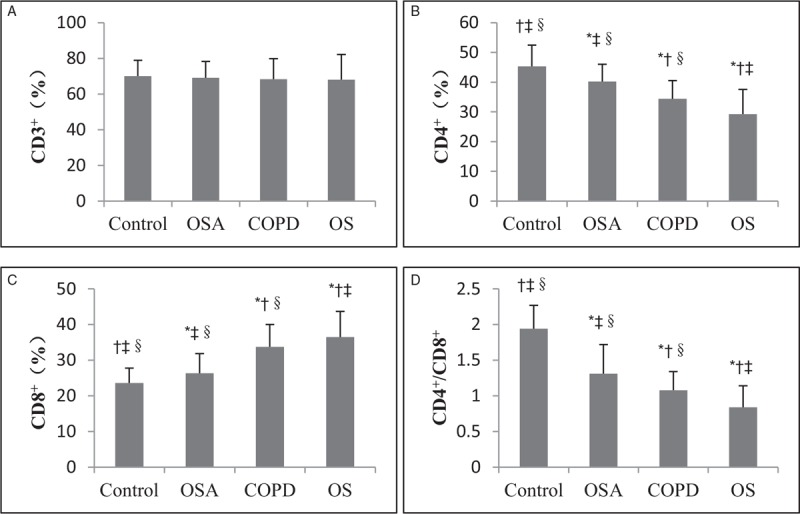
Analysis of T lymphocytes in control, OSA, COPD, and OS groups. (A) No significant difference in the percentage of CD3+ lymphocytes (CD3+%) in the peripheral blood among the four groups was found. (B–D) The percentage of CD4+ lymphocytes (CD4+%) and CD4+/CD8+ lymphocytes were both lower in the OS group than in the other three groups, whereas the percentage of CD8+ lymphocytes (CD8+%) in the OS group was higher than that in the other three groups. ∗P < 0.05 vs. control, †P < 0.05 vs. OSA, ‡P < 0.05 vs. COPD, §P < 0.05 vs. OS. COPD: Chronic obstructive pulmonary disease; OS: Overlap syndrome; OSA: Obstructive sleep apnea.
Correlations in sVCAM-1 and TNF-α with CD4+/CD8+ lymphocytes
There were significant negative correlations in the levels of sVCAM-1 and TNF-α with CD4+/CD8+ lymphocytes (r = –0.77, P < 0.05 and r = –0.83, P < 0.05, respectively), as shown in Figures 3 and 4.
Figure 3.
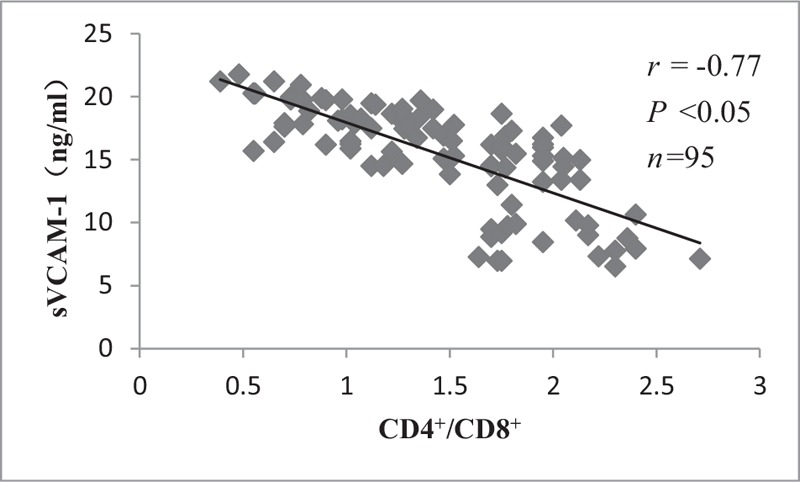
Correlation between CD4+/CD8+ lymphocytes and sVCAM-1. There was significant negative correlation between CD4+/CD8+ lymphocytes and sVCAM-1. sVCAM-1: Soluble vascular cell adhesion molecule-1.
Figure 4.
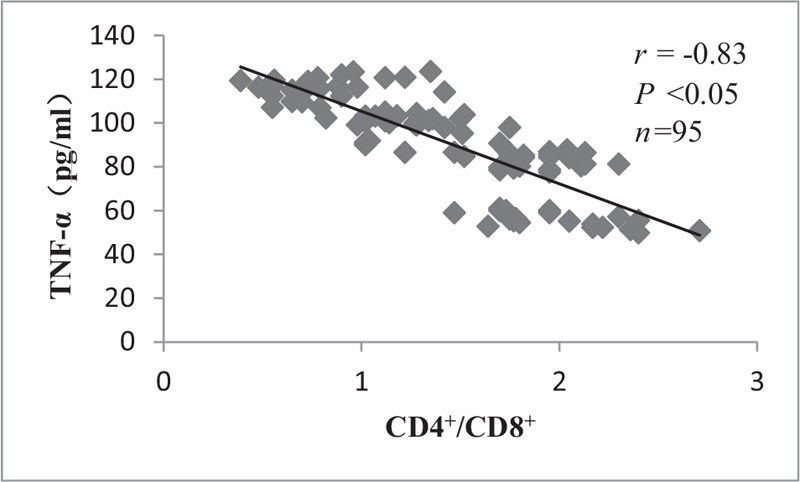
Correlation between CD4+/CD8+ lymphocytes and TNF-α. There was significant negative correlation between CD4+/CD8+ lymphocytes and TNF-α. TNF-α: Tumor necrosis factor-α.
Discussion
The coexistence of COPD and OSA is referred to as OS. Patients with either OSA or COPD are at an increased risk of cardiovascular complications. Previous studies have shown that the prevalence of hypertension, CHD, and diabetes is significantly higher in OS patients than in patients with either OSA or COPD alone.[8–10] However, there are few reports that evaluate the pathogenesis of the increased incidence of cardiovascular complications in OS. Studies have shown that any factor leading to vascular endothelial cell injury can cause endothelial dysfunction, which in turn triggers cardiovascular disease.[11] Vascular endothelial cells are a continuous layer of flat cells lining the inner layer of blood vessel walls. They form a natural biological barrier between the blood and endothelium that can help maintain blood flow balance and stabilize endothelial function. At the same time, they also secrete a variety of vasoactive factors that are involved in inflammation, cell migration, and blood vessel proliferation and remodeling in the vascular endothelium.[12] Vascular cell adhesion molecule-1 (VCAM-1) is expressed at a low level in normal endothelial cells. In response to certain biological triggers such as hypoxia, oxidative damage, and inflammation, particularly with inflammatory factors such as TNF-α, the expression of VCAM-1 and TNF-α can both drastically increase. The surface receptor of VCAM-1 can induce the adherence of lymphocytes and neutrophils to the blood vessel wall, which is believed to be an important part of the inflammatory response. sVCAM-1 is the soluble form of VCAM-1 and sVCAM-1 is believed to be a serum marker for assessing endothelial dysfunction or inflammatory reactions of endothelial cells.[13,14] Therefore, the extent of endothelial dysfunction can be assessed through detecting sVCAM-1. As an important chemokine secreted by neutrophils, TNF-α can promote the synthesis of inflammatory cells and the release of inflammatory mediators through activating neutrophils, macrophages, and lymphocytes.[15] Meanwhile, TNF-α can stimulate airway epithelial cells to increase the transcription of intercellular adhesion molecule 1 (ICAM-1) mRNA, suggesting that the systemic inflammatory response can up-regulate the expression of cell adhesion molecules, which may play an important role in endothelial dysfunction.[16] Peripheral blood T lymphocyte subsets can reflect the status of cellular immunity in patients. Specifically, CD4+ helper T cells secrete lymphokines to enhance and expand the immune response, while CD8+ helper T cells suppress B and T lymphocytes to maintain immune homeostasis. The ratio of CD4+/CD8+ T lymphocytes can directly reflect the abnormalities in T lymphocyte subsets in patients, which allows us to understand the state of cellular immunity to a certain extent.[17] Another important function of T lymphocytes is its ability to modify the microvascular environment. Under the influence of cytokines secreted by antigen-activated T cells or contact-dependent signals, T lymphocytes promote the adhesion of leukocytes to the endothelial cells in post-capillary veins, leading to morphological changes of the endothelial cells and vascular remodeling.[18] Therefore, sVCAM-1, TNF-α, and T-lymphocyte subsets were selected to be as observational indicators.
In our study, we enrolled four groups of age- and sex-matched patients with a similar number of cases per condition. Analyses of clinical characteristics showed that the differences in BMI were statistically significant among the four groups. The BMI of the OSA, COPD, and OS groups were higher than that of the control group, with the BMI of the OSA group being the highest. There was no significant difference in BMI between the COPD group and the OS group. Our study also confirmed that the prevalence of hypertension and CHD was higher in the OS studies. This result was similar to the previous study. The serum levels of sVCAM-1 are elevated in patients with COPD as well as in patients with OSA which have been demonstrated in previous researches.[19,20] Our study also confirmed that the sVCAM-1 levels in the COPD and OSA groups were higher compared with the control group. In addition, the level of sVCAM-1 was higher in the COPD group than in the OSA group. The highest sVCAM-1 level was observed in the OS group, which was significantly higher than the other three groups. These results suggested that patients with OS, COPD, and OSA all exhibited endothelial dysfunction, and the endothelial injury was the most severe in patients with OS. This observation was in line with the clinical characteristics, in which the incidence of hypertension and CHD was the highest in the OS group.
It has been shown that the expression of TNF-α is elevated in both COPD and OSA patients.[21,22] Elevated levels of TNF-α in serum and endothelial cells have also been demonstrated in animal models of COPD and OSA.[23] In our study, the changes of TNF-α were similar to that of sVCAM-1. There was a gradual increasing trend in the control, OSA, COPD, and OS groups, suggesting that a systemic inflammatory response existed in all patients with OSA, COPD, and OS. Compared with patients with OSA and COPD, patients with OS exhibited a stronger inflammatory response, which was consistent with the trends in sVCAM-1 level changes. Dyugovskaya et al[24] showed that the functional and morphological changes of CD4+ and CD8+ T lymphocytes in patients with OSA enhanced the function of CD8+ T lymphocytes, which became cytotoxic. This could lead to an increased incidence of cardiovascular events. Bhat et al[25] investigated the phenotype of immune cells in patients with COPD and supported the conclusion that immune dysfunction leads to exacerbations and disease severity in COPD. Our findings demonstrated that compared with the control group, the OSA, COPD, and OS groups had increased levels of CD8+ T lymphocytes and reduced levels of CD4+ T lymphocytes and CD4+/CD8+ T lymphocyte ratios. The increase in CD8+ T lymphocytes was the most significant in the OS group, which also showed the most significant reduction in CD4+ T lymphocytes and CD4+/CD8+ ratios. These results suggest that while all patients with OSA, COPD, and OS had abnormal immune functions, the condition was the most severe in patients with OS. At the same time, our study found that sVCAM-1 and TNF-α were significantly and negatively correlated with CD4+/CD8+ lymphocytes, which suggests a decrease in cellular immune function, accompanied by an increase in inflammatory response and endothelial dysfunction. As shown previously, systemic inflammatory response and cell adhesion were both important factors in endothelial dysfunction and T lymphocytes might play a role in morphological changes of the endothelial cells and vascular remodeling. Our data has shown the relationship between sVCAM-1, TNF-α, and CD4+/CD8+ lymphocytes. This result suggested T lymphocytes were related to endothelial dysfunction.
In summary, this study examined if cardiovascular complications occurred more frequently in patients with OS, as well as the possible pathogenesis underlying the phenomenon. The data showed that the prevalence of hypertension and CHD was higher in patients with OS than in patients with either OSA or COPD alone. Patients with OS exhibited more severe vascular endothelial injury, a stronger inflammatory response, and a weaker cellular immune function. These could be the mechanism underlying the high incidence of cardiovascular complications in patients with OS. In particular, the cellular immune function mediated by T lymphocytes should not be neglected, as it could be related to inflammation and vascular endothelial function. The limitation of our study is the small sample size. Although it is compliance with the statistical requirement, enlarging the sample size will improve the test efficiency. This will be achieved in our further research. And another research idea will be whether modifying T lymphocyte subsets can reduce the prevalence of cardiovascular disease in patients with OS, the elucidation of which may provide a new direction for future treatment in clinical settings.
Acknowledgement
This work was supported by a grant of the National Natural Science Foundation of China (No. 81670084).
Conflicts of interest
None.
Footnotes
How to cite this article: Wang J, Li X, Hou WJ, Dong LX, Cao J. Endothelial function and T-lymphocyte subsets in patients with overlap syndrome of chronic obstructive pulmonary disease and obstructive sleep apnea. Chin Med J 2019;132:1654–1659. doi: 10.1097/CM9.0000000000000312
References
- 1.Shawon MS, Perret JL, Senaratna CV, Lodge C, Hamilton GS, Dharmage SC. Current evidence on prevalence and clinical outcomes of co-morbid obstructive sleep apnea and chronic obstructive pulmonary disease: a systematic review. Sleep Med Rev 2017; 32:58–68. doi: 10.1016/j.smrv.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Lombardi C, Tobaldini E, Montano N, Losurdo A, Parati G. Obstructive sleep apnea syndrome (OSAS) and cardiovascular system. Med Lav 2017; 108:276–282. doi: 10.23749/mdl.v108i4.6427. [DOI] [PubMed] [Google Scholar]
- 3.Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Khayat R, Javaheri S, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol 2017; 69:841–858. doi: 10.1016/j.jacc.2016.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubinsztajn R, Przybyłowski T, Grabicki M, Karwat K, Maskey-Warzęchowska M, Batura-Gabryel H, et al. Comorbidities in chronic obstructive pulmonary disease: results of a national multicenter research project. Adv Clin Exp Med 2019; 28:319–324. doi: 10.17219/acem/78024. [DOI] [PubMed] [Google Scholar]
- 5.McNicholas WT. Comorbid obstructive sleep apnoea and chronic obstructive pulmonary disease and the risk of cardiovascular disease. J Thorac Dis 2018; 10 Suppl 34:S4253–S4261. doi: 10.21037/jtd.2018.10.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Incalza MA, D’Oria R, Natalicchio A, Perrini S, Laviola L, Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol 2018; 100:1–19. doi: 10.1016/j.vph.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Hansson GK. Inflammation atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352:429–430. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 8.Kendzerska T, Leung RS, Aaron SD, Ayas N, Sandoz JS, Gershon AS. Cardiovascular outcomes and all-cause mortality in patients with obstructive sleep apnea and chronic obstructive pulmonary disease (overlap syndrome). Ann Am Thorac Soc 2019; 16:71–81. doi: 10.1513/AnnalsATS.201802-136OC. [DOI] [PubMed] [Google Scholar]
- 9.Steveling EH, Clarenbach CF, Miedinger D, Enz C, Dürr S, Maier S, et al. Predictors of the overlap syndrome and its association with comorbidities in patients with chronic obstructive pulmonary disease. Respiration 2014; 88:451–457. doi: 10.1159/000368615. [DOI] [PubMed] [Google Scholar]
- 10.Ganga HV, Nair SU, Puppala VK, Miller WL. Risk of new-onset atrial fibrillation in elderly patients with the overlap syndrome: a retrospective cohort study. J Geriatr Cardiol 2013; 10:129–134. doi: 10.3969/j.issn.1671-5411.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castellon X, Bogdanova V. Chronic inflammatory diseases and endothelial dysfunction. Aging Dis 2016; 7:81–89. doi: 10.14336/AD.2015.0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang BY, Jin Z, Zhao Z. Long intergenic noncoding RNA 00305 sponges miR-136 to regulate the hypoxia induced apoptosis of vascular endothelial cells. Biomed Pharmacother 2017; 94:238–243. doi: 10.1016/j.biopha.2017.07.099. [DOI] [PubMed] [Google Scholar]
- 13.Attia EF, Jolley SE, Crothers K, Schnapp LM, Liles WC. Soluble vascular cell adhesion molecule-1 (sVCAM-1) is elevated in bronchoalveolar lavage fluid of patients with acute respiratory distress syndrome. PLoS One 2016; 11:e0149687.doi: 10.1371/journal.pone.0149687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kjaergaard AG, Dige A, Krog J, Tønnesen E, Wogensen L. Soluble adhesion molecules correlate with surface expression in an in vitro model of endothelial activation. Basic Clin Pharmacol Toxicol 2013; 113:273–279. doi: 10.1111/bcpt.12091. [DOI] [PubMed] [Google Scholar]
- 15.Aaron SD, Angel JB, Lunau M, Wright K, Fex C, Le Saux N, et al. Granulocyte inflammatory markers and airway infection during acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163:349–355. doi: 10.1164/ajrccm.163.2.2003122. [DOI] [PubMed] [Google Scholar]
- 16.Higashimoto Y, Elliott WM, Behzad AR, Sedgwick EG, Takei T, Hogg JC, et al. Inflammatory mediator mRNA expression by adenovirus E1A-transfected bronchial epithelial cells. Am J Respir Crit Care Med 2002; 166:200–207. doi: 10.1164/rccm.2111032. [DOI] [PubMed] [Google Scholar]
- 17.Lu W, Mehraj V, Vyboh K, Cao W, Li T, Routy JP. CD4:CD8 ratio as a frontier marker for clinical outcome, immune dysfunction and viral reservoir size in virologically suppressed HIV-positive patients. J Int AIDS Soc 2015; 18:20052.doi: 10.7448/IAS.18.1.20052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J, Song KH, Kim T, Doh J. Endothelial cell focal adhesion regulates transendothelial migration and subendothelial crawling of T cells. Front Immunol 2018; 9:48.doi: 10.3389/fimmu.2018.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imes CC, Baniak LM, Choi J, Luyster FS, Morris JL, Ren D, et al. Correlates of endothelial function in older adults with untreated obstructive sleep apnea and cardiovascular disease. J Cardiovasc Nurs 2019; 34:E1–E7. doi: 10.1097/JCN.0000000000000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blidberg K, Palmberg L, James A, Billing B, Henriksson E, Lantz AS, et al. Adhesion molecules in subjects with COPD and healthy non-smokers: a cross sectional parallel group study. Respir Res 2013; 14:47.doi: 10.1186/1465-9921-14-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitra A, Vishweswaraiah S, Thimraj TA, Maheswarappa M, Krishnarao CS, Sundararaja Lokesh K, et al. Association of elevated serum GM-CSF, IFN-γ, IL-4, and TNF-α concentration with tobacco smoke induced chronic obstructive pulmonary disease in a South Indian population. Int J Inflam 2018; 2018:2027856.doi: 10.1155/2018/2027856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Q, Zheng X. Tumor necrosis factor alpha is a promising circulating biomarker for the development of obstructive sleep apnea syndrome: a meta-analysis. Oncotarget 2017; 8:27616–27626. doi: 10.18632/oncotarget.15203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang QC, Sun X, Wang YM, Wu Q, Feng J, Chen BY. Systematic and endothelial inflammation and endothelial progenitor cell levels in emphysematous rats exposed to intermittent hypoxia. Respir Care 2015; 60:279–289. doi: 10.4187/respcare.03492. [DOI] [PubMed] [Google Scholar]
- 24.Dyugovskaya L, Lavie P, Lavie L. Lymphocyte activation as a possible measure of atherosclerotic risk in patients with sleep apnea. Ann N Y Acad Sci 2005; 1051:340–350. doi: 10.1196/annals.1361.076. [DOI] [PubMed] [Google Scholar]
- 25.Bhat TA, Panzica L, Kalathil SG, Thanavala Y. Immune dysfunction in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc 2015; 12 Suppl 2:S169–S175. doi: 10.1513/AnnalsATS.201503-126AW. [DOI] [PMC free article] [PubMed] [Google Scholar]


